Abstract
Context
Prescription drugs are instrumental to managing and preventing chronic disease. Recent changes in cost-sharing could affect access to them.
Objective
To synthesize published evidence on the associations among cost-sharing features of prescription drug benefits and use of prescription drugs, use of nonpharmaceutical services, and health outcomes.
Data Sources
We searched PubMed for studies published in English between 1985 and 2006.
Study Selection & Data Extraction
Among 923 articles found in the search, we identified 132 articles examining the associations between prescription drug plan cost-containment measures, including co-payments, tiering, or coinsurance (n = 65), pharmacy benefit caps or monthly prescription limits (n = 11), formulary restrictions (n = 41), and reference pricing (n = 16), and salient outcomes, including pharmacy utilization and spending, medical care utilization and spending, and health outcomes.
Data Synthesis
Increased cost-sharing is associated with lower rates of drug treatment, worse compliance among existing users, and more frequent discontinuation of therapy. For each 10% increase in cost-sharing, prescription drug spending falls by 2% to 6%, depending on the class of drugs and the patient’s condition. The reduction in use associated with a benefit cap, which limits either the coverage amount or the number of covered prescriptions, is consistent with other cost-sharing features. For some chronic conditions, higher cost-sharing is associated with increased use of medical services, at least for patients with congestive heart failure, lipid disorders, diabetes, and schizophrenia. While low-income groups may be more sensitive to increased cost-sharing, there is little evidence to support this contention.
Conclusions
Pharmacy benefit design represents an important public health tool for improving patient treatment and adherence. While increased cost-sharing is highly correlated with reductions in pharmacy use, the long-term consequences of benefit changes on health are still uncertain. guidance on conducting a systematic evidence review.
INTRODUCTION
Medical practice in the United States has changed dramatically in the last several decades, including an increase in use of prescription drugs. More and better-quality drugs are available to prevent and manage chronic illness, and these drugs reduce mortality, forestall complications, and make patients more productive.1 Thus, access to outpatient drugs is now a cornerstone of an efficient health care system.
But with recent increases in pharmacy spending, pharmacy benefit managers and health plans have adopted benefit changes designed to reduce pharmaceutical use or steer patients to less-expensive alternatives. The rapid proliferation of mail-order pharmacies, mandatory generic substitution, coinsurance plans, and multitiered formularies has transformed the benefit landscape. In this review, we analyze how the salient cost-sharing features of prescription drug benefits may affect access to prescription drugs and synthesize what is known about how these features may affect medical spending and health outcomes.
SCOPE OF BENEFIT CHANGES
Most beneficiaries are now covered by incentive-based formularies in which drugs are assigned to one of several tiers based on their cost to the health plan, the number of close substitutes, and other factors.2 For example, generics, preferred brands, and nonpreferred brands might have co-payments of $5, $15, and $35, respectively. In contrast, plans may require beneficiaries to pay coinsurance—ie, a percentage of the total cost of the dispensed prescription. The purpose of tiered co-payments and coinsurance is to give beneficiaries an incentive to use generic or low-cost brand-name medications and to encourage manufacturers to offer price discounts in exchange for inclusion of their brand-name products in a preferred tier. By 2005, most workers with employer-sponsored coverage (74%) were enrolled in plans with 3 or more tiers, nearly 3 times the rate in 2000 (27%).3
Some plans also impose benefit caps that limit either the coverage amount or the number of covered prescriptions. For example, the standard Medicare Part D benefit offers beneficiaries coverage of up to $2400 in spending in 2007, at which point coverage stops until beneficiaries reach a catastrophic cap ($5451). Once the catastrophic cap is reached, coverage resumes with minimal cost sharing. Prior to the introduction of Part D, benefit caps—without this catastrophic limit—were a standard feature of Medicare + Choice plans (now known as Medicare Advantage) and some retiree plans. As of 2002, 94% of Medicare + Choice plans that covered branded drugs had an annual dollar cap ranging from $750 to $2000 per year.4 Analogous policies used by state Medicaid programs place limits on the number of prescriptions dispensed per patient per month. Because benefit caps represent an extreme version of cost sharing—patients who reach them must pay all additional pharmacy costs out of pocket—and their central role in Part D, we include them in our review.
Additional cost-saving measures include prior authorization (requiring permission before certain drugs can be dispensed), step therapy (requiring use of lower-cost medications before providing coverage for more expensive alternatives), closed formularies, mandatory generic substitution, and reference pricing (a cap on the amount a plan will pay for a prescription within a specific therapeutic class). There is a growing literature on each of these cost-containment measures.
METHODS
We conducted electronic searches of PubMed for studies published in English between 1985 and 2006. The primary search was based on combinations of 2 sets of key words. The first set included various terms for drug cost sharing: cost-sharing, incentive-based, copay, coinsurance, tiered benefit, benefit cap, patient charge/fee, user charge/fee, prescription charge/fee, step therapy, reference pricing, prior authorization, formulary, formulary restriction, formulary limit, closed formulary, open formulary, and generic only. The second set included drug spending, drug cost, prescription drug, medication, and pharmacy benefit. Articles that contained at least 1 term were included. We performed another search specifically for Medicaid-related drug cost sharing measures by combining one of the terms access restriction, drug/prescription/reimbursement limit, or preferred drug list with Medicaid and with one of the terms spending, use, or cost. We excluded issue briefs, comments, letters, editorials, essays, articles without author names, and reviews. This process yielded 923 studies. We then screened these studies based on titles, abstracts, and, in a few cases, the full text, as described in the Figure.
A study was included in this review if (1) the article was published in a peer-reviewed journal; (2) it examined the effects of cost sharing (co-payments, tiers, coinsurance, reference pricing, formulary restrictions, or benefit caps) on at least 1 of the relevant outcomes (prescription drug utilization or spending, medical utilization or spending, or health outcomes); and (3) it analyzed primary or secondary data (to exclude simulations).
Among the 923 studies, 111 met these criteria. An additional 21 studies were added based on reference lists, resulting in 132 studies for final analysis. Sixty-five studies examined co-payments, tiers, or coinsurance5–69 11 examined benefit caps4, 43, 70–78 41 examined formulary restrictions79–119 and 16 examined reference pricing.120–135 (One study examined the effects of co-payments and benefit caps.43)
Because the majority of these studies analyze observational data, it is very important to understand how the researchers captured the effects of cost-sharing. We classified study designs as follows:
(Aggregated) time series. Analyzed changes over time in data aggregated at the geographic or plan level, with the data spanning a period when benefits changed;
Cross-sectional. Analyzed individual-level data at a point in time for multiple benefit designs—for example, across health plans;
Repeated cross-sectional. Analyzed cross-sectional data from multiple time periods
Longitudinal. Analyzed individual-level data with repeated observations for the same subjects over time; and
Before and after. Compared outcomes at two points in time, before and after a benefit change.
Randomized trial
The literature on cost-sharing is much more diffused than many medical interventions, which benefit from clear delineation of primary and secondary clinical endpoints. For example, some papers examine pharmaceutical spending, while others look at utilization. And, among the latter, utilization is measured in at least five different ways: the medication possession ratio, the proportion of days covered, the cumulative multiple-refill gap, number of prescriptions, and aggregate days supplied. This problem is further exacerbated by the wide range of “treatments”—adding a second or third tier, raising co-payments, requiring co-insurance, to name a few—and treated populations with very different diseases. The result is tremendous heterogeneity in benefit changes, the way results are reported, and for which affected populations. Thus, many of the conclusions of this review are necessarily qualitative.
RESULTS
Impact of Co-Payments and Co-Insurance on Pharmacy Spending
The evidence from the 656,69 studies that examined the relationship between 3 of the most important features of drug benefits—co-payments, tiering, and coinsurance—and pharmacy utilization and costs is summarized in Table 1.
Table 1.
Studies examining the impact of co-payment, tiering or coinsurance on prescription drug utilization and spending, medical utilization and spending (65 studies)
| Author | Study sample | Study design | Drug benefit variation | Outcomes | Key findings |
|---|---|---|---|---|---|
| Studies that examine the impact on prescription drug utilization only | |||||
| Andersson K et al., 2006 | Delivers of pharmaceuticals to the Swedish population from Jan 1986 to Dec 2002, at the chemical subgroups level ( Aggregated data from National Corporation of Swedish Pharmacies ) | Time series | Three national policies (Jan 1, 1991; Jan 1, 1995 and January 1, 1999) in Sweden increasing patient drug co-payment | Total defined daily doses (DDDs) Total drug costs |
Co-payment increases were not associated with changed level or slope of drug cost or volume |
| Dormuth CR, 2006 | 173,076 elderly patients with COPD or asthma in British Columbia (Pharmacy claims and administrative medical records 1997–2004) |
Before-after no control group |
Drug policies in three periods for BC elderly on patients’ prescription payment: 1) 100% dispensing fee up to an annual ceiling of Can $200 (before 2002); 2) $10 or $25 drug co-payment with annual ceilings of $200 or $275, depending on income (Jan 2002-Apr 2003); 3) 25% coinsurance + income based deductible + revised income-based annual ceilings(Since May 2003) | DDDs per 10,000 patient months Drug initiation and cessation |
Drug policy changes in 2002–2003 for BC elderly were associated with significant reductions in the use of inhaled medications (−12.3% to −5.8%), Newly diagnosed patients were 25% less likely to initiate treatment in period 2) or 3), compared to period 1). Chronic users were 47% and 22% more likely to cease treatment during period 2) and 3), compared to period 1). |
| Gibson TB et al., 2006 | 234,685 statin users continuously enrolled in a health plan during 2001–2003 (Enrollment data, pharmacy and medical claims 2001 – 2003) |
Longitudinal | Variation in statin co-payments across health plans and over time | MPR | A 100% co-payment increase lowered monthly adherence rates for statin medications by 2.6 and 1.1 percentage points among new and continuing users, respectively. Those who recently initiated satins therapy were more price-sensitive. |
| Goldman DP et al., 2006 | 62,774 adults continuously enrolled in a health plan for at least 1 year before and after initiating cholesterol therapy (Pharmacy claims and medical claims data 1997–2002) |
Repeated cross-sectional | Variation in statin co-payments across health plans | MPR |
A 100% co-payment increase lowered the fraction of fully compliant patients for cholesterol therapies by 10 to 6 percentage points, depending on patient risk. Eliminating co-payments for high- and medium- risk patients, while raising them (from $10 to $22) for low-risk patients is predicted to avert 79,837 hospitalizations and 31,411 ED visits annually among a national sample of 6.3 million adults on CL therapy. |
| Goldman DP et al., 2006 | Patients with at lest two primary diagnoses for cancer, kidney disease, Rheumatoid Arthritis(RA) or Multiple Sclerosis(MS) among 1.5 million private insurance enrollees. (Pharmacy claims and medical claims data 2003–2004) | Repeated Cross-sectional | Variation in drug coverage generosity (ratio of total out-of-pocket payments relative to total payments, for a specific drug category) across health plans and over time (2003–2004) | Rx spending | A 100% increase in effective coinsurance rate was associated with 7% decrease in MS total drug spending and 21% decrease in RA drug spending. The spending reductions for cancer drugs and Kidney disease drugs were smaller: 1% and 11% respectively and were not statistically significant. |
| Li X et al., 2006 | 8,017 elderly British Columbia residents with rheumatoid arthritis (Administrative data 2001–2002) |
Before-after no control group |
Drug policy changes for BC elderly on patients’ prescription payment: 1) 100% dispensing fee up to annual ceilings of Can $200 (before 2002); 2) $10 or $25 drug co-payment with annual ceilings of $200 or $275, depending on income (Jan 2002-Apr 2003) | Number of prescriptions filled Physician visits |
100% increase in effective drug price (the price an individual would face under the new cost-sharing policy if their consumption remained at the pre-policy level) was associated with 20% to 11% reduction in drug use, and 4% to 6% increase in physician visits, for low-income seniors and other seniors, respectively. |
| Taira DA et al., 2006 | 114,232 hypertension patients who filled prescriptions for hypertensive medications between Jan 1999 to June 2004 (Administrative data and pharmacy claims data from a MCO 1999–2004) |
Repeated cross-sectional | Three co-payment levels in a tiered formulary: $5, $20, and $20–165 | Adherence (MPR >= 0.8) | Relative to medications with a $5 co-payment, the odds ratio for compliance with drugs having a $20 co-payment was 0.76; for drugs requiring a $20 to $165 co-payment, the odds ratio for compliance was 0.48. |
| Wang J et al., 2006 | 47,115 adult prescription users in Medical Expenditures Panel Survey (MEPS) 1996–2001 | Repeated cross-sectional | Cross-sectional variation in generosity of drug benefit (share of annual drug cost paid by insurance) | Number of filled prescriptions | Non-Hispanic blacks were less likely than non-Hispanic whites to receive essential new drugs. The number of essential new drugs acquired is negatively correlated with co-payments. |
| Anis AH, et al., 2005 | 2,968 elderly British Columbia residents with rheumatoid arthritis (Pharmacy claims data and administrative medical records 1996–2000) |
Before-after no control group |
Periods before and after annual drug co-payments reached the maximum, within a calendar year | Number of filled prescriptions Hospital admissions |
Among elderly patients with rheumatoid arthritis and who had exceeded the maximum annual co-payment of Can $200 at least once during the period of 1997–2000, there were 0.38 more physician visits per month, 0.50 fewer prescriptions filled per month and 0.52 fewer prescriptions filled per physician visit, during the “cost-sharing” period, compared to the “free” period. Frequency of hospital admissions didn’t differ. |
| Contoyannis P et al., 2005 | 573, 426 elderly randomly selected from the population of Quebec Pharmacare beneficiaries from Aug 1993 – June 1997 (Administrative data 1993 – 1997) |
Before-after no control group |
Two drug policy changes in Quebec Pharmacare program: Before Aug 1996, low-income elderly had free drug coverage while other elderly paid $2 per prescription. Since Aug 1996 all paid %25 coinsurance with income-based annual ceilings. Beginning Jan 1997 a quarterly deductible was added and an annual ceiling was applied per quarter and still varied by income. | Rx spending | A 100% increase in effective drug price (the price an individual would face under the new cost-sharing policy if their consumption remained at the pre-policy level) was associated with 16% to %12% reduction in total drug spending in a given period. |
| Gibson TB et al., 2005 | 114,232 employees in two firms (Pharmacy claims and medical claims 1995–1998) |
Before–after with a control group |
Co-payment level in one firm changed from $2 to $2, $7 for generics and brand-name drugs, respectively; co-payment level in the other firm remained unchanged | Number of filled prescriptions Rx spending |
A 100% co-payment increase in brand drugs was associated with a 4% decrease in total drug use, 27% decrease in the use of multi-source brand drugs and a 32% decrease in the use of single source brands. Total drug expenditures decreased by about 10%. Enrollees with a newly diagnosed chronic condition were less price-sensitive. |
| Hansen RA et al., 2005 | 9,819 privately insured PPI users in year 1998 (Administrative claims data 1997–1998, DTC advertising expenditure data) |
Cross-sectional | Whether or not a plan has > $5 copay for a brand-name PPI prescription across multiple drug benefit plans | Rx switching | Patients paying >$5 copay for brand-name PPI prescription were 12% less likely to switch from lansoprazole to omeprazole than patients paying lower copayments. |
| Huskamp HA et al., 2005 | 36,102 children continuously enrolled for 33 months as dependents in two employer-sponsored managed care plans (Eligibility file and pharmacy claims data 1999–2001) | Before-after with control group |
One employer changed formulary from 1-tier to 3-tier and increased co-payments in all tiers. The other employer had a stable 2-tier formulary | Initiation of drug therapy Discontinuation rate Rx spending OOP and plan Rx spending |
Adding a third tier with a $30 co-payment decreased the probability that children received a drug for attention-deficit/hyperactivity disorder by 17%, decreased total medication spending by 20% and shifted more medication costs to patients. |
| Landsman PB et al., 2005 | Users of nine drug classes continuously enrolled for two years in one of four managed care plans with total members of 1,630,000 (Enrollment and pharmacy claims data 1999–2001) |
Before-after with control group |
Three plans changed from 2-tier formulary to 3-tier formulary while one plan had a stable 2-tier formulary | MPR Discontinuation rate Rx switching Number of filled prescriptions |
Patients had statistically significant decreases in MPRs in seven out of nine drug classes. A 100% co-payment increase lowered the number of monthly filled prescriptions in each of the nine drug classes. Reductions ranged from 60 percent to 10 percent. |
| Roblin DW et al., 2005 | 26,220 12-month episodes of oral hypoglycemic (OH) from 5 MCOs (Enrollment and pharmacy claims data 1997–1999) |
Time series | Variations in over time co-payment increase ($0 to $10 or more) across 5 MCOs | Standard OH average daily dose (ADD) per month | >$10 co-payment increase decreased use of oral hypoglycemic (OH) medications by 18.5%. Smaller co-payment increases had no significant effect on OH spending. |
| Briesacher B et al., 2004 | 20,868 patients with arthritis, enrolled in 32 employer-sponsored drug plans and used NSAIDs during year 2000 (Pharmacy claims, medical claims and encounter data 2000) |
Cross-sectional | Variation in drug tiers and co-payments for COX II selective inhibitors across drug plans | Probability of using COX II selective inhibitors | The odds of using COX II selective inhibitors were significantly lower (odds ratio 0.36) if their drug formulary designated COX II as only non-preferred products compared with patients with 1-tier drug coverage; Co-payments exceeding $15 were also associated with lower odds (0.49) of drug initiation, relative to co-payments of $5 or less. Such relationship persists even for patients who had GI comorbidities. |
| Crown WH et al., 2004 | 63,231 asthma patients with employer-sponsored drug plans (Pharmacy claims, medical claims and encounter data 1995–2000) |
Repeated cross-sectional | Cross-sectional variations of drug co-payments | Initiation of Rx therapy Days of supply Controller-to-Reliever ratio of asthma drugs |
The level of patient cost-sharing did not affect the use of asthma medications. However, physician/practice prescribing patterns strongly influenced patient-level treatment patterns. |
| Ellis JJ et al., 2004 | 4,802 non-Medicaid enrollees with statin prescriptions in one MCO and between Jan 1998 to Nov 2001 (Pharmacy and medical claims 1998–2001) |
Repeated cross-sectional | Cross-sectional variations of drug co-payments | Cumulative multiple refill-interval gap (CMG) Discontinuation rate |
The mediums duration for statin therapy were 3.9 years, 2.2 years, and 1.0 years for patients whose average monthly statin co-payments were <$10, $10–20, and>$20, respectively. |
| Goldman DP et al., 2004 | 528,969 privately insured beneficiaries aged 18–64 and enrolled from 1 to 4 years in one of 52 health plans (Pharmacy and medical claims data 1997–2000) |
Repeated cross-sectional | Cross-sectional variations of indexes of drug plan generosity | Days of supply | 100% co-payment increase in a two-tier plan lowered utilizations in each of 8 therapeutic classes. Reductions range from 25% to 45%. Largest reductions were for drugs with close OTC substitutes. |
| Kamal-Bahl S et al., 2004 | 149,243 hypertension patients who had prescriptions for at least one of the five classes of drugs during year 1999 (Pharmacy claims, medical claims and encounter data 1999) |
Cross-sectional | Cross-sectional variations in co-payments within 1, 2, or 3 tiered formularies | Initiation of drug therapy Rx spending OOP and plan Rx spending |
Lower likelihood of using ACE inhibitors and angiotensin II receptor blockers with co-payment differences of at least $10 between generic and brand drugs. A 100% increases in drug-co-payment was associated with a predicted decrease of 8.9% in total drug spending in a 1-tier plan. |
| Liu SZ et al., 2004 | More than 3 million prescriptions for a sample of elderly patients randomly drawn from 21 hospitals in Taipei (Administrative data 1998 – 2000) |
Before-after with control group |
Since Aug 1999, prescription drug policy in Taiwan changed from full coverage to 20% coinsurance with a maximum of $15.625 per prescription, for prescriptions costing more than $3.125. Selected groups were exempted | Average prescription cost | Compared to the non-cost sharing group, cost sharing group experienced a lower growth of average prescription cost since drug policy change. Elderly with non-chronic diseases were more price-sensitive. |
| Lurk JT et al., 2004 |
Aggregated monthly data Nov 1999 to Dec 2002 in one safety-net provider |
Before-after no control group |
Over-time change in drug co-payments | Number of filled prescriptions OOP and plan Rx spending |
An average $5 increase in co-payment was associated with reduced drug utilization and a $26.07 decrease in prescription drug cost to the clinic per visit per month, in an ambulatory care safety-net provider setting. |
| Meissner BL et al., 2004 | 8,463 beneficiaries continuously enrolled in a public employer health plan from 1998–1999 (Pharmacy claims data 1998–1999) |
Before-after no control group |
Over-time change in drug co-payments | Days of supply Number of filled prescriptions Plan Rx spending |
An average $10 co-payment increase for two classes of allergy medications was not associated with significant change in combined lower-sedating antihistamines (LSA) and nasal steroids (NS). In stead, it was associated with 13% reduction in plan drug cost for allergic rhinitis patients. Unadjusted Arc elasticity is 0.39 for LSA and −0.22 for NS. |
| Blais L et al., 2003 | 34,627 Quebec residents receiving social assistance, aged 64 or less and had any prescription for medications under study (Quebec Administrative claims data 1992–1997) |
Time series | Drug policy changed for Quebec elderly in 1996–1997: from zero or $2 drug co-pay to %25 coinsurance plus a income-based annual ceiling of $200-$925; A control group included privately insured individuals | Total number of prescriptions dispensed per month | Quebec drug policy change didn’t reduce the total monthly consumption for neuroleptics and anticonvulsants, but reduced total monthly consumption for inhaled corticosteroids by 37%. |
| Huskamp HA et al., 2003 | 151,222 enrollees covered by two employers and were users of one of the following three classes of drugs: ACE inhibitors, PPIs and statins (Eligibility file and pharmacy claims data 1999–2001) |
Before-after with control group |
Employer A changed drug co-pay from $7/$15 to $8/$15/$30; employer B changed from $6/$12 to $6/$12/$24. Enrollees from other employers with stable 2-tier benefits were chosen as control groups | Initiation of drug therapy Adherence/compliance/MPR Rx switching Discontinuation rate Rx spending OOP and plan Rx spending |
Dramatic increases in drug co-payments were associated with higher rate of discontinuation with drug therapy (21% versus 11 %) and higher Rx switching to lower cost medications (49% versus 17%), in all three drug classes. A more moderate increase in drug co-payments were associated with higher Rx switching but not higher discontinuation rates. There were no consistent effects of co-payment increase on total drug spending in three drug classes |
| Liu SZ et al., 2003 | More than 1.6 million prescriptions for a sample of elderly patients randomly drawn from 21 hospitals in Taipei (Administrative data 1998 – 2000) |
Before-after no control group |
Since Aug 1999, prescription drug policy in Taiwan changed from full coverage to 20% coinsurance with a maximum of $15.625 per prescription, for prescriptions costing more than $3.125. Selected groups were exempted | Rx spending | Imposing cost-sharing was associated with a 12.86% increase in total prescription drug costs in the cost-sharing group, mainly due to an increase in average drug costs per prescription (explaining 69.20% of the variance) |
| Nair KV et al., 2003 | 8,312 patients with chronic conditions in a managed care plan (Membership data and pharmacy Claims data 2000 – 2001) |
Before-after with control group |
Intervention group had drug benefit changing from 2-tier to 3-tier; two control groups had stable 2 or 3 tier drug benefits |
Rx switching Formulary compliance rate Discontinuation rate |
Moving from a two-tier to a three-tier drug benefit was associated with an increased use of generic drugs (6 to 8 percentage points) and formulary compliance. |
| Rector TS et al., 2003 | Pharmacy claims for three therapeutic classes (ACE inhibitors, PPIs and statins) in four independent physician practice association model health plans (1998–1999) | Before-after with control group |
Four health plans changed drug benefits from two-tiered plans to three-tiered plans in different quarters during 1998–1999 | Use of preferred brands | Moving from a two-tier to a three-tier drug benefit led to increases in the percentage use of preferred brands for ACEI, PPI and statins by 13.3, 8.9, and 6.0 percentage points, respectively, over a 21-month period. |
| Ong M et al., 2003 | Monthly drug-use data for three therapeutic classes (antidepressants, anxiolytics, and sedatives) from July 1990 through Dec 1999 in Sweden | Time series | Drug co-payment increases in 1995 and 1997 | Defined daily doses (DDD) per 1,000 inhabitants | Permanent increases in male antidepressants and sedatives occurred before the 1995 reform; only female antidepressant use was permanently reduced by the 1997 reform. |
| Artz MB et al., 2002 | 6,237 elderly covered by Medicare (Medicare Current Beneficiary Survey 1995) |
Cross-sectional | Cross-sectional variation in drug coverage generosity | Number of filled prescriptions Rx spending |
Prescription drug spending increased with drug plan generosity across a range of insurance types. |
| Joyce G et al., 2002 | 420,786 primary beneficiaries aged 18–64 with employer-provided drug benefits (Pharmacy and medical claims data 1997–1999) |
Repeated cross-sectional | Cross-sectional variations of drug benefits (number of tiers, co-payments and coinsurance rates) | Rx spending OOP and plan Rx spending |
Doubling co-payments decreased annual drug spending by 22% to 33% and increased the fraction beneficiaries paid out-of-pocket from 17.6% to 25.6% in a two-tier plan. |
| Pilote L et al., 2002 | 22,066 Quebec elderly patients who experienced acute myocardial infarction between 1994–1998 (Quebec Administrative claims data 1994–1998) |
Before-after no control group |
Drug policy changed for Quebec elderly in 1996–1997: from zero or $2 drug co-pay to %25 coinsurance plus a income-based annual out-of-pocket maximum of $200-$925 | Initiation of drug therapy Medication persistence Rx switching Hospital admissions, ED visits and physician visits Mortality |
Quebec drug policy change didn’t reduce use for essential cardiac medications among Quebec elderly who experienced acute myocardial infarction, nor medical utilizations. The findings did not vary by sex or socioeconomic status. |
| Thomas CP et al., 2002 | 29,435 elderly with employer-based drug benefit plans for retirees (Pharmacy claims data 2001) |
Cross-sectional | Variation in drug formulary tiers, co-payment and coinsurance rates across 96 health plans | Number of filled prescriptions Rx switching Prescription size(mail/retail) Rx spending OOP Rx spending |
Increased patient cost-sharing and formulary restrictions were associated with lower drug spending, higher out-of-pocket costs, and a shift to lower cost medications (generics and mail-order). |
| Blais L et al., 2001 | 259,616 Quebec elderly residents who had any prescription for the medicines under study during the whole study period: Aug 1992 to Aug 1997 (Quebec Administrative claims data 1992–1997) |
Time series | Drug policy changed for Quebec elderly in 1996–1997: from zero or $2 drug co-pay to %25 coinsurance with maximum OOP payment ceiling, plus a income-based annual out-of-pocket maximum of $200-$925 | Total number of prescriptions dispensed per month | Quebec drug policy change didn’t reduce total number of prescriptions dispensed per month for nitrates, antihypertensive agents, benzodiazepines, and anticoagulants. |
| Kozyrskyj AL et al., 2001 (a) | 10,703 school-aged children in Manitoba who had asthma (Administrative data Apr 1995 – Apr 1998) |
Before-after with control group |
Before Apr 1996, Manitoba’s drug benefit program required a fixed deductible payment of $237 per family plus 40% co-payment on prescription costs above $237. Since April 1996 this policy was replaced by income-based deductibles with low-income family pay up to 2% of their income as deductible and high-income family pay up to 3%. | Initiation of drug therapy Number of prescription filled |
Implementation of income-based deductible in Monitoba’s drug benefit policy was associated with a decrease in the use of inhaled corticosteroids by high-income children with severe asthma and did not improve use of these drugs by low-income children |
| Kozyrskyj AL et al., 2001 (b) | 12,481 school-aged children in Manitoba who had asthma (Administrative data July 1995 – March 1998) |
Repeated cross-section | Same as Kozyrskyj AL et al., 2001 (a) | Initiation of drug therapy | In comparison with higher-income children with asthma, odds ratio of receiving inhaled corticosteroid prescriptions was 0.82 – 0.88 for low-income children with asthma, controlling for asthma severity, type of drug insurance, or health care utilization patterns. |
| Hillman AL et al., 1999 | 134,937 non-elderly enrollees of nine managed care plans (Pharmacy claims data 1990–1992) |
Repeated cross-section |
Variation of drug co-payments both across and within health plans | Initiation of drug therapy Days of supply Rx spending |
Higher co-payments for prescription drugs were associated with lower drug spending in independent practice associations (IPAs) but not in network models where physicians bear financial risk for prescription drug costs. |
| Motheral BR et al., 1999 | 3,184 individuals continuously enrolled in commercial plans from 1996 to 1997 (Pharmacy claims data 1996–1997) |
Before-after with control group |
Enrollees in two different employer plans experienced brand copay increase from $10 to $15, while those in the control group had brand copay of $10 during the study period | Initiation of drug therapy Number of filled prescriptions Rx switching Discontinuation rate Rx spending OOP and plan Rx spending |
Increasing the co-payment from $10 to $15 was associated with lower use of brand drugs, lower plan drug spending and lower total ingredient costs. But There was no statistically significant difference in overall utilization or discontinuation rates for chronic medications. |
| Stuart B et al., 1999 | 1,302 elderly and disabled Medicaid recipients (Medicare Current Beneficiary Survey 1992) |
Cross-sectional | Variation of drug co-payments across state Medicaid programs | Initiation of drug therapy Number of prescriptions filled OOP Rx spending |
Imposing $0.50 - $3.0 drug co-payments in state Medicaid programs reduced drug use among elderly and disabled Medicaid recipients by 15.5% in 1992. Primary effect of co-payments is to reduce the likelihood of any prescription filling (by 7.7 percentage points). Those reporting poor health status were most adversely affected by co-payments. |
| Grootendorst PV et al., 1997 | 5,743 Ontario residents aged 55–75 (Survey data) |
Cross-sectional | Discontinuity in drug benefit availability: the provision of first-dollar prescription drug insurance coverage for Ontario residents at age 65 | Initiation of drug therapy Number of prescriptions filled |
The provision of first-dollar prescription drug insurance coverage at age 65 increased drug use, primarily among individuals with lower levels of health status. Most of the increased use was due to the increased level of use among drug users rather than an increase in the probability of use. |
| Hong SH et al., 1996 | 3,144 children enrolled in five drug benefit plans during Dec 1992 to Dec 1993 (Pharmacy claims and enrollment database 1992–1993) |
Cross-sectional | Variations in drug co-payment and cost-sharing differentials between generic and brand name drugs across five drug benefit plans | Rx initiation Number of filled prescriptions Rx spending OOP Rx spending |
Higher levels of cost-sharing per prescription were associated with higher drug utilization. Larger cost-sharing differentials between generic and brand name drugs were associated with higher rates of generic drug use but were not always associated with lower expenditure rates |
| McManus P et al., 1996 | Summary statistics on total number of prescriptions (Administrative data 1987–1994) |
Time series | In Nov 1990 in Australia’s Pharmaceutical Benefits Scheme (PBS), patient contribution increased from $A11 to $A15 for the general population. In Jan 1992, a $A2.50 co-payment was required for returned service men and women. | Total monthly number of prescriptions | Increasing drug co-payment was associated with decreased level of drug consumption, but not associated with a changing trend, for both the general population and the returned service men and women. The effect was larger for “discretionary drugs”, relative to “essential drugs”. |
| Coulson NE et al., 1995 | 4,508 elderly Medicare beneficiaries in Pennsylvania (Survey data linked with administrative claims data 1989) |
Cross-sectional | Variation in drug coverage generosity by different insurance types among the elderly. | Number of prescriptions filled | low-income elderly (<$12,000 single or <$15,000 married) in Pennsylvania were covered by the program of Pharmaceutical Assistance Contract for the Elderly (PACE) and only paid $4 per 30 day dosage. Enrollees of the PACE program had 0.29 more prescriptions per two-week period than did elderly who had no prescription drug coverage. |
| Hughes D et al., 1995 | Monthly statistics in England in 1969–1992 (Published government statistics) |
Time series | Over-time variation of drug co-payments in UK National Health Service | Number of non-exempt dispensed prescriptions per year per capita |
A 10% increase in prescription charge was associated with a 3.2% decrease in per capita utilization of drugs within the non-exempt category |
| Smith DG et al., 1993 | Aggregated data on use and costs of prescription drugs for 212 employer-groups covered by one managed care company in 1989 | Cross-sectional | Variation of drug co-payments ($1 to $8) across employer-groups | Number of filled prescriptions Rx spending OOP and plan Rx spending |
Increasing co-payments from $3 to $5 was associated with a 5% decrease in the number of filled prescriptions and a 10% decrease in employer drug spending. |
| Ryan M et al., 1991 | Monthly statistics in England in 1979–1985 (Published government statistics) |
Time series | Over-time variation of drug co-payments in UK National Health Service | Number of non-exempt dispensed prescriptions per month per capita Rx spending |
10% increase in prescription drug charge was associated with 1% reduction in per capita drug utilization during the period of 1979 – 1985. Approximately two thirds of the government expenditure savings were due to reduction in utilization as opposed to increased charges on per item of drugs. |
| Harris BL et al., 1990 | 43,146 beneficiaries continuously enrolled in an HMO for a four-year period (Administrative pharmacy data 1982–1986) |
Before-after with control group |
The intervention group experienced co-payment rates of $1.50, $1.30, $3,00 plus other benefit changes during a three-year period while the control group of the same plan had no drug co-payment during the whole period | Number of filled prescriptions Rx spending |
Graduated increases in drug co-payments (from $0 to $1.50 to $3) plus other formulary restrictions were associated with 10% to 12% reductions in the number of prescriptions and 6.7% reduction in per capita drug costs |
| Lavers RJ 1989 | Monthly statistics in England and Wales in 1971 – 1982 (Published government statistics) |
Time series | Over-time variation of drug co-payments in UK National Health Service | Number of non-exempt dispensed prescriptions per month | 10% increase in prescription drug charge was associated with a 2.0% to 1.5% fall in the monthly volume of non-exempt items during the period of 1971–1982, |
| O’Brien B, 1989 | Monthly statistics in England in 1969–1986 (Published government statistics) |
Time series | Over-time variation of drug co-payments in UK National Health Service | Number of non-exempt dispensed prescriptions per month | 10% increase in prescription drug charge was associated with a 3.3% fall in the volume of non-exempt items during the period of 1969–1986, the reduction was 2.3% in the sub-period 1969–1977 and 6.4% in the later period 1978–1986. Cross-price elasticity on exempted items was |
| Foxman B, 1987 | 5,765 non-elderly enrollees who participated the entire second year of RAND HIE on the fee-for-service plans in six sites | Randomized trial | Participants were randomly assigned into health plans with %0, %25, %50, %95 coinsurance rates or an individual deductible plan | Number of filled prescriptions | People with free medical care used 85% more antibiotics than those required to pay some portion of their medical bills. |
| Birch S 1986 | Annual statistics in NHS in 1979 – 1983 (Published government statistics) |
Time series | NHS patient charges on pharmaceuticals increased from 1979 to 1983. Part of the population were required to pay the charges while others were exempted from the charges. | Number of items dispensed per capita per year | During the period of 1979–1982, the per capita consumption of prescriptions in non-exempt group decreased by 7.5% while the per-capita consumption in exempted group increased by 1%. |
| Leibowitz A et al., 1985 | 3,860 non-elderly enrollees who participated the entire first year of RAND HIE on the fee-for-service plans in three sites | Randomized trial | Participants were randomly assigned into health plans with %0, %25, %50, %95 coinsurance rates or an individual deductible plan | Number of filled prescriptions Rx switching Samples from physicians Rx spending |
Consumers facing a 95% coinsurance rate for prescription drugs (up to a maximum dollar expenditure) spent 57% as much as those in a free-care plan. |
| Reeder CE et al., 1985 | 62,176 Medicaid recipients in South Carolina (claims data 1976–1979) |
Time series | Change in Medicaid outpatient drug co-payments since Jan 1977: from $0.0 to $0.50 per prescription | Rx spending | Imposing a $0.50 co-payment for outpatient prescriptions covered by South Carolina Medicaid programs had differential effects on the utilizations of drugs in ten various therapeutic categories. Drug utilizations dropped immediately after the co-payment increase in eight out of ten classes (except analgesics and sedatives/hypnotics). In the long-term the utilization trends in four therapeutic classes were significantly changed after the co-payment increase. |
| Studies that also examine the impact on medical utilization and spending | |||||
| Cole JA et al., 2006 | 12,776 CHF patients taking ACE inhibitors, Beta blockers or both in 2002 (Claims data 2002, 2003) |
Cross-sectional | Variation in drug co-payments across health plans | Medication possession ratio (MPR) Total medical costs CHF-related hospitalizations |
A $10 increase in drug co-payment is associated with 2.6% and 1.8% decreases in MPRs for patients taking ACE inhibitors and Beta blockers, respectively. Such decreases were associated with predicted increases of CHF-related hospitalizations by 6.1% and 8.7%. Predicted total medical costs were not affected. |
| Gibson TB et al., 2006 | 117,366 statin users continuously enrolled in a health plan during 2000–2003 (Pharmacy and medical claims data 2000–2003) |
Repeated cross-sectional | Variation in statin co-payments across health plans | MPR Hospital admissions ED visits Physician visits |
A $10 increase in co-payment resulted in a 1.8 and 3.0 percentage point reduction in adherence, among new and continuing statin users, respectively. For continuing users, higher statin adherence was associated with lower negative events (hospital admissions and ED visits) but not with total costs. |
| Mahoney JJ et al., 2005 | Diabetes-related claims and drug use and cost statistics in one company (2001–2003) | Before-after no control group |
One company reduced coinsurance rates on diabetes drugs to 10% (from 25% to 50%) in Jan 2002. | Adherence Rx spending Rx + Medical spending ED visits |
From 2001 to 2003, adherence and use of fixed-combination therapy had increased among diabetes patients. Average total pharmacy costs had decreased by 7% and overall medical costs decreased by 6%. ED visits had decreased by 26%. |
| Winkelmann R, 2004 | 37,319 individuals in Germany (Survey data 1995–1999) |
Before-after with control group |
Co-payment for prescriptions increased by 6 DM in 1997. Certain groups were exempted from such an increase and served as the “control” group | Physician visits | In Germany, An additional 6 DM prescription fee reduced the number of doctor visits by 10% on average. |
| Fairman KA et al., 2003 | 7,709 enrollees in a PPO (Pharmacy and medical claims data 1997–2000) |
Before-after with control group |
Enrollees in the intervention group experienced a formulary change from 2-tier to 3-tier; Enrollees in the control group had stable 2-tier formulary | Number of filled prescriptions Rx Continuation rate Rx spending OOP and plan Rx spending Hospitalizations, ED visits and ambulatory visits |
Moving from a two-tier to a three-tier drug benefit was associated with reduced growth in plan cost and lowered utilization of non-formulary medications, but not associated with lower growths of total prescription claims or total drug spending. The associations between adding tiers and drug continuation rates were mixed for four classes of chronic medications. Such drug benefit change was not associated with number of hospitalizations, ED visits or office visits |
| Balkrishnan R et al., 2001 | 2,411 Medicare HMO enrollees in 1998 and 1999 (Data source unknown) |
Before-after no control group |
In 1998, co-payments were $7/$15, for generics and brand names, separately, with quarter OOP maximum of $200. In 1999, there was unlimited coverage for generics and limited coverage for brand drugs. | Plan Rx spending Plan Rx + medical spending Physician visits |
Changing to a drug policy with unlimited coverage for generics and limited coverage for brand drugs was associated with 27% decrease in plan drug costs, 4% decrease in physician visits and 5% decrease in plan total costs |
| Motheral BR et al., 2001 | 20,160 individuals continuously enrolled in a PPO from Jan 1997 to Dec 1999. (Pharmacy and medical claims data 1997–1999) |
Before-after with control group |
Enrollees of the intervention group had drug benefit changed from 2-tier to 3-tier. Those in the control group has stable 2-tier benefit | Initiation of drug therapy Number of filled prescriptions Rx Discontinuation rate Rx spending OOP and plan Rx spending Hospitalizations, ED visits and ambulatory visits |
Moving from a two-tier benefit with co-payments of $7/$12 to a three-tier benefit with co-payments of $8/$15/$25 was associated with slower growth in prescription drug utilization and drug spending (15% vs. 22%). Adding tiers were not consistently associated with medication discontinuation rates of four chronic therapy classes, and not associated with hospitalizations, ED visits or office visits |
| Tamblyn R et al., 2001 | 149,283 Quebec residents 65 years and older or receiving welfare (Administrative data 1993–1997) |
Time series | Drug policy changed for Quebec elderly in 1996–1997: from zero or $2 drug co-pay to %25 coinsurance plus a income-based annual out-of-pocket maximum of $200-$925 | Mean daily drug use Serious adverse events (acute care hospitalizations, long-term care admission, or death) |
Quebec drug policy change was associated with a 9% and 14% reduction in use of essential drugs, for elderly and welfare recipients respectively. Such reductions were associated with increased number of serious adverse events and ED visits. Use of less essential drugs decreased by 15% and 22%. |
| Berndt ER et al., 1997 | 3,470 privately insured individuals from 26 plans treated for depression in 1993 (Medical and pharmacy claims 1993) |
Cross-sectional | Variations of drug copayment across 26 health benefit plans | Initiation of drug therapy Hospitalizations |
Among depressed patients receiving outpatient treatment, higher prescription drug copayment was associated higher share of SSRI utilizations in all anti-depressant medications; higher drug copayment was not associated with higher probability of hospitalizations |
| Johnson RE et al., 1997(a) | Elderly HMO members during a four-year period (Administrative data 1987–1991) |
Before-after with control group |
Two Medicare risk groups in an HMO setting had their co-payments and coinsurance rates increased in different years during a three-year period | Initiation of drug therapy Days of supply Rx spending Health status index |
Graduated increases in co-payments from $1 to $5 and coinsurance (from 50% to 70%, with a $25 max) did not reduce prescription drug utilization and costs in a consistent manner among each of twenty-two therapeutic drug classes. Health status may have been adversely affected as measured by Combined Chronic Disease Score and Diagnostic Cost Groups. |
| Johnson RE et al., 1997(b) | Elderly HMO members during a four-year period (Administrative data 1987–1991) |
Before-after with control group |
Two Medicare risk groups in an HMO setting had their co-payments and coinsurance rates increased in different years during a three-year period | Number of filled prescriptions Rx spending OOP Rx spending Hospitalizations, ED visits and ambulatory visits Rx + Medical spending |
Graduated increases in co-payments from $1 to $5 and coinsurance (from 50% to 70%, with a $25 max) resulted in lower prescription drug uses and expenses, and did not affect medical care utilization and expenses in a consistent manner. |
| Lingle EW et al., 1987 | 9,966 elderly Medicare beneficiaries and not eligible for Medicaid benefits (1975 and 1979) (Medicare claims data 1975,1979) |
Before-after with control group |
The intervention group includes Medicare beneficiaries covered by New Jersey’s Pharmaceutical Assistance for the Aged (PAA); the control group includes beneficiaries in eastern Pensylvania | Medical utilization Plan medical spending |
Re-imbursement for in-patient care for New Jersey’s PAA recipients was on average 238.50 lower than that in eastern Pensylvania. There was no significant increase in total medical costs reimbursed by Medicare among New Jersey’s PAA recipitents. |
Most of the evidence comes from observational studies, with the exception of two studies of the RAND Health Insurance Experiment (HIE). The HIE randomized 2,750 families to different levels of cost-sharing ranging from free care to 95% coinsurance. The HIE demonstrated that individuals subject to higher coinsurance rates reduced their demand for care, and that the cost sharing response for prescription drugs was similar to the response for all ambulatory care.66, 68 However, data from the HIE are more than three decades old. In addition, the health insurance packages in the HIE did not vary by prescription drug benefit. Thus, it is unclear whether the higher drug expenditures among people with more generous coverage were due to lower out-of-pocket costs for drugs or lower cost-sharing for office visits and other medical services that are the usual pathways for receiving prescriptions.
All the remaining studies are observational. Key features of the best studies include large sample sizes, variation in benefit design both across plans and over time, and attempts to control for other factors known to affect pharmaceutical use.19, 20, 22–24, 27, 36, 38, 42 Of particular value are studies that employ data from multiple plans and control for medical benefits, especially when they may be changing in concert with the pharmacy benefit.8, 9, 26, 34, 40, 49, 50, 52 For example, changing office visit co-payments will affect how frequently patients see the doctor, and hence the number of prescriptions they receive. Poor features include analyses that do not control for other factors that might be changing, including most of the designs looking before and after a benefit change with no control group. These designs include (but are not limited to) many international studies where co-payments change for the entire population.5,7,12,15,16,29,30,35,37,41,44,57,59,61,64,65,67
Some of the studies find relatively small changes in utilization in response to higher cost-sharing,17, 51, 55, 60, 62, 69 but these focused on small changes in co-payments. In some studies, the control groups have very different characteristics,28, 32, 39, 45, 46, 53, 67 patients may self-select into the treatment group on the basis of medication choice,13 or the source of co-payment variation is not clear.25 Given the evidence that there is differential response by condition or class of medication, studies that restrict attention to a specific patient population or conduct subgroup analysis can yield additional insight.
The effects of cost-sharing can be summarized using the price elasticity of demand. This measure represents the percentage change in drug spending that would be associated with a 1% increase in cost-sharing. When we exclude the studies that involve very small cost-sharing changes or do not have an adequate control group, we find elasticites ranging from −0.2 to −0.6, indicating that cost-sharing increases of 10% (either through higher co-payments or coinsurance) would be associated with a 2% to 6% decline in prescription drug use or expenditures.
Eleven of the 65 studies in our review explicitly looked at changes in coinsurance rates, 28,32,35,41,42,44,48,54,55,66,68,57 with two of these coming from the HIE and 4 from a benefit change in Quebec in 1996. Overall, the impact of increasing co-insurance is at the low end of our range of −0.2 to −0.6; but these effects are clearly muted by the simultaneous imposition of out-of-pocket maximums in most of these studies.
Differential Responses by Therapeutic Class
Several studies suggest that consumer sensitivity to cost-sharing depends upon a drug’s therapeutic class and that increased cost-sharing may decrease “non-essential” drug use —e.g., antihistamines—more than “essential” drug use such as anti-hypertensives and oral hypoglycemics. However, the empirical evidence in this area is mixed. Harris et al 62 found substantially larger reductions in the use of discretionary medications than essential medications in response to a modest increase in co-payments. More recently, Goldman et al 26 found that doubling co-payments was associated with lower use of eight classes of medication by 25% (antidiabetics) to 45% (antiinflammatories). Patients were less likely to reduce use of these drugs if they were receiving ongoing care from a physician for the disorder, ranging from 8% (antidepressants) to 31% (antihistamines). Landsman et al found similar price responses across nine therapeutic classes.20 Several other studies found modest, but inconsistent, effects of higher co-payments on use of essential and non-essential drug classes.33, 47, 50, 55, 69
Benefit Caps, Prescription Drug Use, and Costs
Information from the 11 studies4,43,70,78 that examined the association between benefit caps, including caps that limit the number of prescriptions and caps on an annual pharmacy benefit, and drug use and drug costs is summarized in Table 2.
Table 2.
Studies examining the impact of benefit caps on prescription drug utilization and spending, medical utilization and spending (11 studies)
| Author | Study sample | Study design | Drug benefit variation | Outcomes | Key findings |
|---|---|---|---|---|---|
| Studies that examine the impact on prescription drug utilization only | |||||
| Tseng CW et al., 2004 | 1,308 Medicare managed care enrollees in 2001 whose drug benefits were capped and annual spending exceeded annual caps of $750 or $1,200 (Survey data 2002) |
Cross-sectional | Variations in annual drug benefit caps across counties | Under-use due to cost Rx switching |
Medicare + Choice beneficiaries exceeding their annual drug benefit cap were more likely than those that did not exceed the cap to switch medications (15% vs. 9%), use samples (34% vs. 27%) and report difficulty paying for prescriptions (62% vs. 37%). |
| Tseng CW et al., 2003 | 438,802 Medicare managed care enrollees in 2001 whose drug benefits were capped at $750, $1,000 or $2,000. (Pharmacy claims data 2001) |
Cross-sectional | Levels of annual drug benefit caps: $750, $1,000, or $2,000. | % exceeding benefit caps OOP Rx spending |
A total of 22%, 14%, and 4% of Medicare patients exceeded annual drug benefit caps of $750, $1,000, and $2,000, respectively. |
| Cox ER et al., 2002 | 212 Medicare+Choice beneficiaries with capped annual prescription drug benefits of $500 or $1,000 in year 2000 (Survey data) |
Cross-sectional | Capped drug benefits | Adherence Discontinuation |
For those who exceeded their cap prior to Oct 2000, they were more likely to stop taking one or more medications or took less than prescribed amount, after reaching the cap, compared to the pre-cap period. But these differences were not statistically significant. |
| Balkrishnan R et al., 2001 | 259 Medicare HMO enrollees in 1997–1998 (Data source unknown) |
Before-after no control group |
Change of drug benefit policy from 1997–1998: benefit cap increased from $500 per year to $200 per quarter; and co-payments changed from $6/$12 to $7/$15, for generics and brand names, separately | Plan Rx spending Plan Rx + medical spending |
Drug benefit cap from $500 per year to $200 per quarter and increased drug co-payments were associated with a 29% increase in plan Rx costs and 38% increase in total plan costs. |
| Cox ER et al., 2001 | 378 Medicare HMO enrollees who had reached >=60% of their prescription drug cap in 1997 (Survey data) |
Cross-sectional | Capped drug benefits ($750 for rural counties, $1,500 for urban counties) | Initiation of drug therapy Adherence/compliance/MPR Rx switching |
Those who reached their prescription cap were more likely to reduce drug use (OR, 2.83), to discontinue a medication (OR, 3.36), and to obtain samples from their physician (OR, 2.02), compared to those who had not reached their cap. |
| Fortess EE et al., 2001 | 343 chronically ill Medicaid enrollees (Pharmacy claims data) |
Before-after no control group |
A state Medicaid program imposed a three-prescription monthly reimbursement limit(12 months pre- and 6 months after policy change) | Standard monthly doses for essential medications | A three-prescription monthly reimbursement limit (cap) in the New Hampshire Medicaid program was associated with a 34.4% reduction in the use of essential medications. |
| Martin BC et al., 1996 | 743 Medicaid enrollees (Pharmacy claims data 1991–1992) |
Time series | A state Medicaid program reduced monthly reimbursement limit of prescriptions from six to five (6 months pre- and 6 months after policy change) | Number of filled prescriptions Rx spending OOP and plan Rx spending |
Reducing the maximum number of monthly reimbursable prescriptions from 6 to 5 was associated with a 6.6% reduction in total prescriptions among Georgia Medicaid beneficiaries with high use of prescription drugs. |
| Soumerai SB et al., 1987 | 10,734 Medicaid enrollees (Pharmacy claims data 1980–1983) |
Time series | New Hampshire Medicaid program imposed a three-prescription monthly reimbursement limit (cap) on Sep 1981, but later discontinued the policy (20 months pre-policy change; 11 months post-policy change; 17 months after the limit was replaced by $1 co-payment). | Number of filled prescriptions | A three-prescription monthly reimbursement limit (cap) in the New Hampshire Medicaid program was associated with a 30% reduction in the number of prescriptions filled. Utilization approached pre-cap levels after the cap was replaced with a $1 co-payment. |
| Studies that also examine the impact on medical utilization and spending | |||||
| Hsu et al., 2006 | 199,179 Medicare managed care enrollees in 2003 (Administrative data 2003) |
Cross-sectional | In 2003, 157,275 Medicare + Choice enrollees had annual drug benefit capped at $1,000; Another 41,904 enrollees had unlimited drug coverage due to employee supplements | Adherence Rx and medical spending Hospitalizations, ED visits, and ambulatory visits Blood pressure, LDL, Glycated hemoglobin, and mortality |
Subjects facing a $1,000 Rx benefit cap had 31% lower pharmacy costs, higher rates of drug non-adherence (Odd ratios of 1.27 to 1.33), ED visits (RR 1.09), non-elective hospitalizations (RR 1.13), and death (RR 1.22). Their total medical costs were not significantly different from those without Rx benefit cap. |
| Soumerai SB et al., 1994 | 2,227 Medicaid enrollees with Schizophrenia (Pharmacy and medical claims data 1980–1983) |
Time series | New Hampshire Medicaid program imposed a three-prescription monthly reimbursement limit (cap) on Sep 1981, but later discontinued the policy (14 months pre-policy change; 11 months post-policy change; 17 months after the limit was replaced by $1 co-payment). | Days of supply Plan Rx spending Plan medical spending Ambulatory visits Hospitalizations |
A three-prescription monthly reimbursement limit (cap) in the New Hampshire Medicaid program was associated with an immediate reduction (ranged 15% to 49%) in the use of psychotropic drugs, a significant increase in the use of emergency mental health services and partial hospitalization, but not associated with hospital admissions. Drug and medical utilizations approached pre-cap levels after the cap was replaced with $1 co-payment. |
| Soumerai SB et al., 1991 | 1,786 Medicaid enrollees who in a baseline year had been taken 3 or more prescriptions per month (Pharmacy and medical claims data 1980–1983) |
Time series | New Hampshire Medicaid program imposed a three-prescription monthly reimbursement limit (cap) on Sep 1981, but later discontinued the policy. | Days of supply Nursing home admissions Hospitalizations |
A three-prescription monthly reimbursement limit (cap) in the New Hampshire Medicaid program was associated with a 35% reduction of drug utilizations, increased risk of nursing home admissions, but not with hospitalizations, among older patients (60 and older) and who were frequent Rx users. |
Soumerai et al 77 compared Medicaid patients in New Hampshire—for whom the program had imposed a three-drug limit per patient per month—with those of New Jersey where no such cap was introduced. They found a 35% reduction in drug use relative to the control group. For patients on psychotropic medications, they found the cap was associated with a 15% to 49% reduction in the use of these drugs.76
The most salient evidence on the impact of benefit caps comes from an analysis of medical and pharmacy claims from a single, closed-network health maintenance organization.70 Members whose benefits were capped at $1,000 had 31% lower pharmacy costs than comparable enrollees not subject to a cap. One survey of Medicare beneficiaries suggest that elderly individuals who experience gaps in coverage report using fewer medications, are more likely to switch to generics or lower cost medications, and rely more on drug samples from their physicians.71 Two other studies found that patients exceeding the cap were two-to-three times more likely to discontinuation a medication 73 and unenroll from the plan.136
Reference Pricing
Information from the 16 studies121,135 examining reference pricing, wherein insurers cap the amount they will pay for a prescription within a specific therapeutic class, is summarized in Table 3.
Table-3.
Studies examining the impact of reference pricing (RP) on prescription drug utilization and spending, medical utilization and spending (16 studies)
| Author | Study sample | Study design | Drug benefit variation | Outcomes | Key findings |
|---|---|---|---|---|---|
| Studies that examine the impact on prescription drug utilization only | |||||
| Mabasa VH et al., 2006 | PPI prescriptions for Canadians with private employer-sponsored drug plans (Claims data June 2002-May 2005) | Before-after with control group |
One employer group adopted reference pricing for PPIs since June 2003, while other employer groups didn’t have reference pricing for PPIs through the whole study period | Number of days supplied Rx spending |
Introduction of reference-based pricing for PPIs in one employer in Canada reduced plan spending on PPIs by approximately 26%. Less than one third of the reduction was attributed to average price of PPIs and more than two thirds to a decline of utilization of PPIs. |
| Grootendorst PV et al., 2005 | British Columbia Pharmacare for the elderly (Aggregated data 1993–2001) |
Time series | Pharmacare introduced two types of RP for NSAIDs: Type I in Apr 1994 and Type II in Nov 1995. Under Type I RP, generic and brand versions of the same NSAIDs were exchangeable, under Type II RP, different NSAIDs were considered interchangeable. | Rx plan spending | Imposing reference pricing among all NSAIDS (Type 2 RP) achieved more savings compared to a reference pricing among each NSAID(Type 1 RP). After Type 2 RP, annual plan expenditures for NSAID were cut by $4 million (50%). Most savings accrued from the substitution of low-cost NSAIDs for most costly alternatives. About 20 percent of savings represented expenditures by seniors who paid cost-sharing NSAIDs. |
| Schneeweiss S et al., 2004 | British Columbia Pharmacare for the elderly (Aggregated data 1995–1998) |
Before-after no control group |
Introduction of reference pricing to ACE inhibitors in elderly BC residents in 1997 | Rx plan spending | RP to ACE inhibitors in elderly BC residents was associated with savings of CAN $6 million among continuing users and $0.2 million among new users, during the first year of the implementation. Approximately five sixths were achieved by utilization changes and one sixth by cost shifting to patients. There were no savings through drug price changes |
| Marshall JK et al., 2002 | BC Pharmacare beneficiaries (Aggregated data 1993–1999) |
Time series | Introduction of reference pricing to H2RAs and special authority for PPIs in elderly BC residents in 1995 | Number of defined daily doses per 10,000 beneficiaries OOP and plan drug spending per 10,000 beneficiaries |
RP for H2RAs and special authority for PPIs reduced plan expenditures by $1.8 to $3.2 million per year for H2RAs and $5.5 million per year for PPIs. Beneficiary contributions for H2RAs increased from negligible amount to approximately 16% of total drug expenditures |
| Schneeweiss S et al., 2002 | 119,074 BC Pharmacare beneficiaries who used ACE inhibitors from 1995–1998 (Administrative data 1995–1998) |
Longitudinal | Introduction of reference pricing to ACE inhibitors in elderly BC residents in 1997 | Number of prescriptions Plan Rx spending Rx switching Discontinuation rates |
RP for ACE inhibitors was associated with 11% reduction in use of all ACE inhibitors. But the use of overall antihypertensives was unchanged. The policy saved $6.7 million in pharmaceutical expenditures for existing users during its first 12 months. Relative to high-income patients, patients with low-income status were more likely to stop all antihypertensive therapy (OR 1.65) |
| Aronsson T et al., 2001 | Quarterly time-series data on prices and quantities for twelve brand-name drugs and their generic substitutes from 1972–1996 | Time series | Introduction of reference pricing in 1993 which specifies that any costs exceeding the price of the least expensive generic substitute by more than 10% must be borne by patients | Market share of brand-name drugs Relative price of brand-name versus generics |
Introduction of reference pricing was negatively associated with market shares for three brand-name drugs while positively associated with market shares for other two. Reference pricing was also associated with decreased relative price of brand-name versus generics. |
| Grootendorst PV et al., 2001 | BC Pharmacare for the elderly (Aggregated data 1994 – 1999) |
Before-after no control group |
Introduction of reference pricing to nitrates in elderly BC residents in 1995 | Monthly total number of prescriptions Plan and OOP Rx spending |
During three and a half years after introduction of RP for nitrates, BC Pharmacare expenditures on nitrates for the elderly declined by $14.9 million. Most of these savings were due to the lower prices that Pharmacare paid for restricted nitrates. Prescribing of reference-standard nitrates increased immediately after the policy was introduced but later dropped after nitroglycerin patch was exempted from additional charges. $1.2 million of the savings represented expenditures by senior citizens who bought restricted nitrates. There were no compensatory increases in expenditures for other anti-angina drugs |
| McManus P et al., 2001 | Prescriptions in Australia which were under government subsidy (Claims data 1990, 1994 and 1999) |
Before-after no control group |
Introduction of minimum pricing policy in Australia in 1990 and generic substitution policy in 1994 | Rx switching | After implementation of minimum pricing, share of generic drugs increased from zero in 1990 to 17% in 1994. Generic substitution policy further increased the share to 45% in 1999. |
| Narine L et al., 2001 | BC Pharmacare for the elderly (Aggregated data 1994–1996) |
Before-after no control group |
In 1995, the BC Pharmacare introduced a reference-based pricing (RBP) system for H2 antagonists, nitrates, and NSAIDs | Plan Rx spending Total number of prescriptions Rx switching |
Introduction of RBP was associated with a 44% decrease in Pharmacare drug costs. Total number of prescriptions for H2 antagonists and nitrates decreased by 5.2% and 2.5%, respectively. A significant number of patients were switching from one drug to the other after introduction of RBP. |
| Narine L et al., 1999 | BC Pharmacare (Aggregated data 1994 – 1996) |
Before-after no control group |
Introduction of reference pricing to H2RAs in elderly BC residents in 1995 | Annual total number of prescriptions Plan Rx spending |
In the year following the introduction of RP for H2RAs, the total number of prescriptions decreased by 5.2%, the market share of reference drug increased by 410%. Pharmacare expenditures for Histamine-2 receptor antagonists decreased by 38%. There was no substantial changes in drug prices |
| Jonsson B., 1994 | Swedish reimbursement system for drugs (Aggregated data 1992–1993) | Before-after no control group |
Introduction of a reference price system on Jan 1993 | Plan Rx spending OOP Rx spending |
During the first three months of the introduction of a reference system in Sweden, relative to the same period in the previous year, there was a slight decrease (1.6%) in total expenditure for the reimbursement scheme (NSIB) but a 14% increase for patient co-payments. |
| Studies that also examine the impact on medical utilization and spending | |||||
| Schneeweiss S et al., 2006 | 5 million BC elderly residents (Administrative data Jan 2002 to June 2004) | Longitudinal | Beginning at 2003, BC Pharmacare program only covered one PPI: rabeprazole, and imposed access restrictions on three leading PPIs | Defined daily doses per month Rx discontinuation rates Rx spending Gastrointestinal hemorrhage rates |
Within 6 months after policy change, 45% of all PPI users switched to the covered PPIs, and the provincial health plan saved at least Can $2.9 millions. There were no increased use of H2 blockers, discontinuation of gastroprotective drugs or hospitalizations for hemorrhage. |
| Schneeweiss S et al., 2004 | 5,463 patients covered by British Columbia Pharmacare with at least one prescriptions for a nebulised respiratory drug in the preceding 12 months (Administrative data Sep 1997 - Aug 1999) |
Randomized controlled trial Observational time series |
Since March 1999, Pharmacare restricted reimbursement for nebulised respiratory medications to patients with doctor’s exemption. Patients in the intervention group in a randomized control trial were not subject to this restriction for six months. | Rx utilization Rx spending Contacts with doctors and services Emergent admissions to hospitals |
both in the randomized control trial and the observational analysis found that restricting reimbursement for nebulised respiratory drugs was not associated with increase of unintended health outcomes, |
| Schneeweiss S et al., 2003 | 61,763 elderly British Columbia residents who were dihydropyridine CCBs users and covered by Pharmacare (Administrative data 1995–1997) |
Longitudinal | Introduction of reference pricing to dihydropyridine CCBs in elderly BC residents in 1997 | Median monthly doses Rx switching Hospital admissions ED visits Admissions to long-term care facilities |
RP to dihydropyridine CCBs was associated with increased use of fully-covered dihydropyridine CCBs and reduced total medical costs by Canadian $1.6 million in the first 12 months of implementation. Overall antihypertensive use did not decline, and there were no increases in hospitalizations, ED visits or long-term care admissions. |
| Hazlet TK et al., 2002 | 20,000 British Columbia Pharmacare beneficiaries exposed to Histamine-2 receptor antagonists (H2RAs) and other antisecretory drugs from 1993 through 1996 (Administrative data 1993–1996) |
Longitudinal | Introduction of reference pricing to H2RAs in elderly BC residents in 1995 | Number of prescriptions filled Hospital visits ED visits Hospital admissions Length of hospital stay |
RP to H2RAs in elderly BC residents was not associated with worsening health outcomes among antisecretory drug users. |
| Schneeweiss S et al., 2002 | 37,362 BC Pharmacare beneficiaries who used selective ACE inhibitors before the RP policy for ACE inhibitors (Administrative data 1995–1998) |
Longitudinal | Introduction of reference pricing to ACE inhibitors in elderly BC residents in 1997 | Hospital admissions ED visits Admissions to long0term care facilities RX plan spending |
RP for ACE inhibitors was not associated with cessation of treatment or changes in the rates of visits to physicians, hospitalizations, admissions to long-term care facilities, or mortality. Net savings were estimated to be $6 million during the first 12 months of reference pricing |
Few health plans in the U.S. have adopted reference pricing so far. However, it is widely used in parts of Canada and Europe. In general, almost all of the studies find large increases in use of drugs priced at or below the reference price and sharp declines in use of higher cost drugs that require some patient cost-sharing. In a series of studies of reference pricing in British Columbia (BC), Schneeweiss and colleagues found that an increase in copayments for the most expensive angiotensin-converting enzyme (ACE) inhibitors (drugs priced above the “reference price”) was not associated with stopping treatment for hypertension or higher health care utilization.123, 128, 129 They found similar effects on use of calcium channel blockers125 and proton pump inhibitors.121 The only potential concern raised by these studies was that low-income patients were more likely to stop hypertensive therapy than high- income patients (odds ratio, 1.65; 95% confidence interval, 1.43–1.89). Grootendorst and colleagues122, 131 examined similar policies for non-steroidal anti-inflammatory drugs (NSAIDs) and nitrates. They found that most of the savings to BC’s Pharmacare program could be explained by the substitution of low cost drugs and higher patient cost-sharing for restricted medications. The remaining studies listed in Table 3 found that reference pricing was only weakly correlated with overall use within the restricted drug class and uncorrelated with medical service use.
Prior Authorization and Formulary Restrictions
Increasingly, public and private health plans are imposing prior authorization and/or fail-first requirements on non-preferred prescription drugs. These programs require use of older or less expensive medications before covering newer therapies. For example, a plan may require a patient to try at least one generic non-steroidal anti-inflammatory drug before paying for a cox-2 inhibitor. The main concern about these cost containment policies is that patients may switch to less effective medications or become noncompliant, and, as a result, experience adverse health effects. Several studies, as summarized in Table 4, support such concerns. Two of the studies found that Medicaid beneficiaries taking a restricted statin medication filled fewer prescriptions and were more likely to be non-adherent than unrestricted patients.79, 84 Similar effects were found for antihypertensive medications.95 Another study found that a preferred drug list for cardiovascular medications was associated with an increase in outpatient visits in the first six months of implementation, but these differences did not persist over time.93
Table 4.
Studies examining the impact of prior authorization or formulary restrictions on drug utilization and spending, medical utilization and spending (41 studies)
| Author | Study sample | Study design | Drug benefit variation | Outcomes | Key findings |
|---|---|---|---|---|---|
| Studies that examine the impact on prescription drug utilization only | |||||
| Abdelgawad T et al., 2006 | Aggregated Measures (at county level) about prescriptions for Statins filled between Apr 2003 and May 2005 and paid by Medicaid in six states (Retail Pharmacy transaction records 2003–2005) |
Before-after with control group |
Three state Medicaid programs implemented Preferred Drug Lists (PDLs) for statins in Feb-Apr 2004 while the other three states didn’t. | Number of prescriptions filled |
Imposing PDLs for statins associated with reduced Medicaid prescription fills for statins |
| Carroll NV et al., 2006 | 104,568 fee-for-service patients enrolled in Medicaid programs of two states during year 2002 to 2003 |
Before-after with control group |
The state of Missouri initiated a prior authorization program for COX-2 inhibitors while the Medicaid program of a controlled state didn’t. | Rx spending Number of prescriptions filled |
Initiating a PA program for COX-2 inhibitors resulted in reduced use and expenditures for COX-2 inhibitors and reduced net expenditures for all pain and GI-protective medications. These effects were greatest for patients at low risk for GI complications |
| Dunn JD et al., 2006 | 191,002 HMO enrollees (Administrative claims data 2004–2005) |
Before-after with control group |
Step-therapy for generic antidepressants was implemented in an HMO in Jan 2005 |
Days of supply Rx spending |
Requiring HMO members to use a generic antidepressant as the first-line therapy reduced spending for antidepressants by 9%. The decrease of use of antidepressants was 1.5%, smaller than the 5% decrease in a comparison group. |
| Kahan NR et al., 2006 | Prescriptions for cefuroxime during 9 months in a managed care organization in Isreal | Before-after no control group |
One MCO initiated PA program for cefuroxime and later revoked the program | Number of prescriptions filled | Implementation of a prior authorization requirement significantly reduced the proportion of cefuroxime in antibiotic prescriptions (From 8% to 1.2%). After the revocation of the PA program the proportion rose to 4.3%. |
| Ridley DB et al., 2006 | 13,517 statin users covered by Medicaid programs in North Carolina and Alabama (Retail Pharmacy transaction records 2001–2005) |
Before-after with control group |
Alabama Medicaid program implemented PDL for statins in 2004 while North Carolina Medicaid program didn’t. | Discontinuation rate Rx switching |
Implementation of PDL for statins in Alabama Medicaid program was associated with higher non-adherence rate for statin users (odds ratio 1.82). In addition, patients taking restricted statins and elderly patients were more likely to be non-adherent (odds ratios were 1.42 and 1.33, respectively). |
| Roughead EE et al., 2006 | Utilizations of COX-II inhibitors and non-selective NSAID for 35 State Medicaid programs (quarterly aggregated data from CMS 1996–2003) | Before-after with control group |
Some State Medicaid programs implemented PA programs for COX II inhibitors at different time (market entry or two years after market entry), while others didn’t implement such a PA program. | Defined daily doses per 1000 population per day | States implementing prior –authorization (PA) policy for Cox II inhibitors at market entry had the lowest use of uptake (10.9 DDD/1000/d); states implementing PA more than two years after market entry experienced 40% drop in utilization (23.0 to 13.9 DDD/1000/d); states that never restricted access had the highest utilization, averaging 29.0 DDD/1000/d |
| Spence MM et al., 2006 | 1,624 Kaiser Permanente elderly patients Who had a diagnosis of COPD and received at least 1 prescription for COPD-related medication during 2003 (Survey data) |
Cross-sectional | Cross-sectional variations in types of pharmacy benefit: generic-only, single copayement tier and 2 copayment tiers | Adherence Discontinuation rate |
COPD patients with generic-only benefits are significantly more likely to report that they had taken less than the prescribed amount of medication(Odds ratio = 1.70) and that they stopped taking one or more of their regular medications (Odds ratio = 1.77) |
| Tseng CW et al., 2006 | 611 elderly Medicare managed care enrollees (Survey data 2002) |
Before-after no control group |
Elderly enrollees in a Medicare managed care plan in one state had $2,000 capped brand name benefits in 2001 and generic-only drug coverage in 2002 | Rx switching Discontinuation rate |
Generic-only drug coverage decreased medication use and increased switching rates |
| West DS et al., 2006 | 127,495 State employees (Claims data Dec 2002-May 2005) |
Before-after no control group |
Coverage of omeprazole OTC and an increase in pharmacy reimbursement for omeprazole were implemented on March 2004 in a State employee health plan | Days of supply Plan Rx spending |
Coverage of omeprazole OTC and an increase in pharmacy reimbursement for omeprazole resulted in a 38% savings to a state employee health plan, despite a 6% increase in PPI utilization. |
| Cunningham PJ et al., 2005 | 3,200 Medicaid enrollees (Community tracking survey 2000, 2001, 2003) |
Repeated cross-sectional | Variations of Medicaid cost-containment strategies across states and over time | Initiation of a drug therapy | Medicaid cost-containment strategies, including prior authorization, step therapy, generic mandates, co-payments and spending limits, reduced the probability of receiving a drug |
| Lichtenberg FR, 2005 | Medicaid and Non-Medicaid prescriptions (Pharmacy claims data 2001, 2003) |
Time series |
Variations of Medicaid drug access restrictions (PDLs) across states; with the non-Medicaid prescriptions as the control group | Use of innovative drugs | Medicaid restrictions such as a preferred drug list (PDL) increased the average age or "vintage" of prescribed drugs in 6 therapeutic classes. |
| Virabhak S et al., 2005 | Prescriber-level and payer/prescriber level data on prescriptions for four states (2002 and 3003) |
Time series | Two states (Illinois and Louisiana) implemented PDL in 2002–2003, while two other states (New York and Mississippi) didn’t. | Share of off-PDL drugs | Introduction of PDL was associated with 67.7% to 40.5% decrease for off-PDL Medicaid prescriptions, and 6.8% to 8.6% decreases for off-PDL prescriptions in the third party insurance market, for the states of Illinois and Louisiana, respectively. For physicians whose practices were more than 50% Medicaid, average third party shares of off-PDL products fell by 37.5%. |
| Wilson J et al., 2005 | 5,798 Medicaid enrollees in one state (Pharmacy claims data 2000–2003) |
Before-after with control group |
One state Medicaid program implemented a PDL on June 2002 | Discontinue rate Rx switching Medications added-on |
A preferred drug list (PDL) in a state Medicaid program increased discontinuation rates of antihypertensive medications (OR 1.39) compared to 1 year earlier. |
| Fischer MA et al., 2004 | NSAID prescriptions covered by 50 state Medicaid programs (Aggregated data 1999–2003) |
Time series | 22 states implemented PA programs for selective COX II inhibitors during the study period | Rx spending Proportion of Coxibs uses among NSAIDs |
Prior authorization for selective COX II inhibitors reduced the proportion of NSAID doses made up of coxibs by 15% and decreased the cost per NSAID prescription by $10.28. |
| Harris BN et al., 2004 | 28,162 State employees (Administrative claims data Jan 2004 – Apr 2004) |
Before-after no control group |
Drug benefit change of one state Employee Benefit Division since March 2004: inclusion of omeprazole OTC in drug coverage nad an increase in pharmacy reimbursement for omeprazole | Plan and OOP Rx spending Rx switching |
Coverage of omeprazole OTC and an increase in pharmacy reimbursement for omeprazole lowered the average copayment for a PPI by $4.20. Total costs of PPI drugs for Arkansas State Employee Benefit Division were reduced by as much as 50%. OTC omeprazole represented 60% of all PPI claims within 2 months of the change. |
| Motheral BR et al., 2004 | 20,000 enrollees in one employer-sponsored drug plan and a comparison group with 1.9 million members who were commercially insured. (Claims 2001–2003 and mailed survey) |
Before-after with control group |
The intervention group implemented 3 step-therapy programs for PPIs, SSRIs and NSAIDs in Sep 2002. A random sample of members from commercial plans without step-therapy programs | Per-member-per-month (PMPM) net cost Experience with step-therapy |
A step-therapy program covering 3 drug classes was associated with a reduction in plan drug spending by $0.93 per member per month. Under this program, 30% of patients received a generic drug, 23% were granted a medical exception for the brand, 17% received no medication and 16% paid the full retail price. |
| Campbell CA et al., 2003 | Elderly enrollees in Nova Scotia Seniors’ Pharmacare Program in Canada (Administrative data 1999 to 2001) |
Time series | Beginning at Apr 2000, all but two combination topical corticosteroid products were removed from the covered list | Number of prescriptions filled Plan Rx spending |
Prescribing of topical corticosteroid combination products decreased after the formulary restriction while prescribing of preferred potent topical corticosteroid increased during the same period. |
| Huskamp HA et al., 2003 | The Veterans Health Administration (VHA) aggregated monthly market share data for six drug classes, monthly VISN-level price data and aggregated spending data for each drug product in these classes (1995–1999) | Time series | Change of formulary status overtime - closed, preferred or open – for a certain drug. | Market share Rx spending |
Imposing a closed formulary on certain drug classes was effective at shifting prescribing behaviors toward the selected drugs, achieving lower drug prices from manufacturers, and greatly decreasing drug spending. |
| Wang YR et al., 2003 | PPI prescriptions in three states (Claims data 2000 – 2001) |
Time series | Maine Medicaid drug program implemented restricted formulary for PPIs, with pantoprazole as the only preferred drug. New Hampshire and Vermont both had open formulary for Medicaid programs and serve as control states | Pantoprazole’s market share of all PPI prescriptions by month | Restricting coverage of PPIs to pantoprazole in Maine’s Medicaid drug program was associated with 72% increase in Pantorazole share among Medicaid prescriptions. For each 10% increase in Medicaid share of preactice, pantoprazole’s market share increased 1.8% among cash prescriptions and 1.4% among third-party payer prescriptions. |
| Mccombs JS et al., 2002 | 6,409 treatment periods for California Medi-cal recipients with a diagnosis of major depressive disorder from Sep 1994 to Jan 1999 (Pharmacy claims 1994–1999) |
Before-after no control group |
The California Medi-cal removed prior authorization restrictions for 2 SSRIs in May 1996 | Therapy completion rate Rx switching |
Removing prior-authorization restrictions for 2 selective SSRIs was associated with reduction in the likelihood of completed therapy without increasing in switching. |
| Motheral BR, 1999 | 5,890 government employees (Pharmacy claims data 1996–1998) |
Before-after with control group |
An employer plan implemented a closed formulary in July 1997, while the other employer plan had no drug benefit change during the study period | Initiation of drug therapy Number of filled prescriptions Discontinuation rate Rx spending |
A closed formulary was associated with slower growth in drug utilization and spending and lower rates of medication continuation with chronic conditions in the 9 months following implementation. |
| Streja DA et al., 1999 | 187 patients who were newly prescribed SSRIs and are enrollees of two HMOs (Administrative pharmacy data and chart review in physicans’ office 1996–1997) |
Cross-sectional | 82 patients were in a HMO with a single preferred SSRI while 105 were in the other HMO with 2 preferred SSRIs | Discontinuation rate | Patients from the HMO with a single preferred SSRI(paroxetine) were 80% less likely to complete therapy than were patients from the HMO with 2 preferred SSRIs (fluoxetine and paroxetine) |
| Phillips CR et al., 1997 | Iowa Medicaid drug PA program (Monthly prescription claims summaries 1990–1992, 1995; Program operation records during a two-week period in 1995) |
Before-after no control group |
In 1992–1993, Iowa Medicaid drug program initiated PA. | PA pperational performance Rx spending |
82.9% of new and extension PA requests were approved for coverage. The total net savings (savings in drug spending minus PA administrative costs) for four classes of drugs ranged from $2.51 million to $3.83 million. |
| Jones DL et al., 1996 | NSAID prescriptions at two military medical centers (1992–1994) Questionnaire to 203 clinicians |
Before-after 21 month trial with one study site and two control sites | One site implemented NSAID prescribing protocol requiring a trial of either ibuprofen or indomethacin before new prescription of more expensive NSAIDs. There were two control sites | Proportion of expensive NSAIDs prescribed Total NSAID costs Clinician acceptance |
Quarterly use of expensive NSAIDs fell from 34% to 21%, decreasing total NSAID costs by 30%, while one control site experienced 5% decrease and the other had a 2% increase. Surveyed clinicians reported very few protocol-reported patient problems |
| Kotzan JA et al., 1996 | Medicaid and cash prescriptions in Georgia (Pharmacy claims data Jan – Mar 1994) |
Cross-sectional | Georgia Medicaid drug program initiated PA before year 1994. Private-paid prescriptions are considered as the control group | Market share of PA products | Controlling for age and gender, the odds ratio of private-pay patients getting a PA prescription is 2.26, relative to Medicaid patients. |
| Kreling DH et al., 1989 | Prescriptions for internal analgesic products dispensed to Medicaid recipients during Apr – June 1984 and Apr – June 1985 (Pharmacy claims data 1984 – 1985) |
Before-after no control group |
Since Feb 1985, Wisconsin Medicaid drug program no longer covered propoxyphene napsylate | Rx spending | Removing propoxyphene napsylate from covered drug list was not associated with decreased expenditures for internal analgesic drugs, measured either by overall expenditures or per recipient expenditures. |
| Studies that also examine the impact on medical utilization and spending | |||||
| Ackman ML et al., 2006 | 112 elderly patients who received a coronary setnt between Sep 1, 2001 and Aug 31, 2002 at one hospital and who were eligible for Alberta Blue Cross coverage in Canada | Before-after no control group |
A prior authorization (PA) process for patients prescribed clopidogrel following stent insertion was changed to an authorized prescriber (AP) list process in Mar 2002 | Initiation of drug therapy Medical utilization |
Patients in the PA period were less likely to have their prescriptions filled on the day of charge (31% versus 54%) and the median time to fill was longer (4 days versus 0 days); the filling rate after 28 days of discharge was not significantly different between two periods. Two repeated revascularization procedures were necessary within six weeks after stent placement, both in PA period patients who delayed or failed to fill their prescription. |
| Delate T et al., 2005 | More than 1.2 million Medicaid enrollees (Pharmacy and medical claims data 2001 to 2003) | Time series | Medicaid program required that PA to be obtained for all PPI prescriptions since Feb 2002 | Plan Rx spending Rx switching Plan medical spending |
In the month immediately following the implementation of the prior authorization for PPIs, Medicaid spending for PPIs reduced by 91% while spending for Histamine2-receptor antagonist (H2RAs) increased by 223%. Enrollees who received an H2RAs or no antisecretory drugs were no more likely to have incurred greater total medical care expenditures than those who received a PPI during the year following the 6-month post-policy period. |
| Gleason PP et al., 2005 | 737 COX-2 inhibitor users who were continuously enrolled in an employer-sponsored health insurance plan (Pharmacy and medical claims data 2002–2003) |
Before-after no control group |
A PA program for COX II inhibitors was implemented on Jan 2003. (Study period included 3-month pre- and 12-month after- policy change) | Initiation of a drug therapy Rx spending Medical spending |
For patients denied coverage for COX II inhibitors after implementation of a PA program, pharmacy costs declined without an increase in gastrointestinal-related medical cost. |
| Murawski MM et al., 2005 | 3,250 Medicaid and 3,788 non-Medicaid cardiovascular patients in one state (Pharmacy and medical claims data 2001–2003) |
Before-after with control group |
One state Medicaid program implemented a PDL on June 2002 | Hospital visits Physician visits |
A preferred drug list (PDL) in one state Medicaid program was associated with increased outpatient hospital visits and physician visits among cardiovascular patients during the first 6-month of implementation. But such an increase became insignificant during the second 6 months after PDL implementation |
| Christian-Herman et al., 2004 | 957,500 Medicare HMO enrollees (Administrative data 2001–2002) | Before-after with control group |
In 2002, part of the enrollees in a Medicare HMO had drug benefit changed to generic-only coverage, while other enrollees in the same HMO continued to have drug benefits in 2001 | Number of filled prescriptions Switching rate Adherence/compliance/MPR Hospitalizations Rx spending Plan and OOP Rx spending |
Generic-only drug coverage was associated with lower health plan pharmacy costs, increased hospitalizations and lower quality measures for some conditions. |
| Hartung DM et al., 2004 | Monthly aggregated claims data for one Medicaid managed care organization (Pharmacy and medical claims 1999–2000) |
Time series | Implementation of a PA program for celecoxib in one Medicaid managed care organization during the 22-month study period | Days of supply Hospitalizations ED visits Ambulatory visits |
After the implementation of Prior-authorization policy for celecoxib, use of colecoxib was immediately reduced by 50% and the monthly rate of increase was also reduced. No important changes in use of other related drug classes were detected. No significant changes in medical service utilizations were found. |
| Cromwell DM et al., 1999 | Quarterly summary from Medicaid drug claims and eligibility data (1989–1993) Acute care hospital discharge abstract data (1989–1993) |
Before-after no control group |
Beginning Aug 1991, the Florida Medicaid program initiated a policy restricting reimbursement for anti-ulcer medicines | Number of prescriptions reimbursed Peptic-related hospitalizations |
Restricting Medicaid reimbursement for anti-ulcer drugs was associated with 33% reduction in quarterly number of prescriptions reimbursed. No associated increase occurred in the rate of Medicaid peptic-related hospitalizations. |
| Horn SD et al., 1998 |
12,997 HMO enrollees (Data collected in a clinical practice improvement study in 1992) |
Cross-sectional | Variations of formulary restrictions across six HMOs | Number of filled prescriptions Hospitalizations, ED visits Ambulatory services |
Formulary restrictions were associated with higher rates of ED visits and hospital admissions for most conditions. |
| White AC et al., 1997 | Use of antimicrobials in a urban, county teaching hospital in July-December period in 1993 and 1994 (Administrative data) |
Before-after no control group |
In Jan 1994 the hospital implemented a prior authorization program for selected parenteral antimicrobial agents | Rx spending Susceptibilities to antibiotics |
Implementing a PA program for selected antimicrobials reduced total parenteral antimicrobial expenditures by 32% and improved susceptibilities to antibiotics without compromising patient outcomes or length of hospital stay. |
| Smalley WE et al., 1995 | 495,821 Tennessee Medicaid recipients (Pharmacy and medical claims data 1988–1991) | Before-after no control group |
The Tennessee Medicaid program required PA for NSAIDs since Oct 1989. | Number of prescriptions filled Rx switching Plan Rx spending Plan medical spending |
Prior-authorization requirement for NSAID was associated with a 53% decrease in Medicaid expenditures for NSAIDs. The reduction resulted from the increased use of generic NSAIDs (generic rate increased from 43% to 79%), as well as from a 19 percent decrease in overall NSAID use. There was no concomitant increase in Medicaid expenditure for other medical use. |
| Kotzan JA et al. 1993a | 80,064 Medicaid NSAID patients from Jan 1989 to July 1990 (Pharmacy and medical claims data 1989–1990) |
Time series | The Georgia Medicaid program initiated a prior authorization program for single-source NSAIDs beginning Jan 1990 | Rx spending Physician visits Medical spending |
Total costs for NSAID therapy decreased by more than $3 million during the first 7 month of a PA program for NSAIDs. No additional medical or physician costs were observed for the seven months of the program |
| Kotzan JA et al. 1993b | 39,604 continuously eligible H2 antagonist recipients (Pharmacy and medical claims data 1989–1990) |
Time series | The Georgia Medicaid program imposed a maintenance dosage program for 4 single-source H2 antagonist products beginning Jan 1990 | Physician visits Inpatient and outpatient claims GI endoscopic procedure claims |
Total costs for H2 antagonist drugs were reduced by $1.4 million during the first 7 month of a maintenance dosage program for 4 single-source H2 antagonist products. No significant change in medical utilizations was observed. |
| Moore WJ et al., 1993 | 47 State Medicaid programs (Summary statistics from National Pharmaceutical Council 1985–1989) |
Cross-sectional | 20 state Medicaid programs initiated restricted formulary before 1985 while the others had open formulary. | Plan Rx spending Plan medical + Rx spending |
Restricted formulary in state Medicaid programs was associated with lower per-capita drug spending, but not associated with lower total Medicaid spending. |
| Kozma CM et al., 1990 | 12,139 prescription drug users continuously enrolled in South Carolina Medicaid program for two years (Data source unknown 1983 – 1986) |
Longitudinal | On Oct 1984, South Carolina Medicaid Drug program changed from a restrictive drug formulary to a non-restrictive drug formulary | Number of Rx claims Physician visits Outpatient visits Hospitalizations |
Changing a restrictive drug formulary to an open formulary was associated with increased number of Rx claims, physician visits and outpatient visits, and reduced number of hospitalizations. |
| Bloom BS et al., 1985 | Patients with peptic ulcer diseases in West Virginia Medicaid program (Pharmacy and medical claims1981–1983) |
Before-after no control group |
Since March 1982, a closed drug formulary was imposed in the West Virginia Medicaid program | Plan Rx spending Physician payments Hospital inpatient costs |
Introduction of a closed formulary was related to 79% decrease in drug costs, 3.1% increase in physician payments and 23% increase in hospital costs |
Most other studies, in contrast, suggest that the outcomes associated with prior authorization and step therapy requirements are modest, although these policies can have strong associations with use of restricted medications. For example, Tennessee Medicaid’s expenditures for NSAIDs declined by 53% following implementation of prior authorization and fail-first requirements for brand-name NSAIDs.113 The reduction in spending was associated with higher use of generic NSAIDs and a 19% decline in overall NSAID use. The findings of Kotzan et al114,115 were similar. More generally, prior authorization programs are associated with lower drug spending within the restricted class but are uncorrelated with medical care utilization and spending.90,91,99 Two studies on step therapy82,111 also reported decreased drug spending without adverse effects on drug utilization or physician concerns.
The outcomes associated with closed formularies or generic-only drug coverage may differ substantially from those of prior authorization requirements. One study found that a closed formulary was associated with lower rates of medication continuation among patients with chronic conditions.106 Two other studies found that limiting coverage to generic drugs was associated with decreased medication use87 and increased hospitalizations.96 Another study108 found that the degree of formulary restrictions was positively correlated with higher drug costs, more office visits, and high likelihood of hospitalization among patients with certain diseases.
Drug Cost-Sharing, Medical Costs, and Health Outcomes
The evidence clearly demonstrates that increased cost-sharing is associated with lower pharmaceutical use. These effects can be quite large—even for chronic medications—suggesting there will be long-term health consequences. In fact, the direct evidence on the link between cost-sharing and health is rather limited. Most studies examine important proxies for health (and medical spending) such as emergency department use and hospitalizations. The findings from studies focusing solely on the chronically-ill are unambiguous: for patients with congestive heart failure,6 lipid disorders,8, 10 diabetes,21 and schizophrenia,76 greater use of inpatient and emergency medical services are associated with higher copayments cost-sharing for prescription drugs or benefit caps. These findings are corroborated by the one paper that looked at clinical outcomes for a population with benefit caps.70
By contrast, studies that look at the effects of cost-sharing more broadly (on all drugs or a wide range of classes)—are ambiguous in their findings. Some find that higher cost-sharing is associated with adverse outcomes,137 particularly among vulnerable populations such as the elderly and poor.48, 77 But most find that—when the population is not limited to certain chronic illnesses—the effects of prescription drug cost containment policies are mostly benign. For example, studies by Fairman et al,33 Motheral et al,47 Johnson et al 54 and Smith and Kirking 138 found that increased co-payments were not associated with more outpatient visits, hospitalizations, or emergency department visits.
Socioeconomic Differences and Cost-Sharing
Although there is ample evidence that the demand for pharmaceuticals declines with higher co-payments, there is concern that low-income beneficiaries will be more responsive to cost sharing. Most evidence on this point comes from nonexperimental studies of state Medicaid programs that introduced very modest co-payments. Medicaid enrollees in South Carolina used significantly fewer drugs after the imposition of a $0.50 co-payment.69 A more recent study found that elderly Medicaid recipients residing in states with co-payment provisions consumed fewer drugs and were less likely to fill any prescriptions during the year than those in states without co-payments.51 Survey data indicate that 1 in 4 Medicaid patients aged 18 to 64 years could not afford to fill at least 1 prescription in the past year compared with less than 1 in 10 among privately insured individuals.139 A study of Medicare beneficiaries in Pennsylvania found that elderly individuals with annual incomes of more than $18 000 were 18% more likely to treat medical problems with prescription drugs than those with incomes of less than $6000.140
DISCUSSION
We reviewed studies examining the impact of co-payments and other salient benefit features on pharmaceutical utilization and spending, as well as their impact on non-pharmaceutical services and health outcomes. The evidence summarized here suggests that for each 10% increase in cost-sharing, overall prescription drug spending falls by 2% to 6%, depending on the class of drugs and the patient’s condition. Benefit changes are not without consequences: for some chronic conditions, we found that higher cost-sharing for prescription drugs was associated with greater use of expensive medical services.
It is interesting to compare these effects with other interventions designed to improve use of chronic medications. A 2002 JAMA review, for example, identified 33 interventions designed to improve patient adherence to prescribed medications.141 Even the most successful interventions did not result in large improvements in adherence, and generally relied on complicated, labor-intensive regimens of uncertain effectiveness. Thus, pharmacy benefit design represents one of the most important public health tools for improving patient treatment and adherence.
Several key research issues remain unresolved. First, while greater cost-sharing is clearly associated with reduced access, the precise mechanisms are not clear. Less pharmaceutical use could come about through three different behavioral pathways: reduced initiation of prescription drug treatment, worse compliance among existing users, or more frequent discontinuation of therapy (Although the latter could be interpreted as an extreme example of poor compliance among users). Distinguishing between these hypotheses is important because it affects the advice and monitoring that physicians and plans should use to counteract any adverse consequences of plan design changes. We found evidence that all three may be complicit when cost-sharing rises, although compliance among existing users seems to be the primary mechanism. On the other hand, if one accepts the criteria in current national guidelines, then even small affects of cost-sharing on the likelihood of initiating therapy could have dramatic health consequences. Topol,142 for example, notes that 36 million people in the United States should be taking a statin, but only 11 million are currently being treated.
Second, increased cost-sharing is associated with adverse medical events such as hospitalizations and worsening clinical outcomes over one to two years for patients with congestive heart failure, lipid disorders, diabetes, and schizophrenia. Additional evidence suggests there may be adverse consequences for asthma as well. Because patients leave employers and plans with relative frequency—and plan benefit designs change rapidly—it is difficult to isolate the long-term health consequences of changes in cost-sharing using existing study designs. A key challenge in this type of analysis is the fact that disease severity cannot be measured directly and that people who are more severely ill tend to use more drugs and more of other services. If this tendency is not properly accounted for in the data analysis, estimates of the effects of prescription drug use on other costs will demonstrate little or no cost savings. In fact, this spurious correlation probably has limited past efforts in this area. This is especially problematic when patients have a choice of drug plans—a feature which introduces bias in the same way that patients self-select into treatment regimens. Some of this bias is mitigated because, while many employers offer employees a choice of medical plans, the vast majority standardize on one drug benefit regardless of medical plan choice. Ultimately, though, the long-term consequences of benefit changes remain elusive.
Third, if cost containment policies have adverse effects, those effects are likely to be magnified among low-income groups, whose high rate of chronic health problems and low incomes may result in more price-sensitive behavior. Recent survey data indicate that nearly 40% of chronically ill low-income people with public insurance and 35% with private coverage were unable to fill at least one prescription because of cost concerns.143 One of the severe limitations of analyses with claims data is that they do not include information on race, ethnicity, income, education, and wealth, and, when economic status is included using national survey data, there is substantial bias in its measurement.144 Thus, while it is often claimed that low-income groups are most sensitive to cost-sharing changes, there is little reliable evidence to support this contention.
Fourth, the introduction of Medicare Part D has initiated a bold experiment with benefit caps. While benefit caps clearly act in a manner consistent with other cost-sharing features, little is known about the dynamics of these changes. For example, if patients do discontinue therapy or take their medicine less frequently once they hit their benefit cap, how quickly—if ever—do they reinitiate drug therapy once coverage resumes in the next benefit year? Benefit caps also provide a counterpoint to consumer-directed health plans that encompass high-deductible catastrophic coverage. With these plans, patients must pay all the costs until a cap is reached, beyond which they pay nothing. Benefit caps, in contrast, provide coverage up to a specified limit. A comparison of these financing alternatives is needed, especially with regard to how they might affect those with chronic illness.
Fifth, there has been a dramatic increase in the use of specialty drugs—i.e., agents targeting a gene or protein that are typically injected or infused. They are often used to treat complex, chronic conditions such as anemia, cancer, growth hormone deficiency, and multiple sclerosis, but at prices that can be substantially higher than traditional medications. Historically only a small percentage of plan members suffered from these conditions, and so the total population of specialty drug users was quite small. However, costs of specialty drugs are expected to increase substantially in the near future as new drugs enter the market for the treatment of diabetes, osteoporosis, and rheumatoid arthritis—diseases that affect much larger populations. Many insurers are contemplating a variety of cost-sharing strategies to control their use and costs. There is some evidence that patients are less price-responsive for these products than for traditional oral agents11 perhaps because of relatively few alternative therapeutic options. Whatever the reason, it is clear that this area may be the next frontier where we observe dramatic changes in benefit design, and it will be important to assess the consequences for spending and health.
The majority of papers we examined were outcomes studies conducted using administrative data. The researchers typically isolated a plan or plans that changed their benefit design and analyzed the resulting patterns of prescription drug use, and (less frequently) medical utilization, with the best designs including a control plan that did not change benefits over the same period. Such data are rich in sample size and measures of utilization, but they have limitations beyond the lack of important clinical detail. There is no information on socioeconomic status and one cannot control for key health-related behaviors such as diet, exercise, and smoking. Physician prescribing practices—especially whether a prescription was written but not filled—are unobserved. Further, long term follow-up is difficult because plan enrollment often changes over the course of the study.
In sum, the evidence suggests that patients are very responsive to the cost-sharing arrangements in prescription drug plans—even among the chronically-ill. And, for certain conditions, the evidence clearly indicates that more cost-sharing is associated with increased use of other medical services such as hospitalizations and emergency department visits. These findings make benefit design an important public health tool for improving population health. The challenge for public and private plans is to make patients more sensitive to the cost of treatment without encouraging them to forego cost-effective care. This requires not only knowing how patients respond to different incentives, but also cataloging the net benefits of alternative therapies—not only for health, but also current and future health care costs, productivity, and patient utility.
Figure 1.
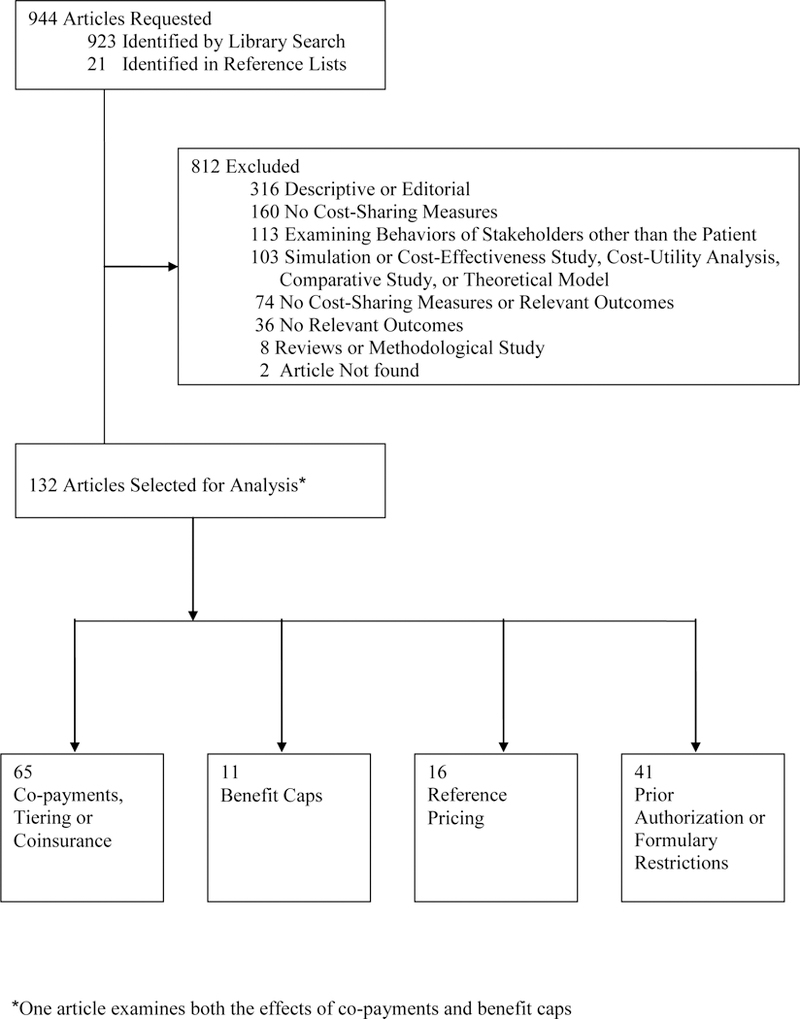
Study Design
ACKNOWLEDGEMENTS
This research was sponsored by the National Institute on Aging through its support of the RAND Roybal Center for Health Policy Simulation and by the Peter Bing Center for Health Economics. None of the sponsors had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Goldman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors have any relevant conflicts of interests. We are grateful to Paul Shekelle, MD, PhD, RAND Corporation, and Veterans Affairs Health Services Research and Development Service, Los Angeles, California, for his guidance on conducting a systematic evidence review.
REFERENCES
- 1.Lichtenberg FR. Are the benefits of newer drugs worth their cost? Evidence from the 1996 MEPS. Health Aff (Millwood) Sep-Oct 2001;20(5):241–251. [DOI] [PubMed] [Google Scholar]
- 2.Gabel J, Levitt L, Holve E, et al. Job-based health benefits in 2002: some important trends. Health Aff (Millwood) Sep-Oct 2002;21(5):143–151. [DOI] [PubMed] [Google Scholar]
- 3.Prescription Drug Trends Menlo Park, CA: Kaiser Family Foundation; June 2006. 3057–05. [Google Scholar]
- 4.Tseng CW, Brook RH, Keeler E, Mangione CM. Impact of an annual dollar limit or “cap” on prescription drug benefits for Medicare patients. Jama July 9 2003;290(2):222–227. [DOI] [PubMed] [Google Scholar]
- 5.Andersson K, Petzold MG, Sonesson C, Lonnroth K, Carlsten A. Do policy changes in the pharmaceutical reimbursement schedule affect drug expenditures? Interrupted time series analysis of cost, volume and cost per volume trends in Sweden 1986–2002. Health Policy December 2006;79(2–3):231–243. [DOI] [PubMed] [Google Scholar]
- 6.Cole JA, Norman H, Weatherby LB, Walker AM. Drug copayment and adherence in chronic heart failure: effect on cost and outcomes. Pharmacotherapy August 2006;26(8):1157–1164. [DOI] [PubMed] [Google Scholar]
- 7.Dormuth CR, Glynn RJ, Neumann P, Maclure M, Brookhart AM, Schneeweiss S. Impact of two sequential drug cost-sharing policies on the use of inhaled medications in older patients with chronic obstructive pulmonary disease or asthma. Clin Ther June 2006;28(6):964–978; discussion 962–963. [DOI] [PubMed] [Google Scholar]
- 8.Gibson TB, Mark TL, Axelsen K, Baser O, Rublee DA, McGuigan KA. Impact of statin copayments on adherence and medical care utilization and expenditures. Am J Manag Care December 2006;12 Spec no.:SP11–19. [PubMed] [Google Scholar]
- 9.Gibson TB, Mark TL, McGuigan KA, Axelsen K, Wang S. The effects of prescription drug copayments on statin adherence. Am J Manag Care September 2006;12(9):509–517. [PubMed] [Google Scholar]
- 10.Goldman DP, Joyce GF, Karaca-Mandic P. Varying pharmacy benefits with clinical status: the case of cholesterol-lowering therapy. Am J Manag Care January 2006;12(1):21–28. [PubMed] [Google Scholar]
- 11.Goldman DP, Joyce GF, Lawless G, Crown WH, Willey V. Benefit design and specialty drug use. Health Aff (Millwood) Sep-Oct 2006;25(5):1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Guh D, Lacaille D, Esdaile J, Anis AH. The impact of cost sharing of prescription drug expenditures on health care utilization by the elderly: Own- and cross-price elasticities. Health Policy November 27 2006. [DOI] [PubMed]
- 13.Taira DA, Wong KS, Frech-Tamas F, Chung RS. Copayment level and compliance with antihypertensive medication: analysis and policy implications for managed care. Am J Manag Care November 2006;12(11):678–683. [PubMed] [Google Scholar]
- 14.Wang J, Noel JM, Zuckerman IH, Miller NA, Shaya FT, Mullins CD. Disparities in access to essential new prescription drugs between non-Hispanic whites, non-Hispanic blacks, and Hispanic whites. Med Care Res Rev December 2006;63(6):742–763. [DOI] [PubMed] [Google Scholar]
- 15.Anis AH, Guh DP, Lacaille D, et al. When patients have to pay a share of drug costs: effects on frequency of physician visits, hospital admissions and filling of prescriptions. Cmaj November 22 2005;173(11):1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contoyannis P, Hurley J, Grootendorst P, Jeon SH, Tamblyn R. Estimating the price elasticity of expenditure for prescription drugs in the presence of non-linear price schedules: an illustration from Quebec, Canada. Health Econ September 2005;14(9):909–923. [DOI] [PubMed] [Google Scholar]
- 17.Gibson TB, McLaughlin CG, Smith DG. A copayment increase for prescription drugs: the long-term and short-term effects on use and expenditures. Inquiry. Fall 2005;42(3):293–310. [DOI] [PubMed] [Google Scholar]
- 18.Hansen RA, Shaheen NJ, Schommer JC. Factors influencing the shift of patients from one proton pump inhibitor to another: the effect of direct-to-consumer advertising. Clin Ther September 2005;27(9):1478–1487. [DOI] [PubMed] [Google Scholar]
- 19.Huskamp HA, Deverka PA, Epstein AM, et al. Impact of 3-tier formularies on drug treatment of attention-deficit/hyperactivity disorder in children. Arch Gen Psychiatry April 2005;62(4):435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landsman PB, Yu W, Liu X, Teutsch SM, Berger ML. Impact of 3-tier pharmacy benefit design and increased consumer cost-sharing on drug utilization. Am J Manag Care October 2005;11(10):621–628. [PubMed] [Google Scholar]
- 21.Mahoney JJ. Reducing patient drug acquisition costs can lower diabetes health claims. Am J Manag Care August 2005;11(5 Suppl):S170–176. [PubMed] [Google Scholar]
- 22.Roblin DW, Platt R, Goodman MJ, et al. Effect of increased cost-sharing on oral hypoglycemic use in five managed care organizations: how much is too much? Med Care October 2005;43(10):951–959. [DOI] [PubMed] [Google Scholar]
- 23.Briesacher B, Kamal-Bahl S, Hochberg M, Orwig D, Kahler KH. Three-tiered-copayment drug coverage and use of nonsteroidal anti-inflammatory drugs. Arch Intern Med August 9–23 2004;164(15):1679–1684. [DOI] [PubMed] [Google Scholar]
- 24.Crown WH, Berndt ER, Baser O, et al. Benefit plan design and prescription drug utilization among asthmatics: do patient copayments matter? Front Health Policy Res 2004;7:95–127. [DOI] [PubMed] [Google Scholar]
- 25.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med June 2004;19(6):638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman DP, Joyce GF, Escarce JJ, et al. Pharmacy benefits and the use of drugs by the chronically ill. Jama May 19 2004;291(19):2344–2350. [DOI] [PubMed] [Google Scholar]
- 27.Kamal-Bahl S, Briesacher B. How do incentive-based formularies influence drug selection and spending for hypertension? Health Aff (Millwood) Jan-Feb 2004;23(1):227–236. [DOI] [PubMed] [Google Scholar]
- 28.Liu SZ, Romeis JC. Changes in drug utilization following the outpatient prescription drug cost-sharing program--evidence from Taiwan’s elderly. Health Policy June 2004;68(3):277–287. [DOI] [PubMed] [Google Scholar]
- 29.Lurk JT, DeJong DJ, Woods TM, Knell ME, Carroll CA. Effects of changes in patient cost sharing and drug sample policies on prescription drug costs and utilization in a safety-net-provider setting. Am J Health Syst Pharm February 1 2004;61(3):267–272. [DOI] [PubMed] [Google Scholar]
- 30.Meissner BL, Moore WM, Shinogle JA, Reeder CE, Little JM. Effects of an increase in prescription copayment on utilization of low-sedating antihistamines and nasal steroids. J Manag Care Pharm May-Jun 2004;10(3):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkelmann R Co-payments for prescription drugs and the demand for doctor visits--evidence from a natural experiment. Health Econ November 2004;13(11):1081–1089. [DOI] [PubMed] [Google Scholar]
- 32.Blais L, Couture J, Rahme E, LeLorier J. Impact of a cost sharing drug insurance plan on drug utilization among individuals receiving social assistance. Health Policy May 2003;64(2):163–172. [DOI] [PubMed] [Google Scholar]
- 33.Fairman KA, Motheral BR, Henderson RR. Retrospective, long-term follow-up study of the effect of a three-tier prescription drug copayment system on pharmaceutical and other medical utilization and costs. Clin Ther December 2003;25(12):3147–3161; discussion 3144–3146. [DOI] [PubMed] [Google Scholar]
- 34.Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan KA, Frank RG. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med December 4 2003;349(23):2224–2232. [DOI] [PubMed] [Google Scholar]
- 35.Liu SZ, Romeis JC. Assessing the effect of Taiwan’s outpatient prescription drug copayment policy in the elderly. Med Care December 2003;41(12):1331–1342. [DOI] [PubMed] [Google Scholar]
- 36.Nair KV, Wolfe P, Valuck RJ, McCollum MM, Ganther JM, Lewis SJ. Effects of a 3-tier pharmacy benefit design on the prescription purchasing behavior of individuals with chronic disease. J Manag Care Pharm Mar-Apr 2003;9(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong M, Catalano R, Hartig T. A time-series analysis of the effect of increased copayments on the prescription of antidepressants, anxiolytics, and sedatives in Sweden from 1990 to 1999. Clin Ther April 2003;25(4):1262–1275. [DOI] [PubMed] [Google Scholar]
- 38.Rector TS, Finch MD, Danzon PM, Pauly MV, Manda BS. Effect of tiered prescription copayments on the use of preferred brand medications. Med Care March 2003;41(3):398–406. [DOI] [PubMed] [Google Scholar]
- 39.Artz MB, Hadsall RS, Schondelmeyer SW. Impact of generosity level of outpatient prescription drug coverage on prescription drug events and expenditure among older persons. Am J Public Health August 2002;92(8):1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joyce GF, Escarce JJ, Solomon MD, Goldman DP. Employer drug benefit plans and spending on prescription drugs. Jama October 9 2002;288(14):1733–1739. [DOI] [PubMed] [Google Scholar]
- 41.Pilote L, Beck C, Richard H, Eisenberg MJ. The effects of cost-sharing on essential drug prescriptions, utilization of medical care and outcomes after acute myocardial infarction in elderly patients. Cmaj August 6 2002;167(3):246–252. [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas CP, Wallack SS, Lee S, Ritter GA. Impact of health plan design and management on retirees’ prescription drug use and spending, 2001. Health Aff (Millwood) Jul-Dec 2002;Suppl Web Exclusives:W408–419. [DOI] [PubMed]
- 43.Balkrishnan R, Byerly WG, Camacho FT, Shrestha A, Anderson RT. Effect of prescription benefit changes on medical care utilization in a Medicare HMO population. Am J Manag Care November 2001;7(11):1093–1100. [PubMed] [Google Scholar]
- 44.Blais L, Boucher JM, Couture J, Rahme E, LeLorier J. Impact of a cost-sharing drug insurance plan on drug utilization among older people. J Am Geriatr Soc April 2001;49(4):410–414. [DOI] [PubMed] [Google Scholar]
- 45.Kozyrskyj AL, Mustard CA, Cheang MS, Simons FE. Income-based drug benefit policy: impact on receipt of inhaled corticosteroid prescriptions by Manitoba children with asthma. Cmaj October 2 2001;165(7):897–902. [PMC free article] [PubMed] [Google Scholar]
- 46.Kozyrskyj AL, Mustard CA, Simons FE. Socioeconomic status, drug insurance benefits, and new prescriptions for inhaled corticosteroids in schoolchildren with asthma. Arch Pediatr Adolesc Med November 2001;155(11):1219–1224. [DOI] [PubMed] [Google Scholar]
- 47.Motheral B, Fairman KA. Effect of a three-tier prescription copay on pharmaceutical and other medical utilization. Med Care December 2001;39(12):1293–1304. [DOI] [PubMed] [Google Scholar]
- 48.Tamblyn R, Laprise R, Hanley JA, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. Jama January 24–31 2001;285(4):421–429. [DOI] [PubMed] [Google Scholar]
- 49.Hillman AL, Pauly MV, Escarce JJ, et al. Financial incentives and drug spending in managed care. Health Aff (Millwood) Mar-Apr 1999;18(2):189–200. [DOI] [PubMed] [Google Scholar]
- 50.Motheral BR, Henderson R. The effect of a copay increase on pharmaceutical utilization, expenditures, and treatment continuation. Am J Manag Care November 1999;5(11):1383–1394. [PubMed] [Google Scholar]
- 51.Stuart B, Zacker C. Who bears the burden of Medicaid drug copayment policies? Health Aff (Millwood) Mar-Apr 1999;18(2):201–212. [DOI] [PubMed] [Google Scholar]
- 52.Berndt ER, Frank RG, McGuire TG. Alternative insurance arrangements and the treatment of depression: what are the facts? Am J Manag Care February 1997;3(2):243–250. [PubMed] [Google Scholar]
- 53.Grootendorst PV, O’Brien BJ, Anderson GM. On becoming 65 in Ontario. Effects of drug plan eligibility on use of prescription medicines. Med Care April 1997;35(4):386–398. [DOI] [PubMed] [Google Scholar]
- 54.Johnson RE, Goodman MJ, Hornbrook MC, Eldredge MB. The effect of increased prescription drug cost-sharing on medical care utilization and expenses of elderly health maintenance organization members. Med Care November 1997;35(11):1119–1131. [DOI] [PubMed] [Google Scholar]
- 55.Johnson RE, Goodman MJ, Hornbrook MC, Eldredge MB. The impact of increasing patient prescription drug cost sharing on therapeutic classes of drugs received and on the health status of elderly HMO members. Health Serv Res April 1997;32(1):103–122. [PMC free article] [PubMed] [Google Scholar]
- 56.Hong SH, Shepherd MD. Outpatient prescription drug use by children enrolled in five drug benefit plans. Clin Ther May-Jun 1996;18(3):528–545. [DOI] [PubMed] [Google Scholar]
- 57.McManus P, Donnelly N, Henry D, Hall W, Primrose J, Lindner J. Prescription drug utilization following patient co-payment changes in Australia. Pharmacoepidemiol Drug Saf November 1996;5(6):385–392. [DOI] [PubMed] [Google Scholar]
- 58.Coulson NE, Stuart BC. Insurance Choice and the Demand for Prescription Drugs. Southern Economic Journal 1995;61(4):1146–1157. [Google Scholar]
- 59.Hughes D, McGuire A. Patient charges and the utilisation of NHS prescription medicines: some estimates using a cointegration procedure. Health Econ May-Jun 1995;4(3):213–220. [DOI] [PubMed] [Google Scholar]
- 60.Smith DG. The effects of copayments and generic substitution on the use and costs of prescription drugs. Inquiry. Summer 1993;30(2):189–198. [PubMed] [Google Scholar]
- 61.Ryan M, Birch S. Charging for health care: evidence on the utilisation of NHS prescribed drugs. Soc Sci Med 1991;33(6):681–687. [DOI] [PubMed] [Google Scholar]
- 62.Harris BL, Stergachis A, Ried LD. The effect of drug co-payments on utilization and cost of pharmaceuticals in a health maintenance organization. Med Care October 1990;28(10):907–917. [DOI] [PubMed] [Google Scholar]
- 63.Lingle E, Reeder C, Kozma C. Impact of an open formulary system on the utilization of medical services. J Res Pharm Econ 1990;2:93–123. [Google Scholar]
- 64.Lavers RJ. Prescription Charges, the Demand for Prescriptions and Morbidity. Applied Economics 1989;21(8):1043–1052. [Google Scholar]
- 65.O’Brien B The effect of patient charges on the utilisation of prescription medicines. J Health Econ March 1989;8(1):109–132. [DOI] [PubMed] [Google Scholar]
- 66.Foxman B, Valdez RB, Lohr KN, Goldberg GA, Newhouse JP, Brook RH. The effect of cost sharing on the use of antibiotics in ambulatory care: results from a population-based randomized controlled trial. J Chronic Dis 1987;40(5):429–437. [DOI] [PubMed] [Google Scholar]
- 67.Birch S Relationship between increasing prescription charges and consumption in groups not exempt from charges. J R Coll Gen Pract April 1986;36(285):154–156. [PMC free article] [PubMed] [Google Scholar]
- 68.Leibowitz A, Manning WG, Newhouse JP. The demand for prescription drugs as a function of cost-sharing. Soc Sci Med 1985;21(10):1063–1069. [DOI] [PubMed] [Google Scholar]
- 69.Reeder CE, Nelson AA. The differential impact of copayment on drug use in a Medicaid population. Inquiry. Winter 1985;22(4):396–403. [PubMed] [Google Scholar]
- 70.Hsu J, Price M, Huang J, et al. Unintended consequences of caps on Medicare drug benefits. N Engl J Med June 1 2006;354(22):2349–2359. [DOI] [PubMed] [Google Scholar]
- 71.Tseng CW, Brook RH, Keeler E, Steers WN, Mangione CM. Cost-lowering strategies used by medicare beneficiaries who exceed drug benefit caps and have a gap in drug coverage. Jama August 25 2004;292(8):952–960. [DOI] [PubMed] [Google Scholar]
- 72.Cox ER, Henderson RR. Prescription use behavior among medicare beneficiaries with capped prescription benefits. J Manag Care Pharm Sep-Oct 2002;8(5):360–364. [DOI] [PubMed] [Google Scholar]
- 73.Cox ER, Jernigan C, Coons SJ, Draugalis JL. Medicare beneficiaries’ management of capped prescription benefits. Med Care March 2001;39(3):296–301. [DOI] [PubMed] [Google Scholar]
- 74.Fortess EE, Soumerai SB, McLaughlin TJ, Ross-Degnan D. Utilization of essential medications by vulnerable older people after a drug benefit cap: importance of mental disorders, chronic pain, and practice setting. J Am Geriatr Soc June 2001;49(6):793–797. [DOI] [PubMed] [Google Scholar]
- 75.Martin BC, McMillan JA. The impact of implementing a more restrictive prescription limit on Medicaid recipients. Effects on cost, therapy, and out-of-pocket expenditures. Med Care July 1996;34(7):686–701. [DOI] [PubMed] [Google Scholar]
- 76.Soumerai SB, McLaughlin TJ, Ross-Degnan D, Casteris CS, Bollini P. Effects of a limit on Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med September 8 1994;331(10):650–655. [DOI] [PubMed] [Google Scholar]
- 77.Soumerai SB, Ross-Degnan D, Avorn J, McLaughlin T, Choodnovskiy I. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med October 10 1991;325(15):1072–1077. [DOI] [PubMed] [Google Scholar]
- 78.Soumerai SB, Avorn J, Ross-Degnan D, Gortmaker S. Payment restrictions for prescription drugs under Medicaid. Effects on therapy, cost, and equity. N Engl J Med August 27 1987;317(9):550–556. [DOI] [PubMed] [Google Scholar]
- 79.Abdelgawad T, Egbuonu-Davis L. Preferred drug lists and medicaid prescriptions. Pharmacoeconomics 2006;24 Suppl 3:55–63. [DOI] [PubMed] [Google Scholar]
- 80.Ackman ML, Graham MM, Hui C, Tsuyuki RT. Effect of a prior authorization process on antiplatelet therapy and outcomes in patients prescribed clopidogrel following coronary stenting. Can J Cardiol December 2006;22(14):1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carroll NV, Smith JC, Berringer RA, Oestreich GL. Evaluation of an automated system for prior authorization: a COX-2 inhibitor example. Am J Manag Care September 2006;12(9):501–508. [PubMed] [Google Scholar]
- 82.Dunn JD, Cannon E, Mitchell MP, Curtiss FR. Utilization and drug cost outcomes of a step-therapy edit for generic antidepressants in an HMO in an integrated health system. J Manag Care Pharm May 2006;12(4):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kahan NR, Chinitz DP, Waitman DA, Kahan E. When gatekeepers meet the sentinel: the impact of a prior authorization requirement for cefuroxime on the prescribing behaviour of community-based physicians. Br J Clin Pharmacol March 2006;61(3):341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ridley DB, Axelsen KJ. Impact of medicaid preferred drug lists on therapeutic adherence. Pharmacoeconomics 2006;24 Suppl 3:65–78. [DOI] [PubMed] [Google Scholar]
- 85.Roughead EE, Zhang F, Ross-Degnan D, Soumerai S. Differential effect of early or late implementation of prior authorization policies on the use of Cox II inhibitors. Med Care April 2006;44(4):378–382. [DOI] [PubMed] [Google Scholar]
- 86.Spence MM, Hui R, Chan J. Cost reduction strategies used by elderly patients with chronic obstructive pulmonary disease to cope with a generic-only pharmacy benefit. J Manag Care Pharm June 2006;12(5):377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tseng CW, Brook RH, Keeler E, Steers WN, Waitzfelder BE, Mangione CM. Effect of generic-only drug benefits on seniors’ medication use and financial burden. Am J Manag Care September 2006;12(9):525–532. [PubMed] [Google Scholar]
- 88.West DS, Johnson JT, Hong SH. A 30-month evaluation of the effects on the cost and utilization of proton pump inhibitors from adding omeprazole OTC to drug benefit coverage in a state employee health plan. J Manag Care Pharm Jan-Feb 2006;12(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cunningham PJ. Medicaid cost containment and access to prescription drugs. Health Aff (Millwood) May-Jun 2005;24(3):780–789. [DOI] [PubMed] [Google Scholar]
- 90.Delate T, Mager DE, Sheth J, Motheral BR. Clinical and financial outcomes associated with a proton pump inhibitor prior-authorization program in a Medicaid population. Am J Manag Care January 2005;11(1):29–36. [PubMed] [Google Scholar]
- 91.Gleason PP, Williams C, Hrdy S, Hartwig SC, Lassen D. Medical and pharmacy expenditures after implementation of a cyclooxygenase-2 inhibitor prior authorization program. Pharmacotherapy July 2005;25(7):924–934. [DOI] [PubMed] [Google Scholar]
- 92.Lichtenberg FR. The effect of access restrictions on the vintage of drugs used by Medicaid enrollees. Am J Manag Care January 2005;11 Spec No:SP7–13. [PubMed] [Google Scholar]
- 93.Murawski MM, Abdelgawad T. Exploration of the impact of preferred drug lists on hospital and physician visits and the costs to Medicaid. Am J Manag Care January 2005;11 Spec No:SP35–42. [PubMed] [Google Scholar]
- 94.Virabhak S, Shinogle JA. Physicians’ prescribing responses to a restricted formulary: the impact of Medicaid preferred drug lists in Illinois and Louisiana. Am J Manag Care January 2005;11 Spec No:SP14–20. [PubMed] [Google Scholar]
- 95.Wilson J, Axelsen K, Tang S. Medicaid prescription drug access restrictions: exploring the effect on patient persistence with hypertension medications. Am J Manag Care January 2005;11 Spec No:SP27–34. [PubMed] [Google Scholar]
- 96.Christian-Herman J, Emons M, George D. Effects of generic-only drug coverage in a Medicare HMO. Health Aff (Millwood) Jul-Dec 2004;Suppl Web Exclusives:W4–455-468. [DOI] [PubMed]
- 97.Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid prior-authorization programs and the use of cyclooxygenase-2 inhibitors. N Engl J Med November 18 2004;351(21):2187–2194. [DOI] [PubMed] [Google Scholar]
- 98.Harris BN, West DS, Johnson J, Hong SH, Stowe CD. Effects on the cost and utilization of proton pump inhibitors from adding over-the counter omeprazole to drug benefit coverage in a state employee health plan. J Manag Care Pharm Sep-Oct 2004;10(5):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hartung DM, Touchette DR, Ketchum KL, Haxby DG, Goldberg BW. Effects of a prior-authorization policy for celecoxib on medical service and prescription drug use in a managed care Medicaid population. Clin Ther September 2004;26(9):1518–1532. [DOI] [PubMed] [Google Scholar]
- 100.Motheral BR, Henderson R, Cox ER. Plan-sponsor savings and member experience with point-of-service prescription step therapy. Am J Manag Care July 2004;10(7 Pt 1):457–464. [PubMed] [Google Scholar]
- 101.Campbell CA, Cooke CA, Weerasinghe SD, Sketris IS, McLean-Veysey PR, Skedgel CD. Topical corticosteroid prescribing patterns following changes in drug benefit status. Ann Pharmacother June 2003;37(6):787–793. [DOI] [PubMed] [Google Scholar]
- 102.Huskamp HA, Epstein AM, Blumenthal D. The impact of a national prescription drug formulary on prices, market share, and spending: lessons for Medicare? Health Aff (Millwood) May-Jun 2003;22(3):149–158. [DOI] [PubMed] [Google Scholar]
- 103.Wang YR, Pauly MV, Lin YA. Impact of Maine’s Medicaid drug formulary change on non-Medicaid markets: spillover effects of a restrictive drug formulary. Am J Manag Care October 2003;9(10):686–696. [PubMed] [Google Scholar]
- 104.McCombs JS, Shi L, Stimmel GL, Croghan TW. A retrospective analysis of the revocation of prior authorization restrictions and the use of antidepressant medications for treating major depressive disorder. Clin Ther November 2002;24(11):1939–1959; discussion 1938. [DOI] [PubMed] [Google Scholar]
- 105.Cromwell DM, Bass EB, Steinberg EP, et al. Can restrictions on reimbursement for anti-ulcer drugs decrease Medicaid pharmacy costs without increasing hospitalizations? Health Serv Res February 1999;33(6):1593–1610. [PMC free article] [PubMed] [Google Scholar]
- 106.Motheral BR, Henderson R. The effect of a closed formulary on prescription drug use and costs. Inquiry. Winter 1999;36(4):481–491. [PubMed] [Google Scholar]
- 107.Streja DA, Hui RL, Streja E, McCombs JS. Selective contracting and patient outcomes: a case study of formulary restrictions for selective serotonin reuptake inhibitor antidepressants. Am J Manag Care September 1999;5(9):1133–1142. [PubMed] [Google Scholar]
- 108.Horn SD, Sharkey PD, Phillips-Harris C. Formulary limitations and the elderly: results from the Managed Care Outcomes Project. Am J Manag Care August 1998;4(8):1105–1113. [PubMed] [Google Scholar]
- 109.Phillips CR, Larson LN. Evaluating the Operational Performance and Financial Effects of a Drug Prior Authorization Program. J Managed Care Pharm 1997(3):699–706. [Google Scholar]
- 110.White AC Jr., Atmar RL, Wilson J, Cate TR, Stager CE, Greenberg SB. Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis August 1997;25(2):230–239. [DOI] [PubMed] [Google Scholar]
- 111.Jones DL, Kroenke K, Landry FJ, Tomich DJ, Ferrel RJ. Cost savings using a stepped-care prescribing protocol for nonsteroidal anti-inflammatory drugs. Jama March 27 1996;275(12):926–930. [PubMed] [Google Scholar]
- 112.Kotzan J, Perri M, Martin B. Assessment of Medicaid prior-approval policies on prescription expenditures: Market-share analysis of Medicaid and cash prescriptions. J Managed Care Pharm 1996;2(6):651–656. [Google Scholar]
- 113.Smalley WE, Griffin MR, Fought RL, Sullivan L, Ray WA. Effect of a prior-authorization requirement on the use of nonsteroidal antiinflammatory drugs by Medicaid patients. N Engl J Med June 15 1995;332(24):1612–1617. [DOI] [PubMed] [Google Scholar]
- 114.Kotzan JA, et al. Initial Impact of a Medicaid Prior Authorization Program for NSAID Prescriptions. Journal of Research in Pharmaceutical Economics 1993;5(1):25–41. [Google Scholar]
- 115.Kotzan JA, et al. Initial Impact of a Medicaid Maintenance Dose Program for H2 Antagonist Prescriptions. Journal of Research in Pharmaceutical Economics 1993;5(1):43–58. [Google Scholar]
- 116.Moore WJ, Newman RJ. Drug Formulary Restrictions as a Cost-Containment Policy in Medicaid Programs. Journal of Law and Economics 1993;36(1):71–97. [Google Scholar]
- 117.Kozma CM, Reeder CE, Lingle EW. Expanding Medicaid drug formulary coverage. Effects on utilization of related services. Med Care October 1990;28(10):963–977. [DOI] [PubMed] [Google Scholar]
- 118.Kreling DH, Knocke DJ, Hammel RW. The effects of an internal analgesic formulary restriction on Medicaid drug expenditures in Wisconsin. Med Care January 1989;27(1):34–44. [DOI] [PubMed] [Google Scholar]
- 119.Bloom BS, Jacobs J. Cost effects of restricting cost-effective therapy. Med Care July 1985;23(7):872–880. [DOI] [PubMed] [Google Scholar]
- 120.Mabasa VH, Ma J. Effect of a therapeutic maximum allowable cost (MAC) program on the cost and utilization of proton pump inhibitors in an employer-sponsored drug plan in Canada. J Manag Care Pharm June 2006;12(5):371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schneeweiss S, Maclure M, Dormuth CR, Glynn RJ, Canning C, Avorn J. A therapeutic substitution policy for proton pump inhibitors: clinical and economic consequences. Clin Pharmacol Ther April 2006;79(4):379–388. [DOI] [PubMed] [Google Scholar]
- 122.Grootendorst PV, Marshall JK, Holbrook AM, Dolovich LR, O’Brien BJ, Levy AR. The impact of reference pricing of nonsteroidal anti-inflammatory agents on the use and costs of analgesic drugs. Health Serv Res October 2005;40(5 Pt 1):1297–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schneeweiss S, Dormuth C, Grootendorst P, Soumerai SB, Maclure M. Net health plan savings from reference pricing for angiotensin-converting enzyme inhibitors in elderly British Columbia residents. Med Care July 2004;42(7):653–660. [DOI] [PubMed] [Google Scholar]
- 124.Schneeweiss S, Maclure M, Carleton B, Glynn RJ, Avorn J. Clinical and economic consequences of a reimbursement restriction of nebulised respiratory therapy in adults: direct comparison of randomised and observational evaluations. Bmj March 6 2004;328(7439):560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schneeweiss S, Soumerai SB, Maclure M, Dormuth C, Walker AM, Glynn RJ. Clinical and economic consequences of reference pricing for dihydropyridine calcium channel blockers. Clin Pharmacol Ther October 2003;74(4):388–400. [DOI] [PubMed] [Google Scholar]
- 126.Hazlet TK, Blough DK. Health services utilization with reference drug pricing of histamine(2) receptor antagonists in British Columbia elderly. Med Care August 2002;40(8):640–649. [DOI] [PubMed] [Google Scholar]
- 127.Marshall JK, Grootendorst PV, O’Brien BJ, Dolovich LR, Holbrook AM, Levy AR. Impact of reference-based pricing for histamine-2 receptor antagonists and restricted access for proton pump inhibitors in British Columbia. Cmaj June 25 2002;166(13):1655–1662. [PMC free article] [PubMed] [Google Scholar]
- 128.Schneeweiss S, Soumerai SB, Glynn RJ, Maclure M, Dormuth C, Walker AM. Impact of reference-based pricing for angiotensin-converting enzyme inhibitors on drug utilization. Cmaj March 19 2002;166(6):737–745. [PMC free article] [PubMed] [Google Scholar]
- 129.Schneeweiss S, Walker AM, Glynn RJ, Maclure M, Dormuth C, Soumerai SB. Outcomes of reference pricing for angiotensin-converting-enzyme inhibitors. N Engl J Med March 14 2002;346(11):822–829. [DOI] [PubMed] [Google Scholar]
- 130.Aronsson T, Bergman MA, Rudholm N. The Impact of Generic Drug Competition on Brand Name Market Shares--Evidence from Micro Data. Review of Industrial Organization 2001;19(4):425–435. [Google Scholar]
- 131.Grootendorst PV, Dolovich LR, O’Brien BJ, Holbrook AM, Levy AR. Impact of reference-based pricing of nitrates on the use and costs of anti-anginal drugs. Cmaj October 16 2001;165(8):1011–1019. [PMC free article] [PubMed] [Google Scholar]
- 132.McManus P, Birkett DJ, Dudley J, Stevens A. Impact of the Minimum Pricing Policy and introduction of brand (generic) substitution into the Pharmaceutical Benefits Scheme in Australia. Pharmacoepidemiol Drug Saf Jun-Jul 2001;10(4):295–300. [DOI] [PubMed] [Google Scholar]
- 133.Narine L, Senathirajah M, Smith T. An Assessment of the Impact of Reference-Based Pricing Policies on the H2 Antagonist Market in British Columbia, Canada. Journal of Research in Pharmaceutical Economics 2001;11(1):63–78. [Google Scholar]
- 134.Narine L, Senathirajah M, Smith T. Evaluating reference-based pricing: initial findings and prospects. Cmaj August 10 1999;161(3):286–288. [PMC free article] [PubMed] [Google Scholar]
- 135.Jonsson B.Pricing and reimbursement of pharmaceuticals in Sweden. Pharmacoeconomics 1994;6 Suppl 1:51–60. [DOI] [PubMed] [Google Scholar]
- 136.Rector TS. Exhaustion of drug benefits and disenrollment of medicare beneficiaries from managed care organizations. Jama April 26 2000;283(16):2163–2167. [DOI] [PubMed] [Google Scholar]
- 137.Lingle EW Jr., Kirk KW, Kelly WR. The impact of outpatient drug benefits on the use and costs of health care services for the elderly. Inquiry. Fall 1987;24(3):203–211. [PubMed] [Google Scholar]
- 138.Smith DG, Kirking DM. Impact of consumer fees on drug utilisation. Pharmacoeconomics October 1992;2(4):335–342. [DOI] [PubMed] [Google Scholar]
- 139.Cunningham PJ. Prescription drug access: not just a Medicare problem. Issue Brief Cent Stud Health Syst Change April 2002(51):1–4. [PubMed] [Google Scholar]
- 140.Stuart B, Grana J. Ability to pay and the decision to medicate. Med Care February 1998;36(2):202–211. [DOI] [PubMed] [Google Scholar]
- 141.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. Jama December 11 2002;288(22):2868–2879. [DOI] [PubMed] [Google Scholar]
- 142.Topol EJ. Intensive statin therapy--a sea change in cardiovascular prevention. N Engl J Med April 8 2004;350(15):1562–1564. [DOI] [PubMed] [Google Scholar]
- 143.Reed MC. An Update on Americans’ Access to Prescription Drugs Washington, DC: Center for Styding Health System Change; May 2005. Issue Brief 95. http://www.hschange.com/CONTENT/738/ [PubMed] [Google Scholar]
- 144.Goldman DP, Smith JP. Methodological biases in estimating the burden of out-of-pocket expenses. Health Serv Res 2001;35(6):1357–136511221824 [PMC free article] [PubMed] [Google Scholar]


