Abstract
Human toxoplasmosis, a protozoonosis caused by Toxoplasma gondii, has been described as a worldwide foodborne disease with important public health impact. Despite infection has reportedly varied due to differences in alimentary, cultural and hygienic habits and geographic region, social vulnerability influence on toxoplasmosis distribution remains to be fully established. Accordingly, the present study has aimed to assess T. gondii seroprevalence and factors associated to social vulnerability for infection in households of Ivaiporã, southern Brazil, with 33.6% population making half minimum wage or less, ranked 1,055th in population (31,816 habitants), 1,406th in per capita income (U$ 211.80 per month) and 1,021st in HDI (0.764) out of 5,570 Brazilian cities. Serum samples and epidemiological questionnaires were obtained from citizen volunteers with official City Secretary of Health assistance in 2015 and 2016. In overall, serosurvey has revealed 526/715 (73.57%) positive samples for anti-T. gondii antibodies by Indirect Fluorescent Antibody Test. Logistic regression has shown a significant increase associated to adults (p = 0.021) and elderly (p = 0.014) people, illiterates (p = 0.025), unemployment (p <0.001) and lack of household water tank (p = 0.039). On the other hand, sex (male or female), living area (urban or rural), yard hygiene, meat ingestion, sand or land contact, owning pets (dog, cat or both) were not significant variables of positivity for anti-T. gondii antibodies in the surveyed population. Although no significant spatial cluster was found, high intensity areas of seropositive individuals were located in the Kernel map where the suburban neighborhoods are located. In conclusion, socioeconomic vulnerability determinants may be associated to Toxoplasma gondii exposure. The increased risk due to illiteracy, adult or elderly age, unemployment and lack of household water tank were confirmed by multivariate analysis and the influence of low family income for seropositivity by the spatial analysis.
Introduction
Human toxoplasmosis, a protozoonosis caused by intracellular parasite Toxoplasma gondii, has been described as a worldwide foodborne disease with important public health impact [1,2]. The foodborne transmission may occur by intake of shedding oocysts from felid feces and contaminate water, infecting a wide range of intermediate hosts including dogs and human beings [3]. Transmission occurs through consumption of vegetables contaminated with faecal oocysts, uncooked meat, fresh milk from acutely infected goats, trans-placental infection during pregnancy, organ transplantation or blood transfusion [4,5]. Cases of toxoplasmosis in immunocompetent patients has been mostly asymptomatic; in immunocompromised individuals, shown a tendency of more severe clinical manifestations. Pregnant women may be an important risk group, since vertical transmission may trigger reproductive disorders, abortion, congenital disease with unspecific systemic symptoms, neurological and severe ocular damage in fetuses, auditive impairment, psychomotor development, hyperactivity and attention deficit [6–9].
Prevalence of T. gondii seropositivity may vary worldwide from 10% to 90%, mainly due to regional variations [1], with lifetime persistence of infection, typically asymptomatic, potentially latent and associated to psychiatric disorders [10–12] or including death in immunocompromised patients [13–15]. Associated factors for toxoplasmosis have been shown relevance on T. gondii seroprevalence, including school level [16,17] and low family income in latent toxoplasmosis related to cognitive deficit [18].
Spatial analysis has been recently applied to epidemiologic investigation of affected individuals in urban and rural settings, providing a clear view of territory spreading and a better understanding of disease distribution. Identification of factors associated to disease in considered populations may contribute to extrapolation and development of effective prophylactic strategies [19,20].
Despite infection has reportedly varied due to differences in alimentary, cultural and hygienic habits and geographic region, social vulnerability influence on distribution remains to be fully established. Accordingly, the present study has aimed to assess T. gondii seroprevalence and factors associated to social vulnerability for infection in households of Ivaiporã, southern Brazil, 33.6% of its population, ranked 1,055th in population (31,816 habitants), 1,406th in per capita income (U$ 211.80 per month) and 1,021st in HDI (0.764) out of 5,570 Brazilian cities.
Materials and methods
The present study has been approved by the Ethics Committee of Research Involving Human Beings at the Londrina State University (protocol 1,177,975/2015) and conducted as part of the official activities coordinated by the City Secretary of Health. Consent was obtained by the signature of a Free Prior Informed Consent Form
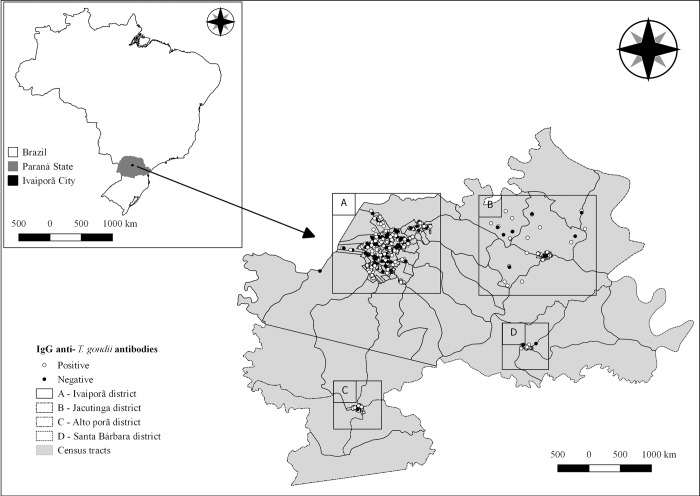
Ivaiporã city (24°14'52"S and 51°41'06"W), located in Paraná State, southern Brazil (Fig 1), composed of central area and districts of Jacutinga, Alto Porã and Santa Bárbara, has been characterized by distinct rural and urban areas. Situated within the Atlantic Forest biome with humid subtropical climate (Cfa), has historically presented an average pluviosity of 168 mm, 76% humidity and temperatures varying from 15°C to 26°C, [21]. The estimated population at the time of survey was 31,816 habitants (ranked 1,055th in population out of 5,570 Brazilian cities), with majority of 27,438 (86.20%) people living in urban area.
Fig 1. Location of Ivaiporã city, Paraná State, Brazil, including the serology results for IgG anti-T. gondii antibodies in 715 human samples tested by IFAT, from 2015 to 2016.
Despite the city has established a public treated water system and presented relatively high (0.730) human development index (HDI) at the time of survey (ranked 1,021st out of 5,570 Brazilian cities), Gini index related to economic level inequality was intermediate (0.4882) [21], and social vulnerability index (SVI) was classified as low (0.263) [22]. The State minimum wage at the time was of R$834.00 (U$ 243.15 at 3.43 exchange rate in 2016), with city ranked as 1,406th in per capita income (U$ 211.80 per month), with 33.6% of the population with a monthly income of up to ½ minimum wage, out of 5,570 Brazilian cities.
Minimum sampling of 570 human beings was determined by the OpenEpi software [23], based on estimated population [21] and with an expected prevalence of 50%, confidence level of 95%, error of 5% and Deff of 1.5. A multidisciplinary taskforce conducted by the city hall and involving the Basic Units for Health (UBS) was organized and conducted throughout 2015 and 2016 in several regions of the city. This taskforce was announced through print media, electronic media and sound cars, inviting the entire population to participate. On the scheduled date for each region, immediately after a zoonosis prevention program, the taskforce professionals collected blood samples and applied questionnaires (S1 File) to all those who agreed to participate voluntarily. Therefore, the sampling of this study is considered a convenience sampling. Serum samples were voluntarily obtained and kept at -20° C until testing for IgG anti-T gondii antibodies by Indirect Fluorescent Antibody Test (IFAT), as previously described [24]. Tachyzoites of RH strain were used as antigens, a commercially available anti-antibody species-specific conjugated with fluorescein isothiocyanate was used (Sigma-Aldrich, Saint Louis, MO, USA). Negative and positive controls were added in each slide, the considered cut-off was 1:16, reactive samples in higher dilutions were considered positive.
A questionnaire containing volunteer information (age, sex, address) along with questions on volunteer habits and intra and extra-domiciliary environments was used as previously described [25], in addition to questions related to socioeconomical conditions, alimentary and sanitary habits, own pet relationship and epidemiological factor for T. gondii infection.
Data was gathered and analyzed by a free software package (R version 3.4.2, R Core Team, Vienna, Austria). First, a univariate analysis was performed to test variable association to seropositivity using the Q-square or Exact Fisher Tests, Odds Ratio (OR) calculation and Confidence Interval (CI) of OR, with 5% of confidence level (α). Following, a multivariate analysis was performed, including associated risk factors with p-value of < 0.020 in a logistic regression model. Interactions between independent variables were tested and included in the model.
The geographical points were obtained by spatial coordinates, based on the residential address given during questionnaire interview. A purely spatial analysis of cluster using Bernoulli model [26] was performed in each city region, using a commercial software (SaTScanTM version 9.4.4, Boston, MA, USA) with a 5% significance level. A Kernel intensity map was applied for evaluation of T. gondii seropositivity distribution. All maps were constructed using a commercial software [27].
Results
The sampled population was of 715 people, representing 2.25% of overall Ivaiporã City population. No clinical complaint related to disease was made at the time of survey. Serosurvey has revealed 526/715 (73.57%) positive samples for anti-T. gondii antibodies by Indirect Fluorescent Antibody Test. The resulting seroprevalence of 73.57% presented a 95% confidence interval (CI) between 70.33% and 76.80%. Considering only the woman of childbearing age (15 to 49 years old), 171/248 were positive, with a seroprevalence of 68.95% (95% CI: 65.56–72.34).
The volunteer profile was heterogeneous, with 530/715 (74.13%) living on urban area, 650/715 (90.91%) over 18-years old, 495/715 (69.23%) females, 486/715 (67.97%) with basic school level, 667/715 (93.29%) with low income (<3 minimal wage), 393/715 (54.97%) with some kind of occupation, 564/715 (78.88%) owning pets, of which 532/564 (94.33%) dog owners, 197/564 (34.93%) cat owners and 165/564 (29.26%) owning both and most people lacked household water tank (417/715, 58.32%) (Table 1).
Table 1. Results of univariate analysis of associated risk factors for seropositivity of IgG anti-T. gondii antibodies in 715 human samples tested by IFAT, from 2015 to 2016, in Ivaiporã city, Paraná State, Brazil.
| Variable | Descriptive | Positive | OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Yes/Total (%) | Yes/Total (%) | ||||
| Gender | |||||
| Male | 220/715 (30.77) | 165/220 (75.00) | 0.898 | 0.62–1.29 | 0.562 |
| Female | 495/715 (69.23) | 361/495 (72.93) | |||
| Age range | |||||
| Young (< = 18 years old) | 65/715 (9.09) | 35/65 (53.85) | 2.647 | 1.58–4.45 | <0.001* |
| Adult (> 18 years old) | 650/715 (90.91) | 491/650 (75.54) | |||
| Schooling | |||||
| Low (up to elementary school) | 486/715 (67.97) | 380/486 (78.19) | 2.038 | 1.44–2.88 | <0.001* |
| High (high school or higher education) | 229/715 (32.03) | 146/229 (63.76) | |||
| Monthly income | |||||
| < = 3 minimum wage | 667/715 (93.29) | 497/667 (74.51) | 1.915 | 1.05–3.51 | 0.035* |
| > 3 minimum wage | 48/715 (6.71) | 29/48 (60.42) | |||
| Occupation | |||||
| No (retired, unemployed people and homemakers) | 322/715 (45.03) | 269/322 (83.54) | 2.686 | 1.87–3.85 | <0.001* |
| Yes | 393/715 (54.97) | 257/393 (65.39) | |||
| Area | |||||
| Rural | 48/715 (6.71) | 35/48 (72.92) | 0.965 | 0.50–1.87 | 0.916 |
| Urban | 667/715 (93.29) | 491/667 (73.61) | |||
| Source of drinking water | |||||
| Public system | 662/715 (92.59) | 488/662 (73.72) | 0.903 | 0.49–1.68 | 0.749 |
| Other | 53/715 (7.41) | 38/53 (71.70) | |||
| Presence of water tank | |||||
| Yes | 298/715 (41.68) | 206/298 (69.13) | 1.473 | 1.05–2.06 | 0.023* |
| No | 417/715 (58.32) | 320/417 (76.74) | |||
| Covers water tank | |||||
| Yes | 293/296 (98.99) | 203/293 (69.28) | 0.222 | 0.02–2.48 | 0.221 |
| No | 3/296 (1.01) | 1/3 (33.33) | |||
| Cleans water tank | |||||
| Yes | 261/296 (88.18) | 179/261 (68.58) | 1.145 | 0.53–2.50 | 0.732 |
| No | 35/296 (11.82) | 25/35 (71.43) | |||
| Cleaning frequency of water tank | |||||
| At least once a year | 202/296 (68.24) | 137/202 (67.82) | 1.177 | 0.69–2.01 | 0.550 |
| Do not know or do not clean | 94/296 (31.76) | 67/94 (71.28) | |||
| Sewer system | |||||
| Public system | 59/714 (8.26) | 41/59 (69.49) | 1.243 | 0.70–2.22 | 0.464 |
| Other | 655/714 (91.74) | 484/655 (73.89) | |||
| Garbage disposal | |||||
| Correct | 690/715 (96.50) | 504/690 (73.04) | 2.706 | 0.80–9.15 | 0.109* |
| Incorrect | 25/715 (3.50) | 22/25 (88.00) | |||
| Backyard | |||||
| Clean | 455/715 (63.64) | 336/455 (73.85) | 0.961 | 0.68–1.36 | 0.823 |
| Dirty | 260/715 (36.36) | 190/260 (73.08) | |||
| Cleaning frequency of backyard | |||||
| Low (fortnightly, monthly or occasionally | 122/714 (17.09) | 88/122 (72.13) | 0.918 | 0.59–1.42 | 0.701 |
| High (at least once a week) | 592/714 (82.91) | 437/592 (73.82) | |||
| Washes fruits and vegetables | |||||
| Yes | 707/715 (98.88) | 520/707 (73.55) | 1.079 | 0.22–5.39 | 0.926 |
| No | 8/715 (1.12) | 6/8 (75.00) | |||
| Product used to wash fruits and vegetables | |||||
| Just water | 528/707 (74.68) | 393/528 (74.43) | 1.192 | 0.82–1.74 | 0.362 |
| Sanitary water or vinegar | 179/707 (25.32) | 127/179 (70.95) | |||
| Washes hands prior to meals | |||||
| Always | 637/715 (89.09) | 467/637 (73.31) | 1.131 | 0.66–1.95 | 0.660 |
| Sometimes or never | 78/715 (10.91) | 59/78 (75.64) | |||
| Meat consumption | |||||
| Yes | 709/715 (99.16) | 522/709 (73.62) | 1.396 | 0.25–7.68 | 0.702 |
| No | 6/715 (0.84) | 4/6 (66.67) | |||
| Beef meat consumption | |||||
| Yes | 621/709 (87.59) | 458/621 (73.75) | 1.054 | 0.64–1.74 | 0.838 |
| No | 88/709 (12.41) | 64/88 (72.73) | |||
| Pork meat consumption | |||||
| Yes | 596/709 (84.06) | 440/596 (73.83) | 1.066 | 0.68–1.68 | 0.781 |
| No | 113/709 (15.94) | 82/113 (72.57) | |||
| Sheep meat consumption | |||||
| Yes | 177/708 (25.00) | 128/177 (72.32) | 0.917 | 0.63–1.34 | 0.658 |
| No | 531/708 (75.00) | 393/531 (74.01) | |||
| Poultry meat consumption | |||||
| Yes | 646/709 (91.11) | 473/646 (73.22) | 0.781 | 0.42–1.45 | 0.434 |
| No | 63/709 (8.89) | 49/63 (77.78) | |||
| Fish consumption | |||||
| Yes | 448/708 (63.28) | 326/448 (72.77) | 0.891 | 0.63–1.26 | 0.516 |
| No | 260/708 (36.72) | 195/260 (75.00) | |||
| Raw or undercooked meat consumption | |||||
| Yes | 126/709 (17.77) | 94/126 (74.60) | 1.064 | 0.68–1.65 | 0.784 |
| No | 583/709 (82.23) | 428/583 (73.41) | |||
| Raw kebab consumption | |||||
| Yes | 50/708 (7.06) | 33/50 (66.00) | 0.676 | 0.37–1.25 | 0.209 |
| No | 658/708 (92.94) | 488/658 (74.16) | |||
| Barbecue undercooked meat consumption | |||||
| Yes | 190/709 (26.80) | 142/190 (74.74) | 1.082 | 0.74–1.58 | 0.684 |
| No | 519/709 (73.20) | 380/519 (73.22) | |||
| Smoked sausage consumption | |||||
| Yes | 424/709 (59.80) | 311/424 (73.35) | 0.965 | 0.69–1.36 | 0.839 |
| No | 285/709 (40.20) | 211/285 (74.04) | |||
| Fresh sausage consumption | |||||
| Yes | 506/709 (71.37) | 375/506 (74.11) | 1.091 | 0.76–1.57 | 0.643 |
| No | 203/709 (28.63) | 147/203 (72.41) | |||
| Salami consumption | |||||
| Yes | 222/709 (31.31) | 157/222 (70.72) | 0.807 | 0.57–1.15 | 0.237 |
| No | 487/709 (68.69) | 365/487 (74.95) | |||
| Raw milk | |||||
| Yes | 135/715 (18.88) | 100/135 (74.07) | 1.033 | 0.67–1.58 | 0.882 |
| No | 580/715 (81.12) | 426/580 (73.45) | |||
| Sand/soil contact | |||||
| Yes | 453/715 (63.36) | 343/453 (75.72) | 1.346 | 0.96–1.89 | 0.087* |
| No | 262/715 (36.64) | 183/262 (69.85) | |||
| Own animals | |||||
| Yes | 564/715 (78.88) | 420/564 (74.47) | 1.238 | 0.83–1.84 | 0.291 |
| No | 151/715 (21.12) | 106/151 (70.20) | |||
| Own dogs | |||||
| Yes | 532/564 (94.33) | 397/532 (74.62) | 1.151 | 0.52–2.55 | 0.729 |
| No | 32/564 (5.67) | 23/32 (71.88) | |||
| Own cats | |||||
| Yes | 197/564 (34.93) | 148/197 (75.13) | 1.055 | 0.71–1.57 | 0.793 |
| No | 367/564 (65.07) | 272/367 (74.11) |
p<0.05, Q-square or Exact Fisher Tests, OR: odds ratio, CI: Confidence Interval, MW: the monthly State Minimum Wage at the time of survey was R$834.00, equivalent to US$ 243.15 with an exchange rate of 3.43 in 2016 for US$ Dollar to R$ Real.
* Variables included in the logistic model.
In the univariate analysis, the risk factors associated with toxoplasmosis seroprevalence (p-value <0.05) included not having occupation outside home (retired or unemployed people and own housekeeper) (OR 2.69, CI: 1.87–3.85), older than 18 years (OR 2.65, CI: 1.58–4.45), none or low school level (OR 2.04 CI: 1.45–2.88), low income (OR 1.92, CI: 1.05–3.51) and lack of household water tank (OR 1.47, CI 1.05–2.06) (Table 1). Other analyzed risk factors such as sex (male or female), living area (urban or rural), yard hygiene, meat ingestion, contact with sand or land and owning pets (dog, cat or both) were not associated with seropositivity on surveyed population.
Multivariate analysis has shown a significant increased risk associated with unemployed people (OR 1.67, CI: 1.18–2.64), older than 18 years, adults (18 to 59 years old) (OR 2.60, 95% CI: 1.15–5.84) and elderly (over 60 years old) (OR 3.10, 95% CI: 1.26–7.67) people and lacking household water tank (OR 1.46; 95% CI:1.02–2.08). Regarding school level, only illiteracy was a significant risk factor (OR 4.37; 95% CI: 1,21–15,88) (Table 2). All other risk factors included in the model (income, garbage disposal, contact with sand or land and presence of bathroom, toilet and piped water in the household) were not statistically significant.
Table 2. Final logistic model for the analysis of risk factors associate for seropositivity of IgG anti-T. gondii antibodies in 715 human samples tested by IFAT, from 2015 to 2016, in the city of Ivaiporã, Paraná State, Brazil.
| Variables | Adjusted OR | CI | p-value |
|---|---|---|---|
| Age range (Ref = child up to 11 years old) | |||
| Adolescent (12 to 18 years old) | 1.67 | 0.6–4.68 | 0.330 |
| Adult (19 to 59 years old) | 2.60 | 1.15–5.84 | 0.021* |
| Elderly (above 60 years old) | 3.10 | 1.26–7.67 | 0.014* |
| Schooling (Ref = higher education) | |||
| High School | 0.80 | 0.38–1.69 | 0.558 |
| Elementary School | 1.24 | 0.59–2.61 | 0.573 |
| Illiterate | 4.37 | 1.21–15.88 | 0.025* |
| Occupation (retired, unemployed people and homemakers) | 1.67 | 1.18–2.64 | 0.006* |
| Household lacking water tank | 1.46 | 1.02–2.08 | 0.039* |
OR: odds ratio, CI: Confidence Interval.
* Statistically significant variables
Spatial analysis has shown no significant cluster on studied area. However, most heat areas of Kernel map has shown occurrence of higher intensity number of seropositive individuals concentrated on peri-urban areas (Fig 2).
Fig 2. Kernel map of the seropositivity results for IgG anti-T. gondii antibodies in 715 human samples tested by IFAT, from 2015 to 2016 in the city of Ivaiporã, Paraná State, Brazil”.
Letters represent the four municipal districts: A: Ivaiporã; B: Jacutinga; C: Alto Porã; D: Santa Bárbara.
Discussion
Prevalence of anti-T. gondii on vulnerable population herein (73.57%) has been higher than 41.54% on northern-central [25], 213/356 (59.8%) general population people and 40/66 (60.6%) pregnant women of northern [28] Paraná State, but similar to 527/599 (87.97%) elderly people from Rio Grande do Sul State [29]. Prevalence was expected similar to overall 196/280 (70.00%) seropositivity with 143/207 (69.08%) in rural and 53/73 (72.60%) in urban areas of a slum community about 100 miles from Ivaiporã [19] due to similarities in climate, population dynamics and definitive / intermediate (preys) hosts [30].
The epidemiological profile of higher risk of seropositivity for T. gondii was characterized by more years of life, fewer years of study, lower family income, no occupation outside home and living in household lacking water tank. Thus, risk factors herein were more likely associated to socioeconomic conditions than well-established alimentary habits such as consumption of raw or undercooked meat [4], unwashed vegetables [31] and cat ownership and soil handling [32,33]. In addition, spatial distribution of seropositive cases has demonstrated a higher toxoplasmosis occurrence in suburban than central city areas, where mostly low-income communities have been located.
The odds ratio of adult and elderly seropositivity to IgG anti-T. gondii antibodies were respectively 2.60 and 3.10 higher than in people aged 18 years old or younger. Such association have probably occurred due to longer risk exposure, increasing the individual chances of been infected over time [29,34,35]. The disease manifestation may not be related to latent stage but the loss of disability-adjusted life years (DALY) has been suggested on long term [10]. Previous studies have indicated an association of seropositivity with psychiatric disorders such as bipolarity, obsessive-compulsive disorder, schizophrenia, suicide attempting, compromised memory in elderly people and Parkinson [8,36,37].
On the other hand, the seronegative results of younger and adolescent populations should be considered on social risk of adolescence pregnancy, with higher chances of primo-infection by T. gondii on beginning of woman fertile period. Transplacental transmission of parasite to fetus may trigger the development of serious congenital damages and sequels over the years [6,38]. A cohort study on congenital toxoplasmosis has shown that lower socioeconomic level was a risk factor for non-screening participation during pregnancy, resulting in higher likelihood on disease onset through pregnancy and neonatal in low-income mothers; disease risk was reduced in pregnant women with higher socioeconomic level [39].
Low level of school years was observed in high prevalence of IgG anti-T. gondii antibodies herein, with illiteracy associated to 4.37-fold higher odds ratio for IgG anti-T. gondii antibodies. Lack of information about potential transmission ways may have exposed vulnerable populations to infection [40], and may indicate the importance of education as health promotion and disease prevention. School years and toxoplasmosis were statistically associated (p = 0.010) in a survey with 712 pregnant women from northeastern and 229 (p = 0.049; OR:2.52) from central-western Brazil [34,35], highlighting that prevalence may be higher in illiterate women. As expected, education in public health for pregnant women has shown to be an effective prevention measure [41]. In addition, detection of such variable obtained herein in non-pregnant population may suggest similar results in all populational extracts, supporting educational programs as public health strategy for toxoplasmosis prevention.
Lacking of household water tank was statistically associated to seropositivity for anti-T. gondii antibodies, and previously recognized by the Paraná state as an indicator of social vulnerability as mirror of low-income status, linked to lack of resources for basic sanitary conditions [22]. However, presence of water tank without adequate seal or maintenance may fail to prevent felid feces contamination, as oocysts may environmentally survive for years [42]. Not surprisingly, the biggest world outbreak of toxoplasmosis was reported in nearby northern Parana State in 2001, with the city water reservoir as the source for oocyst spreading and infection of at least 426 inhabitants [43].
Lower socioeconomic level has been associated to T. gondii infection due to non-treated and non-filtrated drinking water as the main risk factor for low-income populations [44]. Despite hand and food washing have been associated to toxoplasmosis prevention [5], hygiene habits were assessed only by self-evaluation questionnaire and therefore may have been overestimated in the present study. Regardless, occupation classified by retired, unemployed people and housekeeping individuals herein were more likely to present anti-T. gondii antibodies (p<0.001), clearly demonstrating that T. gondii exposure has been influenced by socioeconomic issues.
It should be noted that even with an adequate sample size, low number of negatives (189/715) can affect the results when subcategory analysis are performed. For example, the categories of age (above 60 years old) and schooling (illiterate) showed wider confidence intervals and some variables such as meat consumption and garbage disposal showed wider confidence intervals but non-significant association with toxoplasmosis.
Spatial distribution of individuals with anti-T. gondii antibodies has shown two areas with high density of points in the Kernel map, one with the lowest reported family income and the other with unreported income due to recent invasion settlement. Nonetheless, both areas were characterized by inadequate constructions and lack of potable water supply. A previous study in a bigger city within the same northern state region has shown one area only of cluster, which was also correlated to low-income families [25]. In a rural area of northern France [45] and an urban area of northeastern Mainland China [46], despite the spatial analysis approach of soil samples has shown environmental contamination of T. gondii oocysts, no socioeconomic aspects or vulnerability associated risks for infection were tested. In the present study, vulnerability indicators have been directly associated to presence of anti-T. gondii antibodies.
Differences on seroprevalence found herein may be associated to socioeconomic conditions, which may influence on alimentary and hygiene habits [47,48]. Moreover, vulnerability as a group of risk factors may be a major common denominator which has a daily impact on individual exposure to pathogens [40]. In such scenario, epidemiology of zoonotic diseases may demand a comprehensive and holistic approach, denominated as One Health and contemplating human, animal and environmental health [48].
Finally, to the author’s knowledge, no study has yet attempted to assess social vulnerability, either alone or in a multi-factorial investigation, and its association to T. gondii infection. Surprisingly, toxoplasmosis has already been ranked as the second among foodborne parasitic diseases, particularly in vulnerable populations [49].
Conclusions
Socioeconomic vulnerability was statistically associated to Toxoplasma gondii exposure, which included adult and elderly ages, illiteracy, no occupation (retired, unemployed and own housekeeping people) and lack of household water tank were demonstrated by multivariate analysis; influence of low-income family was demonstrated by spatial analysis. On the other hand, variables well established as associated risk factors for toxoplasmosis as living area, yard hygiene, meat ingestion and pet ownership were not statistically significant in the multivariate model and may play a secondary role in such communities.
Supporting information
(DOCX)
Acknowledgments
Authors are kindly thankful to the personal of Ivaiporã Secretary of Health for logistic assistance throughout the survey.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. International Journal for Parasitology. 2009;39: 1385–1394. 10.1016/j.ijpara.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 2.Robert-Gangneux F, Darde M-L, Dardé M-LMM-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews. 2012;25: 264–296. 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey JP, Lago EG, Gennari SM, Su C, JONES JL. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. Cambridge University Press; 2012;139: 1375–1424. 10.1017/S0031182012000765 [DOI] [PubMed] [Google Scholar]
- 4.Belluco S, Mancin M, Conficoni D, Simonato G, Pietrobelli M, Ricci A. Investigating the Determinants of Toxoplasma gondii Prevalence in Meat: A Systematic Review and Meta-Regression. Lepczyk CA, editor. PLOS ONE. 1160 BATTERY STREET, STE 100, SAN FRANCISCO, CA 94111 USA: PUBLIC LIBRARY SCIENCE; 2016;11: e0153856 10.1371/journal.pone.0153856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bresciani KDS, Galvão ALB, Vasconcellos AL de, Soares JA, Matos LVS de, Pierucci JC, et al. Relevant aspects of human toxoplasmosis. Research Journal of Infectious Diseases. 2013;1: 7 10.7243/2052-5958-1-7 [DOI] [Google Scholar]
- 6.Capobiango JD, Mitsuka Breganó R, Navarro IT, Rezende Neto CP, Barbante Casella AÔM, Ruiz Lopes Mori FM, et al. Congenital toxoplasmosis in a reference center of Paraná, Southern Brazil. The Brazilian Journal of Infectious Diseases. Elsevier Editora Ltda; 2014;18: 364–371. 10.1016/j.bjid.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carellos EVM, De Andrade GMQ, Vasconcelos-Santos DV, Januário JN, Romanelli RMC, Abreu MNS, et al. Adverse socioeconomic conditions and oocyst-related factors are associated with congenital toxoplasmosis in a population-based study in Minas Gerais, Brazil. PLOS ONE. 2014;9 10.1371/journal.pone.0088588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallahi S, Rostami A, Shiadeh MN, Behniafar H, Paktinat S, Nourollahpour Shiadeh M, et al. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. Journal of Gynecology Obstetrics and Human Reproduction. France; 2018;47: 133–140. 10.1016/j.jogoh.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 9.Gilbert RE, Freeman K, Lago EG, Bahia-Oliveira LMG, Tan HK, Wallon M, et al. Ocular Sequelae of Congenital Toxoplasmosis in Brazil Compared with Europe. Campos MAS, editor. PLOS Neglected Tropical Diseases. 2008;2: e277 10.1371/journal.pntd.0000277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flegr J, Dama M. Does the prevalence of latent toxoplasmosis and frequency of Rhesus-negative subjects correlate with the nationwide rate of traffic accidents? Folia parasitologica. Czech Republic; 2014;61: 485–494. [PubMed] [Google Scholar]
- 11.Flegr J, Horáček J. Toxoplasma-infected subjects report an Obsessive-Compulsive Disorder diagnosis more often and score higher in Obsessive-Compulsive Inventory. European Psychiatry. 2017;40: 82–87. 10.1016/j.eurpsy.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 12.Sugden K, Moffitt TE, Pinto L, Poulton R, Williams BS, Caspi A. Is Toxoplasma gondii Infection related to brain and behavior impairments in humans? Evidence from a population-representative birth cohort. PLOS ONE. 2016;11: 1–14. 10.1371/journal.pone.0148435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Liu LN, Wang P, Lv TT, Fan YG, Pan HF. Elevated seroprevalence of Toxoplasma gondii in AIDS/HIV patients: A meta-analysis. Acta Tropica. Elsevier B.V.; 2017;176: 162–167. 10.1016/j.actatropica.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 14.Wang Z-D, Wang S-C, Liu H-H, Ma H-Y, Li Z-Y, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. The Lancet HIV. 2017;4: e177–e188. 10.1016/S2352-3018(17)30005-X [DOI] [PubMed] [Google Scholar]
- 15.Dard C, Marty P, Brenier-Pinchart M-P, Garnaud C, Fricker-Hidalgo H, Pelloux H, et al. Management of toxoplasmosis in transplant recipients: an update. Expert Review of Anti-infective Therapy. Taylor & Francis; 2018;16: 447–460. 10.1080/14787210.2018.1483721 [DOI] [PubMed] [Google Scholar]
- 16.Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: A systematic review and meta-analysis. Acta Tropica. 2014;137: 185–194. 10.1016/j.actatropica.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 17.Alvarado-Esquivel C, Hernández-Tinoco J, Sánchez-Anguiano LF, Ramos-Nevárez A, Cerrillo-Soto SM, Sáenz-Soto L, et al. High seroprevalence of Toxoplasma gondii infection in inmates: A case control study in Durango City, Mexico. European Journal of Microbiology and Immunology. 2014;4: 76–82. 10.1556/EuJMI.4.2014.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce BD, Kruszon-Moranb D, Jones JL. The association of Toxoplasma gondii infection with neurocognitive deficits in a population based analysis Brad. Accounts of Chemical Research. 2014;49: 1001–1010. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benitez A do N, Gonçalves DD, Nino B de SL, Caldart ET, Freire RL, Navarro IT. Seroepidemiology of Toxoplasmosis in Humans and Dogs From a Small Municipality in Parana, Brazil. Ciência Animal Brasileira. 2017;18: 1–9. 10.1590/1089-6891v18e-42102 [DOI] [Google Scholar]
- 20.Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Scientific Reports. 2016;6: 22551 10.1038/srep22551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IBGE. Ivaiporã - Panorama geral [Internet]. 2010. Available: https://cidades.ibge.gov.br/brasil/pr/ivaipora/panorama
- 22.IPEA. Atlas da Vulnerabilidade Social nos Municípios Brasileiros. Ipea. 2015. 10.1017/CBO9781107415324.004 [DOI]
- 23.Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH, et al. Epi Info, Version 6: a word processing, data bases, and statistic program for epidemiology on microcomputers. Atlanta, Georgia: Center for Diseases Control and Prevention; 1994. [Google Scholar]
- 24.Camargo ME. Improved technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Revista do Instituto de Medicina Tropical de São Paulo. 1964;6: 117–118. Available: http://www.imt.usp.br/wp-content/uploads/revista/vol06/117-118.pdf [PubMed] [Google Scholar]
- 25.Benitez A do N, Martins FDC, Mareze M, Santos NJR, Ferreira FP, Martins CM, et al. Spatial and simultaneous representative seroprevalence of anti-Toxoplasma gondii antibodies in owners and their domiciled dogs in a major city of southern Brazil. de Boer WF, editor. PLOS ONE. 2017;12: e0180906 10.1371/journal.pone.0180906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulldorff M. A spatial scan statistic. Communications in Statistics—Theory and Methods. Taylor & Francis; 1997;26: 1481–1496. 10.1080/03610929708831995 [DOI] [Google Scholar]
- 27.Team QD. QGIS geographic information system. Open source geospatial foundation project. online; 2016.
- 28.Bittencourt LHF de B, Navarro IT, Pinto SB, Freire RL, Valentim-Zabott M, Mitsuka-Breganó R, et al. Seroepidemiology of toxoplasmosis in pregnant women from the implementation of the Surveillance Program for Acquired and Congenital Toxoplasmosis in municipalities in the western region of Paraná. Brazilian Journal of Gynecology and Obstetrics; 2012;34: 63–68. Available: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-72032012000200004&lang=pt [PubMed] [Google Scholar]
- 29.Engroff P, Ely LS, Guiselli SR, Goularte FH, Gomes I, Viegas K, et al. Soroepidemiologia de Toxoplasma gondii em idosos atendidos pela Estratégia Saúde da Família, Porto Alegre, Rio Grande do Sul, Brasil. Ciência & Saúde Coletiva. 2014;19: 3385–3393. 10.1590/1413-81232014198.12402013 [DOI] [PubMed] [Google Scholar]
- 30.Afonso E, Germain E, Poulle M-LL, Ruette S, Devillard S, Say L, et al. Environmental determinants of spatial and temporal variations in the transmission of Toxoplasma gondii in its definitive hosts. International Journal for Parasitology: Parasites and Wildlife. Australian Society for Parasitology; 2013;2: 278–285. 10.1016/j.ijppaw.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira FP, Caldart ET, Freire RL, Freitas FM De, Miura AC, Mareze M, et al. The effect of water source and soil supplementation on parasite contamination in organic vegetable gardens. Brazilian Journal of Veterinary Parasitology. 2018;2961: 1–11. [DOI] [PubMed] [Google Scholar]
- 32.Carellos EVM, De Andrade GMQ, Vasconcelos-Santos D V, Januário JN, Romanelli RMC, Abreu MNS, et al. Adverse socioeconomic conditions and oocyst-related factors are associated with congenital toxoplasmosis in a population-based study in Minas Gerais, Brazil. PLOS ONE. 2014;9 10.1371/journal.pone.0088588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egorov AI, Converse R, Griffin SM, Styles J, Klein E, Sams E, et al. Environmental risk factors for Toxoplasma gondii infections and the impact of latent infections on allostatic load in residents of Central North Carolina. BMC Infectious Diseases. BMC Infectious Diseases; 2018;18: 1–11. 10.1186/s12879-017-2892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avelar MV, Martinez VO, Moura DL de, Barros IA, Primo AA da S, Duarte AO, et al. Association between seroprevalence of IgG anti-Toxoplasma gondii and risk factors for infection among pregnant women in climério de oliveira maternity, Salvador, Bahia, Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 2017;59 10.1590/S1678-9946201759090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avelar JB, Da Silva MG, Rezende HHA, Storchilo HR, Do Amaral WN, Xavier IR, et al. Epidemiological factors associated with Toxoplasma gondii infection in postpartum women treated in the public healthcare system of goiânia, state of Goiás, Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2018;51: 57–62. 10.1590/0037-8682-0112-2017 [DOI] [PubMed] [Google Scholar]
- 36.Coryell W, Yolken R, Butcher B, Burns T, Dindo L, Schlechte J, et al. Toxoplasmosis Titers and Past Suicide Attempts Among Older Adolescents Initiating SSRI Treatment. 2016;20: 605–613. 10.1080/13811118.2016.1158677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangot AG. Psychiatric aspects of toxoplasmosis: an Indian perspective. Journal of parasitic diseases: official organ of the Indian Society for Parasitology. India; 2016;40: 1636–1639. 10.1007/s12639-015-0684-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsuka-Breganó R, Lopes-Mori FMR, Navarro IT. Toxoplasmose adquirida na gestação e congênita: vigilância em saúde, diagnóstico, tratamento e condutas. 1st ed. Londrina: EDUEL; 2010. [Google Scholar]
- 39.Lange AE, Thyrian JR, Wetzka S, Flessa S, Hoffmann W, Zygmunt M, et al. The impact of socioeconomic factors on the efficiency of voluntary toxoplasmosis screening during pregnancy: A population-based study. BMC Pregnancy and Childbirth. BMC Pregnancy and Childbirth; 2016;16: 1–9. 10.1186/s12884-015-0735-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmo ME do Guizardi FL. O conceito de vulnerabilidade e seus sentidos para as políticas públicas de saúde e assistência social. Cadernos de Saúde Pública. 2018;34 10.1590/0102-311x00101417 [DOI] [PubMed] [Google Scholar]
- 41.Moura FL de Goulart PRM, Moura APP de, Souza TS de, Fonseca ABM, Amendoeira MRR, et al. Fatores associados ao conhecimento sobre a toxoplasmose entre gestantes atendidas na rede pública de saúde do município de Niterói, Rio de Janeiro, 2013–2015*. Epidemiologia e Serviços de Saúde. 2016;25: 655–661. 10.5123/S1679-49742016000300022 [DOI] [PubMed] [Google Scholar]
- 42.Dubey JP. Toxoplasmosis—a waterborne zoonosis. Veterinary parasitology. Netherlands; 2004;126: 57–72. 10.1016/j.vetpar.2004.09.005 [DOI] [PubMed] [Google Scholar]
- 43.Vaudaux JD, Muccioli C, James ER, Silveira C, Magargal SL, Jung C, et al. Identification of an atypical strain of Toxoplasma gondii as the cause of a waterborne outbreak of toxoplasmosis in Santa Isabel do Ivai, Brazil. The Journal of infectious diseases. United States; 2010;202: 1226–1233. 10.1086/656397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahia-Oliveira LMG, Jones JL, Azevedo-Silva J, Alves CCF, Oréfice F, Addiss DG. Highly Endemic, Waterborne Toxoplasmosis in North Rio de Janeiro State, Brazil. Emerging Infectious Diseases. 2003;9: 55–62. 10.3201/eid0901.020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotteland C, McFerrin BM, Zhao X, Gilot-Fromont E, Lélu M. Agricultural landscape and spatial distribution of Toxoplasma gondii in rural environment: An agent-based model. International Journal of Health Geographics. 2014;13: 1–11. 10.1186/1476-072X-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao X, Wang, Wang, Qin H, Xiao J. Land use and soil contamination with Toxoplasma gondii oocysts in urban areas. Science of the Total Environment. Elsevier B.V.; 2016;568: 1086–1091. 10.1016/j.scitotenv.2016.06.165 [DOI] [PubMed] [Google Scholar]
- 47.IPEA. Perfil das Organizações da Sociedade Civil do Brasil [Internet]. 2018. Available: http://www.ipea.gov.br/portal/images/stories/PDFs/livros/livros/180607_livro_perfil_das_organizacoes_da_sociedade_civil_no_brasil.pdf
- 48.WHO. WHO | One Health. WHO. World Health Organization; 2017; Available: http://www.who.int/features/qa/one-health/en/
- 49.WHO. WHO Initiative to Estimative the Global Burden of Foodborne Diseases. 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.