Abstract
BACKGROUND
Accurate assessment of the need for glucocorticoid therapy is essential after transsphenoidal surgery (TSS) for pituitary tumors. Agreement on the best test to use in the early postoperative setting is lacking.
OBJECTIVE
To examine recovery room (RR) cortisol as a predictor of long-term need for glucocorticoids.
METHODS
We conducted a retrospective cohort study of 149 patients who underwent TSS for pituitary tumors between January 2007 and December 2014. Pathological tumor diagnoses were confirmed. Endocrinologists assessed the need for glucocorticoid supplementation within 6 to 8 wk after TSS. We extracted data on preoperative, RR, and day 1 to 3 post-TSS morning serum cortisol (MSC). We reported areas under the receiver operating characteristic curve (AUC) and diagnostic measures for different cortisol measures. We also conducted a logistic regression to identify the most predictive variables.
RESULTS
Eighteen patients required glucocorticoid supplementation at follow-up. RR cortisol was the most accurate measurement in the early postoperative period (AUC [95% confidence interval (CI)], .92 [.85-.99]; P < .001), followed by day 1, 2, and 3 post-TSS MSC, respectively. A threshold RR cortisol of 744.0 nmol/L (26.97 μg/dL) had 90.9% sensitivity and 73.7% specificity for detecting patients in the hypocortisolism group, while 757.5 nmol/L (27.46 μg/dL) had 100% and 70.0%, respectively. The logistic regression identified RR cortisol as the sole significant predictor (odds ratio [CI], .36[.18-.71] for every 100 nmol/L increase; P = .0033).
CONCLUSION
The RR cortisol is accurate in predicting long-term glucocorticoid supplementation and may be the best early postoperative measure. Future larger studies should validate these findings and derive optimal RR cortisol threshold values.
Keywords: Glucocorticoid, Transsphenoidal surgery, Neurosurgery, Pituitary resection, Pituitary tumors
ABBREVIATIONS
- ACTH
adrenocorticotropic hormone
- AUC
area under the curve
- CI
confidence interval
- GC
glucocorticoid
- HPA
hypothalamic–pituitary–adrenal
- IQR
interquartile ranges
- ITT
insulin tolerance test
- MSC
morning serum cortisol
- NPV
negative predictive value
- PPV
positive predictive value
- RCC
Rathke's cleft cysts
- RR
recovery room
- ROC
receiver operating characteristic
- TSS
transsphenoidal surgery
- SD
standard deviation
Acquired anterior pituitary insufficiency is a complication of transsphenoidal surgery (TSS) for pituitary tumors.1-7 Disruptions to the hypothalamic–pituitary–adrenal (HPA) axis can cause adrenocorticotropic hormone (ACTH) deficiency, potentially leading to fatigue, hypotension, and even death.8 Postoperative glucocorticoid (GC) supplementation was historically given to all patients undergoing TSS. Although identifying patients with a deficiency is a higher priority, administering GC in high doses or with long-term use puts patients at risk of developing hyperglycemia, delayed wound healing, hypertension, and bone loss.9-11 Hence, accurately assessing postoperative HPA axis function is essential in deciding which patients require GC supplementation.
The insulin tolerance test (ITT) is considered the gold standard for assessing HPA axis integrity,8,12-14 but is limited in the early postoperative period.13,15 The physiological stress associated with hypoglycemia confers patients to several risks.13,15 Additionally, the ITT is contraindicated in elderly patients and patients with epilepsy or cardiovascular disease.13 Other assays, such as the cosyntropin ACTH stimulation test, are also inadequate in the early postoperative period.16 Hence, these tests are usually delayed for 4 to 6 wk after TSS.13,15,16 Several centers have thus measured early postoperative serum cortisol as a surrogate marker. Despite emerging evidence for this practice,16-22 controversy remains as to which point in the postoperative period is most useful and the most accurate threshold cortisol value to predict the need for GC supplementation.
There is currently no accepted, consistent timing or threshold for early post-TSS morning serum cortisol (MSC) to assess HPA axis function. Suggested day 1 or 2 thresholds range from >110 nmol/L (>4 μg/dL) to >414 nmol/L (>15 μg/dL).16-20 Similar large variability exists for day 5 to 7 MSC thresholds.16,21,22 Only one study by Marko et al. investigated the utility of recovery room (RR) cortisol immediately after TSS, and used a low-dose cortrosyn stimulation test 4 to 6 wk postoperatively as the gold standard.23 They found that RR cortisol ≥414 nmol/L was predictive of normal HPA axis function (sensitivity 98%, positive predictive value [PPV] 99%, accuracy 97%), and that RR cortisol was more accurate than day 1 post-TSS MSC.23
We studied RR cortisol as a predictor of long-term GC therapy, and compared it to other early post-TSS MSC measurements. Our investigation differs from that of Marko et al,23 as we used a different standard and used additional variables to predict which patients require GC. We also conducted a logistic regression to model which variables best predict the need for long-term GC. Moreover, 99% of patients in that study had normal postoperative HPA function, compared to 87.9% of patients in our study, allowing us to better predict which patients require GC. We hypothesized that a blunted RR cortisol response to the stress of surgery was the best predictor of long-term need for GC replacement after TSS.
METHODS
This study was approved by the Research Ethics Board at our hospital. As this was a retrospective study, consent from individual patients was not required. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology Statement in reporting this study.24
Patient Population
We retrospectively reviewed consecutive patients between January 2007 and December 2014 that underwent endoscopic or microscopic TSS for pituitary adenomas or Rathke's cleft cysts (RCC) using previously described techniques.25-27 Most patients had serum cortisol measured in the postanesthesia care unit or “recovery room” after surgery as a standard order. We excluded patients if they received preoperative or intraoperative GC therapy, patients with ACTH-secreting adenomas, patients who received exogenous estrogen or other steroids that can interfere with serum cortisol values, and patients who did not follow-up for postoperative assessments. The need for preoperative or intraoperative GC was based on a preoperative MSC < 220 nmol/L and at the discretion of the treating neurosurgery and endocrinology teams. Patients had daily 0800h postoperative MSC drawn in the early postoperative period. We initiated long-term GC therapy in patients with a postoperative day 1 to 3 0800h MSC lower than 100 nmol/L (3.62 μg/dL), daily cortisol replacement in asymptomatic patients with 100 to 250 nmol/L, stress dosing in patients with 250 to 350 nmol/L (9.06-12.70 μg/dL), and discharged asymptomatic patients with no GC replacement if cortisol levels exceeded 350 nmol/L.
Endocrinologists reassessed patients by clinical evaluation and 0800h MSC within 6 to 8 wk of surgery. The decision to initiate or continue GC therapy was made at the discretion of the treating endocrinologist. Endocrinologists used the following as a guideline: patients without clinical features of cortisol deficiency (eg chronic fatigue, weight loss, nausea, and hypoglycemia) and with follow-up MSC over 350 nmol/L were considered to have no deficiency in the HPA axis. Patients with: (1) clinical features of cortisol deficiency and MSC between 350 and 500 nmol/L (12.70-18.12 μg/dL), or (2) MSC between 100 and 350 nmol/L (with or without symptoms) underwent an ITT (or 250 μg ACTH stimulation test if ITT was contraindicated). A threshold value of >500 nmol/L was considered normal for both tests. Patients with MSC under 100 nmol/L remained on long-term GC replacement. We considered patients to have hypocortisolism if they were initiated or remained on long-term GC therapy during follow-up. This approach represents a standard considering the entire clinical and biochemical picture, tailored to each individual patient's needs.
Data Extraction
Preoperative MSC, RR cortisol, and day 1 to 3 post-TSS MSC were obtained from a central database. We defined RR cortisol as serum cortisol measured within 4 h after leaving the operating room and at least 1 h after resection of the tumor. Serum cortisol level was assessed by ADVIA Centaur® (Siemens Health Care, Erlangen, Germany) competitive immunoassay using direct chemiluminescent technology. This assay is highly specific for cortisol, and has a sensitivity and assay range of 5.5 to 2069 nmol/L (.20-75 μg/dL). In addition, we extracted patient age, sex, surgery type, tumor pathology, average number of preoperative pituitary deficiencies, Knosp score,28 if the tumor was a macroadenoma, if the tumor was recurrent, and postoperative complications. The tumors were investigated in detail by histology, immunohistochemistry and in many cases, by transmission electron microscopy. A pathological diagnosis was achieved based on these findings.
Statistical Analysis
Statistics were reported as mean and standard deviation (SD) or as frequencies. We reported medians and interquartile ranges (IQR) for variables that were not normally distributed.29 We compared means between groups using an independent t-test, and medians with a Wilcoxon 2-sample test. We analyzed categorical variables using Fisher's exact test. We assessed the accuracy of serum cortisol to predict GC need using the area under the curve (AUC) of the corresponding receiver operating characteristic (ROC) curve. We used the ROC curves to derive threshold cortisol values, and calculated the corresponding sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR–), PPV, and negative predictive value (NPV). We also conducted a logistic regression using demographic data, preoperative variables, and early postoperative serum measures using a stepwise selection method. Statistical significance was taken at the 2-tailed 5% level. Analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, North Carolina), and ROC curves using SPSS 20 (SPSS Inc, IBM, Armonk, New York).
RESULTS
There were 362 patients throughout the study period. Patients were excluded as follows: 104 with ACTH-secreting adenoma or preoperative/intraoperative GC use, 33 with exogenous estrogen or other steroid use, 27 for lack of follow-up, 20 for lack of data on early postoperative measures, 17 for tumors other than adenomas or RCC, and 12 for inaccessible data. The remaining 149 patients were included in this study.
Total Sample Characteristics
Patient characteristics are presented in Table 1. The mean (SD) age was 52.2 (14.8) years, and 79 (53.0%) patients were male; 134 (89.9%) patients had endoscopic surgery; 24 (16.1%) patients had recurrent tumors (Table 1). The following number of patients had data for different serum cortisol measures: 128 preoperative, 101 RR, 142 day-1, 127 day-2, and 78 day-3.
TABLE 1.
Characteristics of Study Patients
| Variablea | Total | Hypocortisolism | Nonhypocortisolism | P value |
|---|---|---|---|---|
| Number of patients (%) | 149 | 18 (12.1) | 131 (87.9) | – |
| Sex, n (%) | ||||
| Male | 79 (53.0) | 14 (77.8) | 65 (49.6) | .042 |
| Female | 70 (47.0) | 4 (22.2) | 66 (50.4) | .042 |
| Age, yr | 52.2 (14.8) | 54.3 (12.4) | 51.9 (15.1) | .51 |
| Tumor pathology, n (%) | ||||
| Nonfunctioning | 69 (46.3) | 9 (50.0) | 60 (45.8) | .80 |
| Somatotroph | 35 (23.5) | 2 (11.1) | 33 (25.2) | .24 |
| Lactotroph | 5 (3.4) | 0 | 5 (3.8) | >.99 |
| Gonadotroph | 23 (15.4) | 3 (16.7) | 20 (15.3) | >.99 |
| Oncocytoma | 9 (6.0) | 3 (16.7) | 6 (4.6) | .079 |
| Rathke's cyst | 8 (5.4) | 1 (5.6) | 7 (5.3) | >.99 |
| Macroadenoma, n (%)c | 97 (68.8) | 14 (82.4) | 83 (66.9) | .27 |
| Knosp score, n (%)c | ||||
| 0 | 28 (19.9) | 1 (5.9) | 27 (21.8) | .19 |
| 1 | 34 (24.1) | 1 (5.9) | 33 (26.6) | .12 |
| 2 | 34 (24.1) | 3 (17.6) | 31 (25) | .76 |
| 3 | 36 (25.5) | 9 (52.9) | 27 (21.8) | .014 |
| 4 | 9 (6.4) | 3 (17.6) | 6 (4.8) | .078 |
| Preoperative deficiencies | 1.55 (1.51) | 2.17 (1.54) | 1.47 (1.49) | .065 |
| Recurrent tumor, n (%) | 24 (16.1) | 3 (16.7) | 21 (16.0) | >.99 |
| Surgery type, n (%) | ||||
| Endoscopic | 134 (89.9) | 14 (77.8) | 120 (91.6) | .087 |
| Microscopic | 15 (10.1) | 4 (22.2) | 11 (8.4) | .087 |
| Complications, n (%) | ||||
| CSF leak | 5 (3.4) | 3 (16.7) | 2 (1.5) | .013 |
| Pituitary apoplexy | 3 (2.0) | 3 (16.7) | 0 | .0015 |
| Hypogonadism | 5 (3.4) | 0 | 5 (3.8) | >.99 |
| Diabetes insipidusb | 13 (8.7) | 2 (11.1) | 11 (8.4) | .66 |
| Requiring THS | 27 (18.1) | 7 (38.9) | 20 (15.3) | .023 |
CSF, cerebrospinal fluid; SD, standard deviation; THS, thyroid hormone supplementation.
aAll values are reported as frequency (%) or mean (SD).
bRepresents nontransient diabetes insipidus.
cDoes not include Rathke's cleft cysts as they are not applicable.
Comparison Between Groups
Comparisons between groups are presented in Table 1. Hypocortisolism developed postoperatively in 18 (12.1%) patients. Of 131 patients in the nonhypocortisolism group, 34 had to undergo dynamic testing (they remained classified in that group). The hypocortisolism group had significantly more males (77.8% vs 49.6%; P = .042). Macroadenomas were more frequent in the hypocortisolism group, but this was not statistically significant (82.4% vs 66.9%; P = .27). The Knosp score was generally higher in the hypocortisolism group (Table 1). Of the complications, cerebrospinal fluid leak, pituitary apoplexy, and requiring thyroid hormone supplementation were significantly more frequent in the hypocortisolism group. The 48 patients who did not have RR serum cortisol values did not differ in clinical, operative, or tumor characteristics compared to the 101 patients who had RR cortisol values. This was also true for patients who had missing values for day 1, 2, and 3 post-TSS MSC. Six patients were initiated on GC in the hospital based on day 1 to 3 post-TSS MSC, with RR cortisol values significantly lower in these patients (mean ± SD in nmol/L, 435.8 ± 343.8 vs 886.7 ± 253.5; P < .001).
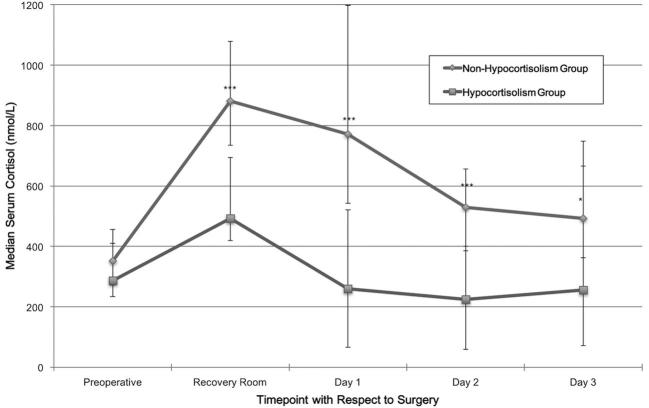
The preoperative MSC values were normally distributed. They were not significantly different between groups (mean ± SD in nmol/L, 313.4 ± 126.9 vs 375.5 ± 149.0; P = .15). All early postoperative cortisol measures were not normally distributed. The hypocortisolism group had a significantly lower serum cortisol in the RR (median [IQR], 493.0 [419.9-693.9] vs 880.4 [734.7-1079.3]; P < .001), at day 1 post-TSS (259.1 [65.4-520.6] vs 770.9 [541.9-1198.0]; P < .001), day 2 (224.0 [59.0-400.6] vs 529.5 [385.4-656.9]; P < .001), and day 3 (256.0 [70.9-748.0] vs 493.0 [362.0-666.0]; P = .037). Figure 1 illustrates these results.
FIGURE 1.
Median serum cortisol comparison between the hypocortisolism and nonhypocortisolism groups at different time points before and after the surgery (length of time between time points is not equivalent). The error bars represent IQR, and not standard deviations or errors. *** denotes P < .001; * denotes P = .037.
Accuracy and Diagnostic Measures
We evaluated the accuracy for each of the cortisol measures to predict the need for GC therapy using the AUC–ROC (Table 2). Of the early postoperative cortisol measures, RR cortisol was most accurate (AUC [95% confidence interval (CI)], .92 [.85-.99]; P < .001; Figure 2), followed by day 1, 2, and 3 post-TSS MSC, respectively (Table 2). Preoperative MSC was not statistically significant (.61 [.45-.77]; P = .20).
TABLE 2.
Accuracy of Different Cortisol Measures
| Cortisol measure | Sample size | AUC | 95% CI | P value |
|---|---|---|---|---|
| Preoperative | 128 | .61 | .45-.77 | .20 |
| RR | 101 | .92 | .85-.99 | <.001 |
| Day 1 AM | 142 | .84 | .72-.95 | <.001 |
| Day 2 AM | 127 | .81 | .67-.95 | <.001 |
| Day 3 AM | 78 | .70 | .46-.94 | .037 |
| In patients with both RR and day 1 a.m. values | ||||
| RR | 95 | .92 | .84-1.00 | <.001 |
| Day 1 a.m. | 95 | .85 | .69-1.00 | <.001 |
AUC, area under the curve; CI, confidence interval.
FIGURE 2.
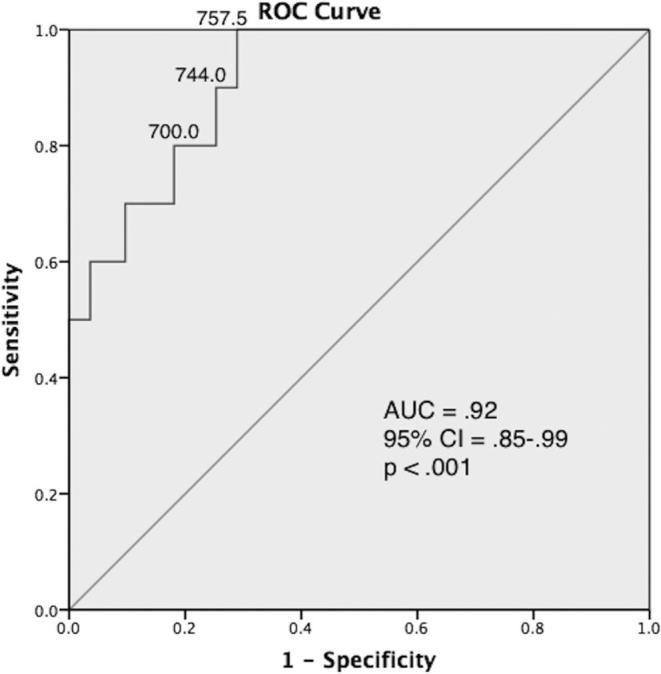
ROC curve for RR cortisol. Cortisol thresholds (nmol/L) are shown on three points on the curve. AUC, area under the curve; CI, confidence interval.
We reported the sensitivity, specificity, LR+, LR–, PPV, and NPV of different cortisol thresholds (Table 3). A RR threshold of 757.5 nmol/L (27.46 μg/dL) resulted in 100% sensitivity and 70.0% specificity. The RR cortisol and day 1 post-TSS MSC were the most useful cortisol measures. Ninety-five patients had both values available. In these patients, the AUC for RR cortisol was .92 (CI, .84-1.00; P < .001), while day 1 post-TSS MSC had an AUC of .85 (.69-1.00; P < .001; Table 2).
TABLE 3.
Diagnostic Measures for Different Cortisol Thresholds at Different Time Points
| Threshold | Sensitivity | Specificity | LR+ | LR– | PPV | NPV |
|---|---|---|---|---|---|---|
| (nmol/L) | [95% CI] | [95% CI] | [95% CI] | [95% CI] | [95% CI] | [95% CI] |
| RR | ||||||
| 700.0 | 81.8% | 82.2% | 4.6 | .22 | 36.0% | 97.4% |
| [48.2-97.7] | [72.7-89.5] | [2.7-7.8] | [.06-.78] | [18.0-57.5] | [90.8-99.7] | |
| 744.0 | 90.9% | 73.3% | 3.4 | .12 | 29.4% | 98.5% |
| [58.7-99.8] | [63.0-82.1] | [2.3-5.0] | [.02-.81] | [15.1-47.5] | [92.0-100] | |
| 757.5 | 100% | 70.0% | 3.3 | .00 | 29.0% | 100% |
| [71.5-100] | [59.4-79.2] | [2.4-4.6] | [.00-.00] | [15.4-45.9] | [94.3-100] | |
| Day 1 | ||||||
| 465.5 | 70.6% | 82.4% | 4.0 | .36 | 35.3% | 95.4% |
| [44.0-89.7] | [74.6-88.6] | [2.5-6.5] | [.17-.75] | [19.8-53.5] | [89.5-98.5] | |
| 469.0 | 76.5% | 81.6% | 4.2 | .29 | 36.1% | 96.2% |
| [50.1-93.2] | [73.7-88.0] | [2.6-6.5] | [.12-.68] | [20.8-53.8] | [90.6-99.0] | |
| 576.5 | 88.2% | 70.4% | 3.0 | .17 | 28.9% | 97.8% |
| [63.6-98.5] | [61.6-78.2] | [2.2-4.1] | [.05-.62] | [17.1-43.1] | [92.2-99.7] | |
| Day 2 | ||||||
| 385.5 | 76.9% | 75.4% | 3.1 | .31 | 26.3% | 96.6% |
| [46.2-95.0] | [66.5-83.0] | [2.0-4.9] | [.11-.83] | [13.4-43.1] | [90.5-99.3] | |
| 416.5 | 84.6% | 69.3% | 2.8 | .22 | 23.9% | 97.5% |
| [54.6-98.1] | [60.0-77.6] | [1.9-4.0] | [.06-.80] | [12.6-38.8] | [91.4-99.7] | |
| 440.5 | 92.3% | 66.7% | 2.8 | .12 | 24.0% | 98.7% |
| [64.0-99.8] | [57.2-75.2] | [2.0-3.8] | [.02-.76] | [13.1-38.2] | [93.0-100] | |
| Day 3 | ||||||
| 257.5 | 54.6% | 89.6% | 5.2 | .51 | 46.2% | 92.3% |
| [23.4-83.3] | [79.7-95.7] | [2.2-12.6] | [.26-.97] | [19.2-74.9] | [83.0-97.5] | |
| 273.5 | 63.6% | 88.1% | 5.3 | .41 | 46.7% | 93.7% |
| [30.8-89.1] | [77.8-94.7] | [2.4-11.7] | [.19-.91] | [21.3-73.4] | [84.5-98.2] | |
| 329.0 | 72.7% | 82.1% | 4.1 | .33 | 40.0% | 94.8% |
| [39.0-94.0] | [70.8-90.4] | [2.2-7.6] | [.13-.88] | [19.1-64.0] | [85.6-98.9] |
CI, confidence interval; LR+, positive likelihood ratio; LR–, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; RR, recovery room.
The likelihood ratios refer to patients in the hypocortisolism group. The PPV also refers to patients in the hypocortisolism group.
The logistic regression model identified RR cortisol as the only significant predictor in the model. We found that the odds of not requiring long-term glucocorticoids were 2.8 times higher for every 100 nmol/L increase in the RR cortisol (odds ratio [CI], .36 [.18-.71]; P = .0033).
DISCUSSION
We retrospectively studied the use of RR cortisol in predicting the need for long-term GC after TSS for pituitary tumors. Of the early postoperative cortisol measurements, we found RR cortisol to be most accurate, followed by day 1, 2, and 3 post-TSS MSC, respectively. As well, we derived optimal cortisol thresholds for the different time-points. A threshold RR cortisol of 744.0 nmol/L (26.97 μg/dL) had 90.9% sensitivity and 73.7% specificity, while 757.5 nmol/L (27.46 μg/dL) had 100% sensitivity and 70.0% specificity. The multivariate logistic regression identified RR cortisol as the only significant predictor in the model.
Study Importance
Accurately evaluating HPA axis integrity after TSS is important. The half-life of ACTH is approximately 10 min, while the half-life of cortisol is approximately 66 min. Since we measured cortisol after at least 1 half-life but still within the period of operative stress, we could detect a blunted stress response in patients who required GC supplementation. Failing to treat patients with an ACTH deficiency can lead to several complications including fatigue, hypotension, and even death,8 while high doses or long-term use of GC can cause hypertension, hyperglycemia, and impaired wound healing.9-11 Identifying patients who are deficient is a higher priority, and some centers test patients’ cortisol levels for several days after surgery for this purpose.16,21,22 The ITT is considered the gold standard for evaluating HPA axis integrity, but is limited in the early postoperative period.13,15 Other tests are also inadequate in this setting.15 Hence, many algorithms use early postoperative serum cortisol as a surrogate for HPA axis integrity.16-23 However, the optimal time-point and threshold remain contested.
Previous Studies
McLaughlin et al18 suggested that a day 1 or 2 post-TSS MSC of >110 nmol/L (>4 μg/dL) was predictive of normal HPA axis function. However, Marko et al19 recommended that patients have at least a day 1 or 2 MSC of ≥414 nmol/L (≥15 μg/dL), while Little et al20 recommended that day 2 MSC should be >276-331 nmol/L (>10-12 μg/dL). Other recommendations differ still, such as the suggestion from Watts et al17 of normal HPA axis function if the day 2 to 3 post-TSS MSC was >248 nmol/L (>9 μg/dL), a requirement for GC supplementation for <83 nmol/L (<3 μg/dL), and dynamic testing for values between 83 and 248 nmol/L. Notably, the discrepancies between these studies are further compounded by the lack of a consistent gold standard. For example, Marko et al19 used definitive HPA axis function testing at 1 to 3 mo postoperatively as their gold standard, whereas Watts et al17 used the ITT 5 to 7 d postoperatively, and Little et al20 relied on whether patients were dependent on GC 1 yr after surgery. Furthermore, the lack of a consistent standard between studies makes it difficult to compare results across them. Our standard considers the entire clinical and biochemical picture, tailored to each individual patient's needs supplemented with appropriate biochemical testing in select patients.
To our knowledge, the study by Marko et al23 is the only published article that used the RR cortisol as a predictor of HPA axis function. One recent study by Asuzu et al30 investigated the utility of early postoperative serum cortisol values for predicting nonremission of Cushing's disease after TSS. The study used these measures to help identify patients with residual adenoma leading to remnant hypercortisolemia.30 Contrastingly, our study aimed to predict which patients would develop a deficiency, and excluded patients with Cushing's disease. Marko et al23 found RR cortisol to be more accurate than the day 1 post-TSS MSC. Our present study found consistent results, as the RR cortisol was more accurate than MSC values on days 1, 2, or 3 following TSS. The hypocortisolism group contained more macroadenomas, more invasive tumors in general, and had a higher complication ratio. This could signify increased surgical trauma in the hypocortisolism group, possibly increasing the likelihood of developing hypocortisolism, but it is difficult to draw definitive conclusions about this with our current data.
Earlier studies demonstrated increased serum cortisol in response to surgical and anesthetic stresses, and formed the background foundation to interpret our findings.31-33 RR cortisol is hypothesized to be better than later postoperative measures because it is measured in closer proximity to a significant and relevant stressor: patients undergoing TSS. If the assessment of cortisol levels is done after at least 1 or 2 half-lives of cortisol, pituitary damage that occurred during surgery will manifest as a blunted response in comparison to those without damage. A greater difference is thus expected between patients who can adequately mount a stress response and those who acquire hypocortisolism. These findings are in keeping with the graded accuracy over time for early postoperative cortisol measurement in which the RR was most predictive, followed by day 1, 2, and 3 post-TSS MSC, respectively.
Clinical Utility of this Study
At our institution, endocrinologists used patients’ clinical presentation and follow-up MSC to assess the need for GC therapy or dynamic testing. Although the ITT is considered the gold standard for assessing HPA axis integrity, it has several disadvantages, including cost, safety issues, time intensiveness, and contraindications in certain patient populations.8,12-15,34-36 It is more helpful for patients with equivocal findings.8,15 Our approach used a standard considering the entire clinical and biochemical picture. We based it on each individual patient's need for long-term GC therapy, and only used dynamic testing when necessary.
We used the ROC curves to determine optimal threshold values for early postoperative cortisol measures. Specifically, we found that a RR cortisol of 744.0 nmol/L (26.97 μg/dL) had 90.9% sensitivity and 73.7% specificity, while 757.5 nmol/L (27.46 μg/dL) had 100% sensitivity and 70.0% specificity for detecting GC therapy need. Having a robust RR cortisol threshold can help in guiding clinical decision-making. In our sample, 757.5 nmol/L had 100% sensitivity in detecting a need for GC therapy. Our cortisol threshold values were high compared to previous studies. This is expected since serum cortisol should rise higher immediately after a significant stressor like TSS as compared with a day or more after TSS. We could not identify any other factors, such as concomitant infections, that could explain the higher values observed. Although these results are promising, this value is likely specific to our patient sample. Future prospective studies must validate these findings before a threshold is recommended in general clinical practice.
Limitations
Our study had some limitations. Firstly, its retrospective nature made it difficult to obtain complete data for all variables. This is especially true given that we had some patients who did not follow-up with endocrinologists 6 to 8 wk after TSS. As well, we could not obtain complete data for every patient included in our study. Future studies could be improved by prospectively evaluating a larger number of patients, which can reduce the bias of missing data. Secondly, the cortisol thresholds derived from the ROC curves are likely specific to this patient sample. Larger studies are required before general clinical recommendations about cortisol thresholds can be made. Finally, not all surgeries were performed in the morning, and this may impact the value of the RR cortisol obtained postoperatively.
CONCLUSION
We showed that RR cortisol is an accurate predictor of the need for long-term GC replacement. An appropriate threshold value for RR cortisol was also derived in our sample. Future larger studies should validate these findings prospectively and derive optimal RR cortisol threshold values.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
Authors are grateful to the Jarislowsky and Lloyd Carr-Harris foundations, and numerous patients and families for their continuous support.
Notes
Part of this work was presented as an oral plenary presentation at the 84th American Association of Neurological Surgeons Annual Meeting in Chicago, Illinois on May 4, 2016 and is published as an abstract with the following citation: Qaddoura A, Shalung T, Meier M, et al. Recovery Room Cortisol Predicts Long-term Glucocorticoid Need after Transsphenoidal Surgery for Pituitary Tumors (Abstract 827). J Neurosurg. 2016;124(4):A1146-209.
COMMENTS
The authors present an interesting observation that may help with glucocorticoid (GC) replacement following transsphenoidal surgery (TSS) based on a retrospective analysis of postoperative serum cortisol data. The authors reviewed the preoperative and postoperative serum cortisol levels in 149 consecutive patients undergoing TSS for pituitary adenomas and Rathke's cleft cysts. Patients with postoperative day 1–3 morning serum cortisol levels less that 3.62 μg/dL received long-term GC replacement. Patients with levels 3.62–9.06 μg/dL received daily GC replacement, while those with levels between 9.06–12.7 μg/dL received stress dosing. For definitive testing of hypocortisolism, endocrinologists depended on clinical assessment and morning serum cortisol (MSC) 6–8 weeks following surgery. Asymptomatic patients with MSC > 12.7 μg/dL were considered eucortisolemic. The rest underwent ACTH stimulation test or Insulin tolerance test to detect adrenal atrophy. Patients that remained or started on long-term GC therapy were considered hypocortisolemic.
The authors then validated the use of serum cortisol level obtained in the recovery room (RR) within 4 hours of leaving the operating room, and at least 1 hour after completing tumor resection. The authors report RR values with median and interquartile range. They report that hypocortisolemic patients had significantly lower RR serum cortisol values. They also found that RR cortisol was the most accurate marker of eventual hypocortisolemia (AUC − 0.92). At 27.46y μg/dL, the RR cortisol value had 100% sensitivity and 70% specificity in detecting eucortisolemia (ie, lack of need for long term GC replacement). The authors discuss the importance and limitations of these findings well.
Overall, this study adds to previous attempts at validating the use of perioperative cortisol data to detect eventual need for hormone replacement.1 This study is based on prior observations that surgical stress leads to a large increase in serum cortisol levels near cessation of the general anesthetic and extubation.2-4 We have utilized a similar strategy to predict early remission following surgery for Cushing's disease.5 The utility of perioperative testing for serum cortisol ultimately is in helping clinicians start GC replacement. The authors present a valid method to help make this decision.
Prashant Chittiboina
Bethesda, Maryland
References
- 1.Marko NF, Hamrahian AH, Weil RJ. Immediate postoperative cortisol levels accurately predict postoperative hypothalamic-pituitary-adrenal axis function after transsphenoidal surgery for pituitary tumors. Pituitary. 2010;13(3):249-255. [DOI] [PubMed] [Google Scholar]
- 2.Sowers JR, Raj RP, Hershman JM, Carlson HE, McCallum RW. The effect of stressful diagnostic studies and surgery on anterior pituitary hormone release in man. Acta Endocrinol (Copenh). 1977;86(1).:25-32 [DOI] [PubMed] [Google Scholar]
- 3.Kaniaris P, Fassoulaki A, Paraschou A. Cortisol levels during enflurane anesthesia in man. Acta Anaesthesiol Belg. 1979;30(4):225-229. [PubMed] [Google Scholar]
- 4.Estep HL, Island DP, Ney RL, Liddle GW. Pituitary-Adrenal Dynamics During Surgical Stress. J Clin Endocrinol Metab. 1963;23(5):419-425. [Google Scholar]
- 5.Asuzu D, Chatain GP, Hayes C, et al. Normalized Early Postoperative Cortisol and ACTH Values Predict Nonremission After Surgery for Cushing Disease. J Clin Endocrinol Metab. 2017;102(7):2179-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
This is a nice and interesting article presenting data from a large study on predictors for long-term cortisone replacement after pituitary surgery. Recovery room cortisol was shown to be an adequate predictor and has the potential to become helpful for clinicians taking care of these patients.
Charlotte Hoybye
Stockholm, Sweden
REFERENCES
- 1. Fatemi N, Dusick J, Mattozo C et al. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008;63(4):709-719. [DOI] [PubMed] [Google Scholar]
- 2. Abosch A, Tyrrell J, Lamborn K, Hannegan L, Applebury C, Wilson C. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab. 1998;83(10):3411-3418. [DOI] [PubMed] [Google Scholar]
- 3. Berker M, Hazer D, Yücel T et al. Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary. 2012;15(3):288-300. [DOI] [PubMed] [Google Scholar]
- 4. Gondim J, Almeida J, Albuquerque L et al. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14(2):174-183. [DOI] [PubMed] [Google Scholar]
- 5. Cappabianca P, Cavallo L, Colao A, Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97(2):293-298. [DOI] [PubMed] [Google Scholar]
- 6. Honegger J, Buchfelder M, Fahlbusch R. Surgical treatment of craniopharyngiomas: endocrinological results. J Neurosurg. 1999;90(2):251-257. [DOI] [PubMed] [Google Scholar]
- 7. Nomikos P, Ladar C, Fahlbusch R, Buchfelder M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas – a study on 721 patients. Acta Neurochir. 2004;146(1):27-35. [DOI] [PubMed] [Google Scholar]
- 8. Cooper M, Stewart P. Diagnosis and treatment of ACTH deficiency. Rev Endocr Metab Disord. 2005;6(1):47-54. [DOI] [PubMed] [Google Scholar]
- 9. Peacey S, Guo C, Robinson A et al. Glucocorticoid replacement therapy: are patients over treated and does it matter? Clin Endocrinol. 1997;46(3):255-261. [DOI] [PubMed] [Google Scholar]
- 10. Sholter D, Armstrong P. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000;16(4):505-511. [PubMed] [Google Scholar]
- 11. Weinstein R. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365(1):62-70. [DOI] [PubMed] [Google Scholar]
- 12. Hartzband P, Van Herle A, Sorger L, Cope D. Assessment of hypothalamic-pituitary-adrenal (HPA) axis dysfunction: comparison of ACTH stimulation, insulin-hypoglycemia and metyrapone. J Endocrinol Invest. 1988;11(11):769-776. [DOI] [PubMed] [Google Scholar]
- 13. Grinspoon S, Biller B. Clinical review 62 : laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923-931. [DOI] [PubMed] [Google Scholar]
- 14. Erturk E, Jaffe C, Barkan A. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350-2354. [DOI] [PubMed] [Google Scholar]
- 15. Ausiello J, Bruce J, Freda P. Postoperative assessment of the patient after transsphenoidal pituitary surgery. Pituitary. 2008;11(4):391-401. [DOI] [PubMed] [Google Scholar]
- 16. Karaca Z, Tanriverdi F, Atmaca H et al. Can basal cortisol measurement be an alternative to the insulin tolerance test in the assessment of the hypothalamic-pituitary-adrenal axis before and after pituitary surgery? Eur J Endocrinol. 2010;163(3):377-382. [DOI] [PubMed] [Google Scholar]
- 17. Watts N, Tindall G. Rapid assessment of corticotropin reserve after pituitary surgery. JAMA. 1988;259(5):708-711. [PubMed] [Google Scholar]
- 18. McLaughlin N, Cohan P, Barnett P, Eisenberg A, Chaloner C, Kelly D. Early morning cortisol levels as predictors of short-term and long-term adrenal function after endonasal transsphenoidal surgery for pituitary adenomas and Rathke's cleft cysts. World Neurosurg. 2013;80(5):569-575. [DOI] [PubMed] [Google Scholar]
- 19. Marko N, Gonugunta V, Hamrahian A, Usmani A, Mayberg M, Weil R. Use of morning serum cortisol level after transsphenoidal resection of pituitary adenoma to predict the need for long-term glucocorticoid supplementation. J Neurosurg. 2009;111(3):540-544. [DOI] [PubMed] [Google Scholar]
- 20. Little A, Oppenlander M, Knecht L, Duick D, White W. Early postoperative serum cortisol measurements guide management in a steroid-sparing protocol and predict need for long-term steroid replacement after resection of nonfunctioning pituitary adenomas. J Biosci Med. 2013;3(1):18-22. [Google Scholar]
- 21. Jayasena C, Gadhvi K, Gohel B et al. Day 5 morning serum cortisol predicts hypothalamic-pituitary-adrenal function after transsphenoidal surgery for pituitary tumors. Clin Chem. 2009;55(5):972-977. [DOI] [PubMed] [Google Scholar]
- 22. Courtney C, McAllister A, McCance D et al. Comparison of one week 0900 h serum cortisol, low and standard dose Synacthen tests with a 4 to 6 week insulin hypoglycaemia test after pituitary surgery in assessing HPA axis. Clin Endocrinol. 2000;53(4):431-436. [DOI] [PubMed] [Google Scholar]
- 23. Marko N, Hamrahian A, Weil R. Immediate postoperative cortisol levels accurately predict postoperative hypothalamic-pituitary-adrenal axis function after transsphenoidal surgery for pituitary tumors. Pituitary. 2010;13(3):249-255. [DOI] [PubMed] [Google Scholar]
- 24. von Elm E, Altman D, Egger M, Pocock S, Gotzsche P, Vandenbroucke J. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-578. [DOI] [PubMed] [Google Scholar]
- 25. Cusimano MD, Fenton RS. A technique for endoscopic pituitary tumor removal. Neurosurg Focus. 1996;1(1):e1-e6. [DOI] [PubMed] [Google Scholar]
- 26. Alahmadi H, Cusimano MD, Woo K et al. Impact of technique on Cushing disease outcome using strict remission criteria. Can J Neurol Sci. 2013;40(3):334-341. [DOI] [PubMed] [Google Scholar]
- 27. Fathalla H, Cusimano MD, Di Ieva A et al. Endoscopic versus microscopic approach for surgical treatment of acromegaly. Neurosurg Rev. 2015;38(3):541-549. [DOI] [PubMed] [Google Scholar]
- 28. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with Invasion of the cavernous sinus space. Neurosurgery. 1993;33(4):610-618. [DOI] [PubMed] [Google Scholar]
- 29. Madadizadeh F, Asar Ezati M, Hosseini M. Common statistical mistakes in descriptive statistics reports of normal and non-normal variables in miomedical sciences. Iran J Public Heal. 2015;44(11):1557-1558. [PMC free article] [PubMed] [Google Scholar]
- 30. Asuzu D, Chatain G, Hayes C et al. Normalized early postoperative cortisol and ACTH values predict nonremission after surgery for cushing disease. J Clin Endocrinol Metab. 2017;102(7):2179-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Estep H, Island D, Ney R, Liddle G. Pituitary-adrenal dynamics during surgical stress. J Clin Endocrinol Metab. 1963;23(5):419-425. [Google Scholar]
- 32. Sowers J, Raj R, Hershman J, Carlson H, McCallum R. The effect of stressful diagnostic studies and surgery on anterior pituitary hormone release in man. Acta Endocrinol. 1977;86(1):25-32. [DOI] [PubMed] [Google Scholar]
- 33. Kaniaris P, Fassoulaki A, Paraschou A. Cortisol levels during enflurane anesthesia in man. Acta Anaesthesiol Belg. 1979;30(4):225-229. [PubMed] [Google Scholar]
- 34. Cozzi R, Lasio G, Cardia A, Felisati G, Montini M, Attanasio R. Perioperative cortisol can predict hypothalamus-pituitary-adrenal status in clinically non-functioning pituitary adenomas. J Endocrinol Invest. 2009;32(5):460-464. [DOI] [PubMed] [Google Scholar]
- 35. Sarlos S, Inder W. Selective use of the insulin tolerance test to diagnose hypopituitarism. Intern Med J. 2013;43(1):89-93. [DOI] [PubMed] [Google Scholar]
- 36. De Tommasi C, Goguen J, Cusimano M. Transphenoidal surgery without steroid replacement in patients with morning serum cortisol below 9 μg/dl (250 Nmol/l). Acta Neurochir. 2012;154(10):1903-1915. [DOI] [PubMed] [Google Scholar]