Abstract
BACKGROUND AND PURPOSE:
A recent study using task-based fMRI demonstrated that the middle frontal gyrus is comparable with Broca's area in its ability to determine language laterality using a measure of verbal fluency. This study investigated whether the middle frontal gyrus can be used as an indicator for language-hemispheric dominance in patients with brain tumors using task-free resting-state fMRI. We hypothesized that no significant difference in language lateralization would occur between the middle frontal gyrus and Broca area and that the middle frontal gyrus can serve as a simple and reliable means of measuring language laterality.
MATERIALS AND METHODS:
Using resting-state fMRI, we compared the middle frontal gyrus with the Broca area in 51 patients with glial neoplasms for voxel activation, the language laterality index, and the effect of tumor grade on the laterality index. The laterality index derived by resting-state fMRI and task-based fMRI was compared in a subset of 40 patients.
RESULTS:
Voxel activations in the left middle frontal gyrus and left Broca area were positively correlated (r = 0.47, P < .001). Positive correlations were seen between the laterality index of the Broca area and middle frontal gyrus regions (r = 0.56, P < .0005). Twenty-seven of 40 patients (67.5%) showed concordance of the laterality index based on the Broca area using resting-state fMRI and the laterality index based on a language task. Thirty of 40 patients (75%) showed concordance of the laterality index based on the middle frontal gyrus using resting-state fMRI and the laterality index based on a language task.
CONCLUSIONS:
The middle frontal gyrus is comparable with the Broca area in its ability to determine hemispheric dominance for language using resting-state fMRI. Our results suggest the addition of resting-state fMRI of the middle frontal gyrus to the list of noninvasive modalities that could be used in patients with gliomas to evaluate hemispheric dominance of language before tumor resection. In patients who cannot participate in traditional task-based fMRI, resting-state fMRI offers a task-free alternate to presurgically map the eloquent cortex.
The determination of hemispheric language dominance is a critical part of the presurgical evaluation of patients with brain tumors. Studies have shown blood oxygen level–dependent (BOLD) task-based functional MR imaging to be an excellent noninvasive alternative to the intracarotid amobarbitol procedure or Wada test.1–4 However, the usefulness and reliability of task-based fMRI are limited in cognitively impaired patients, pediatric patients, and patients with language barriers for whom task completion poses a major challenge. To overcome this limitation, resting-state fMRI (rsfMRI) is emerging as an alternative paradigm-free extraction of brain networks, including language networks, using low-frequency fluctuations in the BOLD signal.5
The Wernicke area and Broca area (BA) are considered primary language centers, and activations in these regions are commonly evaluated for language laterality.1,6,7 However, the presence of abnormal tumor neovasculature and resultant neurovasculature uncoupling can contribute to false-negative signals from the disruption and subsequent diversion of BOLD signals ipsilateral to the tumor.8,9 To overcome this problem, studies are investigating the use of secondary language areas supplementary to, or as substitutes for, primary language areas to determine hemispheric dominance.10 The middle frontal gyrus (MFG) is one of the secondary language areas implicated in nuances of language expression such as semantics, grammar and syntax, verbal fluency, and verbal working memory among other cognitive functions, including attention orientation.11–19 Prior research has noted that the MFG consistently activates during fMRI language tasks,4,20,21 and a recent study using task-based fMRI demonstrated that the MFG is comparable with the BA in its ability to indicate hemispheric dominance for language using a measure of verbal fluency.18
To the best of our knowledge, no previous study has used rsfMRI to estimate language laterality using the MFG. The purpose of this study, therefore, was to investigate whether the MFG can be used as an indicator of language-hemisphere dominance in patients with brain tumors using paradigm-free resting-state fMRI. We hypothesized no significant difference in language lateralization between the MFG and BA and that the MFG can serve as a simple and reliable means of measuring language laterality.
Materials and Methods
Subjects
The study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center. Fifty-one patients with brain tumors (age range, 22–78 years; mean, 51 ± 14.2 years; 31 men) referred for presurgical functional mapping by fMRI were included in this retrospective study. All patients were native English speakers and had no pre-existing language impairment per chart review. Handedness was determined by the Edinburgh Handedness Inventory with 47 patients determined to be right-handed and 4 determined to be left-handed. All patients had subsequent pathologically confirmed intra-axial primary glial tumors. Pathology revealed 20 low-grade (World Health Organization I and II) and 31 high-grade (World Health Organization III and IV) tumors. In 28 patients, the lesion was located in the left hemisphere (7 in the temporal lobe, 15 in the frontal lobe, and 6 in the parietal lobe). In 23 patients, the lesion was located in the right hemisphere (5 in the temporal lobe, 17 in the frontal lobe, and 1 in the parietal lobe).
Data Acquisition
Each patient underwent resting-state fMRI as part of the routine presurgical work-up. Scanning was performed on 3T scanners (GE 750W Discovery, Milwaukee, Wisconsin) using an 24-channel head coil. For task-based and rsfMRI, T2*-weighted images were acquired with a single-shot gradient-echo echo-planar sequence in an axial orientation (TR/TE = 2500/30 ms, flip angle = 80°, slice thickness = 4 mm, FOV = 240 mm2, matrix = 64 × 64) covering the whole brain. 3D T1-weighted images were acquired with a spoiled gradient-recalled sequence (TR/TE = 22/4 ms, matrix = 256 × 256 matrix, flip angle = 30°, slice thickness = 1.5 mm). For the resting-state fMRI scan, patients were instructed to leave their eyes open, focus on looking at a crosshair, and not think about anything during the scan. A total of 160 volumes were acquired. Of the 51 patients included in the study, 40 also performed a silent word-generation task in the same session as part of presurgical language mapping. The language tasks were used to determine language laterality (right-dominant, left-dominant, or bihemispheric dominance) using methodology previously used by Dong et al,18 and the results were documented in the patients' final presurgical mapping report.
Resting-State fMRI Data Analysis
In the current study, we implemented a data-processing scheme as outlined in Bharath et al.22 Data processing was based on SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). In the first step, we discarded the first 5 time points from the functional MR imaging data to allow T1 relaxation. Next head-motion correction was performed to reduce the effect of within-scan head motion. During head-motion correction, we extracted the motion parameters, which describe the subject's motion in 6 different directions. Following motion correction, the subject's fMRI data were coregistered with the subject-specific anatomic images to improve normalization into Montreal Neurological Institute space. Following coregistration, we segmented each subject's anatomic images into gray matter, white matter, and CSF images. During the segmentation procedure, deformation fields were derived to transfer functional images into Montreal Neurological Institute standard space. We performed segmentation using the new segment procedures in SPM12. Each subject's segmentation maps were manually inspected to ensure successful segmentation. Finally, we used this subject-specific deformation field to transform the functional images into standard space images. In the next step, we implemented a linear regression to remove the effects of motion-related noise from the BOLD fMRI data. A general linear model–based regression approach was implemented using 24 motion regressors. This consisted of 6 motion parameters derived in the motion correction step, 6 squared of the original motion parameters, 6 one-time-points delayed version of the motion parameters and finally 6 squared of the delayed motion parameters. No regressors from the CSF or white matter region were included in the regression model. Following regression, residual data were derived and a temporal filtering between the frequency bands of 0.01 and 0.1 Hz was applied. Finally, 6-mm spatial smoothing was applied to the filtered fMRI data.
Spatial Extent of the MFG and BA
To identify active voxels in the BA and MFG regions, we implemented an independent component analysis (ICA)–based approach. In the first step, we performed a single-subject ICA on the filtered fMRI data. For each of the subjects, we performed a separate ICA and extracted 40 independent components. To identify ICAs representing the MFG and BA, we implemented a 2-step process. First, we calculated Dice coefficients between each of the independent components and the BA and MFG mask derived from the Harvard-Oxford Atlas.23,24 Next, we identified the top 5 independent components with the highest Dice coefficient. Each of these 5 independent components was then manually inspected using consensus viewing by 2 neuroradiologists with 4–15 years of fMRI experience to identify independent components representing the functional connectivity of the MFG and BA. After identifying these components, active voxels within the left MFG were calculated as the overlap between the independent component representing the MFG and the MFG region derived through the Harvard-Oxford atlas. The same process was repeated for the right MFG, as well as the left and right BAs to calculate the active voxels for each of the ROIs. Figure 1 illustrates the masks for BA and MFG regions, as well as independent components from a representative patient. The mean activated voxels in the MFG and BA were calculated for each patient. To account for differences in the size of MFG and BA masks, we divided active voxels by the number of voxels in the Harvard-Oxford atlas. We compared the ratio of active voxels using a paired t test. The possible relationship of voxel count between the left MFG and the left BA was shown using a scatterplot, and correlation was tested using the Pearson correlation analysis. For all statistical analyses, a significance level of .001 was used.
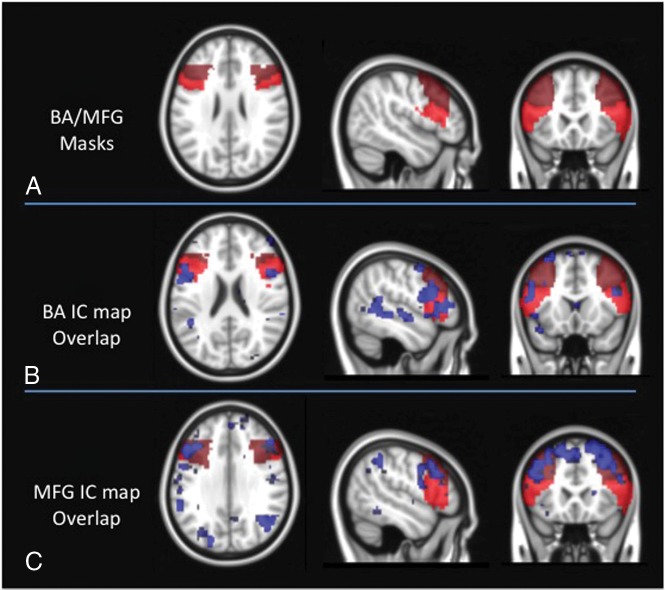
Fig 1.
A, Broca area and middle frontal gyrus masks overlaid on the Montreal Neurological Institute standard brain. B, ICA maps representing the BA network are overlaid on the BA/MFG masks for a representative subject. C, ICA maps representing the MFG network are overlaid on the BA/MFG masks for a representative subject.
Laterality Index in the MFG and BA
The laterality index (LI) for the MFG and BA was calculated using the standard LI formula2,7,25: LI = L − R / L + R, where L and R are the number of active voxels in given ROIs (MFG and BA) in the left and right hemispheres, respectively. The LI ranged from −1 (complete right dominance) to +1 (complete left dominance). Consistent with prior studies,26–28 we defined right-hemispheric laterality as −1 ≤ LI < −0.2, bilaterality as −0.2 ≤ LI ≤ 0.2, and left-hemispheric language laterality as 0.2 < LI ≤ 1.
In addition, we also calculated the functional connectivity between the MFG and BA ROIs. For each of the subjects, the filtered fMRI signal was extracted from the active voxels. The Pearson correlation coefficient was calculated between these time-series to derive functional connectivity between the MFG and BA ROIs.
Effect of Tumor Grade and Location on the LI
Tumors in each of the left and right hemispheres were categorized as high-grade (World Health Organization grades III and IV) or low-grade (World Health Organization grades I and II). For each hemisphere, LIs of the BA and MFG were compared between the high-grade and low-grade tumor groups. Differences in the LI of the BA and MFG between tumors in the left-versus-right hemisphere were also compared. LI differences were assessed using a 2-sample t test with the significance level set at <.05.
Comparison of the LI between rsfMRI and Task-Based fMRI
Forty of the 51 patients included in this study had both rsfMRI and language task–based data obtained in the same session as part of presurgical language mapping. For each of these 40 patients, the LI of the MFG and BA based on rsfMRI was compared with the LI based on task-based fMRI so that a match (eg, left-versus-left) was scored as 100% accurate, while a mismatch (eg, left-versus-right) was scored as 0% accurate.
Results
Spatial Extent of the MFG and BA
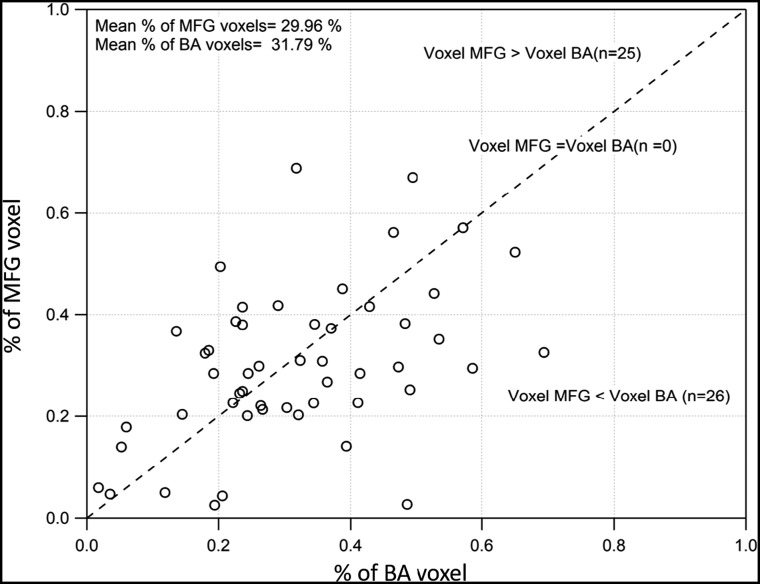
In the first step, we compared the number of active voxels between the left BA and left MFG regions. The scatterplot between the number of active voxels for the left BA and the left middle frontal gyrus is shown in Fig 2. We observed significant correlation for the number of active voxels between the left MFG and left BA (r = 0.47, P < .001). The number of active voxels was found to be significantly higher in the MFG regions (376.92) compared with the BA regions (218.09); however, when we corrected for the size of the MFG and BA masks, these differences were not statistically significant. Similarly, a significantly higher number of voxels was found in the right MFG region (218.09) compared with the right BA region (99.35); however, these differences were also not significant when correcting for the size of the MFG and BA masks (Fig 2).
Fig 2.
Scatterplot between the percentage of BA voxels and the percentage of middle frontal gyrus voxels across participants. Significant correlation was observed between the percentage of MFG and BA voxels across patients.
Laterality Index in the MFG and BA
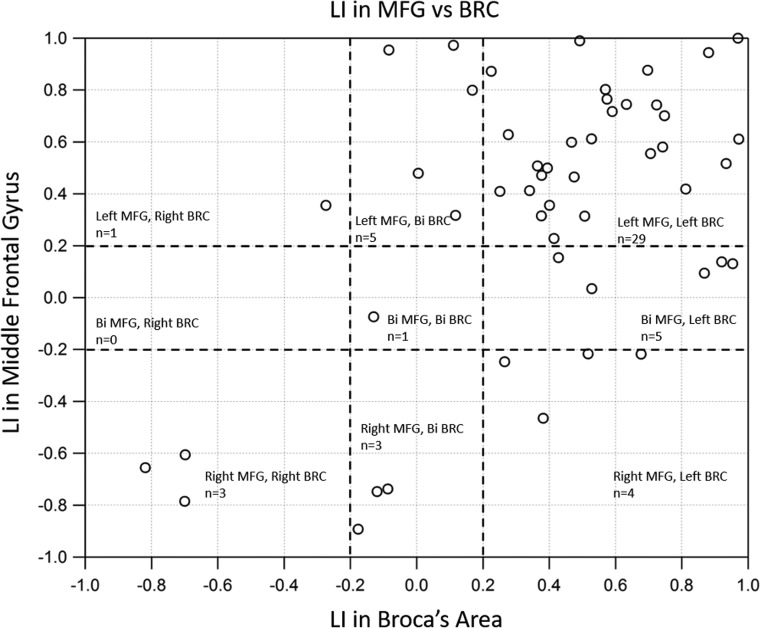
On the basis of the values for the number of active voxels, we calculated laterality indices for both the MFG and BA regions. Figure 3 shows the LI for each of the subjects calculated using these active voxels. We observed significant correlation (r = 0.56, P < .0005) between the LI of the BA and the LI of the MFG regions. As evident from Fig 3, many patients were identified as left-lateralized for both the MFG and BA, while only 3 patients were found to be right-lateralized in both groups.
Fig 3.
Scatterplot of the LIs in the middle frontal gyrus versus the BA. A significant positive correlation was observed between the LI of MFG and BA regions.
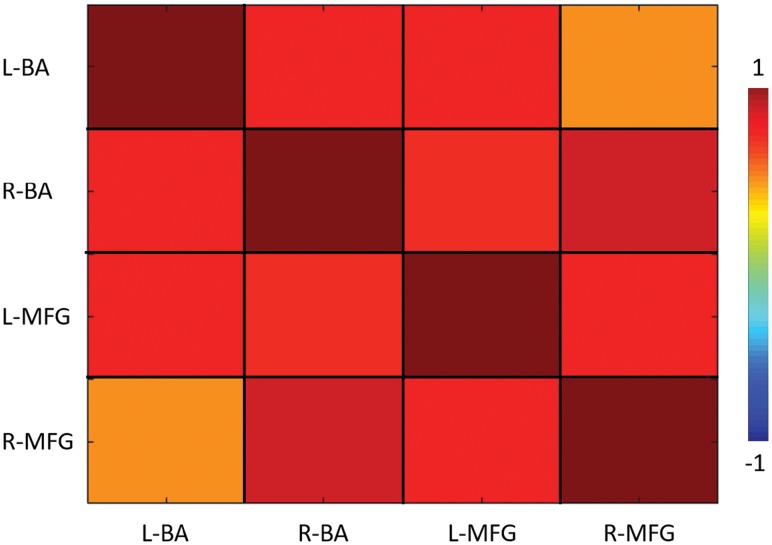
We observed significantly higher functional connectivity between the left and right BAs and the left MFG, while the functional connectivity between the left BA regions and the right MFG was the lowest. Significantly high functional connectivity between right BA regions and the right MFG was noted. Figure 4 depicts group-level functional connectivity between the BA and the MFG regions.
Fig 4.
Group-level functional connectivity between the bilateral BA and MFG regions across patients. Positive functional connectivity was observed between the bilateral BA and bilateral MFG regions.
Effect of Tumor Grade and Location on the LI
In patients with tumors in the left hemisphere, the mean LI in the MFG was 0.06 ± 0.50 for low-grade tumors and 0.26 ± 0.52 for high-grade tumors (P = .3577, two-sample t test), while the mean LI in the BA was 0.26 ± 0.45 for low-grade tumors and 0.30 ± 0.47 for high-grade tumors (P = .8383, two-sample t test). For right-hemisphere tumors, the mean LI in the MFG was 0.38 ± 0.60 for low-grade tumors and 0.58 ± 0.33 for high-grade tumors (P = .3353, two-sample t test), while the mean LI in the BA was 0.56 ± 0.37 for low-grade tumors and 0.43 ± 0.35 for high-grade tumors (P = .3917, two-sample t test).
Tumor location in the left-versus-right hemisphere regardless of grade did not significantly contribute to the LI in both the BA and MFG. The mean LI in the BA for left-hemisphere tumors was 0.29 ± 0.46, and 0.49 ± 0.36 for right-hemisphere tumors (P = .09, two-sample t test). The mean LI in the MFG for left-hemisphere tumors was 0.19 ± 0.52, and 0.48 ± 0.49 for right-hemisphere tumors (P = .04, two-sample t test). However, tumor location in the left hemisphere (frontal-versus-nonfrontal) contributed to differences in the LI in the BA and MFG. The mean LI in the BA for left-frontal tumors was 0.12 ± 0.53, and 0.47 ± 0.28 for left nonfrontal tumors (P = .0472, two-sample t test). The mean LI of the MFG for left-frontal tumors was −0.02 ± 0.49, and 0.44 ± 0.42 for left nonfrontal tumors (P = .0113, 2- sample t test). Tumor location in the right hemisphere (frontal-versus-nonfrontal) also contributed to differences in the LI in the BA but not in the MFG. The mean LI in the BA for right frontal tumors was 0.58 ± 0.26, and 0.14 ± 0.47 for right nonfrontal tumors (P = .0119, two-sample t test). The mean LI in the MFG for right-frontal tumors was 0.56 ± 0.37, and 0.18 ± 0.74 for right nonfrontal tumors (P = .12, two-sample t test).
Comparison of the LI between rsfMRI and Task-Based fMRI
To derive the reliability of the laterality index through resting-state fMRI, we directly compared it with the task-based laterality index. We observed overlap between the laterality index derived from rsfMRI and task-based fMRI. Specifically, when we derived the LI on the basis of the BA using rsfMRI, 27/40 patients (67.5%) showed concordance with the laterality index based on a silent word-generation task. Similarly, when we derived the LI using the MFG and rsfMRI, 30/40 patients (75%) showed concordance with the laterality index based on a silent word-generation task.
Discussion
This study is the first to demonstrate that the MFG is a reliable means of lateralizing language networks using rsfMRI in patients with brain tumors. Our study demonstrated that voxel activation in the MFG correlated with that in the BA. Similarly, the LIs in the MFG correlated with LIs in the BA so that the greater the LI in the BA, the greater it was in the MFG. While the MFG had a slightly higher average LI than the BA, we found no statistically significant differences in language lateralization between the MFG and BA. Most important, LIs calculated from rsfMRI showed significant overlap with the LI determined from task-based fMRI.
Although the MFG is a well-known secondary language area,29–31 research characterizing its exact role in presurgical mapping is limited.11,12,14,32,33 In a study of patients with temporal lobe epilepsy, Lehericy et al4 demonstrated that asymmetric activation in the MFG correlated with hemispheric-dominance determination based on Wada testing. Task-based fMRI has shown similar activation patterns of the MFG in patients with brain tumors who were provided language tasks.20,21 The results of our study support these prior findings and suggest that the MFG can be used as an additional indicator in determining language-hemispheric dominance in clinically difficult cases in which a brain tumor could result in false-negative activation in the BA.
In contrast to prior studies that used task-based paradigms to study the MFG, this study provided a comparative measure of the utility of the MFG relative to the BA in determining language lateralization using task-free resting-state fMRI. In those patients with significant physical or cognitive deficits, performing a task can be challenging; rsfMRI has risen as a promising alternate. This study adds to the growing body of evidence that suggests that rsfMRI can be potentially useful for presurgical mapping of eloquent cortices.5,34–42 Furthermore, in contrast to prior studies that have used a priori seed-based methods for presurgical mapping of language functions with rsfMRI, this study uses a model-free ICA approach that avoided some of the pitfalls of seed-based analysis, including subjective expertise in seed placement.
Our study found no significant effects of tumor grade on language lateralization. However, not unexpectedly, tumor location contributed to language lateralization so that patients with left-hemisphere tumors that were located in the frontal region had lower lateralization compared with those with nonfrontal left-hemisphere tumors. Previous studies have suggested that tumor neovasculature diminished fMRI activation in the tumor hemisphere8,43,44 and consequently affected the fMRI determination of true lateralization for language in patients with brain tumors. Our findings are consistent with previous literature that demonstrated that right-handed patients with neoplasms affecting language areas in the left hemisphere had lower LIs compared with healthy controls.45
Some limitations to this study merit further research. First, the sample size was small, especially in terms of patients with atypical or right-sided language laterality. Second, although we excluded patients with an operation, we acknowledge that this method may not be generalizable to patients after surgery or patients with major structural lesions that may affect the consistency of standardized anatomic templates. Third, we used a model-free ICA approach; specific parameters such as the number of ICA components could have influenced the results of ICA-based functional MR imaging analyses.
Before surgery, it is important to map the eloquent areas close to a tumor to avoid damaging those areas. Generally, direct cortical stimulation has been the method of choice for making this assessment, but it is limited to detecting mainly the cortical surface areas. Moreover, direct cortical stimulation determination of language-hemisphere dominance is not perfect. There is no one method that can provide a completely accurate lateralization of language. Therefore, multiple modalities must be used to determine lateralization of language with as much accuracy and certainty as possible. This recommendation is especially important in cases in which lateralization based on the BA can be misleading and additional markers are needed. Our results suggest the addition of rsfMRI of the MFG to the list of noninvasive modalities that could be used in patients with gliomas to evaluate hemispheric dominance of language before tumor resection.
Conclusions
Activation in the MFG parallels that in the BA in non-task-based rsfMRI assessing hemispheric dominance of language. Task-based and rsfMRI comparisons of the BA and MFG are similar. Therefore, clinical use of rsfMRI for language lateralization, specifically by assessing MFG activity, should be considered in patients with brain tumors.
ABBREVIATIONS:
- BA
Broca area
- BOLD
blood oxygen level–dependent
- ICA
independent component analysis
- LI
laterality index
- MFG
middle frontal gyrus
- rsfMRI
resting-state fMRI
Footnotes
Disclosures: Suril Gohel—UNRELATED: Grants/Grants Pending: National Institutes of Health, Department of Defense. Maria Elena Laino—RELATED: Grant: National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748.* Andrei I. Holodny—RELATED: Grant: National Institutes of Health, Comments: National Institutes of Health–National Institute of Biomedical Imaging and Bioengineering 1R01EB022720–01 (H. Makse and A.I. Holodny, Multiple Principal Investigators), National Institutes of Health–National Cancer Institute 1 R21 CA220144–01 (A.I. Holodny and K. Peck, Multiple Principal Investigators), and subaward on U54 CA 137788 (T. Ahles, Principal Investigator)*; OTHER RELATIONSHIPS: President of fMRI Consulting, LLC, a purely educational entity. Behroze Vachha—RELATED: Grant: National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748, National Cancer Institute/National Institutes of Health award support number R25CA020449.* *Money paid to the institution.
This research was funded, in part, through the National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748. Research reported in this study was also supported by the National Cancer Institute of the National Institutes of Health under award No. R25CA020449 and, in part, by a grant to Maria Elena Laino, Principal Investigator, from the Department of Radiology at Memorial Sloan Kettering Cancer Center for a European School of Radiology Visiting Scholarship in oncologic imaging.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Janecek JK, Swanson SJ, Sabsevitz DS, et al. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia 2013;54:314–22 10.1111/epi.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desmond JE, Sum JM, Wagner AD, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain 1995;118(Pt 6):1411–19 10.1093/brain/118.6.1411 [DOI] [PubMed] [Google Scholar]
- 3. Dym RJ, Burns J, Freeman K, et al. Is functional MR imaging assessment of hemispheric language dominance as good as the Wada test?: a meta-analysis. Radiology 2011;261:446–55 10.1148/radiol.11101344 [DOI] [PubMed] [Google Scholar]
- 4. Lehéricy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology 2000;54:1625–33 10.1212/WNL.54.8.1625 [DOI] [PubMed] [Google Scholar]
- 5. Tie Y, Rigolo L, Norton IH, et al. Defining language networks from resting-state fMRI for surgical planning: a feasibility study. Hum Brain Mapp 2014;35:1018–30 10.1002/hbm.22231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frost JA, Binder JR, Springer JA, et al. Language processing is strongly left lateralized in both sexes: evidence from functional MRI. Brain 1999;122 (Pt 2):199–208 10.1093/brain/122.2.199 [DOI] [PubMed] [Google Scholar]
- 7. Szaflarski JP, Binder JR, Possing ET, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology 2002;59:238–44 10.1212/WNL.59.2.238 [DOI] [PubMed] [Google Scholar]
- 8. Ulmer JL, Hacein-Bey L, Mathews VP, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery 2004;55:569–79, discussion 580–81 [DOI] [PubMed] [Google Scholar]
- 9. Hou BL, Bradbury M, Peck KK, et al. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage 2006;32:489–97 10.1016/j.neuroimage.2006.04.188 [DOI] [PubMed] [Google Scholar]
- 10. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med 2008;358:18–27 10.1056/NEJMoa067819 [DOI] [PubMed] [Google Scholar]
- 11. Brown S, Martinez MJ, Parsons LM. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. Eur J Neurosci 2006;23:2791–803 10.1111/j.1460-9568.2006.04785.x [DOI] [PubMed] [Google Scholar]
- 12. Wang S, Zhu Z, Zhang JX, et al. Broca's area plays a role in syntactic processing during Chinese reading comprehension. Neuropsychologia 2008;46:1371–78 10.1016/j.neuropsychologia.2007.12.020 [DOI] [PubMed] [Google Scholar]
- 13. Abrahams S, Goldstein LH, Simmons A, et al. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp 2003;20:29–40 10.1002/hbm.10126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leung HC, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci 2002;14:659–71 10.1162/08989290260045882 [DOI] [PubMed] [Google Scholar]
- 15. Smolker HR, Depue BE, Reineberg AE, et al. Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Struct Funct 2015;220:1291–306 10.1007/s00429-014-0723-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinmann E, Schmalor A, Prehn-Kristensen A, et al. Developmental changes of neuronal networks associated with strategic social decision-making. Neuropsychologia 2014;56:37–46 10.1016/j.neuropsychologia.2013.12.025 [DOI] [PubMed] [Google Scholar]
- 17. Brennan NP, Peck KK, Holodny A. Language mapping using fMRI and direct cortical stimulation for brain tumor surgery: the good, the bad, and the questionable. Top Magn Reson Imaging 2016;25:1–10 10.1097/RMR.0000000000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong JW, Brennan NM, Izzo G, et al. fMRI activation in the middle frontal gyrus as an indicator of hemispheric dominance for language in brain tumor patients: a comparison with Broca's area. Neuroradiology 2016;58:513–20 10.1007/s00234-016-1655-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Japee S, Holiday K, Satyshur MD, et al. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci 2015;9:23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roux FE, Boulanouar K, Lotterie JA, et al. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery 2003;52:1335–45, discussion 1345–47 [DOI] [PubMed] [Google Scholar]
- 21. Kamada K, Sawamura Y, Takeuchi F, et al. Expressive and receptive language areas determined by a non-invasive reliable method using functional magnetic resonance imaging and magnetoencephalography. Neurosurgery 2007;60:296–305, discussion 305–06 [DOI] [PubMed] [Google Scholar]
- 22. Bharath RD, Munivenkatappa A, Gohel S, et al. Recovery of resting brain connectivity ensuing mild traumatic brain injury. Front Hum Neurosci 2015;9:513 10.3389/fnhum.2015.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor PA, Gohel S, Di X, et al. Functional covariance networks: obtaining resting-state networks from intersubject variability. Brain Connect 2012;2:203–17 10.1089/brain.2012.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor PA, Saad ZS. FATCAT: (an efficient) functional and tractographic connectivity analysis toolbox. Brain Connect 2013;3:523–35 10.1089/brain.2013.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Binder JR, Sabsevitz DS, Swanson SJ, et al. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia 2008;49:1377–94 10.1111/j.1528-1167.2008.01625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging 2008;26:594–601 10.1016/j.mri.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deblaere K, Boon PA, Vandemaele P, et al. MRI language dominance assessment in epilepsy patients at 1.0 T: region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology 2004;46:413–20 [DOI] [PubMed] [Google Scholar]
- 28. Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain 1999;122 (Pt 11):2033–46 10.1093/brain/122.11.2033 [DOI] [PubMed] [Google Scholar]
- 29. Rogalski E, Cobia D, Harrison TM, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci 2011;31:3344–50 10.1523/JNEUROSCI.5544-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fertonani A, Rosini S, Cotelli M, et al. Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res 2010;208:311–18 10.1016/j.bbr.2009.10.030 [DOI] [PubMed] [Google Scholar]
- 31. Grossman M, Cooke A, DeVita C, et al. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage 2002;15:302–17 10.1006/nimg.2001.0971 [DOI] [PubMed] [Google Scholar]
- 32. Acheson DJ, MacDonald MC. Verbal working memory and language production: common approaches to the serial ordering of verbal information. Psychol Bull 2009;135:50–68 10.1037/a0014411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baddeley A. Working memory and language: an overview. J Commun Disord 2003;36:189–208 10.1016/S0021-9924(03)00019-4 [DOI] [PubMed] [Google Scholar]
- 34. DeSalvo MN, Douw L, Takaya S, et al. Task-dependent reorganization of functional connectivity networks during visual semantic decision making. Brain Behav 2014;4:877–85 10.1002/brb3.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitchell TJ, Hacker CD, Breshears JD, et al. A novel data-driven approach to preoperative mapping of functional cortex using resting-state functional magnetic resonance imaging. Neurosurgery 2013;73:969–82, discussion 982–83 10.1227/NEU.0000000000000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosazza C, Aquino D, D'Incerti L, et al. Preoperative mapping of the sensorimotor cortex: comparative assessment of task-based and resting-state FMRI. PLoS One 2014;9:e98860 10.1371/journal.pone.0098860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doucet GE, Pustina D, Skidmore C, et al. Resting-state functional connectivity predicts the strength of hemispheric lateralization for language processing in temporal lobe epilepsy and normals. Hum Brain Mapp 2015;36:288–303 10.1002/hbm.22628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang D, Johnston JM, Fox MD, et al. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: initial experience. Neurosurgery 2009;65(6 Suppl):226–36 10.1227/01.NEU.0000350868.95634.CA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu H, Buckner RL, Talukdar T, et al. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. J Neurosurg 2009;111:746–54 10.3171/2008.10.JNS08846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kokkonen SM, Nikkinen J, Remes J, et al. Preoperative localization of the sensorimotor area using independent component analysis of resting-state fMRI. Magn Reson Imaging 2009;27:733–40 10.1016/j.mri.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 41. Shimony JS, Zhang D, Johnston JM, et al. Resting-state spontaneous fluctuations in brain activity: a new paradigm for presurgical planning using fMRI. Acad Radiol 2009;16:578–83 10.1016/j.acra.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leuthardt EC, Roland J, Breshears JD, et al. Listening to the brain: new techniques in intraoperative brain mapping. Neurosurgery 2013;60(Suppl 1):64–69 10.1227/01.neu.0000430311.63702.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holodny AI, Schulder M, Liu WC, et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 2000;21:1415–22 [PMC free article] [PubMed] [Google Scholar]
- 44. Schreiber A, Hubbe U, Ziyeh S, et al. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-dependent contrast enhancement. AJNR Am J Neuroradiol 2000;21:1055–63 [PMC free article] [PubMed] [Google Scholar]
- 45. Partovi S, Jacobi B, Rapps N, et al. Clinical standardized fMRI reveals altered language lateralization in patients with brain tumor. AJNR Am J Neuroradiol 2012;33:2151–57 10.3174/ajnr.A3137 [DOI] [PMC free article] [PubMed] [Google Scholar]