Abstract
Background:
In mice, restriction of loss of the mitochondrial complex I gene Ndufs4 to glutamatergic neurons confers a profound hypersensitivity to volatile anesthetics similar to that seen with global genetic knockout of Ndufs4. Astrocytes are crucial to glutamatergic synapse functioning during excitatory transmission. Therefore, we examined the role of astrocytes in the anesthetic hypersensitivity of Ndufs4(KO).
Methods.
A tamoxifen activated astrocyte-specific Ndufs4(KO) mouse was constructed. The specificity of the astrocyte-specific inducible model was confirmed by using the green fluorescent protein reporter line Ai6. Approximately 120 astrocyte-specific knockout and control mice were used for the experiments. Mice were anesthetized with varying concentrations of isoflurane or halothane, loss of righting reflex and response to a tail clamp were determined and quantified as the induction and emergence EC50s. Since norepinephrine has been implicated in emergence from anesthesia and astrocytes respond to norepinephrine to release gliotransmitters, we measured norepinephrine levels in the brains of control and knockout Ndufs4 animals.
Results:
The induction EC50s for tail clamp in both isoflurane and halothane were similar between the control and astrocyte-specific Ndufs4(KO) mice at 3 weeks post 4-hydroxy tamoxifen injection (Induction concentration, EC50(ind) - ISO: control=1.27±0.12, astrocyte-specific KO=1.21±0.18, p=0.495; HAL: control=1.28±0.05, astrocyte-specific KO=1.20±0.05, p=0.017). However, the emergent concentrations in both anesthetics for the astrocyte-specific Ndufs4(KO) mice were less than the controls for tail clamp; (Emergence concentration, EC50(em) - ISO: control=1.18±0.10, astrocyte-specific KO=0.67±0.11, p<0.0001; HAL: control=1.08±0.09, astrocyte-specific KO=0.59±0.12, p<0.0001). The induction EC50s for loss of righting reflex were also similar between the control and astrocyte-specific Ndufs4(KO) mice (EC50(ind) - ISO: control=1.02±0.10, astrocyte-specific KO=0.97±0.06, p=0.264; HAL: control=1.03±0.05, astrocyte-specific KO=0.99±0.08, p=0.207). The emergent concentrations for loss of righting reflex in both anesthetics for the astrocyte-specific Ndufs4(KO) mice were less than the control (EC50(em) - ISO: control=1.0±0.07, astrocyte-specific KO=0.62±0.12, p<0.0001; HAL: control=1.0±0.04, astrocyte-specific KO=0.64±0.09, p<0.0001); N≥6 for control and astrocyte-specific Ndufs4(KO) mice. For all tests, similar results were seen at 7 weeks post 4-hydroxy tamoxifen injection. The total norepinephrine content of the brain in global or astrocyte-specific Ndufs4(KO) mice was unchanged compared to control mice.
Conclusion:
The only phenotype of the astrocyte-specific Ndufs4(KO) mouse was a specific impairment in emergence from volatile anesthetic-induced general anesthesia. We conclude that normal mitochondrial function within astrocytes is essential for emergence from anesthesia.
Summary statement:
The mechanisms of action of general anesthesia remain elusive. Mice lacking a mitochondrial complex I gene (Ndufs4) in astrocytes are defective specifically in emergence from anesthesia.
Introduction
The mechanism and identities of cells in the central nervous system which contribute to the anesthetic response are not well elucidated. Inhibition of mitochondrial complex I function has been proposed as a possible molecular mechanism of action of volatile anesthetics 1,2. Both the C. elegans mutant gas-1 and the Drosophila mutant ND23, each defective in a single distinct mitochondrial complex I subunit, display increased sensitivity to volatile anesthetics 1,3. In addition, clinical studies show that some children with complex I defects are hypersensitive to sevoflurane 4. Similarly, a mouse model of complex I dysfunction, Ndufs4(KO), is hypersensitive to volatile anesthetics 5. Furthermore, the function of complex I is inhibited by volatile anesthetics at concentrations that match the whole animal EC50s of normal and mutant mice 1,2. Thus, mitochondrial complex I function profoundly affects anesthetic sensitivity across the animal kingdom.
In the mouse, restriction of Ndufs4 loss to glutamatergic (VGLUT2-expressing) neurons conferred the same profound hypersensitivity to volatile anesthetics as seen with global loss of the protein, with an EC50 one-third that of wild-type mice 6. This finding implicates a role for glutamatergic synaptic transmission in mediating volatile anesthetic hypersensitivity. Glutamatergic synapses have been shown to consist of three cells, a presynaptic and postsynaptic neuron and a supporting astrocyte 7. The configuration is termed the tripartite synapse 7, where the astrocytic roles include both glutamate re-uptake 8,9 as well as modulation of synaptic transmission 10. We therefore questioned whether astrocytic function contributes to the change in anesthetic sensitivity in Ndufs4(KO).
In this study we have investigated the role of astrocytes in mediating the effect of volatile anesthetics by constructing a conditional loss of Ndufs4 in astrocytes. Since this animal loses the gene acutely during adulthood and only in astrocytes, no compensatory changes are expected during development leading to confounding phenotypes. The resulting astrocyte-specific Ndufs4(KO) animal was tested for responses to isoflurane and halothane using two different anesthetic endpoints, loss of righting reflex and response to a tail clamp. We hypothesized that the astrocyte-specific Ndufs4(KO) would be hypersensitive to the volatile anesthetics when compared to control mice.
Materials and Methods
Generation of astrocyte-specific Ndufs4(KO) mice.
All studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the Seattle Children’s Research Institute. Mice with a conditional exon 2-floxed allele of the Ndufs4 gene (Ndufs4lox/lox) were a kind gift from the Palmiter laboratory, University of Washington. The 4-hydroxy tamoxifen inducible Cre-recombinase expressing line, Pgfap (glial fibrillary acidic protein)-CreERT2 was purchased from the Jackson Laboratory, Bar Harbor, ME (Jax stock #012849). Pgfap-CreERT2 mice were crossed to Ndufs4Δ/+ mice following the breeding scheme in Figure 1A. Offspring that were Pgfap-CreERT2/+ and heterozygous for the Ndufs4 deletion (Δ/+) were selected and crossed to Ndufs4 mice floxed at exon 2 to create Pgfap-CreERT2/+;Ndufs4Δ/lox (conditional knockouts of Ndufs4) and Pgfap-CreERT2/+;Ndufs4+/lox mice (controls). The Pgfap promoter driven Cre-ERT2 fusion enzyme requires induction by 4-hydroxy tamoxifen for nuclear translocation and function 11. 4-hydroxy tamoxifen (Sigma, St. Louis, MO) was diluted to a final concentration of 10μg/μl in autoclaved, filtered sunflower seed oil and stored at −20oC until administration. The inducible knockout and control mice were injected intraperitoneally with 4-hydroxy tamoxifen at a dose of 50 μg/g bodyweight daily for a week beginning at post-natal day (PND) 33 and tested for anesthetic behavior in isoflurane and halothane at three and seven weeks post injections. Two mice out of approximately 120 injected mice did not survive the injection regime and were excluded from the analyses.
Figure 1: Generation of the astrocyte-specific Ndufs4(KO) mouse model.
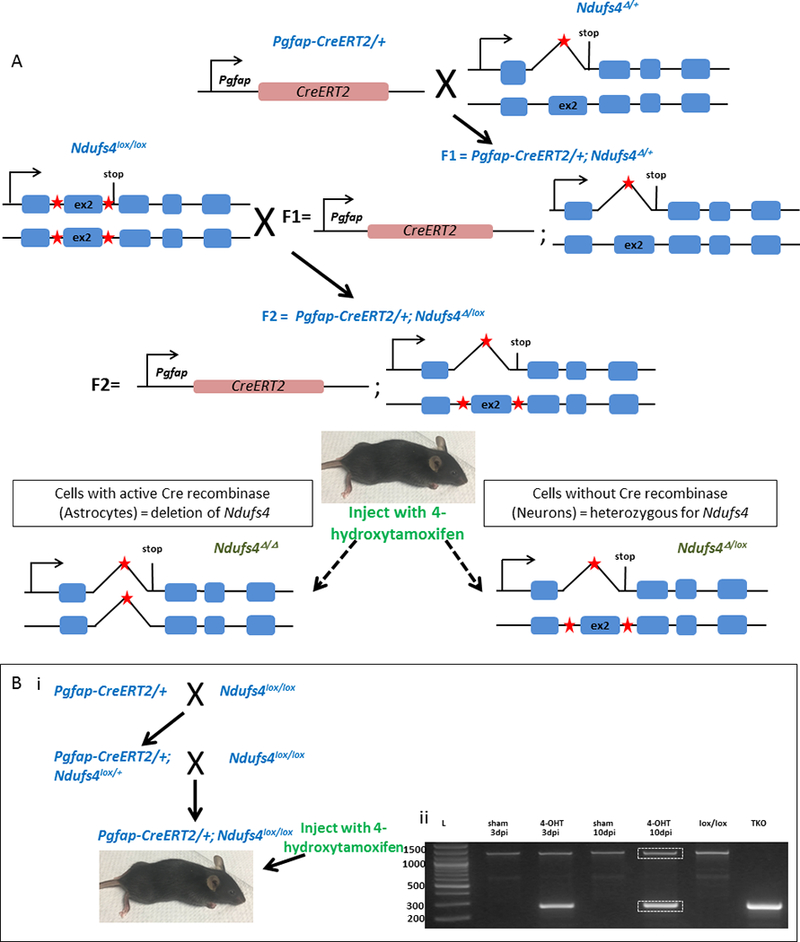
A. Schematic showing the crossing strategy used. The heterozygous Ndufs4Δ/+ mice do not display hypersensitivity to volatile anesthetics or the Leigh syndrome pathogenesis displayed by the global KO, indicating that Ndufs4 is haplo-sufficient. The Pgfap-CreERT2 allele has a dominant phenotype; the breeding recommendation from the JAX lab for these mice is to maintain them as heterozygotes. Pgfap-CreERT2/+ mice were crossed to Ndufs4Δ/+ mice. From the resulting F1, Pgfap-CreERT2/+;Ndufs4Δ/+ offspring were selected and crossed to Ndufs4lox/lox mice to generate F2 Pgfap-CreERT2/+;Ndufs4 Δ/lox (conditional astrocyte-specific Ndufs4(KO)) and Pgfap-CreERT2/+;Ndufs4+/lox mice (controls). We used Ndufs4lox/Δ instead of Ndufs4lox/lox to avoid possible recombination due to the leakiness of the promoter (as seen with the Pvglut2 driven Cre mice). Both genotypes were injected with 4-hydroxytamoxifen intraperitoneally at a concentration of 50 μg/g bodyweight daily from post-natal day 33 to 39. Red stars indicate the loxP sites. B. i: Schematic of generation of mice to confirm loss of full length Ndufs4 upon 4-hydroxy tamoxifen induced activation of Cre recombinase in astrocytes. Pgfap-CreERT2/+ mice were crossed to Ndufs4lox/lox mice; from the progeny, Pgfap-CreERT2/+;Ndufs4lox/+ animals were selected by genotyping and crossed again with Ndufs4lox/lox to generate Pgfap-CreERT2/+;Ndufs4lox/lox mice, which were injected with 4-hydroxy tamoxifen or vehicle control (sham). The brains of the injected mice were harvested after 3 and 10 days post injection (dpi) and genotyped. B. ii: Genotyping gel of brains of Pgfap-CreERT2/+;Ndufs4lox/lox after 4-hydroxy tamoxifen or sham injections, using PCR (lanes 2–5, dpi – days post injection of 4-hydroxy tamoxifen or sham oil control). Sham injections yield only a large uncut band at 1300bp, while 4-hydroxy tamoxifen injections show both the large band (uncut in neurons) as well as a smaller band (excised in astrocytes) at 270bp. Sequencing of excised bands outlined in white rectangles (lane 5), confirmed a full length allele as well as a truncated allele lacking the second exon, as predicted by size, in the 4-hydroxy tamoxifen injected mice. Genomic DNA isolated from the tails of Ndufs4lox/lox mice and total Ndufs4(KO) (TKO) were used as controls (lanes 6 and 7 respectively). Lane 1 shows the DNA ladder size markers (base pairs).
To demonstrate 4-hydroxy tamoxifen induced exon excision by the Cre-recombinase, Pgfap-CreERT2 mice were crossed to Ndufs4lox/lox mice following the breeding scheme in Figure 1B. Pgfap-CreERT2/+;Ndufs4lox/+ offspring were selected from the offspring and crossed to Ndufs4lox/lox to generate Pgfap-CreERT2/+;Ndufs4lox/lox mice. After injecting intraperitoneally with 4-hydroxy tamoxifen as described above, the deletion was confirmed by PCR.
Generation of astrocyte-specific Ai6 reporter mice.
The inducible Cre-recombinase expressing line, Pgfap-CreERT2/+, was crossed to the Ai6 (ai6/ai6) reporter line 12, which produces ZsGreen, a green fluorescent protein (GFP) only in Cre-recombinase expressing cells. When injected with 4-hydroxy tamoxifen, the astrocytes of the Pgfap-CreERT2/+;ai6/+ progeny express Cre-recombinase and ZsGreen. The efficiency of Cre-recombinase induction and recombination by 4-hydroxy tamoxifen was assessed by immunohistochemistry (see below).
Behavioral testing.
Mice (6–7 for each experiment, exact number specified in figure legends) were anesthetized with isoflurane or halothane and assayed for loss of righting reflex or response to a tail clamp as described by Sonner et al. 13. The volatile anesthetics were delivered by inline gas lines to the mouse chamber. Step sizes were 0.1% for both volatile anesthetics in LORR, 0.2% in TC. Equilibration time was 10, 20 or 30 minutes at each step, for separate sets of mice (data shown for 20-minute interval experiments only). For induction and emergence assays (done consecutively), the same step sizes and equilibration times were maintained. Anesthetic concentrations were analyzed by gas chromatography as described 14. Animals were kept warm on a heating pad throughout and allowed to recover for at least 24 h before testing again, with a different anesthetic or for a different endpoint. The observers were blinded to the genotype of the tested mice. The concentration for induction of anesthesia was defined as the average concentrations of gas in the last sample before loss of response and in the first sample at which response was lost. The concentration for emergence was defined as the average concentration of gas in the first sample at which the animal once again responded, and the sample immediately prior. All EC50s for induction and emergence were calculated from the quantal endpoints, i.e. the averages of the induction or emergent concentrations for all the mice in a specific anesthetic. The mice were tested at 3 weeks and 7 weeks post injection of 4-hydroxy tamoxifen and then sacrificed. All animals survived the anesthetic exposure.
Immunohistochemical analysis.
Mice were euthanized using CO2, and isolated brains were fixed in 4% paraformaldehyde overnight at 4oC, cryoprotected in 30% sucrose in PBS for three days and embedded in Optimal Cutting Temperature (OCT) compound (Tissue-tek). Brains were then sliced at 10 μm thickness and mounted on slides. For heat-induced epitope retrieval, slides were then boiled in sodium citrate buffer (pH 6.0) at 100oC for 20 minutes using a water bath. After this treatment, Cre recombinase mediated ZsGreen fluorescence was quenched, but recaptured with anti-GFP antibodies. Slides were then blocked in 10% normal donkey serum in PBS for 1 hour at room temperature. Primary antibodies used were mouse anti-GFAP (1:100, Chemicon) and goat anti-GFP (1:500, Abcam) incubated overnight. Secondary antibodies used were donkey anti-mouse IgG-Alexa Fluor 568 (1:1000, Abcam) and donkey anti-goat IgG-Alexa Fluor 488 (1:2000, Life Technologies), and were incubated for an hour at room temperature.
Norepinephrine assay.
Brains of 40-day old global Ndufs4(KO) and wild-type mice along with those of 60-day old astrocyte-specific KO mice (3 weeks post 4-hydroxy tamoxifen injection), were snap-frozen in dry ice and stored at −80oC. 60-day old astrocyte specific KO animals were used to allow for complete loss of the NDUFS4 protein post 4-hydroxy tamoxifen injection at PND33 15. The brains were thawed, homogenized in PBS containing protease inhibitor cocktail (Sigma), sonicated on ice and centrifuged. After estimating the protein content of the supernatant, 20μg each of the total protein extract was subjected to norepinephrine extraction and measurement following the manufacturer’s protocol (Mouse/rat norepinephrine assay kit, Eagle Biosciences catalogue # NOU39-K010).
Statistics.
The effective concentration for 50% of the animals tested (EC50) for volatile anesthetics, was determined as described by Sonner et al., using an up and down method 13. Values for EC50s were compared between the WT and KO strains using two-tailed two-sample t-tests with unequal variance. No statistical power calculation was conducted prior to the study. The sample sizes were based on the sample sizes necessary to establish significance in previous studies with global and glutamatergic-specific knockouts of Ndufs4 6. For the norepinephrine assay, the norepinephrine concentrations were compared between the WT, global Ndufs4(KO) and astrocyte-specific Ndufs4(KO) brain samples using one-way ANOVA and assessed by constructing a barplot. We set p-value threshold at p<0.01 to lessen the likelihood of reporting a false positive result. Since we compared multiple groups, a Bonferroni correction was performed to determine significance of the p-values. Significance was defined as a p<0.01 for all analyses except where subjected to a Bonferroni correction where the critical p<0.01 was divided by the number of comparisons to derive the corrected cut-off. The measures of variability assessed by standard deviation are indicated in the figures and legends. Outliers, if any, were always included in the analyses. All statistical analyses were performed using R version 3.4.1, and plotted using ggplot2.
Results
Characterization of the inducible Pgfap-CreERT2 model.
We constructed the inducible astrocyte-specific Ndufs4(KO) mouse model, with the Cre-recombinase expressed under the control of an astrocyte-specific promoter Pgfap (Figure 1A). Activation of Cre-recombinase was induced by 4-hydroxy tamoxifen injections at PND 33–40, after gfap expression had ended in neuronal progenitors, thereby temporally limiting the expression and nuclear localization of Cre-recombinase to astrocytic cells. Knockout of Ndufs4 in the brain cells of 4-hydroxy tamoxifen injected Ndufs4lox/lox mice was confirmed by genotyping the mice brains with and without 4-hydroxy tamoxifen injections (Figure 1B). At 3 days and 10 days post injection, we found that Cre-recombinase mediated excision of Ndufs4 was evident in the brains of the 4-hydroxy tamoxifen injected Pgfap-CreERT2/+;Ndufs4lox/lox mice, while it was absent in the brains of sham-injected Pgfap-CreERT2/+;Ndufs4lox/lox mice (Figure 1B). The lox and KO (Δ) allelic PCR amplification bands in the gel from the astrocyte-specific Ndufs4(KO) were excised and sequenced to confirm Cre-recombinase mediated deletion of exon 2. This result confirmed that 4-hydroxy tamoxifen injections resulted in knockout of Ndufs4 in the brain cells. The astrocyte-specific KO mouse was similar to the controls in terms of life span, appearance, grooming characteristics and growth pattern. Unlike the global Ndufs4(KO), the astrocyte-specific KO mice did not become progressively ataxic, lose weight or die young.
The NDUFS4 antibody did not resolve astrocyte-specific loss of the protein in a background in which all other cells in the CNS were fully expressing it. Therefore, to determine the specificity of the 4-hydroxy tamoxifen induced Cre-lox system, we crossed the 4-hydroxy tamoxifen sensitive Pgfap-CreERT2 driver line with the ai6/ai6 reporter mice 12, which harbors a Cre-inducible green fluorescent protein (ZsGreen) expression cassette. After genotyping the progeny, Pgfap-CreERT2/+;ai6/+ mice and control ai6/+ sibling mice were injected daily with 4-hydroxy tamoxifen for a week from PND 33 to 40. When injected with 4-hydroxy tamoxifen, the GFAP expressing astrocytes of Pgfap-CreERT2/+;ai6/+ mice should express induced ZsGreen. Three weeks following the last day of injections, the brains were harvested and immunostained (Figure 2, Supp. Figure 1). Specific expression of Cre-recombinase within astrocytes of the Cre/+;ai6/+ mice was confirmed by the yellow appearance of the co-localized GFAP (labeled red) and ZsGreen (labeled green) under confocal microscopy (Figure 2). The control ai6/+ brain slices did not co-express ZsGreen and hence the astrocytes appeared red.
Figure 2: Confirmation of the specificity of the Cre-lox system by immunohistochemistry of the Ai6 reporter line.
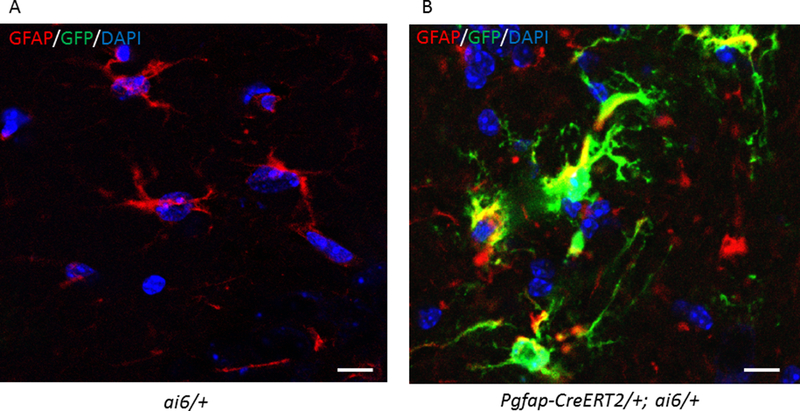
Confocal images of the 4-hydroxy tamoxifen injected Pgfap-CreERT2/+;ai6/+ and ai6/+ mice brain slices. A. ai6/+ mice which only express a green marker (Zsgreen) when activated by the Cre-recombinase were used as controls. Nuclei were stained with DAPI (blue), astrocytes with anti-GFAP (red) and Zsgreen with anti-GFP (green). There is no green seen in astrocytes since there is no Cre-recombinase acting in these animals. B. Immunohistochemistry using anti-GFAP antibody reveals the co-localization of Zsgreen in the astrocytes of Pgfap-CreERT2/+;ai6/+ reporter mice when Cre-recombinase is activated by 4-hydroxy tamoxifen. Thus, the localization of Zsgreen marks those cells with Pgfap driven Cre-recombinase mediated excision of the floxed gene.
Anesthetic sensitivity, Tail Clamp:
The half-life for the NDUFS4 protein in mouse brain has been shown to be 17 days; its half-life in astrocytes is not known 15. To allow for possible tissue-specific differences in protein half-life, we tested the mice at three and seven weeks after knocking out the gene; the phenotype remained stable over time. We subjected the mice to 10, 20 and 30 minute intervals of anesthetic exposure at each step to rule out a pharmacokinetic effect. All data are shown for 20 minutes of exposure of mice to each concentration of the volatile anesthetic. The astrocyte-specific Ndufs4(KO) (Pgfap-creERT2/+;Ndufs4Δ/lox) and control sibling mice were first tested for TC response at 3 weeks and 7 weeks post injection of 4-hydroxy tamoxifen, with increasing concentrations of ISO or HAL. The induction concentrations for the loss of tail flick were not different between the astrocyte-specific Ndufs4(KO) and control animals in both ISO and HAL (Induction concentration, EC50(ind) - ISO: control=1.27±0.12, astrocyte-specific Ndufs4(KO)=1.21±0.18, p=0.495; HAL: control=1.28±0.05, astrocyte-specific Ndufs4(KO)=1.20±0.05, p=0.017 at 3 weeks; ISO: control=1.24±0.07, astrocyte-specific Ndufs4(KO)=1.18±0.13, p=0.305; HAL: control=1.19±0.04, astrocyte-specific Ndufs4(KO)=1.22±0.07, p=0.396 at 7 weeks) (Table 1). Control animals emerged from the anesthetized states at concentrations similar to their induction concentrations. However, the conditional astrocyte-specific Ndufs4(KO)s emerged from the anesthetic state at significantly lower concentrations than did the controls (Emergence concentration, EC50(em) - ISO: control=1.18±0.10, astrocyte-specific Ndufs4(KO)=0.67±0.11, p<0.0001; HAL: control=1.08±0.09, astrocyte-specific Ndufs4(KO)=0.59±0.12, p<0.0001 at 3 weeks; ISO: control=1.20±0.05, astrocyte-specific Ndufs4(KO)=0.67±0.10, p<0.0001; HAL: control=1.15±0.10, astrocyte-specific Ndufs4(KO)=0.66±0.10, p<0.0001 at 7 weeks) (Table 1, Figure 3, Supp. Figure 2). While the difference between the induction/emergence concentrations was not as large as seen with the astrocyte-specific Ndufs4(KO), there was a statistically significant change between the induction versus emergence in control animals in HAL, with 1.28% versus 1.08% respectively at three weeks (p=5.695X10−4). The difference was not seen at 7 weeks (1.19% versus 1.15%, p=0.277).
Table 1:
Effects of astrocyte-specific Ndufs4(KO) on anesthetic sensitivity for response to tail clamp.
| Tail Clamp-ISO Emerge/Induce | Tail Clamp-HAL Emerge/Induce | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Astrocyte-specific Ndufs4(KO) | Control | Astrocyte-specific Ndufs4(KO) | |||||
| Induction | Emergence | Induction | Emergence | Induction | Emergence | Induction | Emergence | |
| 3 weeks | 1.27±0.12 | 1.18±0.10 | 1.21±0.18 | 0.67±0.11* | 1.28±0.05 | 1.08±0.09 | 1.20±0.05 | 0.59±0.12* |
| 7 weeks | 1.24±0.07 | 1.20±0.05 | 1.18±0.13 | 0.67±0.10* | 1.19±0.04 | 1.15±0.10 | 1.22±0.07 | 0.66±0.10* |
In each cell, bold numbers show the ratios of EC50s for emergence and induction for isoflurane or halothane, for control mice or astrocyte-specific Ndufs4(KO), at 3 weeks and 7 weeks post 4-hydroxytamoxifen injections. The endpoint used is response to tail clamp. The lower set of numbers in each cell are the measured values for the EC50s (emergence) and the EC50s (induction), respectively. p-values compare the KO ratio to the corresponding control ratio.
indicates p< 0.005. N=6 for controls, N≥6 for the astrocytic Ndufs4(KO).
Figure 3: Astrocyte-specific knockout of Ndufs4 causes hysteresis between induction and emergence in TC. A.
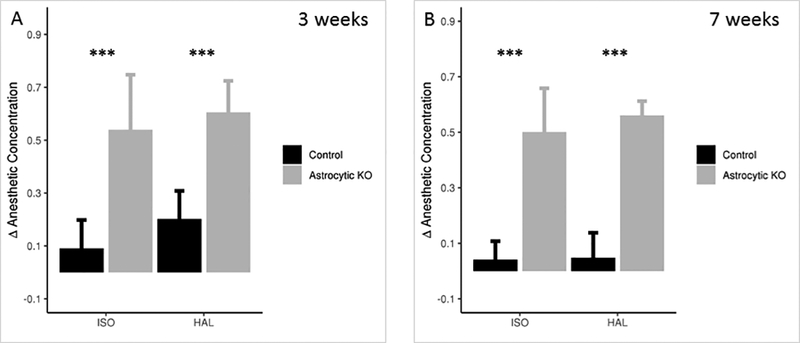
Δ Anesthetic Concentration, the change in the anesthetic concentration between induction and emergence, required to respond to tail clamp before and after exposure to ISO or HAL, at 3 weeks post injection of 4-hydroxy tamoxifen. N=6 for control, N=7 for the astrocyte-specific Ndufs4(KO). B. Δ Anesthetic Concentration required to respond to tail clamp before and after exposure to ISO or HAL, at 7 weeks post injection of 4-hydroxy tamoxifen. N=6 for control and the astrocyte-specific Ndufs4(KO). Tests for significance done on the difference between the EC50s for induction and emergence *** p<0.001. The error bars represent SD.
Anesthetic Sensitivity, Loss of righting reflex.
To determine if the altered concentration for emergence was specific to a behavioral endpoint and possibly a CNS region, we tested a second endpoint, loss of righting reflex (LORR). The anesthetic concentration requirement for LORR (induction) was similar between the control and astrocyte-specific KO mice in ISO and HAL (Induction concentration, EC50(ind) - ISO: control=1.02±0.10, astrocyte-specific Ndufs4(KO)=0.97±0.06, p=0.264; HAL: control=1.03±0.05, astrocyte-specific Ndufs4(KO)=0.99±0.08, p=0.207 at 3 weeks; ISO: control=0.92±0.07, astrocyte-specific Ndufs4(KO)=0.98±0.05, p=0.128; HAL: control=0.98±0.08, astrocyte-specific Ndufs4(KO)=0.93±0.13, p=0.410 at 7 weeks) (Table 2). The control animals regained the righting reflex (emergence) at close to the induction concentration, while the astrocyte-specific Ndufs4(KO)s recovered their righting reflex at significantly lower concentrations than the controls (Emergence concentration, EC50(em) - ISO: control=1.0±0.07, astrocyte-specific Ndufs4(KO)=0.62±0.12, p<0.0001; HAL: control=1.0±0.04, astrocyte-specific Ndufs4(KO)=0.64±0.09, p<0.0001 at 3 weeks; ISO: control=0.89±0.06, astrocyte-specific Ndufs4(KO)=0.68±0.08, p=0.0005; HAL: control=0.94±0.04, astrocyte-specific Ndufs4(KO)=0.55±0.07, p<0.0001 at 7 weeks) (Table 2, Figure 4, Supp. Figure 2). The difference between induction and emergence seen for both LORR and TC were similar for HAL and ISO, and were not altered by prolonged exposure times (30 minutes) at each concentration of the anesthetic.
Table 2:
Effects of astrocyte-specific Ndufs4(KO) on anesthetic sensitivity for loss of righting reflex.
| LORR – ISO Emerge/Induce | LORR – HAL Emerge/Induce | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Astrocyte-specific Ndufs4(KO) | Control | Astrocyte-specific Ndufs4(KO) | |||||
| Induction | Emergence | Induction | Emergence | Induction | Emergence | Induction | Emergence | |
| 3 weeks | 1.02±0.10 | 1.0±0.07 | 0.97±0.06 | 0.62±0.12* | 1.03±0.05 | 1.0±0.04 | 0.99±0.08 | 0.64±0.09 * |
| 7 weeks | 0.92±0.07 | 0.89±0.06 | 0.98±0.05 | 0.68±0.08* | 0.98±0.08 | 0.94±0.04 | 0.93±0.13 | 0.55±0.07* |
In each cell, bold numbers show the ratios of EC50s for emergence and induction for isoflurane or halothane, for control mice or astrocyte specific Ndufs4(KO), at 3 weeks and 7 weeks post 4-hydroxytamoxifen injections. The endpoint used is loss of righting reflex. The lower set of numbers in each cell are the measured values for the EC50s (emergence) and the EC50s (induction), respectively. p-values compare the KO ratio to the corresponding control ratio.
indicates p< 0.005. N=6 for controls, N≥6 for the astrocytic Ndufs4(KO).
Figure 4: Astrocyte-specific Ndufs4(KO) displays hysteresis in LORR.
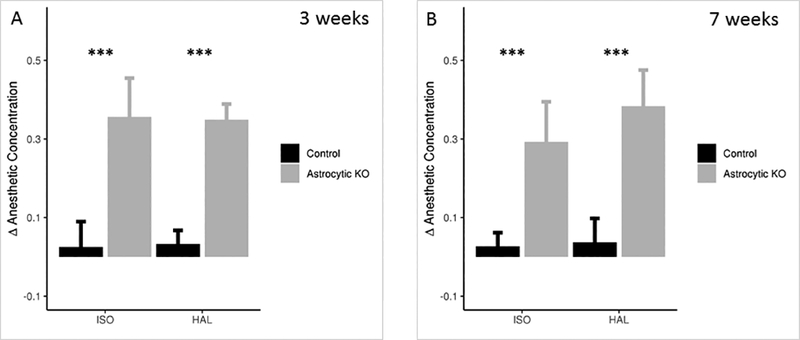
A. Δ Anesthetic Concentration between induction and emergence, required to demonstrate righting reflex before and after exposure to ISO or HAL, at 3 weeks post injection of 4-hydroxy tamoxifen. N=7 for control and the astrocyte-specific Ndufs4(KO). B. Δ Anesthetic Concentration required to demonstrate righting reflex before and after exposure to ISO or HAL, at 7 weeks post injection of 4-hydroxy tamoxifen. N=6 for control and the astrocyte-specific Ndufs4(KO). Tests for significance done on the difference between the EC50s for induction and emergence *** p<0.001. The error bars represent SD.
Norepinephrine assay:
Since norepinephrine is known to activate cortical astrocytes 16, we tested whether the levels of norepinephrine in the global Ndufs4(KO) are reduced when compared to the astrocyte-specific Ndufs4(KO) or control mice. There was no significant difference in the total norepinephrine content in whole brain extracts between the global or astrocyte-specific Ndufs4 KOs, compared to wild-type mice (Figure 5).
Figure 5: Total brain norepinephrine levels are unaffected by Ndufs4 mutation.
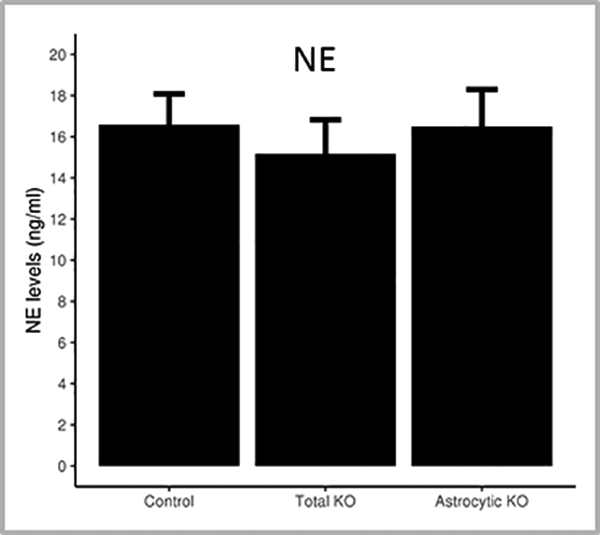
The norepinephrine levels in the brains of mice were assessed using biochemical assay. Norepinephrine content (ng/ml) in the total brains extracts of wildtype, total Ndufs4(KO) and astrocyte-specific Ndufs4(KO) mice. N=6 for wildtype, total Ndufs4(KO) and astrocyte-specific Ndufs4(KO) mice. The error bars represent SD.
Discussion
Astrocytes affect synaptic transmission by multiple mechanisms. During synaptic transmission, glutamate is removed from the synaptic cleft by astrocytes, actively converted to glutamine by the astrocyte-specific glutamine synthetase and supplied back to neurons through glial transporters SN1 and SN2 8,9. In addition, synaptic glutamate release, as well as neuromodulators like norepinephrine, evoke a calcium spike in astrocytes which can be transmitted between adjacent neurons and astrocytes 17,18. This calcium signaling causes the release of neural modulators, called gliotransmitters, from astrocytes into the synapse. Gliotransmitters include ATP, GABA, glutamate and D-serine, all of which modulate local neuronal function and synaptic transmission 10,19,20 at the tripartite synapse (Figure 6). Surprisingly, the acute loss of NDUFS4 in astrocytes specifically lowers the concentrations at which the mutant emerges from anesthesia, without altering the induction concentrations, for both responses to tail clamp and LORR assays. We interpret the data to indicate that the mutant mitochondria in astrocytes are inhibited by volatile anesthetics at lower concentrations than are control mitochondria. This inhibition results in decreased release of excitatory gliotransmitters and/or inhibited glutamate metabolism (Figure 6). Defective gliotransmitter release or glutamate metabolism in mutant astrocytes results in a failure of reactivation of neuronal function until volatile anesthetic concentrations are lowered sufficiently for astrocytic mitochondrial function to recover. Two important caveats must be considered: 1. We have not tested the effects of non-astrocytic non-neuronal cells which may play a role, and 2. While we saw no evidence of compensatory changes in this acute knockout model, the existence of such changes cannot be completely ruled out.
Figure 6. A model of the tripartite synapse.
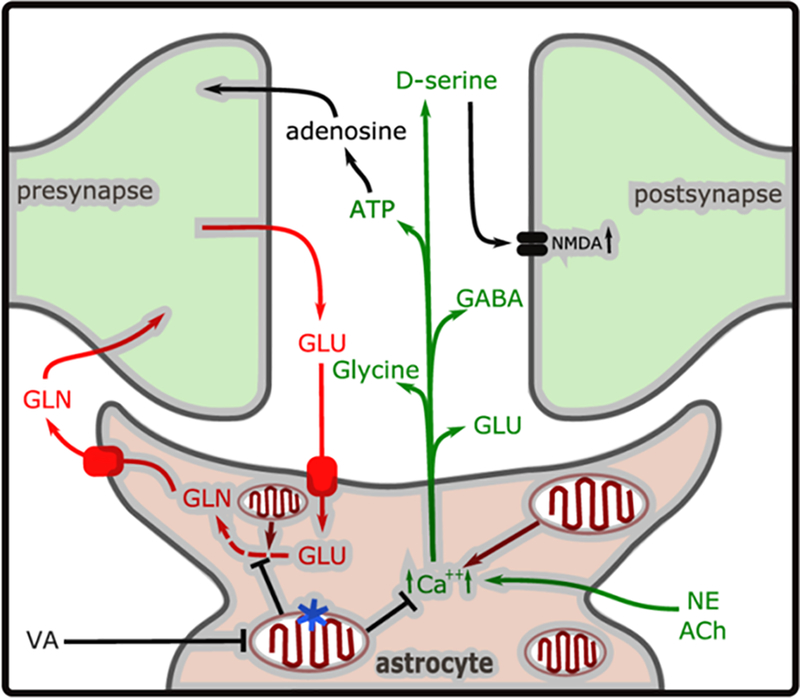
Glutamate (GLU) released during synaptic transmission is taken up by GLT-1 or GLAST transporters, converted to glutamine (GLN) and released by SN1/SN2 (pathway indicated in red). In addition, NE (norepinephrine) or ACh (acetylcholine) stimulation during emergence causes increased intracellular Ca2+ concentration ([Ca2+]i), causing release of the gliotransmitters ATP, GLU, and D-serine, glycine and GABA (in green). The Ca2+ homeostasis or glutamate recycling may be dysregulated in the astrocyte-specific mitochondrial mutant (indicated by blue star). The volatile anesthetics (ISO/HAL) inhibit mitochondrial complex I function at a lower concentration in Ndufs4(KO) mitochondria than in control mitochondria. In the case of the astrocyte specific Ndufs4(KO) (mutation indicated by the blue star in one mitochondrion), this leads to mitochondrial inhibition persisting in the astrocyte, compared to the neurons, during reduction of anesthetic concentration. We propose that this leads to neuronal inhibition from decreased excitatory gliotransmitter release or defective glutamate recycling, resulting in delayed emergence.
The response to tail clamp during volatile anesthetic exposure in rodents is mediated by the spinal cord with supraspinal modification 21,22. Previous studies have implicated the spinal cord in mediating nociception and the tail clamp reflex 21,23. While the spinal cord astrocytes have been implicated in modulating nociception 24 their role has been mostly studied in relation to chronic neuropathic pain and allodynia. Inhibition of the astrocytic glutamate-glutamine shuttle attenuates nociceptive neuronal responsiveness in response to inflammatory and nociceptive stimuli in the medulla 25. Alterations of these pathways due to mitochondrial dysfunction may play a part in causing the hysteresis that we observed.
In contrast to tail clamp, the loss of righting reflex (LORR) is likely mediated by interactions between the brainstem 26 and thalamo-cortical pathways 27–29. These two behavioral endpoints, primarily determined by different regions of the CNS, model different human anesthetic responses (minimal alveolar concentration versus loss of consciousness). Since the significant difference between induction and emergence concentrations (increased anesthetic hysteresis) was found with both of the behavioral endpoints, the emergence mechanism involved is unlikely to be dependent on one specific neuronal pathway. Instead, an astrocyte-specific mechanism is necessary for emergence with both endpoints. In addition, structurally different anesthetics isoflurane and halothane showed similar reductions in the concentration needed to be attained before the animals could emerge from anesthesia suggesting that dependence of emergence on astrocytes may be a general feature of volatile anesthetic response.
Control animals and global Ndufs4(KO) do not display marked hysteresis presumably since both neurons and astrocytes have equal mitochondrial functions in each genotype. Mice with Ndufs4 loss restricted to glutamatergic neurons do not display overt hysteresis either, potentially since the concentration necessary for induction is so low as to obscure the role of astrocytes in emergence. The loss of NDUFS4 solely in astrocytes reveals the critical role for this cell type in restoring consciousness.
While the response to the tail clamp was abrupt and often strong, the return to normal activity was qualitatively different in the astrocyte-specific Ndufs4(KO). Unlike the control mice, most astrocyte-specific Ndufs4(KO) mice displayed ataxia during the arousal period. Comparatively, the transition to fully awake state after regaining of righting reflex was without overt ataxia presumably due to the induction concentrations for TC being higher than for LORR. Extending our observations to the clinical setting is difficult but our findings suggest that there could be a link between post-operative emergence trajectory and changes in glial metabolism in some individuals.
Thrane et al. showed that anesthetic agents (ketamine/xylazine, isoflurane, urethane) depress widespread astrocytic IP3R2 (inositol 1,4,5-triphosphate type 2 receptor)-dependent Ca2+ transients associated with arousal in mice 30. One model of arousal argues that there is an evolutionarily conserved single arousal switch governing both loss and recovery of consciousness 31–33. However, anesthesia-induced loss and recovery of consciousness have also been suggested to be two distinct states clearly defined by separate anesthetic concentration response curves 34,35. Kelz’s group first described a difference between the concentrations needed for induction and emergence from the state of general anesthesia in Drosophila and in mice 34,35. In their pioneering work, they characterized this as a hysteresis, e.g. arousal was in some way state dependent. They termed this phenomenon neural inertia 34. Our results suggest that the neural inertia may be mediated by astrocytes and indicates that the restoration of consciousness is an energetically demanding process, inhibited by volatile anesthetics independent from the mechanisms causing initial loss of consciousness. The difference in emergence versus induction concentrations of isoflurane for the astrocyte-specific Ndufs4(KO) was similar to that described by Kelz’s group for mice lacking dopamine β-hydroxylase 34. However, LORR for wildtype mice in HAL was reported by Friedman et. al. to reduce the emergence/induction ratio to less than half 34. In the present report, control mice did not display statistically significant hysteresis under HAL or ISO for LORR. Whether this disparity was due to a different stepwise decrease in halothane concentration (.04% versus our.1%), the manner in which LORR was measured, the genetic background of the mouse strain or some other technical difference is not clear.
Importantly, astrocytes modulate both excitatory and inhibitory neuronal firing and are likely themselves regulated by metabolic status 36–38. Astrocyte mediated inhibition of neuronal firing can be dysregulated by astrocytic metabolic dysfunction and has been shown to result in seizure-like events in mouse cortex, counteracted by etomidate or propofol 38. These drugs mediate their effects by accentuating GABAergic neurotransmission. Volatile anesthetics primarily mediate their effects by inhibiting excitatory neurotransmission 37,38; our results are more consistent with astrocytic effects on excitatory synapses. The use of volatile anesthetic sensitivity as an endpoint in our studies appears to isolate the anesthetic effects on excitatory rather than inhibitory synapses. Since we previously did not see a major role for loss of NDUFS4 in GABAergic neurons in mediating anesthetic sensitivity 39, any effects of astrocytes on inhibitory neurotransmission is unlikely to be observed with our anesthetic endpoints. In the absence of anesthetic, the sub-optimal mitochondrial function in mutant astrocytes appears to be sufficient to avoid the excitatory effects seen by others with more profound defective astrocyte function36,38. The emergence defect of the astrocyte-specific Ndufs4(KO) reflects an inability of the astrocytic metabolic mutant to re-initiate excitatory firing post induction probably due to a persistent mitochondrial inhibition in the astrocyte at relatively low volatile anesthetic concentrations.
The simplest explanation of our results is that lack of ATP in astrocytes directly alters gliotransmission, resulting in downstream inability to regain excitatory synaptic function in the presence of low concentrations of volatile anesthetic. However, whether the mitochondrial dysfunction alters ATP levels, or disrupts either Ca2+ homeostasis or glutamate recycling, is not known. Although astrocytes are classically thought to rely on aerobic glycolysis 40,41 during synaptic transmission, the astrocytic somas contain the same volume of mitochondria as neuronal cell bodies 42 implying important mitochondrial functions in astrocytes 43–45. During resting states, astrocytic oxidative metabolism accounts for 30% of the cortical oxygen consumption, while occupying 20–25% of the total volume 46,47. The recognition that astrocytes are equivalent to neurons in their oxidative capacities signals a shift in the classical perception of astrocytes as primarily glycolytic, with broad impacts for the functional roles of the cells during resting and active states.
We also explored whether adrenergic signaling is affected by the Ndufs4(KO). Norepinephrine and acetyl choline are important extracellular activating signals to cortical astrocytes 48,49, and have been associated with increasing astrocytic Ca2+ transients 16,50,51 and inducing the release of gliotransmitters 19,52. Selective activation of the locus coeruleus neurons in rats under deep isoflurane anesthesia was shown to reduce burst suppression and shift EEG power from δ to θ, indicative of cortical arousal, and accelerate behavioral emergence 53. We observed that the total norepinephrine content is unaltered between the global and astrocyte-specific Ndufs4 knockouts and wild-type mice, it is still possible that the release or regional distribution of norepinephrine might be affected, rather than its production.
In summary, we have discovered an unexpected phenotype from acute loss of Ndufs4 in astrocytes. Loss of Ndufs4 in astrocytes did not change the volatile anesthetic concentrations required to induce anesthesia. However, the concentrations at which emergence occurred were significantly lower in the astrocyte-specific Ndufs4(KO) indicating a hysteresis in anesthetic sensitivity, produced by astrocytes alone.
Supplementary Material
Suppl. Figure 1: Specificity of the Cre-lox system by immunohistochemistry of the Ai6 reporter line. Low power fluorescence images of the 4-hydroxy tamoxifen injected Pgfap-CreERT2/+;ai6/+ and ai6/+ mice brain slices. A. Anterior – secondary motor cortex B. Mid - striatum C. Posterior – parabrachial nucleus. Immunohistochemistry using anti-GFAP antibody shows co-localization of Zsgreen in the anti-GFAP stained astrocytes of Pgfap-CreERT2/+;ai6/+ reporter mice, due to Cre-recombinase activation by 4-hydroxy tamoxifen.
Suppl. Figure 2: Astrocyte-specific knockout of Ndufs4 causes hysteresis between induction and emergence in LORR and TC. Scatter plots for the Δ Anesthetic Concentration (the change in the anesthetic concentration between induction and emergence) values, before and after exposure to ISO or HAL, at 3 and 7 weeks post injection of 4-hydroxy tamoxifen. The error bars represent SD.
Acknowledgements:
We thank Dr. Beatrice Predoi, Hailey Worstman, Jake Nealon and Julia Stokes for technical support, and Dr. Ernst-Bernhard Kayser, Christian Woods and Dr. Pavel Zimin for meaningful discussions and critical advice.
Funding statement: This work was supported by R01GM105696 to Dr. Margaret M. Sedensky, and by The Northwest Mitochondrial Research Guild.
Footnotes
Conflicts of interest: The authors declare no competing interests.
Prior Presentations: Title: “Effects of astrocytic knock out of the mitochondrial protein, NDUFS4, on volatile anesthetic response”, Abstract #A1153, Anesthesiology 2016 (October 22nd 2016), sponsored by American Society of Anesthesiologists (ASA), Chicago, IL, USA.
Clinical trial number and registry URL: Not applicable
References
- 1.Kayser EB, Suthammarak W, Morgan PG, Sedensky MM: Isoflurane selectively inhibits distal mitochondrial complex I in Caenorhabditis elegans. Anesth Analg 2011; 112: 1321–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanley PJ, Ray J, Brandt U, Daut J: Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol 2002; 544: 687–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olufs ZPG, Loewen CA, Ganetzky B, Wassarman DA, Perouansky M: Genetic variability affects absolute and relative potencies and kinetics of the anesthetics isoflurane and sevoflurane in Drosophila melanogaster. Sci Rep 2018; 8: 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan PG, Hoppel CL, Sedensky MM: Mitochondrial defects and anesthetic sensitivity. Anesthesiology 2002; 96: 1268–70 [DOI] [PubMed] [Google Scholar]
- 5.Quintana A, Morgan PG, Kruse SE, Palmiter RD, Sedensky MM: Altered anesthetic sensitivity of mice lacking Ndufs4, a subunit of mitochondrial complex I. PLoS One 2012; 7: e42904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimin PI, Woods CB, Quintana A, Ramirez JM, Morgan PG, Sedensky MM: Glutamatergic Neurotransmission Links Sensitivity to Volatile Anesthetics with Mitochondrial Function. Curr Biol 2016; 26: 2194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araque A, Parpura V, Sanzgiri RP, Haydon PG: Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 1999; 22: 208–15 [DOI] [PubMed] [Google Scholar]
- 8.Danbolt NC: Glutamate uptake. Prog Neurobiol 2001; 65: 1–105 [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry FA, Reimer RJ, Edwards RH: The glutamine commute: take the N line and transfer to the A. J Cell Biol 2002; 157: 349–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellin T, Sul JY, D’Ascenzo M, Takano H, Pascual O, Haydon PG: Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP. Novartis Found Symp 2006; 276: 208–17; discussion 217–21, 233–7, 275–81 [DOI] [PubMed] [Google Scholar]
- 11.Chow LM, Zhang J, Baker SJ: Inducible Cre recombinase activity in mouse mature astrocytes and adult neural precursor cells. Transgenic Res 2008; 17: 919–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H: A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13: 133–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonner JM, Werner DF, Elsen FP, Xing Y, Liao M, Harris RA, Harrison NL, Fanselow MS, Eger EI 2nd, Homanics GE: Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express alpha1 gamma-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology 2007; 106: 107–13 [DOI] [PubMed] [Google Scholar]
- 14.Morgan SE, Frink EJ, Gandolfi AJ: A simplified gas chromatographic method for quantifying the sevoflurane metabolite hexafluoroisopropanol. Anesthesiology 1994; 80: 201–5 [DOI] [PubMed] [Google Scholar]
- 15.Karunadharma PP, Basisty N, Chiao YA, Dai DF, Drake R, Levy N, Koh WJ, Emond MJ, Kruse S, Marcinek D, Maccoss MJ, Rabinovitch PS: Respiratory chain protein turnover rates in mice are highly heterogeneous but strikingly conserved across tissues, ages, and treatments. Faseb j 2015; 29: 3582–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekar LK, He W, Nedergaard M: Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 2008; 18: 2789–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nedergaard M: Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 1994; 263: 1768–71 [DOI] [PubMed] [Google Scholar]
- 18.Hirase H, Qian L, Bartho P, Buzsaki G: Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol 2004; 2: E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henneberger C, Papouin T, Oliet SH, Rusakov DA: Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010; 463: 232–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ: Channel-mediated tonic GABA release from glia. Science 2010; 330: 790–6 [DOI] [PubMed] [Google Scholar]
- 21.Antognini JF, Schwartz K: Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology 1993; 79: 1244–9 [DOI] [PubMed] [Google Scholar]
- 22.Jinks SL, Bravo M, Hayes SG: Volatile anesthetic effects on midbrain-elicited locomotion suggest that the locomotor network in the ventral spinal cord is the primary site for immobility. Anesthesiology 2008; 108: 1016–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rampil IJ, King BS: Volatile anesthetics depress spinal motor neurons. Anesthesiology 1996; 85: 129–34 [DOI] [PubMed] [Google Scholar]
- 24.Ren K: Emerging role of astroglia in pain hypersensitivity. Jpn Dent Sci Rev 2010; 46: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ: Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci 2007; 27: 9068–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devor M, Zalkind V: Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain 2001; 94: 101–12 [DOI] [PubMed] [Google Scholar]
- 27.Ramadasan-Nair R, Hui J, Zimin PI, Itsara LS, Morgan PG, Sedensky MM: Regional knockdown of NDUFS4 implicates a thalamocortical circuit mediating anesthetic sensitivity. PLoS One 2017; 12: e0188087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin TJ, Cho D, Ham J, Choi DH, Kim S, Jeong S, Kim HI, Kim JG, Lee B: Changes in thalamo-frontal interaction under different levels of anesthesia in rats. Neurosci Lett 2016; 627: 18–23 [DOI] [PubMed] [Google Scholar]
- 29.Akeju O, Loggia ML, Catana C, Pavone KJ, Vazquez R, Rhee J, Contreras Ramirez V, Chonde DB, Izquierdo-Garcia D, Arabasz G, Hsu S, Habeeb K, Hooker JM, Napadow V, Brown EN, Purdon PL: Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. Elife 2014; 3: e04499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thrane AS, Rangroo Thrane V, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M: General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci U S A 2012; 109: 18974–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allada R, Nash HA: Drosophila melanogaster as a model for study of general anesthesia: the quantitative response to clinical anesthetics and alkanes. Anesth Analg 1993; 77: 19–268317731 [Google Scholar]
- 32.Alkire MT, Asher CD, Franciscus AM, Hahn EL: Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology 2009; 110: 766–73 [DOI] [PubMed] [Google Scholar]
- 33.Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, Morgan PG, Nash HA: A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr Biol 2007; 17: 624–9 [DOI] [PubMed] [Google Scholar]
- 34.Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB: A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One 2010; 5: e11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joiner WJ, Friedman EB, Hung HT, Koh K, Sowcik M, Sehgal A, Kelz MB: Genetic and anatomical basis of the barrier separating wakefulness and anesthetic-induced unresponsiveness. PLoS Genet 2013; 9: e1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keyser DO, Pellmar TC: Synaptic transmission in the hippocampus: critical role for glial cells. Glia 1994; 10: 237–43 [DOI] [PubMed] [Google Scholar]
- 37.Keyser DO, Pellmar TC: Regional differences in glial cell modulation of synaptic transmission. Hippocampus 1997; 7: 73–7 [DOI] [PubMed] [Google Scholar]
- 38.Voss LJ, Harvey MG, Sleigh JW: Inhibition of astrocyte metabolism is not the primary mechanism for anaesthetic hypnosis. Springerplus 2016; 5: 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimin PI, Woods CB, Kayser EB, Ramirez JM, Morgan PG, Sedensky MM: Isoflurane disrupts excitatory neurotransmitter dynamics via inhibition of mitochondrial complex I. Br J Anaesth 2018; 120: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazucanti CH, Kawamoto EM, Mattson MP, Scavone C, Camandola S: Activity-dependent neuronal Klotho enhances astrocytic aerobic glycolysis. J Cereb Blood Flow Metab 2018: 271678×18762700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koppenol WH, Bounds PL, Dang CV: Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011; 11: 325–37 [DOI] [PubMed] [Google Scholar]
- 42.Pysh JJ, Khan T: Variations in mitochondrial structure and content of neurons and neuroglia in rat brain: an electron microscopic study. Brain Res 1972; 36: 1–18 [DOI] [PubMed] [Google Scholar]
- 43.Balaban RS, Nemoto S, Finkel T: Mitochondria, oxidants, and aging. Cell 2005; 120: 483–95 [DOI] [PubMed] [Google Scholar]
- 44.Mitchell P: Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. 1966. Biochim Biophys Acta 2011; 1807: 1507–38 [DOI] [PubMed] [Google Scholar]
- 45.Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P: Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2017 [DOI] [PubMed] [Google Scholar]
- 46.Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R: Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci 2004; 24: 11273–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL: Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 2002; 22: 1523–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE: Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 2014; 82: 1263–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papouin T, Dunphy JM, Tolman M, Dineley KT, Haydon PG: Septal Cholinergic Neuromodulation Tunes the Astrocyte-Dependent Gating of Hippocampal NMDA Receptors to Wakefulness. Neuron 2017; 94: 840–854.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M: alpha1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 2013; 54: 387–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duffy S, MacVicar BA: Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci 1995; 15: 5535–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS: Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 2005; 8: 1078–86 [DOI] [PubMed] [Google Scholar]
- 53.Vazey EM, Aston-Jones G: Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A 2014; 111: 3859–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1: Specificity of the Cre-lox system by immunohistochemistry of the Ai6 reporter line. Low power fluorescence images of the 4-hydroxy tamoxifen injected Pgfap-CreERT2/+;ai6/+ and ai6/+ mice brain slices. A. Anterior – secondary motor cortex B. Mid - striatum C. Posterior – parabrachial nucleus. Immunohistochemistry using anti-GFAP antibody shows co-localization of Zsgreen in the anti-GFAP stained astrocytes of Pgfap-CreERT2/+;ai6/+ reporter mice, due to Cre-recombinase activation by 4-hydroxy tamoxifen.
Suppl. Figure 2: Astrocyte-specific knockout of Ndufs4 causes hysteresis between induction and emergence in LORR and TC. Scatter plots for the Δ Anesthetic Concentration (the change in the anesthetic concentration between induction and emergence) values, before and after exposure to ISO or HAL, at 3 and 7 weeks post injection of 4-hydroxy tamoxifen. The error bars represent SD.


