SUMMARY
Infants are more susceptible to enteric infections due to immature immune systems and impaired colonization resistance mediated by the microbiota. Maternal antibodies can provide immunity, with maternal vaccination offering a protective strategy. We find that oral infection of adult females with the enteric pathogen Citrobacter rodentium protects dams and offspring against oral challenge. Parenteral immunization of dams with heat-inactivated C. rodentium reduces pathogen loads and mortality in offspring but not mothers. IgG, but not IgA or IgM, transferred through breast milk to the intestinal lumen of suckling offspring coats the pathogen and reduces intestinal colonization. Protective IgG largely recognizes virulence factors encoded within the locus of enterocyte effacement (LEE) pathogenicity island, including the adhesin Intimin and T3SS filament EspA, which are major antigens conferring protection. Thus, pathogen-specific IgG in breast milk induced during maternal infection or immunization protects neonates against infection with an attaching and effacing pathogen.
Keywords: Maternal vaccination, enteric infection, C. rodentium, EPEC, IgG, LEE-virulence factors
Graphical Abstract
Caballero-Flores et al. demonstrate that maternal immunization with heat inactivated C. rodentium or surface pathogen antigens protects neonatal mice against pathogen oral challenge. Protection was mediated by the delivery of pathogen-specific IgG through breast milk which coated the pathogen, increased its phagocytosis and decreased epithelial attachment in the gut.

INTRODUCTION
Enteric and diarrheal diseases are important causes of childhood death worldwide, leading to nearly 1 million deaths per year in children under 5 years old, and ranking as the second cause of death in this age group (Lanata et al., 2013; Croxen et al., 2013). The increased susceptibility of newborns and infants to enteric infections has been ascribed to immaturity of their immune system and impaired colonization resistance conferred by the neonatal microbiota (Simon et al., 2015; Kim, et al., 2017). Thus, neonates rely, at least in part, on maternal antibodies for immunity against enteric infection by pathogens that often gain access to the gastrointestinal tract (PrabhuDas et al., 2011; Kollmann et al., 2012). For this reason, maternal vaccination has emerged as a valuable tool in covering the gap between birth and the maturation of the immune system, since it represents a safe and effective strategy to transfer protective maternal antibodies to the offspring during pregnancy (Jennewein et al., 2017; Riccardo et al., 2017).
There are currently various vaccines recommended for use on all pregnant women, such as those against Pertussis, Tetanus and Influenza, whereas others including those against Hepatitis, Pneumococcus, Meningococcus, Smallpox, Varicella and Rubella are only indicated for pregnant women with specific risk factors (Riccardo et al., 2017). However, even though maternal vaccination has proven itself as a safe and effective strategy for prevention of life-threatening infections in neonates, there are no licensed maternal vaccines for protection against bacterial enteric pathogens, including Enteropathogenic Escherichia coli (EPEC), Vibrio cholerae and Shigella, which remain important causes of childhood death worldwide (Czerkinsky and Holmgren, 2015; Riccardo et al., 2017). EPEC, a food- and water-borne non-invasive enteric pathogen, is a common cause of diarrheal deaths in newborns and infants (Lanata et al., 2013; Kotloff et al., 2013), whereas infections in adults are rarely observed (Croxen et al., 2013). EPEC attaches to and colonizes the intestinal tract by inducing characteristic “attaching and effacing” (A/E) lesions on the intestinal epithelium (Kaper et al., 2004; Mundy et al., 2005). The A/E phenotype is conferred by the locus of enterocyte effacement (LEE), a pathogenicity island encoding a specialized type III secretion system (T3SS), as well as translocators, secreted effector proteins, bacterial adhesins and Ler, the global activator of all LEE genes (Frankel and Philips, 2008; Gaytán et al., 2016). Unfortunately, antibiotic therapy for EPEC infection is not effective and may worsen disease (Croxen et al., 2013). Thus, there is an urgent need for novel therapies to prevent or treat infections by this pathogen, particularly in infants and young children.
Citrobacter rodentium, a mouse pathogen that also harbors the LEE and induces A/E intestinal lesions, has been extensively used as a model for studying the pathogenesis of EPEC infection in humans (Law et al., 2013; Collins et al., 2014). Previous studies have demonstrated that C. rodentium-specific IgG antibodies, but not IgA or IgM, are required for pathogen clearance and host survival in adult mice (Bry and Brenner, 2004; Maaser et al., 2004; Kamada et al., 2015). In contrast, the role of maternal IgG in neonatal host defense against C. rodentium or other enteric pathogens remains poorly understood. During pregnancy, maternal IgG is transported through the placenta to the fetus by the neonatal Fc receptor (FcRn) that also regulates the stability of plasma IgG and mediates the neonatal uptake of maternally derived IgG from ingested milk into the small intestine (Rath et al., 2013; Pyzik et al., 2015).
In this study, we examined the mechanism by which maternal antibodies protect the offspring against A/E pathogens during the neonatal period. We show that maternal pathogen-specific IgG, but not IgA or IgM, plays a critical role in neonatal protection, and that protective IgG is mainly delivered through breast milk into the neonatal intestinal lumen. Furthermore, we observed that maternal protective IgG was produced against LEE virulence factors and identified LEE-encoded Intimin and EspA as major antigens for maternal vaccination and protection of the offspring.
RESULTS
Oral infection with C. rodentium, but not parenteral immunization, confers protection against secondary pathogen challenge in adult mice
Initial experiments showed that oral infection with C. rodentium, but not intraperitoneal (IP) immunization with heat-inactivated bacteria, conferred full protection in adult mice against pathogen colonization after a second oral challenge (Figure 1A), even though both routes of immunization induced similar levels of pathogen-specific IgG antibodies in serum that were largely induced against Ler-regulated virulence proteins (Figure 1B and C). In contrast, fecal IgG1, IgG2b, and total IgG specific for C. rodentium were observed only after oral infection, but not IP immunization (Figure 1D). The increased amount of C. rodentium-reactive IgG observed in the feces after oral infection, compared with that obtained after IP immunization, was maintained on day 2 and 7 after a second oral pathogen challenge (Figure S1A and B). Likewise, oral infection but not IP immunization, induced fecal C. rodentium-specific IgA that was also observed after a second pathogen challenge (Figure S1C and D). These results indicate that protective immunity against enteric infection is induced after oral infection but not IP immunization in adult mice, which correlates with the presence of pathogen-specific immunoglobulins in the intestinal lumen.
Figure 1. Parenteral immunization protects the offspring, but not the dam, against pathogen oral challenge.
(A) Adult C57BL/6 mice were orally- or IP-inoculated with alive or heat-inactivated C. rodentium, respectively. After one month, mice were challenged orally with 5×108 CFU and bacterial counts in feces were determined on the indicated days after infection. Naïve, untreated animals. (B) Serum α-C. rodentium IgG subtypes in orally infected or IP immunized adult mice determined by ELISA two weeks after inoculation (n= 4 mice per group). (C) Immunoblotting of C. rodentium WT and Δler bacterial lysates using serum from orally infected or IP immunized adult mice. Proteins recognized by antibodies in serum were revealed with HRP-anti-mouse IgG. SDS-PAGE gel stained with Coomassie blue is shown to reveal total amounts of cell lysates loaded. (D) Fecal α-C. rodentium IgG subtypes in orally infected or IP immunized adult mice determined by ELISA two weeks after inoculation (n= 4 mice per group). (E) Adult mice (6-week old) and pups (18-day old) were orally infected with 5×108, 5×107 or 5×106 CFU and mouse survival was determined over the indicated time after infection. (F) 18-day old pups from orally infected or IP immunized mothers were challenged orally with 5×108 CFU of C. rodentium and survival rates were determined. Naïve, pups from untreated dams. Representative results from two independent experiments are shown. Data are represented as mean ± SD (A-B, and D). *p < 0.05, **p < 0.01, ***, p<0.001 by Mann-Whitney U test (B and D) or log rank test (E and F, compared to control groups indicated in red). Only statistically significant differences are shown. See also Figure S1.
Parenteral maternal immunization reduces pathogen loads and intestinal inflammation in the offspring after oral challenge
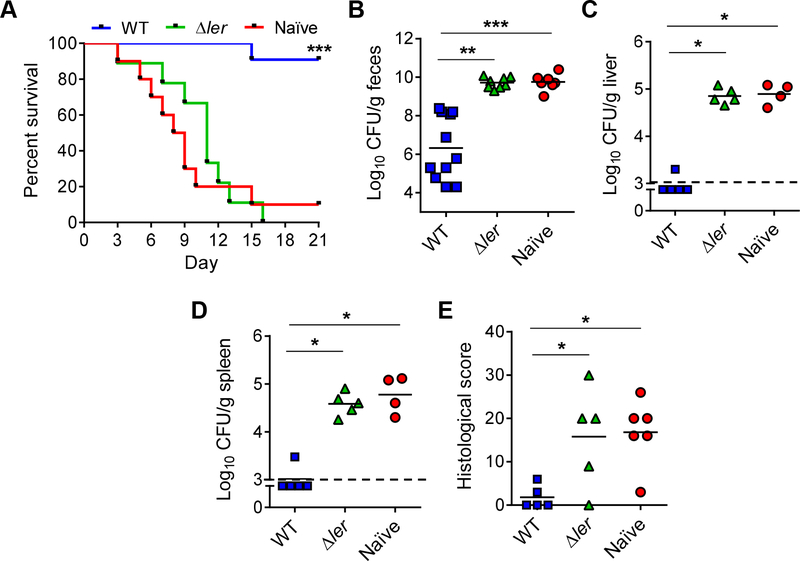
To determine whether oral infection and/or IP immunization of adult females confer protection to the offspring against C. rodentium, we first optimized a lethal infection model in neonatal mice by inoculating 18-day old pups with different doses of C. rodentium. We used 5×108 colony-forming units (CFU), a dose previously used for inoculation of adult mice (Maaser et al., 2004), as well as inocula containing 10-fold and 100-fold less bacteria (5×107 and 5×106 CFU, respectively). As expected, all adult mice survived after infection with the highest dose of 5×108 CFU, while 100% of the pups succumbed to infection with both 5×108 and 5×107 bacteria, and only ~ 50% of the pups survived after 5×106-challenge (Figure 1E). After challenging the offspring of both orally and IP inoculated dams, we found that both routes of maternal immunization protected the offspring against oral challenge with 5×108 CFU whereas the same dose resulted in lethality in age-matched naïve pups (Figure 1F). These data indicate that maternal immunization via both the oral and parenteral routes is effective in protecting neonates against enteric infection. Because maternal immunization with heat-killed C. rodentium, hereinafter referred to as “HK-Cr”, promoted offspring survival in the absence of infection in the dam (Figure 1F), we further studied the protective phenotype in the offspring of such maternally immunized pups after challenge with the pathogen. Consistently with results shown in Figure 1F, HKCr maternal immunization led to ~90% survival of pups after challenge with 5×108 CFU of C. rodentium, while all pups from naïve dams succumbed to this pathogen dose (Figure 2A). These results correlated with lower pathogen loads in feces, liver and spleen (Figure 2B to D), as well as reduced inflammation and pathology scores in the intestine of the maternally immunized pups compared to naïve mice (Figure 2E and F). In contrast, HKCr treatment did not reduce pathogen loads or intestinal pathology in the immunized dams after pathogen challenge when compared to naïve mice (Figure S2A to E). These results confirm the previous observations that parenteral HK-Cr immunization does not protect adult mice against enteric C. rodentium infection, but it is effective in transferring passive immunity to the offspring through maternal immunization.
Figure 2. Parenteral maternal immunization reduces pathogen loads and intestinal inflammation in the offspring after oral challenge.
(A) 18-day old pups from naïve or HK-Cr immunized mothers were challenged orally with 5×108 CFU of C. rodentium and mouse survival was monitored at the indicated days post-infection (dpi). (B-D) Pathogen load in feces at 5 dpi (B), liver (C) and spleen (D) (7 dpi), in pups from naïve and HK-Cr immunized mothers after C. rodentium challenge. (E-F) Representative H&E staining sections (E) and pathology scores (F) of colonic tissue harvested from pathogen challenged pups depicted in B-D. Arrows indicate areas with marked epithelial damage and inflammation and arrowheads pathogen foci in the mucosa. Scale bars, 400 μm. Data show representative (E) or combined (A-D, and F) results from at least two independent experiments. Each dot represents one mouse (B-D, and F). Dotted line indicates the limit of detection. *p < 0.05, **p < 0.01, ***, p<0.001 by log rank test (A), unpaired two-tailed T- test (B) or Mann-Whitney U test (C-D, and F). See also Figure S2.
Passive immunity is transferred from the mother to the offspring mainly through breast milk
In mice, maternal antibody transfer occurs during pregnancy and after birth through the placenta and breast milk, respectively (Hurley and Theil, 2011; Ma et al., 2018). To determine whether protective maternal antibodies are transferred to the offspring pre- or postnatally, we performed cross-fostering experiments in which 1- to 2-day old pups from HK-Cr immunized mothers were cross-fostered and nursed by naïve dams (prenatal antibody transfer), while newly born pups from such naïve mothers were nursed by immunized dams (postnatal antibody transfer) (Figure 3A). We observed that ~60% of pups receiving C. rodentium-specific antibodies postnatally survived pathogen challenge (Figure 3B), while only ~10% of pups that received maternal antibodies prenatally, and none of the naïve (non-cross fostered) mice, survived the infection (Figure 3B). The increased survival observed in the postnatal group correlated with reduced pathogen loads and pathology scores compared to the prenatal and naïve groups (Figure 3C to F). These results also correlated with increased detection of C. rodentium-specific IgG antibodies in breast milk of HK-Cr immunized mothers compared to milk samples of naïve dams as detected by immunoblotting (Figure 3G). As previously observed in the serum of infected mice (Figure 1C), pathogen-specific IgG antibodies in breast milk were mainly produced against Ler-dependent virulence factors (Figure 3G). Altogether, these data suggest that postnatal transfer of maternal antibodies through breast milk is critical for protection of the offspring, whereas prenatal transfer during pregnancy confers only a slight degree of protection to the offspring.
Figure 3. Passive immunity is transferred from the mother to the offspring mainly through breast milk.
(A) Experimental design. Newborn pups from naïve and HK-Cr immunized dams were cross-fostered and nursed by the indicated recipient mother. Pups were then challenged with 5×108 CFU of C. rodentium when they were 18-day old. (B) Survival rates of the challenged pups over the indicated days post-infection (dpi). Prenatal, pups from immunized mothers nursed by naïve dams; Postnatal, pups from naïve mothers nursed by immunized dams; Naïve, non-fostered pups from untreated mothers. (C-E) Pathogen load in feces at 5 dpi (C), liver (D) and spleen (E) (7 dpi) of the pathogen challenged pups. (F) Pathology scores of cecal and colonic tissues from pups depicted in B-E. (G) Immunoblotting of C. rodentium WT and Δler bacterial lysates with breast milk from naïve or HK-Cr immunized mothers. Proteins recognized by antibodies in milk were revealed with HRP-anti-mouse IgG. SDS-PAGE gel stained with Coomassie blue is shown to reveal total amounts of cell lysates loaded. Data show representative (G) or combined (B-F) results from at least two independent experiments. Each dot represents one mouse (C-F). Dotted line indicates the limit of detection. *p < 0.05, **p < 0.01, ***, p<0.001 by log rank test (B), one-way ANOVA (C) or Kruskal-Wallis test (D-F). Only statistically significant differences are indicated.
Maternal IgG, but not IgA or IgM, are required for protection of the offspring
We next tested the role of maternal IgG, IgA and IgM in protecting the offspring against C. rodentium infection. In these experiments, we performed maternal HK-Cr immunization experiments in mutant mice lacking IgA (Igha−/−), FcRn (Fcgrt−/−) or pIgR (Pigr−/−), the transporter of secretory IgA and IgM. We found that HK-Cr immunization of IgA-deficient dams resulted in protection of the corresponding Igha−/− offspring against oral pathogen challenge (Figure 4A), which correlated with reduced pathogen loads in feces, liver and spleen (Figure 4B to D), as well as lower intestinal pathology scores compared to naïve Igha−/− pups that succumbed to infection (Figure 4E and Figure S3). Likewise, immunization of pIgR-deficient females also protected the Pigr−/− offspring against pathogen challenge compared to naïve Pigr−/− pups (Figure 4F to J and Figure S3). As expected, we did not detect C. rodentium-specific IgA in breast milk from Igha−/− immunized dams, whereas IgA and IgM were highly reduced in samples from Pigr−/− mice compared to WT dams (Figure S4B and C). The levels of anti-pathogen IgM in breast milk were increased in Igha−/− mice perhaps reflecting a compensatory response (Harriman et al., 1999; Furuya et al., 2013). Additionally, we did not observe significant differences in susceptibility of naïve Igha−/−or Pigr−/− pups compared to naïve wild-type (WT) pups (compare Figure 2A with Figure 4A and F). Altogether, these results indicate that maternal IgA and IgM do not contribute significantly to protection of the offspring. In contrast, both maternally immunized and naïve Fcgrt−/− pups from FcRn-deficient dams succumbed to infection (Figure 4K), and showed comparable pathogen loads in feces, liver and spleen (Figure 4L to N), as well as comparable intestinal pathology scores after C. rodentium challenge (Figure 4O and Figure S3). FcRn mediates the transfer of maternal IgG across the placenta and neonatal intestine, as well as regulates plasma IgG levels by protecting IgG from catabolism (Israel et al., 1995; Ghetie et al., 1996; Roopenian et al., 2003). However, its role in IgG transfer from plasma into breast milk remains controversial (Israel et al., 1995; Cianga et al., 1999; Ma et al., 2018). Although we found a reduction of total anti-C. rodentium IgG in breast milk of Fcgrt−/−dams compared to WT mice after HKCr immunization, the difference was not significant compared to the other experimental groups (Figure S4A). However, immunoblotting analysis confirmed that C. rodentium-specific IgG antibodies, including those against Ler-dependent virulence factors, were reduced in breast milk of immunized Fcgrt−/−dams compared to WT mice (Figure S4D). We observed a similar reduction of pathogen-specific IgG in the serum of such Fcgrt−/−mice after immunization (Figure S4E), indicating that FcRn is required for a robust IgG response after immunization, as reported for other foreign antigens (Roopenian et al., 2003; Cervenak, et al., 2011). Consistently, we found that Fcgrt+/− heterozygous pups from HK-Cr immunized Fcgrt−/− mothers succumbed to pathogen challenge and showed comparable pathogen burdens than naïve or maternally immunized Fcgrt−/−pups (Figure S5A to D). Altogether, these results indicate that an efficient IgG, but not IgA or IgM, immune response in the dam is important for protection of the offspring after maternal immunization.
Figure 4. Maternal IgG, but not IgA or IgM, is required for protection of the offspring.
(A) 18-day old pups from naïve or HK-Cr immunized IgA-deficient (Igha−/−) mothers were challenged with 5×108 CFU of C. rodentium and survival rates were determined over the indicated days post-infection (dpi). (B-D) Pathogen load in feces at 5 dpi (B), liver (C) and spleen (D) (7 dpi) in pups from naïve and HK-Cr immunized Igha−/− mothers after pathogen challenge. (E) Pathology scores of cecal and colonic tissue harvested from infected pups depicted in B-D. (F) Pups from naïve or HK-Cr immunized pIgR-deficient (Pigr−/−) mothers were challenged and mouse survival recorded as in A). (G-I) Pathogen load in feces at 5 dpi (G), liver (H) and spleen (I) (7 dpi), in pups from naïve and HK-Cr immunized Pigr−/− mothers after C. rodentium challenge. (J) Pathology scores of cecal and colonic tissue harvested from infected pups depicted in G-I. (K) Pups from naïve or HK-Cr immunized FcRn−/−-deficient (Fcgrt−/−) mothers were challenged and mouse survival recorded as in A). (L-N) Pathogen load in feces at 5 dpi (L), liver (M) and spleen (N) (7 dpi), in pups from naïve and HK-Cr immunized Fcgrt−/− mothers after C. rodentium challenge. (O) Pathology scores of cecal and colonic tissue harvested from infected pups depicted in L-N. Data represent combined results from two independent experiments. Each dot represents one mouse (B-E, G-J, L-N). Dotted line indicates the limit of detection. *p < 0.05, **p < 0.01, ***, p<0.001 by log rank test (A, F and K) and Mann-Whitney U test (BE, G-J, L-O). ns, not significant. See also Figures S3–5.
Maternal IgG coats C. rodentium, promotes phagocytosis of virulent bacteria and reduces pathogen attachment to intestinal mucosa
To determine the mechanism by which maternal IgG confers protection to the offspring, we first evaluated whether maternal C. rodentium-specific IgG can coat the pathogen both in vitro and during the infection. We found that >90% of C. rodentium were coated by IgG after incubation in vitro with milk samples from HK-Cr immunized dams, whereas incubation with milk from naïve mothers did not lead to pathogen coating (Figure 5A). These results correlated with increased levels (~50%) of pathogen-IgG coating in the intestinal lumen of maternally immunized pups compared to naïve pups (<1% coating) after oral challenge with the pathogen (Figure 5B). Because Ler-dependent factors are targeted by maternal IgG (Figure 3G), we tested next whether pathogen coating by IgG in breast milk promotes selective phagocytosis of Ler-positive (WT) C. rodentium by neutrophils, which have been shown to be involved in intestinal pathogen eradication in adult mice (Kamada et al., 2015). We found that incubation of the pathogen with milk samples from HK-Cr immunized dams increased selectively the intracellular loads of Ler-expressing bacteria about seven-fold compared to incubation with naïve milk (Figure 5C). To evaluate whether maternal immunization increases the phagocytosis of Ler-expressing C. rodentium in vivo, WT and ler-deficient C. rodentium were co-injected in the neutrophil-rich peritoneal cavity of naïve and maternally immunized pups. There were increased intracellular loads of WT C. rodentium, but not ler mutant bacteria, in neutrophils harvested from the peritoneum of maternally immunized pups compared to naïve pups (Figure 5D). We also observed reduced pathogen attachment to the intestinal mucosa of maternally immunized pups compared to naïve pups after oral infection, both by using a C. rodentium luciferase reporter strain (Figure 5E), and by counting the number of mucosa-attached bacteria (Figure 5F). Collectively, these results suggest that maternal IgG opsonizes virulent C. rodentium, increases its phagocytosis by neutrophils and reduces pathogen attachment to the intestinal epithelium during the neonatal period.
Figure 5. Maternal IgG coats C. rodentium in the intestine of the offspring, promotes phagocytosis of virulent bacteria, and reduces pathogen attachment.
(A) IgG coating of C. rodentium-GFP incubated in vitro with breast milk from naïve or HK-Cr immunized mothers. Left panel, representative flow cytometry profiles; right panel, percentage of IgG-coated C. rodentium-GFP (naïve, n= 4 mice; immunized, n= 5 mice). (B) IgG coating of C. rodentium-GFP in intestinal contents of infected pups from naïve or HKCr immunized mothers. Left panel, representative flow cytometry profiles; right panel, percentage of IgG-coated C. rodentium-GFP (naïve, n= 4 mice; immunized, n= 5 mice). (C) Neutrophil phagocytosis assays in vitro. WT/Δler bacterial mixtures (1:1) were treated with breast milk from naïve or HK-Cr immunized dams and incubated with neutrophils harvested from the peritoneal cavity of naïve mice. Ratios (WT/Δler) of intracellular bacteria in gentamicin-treated neutrophils are shown (naïve, n= 5 mice; immunized, n= 6 mice). (D) Neutrophil phagocytosis assays in vivo. WT/Δler mixtures (1:1) were IP-injected into the neutrophil-rich peritoneal cavity of pups from naïve or HK-Cr immunized mothers. Ratios (WT/ler) of intracellular bacteria in gentamicin-treated neutrophils are shown (naïve, n= 6 mice; immunized, n= 6 mice). (E-F) Levels of C. rodentium attached to intestinal epithelium. Pups from naïve or HK-Cr immunized mothers were orally inoculated with 5×108 CFU of a C. rodentium-lux reporter strain. Cecal and colonic tissues were harvested, washed to remove non-adherent bacteria, and used to assess bacterial bioluminescence (E) and total bacteria in tissue homogenates (F). Data show representative (A and B, left panels; and E) or combined (A and B, right panels; C, D and F) results from two independent experiments. Data are represented as mean ± SD (A-B, right panels; C and D). Each dot represents one mouse (F). *p < 0.05, **p < 0.01, ***, p<0.001 by Mann-Whitney U test (A to D) or unpaired two-tailed T-test (F).
LEE virulence factors are required to confer passive immunity against C. rodentium
Our results suggest that Ler-dependent LEE virulence factors are required for an efficient antibody response against C. rodentium in the immunized dams (Figure 1C and3G). Thus, we determined whether expression of the LEE-encoded T3SS is required to confer passive immunity to the offspring after maternal immunization. To test this, we immunized adult females with WT or Δler HK-Cr (that does not express LEE). We found that maternal immunization with the WT strain, but not the Δler mutant, conferred protection to the offspring against oral pathogen challenge (Figure 6A) that was associated with lower pathogen loads in feces, liver and spleen (Figure 6B to D), as well as reduced pathology scores in the intestine (Figure 6E). These results indicate that LEE-encoded virulence factors are the main C. rodentium antigens that promote production of protective IgG antibodies after maternal immunization.
Figure 6. LEE virulence factors are required to confer passive immunity against C. rodentium.
(A) 18-day old pups from mothers immunized with WT or Δler HK-Cr were challenged with 5×108 CFU of C. rodentium and mouse survival was determined over the indicated days post-infection (dpi). Naïve, pups from untreated dams. (B-D) Pathogen load in feces at 5 dpi (B), liver (C) and spleen (D) (7 dpi) in pups from naïve and HK-Cr immunized mothers after C. rodentium challenge. (E) Pathology scores of cecal and colonic tissue harvested from infected pups depicted in B-D. Data represent combined results from two independent experiments. Each dot represents one mouse (B-E). Dotted line indicates the limit of detection. *p < 0.05, **p < 0.01, ***, p<0.001 by log rank test (A, compared to naïve group), one-way ANOVA (B) or Kruskal-Wallis test (C-E). Only statistically significant differences are indicated.
Maternal vaccination with purified LEE antigens confers protection against C. rodentium
To identify LEE virulence factors that promote the production of protective IgG maternal antibodies for the offspring, we purified two LEE-encoded antigens: a non-fimbrial adhesin (Intimin) and a component of the T3SS filament (EspA), which are both exposed on the bacterial surface of the pathogen (Gaytán et al., 2016). We found that both proteins were recognized by IgG in the serum and milk of HK-Cr immunized dams, but not by the serum of dams immunized with the Δler mutant (Figure 7A). Vaccination of adult females with purified Intimin in the presence of the adjuvant Alum elicited mainly antigen-specific IgG1 antibodies, while EspA immunization induced IgG1, IgG2b and IgG3 (Figure 7B). In contrast, HK-Cr immunization elicited IgG1, IgG2b and IgG3 against both Intimin and EspA albeit at lower levels compared to single protein vaccinations (Figure S6A and B). Both Intimin- and EspA-maternal vaccinations promoted higher survival rates in the offspring (Figure 7C) that correlated with reduced pathogen loads (Figure 7D to F), and pathology scores (Figure 7G) compared to pups maternally vaccinated with ovalbumin (OVA), an irrelevant protein used as a negative control. However, the level of protection observed with Intimin- and EspA-maternal vaccinations was lower than that observed with HK-Cr immunization (see Figure 2), which might be due to the differential IgG responses against the pathogen elicited by both immunization approaches (Figure S6C). Likewise, maternal vaccination with purified EspB, a pore component of the T3SS, or a triple vaccine comprising Intimin, EspA and EspB, conferred similar levels of protection as Intimin and EspA single immunizations (Figure S7A to E). These results indicate that maternal vaccination with single LEE-antigens confers effective but partial protection to the offspring against C. rodentium.
Figure 7. Maternal vaccination with purified LEE antigens confers protection to the offspring against C. rodentium.
(A) Immunoblotting of purified Intimin and EspA using serum or breast milk from mothers immunized with WT or Δler HK-Cr. Proteins recognized by antibodies in serum and milk were revealed with HRP-anti-mouse IgG. SDS-PAGE gel stained with Coomassie blue is shown to reveal total amounts of protein loaded. (B) Antigen-specific IgG subtypes determined by ELISA using serum from dams immunized with the indicated proteins. OVA, Ovalbumin. (C) 19-days old pups from dams immunized with the indicated proteins were challenged with 5×107 CFU of C. rodentium and survival rates were determined over the indicated days post-infection (dpi). (D-F) Pathogen load in feces at 5 dpi (D), liver (E) and spleen (F) (7 dpi) in the pathogen challenged pups. (G) Pathology scores of cecal and colonic tissue harvested from the infected pups depicted in D-F. Data show representative (A and B) or combined (C-G) results from two independent experiments. Each dot represents one mouse (D-G). Dotted line indicates the limit of detection. *p < 0.05, **p < 0.01, ***, p<0.001 by log rank test (C, compared to OVA group), one-way ANOVA (D and G) or Kruskal-Wallis test (E and F). Only statistically significant differences are indicated. See also Figures S6–7.
DISCUSSION
Maternal vaccination has proven to be an effective strategy for prevention of life-threatening infections in neonates and infants. Although various maternal vaccines are currently available against pertussis, neonatal tetanus and influenza (Riccardo et al., 2017), no licensed maternal vaccines against enteric bacterial pathogens such as EPEC are available despite the fact that enteric bacterial infection remains a major cause of childhood mortality worldwide. In this study, we report that maternal immunization with HK-Cr or pathogen-specific purified surface proteins confers protection to the offspring but not to the dam against enteric infection with the pathogen. We found that oral infection, but not parenteral immunization, with C. rodentium protected adult mice against a subsequent oral challenge with the pathogen. Although such a difference in protection could be potentially explained by different antibody responses elicited by oral and parenteral routes of immunization, we observed similar levels of anti-C. rodentium IgG antibodies in the serum of both groups of animals. However, fecal pathogen-specific IgG was only detected after oral infection, providing a plausible mechanism to account for the differential protective effect of oral vs IP immunization. Previous studies have demonstrated that natural (oral) infection by various pathogens confers protection against disease caused by subsequent infection with the same pathogen (Czerkinsky and Jan Holmgren, 2015). This long-lasting immunological memory relies on the generation of B memory (BM) cells in the germinal centers of intestinal Peyer’s patches and lymphoid follicles, these BM cells express gut homing properties and differentiate into antibody-secreting plasma cells that produce mucosal antibodies in the gut (Pasetti et al., 2011; Czerkinsky and Holmgren, 2015). In contrast, systemic antibodies, such as those produced after IP immunization, are unable to reach the intestinal lumen under homeostatic conditions since most of the intestine is not permeable to serum antibodies in the absence of intestinal damage (Czerkinsky and Holmgren, 2015). This may explain why parenteral immunization did not confer protection against oral challenge with C. rodentium in adult mice because IP immunization did not induce the presence of IgG against the pathogen in the gut. Thus, plasma antibodies elicited via the parenteral route might be primarily effective against enteric pathogens that cross the epithelial barrier. Consistently, parenteral immunization with murein lipoprotein, an outer membrane protein of Gram-negative bacteria, conferred protection against systemic infections by E. coli and Salmonella through IgG-coating of the pathogen to promote its killing by phagocytes (Zeng et al., 2016). Although C. rodentium-specific IgA was also observed in the feces after oral infection, but not IP immunization, orally infected IgA-deficient mice develop effective protective immunity against a secondary challenge with C. rodentium (Maaser et al., 2004). Thus, IgA is not important for the development of protective immunity against the pathogen. Altogether, these data suggest that luminal C. rodentium-specific IgG, but not IgA, generated during oral infection is essential to confer protection against reinfection with the pathogen.
Although secretory IgA and IgM can confer protection against various enteric pathogens by preventing colonization and/or replication of virulent bacteria (Boullier et al., 2009; Brandtzaeg, 2009), we found that genetic deficiency of IgA, or the reduction of passive transfer of maternal IgA and IgM through breast milk in pIgR-deficient mice, did not have any effect in protection of the offspring against pathogen challenge. In contrast, the absence of the neonatal IgG-specific transporter FcRn abolished the protection conferred by maternal immunization due to an impaired production and/or maintenance of IgG in the dams after immunization. Consistently, in addition to its role in regulating IgG stability and the passive transfer of maternal IgG to the offspring, FcRn is also important for the generation of a potent IgG response against foreign antigens in adult mice (Roopenian et al., 2003; Cervenak et al., 2011). Additionally, a previous study using transgenic mice constitutively expressing FcRn, whose natural expression is turned off after weaning in rodents (Yoshida et al., 2006), showed that selective expression of FcRn in the intestinal epithelium of adult mice was associated with increased secretion of IgG into the lumen and reduced susceptibility against C. rodentium (Yoshida et al., 2006). Altogether, these observations highlight the essential role of IgG and its transporter FcRn in protection against C. rodentium infection in both adult and neonatal mice.
Several studies have addressed the role of maternal immunization in generation of passive immunity in the offspring against enteric pathogens such as Shigella (Mitra et al., 2012), Enterotoxigenic E. coli (ETEC) (Luiz et al., 2015) and Enterohemorragic E. coli (EHEC) (Dean-Nystrom et al., 2002; Rabinovitz et al., 2016). However, the protective immunoglobulin, the route of delivery, and the mechanism by which maternal immunization conferred protection to the offspring were not determined in such studies. Additionally, in the case of EHEC, the lack of a lethal and/or natural model of infection resulted in only subtle differences in susceptibility between the immunized and control groups (Dean-Nystrom et al., 2002; Rabinovitz et al., 2016). In this study, we showed that maternal pathogen-specific IgG delivered through breast milk confers protection to the offspring and demonstrated that protective IgG coats the pathogen in the intestinal lumen of the suckling pup, thus reducing its attachment to the mucosa and preventing epithelial damage.
Our studies indicate that LEE-specific antigens are essential for protection of the offspring against C. rodentium infection, since immunization with a Ler mutant that does not express LEE virulence factors did not confer protection to the offspring. We identified EspA, Intimin and EspB, three extracellular components of the T3SS encoded by LEE (Gaytán et al., 2016), as major antigens to confer passive immunity in the offspring after maternal immunization. Notably, antibodies reactive to these T3SS components have been detected in serum (Jenkins et al., 2000; Li et al., 2000; Martinez et al., 1999) and milk samples (Manjarrez-Hernandez et al., 2000; Gavilanes-Parra et al., 2013; Altman et al., 2017) of patients infected with EPEC. The observation that maternal vaccination with single LEE-antigens (Intimin, EspA or EspB) or their combination conferred similar levels of protection in the offspring suggests that antibodies against these antigens might act through a similar mechanism to confer protection. Such antibodies could act by promoting pathogen opsonization and killing by neutrophils in the intestine as previously reported for adult mice (Kamada et al., 2015) and/or by interfering with the intimate binding of the pathogen to the intestinal epithelium promoted by these LEE proteins (Deng et al., 2004; Gaytán et al., 2016). Because protection obtained with single protein vaccinations was lower than that observed after HK-Cr immunizations, it is also possible that naturally folded LEE antigens, and/or additional LEE proteins, are required for the induction of an optimal IgG response and full protection after maternal vaccination. In addition, we cannot rule out the possibility that non-LEE factors may contribute to the full protective response conferred by LEE antigens, for example, by acting as natural adjuvants.
In summary, our results provide insights into the mechanism by which maternal antibodies, in particular IgG in breast milk, protect mice from enteric infection in neonates, a population highly susceptible to diarrheal disease, including EPEC infection. These results may lead to the development of better approaches to treat enteric infections during the neonatal period including the delivery of protective IgG to the intestine of infants via breast milk after maternal vaccination and/or through enriched formula containing pathogen-specific IgG antibodies.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCING SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Gabriel Nunez (gabriel.nunez@umich.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
The WT and FcRn-deficient (Fcgrt−/−) mice were purchased from The Jackson Laboratory. The IgA-deficient (Igha−/−) mice were provided by Dr. Bernard Arulanandam (UT Health San Antonio; Harriman et al., 1999). The pIgR-deficient (Pigr−/−) mice were provided by Dr. Christiane E. Wobus (University of Michigan; Uren et al., 2003). All mice were in the C57BL/6 background and bred/kept under the same specific-pathogen-free (SPF) conditions in our mouse Facility at the University of Michigan. Adult females (6–8-week old) and neonatal mice (18–19-day old, males and females) were used for immunization and/or pathogen challenge. All animal studies were approved by the University of Michigan Committee on the Care and Use of Animals.
Bacterial strains
The kanamycin-resistant (KanR) C. rodentium strain DBS120 (pCRP1::Tn5) was originally provided by Dr. David Schauer (Schauer and Falkow, 1993). The isogenic C. rodentium Δler mutant (KanR), the ampicillin-resistant (AmpR) C. rodentium-lux, and the chloramphenicol-resistant (ChlR) C. rodentium-GFP reporter strains have been previously described (Deng et al., 2004; Bergstrom et al., 2010; Kamada et al., 2015). The E. coli strain BL21 was purchased from New England Biolabs. All bacteria were routinely grown in Luria-Bertani broth (LB, Invitrogen) or Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific). Media were supplemented with Kan (50 μg/ml), Amp (200 μg/ml) or Chl (35 μg/ml) as required.
METHOD DETAILS
Mouse immunization and infection.
For HK-Cr immunizations, bacteria were grown in DMEM (5% CO2, 37°C, static) and concentrated to ~5×108 CFU/ml in phosphate buffered saline (PBS 1X, Thermo Fisher Scientific). Bacterial suspensions were then heat-inactivated at 75°C for 45–60 min, and 0.2 ml were IP injected into adult females. After two weeks, a second HK-Cr injection was administered. For protein immunizations, 50 μg of the purified proteins were mixed 1:1 (v/v) with Imject Alum (Thermo Fisher Scientific) and IP injected into adult females. A second protein/Alum injection, and a final antigen immunization (100 μg) without adjuvant were administered at 2-week intervals. For both HK-Cr and protein immunizations, the females were mated with naïve males two weeks after last immunization, and the resulting offspring was orally challenged with C. rodentium as described below.
For oral C. rodentium challenge, bacteria were grown in LB broth with shaking at 37°C until cultures reached an optical density at 600 nm (O.D.600) of ~1.0. Bacterial cultures were then used to prepare bacterial suspensions containing ~5×109, 5×108 or 5×107 CFU/ml in PBS 1X and 0.1 ml were administered by oral gavage into adult or neonatal mice. Pathogen counts in feces were determined at different time points after infection through serial dilution plating. Some mice were sacrificed between day 7 and 10 after infection to determine pathogen loads in liver and spleen, and to process colonic and cecal tissues for Haemotoxylin and Eosin (H&E) staining, whereas the rest of the mice were used to monitor mouse survival.
Histological scoring
The pathology score was evaluated blindly by one pathologist using a previously described scoring system (Chen et al., 2008). Briefly, severity of inflammation (0= none, 1= mild, 2= moderate, 3= severe), level of involvement (0= none, 1= mucosa, 2= mucosa and submucosa, 3= transmural), and extent of epithelial/crypt damage (0= none, 1= basal 1/3, 2= basal 2/3, 3= crypt loss, 4= crypt and surface epithelial destruction) were determined. Each variable was then multiplied by a factor reflecting the percentage of the cecum/colon involved (0–25%, 26–50%, 51–75%, 76–100%), and then summed to obtain the overall score.
Protein purifications
The full nucleotide coding regions of EspA and EspB were amplified using the sets of primers pQE60-EspA and pQE60-EspB, respectively (see Key Resources Table), and then cloned into the plasmid pQE60 (QIAGEN). The plasmid p6his-Intb385 containing the DNA region encoding the extracellular C-terminal 385 amino acids of Intimin-β (6xHis-tagged-Intimin-β385) cloned into the vector pQE32 was kindly provided by Alison D. O’Brien (Sinclair and O’Brien, 2004). The recombinant plasmids were transformed into E. coli BL21 containing the plasmid pREP4 (QIAGEN) and protein overexpression was induced by adding 1mM isopropyl β-D-1-thiogalactopyranoside (IPTG; Sigma-Aldrich) into the bacterial cultures. Recombinant proteins were purified under denaturing conditions using nickel nitrilotriacetic acid (Ni-NTA) agarose (QIAGEN), and then dialyzed in PBS 1X. Protein concentration was determined using the Quick Start Bradford Protein Assay (Bio-Rad) and the purity of the samples was analyzed by electrophoresis into 12% SDS-PAGE. Aliquots of the proteins were stored at −20°C until use.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Biotin Rat anti-Mouse IgG1 | BD Biosciences | Cat# 553441; RRID: AB_394861 |
| Biotin Rat anti-Mouse IgG2a | BD Biosciences | Cat# 553388; RRID: AB_394826 |
| Biotin Rat anti-Mouse IgG2b | BD Biosciences | Cat# 553393; RRID: AB_394831 |
| Biotin Rat anti-Mouse IgG3 | BD Biosciences | Cat# 553401; RRID: AB_394838 |
| Goat anti-Mouse IgA, Human ads-AP | SouthernBiotech | Cat# 1040–04; RRID: AB_619826 |
| Goat anti-Mouse IgG1, Human ads-AP | SouthernBiotech | Cat# 1070–04 |
| Goat anti-Mouse IgG2a, Human ads-AP | SouthernBiotech | Cat# 1080–04; RRID: AB_2692322 |
| Goat anti-Mouse IgG2b, Human ads-AP | SouthernBiotech | Cat# 1090–04; RRID: AB_619828 |
| Goat anti-Mouse IgG3, Human ads-AP | SouthernBiotech | Cat# 1100–04 |
| Goat anti-Mouse IgM, Human ads-AP | SouthernBiotech | Cat# 1020–04; RRID: AB_619829 |
| Peroxidase AffiniPure Rabbit Anti-Mouse IgG, F(ab’)2 fragment specific | Jackson ImmunoResearch | Cat# 315–035-047; RRID: AB_2340068 |
| Bacterial and Virus Strains | ||
| Citrobacter rodentium DBS120 (pCRP1::Tn5) | Dr. David Schauer (Schauer and Falkow, 1993) | N/A |
| Citrobacter rodentium DBS100 Δler | Dr. José Luis Puente (Deng et al., 2004) |
N/A |
| Citrobacter rodentium DBS100-lux | Dr. Bruce A. Vallance (Bergstrom et al., 2010) |
N/A |
| Citrobacter rodentium DBS100-GFP | Dr. Bruce A. Vallance (Bergstrom et al., 2010) | N/A |
| Escherichia coli BL21 | New England Biolabs | Cat# C2530H |
| Chemicals, Peptides, and Recombinant Proteins | ||
| APC-streptavidin | Biolegend | Cat#: 405207 |
| Dulbecco’s Modified Eagle Medium | Thermo Fisher Scientific | Cat#: 11995065 |
| EspA-6xHis-tagged | This paper | N/A |
| EspB-6xHis-tagged | This paper | N/A |
| Fluriso (Isofluorane) | VetOne | Cat#: 501017 |
| Gentamicin | Thermo Fisher Scientific | Cat#: 15710064 |
| Imject Alum | Thermo Fisher Scientific | Cat#: 77161 |
| Ni-NTA Agarose | QIAGEN | Cat#: 30210 |
| NP-Ovalbumin | Biosearch Technologies | Cat#: N-5051 |
| Oxytocin | VetOne | Cat#: 501013 |
| pNPP Substrate, Tablets | SouthernBiotech | Cat#: 0201–01 |
| Thyoglicolate Broth | Sigma-Aldrich | Cat# 70157 |
| 6xHis-tagged-Intimin-β385 | This paper & Sinclair and O’Brien, 2004 | N/A |
| Critical Commercial Assays | ||
| Quick Start Bradford Protein Assay |
Bio-Rad | Cat#: 500–0205 |
| Pierce ECL Western Blotting Substrate | Thermo Fisher Scientific | Cat#: 32106 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J: WT | The Jackson Laboratory | Cat#: 000664; RRID: IMSR_JAX:000664 |
| Mouse: FcRn-deficient (Fcgrt−/−): B6.129X1-Fcgrttm1Dcr | The Jackson Laboratory | Cat#: 003982; RRID: IMSR_JAX:003982 |
| Mouse: IgA-deficient (Igha−/−): C.129S7(B6)-Igh-2tm1Grh | Dr. Bernard Arulanandam (Harriman et al., 1999) | Cat#: 031020-MU; RRID: MMRRC_031020-MU |
| Mouse: pIgR-deficient (Pigr−/−): B6.129P2-Pigrtm1Fejo | Dr. Christiane E. Wobus (Uren et al., 2003). | Cat#: 030988-MU; RRID: MMRRC_030988-MU |
| Oligonucleotides | ||
| pQE60-EspA-F: GGTTTACCCATGGATACATCAACTATG | This paper | N/A |
| pQE60-EspA-R: TATCTCGGATCCTTTGCCAATGGGTATTGCTG |
This paper | N/A |
| pQE60-EspB-F: CGAGACCATGGATACTATCGATTATAACAATGC | This paper | N/A |
| pQE60-EspB-R: AATGTTGGATCCCCCTGCTAAACGAGCCGATTG |
This paper | N/A |
| Recombinant DNA | ||
| Plasmid pQE60 | QIAGEN | Cat#: 32903 |
| Plasmid pQE60-EspA | This paper | N/A |
| Plasmid pQE60-EspB | This paper | N/A |
| Plasmid pREP4 | QIAGEN | N/A |
| Plasmid p6his-Intb385 | Dr. Alison D. O’Brien (Sinclair and O’Brien, 2004) | N/A |
| Software and Algorithms | ||
| GraphPad Prism version 7 | GraphPad Software | https://www.graphpad.com/ |
| Living Image Software | PerkinElmer | http://www.perkinelmer.com/lab-products-and-services/resources/in-vivo-imaging-software-downloads.html#LivingImage |
Milk collection from mice
Milk collection was performed as previously reported (Gómez-Gallego, et al., 2013). Briefly, adult female mice with nursing litters were separated from their pups for 4–6 h to allow accumulation of milk in the mammary glands. Dams were then anesthetized using 2% isoflurane (VetOne), and 2–8 units per mouse of oxytocin (VetOne) was administered intraperitoneally between the left and right inguinal nipples to induce milk flow. Samples were collected using an electric human breast pump that was modified to accommodate mouse nipples and to handle small liquid volumes (Gómez-Gallego, et al., 2013). When no more milk was recovered from various nipples, the milking machine was stopped, and the milk stored at −20°C until use.
Immunoblotting and ELISA
For detection of pathogen-specific IgG by immunoblotting, bacterial lysates of WT and Δler C. rodentium, or the indicated purified proteins, were separated by electrophoresis into 12% SDS-PAGE and then transferred to Immobilon-P membranes (Millipore). Blots were incubated overnight at 4°C with mouse serum (1:10,000 dilution) or milk (1:1,000) in blocking buffer (PBS 1X, 0.1% Tween 20, 5% dry milk). Membranes were washed with PBS-T buffer (PBS 1X, 0.1% Tween), and then incubated for 1 h at room temperature with peroxidase rabbit anti-mouse IgG (1:10,000; Jackson ImmunoResearch) in blocking buffer. Bound IgG was detected with Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific) following the manufacturer’s instructions.
For measurement of antigen-specific antibodies by ELISA, 96-well plates were coated with a suspension containing ~1×107 CFU of HK-Cr, or 1 μg of purified protein, in PBS 1X. Wells were blocked with PBS-A buffer (PBS 1X, 2% bovine serum albumin), and incubated overnight at 4°C with mouse serum (1:1,000 to 1:10,000), milk (1:100 to 1:1,000) or luminal contents (0.1 to 0.01 μg/ml) in PBS-A. Wells were washed with PBS-T, and then incubated 1 h with goat alkaline phosphatase (AP)-conjugated anti-mouse IgG, IgA or IgM (1:500; SouthernBiotech). Binding of specific IgG subtypes or IgA was detected by measuring the O.D. at 405 nm after adding p-nitrophenyl phosphate (pNPP) substrate (SouthernBiotech).
Cross-fostering experiments
Naïve or HK-Cr immunized females were pair-housed with naïve males at the same day (two weeks after last immunization of the treated group). Once pregnancy was noticed, the male was removed from the cage and the pregnant females were monitored daily for delivery. Age matched (1- to 2-day old) newly born pups were then reciprocally cross-fostered between naïve and HK-Cr immunized dams. Pups were left undisturbed with the recipient mother until they were 18- to 19-day old, and then challenged with C. rodentium as described above. Age matched naïve (non-cross-fostered) pups were used as controls in these experiments.
Neutrophil phagocytosis assays
For the in vitro phagocytosis assays, 1×106 CFU of WT C. rodentium (ChlR) and Δler mutant (KanR), previously grown in DMEM to induce expression of virulence factors, were mixed 1:1 and incubated with milk from naive or HK-Cr immunized dams for 30 min at room temperature. Bacterial mixtures were then incubated (30 min, 37°C, 5% CO2) with 1×106 neutrophil cells harvested from the peritoneum of naïve mice previously treated with 4% thioglycolate broth (Sigma-Aldrich) to induce neutrophil recruitment in the peritoneal cavity. Neutrophils-bacteria mixtures were then treated with gentamycin (10 μg/ml) for 30 min to kill extracellular bacteria. Cells were washed with PBS 1X to remove the excess of gentamicin in the media, and then permeabilized with 0.1% triton-X100. The number of intracellular bacteria was determined by plating serial dilutions of the permeabilized cell samples in LB agar plus Chl or Kan. For the in vivo phagocytosis assays, 1 ×106 CFU of the WT and Δler strains were co-injected into the neutrophil-rich peritoneal cavity of naïve or maternally immunized pups, previously treated with 4% thioglycolate. After 30 min, neutrophils were harvested from the peritoneal cavity and the number of intracellular bacteria was determined as described above.
Measurement of mucosa-associated bacteria
For detection of mucosa-attached pathogen, naïve or maternally immunized pups were infected with ~5×108 CFU of the luciferase reporter strain C. rodentium-lux and cecal/colonic tissue were harvested four days after infection. To remove non-adherent bacteria, intestinal tissues were flushed with PBS 1X using an oral gavage needle, and further washed by vortex in a conical tube containing sterile PBS 1X. Washed tissues were immediately placed into the light-tight chamber of a CCD in vivo imaging system (IVIS 200, PerkinElmer) and bacterial bioluminescence in the tissue was captured using the software Living Image (PerkinElmer). Both control and treatment groups were analyzed at the same time and using the same software settings. After capturing the images, ceca and colons were separately homogenized in sterile PBS 1X (0.1 mg tissue/μl buffer), and serial dilutions of the tissue homogenates were plated on LB agar plus Amp to determine the total number of attached bacteria.
IgG coating of luminal C. rodentium
For IgG coating of C. rodentium in vivo, naïve and maternally immunized pups were infected with the reporter strain C. rodentium-GFP. Two to four hours after infection, gastrointestinal (GI) contents were collected, resuspended in PBS 1X and filtrated sequentially through 100 μm, 70 μm and 40 μm strainers (Falcon). The filtrated luminal contents (cell pellets) were then washed with FACS buffer (PBS1 X, 1% BSA, 0.1% NaN3) and stained with biotin rat anti-mouse IgG (eBiosciences). Samples were washed with FACS buffer and then incubated with APC-streptavidin (Biolegend). After a second washing, Immunoglobulin bound GFP+-bacteria were detected by Flow Cytometry using FACSCalibur or FACSCanto II (BD Biosciences). For IgG coating of C. rodentium in vitro, bacteria were cultured in DMEM, incubated for 30 min with milk samples from naïve or HK-Cr immunized dams, and then stained and analyzed by Flow Cytometry as described above.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed using GraphPad Prism version 7 (GraphPad Software). Differences between two groups were evaluated using Student’s t test (parametric) or Mann-Whitney U test (non-parametric). For multiple comparisons, statistical analyses were performed using one-way ANOVA (parametric) or Kruskal-Wallis test (non-parametric), and then Bonferroni test for parametric samples, or Dunn’s test for non-parametric samples as post hoc tests. Differences at or below p < 0.05 were considered significant. If not otherwise stated, data are shown as mean ± standard deviation (SD). Information regarding exact number of animals (n= “X”), experimental replication, dispersion measures, and statistical tests used are described in the figure legends.
Supplementary Material
HIGHLIGHTS:
Maternal immunization confers protection to offspring against C. rodentium infection.
Maternal IgG in breast milk, but not IgA or IgM, is required for neonatal protection.
Maternal IgG coats the pathogen and increases its engulfment by neutrophils.
LEE-specific antibodies are required to confer protection in the offspring.
ACKNOWLEDGMENTS
The authors thank the University of Michigan Flow Cytometry Core, the Research Histology and Immunohistochemistry Core, and the Center for Molecular Imaging for technical support; Joseph Pickard and José Luis Puente for manuscript review; Caitlin Reynolds for animal care and husbandry; and Tailor Mathes for lab management. G.C.-F. and V.M-A were supported by a postdoctoral (454848) and PhD (253771) fellowship respectively, from the “Consejo Nacional de Ciencia y Tecnología” of Mexico (CONACYT). K.S. was supported by fellowships from the Japan Society for the Promotion of Science, the Kanae Foundation for the Promotion of Medical Science, and the Mishima Kaiun Memorial Foundation. M.Z. was supported by NIH training grants T32DK094775, T32HL007517 and the University of Michigan Center for Gastrointestinal Research (NIH 5P30DK034933). This work was supported by NIH grants DK091191 and DK095782 to G.N. The first author (G.C.-F.) dedicates this paper to the bright memory of Santiago Caballero-Matus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Altman SPN, Tino-De-Franco M, Carbonare CB, Palmeira P, and Carbonare SB (2017). Placental and colostral transfer of antibodies reactive with enteropathogenic Escherichia coli intimins alpha, beta, or gamma. J. Pediatr. (Rio J) 93, 568–575. [DOI] [PubMed] [Google Scholar]
- Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et al. (2010). Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 6, e1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthésy B, and Phalipon A (2009). Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J. Immunol 183, 5879–5885. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P (2009). Mucosal immunity: induction, dissemination, and effector functions. Scand. J. Immunol 70, 505–515. [DOI] [PubMed] [Google Scholar]
- Bry L, and Brenner MB (2004). Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol 172, 433–441. [DOI] [PubMed] [Google Scholar]
- Cervenak J, Bender B, Schneider Z, Magna M, Carstea BV, Liliom K, Erdei A, Bosze Z, and Kacskovics I (2011). Neonatal FcR overexpression boosts humoral immune response in transgenic mice. J. Immunol 186, 959–968. [DOI] [PubMed] [Google Scholar]
- Chen GY, Shaw MH, Redondo G, and Núñez G (2008). The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 68, 10060–10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianga P, Medesan C, Richardson JA, Ghetie V, and Ward ES (1999). Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur. J. Immunol 29, 2515–2523. [DOI] [PubMed] [Google Scholar]
- Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, and Frankel G (2014). Citrobacter rodentium: infection, inflammation and the microbiota. Nat. Rev. Microbiol 12, 612–623. [DOI] [PubMed] [Google Scholar]
- Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, and Finlay BB (2013). Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev 26, 822–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C, and Holmgren J (2015). Vaccines against enteric infections for the developing world. Philos. Trans. R. Soc. Lond. B. Biol. Sci 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean-Nystrom EA, Gansheroff LJ, Mills M, Moon HW, and O’Brien AD (2002). Vaccination of pregnant dams with intimin(O157) protects suckling piglets from Escherichia coli O157:H7 infection. Infect. Immun 70, 2414–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vázquez A, Barba J, Ibarra JA, O’Donnell P, Metalnikov P, et al. (2004). Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 101, 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel G, and Phillips AD (2008). Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell. Microbiol 10, 549–556. [DOI] [PubMed] [Google Scholar]
- Furuya Y, Kirimanjeswara GS, Roberts S, and Metzger DW (2013). Increased susceptibility of IgA-deficient mice to pulmonary Francisella tularensis live vaccine strain infection. Infect. Immun 81, 3434–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilanes-Parra S, Mendoza-Hernández G, Chávez-Berrocal ME, Girón JA, Orozco-Hoyuela G, and Manjarrez-Hernández A (2013). Identification of secretory immunoglobulin A antibody targets from human milk in cultured cells infected with enteropathogenic Escherichia coli (EPEC). Microb. Pathog 64, 48–56. [DOI] [PubMed] [Google Scholar]
- Gaytán MO, Martínez-Santos VI, Soto E, and González-Pedrajo B (2016). Type three secretion system in attaching and effacing pathogens. Front. Cell. Infect. Microbiol 6, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, and Ward ES (1996). Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol 26, 690–696. [DOI] [PubMed] [Google Scholar]
- Gómez-Gallego C, Ilo T, Ulla-Marjut J, Salminen S, Periago MJ, Ros G, and Frias R (2013). A method to collect high volumes of milk from mice (Mus musculus). An. Vet. (Murcia) 29, 55–61. [Google Scholar]
- Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, and Mbawuike IN (1999). Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol 162, 2521–2529. [PubMed] [Google Scholar]
- Hurley WL, and Theil PK (2011). Perspectives on immunoglobulins in colostrum and milk. Nutrients 3, 442–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel EJ, Patel VK, Taylor SF, Marshak-Rothstein A, and Simister NE (1995). Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J. Immunol 154, 6246–6251. [PubMed] [Google Scholar]
- Jenkins C, Chart H, Smith HR, Hartland EL, Batchelor M, Delahay RM, Dougan G, and Frankel G (2000). Antibody response of patients infected with verocytotoxin-producing Escherichia coli to protein antigens encoded on the LEE locus. J. Med. Microbiol 49, 97–101. [DOI] [PubMed] [Google Scholar]
- Jennewein MF, Abu-Raya B, Jiang Y, Alter G, and Marchant A (2017). Transfer of maternal immunity and programming of the newborn immune system. Semin. Immunopathol 39, 605–613. [DOI] [PubMed] [Google Scholar]
- Kamada N, Sakamoto K, Seo SU, Zeng MY, Kim YG, Cascalho M, Vallance BA, Puente JL, and Núñez G (2015). Humoral immunity in the gut selectively targets phenotypically virulent attaching-and-effacing bacteria for intraluminal elimination. Cell Host Microbe 17, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, and Mobley HL (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol 2, 123–140. [DOI] [PubMed] [Google Scholar]
- Kim YG, Sakamoto K, Seo SU, Pickard JM, Gillilland MG 3rd, Pudlo NA, Hoostal M, Li X, Wang TD, Feehley T, et al. (2017). Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science 356, 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann TR, Levy O, Montgomery RR, and Goriely S (2012). Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. (2013). Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382, 209–222. [DOI] [PubMed] [Google Scholar]
- Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, Child Health Epidemiology Reference Group of the World Health, O., and Unicef (2013). Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 8, e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RJ, Gur-Arie L, Rosenshine I, and Finlay BB (2013). In vitro and in vivo model systems for studying enteropathogenic Escherichia coli infections. Cold Spring Harb. Perspect. Med 3, a009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Frey E, Mackenzie AM, and Finlay BB (2000). Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun 68, 5090–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiz WB, Rodrigues JF, Crabb JH, Savarino SJ, and Ferreira LC (2015). Maternal vaccination with a fimbrial tip adhesin and passive protection of neonatal mice against lethal human enterotoxigenic Escherichia coli challenge. Infect. Immun 83, 4555–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Ke C, Wan Z, Li Z, Cheng X, Wang X, Zhao J, Ma Y, Ren L, Han H, et al. (2018). Truncation of the murine neonatal Fc receptor cytoplasmic tail does not alter IgG metabolism or transport in vivo. J. Immunol 200, 1413–1424. [DOI] [PubMed] [Google Scholar]
- Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, and Eckmann L (2004). Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun 72, 3315–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjarrez-Hernandez HA, Gavilanes-Parra S, Chavez-Berrocal E, Navarro-Ocaña A, and Cravioto A (2000). Antigen detection in enteropathogenic Escherichia coli using secretory immunoglobulin A antibodies isolated from human breast milk. Infect. Immun 68, 5030–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MB, Taddei CR, Ruiz-Tagle A, Trabulsi LR, and Girón JA (1999). Antibody response of children with enteropathogenic Escherichia coli infection to the bundle-forming pilus and locus of enterocyte effacement-encoded virulence determinants. J. Infect. Dis 179, 269–274. [DOI] [PubMed] [Google Scholar]
- Mitra S, Barman S, Nag D, Sinha R, Saha DR, and Koley H (2012). Outer membrane vesicles of Shigella boydii type 4 induce passive immunity in neonatal mice. FEMS Immunol. Med. Microbiol 66, 240–250. [DOI] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, and Wiles S (2005). Citrobacter rodentium of mice and man. Cell. Microbiol 7, 1697–1706. [DOI] [PubMed] [Google Scholar]
- Pasetti MF, Simon JK, Sztein MB, and Levine MM (2011). Immunology of gut mucosal vaccines. Immunol. Rev 239, 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, and Siegrist CA (2011). Challenges in infant immunity: implications for responses to infection and vaccines. Nat. Immunol 12, 189–194. [DOI] [PubMed] [Google Scholar]
- Pyzik M, Rath T, Lencer WI, Baker K, and Blumberg RS (2015). FcRn: The architect behind the immune and nonimmune functions of IgG and albumin. J. Immunol 194, 4595–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz BC, Larzábal M, Vilte DA, Cataldi A, and Mercado EC (2016). The intranasal vaccination of pregnant dams with Intimin and EspB confers protection in neonatal mice from Escherichia coli (EHEC) O157:H7 infection. Vaccine 34, 2793–2797. [DOI] [PubMed] [Google Scholar]
- Rath T, Kuo TT, Baker K, Qiao SW, Kobayashi K, Yoshida M, Roopenian D, Fiebiger E, Lencer WI, and Blumberg RS (2013). The immunologic functions of the neonatal Fc receptor for IgG. J. Clin. Immunol 33 Suppl 1, S9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardo F, Réal A, Voena C, Chiarle R, Cavallo F, and Barutello G (2017). Maternal immunization: new perspectives on its application against non-infectious related diseases in newborns. Vaccines (Basel) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, et al. (2003). The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol 170, 3528–3533. [DOI] [PubMed] [Google Scholar]
- Schauer DB, and Falkow S (1993). The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun 61, 4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AK, Hollander GA, and McMichael A (2015). Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci 282, 20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JF, and O’Brien AD (2004). Intimin types alpha, beta, and gamma bind to nucleolin with equivalent affinity but lower avidity than to the translocated intimin receptor. J. Biol. Chem 279, 33751–33758. [DOI] [PubMed] [Google Scholar]
- Uren TK, Johansen FE, Wijburg OL, Koentgen F, Brandtzaeg P, and Strugnell RA (2003). Role of the polymeric Ig receptor in mucosal B cell homeostasis. J. Immunol 170, 2531–2539. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, et al. (2006). Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest 116, 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, and Núñez G (2016). Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44, 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.