Abstract
The aim of this study was to assess the associations of intake of different types of meat with semen parameters and reproductive hormones in healthy young men. This cross-sectional study included 206 men, 18–23 years, from Southern Spain. All men completed a validated food frequency questionnaire, underwent a physical examination, and provided blood and semen samples. Multivariable linear regression was used to evaluate the associations between meat intake with semen quality parameters and reproductive hormones. Total meat intake was unrelated to semen quality or reproductive hormone levels. When subgroups of meat were separately considered, however, shellfish intake was positively related to progressive motility. The adjusted percentages of progressively motile spermatozoa for men in increasing quartiles of shellfish intake were 45.2%, 42.0%, 49.4%, and 53.2% with a significant linear trend across quartiles (p-trend=<0.001). In contrast, men who consumed organ meats had significantly lower progressive sperm motility (51.5% vs. 42.8%; p=0.001) and higher LH levels (4.0 IU/L vs. 4.6 IU/L; p=0.03) than men who did not consume organ meats. Intake of shellfish and organ meats was low in this population, however. Given the scarcity of data on the relation between specific types of meat with semen quality and reproductive hormone levels, additional research is needed to confirm or refute these findings.
Keywords: Meat, Fish, Shellfish, Semen motility, Semen quality parameters
Introduction
Decreasing trends in sperm counts during the last decades have been reported in multiple studies (1–4). The reasons for this downward trend have not been fully elucidated, but exposures adversely affecting prenatal testicular development as well as exposures and lifestyle factors during childhood and young adulthood have been suggested(4, 5). However, relatively little work has been devoted to understanding the modifiable determinants of semen quality, as a marker of men’s reproductive potential,(6) which could lead to the design of clinical and public health interventions(7).
Diet is a modifiable risk factor that can be assessed in clinical practice and inform actions for disease prevention.(8) Previous epidemiologic studies suggest that diet may be associated with semen parameters(9–14). Animal food intake, and in particular intake of red meats and fish, has received particular attention since their nutritional profile and their contamination with persistent organic pollutants and hormonal residues may affect testicular function(11, 15–19). Although literature is scarce, red meats have consistently been associated to worse semen quality, while fish intake appears to have the opposite relation(19–22). However, these studies have been conducted in different populations and settings, with diverse age and health status, and only one previous work was performed in healthy young men (19). Therefore, the aim of this study is to investigate the associations of intakes of meats, including red, white, processed and fish meats, with semen quality parameters and reproductive hormones among healthy young men from Spain.
Methods
Study population
The Murcia Young Men’s Study (MYMS) was a cross-sectional study conducted in 2010–2011 that enrolled healthy men between 18 and 23 years of age at University of Murcia (Region of Murcia, Spain). MYMS is part of a multi-center international study (Finland, Denmark, Spain and USA) aimed at evaluating the role of environmental contaminants on semen quality. The same study population has been used in previously published works(10, 12, 23, 24). Participants were recruited through flyers posted at university campuses. Men were eligible to participate if they were born in Spain after 31 December 1987 and were able to contact their mother and ask her to complete a questionnaire.
A total of 240 men contacted the study staff. Of these, 223 (92.9%) met the eligibility criteria and 215 completed a study visit and agreed to participate in the study. During the visit, men underwent a physical examination, completed questionnaires concerning diet, lifestyle, medical and reproductive history, and provided semen and blood samples. Six men who reported implausible total caloric intake (>5000kcal/day) and three who had a hydrocele were excluded from the analysis. The final analytical sample comprised 206 men (85.8%). Participants received a €50 gift card for their participation in the study. Written informed consent was obtained from all subjects. The Research Ethics Committee of the University of Murcia approved this study (No. 495/2010, approved 14 May 2010).
Physical examination
Men’s height and weight were measured using a wall stadiometer and digital scale (Tanita SC 330- S, London, UK) to determine body mass index (BMI). The presence of varicocele, hydrocele or other scrotal abnormalities were recorded. Testicular volume was assessed with a Prader orchidometer (Andrology Australia, Clayton, Victoria, Australia). All physical examinations were performed by the same investigator (J.M.) to minimize variability in study measures.
Dietary assessment
Participants completed a 101-item semi-quantitative food frequency questionnaire (FFQ) previously validated in several adult populations in Spain(25, 26). Men reported their usual intake of foods and supplements over the past year. The FFQ had nine categories for frequency of consumption, ranging from never to ≥6 times per day. Total meat intake was defined as the sum of red meat, white meat and fish. We grouped specific meat types in 7 groups (Supplemental Table 1); 3 types of red meat, 1 of white meat and 3 of fish.
Semen analysis
Men were instructed to abstain from ejaculation for 48 h before sample collection, but were not excluded if this was not the case. At the time of sample collection they also provided information on time of the previous ejaculation. Semen samples were obtained by masturbation at the clinic, and lubricants were not used. Ejaculate volume was estimated by sample weight, assuming a semen density of 1.0 g/ml. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific, Inc., Horsham, PA, USA). Sperm motility was evaluated as either motile or immotile according to the World Health Organization criteria(27) and the percentage of total motile sperm and progressive motility were calculated. Smears for morphology were made, air-dried, fixed, Papanicolaou stained and evaluated using strict criteria(28). Total sperm count (volume x sperm concentration) was also calculated. The same specialist biologist carried out all the semen analyses. An external quality control on semen samples throughout the study period was carried out in collaboration with the University of Copenhagen’s Department of Growth and Reproduction. In order to assess inter-laboratory variation in sperm concentration analysis, five sets of duplicate semen samples (600 µleach) were sent by mail during the study period from the University of Copenhagen’s Department of Growth and Reproduction to the Murcia Andrology Laboratory. The samples were blinded, non-diluted fresh sperm specimens from regular semen donors that were preserved by adding 10 µlof a 3 M sodium azide solution per 1 ml of the ejaculate after liquefaction. Results showed no systematic differences. The mean inter-examiner coefficient of variation was 4.0%, ranging between 1.7 and 7.1%.
Reproductive hormones measurement
Blood samples were drawn from men’s cubital veins and centrifuged; the serum was separated, stored and frozen at −80° C. Serum samples were shipped to Rigshospitalet (Copenhagen, Denmark) and stored at −20° C until hormone analysis was performed. The methods have been outlined previously(29). Briefly, hormone assessments were performed simultaneously to reduce intralaboratory variations. Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH) and sex hormone-binding globulin (SHBG) were determined using time-resolved immunofluorometric assays (DELFIA; PerkinElmer, Skovlund, Denmark). Intra- and inter-assay variations were <5% in each of the three assays. Serum total testosterone (TT) levels were determined using a time-resolved fluoroimmunoassay (DELFIA; PerkinElmer) with intra- and interassay variations of < 8%. Estradiol (E2) was measured by radioimmunoassay (Pantex, Santa Monica,CA) with an intra-assay variation of <8% and an inter-assay variation of <13%. Inhibin B levels were determined by a specific two-sided enzyme immunometric assay (Oxford Bio-Innovation Ltd, Bicester, UK) with intra and inter-assay variations of 13 and 18%, respectively. When inhibin B was above approximately 100pg/mL, the intra-assay variation was <7% and the inter-assay variation was <6%. The majority of the men had levels above 100 pg/mL. Calculated free testosterone (FT) levels was determined using the equation of Vermeulen et al.(30) assuming a fixed albumin of 43.8 g/l.
Statistical analysis
Sample size was calculated assuming anticipating an effect size of 0.15, for a prespecified alpha and power level of 5% and 80%, respectively. With a non-response rate of 20%, a minimum sample size of 157 subjects was needed(31). Participants’ characteristics were summarized using the median and interquartile range (IQR) and absolute frequency and percentage across quartiles of total meat intake. Statistical differences across quartiles of meat intake, red meat intake and fish meat intake were assessed using Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables. Semen volume, sperm concentration, total sperm count, morphologically normal sperm, FSH levels, and estradiol levels were log (ln) transformed before analysis to improve normality. After analysis, we back-transformed them to show values in original scale. Multivariate linear regression models were used to investigate the association of each group of meat intake (in categories) with semen quality parameters and reproductive hormones with raw data and after adjusting for confounders. We used analysis of covariance (ANCOVA) to calculate adjusted reproductive outcomes and 95% confidence intervals (CI) as dependent continuous variables for each category of meat intake and covariates as independent variables. An association was considered when we found a statistically significant linear trend among categories of meat intake as ordinal variable. Explicit substitution analyses were performed for the foods for which the unspecified substitution was statistically significant.
Confounding was assessed based in participants’ characteristics associated with meat intake and semen parameters or reproductive hormones. Based on this criteria, models were adjusted for age (years), BMI (kg/m2), smoking (current smoker versus not current smoker), physical activity (hours per week), TV watching (hours per week) and total calorie intake (kcal/day). The remaining types of meat (continuous) and dietary patterns (continuous) were also included into the models to address the possibility that observed associations would be explained by overall food choices rather than intake of specific meats. Specifically, as described elsewhere in this population,(23) we used principal components analysis (PCA) with orthogonal transformation based on predefined food groups(32)to obtain uncorrelated factors (dietary patterns) with simpler structures. Terms for intake of meats were excluded from this analysis. We retained the first two factors (Supplemental Table 2) based on the amount of variance explained by the factor, the scree plot and the interpretability of the factors. Every subject was given a score for the two identified patterns according to their food consumption, and each participant appears in results for both dietary patterns. All analyses of semen parameters were also adjusted for abstinence time. Analyses of sperm motility were additionally adjusted for time to start semen analysis, and analyses of reproductive hormones were adjusted for time of blood draw. Analyses were performed with the statistical software IBM SPSS 21.0 (IBM Corporation, Armonk, NY, USA).
Results
Participants were predominantly Caucasian (97.6%), and non-smokers (68.1%). They had a median (interquartile range [IQR]) age of 20.5 (19.6–21.5) years and BMI of 23.7 (21.8–25.4) kg/m2. The median physical activity and TV watching was 9 and 20 h/week, respectively (Table 1, Supplemental Table 3). Seventy-one subjects had a surgical scar in genital area or lower abdomen mostly due to frenectomies or circumcision (53.5%), appendectomy (16.9%) and inguinal hernia repair (9.9%) (data not shown). The most consumed meat product was processed red meat (29%), followed by dark meat fish (22%), white meat (18%), unprocessed red meat (12%), white meat fish (11%), shellfish (5%) and organ meats (3%). Men in highest quartile of total meat intake had a higher total calorie intake. Meat intake was positively related to physical activity, and to the Mediterranean and Western dietary patterns, and inversely related to TV watching. Red meat intake was positively related to caloric intake and the Western dietary pattern, and negatively related to TV watching. Fish intake was positively associated with calorie intake, the Mediterranean dietary pattern and physical activity. No other subject characteristics were significantly different across quartiles of total meat intake, red meat or fish intake (Table 1, Supplemental Table 3). Semen parameters and reproductive hormone levels were within normal limits for healthy adult men (Table 1, Supplemental Table 3).
Table 1.
Participant characteristics according to intakes of red meat or fisha. Murcia Young Men’s Study (n=206)
| Total cohort (n = 206) | Red meats | Fish | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n= 53) (lowest) | Q4 (n= 51) (highest) | P‡ | Q1 (n= 51) (lowest) | Q4 (n= 51) (highest) | P‡ | |||||||
| Range, servings/day | 0 – 0.72 | 1.43 – 4.25 | 0 – 0.53 | 1.25 – 4.66 | ||||||||
| Age (years) | 20.5 | (19.6–21.5) | 20.7 | (19.6–21.4) | 20.4 | (19.6–21.6) | 0.28 | 20.7 | (19.6–21.3) | 20.4 | (19.7–21.9) | 0.58 |
| Caucasian | 201 | (97.6) | 52 | (100) | 49 | (96.1) | 0.52 | 51 | (96.2) | 50 | (100) | 0.54 |
| Body mass index (Kg/m2) | 23.7 | (21.8–25.4) | 23.9 | (22.1–25.1) | 23.1 | (21.9–25.6) | 0.63 | 24.4 | (22.1–25.6) | 23.8 | (22.1–25.7) | 0.44 |
| Smoking | 65 | (31.9) | 14 | (28.0) | 17 | (33.3) | 0.92 | 22 | (41.5) | 15 | (30.6) | 0.35 |
| Testicular volume (ml) | 21.0 | (19.5–24.0) | 21.0 | (19.0–24.3) | 20.5 | (19–23) | 0.23 | 21.0 | (19.8–24.3) | 22.3 | (20.0–24.5) | 0.22 |
| History of cryptorchidism | 4 | (1.9) | 2 | (3.8) | 0 | (0.0) | 0.94 | 1 | (1.9) | 0 | (0.0) | 0.30 |
| Varicocele, n (%) | 14 | (6.8) | 4 | (7.7) | 4 | (7.8) | 0.35 | 4 | (7.5) | 2 | (4.0) | 0.74 |
| Inguinal hernia repairb | 7 | (3.4) | 2 | (3.8) | 1 | (2.0) | 0.93 | 3 | (5.7) | 2 | (4.0) | 0.42 |
| Surgical scarb | 71 | (34.5) | 14 | (26.9) | 24 | (47.1) | 0.12 | 18 | (34.0) | 15 | (30.0) | 0.77 |
| Use of hormonesc | 1 | (0.5) | 0 | (0.0) | 0 | (0.0) | 0.37 | 1 | (1.9) | 0 | (0.0) | 0.42 |
| Use of dietary supplementsd | 29 | (14.1) | 7 | (13.5) | 7 | (13.7) | 0.32 | 6 | (113) | 6 | (12.0) | 0.57 |
| Calories intake (kcal/day) | 2281.0 | (1894.4–2928.6) | 1886.3 | (1482.5–2274.3) 2845.4 | 2845.4 | (2394.2–3536.8) | <0.001 | 2197.5 | (1728.3–2754.9) | 2835.7 | (2278.6–3349.7) | <0.001 |
| Physical activity (h/week) | 9.0 | (6.0–13.0) | 8.0 | (5.0–12.0) | 9.0 | (6.0–15.0) | 0.36 | 7.0 | (5.0–10.0) | 10.0 | (6.8–14.3) | 0.07 |
| TV watching (h/week) | 20.0 | (14.0–41.0) | 29.0 | (14.0–41.0) | 20.0 | (14.0–35.0) | 0.07 | 26.0 | (14.0–47.0) | 20.0 | (14.0–36.5) | 0.24 |
| Abstinence time (h) | 71.0 | (59.8–92.0) | 71.0 | (58.8–98.5) | 67.0 | (50.0–93.0) | 0.68 | 72.0 | (64.5–93.0) | 72.0 | (61.3–92.5) | 0.66 |
| Time to semen analysis (min) | 35.0 | (30.0–45.0) | 32.5 | (30.0–40.0) | 40.0 | (30.0–45.0) | 0.10 | 35.0 | (30.0–45.0) | 40.0 | (30.0–45.0) | 0.33 |
| Time of blood draw (min) | 245.0 | (112.5–270.0) | 255.0 | (142.5–288.8) | 240.0 | (125–270) | 0.73 | 255.0 | (157.5–277.5) | 242.5 | (101.3–281.3) | 0.23 |
| Mediterranean diet pattern scoree | −0.2 | (−0.7 to 0.4) | −0.3 | (−0.8 to 0.1) | −0.2 | (−0.7–0.7) | 0.30 | −0.7 | (−1.0–0.2) | 0.3 | (−0.1–1.1) | <0.001 |
| Western diet pattern scoree | −0.2 | (−0.7 to 0.5) | −0.5 | (−0.9 to 0.0) | 0.2 | (−0.3 to 1.0) | <0.001 | 0.0 | (−0.5 to 0.6) | −0.2 | (−0.8 to 0.8) | 0.34 |
| Semen volume (ml) | 3.0 | (2.0–4.0) | 3.2 | (2.3–4.4) | 2.7 | (1.9–3.7) | 0.42 | 3.0 | (2.1–3.8) | 3.0 | (2.0–4.1) | 0.21 |
| Sperm concentration (millions/ml) | 43.4 | (21.9–72.3) | 46.2 | (23.0–74.1) | 48.7 | (18.7–79.3) | 0.96 | 43.4 | (25.6–74.7) | 55.6 | (19.1–80.5) | 0.81 |
| Total sperm count (millions) | 120.1 | (63.3–212.5) | 132.2 | (90.2–210.1) | 106.3 | (46.8–196.2) | 0.60 | 129.5 | (75.1–196.4) | 115.1 | (67.6–249.8) | 0.43 |
| Sperm motility (%) | 57.1 | (50.7–63.8) | 57.6 | (46.8–63.9) | 59.0 | (54.3–65.7) | 0.28 | 59.2 | (51–66.2) | 54.9 | (50.7–63.1) | 0.40 |
| Progressive motility (%) | 48.3 | (41.3–55.2) | 47.0 | (39.8–56.5) | 50.3 | (42.4–58.3) | 0.10 | 50.3 | (42.4–56.9) | 44.7 | (38.5–51.0) | 0.10 |
| Morphologically normal sperm (%) | 9.0 | (6.0–14.0) | 8.0 | (6.0–12.8) | 7.5 | (5.0–13.8) | 0.58 | 9.0 | (5.5–15.0) | 9.0 | (5.0–14.0) | 0.95 |
| Luteinizing hormone (IU/L) | 4.0 | (2.8–5.3) | 3.9 | (2.7–5.1) | 4.3 | (3.2–5.6) | 0.49 | 4.1 | (2.8–5.3) | 3.9 | (2.6–5.0) | 0.52 |
| Follicle-stimulating hormone (IU/L) | 2.2 | (1.6–3.3) | 2.3 | (1.5–3.0) | 2.4 | (1.9–3.5) | 0.68 | 2.0 | (1.5–3.0) | 2.1 | (1.3–3.1) | 0.03 |
| Estradiol (pmol/L) | 76.0 | (63.0–91.2) | 75.5 | (61.0–86.0) | 80.0 | (67.0–106.0) | 0.05 | 72.0 | (59.0–93.0) | 77.0 | (60.8–95.3) | 0.59 |
| Calculated free testosterone (nmol/L) | 13.4 | (10.7–17.1) | 13.7 | (10.3–16.9) | 15.5 | (11.3–18.7) | 0.05 | 12.7 | (10.5–17.0) | 14.1 | (10.4–18.7) | 0.72 |
| Total testosterone (nmol/L) | 21.2 | (17.1–26.6) | 20.9 | (16.6–27.2) | 22.0 | (18.8–27.2) | 0.11 | 20.1 | (16.6–25.5) | 21.4 | (16.6–28.1) | 0.52 |
| Inhibin B (pg/mL) | 193.0 | (147.0–246.0) | 181.5 | (145.3–239.3) | 184.0 | (145.0–227.0) | 0.65 | 203.0 | (146.5–249.5) | 201.0 | (157.5–266.5) | 0.21 |
| Sex hormone-binding globulin (nmol/L) | 30.0 | (23.0–39.0) | 29.5 | (21.3–40.5) | 30.0 | (24.0–36.0) | 0.93 | 27.0 | (20.5–39.5) | 29.5 | (24.0–37.3) | 0.22 |
Continuous variables are shown as median and interquartile range unless otherwise indicated.
Total red meat: includes processed and unprocessed red meats, and organ meat; Total fish meat: includes white fish meat, dark fish meat and shellfish.
Physical examination in the genital area (including lower abdomen)
Self-report of any use of dehydroepiandrostendione, androstenedione, creatinine, steroids or other muscle buildings;
Vitamins and minerals;
Dietary pattern scores without total meat intake
Kruskal-Wallis test for continuous variables and χ2 test for categorical variables
Total meat intake was unrelated to semen quality parameters (Table 2, Supplemental Table 4) or reproductive hormone levels (Table 3, Supplemental Table 5). Similarly, when the major categories of meat were examined, we found no associations of intakes of red meats, white meats or fish with semen quality parameters (Table 2) or reproductive hormone levels (Table 3).
Table 2.
Multivariable adjusteda semen parameters (95% Confidence interval) in relation to meat intake. Murcia Young Men’s Study (n=206)
| Meat intake (servings/day); range |
Volume* | Sperm concentration* | Total sperm count* | Motile spermb | Progressive motilityb |
Morphologically normal sperm* |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | ml | 95% CI | 106/ml | 95% CI | 106 | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Total meat intake | |||||||||||||
| Q1 (0–1.78) | 53 | 2.8 | 2.3–3.4 | 33.0 | 24.1–45.2 | 92.7 | 65.6–130.8 | 54.9 | 51.5–58.2 | 45.2 | 41.8–48.7 | 7.6 | 6.3–9.3 |
| Q2 (>1.78–2.38) | 51 | 2.6 | 2.1–3.1 | 40.3 | 30.3–53.6 | 103.1 | 75.0–141.7 | 57.7 | 54.7–60.8 | 48.6 | 45.5–51.8 | 8.6 | 7.2–10.4 |
| Q3 (>2.38–3.23) | 51 | 3.1 | 2.6–3.7 | 39.0 | 29.5–51.7 | 121.0 | 88.8–165.0 | 56.6 | 53.6–59.6 | 49.1 | 45.9–52.2 | 8.7 | 7.3–10.4 |
| Q4 (>3.23–9.12) | 51 | 2.6 | 2.1–3.3 | 39.7 | 28.6–55.0 | 105.1 | 73.3–150.5 | 58.7 | 55.3–62.2 | 49.8 | 46.1–53.4 | 10.2 | 8.3–12.5 |
| Ptrend | 0.93 | 0.52 | 0.52 | 0.24 | 0.12 | 0.10 | |||||||
| Total red meat intakec | |||||||||||||
| Q1 (0–0.72) | 52 | 2.8 | 2.3–3.4 | 38.1 | 28.1–51.8 | 105.3 | 74.6–148.6 | 55.8 | 52.6–59.0 | 46.3 | 43.0–49.6 | 8.4 | 6.9–10.2 |
| Q2 (>0.72–1.01) | 53 | 2.8 | 2.3–3.3 | 37.2 | 28.1–49.2 | 103.1 | 75.6–140.6 | 55.5 | 52.6–58.4 | 46.1 | 43.1–49.1 | 8.9 | 7.5–10.6 |
| Q3 (>1.01–1.42) | 50 | 2.7 | 2.3–3.3 | 38.2 | 28.7–51.0 | 104.3 | 75.9–143.3 | 58.1 | 55.1–61.1 | 50.3 | 47.2–53.3 | 9.3 | 7.8–11.2 |
| Q4 (>1.42–4.25) | 51 | 2.8 | 2.3–3.4 | 37.7 | 27.6–51.6 | 106.4 | 75.2–150.7 | 58.4 | 55.1–61.7 | 49.9 | 46.5–53.3 | 8.4 | 6.9–10.2 |
| Ptrend | 0.98 | 0.97 | 0.96 | 0.20 | 0.07 | 0.89 | |||||||
| Total white meat intaked | |||||||||||||
| Q1 (0–0.21) | 58 | 2.7 | 2.3–3.3 | 37.9 | 28.8–49.8 | 104.3 | 76.9–141.5 | 55.2 | 52.4–58.1 | 46.9 | 44.0–49.9 | 9.7 | 8.1–11.6 |
| Q2 (>0.21–0.35) | 43 | 2.9 | 2.4–3.5 | 28.7 | 21.2–38.8 | 83.5 | 59.6–117.0 | 56.6 | 53.5–59.7 | 48.0 | 44.8–51.2 | 7.7 | 6.4–9.4 |
| Q3 (>0.35–0.57) | 62 | 3.1 | 2.6–3.6 | 37.3 | 28.9–48.3 | 113.9 | 85.4–152.0 | 56.8 | 54.1–59.4 | 47.9 | 45.1–50.6 | 9.2 | 7.8–10.8 |
| Q4 (>0.57–2.57) | 43 | 2.3 | 1.9–2.9 | 50.8 | 36.2–71.1 | 118.5 | 81.4–172.6 | 59.9 | 56.4–63.5 | 50.2 | 46.5–53.8 | 8.0 | 6.4–10.0 |
| Ptrend | 0.59 | 0.23 | 0.44 | 0.09 | 0.26 | 0.43 | |||||||
| Total fish intake | |||||||||||||
| Q1 (0–0.53) | 53 | 2.7 | 2.2–3.2 | 38.0 | 28.6–50.6 | 101.4 | 74.1–138.9 | 56.5 | 53.5–59.5 | 47.6 | 44.5–50.7 | 8.0 | 6.7–9.7 |
| Q2 (>0.53–0.77) | 53 | 2.6 | 2.1–3.1 | 33.0 | 24.8–44.0 | 84.4 | 61.6–115.8 | 56.2 | 53.2–59.2 | 46.8 | 43.7–49.9 | 8.4 | 7.0–10.1 |
| Q3 (>0.77–1.24) | 50 | 3.3 | 2.8–4.0 | 34.8 | 26.1–46.4 | 114.7 | 83.3–157.7 | 57.9 | 54.8–60.9 | 50.3 | 47.1–53.5 | 8.0 | 6.7–9.6 |
| Q4 (>1.24–4.66) | 50 | 2.6 | 2.1–3.3 | 46.9 | 33.7–65.4 | 124.1 | 86.2–178.8 | 57.3 | 53.8–60.8 | 47.9 | 44.3–51.5 | 10.8 | 8.8–13.3 |
| Ptrend | 0.52 | 0.51 | 0.33 | 0.58 | 0.53 | 0.13 | |||||||
CI, confidence interval
Adjusted for calories intake, intakes of the remaining meats, dietary patterns, age, body mass index, smoking, physical activity, TV watching and abstinence time
Additionally adjusted for time to start semen analysis (minutes)
Includes processed and unprocessed red meat, and organ meat
Includes chicken with and without skin, rabbit, quail and duck
Back-transformed to original scale
Table 3.
Multivariable adjusteda reproductive hormone levels (95% Confidence interval) in relation to meat intake. Murcia Young Men s Study (n=206)
| Meat intake (servings/day); range |
LH | FSH* | Estradiol* | Free Testosterone |
Total testosterone | Inhibin B | SHBG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IU/L | 95% CI | IU/L | 95% CI | pmol/L | 95% CI | nmol/L | 95% CI | nmol/L | 95% CI | pg/mL | 95% CI | nmol/L | 95% CI | |
| Total meat intake | |||||||||||||||
| Q1 (0–1.78) | 53 | 4.4 | 3.9–5.0 | 2.3 | 1.9–2.7 | 75.2 | 68.6–82.4 | 14.7 | 13.2–16.2 | 22.3 | 20.2–24.4 | 212.9 | 188.4–237.4 | 30.6 | 27.4–33.9 |
| Q2 (>1.78–2.38) | 51 | 3.9 | 3.4–4.3 | 2.3 | 1.9–2.7 | 78.5 | 72.4–85.2 | 13.7 | 12.3–15.1 | 21.8 | 19.9–23.7 | 197.8 | 175.7–219.8 | 33.8 | 30.9–36.7 |
| Q3 (>2.38–3.23) | 51 | 4.5 | 4.1–5.0 | 2.2 | 1.9–2.6 | 77.9 | 71.7–84.5 | 14.8 | 13.5–16.2 | 22.4 | 20.5–24.3 | 203.1 | 181.0–225.2 | 31.1 | 28.2–34.0 |
| Q4 (>3.23–9.12) | 51 | 4.1 | 3.5–4.7 | 2.4 | 2.0–2.9 | 71.7 | 65.1–78.9 | 13.7 | 12.1–15.4 | 20.8 | 18.6–23.0 | 196.9 | 171.0–222.8 | 30.6 | 27.2–34.0 |
| Ptrend | 0.87 | 0.91 | 0.59 | 0.73 | 0.52 | 0.75 | 0.89 | ||||||||
| Total red meat intakeb | |||||||||||||||
| Q1 (0–0.72) | 52 | 4.1 | 3.6–4.6 | 2.2 | 1.8–2.5 | 74.1 | 68–80.7 | 14.4 | 12.9–15.8 | 22 | 20.0–23.9 | 209.9 | 186.8–232.9 | 31.4 | 28.3–34.5 |
| Q2 (>0.72–1.01) | 53 | 4.1 | 3.7–4.6 | 2.2 | 1.9–2.6 | 76.7 | 70.9–83.1 | 14.5 | 13.2–15.8 | 21.9 | 20.1–23.8 | 213.8 | 192.4–235.2 | 30.6 | 27.8–33.5 |
| Q3 (>1.01–1.42) | 50 | 4.4 | 3.9–4.9 | 2.2 | 1.9–2.6 | 71.2 | 65.7–77.3 | 12.7 | 11.3–14.1 | 20.1 | 18.2–22.0 | 207.5 | 185.7–229.4 | 32.7 | 29.8–35.7 |
| Q4 (>1.42–4.25) | 51 | 4.3 | 3.8–4.9 | 2.6 | 2.2–3.1 | 80.9 | 74–88.4 | 15.4 | 13.8–16.9 | 23.2 | 21.2–25.3 | 179.8 | 155.7–203.9 | 31.3 | 28.1–34.6 |
| Ptrend | 0.51 | 0.21 | 0.44 | 0.86 | 0.80 | 0.12 | 0.79 | ||||||||
| Total white meat intakec | |||||||||||||||
| Q1 (0–0.21) | 58 | 4.1 | 3.7–4.6 | 2.4 | 2.0–2.7 | 72.0 | 66.6–77.9 | 13.7 | 12.4–15.0 | 20.9 | 19.1–22.7 | 215.2 | 194.2–236.2 | 30.7 | 27.9–33.5 |
| Q2 (>0.21–0.35) | 43 | 4.5 | 4.0–5.0 | 2.5 | 2.1–2.9 | 75.7 | 69.4–82.7 | 14.1 | 12.7–15.6 | 21.7 | 19.6–23.7 | 190.2 | 166.6–213.7 | 31.2 | 28.0–34.3 |
| Q3 (>0.35–0.57) | 62 | 4.3 | 3.8–4.7 | 2.1 | 1.9–2.5 | 76.3 | 70.9–82.2 | 14.8 | 13.5–16.0 | 22.5 | 20.8–24.2 | 195.8 | 176.0–215.5 | 31.8 | 29.1–34.4 |
| Q4 (>0.57–2.57) | 43 | 4.1 | 3.5–4.7 | 2.2 | 1.9–2.7 | 80.1 | 72.7–88.2 | 14.3 | 12.7–15.9 | 22.3 | 20.0–24.5 | 208.7 | 182.9–234.5 | 32.5 | 29.1–36.0 |
| Ptrend | 0.92 | 0.42 | 0.11 | 0.41 | 0.26 | 0.56 | 0.42 | ||||||||
| Total fish meat intake | |||||||||||||||
| Q1 (0–0.53) | 53 | 4.3 | 3.8–4.8 | 2.1 | 1.8–2.5 | 75 | 69.1–81.3 | 14.4 | 13.0–15.8 | 22.1 | 20.2–24.0 | 208.5 | 187.2–229.8 | 32.2 | 29.3–35.1 |
| Q2 (>0.53–0.77) | 53 | 4.5 | 4.0–5.0 | 2.7 | 2.3–3,2 | 79.6 | 73.4–86.2 | 14.4 | 13.0–15.8 | 22.8 | 20.9–24.7 | 181.2 | 160.0–202.4 | 33.8 | 30.9–36.7 |
| Q3 (>0.77–1.24) | 50 | 4.3 | 3.8–4.8 | 2.4 | 2.1–2.9 | 75 | 69.1–81.5 | 13.7 | 12.3–15.1 | 20.6 | 18.7–22.5 | 202.4 | 180.7–224.2 | 29.6 | 26.6–32.5 |
| Q4 (>1.24–4.66) | 50 | 3.8 | 3.3–4.3 | 1.9 | 1.6–2.3 | 73.3 | 67–80.2 | 14.4 | 12.9–16.0 | 21.7 | 19.6–23.8 | 219.8 | 196.0–243.6 | 30.3 | 27.0–33.5 |
| Ptrend | 0.27 | 0.54 | 0.63 | 0.82 | 0.49 | 0.46 | 0.21 | ||||||||
CI, confidence interval; LH, luteinizing hormone; FSH, follicle-stimulating hormone; SHBG, sex hormone-binding globulin
Adjusted for calories intake, intakes of the remaining meats, dietary patterns, age, body mass index, smoking, physical activity, TV watching and time of blood draw
Includes processed and unprocessed red meat, and organ meat
Includes chicken with and without skin, rabbit, quail and duck
Back-transformed to original scale
We then evaluated intake of sub-categories of meat in relation to semen quality and reproductive hormones (Supplemental Tables 6–9). In these analyses, shellfish intake was positively related to progressive sperm motility (Supplemental Tables 6, 8). The multivariate adjusted progressive sperm motility (95% Confidence Interval) for men in increasing quartiles of shellfish intake was 45.2%(42.1–48.3%), 42.0% (38.2–45.7%), 49.4%(46.9–51.8%), and 53.2%(50.0–56.4%), p-trend <0.001 (Fig. 1, Supplemental Table 8).This association was similar when we used men who reported no intake of shellfish as the reference category and divided the remaining men in quartiles of intake. In this analysis, the multivariable adjusted progressive sperm motility for men who did not consume shellfish and men in increasing quartiles of intake was 44.8%, 44.7%, 42.2%, 49.4% and 53.2%, respectively (p-trend < 0.001), and similar pattern was found for total sperm motility. Furthermore, there was an inverse relation between shellfish intake and E2 levels (Supplemental Table 4). The multivariable-adjusted serum E2 concentrations for men in increasing quartiles of shellfish intake were 77.2 pmol/L, 86.6 pmol/L, 76.1 pmol/L, and 67.8 pmol/L, p-trend = 0.02 (Supplemental Table 4). Last, we examined the difference in semen quality parameters associated with the substitution of other types of meat with shellfish. Consuming shellfish instead of organ meats was associated with higher total and progressive sperm motility (p-trend < 0.001). A similar association was observed when shellfish was eaten instead of white meat fishs (motile sperm, p- trend = 0.03; progressive motility, p-trend = 0.004) or dark meat fishs (motile sperm, p-trend = 0.03; progressive motility, p-trend = 0.006) (data not shown).
Fig. 1.
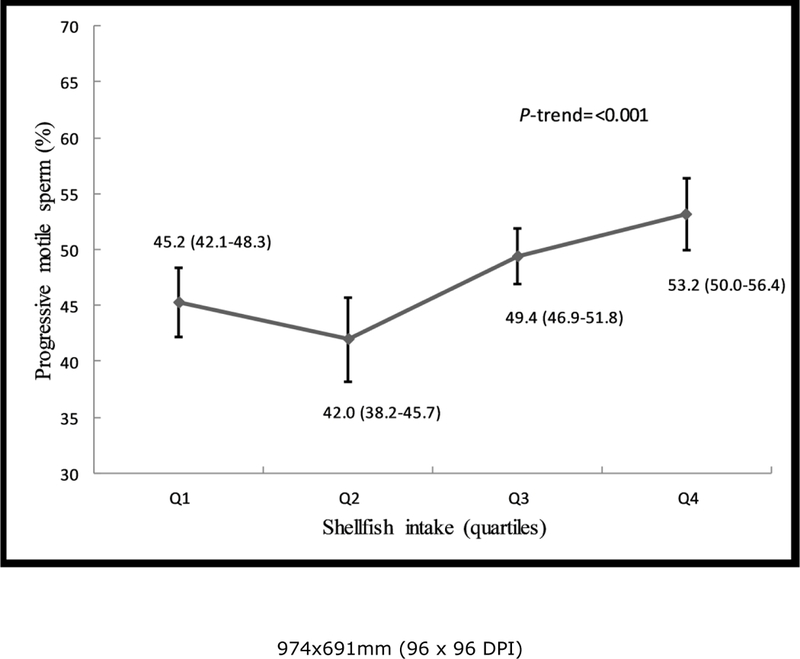
Shellfish intake in relation to progressive sperm motility among healthy young men. Estimates are adjusted for total calorie intake, intakes of other meats, dietary patterns, age, body mass index, smoking, physical activity, TV watching, abstinence time and time to start semen analysis.
We also found an inverse association between organ meat intake and sperm motility (Supplemental Table 6, 8). The multivariate percentage of progressively motile sperm (95% CI) for men who did not consume organ meats was 50.2% (48.3–52.1%) compared to 44.8% (42.4–47.2%) for men who consumed organ meats (p=0.001) (Supplemental Table 8). LH levels for organ meat consumers were higher than those of non-consumers (4.6IU/L vs. 4.0 IU/L) after multivariable adjustment (p=0.03). Levels of total testosterone, free testosterone and estradiol, as well as the TT/LH and FT/LH ratios were unrelated to organ meat intake (Data not shown). In addition, unprocessed red meat intake was positively associated with LH (p-trend = 0.02) and SHBG (p-trend = 0.001) in adjusted models. No other associations were identified with semen quality parameters (Supplemental Table3) or reproductive hormone levels (Supplemental Table4).
As shellfish intake was related to higher progressive motility and lower estradiol levels, and organ meat to lower progressive motility and higher LH levels, we examined whether LH or E2 were related to progressive motility in this population, and whether further adjustment for these hormones affected the associations of shellfish and organ meat intake with semen quality. LH and E2 were unrelated to semen quality in this population, and additional adjustment did not change the previously observed associations (data not shown).
Discussion
In this cross-sectional study of healthy young men, we found that total meat intake was unrelated to semen quality or reproductive hormone levels. When subgroups of meat were separately considered, however, shellfish intake was associated with higher progressive motility and organ meat intake was inversely related to this outcome. We also found associations with reproductive hormones that did not explain the associations with semen quality. The remaining types of meat were unrelated to semen quality parameters or reproductive hormone levels. The relevance of the observed associations of shellfish and organ meat intakes with semen quality and reproductive hormones should be further evaluated given that these specific relations have only been evaluated in a small number of previous studies (19).
Our findings are consistent with those of previous studies that explored the association between fish and seafood intake with semen quality. Two case-control studies among men in fertility clinics have reported significantly higher shellfish intake among normospermic men than among oligoasthenoteratospermic patients(19) and greater seafood intakes as a protective factor for asthenozoospermia(21). Greater sperm motility has also been previously related to higher consumption of fish and other seafood(33), while in other study no associations were found with any semen indicators(34). Afeiche and colleagues reported a positive association between intake of dark meat fish intake and total sperm count, as well as a positive relation between white meat fish intake and morphologically normal sperm among men presenting to a fertility clinic(22), but not among healthy young men(19).
Shellfish is an important source of polyunsaturated fatty acids (PUFA)(35) and previous work has related higher consumption of omega-3 PUFAs to better sperm motility(36). However, because we did not observe an association between dark meat fish intake and semen quality in this study, and previous work in this cohort has not found associations between intakes of omega-3 and semen quality parameters or reproductive hormones(12, 24), intake of omega-3 PUFAs is unlikely to explain our findings. Instead, the relation between shellfish intake with sperm motility may be due to intake of micronutrients highly concentrated in shellfish such as zinc(35). Zinc is involved in male reproductive function including spermatogenesis, sperm maturation and sperm activation(37). A recent meta-analysis showed that zinc supplementation in infertile men was associated to better semen quality parameters, including sperm motility(37).
To our knowledge, only three previous studies have addressed the association between intake of organ meat and semen parameters. Organ meat intake has been previously hypothesized to lower semen quality(19). Contrary to our findings, a similar study among young men in the United States found organ consumption to be positively related to sperm motility(19). Previous studies among infertile men have not found a relationship between organ consumption and sperm motility(19, 22). As literature is scarce and inconsistent, this association may represent a chance finding and more studies are needed to clarify this discrepancy.
Several limitations should be discussed. The cross-sectional design is not strong to distinguish causal from non-causal relationships and the small sample size could result in missing true associations. However, results of the semen analysis were unknown to participants, essentially blinding them to the study outcome, and thus decreasing the likelihood of reverse causation. Diet was assessed using a FFQ and like all other diet assessment tools, FFQs are subject to measurement error. However, this questionnaire has been validated previously in Spanish populations(27). In addition, FFQs are known to be better at ranking than at estimating exact intakes. Our analytic strategy of making comparisons between quantiles of intake rather than relying on continuous measures of intake is not only better aligned with this characteristic of FFQs but also protects against the influence of individuals with very high intakes thus resulting in conservative estimates of the associations examined. Moreover, as the consumption of shellfish and organ meats was very low (3–5%) and the multiple comparisons developed in the statistical analysis, we cannot rule out the possibility of residual confounding or chance findings that could explain the associations reported. However, we adjusted for a large number of potential confounders, including known predictors of semen quality as well as lifestyle factors associated with meat intake including other dietary behaviors as captured by data-derived dietary patterns. Finally, although the homogeneity of study participants may have increased the internal validity of the study, it may limit the generalizability of our results to men facing difficulties with fertility.
In conclusion, we found no relation of total meat intake nor intake of major types of meat (red meats, white meats, fish) with markers of testicular function. Nevertheless, analyses of subgroups of meat suggests that intake of shellfish may be beneficial for sperm motility whereas the intake of organ meats may have the opposite relation. Due to the scarcity of literature in this population and in specific types of meats, additional research is needed to confirm or refute these findings.
Supplementary Material
Acknowledgments
Financial Support
This work was supported by The Seneca Foundation, Regional Agency of Science and Technology, Grant No. 08808/PI/08; Ministerio de Ciencia e Innovación, Instituto Carlos III (FIS), Grant No. PI10/00985; National Institutes of Health, Grant No. P30DK046200.
References
- 1.Swan SH, Elkin EP & Fenster L (2000) The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect 108, 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar N & Singh AK (2015) Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum ReprodSci 8,191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine H, Jørgensen N, Martino-Andrade A et al. (2017) Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 23, 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virtanen HE, Jørgensen N & Toppari J (2017) Semen quality in the 21(st) century. Nat Rev Urol 14, 120–130. [DOI] [PubMed] [Google Scholar]
- 5.Nordkap L, Joensen UN, Blomberg Jensen M et al. (2012) Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol 355, 221–230. [DOI] [PubMed] [Google Scholar]
- 6.Povey AC, Clyma JA, McNamee R et al. (2012) Modifiable and non-modifiable risk factors for poor semen quality: a case-referent study. Hum Reprod 27, 2799–2806. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (2014) National Public Health Action Plan for the Detection, Prevention, and Management ofInfertility. Atlanta: CDC. [Google Scholar]
- 8.Mahe G, Carsin M, Zeeny M et al. (2011) Dietary pattern, a modifiable risk factor that can be easily assessed for atherosclerosis vascular disease prevention in clinical practice. Public Health Nutr 14, 319–326. [DOI] [PubMed] [Google Scholar]
- 9.Jensen TK, Swan SH, Skakkebaek NE et al. (2010) Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol 171, 883–891. [DOI] [PubMed] [Google Scholar]
- 10.Mínguez-Alarcón L, Mendiola J, López-Espín JJ et al. (2012) Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod 27, 2807–2814. [DOI] [PubMed] [Google Scholar]
- 11.Jensen TK, Heitmann BL, Blomberg Jensen M et al. (2013) High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr 97, 411–418. [DOI] [PubMed] [Google Scholar]
- 12.Chavarro JE, Mínguez-Alarcón L, Mendiola J et al. (2014) Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod 29, 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giahi L, Mohammadmoradi S, Javidan A et al. (2016) Nutritional modifications in male infertility: a systematic review covering 2 decades Nutr Rev 74, 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salas-Huetos A, Bulló M & Salas-Salvadó J (2017) Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update 23, 371–389. [DOI] [PubMed] [Google Scholar]
- 15.Swan SH, Liu F, Overstreet JW et al. (2007) Semen quality of fertile US males in relation to their mothers’ beef consumption during pregnancy. Hum Reprod 22,1497–1502. [DOI] [PubMed] [Google Scholar]
- 16.Gasull M, Bosch de Basea M, Puigdomènech E et al. (2011) Empirical analyses of the influence of diet on human concentrations of persistent organic pollutants: a systematic review of all studies conducted in Spain. Environ Int 37,1226–1235. [DOI] [PubMed] [Google Scholar]
- 17.Attaman JA, Toth TL, Furtado J et al. (2012) Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 27, 1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vested A, Giwercman A, Bonde JP et al. (2014) Persistent organic pollutants and male reproductive health. Asian JAndrol 16, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afeiche MC, Williams PL, Gaskins AJ et al. (2014) Meat intake and reproductive parameters among young men. Epidemiology 25, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendiola J, Torres-Cantero AM, Moreno-Grau JM et al. (2009) Food intake and its relationship with semen quality: a case-control study. Fertil Steril 91, 812–818. [DOI] [PubMed] [Google Scholar]
- 21.Eslamian G, Amirjannati N, Rashidkhani B et al. (2012) Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod 27, 3328–3336. [DOI] [PubMed] [Google Scholar]
- 22.Afeiche MC, Gaskins AJ, Williams PL et al. (2014) Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr 144,1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutillas-Tolin A, Minguez-Alarcon L, Mendiola J et al. (2015) Mediterranean and western dietary patterns are related to markers of testicular function among healthy men. Hum Reprod 30, 2945–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minguez-Alarcon L, Chavarro JE, Mendiola J, et al. (2017) Fatty acid intake in relation to reproductive hormones and testicular volume among young healthy men. Asian JAndrol 19, 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vioque J & Gonzalez L (1991) Validity of a food frequency questionnaire (preliminary results). Eur J Cancer Prev 1, 19–20. [Google Scholar]
- 26.Vioque J, Navarrete-Muñoz EM, Gimenez-Monzó D et al. (2013) Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization (2010) WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed Geneva: WHO. [Google Scholar]
- 28.Menkveld R, Stander FS, Kotze TJ et al. (1990) The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 5, 586–592. [DOI] [PubMed] [Google Scholar]
- 29.Asklund C, J0rgensen N, Skakkebaek NE et al. (2007) Increased frequency of reproductive health problems among fathers of boys with hypospadias. Hum Reprod 22, 2639–2646. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen A, Verdonck L & Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin EndocrinolMetab 84, 3666–3672. [DOI] [PubMed] [Google Scholar]
- 31.Soper DS (2018) Free Statistics Calculators - A-priori Sample Size Calculator for Multiple Regression http://www.danielsoper.com/statcalc (accessed January 2018).
- 32.Hu FB, Rimm EB, Stampfer MJ et al. (2000) Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 72, 912–921. [DOI] [PubMed] [Google Scholar]
- 33.Vujkovic M, de Vries JH, Dohle GR et al. (2009) Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod 24, 1304–1312. [DOI] [PubMed] [Google Scholar]
- 34.Braga DP, Halpern G, Figueira R de C et al. (2012) Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril 97, 53–59. [DOI] [PubMed] [Google Scholar]
- 35.Dong FM (2009) The Nutritional Value of Shellfish. WA: University of Washington. [Google Scholar]
- 36.Safarinejad MR (2011) Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia 43, 38–47. [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Dong X, Hu X et al. (2016) Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. SciRep 6, 22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


