Abstract
Background:
Prevalence of osteoporosis and fracture is increased among older people with HIV. We compared the effects of Low (1000 IU) vs Moderate (3000 IU) Vitamin D3 (VItD) supplementation on areal and volumetric bone mineral density (aBMD and vBMD) in African American and Hispanic postmenopausal women with HIV on antiretroviral therapy.
Methods:
We performed a 12-month prospective, randomized, double-blind, placebo-controlled study with primary outcomes of change in aBMD by dual-energy X-ray absorptiometry (DXA) and secondary outcomes of change in vBMD by quantitative computed tomography and bone turnover markers. An intent to treat analysis was performed on 85 randomized subjects (43 Low and 42 Moderate) for primary DXA outcomes, and complete case analysis performed for secondary outcomes.
Results:
Mean age was 56±5 years, median CD4 count 722 cells/mm3 and 74% had HIV RNA≤50 copies/ml. Serum 25-OHD was higher in the Moderate than Low VitD group at 6 months (33.1±10.3 vs 27.8±8.1 ng/ml, p=0.03) and 12 months, but PTH levels remained similar. Percent change in aBMD, vBMD and bone turnover markers did not differ between Low and Moderate VitD groups before or after adjustment for baseline aBMD.
Conclusion:
VitD supplementation at 3000 IU daily increased mean total 25-OHD levels in postmenopausal women with HIV, but we did not find evidence of an effect on BMD beyond those observed with 1000 IU daily. Future studies are necessary to determine whether VitD supplementation is beneficial in this patient population, and if so, what dose is optimal for skeletal health.
Introduction
Prevalence of fracture is 2 to 3-fold higher in women with HIV over age 50 than in the general population,1. We previously found lower areal bone mineral density (aBMD), higher bone turnover markers (BTMs), and higher rates of bone loss in postmenopausal minority women with HIV than uninfected controls with similar age and race/ethnicity.2 We also found that vitamin D (VitD) deficiency and insufficiency were common among postmenopausal minority women (76%), but their prevalence did not differ by HIV status;3 however, lower baseline serum 25-hydroxyvitamin D (25-OHD) was associated with secondary hyperparathyroidism and a trend toward more bone loss, particularly at the forearm.
VitD and calcium supplementation have been shown to mitigate bone loss associated with antiretroviral therapy (ART) initiation,4 but have not been studied in older individuals on stable ART regimens, who are at higher risk of fracture. Provision of adequate calcium and VitD is the cornerstone of effective prevention of osteoporosis and its consequences, as VitD in particular has been shown to reduce bone loss and falls and lower the risk of hip fracture.5–7. The purpose of this study was to compare the effects of two doses of vitamin D3 repletion (3000 IU Vs 1000 IU) on bone turnover and change in areal and volumetric bone mass and microarchitecture in postmenopausal women with HIV.
Methods
Study population
Postmenopausal African American or Hispanic women with HIV between ages 40–70 were recruited from 4 sites in New York City (Columbia University Medical Center, Mt Sinai St. Luke’s and Mt. Sinai West, Bronx Lebanon Hospital Center, and Montefiore Medical Center). All participants had serum 25-OHD ≥10 ng/mL and ≤ 32 ng/mL and were on stable ART for at least 2 years with 2 or more HIV RNA levels <400 copies/ml within the last year prior to enrollment. Exclusion criteria included: metabolic bone diseases; renal insufficiency (serum creatinine>1.5 mg/dl or eGFR<45 ml/min), current use of glucocorticoids or hormone replacement therapy; current or past osteoporosis therapy; history of fragility fracture or T score<−3.0; history of hypercalcemia or calcium-containing kidney stones; and weight over 300 lbs.
Participants were randomized to 3000 vs 1000 IU cholecalciferol (vitamin D3) daily (Tishcon Corporation, Westbury, NY) plus 500mg calcium carbonate twice daily and counseled to take with food to facilitate absorption. Participants were randomized in a 1:1 ratio using permuted blocks stratified by screening serum 25-OHD (≤ 20 and >20 ng/mL). Adherence to VitD and Calcium was monitored by counts of returned pills at each 3-month study visit. The Institutional Review Boards of all participating sites approved the study.
Primary outcomes were percentage change in lumbar spine, total hip and 1/3 distal radius areal BMD (aBMD) by DXA from baseline to 12 months. Secondary outcomes included percentage change in volumetric BMD (vBMD) of the lumbar spine and proximal femur by central quantitative computed tomography (cQCT) and vBMD and microarchitecture at the distal radius and tibia by high resolution peripheral QCT (HRpQCT), and bone turnover markers at 12 months. Changes in plasma 25-OHD, parathyroid hormone (PTH), soluble inflammatory biomarkers over 12 month were also examined in relation to the bone imaging outcomes. Incidence of hypercalcemia and nephrolithiasis were monitored. All DXA, cQCT and HRpQCT scans were performed at CUMC using a standardized protocol.
Bone Density Measurements
Areal BMD of the lumbar spine (LS; L1–4), femoral neck, total hip, non-dominant 1/3 radius, ultradistal radius, and body composition were measured by DXA on a QDR 4500 bone densitometer (Hologic, Inc., Bedford, MA) at CUMC. Short term in vivo precision is 0.68% for the lumbar spine, 1.36% for the total hip, and 0.70% for the radius. T-scores, which compare subjects to the peak bone mass of young individuals of the same sex and race, were derived for the hip NHANES III and the manufacturer’s normative database. Osteoporosis and osteopenia were defined by World Health Organization criteria for postmenopausal Caucasian women: T-scores ≤−2.5 represent osteoporosis; T-scores between −1.0 and −2.49 represent osteopenia.8 Height and weight were measured by Harpenden stadiometer and balance beam scale, respectively.
Central Skeleton: Cortical and Trabecular vBMD and Microarchitecture
Participants underwent helical (pitch = 1) cQCT scanning of the proximal femur and spine (L1-L2) at 80 kVp, 2.5 mm slice thickness (GE CTi and GE Light Speed VCT 64; General Electric Medical Systems, Milwaukee WI) with a calibration phantom (3-Bar; Image Analysis, Columbia, KY) used to calibrate cQCT images to an equivalent concentration of calcium hydroxyapatite. Specialized image analysis software 9 was used to compute total, cortical, and trabecular volumetric density of the proximal femur and femoral neck bilaterally, and total (integral) and trabecular volumetric density of L1-L2. Cortical bone regions were determined by applying a threshold of 0.35 g/cm3 to voxels falling outside the trabecular regions but within the integral regions. The spinal trabecular ROI was a semicircular region within the anterior vertebral body, centered on the midvertebral level, encompassing 70% of the volume between vertebral endplates. Trabecular regions at the proximal femur and femoral neck were determined by eroding the integral bone regions to produce regions of the same shape but fully contained within the medullary volumes.
Peripheral Skeleton: Cortical and Trabecular vBMD and Microarchitecture
Volumetric BMD and microarchitecture were assessed in vivo at the non-dominant distal radius and tibia using HRpQCT (Scanco Medical AG, Brüttisellen, Switzerland) with an isotropic voxel size of 82 μm using the standard manufacturer imaging protocol.10 In case of a contraindication on the non-dominant limb, the dominant limb was scanned. The region of interest (ROI) consisted of a 9.02 mm section (110 slices) beginning at 9.5 mm and 22.5 mm proximal to the reference line at the radius and tibia respectively. The volume of interest was separated into cortical and trabecular regions as previously described 11–14 and included mTheean cortical thickness (Ct.Th; μm); total, cortical and trabecular bone density (Tt.vBMD, Ct.vBMD and Tb.vBMD; mg HA/cm3); trabecular number (Tb.N; mm−1), thickness (Tb.Th; μm) and separation (Tb.Sp; μm). Towards the end of the study, we transitioned from the first generation (XCT1) to the second generation HRpQCT (XCT2) scanner with a higher nominal isotropic resolution of 61 μm. A similar scanning protocol was followed,15 generating an ROI of 10.24 mm(168 slices) starting at 9.0 mm and 22.0 mm proximal to the reference line, thus centered on the XCT1 region. The XCT2 data was calibrated to XCT1 based on a separate study in which we compared 51 adults and found excellent agreement between both generations of scanner (R2 > 0.9) for most measurements.16 Our study compared agreement for both cross-sectional (XCT1 vs. XCT2) as well as longitudinal (XCT1 vs. XCT2D) cases. For longitudinal comparison we downscaled the XCT2 scans to XCT1 resolution, slice-matched with the corresponding XCT1 scans and then used regression for calibration.
Biomarker Assays
Serum samples were stored at −70˚C until batched analysis at the Irving Institute Biomarkers Core at Columbia University Medical Center. We measured 25-OHD2 and D3 by liquid chromatography tandem mass spectrometry; intact PTH by radioimmunoassay (Scantibodies, Santee, CA); N-terminal propeptide of procollagen type 1 (P1NP; RIA; IDS, Scottsdale, AZ); C-telopeptide (CTX; ELISA; IDS Scottsdale, AZ); IL-6 (ELISA; R&D Systems, Minneapolis, MN); soluble receptors of TNFα (sTNFr-I and –II; ELISA; R&D Systems, Minneapolis, MN), and soluble CD14 (sCD14, ELISA; R&D Systems, Minneapolis, MN). Except for 25-OHD, biomarkers were measured in duplicate and values averaged for analysis.
Statistical Analysis
To provide an intent to treat approach for the primary outcome, evaluations were performed regardless of treatment change or discontinuation on randomized subjects (n=85; 43 Low and 42 Moderate). A complete case approach was utilized for secondary outcomes in participants with aBMD data at 12 months (n=69). Stratified Wilcoxon rank sum tests were used to test for distribution shifts between the treatment groups. Fisher’s exact tests and Wilcoxon rank sum tests were used to evaluate for differences between groups for categorical and continuous secondary outcomes, respectively. Wilcoxon signed rank test was used to evaluate within treatment group change. The 95% confidence intervals for median changes within treatment group were estimated using distribution-free method via percentiles. Analysis of the primary outcomes of change in lumbar spine, total hip, and 1/3 distal radius aBMD from baseline to 48 weeks utilized a predictive mean matching multiple imputation approach to impute missing data.17 We did not utilize imputation for secondary outcomes. Using 100 independent imputations, we tested between-group differences in 48-week changes in each of the aBMD sites. All statistical tests were two-sided and interpreted at the 5% level of significance without adjustment for multiple comparisons. Analyses were performed using R 3.4.0.
Results
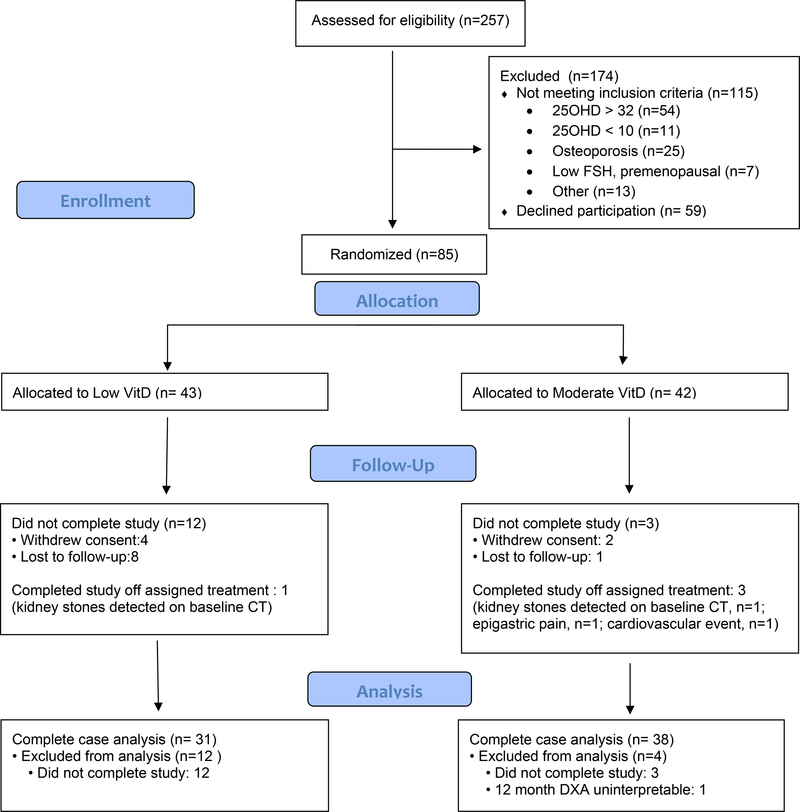
Between 2011 and 2016, we enrolled and randomized 85 participants who were included in the intent to treat analysis. Among all participants, 82 received at least one dose of the allocated intervention (41 in low VitD and 41 moderate VitD). Overall, 69 subjects (31 in the low VitD and 38 in the moderate vitD groups) completed the 12 month study with interpretable DXA data and were included in the complete-case analysis. Four participants discontinued study drug due to incidental findings of asymptomatic kidney stones on cQCT (n=2), epigastric pain (n=1) and cardiovascular event (n=1). Six participants withdrew from the study because they did not want to continue with study visits or study medications, and 9 moved or changed contact information (Figure 1- consort diagram)
Figure 1.
CONSORT Flow Diagram
Baseline demographic, immunologic and other parameters are summarized in Table 1 for the 85 participants in the intent-to-treat analysis. Overall, the mean age was 56 years; 43% were African American, 57% Hispanic; median CD4 count 738 cells/mm3 and 74% had HIV RNA≤50 copies/ml. Age, race/ethnicity, proportion with diabetes, and HIV-specific parameters such as current/nadir CD4, history of AIDS defining illness, ART class and proportion with HIV RNA levels<50 copies/ml were similar between groups. However at baseline, the Moderate VitD group had lower BMI, total body fat and trunk fat (Table 1). Notably, there were no significant differences between groups in ART regimens or utilization of tenofovir disoproxil fumarate (TDF) at baseline; none of the partipants received tenofovir alafenamide fumarate (TAF) containing regimens or switched ART regimens during the study. Mean 25-OHD and PTH did not differ between treatment groups; 51.2% and 47.5% had vitamin D deficiency with 25-OHD<20 ng/ml in the Moderate VitD and low VitD groups respectively (p=0.91). Serum levels of bone turnover markers (P1NP, CTX) and inflammatory biomarkers (TNF-α, IL-6, sCD14) did not differ between treatment groups.
Table 1.
Baseline characteristics of subjects who received allocated intervention (N=85)
| Low VitD Group A (N=43) | Moderate VitD Group B (N=42) | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | P value | |
| Patient Characteristics | |||
| Age | 56.2 ± 4.9 | 56.5 ± 5.6 | 0.85 |
| Race/Ethnicity | 0.59 | ||
| African American | 17 (40%) | 20 (48%) | |
| Hispanic | 26 (60%) | 22 (52%) | |
| Height (cm) | 158.5 ± 7.8 | 161.8 ± 7.3 | 0.047 |
| Weight (kg) | 82.6 ± 18.1 | 77.4 ± 15.7 | 0.16 |
| Body mass index (kg/cm2) | 32.5 ± 6.9 | 29.4 ± 5.5 | 0.02 |
| Current smoking | 11 (30%) | 16 (41%) | 0.43 |
| Diabetes | 5 (14%) | 3 (8%) | 0.47 |
| HIV Parameters | |||
| Current CD4 (cells/mm3) | 673 ± 271 | 781 ± 412 | 0.16 |
| Nadir CD4 (cells/mm3) | 191 ± 139 | 254 ± 225 | 0.13 |
| History of AIDS | 23 (56%) | 21 (51%) | 0.82 |
| Current HIV VL ≤ 50 | 31 (76%) | 29 (71%) | 0.80 |
| Current HIV VL<400 copies/ul | 41 (100%) | 41 (100%) | NA |
| ART Class | 0.29 | ||
| NNRTI-based | 18 (45%) | 20 (49%) | |
| PI-based | 14 (35%) | 18 (44%) | |
| Integrase-based | 5 (13%) | 3 (7%) | |
| Tenofovir-containing regimen | 29 (67%) | 30 (71%) | |
| Baseline DXA and Body composition | |||
| Lumbar spine T score | -0.92 ± 1.11 | -0.59 ± 1.25 | 0.18 |
| Femoral neck T score | -0.90 ± 0.87 | -0.67 ± 0.92 | 0.25 |
| Total hip T score | -0.40 ± 0.83 | -0.34 ± 0.91 | 0.77 |
| Distal 1/3 radius T score | -0.32 ± 1.05 | -0.03 ± 1.37 | 0.28 |
| Ultradistal radius T score | -0.14 ± 0.88 | -0.41 ± 1.04 | 0.21 |
| Total Body Fat (kg) | 35.0 ± 12.6 | 29.6 ± 10.3 | 0.03 |
| Trunk Fat (kg) | 17.3 ± 6.5 | 14.3 ± 5.2 | 0.02 |
| Total Body Lean Mass (kg) | 47.3 ± 6.8 | 46.8 ± 7.7 | 0.75 |
| Trunk Lean Mass (kg) | 23.1 ± 3.6 | 23.0 ± 4.0 | 0.92 |
| Biochemical markers | |||
| Total 25-hydroxyvitamin D (ng/ml) | 19.7 ± 5.5 | 20.0 ± 6.6 | 0.814 |
| 25-hydroxyvitamin D2 (ng/ml) | 3.0 ± 4.4 | 2.6 ± 3.2 | 0.618 |
| 25-hydroxyvitamin D3 (ng/ml) | 16.7 ± 5.2 | 17.4 ± 6.6 | 0.574 |
| Parathyroid hormone (pg/ml) | 61.1 ± 51.0 | 58.9 ± 37.4 | 0.83 |
| CTX (ng/ml) | 0.60 ± 0.31 | 0.71 ± 0.39 | 0.16 |
| P1NP (ng/ml) | 63.9 ± 30.0 | 65.8 ± 28.6 | 0.77 |
| TNFα (pg/ml) | 9.8 ± 5.8 | 8.6 ± 4.0 | 0.29 |
| IL6 (pg/ml) | 3.6 ± 2.3 | 3.7 ± 3.2 | 0.81 |
| Soluble CD14 (ng/ml) | 368 ± 670 | 403 ± 862 | 0.84 |
Abbreviations: NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; CTX, C-telopeptide; P1NP, pro-collagen type 1 amino-terminal propeptide,
Comparison of between group differences in participants who completed the study (N=69) versus those with incomplete follow up (N=16) reveals no significant differences in baseline age, race/ethcnity, BMI, smoking and diabetes status, CD4, history of AIDS, HIV VL or ART regimen (data not shown).
Change in 25-OHD, PTH and bone turnover markers
Adherence to VitD supplementation by pill count did not differ between groups, with mean utilization over 80% in the Low vs Moderate VitD groups from 0–6 months (81 ± 21% vs 84 ± 15%, p=0.48) and from 6–12 months (84 ± 21% vs 81 ± 20%, p=0.60). Serum levels of total 25-OHD were higher in the Moderate than Low VitD group at 6 months (33.1 ± 10.3 vs 27.8 ± 8.1 ng/ml, p=0.03) and 12 months (30.2 ± 9.6 vs 24.3 ± 7.6 ng/ml, p=0.007). Similarly, percentage change from baseline in serum 25-OHD levels were greater in the Moderate than Low VitD groups at 6 months (73.6 ± 74% vs 47.4 ± 48%, p=0.08) and 12 months (64.1 ± 69% vs 33.7 ± 42%, p=0.03). Overall, PTH levels decreased in both groups, but the decrease did not differ between Moderate and Low VitD groups (Table 2)
Table 2.
Percent change from baseline in aBMD, 25-hydroxyvitamin D, parathyroid hormone,and bone turnover markers among participants with complete data (N=69) (Mean±SD)
| 6 Months | 12 Months | |||||
|---|---|---|---|---|---|---|
| Percent Change | P Value | P Adjusted for TBF | Percent Change | P Value | P Adjusted for TBF | |
| DXA parameters | ||||||
| Lumbar spine aBMD | ||||||
| Low VitD | 0.5 ± 3.1 | 0.34 | 0.51 | 0.4 ± 4.4 | 0.26 | 0.36 |
| Moderate VitD | -0.3 ± 3.5 | -0.8 ± 4.6 | ||||
| Total hip aBMD | ||||||
| Low VitD | 0.4 ± 2.9 | 0.47 | 0.21 | -0.5 ± 3.1 | 0.74 | 0.51 |
| Moderate VitD | -0.1 ± 2.8 | -0.8 ± 3.2 | ||||
| Femoral neck aBMD | ||||||
| Low VitD | -0.7 ± 3.9 | 0.79 | 0.90 | -1.1 ± 3.5 | 0.96 | 0.78 |
| Moderate VitD | -0.4 ± 3.6 | -1.1 ± 5.1 | ||||
| Distal 1/3 radius aBMD | ||||||
| Low VitD | -1.6* ± 2.7 | 0.10 | 0.17 | -1.3* ± 2.8 | 0.69 | 0.67 |
| Moderate VitD | -0.5 ± 2.9 | -1.6* ± 3.5 | ||||
| Ultradistal radius aBMD | ||||||
| Low VitD | -1.3* ± 3.1 | 0.73 | 0.53 | -1.6* ± 4.2 | 0.39 | 0.42 |
| Moderate VitD | -1.0 ± 4.2 | -0.7 ± 4.7 | ||||
| Vitamin D and bone turnover markers | ||||||
| 25-hydroxyvitamin D | ||||||
| Low VitD | 45.1* ± 44.1 | 0.041 | 28.1* ± 41.9 | 0.031 | ||
| Moderate VitD | 66.7* ± 78.0 | 51.2* ± 68.9 | ||||
| Parathyroid hormone | ||||||
| Low VitD | -17.7* ± 36.2 | 0.721 | -15.9 ± 71.4 | 0.568 | ||
| Moderate VitD | -22.5* ± 31.8 | -24.2* ± 32.7 | ||||
| P1NP | ||||||
| Low VitD | -13.4* ± 38.4 | 0.527 | -8.1 ± 37.3 | 0.726 | ||
| Moderate VitD | -6.8 ± 35.9 | −4.0 ± 41.1 | ||||
| CTX | ||||||
| Low VitD | -4.8 ± 51.0 | 0.315 | -9.9 ± 65.3 | 0.388 | ||
| Moderate VitD | -17.0* ± 37.3 | -21.3* ± 42.0 | ||||
Abbreviations: TBF: total body fat; CTX, C-telopeptide; P1NP, pro-collagen type 1 amino-terminal propeptide
p<0.05 for within-group change from baseline
In both groups, there were significant within-group decreases in CTX levels from baseline to 6 and 12 months, but change in CTX did not differ between groups (Table 2). Similarly, there were no significant between-group differences in change in P1NP levels from baseline to either 6 or 12 months.
Change in aBMD by DXA at 12 months
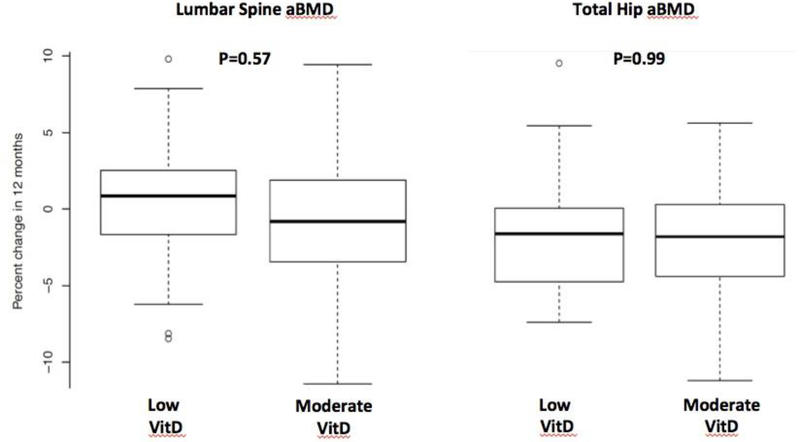
At baseline, the mean aBMD T scores were similar between the two groups at the all bone sites (Table 1). There were significant within-group decreases in aBMD at 1/3 distal radius in both moderate and low VitD groups at 12 months, and significant within-group decreases in the ultradistal radius in the low VitD group (Table 2). However, there were no significant between-group differences in change in aBMD at any site from baseline to 12 months by ITT with multiple imputation. Percent change in aBMD between Low vs Moderate VitD groups did not differ at the lumbar spine (0.4 ± 4.4% vs -0.8± 4.6 %, p=0.57), total hip (−0.5 ± 3.1% vs -0.8 ± 3.2%, p=0.99) or 1/3 distal radius (−1.3 ± 2.8% vs -1.6 ± 3.5%, p=0.83) before or after adjustment for baseline aBMD (Figure 2), or after adjustment for total body fat (p=0.21 to 0.90) (Table 2).
Figure 2:
Percent change in lumbar spine (LS) and total hip (TH) aBMD after 12 months in Low versus Moderate Vitamin D groups. P-values were calculated based on intent to treat analysis, after adjusting for the baseline aBMD.
Among participants with 25-OHD<20ng/ml at baseline, change in aBMD also did not differ between Moderate and Low VitD groups at any site. However, in regression analyses, lower baseline 25-OHD in the Moderate VitD group was associated with greater percent increase in aBMD at the femoral neck (p=0.04) and ultradistal radius (p=0.045) at 12 months.
Change in vBMD and microarchitecture by cQCT and HRpQCT at 12 months
We next compared between-group changes in lumbar spine (L1 and L2 combined) and total hip vBMD by cQCT and distal radius and tibia vBMD, and microarchitecture by HRpQCT. Among participants with complete data on cQCT at baseline and 12 months, we observed no significant between-group differences in total vBMD or trabecular vBMD at the lumbar spine or in total, trabecular or cortical vBMD at the total hip (Table 3). Similarly, by HRpQCT, we observed no significant between-group differences in total and trabecular vBMD, cortical thickness, or trabecular thickness, number at separation at the tibia (Table 3) and radius (data not shown).
Table 3.
Baseline and change in Quantitative CT parameters at 12 months
| N | Baseline | P value | % Change | P value | |
|---|---|---|---|---|---|
| Central QCT | |||||
| Lumbar spine (L1L2) vBMD (mg/cm3) | 49 | ||||
| Low VitD | 230 ± 30 | 0.30 | 0.0 ± 7.7 | 0.87 | |
| Moderate VitD | 240 ± 30 | 0.4 ± 5.3 | |||
| Lumbar spine trabecular vBMD (mg/cm3) | 49 | ||||
| Low VitD | 140 ± 40 | 0.29 | -1.2 ± 13.4 | 0.86 | |
| Moderate VitD | 150 ± 30 | -1.8 ± 11.8 | |||
| Proximal Femur Total vBMD (mg/cm3) | 49 | ||||
| Low VitD | 280 ± 40 | 0.32 | -0.9 ± 9.3 | 0.14 | |
| Moderate VitD | 290 ± 40 | 2.9 ± 7.5 | |||
| Proximal Femur Trabecular vBMD (mg/cm3) | 49 | ||||
| Low VitD | 130 ± 40 | 0.34 | -5.0 ± 18.7 | 0.11 | |
| Moderate VitD | 140 ± 40 | 3.3 ± 14.9 | |||
| Proximal Femur Cortical vBMD (mg/cm3) | 49 | ||||
| Low VitD | 500 ± 30 | 0.38 | 0.4 ± 6.0 | 0.38 | |
| Moderate VitD | 500 ± 40 | 1.8 ± 4.7 | |||
| HRpQCT of Tibia | |||||
| Total vBMD | 62 | ||||
| Low VitD | 272 ± 50 | 0.30 | -0.3 ± 2.9 | 0.39 | |
| Moderate VitD | 258 ± 56 | -0.9 ± 2.5 | |||
| Cortical vBMD (mg/cm3) | 62 | ||||
| Low VitD | 841 ± 57 | 0.42 | -0.1 ± 1.8 | 0.15 | |
| Moderate VitD | 829 ± 60 | -0.7 ± 1.5 | |||
| Trabecular vBMD (mg/cm3) | 62 | ||||
| Low VitD | 140 ± 38 | 0.92 | 0.5 ± 3.5 | 0.11 | |
| Moderate VitD | 141 ± 36 | -0.9 ± 3.0 | |||
| Cortical thickness (μm) | 62 | ||||
| Low VitD | 1.1 ± 0.2 | 0.34 | -1.4 ± 4.0 | 0.40 | |
| Moderate VitD | 1.1 ± 0.2 | -0.6 ± 3.7 | |||
| Trabecular thickness (μm) | 62 | ||||
| Low VitD | 0.1 ± 0.01 | 0.41 | -1.9 ± 11.5 | 0.79 | |
| Moderate VitD | 0.1 ± 0.01 | -1.2 ± 9.0 | |||
| Trabecular number (1/mm) | 62 | ||||
| Low VitD | 1.7 ± 0.4 | 0.34 | 3.1 ± 14.0 | 0.56 | |
| Moderate VitD | 1.8 ± 0.3 | 1.2 ± 9.2 | |||
| Trabecular separation (mm) | 62 | ||||
| Low VitD | 0.6 ± 0.2 | 0.19 | -2.0 ± 11.9 | 0.55 | |
| Moderate VitD | 0.5 ± 0.1 | -0.3 ± 9.2 | |||
Safety
Overall, 16 participants (19%) reported at least one adverse event during the study. Two participants (one in each group) discontinued study drug based upon incidental findings of kidney stones on the cQCT scan performed at baseline. In the Moderate VitD group, one additional participant discontinued study drug because of epigastric pain at one month and another because of a myocardial infarction at six months. There were four cases of asymptomatic hypercalcemia above the upper limit of normal (10.5 mg/dl).
Discussion:
In this study, we found that VitD supplementation at 3000 IU daily increased mean total 25-OHD levels to ≥ 30 ng/ml in minority postmenopusal women with HIV. However, VitD supplementation with 3000 IU did not result in either greater increases in areal BMD by DXA or volumetric BMD by cQCT or HRpQCT, or suppression of bone turnover markers, when compared to VitD supplementation with 1000 IU daily.
There has been considerable interest in testing whether and to what extent various VitD supplementation regimens raise serum 25-OHD levels in people with HIV, since VitD deficiency is commonly reported and because decreases in 25-OHD have been reported in association with efavirenz use.18 Several recent studies have documented successful VitD supplementation using various doses of VitD3 among a wide age range of people with HIV and found no significant differences by ART regimen, including efavirenz-based regimens.19–21 Lerma-Chippiraz et al. reported that over 80% of patients with serum 25-OHD concentrations below 20 ng/ml, who were supplemented with 16,000 IU weekly or every two weeks, achieved levels above 20 ng/ml over a median follow up of two years.19 In adults with serum 25-OHD concentrations below 20ng/ml, Noe et al. found that weekly supplementation with 20,000 IU of VitD3 resulted in a median serum 25-OHD concentration of 28.5 ng/ml.20 In U.S. children and young adults with serum 25-OHD concentrations below 30 ng/ml, Eckhard et al. found that monthly supplementation with 18,000 IU of VitD resulted in serum 25-OHD concentrations above 30 ng/ml in 82% of participants, with similar increases observed amongst people with HIV and uninfected controls.22
A major focus of VitD supplementation studies in persons with HIV has been to address musculoskeletal complications, such as bone loss and fractures; however, the clinical impact of vitamin D supplementation remains uncertain. Rovner et al. recently reported a randomized trial of high dose VitD3 supplementation (7000 IU/day versus placebo) for 12 months in children and young adults between ages 5 and 25 years with perinatally- and behaviorally-acquired HIV infection.21 Despite a significant 12 month increase in serum 25-OHD to a mean of 26.7 ng/ml in the VitD supplemented group, there were no significant between-group differences in bone density or body composition outcomes by DXA or peripheral QCT. The authors did not address whether the modest increase in 25-OHD levels to such a high dose of VitD supplementation was due to poor adherence, but theorized that perhaps concentrations above 30 ng/ml were necessary to optimize bone health in at risk populations. In another trial of VitD supplementation in young adults with HIV (aged 16–24 years), Havens et al randomized 214 adolescents and young adults on TDF-containing ART to receive 50,000 IU VitD3 monthly by directly observed therapy or placebo; both groups received a multivitamin with 400 IU of VitD.23 At 48 weeks, serum 25-OHD levels were higher in the VitD than placebo group (36.9 versus 20.6 ng/ml). There was a significant within-group increase in lumbar spine BMD from baseline in the VitD but not placebo group, but no significant between group difference (1.15% versus 0.09%, p=0.12). It is interesting to contrast these results with VitD supplementation during ART initiation, which is a more dynamic period of bone loss.24–27 Overton et al. randomized 168 adults with HIV initiating efavirenz/TDF/emtricitabine to daily supplementation with 4000 IU VitD3 and 1000 mg calcium carbonate versus placebo, for both VitD and calcium.4 At 48 weeeks, 25-OHD levels were higher in the VitD group than placebo (56.5 versus 26.2 ng/ml), and there was significantly,less bone loss at the total hip (−1.36% versus −3.22%, p=0.004) and smaller increases in bone turnover markers in the VitD group than placebo.
In our study, median 25-OHD levels were ≥30 ng/ml at both the 6 and 12 month timepoints in the Moderate VitD treatment arm, with over 56% and 53% of women achieving levels >30ng/ml at 6 and 12 months, respectively. From a previous observational study of postmenopausal minority women with HIV at our institution with a similar mean age of 56, we found an annualized rate of bone loss of −1.2 ± 0.3% at the lumbar spine, −1.6 ± 0.4% at the total hip, −1.2 ± 0.4% at the femoral neck, and −1.1 ± 0.2% at the 1/3 distal radius 28. In the current study, we observed a maximum of - 0.8% loss at the lumbar spine and total hip at 12 months, and a maximum loss of −1.6% at the ultradistal radius site, suggesting that VitD supplementation at either 1000 or 3000 IU mitigates bone loss at some but not all bone sites. However, we found no evidence of a difference between the Low and Moderate VitD groups in rates of change in bone mass or structure by DXA or QCT.
There are several potential explanations for our results. First, we were underpowered to detect between-group differences. Although we had originally planned for a larger sample size (N=100, 50 per treatment arm), it was not possible to recruit the required number of participants over an extended period despite the addition of several clinical sites. The majority of our potential participants screened out because baseline 25-OHD levels were outside of our prespecified eligibility range (Figure 1). Also, we had a higher rate of study discontinuation than anticipated; both may have contributed to the negative results. Our post-hoc power analysis indicated that we had only 20% power to detect a significant difference in 0–12 month percent change in lumbar spine BMD between the two groups. Second, the 25-OHD levels at baseline may not have been sufficiently low to cause bone loss or to be able to detect an significant improvement with supplementation. Third, was the lack of a true placebo or a sufficiently large difference between the low and moderate VitD doses. At the time the study was conceived, a large amount of observational data suggested that VitD supplementation was beneficial in multiple domains, and there were ethical concerns about randomizing severely VitD deficient postmenopausal women to placebo for 12 months. Therefore, we chose to compare a lower (1000 IU/day) dose with a higher (3000 IU/day) dose that was below the 4000 IU set by the Institute of Medicine guidelines as the upper limit of daily VitD supplementation.29 Fourth, in postmenopausal women, VitD and calcium supplementation alone may be insufficient to reverse the bone loss that occurs during the course of HIV infection, particularly if the pathogenetic mechanisms of the bone loss are not solely related to VitD deficiency, and are due to other causes, such as menopause, HIV, or ART per se. Lastly, unlike in the aforementioned studies by Havens et al and Overton et al, our participants were on a variety of established ART regimens, many of which did not contain either TDF or efavirenz, which are known to be associated with bone loss and/or effects on VitD metabolism.30,31
In conclusion, our study found that moderate dose VitD (3000 IU) supplementation in minority postmenopausal women with HIV on established ART did not appear to have a greater impact on BMD or bone turnover than low dose VitD supplementation (1000 IU). We cannot determine whether the lack of between-group difference was due to an insufficient sample size, participants with baseline 25-OHD at levels too high to benefit or truly a lack of effect for vitD supplementation beyond 1000 IU in postmenopausal women with HIV. Future studies are necessary to determine whether VitD supplementation is beneficial in this patient population, and if so, what dose provides the maximum benefit in terms of musculoskelal health in persons aging with HIV.
Acknowledgments
Funding:
This work was supported by the National Institutes of Health through Grants AI059884 (MTY), AI1065200 (ES), AR06199301 (AS) and the National Center for Advancing Translation Sciences, through Grant Number UL1TR001873. This content is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
Footnotes
Conflicts of Interest:
MTY has served as a consultant for Gilead Sciences and Viiv. The other authors have no conflicts to declare.
References:
- 1.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. The Journal of clinical endocrinology and metabolism. September 2008;93(9):3499–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin MT, McMahon DJ, Ferris DC, et al. Low Bone Mass and High Bone Turnover in Postmenopausal Human Immunodeficiency Virus-Infected Women. J Clin Endocrinol Metab. December 4 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein EM, Yin MT, McMahon DJ, et al. Vitamin D deficiency in HIV-infected postmenopausal Hispanic and African-American women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. February 2011;22(2):477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overton ET, Chan ES, Brown TT, et al. Vitamin D and Calcium Attenuate Bone Loss With Antiretroviral Therapy Initiation: A Randomized Trial. Annals of internal medicine. June 16 2015;162(12):815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. April 28 2004;291(16):1999–2006. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. May 11 2005;293(18):2257–2264. [DOI] [PubMed] [Google Scholar]
- 7.Dawson-Hughes B, Bischoff-Ferrari HA. Therapy of osteoporosis with calcium and vitamin D. J Bone Miner Res. December 2007;22 Suppl 2:V59–63. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. June 1 2002;359(9321):1929–1936. [DOI] [PubMed] [Google Scholar]
- 9.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. June 2004;19(6):1006–1012. [DOI] [PubMed] [Google Scholar]
- 10.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. December 2005;90(12):6508–6515. [DOI] [PubMed] [Google Scholar]
- 11.Liu XS, Zhang XH, Sekhon KK, et al. High-resolution peripheral quantitative computed tomography can assess microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res. April 2010;25(4):746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. The Journal of clinical endocrinology and metabolism. December 2005;90(12):6508–6515. [DOI] [PubMed] [Google Scholar]
- 13.Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. December 2010;25(12):2572–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickolas TL, Stein E, Cohen A, et al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol. August 2010;21(8):1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manske SL, Zhu Y, Sandino C, Boyd SK. Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone. 2015;79:213–221. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal S, Rosete F, Zhang C, et al. In vivo assessment of bone structure and estimated bone strength by first- and second-generation HR-pQCT. Osteoporosis International. 2016:1–12. [DOI] [PubMed] [Google Scholar]
- 17.Schafer JL. Analysis of multivarate incomplete data. London: Chapman & Hall; 1997. [Google Scholar]
- 18.Brown TT, McComsey G. Association between initiation of antiretroviral therapy with efavirenz and decrease in 25-hydroxyvitamin D. Antiviral therapy. 2010;15(3):425–429. [DOI] [PubMed] [Google Scholar]
- 19.Lerma-Chippirraz E, Guerri-Fernandez R, Villar Garcia J, et al. Validation Protocol of Vitamin D Supplementation in Patients with HIV-Infection. AIDS research and treatment. 2016;2016:5120831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noe S, Heldwein S, Pascucchi R, et al. Cholecalciferol 20 000 IU Once Weekly in HIV-Positive Patients with Low Vitamin D Levels: Result from a Cohort Study. Journal of the International Association of Providers of AIDS Care. Jul-Aug 2017;16(4):315–320. [DOI] [PubMed] [Google Scholar]
- 21.Rovner AJ, Stallings VA, Rutstein R, Schall JI, Leonard MB, Zemel BS. Effect of high-dose cholecalciferol (vitamin D3) on bone and body composition in children and young adults with HIV infection: a randomized, double-blind, placebo-controlled trial. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. January 2017;28(1):201–209. [DOI] [PubMed] [Google Scholar]
- 22.Eckard AR, Thierry-Palmer M, Silvestrov N, et al. Effects of cholecalciferol supplementation on serum and urinary vitamin D metabolites and binding protein in HIV-infected youth. The Journal of steroid biochemistry and molecular biology. April 2017;168:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havens PL, Stephensen CB, Van Loan MD, et al. Vitamin D3 Supplementation Increases Spine Bone Mineral Density in Adolescents and Young Adults With Human Immunodeficiency Virus Infection Being Treated With Tenofovir Disoproxil Fumarate: A Randomized, Placebo-Controlled Trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. January 6 2018;66(2):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. The Journal of infectious diseases. June 15 2011;203(12):1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoy JF, Grund B, Roediger M, et al. Immediate Initiation of Antiretroviral Therapy for HIV Infection Accelerates Bone Loss Relative to Deferring Therapy: Findings from the START Bone Mineral Density Substudy, a Randomized Trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. September 2017;32(9):1945–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taiwo BO, Chan ES, Fichtenbaum CJ, et al. Less Bone Loss With Maraviroc- Versus Tenofovir-Containing Antiretroviral Therapy in the AIDS Clinical Trials Group A5303 Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. October 1 2015;61(7):1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown TT, Moser C, Currier JS, et al. Changes in Bone Mineral Density After Initiation of Antiretroviral Treatment With Tenofovir Disoproxil Fumarate/Emtricitabine Plus Atazanavir/Ritonavir, Darunavir/Ritonavir, or Raltegravir. The Journal of infectious diseases. October 15 2015;212(8):1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin MT, Zhang CA, McMahon DJ, et al. Higher rates of bone loss in postmenopausal HIV-infected women: a longitudinal study. The Journal of clinical endocrinology and metabolism. February 2012;97(2):554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. The Journal of clinical endocrinology and metabolism. January 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antiviral therapy. 2010;15(3):425–429. [DOI] [PubMed] [Google Scholar]
- 31.Havens PL, Kiser JJ, Stephensen CB, et al. Association of higher plasma vitamin D binding protein and lower free calcitriol levels with tenofovir disoproxil fumarate use and plasma and intracellular tenofovir pharmacokinetics: cause of a functional vitamin D deficiency? Antimicrobial agents and chemotherapy. November 2013;57(11):5619–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]