Abstract
Preparing a single cell suspension is a critical step in any solid tissue flow cytometry experiment. Tissue dissection, enzymatic digestion, and mechanical dissociation are three significant steps leading to the degradation of the extracellular matrix and the isolation of single cells, allowing the generation of high-quality flow cytometry data. Cells and the extracellular matrix contain various proteins and other structures which must be considered when designing a tissue digestion protocol to preserve the viability of cells and the presence of relevant antigens while digesting matrix components and cleaving cell-cell junctions. Evaluation of the single cell suspension is essential before proceeding with the labeling of the cells as high viability and absence of cell debris and aggregates are critical for flow cytometry. The information presented should be used as a general guide of steps to consider when preparing a single cell suspension from solid tissues for flow cytometry experiments.
Purpose
The purpose of this paper is to define the critical steps in the segregation of solid tissues by describing the composition of tissues and the common digestive enzymes used to digest extracellular matrix and cleave cell-cell junctions to obtain a single cell suspension.
Keywords: single cell suspension, tissue digestion, disaggregation, flow cytometry
Introduction
The preparation of single cells is a crucial part of any solid tissue flow cytometry experiment. The purpose of preparing a single cell suspension is to quickly isolate cells from tissues and stain or label them for flow cytometric acquisition while avoiding cell death and aggregation. This procedure should yield a cell population with high viability, minimal cell debris or aggregates, and preserved cell surface antigens for flow cytometric immunophenotyping to be effective 1. The basic steps in preparing a single cell suspension include 1) increasing the surface area of the starting solid tissue material in order to maximize contact between the tissues and digestive enzymes, 2) digesting the extracellular matrix by introducing these enzymes to the masses, and 3) cleaving cell-cell junctions. These steps must be carried out while ensuring that the cells remain as intact as possible, as a single cell suspension should include minimal amounts of DNA released by dying cells or fragmented cells.
This paper is focused on single cell preparation from solid tissues. Liquid tissues containing hematopoietic cells such as blood or bone marrow can be prepared relatively easily using density gradient separation or red blood cell lysis. These techniques allow for the separation of lymphocytes or specific mononuclear cells from whole blood or other liquid tissues with a high yield and purity. However, preparation of a single cell suspension from liquid tissues is not the purpose of this paper.
Information and procedures regarding the best practices for preparing a single cell suspension specifically for mass cytometry experiments has been described 2. This paper will focus on best practices for flow cytometry experiments only.
Tissue Composition
Tissues are composed of cells embedded in an extracellular matrix, within which neighboring cells are anchored to each other via cell-cell junctions on the cell membrane.
Extracellular Matrix
Throughout an organism, all tissues and organs are composed of cells surrounded by a non-cellular component known as the extracellular matrix. The function of the extracellular matrix is to provide scaffolding and structural support to tissues while also initiating biochemical and biomechanical cues within each specific tissue 3. The extracellular matrix is made up of components belonging to three major categories of biological molecules: collagens, proteoglycans, and glycoproteins 4, 5.
Collagens are the most abundant fibrous protein in the extracellular matrix. As a result, collagens are the primary structural element of this matrix, providing tensile strength and regulating cell adhesion. Collagens also respond to biochemical cues in the extracellular matrix by supporting chemotaxis and migration while directing tissue development 6. Proteoglycans are biological molecules made up of a protein core bonded to glycosaminoglycan chains. The roles of proteoglycans in the extracellular matrix include organizing matrix assembly, regulating the signaling of cytokines and growth factors in tissues, and activating cell-surface receptors to affect the function and development of cells and whole organs 7. Common types of proteoglycans found in tissues include decorin, versican, and hyaluronan. Decorin and versican are present in all tissues throughout an organism. Hyaluronan is found in most tissues acting as a structural component and signaling molecule but lacks the protein core typically indicative of proteoglycans 8,7. Many other types of proteoglycans are present in only specific tissue types throughout an organism and will therefore not be reviewed in this paper. Glycoproteins are any protein composed of a polypeptide chain attached to a carbohydrate group. One common glycoprotein found in the extracellular matrix is fibronectin, which plays a structural role by binding to collagen, thrombospondin, integrins, fibrins, and glycosaminoglycans 9. Laminin also contributes to the structure of the extracellular matrix by actively modulating the behavior of associated cells in regards to adhesion, differentiation, migration, phenotype stability, and apoptosis resistance 5. Elastin is the main component of elastic fibers in the extracellular matrix of tissues and is the main contributor to the elasticity of these fibers 10,11. Elastin is typically found in skin, lungs, ligaments, tendons, and vascular tissues 12. Other glycoproteins commonly found in the extracellular matrix include fibrinogen, fibrillins, fibulins, tenascins, thrombospondins, and cartilage oligomeric complex proteins 5.
Cell Membrane
The cell membrane is a phospholipid bilayer which serves as a hydrophobic barrier surrounding the cell, protecting the interior of the cell and its organelles 13. The cell membrane contains various membrane proteins which are vital in the development and survival of a cell. Receptor proteins facilitate the relay of signals across the cell membrane which allows the cell to respond to cues from its environment, while channels and transporter proteins embedded in the cell membrane enable the passage of specific ions into or out of the cell using active and passive processes 13, 14. Enzyme proteins are also present throughout the cell membrane, catalyzing chemical reactions in and around the cell. Digestive enzyme selection and concentration are vital in the preparation of a single cell suspension from solid tissues for flow cytometry, as cell surface receptors or membrane proteins can be cleaved by the proteolytic activity of enzymes such as trypsin 15, resulting in falsely negative results in immunophenotyping experiments.
Cell-Cell Junctions
Cell-cell junctions are a major structural and functional component of tissues which must be cleaved to prepare a single cell suspension that will yield usable data in a flow cytometry experiment. The three major categories of cell-cell junctions that are relevant to the preparation of a single cell suspension are 1) occluding junctions, 2) communicating junctions, and 3) anchoring junctions. Occluding junctions, commonly known as tight junctions, maintain a continuous circumferential seal near the apex of endothelial and epithelial cells 16. These junctions appear as a series of discrete sites of apparent fusion with the cell membranes of neighboring cells 17. Tight junctions present with variation in structure and are composed of transmembrane proteins including claudins, junctional adhesion molecule family proteins, occludin, nectins, and endothelial-cell-selective adhesion molecules 16, 18. The function of tight junctions is to form a barrier which can control the movement of water and solutes across the paracellular space while also maintaining the distribution of ion channels, pumps, and carrier proteins present throughout the cell membrane 17. Communicating junctions, commonly known as gap junctions, function to allow the cytoplasmic exchange of metabolites and ions between adjacent cells 19. These gap junctions are comprised of innexin proteins in invertebrate organisms and connexin proteins in vertebrate animals, where six connexin proteins unite to form one connexon 20. Anchoring junctions include adherens junctions, desmosomes, and hemidesmosomes, which act to mediate cell adhesion between adjacent cells and to transfer intracellular signals 18. Anchoring junctions are composed of cadherin proteins and form a zipper-like structure that allows the stable adhesion of neighboring cells within tissues.
Tissue Disaggregation
Mincing Tissues
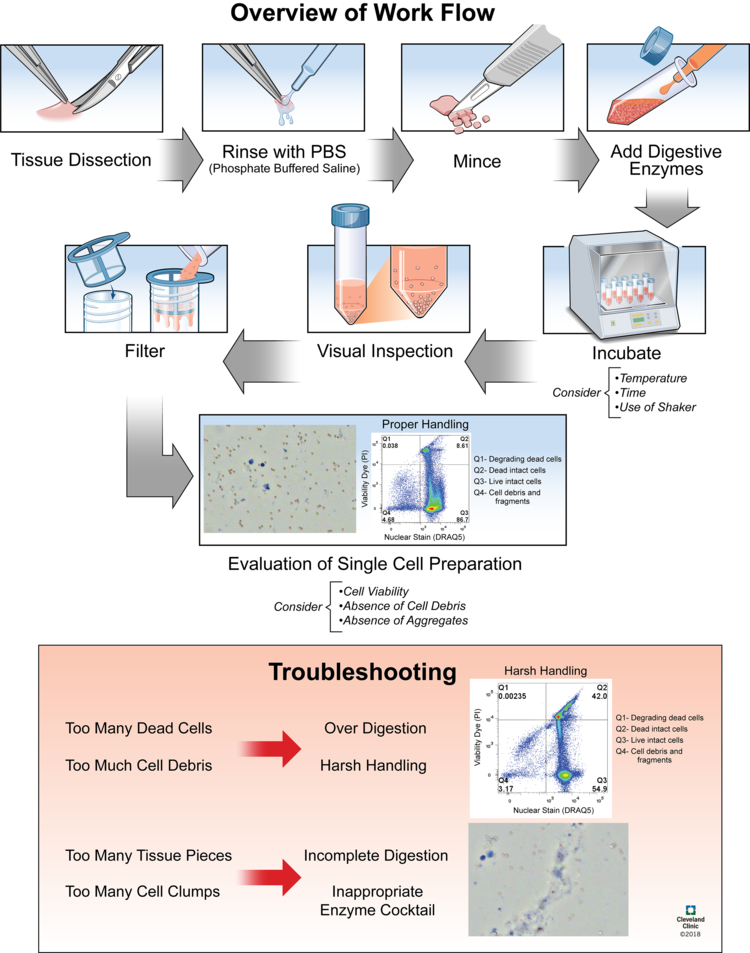
After dissection of solid tissues from an organism, before introducing the digestive enzymes, tissues should be rinsed to clean off any blood or other unwanted material. The tissues should then be minced and dispersed with scissors, a scalpel, or a blade to increase total surface area. This increases contact between the enzymes and the surface of the tissues, leading to more efficient and complete digestion while shortening the time required for digestion.
Enzymes
Tissues hold cells together, supported by extracellular matrix and cell-cell junctions made up of a diverse set of proteins and other biological molecules which require specific enzymes for proper digestion and removal from the cell suspension. The digestive enzymes that play an essential role in the disaggregation of solid tissues are summarized in Table 1. The first category of enzymes to consider are those enzymes that break down the extracellular matrix. Dispase is a commonly used neutral protease isolated from bacteria, with a high level of enzymatic specificity for collagen IV and fibronectin 21,22. Dispase is useful in the detachment of cell colonies and the dissociation of tissue pieces into small clumps of cells, as it works to cleave attachments between cells and the extracellular matrix without affecting cell-cell junctions 23. Caution should be used when digesting tissues with dispase however, as it is able to cleave specific relevant surface molecules or antigens, such as those connected to T cell analysis 24,25. Therefore, omitting dispase from the digestion buffer may be helpful if a loss of epitopes is observed. Also effective in the disaggregation of the extracellular matrix is collagenase. Collagenase is able to break the peptide bonds present in collagen which helps to digest the extracellular matrix, releasing cells into suspension. It is important to note that purified collagenase enzymes are more effective than traditional collagenase naturally derived from bacteria as there is less variability in the composition of the purified enzyme, increasing the stability of the cells throughout the tissue digestion 26. The final enzyme to consider in the digestion of the extracellular matrix in solid tissues is hyaluronidase. Hyaluronan, a structural proteoglycan in the extracellular matrix, is degraded by the hyaluronidase family of enzymes, which are produced in both bacterial and vertebrate organisms. These hyaluronidase enzymes cleave the β1,4 glycosidic bond present in the glycosaminoglycan portion of hyaluronan 27, contributing to the digestion of the extracellular matrix.
Table 1.
Digestive Enzymes in Solid Tissue Disaggregation
| ENZYME | PURPOSE |
|---|---|
| Dispase | -Breaks down extracellular matrix |
| -Detaches cell colonies | |
| -Cleaves attachments between cells and extracellular matrix | |
| Collagenase | -Breaks down extracellular matrix |
| -Breaks peptide bonds present in collagen | |
| Hyaluronidase | -Breaks down extracellular matrix |
| -Cleaves glycosidic bonds in hyaluronan | |
| Papain | -Degrades proteins which make up tight junctions between cells |
| DNase-I | -Degrades free-DNA |
| -Prevents cell aggregation | |
| Accutase | -Proteolytic, collagenolytic, and DNase activity |
| TrypLE | -Cleaves cell-cell junctions |
| -Does not alter antigen expression as trypsin would |
The next enzyme group to consider in the preparation of a single cell suspension includes the enzymes that break cell-cell junctions. Trypsin is a natural protease synthesized in the digestive system of vertebrate organisms. Although it is useful in degrading certain proteins present in cell-cell junctions, trypsin also has a very harsh effect on cell membrane proteins 1. Also, trypsin has been shown to lead to free-DNA induced aggregation of cells, which indicates that cell lysis is occurring within the suspension 28. Therefore, trypsin is traditionally avoided in the preparation of a single cell suspension from solid tissues for flow cytometry experiments. Papain is an alternative protease derived from the papaya plant. Papain is known to degrade the proteins that make up tight junctions between cells 29. However, like trypsin, papain has been shown to lead to free-DNA induced aggregation of cells due to the cell lysis that occurs during enzymatic digestion 28.
The final enzyme type to consider in the preparation of a single cell suspension is deoxyribonuclease (DNase) which acts to cleave the phosphodiester linkages of the DNA backbone. The two major types of DNase are DNase-I and DNase-II, which possess slightly different enzymatic functions. DNase-II is not suitable for the preparation of a single cell suspension as it plays a role in engulfment-mediated DNA degradation pathways involved in apoptosis 30,31. DNase-I is appropriate for tissue digestion and preparation of a single cell suspension as it prevents cell aggregation by degrading free-DNA released through dead cell lysis during the enzymatic digestion without initiating apoptotic pathways. Calcium chloride (CaCl2) acts as an enzyme activator of DNase I and is therefore introduced into the digestion cocktail during enzymatic digestion. Calcium ions (Ca2+) bind tightly to the DNase-I enzyme to stabilize its active conformation and allow for the proper degradation of free-DNA 32.
To avoid the possible problems caused by adding the above enzymes to a tissue digestion cocktail, commercially available digestion cocktails have been developed and optimized. Accutase is a commercially available protease and collagenase blend which mimics the action of trypsin and collagenase but does so at a much lower concentration than is needed when using the standard enzymes. Accutase contains a mixture of enzymes with proteolytic, collagenolytic, and DNase activity and produces a higher total cell yield and improved overall antigen preservation when compared to using a cocktail of similar enzymes for tissue digestion 33, 34. TrypLE is another commercially available enzyme cocktail containing purified, recombinant enzymes which mimic the activity of trypsin without altering the expression of cell surface antigens 35. This product avoids the issues that arise with trypsin use in tissue digestion and allows for improved cell survival and more efficient single cell suspension preparation.
Enzymatic and Mechanical Dissociation
Enzymatic dissociation is carried out by introducing a digestion cocktail to minced, solid tissues and incubating at specific temperatures, based on the enzyme cocktail being used. Enzymes may be temperature specific, and therefore work with maximum speed and efficiency at a given temperature, commonly 37°C. Depending on the specific enzymes, enzymatic dissociation may also be carried out at 4° C or on ice. These lower temperatures will likely slow the reaction rate of the enzymes and extend the incubation period but can help to minimize cell death. Enzyme strength and enzyme concentration are the two most important factors to consider when choosing a digestion cocktail for the preparation of a single cell suspension for flow cytometry. Enzymes with high strength or high concentration may compromise cell surface markers present on the cells, which can affect the availability of these markers and the viability of the cells in further experiments. Therefore, lightly adherent cells such as lymphocytes should be isolated using a short digestion period with a gentle or mild enzyme to avoid these issues 36. Determining the optimal strength and concentration of the enzymes being used in enzymatic dissociation is empirical and critical for proper isolation of cells and successful digestion of tissues.
Mechanical dissociation plays a role in the preparation of a single cell suspension from solid tissues throughout the enzymatic dissociation. To assist in the mechanical dissociation of the tissues, by which cells are released from the extracellular matrix into suspension, enzymatic digestion may be carried out on an orbital shaker. Following enzymatic dissociation, the suspension should be filtered in order to exclude any undigested tissue pieces or aggregates from the newly prepared single cell suspension.
Evaluation of a Single Cell Suspension
Evaluating a single cell suspension obtained from solid tissues is an important step to take before using cells for a flow cytometry experiment. The three critical parameters to assess following enzymatic and mechanical dissociation of solid tissues are 1) cell viability, 2) an absence of cell debris, and 3) an absence of aggregates. Cell viability in a single cell suspension can easily be screened for using the trypan blue cell viability exclusion assay in which dead cells absorb trypan blue into their cytoplasm while live cells retain their selectively permeable membrane and prevent the trypan blue from entering the cytoplasm 37. The relative amount of live and dead cells in the single cell suspension can then be evaluated using light microscopy. Cell debris and cell aggregates can also quickly be evaluated using light microscopy. Running the single cell suspension on a flow cytometer is also highly recommended for the evaluation of the single cell preparation. Adding a nuclear stain will discriminate intact cells from cell debris, and the inclusion of a viability dye will allow quantification of the percentage of dead cells.
Viability Improvement and Removal of Cell Debris
Excluding cell debris and dead cells from flow cytometry data is the best practice for an accurate and efficient study of the cell types or antigens of interest. Over the course of the tissue digestion and immunostaining of a single cell suspension, some cells will die as a result of natural cell death as well as injury caused by enzymatic and mechanical dissociation. High cell viability should be the goal of every tissue digestion, but taking the steps necessary to exclude from the final cell preparation those cells which do die and create debris is also needed. One method of removing cell debris and dead cells from a single cell suspension is to use a dead cell removal kit to “clean-up” the sample population before running samples on a flow cytometer. These kits are available from vendors such as Miltenyi Biotec Inc. (Auburn, CA) and STEMCELL Technologies Inc. (Cambridge, MA), and employ microbeads and specific binding buffers to magnetically label cell debris, dead cells, or dying cells, allowing for a negative selection of these unwanted populations by means of magnetic separation.
Cryopreservation of a Single Cell Suspension
Flow cytometry experiments involving functional assays should be conducted shortly after the preparation of a single cell suspension or on cryopreserved live cells. Potential effects of cryopreservation should be tested in pilot experiments. For batch analyses of samples at a later time point, a mild fixation with paraformaldehyde can be used to avoid altering the expression or presence of antigens during cryopreservation 1. However, fixation itself may affect antigenicity by changing protein conformation, so antibody recognition should also be evaluated in pilot experiments. Fixation physically stabilizes cells in their current state while preventing the fragmentation of the newly dead cells 38. Mild paraformaldehyde fixation also preserves light scatter properties critical to heterogeneous cell populations and helps to prevent cells from sticking together or to the plate or tube in which they are stained. Fixation has been shown to be especially crucial for fragile/heterogeneous populations such as lung single cells 38. Cryopreservable live/dead dye should be applied to cells before fixing to allow for discrimination of these two populations during data analysis.
Common Pitfalls and Considerations
Several common mistakes and pitfalls can occur throughout the process of preparing a single cell suspension from solid tissues for flow cytometry due to improper techniques. These improper techniques (Don’ts) and their corresponding best practices (Dos) are summarized in Table 2. Comparative data between best practices and improper techniques using a trypan blue viability assay and a flow cytometric DRAQ5 and Propidium Iodide panel (described below and in Supplemental File 1) are summarized in Table 3. Raw data files and analysis workspaces for the flow cytometry experiment are available on FlowRepository.org, Experiment ID: FR-FCM-ZYQP.
Table 2.
Preparation of a Single Cell Suspension Dos and Don’ts
| PROTOCOL STEPS | DOS | DON’TS |
|---|---|---|
| Tissue Dissection | -Rinse with PBS | -Freeze tissues |
| Mince | -Use scalpel, blade, or scissors to increase total surface area | -Use tissue homogenizer |
| -Vortex tissues | ||
| Enzymatic Digestion | -Determine optimal strength and concentration of enzymes | -Use trypsin if evaluating cell surface proteins |
| -Determine optimal temperature for digestion | ||
| -Include DNase-I and EDTA in the digestion cocktail | ||
| Incubation | -Use orbital shaker to assist in mechanical dissociation | -Over or under incubate in digestive enzymes |
| Filtration | -Remove undigested tissue pieces or aggregates | |
| Centrifugation | -Use RCF units -Centrifuge cells at 300-900 RCF |
-Use RPM units if using multiple centrifuges |
| Resuspension | -Pipette gently | -Vortex cells |
| -Avoid bubbles | -Create bubbles | |
| Evaluation | -Check cell viability and evaluate cell suspension for total cell yield, cell debris, and aggregates | -Proceed with preparation with low viability or high levels of cell debris and aggregates |
| Viability Improvement/Debris Removal | -Use a “clean-up” kit to remove debris and dead cells -Use red blood cell lysis buffer if sample remains red |
|
| Material Selection | -Use polypropylene tubes and plates to avoid cell adhesion | -Use polystyrene tubes and plates |
Table 3.
Comparison of Proper and Improper Techniques
| TREATMENT | Best Practice |
Frozen Tissue |
Tissue Homogenizer |
Vortexed Cells |
Under Digestion |
Over Digestion |
|---|---|---|---|---|---|---|
| YIELD | 100±0% | 54.0±6.0%* | 50.0±18%* | 98.0±9.2% | 98.0±3.5% | 90.0±21% |
| VIABILITY: TRYPAN BLUE ASSAY | 81.3±17% | 33.6±3.8%* | 53.7±13%* | 40.3±8.7%* | 67.3±4.2% | 47.4±11%* |
| AGGREGATES | 0.3±0.6 | 0.0±0 | 0.0±0 | 3.0±1.0 | 50.7±10* | 1.3±1.2 |
| VIABILITY: FLOW CYTOMETRY | 75.1±1.2% | 46.0±1.9%* | 60.3±1.0%* | 39.6±1.8%* | 72.2±1.1% | 26.1±0.8%* |
| CELL DEBRIS AND FRAGMENTS | 6.8±0.9% | 7.1±1.0% | 1.3±0.1%* | 14.0±1.8%* | 9.2±0.7%* | 19.3±0.6%* |
Use of animals was approved by local IACUC. All experiments were conducted using the left lung lobe of age and gender matched mice. Mean ± Standard Error of three experiments is shown for each condition.
denotes p<0.05 (t-test) compared with Best Practice. Aggregates were quantified as count per 100 nL volume (1 square of Hemocytometer chamber at 0.1 mm depth). Yield calculated as (Recovered Treatment Specific Cell Count) / (Recovered Best Practice Cell Count)*100.
Freezing of dissected tissues by either slow-freeze or snap-freeze methods has been shown to decrease total cell recovery when compared to the isolation of cells from fresh tissues 39. This is evident in the comparative data in Table 3, where lung tissue which was stored at −80°C for 72 hours following dissection and prior to digestion showed a decreased cell yield and decreased cell viability. Therefore, it is the best practice to isolate and process cells from fresh tissues when possible.
Forceful mechanical dissociation using a tissue homogenizer is not suitable for the preparation of a single cell suspension as it instead creates a broth of cell and tissue components and is intended for isolating protein for other methods of analysis 40. Therefore, the cells would not remain viable or intact when using this method and the use of a tissue homogenizer should be avoided. The data in Table 3 show that using a tissue homogenizer in place of mincing tissue resulted in decreased cell yield and viability. The decrease seen in the relative amount of cell debris and fragments is a result of the centrifugation which occurred throughout the protocol, during which debris and fragments created by the tissue homogenizer remained in suspension and were aspirated.
Vortexing cells is another form of vigorous cell handling that must be avoided to preserve the viability of the single cell suspension and to prevent cellular disintegration. Vortexing cells resulted in decreased cell viability and increased cell debris and aggregates in the experiments outlined in Table 3. Rather than vortexing, gentle pipetting to resuspend the cells or cell pellet is preferred. Avoiding bubbles in the suspension when pipetting is equally important.
Centrifugation is another area in which errors can be made, leading to poor results from a solid tissue single cell suspension. In general, cells should be centrifuged between 300 and 900 relative centrifugal force (RCF), with 900 RCF being more suitable for fixed cells and lower force values being used for live, unfixed cells. It is important to note that these values are presented in relative centrifugal force and not rotations per minute (RPM), as RPM denotes a variable force dependent upon the radius of the rotor on each specific centrifuge. Centrifuging cells at too high of a speed can lead to compact pelleting and damage to the cell membrane, while centrifuging at too low of a speed will allow cells to remain in suspension and be lost when the supernatant is aspirated.
Incubation time in the digestion cocktail was shown to play a role in cell viability and aggregation in the experiments summarized in Table 3. Under digestion, in which cells were removed from the digestion cocktail after twenty minutes, resulted in an increase in aggregates and an increase in cell debris and fragments. Over digestion, in which cells remained in the digestion cocktail for five hours, resulted in significantly decreased cell viability as well as increased cell debris and fragments in the suspension. It is therefore critical to determine the correct incubation time for the specific digestive enzymes and tissues involved in each experiment.
If possible, unfixed cells should be stained on ice to preserve viability and avoid cell death over the course of immunostaining. Throughout the digestion, all buffers should contain DNase-I to degrade free-DNA released by lysed cells, preventing cell aggregation. Ethylenediaminetetraacetic acid (EDTA) is a chelating agent that sequesters divalent metal ions, including those necessary for integrin mediated cell adhesion to the extracellular matrix 41. Therefore, including EDTA in the digestion cocktail is important in limiting the adhesion of naturally adherent cells such as epithelial cells and macrophages.
Material selection also plays a role in restricting adhesion. Polypropylene tubes or plates are most suitable for preparing a single cell suspension due to the decreased affinity between cells and the surface of polypropylene, diminishing the likelihood of cells adhering to the tube or plate and being lost from the suspension 42. Polystyrene is designed to promote cell adhesion and spreading and should be avoided in flow cytometry experiments 43.
Checking cell viability and evaluating the single cell suspension for total cell yield and the absence of cell debris and aggregates is very important before attempting to acquire and analyze flow cytometry data. A simple protocol employing a nuclear stain (DRAQ5) and a cell viability stain (Propidium Iodide) for this purpose is available in Supplemental File 1. If cell suspensions are contaminated with excess red blood cells, a red blood cell lysis buffer should be used to remove this contaminant and ensure that only the cell types of interest are present in the suspension for immunostaining and flow cytometric acquisition. If used, this treatment should be applied to all samples in an experiment to keep the cell preparation procedure consistent. The protocol for making red blood cell lysis buffer suitable for this purpose is available in Supplemental File 2.
Each flow cytometry experiment focuses on specific cell types and antigens of interest, dependent upon the aims of the study. However, some general considerations should always be taken into account as the dissociation procedure itself can impact the cells and pathways being studied. Users should be cautious and validate that their digestion protocol does not affect epitope expression for the specific markers being studied in their panel as some enzymes can alter expression of some markers relevant to phenotypic analysis 24. For experiments involving intracellular cytokine detection, protein transport inhibitors are recommended to block secretion of cytokines 44. Tissue dissociation has also been shown to affect RNA expression in part due to the upregulation of microRNA which plays a role in limiting cellular activity when the cell becomes isolated from its surrounding tissue 45. This downregulation of RNA should be investigated prior to evaluating RNA expression by flow cytometry. RNase inhibitors may also be included to avoid the degradation of the RNA transcripts of interest. Phospho-flow studies are meant to measure the phosphorylation state of intracellular proteins by investigating kinase signaling pathways 46. Phosphatase inhibitors should be included in the enzyme cocktail for these studies in order to preserve phosphorylation signals and ensure accurate results. Finally, damaged cells may release proteases capable of cleaving epitopes of interest. Protease inhibitors are able to alleviate this epitope loss when added to the digestion cocktail.
Supplementary Material
Acknowledgments
The authors thank Dave Schumick of the Cleveland Clinic Center for Medical Art and Photography for his illustration work on Figure 1, John Peterson, PhD of the Lerner Research Institute Imaging Core for his assistance with light microscopy imaging for Figure 1, Joseph Gerow, Jena Korecky, and Eric Schultz of the Lerner Research Institute Flow Cytometry Core for their assistance with instrument quality control, and Mario Alemagno, Matthew Frimel, Nicholas Wanner, and Kelly Weiss for their excellent technical assistance.
Figure 1. Critical Steps and Troubleshooting in the Preparation of a Single Cell Suspension from Solid Tissue.
Microscopy images stained with live/dead viability dye (Trypan Blue). Staining protocol for flow cytometry figures is available in Supplemental File 1.
Grant Support: Supported by NIH grants HL103453, HL081064, and HL109250
References
- 1.Khan MR, Chandrashekran A, Smith RK and Dudhia J. Immunophenotypic characterization of ovine mesenchymal stem cells. Cytometry A. 2016;89:443–50. [DOI] [PubMed] [Google Scholar]
- 2.Leelatian N, Doxie DB, Greenplate AR, Sinnaeve J, Ihrie RA and Irish JM. Preparing Viable Single Cells from Human Tissue and Tumors for Cytomic Analysis. Curr Protoc Mol Biol. 2017;118:25C 1 1–25C 1 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frantz C, Stewart KM and Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oskarsson T Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22 Suppl 2:S66–72. [DOI] [PubMed] [Google Scholar]
- 5.Halper J and Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. [DOI] [PubMed] [Google Scholar]
- 6.Rozario T and DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer L and Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339:237–46. [DOI] [PubMed] [Google Scholar]
- 8.Jiang D, Liang J and Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. [DOI] [PubMed] [Google Scholar]
- 9.Potts JR and Campbell ID. Fibronectin structure and assembly. Curr Opin Cell Biol. 1994;6:648–55. [DOI] [PubMed] [Google Scholar]
- 10.Muiznieks LD, Weiss AS and Keeley FW. Structural disorder and dynamics of elastin. Biochem Cell Biol. 2010;88:239–50. [DOI] [PubMed] [Google Scholar]
- 11.Mithieux SM, Wise SG and Weiss AS. Tropoelastin--a multifaceted naturally smart material. Adv Drug Deliv Rev. 2013;65:421–8. [DOI] [PubMed] [Google Scholar]
- 12.Chung MI, Miao M, Stahl RJ, Chan E, Parkinson J and Keeley FW. Sequences and domain structures of mammalian, avian, amphibian and teleost tropoelastins: Clues to the evolutionary history of elastins. Matrix Biol. 2006;25:492–504. [DOI] [PubMed] [Google Scholar]
- 13.Forrest LR. Structural Symmetry in Membrane Proteins. Annu Rev Biophys. 2015;44:311–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almen MS, Nordstrom KJ, Fredriksson R and Schioth HB. Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang HL, Hsing HW, Lai TC, Chen YW, Lee TR, Chan HT, Lyu PC, Wu CL, Lu YC, Lin ST, Lin CW, Lai CH, Chang HT, Chou HC and Chan HL. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J Biomed Sci. 2010;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneeberger EE and Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–61. [DOI] [PubMed] [Google Scholar]
- 17.Farquhar MG and Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejana E and Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci. 2013;126:2545–9. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M and Holstein-Rathlou NH. Gap junctions. Compr Physiol. 2012;2:1981–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phelan P Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–45. [DOI] [PubMed] [Google Scholar]
- 21.Stenn KS, Link R, Moellmann G, Madri J and Kuklinska E. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93:287–90. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimi Dastgurdi M, Ejeian F, Nematollahi M, Motaghi A and Nasr-Esfahani MH. Comparison of two digestion strategies on characteristics and differentiation potential of human dental pulp stem cells. Arch Oral Biol. 2018;93:74–79. [DOI] [PubMed] [Google Scholar]
- 23.Rao MV and Zaidel-Bar R. Formin-mediated actin polymerization at cell-cell junctions stabilizes E-cadherin and maintains monolayer integrity during wound repair. Mol Biol Cell. 2016;27:2844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Autengruber A, Gereke M, Hansen G, Hennig C and Bruder D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur J Microbiol Immunol (Bp). 2012;2:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronke K, Kofoed-Nielsen M and Diefenbach A. Isolation and Flow Cytometry Analysis of Innate Lymphoid Cells from the Intestinal Lamina Propria. Methods Mol Biol. 2017;1559:255–265. [DOI] [PubMed] [Google Scholar]
- 26.Cavanagh TJ, Lakey JR, Wright MJ, Fetterhoff T and Wile K. Crude collagenase loses islet-isolating efficacy regardless of storage conditions. Transplant Proc. 1997;29:1942–4. [DOI] [PubMed] [Google Scholar]
- 27.Stern R and Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106:818–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panchision DM, Chen HL, Pistollato F, Papini D, Ni HT and Hawley TS. Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing CD133, CD15, and CD24. Stem Cells. 2007;25:1560–70. [DOI] [PubMed] [Google Scholar]
- 29.Stremnitzer C, Manzano-Szalai K, Willensdorfer A, Starkl P, Pieper M, Konig P, Mildner M, Tschachler E, Reichart U and Jensen-Jarolim E. Papain Degrades Tight Junction Proteins of Human Keratinocytes In Vitro and Sensitizes C57BL/6 Mice via the Skin Independent of its Enzymatic Activity or TLR4 Activation. J Invest Dermatol. 2015;135:1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torriglia A, Perani P, Brossas JY, Chaudun E, Treton J, Courtois Y and Counis MF. L-DNase II, a molecule that links proteases and endonucleases in apoptosis, derives from the ubiquitous serpin leukocyte elastase inhibitor. Mol Cell Biol. 1998;18:3612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolarevic A, Yancheva D, Kocic G and Smelcerovic A. Deoxyribonuclease inhibitors. Eur J Med Chem. 2014;88:101–11. [DOI] [PubMed] [Google Scholar]
- 32.Price PA. The essential role of Ca2+ in the activity of bovine pancreatic deoxyribonuclease. J Biol Chem. 1975;250:1981–6. [PubMed] [Google Scholar]
- 33.Bajpai R, Lesperance J, Kim M and Terskikh AV. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Mol Reprod Dev. 2008;75:818–27. [DOI] [PubMed] [Google Scholar]
- 34.Robinson AP, Rodgers JM, Goings GE and Miller SD. Characterization of oligodendroglial populations in mouse demyelinating disease using flow cytometry: clues for MS pathogenesis. PLoS One. 2014;9:e107649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuji K, Ojima M, Otabe K, Horie M, Koga H, Sekiya I and Muneta T. Effects of Different Cell-Detaching Methods on the Viability and Cell Surface Antigen Expression of Synovial Mesenchymal Stem Cells. Cell Transplant. 2017;26:1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nayar S, Campos J, Steinthal N and Barone F. Tissue Digestion for Stromal Cell and Leukocyte Isolation. Methods Mol Biol. 2017;1591:225–234. [DOI] [PubMed] [Google Scholar]
- 37.Tennant JR. Evaluation of the Trypan Blue Technique for Determination of Cell Viability. Transplantation. 1964;2:685–94. [DOI] [PubMed] [Google Scholar]
- 38.Reichard A, Wanner N, Stuehr E, Alemagno M, Weiss K, Queisser K, Erzurum S and Asosingh K. Quantification of airway fibrosis in asthma by flow cytometry. Cytometry A. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyler CJ, Perez-Jeldres T, Ehinger E, Capaldo B, Karuppuchamy T, Boyer JD, Patel D, Dulai P, Boland BS, Lannigan J, Eckmann L, Ernst PB, Sandborn WJ, Ho SB and Rivera-Nieves J. Implementation of Mass Cytometry as a Tool for Mechanism of Action Studies in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folley SJ and Watson SC. A high-speed tissue homogenizer. Biochem J. 1948;42:204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moiseeva EP, Straatman KR, Leyland ML and Bradding P. CADM1 controls actin cytoskeleton assembly and regulates extracellular matrix adhesion in human mast cells. PLoS One. 2014;9:e85980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majka SM, Miller HL, Helm KM, Acosta AS, Childs CR, Kong R and Klemm DJ. Analysis and isolation of adipocytes by flow cytometry. Methods Enzymol. 2014;537:281–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis AS, Forrester JV, McInnes C and Lawrie F. Adhesion of cells to polystyrene surfaces. J Cell Biol. 1983;97:1500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin Y, Mitson-Salazar A and Prussin C. Detection of Intracellular Cytokines by Flow Cytometry. Curr Protoc Immunol. 2015;110:6 24 1–18. [DOI] [PubMed] [Google Scholar]
- 45.Richardson GM, Lannigan J and Macara IG. Does FACS perturb gene expression? Cytometry A. 2015;87:166–75. [DOI] [PubMed] [Google Scholar]
- 46.Krutzik PO, Trejo A, Schulz KR and Nolan GP. Phospho flow cytometry methods for the analysis of kinase signaling in cell lines and primary human blood samples. Methods Mol Biol. 2011;699:179–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.