Abstract
Background:
Neonatal dynamic tracheal collapse (tracheomalacia, TM) is a common and serious comorbidity in infants, particularly those with chronic lung disease of prematurity (bronchopulmonary dysplasia, BPD) or congenital airway or lung-related conditions such as congenital diaphragmatic hernia (CDH), but the underlying pathology, impact on clinical outcomes, and response to therapy are not well understood. There is a pressing clinical need for an accurate, objective, and safe assessment of neonatal TM.
Purpose:
To use retrospectively respiratory-gated ultrashort echo-time (UTE) MRI to non-invasively analyze moving tracheal anatomy for regional, quantitative evaluation of dynamic airway collapse in quiet-breathing, non-sedated neonates.
Study Type:
Prospective.
Population/Subjects:
27 neonatal subjects with varying respiratory morbidities (control, BPD, CDH, abnormal polysomnogram)
Field Strength/Sequence:
High-resolution 3D radial UTE MRI (0.7 mm isotropic) on NICU-sited 1.5T scanner.
Assessment:
Images were retrospectively respiratory-gated using the motion-modulated time-course of the k-space center. Tracheal surfaces were generated from segmentations of end-expiration/inspiration images and analyzed geometrically along the tracheal length to calculate percent-change in luminal cross-sectional area (A%) and ratio of minor-to-major diameters at end-expiration (rD,exp). Geometric results were compared to clinically-available bronchoscopic findings (N=14).
Statistical Tests:
Two-sample t-test.
Results:
Maximum A% significantly identified subjects with/without bronchoscopic TM diagnosis (with: 46.9±10.0%; without: 27.0±5.8%; P<0.001), as did minimum rD,exp (with: 0.346±0.146; without: 0.671±0.218; P=0.008). Subjects with severe BPD exhibited a far larger range of minimum rD,exp than subjects with mild/moderate BPD or controls (0.631±0.222, 0.782±0.075, and 0.776±0.030, respectively), while minimum rD,exp was reduced in CDH subjects (0.331±0.171) compared to controls (P<0.001).
Data Conclusion:
Respiratory-gated UTE MRI can quantitatively and safely evaluate neonatal dynamic tracheal collapse, as validated with the clinical standard of bronchoscopy, without requiring invasive procedures, anesthesia, or ionizing radiation.
Keywords: neonatal, dynamic airway collapse, tracheomalacia, airway MRI, respiratory-gated imaging
Introduction:
Excessive dynamic tracheal collapse (tracheomalacia, TM) is a common complication in infants born prematurely or with congenital respiratory disorders (1–3), due to immature or abnormal structure of the airway tissue and airway damage induced by positive pressure ventilation (4). Two mechanisms may contribute to this airway narrowing: weakened tissue of the airway wall (malacia) (5), and increased pleural pressures acting on the airway wall because of parenchymal lung abnormalities (6). However, the underlying pathology, impact on clinical outcome, and response to therapy are not well understood. Infants with TM often face longer hospitalizations and increased medical comorbidities due to increased airway compliance during respiration, as well as higher rates of tracheostomy and increased needs for respiratory support at discharge (2, 3, 7). TM is often comorbid with other lung abnormalities, such as bronchopulmonary dysplasia (BPD) and esophageal atresia/tracheoesophageal fistula (EA/TEF), and has been seen in congenital diaphragmatic hernia (CDH), but the impact of airway issues on overall pulmonary disease severity is not well appreciated, with little clinical ability to differentiate between the contributions of lung and airway morbidities and therefore to devise personalized treatment strategies (8).
Bronchoscopy is the clinical standard for diagnosing presence and degree of airway collapse, but it has multiple limitations: poor inter-reader reliability in children (9); inconsistencies between rigid and flexible scopes (10); lack of quantitative evaluation (11); and invasiveness, which not only increases risk to patients but can alter the dynamics of the anatomy and airflow within. Bronchoscopic readings currently lack a standardized definition for the range of collapse severity, with no established baseline for reference, in part because of measurement artifacts and biases from the varying depths of view of anatomy on bronchoscopic images, which are cumbersome to correct (11). Computed tomography (CT) can be used to visualize collapse but has a time resolution limited by gantry rotation. CT also often requires endotracheal intubation in neonates, and even with recent developments in low-dose protocols, there are concerns over the ionizing radiation exposure from CT, especially in infants and young children (12). Furthermore, both bronchoscopy and CT typically require anesthesia, which also can affect the trachealis and obscure the airway dynamics under evaluation (13, 14).
As a non-ionizing modality, MRI is an ideal diagnostic technique to address this clinical gap, particularly for longitudinal monitoring of disease and treatment efficacy in pediatrics, and also allows for population studies that include patients for whom there is no clinical indication for invasive airway evaluation. Recent developments in pulmonary MRI have yielded 3D high-resolution images of lung parenchyma using ultrashort echo-time (UTE) sequences, with good signal-to-noise contrast in soft and lung tissue (SNR ≈ 20 and 10 in neonates, respectively) and sub-millimeter resolution comparable to that of CT (15–19). The radial k-space acquisition of UTE sequences can yield retrospective motion-tracking (20–22), which has been used in non-sedated infants both for discarding of data acquired during bulk-motion and also for respiratory-gating of lung images for measuring tidal volumes and diaphragmatic function (21–23). However, this retrospective respiratory-gating technique has yet to be applied to airway dynamics throughout the respiratory cycle. Standard cine MRI acquisitions do not yield sufficient temporal and 3D spatial resolution in this very small, rapidly breathing neonatal population (typical tracheal cross-sectional areas of ~15 mm2 and respiratory rates of ~1 Hz), thus necessitating a more novel respiratory-gating method, such as that available with UTE MRI.
In this study, we demonstrate 3D MR images of the dynamic neonatal trachea and develop a novel method for non-invasive, quantitative, regional assessment of neonatal TM under natural, restful breathing conditions via retrospectively respiratory-gated UTE MRI. This technique does not require anesthesia or ionizing radiation and compares well to clinical bronchoscopic findings.
Methods:
Study subjects
This study cohort included 27 neonatal patients (15M/12F) recruited from the neonatal intensive care unit (NICU) at Cincinnati Children’s Hospital with a mean post-menstrual age (PMA) at MRI of 39 ± 3 weeks. All study procedures were performed with Institutional Review Board (IRB) approval and informed parental consent. Subjects’ clinical diagnoses were: BPD (N = 17 [6 mild/moderate, 11 severe]); CDH (N = 3 [1 pre-repair, 2 post-repair]); abnormal polysomnogram (PSG, a sleep study) with prematurity but without diagnosis of BPD (N = 1); and controls with no suspected respiratory abnormalities (e.g., non-respiratory diagnoses of gastroschisis or abdominal mass) (N = 6).
A subset of subjects (N = 14) underwent bronchoscopy as part of their routine clinical care (10 BPD, 3 CDH, and 1 abnormal PSG). The mean interval between bronchoscopy and MRI procedures was 16 ± 16 days, with an interval of ≤7 days for 8 of the 14 subjects. 6 subjects from this subset were clinically diagnosed with TM, while 8 were diagnosed with no TM.
Subjects were fed, swaddled, and equipped with standard hearing protection prior to imaging, with continuous monitoring of heart rates and SpO2 levels performed by NICU staff throughout the MRI exam. Subjects were restfully breathing during scanning and were not sedated specifically for the research exam, although sedation may have been administered as part of ongoing clinical care (N = 11). Subjects who clinically required respiratory support were maintained on their supplemental oxygen or MR-compatible mechanical ventilator throughout the MR exam.
MRI Acquisition and Retrospective Respiratory Gating
MRI was acquired on a neonatal-sized 1.5T scanner (orthopedic scanner hardware from ONI Medical Systems, Wilmington, MA; currently adapted to run software from GE Healthcare, Waukesha, WI) sited within the NICU at Cincinnati Children’s Hospital (21, 24–26). This scanner features an ~18-cm bore with quadrature body coil inserted and can accommodate infants up to ~4.5 kg. MR images were acquired with a 3D radial UTE sequence adapted for neonatal subjects (17, 18). Typical UTE parameters were: repetition time (TR) ≈ 5 ms, echo time (TE) ≈ 200 μs, flip angle (FA) = 5°, field of view (FOV) = 18 cm, number of radial projections ≈ 200,000, 3D isotropic voxel resolution ≈ 0.7 mm, and scan time ≈ 16 min.
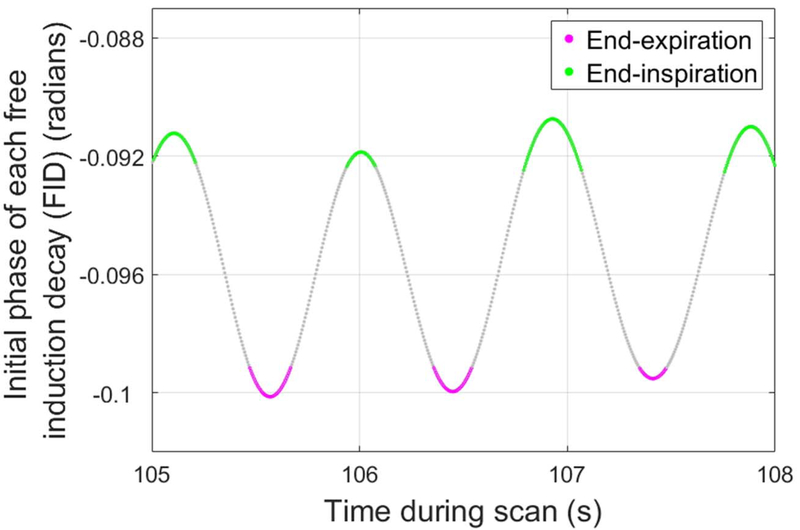
Using previously published methods (21), a retrospective motion-tracking and respiratory-gating waveform was generated from the time-course of the first point on each UTE free induction decay (FID), i.e. the center of k-space (k0), which is modulated by both bulk motion events and quiescent breathing. Data acquired during periods of bulk motion were semi-automatically identified and discarded, while remaining quiescent data were assigned to bins (end-inspiration, end-expiration, or mid-breath) using a sliding-window binning algorithm in MATLAB (The MathWorks, Inc., Natick, Massachusetts) (21). The end-inspiratory and end-expiratory bins each consisted of data acquired over intervals at end-inspiration and end-expiration, respectively, with each bin representing 12.5% of the duration of the respiratory cycle (Figure 1). Bin sizes of 12.5% were chosen as a trade-off between signal-to-noise and temporal gating resolution. The mid-breath data was not used for subsequent analysis in this study. Respiratory-binned data were then used to reconstruct cine-like respiratory-gated images showing the airway anatomy at the end-inspiratory and end-expiratory phases of the respiratory cycle (Figure 2). For reconstruction, iteratively estimated sampling density compensation weights were applied to undersampled, non-uniformly sampled k-space, which was then interpolated onto a Cartesian grid and transformed via inverse Fourier transform into the MR images (17).
Figure 1.
A representative respiratory waveform using the initial phase of each 3D radial free induction decay (FID) from a neonatal ultrashort echo-time (UTE) MRI scan, shown over a 3 s duration, with a temporal resolution of 5 ms. Data acquired during the 12.5% of the respiratory cycle associated with endexpiration (pink) and end-inspiration (green) were binned to reconstruct respiratory-gated UTE MR images.
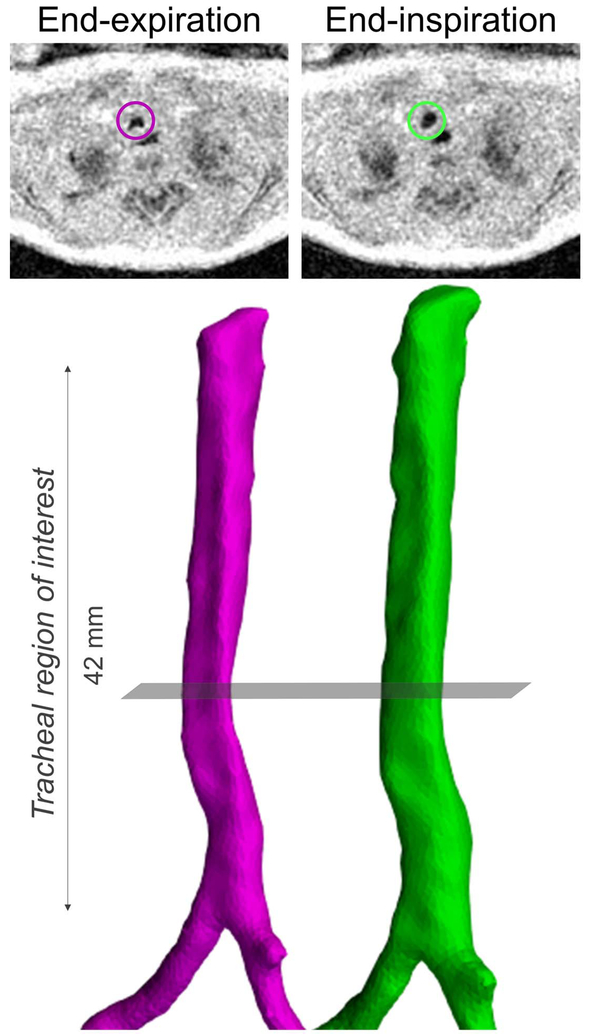
Figure 2.
Tracheal surface renderings created from segmentations from retrospective respiratory-gated ultrashort echo-time (UTE) MRI (pink - end-expiration; green - end-inspiration) in a neonatal subject with congenital diaphragmatic hernia (CDH). Axial MR images shown above each airway rendering correspond to the same part of the respiratory cycle (end-expiration or end-inspiration) and are at the level of the grey plane. In both the MR images and airway renderings, the regional collapse of the trachea occurring at endexpirationis clearly visible.
Image Analysis
End-expiration and end-inspiration tracheal anatomy was semi-automatically segmented from respiratory-gated UTE MR images using the user-guided 3D active contour segmentation technique described by Yushkevich et al. (27) and implemented in ITK-SNAP (3.6.0, Penn Image Computing and Science Laboratory, USA) based on an initial thresholding at an image intensity halfway between that found in the tracheal lumen and the tissue surrounding the airway. From this segmentation, a virtual airway surface was produced, also in ITK-SNAP (Figure 2) . A center-line was defined through the virtual airway surface created from each respiratory-gated image via VMTK 1.2 (Orobix, Bergamo, Italy) (Figure 3) (28). A center-line was used because it most closely followed the path of air through the trachea. Furthermore, subjects’ tracheas were typically not aligned with any of the imaging planes, so the center-line accounted for both airway curvature and axis misalignment in order to avoid errors in area and shape of the airway lumen. A series of luminal cross-sections bounded by the virtual tracheal surface and orthogonal to the center-line were defined at 1-mm intervals using in-house MATLAB software (Figure 3).
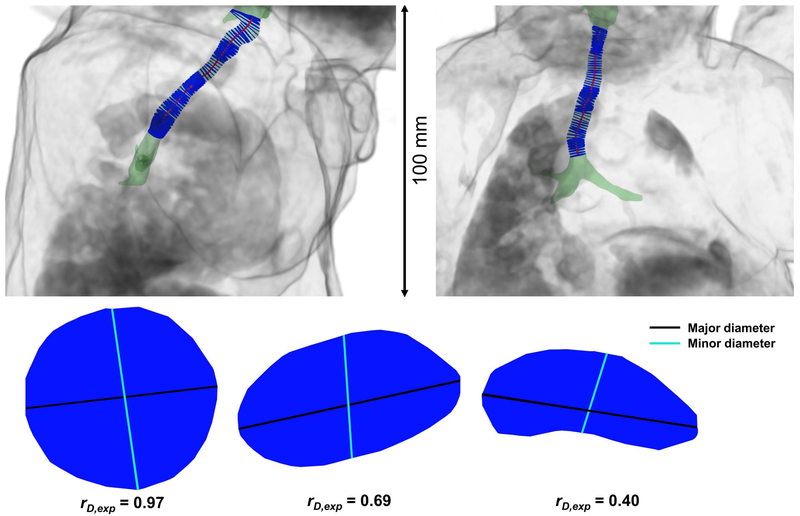
Figure 3.
A neonatal airway surface rendering (green) shown within a body volume rendering (grey) from a neonatal ultrashort echo-time (UTE) MRI scan, retrospectively respiratory-gated to end-inspiration. A center-line is defined along the airway (red), with a series of luminal cross-sections (blue) bounded by the airway and orthogonal to the center-line. Sagittal and coronal views are shown at left and right, respectively. Note that this subject’s trachea is not aligned with any of the imaging planes, demonstrating the need to define a tracheal center-line for accurate cross-sections of the airway lumen. Three example luminal cross-sections are shown below, demonstrating three different values of rD,exp, with descending values from left to right. The major diameters are shown in black, and the minor diameters are shown in cyan.
The area of each luminal cross-section (CSA) was calculated along the length of the trachea at both end-expiration and end-inspiration, with collapse reduction percentage (A%) defined here as:
such that A% = 0% with no dynamic collapse and A% = 100% with complete dynamic collapse at end-expiration. The maximum value of A% along the trachea (cricoid cartilage to carina, as identified from MR images by consultant radiologist) was also determined for each subject.
Two diameters were calculated for each luminal cross-section at end-expiration. The major diameter was defined as the distance between the two points which are furthest apart on the perimeter of the luminal cross-section. The minor diameter was defined as the distance between the two points on the luminal cross-section perimeter which also lie on a line 90° to the major diameter at its midpoint. Finally, the ratio of minor-to-major diameters at end-expiration, rD,exp, was calculated as an indicator of the shape of the tracheal cross-section. A perfectly round trachea would yield a ratio of rD,exp = 1, with the value of rD,exp decreasing toward zero in less round tracheal sections (Figure 3). The minimum value of rD,exp along the trachea (cricoid cartilage to carina) was also determined for each subject.
A two-sample t-test was used to compare geometric MRI results (maximum A% and minimum rD,exp) to clinical diagnoses of respiratory morbidity (control, mild/moderate BPD, severe BPD, CDH, and abnormal PSG) and to bronchoscopic findings (TM or no TM). P < 0.05 was considered to indicate statistical significance
Results:
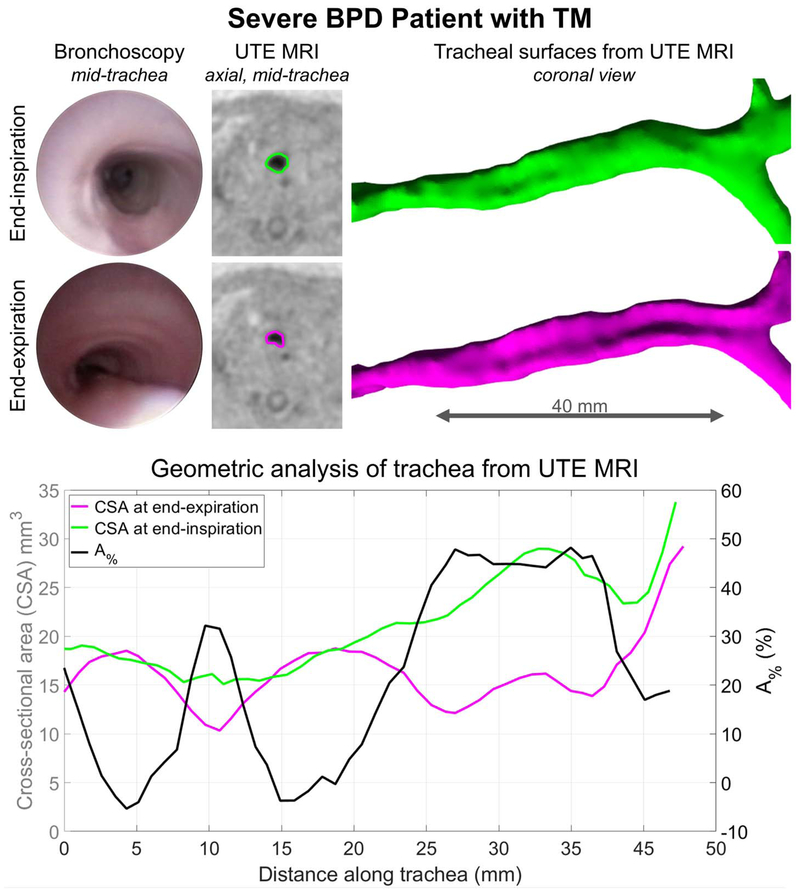
Representative MRI results from one subject with diagnosed with severe BPD and TM are presented in Figure 4, with qualitative comparison to clinical bronchoscopy. MRI-based geometric analysis of this subject’s trachea demonstrated dynamic collapse in the middle and lower trachea. These results regionally agree with qualitative findings from clinical bronchoscopy.
Figure 4.
Representative MRI results for a neonatal patient with severe bronchopulmonary dysplasia (BPD) and bronchoscopically-diagnosed tracheomalacia (TM). Collapse of the posterior tracheal wall at endexpiration is clear on both clinically-obtained bronchoscopic images and retrospectively respiratory-gated ultrashort echo-time (UTE) MRI results. The tracheal lumens are outlined on end-expiration and endinspiration UTE axial images in pink and green, respectively. Gated UTE-based tracheal surfaces are shown, along with corresponding geometric analysis along the intrathoracic trachea: cross-sectional areas (CSA) at end-expiration (pink) and end-inspiration (green); and percentage reduction in CSA (A%, black). Quantitative MRI results of dynamic collapse, particularly notable throughout the middle and lower tracheal regions, agree with qualitative bronchoscopic results.
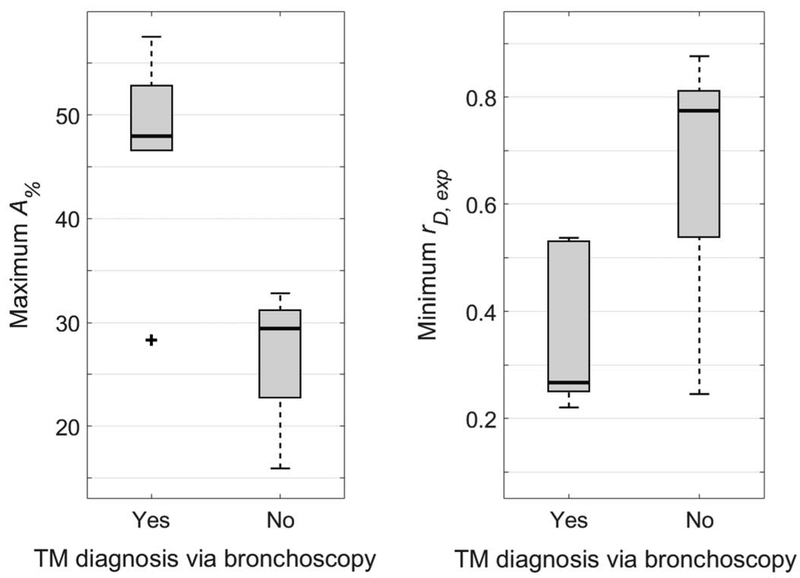
Figure 5 shows the distribution of maximum A% values (left) and minimum rD,exp values (right) for subjects who had either positive or negative TM findings via bronchoscopy (maximum A% = 46.9 ± 10.0% and minimum rD,exp = 0.346 ± 0.146 for positive TM findings; maximum A% = 27.0 ± 5.8% and minimum rD,exp = 0.671 ± 0.218 for negative TM findings). Maximum A% was found to significantly distinguish between these two groups (P < 0.001), as did minimum rD,exp (P = 0.008).
Figure 5.
Comparison of maximum tracheal cross-sectional area reduction percentage between endexpiration and end-inspiration (A% - left) and minimum ratio of tracheal minor-to-major diameter at endexpiration (rD,exp - right) to positive or negative diagnosis of tracheomalacia (TM) via clinical bronchoscopy. Both measures were found to correlate with clinical diagnosis of TM (P < 0.001 and P = 0.008 for A% and rD,exp, respectively). Plot elements are represented as follows: median (black line), interquartile range (grey box), data within 1.5 times the interquartile range below 25% and 75% (black whiskers), and data outside this range (black cross).
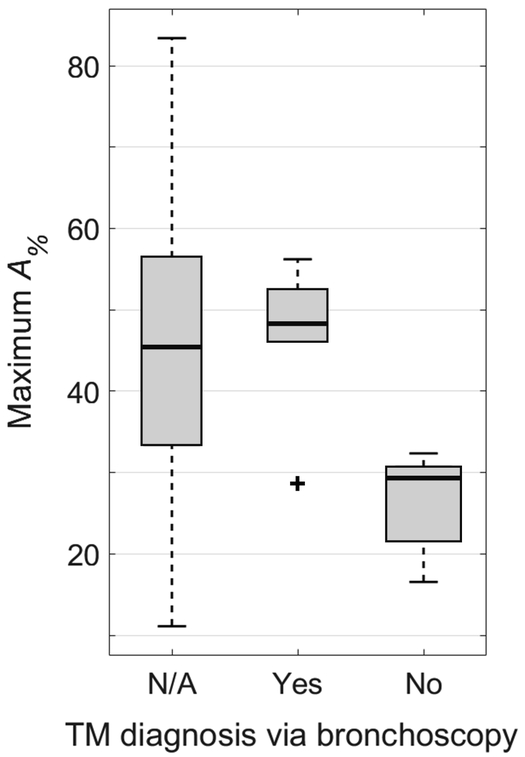
Figure 6 shows the distribution of maximum A% values for those subjects who did not receive a clinical bronchoscopy (maximum A% = 43.6 ± 19.4%). There is a very large range for this subject group, with clear overlap between this group and groups that had positive and negative TM findings via bronchoscopy. There is no significant difference between this group without bronchoscopy compared to the group with positive TM findings via bronchoscopy (P = 0.707), and there is a significant difference between this group without bronchoscopy and the group with negative TM findings (P = 0.032).
Figure 6.
Comparison of maximum tracheal cross-sectional area reduction percentage between endexpiration and end-inspiration (A%) between subjects with positive findings of TM via clinical bronchoscopy, subjects with negative findings of TM via clinical bronchoscopy, and subjects who did not undergo a clinical bronchoscopy (Yes, No, and N/A groups, respectively). There is a statistically significant difference between the No and N/A groups (P = 0.032) and no statistically significant difference between the Yes and N/A groups (P = 0.707). The range of A% in the N/A group spans across all values associated with both positive and negative findings of TM, suggesting that several patients may have undiagnosed TM and would stand to benefit from tracheal collapse evaluation. Plot elements are represented as follows: median (black line), interquartile range (grey box), data within 1.5 times the interquartile range below 25% and 75% (black whiskers), and data outside this range (black cross).
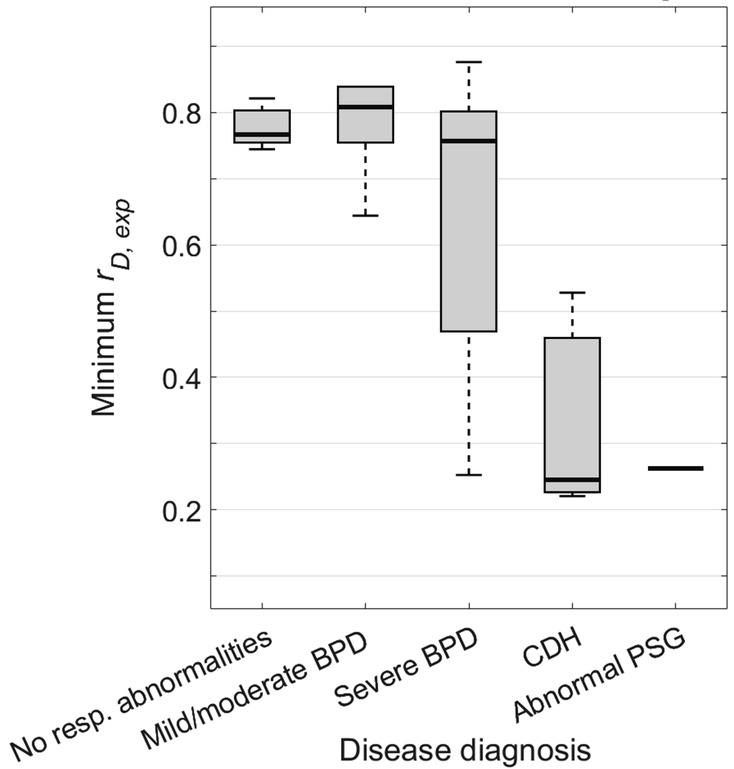
Figure 7 shows the distribution of minimum rD,exp for the various respiratory morbidity groups: minimum rD,exp = 0.776 ± 0.030 for controls, 0.782 ± 0.075 for mild/moderate BPD, 0.631 ± 0.222 for severe BPD, 0.331 ± 0.171 for CDH, and 0.264 ± 0 for abnormal PSG. This measure did not significantly distinguish between subjects with mild/moderate BPD and control subjects (P = 0.859). Subjects with severe BPD exhibited a far greater range of minimum rD,exp than subjects with control and mild/moderate BPD subjects, with a significant difference in comparing the severe BPD group to a combined control and mild/moderate BPD group (P = 0.036), although without significant differences in comparing the severe BPD group to separated control and mild/moderate groups (P = 0.138 and 0.132, respectively). Values of minimum rD,exp were significantly lower for subjects with CDH compared with control subjects (P < 0.001).
Figure 7.
Comparison of minimum ratio of tracheal minor-to-major diameter at end-expiration (rD,exp) to subjects’ respiratory morbidity group. This measure did not statistically distinguish between subjects with mild/moderate BPD and control subjects (P = 0.859). Subjects with severe BPD have a larger range of minimum rD,exp than control subjects with no respiratory abnormalities and subjects with mild/moderate BPD, though without statistically significant separation (P = 0.138 and 0.132), suggesting that airway disease may be comorbid with lung disease in some but not all severe BPD patients. However, there is a statistically significant difference between the severe BPD group and a combined control and mild/moderate BPD group (P = 0.036). Subjects with CDH also have a lower minimum rD,exp value that is significantly different from control subjects (P < 0.001). Plot elements are represented as follows: median (black line), interquartile range (grey box), and data within 1.5 times the interquartile range below 25% and 75% (black whiskers).
Discussion:
Geometric analysis of retrospectively respiratory-gated UTE MRI provides an innovative new platform for quantitative and regional assessment of dynamic tracheal morbidities in neonates while they breathe in their natural, restful state. This method does not require anesthesia or invasive procedures, which can alter the aerodynamics of collapse, nor does it require ionizing radiation, which is of particular concern in pediatric imaging. Therefore, this technique is suitable for longitudinally monitoring airway disease trajectory during neonatal development or in response to therapy.
MRI-based evaluations of dynamic tracheal collapse compare well to clinical bronchoscopy. While rigorous validation with bronchoscopic findings is required to establish accuracy, this technique overcomes the current barriers associated with existing diagnostic standards, yielding objective and quantitative neonatal airway biomarkers currently unavailable through any other method. Furthermore, this technique poses no additional risks to patients and so offers a unique opportunity for serial assessment to guide use of pharmacologic therapy and response to surgical interventions for airway collapse. For example, neonatal patients with severe TM may undergo aortopexy or posterior tracheopexy (29), with no current non-invasive or non-ionizing option for assessing tracheal dynamics before and after surgery. Additionally, Panitch et al. (30) demonstrated in a small cohort that cholinergic agents can improve respiratory mechanics in infants with TM, suggesting that stimulation of the trachealis may increase tracheal stability; however, treatment with albuterol caused a decrease in peak expiratory flow. The airway MRI technique proposed in this work allows for safe quantification of interventional efficacy for dynamic neonatal airway collapse in broad cross-sectional and longitudinal studies.
The results show that two measures generated by this method, maximum A% and minimum rD,exp at end-expiration, both produce significant agreement with diagnosis of TM with clinical bronchoscopy. An advantage of using rD,exp over A% is that it does not require images to be analyzed at both end-inspiration and end-expiration, meaning it may be achievable with traditional plethysmographic respiratory gating, rather than the retrospective approach detailed here, and therefore may be more widely available in a clinical setting.
The results show that the range of minimum rD,exp values in subjects with severe BPD is far greater than control subjects or those with mild/moderate BPD. This result has two potential implications. The first is that there are potentially different phenotypes of severe BPD, specifically those whose respiratory symptoms are elevated due to tracheal collapse, compared with those whose issues are largely due to diseased parenchymal tissue. These phenotypes would account for the large range of minimum rD,exp for subjects with severe BPD, but also the similar means between this group and those with less severe symptoms. A second possibility is that some patients have to exert more effort to inhale and exhale, creating a larger range of pleural pressures and therefore subjecting the trachea to a larger range of transmural pressure forces, which may cause an exaggerated range of tracheal motion despite a normal trachea.
In order to investigate these possibilities in each patient, the internal pressure forces that act on the tracheal walls and the effect that tracheal motion has on subjects’ breathing effort can be calculated through computational fluid dynamics (CFD) simulations. Previously, CFD simulations have been used to calculate the increased tracheal work of breathing in adult subjects with goiters (31, 32) and a transplanted trachea (33). These simulations rely on high-resolution imaging to provide the anatomical boundaries of the airway structure, and there is increasing recognition that the accuracy of CFD simulations suffers when airway wall motion is ignored and simplified into a static model. (34, 35)
While the present work utilized UTE MRI data acquired only during end-expiratory and end-inspiratory stages of the respiratory cycle, the single UTE scan for each subject also acquired data in between these two stages, i.e. during inhalation and exhalation. Future studies could use this additional data to infer further information about the trachea at other respiratory stages, such as the duration of tracheal narrowing and how collapse metrics relate to respiratory flow rates or pleural pressures. Moving airway wall CFD simulations could utilize data acquired throughout the breath in order to capture the necessary tracheal motion for boundary conditions, as has been done previously on real-time cine imaging (34). Therefore, the techniques detailed here can generate respiratory-gated image reconstructions throughout the whole breathing cycle at no additional data acquisition cost. Thus, the novel combination of this study’s MRI-based measurements of the moving airway with CFD applications has high potential for yielding measurements of respiratory effort and energy expenditure related to airway collapse in neonates with tracheal abnormalities.
UTE MRI has generally been used to image species with very short T2* values, such as lung or bone tissue. However, in the development of this moving airway imaging technique, we were interested in achieving high 3D spatial resolution and retrospective respiratory gating using k0, both of which can be provided by 3D UTE MRI. Previous work has performed similar gating via k0 using conventional 3D Cartesian sequences (36), but these methods are often implemented with a slice thickness several times greater than the in-plane resolution and so are prohibitive for imaging small 3D structures. Additionally, scan times from gated Cartesian acquisitions are typically extended due to the insertion of a k0 navigator module into each repetition and the multiple acquisitions necessary to fully encode the 3D data set (36). In the context of imaging patients with comorbid lung and airway abnormalities, 3D UTE MRI with respiratory gating is an advantageous choice; the 3D FOV of UTE is large enough to simultaneously acquire lung and airway data from the same scan, such that there is potential to evaluate multiple aspects of a patient’s respiratory system from a single scan (19).
Radial zero echo-time (ZTE) MRI has previously been used in lung imaging (37) and has the advantage of being virtually silent. However, ZTE acquisitions inherently miss the center of k-space (38), with this data gap typically addressed via acquisition oversampling or algebraic reconstruction. As such, ZTE techniques are not sensitive to the respiratory-related variation of k0 as a function of time throughout the scan. Given the proper hearing protection, scanner noise is not likely to be a concern in applying MRI to the infant population (39), and thus UTE MRI, while not acoustically silent, is still preferred over ZTE in respiratory gating applications.
This respiratory-gated UTE MRI technique for evaluating neonatal dynamic tracheal collapse could be implemented at many institutions. While this study was performed using a unique neonatal-sized MRI scanner operating with GE software, this technique does not require a specialized MRI system, and 3D radial sequences are becoming more accessible on all major MR platforms. Furthermore, these MR acquisitions can be performed on any conventional adult-sized scanner with appropriate coils for neonatal airway imaging, and the respiratory gating is a purely software-based method so does not require specialized gating hardware. The location of the NICU-sited scanner used in this study is logistically advantageous due to the proximity to NICU staff and resources but is not necessary for imaging infants.
Clinical bronchoscopy is not common in the neonatal population, and these MRI-based results suggest that many subjects who do not currently undergo bronchoscopic evaluation have a significant degree of collapse seen on MRI and may have undiagnosed TM. Neonatal MRI is an emerging modality, and since the proposed MRI method poses fewer risks than bronchoscopy, the present technique may be feasible on a broad scale. Patients with potentially undiagnosed TM may see great benefit with this technique since they would not otherwise receive any assessment of tracheal collapse.
The limitations of this study include the MR image resolution; although comparable to CT, the isotropic resolution of 0.7 mm means cross-sectional areas can only be measured to the nearest 0.49 mm2. The segmentation process may also be a source of error, as the tissue-lumen boundary at the edge of the trachea may be blurred due to partial volume effects and/or anatomical motion occurring on timescales shorter than the temporal resolution of the retrospective gating (12.5% of the duration of a breath). The retrospective gating provides an average representation of the airway shape throughout the respiratory cycle over a period of approximately 16 minutes, and therefore tracheal motion and collapses which do not occur on typical breaths are not captured and may instead cause image blurring. Recently reported image reconstruction methods that maintain signal-to-noise ratio with higher temporal resolution at potentially shorter scan times can address some of these limitations (40). Additionally, the comparison between MRI and bronchoscopy results may be affected by the interval between the two procedures (mean interval of 16 ± 16 days). Due to the lack of options for non-invasive serial monitoring, there is not yet a clear understanding of the change in tracheal dynamics over time in the neonatal population. As such, this MRI-based method offers a unique opportunity to longitudinally monitor airway disease trajectories more closely than currently available clinical techniques.
In this study we have demonstrated an innovative, new approach for quantitative evaluation of neonatal dynamic tracheal collapse during quiet breathing using respiratory-gated UTE MRI, without requiring invasive procedures, anesthesia, or ionizing radiation. Looking forward, if regional and dynamic tracheal biomarkers from geometric analysis of airway MRI can be associated with clinical outcomes, such as length of hospital stay or need for tracheostomy, it would allow for direct translation of MRI to the clinical care of neonatal patients with airway complications. This may have impact for subjects with BPD, in whom the contributions of pulmonary and airway disease are not yet well characterized. Ultimately, this approach has the potential to significantly advance understanding and treatment of airway morbidities in this vulnerable neonatal population.
Acknowledgements:
The authors would like to thank the patients and families who participated in the study.
GRANT SUPPORT:
The authors thank The Perinatal Institute at Cincinnati Children’s Hospital, The Research Innovation and Pilot Funding Program at Cincinnati Children’s Hospital, The Hartwell Foundation at University of Wisconsin-Madison, The Departments of Radiology and Medical Physics and the School of Medicine and Public Health at University of Wisconsin-Madison, and NIH P01 HD093363 for research funding and support.
ABBREVIATION KEY
- A%:
percent reduction of tracheal cross-sectional area between end-expiration and end-inspiration
- BPD:
bronchopulmonary dysplasia
- CDH:
congenital diaphragmatic hernia
- CFD:
computational fluid dynamics
- CSA:
cross-sectional area
- CT:
X-ray computed tomography
- EA/TEF:
esophageal atresia/tracheoesophageal fistula
- FA:
flip angle
- FID:
free induction decay
- FOV:
field of view
- IRB:
Institutional Review Board
- k0:
k-space center
- MRI:
magnetic resonance imaging
- NICU:
neonatal intensive care unit
- PMA:
post-menstrual age
- PSG:
polysomnogram (sleep study)
- RF:
radio frequency
- rD,exp:
ratio of minor-to-major tracheal diameters
- T:
tesla
- TE:
echo time
- TM:
tracheomalacia
- TR:
pulse repetition time
- UTE:
ultrashort echo-time
- ZTE:
zero echo-time
REFERENCES:
- 1.McCubbin M, Frey EE, Wagener JS, Tribby R, Smith WL: Large airway collapse in bronchopulmonary dysplasia. J Pediatr 1989; 114:304–307. [DOI] [PubMed] [Google Scholar]
- 2.Hysinger EB, Friedman NL, Padula MA, et al. : Tracheobronchomalacia is associated with increased morbidity in bronchopulmonary dysplasia. Ann Am Thorac Soc 2017; 14:1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hysinger EB, Panitch HB: Paediatric Tracheomalacia. Paediatr Respir Rev 2016:9–15. [DOI] [PubMed] [Google Scholar]
- 4.Bhutani VK, Rubenstein D, Shaffer TH: Pressure-induced deformation in immature airways. Pediatr Res 1981; 15:829–832. [PubMed] [Google Scholar]
- 5.Carden KA, Boiselle PM, Waltz DA, Ernst A: Tracheomalacia and Tracheobronchomalacia in Children and Adults. Chest 2018; 127:984–1005. [DOI] [PubMed] [Google Scholar]
- 6.Loring SH, O’Donnell CR, Behazin N, et al. : Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol 2010; 108:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter JD, Dunbar JS: Tracheomalacia. Ann Otol Rhinol Laryngol 1963:1013:23. [DOI] [PubMed] [Google Scholar]
- 8.Collaco JM, McGrath-Morrow SA: Respiratory Phenotypes for Preterm Infants, Children, and Adults: Bronchopulmonary Dysplasia and More. Ann Am Thorac Soc 2018:AnnalsATS.201709–756FR. [DOI] [PubMed] [Google Scholar]
- 9.Burg G, Hysinger E, Hossain M, Wood R: Evaluation of Agreement on Presence and Severity of Tracheobronchomalacia in Pediatric Patients by Dynamic Flexible Bronchoscopy. In Proc Am Thorac Soc; . [DOI] [PubMed] [Google Scholar]
- 10.Wood RE, Postma D: Endoscopy of the airway in infants and children. J Pediatr 1988; 112:1–6. [DOI] [PubMed] [Google Scholar]
- 11.Masters IB, Eastburn MM, Wootton R, et al. : A new method for objective identification and measurement of airway lumen in paediatric flexible videobronchoscopy. Thorax 2005; 60:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miglioretti DL, Johnson E, Williams A, et al. : The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 2013; 167:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirota K, Sato T, Hashimoto Y, et al. : Relaxant effect of propofol on the airway in dogs. Br J Anaesth 1999; 83:292–295. [DOI] [PubMed] [Google Scholar]
- 14.Hillman DR, Walsh JH, Maddison KJ, et al. : Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology 2009; 111:63–71. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KM, Fain SB, Schiebler ML, Nagle S: Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med 2013; 70:1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lederlin M, Crémillieux Y: Three-dimensional assessment of lung tissue density using a clinical ultrashort echo time at 3 tesla: A feasibility study in healthy subjects. J Magn Reson Imaging 2013; 40:839–847. [DOI] [PubMed] [Google Scholar]
- 17.Hahn AD, Higano NS, Walkup LL, et al. : Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging 2017; 45:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higano NS, Fleck RJ, Spielberg DR, et al. : Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. J Magn Reson Imaging 2017; 46:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higano NS, Spielberg DR, Fleck RJ, et al. : Neonatal Pulmonary MRI of Bronchopulmonary Dysplasia Predicts Short-term Clinical Outcomes. Am J Respir Crit Care Med 2018:rccm.201711–2287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibiletti M, Paul J, Bianchi A, et al. : Multistage three-dimensional UTE lung imaging by image-based self-gating. Magn Reson Med 2016; 75:1324–1332. [DOI] [PubMed] [Google Scholar]
- 21.Higano NS, Hahn AD, Tkach JA, et al. : Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med 2017; 77:1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higano NS, Bates AJ, Tkach JA, et al. : Pre- and post-surgical repair visualization of neonatal esophageal atresia/tracheoesophageal fistula via magnetic resonance imaging: A case series. J Pediatr Surg Case Reports 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoder LM, Higano NS, Schapiro AH, et al. : Elevated Lung Volumes in Neonates with Bronchopulmonary Dysplasia Measured Via Free-Breathing MRI. In Proc Am Thorac Soc; 2018. [DOI] [PubMed] [Google Scholar]
- 24.Tkach JA, Hillman NH, Jobe AH, et al. : An MRI system for imaging neonates in the NICU: Initial feasibility study. Pediatr Radiol 2012; 42:1347–1356. [DOI] [PubMed] [Google Scholar]
- 25.Tkach JA, Merhar SL, Kline-Fath BM, et al. : MRI in the neonatal ICU: Initial experience using a small-footprint 1.5-T system. Am J Roentgenol 2014; 202. [DOI] [PubMed] [Google Scholar]
- 26.Merhar SL, Tkach JA, Woods JC, et al. : Neonatal imaging using an on-site small footprint MR scanner. Pediatr Radiol 2017; 47:1001–1011. [DOI] [PubMed] [Google Scholar]
- 27.Yushkevich PA, Piven J, Hazlett HC, et al. : User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006; 31:1116–1128. [DOI] [PubMed] [Google Scholar]
- 28.Piccinelli M, Veneziani A, Steinman DA, Remuzzi A, Antiga L: A framework for geometric analysis of vascular structures: Application to cerebral aneurysms. IEEE Trans Med Imaging 2009; 28:1141–1155. [DOI] [PubMed] [Google Scholar]
- 29.Shieh HF, Smithers CJ, Hamilton TE, et al. : Posterior tracheopexy for severe tracheomalacia. J Pediatr Surg 2017. [DOI] [PubMed] [Google Scholar]
- 30.Panitch HB, Keklikian EN, Motley RA, Wolfson MR, Schidlow DV.: Effect of altering smooth muscle tone on maximal expiratory flows in patients with tracheomalaci. Pediatr Pulmonol 1990. [DOI] [PubMed] [Google Scholar]
- 31.Bates AJ, Comerford A, Cetto R, Schroter RC, Tolley NS, Doorly DJ: Power loss mechanisms in pathological tracheas. J Biomech 2016; 49:2187–2192. [DOI] [PubMed] [Google Scholar]
- 32.Bates AJ, Cetto R, Doorly DJ, Schroter RC, Tolley NS, Comerford A: The effects of curvature and constriction on airflow and energy loss in pathological tracheas. Respir Physiol Neurobiol 2016; 234:69–78. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton NJ, Kanani M, Roebuck DJ, et al. : Tissue-Engineered Tracheal Replacement in a Child: A 4-Year Follow-Up Study. Am J Transplant 2015; 15:2750–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates AJ, Schuh A, Amine-Eddine G, et al. : Assessing the relationship between movement and airflow in the upper airway using computational fluid dynamics with motion determined from magnetic resonance imaging. Clin Biomech 2017; 0. [DOI] [PubMed] [Google Scholar]
- 35.Miyawaki S, Hoffman EA, Lin CL: Effect of static vs. dynamic imaging on particle transport in CT-based numerical models of human central airways. J Aerosol Sci 2016; 100:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weick S, Breuer FA, Ehses P, et al. : DC-gated high resolution three-dimensional lung imaging during free-breathing. J Magn Reson Imaging 2013. [DOI] [PubMed] [Google Scholar]
- 37.Gibiino F, Sacolick L, Menini A, Landini L, Wiesinger F: Free-breathing, zero-TE MR lung imaging. Magn Reson Mater Physics, Biol Med 2015. [DOI] [PubMed] [Google Scholar]
- 38.Weiger M, Pruessmann KP: MRI with Zero Echo Time. In Encycl Magn Reson; 2012. [DOI] [PubMed] [Google Scholar]
- 39.Tkach JA, Li Y, Pratt RG, et al. : Characterization of acoustic noise in a neonatal intensive care unit MRI system. Pediatr Radiol 2014; 44:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang W, Ong F, Johnson KM, et al. : Motion robust high resolution 3D free-breathing pulmonary MRI using dynamic 3D image self-navigator. Magn Reson Med 2018; 79:2954–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]