Abstract
Rationale:
Congenital heart disease can lead to life-threatening right ventricular heart failure (RVHF). Results from clinical trials support expanding cardiac progenitor cell (CPC) based therapies. However, our recent data show that CPCs lose function as they age, starting as early as 1 year.
Objective:
To determine whether the aggregation of child (1 to 5-year-old) CPCs into scaffold-free spheres can improve differentiation by enhancing Notch signaling, a known regulator of CPC fate. We hypothesized that aggregated (3D) CPCs will repair RVHF better than monolayer (2D) CPCs.
Methods and Results:
Spheres were produced with 1500 CPCs each using a microwell array. CPC aggregation significantly increased gene expression of Notch1 compared to 2D CPCs, accompanied by significant upregulation of cardiogenic transcription factors (GATA4, HAND1, MEF2C, NKX2.5, and TBX5) and endothelial markers (CD31, FLK1, FLT1, vWF). Blocking Notch receptor activation with the γ-secretase inhibitor N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) diminished these effects. To evaluate the therapeutic improvements of CPC aggregation, RVHF was induced in athymic rats by pulmonary artery banding and cells were implanted into the RV free wall. Echocardiographic measurements 28 days post-implantation showed significantly improved RV function with 3D compared to 2D CPCs. Tracking implanted CPCs via DiR-labeling showed improved retention of 3D CPCs. Transducing 3D CPCs with Notch1-shRNA did not reduce retention, but significantly reduced RV functional improvements. Histological analyses showed 3D treatment reduced RV fibrosis and increased angiogenesis. While 3D CPCs formed CD31+ vessel-like cells in vivo, these effects are more likely due to improved 3D CPC exosome function compared to 2D CPC exosomes.
Conclusion:
Spherical aggregation improves child CPC function in a Notch-dependent manner. The strong reparative ability of CPC spheres warrants further investigation as a treatment for pediatric HF, especially in older children where reparative ability may be reduced.
Keywords: Cardiac progenitor cell, Notch signaling, right ventricular heart failure, spherical aggregation, cellular transplantation, congenital cardiac defect
Subject Terms: Basic Science Research, Cell Therapy, Congenital Heart Disease, Heart Failure, Stem Cells
INTRODUCTION
Congenital heart disease (CHD) occurs in 1 in every 100 live births and has the highest mortality rate of all birth defects.1 Advances in surgical management, such as through the Single Ventricle Reconstruction Trial for hypoplastic left heart syndrome (HLHS), have been transformative, enabling babies born with previously fatal defects to enter early adulthood.2 However, right ventricular dysfunction remains a common problem in the clinical care of patients with CHD, contributing to considerable burden of disease.3, 4 Through the improved survival of CHD patients, an epidemic of RV heart failure (RVHF) has begun to emerge, necessitating the development of new regenerative medicine and stem cell-based therapies for the treatment of RVHF.
Cardiac progenitor cells (CPCs) were initially thought to repair the heart by contributing directly to cardiac turnover.5 More recent studies have suggested that indirect paracrine effects, particularly through the release of exosomes and reparative factors, play a larger role in CPC-mediated cardiac repair.6, 7 Nonetheless, clinical trials testing CPCs and cardiosphere-derived cells (CDCs) for ventricular dysfunction in both adults and pediatric populations have demonstrated safety, feasibility, and modest cardiac function improvement.8–10 However, stem cell therapies to improve cardiac function have been limited by low differentiation rates and many studies have shown that upwards of 90% of cells are lost within a few days post-transplantation.11–17 Most importantly, we have shown in a recent publication that CPCs lose their therapeutic functionality as they age. By as early as 1 year old (defined as child CPCs), CPCs have a reduced ability to improve heart function in a rat RVHF model, reduced proliferation rate, and are less effective at inducing cell migration and angiogenesis.18 Based on these limitations, current autologous stem-cell therapies for HF may not be as effective as they need to be.
The cardiac stem cell niche is complex and highly dynamic. Among other signaling processes, CPCs express Notch1 receptor to participate in bidirectional signaling with supporting cells expressing the Notch-activating ligand, Jagged1.19, 20 We and others have shown Notch1 signaling to be an important mediator of CPC cell fate.19–22 Replicating the microenvironment of stem cell niches by spherical aggregation has been shown to improve the differentiation of human embryonic stem cells, human induced pluripotent stem cells (hiPSCs), and mesenchymal stem cells (MSCs).23–27 Additionally, spheroids of cardiosphere-derived cells maintain greater differentiation potential.28 Based on these observations, we hypothesized that aggregating child CPCs into spheres may recapitulate signaling processes within the CSC niche, especially Notch1 signaling, that will improve child CPC therapeutic functionality. In this report, we tested the effect of spherical aggregation on Notch signaling and CPC differentiation. We then used a rat RVHF model to study the effect of CPC aggregation on cardiac repair.
METHODS
Due to the fact that this study involves data collected under IRB approval, the data that support the findings of this study are available from the corresponding author upon reasonable request at michael.davis@bme.emory.edu.
Human sample acquisition and isolation of human CPCs.
This study was approved by the Institutional Review Board at Children’s Healthcare of Atlanta and Emory University. Human c-Kit+ CPCs used in this study were isolated from right atrial (RA) appendage tissue routinely removed during surgical repair of congenital heart defects as previously described.18 Child CPCs are categorized as being isolated from patients aged 12 months to 5 years. Additional details about CPC donor patients are found in Supplemental Table I.
CPC culture and sphere formation.
Cells were used between passages 5 and 9. CPC spheres were formed by seeding 1500 cells per microwell in an AggreWell400 microwell array (Stemcell Technologies, Vancouver, BC, Canada) and culturing overnight. CPC spheres were cultured in rotary orbital suspension.
Animal experiments.
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee. Male adolescent (6 week old) athymic rats (Crl:NIH-Foxn1rnu) (~150 g) were obtained from Charles River Laboratories (Wilmington, MA). Nonischemic HF was induced by pulmonary artery banding (PAB) at 7–8 weeks old as previously described.18 Animals were randomized to treatment groups and 500,000 monolayer cultured CPCs (2D), or approximately 400 freshly formed (Day 0) CPC spheres (3D) totaling ~500,000 CPCs, were delivered two weeks after banding (Supplemental Figure I). To minimize potential differences between CPC donors, animals received injections containing equal parts of all child CPC populations. Cells were labeled with DiR (Thermo Fisher Scientific Life Sciences, Waltham, MA) per manufacturer’s protocol and cell retention was tracked using an IVIS Spectrum in vivo imager (Perkin Elmer, Waltham, MA). To evaluate RV function, echocardiography was performed on PAB and sham rats on the day of surgery, immediately after treatment 2 weeks later, and then every week thereafter up to 4 weeks after treatment. Data were collected and analyzed by blinded researchers. Additional details on randomization and blinding process are given in the Supplemental Methods. No animals were excluded from the analysis.
Conditioned media and exosome generation.
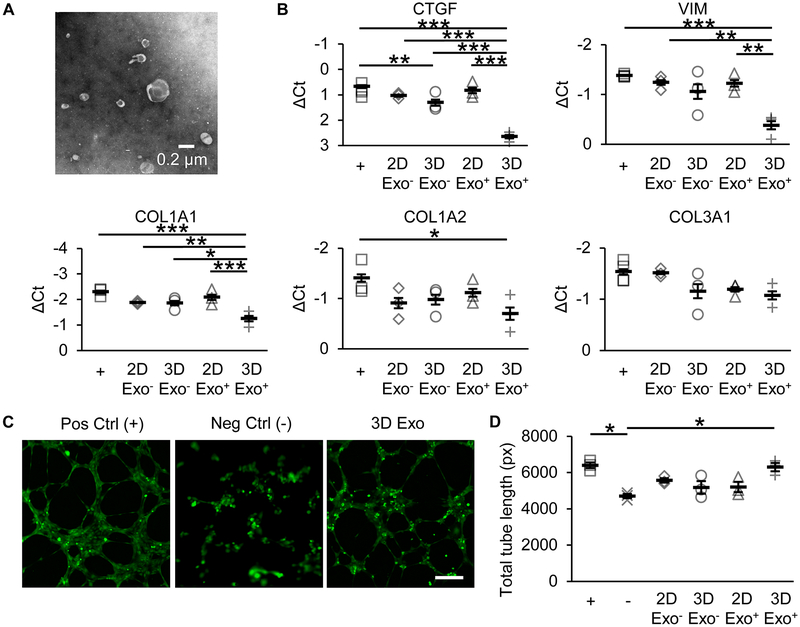
Exosomes were generated as previously described.29 Briefly, after 7 days in culture, 2D and 3D CPCs were quiesced and cultured in FBS-free media for 12 hours to generated conditioned media. Exosomes were generated from conditioned media by sequential ultracentrifugation (Optima XPN-100, Beckman Coulter SW32Ti rotor) at 10,000xg for 35 minutes to remove cell debris and then at 100,000xg for 70 minutes to concentrate exosomes. Successful exosome isolation was confirmed by electron microscopy (Figure 8A). Protein content of the conditioned media and exosome suspension were quantified by A280 measurements (NanoDrop 2000).
Figure 8. Exosomes from aggregated CPCs reduce fibrotic gene expression and improve tube formation.
(A) Representative images of exosomes isolated from CPC conditioned media by ultracentrifugation. (B) Fibrotic gene expression in TGF-β stimulated rat cardiac fibroblasts after treatment with 2D or 3D exosome-free conditioned media (Exo−) or purified exosomes (Exo+). N=3 CPC populations. *p≤0.05, **p≤0.01, ***p≤0.001 with one-way ANOVA with Bonferroni post-hoc analysis. (C) Representative images of blood vessel-like structures formed by human umbilical vein endothelial cells stained with Calcein on Geltrex. Scale bar=100 μm. (D) Total tube length (in pixels) formed by HUVECs treated with growth factor-rich positive control media (+), growth factor-devoid negative control media (−), 2D Exo−, 3D Exo−, 2D Exo+, and 3D Exo+. N=3 CPC populations. *p≤0.05 with one-way ANOVA with Bonferroni post-hoc analysis.
An expanded Methods section is available in the Supplemental Information file.
RESULTS
CPC sphere growth and characterization.
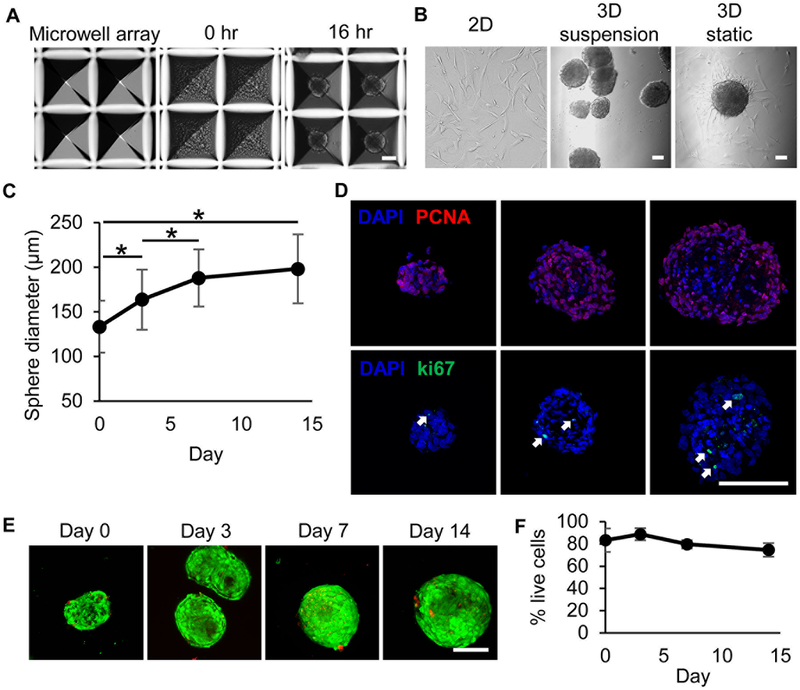
Cell populations isolated and expanded from RA tissue from child donors by c-kit+ magnetic bead sorting were enriched for Nkx2.5 and Gata4, as previously characterized by flow cytometry.18 Seeding 1500 CPCs/microwell consistently formed spheres between 120–150 μm in diameter within 16 hours (Figure 1A–B). Dissociating and counting CPC spheres shows that each sphere contains on average 83% of seeded cells, or 1250 cells (Supplemental Figure I). Cell spheres were maintained in rotary orbital suspension culture for up to 14 days. 3D CPCs cultured under static conditions on standard tissue culture-treated plastic induced sphere adhesion and spreading (Figure 1B). At day 7, CPC spheres had grown up to 180–200 μm in diameter (Figure 1C). CPC sphere growth plateaued after day 7 in culture and did not significantly increase between days 7 and 14. Interestingly, CPC spheres formed from seeding 500 cells per microwell were initially 70–90 μm in diameter, but also grew to 160–190 μm by day 14 (Supplemental Figure II). This universal growth limitation may reflect nutrient and gas diffusion limitations within the densely packed sphere. Immunohistochemical staining of multiple sphere sections (1500 cells/sphere) for ki67 and PCNA revealed robust expression in CPC nuclei throughout the sphere on day 7, suggesting that the growth in sphere size is due primarily to cellular proliferation (Figure 1D). These data were confirmed by manual cell counting and EdU labeling (Supplemental Figure I). Live-dead staining of CPC spheres demonstrated a high ratio of live cells maintained through culture day 14 (Figures 1E–F). CPC spheres formed from 1500 cells/sphere were resistant to dissociation, pliant, and maintained their size and shape when passed through a 28-gauge needle (Supplemental Figure III).
Figure 1. CPCs continue to proliferate within spheres.
(A) Representative bright-field microscopy images of microwells, CPC-loaded microwells, and sphere formation (left to right). (B) Representative bright-field microscopy images of monolayer (2D) CPCs, CPC spheres (3D) in rotary orbital suspension culture, and 3D CPCs in static culture on standard tissue-culture treated plates (left to right). (C) Sphere diameters measured in culture over time presented as average±standard deviation. N=3 CPC populations with ≥30 observations each. *p≤0.05 by one-way repeated measures ANOVA with Bonferroni post-hoc analysis. (D) Representative images of 3 sphere section depths labeled for nuclei (blue), ki67 (green), and PCNA (red). Images were obtained by confocal microscopy and represent z-axis projections. White arrows indicate cells with nuclear ki67 expression. (E) Live-dead staining of 3D CPCs using Calcein-AM (live, green) and ethidium homodimer-1 (dead, red). (F) Live cells were counted and graphed as percent live cells ± standard deviation. N=3 CPC populations. No significant differences were detected using one-way repeated measures ANOVA. Scale bars=100 μm.
While CPC spheres with 1500 cells/sphere and CPC spheres with 500 cells/sphere were not significantly different in viability and size at day 7 in culture, real time-PCR analysis shows significantly reduced NOTCH1 receptor and Notch binding ligand JAG1 with 500 cells/sphere compared to 1500 cells/sphere (Supplemental Figure IIE). Moreover, 3D CPCs with 500 cells/sphere have significantly lower NKX2.5 expression than with 1500 cells/sphere (Supplemental Figure IID). Notch effector HEY1 and endothelial cell marker FLT1 were significantly upregulated in 3D CPCs with 500 cells/sphere compared to 2D CPCs but were not as highly expressed as 3D CPCs with 1500 cells/sphere. Considering these data, we focused our studies on 3D CPCs with 1500 cells/sphere.
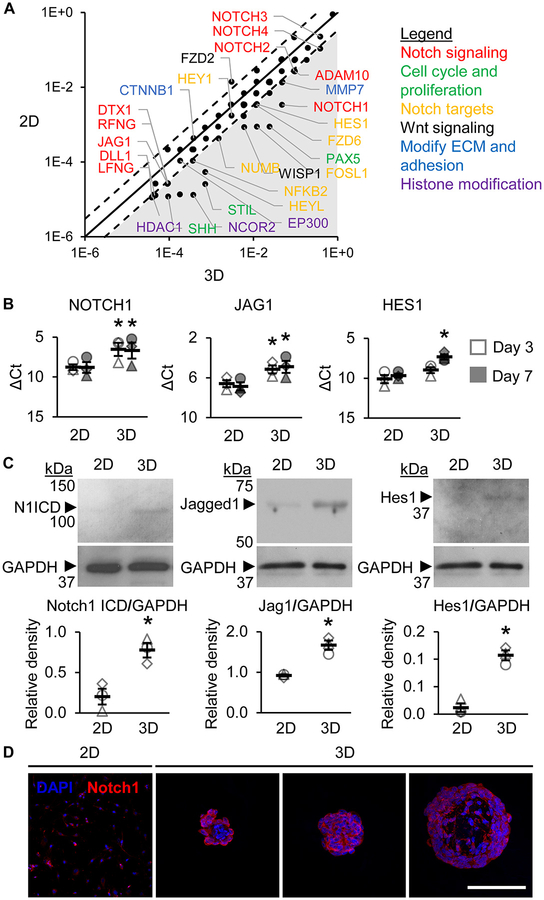
Spherical aggregation increases Notch signaling.
To determine whether spherical aggregation improves Notch signaling, we performed gene analysis on CPC spheres with 1500 cells/sphere. An initial mRNA array of 84 Notch signaling target genes revealed that NOTCH1 and Notch ligands DLL1 and JAG1, among many other Notch signaling-related genes, were highly upregulated in 3D CPCs compared to 2D CPCs at day 3 (Figure 2A). Real-time PCR analysis showed that NOTCH1 and JAG1 transcripts were significantly upregulated in 3D CPCs compared to 2D CPCs at days 3 and 7 in culture, along with HES1, the canonical downstream Notch effector (Figure 2B). Activated Notch1 receptors are cleaved to release Notch1 intracellular domain (N1ICD). Western blotting analysis showed significantly increased N1ICD, Jagged1, and Hes1 in 3D CPCs compared to 2D CPCs at day 7 (Figure 2C). Immunohistochemical analysis of 2D and 3D CPCs also showed robust expression of Notch1 receptor on CPC membranes in 3D spheres, particularly in CPCs found in the sphere periphery (Figure 2D). Together, these data suggest that spherical aggregation increases both the number of Notch1 receptors and Notch1 receptor signal transduction in CPCs.
Figure 2. Spherical aggregation increases Notch signaling in CPCs.
(A) Select genes from mRNA array analysis of 84 Notch signaling target genes. Axes values represent relative expression (log(2−Ct)). The solid line represents equal expression between 2D and 3D. The dotted lines represent a 3-fold change. The shaded area includes all targets that had ≥3-fold change in 3D compared to 2D. N=1 pooled CPC population. (B) Real-time PCR analysis of NOTCH1, JAG1, and HEY1 transcripts at days 3 and 7 in 2D and 3D CPCs. Data presented as difference between cycle threshold of the gene of interest and of housekeeping gene GAPDH (ΔCt). Error bars represent SEM. N=3 CPC populations. *p≤0.05 vs 2D within the same culture day by two-way repeated measures ANOVA with Fisher post-hoc analysis. (C) Western blotting analysis of Notch1 intracellular domain (ICD), Jag1, and Hes1 at day 7 in culture. Data presented as average normalized to GAPDH±SEM with representative blot (right). N=3 CPC populations. *p≤0.05 vs 2D by student’s t-test. (D) Representative z-axis projections of 2D and 3D CPC sphere section depths labeled for nuclei (blue) and Notch1 (red). Images were obtained with confocal microscopy. N=3 CPC populations. Scale bar=100 μm.
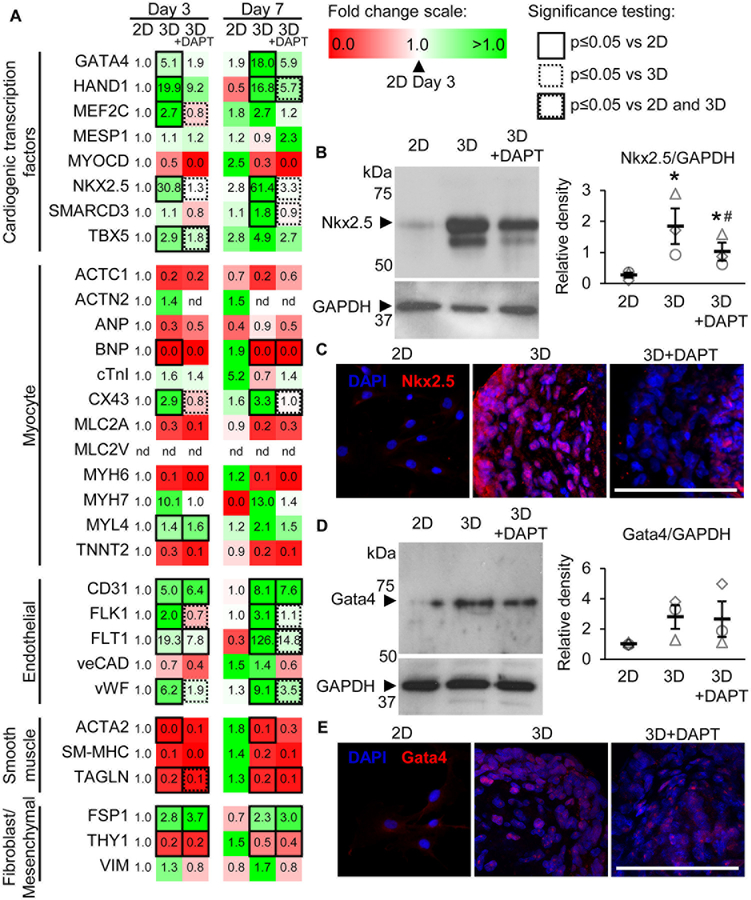
Spherical aggregation promotes Notch-dependent CPC differentiation.
We and others have reported that Notch signaling plays an important role in regulating CPC differentiation.19, 20, 22, 30 To determine the effect of increased Notch signaling in aggregated CPCs on CPC differentiation, we examined the transcript expression of 31 CPC differentiation markers representing cardiogenic transcription factors, myocytes, endothelial cells, smooth muscle cells, and fibroblast/mesenchymal cells by real-time PCR analysis. N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a small molecule inhibitor of γ-secretase that cleaves and releases active NICD, was used to block Notch signal transduction and identify dependent relationships. Treatment with 10 μM DAPT significantly reduced Notch1 receptor and signaling activity without impairing CPC proliferation (Supplemental Figure IV). CPC aggregation significantly upregulated the cardiogenic transcription factors GATA4, HAND1, MEF2C, NKX2.5 and TBX5 at day 3, with MEF2C, NKX2.5 and TBX5 being significantly reduced by DAPT treatment (Figure 3A). At day 7, GATA4, HAND1, and NKX2.5 remained significantly upregulated in 3D CPCs, with additional upregulation of SMARCD3 compared to 2D CPCs. SEM values for the data in Figure 3A can be found in Supplemental Table II. Western blotting analysis of 3D CPC whole cell lysate shows significantly increased Nkx2.5 but not Gata4 protein (Figures 3B and 3D). However, nuclear expression of both Nkx2.5 and Gata4 were significantly increased in 3D CPCs compared to 2D CPCs, as demonstrated by immunohistochemistry (Figures 3C and 3E, Supplemental Figures VIA and VIB). DAPT treatment significantly reduced Nkx2.5 expression.
Figure 3. Spherical aggregation promotes Notch-dependent upregulation of CPC differentiation markers.
(A) Real-time PCR analysis of 31 different CPC differentiation markers at days 3 and 7 in 2D, 3D, and 3D CPCs treated with DAPT to block Notch activation. Data values represent fold change (2−ΔΔCt) relative to 2D data at day 3. N=3 CPC populations. Statistical significance determined using two-way repeated measures ANOVA with Fisher post-hoc analysis. nd=not detected. No significant differences were measured between 2D at day 3 and 2D at day 7. Western blotting analysis of Nkx2.5 (B) and Gata4 (C) at day 7 in culture. Data presented as average normalized to GAPDH±SEM with representative blot (right). N=3 CPC populations. *p≤0.05 vs 2D and #p≤0.05 vs 3D with one-way repeated measures ANOVA with Bonferroni post-hoc analysis. Representative images of 2D and 3D CPC sections labeled for nuclei (blue), Nkx2.5 (D, red), and Gata4 (E, red). Images were obtained with confocal microscopy and represent z-axis projections. N=3 CPC populations. Scale bar=100 μm.
Endothelial markers CD31, FLK1, FLT1, and vWF were significantly upregulated in 3D CPCs at day 3 in a Notch-dependent manner and remained upregulated at day 7 (Figure 3A). Except for CD31, DAPT treatment reduced expression of these endothelial markers at day 7. Interestingly, Notch1-specific inhibition significantly reduced CD31 expression in 3D CPCs at day 7 (Supplemental Figure V). Most myocyte markers investigated were reduced or marginally changed by aggregation. CX43 was significantly upregulated in a Notch-dependent manner on both days 3 and 7 in 3D CPCs. CX43, CD31, and FLT1 (or VEGFR1) transcript data were confirmed by immunohistochemical analysis of the respective proteins in CPC sphere sections (Supplemental Figure VI).
The transcript levels of all smooth muscle markers investigated were decreased by spherical aggregation (Figure 3A). Of the fibroblast and mesenchymal cell markers investigated, FSP1 was significantly upregulated, THY1 was significantly downregulated, and VIM showed no significant change at day 7 (Figure 3A).
Treatment of 2D CPCs with DAPT reduced Notch activity but did not significantly change transcript expression of Notch receptor, Notch binding and processing targets, or cardiogenic differentiation markers, further reinforcing the significance of CPC aggregation on Notch signaling and CPC differentiation (Supplemental Figure VII).
Because DAPT nonspecifically inhibits Notch signaling, the specific contributions of Notch1 and Notch2 were examined using specific RNA interference gene silencing. While Notch1 inhibition using shRNA transduction significantly reduced HAND1, NKX2.5, CD31, and FLT1 transcript expression in 3D CPCs, knockdown of Notch2 in 3D CPCs produced reductions in more cardiogenic markers, including GATA4, and MEF2C (Supplemental Figure V). However, this may likely be attributed to the interesting observation that specific knockdown of Notch2 also reduced Notch1 transcript expression. The greater combined effect of both Notch1 and Notch2 knockdown may represent redundancy between the receptors. Redundant Notch1 and Notch2 receptors have also been described in the differentiation and proper function of neuroprogenitors and other cell types.31, 32
Spherical aggregation improves CPC myocardial retention independent of Notch expression.
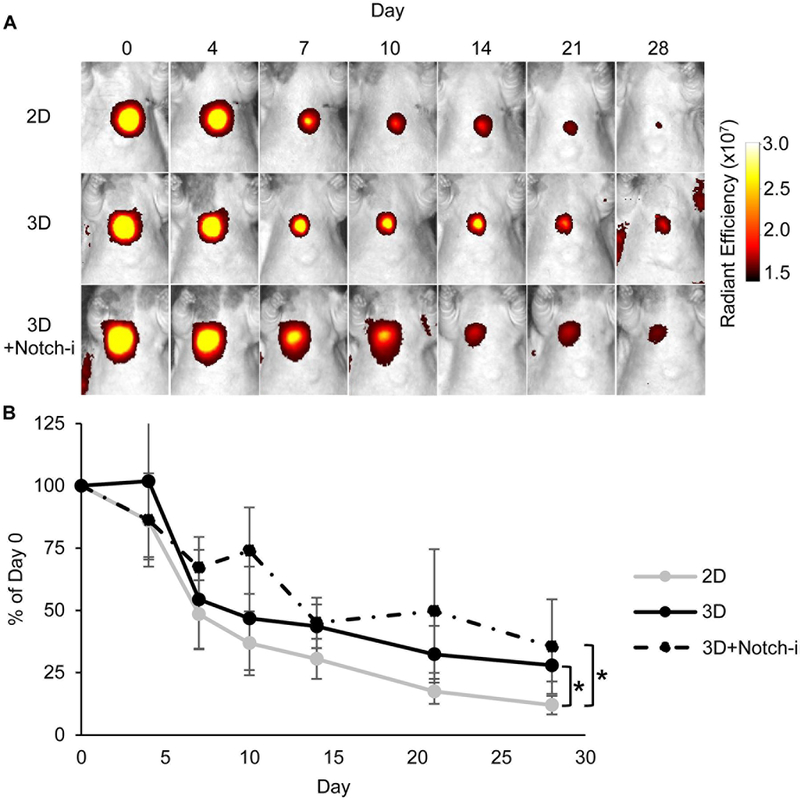
The large size of CPC spheres may inhibit washout via the cardiac venous system, thought to be the most important mechanism of early cell loss.33 To test this hypothesis, the retention of DiR-labeled 2D and 3D CPCs was tracked in vivo. CPC spheres were retained at significantly higher amounts in the RV myocardium compared to 2D CPCs (Figure 4). To test whether Notch may play a role in CPC retention, CPCs were transduced with Notch1-shRNA. CPC spheres transduced with Notch1-shRNA (3D+Notch-i) exhibited significant Notch1 inhibition, without impaired CPC viability and proliferation (Supplemental Figure VIII). The retention of 3D+Notch-i CPCs was still significantly greater than 2D cells and not significantly different from 3D CPCs (Figure 4). 3D CPCs transduced with Control-shRNA (3D+Ctrl-i) also did not have significantly different retention from 3D CPCs (Supplemental Figure IX).
Figure 4. Spherical aggregation increases CPC myocardial retention independent of Notch expression.
(A) DiR fluorescence of 2D CPCs, 3D CPCs, and 3D CPCs transduced with Notch1-shRNA (3D+Notch-i) via whole animal in vivo imaging over 28 days post-injection. (B) Changes in DiR fluorescence were measured over time and are presented as % of day 0 values with 95% confidence intervals. N=7 2D, N=7 3D, N=5 3D+Notch-i. *p≤0.05 for overall difference by treatment group with two-way repeated measures ANOVA with Bonferroni post-hoc analysis.
Improved CPC retention may also be explained by significantly increased release of MMP9 and bFGF at day 3 in 3D CPCs compared to 2D CPCs, factors which have been shown to promote cell engraftment and cell survival (Supplemental Figure X).34, 35
Aggregated CPCs improve RV systolic and diastolic function in PAB rats in a Notch-dependent manner.
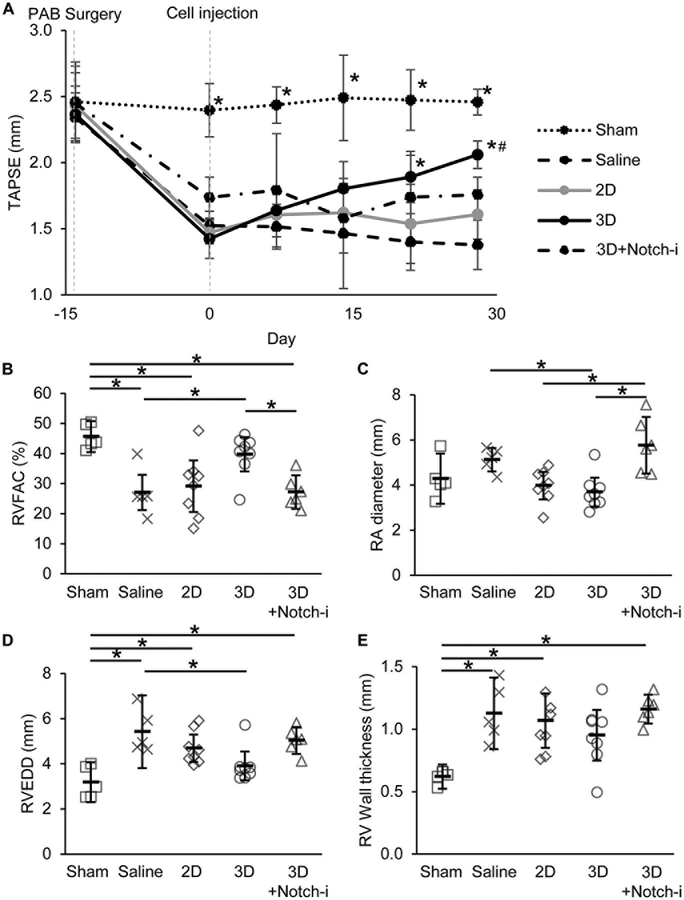
To evaluate the reparative ability of 3D CPCs, RV function was measured longitudinally in athymic rats subjected to PAB surgery. PAB rats demonstrated significantly elevated transpulmonary pressure and velocity gradients and reduced TAPSE, a measure of RV systolic function, by day 14 post-banding (Supplemental Figure XI and Figure 5A). 3D CPC treatment progressively improved TAPSE in PAB rats and was significantly different from saline-control at days 21 and 28 post-injection (Figure 5A). Endpoint measurements on day 28 also showed significantly increased RVFAC, a measure of global RV systolic function, with 3D CPC treatment compared to saline-control (Figure 5B). In contrast, 2D CPC treatment did not significantly improve TAPSE or RVFAC compared to saline (Figure 5A–B). 3D CPC treatment significantly reduced RA diameter compared to saline-control, suggesting that 3D CPC treatment preserves RV diastolic function (Figure 5C). 3D+Notch-i CPC treated rats did not demonstrate significantly improved TAPSE compared to saline-control and had significantly lower RVFAC and greater RA diameter compared to 3D CPC treated rats (Figures 5A–5C).
Figure 5. Aggregated CPCs improve right ventricular function in PAB rats in a Notch-dependent manner.
(A) Tricuspid annular plane systolic excursion (TAPSE) presented as mean with 95% confidence intervals. N=5 Sham, N=5 Saline, N=8 2D, N=8 3D, N=6 3D+Notch-i. *p≤0.05 vs Saline and #=not significant vs Sham at the day level with two-way repeated measures ANOVA with Bonferroni post-hoc analysis. (B) Right ventricle fractional area change (RVFAC), (C) right atrial (RA) diameter, (D) right ventricle end diastolic diameter (RVEDD), and (E) right ventricle wall thickness measured at day 28 post-injection. Data are presented as mean with 95% confidence intervals. *p≤0.05 with one-way ANOVA with Bonferroni post-hoc analysis.
No significant differences in RV function were found between 3D CPC and 3D+Ctrl-i CPC treatment (Supplemental Figures XIIA–C). Moreover, heart rate and left ventricular function were all within normal range and not significantly different among all treatment groups (Supplemental Table III).
Implantation of aggregated CPCs reduces fibrosis and increases vessel density.
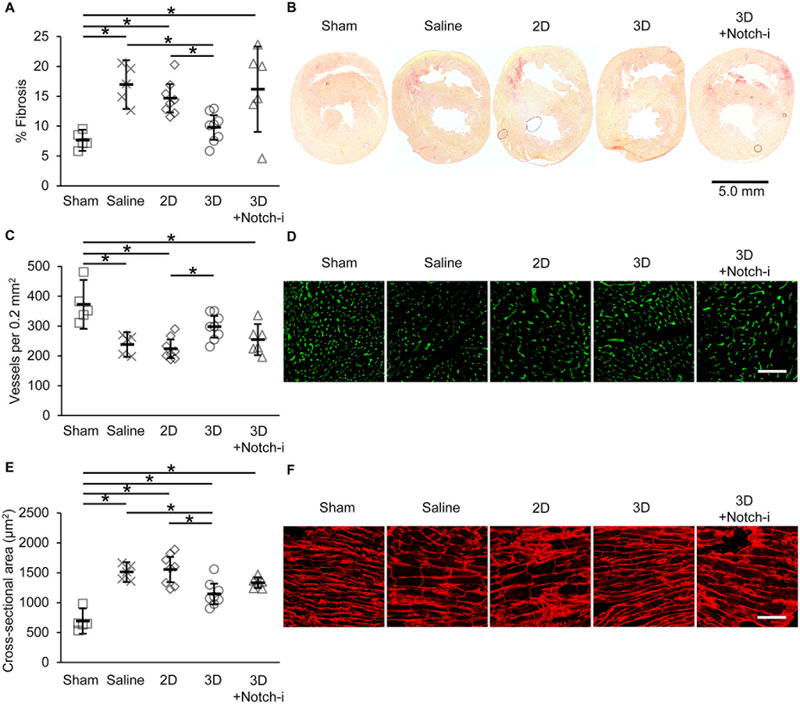
To discern the mechanisms responsible for improved RV function with 3D CPC implantation, we examined fibrosis and vessel density in the RV myocardium 28 days post-cell injection. Picrosirius red staining for collagen fibers revealed significantly reduced RV free wall fibrosis with 3D CPC treatment compared to 2D CPC and saline treatments (Figure 6A and6B). 3D+Notch-i CPC treatment did not significantly reduce fibrosis compared to saline treatment. Similarly, 3D CPC treatment significantly increased density of isolectin-labeled vessels in RV myocardium compared to 2D CPC treatment (Figure 6C and6D). 3D+Notch-i CPC treatment did not significantly increase vessel density compared to 2D CPC and saline treatments.
Figure 6. Histological analyses of right ventricle in PAB rats 28 days post-treatment.
(A) Fibrosis was measured in PAB rat heart sections and is presented as mean percent area of total right ventricular area with 95% confidence intervals. (B) Representative images of picrosirius red-stained fibrosis. (C) Vessels counted per 0.2 mm2 presented as mean with 95% confidence intervals. (D) Representative images isolectin-labeled vessels. (E) Cross-sectional area of myocytes presented as mean with 95% confidence intervals. (F) Representative images of WGA-labeled myocytes. N=5 Sham, N=5 Saline, N=8 2D, N=8 3D, N=6 3D+Notch-i. *p≤0.05 with one-way ANOVA with Bonferroni post-hoc analysis. Scale bar=100 μm unless otherwise noted.
Aggregated CPCs protect against hypertrophy.
To evaluate the effect of CPC aggregation on RV hypertrophy, we examined RV free wall thickness and RV end-diastolic diameter (RVEDD). Unlike 2D CPC treatment, 3D CPC treatment significantly reduced RVEDD compared to saline treatment (Figure 5D). However, RV wall thickness was only modestly reduced by 3D CPC treatment compared to saline treatment. Notch1-shRNA transduction attenuated anti-hypertrophic effects of 3D CPCs on RVEDD and RV wall thickness (Figures 5D and 5E). No significant differences in RVEDD and RV wall thickness were found between 3D CPC and 3D+Ctrl-i CPC treatment groups (Supplemental Figures XIID–E).
We examined myocyte hypertrophy on a cellular level 28 days post-injection by staining RV myocardium with WGA to identify myocyte boundaries. Consistent with echocardiographic data, 3D CPC-treated rats had significantly lower myocyte cross-sectional area compared to myocytes in saline and 2D CPC-treated rats (Figure 6E and6F). 3D+Notch-i treatment was neither different from saline nor 3D treatment. Together with a significantly improved RV systolic function and preserved RV diastolic function, these data suggest that 3D treatment may protect against eccentric hypertrophy.
Implanted CPCs differentiate into vessel-like cells.
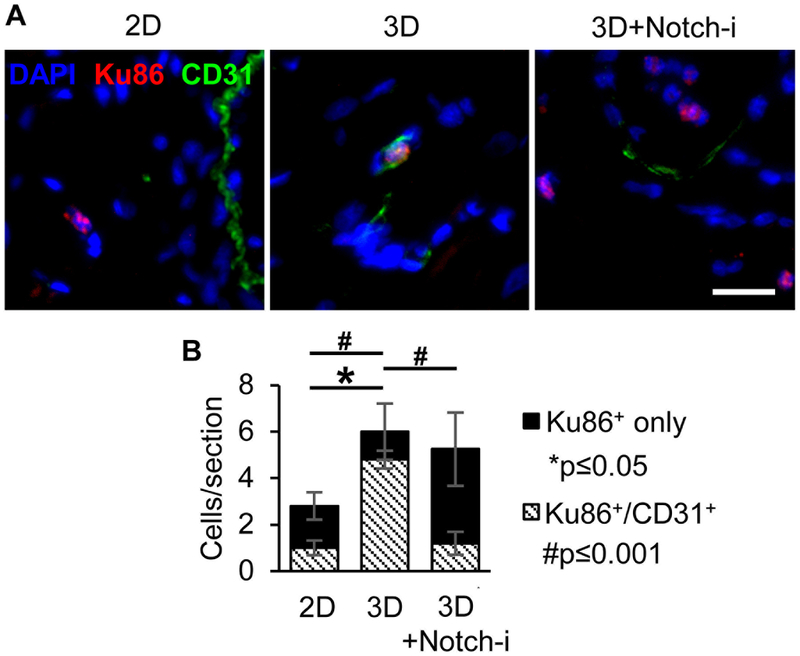
To evaluate the fate of implanted child CPCs, a human specific antibody for Ku86 nuclear protein was used to identify human cells in heart sections 14 days after implantation. Animals implanted with 3D CPCs had significantly higher numbers of Ku86+ cells compared to 2D, but not 3D+Notch-i animals (Figure 7). These data confirm the DiR fluorescence data presented in Figure 4. Additionally, significantly more implanted 3D CPCs were also CD31+ compared to 2D and 3D+Notch-i CPCs.
Figure 7. Aggregated CPCs implanted into PAB rat myocardium differentiate into vessel-like cells.
(A) Representative immunohistochemical images of human CPCs in 14-day rat heart sections at the injection site identified with human-specific Ku86 antibody. Sections were co-stained with DAPI and CD31 antibody. Scale bar=20 μm. (B) Average number of Ku86+ only and Ku86+/CD31+ cells per 10 μm thick heart section. N=3 animals per group, 2 cardiac sections per animal. *p≤0.05 between total Ku86+ cell values and #p≤0.001 between Ku86+/CD31+ cell values via one-way ANOVA with Tukey’s post-hoc analysis.
Exosomes from aggregated CPCs reduce fibrotic gene expression and induce tube formation.
To determine potential paracrine mediators, we isolated conditioned media and exosomes from cultured spheroids for in vitro testing. The effect of exosomes from 2D or 3D cultured cells on rat cardiac fibroblasts was investigated using a fibroblast TGF-β stimulation assay. Treatment of fibroblasts with 3D CPC exosomes significantly reduced fibrotic gene expression, including connective tissue growth factor (CTGF), vimentin (VIM), and collagen type 1 pro-α1 chain (COL1A1), compared to 2D CPC exosomes and untreated fibroblasts (Figure 8B). Treatment of fibroblasts with 2D or 3D exosome-free conditioned media did not reduce fibrotic gene expression. In addition, the effect of exosomes from 2D or 3D cultured cells on human umbilical vein endothelial cells (HUVECs) was investigated using a tube formation assay. Treatment of HUVECs with 3D CPC exosomes significantly improved total tube length compared to 2D CPC exosomes and negative control (Figure 8C). Treatment with 2D or 3D exosome-free conditioned media did not significantly improve tube formation.
DISCUSSION
Growing evidence suggests that CPCs lose their reparative potential as they age, starting as early as 1 year of age.18, 36 We have shown that CPCs isolated from children between 1–5 years of age have a reduced ability to improve RV function in PAB rats compared to CPCs isolated from neonates (0–1 month).18 The present study investigates spherical aggregation as a method for rescuing the reparative potential of child CPCs. Our results demonstrate that aggregated child CPCs have significantly enhanced ability to improve RV function in PAB rats compared to non-aggregated child CPCs. This improvement is mainly attributed to increased Notch1 signaling, which enhances CPC differentiation. This led to a restoration of reparative potential in cells from older donors that was sensitive to Notch inhibition.
Previous approaches for improving the differentiation of isolated CPCs by manipulating them ex vivo focused on genetic engineering or exposure to environmental or chemical treatments. Genetic modifications often employ lentiviruses that pose regulatory and safety concerns.37, 38 Environmental and chemical cues have proven to be a potential strategy, but these face limitations in achieving the precise spatial and temporal regulation required for specific differentiation.39, 40 Hydrogels and biomimetic scaffolds have been employed to enhance CPC engraftment, but such foreign materials may induce inflammation and require further regulatory approvals.41 Moreover, attempting to manipulate CPC function by use of lentiviruses, chemical treatments, or biomimetic substrates typically focuses on only a single or small subset of signaling pathways at a time. Research with iPSCs have demonstrated that cardiac differentiation requires multiple levels of reprogramming stimuli, including growth plate matrix, optimized exposure to growth factors, and temporal modulation of signal transduction pathways.42–44 CPCs may similarly respond positively to multiple levels of stimuli, which may be activated by CPC aggregation. Additionally, our forced aggregation approach using a microwell array enables us to produce natural, scaffold-free CPC spheres and are therefore more conducive to clinical translation due to minimal manipulation guidelines. Compared to other aggregation protocols, such as cell sheets or cardiospheres, our approach produces cell aggregates with less manipulation and in a shorter time.45, 46
Our results demonstrated robust upregulation of several cardiogenic transcription factors by spherical aggregation of child CPCs (Figure 3A). This process of transcription factor upregulation, requiring minimal external manipulation, allows the CPCs to modulate the transcription factors in a dynamic way that may be more beneficial for CPC differentiation and function than constitutive lentiviral-based upregulation. Indeed, these core cardiogenic transcription factors have been shown to regulate each other in complex combinatorial pathways to fine-tune differentiation.47, 48 The expression of these transcription factors was followed by robust expression of endothelial markers and reduced expression of myocyte, smooth muscle, and fibroblast/mesenchymal cell markers in 3D CPCs compared to 2D CPCs, providing evidence that spherical aggregation promotes endothelial lineage commitment. However, the simultaneous upregulation of CX43 and FSP1 suggests that 3D CPCs are not terminally differentiated. 3D CPCs cultured until day 14 did not significantly differ in transcript expression of differentiation markers compared to 7-day 3D CPCs (data not shown).
The present study suggests that Notch1 signaling between aggregated child CPCs is primarily responsible for improved CPC differentiation, likely via the canonical pathway involving Hes1. Interestingly, while spherical aggregation similarly increases HES1 transcript expression in 3D neonate CPCs, both 2D and 3D neonate CPCs have similarly high expression of NOTCH1 and JAG1 transcripts (Supplemental Figure XIII). These data suggest that the high reparative potential of 2D neonate CPCs may be due to innately high Notch1 activity, via a non-canonical pathway not involving Hes1, and that spherical aggregation may be improving child CPC function via the canonical Notch pathway. Although the effect may be modest given the limited upregulation of just NKX2.5, TBX5, and MEF2C in neonate CPCs by spherical aggregation, spherical aggregation could improve the reparative ability of neonate CPCs (Supplemental Figure XIII). The activation of Notch1 signaling in CHO cells suggests that spherical aggregation may likely improve the differentiation of other stem cell types in which Notch signaling plays an important role in cell function (Supplemental Figure XIV).
While our present study focused on the effect of spherical aggregation on Notch1 signaling, the complex microenvironment created by spherical aggregating likely activates several other signaling processes. For example, it is now widely accepted that microenvironmental cues, particularly mechanical signals, can significantly impact stem cell differentiation. In our lab, we have recently shown that hiPSC-derived MSCs can be directed to mature into valve interstitial-like cells by modulating the stiffness of their growth surface.49 The microforces experienced by CPCs within a sphere could also contribute to CPC differentiation. Moreover, researchers have discovered the expression of the mechano-sensing receptors YAP and TAZ on CPC surfaces and demonstrated that these receptors play a key role in CPC fate.50 Additional studies using force probes may elucidate the microforces experienced by CPCs in spheres. We tested the possibility that exosomes could be involved in intra-sphere signaling processes by inhibiting exosome release with the sphingomyelinase inhibitor GW4869. However, inhibition of exosome release failed to significantly reduce the expression of CPC differentiation markers or Notch signaling markers (Supplemental Figure XV). Finally, hypoxic signaling may play a key role in 3D CPC function. Our results demonstrated that the core of a 1500 cell/microwell sphere (approximately inner 50%) exhibited low O2 content (<10 mmHg) (Supplemental Figure XVI). We have previously shown that CPCs cultured in a hypoxic environment produce exosomes enriched with angiogenic and anti-fibrotic miRNAs.29 Furthermore, both hypoxic and normoxic CSC niches have been found in myocardium and this balance in oxygen tension may regulate CSC function.51, 52
Our histological analyses of implanted CPC fate suggest that spherical aggregation modestly improves cell retention. Moreover, implanted 3D CPCs, but not 2D CPCs nor 3D+Notch-i CPCs, form CD31+ cells, providing evidence of Notch-dependent, spherical aggregation-induced differentiation of CPCs into endothelial cells in vivo. However, the total number of cells retained in the RV myocardium is significantly lower than DiR fluorescence estimates. The low number of cells limits the direct effect of CPC-derived endothelial cells on heart function. Additionally, further experiments using computed-tomography angiogram are needed to confirm the vascular function of the CPC-derived endothelial cells. Considering this, the observed improvements in RV function with 3D CPC implantation are likely only minimally attributable to the vascular function of CPC-derived endothelial cells. Instead, the improvements are more likely attributable to paracrine mediators, such as exosomes, released by 3D CPCs. Indeed, growing evidence suggests that exosomes constitute a significant portion of CPC reparative ability.7, 53–55 Our results show that exosomes from 3D CPCs significantly reduce fibrotic gene expression in fibroblasts and improve tube formation (Figure 8). 2D and 3D CPC exosome-free conditioned media had limited effect on fibrotic gene expression or tube formation, consistent with analyses of secreted growth factors (Figure 8 and Supplemental Figure X). 3D CPC exosomes may be responsible for the observed reduction in fibrosis and improvement in vessel density with 3D CPC treatment (Figure 6). The greater retention and engraftment of 3D CPCs likely prolongs the duration of exosome release, compared to 2D CPCs, to improve RV function. Future experiments will further investigate the mechanisms and contents of exosomes from implanted 3D CPCs in vivo.
The strong reparative ability of CPC spheres warrants further investigation as a treatment for pediatric HF, especially in older children where reparative ability may be reduced. Pediatric HF is most commonly caused by congenital heart disease and genetic cardiomyopathy and can result in severe RV failure. We show in the present study, in a RV pressure overload model, that spherical aggregation significantly improves the ability of child CPCs to improve RV contractile function, increase RV vessel density, and reduce RV hypertrophy and RV fibrosis. These effects are likely exosome-mediated, as aggregation improves exosome function. Aggregation also promotes CPC engraftment as CD31+ cells, likely contributing to a prolonged exosome effect. While a Phase I study of CPC therapy in children with HLHS is underway (NCT03406884), there is a potential for rapid translation of this therapy to the clinic, especially in patients that do not respond to traditional cell therapy. While we do not directly show ability to repair the LV, the in vivo mechanisms suggest that this may be a broader therapy for other cardiac indications.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Right ventricular heart failure (RVHF) is a severe problem in the clinical care of patients with congenital heart disease (CHD).
New regenerative medicine and stem cell-based therapies for the treatment of RVHF are promising.
But older cardiac progenitor cells (CPCs), starting as early as 1 year old, have a reduced ability to repair the heart.
What New Information Does This Article Contribute?
Aggregating child (>1 year old) CPCs into spheroids activates Notch signaling and improves endothelial differentiation.
Aggregated child CPCs have an improved ability to repair the right ventricle in a heart failure model.
Aggregated child CPCs likely repair the heart via endothelial cell contribution and exosome signaling effects.
Advances in surgical management have led to improved survival of patients born with CHD, creating a new population of older CHD patients at significant risk for RVHF. New regenerative medicine and stem cell-based therapies for the treatment of RVHF are promising. But older CPCs, starting as early as 1 year old, have a reduced ability to repair the heart. In our studies, we show that aggregating child (>1 year old) CPCs into spheroids activates Notch signaling and improves endothelial differentiation. Aggregated child CPCs have an improved ability to repair the right ventricle in a right ventricular heart failure model. Importantly, we show that aggregated child CPCs likely improve right ventricular function by contributing endothelial cells via direct differentiation and exosome signaling effects. The strong reparative ability of CPC spheres warrants further investigation as a treatment for pediatric HF, especially in older children where reparative ability may be reduced. While a Phase I study of CPC therapy in children with hypoplastic left heart syndrome is underway (NCT03406884), there is a potential for rapid translation of this therapy to the clinic, especially in patients that do not respond to traditional cell therapy.
ACKNOWLEDGMENTS
The authors wish to acknowledge Ming Shen at the Emory Pediatric Animal Physiology Core, the Emory Integrated Cellular Imaging Core, and the Emory Microscopy in Medicine Lab.
SOURCES OF FUNDING
This work was supported by funds from the Betkowski Family Fund and the Children’s Miracle Network awarded to MED, and by the American Heart Association Predoctoral Fellowship (17PRE33460129) awarded to DT.
Nonstandard Abbreviations and Acronyms:
- 3D CPC
cardiac progenitor cells cultured as spheres
- 2D CPC
CPCs cultured as monolayers
- 3D+Notch-i CPC
3D CPCs transduced with Notch1-shRNA
- 3D+Ctrl-I CPC
3D CPCs transduced with Control-shRNA
Footnotes
In November 2018, the average time from submission to first decision for all original research papers submitted to Circulation Research was 12.76 days.
DISCLOSURES
None.
REFERENCES
- 1.Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA and Webb CL. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. [DOI] [PubMed] [Google Scholar]
- 2.Ohye RG, Schranz D and D’Udekem Y. Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions. Circulation. 2016;134:1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chery J, Wong J, Huang S, Wang S and Si MS. Regenerative Medicine Strategies for Hypoplastic Left Heart Syndrome. Tissue engineering Part B, Reviews. 2016;22:459–469. [DOI] [PubMed] [Google Scholar]
- 4.Köhler D, Arnold R, Loukanov T and Gorenflo M. Right Ventricular Failure and Pathobiology in Patients with Congenital Heart Disease – Implications for Long-Term Follow-Up. Frontiers in Pediatrics. 2013;1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B and Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. [DOI] [PubMed] [Google Scholar]
- 6.Maxeiner H, Krehbiehl N, Muller A, Woitasky N, Akinturk H, Muller M, Weigand MA, Abdallah Y, Kasseckert S, Schreckenberg R, Schluter KD and Wenzel S. New insights into paracrine mechanisms of human cardiac progenitor cells. European journal of heart failure. 2010;12:730–7. [DOI] [PubMed] [Google Scholar]
- 7.van Berlo JH and Molkentin JD. Most of the Dust Has Settled: Progenitor Cells Are an Irrelevant Source of Cardiac Myocytes In Vivo. Circulation research. 2016;118:17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC and Marban E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). Journal of the American College of Cardiology. 2014;63:110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JAC, Slaughter MS, Anversa P and Bolli R. Administration of Cardiac Stem Cells in Patients With Ischemic Cardiomyopathy: The SCIPIO Trial: Surgical Aspects and Interim Analysis of Myocardial Function and Viability by Magnetic Resonance. Circulation. 2012;126:S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishigami S, Ohtsuki S, Tarui S, Ousaka D, Eitoku T, Kondo M, Okuyama M, Kobayashi J, Baba K, Arai S, Kawabata T, Yoshizumi K, Tateishi A, Kuroko Y, Iwasaki T, Sato S, Kasahara S, Sano S and Oh H. Intracoronary Autologous Cardiac Progenitor Cell Transfer in Patients With Hypoplastic Left Heart Syndrome: The TICAP Prospective Phase 1 Controlled Trial. Circulation research. 2015;116:653–664. [DOI] [PubMed] [Google Scholar]
- 11.Don CW and Murry CE. Improving survival and efficacy of pluripotent stem cell–derived cardiac grafts. Journal of Cellular and Molecular Medicine. 2013;17:1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG and March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–6. [DOI] [PubMed] [Google Scholar]
- 13.Hudson W, Collins MC, deFreitas D, Sun YS, Muller-Borer B and Kypson AP. Beating and arrested intramyocardial injections are associated with significant mechanical loss: implications for cardiac cell transplantation. The Journal of surgical research. 2007;142:263–7. [DOI] [PubMed] [Google Scholar]
- 14.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW and Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–16. [DOI] [PubMed] [Google Scholar]
- 15.Penn MS and Mangi AA. Genetic Enhancement of Stem Cell Engraftment, Survival, and Efficacy. Circulation research. 2008;102:1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng CJ, Luo J, Chiu RC and Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. The Journal of thoracic and cardiovascular surgery. 2006;132:628–32. [DOI] [PubMed] [Google Scholar]
- 17.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA and Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal U, Smith AW, French KM, Boopathy AV, George A, Trac D, Brown ME, Shen M, Jiang R, Fernandez JD, Kogon BE, Kanter KR, Alsoufi B, Wagner MB, Platt MO and Davis ME. Age-Dependent Effect of Pediatric Cardiac Progenitor Cells After Juvenile Heart Failure. Stem cells translational medicine. 2016;5:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gude N, Joyo E, Toko H, Quijada P, Villanueva M, Hariharan N, Sacchi V, Truffa S, Joyo A, Voelkers M, Alvarez R and Sussman M. Notch activation enhances lineage commitment and protective signaling in cardiac progenitor cells. Basic Res Cardiol. 2015;110:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P and Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15529–15534. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Boopathy AV, Che PL, Somasuntharam I, Fiore VF, Cabigas EB, Ban K, Brown ME, Narui Y, Barker TH, Yoon Y-S, Salaita K, García AJ and Davis ME. The modulation of cardiac progenitor cell function by hydrogel-dependent Notch1 activation. Biomaterials. 2014;35:8103–8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Lu Y, Han R, Yue Q, Song X, Wang F, Wu R, Hou F, Yang L, Xu L, Zhao R and Hu J. Gremlin2 Regulates the Differentiation and Function of Cardiac Progenitor Cells via the Notch Signaling Pathway. Cellular Physiology and Biochemistry. 2018;47:579–589. [DOI] [PubMed] [Google Scholar]
- 23.Baraniak PR and McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell and tissue research. 2012;347:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gothard D, Roberts SJ, Shakesheff KM and Buttery LD. Engineering embryonic stem-cell aggregation allows an enhanced osteogenic differentiation in vitro. Tissue engineering Part C, Methods. 2010;16:583–95. [DOI] [PubMed] [Google Scholar]
- 25.Hamazaki T, Oka M, Yamanaka S and Terada N. Aggregation of embryonic stem cells induces Nanog repression and primitive endoderm differentiation. Journal of cell science. 2004;117:5681–6. [DOI] [PubMed] [Google Scholar]
- 26.Kinney MA and McDevitt TC. Emerging Strategies for Spatiotemporal Control of Stem Cell Fate and Morphogenesis. Trends in biotechnology. 2013;31:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyoda T, Mae S-I, Tanaka H, Kondo Y, Funato M, Hosokawa Y, Sudo T, Kawaguchi Y and Osafune K. Cell aggregation optimizes the differentiation of human ESCs and iPSCs into pancreatic bud-like progenitor cells. Stem Cell Research. 2015;14:185–197. [DOI] [PubMed] [Google Scholar]
- 28.Cho H-J, Lee H-J, Youn S-W, Koh S-J, Won J-Y, Chung Y-J, Cho H-J, Yoon C-H, Lee S-W, Lee EJ, Kwon Y-W, Lee H-Y, Lee SH, Ho W-K, Park Y-B and Kim H-S. Secondary Sphere Formation Enhances the Functionality of Cardiac Progenitor Cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:1750–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD and Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circulation research. 2015;116:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boopathy AV, Martinez MD, Smith AW, Brown ME, Garcia AJ and Davis ME. Intramyocardial Delivery of Notch Ligand-Containing Hydrogels Improves Cardiac Function and Angiogenesis Following Infarction. Tissue engineering Part A. 2015;21:2315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrick DB, Guo Z, Jang W, Schnittke N and Schwob JE. Canonical Notch signaling directs the fate of differentiating neurocompetent progenitors in the mammalian olfactory epithelium. The Journal of Neuroscience. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auderset F, Schuster S, Coutaz M, Koch U, Desgranges F, Merck E, MacDonald HR, Radtke F and Tacchini-Cottier F. Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with Leishmania major. PLoS Pathogens. 2012;8:e1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonios M, Terrovitis J, Chang CY, Engles JM, Higuchi T, Lautamäki R, Yu J, Fox J, Pomper M, Wahl RL, Tsui BM, O’Rourke B, Bengel FM, Marbán E and Abraham MR. Myocardial substrate and route of administration determine acute cardiac retention and lung bio-distribution of cardiosphere-derived cells. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2011;18:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen D, Tang J, Hensley MT, Li T, Caranasos TG, Zhang T, Zhang J and Cheng K. Effects of Matrix Metalloproteinases on the Performance of Platelet Fibrin Gel Spiked With Cardiac Stem Cells in Heart Repair. Stem cells translational medicine. 2016;5:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Guo X and Guan J. A thermosensitive hydrogel capable of releasing bFGF for enhanced differentiation of mesenchymal stem cell into cardiomyocyte-like cells under ischemic conditions. Biomacromolecules. 2012;13:1956–64. [DOI] [PubMed] [Google Scholar]
- 36.Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S and Kaushal S. A Strong Regenerative Ability of Cardiac Stem Cells Derived From Neonatal Hearts. Circulation. 2012;126:S46–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J and Sussman MA. Enhancement of Myocardial Regeneration Through Genetic Engineering of Cardiac Progenitor Cells Expressing Pim-1 Kinase. Circulation. 2009;120:2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowakowski A, Andrzejewska A, Janowski M, Walczak P and Lukomska B. Genetic engineering of stem cells for enhanced therapy. Acta neurobiologiae experimentalis. 2013;73:1–18. [DOI] [PubMed] [Google Scholar]
- 39.Alsberg E, von Recum HA and Mahoney MJ. Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert opinion on biological therapy. 2006;6:847–66. [DOI] [PubMed] [Google Scholar]
- 40.Choi S, Jung S, Asahara T, Suh W, Kwon S-M and Baek S. Direct comparison of distinct cardiomyogenic induction methodologies in human cardiac-derived c-kit positive progenitor cells. Tissue Eng Regen Med. 2012;9:311–319. [Google Scholar]
- 41.Smith RR, Marbán E and Marbán L. Enhancing retention and efficacy of cardiosphere-derived cells administered after myocardial infarction using a hyaluronan-gelatin hydrogel. Biomatter. 2013;3:e24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L and Zambidis ET. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PloS one. 2011;6:e18293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ and Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J and Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circulation research. 2012;111:1125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuda S, Shimizu T, Yamato M and Okano T. Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev. 2008;60:277–85. [DOI] [PubMed] [Google Scholar]
- 46.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G and Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circulation research. 2004;95:911–21. [DOI] [PubMed] [Google Scholar]
- 47.Schlesinger J, Schueler M, Grunert M, Fischer JJ, Zhang Q, Krueger T, Lange M, Tonjes M, Dunkel I and Sperling SR. The cardiac transcription network modulated by Gata4, Mef2a, Nkx2.5, Srf, histone modifications, and microRNAs. PLoS Genet. 2011;7:e1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kathiriya IS, Nora EP and Bruneau BG. Investigating the transcriptional control of cardiovascular development. Circulation research. 2015;116:700–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nachlas ALY, Li S, Jha R, Singh M, Xu C and Davis ME. Human iPSC-derived mesenchymal stem cells encapsulated in PEGDA hydrogels mature into valve interstitial-like cells. Acta biomaterialia. 2018;71:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosqueira D, Pagliari S, Uto K, Ebara M, Romanazzo S, Escobedo-Lucea C, Nakanishi J, Taniguchi A, Franzese O, Di Nardo P, Goumans MJ, Traversa E, Pinto-do OP, Aoyagi T and Forte G. Hippo pathway effectors control cardiac progenitor cell fate by acting as dynamic sensors of substrate mechanics and nanostructure. ACS nano. 2014;8:2033–47. [DOI] [PubMed] [Google Scholar]
- 51.Kimura W and Sadek HA. The cardiac hypoxic niche: emerging role of hypoxic microenvironment in cardiac progenitors. Cardiovascular Diagnosis and Therapy. 2012;2:278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanada F, Kim J, Czarna A, Chan NY-K, Signore S, Ogórek B, Isobe K, Wybieralska E, Borghetti G, Pesapane A, Sorrentino A, Mangano E, Cappetta D, Mangiaracina C, Ricciardi M, Cimini M, Ifedigbo E, Perrella MA, Goichberg P, Choi A, Kajstura J, Hosoda T, Rota M, Anversa P and Leri A. c-kit-Positive Cardiac Stem Cells Nested in Hypoxic Niches are Activated by Stem Cell Factor Reversing the Aging Myopathy. Circulation research. 2014;114:41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.French KM, Maxwell JT, Bhutani S, Ghosh-Choudhary S, Fierro MJ, Johnson TD, Christman KL, Taylor WR and Davis ME. Fibronectin and Cyclic Strain Improve Cardiac Progenitor Cell Regenerative Potential In Vitro. Stem cells international. 2016;2016:8364382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L, Tulapurkar ME, Taylor BS, Yang P, Karathanasis S, Goodlett DR and Kaushal S. A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells. Circulation research. 2017;120:816–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stastna M, Abraham MR and Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS letters. 2009;583:1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.