Abstract
Background
Voluntary medical male circumcision reduces men’s risk of HIV acquisition and may thus increase HIV risk-related sexual behaviors through risk compensation. We analyze longitudinal data from one of Africa’s largest population cohorts using fixed effects panel estimation to measure the effect of incident circumcision on sexual behaviors.
Setting
KwaZulu-Natal, South Africa.
Methods
An open population cohort of men were followed from 2009 to 2015. Men self-reported their circumcision status and sexual behavior annually. We used linear regression models with individual-level fixed effects to measure the effect of incident circumcision on recent sex (past 12 months) and sexual behaviors that increase HIV risk (not using a condom at last sex, never using condoms with the most recent sexual partner, concurrent sexual partners at present, and multiple sexual partners in the past 12 months). We controlled for potential time-varying confounders: calendar year, age, education, and sexual debut.
Results
The 5,127 men in the cohort had a median age of 18 years (IQR 16 to 24) at cohort entry. Over the study period, almost one in five of these men (19.4%) became newly circumcised. Incident circumcision affected neither recent sex (percentage point change [PP] 0.0, 95%CI −1.2 to 1.3) nor sexual behaviors that increase HIV risk (PP −1.6, 95%CI −4.5 to 1.4).
Conclusion
The data from this study strongly reject the hypothesis that circumcision affects sexual risk taking. Risk compensation should not serve as an argument against increased and accelerated scale-up of circumcision in this and similar communities in South Africa.
Keywords: circumcision, risk compensation, sub-Saharan Africa, young males, condom use, sexual behaviors
Introduction
Voluntary medical male circumcision is an attractive HIV prevention approach, because it is a one-time, permanent intervention1–4 that does not rely on the consistent and repeated behaviors required of other HIV prevention interventions, including condom use,5,6 treatment-as-prevention7,8 or pre-exposure prophylaxis.9,10 The effectiveness of circumcision as an HIV prevention intervention, however, may be compromised if circumcision leads to increased sexual risk taking.
Theories of risk compensations predict that circumcision increases sexual risk taking. Risk compensation is a change in behavior in response to a change in perceived level of risk.11 In the context of circumcision, newly circumcised males may adopt sexual behaviors associated with an increased risk of HIV acquisition because they believe that circumcision protects them from HIV infection.3,12–17 Many sub-Saharan African countries have cited risk compensation as a concern for scaling circumcision programs. For instance, the 2013/2014 South African Health Review stated that “risk compensation is a major concern relating to male circumcision … the issue of sexual disinhibition may be especially relevant for young and sexually active populations in high HIV-prevalence areas”.16
Previous studies on the effect of circumcision on male’ HIV risk-related sexual behaviors – some which found no effect14,15,18–24 and others which found evidence of risk compensation3,12,13 – had important limitations. Several of these studies were carried out in the era before the causal effect of circumcision on HIV acquisition had been estab ished.3,18,21–23 Prior to the randomized controlled trials (RCTs) that established that circumcision reduces men’s risk of HIV acquisition,1–3 participants could not have known with certainty that circumcision does indeed protect against HIV acquisition. In the era before the RCT results were known, men may thus not have changed their sexual behaviors following circumcision. Those studies that were conducted in the era after publication of the RCT results mainly used cross-sectional surveys12,13,20 or qualitative interviews14,15 and thus allowed only relatively weak inference on causal effects. One study carried out in the post-RCT era from the Rakai district of Uganda used longitudinal data to identify incident circumcision and to relate this exposure information to sexual behavior.24 This study did not find any evidence of risk compensation.24
In this study, we use longitudinal data from one of Africa’s largest population cohorts in KwaZulu-Natal, South Africa, in the era following the RCTs that established the effect of circumcision on HIV acquisition risk (2009–2015). We strengthen the body of existing evidence by using for the first time fixed effects panel analysis for this estimation. Fixed effects panel analysis allows us to control for all individual-level confounding factors that do not vary over time – including both observed and unobserved factors. Our approach thus completely controls for self-selection into circumcision based on baseline factors. In addition, we are measuring the effect of time since incident circumcision on sexual behavior. Knowledge of the effect of circumcision on HIV risk-related sexual behaviors can be valuable for understanding the full effect of circumcision on HIV prevention and for designing future circumcision programs.
Methods
Study setting
The Africa Health Research Institute (AHRI) has operated a surveillance site in the rural uMkhanyakude district of KwaZulu-Natal, South Africa since 2003.25 The surveillance data includes all households located within a 438-km2 area. KwaZulu-Natal has the highest burden of HIV compared to all the other South African provinces.26–28 In 2014, 34% of all adults and 20% of all men 15 to 49 years in the surveillance area were living with HIV.29 Research assistants collect sociodemographic and health data annually at the surveillance site through individual and household surveys. Since 2009, male participants have been asked to self-report their circumcision status and sexual behaviors. Blood, in the form of dried blood spots, is collected from willing participants for HIV testing annually.
The AHRI surveillance has ethical approval from the Biomedical Research Ethics Committee, University of KwaZulu-Natal. The analyses presented in this paper were exempted from additional review by the Institutional Review Board at the Harvard T.H. Chan School of Public Health because it only uses anonymized secondary data.
Participants
We used surveillance data from 2009 to 2015. Survey respondents were included in our analysis if they were male and 15 years or older, resided in the surveillance area at the time of data collection, and had self-reported their circumcision status at least twice.
Recent sex and HIV risk-related sexual behaviors
We explored the effect of incident circumcision on recent sex and sexual behaviors associated with an increased risk of HIV acquisition. Recent sex was defined as any sex in the past 12 months. We measured the effect of circumcision on recent sex to understand if newly circumcised men engage in more sex, regardless of type, because they no longer feared HIV infection. The HIV risk-related sexual behaviors we measured included: (1) not using a condom at last sex, (2) never using condoms with the most recent sexual partner, (3) concurrent sexual partners at present, and (4) multiple sexual partners in the past 12 months. We also created a binary variable capturing any HIV risk-related sexual behavior. This variable indicates whether men reported at least one of the four individuals HIV risk-related sexual behaviors described above or not a single one. All sexual behavior outcomes were binary and self-reported.
Circumcision status
We used two measurements to capture circumcision status: (i) a binary measure of current circumcision status and (ii) a categorical variable representing different times since incident circumcision. Our time since incident circumcision variable included four categories: more than one year prior to circumcision, within one year prior to circumcision (reference), within one year post circumcision, and more than one year post circumcision. For newly circumcised men, we assumed that incident circumcision occurred midway between the date they last reported being non-circumcised and the date they first reported being circumcised. We included the categorical measure of time since incident circumcision to determine whether changes in sexual behavior due to circumcision varied over time since incident circumcision. Circumcision status was self-reported and has never been validated in the surveillance data. Since traditional circumcision is not practiced by the Zulu culture, the dominant culture in the KwaZulu-Natal region,16 we assumed that majority of incident circumcisions in the surveillance data were voluntary medical male circumcisions.
Statistical methods
We used a linear probability model (LPM) with individual fixed effects to measure the effect of incident circumcision on recent sex and HIV risk-related sexual behaviors. We chose LPM over binary choice models, because the coefficients in LPM are percentage point changes (PP), which are easy to interpret. In addition to the fixed effects, which control for all time-invariant unobserved and observed individual-level confounders, we controlled for a range of time-varying observed confounders: calendar year, participant age (linear and quadratic), participant education (none/primary, 0–7 years; secondary I, 8–9 years; secondary II, 10–11 years, secondary III+, 12+ years), and sexual debut (i.e., a self-report of ever having had sex). Where possible, we computed missing covariate data (i.e., age, education, sexual debut) from previous survey responses. In all other cases, we used the missing indicator method to account for the remaining missing covariates data.30
We conducted two sensitivity analyses (Appendix Tables 1–2,http://links.lww.com/QAI/B247). First, to test the robustness of our model to missing data, we ran complete-case analyses in which we restricted our sample to men for whom all data were available. Second, to understand the effect of errors in reported circumcision status on our outcomes, we dropped all men who reported not being circumcised in years following those years in which they had previously reported being circumcised. We used the same individual fixed effects panel estimation described above for both sensitivity analyses.
To establish heterogeneity in the effect of incident circumcision on sexual behavior, we divided our population into “younger” (age at baseline < median age) and “older” men (age at baseline median ≥ age), and conducted sub-group analyses (Appendix Tables 3–4,http://links.lww.com/QAI/B247). For these analyses we used the individual fixed effects panel estimation described above.
Stata 13.1 (Stata Corporation, College Station, Texas) to conduct all our analyses.
Results
Participants
From 2009 to 2015, 5,127 men in the surveillance data met the inclusion criteria for our analyses. Not all of these men, however, participated in all rounds of annual data collection: 68% participated in two rounds, 25% participated in three rounds, 6% participated in four rounds, and 1% participated in five rounds. A main reason for this differential participation over time is that the cohort that generates our data is dynamic, i.e., over time people “age in”, migrate out, or die. Another reason is refusal to participate despite eligibility to participate. Of all the men in the sample, 1,235 (24%) reported being circumcised and 343 (7%) had errors in their reported circumcision status (i.e., reports of not being circumcised in years following previous reports of being circumcised). Over the study observation period, 897 (17%) men were identified as newly circumcised and our time to incident circumcision variable ranged from six years prior to six years post incident circumcision.
Table 1 describes the sociodemographic characteristics of the men in our study in the year they first entered the surveillance data. The table shows the baseline characteristics of all men, men who were circumcised at cohort entry and thus remained circumcised, men who were never circumcised, and men who became circumcised over the survey years of observation. The majority of all men entered the data before 2011 (51%) and the median age of men when they first appeared in the surveillance data was 18 years (interquartile range 16 to 24 years). HIV prevalence was 8.3% and circumcision prevalence was 6.6% among all men who appeared in the surveillance data for the first time. Almost half of all men in the survey data reported sex in the past year (43%) the first year they entered the surveillance data and 13% of men reported at least one of the sexual behaviors associated with risk of HIV acquisition.
Table 1.
Characteristics of participants the year they first entered the surveillance data*
| Characteristics | All men | Always circumcised | Never circumcised | Became circumcised | |
|---|---|---|---|---|---|
| Year entered data: 2009 |
1,324 (25.8) | 33 (9.8) | 1,153 (29.6) | 138 (15.4) | |
| 2010 | 1,278 (24.9) | 44 (13.0) | 1,016 (26.1) | 218 (24.3) | |
| 2011 | 707 (13.8) | 29 (8.6) | 517 (13.3) | 161 (18.0) | |
| 2012 | 844 (16.5) | 63 (18.6) | 591 (15.2) | 190 (21.2) | |
| 2013 | 624 (12.2) | 93 (27.5) | 408 (10.5) | 123 (13.7) | |
| 2014 | 350 (6.8) | 76 (22.5) | 207 (5.3) | 67 (7.5) | |
| Age (years), median (IQR): | 18 (16 to 24) | 17 (16 to 25) | 18 (16 to 25) | 17 (16 to 19) | |
| Education (years): None/Primary (0–7) |
1,035 (20.7) | 75 (22.3) | 831 (21.9) | 219 (14.7) | |
| Secondary I (8–9) | 1,176 (23.5) | 54 (16.1) | 879 (23.2) | 243 (27.7) | |
| Secondary II (10–11) | 1,638 (32.7) | 126 (37.5) | 1,166 (30.8) | 346 (39.4) | |
| Secondary III (12+) | 1,156 (23.1) | 81 (24.1) | 914 (24.1) | 161 (18.3) | |
| HIV-positive, biologically confirmed | 350 (8.3) | 30 (9.8) | 288 (9.2) | 32 (4.1) | |
| Circumcised | 338 (6.6) | 338 (100.0) | 0 (0.0) | 0 (0.0) | |
| Ever had sex | 2,335 (45.5) | 164 (48.5) | 1,886 (48.5) | 285 (31.8) | |
| Sexually active1 | 2,190 (42.7) | 155 (45.9) | 1,774 (45.6) | 261 (29.1) | |
| Any sexual behavior associated with increased risk of HIV transmission2 |
537 (12.7) | 44 (15.7) | 422 (13.3) | 71 (8.9) | |
| No condom use, last sex | 317 (7.1) | 27 (9.2) | 247 (7.4) | 43 (5.2) | |
| Never use condoms, last partner | 662 (12.9) | 45 (13.3) | 552 (14.2) | 65 (7.3) | |
| Concurrent partners | 339 (6.7) | 23 (6.9) | 263 (6.7) | 53 (6.0) | |
| Multiple partners, past 12 months | 310 (6.2) | 25 (7.6) | 245 (6.5) | 40 (4.6) | |
| Sample size | 5,127 | 338 | 3,892 | 897 | |
Denominators differ slightly due to differences in missing data.
Participants reported sex in the past 12 months.
For this outcome, participants reported at least one of the following: (1) no condom use at last sex; (2) never using condoms with last sexual partner; (3) concurrent sexual partners; (4) multiple sexual partners in the past 12 months.
Several socio-demographic baseline characteristics differed across the various sub-groups that are overall sample can be decomposed into – i.e., men who were always circumcised, never circumcised, or became circumcised: education, HIV status (biologically confirmed), history of sex, and condom use.31 These baseline differences support our choice of analytical approach: fixed effects panel estimation controls for self-selection into circumcision, i.e., confounding, on all individual baseline characteristics – both those that we have observed (and thus can show in Table 1) and those that we have not observed (such as, for instance, attitude towards risk).
Effect of incident circumcision on recent sex and HIV risk-related sexual behaviors
Figure 1 shows the effect of circumcision on recent sex and sexual behaviors associated with an increased risk of HIV acquisition among men in the population cohort. Incident circumcision did not significantly affect whether men reported sex in the past 12 months (percentage point change [PP] 0.0, 95% confidence interval [CI] −1.2 to 1.3, p=0.97), nor did circumcision significantly affect whether men reported any (i.e., at least one of the four) of the HIV risk-related sexual behaviors (PP −1.6, 95% CI −4.5 to 1.4, p=0.30). Incident circumcision did also not affect men’s specific HIV risk-related sexual behaviors: circumcision incidence did not affect men’s condom use at last sex (PP −1.4, 95% CI −3.9 to 1.2, p=0.30), whether they reported never using a condom with their last sexual partner (PP −1.8, 95% CI −4.2 to 0.7, p=0.16), reports of concurrent sexual partners (PP −1.5, 95% CI −3.6 to 0.6, p=0.15), or reports of multiple sexual partners in the past 12 months (PP −0.4, 95% CI −2.4 to 1.6, p=0.67). In our sensitivity (Appendix Table 1,http://links.lww.com/QAI/B247) and sub-group (Appendix Table 3,http://links.lww.com/QAI/B247) analyses, these findings remained essentially the same.
Figure 1. Effect of incidence circumcision on sexual activity (dark blue) and sexual behaviors associated with increased risk of HIV transmission (light blue).
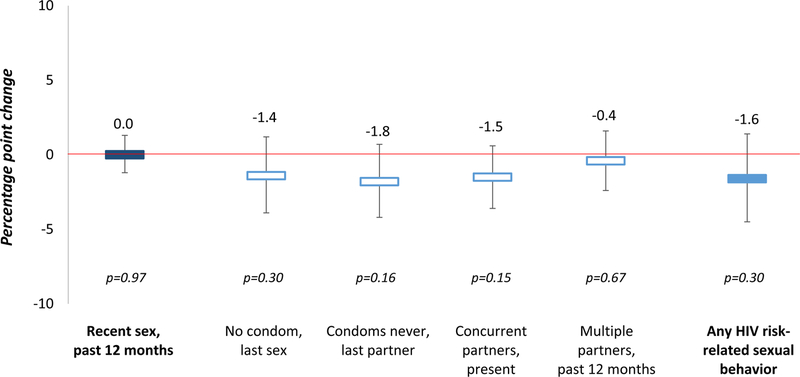
We measured effect sizes (percentage point changes) using linear probability model with individual fixed effects. Sexual behaviors associated with increased risk of HIV transmission included at least one of the following behaviors: no condom use at last sex, never using condoms with the last sexual partner, concurrent sexual partners at present, and multiple sexual partners in the past 12 months (effect size estimates for these in hollow blue).
Effect of time since incident circumcision on recent sex and HIV risk-related sexual behaviors
Figure 2 shows the effect of time since incident circumcision on recent sex and sexual behaviors associated with increased HIV risk among men in the population cohort. Again, we found no effect of time since incident circumcision on men’s report of recent sex or men’s engagement in at least one of the four HIV risk-related sexual behaviors. In the year following incident circumcision, reported sex in the past 12 months decreased by 0.4 percentage points (95% CI −2.0 to 1.2, p=0.61) relative to the year prior incident circumcision, but this effect was not significant. Men’s reports of any sexual behavior associated with an increased HIV risk also decreased by 1.5 percentage points (95% CI −5.5 to 2.6, p=0.40) in the year following incident circumcision relative to the year prior incident circumcision, but this effect was again not significant. Time since incident circumcision also did not significantly affect any of the individual HIV risk-related sexual behaviors. These findings remained essentially unchanged in our sensitivity (Appendix Table 2,http://links.lww.com/QAI/B247) and sub-group (Appendix Table 4,http://links.lww.com/QAI/B247) analyses.
Figure 2. Effect of time since circumcision on (a) sexual activity and (b) sexual behaviors associated with increased risk of HIV transmission.
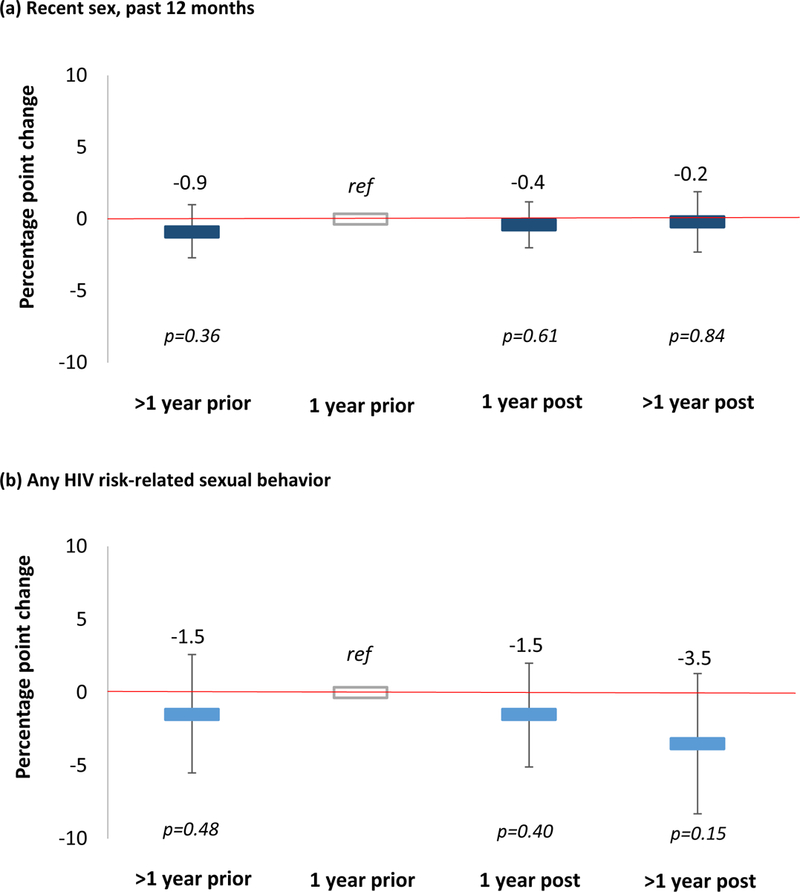
We measured effect sizes (percentage point changes) using linear probability model with individual fixed effects. Our reference category was one year prior to circumcision. Sexually active participants reported having had sex in the past 12 months. Sexual behaviors associated with increased risk of HIV transmission included: no condom use at last sex, never using condoms with the last sexual partner, concurrent sexual partners at present, and multiple sexual partners in the past 12 months.
Discussion
In this longitudinal quasi-experimental study of the effect of incident circumcision and time since incident circumcision on HIV risk-related sexual behaviors in the era following the large circumcision trials in Africa, we fail to find any evidence of sexual risk compensation. Incident circumcision and time since incident circumcision increased neither the frequency nor the riskiness of sex among men living in a community with high HIV prevalence and incidence in rural KwaZulu-Natal, South Africa. These finding are consistent with a number of previous studies14,15,18–24, including the one other study in Uganda that used longitudinal data to explore risk compensation following incident circumcision in the post-RCT era.24 Our study substantially strengthens the evidence on risk compensation following incident circumcision, because we use for the first time fixed effects panel estimation.
Our study findings indicate that biological risk of infection may not be a major driver of sexual risk taking among males. Newly circumcised men in this population cohort did not vary their sexual behavior based on a real biological risk reduction in their per-sex act risk of HIV acquisition. Detailed qualitative research could help us understand why men do not vary their sexual behavior following a biological risk reduction such as circumcision. It is possible that counseling on safe sex often delivered as part of circumcision interventions in the region may have offset changes in risk perception, leading to an overall null effect. It is, however, also possible that the opportunity for sex and psychological motivations – such as attachment, self-control or sensation seeking32–34 – are far more important in determining frequency and riskiness of sex acts than the risk perception. Our results remained the same when we repeated the analyses in the sub-groups of younger and older men. We can thus rule out that our key conclusion – circumcision does not lead to sexual risk compensation – is wrong because our main analysis did not account for effect heterogeneity by age.
The individual fixed effects panel estimation used in this study is a rigorous quasi-experimental method that allows a certain strength of causal inference. Fixed effects panel estimation controls for all baseline and other time-invariant characteristics – including those that we have not observed and thus cannot control for by including them as co-variates in the analysis.34 Factors that we did not observe – but which may be important confounders of the relationship between circumcision and sexual behavior – include psychological characteristics that are likely to guide selection into circumcision, such as attitudes towards risk, future optimism, and locus of control. Fixed effects panel estimation ensures that baseline differences in these factors cannot have biased results.
On the other hand, unlike in a randomized controlled trial we can only control for those time-varying confounders that we have observed – calendar year, age, education, and sexual debut. We cannot rule out time-varying confounders due to unobserved factors, such as individual life experiences and perceptions of peer pressure and societal norms. One powerful approach to further increase the strength of causal inference in estimating the relationship between circumcision and sexual behavior would be to carry out a randomized controlled encouragement trial35 (e.g., using data from a randomized controlled trial of an encouragement to circumcise – such as a financial incentive36 – to estimate the causal effect of circumcision on sexual behavior using instrumental variable analysis).
Our study has a number of strengths. The fixed effects panel analysis for the estimation of the relationship between incident circumcision and sexual behavior, which increases the causal strength of the results compared to the prior evidence on this topic.14,15,18–24 In addition, we estimated for the first time the effect of time since incident circumcision on sexual risk compensation, which provides a more detailed picture of the development of sexual behavior following circumcision than previous studies. Unlike men in experimental settings, the men in our study received realistic levels of pre-circumcision counseling that reflect real-world circumcision interventions and were aware of the benefits of circumcision on HIV acquisition (numerous circumcision communication campaigns were ongoing in KwaZulu-Natal during the surveillance period to increase incident circumcision).37
Our study also has several limitations. First, all sexual behavior outcomes were self-reported. Direct measures of sexual behavior are not feasible and biomarkers for sexual activity are still not established for routine population-based application, because their performance is poor and they are expensive and difficult to collect.38,39 AHRI has recently started to do small-scale, nested sampling of surveillance participants for testing of sexually transmitted infections, but few men who became circumcised were included in this sampling and so far these measurements have only taken place once.40 In the absence of biological measures, self-reported sexual behavior data is the best available option, even though it may be subject to social desirability bias and non-response. Due to the number of promotional campaigns for circumcision that took place KwaZulu-Natal during the study period, there may indeed have been time-varying social desirability bias to report being circumcised. In as far as such time-varying social desirability is shared among the individuals in our study population, however, the calendar year variables in our regressions will have controlled for this potential source of confounding. Second, circumcision status was self-reported and has never been validated in the population in which study took place. However, a study in Kenya found excellent performance of self-reported circumcision status (with 99.0% accuracy evaluated against physically verified circumcision data).41 Finally, since circumcision status is reported only once per year (and for many men even less frequently), our time to circumcision variable is imprecisely measured.
In the absence of an HIV vaccine, and in light of the recent evidence suggesting that the potential population impact of treatment-as-prevention may be difficult to realize,42 circumcision remains an important HIV prevention intervention. We recommend that policy makers continue to invest in circumcision and in encouraging men at risk of HIV infection to get circumcised to reduce the risk of HIV acquisition and work towards and AIDS-free future.43 Based on our results, fears of risk compensation are likely unwarranted and should not stand in the way of rapid progress towards universal circumcision coverage in high HIV incidence communities.
Supplementary Material
Acknowledgements
We would like to acknowledge the study participants and staff at the AHRI surveillance site for their work in providing and collecting these data.
KO, JA, GH and TB conceptualized the paper. KO conducted the analysis and wrote the first draft of the paper. NC, FT and TB oversaw acquisition of data. All authors edited the paper and models and approved the final version.
Sources of support: KO is supported by the NIH (T32AI007535–16A1, PI: George Seage; R01-MH110296, PI: Heffron; R01-MH113572, PIs: Baeten/Ngure). TB was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professorship endowed by the German Federal Ministry of Education and Research. He was also supported by the Wellcome Trust, the European Commission, the Clinton Health Access Initiative, NIAI R01- AI124389 (PIs: Bärnighausen and Tanser), and D43-TW009775 (PIs: Fawzi and Bärnighausen). Both TB and GH are supported by NICHD R01-HD084233 (PIs: Bärnighausen and Tanser). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared that no competing interests exist.
Footnotes
Conference presentation: Ortblad K, Harling G, Salomon J, Tanser F, Pillay D, Mutevedzi T, Bärnighausen T. Male circumcision and risk compensation in a high HIV prevalence population in KwaZulu-Natal, South Africa. Conference on Retroviruses and Opportunistic Infections 2017. Poster presentation. February 2017. Seattle, USA.
The authors report no conflicts of interest related to this work.
References
- 1.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2 [DOI] [PubMed] [Google Scholar]
- 2.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 2007;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4 [DOI] [PubMed] [Google Scholar]
- 3.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk: The ANRS 1265 Trial. PLOS Medicine 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newell M-L, Bärnighausen T. Male circumcision to cut HIV risk in the general population. Lancet 2007;369(9562):617–619. doi: 10.1016/S0140-6736(07)60288-8 [DOI] [PubMed] [Google Scholar]
- 5.Bunnell R, Mermin J, Cock KMD. HIV Prevention for a Threatened Continent: Implementing Positive Prevention in Africa. JAMA 2006;296(7):855–858. doi: 10.1001/jama.296.7.855 [DOI] [PubMed] [Google Scholar]
- 6.Kennedy CE, Medley AM, Sweat MD, O’Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bull World Health Organ 2010;88(8):615–623. doi: 10.1590/S0042-96862010000800014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV WHO; http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Published 2015. Accessed November 14, 2015. [PubMed] [Google Scholar]
- 10.CDC. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2014; a Clinical Practice Guideline Atlanta, GA: Centers for Disease Control and Prevention; 2014. https://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf. [Google Scholar]
- 11.Hallett TB, Singh K, Smith JA, White RG, Abu-Raddad LJ, Garnett GP. Understanding the Impact of Male Circumcision Interventions on the Spread of HIV in Southern Africa. PLoS ONE 2008;3(5). doi: 10.1371/journal.pone.0002212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kibira SPS, Sandøy IF, Daniel M, Atuyambe LM, Makumbi FE. A comparison of sexual risk behaviours and HIV seroprevalence among circumcised and uncircumcised men before and after implementation of the safe male circumcision programme in Uganda. BMC Public Health 2016;16:7. doi: 10.1186/s12889-015-2668-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zungu NP, Simbayi LC, Mabaso M, et al. HIV risk perception and behavior among medically and traditionally circumcised males in South Africa. BMC Public Health 2016;16:357. doi: 10.1186/s12889-016-3024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grund JM, Hennink MM. A qualitative study of sexual behavior change and risk compensation following adult male circumcision in urban Swaziland. AIDS Care 2012;24(2):245–251. doi: 10.1080/09540121.2011.596516 [DOI] [PubMed] [Google Scholar]
- 15.Riess TH, Achieng’ MM, Otieno S, Ndinya-Achola JO, Bailey RC. “When I was circumcised I was taught certain things”: risk compensation and protective sexual behavior among circumcised men in Kisumu, Kenya. PloS ONE 2010;5(8):e12366. doi: 10.1371/journal.pone.0012366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health Systems Trust. South African Health Review 2013/14 Durban, South Afirca: Health Systems Trust; 2014. https://www.health-e.org.za/wp-content/uploads/2014/10/South-African-Health-Review-2013-14.pdf. [Google Scholar]
- 17.Boyle GJ, Hill G. Sub-Saharan African randomised clinical trials into male circumcision and HIV transmission: methodological, ethical and legal concerns. J Law Med 2011;19(2):316–334. [PubMed] [Google Scholar]
- 18.Kong X, Kigozi G, Nalugoda F, et al. Assessment of changes in risk behaviors during 3 years of posttrial follow-up of male circumcision trial participants uncircumcised at trial closure in Rakai, Uganda. Am J Epidemiol 2012;176(10):875–885. doi: 10.1093/aje/kws179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westercamp M, Jaoko W, Mehta S, Abuor P, Siambe P, Bailey RC. Changes in Male Circumcision Prevalence and Risk Compensation in the Kisumu, Kenya Population, 2008–2013. J Acquir Immune Defic Syndr 2017;74(2):e30–e37. doi: 10.1097/QAI.0000000000001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chikutsa A, Ncube A, Mutsau S. Male Circumcision and Risky Sexual Behvaior in Zimbabwe: Evidence from the 2010–2011 Zimbabwe Demographic and Health Survey September 2013. https://www.dhsprogram.com/pubs/pdf/WP102/WP102.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westercamp N, Agot K, Jaoko W, Bailey RC. Risk compensation following male circumcision: results from a two-year prospective cohort study of recently circumcised and uncircumcised men in Nyanza Province, Kenya. AIDS Behav 2014;18(9):1764–1775. doi: 10.1007/s10461-014-0846-4 [DOI] [PubMed] [Google Scholar]
- 22.Mattson CL, Campbell RT, Bailey RC, Agot K, Ndinya-Achola JO, Moses S. Risk Compensation Is Not Associated with Male Circumcision in Kisumu, Kenya: A Multi-Faceted Assessment of Men Enrolled in a Randomized Controlled Trial. PLoS ONE 2008;3(6):e2443. doi: 10.1371/journal.pone.0002443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS 2012;26(5):609–615. doi: 10.1097/QAD.0b013e3283504a3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagaayi J, Kong X, Kigozi G, et al. Self-selection of male circumcision clients and behaviors following circumcision in a service program in Uganda. AIDS 2016;30(13):2125–2129. doi: 10.1097/QAD.0000000000001169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanser F, Hosegood V, Bärnighausen T, et al. Cohort Profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol 2008;37(5):956–962. doi: 10.1093/ije/dym211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naicker N, Kharsany ABM, Werner L, et al. Risk Factors for HIV Acquisition in High Risk Women in a Generalised Epidemic Setting. AIDS Behav 2015;19(7):1305–1316. doi: 10.1007/s10461-015-1002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bärnighausen T, Tanser F, Newell M-L. Lack of a decline in HIV incidence in a rural community with high HIV prevalence in South Africa, 2003–2007. AIDS Res Hum Retroviruses 2009;25(4):405–409. doi: 10.1089/aid.2008.0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bärnighausen T, Hosegood V, Timaeus IM, Newell M-L. The socioeconomic determinants of HIV incidence: evidence from a longitudinal, population-based study in rural South Africa. AIDS 2007;21 Suppl 7:S29–38. doi: 10.1097/01.aids.0000300533.59483.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mossong J, Grapsa E, Tanser F, Bärnighausen T, Newell M-L. Modelling HIV incidence and survival from age-specific seroprevalence after antiretroviral treatment scale-up in rural South Africa. AIDS 2013;27(15):2471–2479. doi: 10.1097/01.aids.0000432475.14992.da [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miettinen O Theoretical Epidemiology John Wiley & Sons; 1985. [Google Scholar]
- 31.Ortblad K, Bärnighausen T, Chimbindi N, Masters S, Salomon J, Harling G. Predictors of male circumcision incidence in a traditionally non-circumcising South African population-based cohor. PLoS ONE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dosch A, Rochat L, Ghisletta P, Favez N, Van der Linden M. Psychological Factors Involved in Sexual Desire, Sexual Activity, and Sexual Satisfaction: A Multi-factorial Perspective. Arch Sex Behav 2016;45(8):2029–2045. doi: 10.1007/s10508-014-0467-z [DOI] [PubMed] [Google Scholar]
- 33.Letamo G, Mokgatlhe LL. Predictors of risky sexual behaviour among young people in the era of HIV/AIDS: evidence from the 2008 Botswana AIDS Impact Survey III. Afr J Reprod Health 2013;17(3):169–181. [PubMed] [Google Scholar]
- 34.Vamos S, Cook R, Chitalu N, Mumbi M, Weiss SM, Jones D. Quality of relationship and sexual risk behaviors among HIV couples in Lusaka, Zambia. AIDS Care 2013;25(9):1102–1108. doi: 10.1080/09540121.2012.749339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West SG, Duan N, Pequegnat W, et al. Alternatives to the Randomized Controlled Trial. Am J Public Health 2008;98(8):1359–1366. doi: 10.2105/AJPH.2007.124446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thirumurthy H, Masters SH, Rao S, et al. Effect of providing conditional economic compensation on uptake of voluntary medical male circumcision in Kenya: a randomized clinical trial. JAMA 2014;312(7):703–711. doi: 10.1001/jama.2014.9087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Govender K, Gavin G, Mucheuki C, Strauss M. Voluntary Medical Male Circumcision in South Africa: Challenges and Opportunities Durban, South Afirca: Health Systems Trust; 2014:127–137. https://www.health-e.org.za/wp-content/uploads/2014/10/South-African-Health-Review-2013-14.pdf. [Google Scholar]
- 38.Corno L, de Paula A. Risky sexual behaviors: Biological markers and self-reported data. CEPR Discuss Pap No DP10271 http://www.ucl.ac.uk/~uctpand/cornodepaula_201707.pdf. [Google Scholar]
- 39.Gallo MF, Steiner MJ, Hobbs MM, Warner L, Jamieson DJ, Macaluso M. Biological Markers of Sexual Activity: Tools for Improving Measurement in HIV/Sexually Transmitted Infection Prevention Research. Sex Transm Dis 2013;40(6). doi: 10.1097/OLQ.0b013e31828b2f77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis SC, Mthiyane TN, Baisley K, et al. Prevalence of sexually transmitted infections among young people in South Africa: A nested survey in a health and demographic surveillance site. PLOS Medicine 2018;15(2):e1002512. doi: 10.1371/journal.pmed.1002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odoyo-June E, Agot K, Mboya E, et al. Agreement of self-reported and physically verified male circumcision status in Kenya. CROI 2017. Seattle, USA; 2017. http://www.croiconference.org/sessions/agreement-self-reported-and-physically-verified-male-circumcision-status-kenya. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial. PLOS Medicine 2016;13(8):e1002107. doi: 10.1371/journal.pmed.1002107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UNAIDS. Ending AIDS: Progress Towards the 90–90-90 Targets Geneva, Switzerland: UNAIDS; 2017. http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


