Abstract
Background:
Perinatally HIV-infected adolescents (PHIVA) are exposed to a chronic systemic infection and long-term antiretroviral therapy (ART), leaving them susceptible to morbidities associated with inflammation, immunodeficiency, and drug toxicity.
Methods:
Data collected 2001–2016 from PHIVA aged 10–19 years within a regional Asian cohort were analysed using competing risk time-to-event and Poisson regression analyses to describe the nature and incidence of morbidity events and hospitalisations, and identify factors associated with disease-related, treatment-related, and overall morbidity. Morbidity was defined according to WHO clinical staging criteria and US National Institutes of Health Division of AIDS criteria.
Results:
A total 3,448 PHIVA contributed 17,778 person-years. Median age at HIV diagnosis was 5.5 years and ART initiation was 6.9 years. There were 2,562 morbidity events and 307 hospitalisations. Cumulative incidence for any morbidity was 51.7% and hospitalisation was 10.0%. Early adolescence was dominated by disease-related infectious morbidity, with a trend toward non-infectious and treatment-related morbidity in later adolescence. Higher overall morbidity rates were associated with a CD4 count <350 cells/μL, HIV viral load ≥10,000 copies/mL, and experiencing prior morbidity at age <10 years. Lower overall morbidity rates were found for those aged 15–19 years compared to 10–14 years, and those who initiated ART at age 5–9 years compared to <5 or ≥10 years.
Conclusions:
Half of our PHIVA cohort experienced a morbidity event, with a trend from disease-related infectious events to treatment-related and non-infectious events as PHIVA age. Antiretroviral therapy initiation to prevent immune system damage, optimise virologic control, and minimise childhood morbidity are key to limiting adolescent morbidity.
Keywords: Adolescent, HIV, morbidity
INTRODUCTION
Adolescents with perinatal HIV infection (PHIVA) are subject to a chronic systemic infection and long-term antiretroviral therapy (ART). As such, they are at risk of an array of morbidities associated with inflammation, immune deficiency, and drug toxicity. There is a growing body of literature detailing morbidities for children and adolescents with perinatally acquired HIV infection (PHIV) that extend beyond the traditionally anticipated conditions associated with low CD4 counts to include non-infectious events that can involve multiple organ systems.1–3
Within the pediatric population surviving beyond infancy with PHIV, there are data to suggest youth bear the highest risk of HIV disease-related morbidity.4 Studies from the United States and the United Kingdom/Ireland demonstrated over half of their PHIVA cohorts had experienced either a CDC class B or C event;5,6 while a Zimbabwean study reported a WHO clinical stage 3 or 4 event in almost three-quarters of its adolescent cohort.7 This high burden of disease-related morbidity is accompanied by an evolving understanding of the vulnerability for those with PHIV to treatment-related complications due to the duration and complexity of ART exposure.8 There have been particular concerns regarding the metabolic impact of long-term ART, initially around lipodystrophy associated with earlier ART agents to dyslipidemia and insulin resistance9,10 associated with protease inhibitors (PIs) and their impact on long-term cardiovascular health.11
Much of the morbidity data to date from low- and middle-income country (LMIC) settings have been presented for broader pediatric populations, with limited data exclusively relating to PHIVA.1,2 There remains a need for more comprehensive data regarding long-term clinical complications PHIVA face,12,13 to assist HIV health care providers to prevent, monitor, and manage them, and optimise models of adolescent HIV care.4 This study aims to describe, report incidence, and identify risk factors for HIV disease- and treatment-related morbidities as well as hospitalisations experienced by PHIVA in an Asian regional cohort.
METHODS
Study population
The TREAT Asia Pediatric HIV Observational Database (TApHOD) of IeDEA Asia-Pacific was established in 2007 to study regional pediatric HIV treatment outcomes in the context of routine care. Detailed methods of the study have previously been reported.14 In brief, data are collected during the course of HIV care across 16 pediatric HIV services in Asia (Cambodia=1, India=1, Indonesia=2, Malaysia=4, Thailand=5, and Vietnam=3), including demographic characteristics, WHO clinical events, laboratory results (e.g., CD4, viral load, haematology, biochemistry, lipid profile), as well as ART regimens, adverse events and reasons for stopping/changing ART agents. Data are de-identified and then transferred to the study’s central data management and biostatistical center at The Kirby Institute (University of New South Wales, Sydney, Australia) on a 6-monthly basis. For this analysis, any PHIVA (ART naïve or experienced) aged 10–19 years at any time during follow-up was eligible for inclusion; data available up to December 2016 were analyzed. Ethics approvals were obtained through the human research ethics committees at the participating sites, The Kirby Institute, and the coordinating center at TREAT Asia/amfAR (Bangkok, Thailand).
Definitions
Morbidity was categorised into two mutually exclusive groups: (1) disease-related morbidity was defined as any reported symptomatic HIV-related clinical event meeting WHO clinical staging criteria;15 and (2) treatment-related morbidity was defined as any reported suspected or confirmed ART-associated adverse event meeting the US National Institutes of Health Division of AIDS (DAIDS) criteria for adverse event.16 Reporting and classifying a disease- or treatment-related morbidity event at the patient level was at the discretion of the treating physician, following the study’s standardized electronic database and data exchange standard. Overall morbidity incorporated both disease- and treatment-related morbidity events. Morbidities were also classified based on the nature of the event, as being either an infection, or an organ specific non-infectious event (additional information on request through corresponding author). Loss to follow-up (LTFU) was determined by either reporting of LTFU by the treating site or a >12-month absence of reported clinical data prior to the time of the last data transfer from the participating site to the central data management center. Transition to an adult HIV service was defined as either a specific report of transfer to adult HIV care, or any transfer to another site when ≥16 years of age. The beginning of follow-up (baseline) was either the subject’s 10th birthday or first clinic visit for those who commenced care after their 10th birthday.
Statistical analysis
Descriptive analyses were used to report the study population characteristics, as well as the number and nature of morbidity events and hospitalisations reported during adolescence. Crude incidence rates with 95% confidence intervals (CI) were calculated for morbidity events and hospitalisations, with person-year observations calculated from baseline to death, LTFU, last reported clinic visit (if it occurred before 20 years of age) or the day prior to their 20th birthday (if remaining in active care at or after 20 years of age). Time-to-event analyses, with death and LTFU as competing events, were used to determine the cumulative incidence for experiencing a first morbidity event or first hospitalisation event during adolescence. Poisson regression was used to determine incidence rate ratios (IRR) and assess for factors associated with disease-related morbidity, treatment-related morbidity, and overall morbidity. Covariates with a p-value <0.1 on univariate analysis were included in multivariate analyses. Multivariate analyses were conducted in a step-wise fashion maintaining covariates that retained a p-value of <0.05. Covariates included age, sex, CD4 count, HIV viral load, age at ART initiation, and having experienced earlier childhood (<10 years of age) disease- or treatment-related morbidity. Age, CD4 count and HIV viral load were analysed as time-dependent variables. Repeated reporting of the same WHO clinical event (of recurrent or chronic nature) was considered a single morbidity event, with date of onset as the earliest date of reporting. Likewise, repeated reporting of the same ART adverse event was considered a single morbidity event if the ART regimen remained unchanged, with date of onset as the earliest date of reporting. All statistical analyses were performed using Stata version 14.2 (StataCorp LP, College Station, Texas, US).
RESULTS
There were 3,448 adolescents included in the analysis, with data collected from 2001 to 2016. The median duration of follow-up during adolescence was 4.7 [IQR 2.3, 7.1] years, with a total 17,778 person-years for the whole cohort. Females represented 51.3% of the study population. Median age at HIV diagnosis was 5.5 [interquartile range (IQR) 2.9, 8.4] years, and median age at ART initiation was 6.9 [IQR 4.1, 9.7] years. There were 163 (4.7%) PHIVA LTFU at a median age of 15.4 [IQR 12.4, 17.4] years, and 119 (3.5%) had died at a median age of 13.0 [IQR 11.1, 15.8] years. A total of 2,328 PHIVA were in active care at a median age of 13.8 [IQR 12.0, 15.9] years at the end of the follow-up period. At baseline, 2,142 (62.1%) PHIVA had a CD4 count ≥500 cells/μL, 354 (10.3%) had a CD4 count of 350–499 cells/μL, 254 (7.4%) had a CD4 count of 200–349 cells/μL, 544 (15.8%) had a CD4 count <200 cells/μL, and 154 (4.5%) had unknown or missing data. The overall baseline median CD4 count was 660 [IQR 362, 940] cells/μL. Baseline HIV viral load characteristics included 1,655 (48.0%) PHIVA with a HIV viral load <400 copies/mL, 63 (1.8%) with a HIV viral load of 400–999 copies/mL, 140 (4.1%) with a HIV viral load of 1,000–9,999 copies/mL, 421 (12.2%) with a HIV viral load ≥10,000 copies/mL, and 1,169 (33.9%) with unknown or missing data. The median frequency of CD4 testing was 175 [IQR 161, 189] days and median frequency of HIV viral load testing was 175 [IQR 118, 329] days. There were 2,213 (64.2%) PHIVA who had experienced a prior disease-related morbidity event and 296 (8.6%) PHIVA who had experienced a prior treatment-related morbidity event before the age of 10 years. Total exposure to nucleoside reverse transcriptase inhibitors (NRTIs) during adolescence included 14,227 person-years for lamivudine, 9,032 for zidovudine, 2,495 person-years for tenofovir, and 2,327 person-years for stavudine. While there was a total 11,245 person-years exposure to non-nucleoside reverse transcriptase inhibitors (NNRTIs), and a total 4,481 person-years exposure to PIs.
Overall morbidity
Overall, there were 2,562 morbidity events experienced by 1,268 (36.8%) PHIVA at a median age of 12.3 [IQR 10.9, 14.4] years. The median interval between CD4 count testing and a morbidity event was 29 [IQR 0, 93] days, and median interval between HIV viral load testing and a morbidity event was 90 [IQR 1, 203] days. The crude incidence rate for experiencing any morbidity event was 14.4 [95%CI 13.9, 15.0] events per 100 person-years. Supplemental Digital Content 1 (table) summarises the relative proportions and crude incidence rates for specific morbidities. Infections represented the highest burden of morbidity (38.6%), followed by metabolic/endocrine events (17.5%) and dermatological/mucosal events (15.3%). The time-to-event analysis, with LTFU and death as competing events, demonstrated the cumulative incidence of overall adolescent morbidity to be 51.7% (Figure 1). Figure 2 shows the cumulative incidence of selected specific morbidities, which demonstrates a plateau in the cumulative incidence of infection and dermatological/mucosal morbidity events; a steady rise for metabolic/endocrine and haematological events; and an upward trend in renal and hepatic events. Multivariate regression analysis showed the following factors to be associated with a higher incidence rate for any morbidity event: pre-event CD4 count <350 cells/μL (200–349 cells/μL IRR 1.9 [95%CI 1.5, 2.4]; <200 cells/μL IRR 2.6 [95%CI 2.1, 3.4]); pre-event HIV viral load ≥10,000 copies/mL (IRR 1.9 [95%CI 1.5, 2.3]); having experienced a prior morbidity event at <10 years of age (IRR 1.9 [95%CI 1.4, 2.5]); and initiating ART age <5 or ≥10 years compared to initiating ART age 5–9 years (<5 years IRR 1.6 [95%CI 1.3, 2.0]; ≥10 years IRR 1.5 [95%CI 1.1, 2.0]) (Table 1). Age 15–19 years was associated with a lower incidence rate for any morbidity event (IRR 0.7 [95%CI 0.6, 0.8]) (Table 1).
Figure 1.
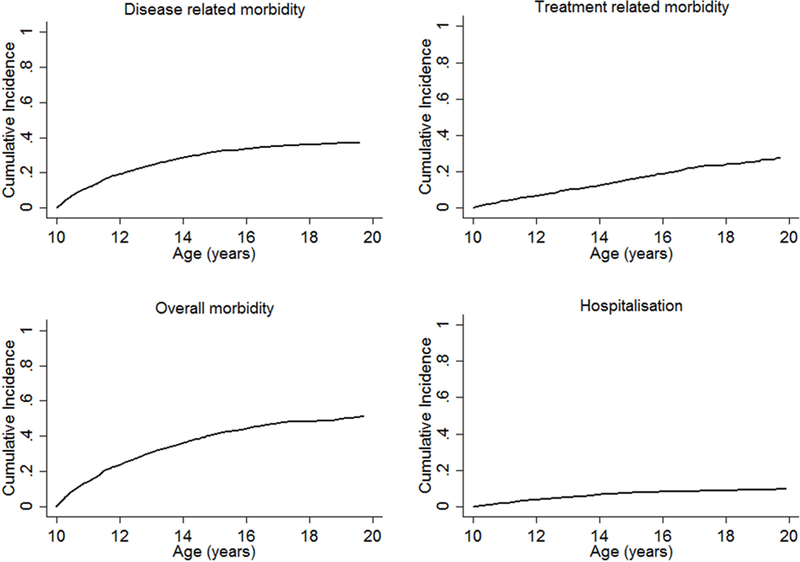
Cumulative incidence of disease-related morbiditya, treatment-related morbidityb, overall morbidityc, and hospitalisations experienced during adolescence.aAny reported symptomatic HIV-related clinical event meeting WHO clinical staging criteria.15 bAny reported suspected or confirmed anitretroviral-associated adverse event meeting the US National Institutes of Health Division of AIDS criteria for adverse events.16 cIncorporates both disease- and treatment-related morbidity.
Figure 2.
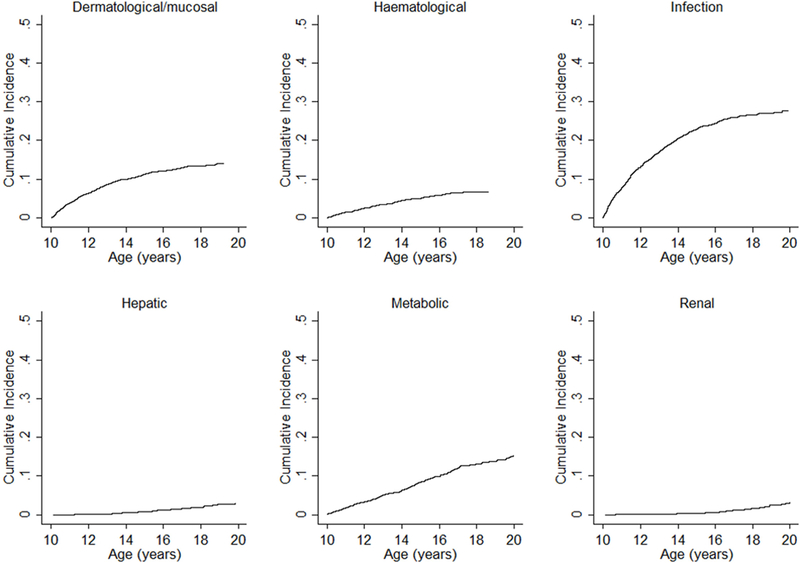
Cumulative incidence of specific morbidities experienced during adolescence.
Table 1.
Adjusted analysis for factors associated with disease-related, treatment-related, and overall morbidity experienced during adolescence.
| Disease-related morbiditya | Treatment-related morbidityb | Overall morbidityc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Event/person-years | IRR [95%CI] | p | Events/person-years | IRR [95%CI] | p | Events/person-years | IRR [95%CI] | p |
| Age (years) | |||||||||
| 10–14 | 1469/12,211 | 1.0 | 580/12,186 | NS | 2,046/12,235 | 1.0 | |||
| 15–19 | 170/5,558 | 0.2 [0.2, 0.3] | <0.001 | 343/5,566 | NS | 513/5,543 | 0.7 [0.6, 0.8] | <0.001 | |
| Sex | |||||||||
| Male | 781/8,378 | NS | 432/8,375 | NS | 1,213/8,382 | NS | |||
| Female | 858/9,392 | NS | 491/9,377 | NS | 1,349/9,396 | NS | |||
| CD4 count (cells/μL) | |||||||||
| ≥500 | 393/11,835 | 1.0 | 590/11,865 | 1.0 | 983/11,838 | 1.0 | |||
| 350–499 | 149/2,404 | 1.3 [1.0, 1.7] | 0.04 | 101/2,424 | 0.8 [0.6, 1.0] | 0.05 | 250/2,390 | 1.0 [0.8, 1.2] | 0.9 |
| 200–349 | 187/1,382 | 2.3 [1.7, 3.0] | <0.001 | 87/1,424 | 1.5 [1.1, 2.0] | 0.02 | 274/1,377 | 1.9 [1.5, 2.4] | <0.001 |
| <200 | 638/1,745 | 5.3 [4.1, 6.8] | <0.001 | 128/1,912 | 1.8 [1.3, 2.4] | <0.001 | 766/1,738 | 2.6 [2.1, 3.4] | <0.001 |
| HIV viral load (copies/mL) | |||||||||
| <400 | 242/11,368 | 1.0 | 619/11,447 | 1.0 | 861/11,363 | 1.0 | |||
| 400–999 | 16/370 | 1.4 [0.8, 2.3] | 0.3 | 22/379 | 0.7 [0.4, 1.2] | 0.2 | 38/369 | 0.9 [0.6, 1.4] | 0.6 |
| 1,000–4,999 | 49/560 | 2.9 [2.0, 4.0] | <0.001 | 21/568 | 0.6 [0.4, 1.1] | 0.08 | 70/560 | 1.2 [0.8, 1.6] | 0.3 |
| 5,000–9,999 | 17/268 | 2.1 [1.2, 3.5] | 0.006 | 23/273 | 1.1 [0.7, 1.9] | 0.7 | 40/269 | 1.3 [0.9, 2.0] | 0.2 |
| ≥10,000 | 390/1,846 | 4.8 [3.8, 6.0] | <0.001 | 107/1,921 | 0.9 [0.7, 1.3] | 0.7 | 497/1,831 | 1.9 [1.5, 2.3] | <0.001 |
| Age at ART initiation (years) | |||||||||
| <5 | 254/4,518 | 1.1 [0.8, 1.4] | 0.5 | 310/4,503 | 1.8 [1.5, 2.3] | <0.001 | 564/4,520 | 1.6 [1.3, 2.0] | <0.001 |
| 5–9 | 448/8,091 | 1.0 | 343/8,090 | 1.0 | 791/8,094 | 1.0 | |||
| ≥10 | 932/5,144 | 1.0 [0.8, 1.2] | 0.9 | 270/5,144 | 1.7 [1.2, 2.4] | 0.006 | 1,202/5,148 | 1.5 [1.1, 2.0] | 0.02 |
| Naive | 5/17 | - | - | - | - | - | 5/17 | - | - |
| Disease-related morbidity <10 years of age | |||||||||
| No | 143/2,699 | NS | 90/2,699 | 1.0 | NA | NA | |||
| Yes | 638/10,343 | NS | 582/10,335 | 1.5 [1.1, 2.1] | 0.005 | NA | NA | ||
| Treatment-related morbidity <10 years of aged | |||||||||
| No | 700/11,741 | NS | 527/11,733 | 1.0 | NA | NA | |||
| Yes | 81/1,301 | NS | 145/1,301 | 2.0 [1.5, 2.6] | <0.001 | NA | NA | ||
| Any morbidity <10 years of age | |||||||||
| No | NA | NA | NA | NA | 228/2,592 | 1.0 | |||
| Yes | NA | NA | NA | NA | 1,225/10,456 | 1.9 [1.4, 2.5] | <0.001 | ||
Adjusted analysis results only reported for characteristics that had a p-value <0.1 on univariate analysis. Bold signifies statistically significant variable.
Any reported symptomatic HIV-related clinical event meeting WHO clinical staging criteria.15
Any reported suspected or confirmed antiretroviral-associated adverse event meeting the US National Institutes of Health Division of AIDS criteria for adverse events.16
Incorporates both disease- and treatment-related morbidity. ART = antiretroviral therapy. CI = confidence interval. IRR = incidence rate ratio. NA = not analysed. NS = not significant on univariate analysis.
Disease-related morbidity
There were 1,639 disease-related morbidity events experienced by 975 (28.3%) adolescents at a median age of 11.7 [IQR 10.7, 13.3] years. The crude incidence rate was 9.2 [95%CI 8.8, 9.7] events per 100 person-years. Of the 1,639 events, 44 (2.7%) were classified as WHO stage I, 813 (49.6%) as WHO stage II, 500 (30.5%) as WHO stage III, and 282 (17.2%) as WHO stage IV. Supplemental Digital Content 1 (table) shows the relative proportions of specific disease-related morbidity events, with infections being the major contributor (60.3%), followed by dermatological/mucosal events (19.2%). Figure 1 shows the cumulative incidence of disease-related morbidity events, demonstrating a plateau with increasing age. On multivariate regression analysis the following factors were found to be associated with a higher incidence rate of disease-related morbidity: CD4 count <500 cells/μL (350–499 cells/μL IRR 1.3 [95%CI 1.0, 1.7]; 200–349 cells/μL IRR 2.3 [95%CI 1.7, 3.0]; <200 cells IRR 5.3 [95%CI 4.1, 6.8]); and a HIV viral load ≥1,000 copies/mL (1,000–4,999 copies/mL IRR 2.9 [95%CI 2.0, 4.0]; 5,000–9,999 copies/mL IRR 2.1 [95%CI 1.2, 3.5]; ≥10,000 copies/mL IRR 4.8 [95%CI 3.8, 6.0]) (Table 1). Age 15–19 years was associated with a lower incidence rate of disease-related morbidity (IRR 0.2 [95%CI 0.2, 0.3]) (Table 1).
Treatment-related morbidity
There were 923 treatment-related morbidity events reported in 556 (16.1%) adolescents. The crude incidence rate of treatment-related morbidity events was 5.2 [95%CI 4.9, 5.5] per 100 person-years. Of the 923 treatment-related morbidity events, 423 (45.8%) were DAIDS grade I/II, 113 (12.2%) were grade III/IV, and 387 (41.9%) were unknown/missing. Supplemental Digital Content 1 (table) shows the relative contributions of specific treatment-related morbidity events, with metabolic/endocrine events representing the main contributor (48.5%) followed by haematological events (12.4%). Figure 1 shows the cumulative incidence of treatment-related morbidity events, which demonstrates a steady increase with age. On multivariate regression analysis, the following factors were associated with a higher incidence rate of treatment-related morbidity: CD4 count <350 cells/μL (200–349 cells/μL IRR 1.5 [95%CI 1.1, 2.0]; <200 cells/μL IRR 1.8 [95%CI 1.3, 2.4]); prior disease-related morbidity at <10 years of age (IRR 1.5 [95%CI 1.1, 2.1]); prior treatment-related morbidity at <10 years of age (IRR 2.0 [95%CI 1.5, 2.6]); and initiating ART age <5 or ≥10 years compared to initiating ART age 5–9 years (<5 years IRR 1.8 [95%CI 1.5, 2.3]; ≥10 years IRR 1.7 [95%CI 1.2, 2.4]) (Table 1).
Hospitalisation
There were 307 morbidity-related hospitalisation events experienced by 234 (6.8%) adolescents, at a median age of 12.2 [IQR 11.0, 13.8] years. The crude hospitalisation rate was 1.8 [95%CI 1.6, 2.0] per 100 person-years. Figure 1 shows the cumulative incidence of hospitalisation during adolescence, which was 10.0%, and demonstrates a plateau in hospitalisations with age. Infections were the main reason for hospitalisation (72.2%), while other causes each contributed <5% to the total (see Table, Supplemental Digital Content 1).
DISCUSSION
This study provides an in-depth overview of the burden and nature of morbidity and hospitalisations experienced by PHIVA in Asia. The cumulative incidence of experiencing a morbidity event during adolescence in our cohort was 50%, which was predominantly disease-related during early adolescence, comprising mainly of opportunistic and other infections. However, as adolescents approached adulthood there was a relative decline in infections and disease-related morbidity in general, and in increase in non-infectious and treatment-related morbidity. This demonstrates an evolving pattern of morbidity from infectious to non-infectious complications associated with HIV and its management. Adolescent HIV health care providers must contend with this shift in HIV-related morbidity, and integrate longer-term treatment and monitoring strategies to minimise treatment-related complications as PHIVA age.17,18 Of note, metabolic/endocrine events dominated treatment-related morbidities in our PHIVA cohort, representing about half of all reported treatment-related events. This is consistent with the established concerns regarding the metabolic effects of ART in children and adolescents,9,11,19 and are an important consideration when designing optimal long-term ART regimens, particularly given the increased risk of cardiovascular disease for adults living with HIV.20,21
The younger adolescent age group had a significantly higher rate of overall morbidity compared to their older counterparts, which is mainly attributable to higher disease-related morbidity. This reflects that at least one-third of our cohort had some degree of immune deficiency or were not virologically suppressed on entry to adolescence, and highlights the need to improve early diagnosis and initiation of ART to gain better disease control throughout childhood. In addition, the comparatively lower rate of morbidity events among the older adolescent age group may also be indicative of those with better disease control surviving into older adolescence who were retained in care and on treatment. Other risk factors identified for experiencing any morbidity event included having a pre-event CD4 count <350 cells/μL, a pre-event HIV viral load ≥10,000 copies/mL, and having experienced a prior morbidity event in earlier childhood (<10 years of age). These factors all relate to poor disease control during childhood or adolescence, which could be a result of delayed diagnosis or treatment, lack of treatment adherence, or virologic failure; and demonstrate the complexities of managing HIV as a chronic disease. Efforts to improve childhood HIV morbidity and mortality has led to the evolution in recommendations for PHIV toward universal early infant diagnosis and ART initiation irrespective of disease status.17,22 However, these efforts need to be followed by long-term strategies to retain patients on effective and durable ART regimens to optomise outcomes.23–26 The impact of CD4 count and HIV viral load was more pronounced for disease-related morbidity, with a pre-event CD4 count <500 cells/μL and a pre-event HIV viral load of ≥1,000 copies/mL shown to be risk factors. This may reflect the inclusion of all WHO clinical stage events, and contributes to the increasing recognition of non AIDS-defining conditions complicating the long-term management of children and adolescents living with PHIV.1,2
Our analysis on the impact of ART showed that after controlling for age at morbidity event, pre-event CD4 count, and pre-event HIV viral load, age at ART initiation was not shown to reduce the incidence rate of disease-related morbidity during adolescence. However, age at ART initiation did have an impact on treatment-related and overall morbidity, with those commencing ART aged 5–9 years having lower incidence rates compared to those commencing ART <5 years or ≥10 years of age. In the context of our cohort having a median age at ART start of 6.9 years, the poorer outcomes for children initiating ART <5 years of age may reflect those presenting for diagnosis and treatment earlier having more rapidly progressing disease with longer ART exposure; while the higher morbidity rates for those ≥10 years of age at ART initiation may also be due to more advanced disease at presentation relative to those 5–9 years of age. A national Thai study found those who commenced ART 5 to <9 years were at lower risk of LTFU and death compared to those who commenced ART <5 years or ≥9 years of age.27 The impact of age at ART initiation in our morbidity analysis, in conjunction with these mortality and LTFU results, not only signify the poorer outcomes for those with PHIV who do not enter care until adolescence, but also the challenges associated with long-term disease control and retention in care for those with PHIV managed from early childhood.
One in 10 adolescents in our cohort required hospitalisation as result of a morbidity event. Consistent with earlier studies among PHIVA,28–30 infection was the main reason for hospitalisation, accounting for almost three-quarters of morbidity-related hospitalisations. Our hospitalisation rate of 1.8 per 100 person-years was much lower than that reported for a US cohort aged 5–16 years (14.7 per 100 person-years) and 17–24 years (34.2 per 100 person-years);29 and higher than that reported in a UK/Ireland cohort aged 0–22 years (0.7 per 100 person-years).31 With the evolving pattern of PHIVA morbidity, further studies evaluating hospitalisations in PHIVA are required to better understand trends in incidence, duration, and nature, in order to guide service provision and clinical management to minimise requirements for hospitalisation.
The main limitations for this analysis include the observational nature of the study and the risk of incomplete and inconsistent data reporting, particularly with regards to hospitalisations, and the variability in diagnosing, grading, and reporting ART adverse events. These limitations could lead to an underestimation of hospitalisation requirements and treatment-related morbidity, as well as promote a bias toward disease-related morbidity over treatment-related morbidity in evaluating overall morbidity. Moreover, our results need to be interpreted in the context of an adolescent population that survived long enough to start treatment at older ages and with more advanced immunodeficiency.
In conclusion, this study shows that our PHIVA cohort were primarily diagnosed later in childhood, and experienced substantial HIV disease- or treatment-related morbidity during adolescence. There was a predominance of opportunistic and other infections in early adolescence followed by a trend towards non-infectious morbidity and treatment-related morbidity in older adolescence. Of particular concern are the emerging metabolic complications, which may have implications for their HIV care in adult life. Timely initiation of ART to prevent immune system damage, optimise virologic control, and minimise childhood morbidity are key to limiting adolescent morbidity.
See Supplemental Digital Content 2 for Acknowlegements.
Supplementary Material
Footnotes
Conflicting interests and sources of funding
AHS has received travel and grant support to her institution from ViiV Healthcare. Other authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute of Mental Health, and National Institute on Drug Abuse as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. AWB received support from an Australian Government Research Training Program Scholarship. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
REFERENCES
- 1.Vreeman RC, Scanlon ML, McHenry MS, Nyandiko WM. The physical and psychological effects of HIV infection and its treatment on perinatally HIV-infected children. J Int AIDS Soc. 2015;18(Suppl 6):20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14(7):627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mofenson LM, Cotton MF. The challenges of success: adolescents with perinatal HIV infection. J Int AIDS Soc. 2013;16:18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neilan AM, Karalius B, Patel K, et al. Association of Risk of Viremia, Immunosuppression, Serious Clinical Events, and Mortality With Increasing Age in Perinatally Human Immunodeficiency Virus-Infected Youth. JAMA Pediatr. 2017;171(5):450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Dyke RB, Patel K, Siberry GK, et al. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. J Acquir Immune Defic Syndr. 2011;57(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster C, Judd A, Tookey P, et al. Young people in the United Kingdom and Ireland with perinatally acquired HIV: the pediatric legacy for adult services. AIDS Patient Care STDs. 2009;23(3):159–166. [DOI] [PubMed] [Google Scholar]
- 7.Makadzange AT, Higgins-Biddle M, Chimukangara B, et al. Clinical, Virologic, Immunologic Outcomes and Emerging HIV Drug Resistance Patterns in Children and Adolescents in Public ART Care in Zimbabwe. PloS One. 2015;10(12):e0144057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlow-Mosha L, Eckard AR, McComsey GA, Musoke PM. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc. 2013;16:18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alam N, Cortina-Borja M, Goetghebuer T, et al. Body fat abnormality in HIV-infected children and adolescents living in Europe: prevalence and risk factors. J Acquir Immune Defic Syndr. 2012;59(3):314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Augustemak de Lima LR, Petroski EL, Moreno YMF, et al. Dyslipidemia, chronic inflammation, and subclinical atherosclerosis in children and adolescents infected with HIV: The PositHIVe Health Study. PloS One. 2018;13(1):e0190785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn AH, Hazra R. The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66 Suppl 2:S144–153. [DOI] [PubMed] [Google Scholar]
- 14.Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol. 2011;40(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO case definitions of HIV surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. World Health Organization, 2007. Available at http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. [Accessed 20 July 2017]. [Google Scholar]
- 16.Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events Version 2.0. US Institutes of Health, November 2014. Available at https://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf. [Accessed 20 July 2017]. [Google Scholar]
- 17.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public helath approach (2nd Edition). World Health Organization, 2016. Available at http://www.who.int/hiv/pub/arv/arv-2016/en/. [Accessed 11 April 2018]. [PubMed] [Google Scholar]
- 18.Bamford A, Turkova A, Lyall H, et al. Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV Med 2015;19(1):e1–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortuny C, Deya-Martinez A, Chiappini E, Galli L, de Martino M, Noguera-Julian A. Metabolic and renal adverse effects of antiretroviral therapy in HIV-infected children and adolescents. Pediatr Infect Dis J 2015;34(5 Suppl 1):S36–43. [DOI] [PubMed] [Google Scholar]
- 20.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44(12):1625–1631. [DOI] [PubMed] [Google Scholar]
- 22.WHO recommendations on the diagnosis of HIV infection in infants and children. World Health Organization, 2010. Available at http://www.who.int/hiv/pub/paediatric/diagnosis/en/. [Accessed 11 April 2018]. [PubMed] [Google Scholar]
- 23.Mark D, Armstrong A, Andrade C, et al. HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub-Saharan African countries. J Int AIDS Soc. 2017;20(Suppl 3):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kranzer K, Bradley J, Musaazi J, et al. Loss to follow-up among children and adolescents growing up with HIV infection: age really matters. J Int AIDS Soc. 2017;20(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey H, Cruz MLS, Songtaweesin WN, Puthanakit T. Adolescents with HIV and transition to adult care in the Caribbean, Central America and South America, Eastern Europe and Asia and Pacific regions. J Int AIDS Soc. 2017;20(Suppl 3):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanoni BC, Archary M, Buchan S, Katz IT, Haberer JE. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: the Cresting Wave. BMJ Glob Health. 2016;1(3):e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teeraananchai S, Kerr SJ, Puthanakit T, et al. Attrition and Mortality of Children Receiving Antiretroviral Treatment through the Universal Coverage Health Program in Thailand. J Pediatr. 2017;188:210–216. [DOI] [PubMed] [Google Scholar]
- 28.Viani RM, Araneta MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39(5):725–731. [DOI] [PubMed] [Google Scholar]
- 29.Berry SA, Gebo KA, Rutstein RM, et al. Trends in hospitalizations among children and young adults with perinatally acquired HIV. Pediatr Infect Dis J. 2014;33(5):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kourtis AP, Bansil P, Posner SF, Johnson C, Jamieson DJ. Trends in hospitalizations of HIV-infected children and adolescents in the United States: analysis of data from the 1994–2003 Nationwide Inpatient Sample. Pediatrics. 2007;120(2):e236–243. [DOI] [PubMed] [Google Scholar]
- 31.Judd A, Doerholt K, Tookey PA, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis. 2007;45(7):918–924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


