Abstract
Neutrophils clear viruses, but excessive neutrophil responses induce tissue injury and worsen disease. Aging increases mortality to influenza infection; however, whether this is due to impaired viral clearance or a pathological host immune response is unknown. Here, we show that aged mice have higher levels of lung neutrophils than younger mice after influenza viral infection. Depleting neutrophils after, but not before, infection substantially improves the survival of aged mice without altering viral clearance. Aged alveolar epithelial cells (AECs) have a higher frequency of senescence and secrete higher levels of the neutrophil-attracting chemokines CXCL1 and CXCL2 during influenza infection. These chemokines are required for age-enhanced neutrophil chemotaxis in vitro. Our work suggests that aging increases mortality from influenza in part because senescent AECs secrete more chemokines, leading to excessive neutrophil recruitment. Therapies that mitigate this pathological immune response in the elderly might improve outcomes of influenza and other respiratory infections.
Introduction
The elderly exhibit a greater mortality and morbidity to influenza virus (IAV) infection than younger people, which poses a considerable burden on health care resources throughout the globe 1–4. Aging of the immune system is a likely reason why older people exhibit this increased mortality. Impaired immunity to influenza infection with aging has been examined mostly in murine models and can be broadly categorized by changes in the innate or adaptive immune system 5, 6. Regarding innate immunity and influenza infection, there is evidence of reduced dendritic cell and natural killer cell activation and impaired in vitro type I interferon responses in human monocytes with aging 7, 8. With respect to adaptive immunity to influenza, aging impairs CD8+ T cell and follicular T cell responses and leads to increased expansion of regulatory T cells 9–12. Overall, these findings indicate that declines in both innate and adaptive immunity with aging compromise host defense to influenza.
Neutrophils are circulating innate immune cells that traffic to sites of infection to contain pathogens 13. Neutrophils are recruited to infected sites by attaching to the vascular endothelium followed by para and transcellular migration through the endothelium into the tissue 14. Not only do neutrophils exhibit effector functions, for example phagocytosis, degranulation and the production of extracellular traps to contain pathogens, but they also guide adaptive immune cells, e.g., CD8+ T cells, to sites of infection to promote pathogen clearance 15. Thus, neutrophils are key innate immune cells that orchestrate innate immunity and regulate adaptive immunity.
In addition to combating bacterial infections, there is evidence that neutrophils control viral infections, including influenza 16–18. The local inflammatory environment within the lung promotes neutrophil recruitment 19. Once neutrophils have arrived within the lung they assist viral clearance, as shown in young mice depleted of neutrophils at the time of infection, which exhibited accelerated mortality and impaired viral clearance 17. Neutrophils promote clearance of influenza virus in the lung by producing extracellular traps, phagocytosing viral particles, and activating the inflammasome 18, 20. However, excessive neutrophil functions beyond viral clearance can promote immune pathology 21–23. To prevent immune pathology during infection, neutrophils are removed by resident macrophages 24. The dual functions of neutrophils during influenza infection were suggested in a prior study in young mice which found that partial depletion of neutrophils, via low dose depleting monoclonal antibody administration, improved survival during influenza viral infection, whereas full depletion of neutrophils with high dose monoclonal antibody accelerated mortality 21.
People over the age of 60 years exhibit cell-intrinsic defects within circulating neutrophils, including decreased phagocytosis of bacteria, reduced production of reactive oxidative species and reduced chemotaxis compared to neutrophils isolated from younger humans 25. Furthermore, with human aging, peripheral blood neutrophils exhibit dysregulated migration 26, 27 and a reduced ability to produce extracellular traps 28. In addition to these cell-intrinsic defects with aging, there is evidence that aging leads to heightened systemic, low-grade inflammation such as increases in TNF-α 29, which could act as cell-extrinsic factors to impact neutrophil response during influenza infection. However, it is not known how aging impacts neutrophil responses to influenza viral infection in vivo.
Results
Aging is associated with increased neutrophil accumulation during influenza infection.
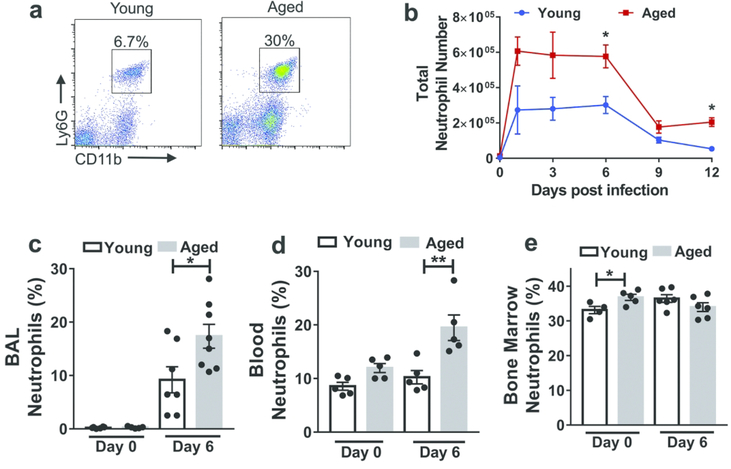
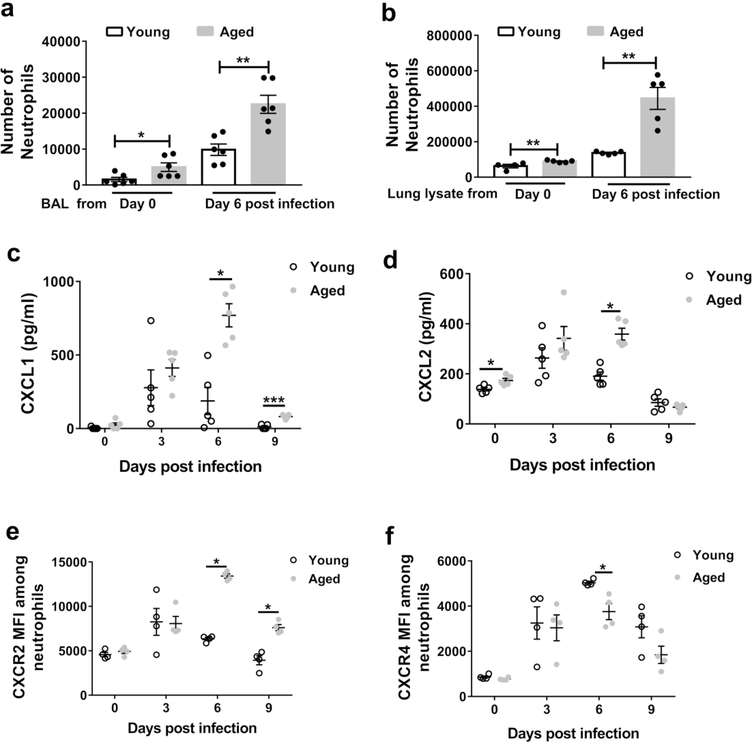
To examine whether aging impacts the role of neutrophils during IAV infection, we employed a murine model that exhibits an age-dependent increase in both morbidity and mortality during IAV infection 12, 30, 31. First, we infected young (2–4 months of age) and aged (18–22 months of age) C57BL/6 mice intranasally with influenza virus (PR8 strain), and used flow cytometric analysis to count the numbers of neutrophils (i.e., Ly6Ghi CD11b+ cells) within whole lungs (Figure 1a). Neutrophil accumulation peaked one day post infection (p.i.) for both aged and young mice. Infected aged mice showed a three fold increase in neutrophil levels six days p.i. compared to infected young (Figure 1a-b). The age-associated increase in neutrophil levels was prominent throughout the first week p.i. (Figure 1b). After the first week p.i., neutrophils numbers declined in both groups, but still remained higher in aged mice compared to young mice at day 12 p.i.
Figure 1. Aging is associated with increased neutrophils within the lung during influenza infection.
Aged (18–22 months of age) and young (2–4 months of age) C57BL/6 mice were intranasally inoculated with PR8 strain influenza virus. Lung tissue was harvested, digested and cells were obtained and suspended to permit incubation with fluorescent monoclonal antibodies followed by flow cytometric analysis.
a Representative flow cytometric plot at day 6 p.i. (post-infection) of a young and aged mouse showing increased proportion of neutrophils (CD11b+, Ly6Ghi) within the lung in the aged mouse than the young mouse. The flow cytometric plots are gated on CD11b+Ly6Ghi cells.
b Absolute number of neutrophils before and during influenza infection in young and aged mice in entire lung (airspace, vasculature and lung interstitium). * P < 0.05, (Mann-Whitney test). n = 3–6 / group/ time point. Data representative of one of two independent experiments. c,d,e Proportion of neutrophils analyzed by flow cytometry in non-infected and infected (day +6 p.i.) young and aged mice in BAL (c) blood (d) and bone marrow (e). * P < 0.05, ** P < 0.01 (Mann-Whitney test). Data representative of one of two independent experiments,which yielded similar results.
To examine whether the increase in neutrophils in the infected lungs was in the airspace compartment, we examined bronchioalveolar lavage (BAL). Before infection, we did not detect neutrophils in the BAL of either young or aged mice (Figure 1c). At day 6 p.i., we detected neutrophils in the BAL of influenza-infected young and aged mice, and the proportion of neutrophils was two-fold higher in aged mice compared to young mice (Figure 1c). Relative to young mice, aged mice also showed higher frequencies of circulating blood neutrophils after infection, but not before (Figure 1d). Lastly, before infection, aged mice exhibited a 5% increase in neutrophil frequency within the bone marrow, compatible with the known myeloid skewing that occurs within the bone marrow with aging 32, although this difference was not evident at day 6 p.i., (Figure 1e). Overall, these data demonstrate that neutrophil levels in the lung airspace are higher in aged infected mice than in young infected mice.
Depletion of neutrophils before or after infection has opposing effects on mortality.
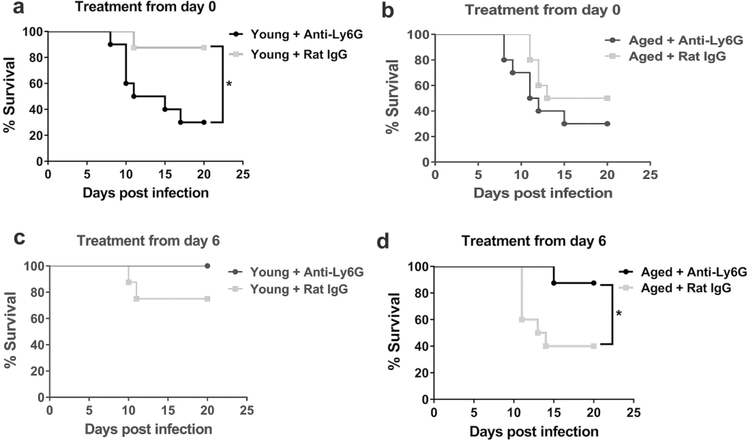
Antibody-mediated depletion of neutrophils administered at the time of viral inoculation increased the mortality of young mice from 10% to 70% at 20 days p.i. (monoclonal antibody IA8, Figure 2a), consistent with prior observations that neutrophils are critical for combatting IAV early during infection 17. However, depletion of neutrophils on day 0 did not significantly increase lethality among aged mice (Figure 2b).
Figure 2. Neutrophil depletion at the end of the first week p.i. (post-infection) enhances survival in aged mice.
a Young mice were administered a neutrophil depleting monoclonal antibody (i.p.) just prior to inoculation with influenza virus and following infection (as described in methods) and survival was monitored. Compared to isotype control treated, infected neutrophil depleted young mice exhibited a significant increase in mortality. * P < 0.05 (Log rank), n= 8–10 per group.
b Aged mice were treated per A and survival post infection was monitored. P value between neutrophil depleted and control group P = 0.23 (Log-Rank). n= 8–10 per group.
c Young mice were administered a neutrophil depleting antibody at day + 6 p.i. then intermittently to day +12 p.i. (as described in Methods) and compared to isotype control, treated young-infected mice. * P = 0.10 (Log-rank), n= 8–10 per group.
d Aged mice were treated according to c and survival was measured post infection. Aged neutrophil, depleted mice exhibited a significant increased survival as compared to control group. * P < 0.05 Log-rank, n= 8–10 per group.
We next examined if depletion of neutrophils after infection of young and aged mice impacted mortality, given our results that aging enhanced the increase in neutrophils after infection. Neutrophil depletion post-infection, from day 6 p.i.−12 p.i., did not impact the survival of young mice (Figure 2c). However, similarly treated infected aged mice showed a substantial and significant increase in survival (Figure 2d). Indeed, depleting neutrophils post-infection in aged mice improved survival to 80% from 40% in controls (Figure 2d). Excessive neutrophil extracellular traps, an important neutrophil effector mechanism, have been implicated in lung injury during influenza viral infection in mice 33. As DNase treatment disrupts these traps 34, we examined if DNase impacted survival in our model. DNase treatment via intranasal administration from day 6–12 p.i. did not significantly enhance survival in either aged or young-infected mice (Supplemental Figure 1a-b). In sum, these data indicate that neutrophils promote survival at the time of viral infection in young mice but substantially promote mortality towards the end of infection in aged infected-mice.
Neutrophil depletion reduces lung damage and lung inflammatory cytokine levels in aged mice without impacting viral clearance during IAV infection.
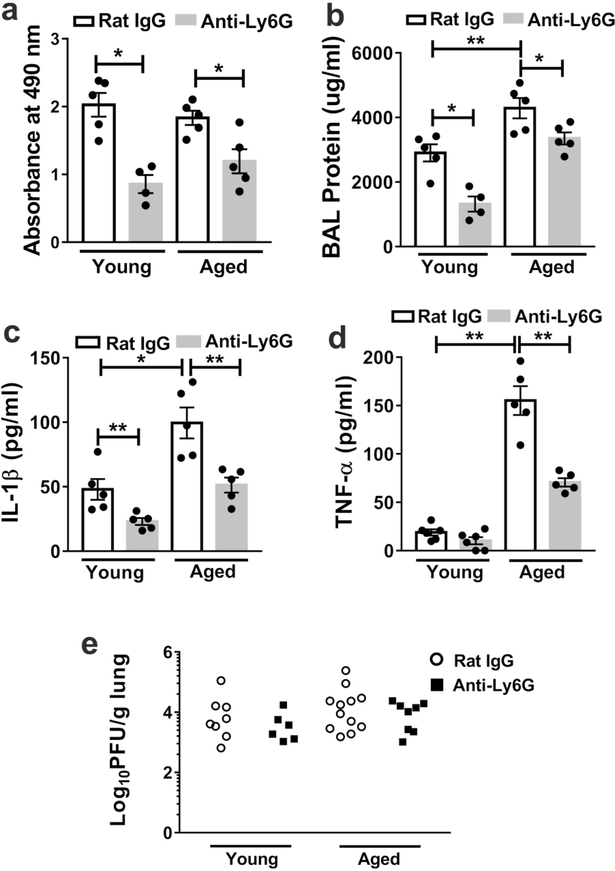
Our prior study demonstrated that aged mice exhibit increased mortality with elevated protein levels in the BAL30, a marker of lung damage. Furthermore, proinflammatory cytokines such as IL1β and TNF-α can either activate neutrophils, be produced by neutrophils or are induced by neutrophils to enhance tissue damage 20, 35, 36. Compared to young mice, aged mice exhibited four-fold higher levels of IL-1β on day 6 p.i., and four-fold higher levels of TNF-α on day 9 p.i. (Supplemental figure 2a-b). We also examined lactate dehydrogenase (LDH), a marker of cell death 37, 38, and found that young and aged mice displayed similar levels of LDH in BAL at day 10 p.i. (Figure 3a). However, BAL protein levels were higher in aged mice compared to in young mice at day 10 p.i. (Figure 3b).
Figure 3. Neutrophil depletion in mice from the first week p.i. (post-infection) reduces lung damage and lung inflammatory cytokines levels without impacting viral clearance.
Aged and young mice were infected with influenza virus and depleted of neutrophils or treated with isotype control at day 6 p.i and then intermittently until day 12 p.i. At day 10 p.i., the BAL was obtained from and LDH (a), protein (b), IL-1β (c), TNF-α (d) measured via ELISA * P < 0.05 ** P = 0.01 (Mann-Whitney test). Data are representative of one of two independent experiments, which yielded similar results. Data are expressed as mean ± SEM eYoung and aged mice were infected with influenza and depleted of neutrophils or treated with isotype control at day 6 p.i and then intermittently until day 10 p.i. At day 10 p.i, lungs were obtained and viral load measured by plaque assay. There were no significant differences in viral load between control and neutrophil depleted groups. P = 0.49 (young Rat IgG vs Anti-Ly6G(Mann-Whitney test) and P = 0.47 (aged Rat IgG vs Anti-Ly6G) (Mann-Whitney test). Data are representative of two independent experiments.
Next, we examined whether depletion of neutrophils during days 6–12 p.i. affected the the levels of proinflammatory cytokines or lung damage. Post-infection neutrophil depletion reduced the BAL levels of LDH, protein and IL-1β in both young and aged mice at day 10 p.i. (Figure 3a-c), and reduced TNF-β levels in aged mice (Figure 3d). However, post-infection neutrophil depletion did not significantly impact viral clearance in either aged or young mice (Figure 3e). These results suggest that, at the end of the first week post IAV infection, neutrophils promote death by increasing lung damage and lung inflammatory cytokine levels without impacting viral clearance.
Neutrophils from aged mice exhibit chemotactic defects in response to CXCL1.
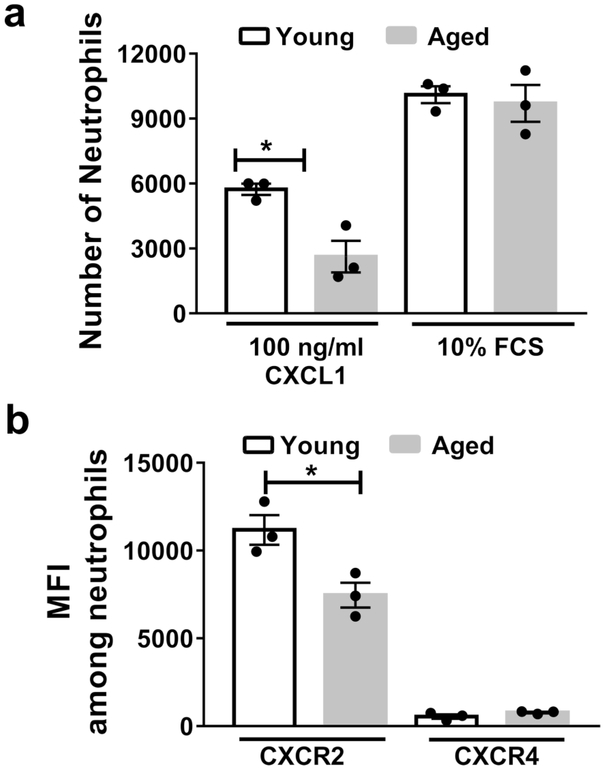
Neutrophils are generated and stored in the bone marrow, where they are poised to traffic to sites of inflammation39. We considered that neutrophils from aged mice have a higher intrinsic ability to migrate towards a chemoattractant, resulting in the increased neutrophil levels in the lungs of aged, infected mice. To test this, we purified neutrophils from the bone marrow of young and aged non-infected mice, and measured their chemotaxis towards CXCL1, a known neutrophil chemoattractant. However, neutrophils purified from bone marrow of aged mice exhibited reduced chemotaxis towards CXCL1 than neutrophils isolated from young mice (Figure 4a). We also examined CXCR2, the chemokine receptor for CXCL1 (Figure 4b), which is critical for neutrophil recruitment during IAV infection in mice 40. We observed reduced surface expression of CXCR2 in aged vs. young bone marrow neutrophils. CXCR4, a chemokine receptor that contributes to neutrophils releasing from and returning to the bone marrow41, was expressed at low levels on neutrophils, with no differences between the age groups (Figure 4b). Fetal calf serum, which contains a variety of neutrophil chemo-attractants, induced a higher degree of chemotaxis than CXCL1, but without any differences between the age groups (Figure 4a). These data are consistent with human studies demonstrating that aged neutrophils exhibit chemotactic defects to specific chemo-attractants 26. Thus, bone marrow neutrophils from aged mice display impaired chemotactic function, even though aged mice exhibited increased neutrophil levels within the lung during viral infection.
Figure 4. Aging impairs bone marrow neutrophil chemotaxis towards CXCL1 and reduces surface expression of CXCR2.
Neutrophils were purified, via negative magnetic separation from the bone marrow of young and aged non-infected mice. a Neutrophil chemotaxis was assessed in response to CXCL1 or fetal calf serum (FCS) at the indicated doses. b Surface expression of CXCR2 and CXCR4 via flow cytometry was assessed on purified bone marrow neutrophils from young and aged mice. Experiments are representative of one experiment repeated twice with consistent results. * P< 0.05 (Mann-Whitney test). Data are expressed as mean ± SEM.
Age-associated changes in chemo-attractants and neutrophil chemoreceptors during infection
We hypothesized that aging alters cell-extrinsic factors that impact neutrophil recruitment towards the lung during IAV infection. To test this, we harvested lung lysate and BAL from young and aged mice before and after infection. We then performed chemotaxis assays with neutrophils isolated from the bone marrow of non-infected, young mice. Neutrophils displayed increased chemotaxis towards the lung lysate and BAL obtained from infected compared to non-infected lungs (Figure 5a-b). Importantly, we observed higher neutrophil chemotaxis towards the lung lysate and BAL harvested from aged mice compared to from young mice, particularly after infection (Figure 5a-b). Consistent with these findings, aged mice exhibited a two to four-fold increase in the BAL levels of the neutrophil chemo-attractants CXCL1 and CXCL2 at day 6 p.i., compared to young mice (Figure 5c-d). Also, on day 6 p.i., aged mice exhibited a three-fold increase in the levels of the proinflammatory cytokine IL-17 (Supplemental Figure 2c), which induces the production of chemokines from lung epithelial cells and promotes immune pathology during IAV infection 42–44. However, aged mice did not exhibit an increase in the chemokine CXCL5 during the course of infection (Supplemental Figure 2d).
Figure 5. Aging increases the levels of CXCL1 and CXCL2 in lung during influenza infection and enhances neutrophil chemotaxis in vitro.
a-b Bone marrow neutrophils were purified from young non-infected mice and added to chemotaxis assays in which the chemoattractant was either the BAL (a) or lung lysate (b) of non-infected or infected (day 6 post-infection), young and aged mice. Young neutrophils exhibited a significant increased chemotaxis towards the fluid obtained from the lungs of aged mice than young mice (before or after infection) * P < 0.05 ** P < 0.01 (Mann-Whitney test). c-d BAL was obtained during the course of influenza infection in young and aged mice and CXCL1 (c) and CXCL2 (d) measured within the BAL via ELISA. n = 5/ time point / group. * P < 0.05 *** P < 0.001 (Mann-Whitney test).
e-f Surface expression of CXCR2 and CXCR4 on neutrophils in the lung before and during the course of influenza infection in young and aged mice. n = 4/ time point / group. * P < 0.05 (Mann-Whitney test).
Next, we examined the surface expression of CXCR2 and CXCR4 on lung neutrophils. CXCR2 on lung neutrophils was higher on days 6 and 9 p.i. of aged mice compared to young mice, whereas the surface expression of CXCR4 on lung neutrophils was diminished in aged mice at day 6 p.i. compared to young mice (Figure 5e-f, Supplemental Figure 3). We also examined bone marrow neutrophils from aged and young mice on day 6 p.i. and observed similar expression of CXCR2, whereas those from aged mice displayed reduced expression of CXCR4 (Supplemental Figure 4). Overall, these data indicate that IAV-infected lungs of aged mice are characterized by increased levels of CXCL1 and CXCL2, and increased CXCR2 but reduced expression of CXCR4 on lung neutrophils.
CXCL1 and CXCL2 in the aged, influenza-infected lung contribute to increased neutrophil chemotaxis
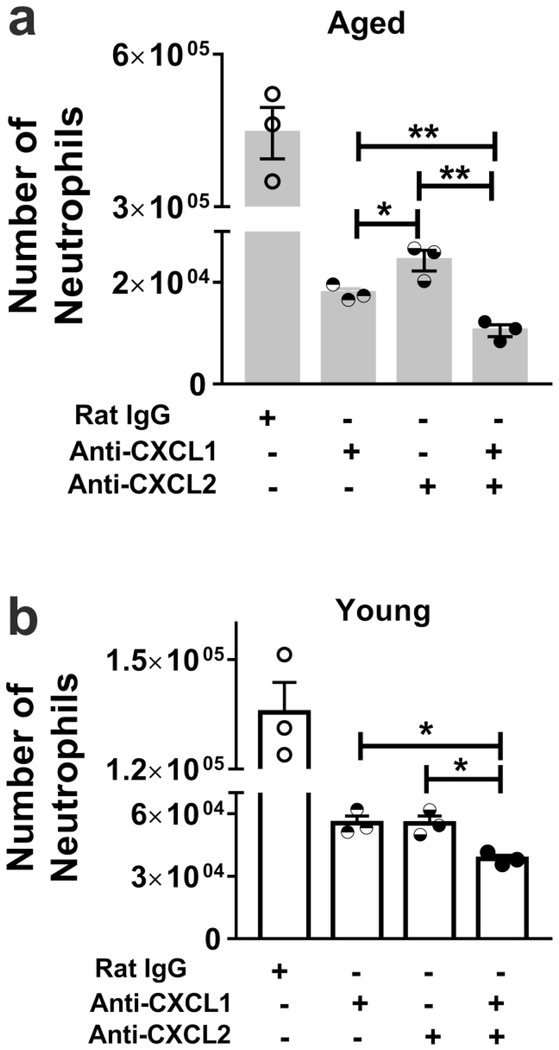
Next, we examined to what extent CXCL1 and CXCL2 contribute to neutrophil chemotaxis in young versus aged infected mice. As above, we purified neutrophils from the bone marrow of young mice and assessed chemotaxis in response to lung lysates isolated from aged or young mice after infection. We found that antibody-mediated blockade of either CXCL1 or CXCL2 significantly reduced neutrophil chemotaxis with the lung lysate from aged, infected mice (Figure 6a). The anti-CXCL1 antibody was significantly more effective at reducing neutrophil chemotaxis induced by the aged, infected lung lysate than the anti-CXCL2 antibody (Figure 6a). Combined blockade of both chemokines further reduced chemotaxis by the aged lung lysate, indicating an additive effect of dual blockade (Figure 6a). In lung lysate from young infected mice, blockade of either chemokine led to a similar reduction in chemotaxis compared to control (Figure 6b). Dual blockade also further reduced chemotaxis induced by the lung lysate from young mice (Figure 6b). Thus, elevated levels of CXCL1 and CXCL2 in the lungs of infected, aged mice enhance neutrophil chemotaxis.
Figure 6. CXCL1 and CXCL2 are critical for chemotaxis of the influenza-infected lung with aging.
The lung lysate from young and aged, infected mice were obtained and employed in chemotaxis assays with neutrophils purified from the bone marrow of young mice. Inhibiting CXCL1 or CXCL2 reduced neutrophil chemotaxis of aged (a) and young (b) lung lysate (P < 0.05 vs. control in age group). n = 3–6. * P < 0.05 (Mann-Whitney test). Isotype control antibody did not significantly reduce neutrophil chemotaxis. Data representative of one of two independent experiments, which yielded similar results. Data are expressed as mean ± SEM.
Alveolar Epithelial Cells (AECs) show an age-associated increase in CXCL1 and CXCL2 secretion during influenza infection.
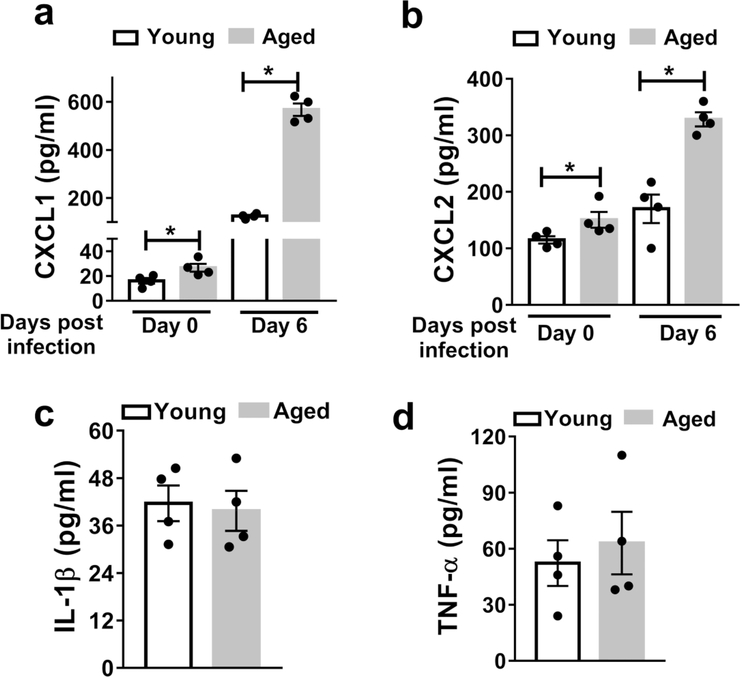
As the alveolar surface is protected by epithelial cells that contribute to the first line of defense against viral infection 45, we hypothesized that AECs are a source of the age-associated increase in CXCL1 and CXCL2 in the lung during influenza infection. To test this hypothesis, we isolated AECs from the lungs of young and aged mice before and after infection, cultured them ex vivo, and measured CXCL1 and CXCL2 in culture supernatants by ELISA. AECs from aged, infected mice produced the highest levels of both CXCL1 and CXCL2 (Figure 7a-b). Importantly, the AECs from aged mice exhibited an over five-fold increase in CXCL1 levels and a two-fold increase in CXCL2 levels upon infection, whereas AECs from young mice showed only a two-fold increase in CXCL1 and a marginal increase in CXCL2 upon infection (Figure 7a-b). AECs from infected mice produced TNFβ and IL-1β, although there were no differences between the age groups (Figure 7c-d). TNFα and IL-1β levels were not detectable in the supernatants of cultured AECs from uninfected mice (data not shown). These results indicate that AECs contribute to the increased levels of CXCL1 and CXCL2 upon IAV infection of aged versus young mice.
Figure 7. AECs secrete higher levels of CXCL1 and CXCL2 with aging during influenza infection.
AECs were purified from young and aged non-infected and infected mice (six days post-infection) andcultured ex vivo. After 36h the supernatants were harvested and CXCL1 (a), CXCL2 (b) TNF-α (c) and IL-1β (d) were measured via ELISA. * P < 0.05 (Mann-Whitney test). Data are representative o ftwo independent experiments, repeated with similar results. Data are expressed as mean ± SEM.
Aged mice display a higher frequency of senescent AECs with aging
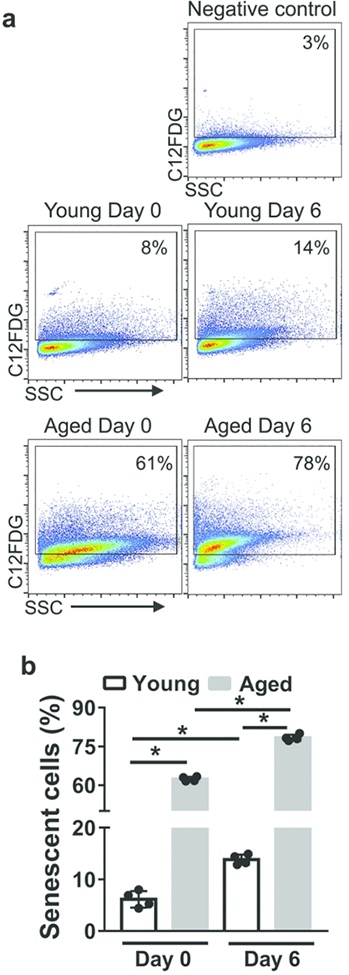
Senescent cells secrete CXCL1 and CXCL2 46. To determine if AECs from aged mice exhibited a higher frequency of senescence than young mice either before or after influenza infection, we measured senescence-associated (SA)-β-galactosidase activity, a well-described marker of senescence 47. We found that aged, infected mice displayed the highest frequency of AECs with SA-β-galactosidase activity, which was 5-fold higher than the frequency of senescent AECs in young, infected mice (Figure 8a-b). Our data indicate that there is a higher frequency of senescent AECs in aged mice both before and after influenza infection, which might contribute to the elevated levels of CXCL1 and CXCL2.
Figure 8. AECs from aged mice upregulate senescence-associated β-galactosidase activity.
AECs were purified from young and aged non-infected and infected mice (six days post-infection) and cultured ex vivo. After 3 h, cells were incubated for 2h with C12FDG, a β-galactosidase substrate that produces fluorescent product upon cleavage. The β-galactosidase activity was measured via flow cytometry. Cells incubated with PBS were used as negative control. a Representative flow cytometry plots gated on cells expressing the cleaved fluorescent product.b Graph indicating the frequency of senescent cells showing β-galactosidase activity. * P<0.05
Discussion
Clinical reports and experimental studies have shown that aging leads to increased mortality during IAV infection, yet the exact cellular mechanisms behind these observations remain unclear 1–3. In our study we focused on neutrophils, as prior work has shown that these cells are critical for clearing influenza virus at the time of infection 17 but can also promote lung damage during influenza infection 23, 33. We found that aged mice exhibit increased levels of neutrophils during the course of IAV infection compared to young mice. The increased neutrophil levels towards the end of the first week of infection were critical for mortality in aged mice, as we found that neutrophil depletion at this time point increased survival to over 80% from less than 40% in controls. Neutrophil depletion was effective at reducing lung damage in young mice but did not extend survival during influenza infection. It is possible that the reduced level of lung damage or an increased tolerance to the level of lung damage in young mice led to a high survival rate during influenza infection without neutrophil depletion. Only a few therapies have been shown to improve outcome in older hosts during active influenza infection, and these therapies have mostly focused on inhibiting viral replication rather than impacting toxic host immune responses to the pathogen48. To our knowledge, our study is the first to demonstrate that reducing an excessive immune response, in this case increased neutrophil accumulation during active influenza infection, substantially and specifically improves survival with aging.
Our study suggests that increased production of CXCL1 and CXCL2 but not CXCL5 contributes to enhanced neutrophil recruitment to the lung during IAV infection with aging. Why aging leads to an impaired increase in CXCL5 levels during influenza infection is not clear and will require future investigation. We previously reported that alveolar macrophages, cells with important functions for host defense and homeostasis 49, are compromised in their ability to uptake and phagocytose apoptotic neutrophils with aging 30. Together, our studies suggest that increased neutrophil accumulation in the lungs during influenza infection with aging arises from both AECs producing higher levels of CXCL1 and CXCL2, and declining neutrophil clearance by alveolar macrophages30. We found that aging impairs chemotaxis of bone marrow-derived neutrophils towards specific chemotactic signals, which is consistent with prior work, predominantly in humans 26, 50. Human neutrophils isolated from aged individuals also exhibit an impaired ability to migrate due to increased activation of Class 1 phosphoinositide 3-kinase (PI3K) activity 27. Despite the observed age-dependent defect in neutrophils chemotaxis, we found that neutrophils accumulated within the lung airspace to a greater degree in aged mice than in young mice during IAV infection. The higher production of both CXCL1 and CXCL2 by aged, IAV-infected lungs was essential for enhanced neutrophil chemotaxis. AECs from aged mice were a source of these elevated chemokines, though we cannot exclude other cellular sources of chemokines, including infiltrating and resident macrophages, dendritic cells, recruited neutrophils, and vascular cells such as endothelial cells. Regardless of the source of CXCL1 and CXCL2, the levels of these chemokines were increased within the lung during IAV with aging. These higher levels of chemokines may have mobilized neutrophils to exit the bone marrow and may explain the increased proportion of circulating neutrophils during infection in aged mice. We also found that bone marrow neutrophils of aged mice downregulated CXCR4 at day 6 p.i. Downregulation of this receptor has been shown to release neutrophils from the bone marrow and to lead to neutrophilia41, 51, 52. Hence the reduced expression of this receptor on bone marrow neutrophils also could have contributed to the increase in peripheral blood neutrophils levels noted with aging during IAV infection.
We found that neutrophils in the lungs of aged mice during influenza infection upregulated cell surface expression of CXCR2 but downregulated CXCR4. CXCR2 was downregulated on aged bone marrow neutrophils before infection but was similarly expressed on bone marrow neutrophils between young and aged mice at day 6 p.i. CXCR4, as stated above, was downregulated on both bone marrow and lung neutrophils with aging during IAV infection. It is possible that the increase maturation status (i.e., increased expression of CXCR2 and reduced expression of CXCR4) on lung neutrophils could be due, in part, to the impaired clearance of neutrophils in the lung by alveolar macrophages. Alternatively but not mutually exclusively, the neutrophils that enter the aged lung during infection may already have down regulated their CXCR4 receptor. Overall, chemokine receptor expression may be impacted by both the aged bone marrow niche and the altered inflammatory environment within the aged lung that occurs during IAV infection. Future investigation will be required to dissect the contributions of the bone marrow and the lung environment on the dysregulated chemokine receptor expression on neutrophils with aging.
Recently, there has been increasing interest in the contribution of the host microbiome to the development of the chronic inflammation observed in aging (“inflamm-aging”) 53. Elderly nursing home residents have elevated inflammatory markers that correlate with distinct fecal microbial signatures compared to community dwelling elderly subjects 54. Similarly, aged mice housed under conventional specific pathogen free conditions demonstrated elevated circulating inflammatory cytokines (e.g., TNF-α, IL-6) that appeared to be associated with increased gut epithelial permeability, whereas germ-free mice did not exhibit age-associated microbial dysbiosis and inflammation 29. Interestingly, co-housing germ-free mice with aged specific pathogen free mice resulted in elevated inflammatory markers, whereas germ-free mice cohoused with young specific pathogen free mice did not 29. Given that the gut microbial community has been shown to play a critical role in immune priming against respiratory pathogens, including influenza and bacteria 55–57 a potential mechanism of the augmented chemokine responses in our aged mice during influenza infection may be aging-induced gut dysbiosis, which will be examined in future studies.
Cellular senescence leads to the production of inflammatory mediators including chemokines and cytokines 46, 58. Importantly, recent experimental and clinical evidence has revealed that senescence of AECs contributes to idiopathic pulmonary fibrosis 59, an age-related disease. Specifically, AECs from patients with idiopathic pulmonary fibrosis or from mice treated with bleomycin exhibit senescence and exhibit an increased inflammatory, pro-fibrotic phenotype59. In our study, we observed a higher frequency of senescent AECs in aged mice than young mice both prior to and after IAV infection. Hence, senescence could explain why AECs from the aged mice secrete high levels of chemokines both before and after influenza infection.
Neutrophils produce extracellular traps, reactive oxidative species such as superoxide, enzymes (e.g., myeloperoxidase and elastase), anti-microbial peptides (e.g., defensins and cathepsins) to kill pathogens 60. Our data suggest that extracellular traps do not contribute to the age-associated increase in mortality, since DNase treatment did not extend survival during infection. Neutrophils also produce inflammatory cytokines and communicate with other innate immune cells, such as alveolar macrophages, to produce cytokines during IAV infection 20. Our results show that during IAV infection, aged mice exhibit higher levels of IL-1β, TNF-α and IL-17 in the BAL compared to young mice. Each of these cytokines can either be produced by neutrophils 61–63 or can activate neutrophils directly 20, 35, 64, or lead to epithelial cells to produce more chemokines to promote neutrophil recruitment 42, 64. IL-1β, TNF-α and IL-17 can also be produced by a variety of cells including macrophages, effector, memory and tissue memory T cells 65–67. Future studies will be required to determine the source of the cytokines in aged hosts and if any of the elevations in these inflammatory cytokines or any of neutrophil’s known other effector functions could contribute to inflammatory tissue damage and mortality during IAV infection with aging.
Our study implies that potential therapies that target AECs, and possibly other lung epithelial cells, neutrophils, and the interaction of between neutrophils and epithelial cells, may provide a therapeutic benefit for older patients infected with influenza. If AECs from older humans exhibit senescence, one potential therapeutic approach to target AECs would be with drugs that remove senescent cells, so called senolytics 68. Indeed, an experimental study has shown that senolytics reduce AEC senescence and ameliorates pulmonary fibrosis 59.
In conclusion, our study has revealed that aging is associated with increased neutrophil accumulation during IAV infection. An age-associated increase in the production of CXCL1 and CXCL2 by AECs may contribute to enhanced neutrophil chemotaxis. Thus, our study has revealed that controlling excessive neutrophil levels may improve outcomes in older people infected with influenza and possibly other respiratory pathogens.
Materials and Methods
Mice and in vivo viral infection
Female C57BL/6 mice of 2–4 months and 18–22 months of age were obtained from the National Institute of Aging rodent facility. Mice were infected with purified influenza virus, A/Puerto Rico/8/34 (H1N1) (PR8) (Advanced Biotechnologies) per our prior study 30. Mice were anesthetized with isoflurane and instilled intranasally (i.n) with 40 μl PBS containing 1 X 104 PFU of PR8 virus. Following infection, mice were monitored daily for changes in weight and mortality. Mice were euthanized when 30% of their original weight was lost, which was recorded as death in survival experiments. No animals were used in the study if they displayed evidence of infection or other illnesses prior to PR8 administration.
Treatment in Mice
Anti-Ly6G treatment:
treatment at day 6 p.i.: mice were treated i.p. with 0.5 mg in 100 μl antiLy6G (clone: 1A8, BioXCell) antibody and also i.n. with 0.25 mg in 50 μl on alternate days starting from day 6 p.i. until day 12 p.i.
day 0 p.i.: mice were treated i.p. with 0.5 mg in 100 μl anti-Ly6G antibody and also i.n. with 0.25 mg in 50 μl on alternate days from day 0 to day 18 p.i., as described before 17, 69. Control mice were treated with Rat IgG.
DNase I treatment:
Mice were treated i.n. with 50 μg/mouse (1 μg/μl) DNase I (Affymetrix), on alternate days starting from day 6 post infection until day 12. Control mice were treated with PBS.
Sample collection and preparation
Mice were euthanized with an overdose of isoflurane. After euthanasia, the trachea was exposed and cannulated using a blunted 23G needle. To harvest lungs, the chest cavity was opened and the lungs were removed. Non-perfused lung tissue were harvested from PR8-infected mice at 1, 3, 6, 9 days p.i. The BAL was obtained by washing the lungs twice with 1 ml cold sterile 1x PBS. Fresh BAL was centrifuged at 1400 rpm for 5 min and supernatant was stored at −80°C. The cells from BAL were used for flow cytometry.
Assessment of tissue injury
Lung injury was assessed by measuring total protein and lactate dehydrogenase (LDH) levels in the BAL fluid. LDH activity was measured with an LDH assay kit (Roche; NY, USA) according to the manufacturer’s instructions. Total protein in BAL was measured using the BCA protein assay kit (Thermo Scientific; MA, USA) following the manufacturer’s instructions.
Viral load measurement
Tissue Preparation:
Lung lysates from left lung lobe from mice were prepared in PBS using TissueLyser II (Qiagen). Lung lysate supernatants were collected by centrifugation at 350 g for 10 min and stored at −80°C. Lung lobe weights were calculated by subtracting weight of tube without lung lobe from weight of tube with lung lobe.
Plaque assay:
Live lung viral titers were determined by plaque assay using Madin Darby canine kidney (MDCK) cells (provided by Dr. Adam Lauring, University of Michigan). MDCK cells were cultured in 6 well plates overnight until the cell monolayer reached >70% confluence. Lung supernatants were thawed at room temperature and diluted to different dilutions (i.e., 10−1-10−6) with 0.1% BSA in 1x PBS. Cells were washed twice with 1x PBS followed by 1h incubation with 400 μl lung supernatants at 37°C with shaking every 15 min. Cells were washed twice with 1x PBS to remove excess virus. The cells were then covered with 2 ml over-lay medium containing a 1:1 mixture of 2% agarose and 2x DMEM medium containing 2 μg/ml (final concentration) acetylated trypsin (Sigma-Aldrich). Plates were incubated at 37°C for 72 h. The agarose was removed from the plates and the cells stained with 0.3% crystal violet for 5 min. The plates were washed and viral plaques were counted. The plaque forming units per gram (pfu/g) were calculated using the formula: (# plaques x dilution factor)/weight of lung lobe.
Flow cytometry
To obtain single-cell suspension, lungs were harvested from animals, minced, and then digested with 1mg/ml Collagenase D (Roche) and 100 U/ml DNase (DN25, Sigma) in PBS without calcium and magnesium for 45 min at 37°C. After digestion, lung tissue was disrupted into single-cell suspension and passaged through a 100 μm sieve followed by 70 μm sieve (Fisherbrand). To remove red blood cells, cells were incubated with red blood cell lysis buffer (Biolegend) for 3 min. Live cells were spun and re-suspended in PBS. Dead cells were stained using LIVE/DEAD fixable aqua dead cell staining kit (ThermoFisher Scientific) or Zombie Aqua fixable viability kit (Biolegend) for 30 min. Cells were washed by centrifugation at 1400 rpm for 5 min at 4°C and resuspended in PBS containing 0.5% BSA (FACS buffer). Fc receptors were blocked by incubating cells with anti-CD16/32 antibody (clone: 93, Biolegend) for 10 min. Cells were thereafter stained with antibodies for the desired surface markers for 20 min. Cells were washed in FACS buffer and fixed in 2% paraformaldehyde for 20 min. Following another wash, cells were stored in FACS buffer until analysis. Flow cytometry data were acquired on Cytoflex for <5 colors (BeckmanCoulter) or Mo-Flo for >5 colors (Beckman-Coulter) and analyzed using FlowJo software. For expression of CXCR2 and CXCR4 median fluorescent intensity (MFI) is shown in arbitrary units.
Antibodies
APC: anti-Ly6G (clone: 1A8, Biolegend); APCeF780: CD8 (clone: 53–6.7, eBioscience), CD45 (clone: 30-F11, eBioscience), CD24 (clone: M1/69, eBioscience); BV421: CXCR4 (clone: L276F12, Biolegend); eF450: CD11b (clone: M1/70, Invitrogen); FITC: Ly6C (clone: HK1.4, Biolegend); PE: CXCR2 (clone: SA044G4, Biolegend), SiglecF (clone: E50–2440, BD bioscience); PECy5: CD19 (clone: 1D3, eBioscience); PECy7: CD4 (clone: GK1.5, eBioscience); PEeF610: CD11c (clone N418, eBioscience); PEVio770: CD64 (clone: REA286, Miltenyi Biotec); PerCPeF710: MHCII (clone: M5/114.15.2, eBioscience).
ELISA
CXCL1, CXCL2, CXCL5, IL-1β, TNF-α and IL-17 levels in BAL and cell culture supernatants were measured with ELISA kits (R&D systems for CXCL1, 2, 5 and IL-17; Invitrogen for IL-1β and TNF-α) following manufacturer’s instructions. Briefly, 96 well plates were coated with capture antibodies overnight for at room temperature for measurement of CXCL1/2/5 and IL-17 and at 4°C for measurement of IL-1β and TNF-α. The unbound antibody was removed by washing plates three times with wash buffer. The unspecific binding was removed by incubating plate with blocking buffer for 2h at room temperature. The test samples and standard were added and the plate was incubated overnight at 4°C. The plate was washed as before and incubated with detection antibody overnight at 4°C or 1 h at room temperature. After washing as before, the bound antibody was detected using strep-avidin and TMB substrate. Reaction was stopped with stop solution (2 N H2SO4). The absorbance was measured at 450 nm using a plate reader (BioTek). The concentration was determined based on absorbance values of the standard. The ELISA kits had following limits of detection: CXCL1, 2, 5 and IL-17 : 15.6 pg/ml, IL-1β and TNF-α: 8 pg/ml.
Cell Migration Assay
Neutrophils were isolated from mouse bone marrow using EasySep Mouse Neutrophil Enrichment Kit (Stem Cell Technologies). Neutrophil migration was analyzed using CytoSelect Cell Migration Kit (Cell Biolabs Inc) containing 12 well plates with polycarbonate membrane of 3 μm pore size as described in the manufacturer’s instructions. Briefly, neutrophils were resuspended in serum free RPMI 1640 medium and added in the upper chamber of the plates. The lower chamber consisted of BAL or lung lysate from experimental mice or neutrophil attracting chemokine as positive control or PBS as negative control. CXCL1 and CXCL2 in lung lysates were blocked using anti-CXCL1 (R&D, clone 48415) and anti-CXCL2 (Invitrogen, clone 40605) neutralizing antibodies. The concentration of the antibodies used for neutralizing was as per the vendor guidelines. The plates were incubated for 1 h at 37°C. The neutrophils that migrated to the lower chamber were counted under light microscope using a Neubauer chamber and then flow cytometric analysis.
AECs Isolation
AEC were isolated by magnetic associated cell sorting (MACS) as previously described 70. Briefly, single cell suspensions from lung were first incubated with CD45 microbeads and CD31 beads in PBS containing 2 mM EDTA and 0.5% BSA. The unbound antibody was removed by centrifuging at 300 g for 5 min at 10°C. Cells were then passed through a MACS column placed in the magnetic field of MidiMACS separator. The number of cells in flow though containing CD45- CD31- cells were counted and the cells were incubated with anti-mouse biotin labeled CD326 (EpCAM) antibody (Miltenyi Biotec) according to manufacturer’s instructions. Cells were washed as before and incubated with anti-biotin microbeads (Miltenyi Biotec). After washing, cells were again passed through a MACS column. The flow through was discarded and CD326+ alveolar epithelial cells attached to the column were removed by firmly applying a plunger. We typically obtained > 84% purity of CD326+ cells. The number of cells were counted and resuspended at a concentration of 1 million cells/ml in DMEM/F12 medium containing 10% FCS and allowed to adhere to tissue culture plates for 24 h followed by DMEM/F12 medium containing 1% BSA overnight. Cell culture supernatants following the overnight culture were stored at −80°C until analysis.
Senescence Associated β-galactosidase Detection by Flow Cytometry
Flow cytometry-based detection of SA- β-galactosidase was performed as described before 47. Briefly, AECs from young and aged non-infected and influenza infected mice were isolated and cultured as described above. Following 36h culture, the cells were treated with Bafilomycin A1 (100 nM, Enzo Life Sciences) for 1h followed by 2h incubation with C12FDG (33 μM, Thermo-Fischer). Cell were harvested, washed once in ice-cold PBS and immediately measured on a flow cytometer (Cytoflex, Beckman-Coulter). Data was analyzed using Flow-Jo software.
Statistics
The Mann-Whitney U (equivalently Wilcoxon Rank Sum) test was used to compare two groups (e.g., Young vs. Aged) for continuous outcomes (e.g., neutrophils). The log rank (Mantel-Cox) test was used for comparing two groups in terms of survival. Statistical analysis was carried out using Prism 7 (GraphPad) software. All results are based on two-sided tests and we evaluate statistical significance at the level of 0.05. Instead of reporting the exact p-values, in figures we present the range of p-values using * =P<0.05; ** = P<0.01; *** =P<0.001. Error bars are presented at mean with SEM.
Regulatory Approval
University of Michigan Institutional Animal Care and Use Committee approved the use of animals in this study. Prior to viral infection, all mice were kept in pathogen-free conditions.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Min Zhang for her expert advice in the statistical analysis of the data. We would like to thank Life Science Editors for their editorial assistance.
The work was supported by NIA AG028082 to D.R.G
Footnotes
Disclosure
The authors have declared that no conflict of interest exists.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ et al. Mortality Associated With Influenza and Respiratory Syncytial Virus in the United States. JAMA: The Journal of the American Medical Association 2003; 289(2): 179–186. [DOI] [PubMed] [Google Scholar]
- 2.Pebody RG, McLean E, Zhao H, Cleary P, Bracebridge S, Foster K et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Eurosurveillance 2010; 15(20): 19571. [PubMed] [Google Scholar]
- 3.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in Hospitalizations for Pneumonia Among Persons Aged 65 Years or Older in the United States, 1988–2002. JAMA 2005; 294(21): 2712–2719. [DOI] [PubMed] [Google Scholar]
- 4.Biggerstaff M, Kniss K, Jernigan DB, Brammer L, Bresee J, Garg S et al. Systematic Assessment of Multiple Routine and Near Real-Time Indicators to Classify the Severity of Influenza Seasons and Pandemics in the United States, 2003–2004 Through 2015–2016. American Journal of Epidemiology 2018; 187(5): 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 2013; 13(12): 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McElhaney JE, Kuchel GA, Zhou X, Swain SL, Haynes L. T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines. Frontiers in Immunology 2016; 7(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillai PS, Molony RD, Martinod K, Dong H, Pang IK, Tal MC et al. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science 2016; 352(6284): 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beli E, Clinthorne JF, Duriancik DM, Hwang I, Kim S, Gardner EM. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mechanisms of Ageing and Development 2011; 132(10): 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang J, Fisher EM, Murasko DM. CD8 T cell responses to influenza virus infection in aged mice. Ageing Research Reviews 2011; 10(4): 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams-Bey Y, Jiang J, Murasko DM. Expansion of regulatory T cells in aged mice following influenza infection. Mech Ageing Dev 2011; 132(4): 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Scientific reports 2016; 6: 25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toapanta F, Ross T. Impaired immune responses in the lungs of aged mice following influenza infection. Respiratory Research 2009; 10(1): 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends in Immunology 2010; 31(8): 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM et al. Intravascular Danger Signals Guide Neutrophils to Sites of Sterile Inflammation. Science 2010; 330(6002): 362–366. [DOI] [PubMed] [Google Scholar]
- 15.Lim K, Hyun Y-M, Lambert-Emo K, Capece T, Bae S, Miller R et al. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science (New York, NY) 2015; 349(6252): aaa4352–aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tate MD, Brooks AG, Reading PC. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir Res 2008; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tate MD, Deng Y-M, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils Ameliorate Lung Injury and the Development of Severe Disease during Influenza Infection. The Journal of Immunology 2009; 183(11): 7441–7450. [DOI] [PubMed] [Google Scholar]
- 18.Camp JV, Jonsson CB. A Role for Neutrophils in Viral Respiratory Disease. Frontiers in Immunology 2017; 8(550). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki M, Kohno S, Newstead MW, Zeng X, Bhan U, Lukacs NW et al. Critical Role of IL-1 Receptor-Associated Kinase-M in Regulating Chemokine-Dependent Deleterious Inflammation in Murine Influenza Pneumonia. The Journal of Immunology 2010; 184(3): 1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peiró T, Patel DF, Akthar S, Gregory LG, Pyle CJ, Harker JA et al. Neutrophils drive alveolar macrophage IL-1β release during respiratory viral infection. Thorax 2018; 73(6): 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandes M, Klauschen F, Kuchen S, Germain Ronald N. A Systems Analysis Identifies a Feedforward Inflammatory Circuit Leading to Lethal Influenza Infection. Cell 2013; 154(1): 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papayannopoulos V Neutrophil extracellular traps in immunity and disease. Nature Reviews Immunology 2017; 18: 134. [DOI] [PubMed] [Google Scholar]
- 23.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G et al. Identification of Oxidative Stress and Toll-like Receptor 4 Signaling as a Key Pathway of Acute Lung Injury. Cell 2008; 133(2): 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nature Reviews Drug Discovery 2016; 15: 551. [DOI] [PubMed] [Google Scholar]
- 25.Wenisch C, Patruta S, Daxböck F, Krause R, Hörl W. Effect of age on human neutrophil function. Journal of Leukocyte Biology 2000; 67(1): 40–45. [DOI] [PubMed] [Google Scholar]
- 26.Fulop T, Larbi A, Douziech N, Fortin C, Guérard K, Lesur O et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004; 3(4): 217–226. [DOI] [PubMed] [Google Scholar]
- 27.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N et al. Phosphoinositide 3kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 2014; 123(2): 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazeldine J, Harris P, Chapple IL, Grant M, Greenwood H, Livesey A et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell 2014; 13(4): 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP et al. AgeAssociated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host & Microbe 2017; 21(4): 455–466.e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong CK, Smith CA, Sakamoto K, Kaminski N, Koff JL, Goldstein DR. Aging Impairs Alveolar Macrophage Phagocytosis and Increases Influenza-Induced Mortality in Mice. The Journal of Immunology 2017; 199(3): 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 Inflammasome Function in Elderly Mice during Influenza Infection Is Rescued by Treatment with Nigericin. The Journal of Immunology 2012; 188(6): 2815–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Frontiers in immunology 2016; 7: 502–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew A-A et al. Excessive Neutrophils and Neutrophil Extracellular Traps Contribute to Acute Lung Injury of Influenza Pneumonitis. The American Journal of Pathology 2011; 179(1): 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto K, Yamada H, Wakana N, Kikai M, Terada K, Wada N et al. Augmented neutrophil extracellular traps formation promotes atherosclerosis development in socially defeated apoE−/− mice. Biochemical and Biophysical Research Communications 2018; 500(2): 490–496. [DOI] [PubMed] [Google Scholar]
- 35.Cowburn AS, Deighton J, Walmsley SR, Chilvers ER. The survival effect of TNF-α in human neutrophils is mediated via NF-κB-dependent IL-8 release. European Journal of Immunology 2004; 34(6): 1733–1743. [DOI] [PubMed] [Google Scholar]
- 36.Netea MG, Simon A, van de Veerdonk F, Kullberg B-J, Van der Meer JWM, Joosten LAB. IL1β Processing in Host Defense: Beyond the Inflammasomes. PLOS Pathogens 2010; 6(2): e1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legrand C, Bour JM, Jacob C, Capiaumont J, Martial A, Marc A et al. Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. Journal of biotechnology 1992; 25(3): 231–243. [DOI] [PubMed] [Google Scholar]
- 38.Maes M, Vanhaecke T, Cogliati B, Yanguas SC, Willebrords J, Rogiers V et al. Measurement of Apoptotic and Necrotic Cell Death in Primary Hepatocyte Cultures. Methods in molecular biology (Clifton, NJ) 2015; 1250: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology 2008; 125(3): 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wareing MD, Shea AL, Inglis CA, Dias PB, Sarawar SR. CXCR2 Is Required for Neutrophil Recruitment to the Lung during Influenza Virus Infection, But Is Not Essential for Viral Clearance. Viral Immunology 2007; 20(3): 369–378. [DOI] [PubMed] [Google Scholar]
- 41.Filippo De. Rankin K CXCR4 SM the master regulator of neutrophil trafficking in homoeostasis and disease. European Journal of Clinical Investigation 2018; 0(0): e12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen K, Eddens T, Trevejo-Nunez G, Way EE, Elsegeiny W, Ricks DM et al. IL-17 Receptor Signaling in the Lung Epithelium Is Required for Mucosal Chemokine Gradients and Pulmonary Host Defense against. Cell Host & Microbe 2016; 20(5): 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI et al. Critical Role of IL-17RA in Immunopathology of Influenza Infection. J Immunol 2009; 183(8): 5301–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurczynski SJ, Moore BB. IL-17 in the lung: the good, the bad, and the ugly. American Journal of Physiology-Lung Cellular and Molecular Physiology 2018; 314(1): L6–L16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason R Biology of alveolar type II cells. Respirology 2006; 11(s1): S12–S15. [DOI] [PubMed] [Google Scholar]
- 46.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013; 123(3): 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nature Protocols 2009; 4: 1798. [DOI] [PubMed] [Google Scholar]
- 48.McGeer A, Green KA, Plevneshi A, Shigayeva A, Siddiqi N, Raboud J et al. Antiviral Therapy and Outcomes of Influenza Requiring Hospitalization in Ontario, Canada. Clinical Infectious Diseases 2007; 45(12): 1568–1575. [DOI] [PubMed] [Google Scholar]
- 49.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 2014; 14(2): 81–93. [DOI] [PubMed] [Google Scholar]
- 50.Kovacs EJ, Palmer JL, Fortin CF, Fülöp T Jr, Goldstein DR, Linton P-J. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends in Immunology 2009; 30(7): 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA et al. Role of the CXCR4/SDF1 chemokine axis in circulating neutrophil homeostasis. Blood 2004; 104(2): 565–571. [DOI] [PubMed] [Google Scholar]
- 52.Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD et al. Pulmonary Stromal-Derived Factor-1 Expression and Effect on Neutrophil Recruitment during Acute Lung Injury. The Journal of Immunology 2007; 178(12): 8148–8157. [DOI] [PubMed] [Google Scholar]
- 53.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longevity & healthspan 2013; 2(1): 8–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488: 178. [DOI] [PubMed] [Google Scholar]
- 55.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nature Medicine 2010; 16: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJTH de Boer JD et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016; 65(4): 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences 2011; 108(13): 5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes 2015; 64(7): 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. The European Respiratory Journal 2017; 50(2): 1602367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayadas TN, Cullere X, Lowell CA. The Multifaceted Functions of Neutrophils. Annual Review of Pathology: Mechanisms of Disease 2014; 9(1): 181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D et al. Neutrophils Produce Interleukin 17A (IL-17A) in a Dectin-1- and IL-23-Dependent Manner during Invasive Fungal Infection. Infection and Immunity 2011; 79(10): 3966–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis and rheumatism 2009; 60(12): 3642–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Frontiers in immunology 2014; 5: 508–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lukacs NW, Strieter RM, Chensue SW, Widmer M, Kunkel SL. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. The Journal of Immunology 1995; 154(10): 5411–5417. [PubMed] [Google Scholar]
- 65.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest 2006; 116(5): 1218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome Joseph JC et al. Distribution and Compartmentalization of Human Circulating and Tissue-Resident Memory T Cell Subsets. Immunity 2013; 38(1): 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21(7): 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017; 21: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 2011; 179(1): 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jansing NL, McClendon J, Kage H, Sunohara M, Alvarez JR, Borok Z et al. Isolation of Rat and Mouse Alveolar Type II Epithelial Cells In: Alper S, Janssen WJ (eds). Lung Innate Immunity and Inflammation: Methods and Protocols. Springer; New York: New York, NY, 2018, pp 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.