Abstract
Costimulatory blockade-induced murine cardiac allograft survival requires intragraft accumulation of CD11b+Ly6CloLy6G- regulatory myeloid cells (MREG) that expand regulatory T cells (TREG) and suppress effector T cells (TEFF). We previously showed that C5aR1 signaling on T cells activates TEFF and inhibits TREG, but whether/how C5aR1 impacts MREG required for transplant survival is unknown. Whereas BALB/c hearts survived >60 days in anti-CD154-(MR1)-treated or CTLA4-Ig-treated wild type (WT) recipients, they were rejected at ~30 days in MR1-treated or CTLA4-Ig-treated recipients selectively-deficient in C5aR1 restricted to myeloid cells (C5ar1fl/flxLysM-Cre). This accelerated rejection was associated with ~2-fold more donor-reactive T cells and ~40% less expansion of donor-reactive TREG. Analysis of graft-infiltrating mononuclear cells on post-transplant day 6 revealed fewer Ly6Clo monocytes in C5ar1fl/flxLysM-Cre recipients. Expression profiling of intragraft Ly6Clo monocytes showed C5aR1 deficiency downregulated genes related to migration/locomotion without changes in genes associated with suppressive function. Co-transfer of C5ar1fl/fl and C5ar1fl/flxLysM-Cre myeloid cells into MR1-treated allograft recipients resulted in less accumulation of C5ar1−/− cells within the allografts and in vitro assays confirmed that Ly6Chi myeloid cells migrate to C5a/C5aR1-initiated signals. Together our results newly link myeloid cell-expressed C5aR1 to intragraft accumulation of myeloid cells required for prolongation of heart transplant survival induced by costimulatory blockade.
INTRODUCTION
Blocking CD40/CD154 and/or CD28/CD80/CD86 interactions promotes murine allograft tolerance (1–4). It prolongs transplant survival, and at the same time allows reduction of immunosuppressant dosing in nonhuman primates (5) and human transplant recipients (6–9).
The pro-tolerogenic immune mechanisms initiated by costimulatory blockade are incompletely understood but experimental evidence supports induction and maintenance of donor-reactive regulatory T cells (TREG) as crucial (3, 10, 11). Studies published since 2008 have additionally implicated a subset of regulatory myeloid cells (MREG) as important contributors to costimulatory blockade-induced transplant survival (2, 4, 12). Myeloid cells capable of suppressing T cell immunity, sometimes referred to as myeloid derived suppressor cells (MDSC), were initially observed in tumor systems (13) and were shown to inhibit anti-tumor T cell immunity. Tumor-associated MDSC produce inducible nitric acid synthase, L-arginase, and IL-10 (among other molecules), can directly inhibit effector T cells (TEFF), and importantly facilitate proliferation and accumulation of TREG at the tumor site (14). In transplantation, MREG were first observed in a rat model of kidney allograft tolerance following costimulatory blockade with anti-CD28 (15). In 2010, the Ochando lab demonstrated that CD11b+CD115+Gr1+ myeloid cells accumulate in heart allografts of MR1-treated recipients and that these MREG are required for MR1-induced long-term allograft survival (2). In further studies the Ochando group showed that the MREG derives from a CD11b+Ly6Chi bone marrow precursor that undergoes CSF1-dependent differentiation into a CD11b+Ly6CloLy6G- subset within the allograft of MR1-treated recipients (4). Functionally, the Ly6Clo MREG require surface expression of DC-SIGN, directly inhibit TEFF (in part by producing IL-10) and facilitate proliferation/expansion of protective TREG (4).
The complement system has been traditionally considered a component of innate immunity. Our cumulative work since 2005 has delineated unanticipated roles for complement, including autocrine C5a/C5aR1 ligations in T cells and dendritic cells (DCs), as crucial signals that activate CD4+ TEFF and inhibit generation, function and stability of TREG, together augmenting T cell immunity (16–25). Absence/blockade of these signals inhibits CD4+ TEFF and enhances generation, function and stability of TREG, favoring immune tolerance. These concepts apply to T cells responding to model antigens, autoantigens, infectious pathogens and transplant antigens (18–20, 22, 23, 26–28). In contrast to the above-noted effects of autocrine C5aR1 signaling as a direct modulator of T cell immunity one 2008 study using a murine tumor system showed that pharmacological C5ar1 blockade enhanced tumor-reactive CD8+ T cell responses and prevented tumor progression (29). Experiments in that system suggested that the dominant mechanism involved inhibition of MDSC function/accumulation which indirectly unleashed protective, tumor-reactive T cell immunity. Direct evidence that C5aR1 impacts MREG is lacking, and whether/how analogous mechanisms apply to MREG in transplantation has not been previously addressed.
Herein, we generated mice in which C5aR1 is conditionally deleted from myeloid cells (with T cell C5aR1 remaining intact). We used the animals to test the impact of disabled myeloid cell C5aR1 signaling on costimulatory blockade-induced allograft survival and to delineate the mechanisms. Our findings demonstrate that myeloid cell C5aR1 is required for costimulatory blockade-induced cardiac allograft survival and newly link C5aR1 expression to MREG accumulation within the allograft, together altering current thinking about how complement impacts alloimmunity and transplant outcomes.
MATERIALS AND METHODS
Mice
C57BL/6 (B6, H-2b) and BALB/c (H-2d) mice 8 weeks of age were purchased from Jackson Laboratory (Bar Harbor, ME) or bred from Jackson-derived animals at Mount Sinai. B6 C5ar1fl/fl mice were generated from ES cells with loxp sites surrounding exon 2 of the C5ar1 gene obtained from the EUCOMM consortium (Figure 1A). Offspring were initially crossed to B6flp/flp mice to delete the FRT sites surrounding the lacZ and neo genes required for selection following homologous recombination, and then crossed offspring to B6 LysM-Cre mice or a B6 S100A8-Cre (Jackson Laboratory). T cell receptor transgenic (Tg) TEa mice [CD4+ reactive to I-Ab + I-Eα52–58] were obtained as a gift from A. Rudensky (Memorial Sloan Kettering Cancer Center, NY, nY) and bred at Mount Sinai. All animals were housed in the Center for Comparative Medicine and Surgery at the Icahn School of Medicine at Mount Sinai under Institutional Animal Care in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International (IACUC). Experiments were performed with age- and sex- matched mice and using animals that were littermates or were maintained in the same room and/or were co-housed within the same cages for >2 weeks to limit potential effects of microbiome differences.
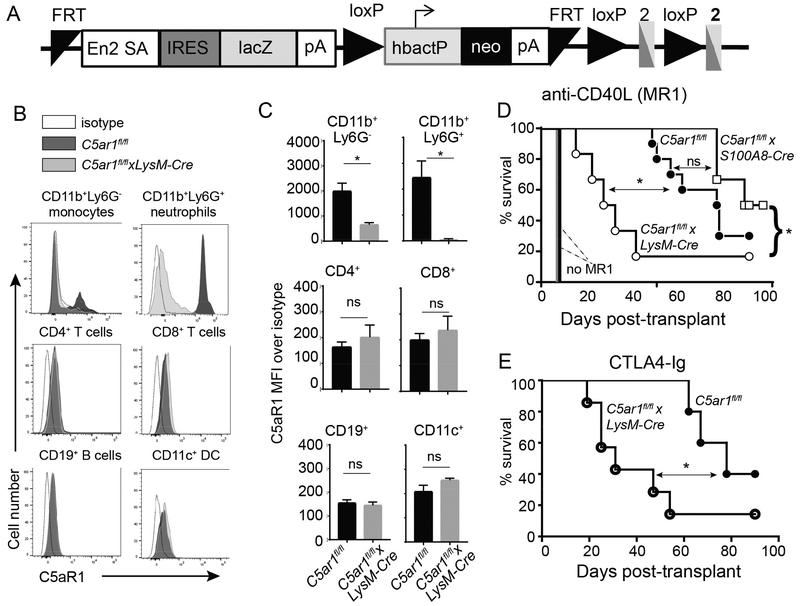
Figure 1.
Myeloid cell deficiency of C5ar1 abrogates costimulatory blockade induced prolonged cardiac allograft survival. A. Schematic representation of the targeting insert to conditionally delete C5ar1. Animals transmitting the insert were crossed with flp/flp mice to remove the genes between the 2 FRT sites. The resultant C5ar1fl/fl mice were crossed to a LysM-Cre transgenic to remove a portion of C5ar1 exon 2 from myeloid cells or to an S100A8-Cre transgenic to remove a portion of C5ar1 exon 2 from neutrophils. B-C. Representative flow cytometry plots (B) and quantified surface expression (MFI) of C5aR1 (C) on monocytes and neutrophils (top row) CD4+ and CD8+ T cells (middle row), and B cells and DCs (bottom row). D. Survival of BALB/c hearts transplanted into C5ar1fl/fl, C5arfl/flxLysM-Cre or C5arfl/flxS100A8-Cre recipients treated with anti-CD40L mAb MR1 250μg on day −1 (n=6–10/group). Survival of BALB/c hearts in untreated C5ar1fl/fl (solid black line, no symbol) and C5arfl/flxLysM-Cre (solid gray line, no symbol, n=4/group) recipients. E. Survival of BALB/c hearts transplanted into C5ar1fl/fl or C5arfl/flxLysM-Cre and treated with CTLA4-Ig (n=5–7/group) (E). *p<0.05 by t-test. ns: nonsignificant. *p<0.05, **p<0.05 by survival analysis (Mantel-Cox log rank test). ns=not significant.
Heterotopic Heart transplantation
Heterotopic Heart transplantation was performed as previously described (4, 19, 30, 31). In some experiments recipients were treated with either 250μg anti-CD40L mAb (clone MR1, BioXcell) i.v. on the day prior surgery or 250μg CTLA4-Ig (abatacept) on day +1. Rejection was defined as the day on which a palpable heartbeat was no longer detectable and was confirmed by histological examination.
Antibodies
Fluorochrome-conjugated monoclonal antibodies (mAbs) specific to mouse, CD11b (M1/70), CD11c (N418), CD45 (30-F11), CD8 (53–6.7), CD4 (GK1.5), Foxp3 (JFK16s), Ly6C (HK1.4), Vα2TCR (B20.1), IFNγ (XMG1.2) and isotype controls were purchased from eBioscience. Fluorochrome-conjugated anti-Ly6G (1A8) mAb and CD115 (CSF1-R; AFS98) were purchased from BioLegend. Two different antibodies were used for C5aR staining: anti-mouse CD88 (20/70, BioLegend and P12/1, Serotec). Fixed Viability Dye eFluor450 or eFluor780 (eBioscience) and PE-Annexin V (BD Biosciences) were used for dead/apoptotic cell analysis.
Cell staining and flow cytometry
Cell washes and monoclonal antibody (mAb) dilutions were performed in phosphate buffered saline (PBS) plus 1% fetal bovine serum at 4°C. For Foxp3 detection, cells were first stained for CD4, permeabilized, and stained for intracellular Foxp3 using the Fixation-and-Permeabilization buffers FoxP3 Kit (eBioscience). Fc receptors were blocked with purified anti-CD16/CD32 (Fc Shield; 2.4G2; TONBO Biosciences).Flow cytometric analysis was performed on FACS CANTO II (BD Biosciences) and analyzed using Cytobank (Cytobank, Santa Clara, CA) or FlowJo software (Tree Star, Inc.).
Isolation and purification of graft infiltrating myeloid cells
Cell suspensions of mouse hearts allografts were prepared as published (19). Cell suspensions were stained with antibodies to CD45, CD11b, Ly6G and Ly6C. Cell sorting was performed at the Flow cytometry core facility at Mount Sinai. To assess suppressive function purified, 5×105 CD11b+Ly6Clo myeloid cells obtained from graft infiltrating lymphocytes were mixed with purified, CFSE-labeled syngeneic T cells (2×105 T cells) purified to >95% by cell sorting (Sony SH800Z) obtained from naïve mice were co-cultures with anti-CD3 (1μg/mL, clone 145–2C11; Thermo Fisher Scientific) and 5×105 APCs in complete RPMI medium for 4 days. For splenic DC isolation, single-cell suspensions of BALB/c (H-2d) splenocytes were incubated with CD11c MicroBeads (Milteny Biotec), following manufacturer’s protocol. T cell proliferation/suppression was measured by flow cytometric analysis of CFSE dilution gated on the CD4+ T cells.
Analysis of donor-reactive IFNγ-producing CD8+ T cells
Recipient spleen cells were stimulated with donor-derived spleen cells overnight, incubated Golgi Plug (BD) for the last 4h of the culture. The cells were then stained for surface CD8, fixed, permeabilized, and stained with fluorochrome-conjugated anti-IFNγ, and analyzed by flow cytometry. In some experiments IFNγ-producing spleen cells were quantified by cytokine ELISPOT assays as previously described (4, 19, 30, 31) and analyzed on ImmunoSpot Series 4 analyzer (CTL, Shaker Heights, OH).
In vivo TREG expansion assays
CD4+ T cells were isolated (>97% purity) from naïve B6 TEa mice by negative selection (Easy Sep Mouse CD4+ T cell Isolation Kit, StemCell Technologies). Aliquots were stained for Foxp3 expression. 20×106 TEa cells were then injected i.v. into MR1-treated B6 recipients on the day prior to transplantation with a BALB/c heart. Spleen cells were harvested one week later and analyzed for Foxp3 expression within the CD4+Vα2+TCR+ gate.
Myeloid cell transfers
Bone marrow monocytes were obtained from C5ar1fl/fl and C5ar1fl/flxLysM-Cre+ mice as published (2, 4) and CD45+CD11b+Ly6Chi monocytes were purified to >95% by cell sorting (Sony SH800Z). The monocytes from each strain were differentially labeled with either 5μM CFSE or 2×10−6M PKH26 (Sigma) and 1×106 cells from each labeled preparation were co-injected i.v. into MR1-treated C5ar1fl/fl recipients of BALB/c hearts 24h post-transplantation. Three days later the mice were sacrificed and graft infiltrating cells were analyzed by flow cytometry.
Microarrays and analysis
Graft infiltrating CD45+CD11b+Ly6CloLy6G- cells were isolated by flow sorting (>95% purity) from MR1-treated C5aR1fl/fl and C5aR1fl/flxLysM-cre recipients of BALB/c hearts at day 6 post-transplantation and immediately placed in TRizol (Ambion, Life Technologies). Total RNA was isolated (>150 pg RNA per sample), and the samples were processed using standard WT Pico protocols and hybridized to Mouse Gene 2.0 ST Arrays. Chips were scanned using a GeneChip Scanner 7G (Affymetrix) at the State University of New York Albany Center for Functional Genomics. Analysis was performed at Mount Sinai. The intensity data at the probe set level were extracted and normalized with the RMA algorithm (32) and data quality was assessed using Expression Console (Affymetrix). The Affymetrix control probe sets as well as the probe sets with low intensity across all samples were excluded from downstream analysis. The limma test (33) was performed on normalized data between comparison groups, and the differentially expressed genes with p<0.05 were identified and visualized using a heat map. Gene ontology enrichment analysis (34, 35) using a Fisher exact test was performed on differentially expressed genes to investigate their associated biological functions or pathways. Iterative analyses were performed on subsets of genes determined by fold-change thresholds ranging from 1.5 to 2.5 to assess the robustness to noise (unspecific genes) and number of genes in the signal. Stable enrichment signals were only detected for the downregulated subset with a strongest signal-to-noise ratio found at a threshold of 2 (70 genes).
Serum donor-reactive alloantibody
Donor reactive alloantibodies were detected as published (36). Briefly, serum samples from recipient mice were diluted in PBS as indicated and incubated for 30 minutes at 4ºC with syngeneic, donor or third-party thymocytes as target cells. Following a wash step with PBS 1% albumin, the bound antibody was detected by incubation with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse IgG (eBioscience) and quantified by flow cytometry.
Real time quantitative polymerase chain reaction (qRT-PCR)
RNA isolation, cDNA synthesis, RT, and real-time RT–polymerase chain reaction (PCR) were performed as described previously (37). PCR products were normalized to the 18S control gene and expressed as fold increase over the mean value of the control group using the DDCt method.
Cell migration assays
Enriched murine bone marrow derived myeloid cells from WT and C5ar1−/− mice were isolated by negative selection (Easy Sep Mouse Monocyte Isolation Kit, StemCell Technologies) and purity assessed by flow cytometry. The isolated monocytes were stimulated overnight in serum free HL1 medium (Lonza) with recombinant mouse C5a (100ng/mL; 5×105 cells/well; R&D Systems) or recombinant murine CXCL9 (1μg/mL; R&D Systems) and CCL2 (2ng/mL; BioLegend) were used as a positive control. A quantitative determination of cell migration was tested using a CytoSelect™ 96-Well Cell Migration Assay Kit (5μm, Fluorometric format; Cell Biolabs) following manufacturer’s instructions. Fluorescence measurement was performed on SpectraMax M3 Fluorometer (Molecular Devices) with 485/538 nm filter set and 530 nm cutoff.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software version 5 (La Jolla, CA). Differences between graft survival rates were assessed by Mantel-Cox log rank test. Group comparisons of paired data were analyzed by paired t tests. One-way repeated measures ANOVA was used for multiple comparisons among treatment groups, with pairwise multiple comparison procedures performed using Bonferroni post hoc tests. All experiments were repeated at least twice. Data are presented as mean values with SD. Statistical significance is expressed as follows: *P < 0.05, NS not significant.
RESULTS
Monocyte-expressed C5aR1 is required for prolonged allograft survival induced by costimulatory blockade
In previous work we identified a subset of regulatory monocyte-derived macrophages that a) develop within the grafts of MR1-treated heart transplant recipients, b) are required for murine heart transplant tolerance (2, 4), and c) are phenotypically and functionally similar to myeloid derived suppressor cells that spontaneously develop in response to tumors. Building upon these findings that blocking C5aR1 limits myeloid derived suppressor cell function in tumor models and causes tumor rejection (29), we tested whether C5aR1 is required for MREG-dependent allograft survival. We generated C5ar1fl/fl mice from EUCOMM embryonic stem cells and crossed them with animals expressing the Cre recombinase (Cre) under a LysM promoter (expresses in myeloid cells, Figure 1A). Comparative flow cytometric phenotyping of spleen cells showed similar frequencies of immune cell subsets between naïve C5ar1fl/fl and C5ar1fl/flxLysM-cre mice (Figure S1). In vitro cultures using spleen cells from naïve animals stimulated overnight with allogeneic BALB/c APCs showed no differences in baseline frequencies of BALB/c-reactive IFNγ-producing CD4+ or CD8+ T cells (Figure S2). We did not detect C5aR1 on splenic monocytes or neutrophils of the C5ar1fl/flxLysM-cre mice (Figure 1B) but C5aR1 was detected on splenic T cells, B cells and dendritic cells at levels comparable to those of C5ar1fl/fl controls (Figure 1C), documenting specific deletion of C5aR1 from myeloid cells in the C5ar1fl/flxLysM-cre mice.
We transplanted groups of MR1-treated C5ar1fl/fl mice and C5ar1fl/flxLysM-cre mice with fully MHC-disparate BALB/c hearts. As anticipated, allografts transplanted into MR1-treated C5ar1fl/fl recipients survived significantly longer than those transplanted into untreated C5ar1fl/fl recipients [median survival time (MST) of 60 vs 8 days, (Figure 1D). Allografts transplanted into MR1-treated C5ar1fl/flxLysM-cre recipients (in which C5aR1 is disabled in myeloid cells) survived for only 30 days (p<0.05 vs. MR1-treated C5ar1fl/fl recipients). Histological examination of the rejected allografts in all MR1-treated recipients confirmed mononuclear cell infiltration consistent with cellular rejection in all cases (data not shown). Untreated C5ar1fl/fl and C5ar1fl/flxLysM-cre recipients each rejected BALB/c hearts with a MST of 7 days (Figure 1D, p=ns). When we transplanted groups of C5ar1fl/fl and C5ar1fl/flxLysM-cre recipients with BALB/c hearts and treated the recipients with CTLA4-Ig (abatacept) we also observed accelerated rejection in the C5ar1fl/flxLysM-cre recipients (Figure 1E), demonstrating that the effects of C5aR1-deficiency on myeloid cells apply to a distinct (and clinically relevant) costimulatory blockade strategy.
The C5ar1fl/flxLysM-cre mice lack C5aR1 in all myeloid cells, including neutrophils. We performed additional heart graft survival experiments in MR1-treated, C5ar1fl/flxS100A8-cre recipients (Figure 1D) that lack C5aR1 solely in neutrophils but otherwise are phenotypically similar to C5ar1fl/fl controls (Figures S2–S4) and functionally, have baseline frequencies of alloreactive T cells that are not different from the C5ar1fl/fl controls (Figure S2). These studies remarkably showed that the MST of heart allografts transplanted into MR1-treated C5ar1fl/flxS100A8-cre recipients (with C5aR1 deficiency restricted to neutrophils) did not differ from the MST of hearts transplanted into MR1-treated C5ar1fl/fl controls (and was significantly longer than the MST of grafts transplanted into MR1-treated C5ar1fl/flxLysM-cre mice). Together the findings support the conclusion that monocyte expression of C5aR1 is required for graft prolongation in this system.
Absence of myeloid cell C5aR1 augments donor-reactive T cell immune responses despite costimulatory blockade
To assess the impact of absent myeloid cell C5aR1 on the donor-reactive T cell response, we transplanted BALB/c hearts into groups of MR1-treated C5ar1fl/fl and C5ar1fl/flxLysM-cre recipients and quantified donor-reactive IFNγ-producing cells in the recipient spleens by ELISPOT (Figure 2A, day 14, all allografts beating). These assays showed ~2-fold higher frequencies of donor-reactive IFNγ-producers in the C5ar1fl/flxLysM-cre recipients vs. the C5ar1fl/fl controls (~250 vs 100 per 5×105 splenocytes, p<0.05).
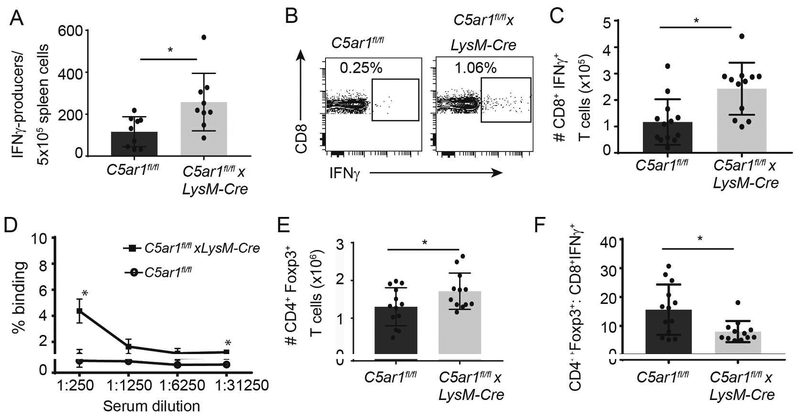
Figure 2.
Absence of myeloid cell expressed C5ar1 augments donor-reactive T cell immune responses in MR1-treated allograft recipients. A. Donor-reactive IFNγ ELISPOTs from C5ar1fl/fl and C5ar1fl/flxLysM-Cre recipients of BALB/c hearts 14 days post-transplant. n=9/group. Frequencies of donor-reactive IFNγ-producers in spleens of untreated C5ar1fl/fl and C5ar1fl/flxLysM-Cre recipients of BALB/c hearts at rejection (day 7) were ~1300/5×105 spleen cells and were not different between groups (not shown). B-C. Representative flow cytometry plots (B) and total numbers per spleen of donor-reactive IFNγ-producing CD8+ T cells (C) from C5ar1fl/fl and C5ar1fl/flxLysM-Cre recipients of BALB/c hearts 14 days post-transplant, n=12/group. D. Serum donor-reactive alloantibodies in C5ar1fl/fl and C5ar1fl/flxLysM-Cre recipients of BALB/c hearts 14 days post-transplant, n=3/group E-F. Total numbers of Foxp3+CD4+ T cells per spleen (E) and calculated ratios of CD4+Foxp3+ cells/donor reactive IFNγ-producers per mouse (TREG:TEFF ratios), n=12/group (F). * p<0.05
To document that the above detected IFNγ derived from recipient T cells we performed mixed lymphocyte responses (MLR) in which recipient spleen cells from day 14 post-transplant recipients were stimulated with BALB/c APCs and analyzed for intracellular IFNγ production within CD8+ T cells by flow cytometry (Figure 2B–C). These assays confirmed higher frequencies of splenic donor-reactive IFNγ-producing CD8+ T cells in the MR1-treated C5ar1fl/flxLysM-cre recipients. Frequencies of donor-reactive IFNγ-producing CD4+ T cells were low (~1%) and did not differ between groups at the 14 day post-transplant time-point tested (n=7/group, 2 different experiments, data not shown). We did detect donor-reactive alloantibodies on day 14 post-transplant in the sera MR1-treated C5ar1fl/flxLysM-cre recipients (titer >1:250) but not in sera from MR1-treated C5ar1fl/fl control recipients (Figure 2D).
Absence of myeloid cell C5aR1 limits TREG expansion
In our published work we previously showed that allograft-derived myeloid cells in MR1-treated recipients mediate their suppressive function by driving expansion of donor-reactive TREG (4). When we quantified frequencies and total numbers of splenic CD4+Foxp3+TREG in the recipients on day 14 post-transplant we observed ~50% higher frequencies of TREG in C5ar1fl/flxLysM-cre recipients (Figure 2E). However, calculated ratios of absolute numbers of TREG:donor reactive IFNγ-producing CD8+ effector T cells in each animal trended lower in the C5ar1fl/flxLysM-cre recipients (Figure 2F).
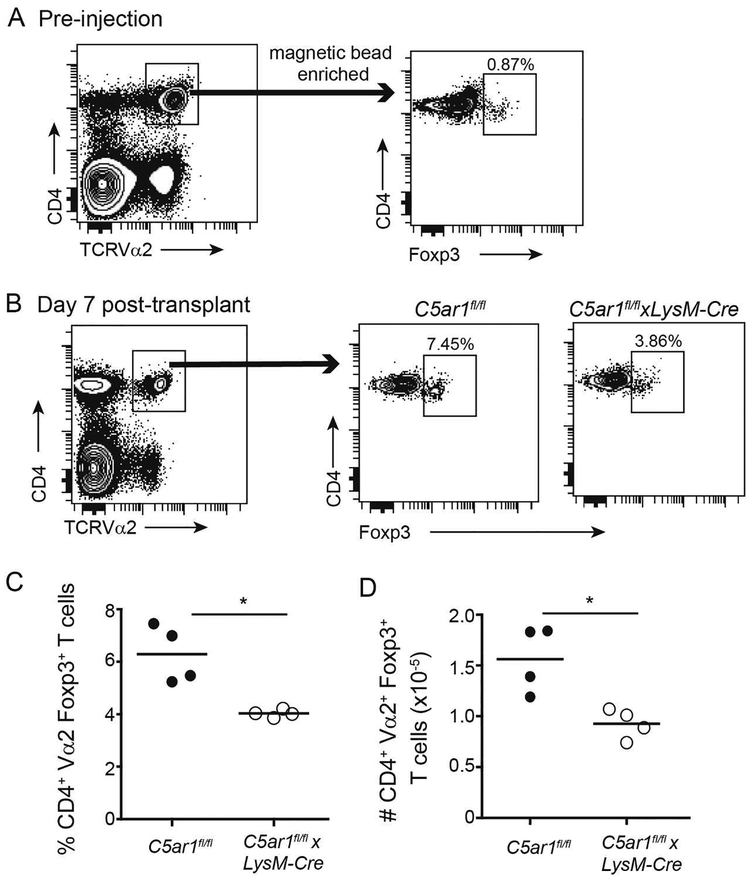
To test whether the absence of myeloid cell expressed C5aR1 inhibits induction/expansion of donor-reactive TREG in vivo, we used TEa TCR Vα2+ transgenic CD4+ cells specific for I-Ab+ the donor-derived peptide, I-Edα52–68 (3). We isolated splenic CD4+ T cells from TEa mice (~0.9% expressed Foxp3, Figure 3A), adoptively transferred them into C5ar1fl/fl and C5ar1fl/flxLysM-cre hosts, treated the recipients with MR1, and 24 h later transplanted them with BALB/c hearts. On day 7 post-transplant, we quantified the percentage and total numbers of splenic CD4+Vα2+ TEa TCR+ FoxP3+ T cells in the recipient spleens (Figure 3B–D). These analyses showed significantly fewer Foxp3+ TEa cells in C5ar1fl/flxLysM-cre recipients (percentage and absolute numbers), indicating that C5aR1 expression on myeloid cells facilitates TREG expansion/induction in MR1-treated allograft recipients.
Figure 3.
Absence of myeloid cell-expressed C5ar1 limits in expansion of donor-reactive TREG in MRI-treated allograft recipients. A. Gating strategy for enriching CD4+Vα2+ TEa T cells from spleens of TEa TCR tg mice (left), confirmation of purity of isolated population (right) demonstrating ~0.9% Foxp3+CD4+ T cells within the sorted TEa+ population. The purified cells were adoptively transferred into C5ar1fl/fl and C5ar1fl/flxLysM-Cre and treated with MR1 (day 0), 24 h later transplanted with BALB/c hearts. B-D. Gating strategy, representative flow cytometry plots (B) and quantified results showing percentages (C) and total numbers (D) of adoptively transferred TEa cells analyzed 7 days post-transplant demonstrating fewer Foxp3+ TEa cells in the C5ar1fl/flxLysM-Cre recipients, n=4/group (from 2 separate experiments). p<0.05
Myeloid cell expression of C5aR1 modulates intragraft accumulation of suppressive, Ly6Clo myeloid cells in MR1-treated recipients
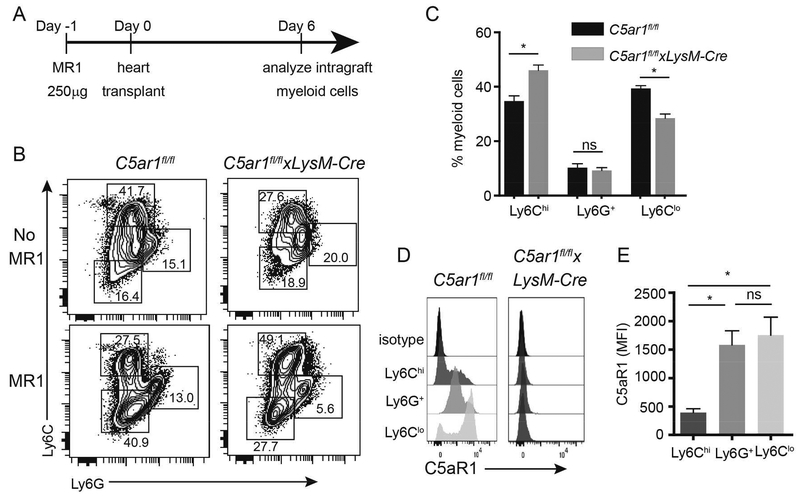
We previously showed that MR1 induces intragraft accumulation of Ly6CloLy6G- myeloid cells that are a) derived from Ly6Chi precursors and b) are required for prolonged allograft survival (2, 4). To assess the impact of myeloid cell C5aR1 on intragraft myeloid cell accumulation we analyzed graft-infiltrating myeloid cell subsets from MR1-treated C5ar1fl/fl and C5ar1fl/flxLysM-cre graft recipients (day 6) by flow cytometry (Figure 4A, schematic). Gating on live CD45+CD11b+ cells and consistent with previous work (2, 4), we discerned three populations based on differential expression patterns of Ly6C and Ly6G: Ly6ChiLy6G-, LyC6intLy6G+ (neutrophils) and Ly6CloLy6G- (contains the suppressive myeloid cells, Figure 4B). Whereas in the C5ar1fl/fl mice MR1 induced an increase in intragraft accumulation of Ly6CloLy6G- myeloid cells compared to untreated controls, we detected significantly fewer intragraft Ly6CloLy6G- myeloid cells in C5ar1fl/flxLysM-cre recipients (p<0.05, Figure 4B–C). We did not observe differences in the frequencies of Ly6G+ cells between groups (Figure 4B–C). Control analyses showed that whereas all intra-graft myeloid cells from C5ar1fl/fl recipients expressed C5aR1, no C5aR1 was detectable on any of the intragraft myeloid cell subsets from the C5ar1fl/flxLysM-cre recipients (Figure 4D–E).
Figure 4.
Myeloid cell-expressed C5ar1 regulates accumulation of Ly6Clo myeloid cells in allografts following recipient treatment with MR1. A. Schematic of experimental design. B. Representative flow cytometry plots of graft-infiltrating anti-Ly6C/Ly6G-stained cells gated on the live CD45+CD11b+ subset from untreated or MR1-treated C5ar1fl/fl and C5ar1fl/flxLysM-Cre recipients of BALB/c hearts. C. Quantified results of MR1 treated recipients (n=8/group). D-E. Representative flow cytometry histograms (D) and quantified MFI results (E) showing absence of C5aR1 expression on graft infiltrating CD11b+ Ly6G/Ly6C subsets from BALB/c allografts transplanted into MR1-treated C5ar1fl/fl and C5ar1fl/flxLysM-Cre (day 6 post-transplant). *p<0.05, ns=not significant.
C5aR1 regulates migration of myeloid cells to the allograft
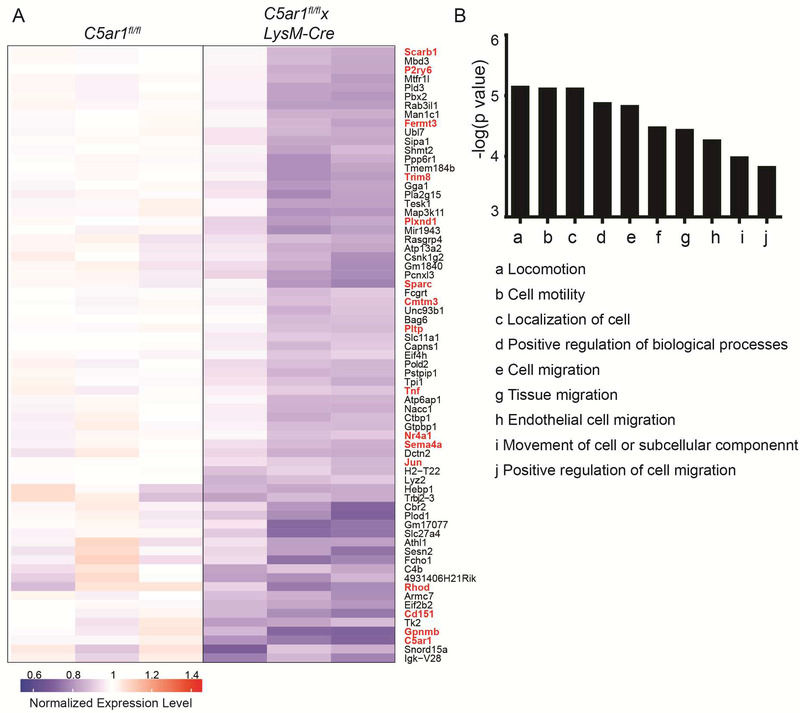
Potential mechanisms to account for less intragraft accumulation of MREG and accelerated rejection in the absence of C5aR1 include defects in myeloid cell migration, defects in intragraft induction/conversion into MREG phenotype, and accelerated myeloid cell death. In an effort to discern these mechanisms (among others), we flow sorted intragraft Ly6CloLy6G- myeloid cells from MR1-treated C5ar1fl/flxLysM-cre and C5ar1fl/fl recipients on day 6 post-transplant, isolated RNA and hybridized the RNA to microarrays. Analysis showed >1.5-fold upregulation of 277 genes and downregulation of 412 genes in the graft-infiltrating myeloid cells of the C5ar1fl/flxLysM-cre recipients (Figure 5A, full dataset available on GEO database). Enrichment analysis of the upregulated genes showed no significant signal (not shown). Enrichment analysis of the downregulated genes implicated gene ontology (GO) term pathways predominantly involved in cell migration, cell motility, locomotion, and localization (Figure 5B). The genes comprising these GO terms include C5ar1 (a receptor for C5a, a known chemoattractant) as well as multiple genes associated with tumor metastasis [Cmtm3, Nr4a1, Sparc, Tnf, Gpnb (38–41)] and with chemotaxis, adhesion and cytoskeletal interactions [Fermt3, Rhod, Cd151, Sema4a, Plxnd1 (42–50)].
Figure 5.
Microarray analysis indicates absence myeloid cell-expressed C5aR1 specifically reduces the expression of genes involved in cell migration and locomotion. Heatmap (A) and enrichment analysis (B) of downregulated genes in microarrays of RNA obtained from flow-sorted intragraft CD11b+Ly6CloLy6G- cells on day 6 after BALB/c hearts were transplanted into MR1-treated C5ar1fl/fl (n=3) and C5ar1fl/flxLysM-Cre (n=4) recipients of BALB/c hearts. The genes that comprise these pathways are depicted in red font in A.
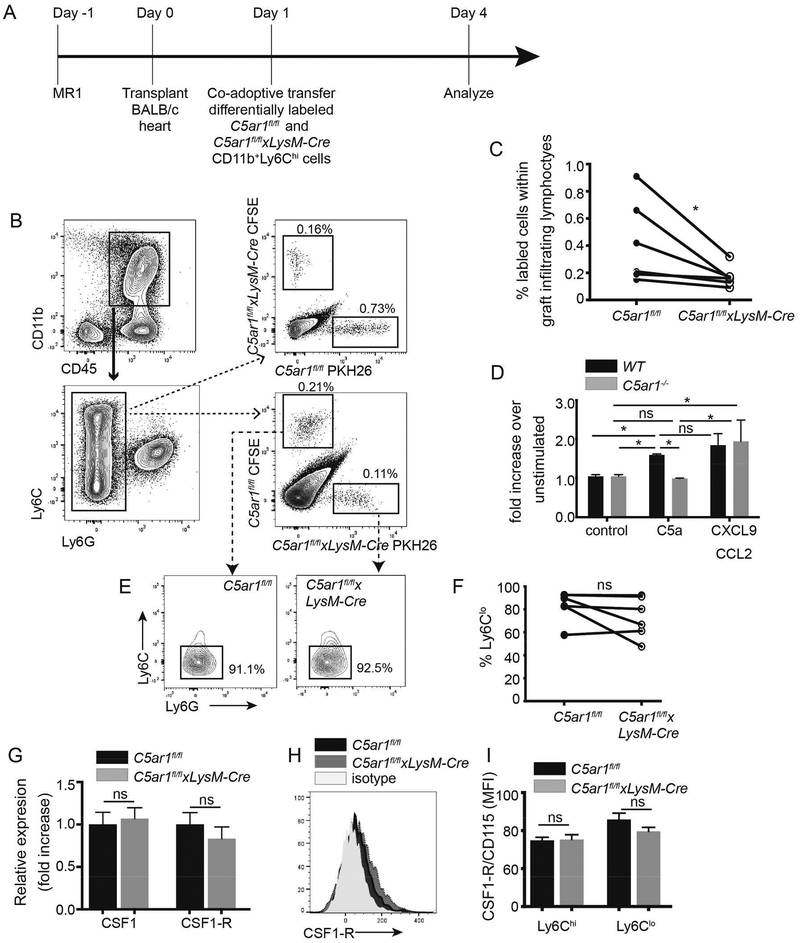
To test for functional evidence of migratory defects in the absence of C5aR1, we flow-sorted CD11b+Ly6Chi cells from bone marrow of C5ar1fl/fl and C5ar1fl/flxLysM-cre mice, differentially labeled them with fluorescent dyes CFSE or PKH26 and co-transferred equal numbers into MR1-treated C5ar1fl/fl recipients of BALB/c hearts (Figure 6A, schematic). We quantified the frequencies of labeled C5ar1fl/fl vs C5ar1fl/flxLysM-cre cells within each graft 3 days later (Figure 6B–C). In 2 separate experiments, these analyses showed significantly fewer C5ar1fl/flxLysM-cre vs control C5ar1fl/fl myeloid cells within the allografts, a result that was independent of the whether the cells were labeled with CFSE or PKH26 (noting that these 2 dyes may differentially result in nonspecific cellular toxicity). In vitro cell migration assays (Figure 6D) demonstrated that while WT murine myeloid cells migrated in response to C5a (and CXCL9/CCL2 as a positive control) C5aR1-deficient murine myeloid cells did not migrate to C5a (p<0.05 vs control) but did respond to CXCL9/CCL2.
Figure 6.
Myeloid cell-expressed C5ar1 is required for optimal migration of myeloid cells to allografts in MR1-treated recipients. A. Schematic of experimental design (see text for details). B Representative flow cytometry plots showing gating strategy and revealing lower frequencies of intragraft, adoptively transferred C5ar1fl/flxLysM-Cre Ly6Glo myeloid cells regardless of label with CFSE or PKH26. C. Quantified frequencies of intragraft myeloid cells in day 4 post-transplant allografts. n=6/group, 2 separate experiments. D. Chemotaxis assays of CD11b+Ly6Chi monocyte WT or C5ar1−/− mice in response to C5a (100 ng/ml) and CXCL9 (1μg/mL) + CCL2 (2ng/mL). E-F. Representative flow cytometry histograms (E) and quantified results (F) depicting percentages of Ly6CloLy6G- myeloid cells within the adoptively transferred intragraft populations. G-I. Quantitative RT-PCR for CSF1 and CSF1-R expression (G), and representative flow plot (H) and quantified surface expression (I) of CSF1-R on intragraft myeloid cells from MR1-treated C5ar1fl/fl and C5ar1fl/flxLysM-Cre recipients of BALB/c hearts. *p<0.05, ns=not significant
To separately assess whether absence of myeloid cell C5aR1 impacts conversion of Ly6Chi myeloid cells to Ly6Clo suppressive myeloid cells (4), we phenotyped the above, adoptively transferred, CFSE-labeled and PHK26-labeled cells within each allograft based on Ly6C/G expression levels (Figure 6E–F). These analyses showed a predominance of Ly6Clo cells with similar elevated ratios of Ly6Clo:Ly6Chi myeloid cells C5ar1fl/fl WT and C5ar1fl/flxLysM-cre Ly6Clo cells within the graft-infiltrating myeloid cells, indicating conversion is unaffected by C5aR1 expression. Moreover, while neutrophil-produced CSF1 binding to CSF1-receptor (CSF1-R) on Ly6Chi myeloid cells is required for conversion to the Ly6Clo regulatory phenotype (4, 12) we did not detect differences in either CSF1 gene expression (Figure 6G) or CSF1-R surface expression on graft infiltrating mononuclear cells between allografts in control C5ar1fl/f vs. C5ar1fl/flxLysM-cre allograft recipients (Figure 6H–I).
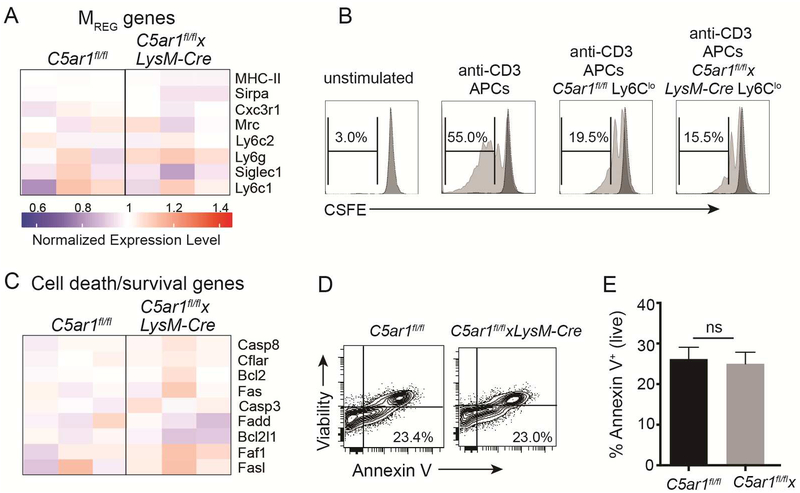
In contrast to the above observed downregulated expression of genes related to migration/locomotion in intragraft C5ar1fl/flxLysM-cre myeloid cells C5aR1 shown in Figure 5, our gene expression profiling revealed no differences in expression of genes/proteins associated with myeloid cell suppressive function [Cx3cr1, Siglec1 (CD169), CD68, Sirpa (CD172), and MHC-II (Figure 7A)] (4). Functionally, intragraft Ly6Clo myeloid cells isolated on day 6 post-transplant from C5ar1fl/f and from C5ar1fl/flxLysM-cre allograft recipients were each able to suppress T cell in vitro stimulated CD4+ T cells (Figure 7B).
Figure 7.
Graft infiltrating C5ar1fl/fl and C5ar1fl/flxLysM-Cre Ly6Clo myeloid cells exhibit equivalent suppressive capacities and rates of apoptosis. A. Heatmap depicting expression levels for MREG-produced genes involved in suppressive function (see Figure 5 for details, n=3–4/group). B. Representative flow cytometry plots of unstimulated or anti-CD3 stimulated WT T cells ± flow sorted CD11b+Ly6CloLy6G- myeloid cells (200,000 T cells, 50,000 CD11c+ APCs and 50,000 myeloid cells) isolated from allografts on day 6 of MR1-treated C5ar1fl/fl (controls) or C5ar1fl/flxLySM-Cre recipients, illustrating suppressive ability of myeloid cells from both genotypes. Representative of results from 2 independent experiments. C. Heatmap depicting expression levels genes involved in cell death/survival (see Figure 5 for details, n=3–4/group). D-E. Representative flow cytometry plot (D) and quantified results (E) depicting frequency of apoptotic (AnnexinV+) CD11b+ Ly6Clo graft-infiltrating cells. *p<0.05, ns=not significant.
When we compared expression levels of genes related to cell death/survival (including Caps8, Bcl2, Fas, Fasl), which we previously showed is one mechanism through which C5aR1 impacts T cell immunity (17, 18), the analyses revealed no differences (Figure 7C). Surface staining of graft infiltrating lymphocytes for annexin-V as a measure of apoptotic cells showed no differences between groups (Figure 7D–E).
DISCUSSION
Our data newly demonstrate that C5aR1 expression on recipient myeloid cells is crucial for costimulatory blockade-induced survival of fully MHC-disparate cardiac allografts. In the absence of myeloid cell-expressed C5aR1, CD11b+ myeloid cells do not optimally migrate to the allograft to become MREG, and as a consequence, do not fully promote TREG expansion, together resulting in enhanced CD8+, IFNγ-producing TEFF expansion in the spleen and accelerated graft rejection.
The gene array analyses performed on RNA derived from graft infiltrating mononuclear cells add to the literature by associating numerous genes previously shown to participate in chemotaxis, angiogenesis, adhesion, and cancer metastasis (38–50) with C5aR1. Additional studies to be performed by our laboratory among others are required to test the specific hypothesis that these gene products, individually or together, are requisite intermediaries of C5a/C5aR1-initiated MREG chemotaxis.
The chemotaxis receptor function of myeloid cell-expressed C5aR1 (in response to C5a ligation) in costimulatory blockade-induced allograft survival has not been previously reported, but C5aR1 signaling has been linked to chemotaxis functions (51–53) and several groups have shown that C5a/C5aR1 ligations on tumor-associated MDSC facilitate MDSC accumulation into the tumor, promote angiogenesis and prevent T cell mediated tumor rejection by augmenting TREG and inhibiting TEFF (29, 54–58). The observations that C5aR1 is required for MREG migration/accumulation into tumors and into transplants, and as a consequence to mediate tumor and transplant survival illustrates commonality of mechanism despite distinct pathophysiological contexts. Complement component C3 has been separately shown to be important in the differentiation of MDSC (59, 60), an effect that has not been attributed to C5aR1. While we have not formally documented the source of C5a in this system, we speculate based on our previous work that it derives from recipient plasma and/or recipient immune cell-produced complement (19, 37, 61).
The findings described herein, in which we show that myeloid cell deficiency of C5aR1 augments alloreactive TEFF and limits TREG expansion following costimulatory blockade are distinct from our previous work in which we demonstrated that global absence/blockade of C5a/C5aR1 interactions inhibits TEFF, promotes TREG induction and function, and inhibits T cell-mediated diseases including transplant rejection (17, 18, 21, 22, 25, 26, 62, 63). This apparent paradox can be explained by cell type-specific differences in C5a/C5aR1-initiated cellular responses. While we showed herein that C5aR1 ligation directly stimulates MREG chemotaxis to the allograft (resulting in immunoregulation), C5a/C5aR1 signaling directly on T cells activates PI-3Kγ-dependent AKT phosphorylation which stimulates T cell proliferation and inhibits apoptosis (17, 18), and simultaneously prevents Foxo1-dependent Foxp3 production to limit TREG induction and function (22, 23, 25). C5a/C5aR1 interactions activate DCs, manifested by increased expression of costimulatory molecule and innate cytokine production, which also contribute to TEFF differentiation and expansion while inhibiting TREG (24, 26). Differential effects of complement/C5aR1 signaling on various cell types likely explains why absence of complement components prevents graft tolerance under some experimental conditions (64, 65) yet promotes tolerance to alloantigens in other circumstances (22, 66–70). While we did not quantify IL-17 or IL-4 production by alloreactive T cells in these experiments, our previous work showed that complement activation augments the strength of the effector response (and inhibits the regulatory response) without altering cytokine profiles (17, 18, 21, 22, 25, 26, 62, 63).
Our observed cell-specific effects of C5aR1 signaling on graft outcome have translational implications for human transplant recipients. CD11b+CD33+HLA-DR- myeloid cells isolated from peripheral blood of kidney transplant recipients have been shown to suppress proliferation of CD4+ T cells and to expand TREG in vitro, and their time-dependent accumulation in transplant recipients directly correlated with increased peripheral blood TREG frequencies, together consistent with an MREG/MDSC phenotype (71). As human CD11b+CD33+HLA-DR- myeloid cells also express C5aR1 (Figure S5), it is important to consider the possibility that pharmacological complement inhibition aimed at blocking antibody-initiated effector functions or T cell-dependent allograft injury could have unanticipated adverse effects on MREG and thereby negatively impact, rather than improve, transplant outcomes.
In conclusion, the studies described herein add to our understanding of the role of complement receptor C5aR1 in transplantation by linking myeloid cell-expressed C5aR1 to intragraft accumulation of MREG required for suppressing pathogenic T cells and prolonging transplant survival. Understanding cell-specific effects of C5aR1 and devising approaches to better target the complement inhibitors to desired cell types will be important considerations as clinical complement inhibition moves into the transplant field.
Supplementary Material
Acknowledgments
The work was supported by NIH grant R01 AI071185 awarded to PSH and MEM. IL is a recipient of a postdoctoral fellowship grant from the American Society of Transplantation. MF is supported by NIH grant U19AI117873. The authors thank Weijia Zhang and Zhengzhi Li (Icahn School of Medicine at Mount Sinai) for assistance with the gene array and the microsurgery core facility (P Boros, Y. Li, J Liu), and the Mouse Genetics Shared Resource facility (K Kelly, Director) at the Icahn School of Medicine at Mount Sinai for their contributions to the studies. Special thanks to N Chun and J Horwitz (Mount Sinai) for their technical assistance with selected experiments.
Abbreviations:
- CFSE
Carboxyfluorescein succinimidyl ester
- C5aR1
C5a receptor
- CSF1
colony stimulating factor 1
- CSF1-R
colony stimulatory factor 1 receptor
- DC
dendritic cell
- IFNγ
interferon gamma
- mAb
monoclonal antibody
- MDSC
myeloid derived suppressor cells
- MHC
major histocompatibility complex
- MLR
mixed lymphocyte response
- MREG
regulatory myeloid cell
- tg
transgenic
- MST
median survival time
- PBS
phosphate buffered saline
- TREG
regulatory T cell
- TEFF
effector T cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996;381(6581):434–438. [DOI] [PubMed] [Google Scholar]
- 2.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest 2010;120(7):2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 2006;7(6):652–662. [DOI] [PubMed] [Google Scholar]
- 4.Conde P, Rodriguez M, van der Touw W, Jimenez A, Burns M, Miller J et al. DC-SIGN(+) Macrophages Control the Induction of Transplantation Tolerance. Immunity 2015;42(6):1143–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson TC, Trambley J, Odom K, Anderson DC, Cowan S, Bray R et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation 2002;74(7):933–940. [DOI] [PubMed] [Google Scholar]
- 6.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 2005;5(3):443–453. [DOI] [PubMed] [Google Scholar]
- 7.Rostaing L, Vincenti F, Grinyo J, Rice KM, Bresnahan B, Steinberg S et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant 2013;13(11):2875–2883. [DOI] [PubMed] [Google Scholar]
- 8.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010;10(3):535–546. [DOI] [PubMed] [Google Scholar]
- 9.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005;353(8):770–781. [DOI] [PubMed] [Google Scholar]
- 10.Burrell BE, Bromberg JS. Fates of CD4+ T cells in a tolerant environment depend on timing and place of antigen exposure. Am J Transplant 2012;12(3):576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krummey SM, Ford ML. New insights into T-cell cosignaling in allograft rejection and survival. Current opinion in organ transplantation 2015;20(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braza MS, Conde P, Garcia M, Cortegano I, Brahmachary M, Pothula V et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. Am J Transplant 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochando JC, Chen SH. Myeloid-derived suppressor cells in transplantation and cancer. Immunol Res 2012;54(1–3):275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 2008;180(12):7898–7906. [DOI] [PubMed] [Google Scholar]
- 16.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med 2005;201(10):1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 2008;112(5):1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 2008;28(3):425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raedler H, Vieyra MB, Leisman S, Lakhani P, Kwan W, Yang M et al. Anti-complement component C5 mAb synergizes with CTLA4Ig to inhibit alloreactive T cells and prolong cardiac allograft survival in mice. Am J Transplant 2011;11(7):1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieyra M, Leisman S, Raedler H, Kwan WH, Yang M, Strainic MG et al. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection. The American journal of pathology 2011;179(2):766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant 2013;13(10):2530–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med 2013;210(2):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS. Cutting Edge: Receptors for C3a and C5a Modulate Stability of Alloantigen-Reactive Induced Regulatory T Cells. J Immunol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheen JH, Strainic MG, Liu J, Zhang W, Yi Z, Medof ME et al. TLR-Induced Murine Dendritic Cell (DC) Activation Requires DC-Intrinsic Complement. J Immunol 2017;199(1):278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol 2013;14(2):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalli PN, Strainic MG, Lin F, Medof ME, Heeger PS. Decay accelerating factor can control T cell differentiation into IFN-gamma-producing effector cells via regulating local C5a-induced IL-12 production. J Immunol 2007;179(9):5793–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Lin F, Strainic MG, An F, Miller RH, Altuntas CZ et al. IFN-gamma and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol 2008;180(9):5882–5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M, Yin N, Murphy B, Medof ME, Segerer S, Heeger PS et al. Immune cell-derived c3 is required for autoimmune diabetes induced by multiple low doses of streptozotocin. Diabetes 2010;59(9):2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C et al. Modulation of the antitumor immune response by complement. Nat Immunol 2008;9(11):1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol 2004;172(9):5456–5466. [DOI] [PubMed] [Google Scholar]
- 31.Fribourg M, Ni J, Nina Papavasiliou F, Yue Z, Heeger PS, Leventhal JS. Allospecific Memory B Cell Responses Are Dependent on Autophagy. Am J Transplant 2018;18(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harr B, Schlotterer C. Comparison of algorithms for the analysis of Affymetrix microarray data as evaluated by co-expression of genes in known operons. Nucleic acids research 2006;34(2):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000;25(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Gene Ontology C Expansion of the Gene Ontology knowledgebase and resources. Nucleic acids research 2017;45(D1):D331–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cravedi P, Lessman DA, Heeger PS. Eosinophils are not required for the induction and maintenance of an alloantibody response. Am J Transplant 2013;13(10):2696–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chun N, Fairchild RL, Li Y, Liu J, Zhang M, Baldwin WM 3rd et al. Complement Dependence of Murine Costimulatory Blockade-Resistant Cellular Cardiac Allograft Rejection. Am J Transplant 2017;17(11):2810–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katerinaki E, Evans GS, Lorigan PC, MacNeil S. TNF-alpha increases human melanoma cell invasion and migration in vitro: the role of proteolytic enzymes. Br J Cancer 2003;89(6):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu F, Yuan W, Wang X, Sheng Z, Yuan Y, Qin C et al. CMTM3 is reduced in prostate cancer and inhibits migration, invasion and growth of LNCaP cells. Clin Transl Oncol 2015;17(8):632–639. [DOI] [PubMed] [Google Scholar]
- 40.Xie J, Yuan Y, Liu Z, Xiao Y, Zhang X, Qin C et al. CMTM3 is frequently reduced in clear cell renal cell carcinoma and exhibits tumor suppressor activities. Clin Transl Oncol 2014;16(4):402–409. [DOI] [PubMed] [Google Scholar]
- 41.Yuan W, Liu B, Wang X, Li T, Xue H, Mo X et al. CMTM3 decreases EGFR expression and EGF-mediated tumorigenicity by promoting Rab5 activity in gastric cancer. Cancer Lett 2017;386:77–86. [DOI] [PubMed] [Google Scholar]
- 42.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med 2009;15(3):300–305. [DOI] [PubMed] [Google Scholar]
- 43.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med 2008;14(3):325–330. [DOI] [PubMed] [Google Scholar]
- 44.Gad AK, Nehru V, Ruusala A, Aspenstrom P. RhoD regulates cytoskeletal dynamics via the actin nucleation-promoting factor WASp homologue associated with actin Golgi membranes and microtubules. Mol Biol Cell 2012;23(24):4807–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meda C, Molla F, De Pizzol M, Regano D, Maione F, Capano S et al. Semaphorin 4A exerts a proangiogenic effect by enhancing vascular endothelial growth factor-A expression in macrophages. J Immunol 2012;188(8):4081–4092. [DOI] [PubMed] [Google Scholar]
- 46.Sun T, Yang L, Kaur H, Pestel J, Looso M, Nolte H et al. A reverse signaling pathway downstream of Sema4A controls cell migration via Scrib. J Cell Biol 2017;216(1):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A et al. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J 2007;26(5):1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fei Y, Wang J, Liu W, Zuo H, Qin J, Wang D et al. CD151 promotes cancer cell metastasis via integrins alpha3beta1 and alpha6beta1 in vitro. Mol Med Rep 2012;6(6):1226–1230. [DOI] [PubMed] [Google Scholar]
- 49.Saito N, Hamada J, Furukawa H, Tsutsumida A, Oyama A, Funayama E et al. Laminin-421 produced by lymphatic endothelial cells induces chemotaxis for human melanoma cells. Pigment Cell Melanoma Res 2009;22(5):601–610. [DOI] [PubMed] [Google Scholar]
- 50.Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin alpha3beta1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell 1998;9(10):2751–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allendorf DJ, Yan J, Ross GD, Hansen RD, Baran JT, Subbarao K et al. C5a-mediated leukotriene B4-amplified neutrophil chemotaxis is essential in tumor immunotherapy facilitated by anti-tumor monoclonal antibody and beta-glucan. J Immunol 2005;174(11):7050–7056. [DOI] [PubMed] [Google Scholar]
- 52.Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol 1996;134(1):255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das D, Barnes MA, Nagy LE. Anaphylatoxin C5a modulates hepatic stellate cell migration. Fibrogenesis Tissue Repair 2014;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Markiewski MM, Lambris JD. Unwelcome complement. Cancer Res 2009;69(16):6367–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corrales L, Ajona D, Rafail S, Lasarte JJ, Riezu-Boj JI, Lambris JD et al. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J Immunol 2012;189(9):4674–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darling VR, Hauke RJ, Tarantolo S, Agrawal DK. Immunological effects and therapeutic role of C5a in cancer. Expert Rev Clin Immunol 2015;11(2):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han X, Zha H, Yang F, Guo B, Zhu B. Tumor-Derived Tissue Factor Aberrantly Activates Complement and Facilitates Lung Tumor Progression via Recruitment of Myeloid-Derived Suppressor Cells. Int J Mol Sci 2017;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nitta H, Murakami Y, Wada Y, Eto M, Baba H, Imamura T. Cancer cells release anaphylatoxin C5a from C5 by serine protease to enhance invasiveness. Oncol Rep 2014;32(4):1715–1719. [DOI] [PubMed] [Google Scholar]
- 59.Downs-Canner S, Magge D, Ravindranathan R, O’Malley ME, Francis L, Liu Z et al. Complement Inhibition: A Novel Form of Immunotherapy for Colon Cancer. Ann Surg Oncol 2016;23(2):655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsieh CC, Chou HS, Yang HR, Lin F, Bhatt S, Qin J et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood 2013;121(10):1760–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keslar K, Rodriguez ER, Tan CD, Starling RC, Heeger PS. Complement gene expression in human cardiac allograft biopsies as a correlate of histologic grade of injury. Transplantation 2008;86(9):1319–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavlov V, Raedler H, Yuan S, Leisman S, Kwan WH, Lalli PN et al. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol 2008;181(7):4580–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant 2009;9(8):1784–1795. [DOI] [PubMed] [Google Scholar]
- 64.Bartel G, Brown K, Phillips R, Peng Q, Zhou W, Sacks SH et al. Donor specific transplant tolerance is dependent on complement receptors. Transpl Int 2013;26(1):99–108. [DOI] [PubMed] [Google Scholar]
- 65.Baskiewicz-Halasa M, Roginska D, Piecyk K, Halasa M, Lejkowska R, Pius-Sadowska E et al. Mixed chimerism and transplant tolerance are not effectively induced in C3a-deficient mice. Exp Hematol 2015;43(1):14–22. [DOI] [PubMed] [Google Scholar]
- 66.Choudhry N, Li K, Zhang T, Wu KY, Song Y, Farrar CA et al. The complement factor 5a receptor 1 has a pathogenic role in chronic inflammation and renal fibrosis in a murine model of chronic pyelonephritis. Kidney Int 2016;90(3):540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Q, Li D, Carreno R, Patenia R, Tsai KY, Xydes-Smith M et al. Complement component C3 mediates Th1/Th17 polarization in human T-cell activation and cutaneous GVHD. Bone Marrow Transplant 2014;49(7):972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma Q, Li D, Nurieva R, Patenia R, Bassett R, Cao W et al. Reduced graft-versus-host disease in C3-deficient mice is associated with decreased donor Th1/Th17 differentiation. Biol Blood Marrow Transplant 2012;18(8):1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol 2006;176(6):3330–3341. [DOI] [PubMed] [Google Scholar]
- 70.Esposito A, Suedekum B, Liu J, An F, Lass J, Strainic MG et al. Decay accelerating factor is essential for successful corneal engraftment. Am J Transplant 2010;10(3):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luan Y, Mosheir E, Menon MC, Wilson D, Woytovich C, Ochando J et al. Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant 2013;13(12):3123–3131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.