Abstract
The objective of this work was to characterize and evaluate wound healing potential of Piper cubeba oil (PO) via self-nanoemulsifying drug delivery system (SNEDDS) in comparison with standard gentamycin. PO loaded SNEDDS was prepared by low energy emulsification technique and characterized for thermodynamic stability, self-emulsification power and various physico-chemical parameters. An optimal formula of PO SNEDDS was subjected to wound healing evaluation, collagen determination and histomorphological examination in female Wistar rats as compared with pure PO and standard antibiotic/gentamycin. An optimal formula of PO SNEDDS showed significant wound healing effects in Wistar female rats in comparison with pure PO. However, wound healing effects of optimized SNEDDS were comparable with standard gentamycin. An optimized formulation also indicated significant enhancement in collagen content (0.82 mg/g) in comparison with pure PO (0.53 mg/g) and negative control (0.33 mg/g). While, the collagen content of SNEDDS (0.82 mg/g) treated rats were comparable with standard gentamycin treated animals (0.98 mg/g). Histopathological examinations of optimized SNEDDS treated animals showed no signs of inflammatory cells which indicated that prepared SNEDDS was safe and nontoxic to rats. The results obtained in this work showed the potential application of SNEDDS in enhancement of the wound healing activity of PO upon oral administration.
Keywords: Collagen estimation, Histopathology, Piper cubeba, SNEDDS, Wound healing
Introduction
Essential oils are very popular group of medicinal plants due to their flavoring properties and they have been investigated for variety of therapeutic activities including from antioxidant to anticancer (Bos et al. 2007; Bakkali et al. 2008; Adorjan 2010; Miguel 2010). The most of the therapeutic activity of essential oils are due to the presence of terpenes (Adorjan 2010). Piper cubeba essential oil (PO) is obtained from Piper cubeba plant which is commonly used in Arab world. Gastroprotective, analgesic, anti-inflammatory, and hepatoprotective activity of PO has been investigated in literature (Choi and Hwang 2003; Perazzo et al. 2003; Morikawa et al. 2004; AlSaid et al. 2014, 2015). More recently, mechanistic anti-inflammatory effects of PO at molecular level have also been reported in animal models (Shakeel et al. 2015).
Various phytoconstituents such as α-thujene, α-pinene, sabinene, trans-sabinene hydrate, limonene, α-cubebene, α-copaene, β-elemene, β-caryophyllene, γ-muurolene, epi-cubebol, cubebol, γ-cadenene and α-cadinol have been reported as the main components of PO (Bos et al. 2007).
Nanomedicine-based drug delivery systems have great potential in enhancing pharmacological effects and pharmacokinetic profile of drug molecules and plant-based bioactive materials (Jaiswal et al. 2015; Shakeel et al. 2015; Odei-Addo et al. 2017; Thiruvengadam et al. 2018; Alam et al. 2018). Recently, oral nanoemulsions and “self-nanoemulsifying drug delivery systems (SNEDDS)” have also been evaluated for the enhancement of pharmacological effects of several therapeutic agents (Jain et al. 2015; Jaiswal et al. 2015; Shakeel et al. 2015; Sharma et al. 2015; Cui et al. 2019; Eleftheriadis et al. 2019). SNEDDS are pre-concentrates of nanoemulsions in which the drug is encapsulated in oil phase in the presence of surfactant/cosurfactant which could form very fine nanosized droplets/nanoemulsions upon mild agitation with an aqueous media (Villar et al. 2012; Jain et al. 2015; Shakeel et al. 2015; Sharma et al. 2015). These systems offer several advantages over other colloidal drug carriers due to their ease of preparation, low preparation cost, thermodynamic stability and nanosized droplets (Villar et al. 2012; Shakeel et al. 2013; Sharma et al. 2015). Recently, wound healing potential of various essential oils such as Commiphora guidottii, Ceylon cinnamon, Croton zehantneri, Cupressus oil, Juniperus oil, Mentha piperita, Cymbopogon citratus, Piper betle, Piper nigrum and Vitex simplicifolia have been reported in different animal models in literature (Cavalcanti et al. 2012; Farhapour and Habibi 2012; Ouoba et al. 2012; Tumen et al. 2012, 2013; Umasankar et al. 2013; Dwivedi and Tripathi 2014; Suntar et al. 2014; Wong and Ling 2014; Gebrehiwot et al. 2015). The wound healing potential of nanoemulsion formulations of clove essential oil and eucalyptus essential oil have recently been proved in literature (Alam et al. 2017, 2018). Nevertheless, the wound healing potential of PO in terms of nanosized formulations had never been investigated in literature. Most of the studies carried out for wound healing evaluation were based on topical administration of pure oil or their respective formulations. Moreover, the wound healing potential of PO has not been investigated via SNEDDS in literature. When, we performed any biological investigation on plant materials or plant phytoconstituent, it is necessary to include positive control/standard to prove the potential of that plant material/phytoconstituent. Positive control may be already marketed product of that particular plant material/phytoconstituent or other marketed product (standard) with similar activity. Because marketed products of PO are not available in the market, gentamycin was used as positive control/standard for comparison purpose. Moreover, gentamycin had broad range of antibacterial activity. For the wound healing effects, the inhibition of bacterial infection is required. If bacterial infection is prevented, wound healing effects will be faster. Therefore, it was selected as positive control in this work. Macroemulsions or ordinary emulsions of PO were not investigated in the present study because oral SNEDDS formulations had lower droplet size as compared with ordinary emulsions and hence SNEDDS have been reported more reliable in enhancing in vivo absorption and therapeutic effects of essential oils (Suqumar et al. 2014; Shakeel et al. 2015; Alam et al. 2017). Advantages of PO-SNEDDS over standard gentamycin tablets are avoidance of adverse effects, self-nanoemulsification efficiency, thermodynamic stability, dose reduction and avoidance of first pass metabolism. Hence, the aim of the present study was to develop suitable SNEDDS formulations of PO and to investigate its wound healing potential in rats in comparison with pure PO and standard gentamycin. The SNEDDS formulation of PO was prepared using safe and nontoxic components such as “Sefsol-218, Triton-X100, Transcutol-HP and water” (Shakeel et al. 2015).
Materials and methods
Plant material and chemicals
The plant of Piper cubeba was procured from local market in “Riyadh, Saudi Arabia” which was authenticated and identified by a taxonomist at “King Saud University”.
The extraction of PO from Piper cubeba plant was carried out according to method described in literature (AlSaid et al. 2015).
Different phytoconstituents of PO were characterized and determined by gas chromatography–mass spectrometry method and results are presented in previously published article of same institution (AlSaid et al. 2015). Sefsol-218 and Transcutol-HP were procured from “Nikko Chemicals (Tokyo, Japan)” and “Gattefossé (Lyon, France)”, respectively. Triton-X100 was procured from “Sigma-Aldrich (St. Louis, MO)”. Deionized water was obtained from “Milli-Q unit” in the laboratory. Other chemicals and reagents used were of analytical grade. These materials were utilized without any further purification.
Preparation of PO loaded SNEDDS
Various SNEDDS formulations of PO were prepared by spontaneous emulsification technique via construction of phase diagrams. The components used for preparation of SNEDDS were Sefsol-218/oil phase, Triton-X100/surfactant, Transcutol-HP/cosurfactant and deionized water/aqueous phase. The detailed preparation and optimization of SNEDDS is presented in our recently published article (Shakeel et al. 2015).
Briefly, 5% w/w of PO was properly mixed with required quantity of Sefsol-218 (5% w/w). The specified quantities of Triton-X100 (25% w/w) and Transcutol-HP (25% w/w) were then incorporated spontaneously into previous oil phase. The mass ratio of surfactant (Triton-X100) to cosurfactant (Transcutol-HP) was 1:1. The specified quantity of deionized water (40% w/w) was added drop by drop till clear and transparent formulation obtained.
Characterization and evaluation of SNEDDS
Optimized SNEDDS of PO was characterized in terms of thermodynamic stability, self-nanoemulsification efficiency, droplet size, polydispersity index (PI), zeta potential (ZP), viscosity, refractive index (RI), percentage of transmittance (% T), and surface morphology. The detailed description of these methodologies is presented in recently published article of PO SNEDDS (Shakeel et al. 2015).
Animals and wound healing evaluation
Female Albino Wistar rats (weighing 200–250 g) were obtained from the “Experimental Animal Care Center at College of Pharmacy, King Saud University, Riyadh, Saudi Arabia”. The animals were given controlled environmental conditions of temperature and humidity. All the animals were provided free access to standard pellet diet and tap water. These studies were performed according to the guidelines of the institute. Wound excision rat model for wound healing activity was used. For excision wound study, the animals were randomly divided into four different groups (each containing six animals). Animals were anaesthetized using anesthetic ether and depilated at the predetermined site before wounding. An excision wound was produced by cutting away approximately 500 mm2 full thickness of the predetermined area on the anterior-dorsal side of each animal (Dash et al. 2001). The treatment to animals was given by oral administration of different formulations.
Group I animals were given oral administration of an optimal SNEDDS F1 without PO and served as control group. Group II animals were given oral administration of pure PO (at dose of 25 mg/kg) and group III animals were given oral administration of an optimal SNEDDS F1 with PO (containing 25 mg/kg of PO). Group IV animals were given oral administration of gentamycin suspension (at dose of 25 mg/kg) that was considered as positive control. Test formulations were administered once daily for the period of 24 days. Wound healing activity of each group was examined in terms of wound contraction percentage and closure time. The wound area was measured every third day by placing a transparent paper over the wound and tracing it out; area of this impression was calculated using the graph sheet and wound contraction was expressed as percentage of contraction (Werner et al. 1994). The closure time for wound time was obtained when total wound healed.
Collagen determination
The piece of skin from the wound area was procured at the end of the experiment (after 24 days) and evaluated for the collagen content. Briefly, 5 ml of TES buffer and 0.1 ml of acid hydrosolate was added in tube, mixed well and incubated at 37 °C for 6 h, the contents were filtered through a syringe filter into clean tube and allowed to stand. To 0.2 ml of test filtrate, 2 ml of ninhydrin reagent was added and boiled for 30 min and cooled at room temperature. Approximately 10 ml of 1-propanol was then added and the absorbance was recorded spectrophotometrically at 570 nm (Moore and Stein 1948; Mandl et al. 1953). The collagen content in mg/g of tissue was determined from spectrophotometric absorbance. The pieces of skin were also procured from untreated rats and used for the analysis of leucine content in untreated rats. The content of leucine (mg/g) in untreated animals was served as negative control.
Histomorphological analysis of tissues
For histomorphological analysis, the healed tissues from the animals of each group were taken at the end of the experiment (after 24 days), fixed in 10% formalin, dehydrated by alcohol and embedded in paraffin blocks. Tissue sections were deparaffinized with the help of xylene. Serial sections of particular diameter were cut with the help of microtome and finally stained using hematoxylin-eosin (HE) and analyzed under light microscopy. In the healed areas, the possibility of the inflammation was estimated in a blind manner by counting the inflammatory cell infiltration/field. The presence of “epithelization, inflammatory cell infiltration, fibroblast proliferation, neovascularization and collagen deposition” on healed area of tissues were determined using a modified 0–5 numerical scale reported in literature (Alam et al. 2018).
Data analysis
The values are presented as mean ± standard error of the mean (SEM). The statistical analysis was carried out by ANOVA and P < 0.05 was considered as significant value.
Results and discussion
Characterization and evaluation of PO-SNEDDS
Various SNEDDS formulations of PO were developed by spontaneous emulsification method via construction of phase diagrams. Phase diagram studies and detailed characterization of these formulations are presented in our recently published article (Shakeel et al. 2015). In spontaneous emulsification method, SNEDDS were prepared based on visual observations, hence there is possibility of getting metastable/unstable SNEDDS. Therefore, various thermodynamic/physical stability tests were performed to remove such formulations. Three different thermodynamic tests such as “centrifugation, heating and cooling cycles, and freeze–pump–thaw cycles were performed and developed SNEDDS formulations were found to be thermodynamically stable at each test (Shakeel et al. 2015).
The use of cosurfactant, i.e., Transcutol-HP along with surfactant (Triton-X100) in SNEDDS was possible for thermodynamic stability of SNEDDS (Shafiq et al. 2007). The presence of Triton-X00 alone is not able to reduce interfacial tension up to the required value. While, the use of Transcutol-HP along with Triton-X100 might reduce the interfacial tension up to required value and proposed SNEDDS become highly stable (Shakeel et al. 2013). Optimized SNEDDS F1 which was investigated in this work passed thermodynamic stability tests in terms of centrifugation, heating and cooling cycles and freeze–pump–thaw cycles. Optimized SNEDDS also passed self-nanoemulsification test with grade A in the presence of various diluents including “0.1 N HCl, water and phosphate buffer”.
The physico-chemical parameters of optimized SNEDDS F1 are discussed in this work. However, the physico-chemical parameters of several SNEDDS formulations of PO are already published in our previous publication (Shakeel et al. 2015). Electron microscopic image of optimized SNEDDS was also published recently which showed spherical droplets of optimized SNEDDS in nanometer range (Shakeel et al. 2015). Generally, the droplet size and viscosity of SNEDDS formulations of PO were found to be decreased with increase in the Sefsol-218 (oil phase) concentration in formulations. However, the concentration of surfactants (Triton-X100 and Transcutol-HP) had little impact on droplet size and viscosity of formulations (Shakeel et al. 2015). The PIs were obtained as less than 0.3 in all prepared SNEDDS which indicated the uniformity of droplets in all formulations.
The droplet size, PI and viscosity of PO-SNEDDS (F1) used in this work were recorded as 7.53 ± 0.56 nm, 0.119 and 12.80 ± 1.09 cp, respectively. The ZP of optimized SNEDDS F1 was recorded as − 28.98 mV. The RI and % T of optimized SNEDDS F1 were recorded as 1.337 ± 0.06 and 98.7 ± 0.3%, respectively. These results supported proper development of SNEDDS formulation of PO. On the basis of the best physico-chemical parameters, thermodynamic stability and self-nanoemulsification efficiency obtained in our previous work, formulation F1 was selected for further wound healing activity in this work.
Wound healing evaluation: wound contraction and epithelization period
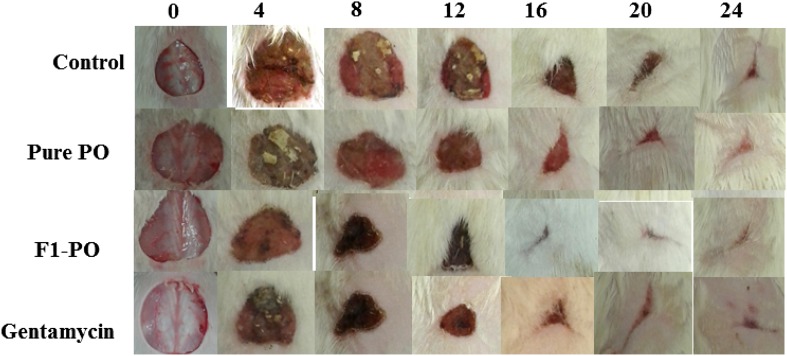
The results of impact of oral administration of pure PO and SNEDDS F1 in comparison with standard gentamycin on wound area contraction of rats are presented in Table 1 and Fig. 1. In this research work, the animals were anaesthetized with the help of diethyl ether because of its major advantages over other anesthetic agents. It was observed that wound contraction was increased till day 24 in both test samples (pure PO and SNEDDS F1) and standard antibiotic treated animals. Pure PO and SNEDDS F1 enhanced wound contraction significantly from day 12 to 24 in comparison with control (P < 0.05). However, the difference in wound contraction between test samples (pure PO and SNEDDS F1) and standard gentamycin was not statistically significant (P > 0.05). The results of impact of oral administration of tests (pure PO and SNEDDS F1) in comparison with standard gentamycin on epithelization period are presented in Table 2. The time for complete epithelization was recorded as 12.20 ± 0.58, 11.60 ± 0.74, 8.40 ± 0.52 and 7.80 ± 0.48 day for control, pure PO, SNEDDS F1 and standard gentamycin, respectively. Epithelization period was significantly shorter in tests (pure PO and SNEDDS F1) and standard gentamycin treated animals in comparison with control group animals (P < 0.05). Moreover, epithelization period was also significantly shorter in SNEDDS F1 and standard gentamycin treated animals in comparison with pure PO treated animals (P < 0.05). However, epithelization period was statistically insignificant in SNEDDS F1 and standard gentamycin treated animals (P > 0.05). Wound contraction effects and epithelization of SNEDDS F1 were comparable with standard gentamycin. These results clearly showed that developed SNEDDS formulation showed greater wound healing effects after oral administration in comparison with pure PO. The enhanced wound contraction by SNEDDS F1 was possible due to nanosized droplets of SNEDDS and the presence of solubilizers namely “Triton-X100 and Transcutol-HP” in formulation F1. The capacity of producing wound contraction by PO and PO loaded SNEDDS recorded in this research work indicated that Piper cubeba plant possesses a definite prohealing action, because 100% of wound healing occurred due to contraction (Ejaz et al. 2009). These wound healing effects of pure PO and PO in SNEDDS could be due to definite enhancement in the proliferation of epithelial cells (Getie et al. 2003). SNEDDS and oral nanoemulsions have been investigated for enhancing therapeutic effects of many drugs and essential oils have good potential for wound healing (Suntar et al. 2011; Tumen et al. 2011; Shakeel et al. 2013; Patel et al. 2019; Tung et al. 2019). Hence, SNEDDS of PO were chosen in this study for the enhancement of wound healing efficacy of PO.
Table 1.
Impact of oral administration of pure PO and an optimized SNEDDS F1 in comparison with standard gentamycin on circular excision wound model in rats at different days of treatment
| Formulations | Wound area of contraction ± SEM (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0 Day | 4th Day | 8th Day | 12th Day | 16th Day | 20th Day | 24th Day | |
| Control | 0.00 ± 0.00 | 18.19 ± 2.40 | 39.49 ± 2.43 | 69.37 ± 1.85 | 85.15 ± 1.55 | 97.46 ± 0.56 | 99.49 ± 0.10 |
| Pure PO | 0.00 ± 0.00 | 19.60 ± 2.16 | 46.97 ± 2.43 | 72.54 ± 1.80 | 86.85 ± 1.30 | 98.65 ± 0.18 | 99.84 ± 0.09 |
| SNEDDS F1 | 0.00 ± 0.00 | 47.72 ± 1.44 | 64.77 ± 2.10 | 89.14 ± 0.70 | 92.67 ± 1.44 | 98.93 ± 0.15 | 100.00 ± 0.00 |
| Gentamycin | 0.00 ± 0.00 | 61.03 ± 0.73 | 78.58 ± 0.43 | 90.25 ± 0.67 | 95.46 ± 0.73 | 99.53 ± 0.14 | 100.00 ± 0.00 |
Fig. 1.
Wound healing effects of pure PO, PO-SNEDDS (F1) and standard antibiotic gentamycin in comparison with control after 0, 4, 8, 12, 16, 20 and 24 days of inducing wound healing
Table 2.
Impact of oral administration of pure PO and an optimized SNEDDS F1 in comparison with standard gentamycin on wound epithelization period in excision wound model in rats
| Formulations | Period of epithelization ± SEM (days) |
|---|---|
| Control | 12.20 ± 0.58 |
| Pure PO | 11.60 ± 0.74 |
| SNEDDS F1 | 8.40 ± 0.52 |
| Gentamycin | 7.80 ± 0.48 |
The interesting finding of our research work is that oral administration of pure PO and PO-SNEDDS once daily for 24 days accelerated the rate of wound closure in excision wound model in rat. The wound healing potential of essential oils have been investigated in literature on animal models (Kumar et al. 2007; de Fatima et al. 2008; Tumen et al. 2011; Suntar et al. 2011). It has been reported that the restoration and the functional integrity of the injured tissue involves several processes such as “inflammation, wound contraction, angiogenesis, extracellular matrix deposition and tissue remodeling”. Single or multiple mechanisms could involve in individual phases of wound healing which can contribute to the overall outcome of the wound healing process (Velnar et al. 2009; Grieb et al. 2011). At day 4 post-wounding, a marked decrease in the swelling and exudates in animals treated with PO-SNEDDS F1 was observed. These effects were comparable to standard gentamycin treated animals and higher than control and pure PO (Fig. 1).
The purpose of this work was to develop SNEDDS of PO to enhance its wound healing effects. SNEDDS are the systems which are capable to self-emulsify with gastrointestinal fluids upon oral administration. Due to this feature, SNEDDS can only be administered by oral route. SNEDDS are not capable to self-emulsify if administered via other routes of administration like topical/transdermal. To obtain similar conditions for statistical comparisons, PO was also administered orally. If we administered PO by other route and SNEDDS via oral route, the comparisons of their biological effects would not be justified. Hence, both PO as well as PO-SNEDDS were administered by oral route. It is known that the internal lipoidal portion of SNEDDS increased the intestinal lymphatic uptake of hydrophobic molecules which can further results in avoidance of pre-systemic metabolism of such drugs (Kalepu et al. 2013). Enhancement in lymphatic uptake of drugs and avoidance of presystemic metabolism could definitely results in rapid absorption of drugs from SNEDDS and finally enhancement in oral bioavailability and therapeutic efficacy of drugs (Kalepu et al. 2013; Sureshkumar et al. 2015). Therefore, the enhanced wound healing effects of PO-SNEDDS were probably due to enhancement in lymphatic uptake of PO from SNEDDS and avoidance of presystemic metabolism.
Collagen content estimation (leucine assay)
Leucine assay is one of the commonly used methods for collagen estimation (Taskiran et al. 1999; Nayak et al. 2006). Leucine content (mg/g of tissue) in the granulation tissues of the rats on day 1 and 10 after oral administration of pure PO, SNEDDS F1 and standard gentamycin are presented in Table 3. Leucine levels of SNEDDS F1 and standard gentamycin treated animals were enhanced significantly on day 10 in comparison with pure PO treated animals and negative control (P < 0.05). In literature, collagen accumulation has been reported as the sum of synthesis and destruction which occur simultaneously during the process of wound healing (Iyyam Pillai et al. 2010). Hence, the enhanced content of leucine in SNEDDS F1 and gentamycin-treated animals indicated enhanced collagen content in these formulations. However, leucine content was lower in case of negative control, which could be due to a prolonged inflammatory phase. In negative control, it was proposed that the degradation of collagen was greater than its synthesis which is responsible for lower level of leucine in negative control. Overall, the formulation of PO in SNEDDS was advantageous in terms of collagen content because the collagen content of rats treated with PO-SNEDDS was higher in comparison with pure PO and negative control. Higher collagen content helps in wound healing effects because they provide strength to repair tissues (Suntar et al. 2014; Gebrehiwot et al. 2015).
Table 3.
Estimation of collagen after oral administration of pure PO and an optimized SNEDDS F1 in comparison with standard gentamycin on circular excision wound model in rats at 1st and 10th day of treatment
| Formulations | Collagen ± SEM (mg of leucine/g of tissue) | ||
|---|---|---|---|
| 1st day | 10th day | Negative control | |
| Pure PO | 0.26 ± 0.06 | 0.53 ± 0.04 | 0.33 ± 0.04 |
| SNEDDS F1 | 0.28 ± 0.03 | 0.82 ± 0.04 | 0.33 ± 0.04 |
| Gentamycin | 0.28 ± 0.03 | 0.98 ± 0.03 | 0.33 ± 0.04 |
Histomorphological analysis of healed tissues
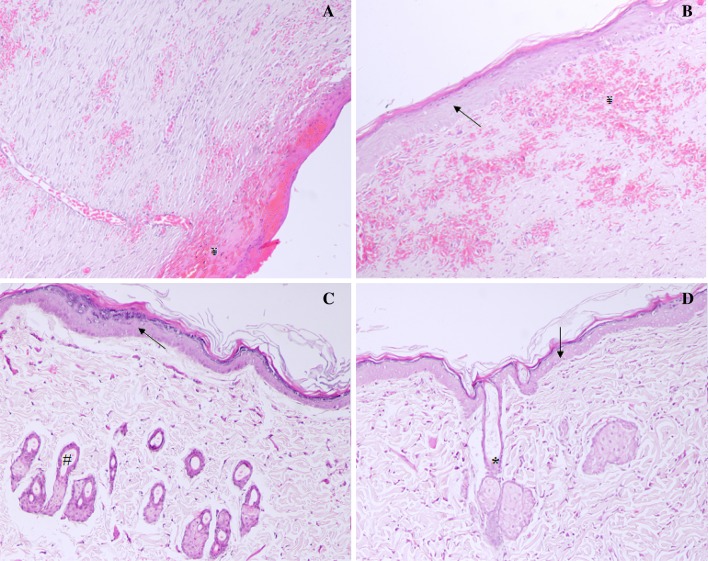
The photomicrographs of histomorphological examination of control, tests (pure PO and SNEDDS F1) and standard gentamycin-treated animals are presented in Fig. 2. Histological evaluation of skin at day 12 with H&E showed the sign of ulceration, edema, epithelization, granulation and abundance of mononuclear cells infiltration in control group animals (Fig. 2a). However, the photomicrographs of pure PO treated rats showing the sign of less ulceration, edema and large amount of granulation as well as sign of healed skin structure with well-formed, near to normal epidermis, restoration of adnexa, and extensive fibrosis and collagen tissue within the dermis (Fig. 2b). PO-SNEDDS (F1) treated rats showed large amount of granulation tissue, small number of mononuclear inflammatory cells, and restoration of adnexa and extensive fibrosis and no sign of ulceration and edema (Fig. 2c). The rats treated with standard antibiotic gentamycin showed healed skin structures with well-formed, near to normal epidermis, restoration of adnexa, and extensive fibrosis and collagen tissue within the dermis (Fig. 2d). The resulting data of histomorphological analysis are presented in Table 4. The above results clearly supported that the wound healing and repair is accelerated by PO. This ability was especially obvious when the data were compared with the other plants as PO is well known for its antioxidant, antibacterial, antifungal and anti-inflammatory activity (Perazzo et al. 2003; Silva et al. 2007). The wound healing is associated with reduction in oxidative stress (Yusufoglu and Alqasoumi 2011).
Fig. 2.
Histopathology of skin at day 12 stained with H&E (100 ×); a control group animals showing, early epithelization, and granulation tissue and abundance of mononuclear inflammatory cells (¥); b pure PO treated rats showing healed skin structures with well-formed, near to normal epidermis, restoration of adnexa (*), and extensive fibrosis and collagen tissue within the dermis (arrow); c PO-SNEDDS (F1) treated rats showing large amount of granulation tissue, small number of mononuclear inflammatory cells, and restoration of adnexa and extensive fibrosis (#); d gentamycin treated rats showing healed skin structures with well-formed, near to normal epidermis, restoration of adnexa, and extensive fibrosis and collagen tissue within the dermis
Table 4.
The median histopathologic scores of wound healing determined after oral administration of pure PO and an optimized SNEDDS F1 in comparison with and control and standard gentamycin using a modified 0–5 numerical scale; the scores were 0 for absence, 1 for occasional presence, 2 for light scattering, 3 for abundance, 4 for confluence of cells and 5 for fibres
| Formulations | Epithelialisation | Inflammatory cell infiltration | Fibroblast proliferation | Neovascularization | Collagen deposition |
|---|---|---|---|---|---|
| Gentamycin | 4.02 ± 0.29 | 1.89 ± 0.51 | 4.10 ± 0.38 | 3.10 ± 0.23 | 4.23 ± 0.61 |
| SNEDDS F1 | 3.93 ± 0.21 | 1.60 ± 0.25 | 4.10 ± 0.50 | 2.83 ± 0.23 | 3.93 ± 0.31 |
| Pure PO | 2.20 ± 0.32 | 2.60 ± 0.32 | 2.53 ± 0.52 | 2.01 ± 0.35 | 2.50 ± 0.25 |
| Control | 0.83 ± 0.41 | 3.17 ± 0.22 | 1.00 ± 0.00 | 0.88 ± 0.35 | 1.01 ± 0.41 |
Conclusions
The potential of SNEDDS formulation of PO for enhancing wound healing effects was investigated in this work. Optimized SNEDDS formulation of PO was characterized physicochemically and subjected to wound healing evaluation in rats in comparison with standard gentamycin and pure PO. Wound healing effects of SNEDDS were found to be significant in comparison with pure PO and control. However, these effects were comparable with standard gentamycin. Moreover, SNEDDS formulation also showed significant enhancement in collagen content in comparison with pure PO and negative control. Histopathological examinations of SNEDDS treated animals showed no signs of inflammatory cells which indicated that prepared SNEDDS was safe and nontoxic to rats. The results obtained in this work showed the potential of SNEDDS for oral delivery of essential oil such as PO for enhancing its wound healing effects in rats. Overall, the treatment with gentamycin was the most successful. This means that apparently inhibition of bacterial infection is the most efficient method to stimulate wound healing. Therefore, the combination of PO and gentamycin especially in the form of SNEDDS would be more interesting for faster wound healing effects in future studies.
Acknowledgements
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the research through research group project number RG-1435-005.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Adorjan B. Biological properties of essential oils: an updated review. Flavour Fragr J. 2010;1:407–426. doi: 10.1002/ffj.2024. [DOI] [Google Scholar]
- Alam P, Ansari MJ, Anwer MK, Raish M, Kamal YKT, Shakeel F. Wound healing effects of nanoemulsion containing clove essential oil. Art Cell Nanomed Biotechnol. 2017;45:591–597. doi: 10.3109/21691401.2016.1163716. [DOI] [PubMed] [Google Scholar]
- Alam P, Shakeel F, Anwer MK, Foudah AH, Alqarni MH. Woumd healing study of eucalyptus essential oil containing nanoemulsion in rat model. J Oleo Sci. 2018;67:957–968. doi: 10.5650/jos.ess18005. [DOI] [PubMed] [Google Scholar]
- AlSaid M, Raish M, Al-Sobaihani M, Al-Yahya M, Rafatullah S. Antiulcerogenic, anti-secretory and cytoprotective effects of Piper cubeba (L.) on experimental ulcer models in rat. Int J Biotechnol Well Ind. 2014;2:173–181. [Google Scholar]
- AlSaid M, Mothanna R, Raish M, Al-Sohaibani M, Al-Yahya M, Ahmad A, Al-Dosari M, Rafatullah S. Evaluation of effectiveness of Piper cubeba extract in the amelioration of CCl4-induced liver injuries and oxidative damage in the Rodent model. Biomed Res Int. 2015;2015:E359358. doi: 10.1155/2015/359358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bos R, Kayser O, Quax WJ. Essential oil constituents of Piper cubeba L. fils. from Indonesia. J Essent Oil Res. 2007;19:14–17. doi: 10.1080/10412905.2007.9699217. [DOI] [Google Scholar]
- Cavalcanti JM, Leal-Cardoso JH, Diniz LRL, Portella VG, Costa CO, Linard CF, Alves K, Rocha MV, Lima CC, Cecatto VM, Coelho-de-Souza AN. The essential oil of Croton zehntneri and trans-anethole improves cutaneous wound healing. J Ethnopharmacol. 2012;144:240–247. doi: 10.1016/j.jep.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Choi EM, Hwang JK. Investigations of anti-inflammatory and antinociceptive activities of Piper cubeba, Physalis angulata and Rosa hybrida. J Ethnopharmacol. 2003;89:171–175. doi: 10.1016/S0378-8741(03)00280-0. [DOI] [PubMed] [Google Scholar]
- Cui W, Zhang S, Zhao H, Luo C, Sun B, Li Z, Sun M, Ye Q, Sun J, He Z. Formulating a single thioether-bridged oleate prodrug into a self-nanoemulsifying drug delivery system to facilitate oral absorption of docetaxel. Biomater Sci DOI. 2019 doi: 10.1039/C8BM00947C. [DOI] [PubMed] [Google Scholar]
- Dash GK, Suresh P, Ganapathy S. Studies on hypoglycemic and wound healing activities of Lantana camara Linn. J Nat Remed. 2001;1:105–110. [Google Scholar]
- de Fatima A, Modolo LV, Sanches AC, Porto RR. Wound healing agents: the role of natural and non-natural products in drug development. Mini Rev Med Chem. 2008;8:879–888. doi: 10.2174/138955708785132738. [DOI] [PubMed] [Google Scholar]
- Dwivedi V, Tripathi S. Review study on potential activity of Piper betle. J Pharmacog Phytochem. 2014;3:93–98. [Google Scholar]
- Ejaz S, Chekarova I, Cho JW, Lee SY, Ashraf S, Lim SW. Effect of aged garlic extract on wound healing: a new frontier in wound management. Drug Chem Toxicol. 2009;32:191–203. doi: 10.1080/01480540902862236. [DOI] [PubMed] [Google Scholar]
- Eleftheriadis GK, Mantelou P, Karavasili C, Chatzopoulou P, Katsantonis D, Irakli M, Mygdalia A, Vizirianakis IS, Fatouros DG. Development and characterization of a self-nanoemulsifying drug delivery system comprised of rice bran oil for poorly soluble drugs. AAPS PharmSciTech. 2019;20:E78. doi: 10.1208/s12249-018-1274-y. [DOI] [PubMed] [Google Scholar]
- Farhapour MR, Habibi M. Evaluation of the wound healing activity of an ethanolic extract of Ceylon cinnamon in mice. Vet Med. 2012;2012:53–57. doi: 10.17221/4972-VETMED. [DOI] [Google Scholar]
- Gebrehiwot M, Asres K, Bisrat D, Mazumder A, Lindemann P, Bucar F. Evaluation of the wound healing property of Commiphora guidottii Chiov. ex. Guid. BMC Compl Alt Med. 2015;15:E282. doi: 10.1186/s12906-015-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getie M, Gebre-Mariam T, Rietz R, Höhne C, Huschka C, Schmidtke M, Abate A, Neubert RH. Evaluation of the antimicrobial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoter. 2003;74:139–143. doi: 10.1016/S0367-326X(02)00315-5. [DOI] [PubMed] [Google Scholar]
- Grieb G, Steffens G, Pallua N, Bernhagen J, Bucala R. Circulating fibrocytes—biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol. 2011;291:1–19. doi: 10.1016/B978-0-12-386035-4.00001-X. [DOI] [PubMed] [Google Scholar]
- Iyyam Pillai S, Palsamy P, Subramanian S, Kandaswamy M. Wound healing properties of Indian propolis studied on excision wound-induced rats. Pharm Biol. 2010;48:1198–1206. doi: 10.3109/13880200903578754. [DOI] [PubMed] [Google Scholar]
- Jain S, Kambam S, Thanki K, Jain AK. Cyclosporine A loaded self-nanoemulsifying drug delivery system (SNEDDS): implication of a functional excipient based co-encapsulation strategy on oral bioavailability and nephrotoxicity. RSC Adv. 2015;5:49633–49642. doi: 10.1039/C5RA04762E. [DOI] [Google Scholar]
- Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalepu S, Manthina M, Padavala V. Oil-based drug delivery systems-a review. Acta Pharm Sin B. 2013;3:361–372. doi: 10.1016/j.apsb.2013.10.001. [DOI] [Google Scholar]
- Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing—exploring medicinal plants of India. J Ethnopharmacol. 2007;114:103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Mandl I, MacLennan JD, Howes EL, DeBellis RH, Sohler A. Isolation and characterization of proteinase and collagenase CL. Histolyticum. J Clin Invest. 1953;32:1323–1329. doi: 10.1172/JCI102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Mol. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Stein WH. Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem. 1948;176:367–388. [PubMed] [Google Scholar]
- Morikawa T, Matsuda H, Yamaguchi I, Pongpiriyadacha Y, Yoshikawa M. New amides and gastroprotective constituents from the fruit of Piper chaba. Planta Med. 2004;70:152–159. doi: 10.1055/s-2004-815493. [DOI] [PubMed] [Google Scholar]
- Nayak S, Nalabothu P, Sandiford S, Bhogadi V, Adogwa A. Evaluation of wound healing activity of Allamanda cathartica L. and Laurus nobilis L. extracts on rats. BMC Complem Altern Med. 2006;6:E12. doi: 10.1186/1472-6882-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odei-Addo F, Shegokar R, Müller RH, Levendal RA, Frost C. Nanoformulation of Leonotis leonurus to improve its bioavailability as a potential antidiabetic drug. 3Biotech. 2017;7:E344. doi: 10.1007/s13205-017-0986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouoba MA, Koudou J, Some N, Ouedraogo S, Guissou IP (2012) Wound healing and antibacterial properties of leaf essential oil of Vitex simplicifolia oliv. from Burkina Faso, Chromatography and its applications, Dr. Sasikumar Dhanarasu (Ed.)
- Patel P, Pailla SR, Rangaraj N, Cheruvu HS, Dodoala S, Sampathi S. Quality by design approach for developing lipid-based nanoformulations of gliclazide to improve oral bioavailability and anti-diabetic activity. AAPS PharmSciTech. 2019;20:E45. doi: 10.1208/s12249-018-1214-x. [DOI] [PubMed] [Google Scholar]
- Perazzo FF, Rodrigues IV, Maistro EL, Souza SM, Nanaykkara NPD, Bastos JK, Carvalho JCT, de Souza DHB. Anti-inflammatory and analgesic evaluation of hydroalcoholic extract and fractions from seeds of Piper cubeba L. (Piperaceae) Pharmacog J. 2003;5:13–16. doi: 10.1016/j.phcgj.2012.12.001. [DOI] [Google Scholar]
- Shaiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66:227–243. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Shakeel F, Haq N, El-Badry M, Alanazi FK, Alsarra IA. Ultra fine super self-nanoemulsifying drug delivery system (SNEDDS) enhanced solubility and dissolution of indomethacin. J Mol Liq. 2013;180:89–94. doi: 10.1016/j.molliq.2013.01.008. [DOI] [Google Scholar]
- Shakeel F, Shazly GA, Raish M, Ahmad A, Kalam MA, Ali N, Ansari MA, Elosaily GM. Biological investigation of supersaturated self-nanoemulsifying drug delivery system of Piper cubeba essential oil. RSC Adv. 2015;5:105206–105217. doi: 10.1039/C5RA22900F. [DOI] [Google Scholar]
- Sharma G, Beg S, Thanki K, Katare OP, Jain S, Kohli K, Singh B. Systematic development of novel cationic self-nanoemulsifying drug delivery systems of candesartan cilexetil with enhanced biopharmaceutical performance. RSC Adv. 2015;5:71500–71513. doi: 10.1039/C5RA11687B. [DOI] [Google Scholar]
- Silva ML, Coímbra HS, Pereira AC, Almeida VA, Lima TC, Costa ES, Vinhólis AH, Royo VA, Silva R, Filho AA, Cunha WR, Furtado NA, Martins CH, Carvalho TC, Bastos JK. Evaluation of Piper cubeba extract,-(cubebin) and its semi-synthetic derivatives against oral pathogens. Phytother Res. 2007;21:420–422. doi: 10.1002/ptr.2088. [DOI] [PubMed] [Google Scholar]
- Suntar I, Akkol EK, Keles H, Oktem A, Baser KH, Yesilada E. A novel wound healing ointment: a formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. J Ethnopharmacol. 2011;134:89–96. doi: 10.1016/j.jep.2010.11.061. [DOI] [PubMed] [Google Scholar]
- Suntar I, Akkol EK, Tosun A, Keles H. Comparative pharmacological and phytochemical investigation on the wound-healing effects of the frequently used essential oils. J Essen Oil Res. 2014;26:41–49. doi: 10.1080/10412905.2013.820672. [DOI] [Google Scholar]
- Suqumar S, Ghosh V, Nirmala MJ, Mukherjee A, Chandrasekaran N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultarason Sonochem. 2014;21:1044–1049. doi: 10.1016/j.ultsonch.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Sureshkumar R, Gowthamarajan K, Bhavani P. Nanoemulsion for lymphatic absorption: investigation of fenofibrate nanoemulsion system for lymphatic uptake. Int J Chem Tech Res. 2015;7:832–841. [Google Scholar]
- Taşkiran D, Taşkiran E, Yercan H, Kutay FZ. Quantification of total collagen in rabbit tendon by the Sirius Red method. Turkish J Med Sci. 1999;29:7–9. [Google Scholar]
- Thiruvengadam M, Rajakumar G, Chung III-M. Nanotechnology: current uses and future applications in the food industry. 3Biotech. 2018;8:E74. doi: 10.1007/s13205-018-1104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumen I, Akkol EK, Suntar I, Keles H. Wound repair and anti-inflammatory potential of essential oils from cones of Pinaceae: preclinical experimental research in animal models. J Ethnopharmacol. 2011;137:1215–1220. doi: 10.1016/j.jep.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Tumen I, Suntar I, Keles H, Akkol EK. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evi Based Compl Alter Med. 2012;2012:E788281. doi: 10.1155/2012/728281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumen I, Suntar I, Eller FJ, Keles H, Akkol EK. Topical wound-healing effects and phytochemical composition of heartwood essential oils of Juniperus virginiana L., Juniperus occidentalis Hook., and Juniperus ashei J. Buchholz. J Med Food. 2013;16:48–55. doi: 10.1089/jmf.2012.2472. [DOI] [PubMed] [Google Scholar]
- Tung NT, Tran CS, Nguyen HA, Nguyen TD, Chi SC, Pham DV, Bui QD, Ho XH. Formulation and biopharmaceutical evaluation of supersaturatable self-nanoemulsifying drug delivery systems containing silymarin. Int J Pharm. 2019;555:63–76. doi: 10.1016/j.ijpharm.2018.11.036. [DOI] [PubMed] [Google Scholar]
- Umasankar K, Nambikkairaj B, Backyavathi MD. Wound healing activity of topical Mentha piperita and Cymbopogan citratus essential oil on streptozotocin induced rats. Asian J Pharm Clin Res. 2013;6:180–183. [Google Scholar]
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- Villar AM, Naveros BC, Campmany AC, Trenchs MA, Rocabert CB, Bellowa LH. Design and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for enhanced dissolution of gemfibrozil. Int J Pharm. 2012;431:161–175. doi: 10.1016/j.ijpharm.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Werner S, Breeden M, Hubner G, Greenhalgh DG, Longaker MT. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Invest Dermatol. 1994;103:469–473. doi: 10.1111/1523-1747.ep12395564. [DOI] [PubMed] [Google Scholar]
- Wong CM, Ling JJ. In vitro study of wound healing potential in black pepper (Piper nigrum L.) UK J Pharm Biosci. 2014;2:5–9. doi: 10.20510/ukjpb/2/i4/91104. [DOI] [Google Scholar]
- Yusufoglu HS, Alqasoumi SI. Anti-inflammatory and wound healing activities of herbal gel containing an antioxidant Tamarix aphylla leaf extract. Int J Pharmacol. 2011;7:829–835. doi: 10.3923/ijp.2011.868.873. [DOI] [Google Scholar]