Abstract
Key points
The expression of miR‐1 is increased in lungs from the Hyp/Su5416 PAH rat model.
Pulmonary artery smooth muscle cells from this animal model are more depolarized and show decreased expression and activity of voltage‐dependent potassium channel (Kv)1.5.
miR‐1 directly targets Kv1.5 channels, reduces Kv1.5 activity and induces membrane depolarization.
Antagomir‐1 prevents Kv1.5 channel downregulation and the depolarization induced by hypoxia/Su5416 exposition.
Abstract
Impairment of the voltage‐dependent potassium channel (Kv) plays a central role in the development of cardiovascular diseases, including pulmonary arterial hypertension (PAH). MicroRNAs are non‐coding RNAs that regulate gene expression by binding to the 3′‐untranslated region region of specific mRNAs. The present study aimed to analyse the effects of miR‐1 on Kv channel function in pulmonary arteries (PA). Kv channel activity was studied in PA from healthy animals transfected with miR‐1 or scrambled‐miR. Kv currents were studied using the whole‐cell configuration of the patch clamp technique. The characterization of the Kv1.5 currents was performed with the selective inhibitor DPO‐1. miR‐1 expression was increased and Kv1.5 channels were decreased in lungs from a rat model of PAH induced by hypoxia and Su5416. miR‐1 transfection increased cell capacitance, reduced Kv1.5 currents and induced membrane depolarization in isolated pulmonary artery smooth muscle cells. A luciferase reporter assay indicated that KCNA5, which encodes Kv1.5 channels, is a direct target gene of miR‐1. Incubation of PA with Su5416 and hypoxia (3% O2) increased miR‐1 and induced a decline in Kv1.5 currents, which was prevented by antagomiR‐1. In conclusion, these data indicate that miR‐1 induces pulmonary artery smooth muscle cell hypertrophy and reduces the activity and expression of Kv channels, suggesting a pathophysiological role in PAH.
Keywords: miR-1, AntagomiR, pulmonary hypertension, Kv1.5 channels
Key points
The expression of miR‐1 is increased in lungs from the Hyp/Su5416 PAH rat model.
Pulmonary artery smooth muscle cells from this animal model are more depolarized and show decreased expression and activity of voltage‐dependent potassium channel (Kv)1.5.
miR‐1 directly targets Kv1.5 channels, reduces Kv1.5 activity and induces membrane depolarization.
Antagomir‐1 prevents Kv1.5 channel downregulation and the depolarization induced by hypoxia/Su5416 exposition.
Introduction
MicroRNAs (miRNAs) are small non‐coding RNAs that regulate gene function by binding to the 3′‐untranslated region (3′‐UTR) of specific mRNAs. The miRNA–3′UTR interaction leads to cleavage of target mRNA or to translational repression, resulting in a decrease in the targeted protein. Mature miRNAs play a key role in global cellular processes, such as differentiation, proliferation, apoptosis and organogenesis, as a result of their capacity to target multiple mRNAs (Bartel, 2004). Dysregulation of miRNAs has been involved in a number of clinically important diseases, ranging from myocardial infarction to cancers, highlighting their potential usefulness as diagnostic and prognostic tools (Sayed & Abdellatif, 2011; Meloche et al. 2014). Moreover, modulating the levels of under‐ and overexpressed miRNAs has recently emerged as a promising therapeutic strategy. miRNA‐based therapeutic strategies (van Rooij & Kauppinen, 2014) can be formulated by either antagonizing (with antagomirs) or restoring (with mimetics) the functions of miRNAs. Mimetics are synthetic RNA duplexes designed to mimic the endogenous functions of the miRNA of interest, with modifications for stability and cellular uptake, whereas antagomirs are modified anti‐sense oligonucleotides harbouring the full or partial complementary reverse sequence of a mature miRNA.
Pulmonary hypertension (PH) is defined as a mean pulmonary arterial pressure of at least 25 mmHg at rest. Class I PH (i.e pulmonary arterial arterial hypertension; PAH) originates from alterations in the small precapillary pulmonary arteries (PA) (Galie et al. 2016). It is a disorder with significant burden in terms of both severity and prevalence, affecting young and middle aged individuals, preferentially women (Galie et al. 2016). The disease progresses with increasing pulmonary vascular resistance, right heart failure and ultimately death. It is characterized by sustained pulmonary vasoconstriction, thrombosis in situ and excessive pulmonary vascular remodelling, involving an increase in medial thickness as a result of hypertrophy and proliferation of pulmonary artery smooth muscle cells (PASMCs) (Meyrick & Reid, 1980; Tajsic & Morrell, 2011). These factors contribute to a reduced lumen diameter and increased peripheral resistance.
Ionic remodelling, comprising the downregulation of voltage‐gated K+ channels (Kv) channels (notably the α subunit Kv1.5), is also considered to be an early contributor to the pathophysiology of the disease (Yuan et al. 1998; Wang et al. 2005; Barnes et al. 2017) via a more depolarized membrane potential in PASMCs, leading to increased intracellular calcium, vasoconstriction, hypertrophy and proliferation (Shimoda et al. 2001; Cogolludo et al. 2007; Burg et al. 2008).
We hypothesized that specific miRNAs are involved in key pathophysiological features of pulmonary vascular diseases, such as ionic remodelling. miR‐1 has been previously reported as a biomarker of PH (Sarrion et al. 2015; Sysol et al. 2018). Therefore, the present study aimed to analyse the effects of miR‐1 on pulmonary Kv channel currents.
Methods
Ethical approval
All experimental procedures utilizing animals were carried out in accordance with the Care and Use of Laboratory Animals and were approved by the institutional Ethical Committees of the Universidad Complutense de Madrid (Madrid, Spain) and the regional Committee for Laboratory Animals Welfare (Comunidad de Madrid, Ref. number PROEX‐251/15). The present study conforms with the principles and regulations described by Grundy (2015). All of the investigators understand the ethical principles under which the journal operates and state that their work complies with the journal's animal ethics checklist.
Animals and models of PAH
Pathogen‐free male Wistar rats were obtained from Envigo (Barcelona, Spain). All animals were kept with free access to standard rat chow and water in an enriched environment throughout the whole experiment period and maintained under a 12:12 h light/dark cycle at 24°C. Rats were killed using CO2.
For the rat model of Su5416 plus hypoxia‐induced PAH (Hyp/Su5416), rats weighing 220 g were injected s.c. with a single dose of the vascular endothelial growth factor (VEGF) receptor type 2 inhibitor Su5416 (20 mg kg–1) or vehicle (Taraseviciene‐Stewart et al. 2001; Gomez‐Arroyo et al. 2012). Then, Su5416‐treated animals were introduced into glass cages and ventilated with 10% O2 (hypoxia) for 3 weeks. CO2 and water vapour produced by the animals were captured with soda lime and silica gel, respectively. Control rats (normoxia) were kept in the same room. Oxygen was monitored using an oxygen sensor (DrDAQ Oxygen Sensor; Pico Technology, St Neots, UK) in the room and in the chamber outflow. The chambers were opened for 20–30 min daily for regular animal care.
Haemodynamic measurements
At the end of the experimental protocol, rats were anaesthetized i.p. with 80 mg kg–1 ketamine plus 8 mg kg–1 xylazine and ventilated with room air (tidal volume 9 mL kg–1, 60 breaths min–1, positive end‐expiratory pressure 2 cmH2O). Right ventricular systolic pressure and diastolic pressure, as well as systolic, diastolic and mean pulmonary arterial pressures, were then measured in open‐chest rats with a pressure transducer via a catheter advanced through the right ventricle into the PA (Morales‐Cano et al. 2014). After haemodynamic measurements, rats were killed by exsanguination under anaesthesia ketamine/xylazine (80 mg/8 mg kg–1, i.p.).
In silico analysis for miR target prediction
Target gene prediction analysis was completed using the microRNA.org target prediction resource, utilizing miRanda sites and miRSVR scoring. The target sites predicted using miRanda are scored for likelihood of mRNA downregulation using a regression model trained on sequence and contextual features of the predicted miRNA:mRNA duplex.
Incubation and transfection of PA
Rat PA were isolated from the lungs and cut into rings. In preliminary experiments, we assessed the transfection efficiency of this protocol using a fluorescent miRNA (miRIDIAN mimic transfection control‐Dy547, CP‐004500‐01‐05; Dharmacon, Lafayette, CO, USA). To increase transfection efficiency into the smooth muscle cells, PAs were first partially digested with a Ca2+‐free solution containing (in mg mL–1): 1.125 collagenase, 0.1 elastase and 1 albumin for 4 min at 4°C followed by 1 min at 37°C. To introduce miR‐1 (hsa‐miR‐1‐3p mirVanaTM, miRNA mimic, MC10617; Applied Biosystems, Foster City, CA, USA), scrambled miR (miRNA mimic negative control, 4464058) and the antagomiR‐1 (hsa‐miR‐1‐3p mirVanaTM miRNA inhibitor, MH10617; Applied Biosystems) into isolated PA, a combination of reverse permeabilization (Moreno et al. 2014) and Lipofectamine RNAiMAX (Life Technologies, Grand Island, NY, USA) was used. miRNA duplexes with a final concentration of 100 nm and Lipofectamine RNAiMAX were mixed with Opti‐MEM (Life Technologies). Briefly, PA were exposed to three successive solutions (4°C) containing (in mm): (i) miRNA duplexes, 10 EGTA, 120 KCl, 5 Na2ATP, 2 MgCl2 and 20 Hepes (pH 6.8; 30 min); (ii) miRNA duplexes, 120 KCl, 5 Na2ATP, 2 MgCl2 and 20 Hepes (pH 6.8; 180 min); and (iii) miRNA duplexes, 120 KCl, 5 Na2ATP, 10 MgCl2 and 20 Hepes (pH 6.8; 30 min). Subsequently, PA were bathed in a fourth solution containing (in mm) 120 NaCl, 5 KCl, 5 Na2ATP, 10 MgCl2, 5.6 glucose and 10 Hepes (pH 7.1, 4°C), in which [Ca2+] was gradually increased from 0.001 to 0.01, 0.1 and 1 mm every 15 min. Vessels were then placed in Dulbecco's modified Eagle's medium (DMEM) supplemented with glucose (1 g L–1), non‐essential amino acid solution (1×), penicillin (100 U mL–1), streptomycin (0.1 mg mL–1), amphotericin B (0.25 μg mL–1) and maintained for 48 h in a normoxic incubator (21% O2 and 5% CO2) or hypoxic incubator (3% O2 and 5% CO2 in the presence of 10 μmol L−1 Su5416). Under these conditions, we achieved a higher efficiency of transfection as determined in pilot experiments with the fluorescent miRNA. It should be noted that Kv currents are downregulated in cells isolated from PA incubated for 48 h in culture by ∼50% compared to the freshly isolated cells, whereas the permeabilization /transfection produced no effect on these current (not shown). In some experiments, miR‐1 was measured as described below in PA placed in DMEM supplemented with glucose (4.5 g L–1), non‐essential amino acid solution (1×), penicillin (100 U mL–1), streptomycin (0.1 mg mL–1), amphotericin B (0.25 μg mL–1) and maintained for 48 h in a normoxic incubator (21% O2 and 5% CO2) or hypoxic incubator (3% O2 and 5% CO2 in the presence of 10 μmol L−1 Su5416).
Electrophysiology
PASMCs were isolated by enzymatic digestion as described previously (Cogolludo et al. 2006). Membrane currents were recorded with an Axopatch 200B and a Digidata 1322A (Axon Instruments, Burlingame, CA, USA) using the whole‐cell configuration of the patch clamp technique. Cells were superfused with an external Ca2+‐free Hepes solution (see above) and a Ca2+‐free pipette (internal) solution containing (mol L−1): 130 KCl, 1.2 MgCl2, 5 Na2ATP, 10 Hepes, 10 EGTA (pH adjusted to 7.3 with KOH). Kv currents were evoked after the application of 200 ms depolarizing pulses from −60 mV to +60 mV in 10 mV increments. To characterize the contribution of Kv1.5 channels to the total Kv current, cells were exposed to the selective inhibitor DPO‐1 (1 μmol L−1) (Lagrutta et al. 2006; Du et al. 2010). Cell capacitance was calculated from the integral of the capacitive transient current elicited by 10 mV hyperpolarizing pulses from a holding potential of −70 mV. Currents were normalized for cell capacitance and expressed as pA pF–1. Membrane potential was recorded under the current clamp mode. All experiments were performed at room temperature (22–24°C).
miRNA extraction and quantitative RT‐PCR
miRNA was extracted from lung tissue using miRNeasy Mini Kit (Qiagen, Hilden, Germany) and a NucleoSpin miRNA‐RNA purification kit (Macherey‐Nagel GmbH & Co. KG, Germany) was used to isolate miRNA from PA, in accordance with the manufacturer's instructions. RNA concentration and quality was checked using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). For miRNA analysis, complementary DNA analysis was synthesized from total RNA using specific stem‐loop reverse‐transcription primers (Taqman MicroRNA Reverse Transcription Kit; Applied Biosystems). Gene expression was determined by quantitative RT‐PCR with a Taqman system (Applied Biosystems) in the Genomic Unit of the Complutense University (Madrid) using specific primers (U6 Ref. 001973 and miR‐1 Ref. 002222). The ΔΔCt method was used to quantify relative changes. miRNA expression was normalized by the expression of U6.
Luciferase gene expression reporter assay
Luciferase activity assays were performed in the African green monkey kidney‐derived cell line (COS‐7) transfected with the 3′‐UTR region of Kcna5 (NM_002234) cloned into pMirTarget vector (OriGene Technologies, Rockville, MD, USA) containing the sequence of a red fluorescent protein (RFP) and a firefly luciferase as a reporter. COS‐7 cells placed into a 96‐well plate were cotransfected with cloned vector (50 μg) and miR‐1 or scrambled miR (both at 10 nm), using Lipofectamine 2000 transfection reagent (Invitrogen, Thermo Fisher Scientific), in accordance with the manufacturer's instructions. Forty‐eight hours after transfection, luciferase activity was measured using the Firefly Luciferase Assay Kit 2.0 (Biotium, Fremont, CA, USA), in a luminometer (Fluoroskan Ascent, Thermo Scientific) for 5 min. Luminescence was normalized to the red fluorescence of RFP.
Western blotting analysis
Homogenates were subjected to SDS‐PAGE. Proteins were transferred to polyvinylidene difluoride membranes, incubated with primary rabbit polyclonal antibodies against Kv1.5 (dilution 1:200; APC‐004; Alomone Labs, Jerusalem, Israel) overnight and then with the secondary peroxidase conjugated antibodies. Antibody binding was detected by an ECL system (Amersham Pharmacia Biotech, Amersham, UK). Blots were imaged using an Odissey Fc System (Li‐Cor, Lincoln, NE, USA) and were quantified by densitometry using Quantity One software (Bio‐Rad, Hercules, CA, USA). Samples were re‐probed for expression of smooth muscle α‐actin (dilution 1:10,000; A2547; Sigma‐Aldrich, Madrid, Spain).
Immunocytochemistry
Enzymatically isolated PASMCs from PAs were fixed in polylysine coated glass coverslips, blocked and permeabilized as described previously (Morales‐Cano et al. 2016). The coverslips were exposed to primary rabbit anti‐Kv1.5 (dilution 1:100; APC‐004; Alomone Labs) antibodies overnight and exposed afterwards to secondary antibody Cy3 conjugated donkey anti‐rabbit (dilution 1:200; Jackson ImmunoResearch, Ely, UK) and stained with 4′,6‐diamidino‐2‐phenylindole (DAPI). Image analysis was performed using a Confocal laser scanning fluorescence microscope (SP2; Leica Microsystems, Wetzlar, Germany) with oil immersion lens ACS APO 40× (Unidad de Microscopía Confocal Hospital General Universitario Gregorio Marañón, Madrid, Spain).
Drugs
All drugs were obtained from Sigma‐Aldrich, except Su5416 (Tocris Bioscience, Bristol, UK).
Statistical analysis
Data are expressed as the mean ± SEM. Statistical comparisons were performed using one‐ or two‐tailed unpaired t tests or two‐way ANOVA followed by a Bonferroni test as appropriate. P < 0.05 was considered statistically significant.
Results
PASMCs from the Hyp/Su5416 model have increased cell capacitance and depolarized membrane potential
We used an established model of PAH triggered by exposition to Su5416 plus hypoxia (10% O2, Hyp/Su5416). As expected, haemodynamic assessment revealed a robust elevation of mean pulmonary arterial pressure in Hyp/Su5416 rats (Fig. 1 A). In addition, PASMCs from Hyp/Su5416 rats had a significantly increased cell capacitance (Fig. 1 B), as an index of PASMC hypertrophy. Moreover PASMCs from these PAH animals had a more depolarized membrane potential (Fig. 1 C).
Figure 1. Hypertrophy and membrane depolarization in PASMCs from the Hyp/Su5416 model.
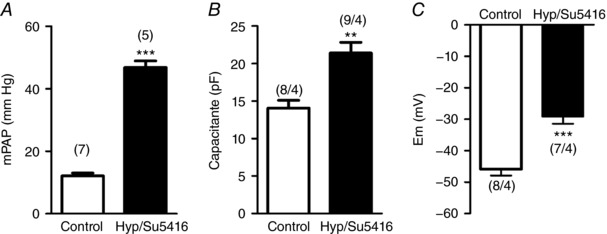
A, mean pulmonary artery pressure (mPAP). B, average cell capacitance of PASMCs from normoxic and Hyp/Su5416 rats. C, resting membrane potential in PASMC measured under current clamp mode. Results are the mean ± SEM. The number of animals in each group is indicated in parenthesis. ** P < 0.01 and *** P < 0.001 vs. normoxia, respectively.
Ionic remodelling in an animal model of PAH
To investigate the possible ionic remodelling, we analysed the total Kv currents in freshly isolated PASMCs from control or Hyp/Su5416 rats. Figure 2 A shows representative original traces of the total Kv currents recorded in freshly isolated PA myocytes under both conditions. Kv currents measured at the end of the 200 ms depolarizing pulses were significantly decreased in PASMCs from Hyp/Su5416 compared to those from control rats (Fig. 2 B). We analysed whether the Kv1.5 current, which represents a main component of the total Kv current in the pulmonary vasculature (Moral‐Sanz et al. 2011), was affected in Hyp/Su5416 rats. We found that the selective Kv1.5 channel inhibitor DPO‐1 (1 μmol L–1) induced a marked reduction of the Kv currents in control and Hyp/Su5416 rat PASMC (Fig. 2 C). Thus, DPO‐1 sensitive currents, which reflect the Kv1.5 channel component, were markedly smaller in Hyp/Su5416 rats (Fig. 2 D). In agreement with these data, the expression of Kv1.5 channels was reduced in lung homogenates from Hyp/Su5416 rats compared to those exposed to normoxia (Fig. 2 E).
Figure 2. Ionic remodelling in Hyp/Su5416 rats.
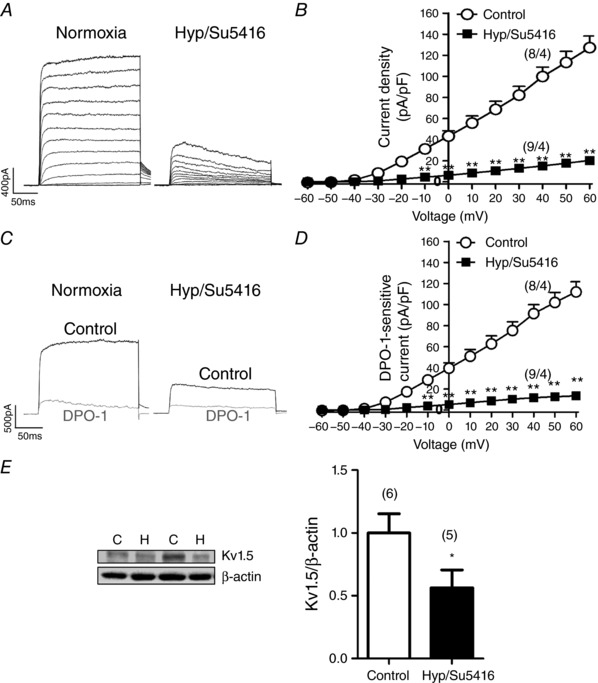
A, representative current traces for 200 ms depolarization pulses from –60 mV to +60 mV in 10 mV increments from a holding potential of ‐60 mV in PASMC. B, average current–voltage relationships of K+ currents, normalized by cell capacitance, measured at the end of the pulse from experiments as those shown in (A). C, representative current traces measured before (black) and after (grey) the addition of the Kv1.5 channel blocker DPO‐1 (1 μmol L−1). D, mean DPO‐1‐sensitive currents obtained by subtracting the current in the absence and in the presence of DPO‐1. E, Kv1.5 protein expression in lung homogenates from rats exposed to normoxia (N) or Hyp/Su5416 (H) analysed by western blotting and normalized by β‐actin expression. The parentheses indicate the number of cells or arteries and the number of animals from which these cells or arteries were obtained, respectively. Results are the mean ± SEM. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. normoxia, respectively.
Expression of Kv1.5 channels is downregulated by miR‐1
We hypothesized that the expression of Kv1.5 could be regulated by miRNAS. Thus, we performed an in silico analysis using the microRNA.org database and identified that KCNA5, which encodes the Kv1.5 channel protein, is a predicted mRNA target of miR‐1 (Fig. 3 A). Remarkably, lungs from Hyp/Su5416 rats showed a marked upregulation of miR‐1 (∼4‐fold increase) (Fig. 3 B). To confirm that miR‐1 binds to the 3′‐UTR of the KCNA5 mRNA, we constructed a vector containing the 3′‐UTR of KCNA5 mRNA and the firefly luciferase. COS‐7 cells were cotransfected with this vector and miR‐1 or a scrambled miR. The luciferase activity of COS‐7 cells was significantly reduced after 48 h of co‐transfection with miR‐1 (Fig. 3 C), confirming that KCNA5 is a direct target of miR‐1. To assess the potential pathophysiological impact of the regulation of KCNA5 by miR‐1 levels, we transfected PA with miR‐1 or a scramble sequence. Transfection of miR‐1 in PAs produced a decrease in the immunostaining of Kv1.5 channels (Fig. 3 D).
Figure 3. Changes in miR‐1 expression in the PAH model.
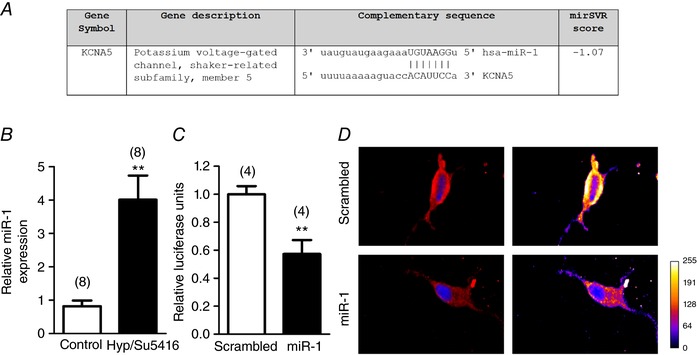
A, predicted sequence alignment between miR‐1 and KCNA5 3′‐UTR mRNA in Homo sapiens from microRNA.org. B, expression of miR‐1 was analysed in lung homogenates from rats with PAH induced by Hyp/Su5416 and controls. C, luciferase activity in Cos7 cells co‐transfected with miR‐1 and the pMirTarget vector containing the 3′‐UTR region of KCNA5 and the luciferase gene. Results are the mean ± SEM. The parentheses indicate the number of experiments. ** P < 0.01 vs. scrambled miR. D, representative confocal images (left) and pseudocolour scale images (right) of PASMCs incubated with rabbit anti‐Kv1.5 (red) and DAPI nuclear staining (blue) following transfection with scrambled miR or miR‐1. Images are representative of cells from three different rats. [Color figure can be viewed at wileyonlinelibrary.com]
miR‐1 reduces Kv1.5 currents and induces membrane depolarization in PASMCs
Interestingly, the transfection of miR‐1 in PAs mimicked the electrophysiological findings observed in the PAH model. Thus, after miR‐1 transfection, PASMC showed an increase in cell capacitance (Fig. 4 A), strongly reduced Kv currents (Fig. 4 B and 4 C) and a more depolarized membrane potential (Fig. 4 D) compared to scrambled miR transfected cells. Moreover, we also found that the selective Kv1.5 channel inhibitor DPO‐1 (1 μmol L−1) induced a smaller reduction of the Kv currents in PASMCs transfected with miR‐1 compared to the scrambled miR (Fig. 5 A). Therefore, in the presence of DPO‐1, the current was similar in cells transfected with the control or with the active miR. Figure 5 B shows that DPO‐1 sensitive current (i.e. the Kv1.5 channel component) was smaller in miR‐1 transfected cells. These data indicate a lower contribution of Kv1.5 channels to whole Kv currents in the presence of miR‐1. DPO‐1 induced a strong depolarization in both scrambled miR and miR‐1 treated cells so that the differences in membrane potential were abolished after Kv1.5 inhibition (Fig. 5C).
Figure 4. miR‐1 reduces Kv currents and induces membrane depolarization in PASMCs.
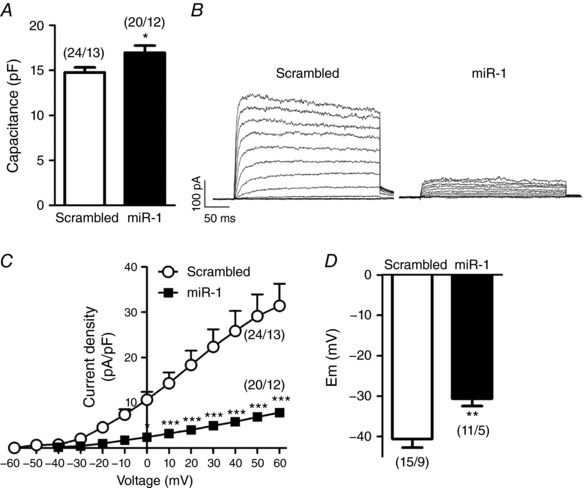
A, average cell capacitance of PASMCs transfected with scrambled miR or miR‐1. B, representative Kv current traces and (C) current–voltage relationships of Kv currents measured at the end of the pulse in PASMCs transfected with scrambled miR or miR‐1. D, resting membrane potential values in PASMCs transfected with scrambled miR or miR‐1. Results are the mean ± SEM. The parentheses indicate the number of cells and the number of animals from which these cells were obtained, respectively. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. scrambled miR, respectively.
Figure 5. miR‐1 reduces Kv1.5 currents in PASMCs.
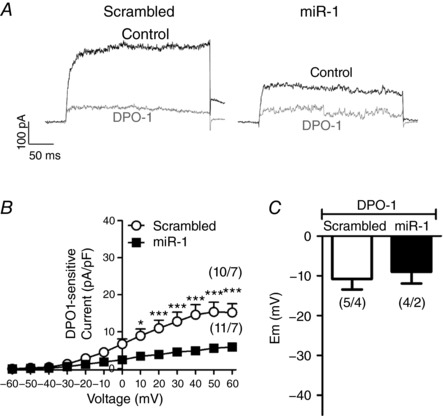
A, representative Kv currents traces recorded in the absence (control, black) and in the presence of the Kv1.5 channel blocker DPO‐1 (1 μmol L−1, grey). B, DPO‐1 sensitive current in PASMCs transfected with scrambled miR and miR‐1. C, resting membrane potential values in PASMCs transfected with scrambled or miR‐1 in the presence of the Kv1.5 channel blocker DPO‐1 (1 μmol L−1). Results are the mean ± SEM. The parentheses indicate the number of cells and the number of animals from which these cells were obtained, respectively. * P < 0.05 and *** P < 0.001 vs. scrambled miR, respectively.
AntagomiR‐1 prevents hypoxia‐induced ionic remodelling
Finally, we evaluated the role of antagomiR‐1 in PA exposed to normoxia and hypoxia (3% O2) plus Su5416 (10 μmol L−1) for 48 h in vitro. We also used scrambled miR as a negative control for antagomir‐1. Under normoxic conditions, antagomiR‐1 transfection did not produce changes in cell capacitance (scrambled: 16.44 ± 0.77 pF and antagomiR‐1: 16.80 ± 1.01 pF, n = 7 and 9, respectively; P > 0.05), in total Kv currents or in membrane potential in PASMCs compared to scrambled miR (Fig. 6 A). We analysed the expression of miR‐1 in PA from rats incubated 48 h in Hyp/Su5416 respect to normoxic conditions. The results showed a significant increase of miR‐1 in PA incubated with Hyp/Su5416 (Fig. 6 B). PASMCs isolated from PA transfected with the inactive miR and exposed to Hyp/Su5416 showed an increase in cell capacitance (23.11 ± 2.21 pF, n = 7; P < 0.05) and a marked reduction in total Kv currents compared to normoxia (Fig. 6 C vs. Fig. 6 A). AntagomiR‐1 transfection prevented the increase in cell capacitance (16.31 ± 2.17 pF, n = 6; P > 0.05), produced a significant increase in Kv currents and hyperpolarized PASMCs isolated from PA exposed to Hyp/Su5416 (Fig. 6 C). DPO‐1 induced a stronger blockade in PASMC from PA transfected with antagomiR‐1 compared to scrambled miR under hypoxia plus Su5416. Therefore, DPO‐1‐sensitive currents were increased by antagomiR‐1 (Fig. 6 D), suggesting that antagomiR‐1 partially prevents the hypoxia plus Su5416‐induced decline in Kv1.5 currents. This was associated with a significantly higher Kv1.5 channel expression in Hyp/Su5416‐exposed PA transfected with antagomiR‐1 compared to those transfected with scrambled miR (Fig. 6 E).
Figure 6. Effects of antagomiR‐1 on hypoxia‐induced ionic remodelling.
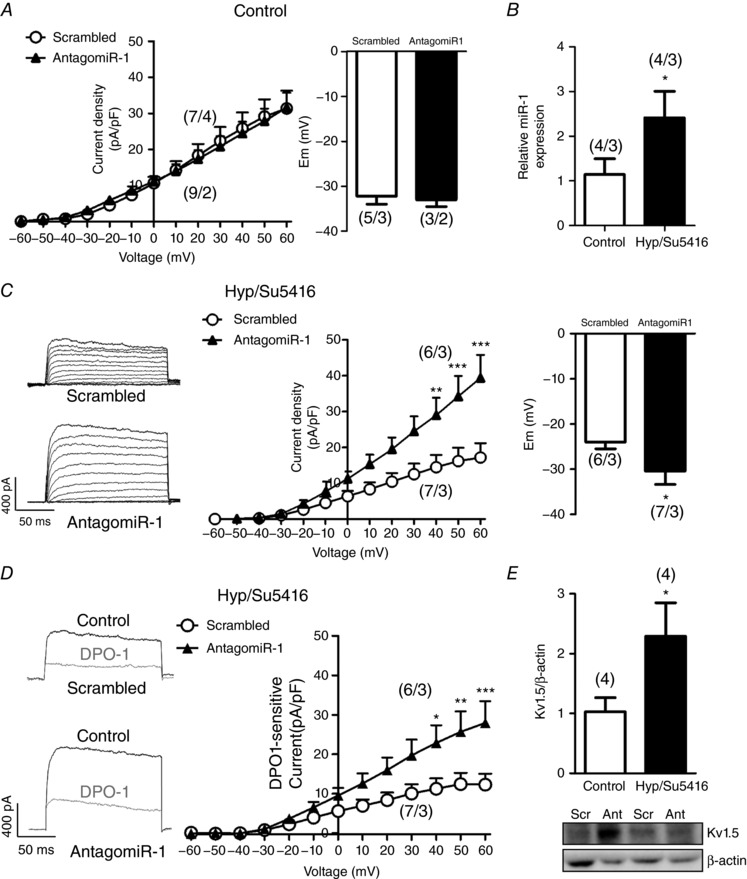
A, current–voltage relationships of Kv currents measured at the end of the pulse and membrane potential values in PASMCs from PAs transfected with scrambled miR or antagomiR‐1. B, expression of miR‐1 was analysed in PA from rats incubated 48 h in Hyp/Su5416 or normoxia. C, representative current traces for 200 ms depolarization pulses from −60 mV to +60 mV in 10 mV increments from a holding potential of −60 mV, current–voltage relationships of Kv currents measured at the end of the pulse and membrane potential in PASMCs isolated from PAs transfected with scrambled miR or antagomiR‐1 maintained in hypoxia (48 h, 3% O2). D, representative Kv current traces measured before (black) and after (grey) the addition of the Kv1.5 channel blocker DPO‐1 (1 μmol L−1) and the DPO‐1‐sensitive currents obtained by subtracting the current in the absence and in the presence of the drug in myocytes from PA transfected with scrambled miR or antagomiR1 maintained in hypoxia. E, Kv1.5 protein expression in Hyp/Su5416‐exposed PA transfected with scrambled (Scr) or antagomir‐1 (Ant) analysed by western blotting and normalized by β‐actin expression. Results are the mean ± SEM. The parentheses indicate the number of cells or arteries and the number of animals from which these cells or arteries were obtained, respectively. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. control, respectively.
Discussion
In the present study, we show that miR‐1 is upregulated and Kv1.5 channels are downregulated in lungs from rats with PAH induced by Su5416 plus hypoxia. Transfection with miR‐1 of PA induced a strong decline in whole Kv currents, which was accompanied by membrane depolarization. We confirmed KCNA5 mRNA, which encodes Kv1.5, as a direct target of miR‐1. Finally, antagomiR‐1 prevented the downregulation of Kv currents and Kv1.5 channel expression, as well as the depolarization induced by Hyp/Su5416 in PA.
Among the miRNAs found in the literature that were increased in PAH but had not been previously functionally characterized with respect to PA function, miR‐1 was the one with the highest upregulation (8‐fold increase in microarray experiments and 12‐fold increase in the validating PCR analysis) in plasma from 12 idiopathic PAH patients (Sarrion et al. 2015). miR‐1 is also upregulated in peripheral blood mononuclear cells in essential hypertensive patients (Kontaraki et al. 2014) and in pre‐eclampsia (Hromadnikova et al. 2015). By contrast, the expression of miR‐1 showed a strong downregulation in the buffy coat of a cohort of 31 subjects with pulmonary hypertension, including all classes of pulmonary hypertension, such as chronic obstructive pulmonary disease, interstitial lung disease, obstructive lung disease, scleroderma, etc. (Wei et al. 2013). Very recently, it was also reported that miR‐1 was downregulated in cultured PASMCs from four patients with idiopathic PAH (Sysol et al. 2018). To further understand these conflicting results and because the expression of miR‐1 has not been analysed in lungs with PAH, we analysed miR‐1 levels in a widely used animal model of PAH, consisting of a combination of hypoxia plus the VEGF antagonist Su5416. The Hyp/Su5416 model, as opposed to hypoxia alone, is considered to best conform to class 1 pulmonary hypertension (i.e. PAH) because it develops complex vascular lesions, including plexiform‐like lesions, and it is irreversible after returning to normoxia (Gomez‐Arroyo et al. 2012; Ryan et al. 2013), rather than pulmonary hypertension as a result of respiratory diseases and hypoxia (i.e. class 3). We found a very strong upregulation of miR‐1 in the lungs of Hyp/Su5416 treated rats. By contrast, the lungs from mice exposed to hypoxia (class 3 PH), as well as cultured human PASMC exposed to hypoxia in vitro, had reduced miR‐1 levels (Sysol et al. 2018). Our in vitro studies show that cultured PA under hypoxia and Su5416 had increased miR‐1 levels. Altogether, the data from human samples and animal models show that changes in miR‐1 are not apparently uniform in pulmonary hypertension, indicating that plasma miR‐1 levels do not appear to be a reliable biomarker of the disease. These variable levels of miR‐1 may contribute to explaining the heterogeneity in the different forms of the pathology.
Kv channels play a fundamental role in controlling PASMC membrane potential and pulmonary arterial tone. Kv channel activity is decreased by several vasoactive factors involved in PAH, such as endothelin‐1, 5‐HT, thromboxane A2 and hypoxia (Shimoda et al. 1998; Cogolludo et al. 2006; Cogolludo et al. 2009). Moreover, decreased PASMC expression of Kv1.5 and Kv2.1 in humans with PAH is considered to play an important pathophysiological role (Yuan et al. 1998; Michelakis et al. 2001; Wang et al. 2005). Thus, diminished transcription of Kv α‐subunits may reduce the number of functional Kv channels in the membrane resulting in the decreased Kv current and leading to pulmonary artery vasoconstriction, hypertrophy and proliferation. Interestingly, we found that PASMC size, as reflected in the measurement of cell capacitance, was increased in PH rats, consistent with PASMC hypertrophy. A similar increase in cell capacitance has been reported previously (Shimoda et al. 2001) in PASMC derived from the chronic hypoxia PH mice model. Moreover, to our knowledge, the present study is the first to show a downregulation of Kv currents in the Hyp/Su5416 model of PAH. This effect is much more evident in this model than in the monocrotaline or in the hypoxic rat model reported previously (Wang et al. 2005; Morales‐Cano et al. 2014). Indeed, the current sensitive to the Kv1.5 channel blocker DPO‐1 (i.e. the component attributable to Kv1.5 channels) was almost completely abolished. In agreement with this finding, we found a lower expression of the Kv1.5 channels in lungs from Hyp/Su5416 rats. A potential limitation of the present study is that the inhibitory effect of DPO‐1 on Kv1.5 channels can be influenced by multiprotein channel composition, such as the presence of the ancillary Kvbeta1.3 subunits (Du et al. 2010). Accordingly, we cannot rule out that changes in the expression of these regulatory subunits could also contribute to the different sensitivity to the drug. Unfortunately, this also appears to occur with other Kv1.5 channel inhibitors (Gonzalez et al. 2002; Decher et al. 2005).
The KCNA5 mRNA, which encodes for the Kv1.5 channel protein, was predicted by microRNA.org to be a target of miR‐1. We subsequently validated this interaction of miR‐1 with the human KCNA5 3′ ‐UTR by a luciferase reporter assay. The data indicate that the levels of Kv1.5 protein may be regulated by miR‐1. Therefore, to analyse the functional consequences of the miR‐1–KCNA5 interaction, we investigated the Kv currents in rat PA transfected with miR‐1. The expression of miR‐1 in the PA myocytes produced an increase in cell capacitance and a dramatic decline of the whole Kv current and specifically of the DPO‐1‐sensitive current. miR‐1 also significantly depolarized the membrane potential. Therefore, miR‐1 transfection mimicked the changes on Kv currents in the Hyp/Su5416 animal model of PAH and in human PAH. Thus, we hypothesized that miR‐1 might play a role in the downregulated Kv currents in PAH. For this purpose, we analysed the effects of the antagomiR‐1 on the PA exposed to hypoxia (3% O2) plus Su5416 for 48 h, an in vitro correlate to the in vivo model. The in vitro exposure to Hyp/Su5416 produced a marked downregulation of the Kv current and increased miR‐1, also mimicking the in vivo model. Interestingly, antagomiR‐1 prevented PASMC hypertrophy and increased Kv currents and Kv1.5 channel expression in Hyp/Su5416‐treated PA. Altogether, these data indicate that miR‐1 downregulates Kv1.5 channels and suggest that that elevated miR‐1 may be implicated in the inhibition of Kv channel expression in the Hyp/Su5416 animal model of PAH.
Kv downregulation for pulmonary hypertension associated with chronic hypoxia has been previously reported to be the result of hypoxia‐inducible factor‐1α (HIF‐1α)‐dependent induction of endothelin‐1 (ET‐1) (Whitman et al. 2008). The results of the present study may represent an alternative mechanism. Bioinformatic analysis and reporter assays revealed miR‐1 binding sites in the 3′‐UTR region of both the HIF‐1α and the ET‐1 mRNAs (Lu et al. 2014). miR‐1 has also demonstrated to downregulate both proteins in colorectal cancer cells (Xu et al. 2017) and hepatocarcinoma cells (Li et al. 2012). Because HIF‐1α and ET‐1 play a fundamental role in pulmonary hypertension, it would be important to understand their relationship with miR‐1 in the context of PAH. We speculate that the reduced miR‐1, as found in some series of patients with pulmonary hypertension and cell culture exposed to hypoxia (discussed above), might allow a higher overexpression of HIF‐1α and ET‐1, finally resulting in reduced Kv1.5. However, although HIF‐1α overproduction in endothelial cells or in the whole lung appears to play a clear pathophysiological role, changes in HIF‐1α in smooth muscle cells are controversial. Reduced Kv1.5 protein expression has been reported to be concurrent with diminished HIF‐1α in the smooth muscle layer of PA from idiopathic PAH (Barnes et al. 2017). Regarding the context of the present study, Su5416 is known to reduce HIF1α expression in several cell types, including PASMCs (Dean et al. 2017). Taken together, Kv downregulation in PASMC may result from at least two mechanisms: (i) HIF‐1α‐ and ET‐1‐dependent and (ii) miR‐1‐dependent pathways. We speculate that the former might be more important in the context of hypoxia and respiratory diseases (class 3 PH) and the later in PAH (i.e. class 1 PH).
In conclusion, these data indicate that miR‐1 reduces the activity and expression of Kv channels. KCNA5 is a direct target of miR‐1 and its downregulation accounts for decreased Kv currents and membrane depolarization in miR‐1 transfected cells. The diminished Kv currents in Hyp/Su5416 treated PA was prevented by antagomiR‐1. All of these data suggest that miR‐1 upregulation may play a pathophysiological role, at least in some forms of PAH.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
GMP, MC, BB, DMC and SE performed and analysed the experiments. GMP and MC drafted the manuscript. AC and FPV conceived the study and designed the experiments. FPV wrote the manuscript with significant conceptual contributions from GMP, MC, LM and AC. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The authors’ research is funded by Ministerio de Economía y Competitividad grants (SAF2014‐55399‐R, SAF2016‐77222R) and from Instituto de Salud Carlos III (PI15/01100) with funds from the European Union (Fondo Europeo de Desarrollo Regional FEDER). MC is supported by UCM‐predoctoral‐grant (CT45/15‐CT46/15).
Acknowledgements
The authors thank Dr Marcos Matamoros for help with amplifying the pMirTarget vector.
Biography
Gema Mondejar‐Parreño and María Callejo are PhD students in the Department of Pharmacology and Toxicology at the Universidad Complutense de Madrid (School of Medicine). Gema Mondejar‐Parreño completed a degree in biochemistry at the University of Valencia and, currently, her research is focused on the alteration of potassium channels in pulmonary hypertension. María Callejo completed a degree in biology at the Complutense University of Madrid and, currently, her research is focused on understanding the pathophysiological mechanisms in pulmonary hypertension at the molecular level.

Mondejar‐Parreño G, Callejo M, Barreira B, et al. miR‐1 is increased in pulmonary hypertension and downregulates Kv1.5 channels in rat pulmonary arteries. J Physiol. 2018;00:00–00. 10.1113/JP276054
Edited by: Harold Schultz and Larissa Shimoda
Linked articles: This article is highlighted in a Perspectives article by Martinez‐Nunez & Aaronson. To read this article, visit https://doi.org/10.1113/JP276390.
This is an Editor's Choice article from the 15 February 2019 issue.
References
- Barnes EA, Chen CH, Sedan O & Cornfield DN. (2017). Loss of smooth muscle cell hypoxia inducible factor‐1alpha underlies increased vascular contractility in pulmonary hypertension. FASEB J 31, 650–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]
- Burg ED, Remillard CV & Yuan JX. (2008). Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol 153 Suppl 1, S99–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Frazziano G, Moral‐Sanz J, Menendez C, Castaneda J, Gonzalez C, Villamor E & Perez‐Vizcaino F. (2009). Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res 82, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, Tamargo J & Perez‐Vizcaino F. (2006). Serotonin inhibits voltage‐gated K+ currents in pulmonary artery smooth muscle cells: role of 5‐HT2A receptors, caveolin‐1, and KV1.5 channel internalization. Circ Res 98, 931–938. [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L & Villamor E. (2007). Mechanisms controlling vascular tone in pulmonary arterial hypertension: implications for vasodilator therapy. Pharmacology 79, 65–75. [DOI] [PubMed] [Google Scholar]
- Dean A, Gregorc T, Docherty CK, Harvey KY, Nilsen M, Morrell NW & MacLean MR. (2017). Role of the aryl hydrocarbon receptor in sugen 5416‐induced experimental pulmonary hypertension. Am J Respir Cell Mol Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decher N, Kumar P, Gonzalez T, Renigunta V & Sanguinetti MC. (2005). Structural basis for competition between drug binding and Kvbeta 1.3 accessory subunit‐induced N‐type inactivation of Kv1.5 channels. Mol Pharmacol 68, 995–1005. [DOI] [PubMed] [Google Scholar]
- Du YM, Zhang XX, Tu DN, Zhao N, Liu YJ, Xiao H, Sanguinetti MC, Zou A & Liao YH. (2010). Molecular determinants of Kv1.5 channel block by diphenyl phosphine oxide‐1. J Mol Cell Cardiol 48, 1111–1120. [DOI] [PubMed] [Google Scholar]
- Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck‐Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H & Luis Zamorano J. (2016). 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37, 67–119.26320113 [Google Scholar]
- Gomez‐Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene‐Stewart L, Sung Y, Kraskauskas D, Farkas D, Conrad DH, Nicolls MR & Voelkel NF. (2012). A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol 302, L977–L991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez T, Navarro‐Polanco R, Arias C, Caballero R, Moreno I, Delpon E, Tamargo J, Tamkun MM & Valenzuela C. (2002). Assembly with the Kvbeta1.3 subunit modulates drug block of hKv1.5 channels. Mol Pharmacol 62, 1456–1463. [DOI] [PubMed] [Google Scholar]
- Grundy D. (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromadnikova I, Kotlabova K, Hympanova L & Krofta L. (2015). Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS ONE 10, e0138383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI & Vardas PE. (2014). Differential expression of vascular smooth muscle‐modulating microRNAs in human peripheral blood mononuclear cells: novel targets in essential hypertension. J Hum Hypertens 28, 510–516. [DOI] [PubMed] [Google Scholar]
- Lagrutta A, Wang J, Fermini B & Salata JJ. (2006). Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther 317, 1054–1063. [DOI] [PubMed] [Google Scholar]
- Li D, Yang P, Li H, Cheng P, Zhang L, Wei D, Su X, Peng J, Gao H, Tan Y, Zhao Z, Li Y, Qi Z, Rui Y & Zhang T. (2012). MicroRNA‐1 inhibits proliferation of hepatocarcinoma cells by targeting endothelin‐1. Life Sci 91, 440–447. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhao FP, Peng Z, Zhang MW, Lin SX, Liang BJ, Zhang B, Liu X, Wang L, Li G, Tian WD, Peng Y, He ML & Li XP. (2014). EZH2 promotes angiogenesis through inhibition of miR‐1/endothelin‐1 axis in nasopharyngeal carcinoma. Oncotarget 5, 11319–11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche J, Pflieger A, Vaillancourt M, Graydon C, Provencher S & Bonnet S. (2014). miRNAs in PAH: biomarker, therapeutic target or both? Drug Discov Today 19, 1264–1269. [DOI] [PubMed] [Google Scholar]
- Meyrick B & Reid L. (1980). Hypoxia‐induced structural changes in the media and adventitia of the rat hilar pulmonary artery and their regression. Am J Pathol 100, 151–178. [PMC free article] [PubMed] [Google Scholar]
- Michelakis ED, Dyck JR, McMurtry MS, Wang S, Wu XC, Moudgil R, Hashimoto K, Puttagunta L & Archer SL. (2001). Gene transfer and metabolic modulators as new therapies for pulmonary hypertension. Increasing expression and activity of potassium channels in rat and human models. Adv Exp Med Biol 502, 401–418. [DOI] [PubMed] [Google Scholar]
- Moral‐Sanz J, Gonzalez T, Menendez C, David M, Moreno L, Macias A, Cortijo J, Valenzuela C, Perez‐Vizcaino F & Cogolludo A. (2011). Ceramide inhibits Kv currents and contributes to TP‐receptor‐induced vasoconstriction in rat and human pulmonary arteries. Am J Physiol Cell Physiol 301, C186–C194. [DOI] [PubMed] [Google Scholar]
- Morales‐Cano D, Menendez C, Moreno E, Moral‐Sanz J, Barreira B, Galindo P, Pandolfi R, Jimenez R, Moreno L, Cogolludo A, Duarte J & Perez‐Vizcaino F. (2014). The flavonoid quercetin reverses pulmonary hypertension in rats. PLoS ONE 9, e114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales‐Cano D, Moreno L, Barreira B, Briones AM, Pandolfi R, Moral‐Sanz J, Callejo M, Mondejar‐Parreno G, Cortijo J, Salaices M, Duarte J, Perez‐Vizcaino F & Cogolludo A. (2016). Activation of PPARbeta/delta prevents hyperglycaemia‐induced impairment of Kv7 channels and cAMP‐mediated relaxation in rat coronary arteries. Clin Sci (Lond) 130, 1823–1836. [DOI] [PubMed] [Google Scholar]
- Moreno L, Moral‐Sanz J, Morales‐Cano D, Barreira B, Moreno E, Ferrarini A, Pandolfi R, Ruperez FJ, Cortijo J, Sanchez‐Luna M, Villamor E, Perez‐Vizcaino F & Cogolludo A. (2014). Ceramide mediates acute oxygen sensing in vascular tissues. Antioxid Redox Signal 20, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JJ, Marsboom G & Archer SL. (2013). Rodent models of group 1 pulmonary hypertension. Handb Exp Pharmacol 218, 105–149. [DOI] [PubMed] [Google Scholar]
- Sarrion I, Milian L, Juan G, Ramon M, Furest I, Carda C, Cortijo Gimeno J & Mata Roig M. (2015). Role of circulating miRNAs as biomarkers in idiopathic pulmonary arterial hypertension: possible relevance of miR‐23a. Oxid Med Cell Longev 2015, 792846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed D & Abdellatif M. (2011). MicroRNAs in development and disease. Physiol Rev 91, 827–887. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Manalo DJ, Sham JS, Semenza GL & Sylvester JT. (2001). Partial HIF‐1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 281, L202–L208. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Sylvester JT & Sham JS. (1998). Inhibition of voltage‐gated K+ current in rat intrapulmonary arterial myocytes by endothelin‐1. Am J Physiol Lung Cell Mol Physiol 274, L842–L853. [DOI] [PubMed] [Google Scholar]
- Sysol JR, Chen J, Singla S, Zhao S, Comhair SAA, Natarajan V & Machado RF. (2018). MicroRNA‐1 is decreased by hypoxia and contributes to the development of pulmonary vascular remodeling via regulation of sphingosine kinase 1. Am J Physiol Lung Cell Mol Physiol 314, L461–L472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajsic T & Morrell NW. (2011). Smooth muscle cell hypertrophy, proliferation, migration and apoptosis in pulmonary hypertension. Compr Physiol 1, 295–317. [DOI] [PubMed] [Google Scholar]
- Taraseviciene‐Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF & Tuder RM. (2001). Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death‐dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15, 427–438. [DOI] [PubMed] [Google Scholar]
- van Rooij E & Kauppinen S. (2014). Development of microRNA therapeutics is coming of age. EMBO Mol Med 6, 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Weigand L, Wang W, Sylvester JT & Shimoda LA. (2005). Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol 288, L1049–L1058. [DOI] [PubMed] [Google Scholar]
- Wei C, Henderson H, Spradley C, Li L, Kim IK, Kumar S, Hong N, Arroliga AC & Gupta S. (2013). Circulating miRNAs as potential marker for pulmonary hypertension. PLoS ONE 8, e64396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL & Shimoda LA. (2008). Endothelin‐1 mediates hypoxia‐induced inhibition of voltage‐gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 294, L309–L318. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhang Z, Zou K, Cheng Y, Yang M, Chen H, Wang H, Zhao J, Chen P, He L, Chen X, Geng L & Gong S. (2017). MiR‐1 suppresses tumor cell proliferation in colorectal cancer by inhibition of Smad3‐mediated tumor glycolysis. Cell Death Dis 8, e2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XJ, Wang J, Juhaszova M, Gaine SP & Rubin LJ. (1998). Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet 351, 726–727. [DOI] [PubMed] [Google Scholar]


