Abstract
The objective of our study was to estimate the seroprevalence of six pathogens transmitted by ticks in HIV-infected persons and blood donors in Poland (B. burgdorferi s.l., A. phagocytophilum, Ehrlichia spp., Babesia spp., Rickettsia spp. Bartonella henselae) to assess the frequency of exposure to such microorganisms in immunocompetent and immunocompromised individuals in endemic regions for I. ricinus ticks. Serum samples were collected from 227 HIV-infected patients and 199 blood donors. All samples were analyzed for antibodies against six tick-borne pathogens and seroprevalence rates were statistically compared between two tested group as well as age, sex and lymphocyte T CD4+ level in HIV infected patients. The seroprevalence of tick-borne infections in HIV-infected patients is higher than that of the healthy population in Poland, although no association between serological status of patients and lymphocyte CD4+ T cell level has been observed. The frequency of tick-borne coinfections and doubtful results of serological tests were significantly higher in HIV-positive individuals. In Poland, the possibility of tick-borne diseases transmission with blood is rather negligible.
Introduction
Recently the experts of the Center for Disease Control and Prevention’s have summarized the alarming increase in the number of vector-borne disease cases reported in the United States and territories from 2004 to 20161. Of the almost 650,000 cases, over 491,000 were tick-borne. However, tick-borne diseases are a large and growing public health problem not only in the United States but also in Europe2. Ixodes ricinus is the most widespread tick species in Europe and constitutes the vector of numerous pathogens, especially Borrelia burgdorferi sl. and Borrelia myiamotoi, Anaplasma phagocytophilum, tick-borne encephalitis virus (TBEV), Babesia spp., as well as some Rickettsia and Ehrlichia species3–6. Lyme borreliosis (LB) is the most common vector-borne disease in temperate zones of the northern hemisphere, and about 85,000 cases are reported annually in Europe7. The estimated incidence of LB in Poland increased dramatically from 20.3 per 100,000 inhabitants in 2007 to 56.0 per 100,000 inhabitants in 2017 (an estimated average increased from 7,735 cases per year in 2007 to 21,516 cases per year in 2017)8. As of today, there are about 100 confirmed or probable cases of anaplasmosis and about 60 cases of babesiosis in Europe9,10, including Poland11–13. The Rickettsia infections and single cases of human granulocytic ehrlichiosis (HGE) have been also noted in Europe14,15. Recent data suggest that ticks could also transmit Bartonella henselae to human16–19. Immunocompetent individuals with tick-borne infections may present with non-specific symptoms, such as fever and a flu-like disease which usually abate spontaneously within a few weeks9,15,20,21. Nevertheless, severe infections in immunocompetent humans have been also noted22–24. Furthermore, asymptomatic tick-borne infections in healthy persons may constitute threats to the safety of the blood supply25,26. However, in individuals with immunologically compromising conditions, including HIV-1 (human immunodeficiency virus type 1)-positive patients, tick-borne pathogens may cause chronic, debilitating opportunistic infection and even death27–32.
Patients diagnosed with HIV-1 are immunodeficient, which is a significant risk factor for diseases caused by specific pathogens, namely those expanding due to the lower level of T lymphocyte (LT) CD4+ cells, since pathogenicity often depends on cellular and humoral immune responses33. In Poland, since 1985, there have been about 22,000 new cases of HIV infection8. As the positive predictive value of serological tests is reduced, in HIV-positive patients diagnostics based on such methods used to be cumbersome34. Improvement in treatment efficacy has resulted in better immune system function of the majority of HIV-positive patients; another consequence has been a significant increase in the positive predictive value (PPV) risk of serology-based methods. Prognosis for patients with HIV-1 has improved pronouncedly since the commencement of HAART (highly active antiretroviral therapy) which involves both antiretroviral drugs and efficient regimens. Consequently, HIV-infected individuals have greater chance of living actively, yet engaging in outdoor activities is a risk factor for tick infestation35,36. Until now, there have been only a few studies concerning occurrence of tick-borne diseases in HIV-positive patients, in contrast to other infections associated with the same virus. Additionally, the studies that had been conducted were analyses of mainly single clinical cases, and only Babesia, Borrelia, A. phagocytophilum and Rickettsia have been detected in HIV-infected individuals in Europe so far, out of the broad spectrum of tick-borne pathogens37–40.
The objective of our study was to estimate the seroprevalence of six pathogens transmitted by ticks (B. burgdorferi s.l., A. phagocytophilum, Ehrlichia spp., Babesia spp., Rickettsia spp. Bartonella henselae) in HIV-infected persons and blood donors (representing the control group) in Poland, to assess the frequency of exposure to such microorganisms in immunocompetent and immunocompromised individuals in endemic regions for I. ricinus ticks41. To the best of our knowledge, this is the first serological study on the occurrence of the most common pathogens transmitted by ticks in HIV-1-infected humans.
Results
Description of the tested group of patients/blood donors
Of the 227 HIV-infected patients included in the study, the medical data (lymphocyte CD4+ T cell level, plasma HIV RNA level, HAART therapy, age and sex, risk group [MSM, injection drug user]) were obtained from 148 patients. In this group, with the mean age of 33 years (range 20–51 years), men predominated (140 patients, 95%). The median lymphocyte CD4+ T cell count was 465/µl with 19% of patients with less than 300/µl. Most of these patients (82%; n = 121) were on HAART.
Of the 199 blood donors, 134 men and 65 women were included with the mean age of 36 (range 18–71 years). The majority of participants (95%; n = 190) inhabit urban areas, however, 32% of them (n = 63) declared contact with ticks, mainly in natural areas (forest). Lyme borreliosis was diagnosed and then treated in the last 10 years in 9 participants (4.5%).
Borrelia burgdorferi s.l. seroprevalence
Of the 227 HIV-infected patients tested for B. burgdorferi s.l. specific IgM and IgG by ELISA test, 66 and 11 were positive, respectively, which corresponds to the seroprevalence rate of 29.1% for IgM and 4.8% for IgG (Table 1). Among 199 serum samples collected from blood donors, 26 (13.1%) for IgM and 10 (5.0%) for IgG were positive, respectively. The IgM seroprevalence noted in HIV-infected patients was significantly higher compared to seroprevalence observed in blood donors (29.1% vs. 13.1%; χ2 = 16.58, df = 1, p = 0.0001); however, IgG seroprevalence did not differ statistically between these two groups (Table 1).
Table 1.
Prevalence of anti-B. burgdorferi, anti-B. microti, anti-A. phagocytophilum, anti-Ehrlichia spp., anti-B. henselae and anti-Rickettsia spp. IgM and IgG antibodies in HIV-infected patients and blood donors in Poland.
| Method | Tested group of patients | IgM | IgG | |||||
|---|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | P | Positive (%) | Negative (%) | P | |||
| Borrelia burgdorferi s.l. | ELISA | HIV-positive | 66/227 (29.1) | 161/227 (70.9) | <10−4 | 11/227 (4.8) | 216/227 (95.2) | 0.932 |
| blood donors | 26/199 (13.1) | 26/199 (86.9) | 10/199 (5.0) | 189/199 (95.0) | ||||
| Babesia microti | IFA | HIV-positive | 21/227 (9.3) | 206/227 (90.7) | <10−4 | 5/227 (2.2) | 222/227 (97.8) | 0.595 |
| blood donors | 2/199 (1.0) | 197/199 (99.0) | 3/199 (1.5) | 196/199 (98.5) | ||||
| Anaplasma phagocytophilum | IFA | HIV-positive | 6/277 (2.6) | 221/227 (97.4) | 0.817 | 6/227 (2.6) | 221/227 (97.4) | 0.410 |
| blood donors | 6/199 (3.0) | 193/199 (97.0) | 3/199 (1.5) | 196/199 (98.5) | ||||
| Ehrlichia spp. | IFA | HIV-positive | 4/227 (1.8) | 223/227 (98.2) | 0.096 | 16/227 (7.0) | 211/227 (93.0) | 0.026 |
| blood donors | 9/199 (4.5) | 190/199 (95.5) | 5/199 (2.5) | 194/199 (97.5) | ||||
| Bartonella henselae | IFA | HIV-positive | 10/227 (4.4) | 217/227 (95.6) | 0.953 | 4/227 (1.8) | 223/227 (95.5) | 0.096 |
| blood donors | 9/199 (4.5) | 190/199 (95.5) | 9/199 (4.5) | 190/199 (95.5) | ||||
| Rickettsia SFG | ELISA | HIV-positive | nd | nd | nd | 4/227 (1.8) | 223/227 (98.2) | 0.503 |
| blood donors | nd | nd | 2/199 (1.0) | 197/199 (99.0) | ||||
All positive results of ELISA tests were confirmed by Western Blot. Out of the IgM and IgG ELISA-positive samples of HIV infected patients, half were confirmed in WB (50% [33/66] and 53.8% [6/11] for IgM and IgG, respectively). Statistically lower IgM ELISA positive results were confirmed in WB in blood donors (23.1% [6/26]; χ2 = 4.49, df = 1, p = 0.034). However, of the 10 IgG ELISA positive results, 8 (80%) were confirmed in WB (statistically not significant).
Due to a sufficient number of B. burgdorferi seropositive cases in WB for HIV-positive patients, the effect of sex, age and lymphocyte CD4+ T cell level on seroprevalence were estimated. IgM seroprevalence increased significantly with age, reaching a maximum of 36.8% for patients aged >35 years (<25 years: 0%; 25–35 years: 11.1%; χ2 = 7.84, df = 2, p = 0.0001). IgG seroprevalence noted for patients aged 25–35 years was almost twice as low as in patients aged >35 years (2.8% vs. 5.3%; no statistical differences). No statistical difference of seroprevalence between men and women was observed (IgM: 15.3% vs 10.0%; IgG: 2.8% vs. 0.0%, respectively). Borrelia IgM and IgG seroprevalence were higher in HIV-infected patients with lymphocyte CD4+ T cell count less than 300/µl (IgM: 19.2% vs. 12.5%; IgG: 3.8% vs. 1.8%, no statistical differences) (Fig. 1).
Figure 1.

Seroprevalence of anti-Borrelia burgdorferi s.l. antibodies in HIV-infected patients with median lymphocyte CD4+ T cell count <or >300/μl.
Babesia microti seroprevalence
The IgM seroprevalence was about 9 times higher in HIV-infected patients (9.3%; 21/227) than in blood donors (1.0%; 2/199) (χ2 = 16.65, df = 1, p = 0.0001) (Table 1). Babesia IgG seroprevalence did not differ statistically between HIV-positive patients and blood donors (2.2% [5/227] vs. 1.5% [3/199]). No significant association was demonstrated between the serological status of the tested group of patients/participants and age, sex or lymphocyte CD4+ T cell level in HIV-positive patients.
Anaplasma phagocytophilum seroprevalence
The IgM and IgG seroprevalence was similar in HIV-infected patients and blood donors (IgM: 2.6% [6/227] vs. 3.0% [6/199], IgG: 2.6% [6/227] vs. 1.5% [3/199], respectively) (Table 1). No significant association was demonstrated between the serological status of the tested group of patients/participants and age, sex or lymphocyte CD4+ T cell level in HIV-positive patients.
Ehrlichia chaffeensis seroprevalence
Of the 227 HIV-positive patients tested for Ehrlichia, 4 (1.8%; 4/227) were positive for specific IgM (Table 1). The IgM seroprevalence in blood donors was 2.5 times higher (4.5%; 9/199), but this difference was not statistically significant. Nevertheless, IgG seroprevalence noted in HIV-infected patients was significantly higher compared to seroprevalence observed in blood donors (7.0% [16/227] vs. 2.5% [5/199]; χ2 = 4.93, df = 1, p = 0.026). No significant association was demonstrated between the serological status of the tested group of patients/participants and age, sex or lymphocyte CD4+ T cell level in HIV-positive patients.
Bartonella henselae seroprevalence
The IgM and IgG seroprevalence was similar in HIV-infected patients and blood donors (IgM: 4.4% [10/227] vs. 4.5% [9/199], IgG: 1.8% [4/227] vs. 4.5% [9/199], respectively) (Table 1). No significant association was demonstrated between the serological status of the tested group of patients/participants and age, sex or lymphocyte CD4+ T cell level in HIV-positive patients.
Rickettsia Spotted-Fever Group seroprevalence
The IgG seroprevalence for Rickettsia SFG did not differ significantly between HIV-positive patients and blood donors (1.8% [4/227] vs. 1.0% [2/199]). No significant association was demonstrated between the serological status of the tested group of patients/participants and age, sex or lymphocyte CD4+ T cell level in HIV-positive patients.
Multiple seropositives
Among 227 HIV-positive patients who were tested for six tick-borne pathogens, the IgM serology was positive for a single pathogen in 26.0% (59/227) and for two pathogens in 3.1% (7/227), especially B. microti together with B. burgdorferi (3/7) or B. henselae (2/7) and A. phagocytophilum with B. henselae (1/7) or E. chaffeensis (1/7) (Fig. 2). The IgM seroprevalence rate was significantly lower in blood donors in whom a single pathogen was noted in 9.5% (19/199) and two pathogens in 1.5% (3/199), in all cases A. phagocytophilum with E. chaffeenisis were involved (χ2 = 22.01, df = 2, p = 0.001).
Figure 2.
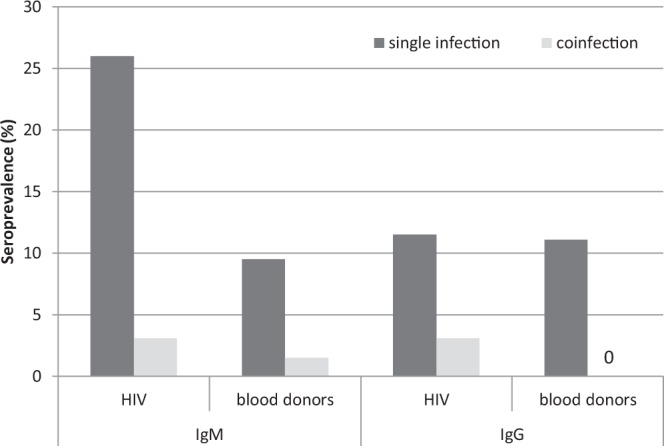
Seroprevalence of specific antibodies in single infection and coinfection with two pathogens in HIV-infected patients and blood donors in Poland.
The rate of IgG seroprevalence of a single pathogen was similar in HIV-positive patients and blood donors (11.5% [26/227] vs. 11.1% [22/199]). However, the infections of two pathogens were noted only in HIV-infected patients (3.1%; 7/227) (χ2 = 8.96, df = 2, p = 0.011): A. phagocytophilum with E. chaffeenisis (6/7) and one case of Rickettsia with B. henselae (Fig. 2). No significant association was demonstrated between the serological status of tested group of patients/participants and age, sex or lymphocyte CD4+ T cell level in HIV-positive patients.
Doubtful results
The frequency of doubtful results was significantly higher in HIV-infected patients than in blood donors (Table 2). In HIV-positive patients the doubtful results for A. phagocytophilum were 9.7% and 6.2%, for E. chaffeensis- 4.4% and 6.6%, for B. henselae- 2.6% and 2.2% for IgM and IgG tests, respectively, whereas the doubtful results for these pathogens were not noted in blood donors. For B. burgdorferi IgM ELISA test, the amount of doubtful results was similar in both tested groups of patients/participants. In HIV-positive patients, the doubtful results for B. burgdorferi IgG ELISA test were observed more often (2.6% vs. 0.5%); however, this difference was not statistically significant.
Table 2.
The frequency of doubtful results in HIV-infected patients and blood donors in Poland.
| Method | Tested group of patients | IgM | IgG | |||
|---|---|---|---|---|---|---|
| Doubtful (%) | P | Doubtful (%) | P | |||
| Borrelia burgdorferi s.l. | ELISA | HIV-positive | 18/227 (7.9) | 0.555 | 6/227 (2.6) | 0.066 |
| blood donors | 19/199 (9.5) | 1/199 (0.5) | ||||
| Babesia microti | IFA | HIV-positive | 0/227 (0.0) | 0.080 | 0/227 (0.0) | 1.000 |
| blood donors | 2/199 (1.0) | 0/199 (0.0) | ||||
| Anaplasma phagocytophilum | IFA | HIV-positive | 22/277 (9.7) | <10−4 | 14/277 (6.2) | <10−4 |
| blood donors | 0/199 (0.0) | 0/199 (0.0) | ||||
| Ehrlichia spp. | IFA | HIV-positive | 10/227 (4.4) | <10−4 | 15/227 (6.6) | <10−4 |
| blood donors | 0/199 (0.0) | 0/199 (0.0) | ||||
| Bartonella henselae | IFA | HIV-positive | 6/227 (2.6) | 0.006 | 5/227 (2.2) | 0.012 |
| blood donors | 0/199 (0.0) | 0/199 (0.0) | ||||
| Rickettsia SFG | ELISA | HIV-positive | nd | nd | 0/227 (0.0) | 1.000 |
| blood donors | nd | 0/199 (0.0) | ||||
Discussion
The large population of immunocompromised patients, including those with HIV/AIDS, is constantly growing. Analysis of the published reports of tick-borne infections shows that the disease in immunocompromised patients is far more severe, prolonged and more likely to be fatal9,14,28. Thus far, investigations of the epidemiology of tick-borne pathogen infections involving serological studies have concentrated on inhabitants of endemic regions who were healthy and whose immunological function was normal. By contrast to those studies or to reviews of clinical findings concerning hospitalized patients, our molecular and retrospective study had a different objective: establishing both the incidence and seroprevalence of tick-borne infections in patients with HIV, compared with the control group of healthy blood donors without clinical symptoms. Both groups were based in an endemic area of I. ricinus ticks, namely Poland. The Polish Society of Epidemiology and Infectious Diseases has issued a recommendation regarding diagnostics of tick-borne diseases to which this study particularly conforms.
In HIV-positive patients, borreliosis is rarely reported as co-infection, with only a few cases so far28,42. Most of them were identified early, with neuroborreliosis confirmed in Dutch and Swedish patients. Our recent molecular study confirmed the asymptomatic B. garinii infection in an HIV-postitive patient with no signs of early or late-stage Lyme borreliosis40. Borrelia burgdorferi was the predominant pathogen in our study, and about 30% and 5% of HIV-infected individuals were recorded as positive for IgM and IgG, respectively. In this group of patients, the IgM seroprevalence rate was significantly higher compared to blood donors. The significantly higher B. burgdorferi seroprevalence was observed in HIV-infected patients aged >35 years and with a median lymphocyte CD4+ T cell less than 300/μl. To the best of our knowledge, as of today, only one serological study of Borrelia infection in HIV-postitive patients was conducted, and the results were comparable – a total of 33% sera were positive43. Antigens of Borrelia and Treponema spirochetes often cross-react with each other. False positive results of serologic tests have been obtained in neurological patients with infections with other bacteria in the same group, for instance T. pallidum42,44. Therefore, our positive results of IgM and IgG ELISA tests were confirmed by Western Blot according to the European guidelines45 in order to exclude false positive results. Only half of positive ELISA results were confirmed in WB in HIV-positive patients. Accordingly, our data strongly recommends confirmatory testing in HIV-infected patients with ELISA positive Lyme screening.
Borrelia seroprevalence among blood donors in Europe does not exceed 10%46–48. In our study, the IgM and IgG seroplevalence in ELISA tests were rather similar (13.1% and 5.0%), and 23% (IgM) and 80% (IgG) of positive/doubtful results were confirmed in WB, respectively. All blood donors with anti-Borrelia IgG antibodies underwent borreliosis in the last 10 years. It is difficult to determine whether tested blood donors were spirochetemic at the time of blood giving, yet most of them declared multiple blood donation in the last decade. Till now, there are only limited and conflicting data on the number of spirochetes in the blood of spirochetemic Lyme disease patients49,50. However, as spirochetemia may occur in Lyme disease, the potential for transfusion-transmitted B. burgdorferi exists but has yet to be reported51. Ginzburg and co-authors have postulated that host-adapted B. burgdorferi survives poorly under blood storage conditions, particularly if the number of organisms per milliliter of human blood is low. We have not confirmed the presence of B. burgdorferi DNA in the blood of the tested group of donors (data not published) and, therefore, we conclude that the risk of transfusion-transmitted B. burgdorferi is rather negligible.
Babesia is transmitted primarily by ixodid ticks, although blood transfusion is another important cause of infection52. To date, babesiosis in HIV-infected individuals has been observed in the United States of America53,54, yet one case has been also recorded in Europe (Spain)29. In those cases, the phase of infection was determined as chronic since it lasted several months. Relapses of babesiosis occurred despite treatment which because of high parasitemia involved blood transfusions54. It is worth to note that recent studies have identified the first case of false-positive HIV serology that was associated with active babesiosis, yet after successful treatment of babesiosis, the positive HIV serology turned negative55. Currently, there is no data on Babesia seroprevalence in HIV-positive individuals. In our study, the IgM B. microti seroprevalence in HIV-positive patients was significantly higher than among blood donors whose estimated IgM and IgG seroprevalence (<2%) was comparable to that reported for B. microti among blood donors in Europe56–58. Nevertheless, IgG seroprevalence among HIV-positive patients and blood donors was similar, and we have not confirmed the presence of Babesia DNA in tested blood samples (data not published). Human babesiosis appears to be rare in Europe9, including Poland11, which is consistent with a low I. ricinus infection rate (1.6–2.8% in Poland, 0.5% in Slovakia, 0.7% in Switzerland)4,59–61.
Human ehrlichiosis and anaplasmosis are acute febrile tick-borne diseases caused by various species from the genera Ehrlichia and Anaplasma. A. phagocytophilum, causing human granulocytotropic anaplasmosis (HGA), and Ehrlichia chaffeensis, the etiologic agent of human monocytotropic ehrlichiosis (HME), are considered as an emerging zoonosis with clinical manifestations ranging from a mild febrile illness to a fulminant disease characterized by multi-organ system failure, especially in immunocompromised individuals62. HME and HGA have similar clinical presentations and both pathogens could be transmitted by transfusion or organ transplantation63,64. Till now, only one case of asymptomatic A. phagocytopilum infection in HIV-positive patient was confirmed40. In our study, HIV-infected patients and blood donors presented a similar rate of A. phagocytophilum seroprevalence (<3%). In Europe, among blood donors, antibodies to A. phagocytophilum vary from 5% in Belgium65 to 22% in Greece66. The low A. phagocytophilum seroprevalence rate found in our study is compatible with the small number of clinical cases identified so far67 as well as the low prevalence in I. ricinus ticks in Poland5.
In persons infected with HIV, ehrlichiosis caused by E. chaffeensis is often life-threatening68. However, the disease responds well to specific, antibiotic therapy, particularly when antibiotics are used at an early stage of infection69. The E. chaffensis seroprevalence in HIV-infected patients in the US was estimated at 1.7% and was similar to the one observed in healthy persons70. Nonetheless, in this group of patients the fatal false-negative results of serological test were reported. Accordingly, the incidence of ehrlichiosis in this population seems to be underestimated68,69. The severe pathology and multi-organ involvement in fatal ehrlichiosis that mimics toxic shock-like syndrome was observed in patients who are immunocompromised due to other infections, such as HIV or chemotherapy, and is thought to be related to dysregulation of the host immune response and immunopathologic mechanism that leads to tissue damage and multi-system organ failure71,72. In our study, the rate of IgG anti-Ehrlichia antibodies was almost three times higher in HIV-infected individuals than among blood donors. Although our positive patients have not declared clinical manifestation characteristic of tick-borne diseases, the diagnosis of HME in HIV-positive patients is complicated since the symptoms of HME often mimic typical findings commonly associated with HIV-infection70. Although in Europe the incidences of HGE are rather rare and the number of study about E. chaffensis infection in I. ricinus is limited, in HIV-positive patients ehrlichiosis should be considered as potential opportunistic infection.
Bacteria of Bartonella genus are distributed in diversified geographic areas and are transmitted by different arthropod vectors, such as sandflies, the human body louse, the cat flies and probably ticks. Contact with animals and vectors seems to be the most important mode of transmission – although the ability of Bartonella to survive in stored blood with the potential for transfusion-associated infection has been shown73. Bartonella infection presents varied clinical symptoms, mainly in HIV-infected patients whose bacillary anigiomatosis and hepatic peliosis are classically associated with AIDS74. Bartonellosis in this group of patients seems to be less frequent today, possibly because of earlier recognition of HIV serostatus and the lesser number of individuals with CD4 lymphocyte cell counts below 50 cells/mm3 75. Our study demonstrated low B. henselae seroprevalence rate among HIV-infected patients and it was similar to that observed in a healthy population of blood donors (<4.5%). Bartonella seroreactivity rates varied between 2% and 30% in studies from Europe and the US with higher prevalence rates in intravenous drug users, homeless people, cat owners and veterinarians76,77. Among HIV-infected patients from Europe, anti-Bartonella antibodies were significantly higher than in our study and varied from 16% to 41% of individuals78,79. The likelihood of false-negative serological results are high in heavy immunocompromised patients with active Bartonella infection80. However, the majority of patients tested in our study have good immunological status – in 81% of tested patients, the level of lymphocyte CD4+ T cells was higher than 300/µl and most of the patients (82%) were on HAART. Furthermore, in contrast to the previous study where the prevalence of anti-Bartonella antibodies is inversely proportional to the number of CD4 lymphocytes74,81, we have not found significant association between the Bartonella serological status and lymphocyte CD4+ T cell level in HIV-positive patients. Our study presented only 19% of patients with low CD4 levels, which could significantly alter our results. Even though the rate of Bartonella infection in ticks in Poland is rather low (<2%82), the serological studies have proved that B. henselae and B. quintana are present and widely distributed in Poland in such specific risk groups as: alcoholics, veterinarians and cats’ owners76. Therefore, the further study determining the Bartonella prevalence among HIV-infected individuals, especially those with a fever of unknown origin, is needed.
Intracellular bacteria of the Rickettsiaceae family (spotted fever group (SFG) are responsible for tick-borne rickettsiosis. In humans, the syndrome manifests clinically mainly through rash, fever and ‘tache noire’, i.e. eschar developing at the site of the tick bite83. In HIV-positive patients, only isolated cases of Mediterranean spotted fever (MSF) have been reported and found to be caused primarily by R. monacensis and R. conorii39. Occassionally, MSF presentation is mimicked by primary HIV infection84. Till now, only few serological studies of Rickettsia infections among HIV-infected patients in Europe were performed. Nogueras et al85. have shown that seroprevalences of R. typhi and R. felis infections do not exceed 7% in this group of patients and were similar to those obtained in healthy subjects from the same region. In our study, the estimated the IgG seroprevalence of SFG Rickettsia was comparable and did not exceed 2% in both groups. Nevertheless, the SFG Rickettsia seroprevalence in Poland seems to be rather high in occupationally exposed populations (forestry and agricultural workers – 36%)86. Moreover, Rickettsia infection in ticks is high and varies from 4% to 53% depending on the tick species (I. ricinus vs. D. reticulatus, respectively)5,87,88. It is likely that, similarly to Bartonella infection, the serologic response in HIV-infected patients with good immunological status could be comparable to that of a healthy population. Therefore, the further study estimating the Rickettsia prevalence among HIV-infected individuals, especially with heavy immunosuppression, should be conducted.
In our study, no significant association was noted between the serological status of patients and their age, sex or lymphocyte CD4+ T cell level in HIV-positive patients. Such medical information about patients was available only for 65% individuals, and it was the primary limitation in our study. However, the previous studies, despite the fact that they possessed all the data, did not find any statistical relations between seropositivities and the assessed variables as well43,79,85,89.
Very few HIV-infected patients and blood donors were seropositive for two of the six studied pathogens. The single infection and coinfections were significantly more often noted in HIV-infected patients whose immunodeficiency significantly increases the risk of infection caused by pathogens. Simultaneous seropositivity for A. phagocytophilum and E. chaffeensis was observed in 59% of all coinfections. It is likely that cross-reactive antigens, shared by Ehrlichia and Anaplasma that induce cross-reactive antibodies, may affect the high rate of false-positive results in serological tests62. Due to this cross-reactivity among ehrlichial species, sera should be tested against both E. chaffeensis and A. phagocytophilium antigens when ascribing a specific etiology. Coinfections with B. burgdorferi and B. microti were the second most frequent combination noted only in HIV-infected patients. Dunn et al.90 observed that coinfection with B. burgdorferi and B. microti significantly increases B. microti parasitemia in mice and that larval ticks become infected with B. microti in greater numbers when fed on coinfected hosts. A possible explanation is that the host immune response to disseminating spirochetes is not restricted to the skin and may interfere with the splenic immune response, which is critical for the control and clearance of B. microti infection. Initial case reports suggested that concurrent Lyme disease and babesiosis are associated with severe illness91. Accordingly, concurrent babesiosis should be considered for any patient with Lyme disease who experiences more severe illness symptoms than expected, especially when the patient does not respond well to recommended antibiotic therapy.
In our study, the frequency of doubtful results for Anaplasma, Ehlichia and Bartonella was significantly higher in HIV-infected patients than in blood donors. The results of the previous studies have shown that the serological evaluation of the presence of IgG Ehrlichia antibodies in patients in a single sample is not sufficient to confirm ehrlichiosis. Non-specific reactions, which are more often observed in the group of patients infected with HIV, may result from the dysfunction of an immunological system, and they might be the consequence of the cross-reaction with other pathogens92. In consideration of the above, examination of many samples using concurrent serological and molecular tests might be crucial to confirm infection with the specific grade of pathogen (for example Ehrlichia or Anaplasma)69,92. Similarly, in the case of serological diagnostic of Bartonella, in patients infected with HIV, the non-specific or false positive results of serological tests are explained by the cross-reaction with the following pathogens: Coxiella burnetti or Chlamydia trachomatis93,94.
In conclusion, our study confirmed that the seroprevalence of tick-borne infections in HIV-infected patients is higher than that of the healthy population in Poland, however no association between serological status of patients and lymphocyte CD4+ T cell level has been observed. The frequency of tick-borne coinfections and doubtful results of serological tests seems to be higher in HIV-positive individuals. Although the advent of HAART had a considerable impact on the incidence of AIDS-associated opportunistic infections, the further studies of tick-borne infection in HIV-infected patients, particularly in patients who do not regain immunological function despite well controlled HIV replication on effective HAART, should be performed95. In this group of patients, tick-borne pathogens may cause chronic, debilitating opportunistic infection and even death. Thus, in clinical care of HIV-positive individuals, detailed history of tick bites in endemic tick areas should be collected via directed anamnesis. The low seroprevalence and negative results of molecular studies of tick-borne pathogens in blood donors (data not published) have suggested that the possibility of tick-borne diseases transmission with blood is rather negligible, which is consistent with the lack of reported cases of transfusion-transmitted tick-borne infections in Poland. Nevertheless, there is still a clear need to further such studies in order to maintain a balance between consideration of the real risk of disease transmission with blood and excessively restrictive approach that eliminates blood donors.
Methods
Selection and recruitment of patients/participants and serum samples
In 2016, serum samples were collected from 199 blood donors (representing the control group) who were diagnosed in AmerLab Ltd. Diagnostic Laboratory of Parasitic Diseases and Zoonotic Infections. Subjects with immunodeficiency were excluded. All participants signed informed consent and obtained a standardized, anonymous questionnaire to record data including age, gender, immunological status, place of residence, the history of tick bite, as well as previously diagnosed borreliosis or other tick-borne diseases. Blood samples obtained from blood donors were stored at room temperature and centrifuged immediately or within a maximum of 12 h after collection. Sera were frozen at −20 °C until further analysis.
The retrospective study was conducted on HIV-positive patients who did not have any known history of tick bite, nor any clinical manifestation characteristic for tick-borne diseases. In 2013, serum samples were collected from 227 patients routinely followed at the HIV Outpatients’ Clinic of the Hospital for Infectious Diseases in Warsaw.
The study protocol followed ethical guidelines of the 2013 Declaration of Helsinki and the study was approved by the Internal Review Board of the Warsaw Medical University (no. AKBE/24/16). Informed consent was obtained from all individual participants included in the study. All ethical approvals for the study have been obtained in accordance with the Polish regulations.
Serological Tests
All serum samples (227 from HIV-positive patients and 199 from blood donors) were analyzed for the presence of antibodies against six tick-borne pathogens by using: (1) Borrelia IgM and IgG ELISA tests (Biomedica Laboratories, Vienna, Austria) for Borrelia burgdorferi s.l.; (2) Western Blot: recomLine Borrelia IgM and recomLine Borrelia IgG (Microgen, Neuried, Germany) for positive or doubtful results of Borrelia ELISA test for confirmation according to the European guidelines (Stanek et al. 2011); (3) Ehrlichia chaffeensis and Anaplasma phagocytophilum IFA IgM Antibody Kit (Fuller Laboratories, California, the USA; positive cut-off 1/512) for A. phagocytophilum i Ehrlichia spp.; (4) Babesia microti IFA IgM and IgG antibody kits (Fuller Laboratories, California, the USA; positive cut-off 1/128 for IgM and 1/512 for IgG) for Babesia spp.; (5) Spotted Fever Rickettsia IgG EIA antibody kit (Fuller Laboratories, California, the USA; the cut-off calibrator is set and the index value for each serum is derived. Indices from 0.9 to 1.1 absorbance units may be considered equivocal, while those above 1.1 are considered positive and those below 0.9 are considered negative) for Rickettsia spp.; (6) Bartonella henselae IFA Human IgM and IgG antibody Kit (Fuller Laboratories, California, the USA; positive cut-off 1/512) with the manufacturer’s interpretation criteria.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics v. 23.0 software. For the analysis of the results, doubtful serological results of tested pathogens were classified as negative. A descriptive analysis of the participants was included and calculations of seroprevalence rates for each pathogen were performed. Seroprevalence rates were compared with the tested group (HIV infected patients/blood donors), age, sex, lymphocyte T CD4+ level (HIV infected patients) using Maximum Likelihood techniques based on log-linear analysis of contingency tables (HILOGLINEAR).
Ethics approval and consent to participate
The study protocol followed ethical guidelines of the 2013 Declaration of Helsinki. The study was approved by the Internal Review Board of the Warsaw Medical University (No. AKBE/24/16). Informed consent was obtained from all individual participants included in the study. All ethical approvals for the study have been obtained according to Polish regulations.
Acknowledgements
This study was supported by the Ministry of Science and Higher Education, Grant Iuventus Plus IP2014050373.
Author Contributions
The study was designed and performed by R.W.F. and A.P. R. W.F., M.B. and A.P. participated in serological and statistical analysis. J.D.K., M.R., B.U.K. and M.B. participated in collecting the material. R.W.F. and A.P. drafted the manuscript. All authors read and approved the final manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenberg R, et al. Trends in Reported Vectorborne Disease Cases — United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018;67:496–501. doi: 10.15585/mmwr.mm6717e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hai VV, et al. Monitoring human tick-borne disease risk and tick bite exposure inEurope: available tools and promising future methods. Ticks Tick Borne Dis. 2014;5:607–619. doi: 10.1016/j.ttbdis.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Heyman P, et al. A clear and present danger: tick-borne diseases in Europe. Expert Rev. Anti. Infect. Ther. 2010;8:33–50. doi: 10.1586/eri.09.118. [DOI] [PubMed] [Google Scholar]
- 4.Welc-Falęciak R, Bajer A, Paziewska-Harris A, Baumann-Popczyk A, Siński E. Diversity of Babesia in Ixodes ricinus ticks in Poland. Adv. Med. Sci. 2012;57:364–369. doi: 10.2478/v10039-012-0023-9. [DOI] [PubMed] [Google Scholar]
- 5.Welc-Falęciak R, et al. Rickettsiaceae and Anaplasmataceae infections in Ixodes ricinus ticks from urban and natural forested areas of Poland. Parasit. Vectors. 2014;7:121. doi: 10.1186/1756-3305-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowalec M, et al. Ticks and the city - are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit. Vectors. 2017;10:573. doi: 10.1186/s13071-017-2391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindgren, E. & Jaenson, T. G. T. Lyme borreliosis in Europe. Influences of climate and climate change, epidemiology, ecology and adaptation measures, World Health Organization, Copenhagen, Denmark (2006).
- 8.National Institute of Public Health – National Institute of Hygiene, Epidemiological reports, www.pzh.gov.pl (2018).
- 9.Hildebrandt A, Gray JS, Hunfeld KP. Human babesiosis in Europe: what clinicians need to know. Infection. 2013;41:1057–1072. doi: 10.1007/s15010-013-0526-8. [DOI] [PubMed] [Google Scholar]
- 10.Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum–a widespread multi-host pathogen with highly adaptive strategies. Front. Cell Infect. Microbiol. 2013;3:31. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welc-Falęciak R, et al. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann. Agric. Environ. Med. 2015;22:51–54. doi: 10.5604/12321966.1141394. [DOI] [PubMed] [Google Scholar]
- 12.Welc-Falęciak R, Kowalec M, Zajkowska J, Pancewicz SA, Siński E. Clinical and molecular features of one case of human infection with Anaplasma phagocytophilum from Podlaskie Province in eastern Poland. Ann. Agric. Environ. Med. 2015;22:414–417. doi: 10.5604/12321966.1167704. [DOI] [PubMed] [Google Scholar]
- 13.Welc-Falęciak R, Hildebrandt A, Siński E. Co-infection with Borrelia species and other tick-borne pathogens in humans: two cases from Poland. Ann. Agric. Environ. Med. 2010;17:309–313. [PubMed] [Google Scholar]
- 14.Blanco JR, Oteo JA. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 2002;8:763–772. doi: 10.1046/j.1469-0691.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- 15.Portillo A, Santibáñez S, García-Álvarez L, Palomar AM, Oteo JA. Rickettsioses in Europe. Microbes Infect. 2015;17:834–838. doi: 10.1016/j.micinf.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Sanogo YO, et al. Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno province, Italy. Emerg. Infect. Dis. 2003;9:329e32. doi: 10.3201/eid0903.020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotté V, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 2008;14:1074e80. doi: 10.3201/eid1407.071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelakis E, et al. Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after tick bite. Clin. Infect. Dis. 2010;50:549e51. doi: 10.1086/650172. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich F, et al. Prevalence of Bartonella henselae and Borrelia burgdorferi sensu lato DNA in Ixodes ricinus ticks in Europe. Appl. Environ. Microbiol. 2010;76:1395e8. doi: 10.1128/AEM.02788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect. Dis. Clin. North Am. 2015;29:341–355. doi: 10.1016/j.idc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moniuszko-Malinowska A, et al. Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect. Dis. (Lond). 2016;48:537–543. doi: 10.3109/23744235.2016.1164339. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez LM, et al. Severe babesiosis in immunocompetent man, Spain, 2011. Emerg. Infect. Dis. 2014;20:724–726. doi: 10.3201/eid2004.131409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genda J, et al. Severe Babesia microti infection in an immunocompetent host in Pennsylvania. J. Investig. Med. High Impact Case Rep. 2016;4:1–4. doi: 10.1177/2324709616663774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripoll, J. G., Rizvi, M. S., King, R. L. & Daniels, C. E. Severe Babesia microti infection presenting as multiorgan failure in an immunocompetent host. BMJ Case Rep. 30, 10.1136/bcr-2018-224647 (2018). [DOI] [PMC free article] [PubMed]
- 25.Cable RG, Leiby DA. Risk and prevention of transfusion-transmitted babesiosis and other tick-borne diseases. Curr. Opin. Hematol. 2003;10:405–411. doi: 10.1097/00062752-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Alhumaidan H, et al. Transfusion-transmitted anaplasmosis from leukoreduced red blood cells. Transfusion. 2013;53:181–186. doi: 10.1111/j.1537-2995.2012.03685.x. [DOI] [PubMed] [Google Scholar]
- 27.Krause PJ, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin. Infect. Dis. 2008;46:370–376. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- 28.van Burgel ND, Oosterloo M, Kroon FP, van Dam AP. Severe course of Lyme neuroborreliosis in an HIV-1 positive patient; case report and review of the literature. BMC Neurol. 2010;10:117. doi: 10.1186/1471-2377-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González LM, et al. First report of Babesia divergens infection in an HIV patient. Int. J. Infect. Dis. 2015;33:202–204. doi: 10.1016/j.ijid.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Ryan MF, Thorn C. Lyme carditis in an immunocompromised patient. Case Rep. Emerg. Med. 2013;2013:380734. doi: 10.1155/2013/380734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wennerås C. Infections with the tick-borne bacterium Candidatus Neoehrlichia mikurensis. Clin. Microbiol. Infect. 2015;21:621–630. doi: 10.1016/j.cmi.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 32.Chmelík V, Chrdle A, Růžek D. Fatal tick-borne encephalitis in an immunosuppressed 12-year-old patient. J. Clin. Virol. 2016;74:73–74. doi: 10.1016/j.jcv.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Chang CC, et al. HIV and co-infections. Immunol. Rev. 2013;254:114–142. doi: 10.1111/imr.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raoult D, Hechemy KE, Baranton G. Cross-reaction with Borrelia burgdorferi antigen of sera from patients with human immunodeficiency virus infection, syphilis, and leptospirosis. J. Clin. Microbiol. 1989;27:2152–2155. doi: 10.1128/jcm.27.10.2152-2155.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuhaus J, et al. Insight Smart and ESPRIT study groups. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with. HIV. AIDS. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mocroft A, et al. EuroSIDA Study in EuroCOORD. The clinical benefits of antiretroviral therapy in severely immunocompromised HIV-1-infected patients with and without complete viral suppression. Antivir. Ther. 2012;17:1291–1300. doi: 10.3851/IMP2407. [DOI] [PubMed] [Google Scholar]
- 37.Cerný R, Machala L, Bojar M, Rozsypal H, Pícha D. Neuroborreliosis in an HIV-1 positive patient. Infection. 2006;34:100–102. doi: 10.1007/s15010-006-4134-8. [DOI] [PubMed] [Google Scholar]
- 38.Machtinger L, et al. Treatment of babesiosis by red blood cell exchange in an HIV-positive, splenectomized patient. J. Clin. Apher. 1993;8:78–81. doi: 10.1002/jca.2920080205. [DOI] [PubMed] [Google Scholar]
- 39.Colomba C, et al. A case of spotted fever rickettsiosis in a human immunodeficiency virus-positive patient. J. Med. Microbiol. 2013;62:1363–1364. doi: 10.1099/jmm.0.053546-0. [DOI] [PubMed] [Google Scholar]
- 40.Welc-Falęciak R, Kowalska JD, Bednarska M, Szatan M, Pawełczyk A. Molecular identification of tick-borne pathogens in asymptomatic individuals with human immunodeficiency virus type 1 (HIV-1) infection: a retrospective study. BMC Infect. Dis. 2018;18:227. doi: 10.1186/s12879-018-3140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.European Centre for Disease Prevention and Control and European Food Safety Authority. Tick maps [Internet]. Stockholm: ECDC; 2018. Available at: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps.
- 42.Bremell D, Säll C, Gisslén M, Hagberg L. Lyme neuroborreliosis in HIV-1 positive men successfully treated with oral doxycycline: a case series and literature review. J. Med. Case Rep. 2011;5:465. doi: 10.1186/1752-1947-5-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oteo Revuelta JA, Elías Calvo C, Martínez de Artola V, Pérez Surribas D. Infection by Borrelia burgdorferi in patients with the human immunodeficiency virus. A diagnostic problem. Med. Clin. (Barc). 1993;101:207–209. [PubMed] [Google Scholar]
- 44.Blatz R, Kühn HJ, Hermann W, Rytter M, Rodloff AC. Neurosyphilis and neuroborreliosis. Retrospective evaluation of 22 cases. Nervenarzt. 2005;76:724–732. doi: 10.1007/s00115-004-1840-2. [DOI] [PubMed] [Google Scholar]
- 45.Stanek G, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011;17:69–79. doi: 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- 46.Hjetland R, Nilsen RM, Grude N, Ulvestad E. Seroprevalence of antibodies to Borrelia burgdorferi sensu lato in healthy adults from western Norway: risk factors and methodological aspects. APMIS. 2014;122:1114–1124. doi: 10.1111/apm.12267. [DOI] [PubMed] [Google Scholar]
- 47.Munro H, Mavin S, Duffy K, Evans R, Jarvis LM. Seroprevalence of lyme borreliosis in Scottish blood donors. Transfus. Med. 2015;25:284–286. doi: 10.1111/tme.12197. [DOI] [PubMed] [Google Scholar]
- 48.Sonnleitner ST, et al. Human seroprevalence against Borrelia burgdorferi sensu lato in two comparable regions of the eastern Alps is not correlated to vector infection rates. Ticks Tick Borne Dis. 2015;6:221–227. doi: 10.1016/j.ttbdis.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Wormser GP, et al. Yield of large-volume blood cultures in patients with early Lyme disease. J. Infect. Dis. 2001;184:1070–1072. doi: 10.1086/323424. [DOI] [PubMed] [Google Scholar]
- 50.Liveris D, et al. Quantitation of cell-associated borrelial DNA in the blood of Lyme disease patients with erythema migrans. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:791–795. doi: 10.1007/s10096-011-1376-x. [DOI] [PubMed] [Google Scholar]
- 51.Ginzburg Y, Kessler D, Kang S, Shaz B, Wormser GP. Why has Borrelia burgdorferi not been transmitted by blood transfusion? Transfusion. 2013;53:2822–2826. doi: 10.1111/trf.12116. [DOI] [PubMed] [Google Scholar]
- 52.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect. Dis. Clin. North Am. 2015;29:357–370. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Froberg MK, Dannen D, Bakken JS. Babesiosis and HIV. Lancet. 2004;363:704. doi: 10.1016/S0140-6736(04)15645-6. [DOI] [PubMed] [Google Scholar]
- 54.Vyas JM, Telford SR, Robbins GK. Treatment of refractory Babesia microti infection with atovaquone-proguanil in an HIV-infected patient: case report. Clin. Infect. Dis. 2007;45:1588–1590. doi: 10.1086/523731. [DOI] [PubMed] [Google Scholar]
- 55.Smotrys M, Magge T, Alkhuja S, Gandotra SD. Babesiosis as a cause of false-positive HIV serology. BMJ Case Rep. 2018;8:2018. doi: 10.1136/bcr-2017-223738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunfeld KP, et al. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. J. Clin. Microbiol. 2002;40:2431–2436. doi: 10.1128/JCM.40.7.2431-2436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chmielewska-Badora J, et al. Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp. and Babesia microti. Ann. Agric. Environ. Med. 2012;19:271–274. [PubMed] [Google Scholar]
- 58.Sonnleitner ST, et al. Risk assessment of transfusion-associated babesiosis in Tyrol: appraisal by seroepidemiology and polymerase chain reaction. Transfusion. 2014;54:1725–1732. doi: 10.1111/trf.12606. [DOI] [PubMed] [Google Scholar]
- 59.Gigandet L, et al. Prevalence of three zoonotic Babesia species in Ixodes ricinus (Linné, 1758) nymphs in a suburban forest in Switzerland. Vector Borne Zoonotic Dis. 2011;11:363–366. doi: 10.1089/vbz.2010.0195. [DOI] [PubMed] [Google Scholar]
- 60.Wójcik-Fatla A, Zając V, Sawczyn A, Cisak E, Dutkiewicz J. Babesia spp. in questing ticks from eastern Poland: prevalence and species diversity. Parasitol. Res. 2015;114:3111–3116. doi: 10.1007/s00436-015-4529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blaňarová L, et al. Presence of Candidatus Neoehrlichia mikurensis and Babesia microti in rodents and two tick species (Ixodes ricinus and Ixodes trianguliceps) in Slovakia. Ticks Tick Borne Dis. 2016;7:319–326. doi: 10.1016/j.ttbdis.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010;30:261–292. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Annen K, et al. Two cases of transfusion-transmitted Anaplasma phagocytophilum. Am. J. Clin. Pathol. 2012;137:562–565. doi: 10.1309/AJCP4E4VQQQOZIAQ. [DOI] [PubMed] [Google Scholar]
- 64.Sachdev SH, Joshi V, Cox ER, Amoroso A, Palekar S. Severe life-threatening Ehrlichia chaffeensis infections transmitted through solid organ transplantation. Transpl. Infect. Dis. 2014;16:119–124. doi: 10.1111/tid.12172. [DOI] [PubMed] [Google Scholar]
- 65.De Keukeleire M, et al. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Francisella tularensis infections in Belgium: results of three population-based samples. Vector Borne Zoonotic Dis. 2017;17:108–115. doi: 10.1089/vbz.2016.1954. [DOI] [PubMed] [Google Scholar]
- 66.Chochlakis D, Papaeustathiou A, Minadakis G, Psaroulaki A, Tselentis Y. A serosurvey of Anaplasma phagocytophilum in blood donors in Crete, Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27:473–475. doi: 10.1007/s10096-007-0457-3. [DOI] [PubMed] [Google Scholar]
- 67.Mączka I, Roguska U, Tylewska-Wierzbanowska S. Prevalence of rickettsioses in Poland in 2006–2012. Przegl. Epidemiol. 2013;67:633–6. [PubMed] [Google Scholar]
- 68.Paddock CD, et al. Fatal seronegative ehrlichiosis in a patient with HIV infection. N. Engl. J. Med. 1993;329:1164–1167. doi: 10.1056/NEJM199310143291605. [DOI] [PubMed] [Google Scholar]
- 69.Paddock CD, et al. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin. Infect. Dis. 2001;33:1586–1594. doi: 10.1086/323981. [DOI] [PubMed] [Google Scholar]
- 70.Talbot TR, Comer JA, Bloch KC. Ehrlichia chaffeensis infections among HIV-infected patients in a human monocytic ehrlichiosis-endemic area. Emerg. Infect. Dis. 2003;9:1123–1127. doi: 10.3201/eid0909.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker DH, Dumler JS. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch. Pathol. Lab. Med. 1997;121:785–791. [PubMed] [Google Scholar]
- 72.Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin. Infect. Dis. 2007;4:45–51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- 73.Magalhães RF, et al. Bartonella henselae survives after the storage period of red blood cell units: is it transmissible by transfusion? Transfus. Med. 2008;18:287–291. doi: 10.1111/j.1365-3148.2008.00871.x. [DOI] [PubMed] [Google Scholar]
- 74.Regnery RL, Childs JE, Koehler JE. Infections associated with Bartonella species in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 1995;21(S1):94–98. doi: 10.1093/clinids/21.Supplement_1.S94. [DOI] [PubMed] [Google Scholar]
- 75.Lamas CC, et al. Bartonella spp. infection in HIV positive individuals, their pets and ectoparasites in Rio de Janeiro, Brazil: serological and molecular study. Acta Trop. 2010;115:137–141. doi: 10.1016/j.actatropica.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 76.Chmielewski T, Podsiadły E, Tylewska-Wierzbanowska S. Presence of Bartonella spp. in various human populations. Pol. J. Microbiol. 2007;56:33–38. [PubMed] [Google Scholar]
- 77.Leibler JH, Zakhour CM, Gadhoke P, Gaeta JM. Zoonotic and vector-borne infections among urban homeless and marginalized people in the United States and Europe, 1990-2014. Vector Borne Zoonotic Dis. 2016;16:435–444. doi: 10.1089/vbz.2015.1863. [DOI] [PubMed] [Google Scholar]
- 78.Pape M, et al. Occurrence of Bartonella henselae and Bartonella quintana among human immunodeficiency virus-infected patients. Ann. N. Y. Acad. Sci. 2005;1063:299–301. doi: 10.1196/annals.1355.047. [DOI] [PubMed] [Google Scholar]
- 79.Pons I, et al. Seroprevalence of Bartonella spp. infection in HIV patients in Catalonia, Spain. BMC Infect. Dis. 2008;8:58. doi: 10.1186/1471-2334-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gasquet S, Maurin M, Brouqui P, Lepidi H, Raoult D. Bacillary angiomatosis in immunocompromised patients. AIDS. 1998;12:1793–1803. doi: 10.1097/00002030-199814000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Comer JA, Flynn C, Regnery RL, Vlahov D, Childs JE. Antibodies to Bartonella species in inner-city intravenous drug users in Baltimore, Md. Arch. Intern. Med. 1996;156:2491–2495. doi: 10.1001/archinte.1996.00440200111014. [DOI] [PubMed] [Google Scholar]
- 82.Zając V, Wójcik-Fatla A, Dutkiewicz J, Szymańska J. Bartonella henselae in eastern Poland: the relationship between tick infection rates and the serological response of individuals occupationally exposed to tick bites. J. Vector. Ecol. 2015;40:75–82. doi: 10.1111/jvec.12135. [DOI] [PubMed] [Google Scholar]
- 83.Faccini-Martínez ÁA, García-Álvarez L, Hidalgo M, Oteo JA. Syndromic classification of rickettsioses: an approach for clinical practice. Int. J. Infect. Dis. 2014;28:126–139. doi: 10.1016/j.ijid.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 84.Segura F, Antón E, Font B, Sala M, Cervantes M. Primary HIV type-1 infection misdiagnosed as Mediterranean spotted fever. Eur. J. Clin. Microbiol. Infect. Dis. 2002;21:478–479. doi: 10.1007/s10096-002-0750-0. [DOI] [PubMed] [Google Scholar]
- 85.Nogueras MM, Pons I, Sanfeliu I, Sala M, Segura F. Serosurvey of Rickettsia typhi and Rickettsia felis in HIV-infected patients. Microbiol. Immunol. 2014;58:257–259. doi: 10.1111/1348-0421.12138. [DOI] [PubMed] [Google Scholar]
- 86.Zając V, et al. Study on tick-borne rickettsiae in eastern Poland. II. Serological response of the occupationally exposed populations. Ann. Agric. Environ. Med. 2013;20:280–282. [PubMed] [Google Scholar]
- 87.Wójcik-Fatla A, et al. Study on tick-borne rickettsiae in eastern Poland. I. Prevalence in Dermacentor reticulatus (Acari: Amblyommidae) Ann. Agric. Environ. Med. 2013;20:276–279. [PubMed] [Google Scholar]
- 88.Mierzejewska EJ, Pawełczyk A, Radkowski M, Welc-Falęciak R, Bajer A. Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit. Vectors. 2015;8:490. doi: 10.1186/s13071-015-1099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blanco JR, et al. Seroepidemiology of Bartonella henselae infection in HIV-infected patients. Enferm. Infecc. Microbiol. Clin. 1999;17:434–438. [PubMed] [Google Scholar]
- 90.Dunn JM, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One. 2014;9:e115494. doi: 10.1371/journal.pone.0115494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wormser GP, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 92.Buller RS, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 93.Rességuier AS, et al. Preauricular lymphadenopathy related to Bartonella henselae. Rev. Med. Interne. 2013;34:770–772. doi: 10.1016/j.revmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 94.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005;84:162–173. doi: 10.1097/01.md.0000165658.82869.17. [DOI] [PubMed] [Google Scholar]
- 95.Pacheco YM, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res. 2015;117:69–74. doi: 10.1016/j.antiviral.2015.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


