Abstract
This paper presents a biodegradation study conducted for 90 days under standardized controlled composting conditions of poly (lactic acid) (PLA) filled with functionalized anatase-titania nanofiller (PLA/TiO2 nanocomposites). The surface morphology, thermal properties, percentage of biodegradation, and molecular weight changes at different incubation times were evaluated via visual inspection, scanning electron microscopy (SEM), X-ray diffraction (XRD), differential scanning calorimetry (DSC), and gel permeation chromatography (GPC) by taking degraded samples from compost at the end of target biodegradation time interval. The rapid increase of crystallinity indicated that the PLA and PLA/TiO2 nanocomposites had heterogeneous degradation mechanisms under controlled composting conditions. The biodegradation rate of PLA/TiO2 nanocomposites was higher than that of pure PLA because water molecules easily penetrated the nanocomposites. The dispersion of the nanoparticles in the PLA/TiO2 nanocomposites affected the biodegradation rate of PLA. Moreover, the biodegradation of PLA could be controlled by adding an amount of dispersed TiO2 nanofillers under controlled composting conditions.
Keywords: Biodegradation, PLA, Functionalized TiO2, Compost
Introduction
Poly (lactic acid) (PLA), a synthetic biodegradable polymer, is investigated worldwide for biomedical and consumer applications because of the increasing need for renewable materials that are sustainable alternatives to petrochemical-derived products [1–4]. PLA is the product that results from the polymerization of lactide or lactic acid, which is the most extensively produced carboxylic acid in nature by microbial fermentation of carbohydrates [5]. However, the applicability of PLA has been relatively limited because its heat distortion temperature, toughness, and degradation rate are unsatisfactory [6, 7]. One of the methods to resolve these drawbacks is to modify PLA by adding inorganic nanoparticles, including typical nanoclay, carbon nanotubes, zinc oxide, and anatase (A-TiO2) [8–15]. Recently, the PLA/TiO2 nanocomposites were prepared by us via melting blending PLA with chemically modified TiO2 (solution lactic acid grafted TiO2, hereafter referred to as g-TiO2) [16]. Results showed that TiO2 nanoparticles had a significant effect on the improvement of the mechanical properties of the PLA/TiO2 blends, such as strain at break and elasticity, compared with pure PLA. At the same time, g-TiO2 nanoparticles had a strong influence on hydrolytic degradation and photodegradation of PLA [17, 18].
The study of biodegradability and biodegradation mechanism of biodegradable materials using laboratory-scale test is an extremely important method from industrial and scientific point of view which provides understanding of the service life of these materials [15]. There are several methods currently available to assess the biodegradability of biodegradable materials, which are in general based on an indirect measurement, such as carbon dioxide production, biogas generation, or oxygen consumption [19, 20].
Biodegradation characteristics of PLA in compost have been studied and reported [21–23]. Composting is an accelerated biodegradation of organic materials in a warm, moist, and aerobic environment under a combination of microbial population and controlled composting conditions [24, 25]. Moreover, the biodegradation of PLA in composting conditions, a temperature- and humidity-dependent process, involves several processes, namely, water uptake, ester cleavage, and formation and dissolution of oligomer fragments [26]. The most accepted mechanism of the PLA biodegradation involves a two-step degradation process. Initially, the heat and moisture in the compost attack the PLA chains and split them apart, thereby producing small Mw polymers and, eventually, lactic acid. Thereafter, the microorganisms in the compost and soil mineralize the oligomer fragments and lactic acid to generate methane and carbon dioxide (CO2) under anaerobic and aerobic conditions, respectively [27–29].
Recently, the effect of fillers on the biodegradation of PLA has attracted great attention and particular attention has been focused on nanofillers, such as nanoclays, carbon nanotubes, and hydroxyapatite [23, 30–38]. Some authors [32–34] found out that adding nanoparticles could accelerate biodegradation of PLA, which was attributed to the high relative hydrophilicity of the nanoparticles, thereby enabling the easy permeability of water into the polymer matrix and triggering hydrolytic degradation. However, other studies [35–38] reported that biodegradation was retarded because of the enhanced barrier properties of the nanocomposites.
Although there have been some literatures focusing on the biodegradation of PLA materials, the role that TiO2 plays in PLA degradation remains controversial. How did the TiO2 nanoparticles affect the biodegradation of PLA was not clear. So, a study of the biodegradation of PLA, modified by TiO2 nanofillers under compost condition, is still needed. The current study, based on the estimation of the evolving CO2, assessed the biodegradation of PLA/TiO2 nanocomposites extensively under controlled laboratory compost conditions, a complement of degradability of the PLA/TiO2 nanocomposites under different degradation conditions, could extend PLA’s use in various end-use applications in the future.
Methods
Materials
PLA (manufactured by Natureworks@ (4032D)) exhibited a weight-average molecular weight (Mw) of 19,600 kDa and polydispersity of 1.89 as determined through gel permeation chromatography (GPC). PLA dried at 65 °C for 24 h under reduced pressure and stored in vacuum with humidity absorber before use. Lactic acid (88%, Guangshui National Chemical Co.) was distilled at 80 °C to remove water before use. The anatase titania nanoparticles, with an average primary particle size of ca. 20 nm, were supplied by Pangang Co., Ltd. Toluene and chloroform were used as received. Chromatographic grade microcrystalline cellulose was supplied by Shanghai Chemical Reagent Co., Ltd. The composting inoculums, which were obtained from an organic fraction of municipal solid waste (MSW), were supplied by the Degradable Plastics Professional Committee of the China Plastics Processing Industry Association (CPPIA).
Sample Preparation
Detailed information on the functionalization of the TiO2 nanoparticles and preparation of the PLA/TiO2 nanocomposites has been reported [16]. G-TiO2 nanofillers were prepared by grafting lactic acid oligomer onto anatase surface. PLA/TiO2 nanocomposites were prepared by melt blending via a corotating twin-screw extruder. Pure PLA was subjected to same mixing treatment so as to have the same thermal history as nanocomposites. The samples with 0, 0.5, 1.0, 2.0, 5.0, 8.0, and 15.0 wt% g-TiO2 were prepared and labeled as PLA, PLA/TiO2–0.5, PLA/TiO2–1, PLA/TiO2–2, PLA/TiO2–5, PLA/TiO2–8, and PLA/TiO2–15 nanocomposites.
Small chip specimens of PLA and g-TiO2 at different ratios were converted into sheets of approximately 0.5 mm in thickness by pressing at 190 °C for 4 min under 10 MPa followed by cooling at room temperature for 5 min under 5 MPa. Thereafter, the compression molded samples were cut into 5 mm × 5 mm size and weighed.
Degradation Tests
A biodegradation test was conducted in a laboratory scale-installation based on standard test methods designed for biodegradable plastics (GB/T19277–2003/ISO 14855-1:2005) (determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions—method by analysis of evolved CO2). Most of the carbon in the metabolized substrates generates energy through chemical transformation to CO2 in aerobic environments [39]. Therefore, measurements of the generation of CO2 are considered the most appropriate measure of biodegradation in most circumstances. The standard specifies a procedure to determine the ultimate aerobic biodegradability by measuring the amount of evolved CO2 and percentage of the biodegradation degree of the test materials under controlled composting conditions. The composting inoculum was obtained from an organic fraction of MSW, which was sieved to sizes under 5 mm. Thereafter, the fine fraction was used as inoculums. Table 1 shows the determined physicochemical properties of the composting inoculums. In each test, a series of composting reactors (each sample in triplicates) was loaded with 15 g of the reference material (i.e., microcrystalline cellulose (MCE), which was suggested by the standard) or test material (each film weighted and labeled before degradation), 85 g of inoculum, and 320 g of dry sea sand (provides good homogeneous conditions and an improved aerobic environment inside the inoculum). Thereafter, the reactors were placed in an incubator without light at 58 ± 2 °C through an experiment time of 90 days. Aeration was initiated using water-saturated CO2-free air; the flow rate through each reactor was set at 25 mL·min−1. The humidity, mixing, and aeration in all the reactors were controlled as established by the GB/T19277–2003/ISO 14855-12,005 requirements. At selected times, three to four specimens of each sample were selected, washed with distilled water, and dried at room temperature at least 24 h to constant weight.
Table 1.
Physicochemical properties of inoculums
| Properties | Value | Test method |
|---|---|---|
| pH | 7.2 | GB/T 19277–2003 ISO 14855-2005 |
| Total dry solid (TS) % | 71.3 | |
| Volatile solids (VS, % on TS) | 19.3 | |
| Residual ash content (RAC, % on TS) | 80.7 | |
| Moisturea (%) | 53.5 | |
| C/N ratio | 15 |
aPercentages are expressed on a dry weight loss
The CO2 that evolved during the biodegradation process was trapped in NaOH solutions and measured at regular intervals using titration method. The NaOH was titrated with standard HCl solution to the phenolphthalein endpoint. The total CO2 evolved during biodegradation was calculated with reference to the control flask. The data reported for each sample was the mean value obtained from three samples.
Characterization
Microscope Examination
Scanning electron microscopy (SEM) images were obtained using a Philips FEI INSPECT F instrument operated at 5 kV. All specimens were sputter coated with gold prior to analysis.
Thermal Analysis
Thermal properties of samples were studied by differential scanning calorimetry (DSC) (TA Q20, TA Instruments). Thermograms were obtained under nitrogen flow (50 mL/min) at a heating and cooling rates of 10 °C/min in the temperature range from room temperature to 200 °C and from 200 to − 50 °C, respectively.
XRD Studies
X-ray diffraction (XRD) analyses were performed using a DX-1000 X-ray diffractometer (Dandong Fanyuan Instrument Co. LTD. China) equipped with a Cu Kα (λ = 0.154 nm) source. The generator was operated at 25 mA and 40 kV. Samples were scanned at different angles (i.e., from 2 to 70°) at a scanning rate of 6°/min.
Determination of the Percent of Biodegradation (Dt, %)
Percent of biodegradation (Dt, %) could be calculated using Eq. 1, which was adopted as for Eq. 2 [1, 40].
| 1 |
where (CO2)T is the amount of CO2 (in g/flask) evolved from the test materials, (CO2)B is the amount of CO2 (in g/flask) evolved in the control flask, and ThCO2 is the theoretical CO2 amount produced by the polymeric materials.
The theoretical CO2 amount that can be produced in each flask (ThCO2, g2/g sample) was calculated using the following equation:
| 2 |
where MTOT is the total weight (g) of the dry polymeric solids material added into the composting flask at the start of the experiment, CTOT is the weight (g) of the total organic carbon in the total dry polymeric solids in the sample, and 44 and 12 are the molecular mass of CO2 and atomic mass of C, respectively.
Molecular Weight Measurement
The molecular weights of the PLA nanocomposites before and after composting were determined through GPC. The GPC system was equipped with a Waters 1515 Isocratic HPLC pump, a Waters 2414 refractive index detector, and Waters 717 plus autosampler. Chloroform was used as eluent at 0.8 mL/min flow rate at 30 °C. Calibration was accomplished with polystyrene standards.
Results and Discussion
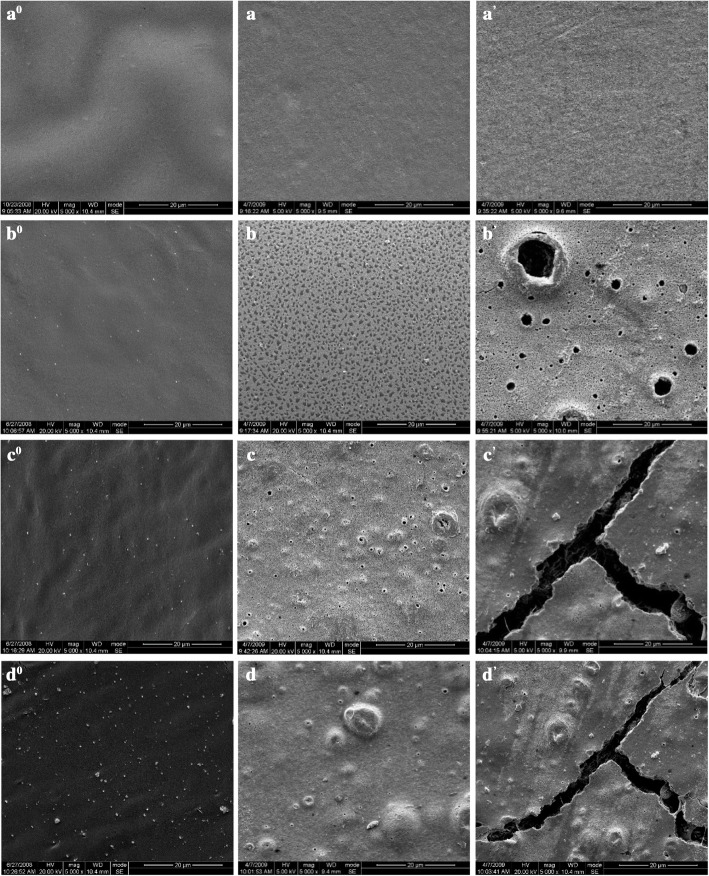
Polymer degradation is associated with changes in characteristics, such as color, surface morphology, and mechanical properties. The temporal changes in the appearance of the pure PLA and PLA/TiO2 nanocomposites were different under laboratory conditions. The pure PLA matrix surfaces, which were initially transparent in agreement with the amorphous structure, became relatively whitish after 2 days of biodegradation [41]. This feature increased with incubation time until complete opacity after 10 days. Yellowy to dark brown plaques, caused by water permeation and microorganisms incubation, began to be observed on the surface of neat PLA films surface after 30 days. However, large area of dark brown plaques emerged on the surface of PLA nanocomposite after 6 days (figure was not shown). The brown spots imply the microorganism colonies and the cracks represent the biodegradation effect. Figure 1 shows the surface morphology of PLA and its TiO2 nanocomposites under SEM observation. Before degradation, the surface of neat and PLA/TiO2 nanocomposites were smooth. Neat PLA did not present significant changes on the surface after biodegradation 5 days in compost conditions. After 20 days, the surface roughness of neat PLA increased (Fig. 1a, a’). However, PLA/TiO2 nanocomposite exhibited progressive changes clearly showing that considerable degradation of PLA/TiO2 composite occurred. Obvious cracks and voids (Fig. 1b, b’; c, c’; and d, d’; respectively) were observed on the surface of nanocomposites. This could be owned to the hydrolysis of PLA and microorganisms activities. With increasing incubation time, the cracks and voids became substantially deep and large (Fig. 1 b’, c’, and d’, respectively), thereby suggesting chain loss and surface erosion as time progresses. The bulk erosion phenomenon for all test materials was similar to the hydrolytic degradation process of the PLA and PLA/TiO2 nanocomposites [17].
Fig. 1.
SEM photography of the surface of pure PLA (a0, a, a’), PLA/TiO2–2 (b0, b, b’), PLA/TiO2–5 (c0, c, c’) and PLA/TiO2–8 (d0, d, d’) nanocomposites as a function of incubation time. a0, b0, c0, d0:0 day; a, b, c, d: 5 days; a’, b’, c’, d’: 20 days
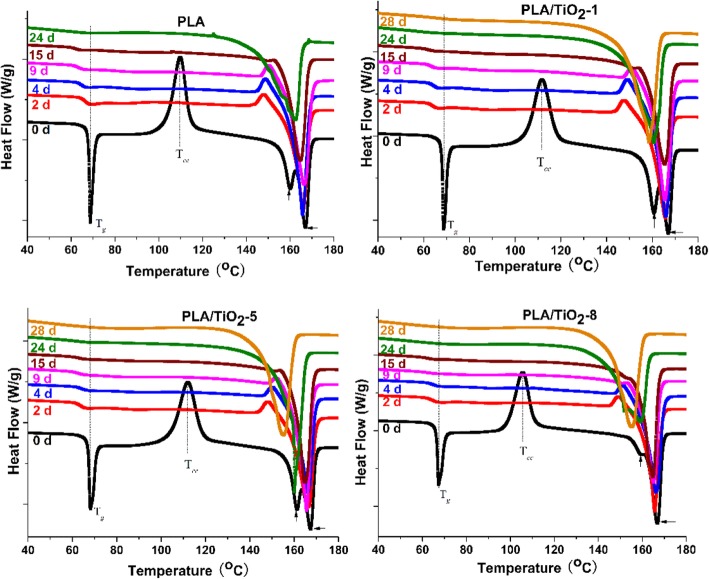
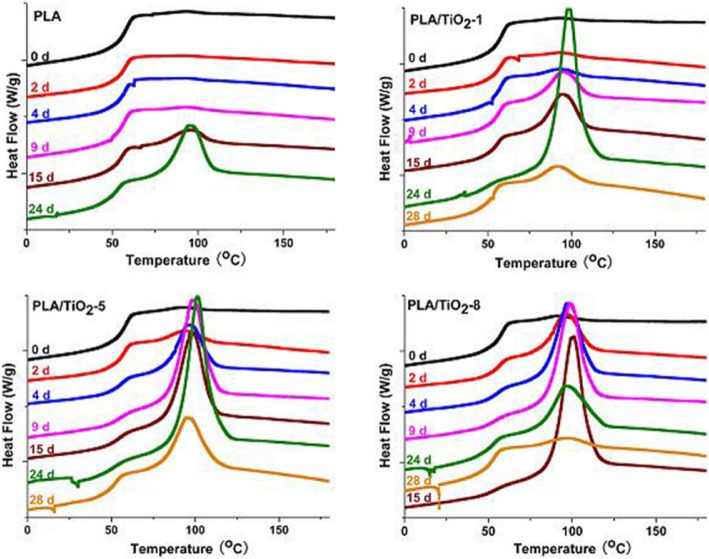
To evaluate the crystallinity of the PLA and PLA/TiO2 nanocomposites during biodegradation, samples selected at different incubation times were analyzed for their thermal properties (Figs. 2 and 3). Figure 2 shows that the glass transition temperature (Tg) decreased slightly for all samples with degradation time. The decrease of Tg was clearly due to an enhanced mobility of the molecules, as a consequence of the hydrolysis process and plasticizing effect of the oligomer fragments and water during biodegradation [33, 42]. The disappearance of cold crystallization peak (Tcc) for all of the samples only after 2 days could be ascribed to the hydrolysis of PLA and the rapid increase in crystallinity of the polymer matrix [43]. The decrease of Tm was ascribed to the rapid molecular mass reduction [44, 45]. The bimodal melting peak gradually changed to monomodal peak, thereby implying that the small and imperfect crystals disappeared with degradation time. This result proved that the degradation of PLA proceeded rapidly in the amorphous regions during the early stage of degradation under controlled composting conditions. The cooling scan (see Fig. 3) shows that the crystallization peak of the neat PLA increased gradually. However, the crystallization peaks of the PLA/TiO2 nanocomposites initially increased significantly and decreased slightly thereafter with an increase in incubation time. Moreover, the higher the nanofillers content was, the earlier the crystallization peaks arrived at its peak. The decrease of crystallization peak further verified that the crystalline region began to degrade after the degradation of the amorphous region. Giuliana and Roberto [42] reported that at short times for PLA sample some amorphous regions change into crystal, then the crystallinity degree increases due to the effect of the erosion of the amorphous parts. Moreover, the crystalline regions undergo hydrolysis at long times.
Fig. 2.
DSC thermograms of biodegradation products of pure PLA and PLA/TiO2 nanocomposites at different incubation times, first heating scan
Fig. 3.
DSC thermograms of biodegraded pure PLA and PLA/TiO2 nanocomposites at different incubation times, cooling scan
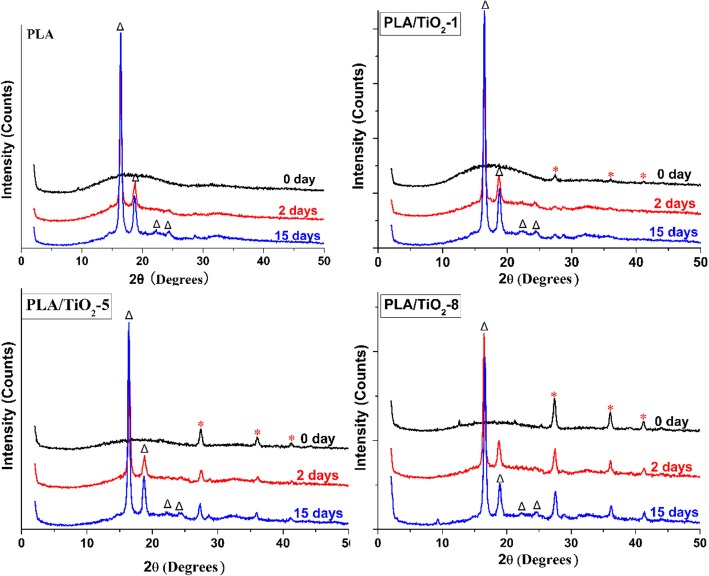
XRD provides an ideal method to monitor changes in the crystallization of polymers during degradation. The XRD patterns of PLA and its nanocomposites (Fig. 4) show that the polymer matrix maintains an amorphous structure before biodegradation. Only after 2 days, two strong peaks at 2θ = 16.4, 18.5°, 20.9°, and 23.6° clearly appeared and their intensity increased with incubation time. This result implied that poly (L-lactide) or poly (D-lactide)-type crystalline structures were formed [46, 47]. The change of crystalline peak indicated that amorphous regions degraded more rapidly than crystalline regions, which increased the crystalline-to-amorphous regions ratio value. This result was consistent with DSC results and the transparency change of the samples.
Fig. 4.
XRD patterns of pure PLA and PLA/TiO2 nanocomposites at different incubation times
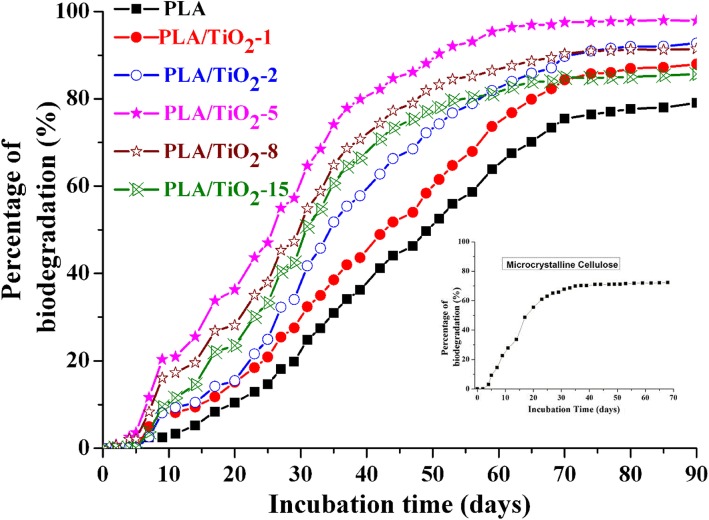
The evaluation of the inoculum validation is crucial during biodegradation under composting conditions. The activity of the inoculum was measured as required by the standard method: Dt of the reference material should be at least 70% at the end of the 45 days of testing. The insert in Fig. 5 shows that MCE begins to biodegrade after 5 days, and the percentage of biodegradation is up to 72% at the end of the 45 days of incubation. These result indicated that MCE in the experiment was effective as a reference material. In the experiment, duplicate composting vessels showed good reproducibility (standard deviation ± 1.3%). Figure 5 shows Dt for neat PLA and PLA/TiO2 nanocomposites during incubation. A similar behavior was observed for the PLA and PLA/TiO2 nanocomposites, that is, a lag phase was first observed which is then followed by a steep linear increase in biodegradation and thereafter by a plateau phase for all samples. The steepness of the increase should be indicative of increased degradation. However, the curves indicated that the lag phase of the nanocomposites was a little shorter than that of pure PLA. This result indicated the presence of TiO2, at some degree, accelerated the initial phase of degradation and increased the percentage of CO2 produced at the end of the incubation period. After 80 days of incubation under controlled composting conditions, Dt for PLA, PLA/TiO2–1, PLA/TiO2–2, PLA/TiO2–5, PLA/TiO2–8, and PLA/TiO2–15 reached up to 78.9, 86.9, 92.0, 97.8, 91.3, and 85.0%, respectively. Kunioka et al. [48] reported that the final biodegradability of PLA was 80%. The results from our experiment showed that Dt of the commercially pure PLA product was also nearly 80% at the end of 80 days. The decrease of Dt beginning from PLA/TiO2–8 is ascribed to the intense agglomeration of TiO2 when its content was beyond 8 wt% [16]. Further details are presented in the following section.
Fig. 5.
Percentage of biodegradation as a function of incubation time for pure PLA and PLA/TiO2 nanocomposites. The insert is the percentage of biodegradation as a function of time for microcrystalline cellulose
The different percentages of biodegradation are related to the different molecular weight changes of the polymer matrix. Figure 6 shows the molecular weight change of the samples as a function of incubation time. The curves show that the changes of Mn in the PLA/TiO2 nanocomposites were similar (i.e., a rapid decrease of Mn followed by a plateau phase of nearly a constant Mn) at least in the determined incubation time. To explore the degradation mechanisms caused by the addition of nanofillers, a model that accounts for autocatalysis by the generated carboxylic acid end groups was used to calculate the catalyzed degradation rate constant according to reference [17, 49]:
| 3 |
where k is catalyzed hydrolytic degradation rate constant, Mn0 is the number-average molecular weight before degradation, Mnt is the number-average molecular weight at any time.
Fig. 6.
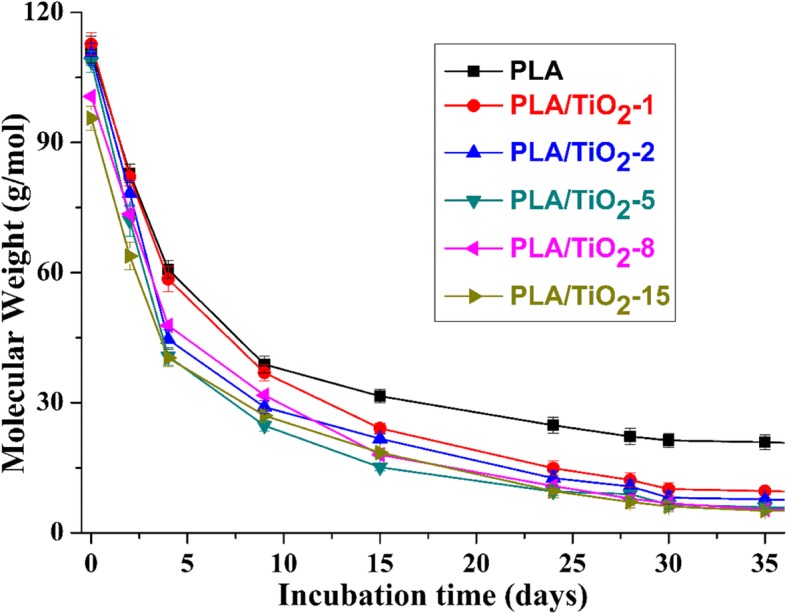
Change of Mn as a function of time for pure PLA and PLA/TiO2 nanocomposites
The k values evaluated by Eq. (3) are plotted in Fig. 7. From Fig. 7, the degradation rate of the PLA and PLA nanocomposites could be identified to two and three phases, respectively. Mn decreased rapidly during the first 8 days and followed by a plateau phase thereafter for neat PLA. For PLA/TiO2 nanocomposites, the highest values of k means that Mn decreased rapidly in the first phase (i.e., from 0 to 4 days). The following 5 to 24 days are ascribed to the second phase, and the values of k decreased slightly compared with the first phase. Few studies [13, 50] showed that the crystalline part of PLA was more resistant to degradation than the amorphous part; thus, the decrease in k in this phase could be caused by the increase of crystallinity of the PLA matrix. After 24 days (i.e., the last phase), the value of k decreased again. At this stage, the polymer completely degraded into oligomer fragments or lactic acid, and the microorganisms mineralized the remaining materials to continuously generate CO2.
Fig. 7.
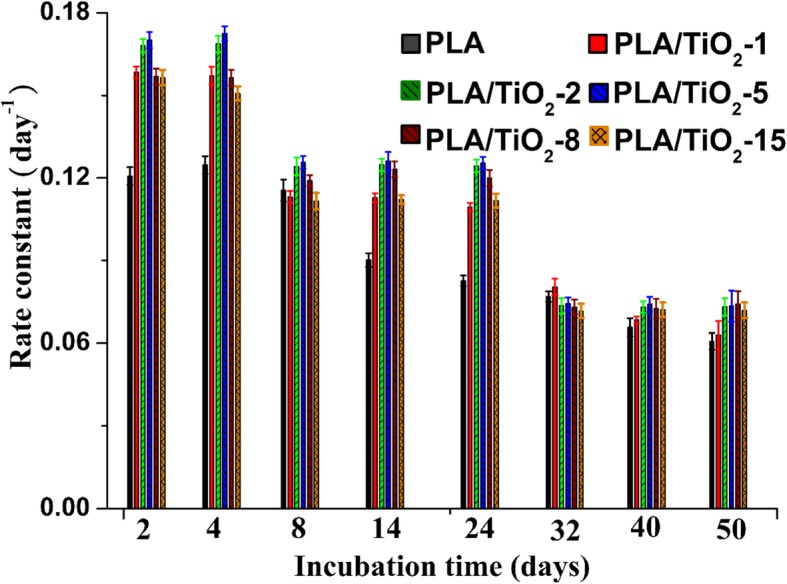
Biodegradation rate versus incubation time for pure PLA and PLA/TiO2 nanocomposites
Under composting conditions, the factors that affect the biodegradation tendency of PLA would control the degradation of the PLA/TiO2 nanocomposites. When an amount of g-TiO2 was homogeneously dispersed in the PLA matrix, the water molecules penetrated easily within the samples to trigger the degradation process [17]. Consequently, Mn decreased substantially in the first phase. The evolution of the lag phase of CO2 for PLA and its nanocomposites during this period indicated that microorganisms need suitable polymer chains to mineralize. With increased incubation time, the polymer chains in amorphous regions degraded and the number of amorphous regions decreased; thus, the percentage of crystalline to the amorphous region (i.e., χc) increased [39], thereby leading to the decrease of k in the second phase. However, the oligomer fragments began to be mineralized by microorganisms in this stage, thereby indicating that the productive phase for the PLA mineralization occurred. With the decrease of the remaining oligomer fragments and the increase of χc, k and Dt decreased and a nearly long plateau phase was observed for k and Dt in the third stage. In our previous study [16], the morphology of each nanocomposite was reported and determined through SEM and TEM; the results showed that the dispersion of g-TiO2 with under 5 wt% in the PLA/TiO2 nanocomposites was better than that obtained with a high concentration of nanofillers. In terms of the dispersion and content of TiO2, PLA/TiO2–5 had the largest k and Dt compared with the other nanocomposites in our experiment.
Conclusions
PLA/TiO2 nanocomposites were prepared (based on PLA and functionalized g-TiO2) and subjected to biodegradation under controlled composting conditions. Using such a standard, the information of patterns on the surface of the samples and the rapid increase of crystallinity indicated that the PLA and PLA/TiO2 nanocomposites had heterogeneous biodegradation mechanisms. The degradation study of nanocomposites under composting conditions showed that the inherent degradable character of PLA remained after the incorporation of functionalized titania nanoparticles (PLA/TiO2). The addition of the TiO2 nanoparticles increased the degradation rate of the PLA matrix because the water molecules easily penetrated the PLA/TiO2 nanocomposites, thereby activating the degradation process. This phenomenon was particularly evident for PLA/TiO2–5 because of its high TiO2 content and good dispersion of TiO2 nanofillers in the PLA matrix compared with other nanocomposites.
Acknowledgements
This work was supported by the Key projects of social science planning in Sichuan Province SC18A013 and Sichuan University Research Cluster for Regional History and Frontier Studies.
Funding
The authors gratefully acknowledge the financial support from High Level Research Team Building Plan of Social Sciences in Sichuan Province (Sichuan Federation of Social Science Association [2017] 43–2) and Advanced Interdisciplinary Innovation Research Project of Sichuan University (skqy201216).
Availability of data and materials
All the data are fully available without restriction.
Abbreviations
- DSC
Differential scanning calorimetry
- Dt
Percent of biodegradation
- GPC
Gel permeation chromatography
- g-TiO2
Grafted TiO2
- MCE
Microcrystalline cellulose
- Mn
Number-average molecular weight
- Mw
Weight-average molecular weight
- PLA
Poly (lactic acid)
- SEM
Scanning electron microscopy
- Tcc
Cold crystallization peak
- Tg
Glass transition temperature
- XRD
X-ray diffraction
Authors’ contributions
LYB contributed to the experimental work, data analysis, and was a major contributor in writing the manuscript. LZC contributed to the experimental work and data analysis. GG analyzed and interpreted the data. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yanbing Luo, Email: luoybs@126.com.
Zicong Lin, Email: lzc33cd@163.com.
Gang Guo, Email: guogang@scu.edu.cn.
References
- 1.Castro-Aguirre E, Iňiguez-Franco F, Samsudin H, Fang X, Auras R. Poly (lactic acid)—mass production, processing, industrial applications, and end of life. Adv Drug Deliv Rev. 2016;107:333–366. doi: 10.1016/j.addr.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Cadar O, Paul M, Roman C, Miclean M, Majdik C. Biodegradation behavior of poly (lactic acid) and (lactic acid-ethylene glycol-malonic or succinic acid) copolymers under controlled composting conditions in a laboratory test system. Polym Degrad and Stab. 2012;97(3):354–357. doi: 10.1016/j.polymdegradstab.2011.12.006. [DOI] [Google Scholar]
- 3.Deroiné M, Le Duigou A, Corre YM, Le Gac PY, Davies P, César G, Bruzaud S. Accelerated ageing of polylactide in aqueous environments: comparative study between distilled water and seawater. Polym Degrad Stab. 2014;108:319–329. doi: 10.1016/j.polymdegradstab.2014.01.020. [DOI] [Google Scholar]
- 4.Pantani R, Sorrentino A. Influence of crystallinity on the biodegradation rate of injection-moulded poly (lactic acid) samples in controlled composting conditions. Polym Degrad Stab. 2013;98:1089–1096. doi: 10.1016/j.polymdegradstab.2013.01.005. [DOI] [Google Scholar]
- 5.Murariu M, Dubois P. PLA composites: from production to properties. Adv Drug Deliv Rev. 2016;107:17–46. doi: 10.1016/j.addr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Sinha RP, Pandey JK, Rutot D, Degeé P, Dubois P. Biodegradation of poly (3-caprolactone)/starch blends and composites in composting and culture environment: the effect of compatibilization on the inherent biodegradability of the host polymer. Carbohydr Res. 2003;338(17):1759–1769. doi: 10.1016/S0008-6215(03)00236-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhou YH, Lei L, Yang B, Li JB, Ren J. Preparation and characterization of polylactic acid (PLA) carbon nanotube nanocomposites. Polym Test. 2018;68:34–38. doi: 10.1016/j.polymertesting.2018.03.044. [DOI] [Google Scholar]
- 8.Cele HM, Ojijo V, Chen H, Kumar S, Land K, Joubert T, de Villiers MFR, Ray SS. Effect of nanoclay on optical properties of PLA/clay composite films. Polym Test. 2014;36:24–31. doi: 10.1016/j.polymertesting.2014.03.010. [DOI] [Google Scholar]
- 9.Hakim RH, Cailloux J, Santana OO, Bou J, Sábchez-Soto M, Odent J, Raquez JM, Dubios P, Carrasco F, Maspoch MLI (2017) PLA/SiO2 composites: influence of the filler modifications on the morphology, crystallization behavior, and mechanical properties. J Appl Polym Sci 134. 10.1002/app.45367
- 10.Costa RGF, Brichi GS, Ribeiro C, Mattoso LHC. Nanocomposite fibers of poly (lactic acid)/titanium dioxide prepared by solution blow spinning. Polym Bull. 2016;73(11):1–13. doi: 10.1007/s00289-016-1635-1. [DOI] [Google Scholar]
- 11.Wu JH, Kuo MC, Chen CW (2015) Physical properties and crystallization behavior of poly (lactide)/poly (methyl methacrylate)/silica composites. J Appl Polym Sci 132. 10.1002/app.42378
- 12.Ramos M, Fortunati E, Peltzer M, Jimenez A, Kenny JM, Garrigós MC. Characterization and disintegrability under composting conditions of PLA-based nanocomposite films with thymol and silver nanoparticles. Polym Degrad Stab. 2016;132:2–10. doi: 10.1016/j.polymdegradstab.2016.05.015. [DOI] [Google Scholar]
- 13.Raquez JM, Habibi Y, Murariu M, Dubois P. Polylactide (PLA)-based nanocomposites. Prog Polym Sci. 2013;38(10):1504–1542. doi: 10.1016/j.progpolymsci.2013.05.014. [DOI] [Google Scholar]
- 14.González A, Dasari A, Herrero B, Plancher E, Santarén J, Esteban A, Lim SH. Fire retardancy behavior of PLA based nanocomposites. Polym Degrad Stab. 2012;97(3):248–256. doi: 10.1016/j.polymdegradstab.2011.12.021. [DOI] [Google Scholar]
- 15.Murariu M, Doumbia A, Bonnaud L, Dechief AL, Paint Y, Ferreira M, Campagne C, Devaux E, Dubois P. High-performance polylactide/ZnO nanocomposites designed for films and fibers with special end-use properties. Biomacromolecules. 2011;12(5):1762–1771. doi: 10.1021/bm2001445. [DOI] [PubMed] [Google Scholar]
- 16.Luo YB, Li WD, Wang XL, Xu DY, Wang YZ. Preparation and properties of nanocomposites based on poly (lactic acid) and functionalized TiO2. Acta Mater. 2009;57(11):3182–3191. doi: 10.1016/j.actamat.2009.03.022. [DOI] [Google Scholar]
- 17.Luo YB, Wang XL, Wang YZ. Effect of TiO2 nanoparticles on the long-term hydrolytic degradation behavior of PLA. Polym Degrad Stab. 2012;97:721–728. doi: 10.1016/j.polymdegradstab.2012.02.011. [DOI] [Google Scholar]
- 18.Luo YB, Cao YZ, Guo G. Effects of TiO2 nanoparticles on the photodegradation of poly (lactic acid) J Appl Polym Sci. 2018;135:1–8. [Google Scholar]
- 19.Pagga U. Testing biodegradability with standardized methods. Chemosphere. 1997;35(12):2953–2972. doi: 10.1016/S0045-6535(97)00262-2. [DOI] [PubMed] [Google Scholar]
- 20.Massardier-Nageotte V, Pestre C, Cruard-Pradet T, Bayard R. Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym Degrad Stab. 2006;91(3):620–627. doi: 10.1016/j.polymdegradstab.2005.02.029. [DOI] [Google Scholar]
- 21.Sedničková M, Pekařová S, Kucharczyk P, Bočkaj J, Janigová I. Changes of physical properties of PLA-based blends during early stage of biodegradation in compost. Int J Biol Macromol. 2018;113:434–442. doi: 10.1016/j.ijbiomac.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 22.Ho KLG, Pometto AL, Gadea A, Briceño JA, Rojas A. Degradation of polylactic acid (PLA) plastic in Costa Rican soil and Iowa state university compost rows. J Environ Polym Degrad. 1999;7(4):173–177. doi: 10.1023/A:1022874530586. [DOI] [Google Scholar]
- 23.Tsuji H, Mizuno A, Ikada Y. Blends of aliphatic polyesters. III. Biodegradation of solution-cast blends from poly (L-lactide) and poly (3-caprolactone) J Appl Polym Sci. 1998;70(11):2259–2268. doi: 10.1002/(SICI)1097-4628(19981212)70:11<2259::AID-APP20>3.0.CO;2-J. [DOI] [Google Scholar]
- 24.Ghorpade VM, Gennadios A, Hanna MA. Laboratory composting of extruded poly (lactic acid) sheets. Bioresour Technol. 2001;76(1):57–61. doi: 10.1016/S0960-8524(00)00077-8. [DOI] [PubMed] [Google Scholar]
- 25.Nampoothiri KM, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresour Technol. 2010;101(22):8493–8501. doi: 10.1016/j.biortech.2010.05.092. [DOI] [PubMed] [Google Scholar]
- 26.Göpferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17(2):103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 27.Hakkarainen M. Aliphatic polyesters: abiotic and biotic degradation and degradation products. Adv Polym Sci. 2002;157:113–138. doi: 10.1007/3-540-45734-8_4. [DOI] [Google Scholar]
- 28.Longieras A, Tanchette JB, Erre D, Braud C, Copinet A. Compostability of poly (lactide): degradation in an inert solid medium. J Polym Environ. 2007;15(3):200–206. doi: 10.1007/s10924-007-0061-8. [DOI] [Google Scholar]
- 29.Saadi Z, Rasmont A, Cesar G, Bewa H, Benguigui L. Fungal degradation of poly(l-lactide) in soil and in compost. J Polym Environ. 2012;20(2):273–282. doi: 10.1007/s10924-011-0399-9. [DOI] [Google Scholar]
- 30.Castro-Aguirre E, Auras R, Selke S, Rubino M, Marsh T. Enhancing the biodegradation rate of poly (lactic acid) films and pla bio-nanocomposites in simulated composting through bioaugmentation. Polym Degrad Stab. 2018;154:46–54. doi: 10.1016/j.polymdegradstab.2018.05.017. [DOI] [Google Scholar]
- 31.Brzezinski M, Biela T. Polylactide nanocomposites with functionalized carbon nanotubes and their stereocomplexes: a focused review. Mater Lett. 2014;121:244–250. doi: 10.1016/j.matlet.2014.01.159. [DOI] [Google Scholar]
- 32.Lee SR, Park HM, Lim H, Kang T, Li X, Cho WJ, et al. Microstructure, tensile properties, and biodegradability of aliphatic polyester/clay nanocomposites. Polymer. 2002;43(8):2495–2500. doi: 10.1016/S0032-3861(02)00012-5. [DOI] [Google Scholar]
- 33.Paul MA, Delcourt C, Alexandre M, Degée P, Monteverde F, Dubois P. Polylactide/ montmorillonite nanocomposites: study of the hydrolytic degradation. Polym Degrad Stab. 2005;87(3):535–542. doi: 10.1016/j.polymdegradstab.2004.10.011. [DOI] [Google Scholar]
- 34.Fukushima K, Abbate C, Tabuani D, Gennari M, Camino G. Biodegradation of poly (lactic acid) and its nanocomposites. Polym Degrad Stab. 2009;94(10):1646–1655. doi: 10.1016/j.polymdegradstab.2009.07.001. [DOI] [Google Scholar]
- 35.Someya Y, Kondo N, Shibata M. Biodegradation of poly (butylene adipate-co-butylene terephthalate)/layered-silicate nanocomposites. J Appl Polym Sci. 2007;106(2):730–736. doi: 10.1002/app.24174. [DOI] [Google Scholar]
- 36.Wu TM, Wu CY. Biodegradable poly (lactic acid)/chitosan-modified montmorillonite nanocomposites: preparation and characterization. Polym Degrad Stab. 2006;91(9):2198–2204. doi: 10.1016/j.polymdegradstab.2006.01.004. [DOI] [Google Scholar]
- 37.Wu KJ, Wu CS, Chang JS. Biodegradability and mechanical properties of polycaprolactone composites encapsulating phosphate-solubilizing bacterium Bacillus sp. PG01. Process Biochem. 2007;42(4):669–675. doi: 10.1016/j.procbio.2006.12.009. [DOI] [Google Scholar]
- 38.Fukushima K, Abbate C, Tabuani D, Gennari M, Rizzarelli P, Camino G. Biodegradation trend of poly(ε-caprolactone) and nanocomposites. Mater Sci Eng C. 2010;30(4):566–574. doi: 10.1016/j.msec.2010.02.012. [DOI] [Google Scholar]
- 39.Selke S, Auras R, Nguyen TA, Aguirre EC, Cheruvathur R, Liu Y. Evaluation of biodegradation-promoting additives for plastics. Environ Sci Technol. 2015;49:3769–3777. doi: 10.1021/es504258u. [DOI] [PubMed] [Google Scholar]
- 40.Kim MC, Masuoda T. Degradation properties of PLA and PHBV films treated with CO2-plasma. React Funct Polym. 2009;69(5):287–292. doi: 10.1016/j.reactfunctpolym.2009.01.013. [DOI] [Google Scholar]
- 41.Lv SS, Zhang YH, Gu JY, Tan HY. Physicochemical evolutions of starch/poly (lactic acid) composite biodegraded in real soil. J Environ Manag. 2018;228:223–231. doi: 10.1016/j.jenvman.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Giuliana G, Roberto P. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: assessment of structural modification and kinetic parameters. Polym Degrad Stab. 2013;98(5):1006–1014. doi: 10.1016/j.polymdegradstab.2013.02.005. [DOI] [Google Scholar]
- 43.Ebadi-Dehaghani H, Barikani M, Borhani S, Bolvardi B, Khonakdar HA, Jafari SH. Biodegradation and hydrolysis studies on polypropylene/polylactide/organo-clay nanocomposites. Polym Bull. 2016;73(12):3287–3304. doi: 10.1007/s00289-016-1655-x. [DOI] [Google Scholar]
- 44.Ratto JA, Stenhouse PJ, Auerbach M, Mitchell J, Farrell R. Processing, performance and biodegradability of a thermoplastic aliphatic polyester/starch system. Polymer. 1999;40(24):6777–6788. doi: 10.1016/S0032-3861(99)00014-2. [DOI] [Google Scholar]
- 45.Hakkarainen M, Karlsson S, Albertsson AC. Rapid (bio) degradation of polylactide by mixed culture of compost microorganisms-low molecular weight products and matrix changes. Polymer. 2000;41(7):2331–2338. doi: 10.1016/S0032-3861(99)00393-6. [DOI] [Google Scholar]
- 46.Li S, McCarthy S. Further investigations on the hydrolytic degradation of poly (DL-lactide) Biomaterials. 1999;20(1):35–44. doi: 10.1016/S0142-9612(97)00226-3. [DOI] [PubMed] [Google Scholar]
- 47.von Recum HA, Cleek RL, Eskin SG, Mikos AG. Degradation of polydispersed poly (L-lactic acid) to modulate lactic acid release. Biomaterials. 1995;16(6):441–447. doi: 10.1016/0142-9612(95)98816-W. [DOI] [PubMed] [Google Scholar]
- 48.Kunioka M, Ninomiya F, Funabashi M. Biodegradation of poly (lactic acid) powders proposed as the reference test materials for the international standard of biodegradation evaluation methods. Polym Degrad Stab. 2006;91(9):1919–1928. doi: 10.1016/j.polymdegradstab.2006.03.003. [DOI] [Google Scholar]
- 49.Biodegradation of poly (lactic acid) powders proposed as the reference test materials for the international standard of biodegradation evaluation methods Polym Degrad Stab. 2006;91(9):1919–1928. doi: 10.1016/j.polymdegradstab.2006.03.003. [DOI] [Google Scholar]
- 50.Tsuji H, Ikada Y. Properties and morphology of poly (L-lactide) 4. Effects of structural parameters on long-term hydrolysis of poly (L-lactide) in phosphate-buffered solution. Polym Degrad Stab. 2000;67(1):179–189. doi: 10.1016/S0141-3910(99)00111-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are fully available without restriction.