Abstract
Key points
We sought to determine the isolated and combined influence of hypovolaemia and hypoxic pulmonary vasoconstriction on the decrease in left ventricular (LV) function and maximal exercise capacity observed under hypobaric hypoxia.
We performed echocardiography and maximal exercise tests at sea level (344 m), and following 5–10 days at the Barcroft Laboratory (3800 m; White Mountain, California) with and without (i) plasma volume expansion to sea level values and (ii) administration of the pulmonary vasodilatator sildenafil in a double‐blinded and placebo‐controlled trial.
The high altitude‐induced reduction in LV filling and ejection was abolished by plasma volume expansion but to a lesser extent by sildenafil administration; however, neither intervention had a positive effect on maximal exercise capacity.
Both hypovolaemia and hypoxic pulmonary vasoconstriction play a role in the reduction of LV filling at 3800 m, but the increase in LV filling does not influence exercise capacity at this moderate altitude.
Abstract
We aimed to determine the isolated and combined contribution of hypovolaemia and hypoxic pulmonary vasoconstriction in limiting left ventricular (LV) function and exercise capacity under chronic hypoxaemia at high altitude. In a double‐blinded, randomised and placebo‐controlled design, 12 healthy participants underwent echocardiography at rest and during submaximal exercise before completing a maximal test to exhaustion at sea level (SL; 344 m) and after 5–10 days at 3800 m. Plasma volume was normalised to SL values, and hypoxic pulmonary vasoconstriction was reversed by administration of sildenafil (50 mg) to create four unique experimental conditions that were compared with SL values: high altitude (HA), Plasma Volume Expansion (HA‐PVX), Sildenafil (HA‐SIL) and Plasma Volume Expansion with Sildenafil (HA‐PVX‐SIL). High altitude exposure reduced plasma volume by 11% (P < 0.01) and increased pulmonary artery systolic pressure (19.6 ± 4.3 vs. 26.0 ± 5.4, P < 0.001); these differences were abolished by PVX and SIL respectively. LV end‐diastolic volume (EDV) and stroke volume (SV) were decreased upon ascent to high altitude, but were comparable to sea level in the HA‐PVX trial. LV EDV and SV were also elevated in the HA‐SIL and HA‐PVX‐SIL trials compared to HA, but to a lesser extent. Neither PVX nor SIL had a significant effect on the LV EDV and SV response to exercise, or the maximal oxygen consumption or peak power output. In summary, at 3800 m both hypovolaemia and hypoxic pulmonary vasoconstriction contribute to the decrease in LV filling, but restoring LV filling does not confer an improvement in maximal exercise performance.
Keywords: hypoxia, high altitude, cardiac function, left ventricular mechanics, hypovolemia, pulmonary vasoconstriction, pulmonary hypertension, diastolic function
Key points
We sought to determine the isolated and combined influence of hypovolaemia and hypoxic pulmonary vasoconstriction on the decrease in left ventricular (LV) function and maximal exercise capacity observed under hypobaric hypoxia.
We performed echocardiography and maximal exercise tests at sea level (344 m), and following 5–10 days at the Barcroft Laboratory (3800 m; White Mountain, California) with and without (i) plasma volume expansion to sea level values and (ii) administration of the pulmonary vasodilatator sildenafil in a double‐blinded and placebo‐controlled trial.
The high altitude‐induced reduction in LV filling and ejection was abolished by plasma volume expansion but to a lesser extent by sildenafil administration; however, neither intervention had a positive effect on maximal exercise capacity.
Both hypovolaemia and hypoxic pulmonary vasoconstriction play a role in the reduction of LV filling at 3800 m, but the increase in LV filling does not influence exercise capacity at this moderate altitude.
Introduction
Following acclimatisation to high altitude, cardiac output at rest and during exercise is comparable to that at sea level, but is achieved through an increased heart rate and a lower stoke volume (Sutton et al. 1988). The reduction in stroke volume (SV) is accompanied by a decrease in left ventricular (LV) filling (Alexander & Grover, 1983; Boussuges et al. 2000; Stembridge et al. 2015). Two mechanisms have been proposed to explain this reduction in SV: (i) decreased blood volume (Alexander et al. 1967; Grover et al. 1976) and (ii) increased pulmonary vascular resistance (PVR) (Allemann et al. 2004; Naeije et al. 2010). A reduction in plasma volume with acclimatisation to high altitude has been consistently reported in the literature over the last 50 years (Pugh, 1964; Alexander et al. 1967; Robach et al. 2000; Ryan et al. 2014), but there is no consensus as to whether the decrease in LV filling is a direct consequence of hypovolaemia. For example, Alexander et al. (1967) observed a reduction in both blood volume and directly measured ventricular filling pressures, but a dextran infusion restored SV in only one of the two subjects tested despite the restoration in right atrial pressure in both. Moreover, Calbet et al. (2004) reported no change in SV and an increase in heart rate following plasma volume expansion after 9 weeks at 5260 m. In contrast, Grover et al. (1976) demonstrated a maintained SV during acclimatisation when subjects (n = 5) were kept isovolaemic by preventing alkalosis via CO2 supplementation, and Siebenmann et al. (2013) restored SV (as estimated by pulse contour analysis) following normalisation of plasma volume via dextran infusion.
The second candidate mechanism for the lower LV filling at high altitude is the increase in PVR secondary to hypoxic pulmonary vasoconstriction. In response to hypoxia, the pulmonary vasculature constricts and creates a greater load against which the right ventricle (RV) must contract (Naeije & Dedobbeleer, 2013). During acute hypoxia, pulmonary pressure is increased, RV systolic function is elevated and LV end‐diastolic volume (EDV) is maintained. Following acclimatisation, pulmonary pressure remains elevated but RV systolic function is slightly decreased (Stembridge et al. 2014) and LV EDV is lower. Indeed, pulmonary artery systolic pressure has been shown to be inversely related to LV diastolic filling at high altitude (Allemann et al. 2004).
Changes in plasma volume and PVR in response to hypoxia have also been investigated as potential limiting factors to maximal exercise performance at high altitude. Pharmacological reversal of hypoxic pulmonary vasoconstriction has been shown in multiple studies to improve maximal exercise performance in acute and chronic hypoxia (Ghofrani et al. 2004; Faoro et al. 2009; Naeije et al. 2010). Plasma volume expansion has been show to improve at high altitude (Robach et al. 2000), although this is not a consistent finding (Calbet et al. 2004). Theoretical analysis has demonstrated that the importance of cardiac output in oxygen transport diminishes with increasing altitude, such that on the summit of Mount Everest (barometric pressure = 253 Torr), is independent of maximal cardiac output (Wagner, 1996). However, at moderate altitudes, any improvement in maximal exercise performance with either plasma volume expansion or pulmonary vasodilatation may be underpinned by changes in cardiac function that are evident during submaximal intensities (Hsu et al. 2006; Kjaergaard et al. 2007). Importantly, however, both interventions may alter arterial oxygen content at maximal exercise via either haemodilution with plasma volume expansion (Duke et al. 2016), or increased diffusion limitation with shorter pulmonary transit times (Wagner, 1996).
Collectively, the available evidence suggests that changes in blood volume and PVR may affect SV and exercise capacity at high altitude either independently or in combination, although this remains to be experimentally determined. The aims of this study are therefore fourfold: to determine the independent and combined effect of high altitude‐induced (i) hypovolaemia and (ii) hypoxic pulmonary vasoconstriction on LV filling and the resultant changes in ventricular function, (iii) to examine how hypovolaemia and hypoxic pulmonary vasoconstriction influence the left and right ventricular response to submaximal exercise and (iv) to explore the potential role of plasma volume normalisation and the reversal of pulmonary vasoconstriction to improve maximal exercise at high altitude. We hypothesised that (i) both plasma volume normalisation and (ii) reversal of hypoxic pulmonary vasoconstriction would increase LV filling at rest and (iii) these responses would be evident during exercise, but (iv) only the reversal of hypoxic pulmonary vasoconstriction would increase maximal exercise performance.
Methods
Ethical approval
Ethical approval was granted by the Clinical Research Ethics Board at the University of British Columbia and conformed to the Declaration of Helsinki, except for registration in a database. Written informed consent was obtained from all volunteers prior to participation in the study. The current study was a standalone experiment that formed part of a high altitude expedition to the Barcroft Research Station (White Mountain, CA, USA; 3800 m). As such, the participants took part in several investigations, although care was taken to ensure no overlap existed between any of the studies performed, and each study addressed a distinct a priori research question.
Participants
Twelve male participants (aged 27 ± 6 years, height 178 ± 4 cm and weight 73 ± 7 kg) were recruited from the expedition team who were normotensive, non‐smokers with no previous history of cardiovascular or respiratory diseases and were taking no prescription medications. Lung function, lung volume and lung diffusing capacity were assessed at baseline in accordance with the American Thoracic Society and European Respiratory Society's joint guidelines (Macintyre et al. 2005; Miller et al. 2005) as previously reported by our group (Tymko et al. 2017). The participants completed baseline trials at sea level (Kelowna, British Columbia, Canada; 344 m) before travelling to California and ascending to 3800 m over the course of 9–10 h by motor vehicle. The high altitude phase of testing was performed between days 5 and 10 after arrival at 3800 m. None of the participants were experiencing any symptoms of acute mountain sickness, as assessed by the Lake Louise questionnaire.
Experimental design
The two phases of testing required three laboratory visits at sea level and three visits at high altitude. Prior to each visit, participants were asked to abstain from exercise, alcohol and caffeine for at least 12 h. The experimental design allowed the examination of cardiac function at rest and during exercise, at sea level (SL) and high altitude (HA) with and without the administration of the pulmonary vasodilator sildenafil (SIL), before and after plasma volume expansion (PVX) to return plasma volume to sea level values. This generated five experimental conditions: Sea Level (SL), High Altitude (HA), High Altitude with Plasma Volume Expansion (HA‐PVX), High Altitude with Sildenafil (HA‐SIL) and High Altitude with both Plasma Volume Expansion and Sildenafil (HA‐PVX‐SIL). In addition, maximal aerobic capacity was assessed in four conditions: Sea Level (SL), High Altitude (HA) and High Altitude with both Plasma Volume Expansion (HA‐PVX) and Sildenafil (HA‐PVX‐SIL). An overview of the experimental design is illustrated in Fig. 1.
Figure 1.
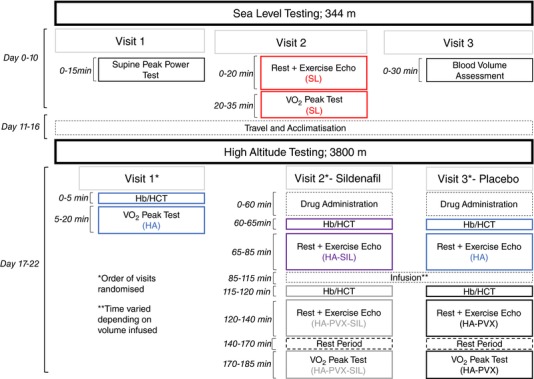
Overview of the experimental design for both the sea level and high altitude protocols
Sea level experimental visits
At sea level, participants completed an incremental test to exhaustion on a semi‐recumbent exercise ergometer (Lode Angio, Lode, Groningen, the Netherlands; see ‘Experimental protocols’ below for full details). The aim of this test was to establish each individual's peak power so that relative intensities could be prescribed in the subsequent visits. All exercise echocardiograms at sea level and high altitude were performed at 50% of the power achieved during this visit in the semi‐recumbent position. During the second visit, echocardiography was performed during rest and at 50% peak power. After 30 min of rest, the participants then completed another incremental test to exhaustion to assess maximal oxygen consumption. The following day, the participants returned to the laboratory for the measurement of absolute blood volume via the carbon monoxide rebreathing method described below. Absolute blood volume was used to calculate the volume of fluid that was required to restore plasma volume in visits 2 and 3 at high altitude.
High altitude experimental visits
To maintain consistency between sea level and high altitude testing, the same equipment was driven from Kelowna, Canada, to The Barcroft Laboratory, USA, including the ultrasound machine and the exercise ergometer. During high altitude testing, an electrical fault developed in our cycle ergometer, so this was replaced by a second electronically braked ergometer from the same manufacturer. Given that the order of visits was randomised, we are confident any differences between devices would be accounted for by our experimental design. At high altitude, the order of experimental visits was randomised to avoid any order effect due to acclimatisation. Before all trials, haemoglobin (Hb) concentration (Hemocue, Radiometer, Copenhagen, Denmark) and haematocrit (HCT; microhaematocrit centrifugation technique) were assessed from a blood sample taken from an indwelling catheter placed in the antecubital vein in order to calculate the percentage change in blood volume from sea level (Dill & Costill, 1974). In visits 2 and 3, the percentage change and the absolute plasma volume from sea level was used to generate a target infusion volume for each individual. Sildenafil was administered orally (50 mg) in a randomised and double‐blinded approach.
During the high altitude visit 1, participants completed a peak test that was used to determine the change in aerobic capacity from sea level. Visits 2 and 3 were identical, apart from the study clinician administering either 50 mg Sildenafil or placebo 60 min before the start of testing. All experimenters and participants were blinded to the conditions except the study clinician (J.A.). The participants underwent an ECG during rest and at 50% peak power. The required target volume of saline was then infused over the course of 20–40 min by infusion pump (Carefusion, San Diego, CA, USA) and Hb and HCT were reassessed to check plasma volume expansion to sea level values had been achieved. The volume required to restore plasma volume in each condition is reported in the results. This was followed by repeating the rest and exercise ECG. A 30‐min rest period then preceded the peak test.
Experimental protocols
Incremental test to exhaustion ( peak test)
The incremental exercise test was performed in the semi‐recumbent position on an electronically braked cycle ergometer (Lode Angio, Lode). Participants were instructed to keep their cadence between 70 and 80 rpm throughout the test. Following a 2 min warm up at 100 W, 20 W was added every minute until volitional exhaustion. Where the test was terminated midway through a stage, the 20 W increment was divided by 60, multiplied by the time completed and added to the power output from the last complete stage. During the incremental tests, ventilation (V E), O2 uptake () and CO2 production () were assessed via breath‐by‐breath online gas analysis (Oxycon Mobile, Carefusion, San Diego, CA, USA), as previously used at high altitude (Faoro et al. 2013). In addition to respiratory analysis, heart rate was recorded via a three‐lead ECG and peripheral oxygen saturation () via finger pulse oximetry.
Rest and exercise ECG
Echocardiographic images were obtained with the participants resting or cycling in the left lateral decubitus position, by the same sonographer (M.S.), using a commercially available ultrasound system (Vivid E9, GE Healthcare, Piscataway, NJ, USA). All images were acquired at end‐expiration. Exercise images were obtained after 2 min of cycling to allow the participant to reach steady state, and were completed within 5 min.
Parasternal long‐ and short‐axis views and apical four‐ and two‐chamber views were recorded for three consecutive cycles and stored for offline analysis (Echopac, GE Healthcare). LV volumes were assessed from apical four‐ and two‐chamber images in accordance with the American Society of Echocardiography guidelines (Lang et al. 2015). Transmitral early (E) and atrial (A) filling was assessed via pulsed‐wave Doppler recordings from an apical four‐chamber view, with the sample volume placed between the tips of the mitral valve leaflets. RV areas were assessed from a modified apical four‐chamber view in line with the RV American Society of Echocardiography guidelines for the assessment of the right heart (Rudski et al. 2010). Pulmonary artery systolic pressure (PASP) was quantified as the maximum systolic pressure gradient across the tricuspid valve added to right atrial pressure estimated from the collapsibility of the inferior vena cava. To derive pressure, the modified Bernoulli equation (4V 2) was applied to the peak systolic regurgitation jet velocity measured via continuous wave Doppler. Unfortunately, due to the difficulty in identifying the regurgitant jet during exercise with a higher ventilation at high altitude, it could only be achieved in 26% of time points during exercise. The missing data meant from our n = 12, we only acquired PASP data in n = 3 across all time points. For this reason, PASP data during exercise is not reported. At rest, PVR was estimated from the ratio of the tricuspid regurgitant jet velocity to the velocity–time integral obtained in the RV outflow tract in a parasternal short‐axis view (Rudski et al. 2010). PVR was estimated from the ratio of the tricuspid regurgitant jet velocity to the velocity–time integral obtained in the RV outflow tract in a parasternal short‐axis view (Abbas et al. 2003; Rudski et al. 2010).
Left and right ventricular mechanics were assessed via two‐dimensional speckle‐tracking echocardiography. LV circumferential strain, rotation, strain rate and rotation velocity were measured from parasternal short‐axis views at the base and apex, as previously described in detail (Stembridge et al. 2014). LV twist, systolic twist velocity and untwisting velocity were calculated by subtracting the apical frame‐by‐frame data from the basal data. Left and right ventricular longitudinal strain and strain rate were analysed from apical four‐chamber views. All images for speckle‐tracking analysis were obtained at the highest possible frame rates (> 70 frames per second) and kept consistent within individuals. Intra‐observer coefficient of variations for the study sonographer (M.S.) have been reported previously (Stembridge et al. 2015), and range from 8.1 to 11% for speckle‐tracking imaging.
Absolute blood volume
Blood volume was assessed at sea level using the carbon monoxide rebreathing method (Schmidt & Prommer, 2005), as previously used at high altitude (Ryan et al. 2014). The participants were instructed to inhale maximally from a custom‐made glass spirometer (Bloodtec, GbR, Germany) attached to a 5‐litre reservoir bag containing 100% O2 whilst carbon monoxide was added (1.0 ml kg−1). The participants then held the full inspiration for 10 s before breathing in and out of the spirometer for 2 min. At the end of the 2 min, the participants exhaled fully into the spirometer before returning to room air. Venous blood samples were taken before and after the rebreathing for the assessment of Hb concentration, HCT and the percentage of Hb bound to carbon monoxide (HbCO; ABL 90, Radiometer, Denmark). Exhaled carbon monoxide was measured using a portable carbon monoxide device (Dräger Pac 3500, Daeger Safety Inc., TX, USA) at baseline, 4 min and 7 min after rebreathing.
Statistical analyses
Results are presented as means ± standard deviation (SD), and each participant has their own unique symbol across Figs 2, 3, 4 so that individual responses can be tracked between experimental conditions. Our four hypotheses stated in the Introduction were approached using the following statistical methods. To explore the effect of the (i) hypovolaemia and (ii) hypoxic pulmonary vasoconstriction at rest, two separate repeated measures ANOVAs were run with paired‐samples t tests used to detect differences where there was a significant main effect; (iii) to explore the response to exercise during the different conditions, a two‐way repeated measures ANOVA was used (condition and exercise intensity) with paired‐samples t tests used to detect differences where there was a significant main effect; and (iv) to examine the differences in maximal exercise performance, a one‐way repeated measures ANOVA was used with paired‐samples t tests used to detect differences where there was a significant main effect. A Bonferroni correction was applied to all post hoc t tests to account for multiple comparisons, with adjusted P values reported. To determine whether meaningful differences were present, effect sizes (Cohen's d) are reported. Data were normally distributed, as confirmed via the Shapiro–Wilk normality test. Where sphericity could not be assumed (Mauchly's) the Greenhouse–Geisser correction was applied.
Figure 2. Left ventricular filling and ejection during rest at sea level (SL) and at high altitude (HA) following plasma volume expansion (HA‐PVX), with sildenafil administration (HA‐SIL) and combined plasma volume expansion and sildenafil administration (HA‐PVX‐SIL).
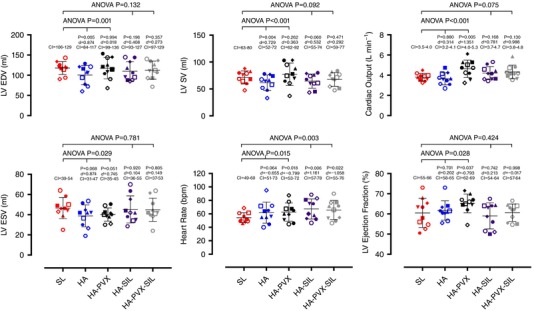
Statistical significance values reported on the figures are from the two separate one‐way ANOVA tests run to compare SL with HA and HA‐PVX and SL with HA‐SIL and HA‐PVX‐SIL. P values in smaller font refer to the post hoc tests where each condition was compared to sea level, and effect sizes (Cohen's d) and 5–95% confidence intervals (CI) are also reported. Each symbol represents the same individual across all conditions.
Figure 3. Left ventricular filling and ejection during 50% peak power supine cycling at sea level (SL) and at high altitude (HA) following plasma volume expansion (HA‐PVX), with sildenafil administration (HA‐SIL) and combined plasma volume expansion and sildenafil administration (HA‐PVX‐SIL).
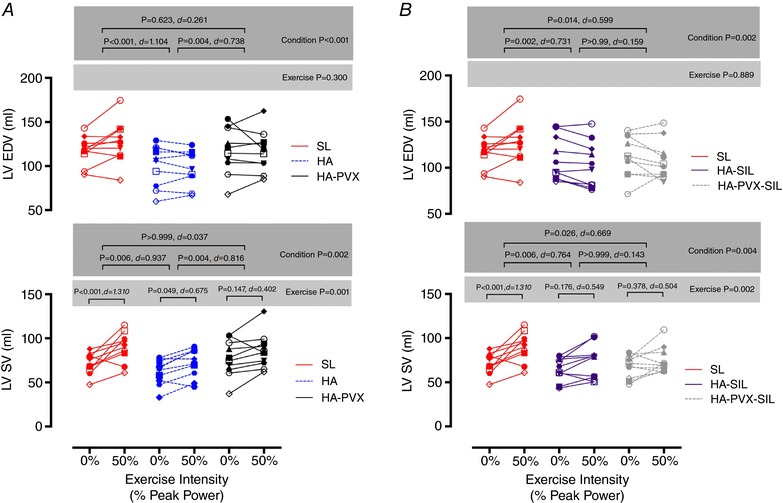
Statistical significance reported on the figures are from the two separate two‐way ANOVA tests run to compare SL with HA and HA‐PVX and SL with HA‐SIL and HA‐PVX‐SIL. P values are reported for Condition and Exercise (e.g. 0 to 50% power), and effect sizes (Cohen's d) are also reported. Each symbol represents the same individual across all conditions.
Figure 4.
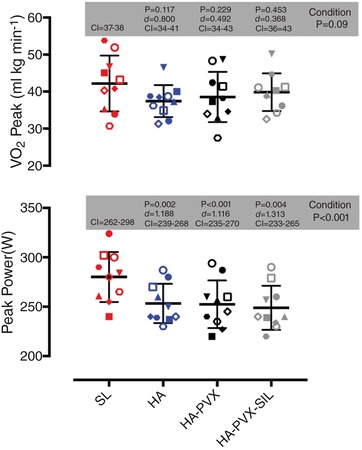
Maximal oxygen consumption ( peak) and peak power achieves during the incremental tests to exhaustion at sea level (SL) and at high altitude (HA) following plasma volume expansion (HA‐PVX) and combined plasma volume expansion and sildenafil administration (HA‐PVX‐SIL)
Statistical significance reported on the figures are from one‐way ANOVA tests run to compare SL with HA, HA‐PVX and HA‐PVX‐SIL, with effect sizes (Cohen's d) and 5–95% confidence intervals (CI) also reported. Each symbol represents the same individual across all conditions.
Results
Effectiveness of interventions
The haematological and pulmonary vascular changes following the interventions are shown in Table 1. Plasma volume was decreased at high altitude by 11 ± 7% and 12 ± 7% in the HA and HA‐SIL trials, respectively. Following the infusion in the HA‐PVX and HA‐PVX‐SIL trials, plasma volume was comparable to that at sea level. The average infusion volume required to restore plasma volume was 398 ± 248 ml in the HA‐PVX trial and 438 ± 247 ml in the HA‐PVX‐SIL trial. PASP and PVR were both elevated in the placebo trials at high altitude (HA and HA‐PVX); however, Sildenafil was effective in reversing this response as there was no significant difference in either PASP or PVR in the Sildenafil trials (HA‐SIL and HA‐PVX‐SIL) when compared to sea level.
Table 1.
Haematological, pulmonary haemodynamic and systemic haemodynamic responses to high altitude, plasma volume expansion and sildenafil administration
| Sea level (244 m) | High altitude (3800 m) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | HA | HA‐PVX | P (ANOVA) | HA‐SIL | HA‐PVX‐SIL | P (ANOVA) | |
| Haematological | |||||||
| (%) | 98 ± 1 | 89 ± 3*** (6.088) | 90 ± 2*** (5.234) | <0.001 | 89 ± 2*** (5.682) | 89 ± 1*** (9.686) | <0.001 |
| Hb concentration (g dl–1) | 148 ± 5 | 160 ± 6** (2.256) | 148 ± 3 (0.084) | <0.001 | 157 ± 8*** (1.443) | 147 ± 7 (0.112) | <0.001 |
| Haematocrit (%) | 44 ± 2 | 47 ± 2** (1.161) | 44 ± 2 (0.205) | <0.001 | 47 ± 3** (0.946) | 44 ± 2 (0.205) | <0.001 |
| Plasma volume (ml) | 3709 ± 573 | – | – | – | – | ||
| Blood volume (ml) | 6249 ± 925 | – | – | – | – | ||
| % Change BV vs. baseline | – | −6 ± 5** | 0.2 ± 3.5 | <0.001 | −7 ± 4*** | 0.4 ± 3.6 | <0.001 |
| % Change PV vs. baseline | – | −11 ± 7** | 0.4 ± 7 | <0.001 | −12 ± 7*** | 1.3 ± 6.3 | <0.001 |
| Pulmonary vascular | |||||||
| PASP (mmHg) | 19.6 ± 4.3 | 26.0 ± 5.4* (1.331) | 25.8 ± 5.1* (1.316) | <0.001 | 22.1 ± 3.9 (0.569) | 21.2 ± 3.3 (0.423) | 0.310 |
| PVR (Woods units) | 1.21 ± 0.14 | 1.51 ± 0.25* (1.544) | 1.54 ± 0.20* (1.950) | 0.007 | 1.37 ± 0.26 (0.807) | 1.30 ± 0.12 (0679) | 0.130 |
| Systemic vascular | |||||||
| Systolic blood pressure | 133 ± 16 | 142 ± 17 (0.618) | 139 ± 12 (0.454) | 0.233 | 136 ± 15 (0.219) | 140 ± 13 (0.502) | 0.376 |
| Diastolic blood pressure | 75 ± 13 | 81 ± 9 (0.567) | 82 ± 11 (0.621) | 0.262 | 81 ± 9 (0.593) | 85 ± 13 (0.803) | 0.106 |
, peripheral oxygen saturation; Hb, haemoglobin; BV, blood volume; PV, plasma volume; PASP, pulmonary artery systolic pressure; PVR, pulmonary vascular resistance. Data are reported as mean ± SD (Cohen's d).
* P < 0.05
** P < 0.01 and
*** P < 0.001 all compared to sea level.
Effects of plasma volume expansion and Sildenafil administration on LV filling and ejection at rest
LV EDV and SV were reduced in the HA condition in comparison to SL (Fig. 2). In addition, there was also a decrease in transmitral E velocity and E/A ratio and an increase in LV twist, apical rotation and apical circumferential strain rate (Table 2). Although there was a significant main effect for RV end‐diastolic (P = 0.045) and end‐systolic area (P = 0.038), post hoc tests failed to reach significance (P = 0.085 and P = 0.054, respectively). The reduction in LV EDV and SV from sea level to high altitude was abolished after HA‐PVX, and transmitral E (P = 0.142) velocity and E/A ratio (P = 0.524) were no longer significantly decreased; however, LV twist and apical circumferential strain rate remained elevated (Table 2).
Table 2.
Left and right ventricular function responses to high altitude, plasma volume expansion and sildenafil administration
| Sea level (244 m) | High altitude (3800 m) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | HA | HA‐PVX | P (ANOVA) | HA‐SIL | HA‐PVX‐SIL | P (ANOVA) | |
| Transmitral E (m s−1) | 0.92 ± 0.12 | 0.81 ± 0.17** (0.755) | 0.87 ± 0.15 (0.347) | 0.003 | 0.81 ± 0.15* (0.826) | 0.80 ± 0.15* (0.844) | 0.006 |
| Transmitral A (ms−1) | 0.39 ± 0.05 | 0.42 ± 0.08 (0.600) | 0.40 ± 0.08 (0.174) | 0.128 | 0.40 ± 0.06 (0.239) | 0.42 ± 0.10 (0.445) | 0.369 |
| Transmitral E/A ratio | 2.41 ± 0.37 | 1.93 ± 0.33** (1.392) | 2.24 ± 0.41 (0.422) | <0.01 | 2.07 ± 0.47* (0.810) | 2.01 ± 0.58* (0.843) | 0.013 |
| LV twist (°) | 15.9 ± 4.0 | 19.4 ± 6.1* (0.697) | 19.5 ± 6.2* (0.697) | <0.05 | 19.2 ± 9.6 (0.484) | 18.2 ± 7.1 (0.411) | 0.231 |
| Systolic twist velocity (° s−1) | 107 ± 18 | 140 ± 28** (1.405) | 132 ± 24* (1.190) | 0.002 | 125 ± 41 (0.620) | 135 ± 31 (1.142) | 0.065 |
| Untwisting velocity (° s−1) | 107 ± 26 | 127 ± 39 (0.594) | 120 ± 20 (0.555) | 0.143 | 125 ± 48 (0.475) | 116 ± 34 (0.285) | 0.278 |
| Basal rotation (°) | 5.4 ± 2.2 | 6.4 ± 3.5 (0.353) | 5.4 ± 2.1 (0.003) | 0.357 | 6.7 ± 3.6 (0.451) | 5.7 ± 2.7 (0.122) | 0.179 |
| Apical rotation (°) | 11.5 ± 4.3 | 14.7 ± 4.8* (0.724) | 14.9 ± 5.5 (0.709) | 0.038 | 13.7 ± 7.1 (0.395) | 13.5 ± 5.9 (0.407) | 0.317 |
| Basal circumferential strain (%) | 19.5 ± 1.7 | 21.1± 3.5 (0.623) | 19.3 ± 3.4 (0.051) | 0.198 | 20.0 ± 3.25 (0.214) | 20.6 ± 3.0 (0.459) | 0.445 |
| Basal circumferential strain rate (%−1) | 1.12 ± 0.13 | 1.32 ± 0.26 (1.010) | 1.32 ± 0.26 (0.657) | 0.062 | 1.23 ± 0.22 (0.608) | 1.24 ± 0.29 (0.537) | 0.180 |
| Apical circumferential strain (%) | 26.6 ± 5.1 | 27.6 ± 5.2 (0.191) | 26.7 ± 5.2 (0.018) | 0.518 | 27.8 ± 0.64 (0.236) | 27.8 ± 5.3 (0.233) | 0.314 |
| Apical circumferential strain rate (%−1) | 1.62 ± 0.42 | 1.99 ± 0.46*** (0.844) | 1.86 ± 0.44** (0.562) | <0.001 | 2.14 ± 0.64** (0.994) | 1.95 ± 0.59** (0.655) | 0.002 |
| LV longitudinal strain (%) | 19.4 ± 1.2 | 19.7 ± 2.8 (0.144) | 20.0 ± 1.8 (0.426) | 0.672 | 19.5 ± 1.9 (0.033) | 19.0 ± 1.9 (0.261) | 0.215 |
| LV longitudinal strain rate (%−1) | 0.99 ± 0.11 | 1.17 ± 0.16 (1.328) | 1.06 ± 0.09 (0.719) | 0.027 | 1.11 ± 0.22 (0.738) | 1.07 ± 0.14 (0.612) | 0.196 |
| End diastolic area (cm2) | 23.7 ± 3.7 | 21.0 ± 2.5 (0.881) | 23.5 ± 2.9 (0.078) | 0.045 | 21.5 ± 3.0 (0.666) | 21.6 ± 3.3 (0.612) | 0.060 |
| End systolic area (cm2) | 13.3 ± 2.1 | 11.4 ± 2.8 (0.784) | 12.2 ± 2.3 (0.493) | 0.038 | 12.7 ± 2.6 (0.288) | 12.0 ± 2.6 (0.572) | 0.150 |
| Fractional area change (%) | 43.6 ± 5.5 | 45.7 ± 11.7 (0.245) | 47.9 ± 7.3 (0.668) | 0.478 | 41.0 ± 8.7 (0.358) | 44.7 ± 4.8 (0.214) | 0.299 |
| Transtricuspid E (m s−1) | 0.60 ± 0.08 | 0.53 ± 0.06 (0.881) | 0.57 ± 0.06 (0.367) | 0.271 | 0.52 ± 0.03 (1.219) | 0.52 ± 0.18 (0.553) | 0.469 |
| Transtricuspid A (m s−1) | 0.35 ± 0.09 | 0.33 ± 0.03(0.302) | 0.30 ± 0.07 (0.703) | 0.490 | 0.33 ± 0.04 (0.341) | 0.36 ± 0.18 (0.035) | 0.696 |
| Transtricuspid E/A ratio | 1.78 ± 0.47 | 1.61 ± 0.23 (0.477) | 2.03 ± 0.54 (0.492) | 0.542 | 1.61 ± 0.20 (0.510) | 1.67 ± 0.63 (0.208) | 0.719 |
| RV longitudinal strain (%) | 23.2 ± 1.8 | 23.9 ± 2.9 (0.318) | 23.0 ± 3.2 (0.050) | 0.510 | 23.3 ± 2.5 (0.069) | 24.3 ± 2.2 (0.559) | 0.303 |
| RV longitudinal strain rate (%−1) | 1.16 ± 0.22 | 1.31 ± 0.21 (0.716) | 1.19 ± 0.12 (0.177) | 0.161 | 1.21 ± 0.2 (0.248) | 1.25 ± 0.19 (0.468) | 0.363 |
E, early filling; A, late filling; LV, left ventricular. Data are reported as mean ± SD (Cohen's d).
* P < 0.05
** P < 0.01 and
*** P < 0.001 compared to sea level.
In the Sildenafil conditions, LV EDV (P = 0.132) and SV (P = 0.092) were not different to sea level values. However, the probability values suggest a trend for SV to be lower, as do post hoc tests between SL and HA‐SIL (P = 0.060). However, this was not the case for HA‐PVX‐SIL (P = 0.471). To explore whether there was a non‐significant but meaningful change between SL and HA‐SIL, effect sizes were calculated and, for completeness, reported for all conditions (Fig. 2). The greatest effect size between SL and the altitude conditions for LV EDV and SV were seen in the HA condition (Cohen's d = 0.874 and 0.729, respectively). The effect sizes for LV EDV and SV indicated a small effect for the HA‐PVX condition (Cohen's d = 0.0.018 and 0.363, respectively), suggesting plasma volume expansion was effective at altering LV EDV and SV. In the Sildenafil trials, effect sizes were larger (Cohen's d = 0.408 and 0.523 for LV EDV and SV, respectively), suggesting a meaningful difference remained despite no significant difference being apparent from difference testing. In the HA‐PVX‐SIL condition, effect sizes were smaller than with HA‐SIL but not as small as with HA‐PVX (Cohen's d = 0.273 and 0.292 for LV EDV and SV, respectively).
The influence of plasma volume expansion and Sildenafil administration on the LV response to exercise
LV EDV did not increase from rest to exercise in either the placebo (main effect for exercise P = 0.300) or Sildenafil (main effect for exercise P = 0.889) trials (Fig. 3). In contrast, both placebo and Sildenafil trials demonstrated an increase in SV from rest to exercise, with post hoc differences evident in the SL and HA trials. When the main effect for condition is considered (pooled rest and exercise data), LV EDV was lower compared to sea level in the HA, HA‐SIL and HA‐PVX‐SIL conditions indicating that LV EDV was most comparable to sea level during the HA‐PVX trial.
Maximal exercise
Peak power was significantly lower during all three high altitude conditions (HA, HA‐PVX and HA‐PVX‐SIL) in comparison to sea level (P < 0.001; Fig. 4); however, there were no differences between the reductions in peak power in the high altitude trials. Differences in peak between SL and HA conditions did not reach statistical significance (ANOVA P = 0.091).
Discussion
In relation to our four hypothesises, we report the following main findings: (i) at 3800 m, normalisation of plasma volume to sea level values restores LV EDV and SV at rest and (ii) the reversal of hypoxic pulmonary vasoconstriction appears to also influence LV EDV in a similar manner, but to a lesser extent; (iii) neither plasma volume expansion nor Sildenafil administration altered the LV EDV and SV response to exercise; and (iv) neither the restoration of plasma volume nor the reduction of PVR ameliorated the reduction in maximal exercise capacity at 3800 m.
LV filling at high altitude
Alexander & Grover (1983) first proposed that the decline in LV filling upon ascent to high altitude was a consequence of the reduction in plasma volume that occurs after the first 3–5 days above 3000 m. The current study demonstrates that normalising plasma volume to sea level values does indeed restore LV EDV and SV. This is in contrast to the previous study of Calbet et al. (2004) who reported no change in SV when participants were made hypervolaemic (via infusion of 1‐litre dextran solution) at high altitude, but in agreement with the more recent work of Siebenmann et al. (2013) who concluded that the reduction in SV can be exclusively explained by the decrease in plasma volume. The differential finding between the work of Calbet et al. (2004) with that of Siebenmann et al. (2013) and ourselves may be explained by important distinctions in the methodological approach. First, the length of exposure varies greatly between the three studies with relatively short durations of ours (5–10 days) and Siebenmann et al. (2013) (26 days), compared to 9 weeks in the study by Calbet et al. (2004). Second, and most importantly, Siebenmann et al. (2013) and the current study were performed at 3454 and 3800 m, respectively, which is a relatively low altitude compared to 5260 m in the work of Calbet and colleagues. The much higher elevation would elicit a greater pulmonary vasoconstriction than observed in the current study, and PVR may therefore play a more substantial role in limiting LV filling at higher altitude and render the impact of hypovolaemia relatively inconsequential.
In the current study, the reversal of hypoxic pulmonary vasoconstriction at high altitude led to there being no statistically significant difference in LV EDV and SV compared to sea level. However, given that probability values of there being a significant difference were close to achieving significance, and effect sizes suggest there was a meaningful difference between sea level and HA‐SIL, reversal of hypoxic pulmonary vasoconstriction may not have been as effective in normalising LV EDV and SV as plasma volume expansion. Curiously, LV EDV and SV were lower in the HA‐PVX‐SIL trial compared to the HA‐PVX trial, despite the same volume normalisation protocol. This latter observation may be explained by the systemic vasodilatory effects of Sildenafil (Vardi et al. 2002), resulting in a greater volume being required within the systemic circulation to maintain the same blood pressure. This would mean the volume infused in the HA‐PVX‐SIL trial would not necessarily present as an enhanced cardiac filling. Despite a reduction in SV, we did not observe any decrement in RV function upon ascent to high altitude. This is in contrast to previous measurements we have taken above 5000 m (Stembridge et al. 2014), differences that might be related to the smaller increase in pulmonary artery systolic pressure observed in the current study at 3800 m (+32%) compared to 5050 m (+43%).
Whilst both plasma volume expansion and reversal of hypoxic pulmonary vasoconstriction increased LV filling, the increase in LV EDV was smaller with the reversal of hypoxic pulmonary vasoconstriction compared to plasma volume expansion. This suggests that the reduction in plasma volume may have a greater effect on LV filling at 3800 m compared to the increase in PVR. Interestingly, when plasma volume expansion and Sildenafil were combined, there was no additive effect. However, given the volume infused was intended to match sea level blood volume, and the pharmacological intervention lowered PVR towards but not below sea level values, there is no reason why LV EDV would increase beyond values recorded at sea level. Collectively, our findings and those from previous investigations suggest that both hypovolaemia and hypoxic pulmonary vasoconstriction decrease LV filling and ejection, but hypoxic pulmonary vasoconstriction may play a greater role at more extreme altitudes.
Restoration of LV filling but persistent changes in LV mechanics
Several recent studies have reported an increase in LV twist at high altitude (Stembridge et al. 2014; Dedobbeleer et al. 2015; Osculati et al. 2016; Maufrais et al. 2017). In our previous work (Stembridge et al. 2015), we hypothesised that the increase in LV twist may be a consequence of the decrease in LV filling observed upon ascent to high altitude. In contrast to our hypothesis, restoring LV EDV in the current study had no effect on LV twist suggesting an alternative mechanism(s) for the elevation of LV mechanics at high altitude. Osculati et al. (2016) have proposed that the increase in LV twist is directly related to subendocardial dysfunction. This has yet to be investigated experimentally, but it is unlikely that the degree of hypoxaemia experienced in our healthy subjects would be sufficient to impair myocardial function and increase twist. More likely is the effects of enhanced inotropic and chronotropic stimulation on LV twist, as circulating catecholamines are known to be increased at high altitude (Mazzeo & Reeves, 2003) and have been shown to substantially increase LV twist (Dong et al. 1999). As such, careful follow‐up experimental investigation is required to delineate these mechanisms.
Hypovolaemia and hypoxic pulmonary vasoconstriction as a limiting factor to exercise capacity
Both the reversal of hypoxic pulmonary vasoconstriction and the restoration of plasma volume have been shown to increase exercise capacity at high altitude (Robach et al. 2000; Ghofrani et al. 2004; Faoro et al. 2009; Naeije et al. 2010), although this is not a universal finding (Calbet et al. 2004; Fischler et al. 2009). We observed no effect of plasma volume expansion or reduction in PVR on maximal exercise capacity in our cohort. Previous studies that have demonstrated the reversal of hypoxic pulmonary vasoconstriction to have a beneficial effect on exercise performance have been performed under more severe normobaric ( 0.10–0.12) and hypobaric (5050–5245 m) hypoxia (Ghofrani et al. 2004; Faoro et al. 2009; Naeije et al. 2010) than used in the current study. As such, similar to LV filling, hypoxic vasoconstriction may play a much greater role in limiting exercise capacity at altitudes above 5000 m. Thus, pharmacological strategies to increase exercise performance at high altitude may only be worthwhile when the athlete or mountaineer reaches a threshold altitude likely to be above 4500–5000 m.
Limitations
Due to the inherent risks, we were not able to directly assess the pulmonary haemodynamic responses to high attitude at rest or during exercise. However, in using echocardiography, we chose a technique that is recommended for the clinical assessment of the pulmonary vasculature (Rudski et al. 2010) and shows good agreement with invasive measures (Yock & Popp, 1984; Currie et al. 1985). Additionally, our cohort is relatively small and that may potentially have led to a type II error. Due to logistical issues associated with field work in remote locations, high altitude studies seldom report data on large cohorts (Ainslie, 2014). However, in support of our data, power analyses were completed a priori based on effect sizes for Hb concentration, LV EDV and PASP from previous work (Stembridge et al. 2014) and they demonstrated that we needed n = 8 as a minimum required sample size to detect a statistically significant effect with alpha set to 0.05 based on a 95% chance of a statistically significant outcome. Notwithstanding this, some of the measures we report are associated with a higher degree of variability (e.g. RV areas), and this may explain why no significant differences were not observed in these measures.
By design, we chose to match absolute workload between conditions as cardiac output is known to be the same for a given power output at sea level and following 3–5 days at high altitude. However, caution must be exercised when interpreting our data, as the higher relative workload will mean our indices of cardiac function were recorded at 50% of peak power at sea level, but 56% and 57% of peak power in the HA‐PVX and HA‐PVX‐SIL trials, respectively. This may therefore overestimate the cardiac response to submaximal exercise at high altitude. As stated in the methods, due to an electrical fault approximately half of the high altitude testing was performed on a different Lode ergometer. However, as the bike was from the same manufacturer and had undergone the same calibration procedures, and the order of visits was randomised, we are confident that this will not have affected the outcomes of the study. We acknowledge that some of the saline infused will leave the vascular compartment. However, following the infusion Hb concentration and haematocrit were checked to ensure a plasma volume expansion had been achieved. Lastly, this study only recruited male participants, as it would not have been possible to match testing days to specific periods of the menstrual cycle given that the experimental design required six visits over a 22‐day period. This is particularly relevant to our study, as chemoreflex stimulation is known to vary depending on menstrual phase (Muza et al. 2001; Usselman et al. 2013). Unfortunately, this is an unavoidable issue with field testing and may therefore limit the broad applicability of our results to the general population.
Conclusion
In summary, this study demonstrates that both plasma volume and hypoxic pulmonary vasoconstriction play a role in regulating LV filling during sojourn to 3800 m above sea level. However, at 3800 m the decrease in plasma volume appears to dominate over the increase in PVR, and there is no additive effect when plasma volume is normalised and hypoxic pulmonary vasoconstriction is reversed. In contrast to LV filling, there is no role of hypovolaemia or hypoxic pulmonary vasoconstriction in limiting exercise capacity at moderate‐high altitude, which may suggest the existence of a ‘threshold altitude’ where by pharmacological strategies to improve exercise capacity at high altitude via administration of pulmonary vasodilatators may only be worthwhile at more extreme altitudes.
Additional information
Competing interests
The authors have no conflicts of interest.
Author contributions
MS, PNA and RS were involved in the conception and design of the work, and all authors were involved in the acquisition, analysis or interpretation of data for the work and drafting the work or revising it critically for important intellectual content. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by the Natural Sciences and Engineering Research Council of Canada (PNA), and the Canadian Foundation for Innovation and a Canada Research Chair (PNA).
Acknowledgements
This study was conducted at the Barcroft high altitude research facility, and we thank the research station's staff for their friendly and helpful hospitality. The authors would also like to take the opportunity to pay tribute to our co‐author, Dr Chris Willie, who tragically died aged just 32 whilst the manuscript was under review. Chris was a dear friend of the entire team, none more so than the expedition leader Professor Ainslie. Chris approached every aspect of his work and life with an insatiable appetite, perfectly personified in his contribution to this study. Future expeditions will never be the same.
Biography
Mike Stembridge completed his PhD in 2015 investigating cardiac adaptation to short‐term and life‐long high altitude exposure under the supervision of Prof. Rob Shave at Cardiff Metropolitan University. During his subsequent postdoctoral work, he extended the hypoxia theme to examine the cardiovascular and cerebrovascular responses to free‐diving with Prof. Philip Ainslie. In September 2017, he was appointed as a Lecturer in Exercise Physiology, where he intends to investigate the mechanisms underpinning cardiovascular and autonomic adaptation to hypoxia through a series of laboratory‐based studies and challenging field expeditions to high altitude locations in South America, Asia and Africa.

Edited by: Harold Schultz and Frank Powell
This is an Editor's Choice article from the 15 February 2019 issue.
Linked articles: This article is highlighted in a Perspectives article by Stickland. To read this article, visit https://doi.org/10.1113/JP276345. The article is also highlighted in a Journal Club article by Karvasarski et al. To read this article, visit https://doi.org/10.1113/JP277301.
References
- Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA & Lester SJ. (2003). A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 41, 1021–1027. [DOI] [PubMed] [Google Scholar]
- Ainslie PN. (2014). On the nature of research at high altitude: packing it all in! Exp Physiol 99, 741–742. [DOI] [PubMed] [Google Scholar]
- Alexander JK & Grover RF. (1983). Mechanism of reduced cardiac stroke volume at high altitude. Clinic Cardiol 6, 301–303. [DOI] [PubMed] [Google Scholar]
- Alexander JK, Hartley LH, Modelski M & Grover RF. (1967). Reduction of stroke volume during exercise in man following ascent to 3,100 m altitude. J Appl Physiol 23, 849–858. [DOI] [PubMed] [Google Scholar]
- Allemann Y, Rotter M, Hutter D, Lipp E, Sartori C, Scherrer U & Seiler C. (2004). Impact of acute hypoxic pulmonary hypertension on LV diastolic function in healthy mountaineers at high altitude. Am J Physiol Heart Circ Physiol 286, H856–862. [DOI] [PubMed] [Google Scholar]
- Boussuges A, Molenat F, Burnet H, Cauchy E, Gardette B, Sainty JM, Jammes Y & Richalet JP. (2000). Operation Everest III (Comex ‘97): modifications of cardiac function secondary to altitude‐induced hypoxia. An echocardiographic and Doppler study. Am J Respir Crit Care Med 161, 264–270. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Radegran G, Boushel R, Sondergaard H, Saltin B & Wagner PD. (2004). Plasma volume expansion does not increase maximal cardiac output or VO2max in lowlanders acclimatized to altitude. Am J Physiol Heart Circ Physiol 287, H1214–1224. [DOI] [PubMed] [Google Scholar]
- Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA & Tajik AJ. (1985). Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler‐catheterization study in 127 patients. J Am Coll Cardiol 6, 750–756. [DOI] [PubMed] [Google Scholar]
- Dedobbeleer C, Hadefi A, Pichon A, Villafuerte F, Naeije R & Unger P. (2015). Left ventricular adaptation to high altitude: speckle tracking echocardiography in lowlanders, healthy highlanders and highlanders with chronic mountain sickness. Int J Cardiovasc Imaging 31, 743–752. [DOI] [PubMed] [Google Scholar]
- Dill DB & Costill DL. (1974). Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37, 247–248. [DOI] [PubMed] [Google Scholar]
- Dong SJ, Hees PS, Huang WM, Buffer SA, Jr , Weiss JL & Shapiro EP. (1999). Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol Heart Circ Physiol 277, H1053–1060. [DOI] [PubMed] [Google Scholar]
- Duke JW, Davis JT, Ryan BJ, Elliott JE, Beasley KM, Hawn JA, Byrnes WC & Lovering AT. (2016). Decreased arterial PO2, not O2 content, increases blood flow through intrapulmonary arteriovenous anastomoses at rest. J Physiol 594, 4981–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faoro V, Boldingh S, Moreels M, Martinez S, Lamotte M, Unger P, Brimioulle S, Huez S & Naeije R. (2009). Bosentan decreases pulmonary vascular resistance and improves exercise capacity in acute hypoxia. Chest 135, 1215–1222. [DOI] [PubMed] [Google Scholar]
- Faoro V, Huez S, Vanderpool RR, Groepenhoff H, de Bisschop C, Martinot JB, Lamotte M, Pavelescu A, Guenard H & Naeije R. (2013). Pulmonary circulation and gas exchange at exercise in sherpas at high altitude. J Appl Physiol 116, 919–926. [DOI] [PubMed] [Google Scholar]
- Fischler M, Maggiorini M, Dorschner L, Debrunner J, Bernheim A, Kiencke S, Mairbaurl H, Bloch KE, Naeije R & Brunner‐La Rocca HP. (2009). Dexamethasone but not tadalafil improves exercise capacity in adults prone to high‐altitude pulmonary edema. Am J Respir Crit Care Med 180, 346–352. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Reichenberger F, Kohstall MG, Mrosek EH, Seeger T, Olschewski H, Seeger W & Grimminger F. (2004). Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mount Everest base camp: a randomized, double‐blind, placebo‐controlled crossover trial. Ann Intern Med 141, 169–177. [DOI] [PubMed] [Google Scholar]
- Grover RF, Reeves JT, Maher JT, McCullough RE, Cruz JC, Denniston JC & Cymerman A. (1976). Maintained stroke volume but impaired arterial oxygenation in man at high altitude with supplemental CO2 . Circ Res 38, 391–396. [DOI] [PubMed] [Google Scholar]
- Hsu AR, Barnholt KE, Grundmann NK, Lin JH, McCallum SW & Friedlander AL. (2006). Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol (1985) 100, 2031–2040. [DOI] [PubMed] [Google Scholar]
- Kjaergaard J, Snyder EM, Hassager C, Olson TP, Oh JK, Johnson BD & Frantz RP. (2007). Right ventricular function with hypoxic exercise: effects of sildenafil. Eur J Appl Physiol 102, 87–95. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W & Voigt JU. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28, 1–39 e14. [DOI] [PubMed] [Google Scholar]
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R & Wanger J. (2005). Standardisation of the single‐breath determination of carbon monoxide uptake in the lung. Eur Respir J 26, 720–735. [DOI] [PubMed] [Google Scholar]
- Maufrais C, Rupp T, Bouzat P, Doucende G, Verges S, Nottin S & Walther G. (2017). Heart mechanics at high altitude: 6 days on the top of Europe. Eur Heart J Cardiovasc Imaging 18, 1369–1377. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS & Reeves JT. (2003). Adrenergic contribution during acclimatization to high altitude: perspectives from Pikes Peak. Exerc Sport Sci Rev 31, 13–18. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J & Force AET. (2005). Standardisation of spirometry. Eur Respir J 26, 319–338. [DOI] [PubMed] [Google Scholar]
- Muza SR, Rock PB, Fulco CS, Zamudio S, Braun B, Cymerman A, Butterfield GE & Moore LG. (2001). Women at altitude: ventilatory acclimatization at 4,300 m. J Appl Physiol (1985) 91, 1791–1799. [DOI] [PubMed] [Google Scholar]
- Naeije R & Dedobbeleer C. (2013). Pulmonary hypertension and the right ventricle in hypoxia. Exp Physiol 98, 1247–1256. [DOI] [PubMed] [Google Scholar]
- Naeije R, Huez S, Lamotte M, Retailleau K, Neupane S, Abramowicz D & Faoro V. (2010). Pulmonary artery pressure limits exercise capacity at high altitude. Eur Respir J 36, 1049–1055. [DOI] [PubMed] [Google Scholar]
- Osculati G, Revera M, Branzi G, Faini A, Malfatto G, Bilo G, Giuliano A, Gregorini F, Ciambellotti F, Lombardi C, Agostoni P, Mancia G & Parati G. (2016). Effects of hypobaric hypoxia exposure at high altitude on left ventricular twist in healthy subjects: data from HIGHCARE study on Mount Everest. Eur Heart J Cardiovasc Imaging 17, 635–643. [DOI] [PubMed] [Google Scholar]
- Pugh LG. (1964). Blood volume and haemoglobin concentration at altitudes above 18,000 ft. (5500 m). J Physiol 170, 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robach P, Dechaux M, Jarrot S, Vaysse J, Schneider JC, Mason NP, Herry JP, Gardette B & Richalet JP. (2000). Operation Everest III: role of plasma volume expansion on during prolonged high‐altitude exposure. J Appl Physiol 89, 29–37. [DOI] [PubMed] [Google Scholar]
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK & Schiller NB. (2010). Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23, 685–713; quiz 786–788. [DOI] [PubMed] [Google Scholar]
- Ryan BJ, Wachsmuth NB, Schmidt WF, Byrnes WC, Julian CG, Lovering AT, Subudhi AW & Roach RC. (2014). AltitudeOmics: rapid hemoglobin mass alterations with early acclimatization to and de‐acclimatization from 5260 m in healthy humans. PLoS One 9, e108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W & Prommer N. (2005). The optimised CO‐rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol 95, 486–495. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Hug M, Keiser S, Muller A, van Lieshout J, Rasmussen P & Lundby C. (2013). Hypovolemia explains the reduced stroke volume at altitude. Physiol Rep 1, e00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stembridge M, Ainslie PN, Hughes MG, Stohr EJ, Cotter JD, Nio AQ & Shave R. (2014). Ventricular structure, function and mechanics at high altitude: chronic remodelling in Sherpa versus short‐term lowlander adaptation. J Appl Physiol (1985) 117, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stembridge M, Ainslie PN, Hughes MG, Stohr EJ, Cotter JD, Tymko MM, Day TA, Bakker A & Shave RE. (2015). Impaired myocardial function does not explain reduced left ventricular filling and stroke volume at rest or during exercise at high altitude. J Appl Physiol (1985) 119, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JR, Reeves JT, Wagner PD, Groves BM, Cymerman A, Malconian MK, Rock PB, Young PM, Walter SD & Houston CS. (1988). Operation Everest II: oxygen transport during exercise at extreme simulated altitude. J Appl Physiol 64, 1309–1321. [DOI] [PubMed] [Google Scholar]
- Tymko MM, Tremblay JC, Hansen AB, Howe CA, Willie CK, Stembridge M, Green DJ, Hoiland RL, Subedi P, Anholm JD & Ainslie PN. (2017). The effect of α1‐adrenergic blockade on post‐exercise brachial artery flow‐mediated dilatation at sea level and high altitude. J Physiol 595, 1671–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usselman CW, Luchyshyn TA, Gimon TI, Nielson CA, Van Uum SH & Shoemaker JK. (2013). Hormone phase dependency of neural responses to chemoreflex‐driven sympathoexcitation in young women using hormonal contraceptives. J Appl Physiol (1985) 115, 1415–1422. [DOI] [PubMed] [Google Scholar]
- Vardi Y, Klein L, Nassar S, Sprecher E & Gruenwald I. (2002). Effects of sildenafil citrate (Viagra) on blood pressure in normotensive and hypertensive men. Urology 59, 747–752. [DOI] [PubMed] [Google Scholar]
- Wagner PD. (1996). A theoretical analysis of factors determining max at sea level and altitude. Respir Physiol 106, 329–343. [DOI] [PubMed] [Google Scholar]
- Yock PG & Popp RL. (1984). Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70, 657–662. [DOI] [PubMed] [Google Scholar]


