Abstract
Background
Evidence suggests that systemic inflammation may have a mechanistic role in age-related frailty, yet prospective data is limited. We examined whether systemic inflammation during midlife was associated with late-life frailty within the community-based Atherosclerosis Risk in Communities Study.
Methods
Plasma levels of four inflammatory markers (fibrinogen, von Willebrand factor, and Factor VIII, and white blood cell count) were measured during Visit 1 (1987–1989; mean age: 52 [5]), standardized into z-scores, and combined to create an inflammation composite score. High-sensitivity C-reactive protein (CRP) was measured 3 (Visit 2, 1990–1992) and 9 (Visit 4, 1996–1999) years later. Frailty was evaluated in 5,760 participants during late life (Visit 5, 2011–2013; mean age: 75 [5]). Analyses were adjusted for demographic and physiological variables, and midlife medical comorbidity using logistic regression.
Results
A 1 SD increase in midlife inflammation composite score was associated with higher odds of frailty 24 years later (odds ratio [OR] = 1.39, 95% confidence interval [CI]: 1.18–1.65). Similarly, each standard deviation increase in Visit 2 CRP (OR = 1.24, 95% CI: 1.09–1.40) and Visit 4 CRP (OR = 1.35, 95% CI: 1.19–1.53) was associated with a higher odds of frailty 21 and 15 years later. Participants who maintained elevated CRP (≥3 mg/L) at Visits 2 and 4 or transitioned to a state of elevated CRP during this period were more likely to subsequently meet frailty criteria compared to those who maintained low CRP. These associations were stronger among white, compared to African American, participants (p-interactions < .038).
Conclusions
Systemic inflammation during midlife may independently promote pathophysiological changes underlying frailty in a subset of the population.
Keywords: Acute-phase proteins, C-reactive protein, Cohort study, Immune system, Risk factor
Frailty is most often defined as an aging-related syndrome of reduced physiologic reserve and resistance to stressors resulting from simultaneous declines in multiple biologic systems (1). Frailty serves as a risk factor for several common geriatric syndromes, is associated with chronic disease, functional disability, and mortality (2), and is more prevalent among women and African Americans (3). Although frailty is associated with chronic disease and medical comorbidity, older adults without chronic medical conditions can also be frail (1,4,5). The physiologic underpinnings of age-related frailty have not yet been identified. However, cross-sectional studies, which report higher levels of circulating proinflammatory cytokines and acute phase proteins among frail older adults, implicate systemic inflammation as a potential mechanism in the pathophysiology of frailty (6–8). While some propose that chronic, low-grade systemic inflammation may lead to increased physiologic vulnerability in later age (9), others have questioned whether inflammation may instead simply be an associative feature of frailty and other aging phenotypes.
To date, the few prospective studies that have examined the association between peripheral inflammatory markers and frailty have been limited by brief follow-up periods and a single assessment of inflammatory markers (7,8,10). As a result, the temporal relationship between systemic inflammation and the development frailty remains poorly understood. Evidence for an association between past systemic inflammation and later frailty in the elderly adults would further support theories proposing a role for systemic inflammation in frailty pathogenesis. Using the prospective design of the Atherosclerosis Risk in Communities (ARIC) Study, we tested the hypothesis that individuals with elevated levels of midlife inflammatory markers are at an increased risk for frailty in later life. We also examined whether race and sex further modified this relationship.
Methods
Study Design and Population
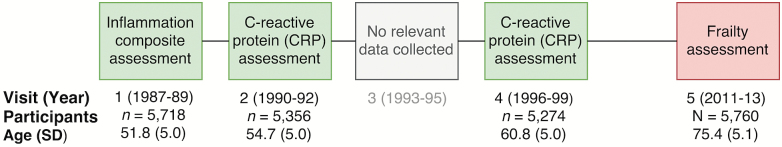
We analyzed data from the ARIC Study, a community-based prospective cohort study, which enrolled individuals (n = 15,792) between ages 45 and 64 years from four communities within the United States: Washington County, MD; Forsyth County, NC; northwestern suburbs of Minneapolis, MN; and Jackson, MS from 1987 to 1989 (Visit 1). As shown in Figure 1, participants were reexamined every 3 years until Visit 4 (1996–1999), and subsequently invited 15 years later for a fifth study examination (2011–2013). Medical examinations and interviews were conducted at each visit. Participants were also contacted annually by phone to obtain information about hospital admission and vital status. The ARIC study protocols were approved by the Institutional Review Boards at each participating center. All participants provided written informed consent.
Figure 1.
Study design and sample characteristics at the time of biomarker measurements and frailty assessment. CRP = C-reactive protein.
Of the participants who completed the Visit 5 examination (n = 6,538), we excluded participants based on the following: missing frailty assessment (n = 715), non-white or non-African American race (n = 12), and missing one or more covariates (n = 51).
Inflammatory Markers
To identify the relationship between midlife systemic inflammation and frailty, blood inflammatory markers from three study visits were utilized. Levels of three acute-phase proteins—fibrinogen, von Willebrand factor (VWF), and Factor VIII (FVIII)—and white blood cell (WBC) count were measured from plasma drawn at Visit 1 and then stored at −70°C until it was analyzed using standard protocols (11). We created a Visit 1 inflammation composite score using the four biomarkers (ie, WBC, fibrinogen, VWF, and FVIII). Visit 1 biomarker values were standardized to z-scores. After log-transforming WBC to correct for skewness, the mean of each participant’s four z-score values was calculated to create the Visit 1 inflammation composite score.
High-sensitivity C-reactive protein (CRP) was measured from blood collected at Visits 2 (1990–1992) and 4. Visit 2 CRP was measured from serum using an immunoturbidimetric assay on the Roche Modular P chemistry analyzer (Roche Diagnostics, Indianapolis, IN) at the University of Minnesota (Minneapolis, MN). Visit 4 CRP was measured from plasma using the nephelometric method on the Siemens Dade Behring BN II analyzer (Siemens Healthcare Diagnostics, Deerfield, IL) at Baylor College of Medicine (Houston, TX). CRP measurement differences between specimen type, laboratories, instrument, assay method, and time of measurement based on laboratory calibration studies were not large enough to warrant calibration (12). All interassay coefficients of variation were below 8%, except VWF (17%–19%).
Frailty Phenotype
All participants who attended Visit 5 were categorized as frail, prefrail, or robust based on the frailty phenotype definition operationalized by the Cardiovascular Health Study (CHS) (1) and previously described within the ARIC Study (13). This frailty definition consists of five component criteria: exhaustion, slowness, low physical activity, weakness, and weight loss. Briefly, exhaustion was classified using participant responses to two questions from the Center for Epidemiological Studies-Depression (CES-D) scale; slowness was classified based on 4 m walking speed using previously validated cutpoints (lowest sex- and height-specific quintile) (1); low physical activity was assessed using the Baecke physical activity questionnaire (lowest sex-specific quintile); weakness was classified using a hydraulic grip strength dynamometer according to previously validated sex- and body mass index (BMI)-specific cutoffs (1); weight loss was defined as a 10% weight loss from Visit 4 to Visit 5, or a BMI at Visit 5 less than 18.5 kg/m2. Participants were classified as “frail” if they met three or more of the criteria, “pre-frail” if they met 1 or 2 of the criteria, and “robust” if no frailty criteria were met. Participants were also classified based on the total number of frailty criteria met (ranging from 0 to 5).
Assessment of Medical Comorbidity and Other Covariates
Medical comorbidity
Prevalent medical conditions listed below were assessed at each of the five Visits. Coronary heart disease (CHD) was defined as a self-reported history or medical record evidence of myocardial infarction, coronary artery bypass graft or angioplasty, or myocardial infarction determined by ECG adjudication. Heart failure was identified based on self-reported heart failure medication use within the past two weeks, or medical record evidence of heart failure related hospitalizations. Hypertension was defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or use of hypertensive medication. Diabetes was defined as fasting glucose of ≥126 mg/dl or non-fasting glucose of ≥200 mg/dl, use of insulin or diabetes medication, or participant report of physician-diagnosed diabetes. Cancer diagnosis was ascertained using information from cancer registries and ARIC hospital surveillance. Chronic obstructive pulmonary disease (COPD) was classified using prebronchodilator spirometry values in accordance with Global Obstructive Lung Disease (GOLD) criteria. Chronic Kidney Disease (CKD) was defined using estimated glomerular filtration rate (GFR) calculated using serum creatinine and demographic characteristics (14) in accordance with National Kidney Foundation guidelines. Previous chronic inflammatory conditions (ie, arthritis, gout, and lupus) were assessed at Visit 4 based on participant report of physician diagnosis. Depressive symptoms were measured at Visit 5 using the CES-D.
Covariates
Race, sex, and education (less than high school; high school/GED/vocational school; or any college) were assessed based on self-report at Visit 1. Indicators of individual socioeconomic status (SES) (ie, total household income, occupational standing, and self-reported community standing) were assessed at Visits 4 and 5. Cognitive status (cognitively normal/mild cognitive impairment/dementia) was adjudicated at Visit 5 by an expert committee (described in Supplementary Methods). Previous long-term anti-inflammatory medication use (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], arthritis medication) was assessed at Visit 5. Time-varying covariates assessed at Visits 1, 2, and 4, concurrent with the measurement of inflammatory markers, included the following: cigarette smoking and alcohol use status (current/former/never) assessed by self-report; total cholesterol and triglycerides measured using the enzymatic method; and total high-density lipoprotein (HDL) cholesterol.
Statistical Analysis
Log-likelihood ratio tests revealed no evidence of a nonlinear relationship between inflammatory marker levels and frailty prevalence; therefore, inflammatory biomarkers were treated as linear, continuous parameters. To examine the association of inflammatory biomarker levels at midlife with late-life frailty status (frail/nonfrail) and the number of frailty characteristics at Visit 5, we used binomial and ordinal logistic regression, respectively. For these analyses, CRP levels were log transformed to correct for skewness.
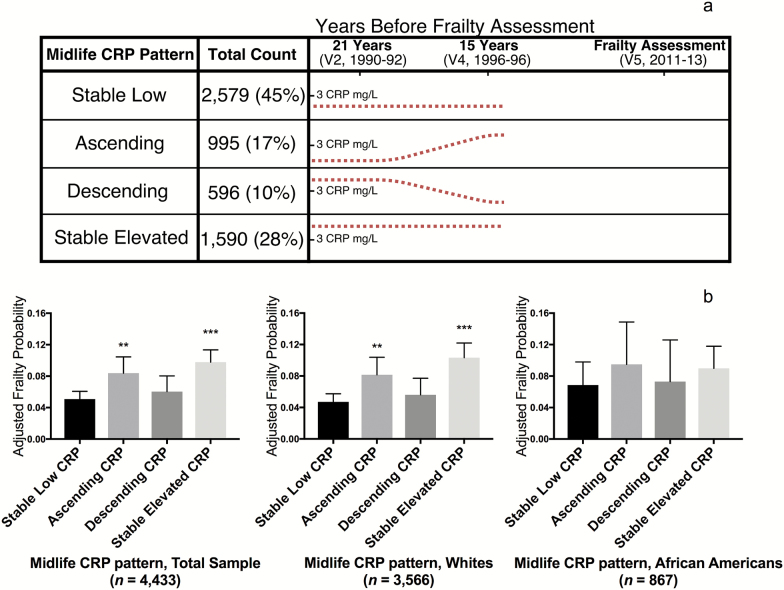
To examine the association of longitudinal patterns of midlife CRP with frailty at Visit 5, participants were categorized as having “low” or “elevated” CRP at Visits 2 and 4 using a cutoff of 3 mg/L, which is commonly used to define systemic inflammation (15). Participants were then categorized into one of four groups using the “low” versus “elevated” dichotomization (see Figure 2a).
Figure 2.
Participant grouping and the adjusted probability of frailty based on longitudinal midlife CRP levels. (a) Using 3 mg/L as the cutoff for elevated versus normal CRP, participants were assigned to one of four groups based on CRP levels at Visits 2 and 4. Estimated CRP patterns for each group are represented by the dotted line. (b) Using multivariable logistic regression, covariate-adjusted probabilities of late-life frailty were calculated for the total sample and for white and African American subgroups. The logistic regression model adjusted for demographic and physiological variables, as well as medical comorbidity at Visit 2. **p < .01, ***p < .001 compared to the Stable Low referent group. CRP = C-reactive protein.
Stable low: low CRP levels at Visits 2 and 4
Ascending: low CRP at Visit 2 and elevated CRP at Visit 4
Descending: elevated CRP at Visit 2 and low CRP at Visit 4
Stable elevated: elevated CRP at Visits 2 and 4
Using the stable low group as the referent, we used multinomial logistic regression to estimate each group’s odds of frailty at Visit 5.
We used two regression models. Model 1 adjusted for potentially confounding demographic and physiological characteristics: age, sex, center-race, education, mid- and late-life SES, total cholesterol, triglycerides, HDL, smoking status, alcohol use, cholesterol-lowering and anti-inflammatory medication use, and late-life cognitive status. Model 2 additionally adjusted for midlife medical comorbidities: hypertension, diabetes, CHD, heart failure, cancer, CKD, COPD, and chronic inflammatory disease. Time-varying covariates were incorporated in the models from the visit concurrent with the inflammatory biomarker assessment. We used multiplicative interaction terms to evaluate effect modification by race and sex.
We conducted several sensitivity analyses. First, we examined the effect of excluding participants with abnormally elevated inflammatory markers, suggestive of an acute inflammatory response (Visit 1 inflammation composite score >2 SD above the sample mean; CRP > 10 mg/L). We also examined the effect of excluding from analyses participants with clinical stroke, and we repeated analyses after additionally adjusting for medical conditions occurring during the follow-up period (between biomarker assessment and frailty assessment), which may lie in the causal pathway between inflammation and frailty (ie, hypertension, diabetes, CHD, heart failure, cancer, CKD, COPD, chronic inflammatory disease, stroke, and depressive symptoms). Last, we used inverse probability of attrition weighting (IPAW) to examine potential selection bias related to participant death and dropout before the frailty assessment. This statistical technique uses the demographic, physiological, and clinical characteristics of observed and unobserved cases to create sampling weights which up-weight observed cases that possess characteristics associated with attrition. We used a two-sided p value < .05 as the cutoff for statistical significance. All analyses were conducted using Stata Version 14 (StataCorp, College Station, TX).
Results
Sample Characteristics
Baseline (1987–1989) participant characteristics are described in Table 1. Among the 5,760 participants, 7%, and 48% met frail and prefrail criteria, respectively, at Visit 5. Compared to robust participants, those who were frail and prefrail were older, more likely female and African American, had lower levels of education, and had greater levels of cardiovascular risk factors and comorbidity during midlife (Visit 1).
Table 1.
Baseline (1987–1989) Participant Characteristics (n = 5,760) Stratified According to Frailty Status at Visit 5 (2011–2013)
| Characteristics | Robust | Prefrail | Frail |
|---|---|---|---|
| N | 2,620 | 2,749 | 391 |
| Demographic Variables | |||
| Agea,b | 50.6 (4.4) | 52.6 (5.1) | 54.3 (5.4) |
| Female (%)a,b | 54.8 | 60.5 | 66.8 |
| African American (%)a,b | 19.7 | 23.4 | 26.3 |
| Median household incomeb,c | 50k–75k | 35k–50k | 25k–35k |
| Education (%) a,b | |||
| Less than high school | 9.5 | 14.9 | 25.4 |
| High school/GED/vocational | 39.4 | 44.1 | 45.8 |
| College/graduate/professional | 51.1 | 41.0 | 28.8 |
| Physiological and Lab Variables | |||
| Body mass index, kg/m2a,b | 26.1 (4.1) | 27.5 (4.8) | 30.2 (6.2) |
| Systolic blood pressure, mm Hga,b | 114.5 (15.1) | 116.9 (15.9) | 121.1 (16.3) |
| Diastolic blood pressure, mm Hga | 72.4 (10.0) | 72.9 (10.4) | 73.9 (11.0) |
| Total cholesterol, mg/dla,b | 207.5 (39.3) | 212.4 (40.0) | 212.2 (39.0) |
| HDL, mg/dlb | 53.8 (17.0) | 52.9 (17.0) | 51.6 (16.7) |
| LDL, mg/dla | 131.2 (37.6) | 135.2 (38.0) | 134.6 (34.7) |
| Triglycerides, mg/dla,b | 114.8 (74.3) | 123.5 (77.4) | 134.1 (108.1) |
| Chronic Medical Conditions (%) | |||
| Hypertensiona,b | 19.2 | 25.3 | 36.9 |
| Diabetes mellitusa,b | 2.4 | 4.6 | 9.0 |
| Coronary heart diseasea,b | 3.2 | 5.4 | 8.7 |
| Heart Failurea,b | 1.3 | 2.9 | 4.2 |
| Cancer | 0.2 | 0.1 | 0.6 |
| Chronic Obstructive Pulmonary Diseasea,b | 13.4 | 16.0 | 17.8 |
| Chronic Kidney Disease | 0.2 | 0.3 | 0.6 |
| Arthritisa,b,c | 29.4 | 40.7 | 57.7 |
| Medication (%) | |||
| Anti-inflammatory (regular use)d | 14.7 | 16.6 | 14.8 |
| Cholesterol lowering (last 2 wk) | 1.8 | 2.5 | 1.7 |
| Cognitive Status (%) a,b,d | |||
| Cognitively normal | 82.6 | 71.7 | 58.3 |
| Mild cognitive impairment | 16.2 | 23.4 | 31.2 |
| Dementia | 1.1 | 4.8 | 10.0 |
Note: Values are displayed as means (SD) for continuous variables, and column percentages for categorical variables. GED = General education diploma.
a p < .05 for significant difference between the prefrail and robust (referent group).
b p < .05 for significant difference between the frail group and robust (referent group).
cAssessed at Visit 4 (1996–1998).
dAssessed at Visit 5 (2011–2013).
Midlife Inflammation and Late-life Frailty
Visit 1 inflammation composite
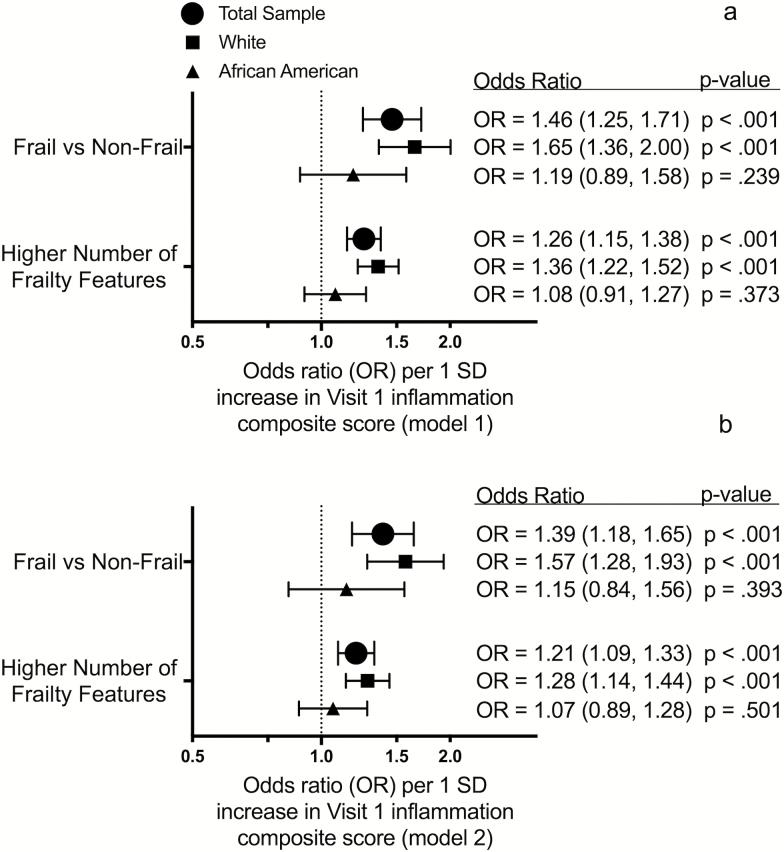
After adjusting for midlife demographic and physiological characteristics, each 1 SD increase in Visit 1 inflammation composite score was associated with a 46% higher odds of frailty and a greater number of frailty characteristics at Visit 5 (24 years of follow-up) (Figure 3a). After further adjustment for concurrent medical comorbidities (Figure 3b), each 1 SD increase was associated with a similar (39%) higher odds of frailty and a greater number of frailty characteristics. Associations between the individual Visit 1 inflammatory markers and frailty risk are provided in Supplementary Figure 1.
Figure 3.
The association between midlife inflammation composite score and odds of late-life frailty. Forest plots display the odds ratios for the association of Visit 1 inflammation composite score with frailty and frailty features at Visit 5 after adjusting for demographic and physiological characteristics (a) and comorbidity (b). OR = Odds ratio.
Visit 2 CRP
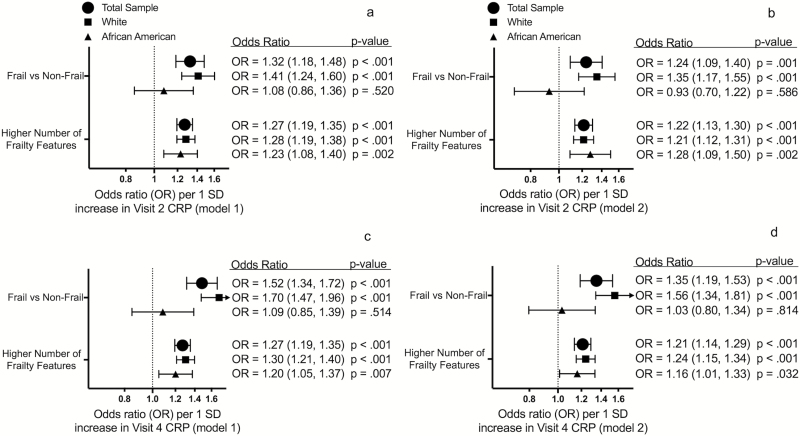
Each 1 SD increase in Visit 2 CRP was associated with a 32% higher odds of frailty and a greater number of frailty features at Visit 5 (21 years of follow-up) after adjusting for demographic and physiological characteristics (Figure 4a). These associations were attenuated, but remained statistically significant, after additionally adjusting for medical comorbidities (Figure 4b).
Figure 4.
The association between midlife CRP and odds of late-life frailty. The first row displays odds ratios for the association of Visit 2 CRP with frailty and frailty features at Visit 5 after adjusting for demographic and physiological characteristics (a) and comorbidity (b). The second row displays odds ratios for the association of Visit 4 CRP with frailty and frailty features at Visit 5 after adjusting for demographic and physiological characteristics (c) and comorbidity (d). CRP = C-reactive protein; OR = Odds ratio.
Visit 4 CRP
Each 1 SD increase in Visit 4 CRP was associated with a 52% higher odds of frailty and a greater number of frailty characteristics at 15 years of follow-up after adjusting for demographic and physiological variables (Figure 4c). The strength of these associations was also attenuated, yet remained statistically significant, after additionally adjusting for medical comorbidities (Figure 4d).
A significant interaction by race on frailty was observed for Visit 1 inflammation composite score (p-interaction = .038), Visit 2 CRP (p-interaction = .005), and Visit 4 CRP (p-interaction < .001). Overall, the associations between midlife inflammatory markers and frailty were stronger for white, compared to African American, participants. No significant interaction by sex was observed.
Longitudinal Pattern of Midlife CRP and Late-life Frailty
Compared to the group with stable low CRP levels, participants with stable elevated midlife CRP (odds ratio [OR]: 2.15; 95% confidence interval [CI]: 1.59, 2.89) and participants who transitioned from low to elevated CRP (ascending) during the same period (OR: 1.78; 95% CI: 1.24, 2.57) had a higher odds of frailty in late-life, after adjusting for demographic and physiological variables, and medical comorbidities (Figure 2b). Stable elevated midlife CRP was more strongly associated with increased frailty risk among white participants (OR, 2.53; CI: 1.80, 3.55) than among African American participants (OR, 1.38; CI: 0.72, 2.68; p-interaction = .023). No interaction by sex was observed.
Sensitivity Analyses
Our findings were robust to the exclusion of participants with abnormally high inflammatory marker levels and the exclusion of participants with clinical stroke (Supplementary Figures 2–5). The association of midlife CRP with frailty was modestly attenuated, but remained robust and statistically significant, after additionally adjusting for potentially mediating medical conditions occurring during the follow-up period (Supplementary Figures 6 and 7). After accounting for potential bias related to selective attrition using IPAW, we observed a stronger association of Visit 1 inflammation composite score with frailty; however, other findings were not substantively changed (Supplementary Figures 8 and 9).
Discussion
In this large community sample, systemic inflammation during midlife, as measured by circulating inflammatory markers, was associated with increased odds of frailty and a higher number of frailty characteristics in older adulthood. While the associations between higher levels of midlife inflammatory markers and frailty were largely confined to white participants, we also found that, among African Americans and whites, higher midlife CRP was associated with having a greater number of frailty characteristics in late-life. In the full sample, each 1 SD increase in the midlife inflammation composite score was associated with a 39% higher odds of frailty approximately 24 years later. Higher midlife CRP levels, and a pattern of persistently elevated or increasing midlife CRP were each associated with an increased odds of frailty decades later. These findings support the hypothesis that midlife, and potentially longstanding, inflammation may play a role in the development of frailty in later life.
Prospective studies have previously reported associations between baseline levels of inflammatory markers, including CRP, D-dimer, fibrinogen, and WBC count, and incident or prevalent frailty over periods ranging from 3 to 10 years among older adults (7,8,10,16). However, null associations between CRP, interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and frailty risk over this same time span have also been reported (7,16). The current findings add to those of previous prospective and cross-sectional (17) studies by providing additional insight into the temporal relationship between systemic inflammation, measured at multiple visits, and subsequent frailty. Specifically, our findings highlight middle adulthood as a potentially important exposure period and, in doing so, provide support for theories that postulate an etiologic role for systemic inflammation in the development of frailty (6). Furthermore, our results suggest that the pathological processes leading to frailty may begin decades prior to frailty onset, in a similar manner to other chronic conditions, including dementia. While our findings align closely with those of translational (18) and genetic (19) studies which provide support for an etiologic role of systemic inflammation, we acknowledge that causation cannot be inferred from the current findings.
Our finding that individuals who maintained elevated systemic inflammation across a 6-year period in midlife were at greatest risk for frailty in late-life is consistent with the view that chronic systemic inflammation is associated with poorer health outcomes years later (6). Our findings suggest that the initiation of systemic inflammation during this same period may also confer risk for later frailty. Middle adulthood may be an especially important exposure period for poor health outcomes in older adults for multiple reasons. First, it is in middle-age when the incidence of common chronic diseases, such as diabetes (20), begins to accelerate. Second, compared to individuals who develop systemic disease and inflammation in late-life, individuals who develop these conditions in midlife may have a longer exposure period and are therefore more susceptible to the deleterious physiological effects. Our observation that the association between midlife inflammatory markers and frailty was robust to adjustment for midlife medical comorbidity, as well as incident medical conditions occurring during the follow-up period, supports the hypothesis that systemic inflammation may play an independent role in promoting the physiological alterations underlying frailty. This is in line with other reports suggesting that disability and comorbidity overlap with frailty, but do not fully explain frailty (5).
Although African Americans are at an increased risk for becoming frail with advancing age (21), research examining potential contributors to frailty risk among African Americans has been sparse. Potential explanations for the race-based differences observed in the present study include racial disparities in the burden of chronic disease, race differences in the regulatory inflammation signaling pathways (22), and race differences in rates of attrition and mortality (see Supplementary Table 1). However, the latter possibility was not supported by our secondary analyses which used IPAW to correct for the higher rates of attrition among African American participants. Additionally, it is possible that the development of frailty among African Americans may be more strongly determined by nonphysiological factors such as SES, access to health care, and health literacy. In support of this notion, we found that SES variables, particularly midlife household income and occupational standing, were more strongly associated with late-life frailty among African Americans, compared to white, participants.
The current study has multiple strengths, including the use of a large, population-based sample, the inclusion of a large number of African American participants, repeat measures of CRP, longitudinal follow-up, and the ability to account for chronic medical conditions and other potential confounding variables. However, the current findings must be considered within the context of several limitations. First, the absence of knowledge about participant frailty status at midlife represents a major limitation. Although the prevalence of frailty is less common during midlife (3), we are unable to ensure that frailty (or a similar condition) was not present when inflammatory markers were assessed. However, the likelihood of confounding from midlife disease or overall poor health status is mitigated in our analyses which are adjusted for a wide range of midlife physiological and disease characteristics thought to be jointly associated with frailty, poor health status, and inflammation. Second, although ARIC is well-characterized with regard to demographic, physiological, and clinical variables known to influence systemic inflammation and frailty, it is possible that the associations between inflammatory marker levels and frailty risk are confounded by unmeasured variables not captured using current methods, such as undiagnosed or subclinical disease, or environmental factors. Third, while many of the inflammatory biomarkers measured in the current study are considered acute-phase reactants involved in peripheral inflammatory signaling, several of the markers (eg, fibrinogen, FVIII) are also involved in additional overlapping biological pathways, including hemostasis. The measurement of inflammatory cytokines and chemokines, which may be more directly involved in the pathogenesis of frailty and other late-life adverse health outcomes, may allow for a more nuanced understanding of the role of systemic inflammation and the identification of novel targets for prevention or treatment.
Despite these limitations, the current study provides further insight into the biological alterations that may underlie frailty by demonstrating an association between systemic inflammatory markers in midlife and increased frailty risk later in life using a large, well-characterized community sample. Taken together, our findings support further study of the causes and consequences of midlife inflammation and suggest that targeting earlier drivers of inflammation rather than employing interventions to reduce inflammation later in life may be important in frailty prevention. In support of this notion, a recent trial of physical activity among older adults, which has been shown to reduce inflammation, failed to show benefit in reducing incident frailty risk in a population at increased risk for functional decline (23). We believe that effective interventions will require a greater understanding of both the pathophysiology and the optimal timing for risk factor mitigation. Specifically, we suggest research efforts focus on identifying disease states, genetics, behaviors, or other triggers of inflammatory pathways and factors that contribute to sustained inflammation over the life course to reduce the risk for frailty with aging. Our findings also highlight the modifying effect race may have on the relationship between inflammation and frailty risk and underscore the need for additional prospective studies to explore early biological and environmental drivers of frailty in multiethnic populations. Targeted and personalized preventive efforts across the life course could lead to the largest public health gains in promoting healthy aging.
Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (grant numbers HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This study was also supported by contracts from the National Institute on Aging (T32 AG027668 to K.A.W., K99-AG052830 to P.P., K24 AG052573 to R.F.G., and P30AG021334 and R01AG050560 to J.W.).
Conflict of Interest
B.G.W. is an investigator/dementia expert on a CMS Coverage with Evidence Development (CED) study, and is an investigator in a clinical trial sponsored by ACADIA Pharmaceutical. B.G.W. and J.W. serve on the Editorial Board for Journal of Gerontology. RFG is an Associate Editor for Neurology®. The other authors declare no competing interests.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 2. Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–149. doi:10.1016/j.archger.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 3. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi:10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 4. Turusheva A, Frolova E, Korystina E, et al. Do commonly used frailty models predict mortality, loss of autonomy and mental decline in older adults in northwestern Russia? A prospective cohort study. BMC Geriatr. 2016;16:98. doi:10.1186/s12877-016-0276-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi:10.1093/gerona/59.3.M255 [DOI] [PubMed] [Google Scholar]
- 6. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi:10.1016/j.arr.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baylis D, Bartlett DB, Syddall HE, et al. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordr). 2013;35:963–971. doi:10.1007/s11357-012-9396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gale CR, Baylis D, Cooper C, Sayer AA. Inflammatory markers and incident frailty in men and women: the English longitudinal study of ageing. Age (Dordr). 2013;35:2493–2501. doi:10.1007/s11357-013-9528-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi:10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 10. Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the cardiovascular health study. Arch Intern Med. 2007;167:635–641. doi:10.1001/archinte.167.7.635 [DOI] [PubMed] [Google Scholar]
- 11. Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study–I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–19. [PubMed] [Google Scholar]
- 12. Parrinello CM, Grams ME, Couper D, et al. Recalibration of blood analytes over 25 years in the Atherosclerosis Risk in Communities Study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61:938–947. doi:10.1373/clinchem. 2015.238873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kucharska-Newton AM, Palta P, Burgard S, et al. Operationalizing frailty in the atherosclerosis risk in communities study cohort. Journals Gerontol Ser A Biol Sci Med Sci. 2016;72:382–388. doi:10.1093/gerona/glw144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi:10.1681/ASN.2004080656 [DOI] [PubMed] [Google Scholar]
- 15. Biasucci LM; CDC; AHA. CDC/AHA workshop on markers of inflammation and cardiovascular disease: Application to clinical and public health practice: Clinical use of inflammatory markers in patients with cardiovascular diseases: A background paper. Circulation. 2004;110:e560–e567. doi:10.1161/01.CIR.0000148983.88334.80 [DOI] [PubMed] [Google Scholar]
- 16. Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf). 2005;63:403–411. doi:10.1111/j.1365-2265.2005.02355.x [DOI] [PubMed] [Google Scholar]
- 17. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi:10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 18. Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA. 2015;112:E6301–E6310. doi:10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mekli K, Nazroo JY, Marshall AD, Kumari M, Pendleton N. Proinflammatory genotype is associated with the frailty phenotype in the English Longitudinal Study of Ageing. Aging Clin Exp Res. 2016;28:413–421. doi:10.1007/s40520-015-0419-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weng W, Liang Y, Kimball ES, et al. Decreasing incidence of type 2 diabetes mellitus in the United States, 2007–2012: Epidemiologic findings from a large US claims database. Diabetes Res Clin Pract. 2016;117:111–118. doi:10.1016/j.diabres.2016.04.043 [DOI] [PubMed] [Google Scholar]
- 21. Hirsch C, Anderson ML, Newman A, et al. ; Cardiovascular Health Study Research Group The association of race with frailty: the cardiovascular health study. Ann Epidemiol. 2006;16:545–553. doi:10.1016/j.annepidem.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 22. Kim CX, Bailey KR, Klee GG, et al. Sex and ethnic differences in 47 candidate proteomic markers of cardiovascular disease: the Mayo Clinic proteomic markers of arteriosclerosis study. Morty RE, ed. PLoS One. 2010;5:e9065. doi:10.1371/journal.pone.0009065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trombetti A, Hars M, Hsu F-C, et al. Effect of physical activity on frailty: secondary analysis of a randomized controlled trial. Ann Intern Med. 2018;168:309–316. doi:10.7326/M16-2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.