Abstract
We evaluated whether the marmoset, a nonhuman primate, can serve as a good model to study aging-related changes in the kidney by employing healthy young and aged marmosets of both sexes. Aging was associated with glomerulosclerosis, interstitial fibrosis, and arteriolosclerosis in both sexes; correspondingly, the content of matrix proteins was increased. Functionally, aging resulted in an increase in urinary albumin and protein excretion. There was a robust correlation between markers of fibrosis and functional changes. We explored signaling pathways as potential mechanistic events. Aging in males, but not in females, was associated with reduced renal cortical activity of AMP-activated protein kinase (AMPK) and a trend toward activation of mechanistic target of rapamycin complex 1 (mTORC1); upstream of AMPK and mTORC1, Akt and IGF-1 receptor were activated. In both sexes, aging promoted kidney activation of transforming growth factor β-1 signaling pathway. While the expression of cystathionine β-synthase (CBS), an enzyme involved hydrogen sulfide (H2S) synthesis, was reduced in both aged males and females, decreased H2S generation was seen in only males. Our studies show that the marmoset is a valid model to study kidney aging; some of the signaling pathways involved in renal senescence differ between male and female marmosets.
Keywords: Primates, Glomerulus, Extracellular matrix, Signaling, Fibrosis
Health span has emerged as the new focus in aging research. Achievement of health span is a composite effort that requires limiting aging-related dysfunction of organ systems including the kidneys. Kidneys contribute to the integrity of milieu interior by regulation of waste disposal, volume and composition of extracellular and intracellular fluid compartments, and, synthesis of hormones such as erythropoietin. Aging phenotype in the kidney in experimental animals is characterized by fibrosis with loss of functioning parenchyma, abnormal albumin loss in urine (albuminuria), and loss of glomerular filtration rate, a measure of kidney waste clearance function (1,2). Not withstanding importance of the kidney in maintaining overall health, mechanisms underlying age-related adverse changes in its structure and function are not well understood. Although kidney fibrosis is a major reason for loss of kidney function in aging, the pathways leading to it in the absence of disease are not well understood.
Proper understanding of aging-induced changes is essential to preserve the integrity of kidney structure and function. This mechanistic approach requires employing animal models that reproduce aging-related changes in the kidney similar to those seen in humans. Nearly all investigations to date have employed rats or mice and have identified oxidative stress, hemodynamic changes, activation of local renin–angiotensin axis and growth factors as mediators of age-related changes (1,2). There are several concerns regarding rodent models employed in the study of kidney aging. (a) In some rodent models, for example, Fisher344 rats, aging rodents develop a chronic nephropathy characterized by progressively worsening glomerular sclerosis, tubulointerstitial nephritis, and fibrosis in association with deterioration of waste clearance function of the kidney (3). Many aged rats develop end stage kidney lesions with severe elevations of blood urea nitrogen and serum creatinine; this abnormal chemical environment can have systemic effects and can interfere with investigation of the aging process (3). These changes are not encountered in aging humans who are otherwise healthy. (b) Aging rodents commonly develop tumors as terminal events (3,4) whereas cardiovascular disease is the most common cause of death in humans. Tumors can have systemic effects and interfere with studies on healthy aging. Study of aging must be separated from study of diseases (5). (c) Tubulointerstitial fibrosis is an important aspect of aging-associated structural change in the human kidney (6). In our experience, this lesion is not easily detectable in aging mice. It is important to explore new models of renal aging because inordinate focus on rodent models can result in perpetuation of limitations of those models in terms of relevance to human kidney aging (5).
Given the evolutionary distance between rodents and humans, studies employing species that are genetically closer to humans such as nonhuman primates would be more relevant to understanding mechanisms of human renal aging. Previous investigators have addressed this deficiency by studying nonhuman primates such as the common marmoset (Callithrix jacchus). Among nonhuman primates marmosets have a number of characteristics that make them attractive for translational biomedical modeling and aging research (7). Their smaller size permits ease of handling. Their higher fertility rate allows studies on greater numbers of animals facilitating sound statistical analyses. Additionally, due to their shorter life span, interventions can be tested over a shorter time span. Previous investigators have reported aging-induced structural changes such as glomerular mesangial matrix expansion and interstitial fibrosis in the kidneys of marmosets (8). However, the mechanisms leading to these changes have not been explored. As marmosets are being increasingly adopted as a model for studies on human aging, it is essential to understand mechanisms underlying senescence-induced changes to test novel interventions to improve health span.
Accumulation of matrix proteins leading to kidney fibrosis involves increase in their synthesis and decreased degradation; however, the regulatory pathways involved in stimulating protein synthesis are unknown in marmosets. Recent investigations have revealed a novel regulatory role for H2S in kidney protein synthesis (9). Kidney disease states characterized by fibrosis are associated with deficiency of H2S generation (9). Kidney constitutively synthesizes significant amounts of H2S via the trans-sulfuration pathway in which conversion of l-homocysteine to l-cystathionine and eventually to l-cysteine releases H2S at several steps; these reactions are catalyzed by cystathionine γ-lyase (CSE) and CBS (9). In addition, mitochondrial 3-mercaptopyruvate sulfotransferase and peroxisomal d-amino acid oxidase also catalyze release of H2S (9). H2S deficiency occurs in the setting of high glucose-induced kidney cell injury, and, sodium hydrosulfide (NaHS), a source of H2S, ameliorates high glucose-induced cell injury including increase in synthesis of matrix proteins by stimulating AMPK and inhibiting mTORC1 (10). Reduced generation of H2S was also observed in aging mice and administration of NaHS to aging mice arrested structural and functional abnormalities (11). Whether H2S dysregulation in the kidney occurs in aging nonhuman primates is not known; accordingly, we examined the status of H2S metabolic pathway in the kidney in aging marmosets.
It is well recognized that females are less susceptible to kidney injury due to hypertension and other diseases (12); whether this sexual dimorphism applies to aging-related changes in the kidney is not well studied in higher order mammals such as nonhuman primates. In view of these deficiencies, we explored whether aging-related changes in the kidney are associated with stimulation of signaling pathways that govern protein synthesis including the status of H2S pathway, and, if there are sex differences in how aging affects the kidney in marmosets.
Method
Materials and Sources
We employed antibodies against the following: Laminin γ1, phospho-Tyr1165/1166-IGF-1 receptor, IGF-1 receptor, phospho-Ser9-glycogen synthase kinase 3 β (GSK3β), GSK3α/β (binds both isoforms), TGFβ1, CSE, CBS (Santa Cruz Biotechnology, Inc., Dallas, TX), type III collagen, fibronectin, nephrin (Abcam Plc, Cambridge, MA), phospho-Ser79-Acetyl-CoA carboxylase (ACC), ACC, phospho-Ser235/236-ribosomal protein S6 (rpS6), rpS6, phospho-Ser423/425-Smad3 (Cell Signaling Technology, Inc., Denvers, MA), Smad3 (Thermo Fisher Scientific, Inc., Waltham, MA), actin (Millipore Sigma, St. Louis, MO). Commercial ELISA kit was used for measuring phospho-Tyr-1150/1151 insulin receptor (IR) (Cell Signaling Technology, Inc, Denvers, MA).
Marmosets
We employed four young male (average age 3.02 years) and four young female (average age 2.8 years) marmosets, and, five aged male (average age 16.08 years) and five aged female (15.86 years) marmosets. We collected urine for 24 hours from each marmoset for estimation of protein and creatinine before sacrifice. The marmosets appeared healthy without evidence of diarrhea and wasting syndrome at the time of sacrifice. This study was approved by the IACUC of the Texas Biomedical Research Institute, San Antonio, TX.
Histology
Kidney samples were fixed in 10% neutral buffered formalin (Millipore Sigma, St. Louis, MO), processed conventionally, embedded in paraffin, and cut at 3 µm. Two slides were prepared from each kidney: one was stained with hematoxylin and eosin and the other with periodic acid-Schiff (PAS). In selected cases, a third slide was stained with Congo Red and evaluated utilizing polarized light. All slides were evaluated by light microscopy by a veterinary pathologist and an MD pathologist. Renal changes were scored using a semi-quantitative system with the pathologist blinded to the age group of the marmosets. There were on an average 65 glomeruli in the sections of kidney examined (average of 18 animals; 46–83 glomeruli per marmoset). Glomerular lesions consisting of segmental or global sclerosis were scored from 0 to 5 by visual estimate utilizing PAS-stained sections with 0 corresponding to absence of sclerosis, 1 corresponding to up to 20% of glomeruli involved, 2 from 21% to 40%, 3 from 41% to 60%, 4 from 61% to 80% and, 5 from 81% to 100%. Segmental and global lesions were defined as involvement of a part of the glomerular tuft or the entire glomerular tuft in individual glomeruli, respectively, and were considered together in scoring. Glomerular mesangial cellularity was defined as clustering of more than three mesangial cells within the glomerular tuft away from the vascular pole (13). Cortical tubular and interstitial lesions were scored for fibrosis from 0 to 5 with 0 being no fibrosis, 1 corresponding to up to 20% of tubulointerstitial area involved, 2 from 21% to 40%, 3 from 41 to 60%, 4 from 61% to 80%, and 5 from 81% to 100%. We looked for evidence of inflammatory cell types in the cortex and medulla. Renal medulla was also examined for evidence of inflammatory cell infiltrate and fibrosis. Glomerular, afferent, efferent, and arcuate vascular morphology was also evaluated.
Clinical Laboratory Assays
We employed a human albumin ELISA kit to measure urinary albumin (Bethyl Laboratories, Inc., Montgomery, TX) and a colorimetric assay to measure urinary creatinine (Enzo Life Sciences, Inc., Farmingdale, NY). Ratio of urinary albumin to creatinine was employed to evaluate albuminuria. We measured urinary protein using Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Inc., Hercules, CA) with BSA standards (Thermo Fisher Scientific, Inc.) and generated urinary protein to creatinine ratio to assess proteinuria. Serum creatinine was measured by an autoanalyzer employing blood samples that were collected about 7 months before sacrifice and kept frozen.
Immunoblot Analysis
Equal amounts of renal cortical protein from homogenates were employed in immunoblotting for individual proteins as described (2). Signal intensities were quantified using the ImageJ software.
H2S Measurements
The assay was performed as described previously with some modification (14). Briefly, equal amounts of renal cortical homogenates were lysed in ice-cold 100 mM potassium phosphate buffer (pH 7.4) using a sonicator. Four hundred fifty micrograms of tissue homogenate was incubated with 400 µM of l-cysteine, 80 µM of pyridoxal 5′-phosphate in 500 µL reaction volume for 3 h at 37°C. Two hundred fifty microliters of zinc acetate (1%, w/v) was added to the reaction tube followed by 10% TCA. One hundred thirty-three microliters of N, N-dimethyl-p-phenylenediamine sulfate (20 µM) in 7.2 M HCl was added followed by incubation with 133 µL of ferric chloride (30 µM) in 1.2 M HCl for 2 hours at room temperature. Total H2S production was measured by a 96-well microplate reader (670 nm, Magellan 6, Tecan Systems, Inc.) with sodium hydrosulfide (NaHS) standard (0.1–50 µM) and expressed as micromoles/g/min.
Statistical Analysis
Data were expressed as mean ± SEM. We log transformed the data and analyses were performed by two-way analysis of variance (ANOVA) and post-test Bonferroni correction (GraphPad Prism). Data were considered statistically significant at p <.05. The semi-quantitative histology data were analyzed by Student’s t test.
Results
Clinical Parameters
Data are shown in Table 1. There were no significant differences in body weight between young and aged marmosets regardless of sex. Fasting blood glucose levels were in the normal range in both young and aged male and female marmosets. There was no significant difference in serum creatinine values between the young and aged marmosets and the values did not differ between males and females. Kidney weight and kidney to body weight ratio showed a nonsignificant trend toward increase in both male and female aged marmosets (Table 1).
Table 1.
Body Weight, Blood Glucose, Serum Creatinine, and Kidney Weight Data
| Male | Female | |||
|---|---|---|---|---|
| Young (n = 4) | Aged (n = 5) | Young (n = 4) | Aged (n = 5) | |
| Age (years) | 3.02 ± 0.33 | 16.08 ± 1.11 | 2.80 ± 0.14 | 15.86 ± 1.04 |
| Body weight (g) | 372.8 ± 14.9 | 361.0 ± 16.0 | 396.3 ± 19.7 | 359.0 ± 11.1 |
| Serum glucose (mg/dL) | 101.8 ± 11.2 | 105.4 ± 9.7 | 104.5 ± 15.0 | 97.0 ± 14.6 |
| Serum creatinine (mg/dL) | 0.45 ± 0.03 | 0.43 ± 0.08 | 0.35 ± 0.05 | 0.30 ± 0.00 |
| Kidney weight (g) | 1.88 ± 0.09 | 2.50 ± 0.27 | 2.40 ± 0.30 | 2.92 ± 0.31 |
| Kidney/body weight (g/kg) | 5.04 ± 0.25 | 6.92 ± 0.63 | 6.02 ± 0.60 | 8.16 ± 0.89 |
Note: No significant differences in parameters were found among the groups by two-way ANOVA.
Histological Changes in the Kidney
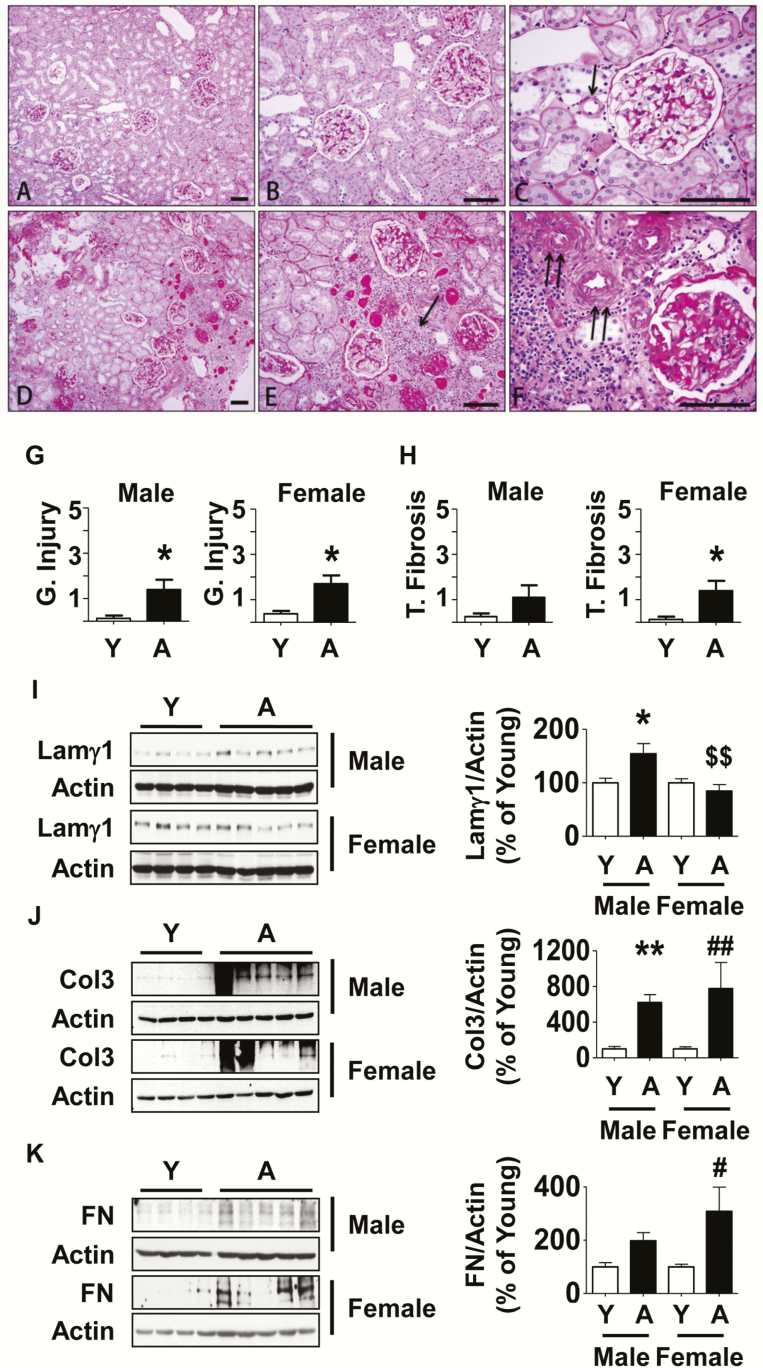
Overall, young male and female marmosets demonstrated normal renal architecture (Figure 1A–C). Renal glomeruli of young marmosets had round to oval configuration with a uniform crescent shaped urinary space (Bowman space) lined by a flat inconspicuous population of parietal epithelial cells. Renal tubules were closely packed in cortex and medulla, lacking intervening deposits of fibrotic tissue, fibroblasts, or inflammation. A few of the young marmosets exhibited minimal tubular accumulation of eosinophilic fluid (casts) ranging from 0 to 6 ectatic tubules per 20× field. Bowman’s capsule, glomerular capillary wall, mesangial matrix, and tubular basement membranes stained positive with PAS stain. Mesangial cellularity was normal and uniform throughout the glomerular tuft and widely separated by uniform-sized thin walled capillary loops with flat endothelial lining. Bowman’s capsule and tubular basement membranes were well demarcated, thin and were of uniform width. Blood vessels were unremarkable.
Figure 1.
Kidney histology and extracellular matrix protein expression in aged marmosets. (A–C) By PAS stain, in young marmosets glomeruli displayed normal cellularity without segmental or global accentuation of mesangial matrix. Tubules were closely packed without atrophy. Blood vessels (arrow in C) were thin walled and lumens were patent. (D–F) In aged marmosets, glomeruli showed either segmental (part of an individual glomerulus) or total (whole of an individual glomerulus) sclerosis. Parietal glomerular basement membrane showed thickening and wrinkling. Tubules showed significant atrophy, loss, thickening of tubular basement membrane and many contained casts. There were rare areas of interstitial nephritis composed of mononuclear cells (arrow in E). Blood vessels showed narrowing of lumen and severe medial hypertrophy and sclerosis (double arrows in F). (G and H) Semi-quantitative scoring of indices of glomerular injury and tubular fibrosis are shown (Y = young, A = aged). Data are shown as mean ± SEM, n = 4 young male and n = 4 young female marmosets, n = 5 aged male and n = 5 aged female marmosets (*p < .05 by t-test). Renal cortical lysates were employed to perform immunoblotting using antibodies against (I) laminin γ1, (J) collagen III, and (K) fibronectin. Actin expression was used to assess loading (Y = young, A = aged). Data are shown as mean ± SEM, n = 4 young male and n = 4 young female marmosets, n = 5 aged male and n = 5 aged female marmosets (*p < .05, **p < .01 vs young male; #p < .05, ##p < .01 vs young female, $$p < .01 vs aged male by two-way ANOVA).
Aged male and female marmosets exhibited several morphologic alterations of varying severity (Figure 1D–F). A striking feature of age-related changes was the heterogeneity in distribution of these abnormalities within the renal parenchyma of individual marmosets suggesting structural changes occurred at a highly variable rate in both males and females. The size and shape of glomeruli of aged marmosets were variable. PAS positive mesangial matrix was increased in both sexes, frequently resulting in segmental or global sclerosis. Glomerular mesangial hypercellularity was not observed. Sclerotic glomeruli frequently showed irregular thickening of Bowman’s capsule and were sometimes surrounded by inflammatory cells (Figure 1F). Interstitial infiltrates of lymphocytes and plasma cells were seen frequently in association with areas of tubular atrophy and tubulointerstitial fibrosis (Figure 1E). PAS positive tubular basement membranes were thickened and distorted. In some tubules, epithelial cells exhibited vacuolar type swelling, intracytoplasmic accumulation of brown pigment or eosinophilic material. There was occasional epithelial regeneration consisting of hypertrophy, increased basophilic cytoplasm, and piling of tubular epithelial cells, particularly in areas of significant tubular loss. Both male and female marmosets exhibited mild to moderate tubular casts ranging from 3 to 20 ectatic cortical tubules per 20× field. The renal medulla was generally less affected, although in some animals, inflammatory infiltrates and increased eosinophilic matrix resulting in separation of collecting ducts could be seen. Extramedullary hematopoiesis within the cortical interstitium was noted in one aged male marmoset. Renal arterioles showed significant medial hypertrophy with narrowed lumens consistent with arteriolosclerosis (Figure 1F).
Semi-quantitative scoring showed that aging was associated with significant glomerular injury in both sexes whereas the tubular fibrosis score was significantly higher in female marmosets although a trend toward increase was seen in males (Figure 1G and H). These injury patterns are in agreement with previous reports (8,15). The histologic changes are similar to aging-related changes (nephrosclerosis) described in humans (6). We did not find significant differences in age-related structural changes between male and female aged marmosets. Important negative findings were lack of glomerulonephritis in young or aged marmosets. In one female aged marmoset, deposits suggestive of amyloid protein were seen; however, they did not show apple green birefringence on Congo Red staining examined under polarizing light (data not shown).
Expression of Matrix Proteins Is Increased in Aging Marmosets
We examined the expression of individual matrix proteins to corroborate histologic findings of matrix expansion. Laminin, fibronectin, and collagens are important components of the renal extracellular matrix and increase in their content contributes to renal fibrosis (16). Laminin is a heterotrimer made of α, β, and γ chains; laminins in different matrix compartments of the kidney have distinct chain compositions. For example, laminin in glomerular basement membrane contains α5, β2, γ1 chains (laminin521). Immunoblotting showed that aging was associated with a significant increase in renal cortical laminin γ1 chain in males but not female marmosets (Figure 1I). Increase in collagens I and III contributes to tubulointerstitial fibrosis in the kidney; aging was associated with increase in the renal cortical expression of collagen type III in both male and female marmosets (Figure 1J). Fibronectin, a component of the glomerular basement membrane, was increased in aged female marmosets with a trend toward increase in aged male animals (Figure 1K).
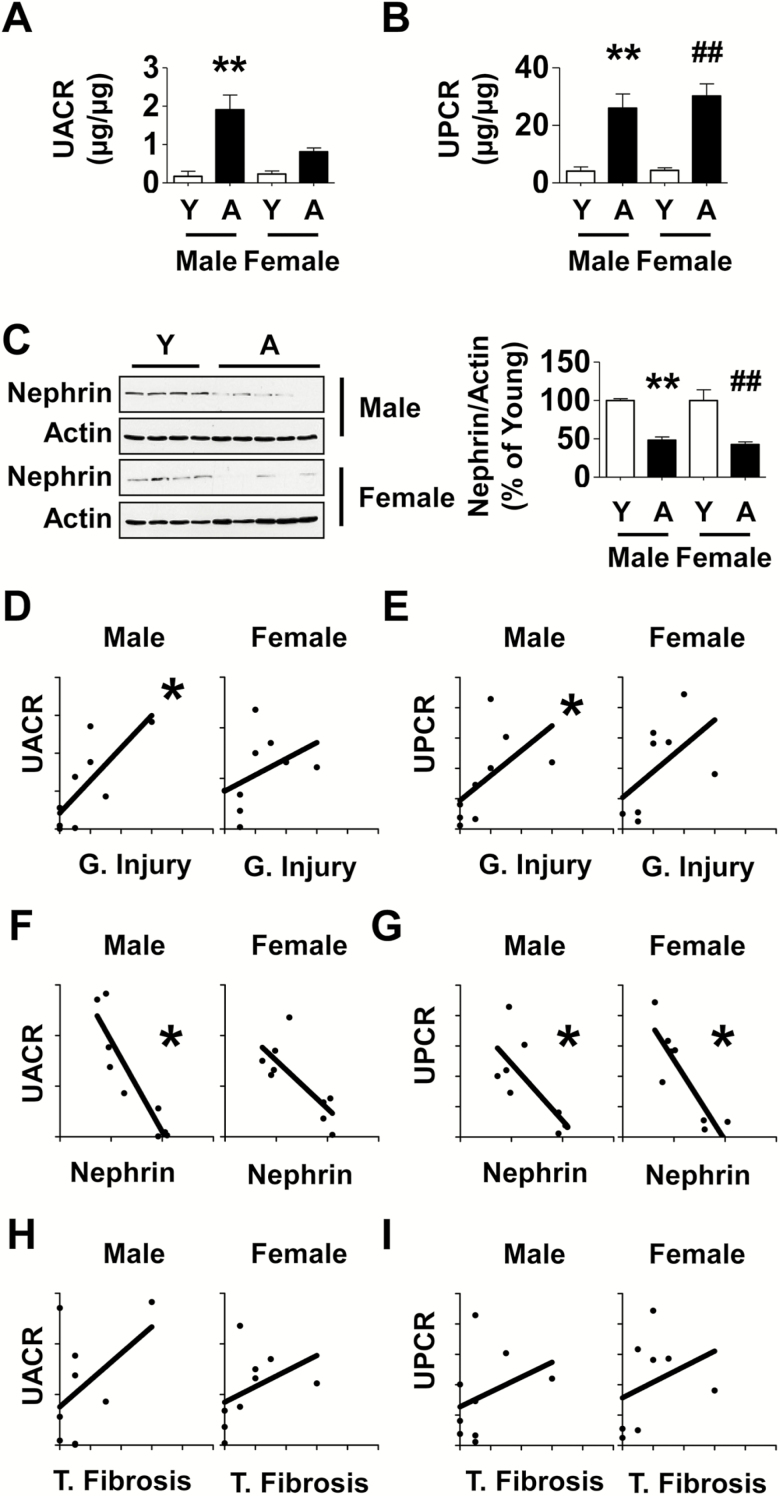
Aging Is Associated With Albuminuria and Proteinuria in Marmosets
Albuminuria measured as the ratio of urinary albumin to urinary creatinine (UACR) is a commonly employed index of kidney dysfunction in clinical medicine. UACR was increased 11-fold in male aged marmosets and a trend toward increase was seen in female aged marmosets (Figure 2A). Because urinary protein profile can include other proteins in addition to albumin we also estimated urinary protein to creatinine ratio (UPCR), another commonly employed clinical tool. UPCR was increased by 6.3- and 7-fold in male and female aged marmosets, respectively (Figure 2B). Nephrin is a component of the slit diaphragm of glomerular podocyte and participates in preventing losses of albumin and other proteins during glomerular ultrafiltration (17). Expression of kidney cortical nephrin was reduced by nearly 50% in both aged male and female marmosets (Figure 2C) providing a mechanistic basis for urinary protein losses associated with aging.
Figure 2.
Aging is associated with changes in kidney function in marmosets and they correlate with histological changes. (A and B) Urinary albumin to creatinine ratio (UACR) and urinary protein to creatinine ratio (UPCR) data in aged marmosets and young marmosets are shown. (C) Immunoblotting of kidney cortical lysates showed reduction in the expression of nephrin in aged marmosets (Y = young, A = aged). Data in A–C are shown as mean ± SEM, n = 4 young male and n = 3–4 young female marmosets, n = 5 aged male and n = 5 aged female marmosets (**p < .01 vs young male; ##p < .01 vs young female by two-way ANOVA). Correlation between glomerular injury (G. Injury) and tubular fibrosis (T. Fibrosis) indices and UACR, UPCR was studied. (D and E) A high degree of correlation existed between glomerular injury and UACR (r = .78, *p = .0172), and UPCR (r = .80, *p = .0138) in aged males; a trend toward correlation between glomerular injury and UPCR (r = .68, p = .0694) but not UACR (r = .58, p = .1080) was seen in aged females. (F and G) A strong negative relationship was present between nephrin expression and UACR (r = −0.88, *p = .0031) and UPCR (r = −.70, *p = .0433) in aged males; in aged females, a negative correlation was present between nephrin and UPCR (r = −0.81, *p = .0218) and a trend with UACR (r = −0.65, p = .0666). (H and I) In aged males, tubular fibrosis did not have a significant relationship with UACR (r = .25, p = .5206) or UPCR (r = .38, p = .3125); in aged females a trend was seen with UACR (r = .67, p = .0589) but not with UPCR (r = .51, p = .1966).
Kidney Histological Changes Correlate With Changes in Function
During filtration the glomerulus prevents losses of large amounts of albumin and other proteins by functioning as a size- and charge-selective barrier although small amounts of albumin do escape. Albumin in the filtrate is subsequently absorbed by the proximal tubules allowing only minimal amounts of albumin to appear in the urine. Thus, in kidney disease, albuminuria and proteinuria could occur by abnormalities in glomerular and/ or tubular function. We explored statistical correlation between scores of glomerular and tubular injury and UACR and UPCR. In male aged marmosets, there was a high degree of correlation between glomerular injury score and UACR (r = .78) and UPCR (r = .80); in female aged marmosets, glomerular injury score showed a trend toward correlation with UPCR (r = .68) but not with UACR (Figure 2D and E). There was a significant negative relationship between nephrin expression and UACR (r = −.88) and UPCR (r = -.70) in aged males; in aged females, while a negative correlation existed between nephrin and UPCR (r = −.81), only a trend was seen with UACR (r = −.65; Figure 2F and G). Tubular fibrosis score did not bear a significant relationship with UPCR in aged males and females; in aged females a trend was seen with UACR (r = .67) but not with aged males (Figure 2H and I). Taken together, these data suggest glomerular injury as the main mechanism of albuminuria and proteinuria in aging; some of the correlations would have probably met significance with greater number of animals.
Signaling Pathways Related to Protein Synthesis Are Activated in the Kidney in Aging Marmosets
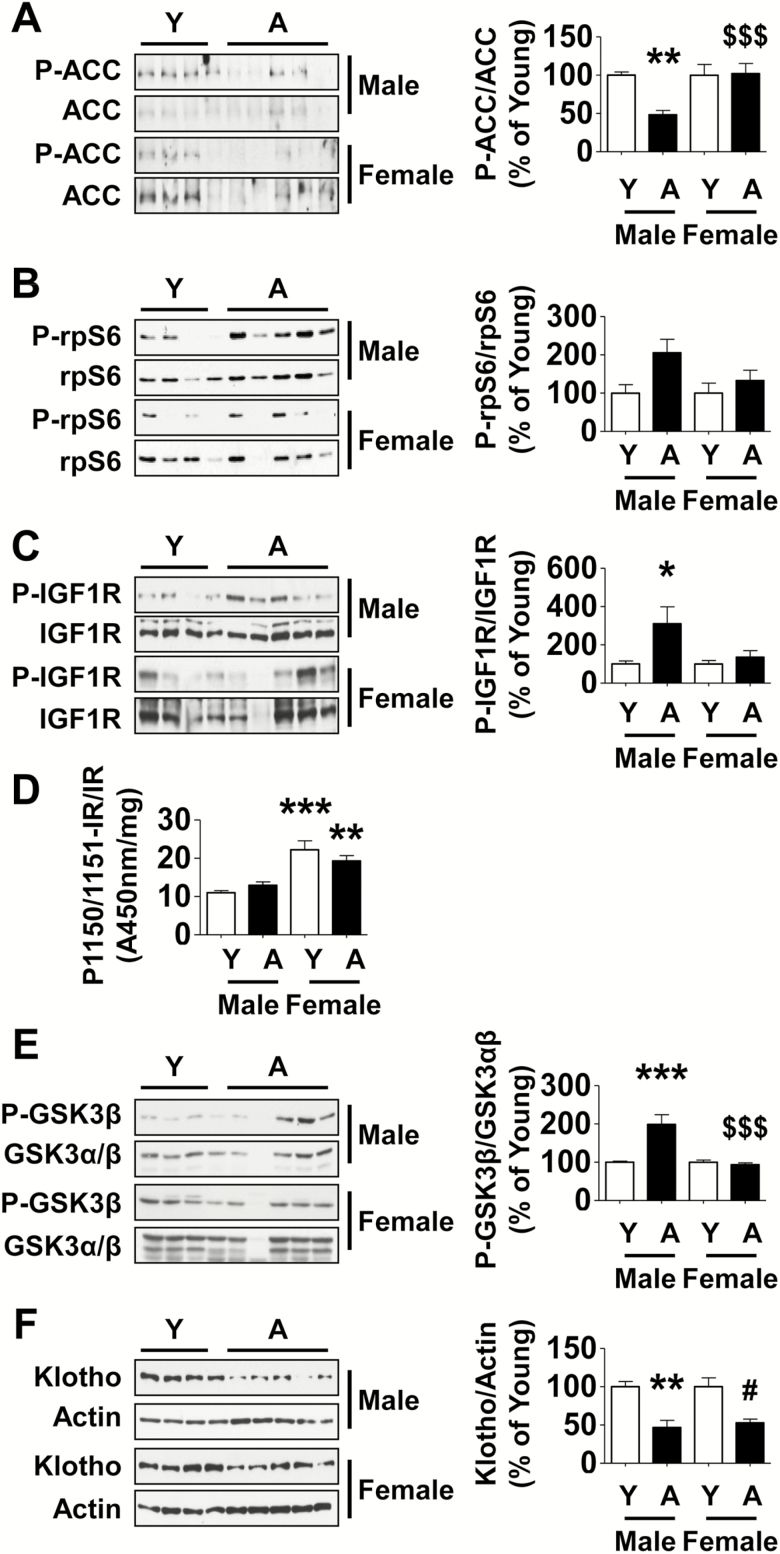
Accumulation of extracellular matrix proteins in the kidney is a dynamic process involving coordinated changes in synthesis and degradation. In terms of synthesis, the regulatory site could be at the level of transcription or at the level of mRNA translation (18–20). Translation of mRNA, an important rate limiting step in protein synthesis, is under the control of signaling pathways which fall into two categories, that is, those that promote and those that inhibit translation. In the kidney, pathways that stimulate mRNA translation commonly involve activation of receptor tyrosine kinases such as the IR, IGF-1 receptor, vascular endothelial growth factor receptor leading to activation of Akt-mTORC1 axis (20–22). Signaling pathways that inhibit mRNA translation in the kidney involve AMPK (10,14,23,24) and GSK3β (25). We examined key signaling events in the kidneys of marmosets for their relevance to aging-related changes in increase in matrix protein expression.
Activity of AMPK was assessed by immunoblotting for Ser-69 phosphorylation of ACC its direct substrate. Compared with young marmosets, ACC phosphorylation was significantly decreased in aged male marmosets but not in aged female marmosets (Figure 3A). When mTORC1 is activated it phosphorylates Thr-389 of p70S6 kinase leading to stimulation of its activity; in turn, p70S6 kinase phosphorylates rpS6 and promotes the initiation phase of mRNA translation (22). Activity of mTORC1 was assessed by phosphorylation of rpS6. Immunoblotting showed a trend toward increase in phosphorylation of rpS6 in the kidney cortex of aged male marmosets but not female marmosets (Figure 3B). We then examined potential upstream signaling events that could stimulate mTORC1. We chose to study IGF-1 receptor and IR because they have been implicated in aging-related changes (26,27); these receptors function as kinases and require phosphorylation of tyrosine residues for activation. Tyrosine phosphorylation of IGF-1 receptor was increased nearly threefold in the renal cortex of aged male marmosets but not aged female marmosets (Figure 3C). ELISA for tyrosine phosphorylation of IR did not show significant changes in either sex of aged marmosets compared with the respective young animals of the same sex. Renal cortical IR tyrosine phosphorylation was higher in both young and old female marmosets compared with the respective male counterparts (Figure 3D). We did not find convincing evidence for stimulation of mTORC1 as a downstream read out of IR activation in young or old female marmosets compared with corresponding males. Stimulation of receptor tyrosine kinases such as IGF-1 receptor leads to activation of Akt, a serine threonine kinase that is upstream of AMPK and mTORC1. Because Akt directly phosphorylates GSK3β on Ser-9, we employed it as a functional index of Akt activation. Immunoblotting revealed a significant increase in Ser-9 phosphorylation of GSK3β in the kidney cortex of aged male but not female marmosets (Figure 3E). These data suggest IGF-1 receptor-Akt-mTORC1 axis may serve as a pathway to stimulate matrix protein synthesis in the kidneys of aging male marmosets. In contrast to males, aging in female marmosets was not associated with activation of these signaling pathways. Although IR tyrosine phosphorylation was increased in both young and old female marmosets, it was not accompanied by Akt activation; more studies are needed to explore differences in IR activity between the sexes.
Figure 3.
Signaling pathways are dysregulated in the kidney cortex of aged male but not female marmosets. Renal cortical lysates were employed to perform immunoblotting using antibodies against (A) phospho-ACC, ACC, (B) phospho-rpS6, rpS6, (C) Tyr-1165/1166 phosphorylated IGF-1 receptor (P-IGF 1R), IGF 1R, (E) Ser-9 phosphorylated GSK 3β, GSK 3β, (F) Klotho and actin. (D) ELISA was performed to assess changes in Tyr-1150/1151 phosphorylation of the IR (Y = young, A = aged). Data are shown as mean ± SEM, n = 4 young male and n = 4 young female marmosets, n = 4–5 aged male and n = 4–5 aged female marmosets (*p < .05, **p < .01, ***p < .001 vs young male; #p < .05 vs young female, $$$p < .001 vs aged male by two-way ANOVA).
Klotho is a determinant of lifespan; mice deficient in klotho age faster whereas klotho transgenic mice demonstrate a slower rate of aging (28). It is robustly expressed in the kidney particularly by distal tubular epithelial cells (29). Because klotho has been shown to inhibit insulin receptor and IGF-1 receptor signaling (28), we examined whether a decrease in klotho expression could be associated with activation of IGF-1 receptor. Klotho expression was significantly decreased in both male and female aged marmosets (Figure 3F). While reduced klotho may contribute to IGF-1 receptor activation in male aged marmosets, its functional significance in female aged marmosets in whom IGF-1 receptor was not activated remains to be defined.
TGFβ Is Activated in the Kidney of Aging Marmosets
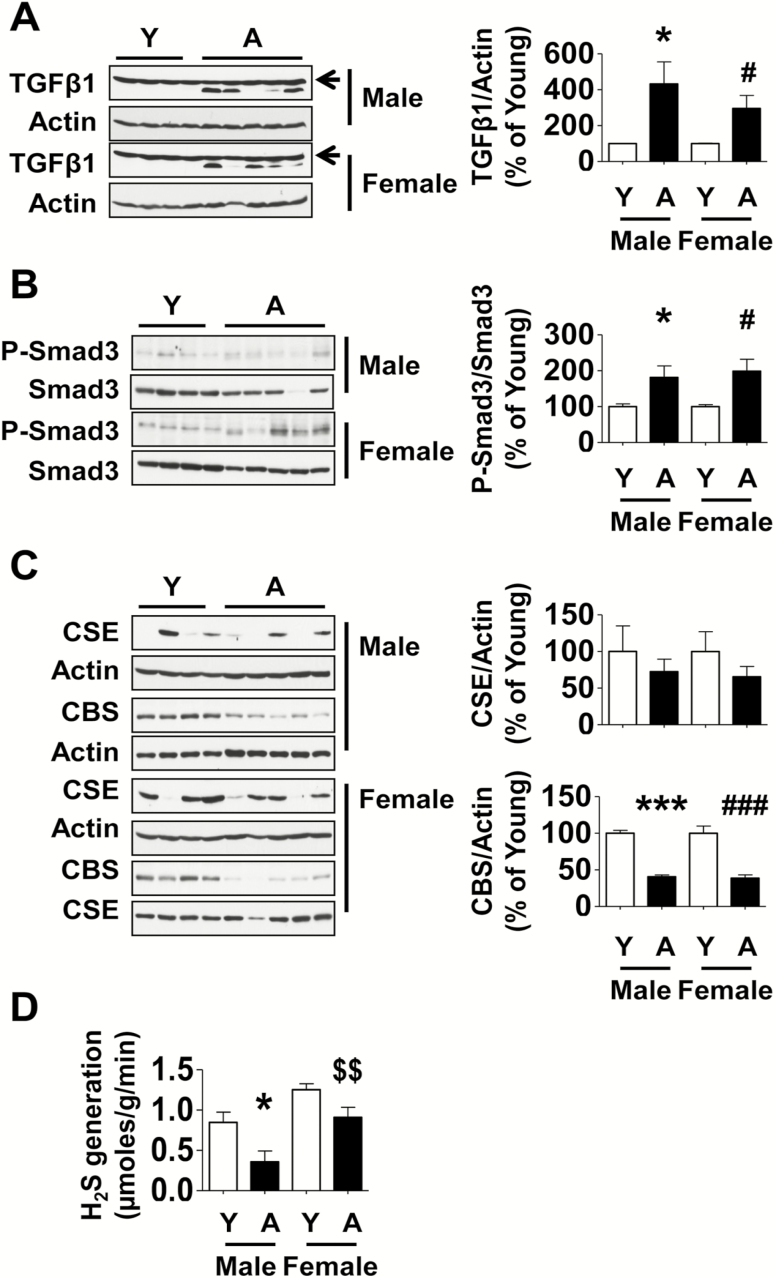
TGFβ, a fibrogenic cytokine, plays an important role in fibrosis of the kidney in a wide variety of pathologic states such as diabetes and genitourinary obstruction; its activation promotes phosphorylation of Smad3, which translocates to nucleus and stimulates transcription of matrix proteins (30). TGFβ expression was significantly increased in the renal cortex of both male and female aged marmosets (Figure 4A). Smad3 phosphorylation was also increased in the kidneys of both male and female aged marmosets (Figure 4B) suggesting activation of TGFβ signaling pathway.
Figure 4.
Changes in TGFβ and H2S pathways in the kidney cortex of aged marmosets. Equal amounts of renal cortex were employed in immunoblotting using antibodies against (A) TGFβ1 and actin (unchanged band above TGFβ1 is nonspecific [arrow]), (B) phospho-Smad3 and Smad3, and (C) CSE and CBS. (D) H2S generation was measured as described in Method section (Y = young, A = aged). Data are shown as mean ± SEM, n = 4 young male and n = 4 young female marmosets, n = 5 aged male and n = 5 aged female marmosets (*p < .05, ***p < .001 vs young male; #p < .05, ###p < .001 vs young female, $$p < .01 vs aged female by two-way ANOVA).
H2S Generation Is Reduced in the Kidney of Aged Marmosets
We previously alluded to the association of H2S deficiency in renal tissue in states of renal fibrosis. We have recently reported that aging-related kidney fibrosis in mice is associated with decrease in H2S generation due to reduction in the expression of CBS and CSE; administration of NaHS to aging mice beginning at the age of 19 months for 5 months resulted in significant amelioration of kidney dysfunction including albuminuria and glomerulosclerosis (11). These data provided rationale for examining the status of H2S in the kidney in aging marmosets.
Expression of CBS in the kidney cortex was significantly reduced in both male and female aged marmosets although there was no change in the content of CSE (Figure 4C). This was associated with a significant 58% reduction in H2S generation in the renal cortex of aged male marmosets; there was a trend toward reduced H2S generation by 27% in the renal cortex of aged female marmosets that did not reach significance (Figure 4D).
Discussion
Our data show that aging-associated structural changes consisting of kidney glomerulosclerosis and tubulointerstitial fibrosis and arteriolosclerosis called nephrosclerosis in the marmosets are similar to changes seen in rodents and humans (2,6,31) although tubulointerstitial fibrosis was more easily observed in the marmosets compared with mice. The morphological lesions that we encountered in aged marmosets in this study differed from other known spontaneous renal disease entities such as nonprogressive glomerulopathy characterized by immunoglobulin deposits but lacking interstitial disease (32). Kidney functional disturbances measured as albuminuria and proteinuria are also similar to that observed in aging rodents (2,31). Some of the signaling abnormalities in kidneys of marmosets were similar to those found in aging mice and rats (11,33), for example, reduced activity of AMPK, stimulation of Akt-mTORC1 axis. Upstream of these signaling events, whereas IR was activated in the kidneys in aged mice (11), activation of IGF-1 receptor was seen in the marmosets. In general terms, aging-associated structural, functional, and signaling changes in the kidney are retained through mammalian evolution. Our data also suggest that the marmoset is a good model to study aging-related changes in the kidney and to evaluate efforts to improve them.
The current study provides a framework for exploring signaling mechanisms underlying aging-related changes in the kidney. Activation of IGF-1 receptor in the renal cortex of aged marmosets may lead to increased protein synthesis via phosphoinositide 3 kinase–Akt activation as suggested by studies in kidney tubular epithelial cells (34). IGF-1 receptor activation may have been facilitated by deficiency of klotho, which is known to inhibit signaling by that receptor (28). Increased TGFβ expression in aging marmoset kidneys could also lead to increase in matrix synthesis by stimulating Akt in a phosphoinositide 3 kinase-dependent manner as suggested by studies in glomerular mesangial cells (35). Akt activation stimulates mTORC1 in three ways. Firstly, it phosphorylates Ser-487 of the α subunit of AMPK and decreases its activity (36). Decrease in AMPK activity is associated with activation of mTORC1 in the kidney (23). Secondly, Akt can phosphorylate and inhibit tuberin (TSC2) removing tonic inhibition of mTORC1 (37). The third mechanism involves Akt phosphorylation of proline rich Akt substrate-40, a component of mTORC1 and its constitutive inhibitor; when phosphorylated by Akt, proline rich Akt substrate-40 dissociates from mTORC1 resulting in activation of the latter (38). As mentioned earlier, mTORC1 phosphorylates Thr-389 of p70S6 kinase stimulating its activity; p70S6 kinase is an important regulator of mRNA translation, the rate-limiting step in protein synthesis (22). Activated p70S6 kinase phosphorylates rpS6 on Ser-235, -236, -240, -244, and -247 (39). Studies employing mice in which these five serines have been replaced by nonphosphorylatable alanine by knock in have shown that compensatory renal hypertrophy following uninephrectomy is inhibited (40). Because increase in protein synthesis is required for kidney hypertrophy, these data show an important role for rpS6 phosphorylation. In the context of aging, rpS6 phosphorylation may serve to augment synthesis of matrix proteins. Previous studies have demonstrated importance of mTORC1 activation in renal matrix accumulation in states of kidney injury, for example, diabetes (19,41). In support of an important role for mTORC1 in kidney dysfunction in aging rats, Shavlakadze and colleagues (42) have shown that RAD001, a rapalog, ameliorates aging-related kidney parenchymal lesions. An additional mechanism of aging-related kidney dysfunction could involve impaired autophagy associated with increase in mTOR activity (43). Recent studies have shown that parabiosis of old mice with young mice results in recovery of autophagy along with amelioration of apoptosis and inflammation, and, aging-associated histologic kidney lesions; these data suggest a mechanistic role for impaired autophagy in renal aging (44). Our finding of mTOR activation in kidney aging is in line with the well-known role of mTOR inhibition by rapamycin in extension of life span (45). Our data support evaluation of rapamycin as an intervention to ameliorate aging-related matrix accumulation in the kidney in marmosets. Tardif and colleagues (46) have reported that long-term administration of oral formulation of rapamycin is well tolerated by marmosets.
The other signaling event of potential translational importance is inhibition of AMPK in the kidney in aging marmosets. AMPK activates TSC2 by phosphorylation on Ser-1227 and -1345 (47). In combination with TSC1, activated TSC2 functions as a GTPase-activating protein and inhibits GTP-bound state of Ras homolog enriched in brain (Rheb) (47). When AMPK is inactivated, TSC2 activity is inhibited leading to retention of Rheb in the GTP-bound state. GTP bound Rheb promotes mTORC1 activation by as yet unknown mechanism (48). Thus, deficient AMPK stimulates mTORC1 and protein synthesis. The mechanism by which aging results in reduced AMPK activity is not clear; however, it may be due to inhibition by Akt that is activated by IGF-1 receptor and/ or TGFβ. Changes in AMP and ATP content can alter AMPK activity. AMPK is inhibited in the kidney in states of deficiency in AMP or increase in ATP content (49). Another contributing factor could be H2S deficiency that is discussed below. Administration of agents that stimulate AMPK, such as metformin, AICAR, NaHS, and tadalafil inhibit high glucose-induced mTORC1 and increase in matrix proteins in kidney cells (10,14,23,41,50,51). Strategies to limit aging-related kidney dysfunction in marmosets could include these agents some of which are already in routine clinical use for other indications, for example, metformin, tadalafil. An additional potential target is klotho; aging male marmosets manifest klotho deficiency in association with activation of IGF-1 receptor (28); thus, administration of klotho could inhibit that receptor and restore integrity of the kidney in aging marmosets.
A novel finding in this study is the detection of reduced kidney H2S generation in aged male marmosets. This appears to be due to reduced expression of CBS. Although the expression of CSE, another H2S generating enzyme in the trans-sulfuration pathway, was unchanged, there could be factors that inhibit its activity in the aging kidney. H2S deficiency also occurs in the kidneys of aged mice (11) suggesting it may be an evolutionarily conserved phenomenon. This observation could have translational value because administration of NaHS ameliorates indices of structural and functional derangement in mice (11). We have reported that H2S augments AMPK activity in renal cells (10,24). H2S deficiency could contribute to inhibition of AMPK activity leading to mTORC1 activation. H2S deficiency is commonly encountered in acute kidney injury due to ischemia (52) and in chronic kidney disease due to diabetes (53), renovascular hypertension (54), or hyperhomocysteinemia (55); administration of agents that provide H2S ameliorates kidney injury in these states (9). As H2S is intolerable to humans due to odor, agents that release H2S, for example, GYY4137 may be employed to test if they ameliorate aging-related dysfunction of the kidney in marmosets. Phosphodiesterase 5 inhibitor, tadalafil, could be tested as an interventional agent because it prevents high glucose-induced matrix protein synthesis in a H2S-dependent manner studies in kidney cells in vitro (14).
Biomedical research has often neglected to evaluate females and potential differences between males and females; we included female animals in addition to males in our study to broadly define kidney aging in the marmoset model. Both genders showed similar structural and functional abnormalities although there were differences in signaling events; unlike male marmosets, the female aged marmosets did not demonstrate changes in AMPK, Akt, mTORC1 activity, and, IGF-1 receptor activation. On the other hand, TGFβ increase and Smad3 phosphorylation were similar as was the reduction in the expression of CBS. H2S generation was not reduced in aged female marmosets suggesting a compensatory adjustment to reduction in CBS expression. These data suggest that there may be differences in signaling pathways that regulate protein synthesis between the sexes. Previous reports suggest that male sex is associated with greater degree of aging-related dysfunction (56).
Although our data catalog a variety of age-related changes in growth factor-protein synthesis signaling pathways, at the present time they are correlational; future studies are needed to explore if they play a causal role. Our study identifies several individual signaling molecules as potential sites for intervention to reduce structural and functional derangements associated with aging. Our study suggests that the marmoset is a reasonable nonhuman primate model for preclinical studies to both understand mechanisms of renal aging and to test interventions to improve kidney health, and thereby the overall health span.
Funding
This work was supported by the Veterans Affairs Biomedical Laboratory Research and Development Service (I01BX001340 to B.S.K., 5I01BX000926 to G.G.C. and DK10412 to M.V.); the National Institutes of Health (DK50190 to G.G.C.); the American Heart Association (15GRNT25700363), and the Veterans Affairs Research Career Scientist Award (IK6BX003611 to G.G.C.).
Acknowledgments
We thank Donna Layne-Colon and Jessica Adams for their dedication to the care of the marmosets at the Southwest National Primate Research Center and help with data collection.
Conflict of Interest
None reported.
Author Contributions
B.S.K. and S.D.T. designed the study. O.G., E.J.D., and M.V. performed histologic examination. H.J.L. and A.D. performed most of the experiments on kidneys with suggestions from G.G.C., D.F., and B.S.K. All authors participated in data analysis and interpretation. B.S.K. wrote the manuscript; all authors approved it.
References
- 1. Choudhury D, Levi M. Kidney aging—inevitable or preventable?Nat Rev Nephrol. 2011;7:706–717. doi: 10.1038/nrneph.2011.104 [DOI] [PubMed] [Google Scholar]
- 2. Sataranatarajan K, Feliers D, Mariappan MM, et al. . Molecular events in matrix protein metabolism in the aging kidney. Aging Cell. 2012;11:1065–1073. doi: 10.1111/acel.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol. 1985;40:671–688. [DOI] [PubMed] [Google Scholar]
- 4. Ikeno Y, Hubbard GB, Lee S, et al. . Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. [DOI] [PubMed] [Google Scholar]
- 5. Weindruch R, Masoro EJ. Concerns about rodent models for aging research. J Gerontol. 1991;46:B87–B88. [DOI] [PubMed] [Google Scholar]
- 6. Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol. 2017;28:2838–2844. doi: 10.1681/ASN.2017040421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. ILAR J. 2011;52:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Isobe K, Adachi K, Hayashi S, et al. . Spontaneous glomerular and tubulointerstitial lesions in common marmosets (Callithrix jacchus). Vet Pathol. 2012;49:839–845. doi: 10.1177/0300985811427151 [DOI] [PubMed] [Google Scholar]
- 9. Kasinath BS, Feliers D, Lee HJ. Hydrogen sulfide as a regulatory factor in kidney health and disease. Biochem Pharmacol. 2018;149:29–41. doi: 10.1016/j.bcp.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 10. Lee HJ, Mariappan MM, Feliers D, et al. . Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. J Biol Chem. 2012;287:4451–4461. doi: 10.1074/jbc.M111.278325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee HJ, Feliers D, Barnes JL, et al. . Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience. 2018;40:163–176. doi: 10.1007/s11357-018-0018-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14:185–201. doi: 10.1038/nrneph.2017.189 [DOI] [PubMed] [Google Scholar]
- 13. Miller SE. Histology for Pathologist. 3rd ed. Lippincott Williams & Wilkins; 2007:839–907. [Google Scholar]
- 14. Lee HJ, Feliers D, Mariappan MM, et al. . Tadalafil integrates nitric oxide-hydrogen sulfide signaling to inhibit high glucose-induced matrix protein synthesis in podocytes. J Biol Chem. 2015;290:12014–12026. doi: 10.1074/jbc.M114.615377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamada N, Sato J, Kanno T, Wako Y, Tsuchitani M. Morphological study of progressive glomerulonephropathy in common marmosets (Callithrix jacchus). Toxicol Pathol. 2013;41:1106–1115. doi: 10.1177/0192623313478206 [DOI] [PubMed] [Google Scholar]
- 16. Lennon R, Byron A, Humphries JD, et al. . Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol. 2014;25:939–951. doi: 10.1681/ASN.2013030233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X, He JC. An update: the role of nephrin inside and outside the kidney. Sci China Life Sci. 2015;58:649–657. doi: 10.1007/s11427-015-4844-1 [DOI] [PubMed] [Google Scholar]
- 18. Ha TS, Barnes JL, Stewart JL, et al. . Regulation of renal laminin in mice with type II diabetes. J Am Soc Nephrol. 1999;10:1931–1939. [DOI] [PubMed] [Google Scholar]
- 19. Sataranatarajan K, Mariappan MM, Lee MJ, et al. . Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol. 2007;171:1733–1742. doi: 10.2353/ajpath.2007.070412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes. 2007;56:476–485. doi: 10.2337/db05-1334 [DOI] [PubMed] [Google Scholar]
- 21. Senthil D, Choudhury GG, McLaurin C, Kasinath BS. Vascular endothelial growth factor induces protein synthesis in renal epithelial cells: a potential role in diabetic nephropathy. Kidney Int. 2003;64:468–479. doi: 10.1046/j.1523-1755.2003.00135.x [DOI] [PubMed] [Google Scholar]
- 22. Kasinath BS, Feliers D, Sataranatarajan K, Ghosh Choudhury G, Lee MJ, Mariappan MM. Regulation of mRNA translation in renal physiology and disease. Am J Physiol Renal Physiol. 2009;297:F1153–F1165. doi: 10.1152/ajprenal.90748.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee MJ, Feliers D, Mariappan MM, et al. . A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617–F627. doi: 10.1152/ajprenal.00278.2006 [DOI] [PubMed] [Google Scholar]
- 24. Lee HJ, Lee DY, Mariappan MM, et al. . Hydrogen sulfide inhibits high glucose-induced NADPH oxidase 4 expression and matrix increase by recruiting inducible nitric oxide synthase in kidney proximal tubular epithelial cells. J Biol Chem. 2017;292:5665–5675. doi: 10.1074/jbc.M116.766758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mariappan MM, Prasad S, D’Silva K, et al. . Activation of glycogen synthase kinase 3β ameliorates diabetes-induced kidney injury. J Biol Chem. 2014;289:35363–35375. doi: 10.1074/jbc.M114.587840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bokov AF, Garg N, Ikeno Y, et al. . Does reduced IGF-1R signaling in Igf1r+/− mice alter aging?PLoS One. 2011;6:e26891. doi: 10.1371/journal.pone.0026891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nelson JF, Strong R, Bokov A, Diaz V, Ward W. Probing the relationship between insulin sensitivity and longevity using genetically modified mice. J Gerontol A Biol Sci Med Sci. 2012;67:1332–1338. doi: 10.1093/gerona/gls199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurosu H, Yamamoto M, Clark JD, et al. . Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol. 2013;9:650–660. doi: 10.1038/nrneph.2013.111 [DOI] [PubMed] [Google Scholar]
- 30. Diamond-Stanic MK, You YH, Sharma K. Sugar, sex, and TGF-β in diabetic nephropathy. Semin Nephrol. 2012;32:261–268. doi: 10.1016/j.semnephrol.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wiggins JE, Goyal M, Sanden SK, et al. . Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488 [DOI] [PubMed] [Google Scholar]
- 32. Collins MG, Rogers NM, Jesudason S, Kireta S, Brealey J, Coates PT. Spontaneous glomerular mesangial lesions in common marmoset monkeys (Callithrix jacchus): a benign non-progressive glomerulopathy. J Med Primatol. 2014;43:477–487. doi: 10.1111/jmp.12134 [DOI] [PubMed] [Google Scholar]
- 33. Park MH, Kim DH, Kim MJ, et al. . Effects of MHY908, a new synthetic PPARα/γ dual agonist, on inflammatory responses and insulin resistance in aged rats. J Gerontol A Biol Sci Med Sci. 2016;71:300–309. doi: 10.1093/gerona/glv043 [DOI] [PubMed] [Google Scholar]
- 34. Senthil D, Choudhury GG, Abboud HE, Sonenberg N, Kasinath BS. Regulation of protein synthesis by IGF-I in proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2002;283:F1226–F1236. doi: 10.1152/ajprenal.00109.2002 [DOI] [PubMed] [Google Scholar]
- 35. Ghosh Choudhury G, Abboud HE. Tyrosine phosphorylation-dependent PI 3 kinase/Akt signal transduction regulates TGFbeta-induced fibronectin expression in mesangial cells. Cell Signal. 2004;16:31–41. [DOI] [PubMed] [Google Scholar]
- 36. Hawley SA, Ross FA, Gowans GJ, Tibarewal P, Leslie NR, Hardie DG. Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells. Biochem J. 2014;459:275–287. doi: 10.1042/BJ20131344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- 38. Sancak Y, Thoreen CC, Peterson TR, et al. . PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 39. Krieg J, Hofsteenge J, Thomas G. Identification of the 40 S ribosomal protein S6 phosphorylation sites induced by cycloheximide. J Biol Chem. 1988;263:11473–11477. [PubMed] [Google Scholar]
- 40. Xu J, Chen J, Dong Z, Meyuhas O, Chen JK. Phosphorylation of ribosomal protein S6 mediates compensatory renal hypertrophy. Kidney Int. 2015;87:543–556. doi: 10.1038/ki.2014.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eid AA, Ford BM, Block K, et al. . AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503–37512. doi: 10.1074/jbc.M110.136796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shavlakadze T, Zhu J, Wang S, et al. . Short-term low-dose mTORC1 inhibition in aged rats counter-regulates age-related gene changes and blocks age-related kidney pathology. J Gerontol A Biol Sci Med Sci. 2018;73:845–852. doi: 10.1093/gerona/glx249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hartleben B, Gödel M, Meyer-Schwesinger C, et al. . Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang Q, Ning Y, Liu D, et al. . A young blood environment decreases aging of senile mice kidneys. J Gerontol A Biol Sci Med Sci. 2018;73:421–428. doi: 10.1093/gerona/glx183 [DOI] [PubMed] [Google Scholar]
- 45. Harrison DE, Strong R, Sharp ZD, et al. . Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tardif S, Ross C, Bergman P, et al. . Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. J Gerontol A Biol Sci Med Sci. 2015;70:577–587. doi: 10.1093/gerona/glu101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. [DOI] [PubMed] [Google Scholar]
- 48. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyamoto S, Hsu CC, Hamm G, et al. . Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine. 2016;7:121–134. doi: 10.1016/j.ebiom.2016.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma K, Ramachandrarao S, Qiu G, et al. . Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee MJ, Feliers D, Sataranatarajan K, et al. . Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell Signal. 2010;22:65–70. doi: 10.1016/j.cellsig.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bos EM, Wang R, Snijder PM, et al. . Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol. 2013;24:759–770. doi: 10.1681/ASN.2012030268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem. 2014;289:28827–28834. doi: 10.1074/jbc.M114.596593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu M, Liu YH, Goh HS, et al. . Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol. 2010;21:993–1002. doi: 10.1681/ASN.2009090949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sen U, Basu P, Abe OA, et al. . Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009;297:F410–F419. doi: 10.1152/ajprenal.00145.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C. The aging kidney revisited: a systematic review. Ageing Res Rev. 2014;14:65–80. doi: 10.1016/j.arr.2014.02.003 [DOI] [PubMed] [Google Scholar]