Abstract
Background
Bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC) present serious reproductive health risks and management challenges, with poor control attributed to survival of treatment-resistant biofilm communities. Boric acid is used in various regimens for non-albicans VVC and recurrent BV. We investigated safety and efficacy of a novel boric acid–based vaginal anti-infective with enhanced antibiofilm activity (TOL-463) in treating BV and VVC.
Methods
In this phase 2 randomized, investigator-blinded trial conducted at 2 sexual health clinics, women with BV or VVC were randomly assigned (1:1) to 7 nights of TOL-463 vaginal gel or insert. The primary test of cure (TOC) was clinical cure at day 9–12; safety was assessed at TOC and day 21–30.
Results
One hundred six participants (53 with BV, 36 VVC, 17 both) were enrolled; most were African American (69%). Clinical cure rate of BV at TOC was 59% (95% confidence interval [CI], 41%–75%) for TOL-463 insert and 50% (95% CI, 31%–69%) for TOL-463 gel, and for VVC, 92% (95% CI, 67%–99%) for TOL-463 insert and 81% (95% CI, 57%–93%) for TOL-463 gel. Both products were safe and well tolerated with no secondary cases of VVC; vulvovaginal burning was the most common adverse event (9.6%).
Conclusions
TOL-463, especially in vaginal insert form, is effective and safe in treating BV and VVC. Future studies should assess the potential role of TOL-463 as a biofilm disrupter in enhancing likelihood of cure relative to approved therapies, reducing recurrence rates, and combined with traditional antimicrobials.
Clinical Trials Registration
Keywords: TOL-463, boric acid, bacterial vaginosis, vulvovaginal candidiasis, vaginal biofilm
TOL-463 vaginal gel or insert, a boric acid–based anti-infective with enhanced antibiofilm activity, was effective and safe in treating Bacterial Vaginosis and Vulvovaginal Candidiasis, with the vaginal insert demonstrating higher efficacy for both conditions.
Bacterial vaginosis (BV) and vulvovaginal candidiasis (VVC) are the 2 most prevalent lower reproductive tract infections, affecting millions of women globally. BV is characterized by a depletion of specific Lactobacillus species necessary for maintaining vaginal health and a dramatic increase in commensal organisms, notably Gardnerella vaginalis and other anaerobes; a local inflammatory response to Candida species (primarily Candida albicans) characterizes VVC. Although current treatments afford reliable symptom relief, failure rates for BV and VVC are as high as 83% and 60%, respectively, and in which the survival of resistant biofilm communities have been increasingly implicated [1–3]. These failure rates represent an important public health concern because these infections are associated with a host of serious complications including increased risk of acquiring and transmitting human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs) and adverse pregnancy outcomes, including preterm premature rupture of membranes, preterm birth, and low birth weight of infants [4–6].
TOL-463 is a boric acid (BA)–based vaginal anti-infective enhanced with ethylenediaminetetraacetic acid (EDTA) specifically targeting vaginal bacterial and fungal biofilms. Boric acid has a long history of clinical use in the treatment of both BV and VVC [7] and, despite the lack of a US Food and Drug Administration (FDA)–approved product, is currently recommended by the US Centers for Disease Control and Prevention [8] for treatment of recurrent BV and non-albicans VVC. Two formulations of TOL-463 are under study: a water-based vaginal gel and polyethylene-glycol-based insert containing 250 mg and 500 mg, respectively, of BA per dose. Each formulation also incorporates EDTA, which has been shown to enhance the antimicrobial activity of BA and provide superior antibiofilm potency against G. vaginalis and Candida biofilm while sparing protective lactobacilli [9–12]. We report the results of a randomized, investigator-blinded study that assessed the efficacy and safety of TOL-463 gel and insert in the treatment of BV and VVC.
METHODS
Study Design
The study was a phase 2 randomized single (investigator)–blinded safety and efficacy trial that enrolled participants at 2 sexual health research clinics in Seattle, Washington and Birmingham, Alabama.
Participants
The study planned to enroll approximately 120 female participants 18–50 years of age to achieve 80 evaluable subjects with BV and/or VVC. Diagnosis of BV was based on presence of all Amsel criteria. Diagnosis of VVC was based on presence of pseudohyphae on potassium hydroxide (KOH) preparation plus at least 1 sign and 1 symptom, each rated based on severity with minimum composite score of 2. Signs included vulvovaginal edema, erythema, and/or excoriation. Symptoms included vulvovaginal itching, burning, and/or irritation. Severity was graded on a scale of 0–3 (absent = 0; mild = 1; moderate = 2; severe = 3).
Randomization and Masking
Participants were randomly assigned (1:1) to vaginal dosing of TOL-463 gel or insert using a permuted blocked randomization scheme stratified by site and infection type: single infections of BV or VVC, or mixed infections. To ensure investigator blinding, participants were instructed not to divulge method of treatment to investigators. In addition, sites delegated responsibility of assessing study product adherence to unblinded site personnel not participating in any assessments of cure.
Procedures
Baseline clinical information was collected on all subjects at enrollment. Vaginal swabs were collected for confirmation of BV and VVC and for excluding other STIs: Gram stain for Nugent scoring, Candida culture, assessment of vaginal pH, saline, and KOH microscopy (clue cells, motile trichomonads, yeast forms), and nucleic acid amplification tests for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. All participants were asked to abstain from anal, oral, and vaginal sexual intercourse during study drug dosing and, if sexually active, to use nonlubricated condoms (in addition to hormonal contraception) throughout study participation. A memory aid was provided to document symptomatic response to treatment, adverse events, and compliance. Study medication was administered vaginally once nightly for 7 days as either a 5 g dose of gel or a 2 g unit-dose insert. Women who administered at least 5 of the 7 doses were considered to be adherent. Treatment response was evaluated at day 9–12 after treatment initiation as the test-of-cure (TOC) visit 2, in conformance with current 2016 FDA guidelines [12, 13], and again at day 21–30 (visit 3).
Outcomes
The primary efficacy endpoint among women with BV at enrollment was the proportion of participants with clinical cure at TOC based on resolution of Amsel criteria: negative KOH whiff test, absence of a homogenous discharge characteristic of BV, and clue cells <20% of vaginal squamous epithelial cells. For VVC, the primary efficacy endpoint was the proportion of participants with clinical cure at TOC, defined as a score of zero (0) for any sign and symptom scored as 1–2 at baseline; or a score of 0–1 for any sign and symptom scored as 3 at baseline and without the need for further treatment at the discretion of the research clinician. These outcome measures were also consistent with current FDA guidelines [13, 14].
Secondary efficacy endpoints included clinical cure of BV and/or VVC at TOC and visit 3 among participants with mixed BV/VVC infections at baseline; clinical cure of BV or VVC at visit 3 among participants with BV or VVC single infections at baseline, microbiologic/mycologic cure and therapeutic cure of BV or VVC at TOC and visit 3; symptom relief and median time to relief during the 7 days of treatment as assessed by the participant; and provision of additional treatment at the discretion of the research clinician.
Microbiologic cure of BV was defined as a Nugent score of 0–3. Mycological cure of VVC was defined as a negative Candida culture (ie, no growth of baseline Candida species). Therapeutic cure for both BV and VVC was defined as a combination of clinical and microbiologic/mycologic cure. Safety was assessed at both follow-up visits by targeted physical examination, including pelvic examination, and the occurrence of secondary VVC among participants with BV at baseline.
Statistical Analysis
The intent was to obtain estimates of efficacy for each TOL-463 formulation in women with BV or VVC and not to formally test differences between formulations. Assuming a 50% clinical cure rate with either formulation, and using the 2-sided 95% Wilson confidence interval, a minimum sample size of 28 per single-infection stratum (ie, 14 per infection type per formulation) in the primary analysis population was calculated to estimate a clinical cure rate with lower confidence bound >25%—the presumed spontaneous cure rate without treatment. The upper limit of evaluable participants per single infection stratum was approximately 38. We estimated 120 enrolled participants would be required to achieve the target of 80 evaluable participants.
For women enrolled with a clinical diagnosis of BV or VVC, evaluable was defined as having an abnormal Gram stain (Nugent score >3) or a positive Candida culture at baseline, respectively. Subjects with C. trachomatis, N. gonorrhoeae, or T. vaginalis or a Pap smear result other than “negative of intraepithelial lesions or malignancy” or “atypical squamous cells of undetermined significance, HPV negative” subsequent to initiation of therapy were considered nonevaluable.
Primary and secondary efficacy endpoints were assessed in the modified intent-to-treat (mITT) analysis populations, which included all evaluable subjects who returned for at least 1 postbaseline visit and had negative STI test results taken at baseline. Efficacy analyses were repeated as secondary analyses in the per-protocol (PP) and intent-to-treat (ITT) analysis populations. The ITT population used the clinically diagnosed baseline infection status, while the mITT and PP populations used the confirmed baseline infection status to assign subjects to an infection stratum.
Endpoints were estimated by infection stratum and treatment arm. Point estimates for the arm-specific proportions and difference in proportions between the TOL-463 gel and TOL-463 insert groups along with corresponding 95% confidence intervals (CIs) were calculated. Additional descriptive analyses of the primary and secondary efficacy outcomes were performed separately by infection stratum and treatment arm. For the PP analysis, subjects whose cure status could not be determined at the TOC visit for any reason were excluded, while those in the mITT and ITT analyses were considered “not cured” for all cure-related endpoints. The cure status of participants whose status could not be determined at visit 3 for any reason was imputed using the cure status at visit 2. Participants receiving at least 1 dose of study medication were included in the safety analyses.
RESULTS
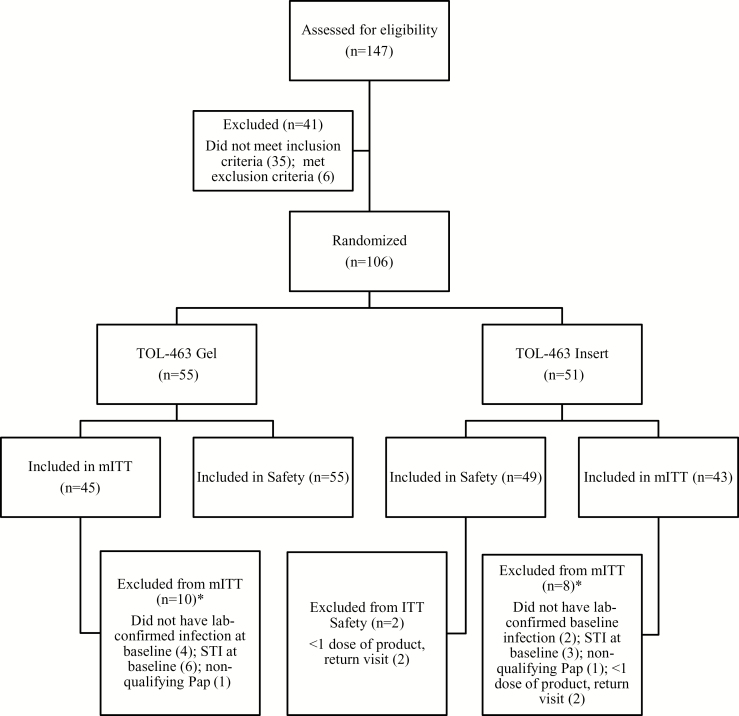
A total of 147 women were screened to enroll 106 participants (Figure 1). The most common reasons for screen failure were lack of all 4 Amsel criteria for BV or for VVC, lack of pseudohyphae on KOH preparation, and a minimum composite score of 2 based on VVC signs and symptoms. Of the 106 enrollees, 53 had a clinical diagnosis of BV, 36 a clinical diagnosis of VVC, and 17 both BV and VVC. Reasons for exclusion by analysis population are detailed in Figure 1.
Figure 1.
Subject disposition. *A participant may have been excluded for >1 reason. Abbreviations: ITT, intent to treat; mITT, modified intent-to-treat primary efficacy analysis population; STI, sexually transmitted infection.
Baseline characteristics of participants are presented in Table 1. Most participants were non-Hispanic (92%) and African American (69%), with mean age 31 years. Among all enrolled with BV, most (75%) were African American compared with 53% of women enrolled with a diagnosis of VVC. Nearly one-quarter of participants had received treatment for BV in the last 60 days, and one-fifth for VVC. Almost all participants with VVC (alone or with BV) had C. albicans identified, and only a few had other species of yeast identified.
Table 1.
Baseline Characteristics of Study Participants, by Randomization Group
| TOL-463 Gel | TOL-463 lnsert | |||||
|---|---|---|---|---|---|---|
| Parameter | BV (n = 28) | VVC (n = 18) | Mixed (n = 9) | BV (n = 25) |
VVC (n = 18) | Mixed (n = 8) |
| Age, y | ||||||
| Mean ± SD (min–max) |
31.5 ± 7.4 (21–46) |
30.2 ± 9.4 (19–47) |
33.3 ± 9.2 (20–47) |
29.6 ± 6.7 (19–49) |
31.4 ± 10.5 (18–48) |
34.3 ± 6.6 (24–41) |
| Ethnicity, No. (%) | ||||||
| Not Hispanic or Latino | 28 (100) | 17 (94) | 9 (100) | 21 (84) | 16 (89) | 6 (75) |
| Hispanic or Latino | 0 (0) | 1 (6) | 0 (0) | 3 (12) | 1 (6) | 1 (13) |
| Not reported or unknown | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (6) | 1 (13) |
| Race, No. (%) | ||||||
| White | 4 (14) | 5 (28) | 0 (0) | 2 (8) | 4 (22) | 1 (13) |
| African American | 18 (64) | 10 (56) | 8 (89) | 22 (88) | 9 (50) | 6 (75) |
| Asian | 2 (7) | 0 (0) | 0 (0) | 0 (0) | 1 (6) | 0 (0) |
| Hawaiian/Pacific Islander | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| American Indian/Alaska Native | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Multiple/other | 3 (11) | 2 (11) | 1 (11) | 1 (4) | 4 (22) | 1 (13) |
| BV/VVC history, No. (%) | ||||||
| Treated for BV in past 60 d | 4 (14) | 2 (11) | 4 (44) | 8 (32) | 2 (11) | 3 (38) |
| Treated for VVC in past 60 d | 3 (11) | 5 (28) | 4 (44) | 4 (16) | 3 (17) | 2 (25) |
| ≥3 BV episodes in past 12 mo | 5 (18) | 0 (0) | 2 (22) | 8 (32) | 0 (0) | 1 (13) |
| ≥3 VVC episodes in past 12 mo | 2 (7) | 5 (28) | 0 (0) | 0 (0) | 2 (11) | 0 (0) |
| ≥3 BV + VVC episodes in past 12 mo | 5 (18) | 3 (17) | 5 (56) | 4 (16) | 3 (17) | 2 (25) |
| Mean BV Nugent scorea | 8.0 | NA | 6.6 | 7.8 | NA | 7.9 |
| Mean VVC scoreb | NA | 6.8 | 6.2 | NA | 7.4 | 5.5 |
Abbreviations: BV, bacterial vaginosis; NA, not applicable; SD, standard deviation; VVC, vulvovaginal candidiasis.
aGram Nugent stain score range 0–10; 4–10 confirmatory for subjects with a clinical diagnosis of BV at entry.
bVVC composite sign/symptom score range 0–18; a minimum score of 2 was required for subjects with a clinical diagnosis of VVC at entry.
Of the 106 subjects enrolled, 104 (98%) received at least 1 dose of study treatment and returned for both follow-up visits. Two subjects were dispensed study treatment but were lost to follow-up without a return visit. Of those receiving treatment, 96 subjects (91%) were adherent.
Primary efficacy results are summarized in Table 2. For BV, 50% (95% CI, 31%–69%) of vaginal gel participants achieved clinical cure at TOC, compared with 59% (95% CI, 41%–75%) in the insert arm. In the ITT analysis, among women randomized to the insert, a clinical cure rate of 68% (95% CI, 48%–83%) was reported; all of these subjects had BV confirmed by Gram stain, including 1 participant with a confounding STI. For VVC, 81% (95% CI, 57%–93%) of gel participants achieved clinical cure, compared with 92% (95% CI, 67%–99%) of those in the insert group. Clinical cure in the mITT analysis at visit 3 was lower for participants with BV, but not for VVC. For BV, only 13% (95% CI, 4%–31%) of participants in the gel group achieved clinical cure, compared with 30% (95% CI, 16%–48%) in the insert group. For VVC, 81% (95% CI, 57%–93%) in the gel group achieved clinical cure, compared with 77% (95% CI, 50%–92%) in the insert group.
Table 2.
Primary Efficacy Results: Percentage of Subjects With Clinical Cure at Test of Cure by Infection Type, Treatment Group, and Analysis Populationa
| Analysis Population | BV | VVC | ||||
|---|---|---|---|---|---|---|
| TOL-463 Gel | TOL-463 Insert | % Difference Gel vs Insert | TOL-463 Gel | TOL-463 Insert | % Difference Gel vs Insert | |
| ITT | 54 (15/28)b [36–70] |
68 (17/25)c [48–83] |
–14 [–38 to 11] |
83 (15/18)d [61–94] |
78 (14/18)e [55–91] |
6 [–21 to 31] |
| mITTf | 50 (12/24) [31–69] |
59 (16/27) [41–75] |
–9 [–34 to 17] |
81 (13/16) [57–93] |
92 (12/13) [67–99] |
–11 [–36 to 17] |
| PP | 47 (8/17) [26–69] |
58 (15/26) [39–74] |
–11 [–37 to 18] |
75 (9/12) [47–91] |
100 (10/10) [72–100] |
–25 [–53 to 7] |
Data are presented as % (no./No.), with 95% confidence intervals in brackets.
Abbreviations: BV, bacterial vaginosis; CI, confidence interval; ITT, intent-to-treat; mITT, modified intent-to-treat; PP, per protocol; VVC, vulvovaginal candidiasis.
aThe denominator for cure rates is based on the number of subjects enrolled with the infection type in the respective treatment group and analysis population.
bOne of 28 patients had a normal Nugent score (0–3) and 3 of 28 had a concomitant STI at baseline.
cZero of 25 patients had a normal Nugent score (0–3) and 1 of 25 had a concomitant STI at baseline.
dThree of 18 had negative baseline Candida cultures.
eTwo of 18 had negative baseline Candida cultures; 2 of 18 were lost to follow-up, and never received study treatment.
fPrimary efficacy analysis population; all subjects had clinical diagnosis of BV or VVC with microbiologic/mycologic confirmation of baseline infection and are classified by confirmed infection.
Relatively few participants enrolled with both BV and VVC had true mixed infections. Of those confirmed (n = 8, mITT), 2 of 5 gel participants achieved clinical cure of BV, compared with none of 3 in the insert group at the TOC visit. In contrast, all 5 of the gel participants achieved clinical cure of VVC, compared with 1 of 3 in the insert arm.
As expected, measures of microbiologic and therapeutic cure paralleled these results, but overall were lower, consistent with other approved vaginitis therapies. For example, microbiologic and therapeutic cure of BV at TOC was 21% (95% CI, 9%–40%) and 21% (95% CI, 9%–40%), respectively, for the gel vs 41% (95% CI, 25%–59%) and 33% (95% CI, 19%–52%) for the insert, and of VVC, 81% (95% CI, 57%–93%) and 63% (95% CI, 39%–82%) for gel and 85% (95% CI, 58%–96%) and 77% (95% CI, 50%–92%) for insert.
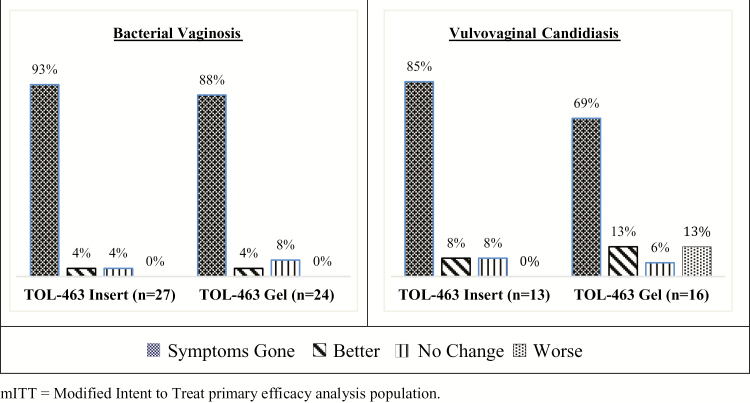
The majority of participants reported symptom resolution by the last day of treatment (Figure 2) (88% and 93% for women with BV and 69% and 85% for women with VVC in the gel and insert groups, respectively) that was sustained through the end of study. At the TOC visit, 83% and 96% of BV subjects and 87% and 85% of those with VVC treated with gel and insert, respectively, reported symptom resolution. By the third visit, 83% and 96% of BV subjects in the gel and insert groups, respectively, and 100% of VVC subjects in either treatment group were asymptomatic. Mean and median time to symptom resolution overall was 6.2 and 7.0 days, respectively.
Figure 2.
Patient-reported symptom relief at the last day of study treatment by infection type and treatment group (modified intent-to-treat primary efficacy analysis population).
Despite high rates of symptom resolution, a significant percentage of BV subjects were prescribed additional treatment, particularly gel-treated subjects, 58%, compared with 41% in the insert group. No criteria were defined a priori for determining need for additional treatment and significant differences notable between the 2 study sites in this regard. Among participants with VVC, need for additional treatment was relatively low (13% and 8% in the gel and insert groups, respectively).
Overall, both study products were safe and well tolerated. Approximately one-fifth (19%) of participants experienced an adverse event related to study product, for a total of 45 adverse events. None were serious or severe or resulted in discontinuation of study treatment. All were mild to moderate in intensity and self-limiting. The majority were described as vulvovaginal burning (Table 3). Moreover, none of the participants enrolled with BV had a subsequent clinical diagnosis of VVC.
Table 3.
Adverse Events Occurring ≥5% of Subjects in Any Treatment Group
| MedDRA Preferred Term | TOL-463 Vaginal Gel (n = 55) | TOL-463 Vaginal Insert (n = 49) | All Subjects (n = 104) | Treatment Related Only |
|---|---|---|---|---|
| Headache | 1 (2) | 4 (8) | 5 (5) | 2 (2) |
| Vulvovaginal burning sensation | 5 (9) | 5 (10) | 10 (10) | 10 (10) |
| Vulvovaginal pruritus | 4 (7) | 3 (6) | 7 (7) | 7 (7) |
Data are presented as No. (%).
Abbreviation: MedDRA, Medical Dictionary for Regulatory Activities.
DISCUSSION
We report here the first study and reporting of the clinical efficacy of TOL-463 vaginal gel and insert in the treatment of BV and VVC. In this randomized, single-blind, 2-center trial, we demonstrated clinical cure rates comparable to those for products recommended for management of these respective infections, none of which are approved for both infections. For example, early clinical cure rates reported for the 2 most recently approved BV treatments were 57.9% and 41.1% for single-dose oral secnidazole (2 g) and single-dose 1.3% metronidazole vaginal gel, respectively [3, 15]. Participants were representative of the population profile of women with these conditions, including a high percentage of African American women, and a high percentage who reported these conditions by self-report or report of prior specific treatment in the recent past. Notably, the proportion of African American women in the BV infection stratum (a more challenging, higher-risk population) was 75% overall, and was generally higher compared with other BV treatment studies [16, 17]. Moreover, the vast majority of women who were clinically diagnosed with BV had high qualifying baseline Nugent scores of 7–10 (98% mITT, 94% ITT). Early clinical cure (the primary endpoint) of BV was achieved by 50% of participants in the vaginal gel group, compared with 59% in the insert arm by mITT. By ITT analysis, 68% of BV subjects in the insert arm, all of whom had Gram stain–confirmed BV, were clinical cures. For VVC, 81% of participants in the vaginal gel arm achieved clinical cure, compared with 92% of those in the vaginal insert group. The majority of these participants had a baseline composite VVC score reflective of moderate to severe disease. Relatively few subjects were confirmed to have mixed infections and thus interpretation of results is limited for this secondary outcome. As expected, measures of microbiologic and therapeutic cure paralleled these results, but overall were lower, consistent with approved vaginitis treatments. The majority of participants reported symptom resolution by the last day of treatment that was sustained through the end of the study. Despite this, need for additional treatment among participants with BV as judged by study clinicians was relatively high at the third visit (notably for participants treated with the lower strength gel) but was low among VVC subjects treated with either dosage form. Overall, both study products were safe and well tolerated, with no severe or serious adverse events deemed related to study product and with high study retention (98%). None of the BV participants developed a secondary VVC infection, a common side effect of approved BV treatments reported in about 10%–15% of women during acute treatment [15, 18] and up to 60% of women on suppressive regimens [19].
This study has limitations. First, the single-blind design necessitated that participants were aware of the type of product delivery they were receiving. We attempted to mitigate concern for this by ensuring that study clinicians charged with determining clinical endpoints, including components of cure, remained blinded to product assignment. Second, only 2 sites participated in the study, with a disproportionate two-thirds of subjects enrolled at 1 center. Moreover, the majority of BV subjects were African American (75% overall). We view this as both a limitation and strength given that African American women represent a higher-risk population more often affected by BV and its associated morbidity (eg, preterm birth, HIV). Molecular studies show distinct differences in the vaginal microbiome by race that may have implications to treatment [20, 21]. Women of African American descent are more likely to present with a BV-like microbiome with greater microbial diversity, fewer lactobacilli, and higher Nugent scores, the latter of which is consistent with this report. Third, this relatively early assessment of this novel class of antimicrobials necessitated a design that did not include traditional antibiotic agents, including other antibacterial or antifungal antimicrobials. Thus, the results do not inform how TOL-463 as a biofilm disrupter might contribute to management of these conditions if it were to be combined with currently recommended antimicrobial regimens. Finally, while an attempt was made to evaluate the utility of TOL-463 in women with both BV and VVC infections as a secondary endpoint, the number of confirmed mixed infections per treatment arm was too low to draw any meaningful conclusions. Nonetheless, women with mixed infections may represent a more challenging management case and closer examination of the microbiology and pathogenesis of this condition is warranted.
In summary, TOL-463 is effective and safe in the treatment of BV and VVC, with the vaginal insert demonstrating a more efficacious profile for both conditions overall. The potential dual indication of TOL-463 likely represents a benefit in the management of infectious vaginitis as diagnosis of these infections is often imprecise. Phase 3 trials will further define the role of TOL-463 in a more representative gynecology population across a broader range of practice settings and patient types. Future studies should evaluate whether this novel, non-azole agent may have a role in enhancing the likelihood of BV and VVC cure relative to approved therapies, reducing rates of recurrent infection, and in treatment regimens that might combine traditional antimicrobials with biofilm disruptors such as this product.
Notes
Financial support. This work was supported by the US National Institutes of Health (NIH) Sexually Transmitted Infections Clinical Trials Group.
Acknowledgments. We thank the study participants, as well as study staff Anna Harrington, Adrienne Kuxhausen, and Hannah Kwak.
Potential conflicts of interest. J. C. D. has received grants from Hologic, ELITech, Quidel, and Curatek, and personal fees from Gilead. A. P. has received grants from the NIH, and other from Toltec Pharmaceuticals. C. P. has received grants from the NIH. J. S. has received other and personal fees from Toltec Pharmaceuticals; grants and personal fees from Symbiomix Pharmaceuticals; and grants from the NIH, Curatek Pharmaceuticals, and Gage Pharmaceuticals. D. D. has received other from Hologic. J. M. M. reports no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Muzny CA, Schwebke JR. Pathogenesis of bacterial vaginosis: discussion of current hypotheses. J Infect Dis 2016; 214(Suppl 1):S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diflucan [package insert]. New York, NY: Pfizer; 2018.
- 3.Nuvessa [package insert]. Irvine, CA: Allergan; 2016.
- 4. Marrazzo JM, Martin DH, Watts DH, et al. . Bacterial vaginosis: identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sex Trans Dis 2010; 37:732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aguin TJ, Sobel JD. Vulvovaginal candidiasis in pregnancy. Curr Infect Dis Rep 2015; 17:462. [DOI] [PubMed] [Google Scholar]
- 6. van de Wijgert JH, Morrison CS, Cornelisse PG, et al. . Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 2008; 48:203–10. [DOI] [PubMed] [Google Scholar]
- 7. Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis 2009; 36:732–4. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC). Sexually transmitted disease surveillance, 2015. Atlanta, GA: CDC, 2017. [Google Scholar]
- 9. Citron DM, Leoncio E, Tyrrell KL, et al. . Antimicrobial activity of boric acid (BA) and TOL-463 against vaginal anaerobes causing bacterial vaginosis (BV) and urinary tract infections (UTIs). In: 12th Biennial Congress of the Anaerobe Society of the Americas, Chicago, IL, 28 June–1 July 2014. [Google Scholar]
- 10. Citron DM, Goldstein EJC. Establishment of the MBC/MFC of boric acid and TOL-463 against culprit vaginitis pathogens and impact on protective vaginal lactobacilli. In: 54th Annual Meeting of Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5–9 September 2014. [Google Scholar]
- 11. Fidel PL, Lilly EA. Activity of TOL-463 against biofilms formed by Candida species in an ex vivo murine vaginitis model. In: IDWeek, San Diego, CA, 7–11 October 2015. [Google Scholar]
- 12. Pulcini E. Effects of boric acid (BA) and TOL-463 against biofilms formed by key vaginitis pathogens Gardnerella vaginalis and Candida albicans. In: 40th Annual Meeting of the Infectious Diseases Society for Obstetrics and Gynecology, Stowe, VT, 7–9 August 2014. [Google Scholar]
- 13. US Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Vulvovaginal candidiasis: development of drugs for treatment, guidance for industry. Silver Spring, MD: CDER, 2016. [Google Scholar]
- 14. US Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Bacterial vaginosis: development of drugs for treatment, guidance for industry. Silver Spring, MD: CDER, 2016. [Google Scholar]
- 15. Solosec [package insert].
- 16. Schwebke JR, Morgan FG Jr, Koltun W, Nyirjesy P. A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis. Am J Obstet Gynecol 2017; 217:678.e1–9. [DOI] [PubMed] [Google Scholar]
- 17. Schwebke JR, Marrazzo J, Beelen AP, Sobel JD. A phase 3, multicenter, randomized, double-blind, vehicle-controlled study evaluating the safety and efficacy of metronidazole vaginal gel 1.3% in the treatment of bacterial vaginosis. Sex Transm Dis 2015; 42:376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clindesse [package insert]. New York: Perrigo Inc.; 2014. [Google Scholar]
- 19. Sobel JD, Ferris D, Schwebke J, et al. . Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006; 194:1283–9. [DOI] [PubMed] [Google Scholar]
- 20. Abdelmaksoud AA, Girerd PH, Garcia EM, et al. . Association between statin use, the vaginal microbiome, and Gardnerella vaginalis vaginolysin-mediated cytotoxicity. PLoS One 2017; 12:e0183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fettweis JM, Brooks JP, Serrano MG, et al. . Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014; 160:2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]