Abstract
There is strong evidence that obesity has deleterious effects on cognitive function of older adults. Previous preclinical studies demonstrate that obesity in aging is associated with a heightened state of systemic inflammation, which exacerbates blood–brain barrier disruption, promoting neuroinflammation and oxidative stress. To test the hypothesis that synergistic effects of obesity and aging on inflammatory processes exert deleterious effects on hippocampal function, young and aged C57BL/6 mice were rendered obese by chronic feeding of a high-fat diet followed by assessment of learning and memory function, measurement of hippocampal long-term potentiation (LTP), assessment of changes in hippocampal expression of genes relevant for synaptic function and determination of synaptic density. Because there is increasing evidence that altered production of lipid mediators modulate LTP, neuroinflammation and neurovascular coupling responses, the effects of obesity on hippocampal levels of relevant eicosanoid mediators were also assessed. We found that aging exacerbates obesity-induced microglia activation, which is associated with deficits in hippocampal-dependent learning and memory tests, impaired LTP, decreased synaptic density, and dysregulation of genes involved in regulation of synaptic plasticity. Obesity in aging also resulted in an altered hippocampal eicosanoid profile, including decreases in vasodilator and pro-LTP epoxy-eicosatrienoic acids (EETs). Collectively, our results taken together with previous findings suggest that obesity in aging promotes hippocampal inflammation, which in turn may contribute to synaptic dysfunction and cognitive impairment.
Keywords: Metabolic syndrome, Vascular cognitive impairment, VCI, Mild cognitive impairment, Inflammaging
In the past half a century the prevalence of obesity has more than doubled to over 37% of individuals aged 65 and older and if the current trend continues, nearly half of the elderly population will be obese in 2030 (1). Prospective and cross-sectional investigations of neurocognitive function demonstrate that obesity has deleterious effects on the brain and neurocognitive function in the elderly (2). For example, in the Framingham Offspring Study, higher waist-hip ratio predicted a significant decline in cognitive function later in life (3). Other longitudinal studies yielded similar results (4). Further, there is strong evidence that obesity-related cognitive deficits increase with age (5). Experimental studies in rodent models confirmed that obesity per se (eg, in the absence of sleep apnea or other comorbid conditions) results in cognitive dysfunction, which is exacerbated with advanced age (6,7).
The cellular mechanisms by which obesity promotes cognitive dysfunction are likely multifaceted and include both dysregulation of cerebral blood flow due to cerebromicrovascular impairment (7) and neuronal dysfunction induced by altered local microenvironment (eg, blood–brain barrier disruption (6), heightened state of neuroinflammation (8,9)) in the cerebral tissue. In the past decade significant progress has been made understanding the pathophysiology of cerebromicrovascular impairment associated with obesity. There is strong evidence that obesity promotes cerebromicrovascular rarefaction, endothelial dysfunction, and neurovascular uncoupling (7). These microvascular alterations likely lead to impaired delivery of oxygen and nutrients to the active brain regions as well as inadequate wash-out of toxic by-products thereby promoting neuronal dysfunction. Obesity also promotes disruption of the blood–brain barrier, microglia activation and oxidative/nitrosative stress in the cortex and hippocampus (6,8–11). Importantly, the adverse cerebromicrovascular effects of obesity are exacerbated in advanced aging (6,10–12). Although increased obesity-induced microvascular alterations and the heightened state of neuroinflammation are likely to be causally linked to age-related exacerbation of adverse neurocognitive effects of obesity (6,7), there are no studies addressing the combined effects of aging and obesity on synaptic function and neuronal phenotype. Long-term synaptic potentiation (LTP), a persistent strengthening of synapses based on recent patterns of activity, is presumed to play an important role in the establishment and storage of stable memories in the hippocampus (13). There is growing evidence that pathophysiological conditions, which promote cognitive decline by impairing the cerebral microcirculation, compromising the blood–brain barrier and promoting neuroinflammation (eg, hypertension (14)), elicit significant impairment of LTP. However, the combined effects of obesity and aging on hippocampal LTP remain still elusive. Decreases in hippocampal synapse number also correlate with cognitive performance (15), yet, it is less understood how obesity affects synaptic density in the aged hippocampus.
The present study was designed to test the hypothesis that obesity in aging exerts deleterious effects on the hippocampus, impairing synaptic plasticity, reducing synaptic density and/or altering hippocampal gene expression profile. To achieve that goal, young and aged C57BL/6 mice were rendered obese by chronic feeding of a high-fat diet (HFD) followed by measurement of LTP in acute hippocampal slices, assessment of changes in hippocampal expression of genes relevant for synaptic function and determination of synaptic density. Because there is increasing evidence that altered production of lipid mediators modulate LTP, neuroinflammation and neurovascular coupling responses (16,17), the effects of obesity on hippocampal levels of relevant eicosanoid mediators were also assessed.
Methods
A detailed description of Materials and Methods is available as supplementary material (Supplementary Methods).
Animals and Diets
Young and aged male C57BL/6 mice (7 and 24 months old at the time of sacrifice, respectively) were placed on either a standard diet (SD) or HFD for 5 months, as described (6,7). The four groups were (a) young animals fed an SD; (b) young animals fed an HFD; (c) old animals fed an SD diet; (d) old animals fed an HFD. All procedures were approved by the Institutional Animal Care and Use Committee of OUHSC.
Spatial Memory Testing of Mice in Y-maze
Hippocampal-dependent contextual memory was tested with the Y-maze two-trial delayed alternation task according to our published protocol (18).
Novel Object Recognition Test
The novel object recognition task was used to evaluate recognition memory (18). Previous studies demonstrate that the novel object recognition test is a sensitive indicator of cognitive dysfunction in mouse models of aging and of age-associated disease.
Electrophysiological Studies for Synaptic Function and LTP
To determine how obesity affects synaptic function, extracellular recordings were performed from acute hippocampal slices as described (14,16,19). Briefly, horizontal hippocampal slices of 325 µm thickness from mice in each cohort were positioned on P5002A multi-electrode arrays (Alpha MED Scientific Inc, Japan). Field excitatory post-synaptic potentials (fEPSPs) were invoked through stimulation of the performant path collaterals (0.2 ms biphasic pulses at 50% strength of maximal activation) and obtained from the dentate gyrus area. LTP was induced using high-frequency stimulation (HFS), which consisted of 100 pulses at 100 Hz applied 4 times with half-minute intervals. fEPSPs were monitored every 30 seconds for 60 minutes following HFS and were recorded with MED-64 system and Mobius software (Alpha MED Scientific Inc). Potentiation was calculated as the percent increase of the mean fEPSP descending slope following HFS and normalized to the mean fEPSP descending slope of baseline recordings.
Synaptic Density Quantification
To compare how HFD-induced obesity and aging affect synaptic density, sections of the hippocampi were immunostained for MAP-2, to label neuronal somata and dendrites and synaptophysin, which is an abundant presynaptic vesicle protein. Confocal images were captured and quantification of the density of synaptophysin-immunoreactive puncta were performed as described recently (14).
Western Blotting
Immunoblotting studies for the AMPA receptor subunits GluR1 (GRIA1) and GluR2 (GRIA2) and the NMDA receptor channel subunits NMDAR1 (GRIN1) and NMDAR2A (GRIN2A) in hippocampal homogenates were performed as described (14).
Transcriptome Analysis Using RNA-Seq
RNA was isolated from hippocampi and stranded RNA-Seq libraries (TrueSeq) were constructed to retain directionality of the transcripts (n = 6 per group), as described (20). Each sequencing library was prepared from RNA isolated from the hippocampus of a single animal. Library sizing was performed by TapeStation (Agilent) and libraries were quantified by qPCR (Kappa Biosystems). The cDNA library was then sequenced using an Illumina Hiseq2500 at the Oklahoma Medical Research Foundation Genomics Facility in a 2 × 100bp fashion.
Following sequencing, raw FASTQ reads were trimmed of their Illumina TruSeq adapter sequences using cutadapt v1.8.3, then aligned using TopHat v2.1.0 to the NCBI mouse transcriptome build 37.2. Raw expression counts were quantified using the samtools “bedcov” utility (version 1.2), and differential expression analysis was performed using DESeq2 1.10.1 using the “parametric” fit type and “Wald” significance testing method. Significantly, differentially expressed genes were obtained using a 0.05 FDR cutoff after Benjamini-Hochberg multiple hypothesis correction. Gene Ontology Enrichment analysis was performed using Fisher’s Exact test. The data will be made available upon request.
Lipidomic Analysis of Eicosanoid Mediators in Hippocampal Samples
Eicosanoid mediators were shown to play a critical role in regulation of synaptic plasticity and neuronal function, as well as regulation of cerebral blood flow (17). To determine how aging and consumption of an HFD affects hippocampal levels of key eicosanoid mediators, high throughput mass spectrometric analysis of eicosanoids in hippocampal samples was performed on an ABI/Sciex 6500 Q-TRAP according to published protocols (21).
Statistical Analysis
Data were analyzed by one-way or two-way analysis of variance with Bonferroni’s Multiple Comparisons Test. A p value less than .05 was considered statistically significant. Data are expressed as mean ± SEM.
Results
Effects of Chronic HFD and Aging on Body Mass
In agreement with previous data (6,7,22), we show that both young and aged mice fed a HFD exhibit significant weight gain over the experimental period although the increase in body mass of HFD-fed young mice was greater as compared with HFD-fed aged animals (Table 1). The effects of chronic HFD feeding on the serum biochemical profile of young and aged C57BL/6 mice are shown in Table 1.
Table 1.
Effects of Consumption of a High-Fat Diet (HFD) on Body Mass and on Various Serum Biomarkers in Young and Aged C57BL/6 Mice
| Parameter | Young (SD) | Young (HFD) | Aged (SD) | Aged (HFD) |
|---|---|---|---|---|
| Body mass (g) | 28.7 ± 0.4 | 52.9 ± 0.7a | 34.0 ± 0.4 | 40.7 ± 0.9a,b,c |
| Glucose (mmol/L) | 6.5 ± 0.3 | 9.4 ± 1.3a | 6.3 ± 0.5 | 8.1 ± 0.5a,c |
| Total triglycerides (mg/dL) | 60.0 ± 4.0 | 106.6 ± 4.4a | 48.0 ± 5.4 | 66.2 ± 3.9c |
| Total cholesterol (mg/dL) | 71.8 ± 3.2 | 135.8 ± 9.6a | 73.0 ± 11.8 | 154.6 ± 23.1a,c |
| BUN (mg/dl) | 31.2 ± 0.9 | 28 ± 0.9 | 33.6 ± 7.2 | 27.6 ± 1.5 |
Note: Body mass was measured at sacrifice. Data are means ± SEM (n = 5–7 for each data point).
a p < .05 vs standard diet (SD)-fed young control mice.
b p < .05 vs HFD-fed young mice.
c p < .05 vs SD-fed aged mice.
Obesity in Aging Elicits Significant Decline in Hippocampal Cognitive Function
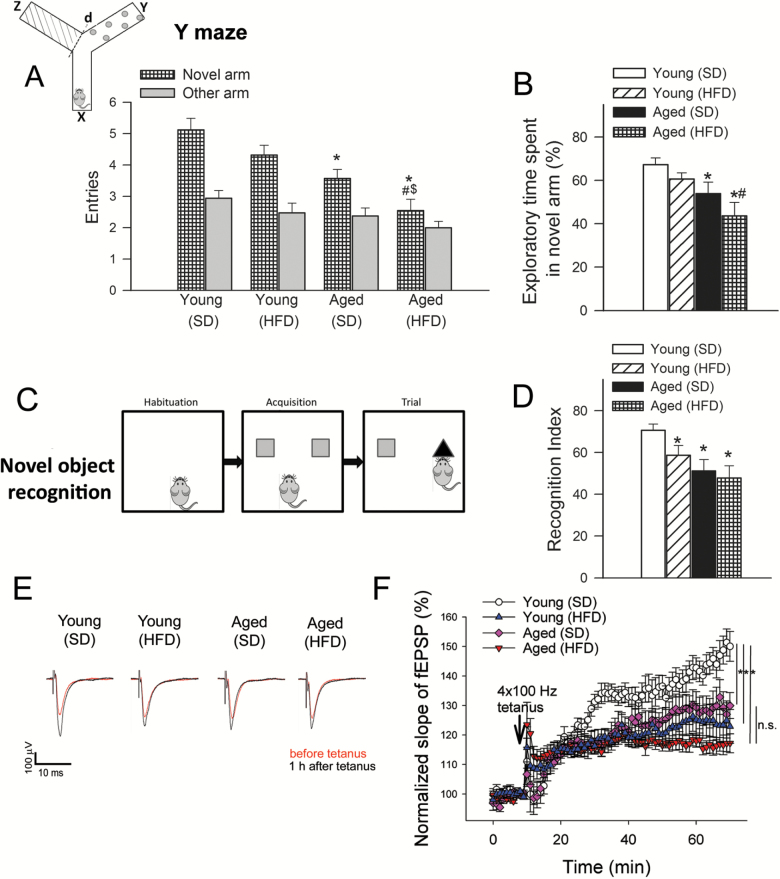
As expected, total number of alternations (Figure 1A) as well as time spent in the novel arm as a percent of total time engaged in exploration (p < .05, Figure 1B) were higher in young SD-fed mice performing a hippocampus-dependent delayed-alternation task using the Y-maze. Both number of entries to the novel arm and percent exploration time spent in the novel arm tended to decrease in young HFD-fed mice and in aged SD-fed mice as compared to young SD-fed mice, although the differences between these group means did not reach statistical significance. Number of entries to the novel arm (Figure 1A) and percent time spent in the novel arm (Figure 1B) were significantly reduced in old HFD-fed mice, suggesting that hippocampus-dependent contextual memory is impaired in this group. By two-way analysis of variance the effects of age (p = .001) and diet (p = .046) were significant, whereas the interaction between diet and age was not significant. Similar negative effects of advanced age and obesity on hippocampus-dependent learning and memory were recently shown using other behavioral tasks (7).
Figure 1.
Detrimental effects of obesity and advanced aging on learning and memory and synaptic function. Panels A–D: Measures of hippocampal-dependent contextual memory. A–B: Number of entries in novel arm of a Y-maze used in a delayed-alternation task (A) and percent exploration time spent in novel arm of the Y maze during the retrieval trial (B) are shown (*p < .05 vs young standard diet [SD]-fed mice; #p < .05 vs young high-fat diet [HFD]-fed mice; $p < .05 vs aged SD-fed mice). The inset shows a schematic picture (x: start arm, y: other arm, z: novel arm, see details in text). HFD-fed aged mice exhibited the greatest impairment of contextual memory as indicated by a similar number of entries in the novel and previously encountered arms, and a significantly reduced percent exploration time spent in the novel arm during retrieval in the delayed-alternation task. Data are means ± SEM (n = 20 in each group). C–D: Measures of hippocampal-dependent recognition memory. (C) Schematic picture of a novel object recognition test that was used to measure hippocampal-dependent recognition memory. (D) average recognition indices (as percent time spent exploring a novel object relative to total time engaged in exploration) are shown as measures of memory of a priorly encountered object. Data are means ± SEM (n = 20 in each group). *p < .05 vs young SD-fed mice. (E) Original recordings showing the effects of aging and chronic feeding of an HFD on field EPSP in the dentate gyrus in response to the stimulation of the perforant pathway on hippocampal brain slices before (10 min) and 1 h after (70 min) 4 × 100Hz tetanus. (F) Long-term potentiation shown as change of field excitatory post-synaptic potential (fEPSP) slope following a 4 × 100Hz tetanic stimulus in the dentate gyrus of the hippocampus. Data are normalized to baseline responses and depicted as means ± SEM (n = 10–14; *p < .05 aged HFD-fed mice vs young SD-fed mice; p < .05 aged SD-fed mice vs young SD-fed mice; p < .05 young HFD-fed mice vs young SD-fed mice.).
To complement these findings, we used the novel object recognition test, which like the delayed-alternation task in the Y maze measures hippocampal-dependent recognition memory (23), but relies on different instinctive and motivational aspects of mouse behavior. We found no significant differences in the time that each group spent exploring two identical objects placed at opposite ends of the arena during the acquisition phase, confirming that the objects used in the task are of equivalent value, and ruling out confounds related to potential differences in value of the position of the objects in the arena. Young SD-fed mice, however, explored a novel object that replaced one of the previously encountered ones in the trial phase (Figure 1C) for a significantly longer time than aged SD-fed mice and aged HFD-fed mice (Figure 1B), indicating impairments in hippocampal-dependent recognition memory for the familiar object in these latter groups (Figure 1C and D). The recognition index (RI) value for aged HFD-fed mice, was the lowest among experimental groups, indicative of severe deficits in hippocampal-dependent recognition memory in this group and consistent with our observations using the delayed-alternation task in the Y-maze (Figure 1D). Differences in mean RI values between young HFD-fed mice and aged SD and HFD-fed mice were not statistically different. The interaction between diet and age was not significant by two-way analysis of variance.
Effects of Obesity and Aging on Synaptic Function
We next examined LTP in the dentate gyrus to determine how obesity in aging impact the phenotype of impaired synaptic plasticity. LTP at hippocampal synapses is considered a crucial component of the cellular basis for learning and memory. In order to characterize the effects of obesity and aging on synaptic function, we measured fEPSP in the dentate gyrus of hippocampi in response to electrical stimulation of the perforant pathway (with 5 µA steps increased up to 100 µA). Original recordings showing field EPSPs in the dentate gyrus in response to the stimulation of the perforant pathway in each group are shown in Figure 1E. We found that each group of mice exhibited normal basal synaptic properties. In particular, the ratio of evoked responses to the presynaptic fiber volley were similar in SD-fed and HFD-fed mice, showing that obesity does not affect neuronal EPSP (data not shown). Following a 4 × 100Hz tetanic stimulation, the fEPSP slope in the dentate gyrus increased significantly less in the HFD-fed young group as compared to the SD-fed young controls during the 60-minute experimental period (Figure 1F). Consistent with previous observations (14), in the hippocampi of aged mice LTP was impaired and was indistinguishable from LTPs obtained in hippocampi of young HFD-fed mice (Figure 1F). When aged mice were fed an HFD, it tended to further decrease LTP (Figure 1F), although the differences between responses in hippocampi of SD-fed aged mice and HFD-fed aged mice did not reach statistical significance.
Effects of Obesity and Aging on Hippocampal Synaptic Density
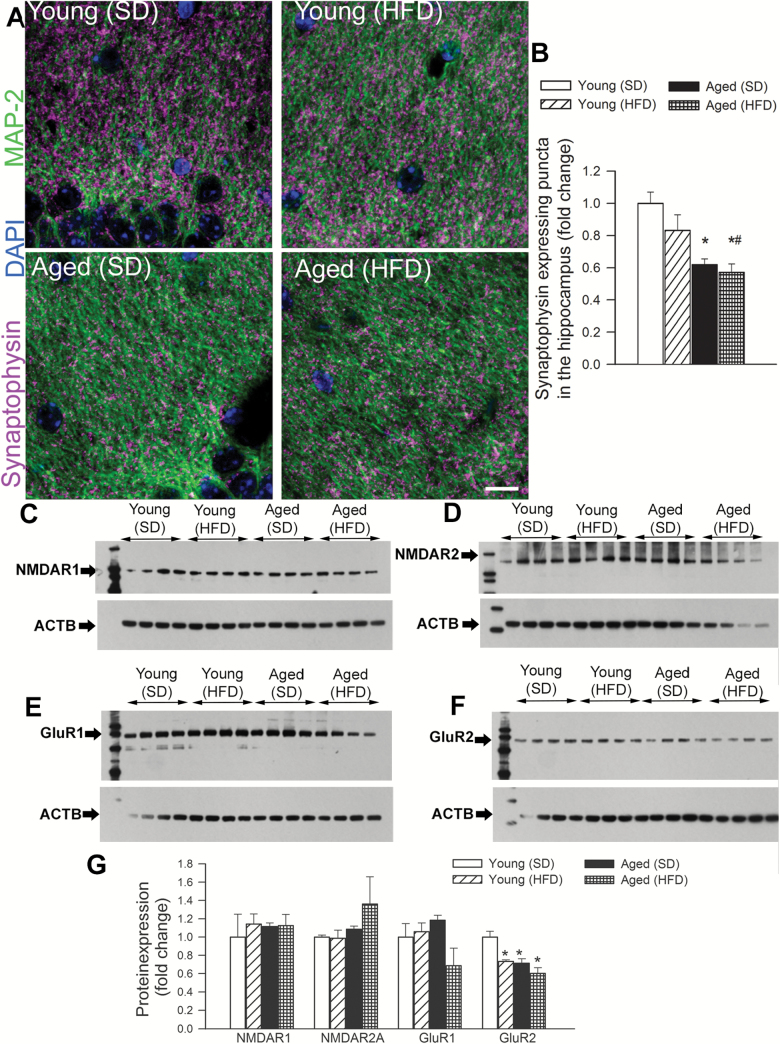
We used immunolabeling against synaptophysin, a protein localized in presynaptic vesicles, to label the density of synapses in mouse hippocampal samples. Double-immunofluorescence labeling against MAP2 demonstrated that synaptophysin-expressing presynaptic puncta were concentrated in the neuropil surrounding the MAP2-immunoreactive somata and dendrites in each group (Figure 2A). We found that synaptophysin-immunoreactive puncta density tended to decrease in the stratum radiatum of the hippocampi of HFD-fed young mice as compared to that in SD-fed young mice (Figure 2B). Density of synaptophysin expressing puncta was also significantly decreased in the stratum radiatum of the hippocampi of aged control mice (Figure 2B). However, when aged mice were fed an HFD, we did not observe an additional decline in the synaptophysin expressing puncta (Figure 2B), reflecting the dominant effect of aging on hippocampal synaptic density.
Figure 2.
Effects of obesity and advanced aging on synaptic density and the expression of post-synaptic neurotransmitter receptors in the mouse hippocampus. Panel A: Representative confocal images of synaptophysin immunoreactivity (purple) in stratum radiatum of the hippocampi of young and aged mice fed a high-fat diet (HFD) or standard diet (SD). Green fluorescence: MAP2 labeled somata and dendrites; blue fluorescence: nuclei. Panel B depicts summary data of relative changes in the number of synaptophysin-positive presynaptic puncta (fold change). Data are mean ± SEM *p < .05 vs young (SD), #p < .05 vs young (HFD). Panel C–F: Original Western blots showing expression of NMDAR1, NMDAR2, GluR1, and GluR2 in the hippocampi of young and aged mice fed a HFD or SD. Summary data are shown in Panel G. Data are mean ± SEM (n = 6 in each group) *p < .05 vs young.
Effects of Obesity and Aging on the Hippocampal Expression of Post-Synaptic Neurotransmitter Receptors
Decreased expression of post-synaptic neurotransmitter receptors may contribute to altered LTP under various pathophysiological conditions. In the present study neither feeding an HFD nor aging were associated with changes in protein expression of GluR1, NMDAR1, and NMDAR2. In contrast, expression of GluR2 tended to decrease in HFD-fed young mice as well as in SD- and HFD-fed aged mice (Figure 2C–G).
Effects of Obesity and Aging on the Hippocampal Expression of Genes Relevant for Neuronal Function and Cerebral Health
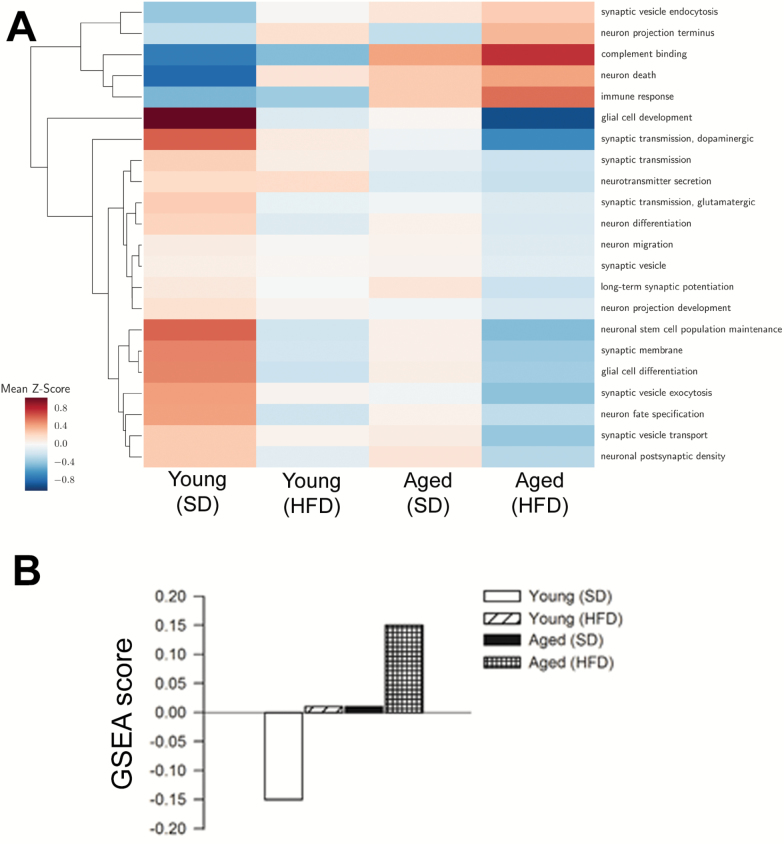
Using RNA-seq to sequence the hippocampus transcriptome a number of genes were identified whose expression is up- or down-regulated with advanced age and/or by consumption of an HFD. Figure 3A depicts the average of gene expression Z-scores for selected Gene Ontology categories relevant for neuronal function and cerebral health. The results suggest that obesity and aging synergistically impair multiple biological processes important for hippocampal memory formation and storage. In particular, we observed that obesity and aging synergistically up-regulated negative regulators of synaptic plasticity (Figure 3B), which may contribute to the observed age- and obesity-dependent alterations in synaptic plasticity.
Figure 3.
Obesity in aging associated with dysregulation of genes affecting multiple aspects of neuronal function and cerebral health. (A) Average of gene expression Z-scores for selected Gene Ontology categories relevant for neuronal function and cerebral health. Each row corresponds to the average Z-score of the genes in the GO categories for each experimental condition (columns). (B) Gene set enrichment analysis (GSEA) scores for negative regulators of synaptic plasticity. Obesity and aging exert synergistic effects on the expression of genes involved in impairment of synaptic plasticity.
Effects of Obesity and Aging on the Hippocampal Expression of Genes Involved in Microglia Activation and Neuroinflammation
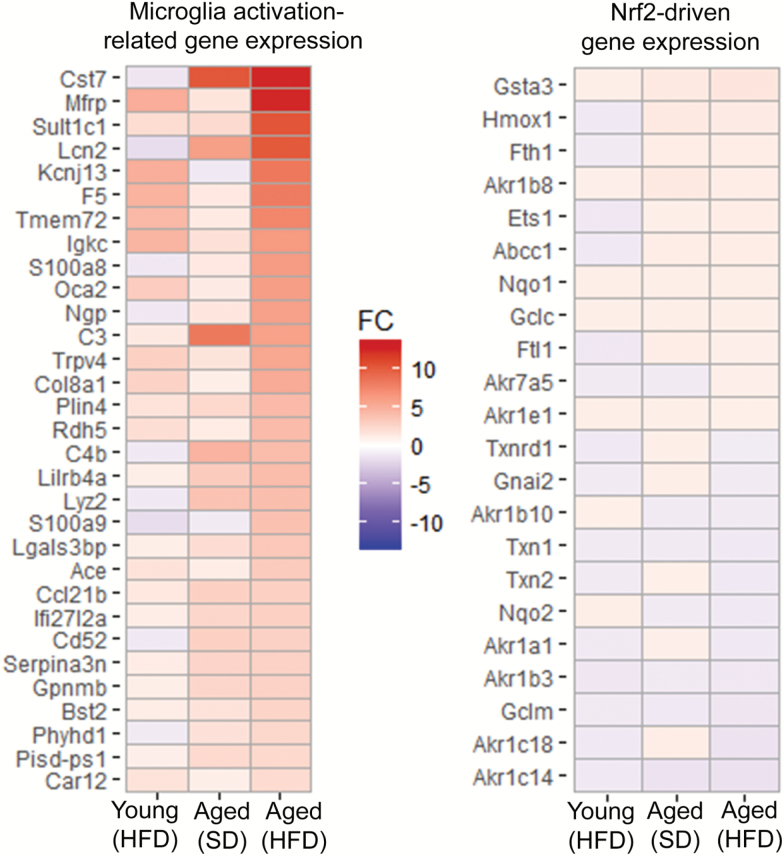
Our findings demonstrate age-related up-regulation of genes involved in microglia activation (Figure 4), extending previous findings both in human (24) and mouse (25,26) hippocampi. Previous studies suggest that obesity and aging exert synergistic effects, exacerbating neuroinflammation by promoting leakage of plasma-derived factors through the damaged BBB, which is a potent stimulus for microglia activation (6). In accordance with this concept we found that in the hippocampi of aged mice obesity promoted significant up-regulation of microglia-enriched pro-inflammatory genes (Figure 4), whereas young mice exhibited significant resistance to obesity-related changes in inflammatory gene expression, extending our previous findings (6).
Figure 4.
Gene expression footprint of microglia activation in the hippocampi of obese aged mice. Panel A: Heat map of expression of 31 microglia activation-related genes in the hippocampi of young and aged mice fed a high-fat diet (HFD) or standard diet (SD). Upregulated gene expression is displayed in red and downregulated gene expression in blue. Each gene was differentially expressed in the hippocampi of aged HFD-fed mice versus that of young control mice. Genes are in decreasing order of fold change (FC) comparing HFD-fed aged to young control. Note that obesity and aging exert synergistic effects on the expression of genes related to microglia activation and neuroinflammation. Panel B: Heat map of Nrf2-driven genes in the hippocampi of young and aged mice fed an HFD or SD.
Consistent with the presence of increased oxidative/nitrosative stress in the brain of obese and aged mice (6,10), there was a discernible trend for increased mRNA expression of gp91phox (encoded by Cybb) in the hippocampi of aged HFD-fed and aged SD-fed mice (5.2- and 4.2-fold, respectively) as well as HFD-fed young mice (1.5-fold), supporting the concept that the effects of age and obesity on brain redox homeostasis are synergistic (10). The effects of aging and HFD feeding on expression of Nrf2-driven genes is shown in Figure 4. We found that increased hippocampal oxidative stress in HFD-fed aged mice is not associated with a gene expression signature consistent with significant compensatory Nrf2 activation.
Lipidomic Analysis of Eicosanoids in Hippocampal Samples
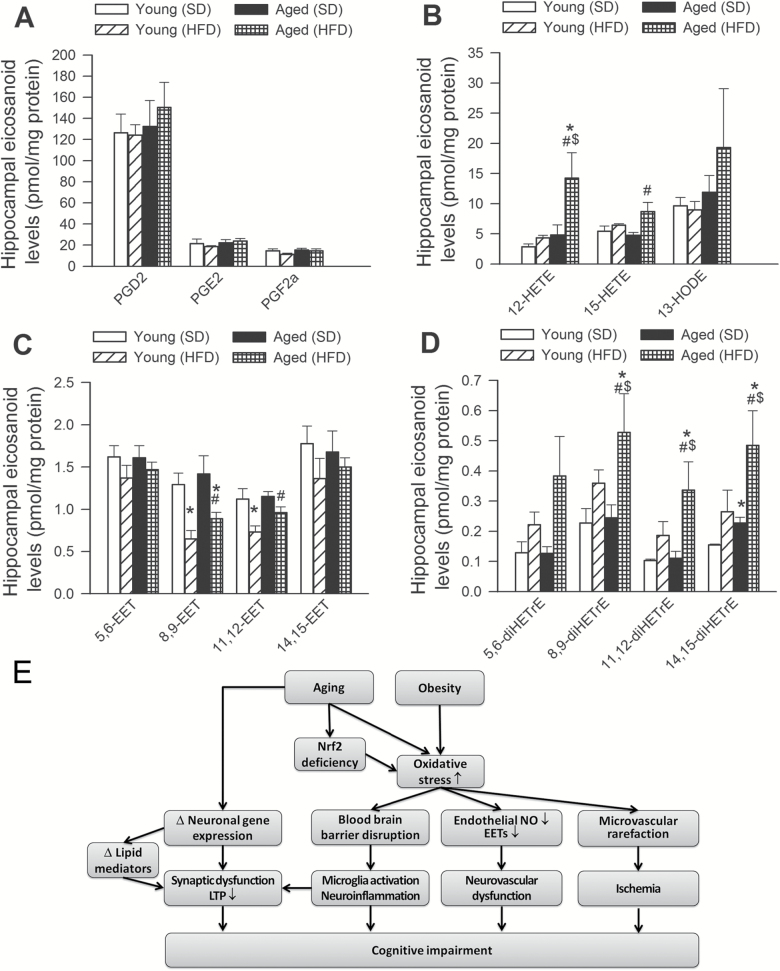
A high-throughput lipidomics methodology was used to determine hippocampal levels of eicosanoids known to be involved in synaptic plasticity and/or neurovascular coupling responses (17). Cyclooxygenase-mediated generation of PGE2, PGF2α, and PGD2 was reported to modulate synaptic plasticity and LTP (27) and to contribute to neurovascular coupling responses (17). We found that neither aging nor obesity affected hippocampal levels of PGE2, PGF2α, and PGD2 (Figure 5A).
Figure 5.
Lipidomic analysis of eicosanoid regulators of long-term potentiation (LTP) and cognitive function in hippocampal samples. HFD- and aging-induced changes in hippocampal levels of cyclooxygenase-derived PGE2, PGD2 and PGF2α (Panel A), the 12/15-lipoxygenase metabolites 12-HETE, 15-HETE, and 13-HODE (Panel B), the cytochrome monooxygenase-derived epoxyeicosatrienoic acids (EETs, Panel C) and the epoxide hydrolase-derived inactive metabolites of EETs (11,12-DiHETrE, 14,15-DiHETrE, 5,6-DiHETrE and 8,9-DiHETrE; Panel D) (n = 6 for each data point; *p < .05 vs young SD-fed mice; #p < .05 vs aged SD-fed mice; $p < .05 vs young HFD). Panel E: Proposed scheme depicting the synergistic roles of age-related exacerbation of obesity-induced oxidative stress, BBB disruption, neuroinflammation, neurovascular dysfunction and synaptic dysfunction, all of which contribute to cognitive impairment in obese elderly subjects.
There is ample evidence that 12/15-lipoxygenase metabolites modulate synaptic function, synaptic integrity and LTP (28). We found that obesity in aged mice increased hippocampal level of the 12/15-lipoxygenase metabolites 12-HETE (12-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid), 15-HETE (15-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid) and 13-HODE (13-hydroxy-9Z,11E-octadecadienoic acid; Figure 5B). In contrast, obesity in young mice had no effect on hippocampal levels of 12/15-lipoxygenase metabolites (Figure 5B).
Epoxyeicosatrienoic acids (EETs) produced by cytochrome (CYP) monooxygenases are important for regulation of neuronal function both directly (29) and indirectly, via regulation of regional cerebral blood flow (16). We found that HFD-induced obesity was associated with significant decline in hippocampal level of 8,9-EET and 11,12-EET both in young and aged mice (Figure 5C), whereas HFD-induced changes in 5,6-EET and 14,15-EET content did not reach statistical significance. Epoxide hydrolases convert the EETs into vicinal diols [11,12-DiHETrE (11,12-dihydroxy-5Z,8Z,14Z-eicosatrienoic acid); 14,15-DiHETrE (14,15-dihydroxy-5Z,8Z,11Z-eicosatrienoic acid); 5,6-DiHETrE (5,6-dihydroxy-8Z,11Z,14Z-eicosatrienoic acid); and 8,9-DiHETrE (8,9-dihydroxy-5Z,11Z,14Z-eicosatrienoic acid], with the concurrent loss of much of their biological activity. The findings that in obese mice reduced EET content was associated with an increased level of EET breakdown products (Figure 5D) indicate that obesity may increase the activity of epoxide hydrolase. This is a potentially interesting finding as up-regulation of epoxide hydrolase was proposed to contribute to cognitive decline (29).
Discussion
The results of this study suggest that exacerbation of obesity-induced neuroinflammation in aged mice is associated with an impairment in synaptic plasticity, altered production of vasodilator and pro-LTP eicosanoids and dysregulation of expression of genes involved in regulation of neuronal function in the hippocampus, all of which may contribute to deficits in cognitive function, which we documented using independent corroborative measures of hippocampal-dependent memory.
On the basis of previous findings (6,7) and the present results, it seems that advanced aging and diet-induced obesity have similar effects on hippocampal neuronal function. Our current understanding of the mechanisms of learning and memory formation in the hippocampus is that changes in synaptic efficacy induced by coincident pre- and postsynaptic activity (the same mechanisms that underlie LTP) have a critical role (13,30). Here we confirm earlier findings that LTP is significantly impaired in aging (14), which likely contributes to age-related defects in learning and memory. Importantly, our findings demonstrate that aging and obesity have similar effects on LTP induced at the dentate gyrus, which parallels their negative effects on cognitive function. The available evidence suggests that obesity-induced impairment of LTP and cognitive decline does not depend on the experimental model used. For example, impairment of hippocampal LTP have been previously reported in obese Zucker rats (31), in leptin-resistant db/db mice (32) and in rats in which obesity was induced by selective knockdown of insulin receptor in the hypothalamus (33). The findings that obesity and aging similarly impair LTP are translationally relevant: it is predicted that if similar synaptic dysfunction is also manifested in the hippocampi of obese elderly patients, it would likely contribute to the pathogenesis of cognitive impairment.
The mechanisms by which obesity impairs synaptic plasticity in the hippocampus are likely multifaceted. We have identified several factors important for synaptic and neuronal function and cerebral health whose expression was significantly altered by aging and obesity. LTP induced at the hippocampal CA3–CA1 and dentate gyrus synapses using HFS in rodents is dependent on the NMDA receptor. While changes in hippocampal expression of NMDA receptors were not evident, we found HFD- and age-related down-regulation of GluR2. Future studies are needed to elucidate the functional consequences of this alteration on AMPAR trafficking in NMDAR-dependent LTP. Through unbiased mRNA sequence analysis of the aged hippocampus we demonstrate that obesity and aging synergistically alter hippocampal LTP–related gene networks and expression of genes relevant for synaptic transmission. Our results extend the findings of previous studies showing that aging significantly alters LTP-related gene expression both in rat (34) and mouse (35) models, which associate with impaired spatial memory retention. Prior analysis of expression profiles of synaptic genes in humans also demonstrate significant expression changes in synapse-related genes in aging (36), affecting multiple aspects of synaptic function. Our findings suggest that that the expression of a common set of synaptic genes is vulnerable to change in aging and obesity. Importantly, caloric restriction was reported to reverse/normalize age-related changes in several LTP-related genes in rodent models (35). Many regard the cellular responses induced by HFD the opposite of the effects induced by caloric restriction. Further studies are evidently needed to elucidate the cellular compartment in the aged brain most affected by HFD, the molecular mechanisms responsible for the dysregulation of the LTP–related gene networks and establish cause-and-effect relationship between the observed transcriptional signature and the physiological alterations. It is likely that loss of synapses only partially accounts for the cognitive decline associated with aging while in obesity it is primarily the molecular changes of existing synapses that reduce their functional capacity.
Multiple lines of evidence support the concept that increased microglia activation and exacerbation of neuroinflammation in obese aged mice significantly contribute to neuronal and synaptic dysfunction, promoting cognitive decline (9,11,37). First, our studies suggest that aging exacerbates obesity-induced microglia activation in the hippocampus (6). Second, microglia-derived pro-inflammatory cytokines, chemokines, proteases, and reactive oxygen species have been demonstrated to promote neuronal dysfunction. Third, elimination of activated microglia was reported to improve cognitive function in various experimental models (38,39). Aging was shown to exacerbate obesity-induced adipose tissue inflammation, which contributes to a marked pro-inflammatory shift in the circulating cytokine profile (22). Mechanisms underlying exacerbated microglia activation in obese aged mice likely include increased circulating levels of inflammatory cytokines derived from the inflamed adipose tissue (22) and disruption of the blood–brain barrier (6). Previous studies provide evidence that in aged obese mice through the damaged BBB plasma constituents enter the brain (6) and that plasma-derived IgG, thrombin, fibrinogen, and inflammatory cytokines are potent stimuli for microglia activation. In addition to promoting microglia activation, disruption of the blood–brain barrier also alters the local microenvironment around neurons, which likely also contribute to impairment of synaptic function. Additional mechanisms that exacerbate obesity-induced neuroinflammation likely include an age-related Nrf2 dysfunction (11). Nrf2 controls several different antioxidants pathways and confers significant anti-inflammatory effects. There is strong evidence that Nrf2 deficiency exacerbates the deleterious microvascular effects associated with consumption of a HFD (40,41), promoting disruption of the blood–brain barrier (42) and thus neuroinflammation. In agreement with the hypothesis that age-related Nrf2 dysfunction exacerbates HFD-induced neuroinflammation, the marked up-regulation of microglia activation-related genes in obese aged mice was not associated with significant induction of Nrf2 target genes, despite the presence of increased oxidative stress in this model (6). It should be noted that Nrf2 activation, metabolic alterations, changes in hippocampal gene expression, synaptic plasticity and/or deficits of learning induced by aging (24) and/or obesity (43) in the mouse brain may be sexually dimorphic. Thus, future studies are warranted to compare outcome measures related to obesity-induced neuroinflammation and cognitive impairment in older female and male subjects. Further studies are also needed to determine how phenotypically heterogeneous microglia populations in different anatomical regions in the aging brain react to obesity.
There is increasing evidence that lipid mediators play an important role in regulation of synaptic plasticity as well as neurovascular coupling responses (17) and inflammatory processes. While cyclooxygenase-mediated generation of PGE2, PGF2α, and PGD2 was reported to modulate synaptic plasticity and LTP and to contribute to neurovascular coupling responses (17), the production of these eicosanoid mediators do not appear to be affected either by obesity of aging. In contrast, obesity in aged mice increased hippocampal level of the 12/15-lipoxygenase metabolites, which are known to modulate synaptic function, synaptic integrity, and LTP. These are potentially important findings as overexpression of 12/15-lipoxygenase was shown to impair hippocampal memory function, whereas pharmacological inhibition of 12/15-lipoxygenase improves cognitive function in mouse models of AD (28,44). Obesity was also associated with significant decline in hippocampal level of EETs, likely due to increased activity of epoxide hydrolases. This finding is significant as EETs facilitate LTPs (45) and up-regulation of epoxide hydrolase was proposed to contribute to cognitive decline (29). In addition, EETs exert important anti-inflammatory effects and also play an important role in neurovascular coupling responses. Thus, their impaired synthesis may also promote neuroinflammation and contribute to dysregulation of local cerebral blood flow in obese aged mice (7). Interestingly, obesity-related suppression of bioavailability of anti-inflammatory EETs has been observed in other tissues as well (46).
Collectively, our present and previous studies as well as investigations by other laboratories demonstrate that obesity in aging promotes hippocampal inflammation by promoting blood–brain barrier disruption, which in turn may contribute to synaptic dysfunction and cognitive impairment (Figure 5E). In addition, obesity also impairs cerebral blood flow, by dysregulating neurovascular coupling responses, impairing endothelial function, and promoting microvascular rarefaction (6,7,42). These microvascular alterations likely impair delivery of oxygen and nutrients to active brain regions, which may also contribute significantly to obesity-induced deterioration of cognitive function. Our findings have important translational relevance. Clinical studies suggest that obesity-induced cognitive decline develops gradually, leaving a time window for therapeutic intervention for prevention. Further studies should determine whether obesity-induced impairment of synaptic plasticity and decline in synapse density are also reversible with anti-obesity treatments (eg, diet (33), exercise, anti-obesity medication, surgery). Importantly, there is increasing epidemiological evidence that there is an association between obesity in aging and the risk for Alzheimer’s disease (AD) -type dementia (2). Experimental studies on rodent models of AD confirm the clinical observations (47). In addition to neuroinflammation, neuronal death, and microvascular pathologies (17,48), synaptic dysfunction also importantly contributes to the pathogenesis of AD-type dementia (49). Our findings combined with these observations highlight a novel mechanism by which obesity in aging may exacerbate the symptoms of AD.
Funding
This work was supported by grants from the American Heart Association (to S.T., M.N.V.A.), the National Institute on Aging (R01-AG055395, R01-AG047879; R01-AG038747), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218, R01-NS100782), NIH-supported Oklahoma Shared Clinical and Translational Resources (U54GM104938), the Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., F.D., A.Y.) and the Presbyterian Health Foundation (to F.D., A.C., U.Z.) and the Oklahoma Nathan Shock Aging Center (P30-AG050911). The authors acknowledge the support from the NIA-funded Geroscience Training Program in Oklahoma (T32AG052363) and the EU-funded EFOP-3.6.1-16-2016-00008 (to Z.U.).
Conflict of Interest
None reported.
Supplementary Material
References
- 1. Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring). 2007;15:2855–2865. doi: 10.1038/oby.2007.339 [DOI] [PubMed] [Google Scholar]
- 2. Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. [DOI] [PubMed] [Google Scholar]
- 4. Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef [DOI] [PubMed] [Google Scholar]
- 5. Stanek KM, Strain G, Devlin M, et al. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology. 2013;27:141–151. doi: 10.1037/a0031988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tucsek Z, Toth P, Sosnowska D, et al. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. doi: 10.1093/gerona/glt177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tucsek Z, Toth P, Tarantini S, et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hao S, Dey A, Yu X, Stranahan AM. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun. 2016;51:230–239. doi: 10.1016/j.bbi.2015.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pistell PJ, Morrison CD, Gupta S, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruce-Keller AJ, White CL, Gupta S, et al. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med. 2010;49:22–30. doi: 10.1016/j.freeradbiomed.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrison CD, Pistell PJ, Ingram DK, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tucsek Z, Gautam T, Sonntag WE, et al. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci. 2013;68:652–660. doi: 10.1093/gerona/gls232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003 [DOI] [PubMed] [Google Scholar]
- 14. Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, et al. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. 2017. doi: 10.1007/s11357-017-9981-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tarantini S, Hertelendy P, Tucsek Z, et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tarantini S, Tran CH, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Csiszar A, Tucsek Z, Toth P, et al. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in β-amyloid generation and Alzheimer’s disease. Am J Physiol Heart Circ Physiol. 2013;305:H1120–H1130. doi: 10.1152/ajpheart.00288.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orock A, Logan S, Deak F. Munc18-1 haploinsufficiency impairs learning and memory by reduced synaptic vesicular release in a model of Ohtahara syndrome. Mol Cell Neurosci. 2018;88:33–42. doi: 10.1016/j.mcn.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imperio CG, McFalls AJ, Colechio EM, et al. Assessment of individual differences in the rat nucleus accumbens transcriptome following taste-heroin extended access. Brain Res Bull. 2016;123:71–80. doi: 10.1016/j.brainresbull.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Armando AM, Quehenberger O, Yan C, Dennis EA. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J Chromatogr A. 2014;1359:60–69. doi: 10.1016/j.chroma.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bailey-Downs LC, Tucsek Z, Toth P, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. doi: 10.1093/gerona/gls238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berchtold NC, Cribbs DH, Coleman PD, et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A. 2008;105:15605–15610. doi: 10.1073/pnas.0806883105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masser DR, Bixler GV, Brucklacher RM, et al. Hippocampal subregions exhibit both distinct and shared transcriptomic responses to aging and nonneurodegenerative cognitive decline. J Gerontol A Biol Sci Med Sci. 2014;69:1311–1324. doi: 10.1093/gerona/glu091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mangold CA, Wronowski B, Du M, et al. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation. 2017;14:141. doi: 10.1186/s12974-017-0920-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piomelli D. Eicosanoids in synaptic transmission. Crit Rev Neurobiol. 1994;8:65–83. [PubMed] [Google Scholar]
- 28. Di Meco A, Li JG, Blass BE, Abou-Gharbia M, Lauretti E, Praticò D. 12/15-Lipoxygenase inhibition reverses cognitive impairment, brain amyloidosis, and tau pathology by stimulating autophagy in aged triple transgenic mice. Biol Psychiatry. 2017;81:92–100. doi: 10.1016/j.biopsych.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 29. Nelson JW, Young JM, Borkar RN, et al. Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins Other Lipid Mediat. 2014;113-115:30–37. doi: 10.1016/j.prostaglandins.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn230 [DOI] [PubMed] [Google Scholar]
- 31. Gerges NZ, Aleisa AM, Alkadhi KA. Impaired long-term potentiation in obese zucker rats: possible involvement of presynaptic mechanism. Neuroscience. 2003;120:535–539. doi:S0306452203002975 [DOI] [PubMed] [Google Scholar]
- 32. Erion JR, Wosiski-Kuhn M, Dey A, et al. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014;34:2618–2631. doi: 10.1523/JNEUROSCI.4200-13.2014.34/7/2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grillo CA, Piroli GG, Evans AN, et al. Obesity/hyperleptinemic phenotype adversely affects hippocampal plasticity: effects of dietary restriction. Physiol Behav. 2011;104:235–241. doi: 10.1016/j.physbeh.2010.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ryan MM, Guévremont D, Luxmanan C, Abraham WC, Williams JM. Aging alters long-term potentiation–related gene networks and impairs synaptic protein synthesis in the rat hippocampus. Neurobiol Aging. 2015;36:1868–1880. doi: 10.1016/j.neurobiolaging.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 35. Schafer MJ, Dolgalev I, Alldred MJ, Heguy A, Ginsberg SD. Calorie restriction suppresses age-dependent hippocampal transcriptional signatures. PLoS One. 2015;10:e0133923. doi: 10.1371/journal.pone.0133923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol Aging. 2013;34:1653–1661. doi: 10.1016/j.neurobiolaging.2012.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White CL, Pistell PJ, Purpera MN, et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho SH, Chen JA, Sayed F, et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1β. J Neurosci. 2015;35:807–818. doi: 10.1523/JNEUROSCI.2939-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Bernhardi R, Eugenín-von Bernhardi L, Eugenín J. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci. 2015;7:124. doi: 10.3389/fnagi.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. doi: 10.1152/ajpheart.00402.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hwang LL, Wang CH, Li TL, et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring). 2010;18:463–469. doi: 10.1038/oby.2009.273 [DOI] [PubMed] [Google Scholar]
- 44. Chu J, Li JG, Giannopoulos PF, et al. Pharmacologic blockade of 12/15-lipoxygenase ameliorates memory deficits, Aβ and tau neuropathology in the triple-transgenic mice. Mol Psychiatry. 2015;20:1329–1338. doi: 10.1038/mp.2014.170 [DOI] [PubMed] [Google Scholar]
- 45. Wu HF, Chen YJ, Wu SZ, et al. Soluble epoxide hydrolase inhibitor and 14,15-epoxyeicosatrienoic acid-facilitated long-term potentiation through cAMP and CaMKII in the hippocampus. Neural Plast. 2017;2017:3467805. doi: 10.1155/2017/3467805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zha W, Edin ML, Vendrov KC, et al. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J Lipid Res. 2014;55:2124–2136. doi: 10.1194/jlr.M053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maesako M, Uemura K, Kubota M, et al. Exercise is more effective than diet control in preventing high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J Biol Chem. 2012;287:23024–23033. doi: 10.1074/jbc.M112.367011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Csiszar A, Tarantini S, Fulop GA, et al. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017. doi: 10.1007/s11357-017-9991-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marcello E, Epis R, Saraceno C, Di Luca M. Synaptic dysfunction in Alzheimer’s disease. Adv Exp Med Biol. 2012;970:573–601. doi: 10.1007/978-3-7091-0932-8_25 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.