Abstract
Background
Understanding how components of frailty change over time and how they can be modeled as time-dependent predictors of mortality could lead to better risk prediction in the dialysis population.
Methods
We measured frailty at baseline, 12 months, and 24 months among 727 patients receiving hemodialysis in Northern California and Atlanta. We examined the likelihood of meeting frailty components (weight loss, exhaustion, low physical activity, weak grip strength, and slow gait speed) as a function of time in logistic regression analysis and association of frailty components with mortality in time-updated multivariable Cox models.
Results
Physical activity and gait speed declined, exhaustion and grip strength did not change, and the odds of meeting the weight loss criterion declined with time. All five components were associated with higher mortality in multivariable analyses, but gait speed was the strongest individual predictor. All frailty components except physical inactivity were independently associated with mortality when all five components were included in the same model. The number of frailty components met was associated with mortality in a gradient that ranged from a hazard ratio of 2.73 for one component to 10.07 for five components met; the model including all five components was the best model based on Akaike information criterion.
Conclusions
Measurement of all frailty components was necessary for optimal mortality prediction, and the number of components met was strongly associated with mortality in this cohort.
Keywords: Hemodialysis, Frailty, Mortality, Gait speed
Approximately one third of patients on hemodialysis are frail, and frailty is associated with mortality (1–4). We recently showed that although the overall degree of frailty changes very little over 1 year, there is considerable change in both directions, with some patients developing worsening frailty and others improving (5). Considering the five components of the Frailty Phenotype developed by Fried and colleagues (6), which include slow gait, weak grip, low physical activity, exhaustion, and weight loss, two thirds of patients in a prevalent hemodialysis cohort either met one or more new components or no longer met one or more components over two successive 1-year periods, and worsening and improvement were relatively balanced (5). However, it is likely that changes in the individual components of frailty do not occur at the same rate, and consideration of the evolution of frailty over time may be important for predicting risk of mortality. In addition, although frailty, defined as meeting any three of the five criteria, is associated with mortality, it is not clear whether each component of frailty is equally and independently associated or whether it might be possible to achieve similar or better mortality prediction using a subset of the components or a score based on the number of components. A better understanding of these issues could help in determining how best to focus efforts to screen and intervene on limitations in physical function and frailty in the hemodialysis population.
We used data from the U.S. Renal Data System (USRDS) Special Study, ACTIVE/ADIPOSE (A Cohort To Investigate the Value of Exercise/Analyses Designed to Investigate be the Paradox of Obesity and Survival in ESRD) (7), to investigate the changes in each of the frailty components over 2 years. We then examined the degree to which individual components of frailty and the number of components were associated with mortality in time-updated analyses. We hypothesized that components of frailty would be independently associated with mortality and that a more granular evaluation of the components of the frailty phenotype rather than the dichotomous determination of frailty versus no frailty would yield improved predictive capacity.
Methods
Study Participants
ACTIVE/ADIPOSE enrolled 771 patients from 14 dialysis facilities in the San Francisco Bay Area and the Atlanta, GA metropolitan area during 2009–2011 and followed them with annual assessments for up to 2 years through 2013 (7). Participants were over 18 years of age, on in-center hemodialysis for at least 3 months, English or Spanish speaking, and able to provide informed consent. The study was approved by the University of California, San Francisco and the Emory University Institutional Review Boards, and patients provided written informed consent for study participation.
Clinical and Laboratory Evaluation
Study coordinators interviewed participants, measured physical performance (gait speed and grip strength), and administered study questionnaires immediately before or during a dialysis session. Coordinators also reviewed participants’ medical records to ascertain the presence of comorbid conditions and the most recent postdialysis weights. Participants’ data were linked to data from the ESRD Medical Evidence Report (Centers for Medicare and Medicaid Services Form 2728) and the patient’s standard analytic file available in the USRDS.
Blood was drawn immediately prior to a dialysis session within 1 month of the annual testing sessions. Samples were centrifuged, aliquoted, stored at −80°C, and transported in batches to the core laboratory where they were stored over liquid nitrogen at −196°C until the time of assay. We used the mean of duplicate measures of serum albumin concentration from a Polychem Autoanalyzer 180 (Polymedco, Cortland Manor, NY).
Components of Frailty
We assessed each of the five components included in the Frailty Phenotype at study enrollment, 12 months, and 24 months as previously described (1,6). Weight loss was determined by asking participants whether they had lost more than 10 pounds in the last year unintentionally. Exhaustion was based on questions about endurance and energy from the Center for Epidemiologic Studies Depression (CES-D) scale (8). Low physical activity was ascertained from the short version of the modified Minnesota Leisure Time Activity (MMLTA) questionnaire, which asks about the frequency and duration of participation in various activities over a 2-week time period (9), using sex-specific cut points (Supplementary Table 1). Participants performed three tests of grip strength with each hand using a handheld dynamometer (Jamar, Lafayette Instrument, Lafayette, IN), and the mean of the strongest hand was used to determine frailty using cutoffs that were developed in the original study of frailty phenotype by Fried and colleagues, which vary according to sex and body mass index (Supplementary Table 1) (6). Gait speed was measured while participants walked at their usual pace over a 15-foot course, and the faster of two trials was recorded. We used sex-specific cut points according to Fried and colleagues (Supplementary Table 1) (6).
Statistical Analyses
Participant characteristics at study enrollment were described according to the number of frailty components met at baseline using mean and SD or median and 25th, 75th percentile and proportions for categorical variables. We used generalized estimating equations to examine change in meeting the individual components of frailty over time (dichotomous outcome) in univariable and multivariable analyses. In univariable analyses, the predictor was time. In the multivariable analyses, we adjusted for age, sex, race, ethnicity, body mass index (in categories including <20, 20 to <25 [reference], 25 to <30, and ≥30 kg/m2), dialysis vintage, history of diabetes, atherosclerotic heart disease, or heart failure, dialysis via a catheter, and serum albumin concentration. We also included change in albumin concentration since baseline and change in catheter status as predictors (5) and tested for interaction with age and race. We also examined annual change in gait speed, physical activity, and grip strength in linear mixed models.
To examine associations among components of frailty and mortality, we used univariable and multivariable Cox models including the same sets of covariates. We did not include inflammation in our primary model because we believe that inflammation is a mediator of change in frailty. However, in a supplementary analysis, we also adjusted for interleukin-6 as a marker of inflammation. We used delayed entry into the model to account for the possibility of survival bias from the time from dialysis initiation (effectively starting follow-up at the time of dialysis initiation). To account for changes over time, frailty and its components were treated as time-varying predictors (ie, was updated at 12 and 24 months). Mortality was ascertained through linkage with the USRDS through December 31, 2014. Patients were censored at the time of receipt of a kidney transplant or at the end of 2014.
We examined frailty components individually and then examined whether considering multiple components provided better prediction than individual components using three strategies. First, we included all components in a single model. Second, we modeled the number of components met as a predictor of mortality. Third, we examined the most common combination of three components (Figure 1) (10). We compared the models using the Akaike information criterion, an estimate of the relative quality of models that takes into account goodness of fit and complexity. To determine whether these more complex constructs were superior to simpler ones, we also compared these models to a model in which frailty was considered as a dichotomous construct and to a model containing only the single component (gait speed) most strongly associated with mortality.
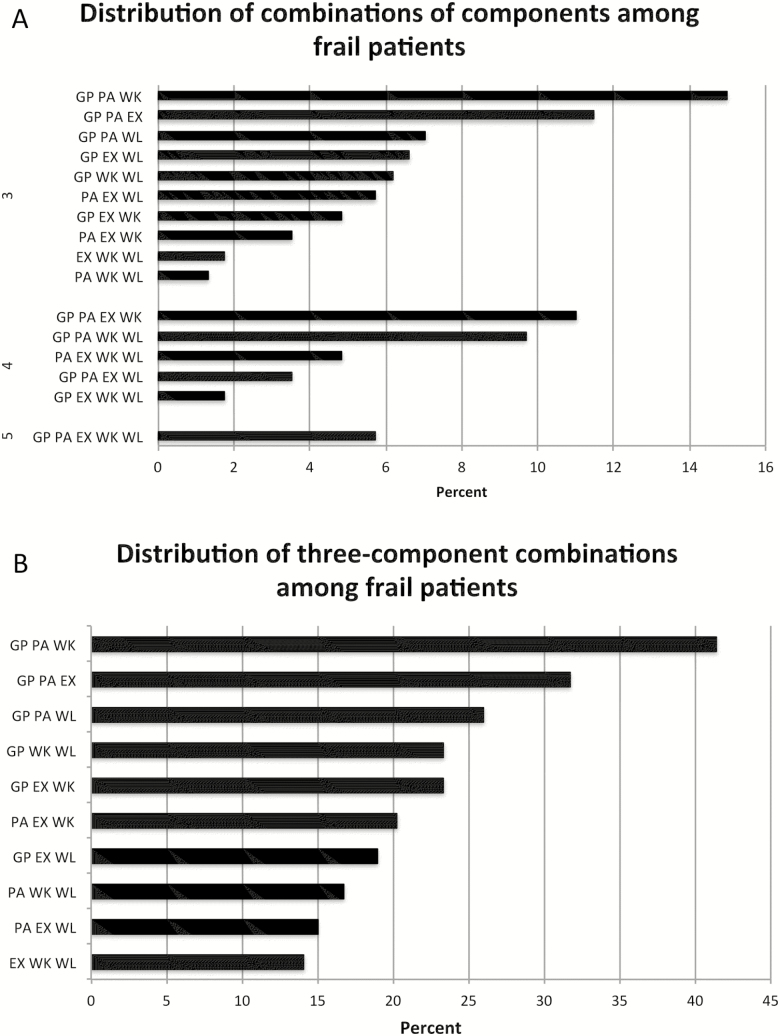
Figure 1.
Distribution of frailty components among frail patients. (A) All possible combinations by which the frailty definition could be met among the 227 frail patients. (B) The percentage of frail patients meeting three-component combinations (with or without also meeting additional components).
In sensitivity analyses, we repeated these analyses using Fine–Gray models to account for kidney transplantation as a competing risk. In addition, although our time-updated frailty variables implicitly incorporate change in frailty components over time, we performed an alternative analysis in which we included both the baseline value and the change from baseline for each frailty construct or component. We used SAS, version 9.4 for all analyses. We considered p values of less than .05 to be statistically significant.
Results
Participants and Baseline Frailty Status
ACTIVE/ADIPOSE enrolled 771 patients. Thirty-four were missing laboratory covariate information, five were missing body mass index, and five were missing one or more frailty components. Seven hundred and twenty-seven (94%) completed frailty assessments, had information available for all covariates, and were included in this study. Participants’ mean age was 57.2 ± 14.3 years, 40.8% were women, 61.7% were black, and 53.1% had diabetes. Patients having more frailty components were older and more likely to be women, to have comorbid conditions, and to have lower serum albumin concentration (Table 1).
Table 1.
Characteristics of Study Participants According to the Number of Frailty Components Met at Baseline
| Characteristic | Number of Frailty Components | p Value | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| (n = 88) | (n = 202) | (n = 207) | (n = 146) | (n = 71) | (n = 13) | ||
| Age, y | 52.2 (12.7) | 54.6 (13.8) | 56.9 (14.2) | 60.7 (14.6) | 63.7 (13.6) | 64.2 (8.3) | <.001 |
| Sex, % female | 32.6 | 37.9 | 40.9 | 38.2 | 60.0 | 61.5 | .005 |
| Race, % | .33 | ||||||
| Black | 68.5 | 67.0 | 59.1 | 52.8 | 65.7 | 61.5 | |
| White | 19.1 | 21.2 | 25.0 | 29.2 | 21.4 | 15.4 | |
| Asian | 10.1 | 10.8 | 12.0 | 11.8 | 8.6 | 15.4 | |
| Other | 2.2 | 1.0 | 3.8 | 6.3 | 4.3 | 7.7 | |
| Ethnicity, % Hispanic | 10.1 | 9.4 | 15.4 | 17.4 | 12.9 | 7.7 | .24 |
| Body mass index, kg/m2 | 26.5 (5.9) | 28.1 (6.7) | 28.1 (6.7) | 28.7 (7.3) | 29.5 (8.0) | 26.5 (5.9) | .14 |
| Vintage, y | 3.1 (1.8, 5.7) | 3.6 (1.4, 7.0) | 2.3 (1.2, 4.9) | 3.4 (0.9, 5.1) | 2.4 (1.7, 5.2) | 2.4 (3.5, 4.0) | .10 |
| Diabetes, % | 27.0 | 46.8 | 55.8 | 61.8 | 71.4 | 84.6 | <.001 |
| Atherosclerotic heart disease, % | 23.6 | 27.1 | 30.3 | 42.4 | 44.3 | 46.2 | .003 |
| Heart failure, % | 29.2 | 26.6 | 33.7 | 41.7 | 44.3 | 76.9 | <.001 |
| Dialyzing via catheter, % | 16.9 | 19.2 | 21.2 | 18.1 | 28.6 | 7.7 | .35 |
| Albumin, g/dL | 4.1 (0.3) | 4.0 (0.3) | 4.0 (0.3) | 3.9 (0.4) | 3.8 (0.4) | 3.7 (0.4) | <.001 |
| Frail, % | |||||||
| Weight loss, % | 0 | 17.2 | 36.5 | 45.1 | 64.3 | 100 | |
| Exhaustion, % | 0 | 25.1 | 36.1 | 53.5 | 68.6 | 100 | |
| Low physical activity, % | 0 | 14.3 | 42.8 | 69.4 | 94.3 | 100 | |
| Weak grip strength, % | 0 | 38.9 | 62.0 | 80.6 | 84.3 | 100 | |
| Slow gait speed, % | 0 | 4.4 | 22.6 | 51.4 | 88.6 | 100 | |
At baseline, 32.2% reported meeting the criterion for unintentional weight loss, 36.3% exhaustion, and 40.9% low physical activity. In addition, 54.5% had weak grip strength and 28.2% slow gait speed. Considering all possible combinations of components meeting the frailty definition, weak grip strength, low physical activity, and slow gait speed were the most common (15%; Figure 1A). Furthermore, 41.4% of all frail patients met these three components (Figure 1B).
Evolution of Frailty Components Over Time
In univariable and multivariable analyses, we examined the odds of meeting each frailty criterion per year over 2 years of follow-up during which frailty was assessed annually. Over time, patients’ physical activity (−231 kcal/wk per year, 95% confidence interval [CI] −319, −144) and gait speed (−0.7 m/s per year, 95% CI −0.08, −0.05) declined significantly such that they were more likely to meet these frailty components (odds ratio 1.15, 95% CI 1.04, 1.26 and 1.35, 95% CI 1.25, 1.46, respectively; Table 2). Exhaustion and grip strength were relatively stable with little change in the odds of meeting these components over time, although there was a tendency for grip strength to weaken over time (−0.67 kg/y, 95% CI −0.99, −0.34). The odds of meeting the weight loss component decreased with time (odds ratio 0.73, 95% CI 0.64, 0.83). Adjustment for demographic and clinical characteristics did not change the estimated trajectory of any of the frailty components, and the trajectory did not vary according to age or race (p > .05 for all interactions).
Table 2.
Results of Generalized Estimating Equations Models Analyzing the Odds of Meeting Each Component of Frailty per Year of Follow-upa
| Frailty Component | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Weight loss | 0.73 (0.64, 0.83) | <.001 | 0.75 (0.65, 0.86) | <.001 |
| Exhaustion | 0.98 (0.89 1.09) | .72 | 0.96 (0.86, 1.07) | .44 |
| Low physical activity | 1.15 (1.04, 1.26) | .006 | 1.17 (1.05, 1.30) | .003 |
| Weak grip strength | 1.06 (0.99, 1.14) | .11 | 1.07 (0.98, 1.17) | .14 |
| Slow gait speed | 1.35 (1.25, 1.46) | <.001 | 1.42 (1.29, 1.57) | <.001 |
Notes: CI = confidence interval; OR = odds ratio.
aAdjusted for age, sex, race, ethnicity, dialysis vintage, body mass index, diabetes, atherosclerotic heart disease, heart failure, dialysis via a catheter, and serum albumin concentration.
Association of Frailty Components With Mortality
Patients were followed for a median of 3.8 years (25th, 75th percentile 2.8–4.4), and there were 204 deaths during follow-up. Frailty as a dichotomous construct was associated with mortality in time-updated analyses (HR 2.25, 95% CI 1.70, 2.97). Each individual frailty component was associated with mortality in univariable and multivariable analyses (Table 3). Of the individual frailty components, gait speed was most strongly associated with mortality (multivariable HR 2.31, 95% CI 1.70, 3.15); other components were associated with 43%–70% higher hazards.
Table 3.
Univariable and Multivariable Time-Updated Cox Models of the Association of Frailty and Its Components, Modeled Individually, Together, and According to the Number of Components, With Mortalitya
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | AIC | ||
| Dichotomous frailty | ||||||
| Frailty phenotype | 2.88 (2.20, 3.79) | <.001 | 2.25 (1.70, 2.97) | <.001 | 1,962.4 | |
| Frailty components modeled individually | ||||||
| Weight loss | 1.56 (1.18, 2.09) | .002 | 1.58 (1.18, 2.12) | .002 | ||
| Exhaustion | 1.52 (1.15, 1.99) | .003 | 1.61 (1.23, 2.12) | <.001 | ||
| Low physical activity | 1.73 (1.31, 2.28) | <.001 | 1.43 (1.08, 1.89) | .01 | ||
| Weak grip strength | 2.22 (1.65, 2.99) | <.001 | 1.70 (1.23, 2.36) | .001 | ||
| Slow gait speed | 2.92 (2.22, 3.83) | <.001 | 2.31 (1.70, 3.15) | <.001 | 1,964.1 | |
| Frailty components modeled together | ||||||
| Weight loss | 1.35 (1.01, 1.80) | .04 | 1.44 (1.07, 1.94) | .02 | 1,950.1 | |
| Exhaustion | 1.42 (1.08, 1.87) | .01 | 1.49 (1.13, 1.96) | .005 | ||
| Low physical activity | 1.23 (0.93, 1.64) | .15 | 1.13 (0.84, 1.51) | .43 | ||
| Weak grip strength | 1.90 (1.40, 2.57) | <.001 | 1.54 (1.10, 2.15) | .01 | ||
| Slow gait speed | 2.42 (1.83, 3.20) | <.001 | 2.04 (1.49, 2.80) | <.001 | ||
| Number of frailty components | ||||||
| 0 (n = 88) | Reference | Reference | 1,953.6 | |||
| 1 (n = 202) | 2.87 (1.22, 6.72) | .02 | 2.73 (1.16, 6.44) | .02 | ||
| 2 (n = 207) | 3.77 (1.63, 8.71) | .002 | 3.51 (1.51, 8.16) | .004 | ||
| 3 (n = 146) | 6.30 (2.75, 14.43) | <.001 | 5.22 (2.28, 11.97) | <.001 | ||
| 4 (n = 71) | 11.97 (5.15, 27.82) | <.001 | 7.98 (3.38, 18.85) | <.001 | ||
| 5 (n = 13) | 13.23 (4.76, 36.78) | <.001 | 10.07 (3.63, 27.94) | <.001 | ||
| Most common combination of three components | ||||||
| GP, PA, WK | 3.37 (2.50, 4.46) | <.001 | 2.24 (1.61, 3.13) | <.001 | 1,969.5 | |
Notes: AIC = Akaike information criterion; CI = confidence interval; GP = grip strength; PA = physical activity; WK = walking time or gait speed.
aAdjusted for age, sex, race, ethnicity, body mass index, diabetes, atherosclerotic heart disease, heart failure, dialysis via a catheter, and serum albumin concentration.
When all components were considered in the same model, all but physical activity remained associated with mortality (Table 3). Although the association of physical inactivity was attenuated substantially when other components were included, the relative lack of attenuation of the other components was remarkable. We further examined the “additive” association of meeting multiple frailty components by examining mortality according to the number of components met, and this model confirmed a rather striking gradient of risk (Table 3). Patients meeting the three most common components of frailty had a similar hazard for mortality (HR 2.24, 95% CI 1.61, 3.13) as those meeting the traditional frailty construct (meeting any three components; HR 2.25, 95% CI 1.70, 2.97).
The model that included all of the components of frailty together had the lowest Akaike information criterion, indicating that it minimized information loss compared with other ways of modeling frailty. The model that included the number of frailty components was the next best model (p = .18 for the difference). Both the model with the number of frailty components and the model with all five components outperformed the models with frailty as a dichotomous construct and with gait speed (or any other individual component) alone (p ≤ .01).
Sensitivity analyses that also adjusted for interleukin-6 resulted in slight attenuation of the associations of different frailty constructs with mortality (Supplementary Table 2). Fine–Gray models with receipt of a kidney transplant as a competing risk were very similar to our primary models (Supplementary Table 3). In our alternative modeling approach in which change was explicitly examined, we found that baseline frailty status and change over time were both associated with mortality for all frailty constructs and for most individual components (Supplementary Table 4).
Discussion
We found that components of frailty did not change to the same degree or in the same direction over 2 years of follow-up with annual assessments. Gait speed and physical activity declined over time, whereas exhaustion and grip strength were relatively stable, and the odds of losing weight declined over time. Examination of time-updated assessments of frailty components with mortality showed that all were associated with higher mortality when considered individually, and all but physical activity were independently associated with mortality when considered together. The model that best-predicted mortality was one that included all five frailty components.
Few studies have examined the rate of change of functional status and frailty components over time among patients receiving hemodialysis. Physical activity, gait speed, and grip strength declined over time, consistent with other reports of declining functional status among older patients (11) and nursing home residents (12) initiating dialysis. However, the decline in grip strength did not result in a significantly higher likelihood of being below the frailty threshold over time. These results support the possibility that muscle wasting and weakness might be less important reasons for declining functional status than physical inactivity. Coordination and balance are important strength-independent determinants of gait speed that have received little attention in the dialysis population and may deserve further consideration as they can potentially be improved by increasing physical activity (13,14).
It was interesting that despite declines in physical activity and gait speed, exhaustion did not appear to change over the 2 years it was assessed. Nevertheless, exhaustion was associated with higher subsequent mortality, independent of the other components of frailty. It is conceivable that exhaustion serves as an indicator of other factors associated with mortality, such as inflammation or depression (15–17). This possibility would be consistent with the conceptual model of frailty as a syndrome in which age, comorbidity, and inflammation could contribute to a state of vulnerability to adverse outcomes (6). It was not entirely surprising that weight loss appeared to improve during follow-up. Ongoing weight loss of more than 10 pounds per year might be likely to indicate severe frailty that could lead to death during follow-up among those whose weight loss did not improve. Moreover, although anorexia is a common concern among patients on dialysis, it is possible that for many patients, a reduction in physical activity may compensate for reductions in calorie intake, rendering patients less susceptible to weight loss. Any loss of metabolically active muscle would in turn lead to a decline in resting energy expenditure, also reducing the likelihood of sustained weight loss. Alternatively, the use of weight alone may not capture fluid accumulation, and loss of body cell mass might be masked without more detailed assessment of body composition.
Although physical inactivity was associated with mortality, the association was not independent of the other components of frailty. There are several possible explanations for this finding. First, physical activity and gait speed were declining in parallel, and changes in these two components of frailty may not be completely independent. To the extent to which changes in physical activity and gait speed do differ, decreases in physical activity that do not result in demonstrable slowing of gait speed may be more transient or less important in predicting longer-term mortality than those associated with changes in gait speed. Second, it is possible that the MMLTA is a less accurate or discriminating instrument for measuring physical activity in the dialysis population than others that have been more strongly associated with mortality (18). Although we are not aware of studies comparing the MMLTA to other instruments in the dialysis population, we have previously shown that recall instruments that focus on moderate and vigorous activities as does the MMLTA did not capture differences in activity at the low end of the spectrum occupied by most patients with ESRD (19). Other physical activity instruments might perform better in frailty assessment in patients with ESRD and may be worth exploring further, as the MMLTA is the most time-consuming aspect of frailty assessment. We have recently developed a shorter instrument, the Low Physical Activity Questionnaire (LoPAQ), that is more focused on walking, compares favorably to the MMLTA, and can be administered in half the time (20). Although it was important to replicate the Frailty Phenotype faithfully in initial assessments of frailty in the dialysis population, it would be reasonable to ascertain whether refinements can increase the utility and feasibility of frailty assessment in future studies.
Of the components of the Frailty Phenotype, gait speed was most strongly associated with mortality in our cohort and was part of the most common frailty triad (weak grip strength, low physical activity, and slow gait speed). However, gait speed as a single frailty-related predictor of mortality and the three “physical” components of frailty were inferior to all other constructs we evaluated, including frailty as a dichotomous predictor, all components together, and a score of the number of components. Furthermore, considering all five components together or the number of frailty components participants met improved prediction of mortality was superior to treating frailty as a dichotomous predictor. Thus, for the purposes of predicting mortality, attempts to streamline the definition of frailty to minimize the burden of data collection resulted in loss of relevant information, and our results suggest that all five components should be considered to best quantify patients’ frailty status.
In light of these findings, perhaps frailty should be considered as part of a standardized mental and physical assessment tool in the dialysis population. The Centers for Medicare and Medicaid Services already requires annual assessment using a standardized tool under its conditions of coverage for dialysis facilities (21), and the Kidney Disease Quality of Life (KDQOL) instrument has become the standard. It may be worthwhile to consider future studies to examine whether assessment of frailty could supplement or replace portions of the KDQOL or be incorporated into a broader functional assessment.
Measurement of frailty according to the Fried Frailty Phenotype, which has become a gold standard for assessing frailty in community-dwelling older individuals (22) and patients with chronic diseases (23–26), including ESRD (1,3,27), is a strength of our study. In addition, longitudinal assessment of frailty over a 2-year period to allow analysis of changes in frailty and its individual components is novel. However, some weaknesses should also be acknowledged. First, we did not include a validation cohort in this study, and our results should be confirmed in future studies. Second, our cohort was slightly younger and included a larger percentage of black patients than the unselected U.S. hemodialysis population (7). However, we did not find that age or race was associated with change in frailty components or that adjustment for these characteristics changed our conclusions.
In summary, of the five components of frailty, physical activity, gait speed, and, to a lesser extent, grip strength, declined over time, exhaustion was relatively stable, and the odds of meeting the weight loss criterion decreased over time in our study. All five components were associated with mortality during follow-up, and all except physical activity were associated with mortality independent of the other components. Comparison of model performance revealed that the model that included all five frailty components provided the most useful information. Thus, our results suggest that measurement of all five components of frailty annually can contribute important risk stratification information beyond routinely collected clinical information among patients on in-center hemodialysis.
Funding
This study was supported by grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK107269 and K24 DK085153 to K.L.J.) and the Department of Veterans Affairs, Clinical Science Research and Development Program under Career Development Award (1IK2CX000527 to C.D.). Dr. Delgado’s contribution is the result of work supported with the resources and the use of facilities at the San Francisco Veterans Affairs Medical Center. Dr. Dalrymple is an employee of Fresenius Medical Care North America.
Supplementary Material
Acknowledgments
The data reported here have been supplied in part by the U.S. Renal Data System (USRDS). The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Conflict of Interest
None reported.
References
- 1. Johansen KL, Dalrymple LS, Delgado C, et al. Association between body composition and frailty among prevalent hemodialysis patients: a US Renal Data System special study. J Am Soc Nephrol. 2014;25:381–389. doi: 10.1681/ASN.2013040431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johansen KL, Dalrymple LS, Glidden D, et al. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol. 2016;11:626–632. doi: 10.2215/cjn.03710415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAdams-Demarco M, Law A, Salter M, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol Med Sci. 2014;69:315–322. doi: 10.1093/gerona/glt109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansen KL, Dalrymple LS, Delgado C, et al. Factors associated with frailty and its trajectory among patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12:1100–1108. doi: 10.2215/cjn.12131116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 7. U.S. Renal Data System. USRDS 2011 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 8. Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi:10.1002/1097-4679(198601)42:1<28::AID-JCLP2270420104>3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- 9. Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi:10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- 10. McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual frailty components and mortality in kidney transplant recipients. Transplantation. 2017;101:2126–2132. doi: 10.1097/tp.0000000000001546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009;361:1612–1613. doi: 10.1056/NEJMc0905289 [DOI] [PubMed] [Google Scholar]
- 12. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shin S, Chung HR, Fitschen PJ, et al. Postural control in hemodialysis patients. Gait Posture. 2014;39:723–727. doi: 10.1016/j.gaitpost.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magnard J, Hristea D, Lefrancois G, Testa A, Paris A, Deschamps T. Implicit postural control strategies in older hemodialysis patients: an objective hallmark feature for clinical balance assessment. Gait Posture. 2014;40:723–726. doi: 10.1016/j.gaitpost.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 15. Bossola M, Di Stasio E, Giungi S, Rosa F, Tazza L. Fatigue is associated with serum interleukin-6 levels and symptoms of depression in patients on chronic hemodialysis. J Pain Symptom Manage. 2015;49:578–585. doi: 10.1016/j.jpainsymman.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 16. Zalai D, Bohra M. Fatigue in chronic kidney disease: definition, assessment and treatment. CANNT J. 2016;26:39–44. [PubMed] [Google Scholar]
- 17. Wang LJ, Wu MS, Hsu HJ, et al. The relationship between psychological factors, inflammation, and nutrition in patients with chronic renal failure undergoing hemodialysis. Int J Psychiatry Med. 2012;44:105–118. doi: 10.2190/PM.44.2.b [DOI] [PubMed] [Google Scholar]
- 18. Johansen KL, Kaysen GA, Dalrymple LS, et al. Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol. 2013;8:248–253. doi: 10.2215/cjn.08560812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59:1121–1127. doi: 10.1046/j.1523-1755.2001.0590031121.x [DOI] [PubMed] [Google Scholar]
- 20. Johansen KL, Painter P, Delgado C, Doyle J. Characterization of physical activity and sitting time among patients on hemodialysis using a new physical activity instrument. J Ren Nutr. 2015;25:25–30. doi: 10.1053/j.jrn.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Department of Health and Human Services. 42 CFR Parts 405, 410, 413 et al. Medicare and Medicaid programs; conditions for coverage for end-stage renal disease facilities; Final rule. Fed Regist. 2008;73:20370–20484. [PubMed] [Google Scholar]
- 22. International Working Group on Sarcopenia. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J Am Med Dir Assoc. 2011;12:249–256. doi:10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onen NF, Agbebi A, Shacham E, Stamm KE, Onen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect. 2009;59:346–352. doi: 10.1016/j.jinf.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 24. Jha SR, Ha HS, Hickman LD, et al. Frailty in advanced heart failure: a systematic review. Heart Failure Rev. 2015;20:553–560. doi: 10.1007/s10741-015-9493-8 [DOI] [PubMed] [Google Scholar]
- 25. Lahousse L, Ziere G, Verlinden VJ, et al. Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci. 2016;71:689–695. doi: 10.1093/gerona/glv154 [DOI] [PubMed] [Google Scholar]
- 26. Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71:988–995. doi: 10.1136/thoraxjnl-2016-208460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Painter P, Kuskowski M. A closer look at frailty in ESRD: getting the measure right. Hemodial Int. 2013;17:41–49. doi: 10.1111/j.1542-4758.2012.00719.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.