Abstract
Background
Herpes simplex virus type 1 (HSV-1) epidemiology in Asia was characterized by assessing seroprevalence levels and extent to which HSV-1 is isolated from clinically diagnosed genital ulcer disease (GUD) and genital herpes.
Methods
HSV-1 reports in Asia were systematically reviewed and synthesized, following PRISMA guidelines. Random-effects meta-analyses estimated pooled mean seroprevalence and proportion of HSV-1 detection in GUD and genital herpes. Random-effects meta-regressions identified predictors of seroprevalence and sources of between-study heterogeneity.
Results
Forty-nine relevant publications were identified. Fifty-four overall seroprevalence measures (182 stratified measures), and 8 and 24 proportions of HSV-1 detection in GUD and in genital herpes, respectively, were extracted. The pooled mean seroprevalence was 50.0% (n = 26; 95% confidence interval [CI], 41.3%–58.7%) for children and 76.5% (n = 151; 73.3%–79.6%) for adults. By age group, the pooled mean was lowest at 55.5% (n = 37; 95% CI, 47.5%–63.4%) in individuals aged <20 years, followed by 67.9% (n = 48; 62.4%–73.3%) in those aged 20–39 and 87.5% (n = 44; 83.4%–91.1%) in those aged ≥40 years. In meta-regression, age was the major predictor of seroprevalence. The mean proportion of HSV-1 detection was 5.6% (n = 8; 95% CI, 0.8%–13.6%) in GUD and 18.8% (n = 24; 12.0%–26.7%) in genital herpes.
Conclusions
HSV-1 epidemiology is transitioning in Asia. HSV-1 is probably playing a significant role as a sexually transmitted infection, explaining one-fifth of genital herpes cases. There is a need for expanded seroprevalence monitoring and GUD/genital herpes etiological surveillance.
Keywords: seroprevalence, genital ulcer disease, genital herpes, synthesis, region
Herpes simplex virus type 1 (HSV-1) epidemiology is transitioning in Asia with lower seroprevalence in youth. Yet, 50% of children and 75% of adults are infected. HSV-1 explained one-fifth of genital herpes and 6% of genital ulcer disease cases.
Herpes simplex virus (HSV) type 1 (HSV-1) infection is widely prevalent [1, 2]. With its persistent shedding [3, 4], HSV-1 is infectious for lifetime, but mostly subclinically and asymptomatically [5–7]. When symptomatic, HSV-1 can cause mild to severe disease [5, 8]. Although infection is often manifested as orolabial herpes [5, 8], the virus can cause a spectrum of diseases such as herpetic whitlow, gingivostomatitis, meningitis, encephalitis, corneal blindness, and neonatal herpes [8, 9].
HSV-1 clinical manifestations are determined by the virus’s initial portal of entry [5, 8]. Although it is predominantly transmitted through oral shedding [5–7], leading to oral manifestations [5, 8], HSV-1 can be transmitted sexually, leading to genital herpes, given the portal of entry [5, 6, 10].
HSV-1 antibody prevalence (seroprevalence) seems to be very high globally, with the majority of affected persons seroconverting by the time they reach puberty [2, 11, 12]. However, with continuing improvement in hygiene and living conditions, seroprevalence seems to have declined, at least in Western countries [11, 13–20]. About half of youth there reach sexual debut before being exposed (nonsexually) to HSV-1 and thus are at risk of acquiring the infection genitally [5, 21]. Evidence indicates a growing role for HSV-1 as a sexually transmitted infection (STI) and as a leading, if not the leading, cause of initial episodes of genital herpes in Western countries [5, 21–25].
Although this striking transition in HSV-1 epidemiology in the West is well documented [5, 7, 26], the extent to which it is occurring elsewhere is unknown. Understanding HSV-1 epidemiology in different regions will help characterize the HSV-1 burden, oral and genital, and target the most affected populations with interventions. To this end, the World Health Organization and global partners are spearheading efforts to accelerate the development of HSV vaccines [27, 28]. A business case is being developed that factors public health needs, pathways of vaccine rollout, impact and cost-effectiveness, and return on investment [27]. To inform this effort, it is critical to establish current infection levels and trends.
Our overarching goals were to assess HSV-1 seroprevalence levels and trends in Asia and the extent to which HSV-1 is the cause of genital ulcer disease (GUD) and genital herpes. We specifically aimed to (1) methodologically review and synthesize available studies on seroprevalence; (2) estimate seroprevalence in different populations and ages by pooling existing measures; (3) assess seroprevalence temporal trend, population-level associations with seroprevalence, and sources of between-study heterogeneity; (4) assess the proportion of HSV-1 viral detection in clinically diagnosed GUD; and (5) assess the proportion of HSV-1 viral detection in clinically diagnosed genital herpes. The distinction between the last 2 aims lies in the denominator—the etiology of GUD includes several indications other than HSV-1 infection (diagnosis of any GUD) [29], and the etiology of genital herpes includes only HSV-1 and HSV type 2 (HSV-2) infections (virological diagnosis of herpes) [30].
MATERIALS AND METHODS
Data Sources and Search Strategy
This systematic review was informed by the Cochrane Collaboration Handbook [31] and followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [32]. The PRISMA checklist is in Supplementary Table 1.
Available HSV-1 publications in PubMed (from 1950) and Embase (from 1974) databases were systematically reviewed until 22 April 2018. For inclusiveness, broad search criteria were used, with MeSH/Emtree terms exploded to cover all subheadings and with no language or year restrictions (Supplementary Box 1). Articles in Chinese, English, French, and Japanese were reviewed in their original language. Articles in other languages were translated. Asia region definition was informed by the World Health Organizations definitions for South-East Asia and Western Pacific regions [33]. The list of included countries/territories is in Supplementary Box 2.
Study Selection and Inclusion/Exclusion Criteria
Search results were imported into Endnote (a reference manager), where duplicate publications were identified and excluded. Titles and abstracts of remaining records were screened for relevance, and full texts of relevant and potentially relevant publications were retrieved for additional screening. References of articles and reviews were also checked to identify further publications that could have been missed.
The inclusion criteria were met for any publication that reported HSV-1 seroprevalence measure(s), based on primary data using type-specific diagnostic assays such as Western blot or type-specific (glycoprotein-G-based) enzyme-linked immunosorbent assays (ELISAs). The inclusion criteria were also met for any publication that reported a proportion of HSV-1 detection by standard viral detection and subtyping methods in GUD or genital herpes—to estimate the “etiological” (or “associative”) fraction for HSV-1 in these clinical conditions. Included studies had to have a sample size of ≥10, regardless of outcome measure.
Exclusion criteria included case reports, case series, reviews, editorials, letters to editors, commentaries, and qualitative studies. Measures reporting seroprevalence in <3-month-old infants were excluded because of maternal antibodies.
For terminology, a “publication” is a document containing a relevant outcome measure, and a “study” or a “measure” indicates all details pertaining to a specific outcome measure—a single publication may contribute multiple measures, and multiple publications of the same data set are deemed a single study.
Data Extraction and Data Synthesis
Extracted variables included author(s), publication title, year(s) of data collection, publication year, country of origin, country of survey, city, study site, study design, study sampling procedure, study population and its characteristics (eg, sex and age), sample size, HSV-1 outcome measures, and diagnostic assay. Data from relevant publications were double extracted by L. K. and M. H., with input from R. O.
Extracted overall outcome measures were substituted with stratified measures, provided the sample size requirement was fulfilled for each stratum. The stratification hierarchy for seroprevalence included population type, age bracket, and age group, for epidemiological relevance and analysis. In age-bracket stratification, we aimed to assess seroprevalence in adults (≥15 years of age) versus children (<15 years). In age-group stratification, we aimed to assess seroprevalence growth with age (<20, 20–39, or ≥40 years); these strata were optimal given reported age-stratified data. Stratification hierarchy for GUD and genital herpes proportions included ethnicity, study site (eg, hospital or STI clinic), and genital herpes episode (first vs recurrent).
Extracted seroprevalence measures were stratified by population type into (1) healthy general populations, consisting of healthy populations such as blood donors, pregnant women, and outpatients with minor health conditions; (2) clinical populations, consisting of any population with a major clinical condition, or a condition related (potentially) to HSV-1 infection; and (3) other populations, consisting of the remaining populations not satisfying the above definitions or populations with an undetermined risk of acquiring HSV-1, such as persons with human immunodeficiency virus infection, sex workers, and men who have sex with men.
Meta-analyses
Meta-analyses were conducted to estimate pooled mean HSV-1 seroprevalence by population type and by age bracket or group and to estimate the pooled mean proportions of HSV-1 detection in GUD and genital herpes.
Pooled means were estimated using DerSimonian-Laird random-effects models [34], provided that ≥3 measures were available. This method accounts for sampling variation and heterogeneity in effect size (seroprevalence or GUD/genital herpes proportion) [34]. The Freeman-Tukey double-arcsine transformation was used for variance stabilization [35].
The Cochran Q statistic was calculated to assess existence of heterogeneity in effect size (P < .10 indicated heterogeneity) [36, 37]. The I2 heterogeneity measure was estimated to assess the percentage of between-study variation in effect size that is due to actual differences in effect size rather than chance [37]. Prediction intervals were calculated to describe the heterogeneity in meta-analyses [36, 37]. Meta-analyses were performed in R software, version 3.4.1 [38] using the meta package [39].
Meta-regression Analyses
Univariable and multivariable random-effects meta-regression analyses were conducted to identify predictors of HSV-1 seroprevalence (including temporal trend) and sources of between-study heterogeneity. The log-transformed proportions were regressed to estimate risk ratios.
Relevant independent variables were specified a priori: age bracket, age group, assay type (Western blot, ELISA, or other), country’s income, population type, sample size (<100 vs ≥100 subjects), sampling method (probability-based vs non–probability-based sampling), sex, year of data collection, and year of publication. Factors associated with seroprevalence at P ≤ .10 in univariable analysis were included in the final multivariable analysis. Factors associated with seroprevalence at P ≤ .05 in the final multivariable analysis were deemed statistically significant.
For the country’s income variable, countries with available data were grouped according to the World Bank classification [40]. For measures that did not include a year of data collection, missing values were imputed using the median of the values calculated by subtracting the year of data collection (when available) from the year of publication. Meta-regression analyses were conducted with Stata/SE software, version 13 [41], using the metareg package [42].
Quality Assessment
For diagnostic methods, diversity, and potential issues of sensitivity or specificity [43, 44], we performed quality assessment with the support of an expert advisor, Rhoda Ashley-Morrow, University of Washington, Seattle. Only publications with sufficiently reliable assays were eligible for inclusion. Study quality was further assessed by conducting risk of bias (ROB) assessment (as informed by the Cochrane approach [31]) and precision assessment.
Studies were categorized as low versus high ROB using 2 quality domains assessing the rigor of sampling method (probability based vs otherwise) and response rate (≥80% vs otherwise). A study was considered to have high (vs low) precision if the sample size was ≥100.
RESULTS
Search Results and Scope of Evidence
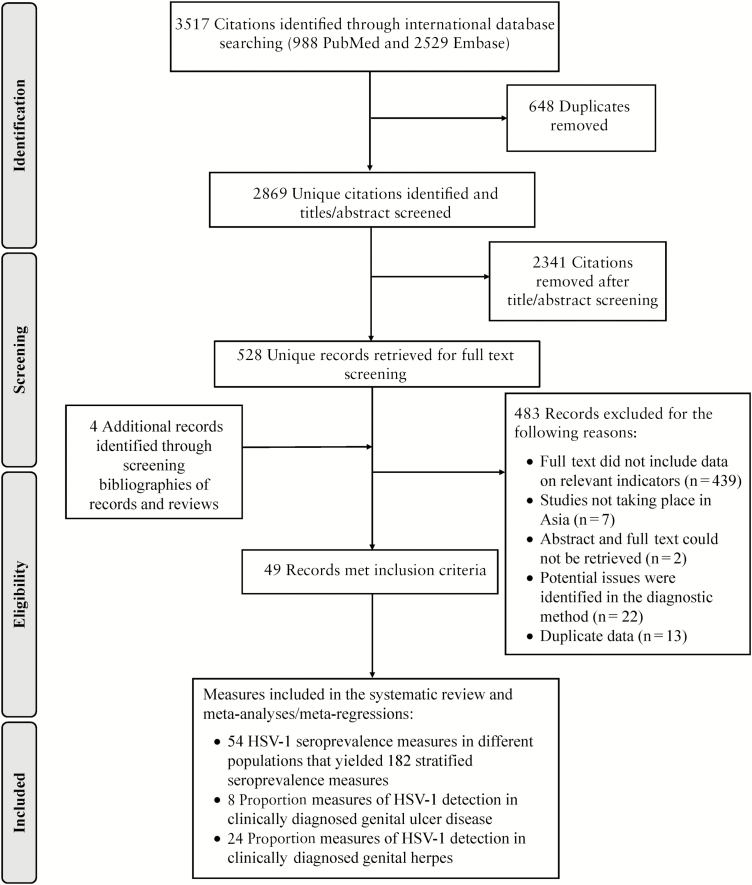
Figure 1 describes the study-selection process based on PRISMA guidelines [32]. A total of 3517 citations were identified (988 through PubMed and 2529 through Embase). Of these, 528 were relevant or potentially relevant after removal of duplicates and screening of titles and abstracts. Eventually, 45 publications were eligible for inclusion after full-text screening. Four additional publications were identified through screening of bibliographies of publications and reviews [45–48].
Figure 1.
Flow chart of article selection for the systematic review of herpes simplex virus type 1 (HSV-1) in Asia, as adapted from the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2009 guidelines [32].
A total of 54 overall seroprevalence measures (distinct overall measures in different populations) were extracted, and these yielded 182 stratified seroprevalence measures. Eight proportions of HSV-1 detection in GUD and 24 proportions in genital herpes were further extracted. Extracted measures originated from 13 of 26 Asian countries/territories.
Seroprevalence Overview
Table 1 summarizes the stratified seroprevalence measures. The earliest measure was published in 1986. Most measures were based on cross-sectional study design (n = 152 measures; 83.5%), and convenience sampling (n = 150; 82.4%).
Table 1.
Studies Reporting Herpes Simplex Virus Type 1 Seroprevalence Among Different Populations in Asia
| Authors (Year) | Year(s) of Data Collection | Country | Study Site | Study Design | Sampling Method | Population | HSV-1 Serological Assay | Sample Size, No. | HSV-1 Seroprevalence, % |
|---|---|---|---|---|---|---|---|---|---|
| Healthy Children Populations (n = 19) | |||||||||
| Bogaerts et al (2001) [49] | 1996–1998 | Bangladesh | Outpatient clinic | CS | Conv | 1–12-y-old children | WB | 79 | 46.0 |
| Chang (1986) [50] | 1984–1986 | China | Hospital | CS | Conv | 7–12-mo-old infants | CFT | 31 | 41.9 |
| Chang (1986) [50] | 1984–1987 | China | Hospital | CS | Conv | 13–24-mo-old children | CFT | 31 | 51.6 |
| Chang (1986) [50] | 1984–1988 | China | Hospital | CS | Conv | 24–35-mo-old children | CFT | 30 | 43.3 |
| Chang (1986) [50] | 1984–1989 | China | Hospital | CS | Conv | 3–4-y-old children | CFT | 31 | 67.7 |
| Chang (1986) [50] | 1984–1990 | China | Hospital | CS | Conv | 5–6-y-old children | CFT | 31 | 48.4 |
| Chang (1986) [50] | 1984–1991 | China | Hospital | CS | Conv | 7–8-y-old children | CFT | 31 | 71.0 |
| Chang (1986) [50] | 1984–1992 | China | Hospital | CS | Conv | 9–14-y-old children | CFT | 31 | 74.2 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 1-y-old children | ELISA | 90 | 11.1 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 2-y-old children | ELISA | 127 | 14.2 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 3-y-old children | ELISA | 92 | 31.5 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 4-y-old children | ELISA | 84 | 23.8 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 5–9-y-old children | ELISA | 111 | 46.8 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 10–14-y-old children | ELISA | 92 | 46.7 |
| Li et al (1990) [52] | 1988–1989 | China | Community | CS | Conv | 1–10-y-old Koreans | PHA | 16 | 38.0 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 5–9-y-old girls | ELISA | 40 | 64.9 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 10–14-y-old girls | ELISA | 45 | 78.3 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 5–9-y-old boys | ELISA | 75 | 59.8 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 10–14-y-old boys | ELISA | 64 | 78.0 |
| Healthy Adult Populations (n = 103) | |||||||||
| Armelia et al (2012) [54] | 2010–2011 | Indonesia | Hospital | CSa | Conv | Kidney donors | Anti-HSV-1 IgG | 23 | 72.7 |
| Ashley et al (2004) [55] | 2000–2001 | Thailand | Community | CS | Conv | ≥15-y-old women in Lampang | WB | 98 | 92.9 |
| Ashley et al (2004) [55] | 2000–2001 | Thailand | Community | CS | Conv | ≥15-y-old women in Songkla | WB | 90 | 61.1 |
| Ashley et al (2004) [55] | 2000–2001 | Vietnam | Community | CS | Conv | ≥15-y-old women in Hanoi | WB | 99 | 100.0 |
| Ashley et al (2004) [55] | 2000–2001 | Vietnam | Community | CS | Conv | ≥15-y-old women in Ho Chi Minh | WB | 100 | 98.0 |
| Bogaerts et al (2001) [49] | 1996–1998 | Bangladesh | Outpatient clinic | CS | Conv | Healthy women | ELISA | 183 | 97.0 |
| Bu et al (2015) [45] | 2012–2013 | China | Hospital | CC | Conv | Healthy individuals | ELISA | 135 | 78.5 |
| Chang (1986) [50] | 1984–1986 | China | Hospital | CS | Conv | >14-y-old adults | CFT | 30 | 93.3 |
| Cowan et al (2003) [56] | 1998–2000 | India | Community | CS | Conv | 15–20-y-old adults | ELISA | 239b | 85.7 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 15–19-y-old adults | ELISA | 115 | 53.0 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 20–29-y-old adults | ELISA | 123 | 69.9 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 30–39-y-old adults | ELISA | 129 | 84.5 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 40–49-y-old adults | ELISA | 100 | 94.0 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 50–59-y-old adults | ELISA | 91 | 98.9 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | 60–69-y-old adult | ELISA | 122 | 100 |
| Chen et al (2013) [51] | 2007 | Taiwan | Community | CS | Conv | >70-y-old adults | ELISA | 96 | 100 |
| Cowan et al (2003) [56] | 1998–2000 | India | Community | CS | Conv | 20–30-y-old adults | ELISA | 239b | 79.9 |
| Cowan et al (2003) [56] | 1998–2000 | India | Community | CS | Conv | 30–35-y-old adults | ELISA | 239b | 80.0 |
| Cowan et al (2003) [56] | 1998–2000 | India | Community | CS | Conv | 25–40-y-old adults | ELISA | 239b | 84.8 |
| Cowan et al (2003) [56] | 1998–2000 | India | Community | CS | Conv | 40–45-y-old adults | ELISA | 239b | 86.2 |
| Cowan et al (2003) [56] | 1998–2000 | India | Community | CS | Conv | >45-y-old adults | ELISA | 239b | 92.5 |
| Doi et al (2009) [57] | 2002 | Japan | Community | CSa | RS | 18–29-y-old women | ELISA | 83 | 45.8 |
| Doi et al (2009) [57] | 2002 | Japan | Community | CSa | RS | 30–39-y-old women | ELISA | 184 | 50.5 |
| Doi et al (2009) [57] | 2002 | Japan | Community | CSa | RS | 40–49-y-old women | ELISA | 198 | 66.7 |
| Doi et al (2009) [57] | 2002 | Japan | Community | CSa | RS | 50–59-y-old women | ELISA | 200 | 79.0 |
| Doi et al (2009) [57] | 2002 | Japan | Community | CSa | RS | 18–29-y-old men | ELISA | 45 | 44.4 |
| Doi et al (2009) [57] | 2002 | Japan | Community | CSa | RS | 30–39-y-old men | ELISA | 129 | 44.2 |
| Doi et al (2009) [57] | 2002 | Japan | Community | CSa | RS | 40–49-y-old men | ELISA | 198 | 49.0 |
| Doi et al (2009) [57] | 2002 | Japan | Community | CSa | RS | 50–59-y-old men | ELISA | 198 | 71.7 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | <30-y-old men blood donors | EIA | 12 | 33.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | 30–50-y-old men blood donors | EIA | 17 | 70.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | >50-y-old men blood donors | EIA | 12 | 92.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | 20–39-y-old healthy women | EIA | 20 | 65.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | 40–99-y-old healthy women | EIA | 28 | 89.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | >50-y-old healthy women | EIA | 27 | 92.5 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | Pregnant women from Tokyo | EIA | 58 | 47.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | Pregnant women from Kagoshima | EIA | 100 | 61.0 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 20–29-y-old men in 1973 | ELISA | 31 | 64.5 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 30–39-y-old men in 1973 | ELISA | 25 | 76.0 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 40–49-y-old men in 1973 | ELISA | 15 | 86.7 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 20–29-y-old men in 1983 | ELISA | 24 | 37.5 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 30–39-y-old men in 1983 | ELISA | 30 | 76.7 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 40–49-y-old men in 1983 | ELISA | 33 | 90.9 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 20–29-y-old men in 1993 | ELISA | 30 | 33.3 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 30–39-y-old men in 1993 | ELISA | 30 | 56.7 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 40–49-y-old men in 1993 | ELISA | 45 | 75.6 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 20–29-y-old women in 1973 | ELISA | 32 | 59.4 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 30–39-y-old women in 1973 | ELISA | 33 | 84.8 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 40–49-y-old women in 1973 | ELISA | 23 | 100.0 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 20–29-y-old women in 1983 | ELISA | 35 | 51.4 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 30–39-y-old women in 1983 | ELISA | 36 | 77.8 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 40–49-y-old women in 1983 | ELISA | 34 | 97.1 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 20–29-y-old women in 1993 | ELISA | 63 | 31.7 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 30–39-y-old women in 1993 | ELISA | 54 | 69.1 |
| Hashido et al (1999) [59] | 1973–1993 | Japan | Community | CS | Conv | 40–49-y-old women in 1993 | ELISA | 41 | 80.5 |
| Kaur et al (1999) [60] | NA | India | Outpatient clinic | CS | Conv | 16–20-y-old pregnant women | EIA | 24 | 50.0 |
| Kaur et al (1999) [60] | NA | India | Outpatient clinic | CS | Conv | 21–25-y-old pregnant women | EIA | 36 | 44.4 |
| Kaur et al (1999) [60] | NA | India | Outpatient clinic | CS | Conv | 26–30-y-old pregnant women | EIA | 34 | 55.8 |
| Kaur et al (1999) [60] | NA | India | Outpatient clinic | CS | Conv | 31–35-y-old pregnant women | EIA | 14 | 14.1 |
| Kaur et al (1999) [60] | NA | India | Outpatient clinic | CS | Conv | >36-y-old pregnant women | EIA | 12 | 83.3 |
| Kaur et al (2005) [61] | NA | India | Outpatient clinic | CS | Conv | 16–20-y-old women | ELISA | 12 | 50.0 |
| Kaur et al (2005) [61] | NA | India | Outpatient clinic | CS | Conv | 21–25-y-old women | ELISA | 17 | 47.1 |
| Kaur et al (2005) [61] | NA | India | Outpatient clinic | CS | Conv | 26–30-y-old women | ELISA | 18 | 50.0 |
| Kaur et al (2005) [61] | NA | India | Outpatient clinic | CS | Conv | 31–40-y-old women | ELISA | 13 | 46.1 |
| Kaur et al (2005) [61] | NA | India | Outpatient clinic | CS | Conv | 16–20-y-old men | ELISA | 13 | 46.1 |
| Kaur et al (2005) [61] | NA | India | Outpatient clinic | CS | Conv | 21–25-y-old men | ELISA | 20 | 25.0 |
| Kaur et al (2005) [61] | NA | India | Outpatient clinic | CS | Conv | 26–30-y-old men | ELISA | 14 | 71.4 |
| Kaur et al (2005) [61] | NA | India | Outpatient clinic | CS | Conv | 31–40-y-old men | ELISA | 13 | 46.1 |
| Li et al (1990) [52] | 1988–1989 | China | Community | CS | Conv | >21-y-old Hans Chinese | PHA | 78 | 99.0 |
| Li et al (1990) [52] | 1988–1989 | China | Community | CS | Conv | >21-y-old Koreans | PHA | 34 | 97.0 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 15–19-y-old women | ELISA | 78 | 87.5 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 20–24-y-old women | ELISA | 101 | 86.1 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 25–29-y-old women | ELISA | 135 | 93.3 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 30–34-y-old women | ELISA | 152 | 96.7 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 35–39-y-old women | ELISA | 154 | 95.5 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 40–44-y-old women | ELISA | 129 | 98.4 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 45–49-y-old women | ELISA | 97 | 98.0 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 50–54-y-old women | ELISA | 101 | 98.1 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 55–60-y-old women | ELISA | 44 | 97.8 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 15–19-y-old men | ELISA | 89 | 76.5 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 20–24-y-old men | ELISA | 93 | 81.9 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 25–29-y-old men | ELISA | 112 | 86.5 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 30–34-y-old men | ELISA | 137 | 90.4 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 35–39-y-old men | ELISA | 144 | 93.7 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 40–44-y-old men | ELISA | 118 | 97.4 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 45–49-y-old men | ELISA | 89 | 96.7 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 50–54-y-old men | ELISA | 82 | 98.7 |
| Lin et al (2011) [53] | 2006 | China | Community | CS | RS | 55–60-y-old men | ELISA | 62 | 98.4 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | India | Community | CS | Conv | <24-y-old Indian men | ELISA | 40 | 40.0 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | India | Community | CS | Conv | 25–29-y-old Indian men | ELISA | 49 | 34.0 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | India | Community | CS | Conv | 30–34-y-old Indian men | ELISA | 50 | 60.0 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | India | Community | CS | Conv | 35–39-y-old Indian men | ELISA | 50 | 36.0 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | India | Community | CS | Conv | 40–44-y-old Indian men | ELISA | 50 | 48.0 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | India | Community | CS | Conv | 45–49-y-old Indian men | ELISA | 50 | 58.0 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | India | Community | CS | Conv | >50-y-old Indian men | ELISA | 35 | 62.0 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | Philippines | Community | CS | Conv | <34-y-old Filipino men | ELISA | 52 | 84.6 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | Philippines | Community | CS | Conv | 35–44-y-old Filipino men | ELISA | 40 | 82.5 |
| Nasrallah GK, Dargham SR, Harfouche M, and Abu-Raddad LJ (2018, unpublished data) | 2013–2016 | Philippines | Community | CS | Conv | >45-y-old Filipino men | ELISA | 28 | 85.7 |
| Patnaik et al (2007) [62] | 1985–2007 | Thailand | Hospital | CC | Conv | Healthy women | WB | 78 | 51.3 |
| Schmid et al (1999) [63] | 1991–1993 | Thailand | Hospital | CS | Conv | >21-y-old army men | WB | 1158 | 77.9 |
| Shivaswamy et al (2005) [64] | 2001–2003 | India | Outpatient clinic | CC | Conv | Healthy individuals | ELISA | 135 | 91.8 |
| Yue (1990) [65] | 1987–1989 | China | Outpatient clinic | CS | Conv | Pregnant women | ELISA | 295 | 82.0 |
| Zegans et al (1999) [66] | 1997 | India | Hospital | CC | Conv | Controls for a study of Mooren ulcer | ELISA | 44 | 64.0 |
| Healthy Mixed-Age Populations (n = 4) | |||||||||
| Li et al (1990) [52] | 1988–1989 | China | Community | CS | Conv | 11–20-y-old Hans Chinese | PHA | 17 | 94.1 |
| Li et al (1990) [52] | 1988–1989 | China | Community | CS | Conv | 11–20-y-old Koreans | PHA | 13 | 85.0 |
| Shen et al (2015) [67] | 2007 | Taiwan | Community | CS | RS | Healthy women | ELISA | 830 | 64.5 |
| Shen et al (2015) [67] | 2007 | Taiwan | Community | CS | RS | Healthy men | ELISA | 581 | 52.0 |
| Clinical Children Populations (n = 7) | |||||||||
| Cowan et al (2003) [56] | 1998–2000 | India | Hospital | CS | Conv | 1–5-y-old children | ELISA | 90b | 40.2 |
| Cowan et al (2003) [56] | 1998–2000 | India | Hospital | CS | Conv | 5–10-y-old children | ELISA | 90b | 68.4 |
| Cowan et al (2003) [56] | 1998–2000 | India | Hospital | CS | Conv | 10–15-y-old children | ELISA | 90b | 75.9 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Hospital | CS | Conv | 1–5-y-old children | ELISA | 144b | 40.5 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Hospital | CS | Conv | 5–10-y-old children | ELISA | 144b | 53.1 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Hospital | CS | Conv | 10–15-y-old children | ELISA | 144b | 74.0 |
| Shymala et al (2008) [68] | 2005–2006 | India | Outpatient clinic | CS | Conv | Infants with congenital cataract | ELISA | 18 | 16.7 |
| Clinical Adult Populations (n = 23) | |||||||||
| Armelia et al (2012) [54] | 2010–2011 | Indonesia | Hospital | CSa | Conv | Pre–kidney transplant patients | Anti-HSV-1 IgG | 23 | 68.2 |
| Bu et al (2015) [45] | 2012–2013 | China | Hospital | CC | Conv | Patients with Alzheimer disease | ELISA | 128 | 85.2 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | <39-y-old patients with STD | EIA | 10 | 60.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | >40-y-old patients with STD | EIA | 16 | 81.2 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | Pregnant Tokyo women with HTLV-1 | EIA | 32 | 56.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | Pregnant Kagoshima women with HTLV-1 | EIA | 100 | 83.0 |
| Kaur et al (2006) [69] | NA | India | Outpatient clinic | CS | Conv | Women attending an STD clinic | ELISA | 52 | 82.7 |
| Kaur et al (2006) [69] | NA | India | Outpatient clinic | CS | Conv | Women attending an STD clinic | ELISA | 76 | 73.7 |
| Patwardhan and Bhalla (2016) [70] | NA | India | Hospital | CS | Conv | Patients with first genital herpes | ELISA | 21 | 42.8 |
| Patwardhan and Bhalla (2016) [70] | NA | India | Hospital | CS | Conv | Patients with recurrent genital herpes | ELISA | 23 | 65.2 |
| Shivaswamy et al (2005) [64] | 2001–2003 | India | Outpatient clinic | CC | Conv | <40-y-old patients in an STI clinic | ELISA | 111 | 90.1 |
| Shivaswamy et al (2005) [64] | 2001–2003 | India | Outpatient clinic | CC | Conv | ≥40-y-old patients in an STI clinic | ELISA | 24 | 95.8 |
| Sun et al (2005) [48] | NA | China | Hospital | CS | Conv | Diabetic inpatients | ELISA | 206 | 46.1 |
| Sun et al (2005) [48] | NA | China | Hospital | CS | Conv | Nondiabetic inpatients | ELISA | 1360 | 36.3 |
| Theng et al (2006) [71] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | <29-y-old men | ELISA | 72 | 47.2 |
| Theng et al (2006) [71] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | 30–39-y-old men | ELISA | 50 | 52.0 |
| Theng et al (2006) [71] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | 40–49-y-old men | ELISA | 41 | 58.8 |
| Theng et al (2006) [71] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | >50-y-old men | ELISA | 37 | 78.4 |
| Theng et al (2006) [71] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | <20-y-old female patients | ELISA | 28 | 32.1 |
| Theng et al (2006) [71] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | 20–29-y-old women | ELISA | 98 | 49.0 |
| Theng et al (2006) [71] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | 30–39-y-old women | ELISA | 40 | 67.5 |
| Theng et al (2006) [71] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | >40-y-old women | ELISA | 32 | 78.2 |
| Zegans et al (1999) [66] | 1999 | India | Hospital | CS | Conv | Patients with Mooren ulcers | ELISA | 21 | 86.0 |
| Clinical Mixed-Age Population (n = 1) | |||||||||
| Lee and Lee (2015) [72] | NA | South Korea | Community | CSa | Conv | >11-y-old patients | Multiplex immunoassay | 2317 | 73.8 |
| Other Populations (n = 25) | |||||||||
| Chu et al (2006) [73] | NA | Thailand | Hospital | CS | Conv | HIV-infected men | ELISA | 66 | 53.0 |
| Chu et al (2006) [73] | NA | Thailand | Hospital | CS | Conv | HIV-infected women | ELISA | 70 | 73.0 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Outpatient clinic | CS | Conv | 15–20-y-old healthy/clinical patients | ELISA | 622b | 74.3 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Outpatient clinic | CS | Conv | 20–30-y-old healthy/clinical patients | ELISA | 622b | 79.2 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Outpatient clinic | CS | Conv | 30–35-y-old health/clinical patients | ELISA | 622b | 74.6 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Outpatient clinic | CS | Conv | 25–40-y-old healthy/clinical patients | ELISA | 622b | 74.5 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Outpatient clinic | CS | Conv | 40–45-y-old healthy/clinical patients | ELISA | 622b | 77.1 |
| Cowan et al (2003) [56] | 1998–2000 | Sri Lanka | Outpatient clinic | CS | Conv | >45-y-old healthy/clinical patients | ELISA | 622b | 82.0 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | Female sex workers | EIA | 70 | 75.7 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | <39-y-old MSM | EIA | 15 | 53.3 |
| Hashido et al (1998) [58] | NA | Japan | Community | CS | Conv | >40-y-old MSM | EIA | 19 | 97.4 |
| Lin et al (2011) [53] | NA | China | Community | CS | Conv | 18–29-y-old HIV-infected patients | ELISA | 191 | 94.3 |
| Lin et al (2011) [53] | NA | China | Community | CS | Conv | 30–39-y-old HIV-infected patients | ELISA | 503 | 92.6 |
| Lin et al (2011) [53] | NA | China | Community | CS | Conv | 40–49-y-old HIV-infected patients | ELISA | 290 | 89.7 |
| Lin et al (2011) [53] | NA | China | Community | CS | Conv | 50–59-y-old HIV-infected patients | ELISA | 96 | 85.4 |
| Lin et al (2011) [53] | NA | China | Community | CS | Conv | 60–94-y-old HIV-infected patients | ELISA | 30 | 93.3 |
| Limpakarnjanara et al (1999) [74] | 1994 | Thailand | Community | CS | Conv | >16-y-old female sex workers | WB | 500 | 91.0 |
| Neal et al (2011) [75] | NA | China | Community | CS | Conv | Sex workers | WB | 273 | 91.9 |
| Qutub and Akhter (2003) [76] | NA | Bangladesh | Community | CSa | Conv | Female sex workers | WB | 463 | 92.7 |
| Theng et al (2006) [77] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | 20–29-y-old sex workers | ELISA | 146 | 80.1 |
| Theng et al (2006) [77] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | 30–39-y-old sex workers | ELISA | 56 | 67.9 |
| Theng et al (2006) [77] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | 40–49-y-old sex workers | ELISA | 60 | 68.3 |
| Theng et al (2006) [77] | 2003–2004 | Singapore | Outpatient clinic | CS | Conv | >50-y-old sex workers | ELISA | 38 | 89.5 |
| Van Griensven et al (2013) [78] | 2006–2010 | Thailand | Community | CS | Conv | >18-y-old MSM | ELISA | 1740 | 56.5 |
| Yap et al (2017) [79] | NA | Malaysia | Hospital | CS | Conv | HIV-infected patients | ELISA | 232 | 70.7 |
Abbreviations: CC, case-control; CFT, complement fixation test; Conv, convenience; CS, cross-sectional; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; HSV-1, herpes simplex virus type 1; HTLV-1, human T-lymphotropic virus 1; MSM, men who have sex with men; NA, not available; PHA, passive hemagglutination assay; RS, random sampling; STD, sexually transmitted disease; STI, sexually transmitted infection; WB, Western blot.
aThe actual study design was cohort, but the extracted seroprevalence measure was for the baseline measurement.
bThe study included overall sample size but no sample sizes for individual strata. Each stratum sample size was assumed to be equal to the overall sample size divided by the number of strata in the study.
Extracted stratified seroprevalence measures varied across and within populations, with a range of 11.1%–100% and a median of 74.1% (Table 2). The range and median for seroprevalence were 11.1%–78.3% and 46.8%, respectively, in populations of healthy children (n = 19), 16.7%–75.9% and 53.1% in clinical populations of children (n = 7), 14.1%–100% and 78.5% in healthy adult populations (n = 103), and 32.1%–95.8% and 67.5% in clinical adult populations (n = 23). Table 2 also includes the ranges and medians for further populations.
Table 2.
Pooled Mean Estimates for Herpes Simplex Virus Type 1 Seroprevalence Among Different Populations in Asia
| Population Type | Outcome Measures, Total No. | Samples, Total No. | HSV-1 Seroprevalence | Pooled Mean HSV-1 Seroprevalence, Mean (95% CI) | Heterogeneity Measuresa | |||
|---|---|---|---|---|---|---|---|---|
| Range | Median | Q (P Value) | I 2 (95% CI), % | Prediction Interval, % | ||||
| Healthy general populations | ||||||||
| Children | 19 | 1131 | 11.1–78.3 | 46.8 | 48.5 (37.8–59.3) | 228.6 (<.001) | 92.1 (89.1–94.3) | 7.1–91.2 |
| Adults | 103 | 9514 | 14.1–100 | 78.5 | 77.4 (73.4–81.1) | 1841.6 (<.001) | 94.5 (93.7–95.1) | 34.9–100 |
| Mixed ages | 4 | 1441 | 52.0–94.1 | 74.8 | 68.9 (56.3–80.3) | 36.5 (<.001) | 91.8 (82.2–96.2) | 16.6–100 |
| All healthy general populations | 126 | 12086 | 11.1–100 | 73.4 | 73.1 (68.9–77.1) | 2955.4 (<.001) | 95.8 (95.3–96.2) | 25.3–100 |
| Clinical populations | ||||||||
| Children | 7 | 720 | 16.7–75.9 | 53.1 | 54.2 (40.5–67.6) | 78.4 (<.001) | 92.3 (86.8–95.6) | 11.0–93.9 |
| Adults | 23 | 2601 | 32.1–95.8 | 67.5 | 67.1 (56.7–76.8) | 456.4 (<.001) | 95.2 (93.8–96.3) | 17.3–100 |
| Mixed ages | 1b | 2317 | - | - | 73.8 (71.9–75.6) | -b | -b | -b |
| All clinical populations | 31 | 5638 | 16.7–95.8 | 67.5 | 64.3 (56.3–71.9) | 809.2 (<.001) | 96.3 (95.5–97.0) | 21.1–97.0 |
| Other populations | ||||||||
| HIV-infected patients | 8 | 1476 | 53.0–94.3 | 87.6 | 83.3 (74.0–91.0) | 119.4 (<.001) | 94.1 (90.6–96.3) | 45.7–100 |
| MSM | 3 | 1774 | 53.3–97.4 | 56.5 | 69.7 (42.9–91.7) | 15.5 (<.001) | 87.1 (63.2–95.5) | 0.0–100 |
| Sex workers | 8 | 1606 | 67.9–92.7 | 84.9 | 84.1 (77.6–89.7) | 63.2 (<.001) | 88.9 (80.5–93.7) | 59.3–98.6 |
| Healthy/ clinical adult populations | 6 | 3732 | 74.3–82.0 | 75.9 | 77.0 (74.4–79.5) | 18.0 (.003) | 72.3 (36.0–88.0) | 68.1–84.8 |
| Age groups | ||||||||
| <20 y | 37 | 3101 | 11.1–94.1 | 51.6 | 55.5 (47.5–63.4) | 654.8 (<.001) | 94.5 (93.3–95.5) | 11.7–94.6 |
| 20–39 y | 48 | 5601 | 14.1–96.7 | 67.7 | 67.9 (62.4–73.3) | 784.3 (<.001) | 94.0 (92.8–95.0) | 23.0–96.0 |
| ≥40 y | 44 | 4966 | 48.0–100 | 89.3 | 87.5 (83.4–91.1) | 633.6 (<.001) | 93.2 (91.7–94.4) | 55.2–100 |
| All children | 26 | 1851 | 11.1–78.3 | 47.6 | 50.0 (41.3–58.7) | 343.6 (<.001) | 92.7 (90.5–94.4) | 10.2–89.8 |
| All adults | 151 | 20705 | 14.1–100 | 77.8 | 76.5 (73.3–79.6) | 3951.1 (<.001) | 96.2 (95.8–96.5) | 34.2–100 |
| All mixed-age groups | 5 | 3758 | 52.0–94.1 | 73.8 | 70.6 (59.4–80.8) | 112.8 (<.001) | 96.5 (94.0–97.9) | 29.6–98.3 |
| All studies/ strata | 182 | 26314 | 11.1–100 | 74.1 | 72.9 (69.8–75.9) | 5038.0 (.001) | 96.4 (96.1–96.7) | 30.3–99.4 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HSV-1, herpes simplex virus type 1; MSM, men who have sex with men.
aThe Cochran Q statistic is a measure assessing the existence of heterogeneity in effect size; I2, a measure that assesses the magnitude of between-study variation due to actual differences in effect size across studies rather than chance; and prediction interval, a measure that estimates the distribution (95% interval) of true effect sizes around the estimated mean.
bNo meta-analysis was done owing to the small number of studies (n < 3).
Pooled Seroprevalence Estimates
Table 2 shows the results of the seroprevalence meta-analyses. Among children, the pooled mean seroprevalence was 48.5% (n = 19; 95% confidence interval [CI], 37.8%–59.3%) for those who were healthy and 54.2% (n = 7; 40.5%–67.6%) for those with clinical conditions. Among adults, the pooled mean was 77.4% (n = 103; 95% CI, 73.4%–81.1%) for healthy adults and 67.1% (n = 23; 56.7%–76.8%) for those with clinical conditions. Table 2 includes pooled results for further populations. By age group, the pooled mean was lowest, at 55.5% (n = 37; 95% CI, 47.5%–63.4%), in individuals aged <20 years, followed by 67.9% (n = 48; 62.4%–73.3%) in those aged 20–39 and 87.5% (n = 44; 83%.4–91.1%) in those aged ≥40 years.
Country-specific meta-analyses were conducted for countries with ≥5 measures for healthy children or adults. For China, the pooled means were 61.3% (n = 12; 95% CI, 53.1%–69.2%) in children and 93.1% (n = 23; 90.0%–95.6%) in adults. For India and Japan, the pooled means were 66.8% (n = 21; 95% CI, 58.6%–74.6%) and 68.1% (n = 34; 61.5%–74.6%), respectively, in healthy adults.
There was strong evidence for heterogeneity in seroprevalence in all meta-analyses (P < .003; Table 2). Most variation was due to true variation in seroprevalence rather than sampling variation (I2 > 50%). The prediction intervals affirmed substantial variation in seroprevalence. Forest plots are shown in Supplementary Figure 1.
Predictors of Seroprevalence and Sources of Between-study Heterogeneity
Table 3 shows the results of the regression analyses. In univariable analyses, age bracket, age group, assay type, country’s income, population type, and sampling method had P values of <.10 and were included in the final multivariable analyses. Age group best explained the seroprevalence variation (adjusted R2 = 21.1%).
Table 3.
Univariable and Multivariable Meta-regression Analyses of Herpes Simplex Virus Type 1 Seroprevalence Among Different Populations in Asia
| Variable | Outcome Measures, Total No. |
Samples, Total No. |
Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) |
P Value | Variance Explained, Adjusted R2, % | Model 1a | Model 2b | |||||
| ARR (95%CI) |
P Value | ARR (95% CI) |
P Value | ||||||
| Age bracket | |||||||||
| Children | 26 | 1851 | 1.0 | … | 1.0 | … | … | … | |
| Adults | 151 | 20705 | 1.5 (1.3–1.7) | <.001 | 1.5 (1.3–1.7) | <.001 | … | … | |
| Mixed ages | 5 | 3758 | 1.4 (1.1–1.9) | .01 | 18.6 | 1.5 (1.1–2.0) | .006 | … | … |
| Age group | |||||||||
| <20 y | 37 | 3101 | 1.0 | … | … | … | 1.0 | … | |
| 20–39 y | 48 | 5601 | 1.2 (1.0–1.4) | .008 | … | … | 1.3 (1.0–1.5) | <.001 | |
| ≥40 y | 44 | 4966 | 1.5 (1.3–1.8) | <.001 | … | … | 1.6 (1.4–1.9) | <.001 | |
| Mixed | 53 | 12646 | 1.3 (1.1–1.5) | <.001 | 21.1 | … | … | 1.3 (1.1–1.5) | <.001 |
| Assay type | |||||||||
| Western blot | 9 | 2859 | 1.0 | … | 1.0 | … | 1.0 | … | |
| ELISA | 137 | 20032 | 0.8 (.6–1.0) | .09 | 0.9 (.8–1.1) | .63 | 0.9 (.7–1.0) | .28 | |
| Others | 36 | 3423 | 0.8 (.6–1.0) | .13 | 0.5 | 1.0 (.8–1.2) | .98 | 1.0 (.8–1.2) | .72 |
| Country’s income | |||||||||
| LMIC | 58 | 8047 | 1.0 | … | 1.0 | … | 1.0 | … | |
| UMIC | 55 | 10084 | 1.2 (1.0–1.3) | .02 | 1.1 (1.0–1.3) | .01 | 1.1 (1.0–1.3) | .03 | |
| HIC | 69 | 8183 | 0.9 (.8–1.1) | .39 | 7.1 | 0.9 (.8–1.2) | .13 | 0.9 (.8–.9) | .01 |
| Population type | |||||||||
| Healthy general populations | 126 | 12086 | 1.0 | … | 1.0 | … | 1.0 | … | |
| Clinical populations | 31 | 5638 | 0.9 (.8–1.0) | .17 | 1.0 (.8–1.1) | .74 | 1.0 (.9–1.1) | .87 | |
| Other populations | 25 | 8590 | 1.1 (1.0–1.3) | .07 | 0.2 | 1.1 (.9–1.2) | .53 | 1.0 (.9–1.2) | .52 |
| Sample sizec | |||||||||
| <100 | 22 | 905 | 1.0 | … | … | … | … | … | |
| ≥100 | 160 | 25409 | 0.9 (.8–1.1) | .65 | 0.0 | … | … | … | … |
| Sampling method | |||||||||
| Probability based | 33 | 7104 | 1.0 | … | 1.0 | … | 1.0 | … | |
| Non–probability based | 149 | 19210 | 0.9 (.8–1.0) | .04 | 1.4 | 1.0 (.9–1.2) | .67 | 1.0 (.8–1.1) | .93 |
| Sex | |||||||||
| Female | 56 | 5665 | 1.0 | … | … | … | … | … | |
| Male | 55 | 6422 | 0.9 (.8–1.1) | .29 | … | … | … | … | |
| Mixed | 71 | 14227 | 0.9 (.8–1.1) | .46 | 1.4 | … | … | … | … |
| Year of data collection | 182 | 26314 | 1.0 (1.0–1.0) | .84 | 0.0 | … | … | … | … |
| Year of publication | 182 | 26314 | 1.0 (1.0–1.0) | .58 | 0.0 | … | … | … | … |
Abbreviations: ARR, adjusted risk ratio; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; HIC, high-income country; LMIC, lower-middle-income country; RR, risk ratio; UMIC, upper-middle-income country.
aThe variance explained by the final multivariable model 1 (adjusted R2) was 26.0%
bThe variance explained by the final multivariable model 2 (adjusted R2) was 33.9%
cSample size denotes the sample size for each study population found in the original publication.
Sample size and sex were not statistically significant. Year of data collection and year of publication were also not statistically significant; strikingly, both risk ratios were 1.0 (95% CI, 1.0–1.0) supporting a flat seroprevalence over time.
Two final multivariable analyses were conducted, instead of one, because of collinearity between age bracket and age group. The model including age bracket, assay type, country’s income, population type, and sampling method explained 26.0% of seroprevalence variation. Seroprevalence in adults was 1.5-fold (95% CI, 1.3–1.7-fold) higher than in children. Seroprevalence in upper-middle-income countries was 1.1-fold (95% CI, 1.0–1.3-fold) higher than in lower-middle-income countries. No association with assay type, population type, and sampling method was found.
The model including age group instead of age bracket explained 33.9% of seroprevalence variation and yielded similar results. Seroprevalence in individuals aged 20–39 years was 1.3-fold (95% CI, 1.0–1.5-fold) higher than in individuals <20, and for those aged ≥40 years, it was 1.6-fold (1.4–1.9-fold) higher.
HSV-1 Detection in GUD and Genital Herpes
Table 4 summarizes the studies reporting proportion of HSV-1 detection in GUD (n = 8) and genital herpes (n = 24). Table 5 shows the results of meta-analyses, with strong evidence for heterogeneity. Forest plots are shown in Supplementary Figure 2.
Table 4.
Studies From Asia Reporting Proportion of Herpes Simplex Virus Type 1 (HSV-1) Viral Detection in Clinically Diagnosed Genital Ulcer Disease, or Proportion of HSV-1 Viral Detection in Clinically Diagnosed Genital Herpes
| Authors (Year) | Year(s) of Data Collection | Country | Study Site | Study Design | Sampling Method |
HSV-1 Biological Assay | Population | Sample Size, No. | Proportion of HSV-1 Detection, % |
|---|---|---|---|---|---|---|---|---|---|
| HSV-1 Detection in Clinically Diagnosed GUD (n = 8) | |||||||||
| Chu et al (2006) [73] | NA | Thailand | Hospital | CS | Conv | PCR | Patients with genital ulcers | 26 | 0.0 |
| Chua and Cheong (1995) [80] | 1993 | Singapore | Outpatient clinic | CS | Conv | CF | Male patients with primary genital ulcers | 121 | 8.3 |
| Chua and Cheong (1995) [80] | 1993 | Singapore | Outpatient clinic | CS | Conv | CF | Female patients with primary genital ulcers | 54 | 27.8 |
| Chua and Cheong (1995) [80] | 1993 | Singapore | Outpatient clinic | CS | Conv | CF | Male patients with recurrent genital ulcer | 181 | 1.6 |
| Chua and Cheong (1995) [80] | 1993 | Singapore | Outpatient clinic | CS | Conv | CF | Female patients with recurrent genital ulcers | 24 | 0.0 |
| Hooi et al (2002) [81] | 1990–1999 | Malaysia | Hospital | CS | Conv | IF | Patients attending a university hospital | 102 | 28.4 |
| Hooi et al (2002) [81] | 1990–1999 | Malaysia | Outpatient clinic | CS | Conv | IF | Patients attending an STD clinic | 204 | 3.4 |
| Thirumoorthy et al (1986) [82] | 1984 | Singapore | Outpatient clinic | CS | Conv | IF | Male patients with penile ulcers | 80 | 0.0 |
| HSV-1 Detection in Clinically Diagnosed Genital Herpes (n = 24) | |||||||||
| Cheong et al (1990) [83] | 1986–1987 | Singapore | Hospital | CS | Conv | IF | First genital herpes episode | 62 | 33.9 |
| Chiam et al (2010) [84] | 1982–2008 | Malaysia | Hospital | CS | Conv | DFA | Malaysian patients | 49 | 61.2 |
| Chiam et al (2010) [84] | 1982–2008 | Malaysia | Hospital | CS | Conv | DFA | Indian patients | 36 | 50.0 |
| Chiam et al (2010) [84] | 1982–2008 | Malaysia | Hospital | CS | Conv | DFA | Chinese patients | 30 | 6.7 |
| Chio et al (2015) [46] | 2014 | Singapore | Outpatient clinic | CS | Conv | PCR | Patients with genital herpes | 193 | 13.9 |
| Chua and Cheong (1995) [80] | 1993 | Singapore | Outpatient clinic | CS | Conv | CF | Male patients with primary genital herpes | 98 | 10.2 |
| Chua and Cheong (1995) [80] | 1993 | Singapore | Outpatient clinic | CS | Conv | CF | Female patients with primary genital herpes | 52 | 28.9 |
| Chua and Cheong (1995) [80] | 1993 | Singapore | Outpatient clinic | CS | Conv | CF | Male patients with recurrent genital herpes | 116 | 2.5 |
| Chua and Cheong (1995) [80] | 1993 | Singapore | Outpatient clinic | CS | Conv | CF | Female patients with recurrent genital herpes | 19 | 0.0 |
| Doraisingham et al (1987) [85] | 1984–1986 | Singapore | Hospital | CS | Conv | IF | Genital lesions positive for HSV | 215 | 21.4 |
| Doraisingham et al (1987) [85] | 1984–1986 | Singapore | Hospital | CS | Conv | IF | Genital HSV isolates | 49 | 32.7 |
| Hooi et al (2002) [81] | 1990–1999 | Malaysia | Hospital | CS | Conv | IF | Patients attending a university hospital | 55 | 52.7 |
| Hooi et al (2002) [81] | 1990–1999 | Malaysia | Outpatient clinic | CS | Conv | IF | Patients attending an STD clinic | 165 | 4.2 |
| Ishiguro et al (1982) [86] | 1975–1978 | Japan | Outpatient clinic | CS | Conv | Nab | Patients with genital herpes | 13 | 53.8 |
| Jacob et al (1989) [87] | 1983–1986 | India | Outpatient clinic | CS | Conv | IF | Patient with primary genital herpes | 10 | 10.0 |
| Jacob et al (1989) [87] | 1983–1986 | India | Outpatient clinic | CS | Conv | IF | Patient with recurrent genital herpes | 42 | 0.0 |
| Kao et al (1991) [88] | 1981–1990 | Taiwan | Hospital | CS | Conv | IF | Genital HSV isolates in men | 53 | 0.0 |
| Kao et al (1991) [88] | 1981–1990 | Taiwan | Hospital | CS | Conv | IF | Genital HSV isolates in women | 96a | 9.4 |
| Kawana et al (1982) [47] | NA | Japan | Outpatient clinic | CS | Conv | Nab | Patients with primary genital herpes | 50 | 62.0 |
| Kawana et al (1982) [47] | NA | Japan | Outpatient clinic | CS | Conv | Nab | Patients with recurrent genital herpes | 49 | 10.2 |
| Puthavathana et al (1998) [89] | 1994–1996 | Thailand | Hospital | CS | Conv | IF | Women with genital herpes | 75 | 18.7 |
| Sen et al (2008) [90] | 1996–2006 | Singapore | Outpatient clinic | CS | Conv | PCR | Patients with genital herpes | 13 | 53.8 |
| Theng and Chan (2004) [91] | 2001 | Singapore | Outpatient clinic | CS | Conv | IF | First genital herpes episode | 114 | 19.3 |
| Theng and Chan (2004) [91] | 2001 | Singapore | Outpatient clinic | CS | Conv | IF | Recurrent genital herpes episode | 127 | 4.7 |
Abbreviations: CF, complement fixation; Conv, convenience; CS, cross-sectional; DFA, direct fluorescent assay; GUD, genital ulcer disease; HSV-1, herpes simplex virus type 1; IF, immunofluorescence; NA, not available; Nab, neutralization antibody test; PCR, polymerase chain reaction; STD, sexually transmitted disease.
aThis population included a mix of patients with clinically diagnosed genital herpes and patients suspected of a viral infection from whom cervical swab samples were collected (n = 47).
Table 5.
Pooled Proportions in Asia of Herpes Simplex Virus Type 1 Viral Detection in Clinically Diagnosed Genital Ulcer Disease or Genital Herpes
| Population Type | Measures, Total No. |
Samples, Total No. |
Proportion of HSV-1 Detection, % | Pooled Proportion of HSV-1 Detection Mean (95% CI), % | Heterogeneity Measurea | |||
|---|---|---|---|---|---|---|---|---|
| Range | Median | Q (P Value) | I 2 (95% CI), % | Prediction Interval, % | ||||
| Patients with clinically diagnosed GUD | 8 | 792 | 0.0–28.4 | 2.5 | 5.6 (.8–13.6) | 91.1 (<.001) | 92.3 (87.2–95.4) | 0.0–43.7 |
| Patients with clinically diagnosed genital herpes | 24 | 1781 | 0.0–62.0 | 16.3 | 18.8 (12.0–26.7) | 330.4 (<.001) | 93.0 (90.8–94.7) | 0.0–62.9 |
Abbreviations: CI, confidence interval; GUD, genital ulcer disease; HSV-1, herpes simplex virus type 1.
aThe Cochran Q statistic is a measure assessing the existence of heterogeneity in effect size; I2, a measure that assesses the magnitude of between-study variation due to actual differences in effect size across studies rather than chance; and prediction interval, a measure that estimates the distribution (95% interval) of true effect sizes around the estimated mean.
The proportion of HSV-1 detection in GUD ranged between 0.0% and 28.4%, with a median of 2.5%. The pooled mean proportion was 5.6% (n = 8; 95% CI, 0.8%–13.6%). The proportion of HSV-1 detection in genital herpes ranged between 0.0% and 62.0%, with a median of 16.3%. The pooled mean proportion was 18.8% (n = 24; 95% CI, 12.0%–26.7%). HSV-1 was more frequently detected in first-episode genital herpes than in recurrent genital herpes (Table 4).
Quality Assessment
Outcomes of the quality assessment are shown in Supplementary Table 2. Overall, seroprevalence studies were of reasonable quality. Of all studies, 70.4% were of high precision, 7.4% had low ROB in the sampling method domain, and 38.9% had low ROB in the response rate domain. Only 7.4% of studies had high ROB in both quality domains.
DISCUSSION
We presented a comprehensive systematic review and synthesis of HSV-1 epidemiology in Asia. Fifty percent of children and 75% of adults were infected. Seroprevalence increased with age, with most infections acquired in childhood. No evidence was found for a temporal trend; seroprevalence appeared stable for 3 decades. Nonetheless, seroprevalence was 60% higher in those aged ≥40 than in those aged <20 years, possibly reflecting a higher exposure risk in earlier times, and an earlier transition toward lower seroprevalence.
As many as 50% of youth reach sexual debut with no protective antibodies against HSV-1, and thus potentially at risk of sexual acquisition. Remarkably, based on virological diagnosis studies, there was a substantial role for HSV-1 in genital herpes and GUD: 19% of genital herpes cases were due to HSV-1 (as opposed to HSV-2), and 6% of GUD cases. These findings suggest an apparently ongoing HSV-1 epidemiological transition, as in Western countries [5, 7, 26], possibly mediated by Asia’s rapid socioeconomic modernization.
The seroprevalence of HSV-1 varied somewhat by country income but was highest in upper-middle-income countries (including China). The weaker socioeconomic association may relate to recent modernization, say for China, and to unexplained low seroprevalence in populations on the Indian subcontinent [92]; seroprevalence in adults was 93% in China but only 67% in India.
Strikingly, there were no differences in seroprevalence by sex, population type, assay type, sampling method, or sample size. Age was the only major predictor of seroprevalence. This speaks for how HSV-1 is a general-population infection that permeates all strata of society. This also demonstrates the ease of sampling a representative sample to measure seroprevalence, provided that the sample age distribution is representative of the underlying population age distribution.
Although seroprevalence was much higher in older than in younger cohorts, there was no evidence for a recent temporal decline in seroprevalence. This finding may be explained by an earlier transition toward lower seroprevalence, or (speculatively) by a demographic effect. HSV-1 seroincidence could be declining, but with rapidly declining fertility and increasing life expectancy rates, the overall seroprevalence could remain stable, masking the decline in seroincidence. Findings from community-based Japanese study (performed over 2 decades) seem to support such a conjecture; seroprevalence in persons aged 20–49 years declined by nearly 10% every decade [59].
Our study has limitations. Data availability varied by country and no data were identified for 13 mostly lower-income countries and territories (Bhutan, Brunei, Cambodia, Hong Kong, Laos, Macau, Mongolia, Myanmar, Nepal, Papua New Guinea, North Korea, Tibet, and Timor-Leste). Seroprevalence showed high heterogeneity, but examined predictors explained only 34% of the variation. Different diagnostic assays were used across studies, but assays may vary by sensitivity and specificity (eg, ELISA vs Western blot) [43, 44], as well as in the differential effect of HSV-2 antibodies—particularly for the classic “relative reactivity” methods [93–95]. However, no evidence was found for differences in seroprevalence by assay type (Table 3).
Similarly, various diagnostic assays were used for viral detection (immunofluorescence, direct fluorescent assay, neutralization antibody test, and nucleic acid amplification test), but these may differ in HSV-1 detection [96]. HSV-1 detection in GUD and genital herpes varied across studies, possibly reflecting variation in the underlying epidemiology. For example, a Malaysian study found >50% HSV-1 detection rates in genital herpes in a university hospital, but <5% in a sexually transmitted disease clinic [81], probably reflecting differences in the populations attending these facilities (general vs sexual high-risk population).
In conclusion, HSV-1 seroprevalence remains high in Asia, with 50% of children and 75% of adults testing seropositive. However, there seems to be an epidemiological transition, with lower seroprevalence in younger cohorts. Close to 50% of youth reach sexual debut uninfected and potentially at risk of sexual acquisition. HSV-1 is possibly playing an influential role as an STI, explaining a fraction of GUD and genital herpes diagnoses. These findings demonstrate the importance of seroprevalence monitoring and GUD/genital herpes etiological surveillance, as well as expansion of HSV-1 epidemiology research in different age groups and countries; for half of countries, no data were available. These findings also highlight the need to accelerate HSV-1 vaccine development to control transmission and prevent associated clinical and psychosocial disease burden.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. L. K. and M. H. conducted the systematic search, screening, data extraction, and data analysis. R. O. contributed to data extraction. G. S. contributed to the statistical analysis. H. C. provided support in study design and data extraction. L. J. A.-R. conceived the study and supervised study conduct and analyses. L. K., M. H., and L. J. A.-R. wrote the first draft of the manuscript. All authors have read and approved the final manuscript.
Acknowledgments. We gratefully acknowledge Rhoda Ashley Morrow from the University of Washington, for her support in assessing the quality of study diagnostic methods and for critically reviewing the manuscript. We are also grateful to Adona Canlas for administrative support and to Fang Yu for providing Chinese translations.
Disclaimer. The findings reported herein are solely the responsibility of the authors.
Financial support. This work was supported by the Qatar National Research Fund (member of the Qatar Foundation; grant NPRP 9-040-3-008) and by pilot funding from the Biomedical Research Program and infrastructure support from the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine in Qatar.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Herpes simplex virus. 2017. Available at: http://www.who.int/mediacentre/factsheets/fs400/en/#hsv1. Accessed 18 October 2017.
- 2. Looker KJ, Magaret AS, May MT, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One 2015; 10:e0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramchandani M, Kong M, Tronstein E, et al. Herpes simplex virus type 1 shedding in tears and nasal and oral mucosa of healthy adults. Sex Transm Dis 2016; 43:756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mark KE, Wald A, Magaret AS, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 2008; 198:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernstein DI, Bellamy AR, Hook EW III, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis 2013; 56:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gnann JW Jr, Whitley RJ. Genital herpes. N Engl J Med 2016; 375:666–74. [DOI] [PubMed] [Google Scholar]
- 7. Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999-2010. J Infect Dis 2014; 209:325–33. [DOI] [PubMed] [Google Scholar]
- 8. Brady RC, Bernstein DI. Treatment of herpes simplex virus infections. Antiviral Res 2004; 61:73–81. [DOI] [PubMed] [Google Scholar]
- 9. Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol 2007; 57:737–63; quiz 764–6. [DOI] [PubMed] [Google Scholar]
- 10. Ryder N, Jin F, McNulty AM, Grulich AE, Donovan B. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992-2006. Sex Transm Infect 2009; 85:416–9. [DOI] [PubMed] [Google Scholar]
- 11. Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 2002; 186(suppl 1):S3–28. [DOI] [PubMed] [Google Scholar]
- 12. Nahmias AJ, Lee FK, Beckman-Nahmias S. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl 1990; 69:19–36. [PubMed] [Google Scholar]
- 13. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006; 296:964–73. [DOI] [PubMed] [Google Scholar]
- 14. Kramer MA, Uitenbroek DG, Ujcic-Voortman JK, et al. Ethnic differences in HSV1 and HSV2 seroprevalence in Amsterdam, the Netherlands. Euro Surveill 2008; 13:pii: 18904. [PubMed] [Google Scholar]
- 15. Xu F, Lee FK, Morrow RA, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. J Pediatr 2007; 151:374–7. [DOI] [PubMed] [Google Scholar]
- 16. Wutzler P, Doerr HW, Färber I, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations-relevance for the incidence of genital herpes. J Med Virol 2000; 61:201–7. [DOI] [PubMed] [Google Scholar]
- 17. Sauerbrei A, Schmitt S, Scheper T, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in Thuringia, Germany, 1999 to 2006. Euro Surveill 2011; 16:pii: 20005. [PubMed] [Google Scholar]
- 18. Pebody RG, Andrews N, Brown D, et al. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect 2004; 80:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aarnisalo J, Ilonen J, Vainionpää R, Volanen I, Kaitosaari T, Simell O. Development of antibodies against cytomegalovirus, varicella-zoster virus and herpes simplex virus in Finland during the first eight years of life: a prospective study. Scand J Infect Dis 2003; 35:750–3. [DOI] [PubMed] [Google Scholar]
- 20. Vyse AJ, Gay NJ, Slomka MJ, et al. The burden of infection with HSV-1 and HSV-2 in England and Wales: implications for the changing epidemiology of genital herpes. Sex Transm Infect 2000; 76:183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 2003; 30:797–800. [DOI] [PubMed] [Google Scholar]
- 22. Löwhagen GB, Tunbäck P, Andersson K, Bergström T, Johannisson G. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex Transm Infect 2000; 76:179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nilsen A, Myrmel H. Changing trends in genital herpes simplex virus infection in Bergen, Norway. Acta Obstet Gynecol Scand 2000; 79:693–6. [PubMed] [Google Scholar]
- 24. Samra Z, Scherf E, Dan M. Herpes simplex virus type 1 is the prevailing cause of genital herpes in the Tel Aviv area, Israel. Sex Transm Dis 2003; 30:794–6. [DOI] [PubMed] [Google Scholar]
- 25. Gilbert M, Li X, Petric M, et al. Using centralized laboratory data to monitor trends in herpes simplex virus type 1 and 2 infection in British Columbia and the changing etiology of genital herpes. Can J Public Health 2011; 102:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whitley RJ. Changing epidemiology of herpes simplex virus infections. Clin Infect Dis 2013; 56:352–3. [DOI] [PubMed] [Google Scholar]
- 27. Gottlieb SL, Giersing B, Boily MC, et al. Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: key findings from the World Health Organization consultation on HSV vaccine impact modelling. Vaccine 2017. doi: 10.1016/j.vaccine.2017.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gottlieb SL, Deal CD, Giersing B, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps. Vaccine 2016; 34:2939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Risbud A, Chan-Tack K, Gadkari D, et al. The etiology of genital ulcer disease by multiplex polymerase chain reaction and relationship to HIV infection among patients attending sexually transmitted disease clinics in Pune, India. Sex Transm Dis 1999; 26:55–62. [DOI] [PubMed] [Google Scholar]
- 30. Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med 1983; 98:958–72. [DOI] [PubMed] [Google Scholar]
- 31. Higgins JP, Green S.. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons, 2011. [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62:1006–12. [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization. WHO regional offices. Available at: http://www.who.int/about/regions/en/. Accessed 20 May 2017. [Google Scholar]
- 34. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR.. Front matter. In: Borenstein M, Hedges LV, Higgins JP, Rothstein HR, eds. Introduction to meta-analysis. Chichester, UK: John Wiley & Sons, 2009. [Google Scholar]
- 35. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat 1950; 607–11. [Google Scholar]
- 36. Borenstein M. Introduction to meta-analysis. Chichester, UK: John Wiley & Sons, 2009. [Google Scholar]
- 37. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. RStudio Team. RStudio: integrated development for R. Boston, MA: RStudio; Available at: http://www.rstudio.com/. 2015. Accessed 10 June 2017. [Google Scholar]
- 39. Schwarzer G. meta: an R package for meta-analysis. R News 2007; 7:40–5. [Google Scholar]
- 40. World Bank. World Bank country and lending groups. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 10 June 2017. [Google Scholar]
- 41. StataCorp. Stata Statistical Software: release 14. College Station, TX: StataCorp, 2015. [Google Scholar]
- 42. Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J 2008; 8:493–519. [Google Scholar]
- 43.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 1988; 26:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ashley RL. Performance and use of HSV type-specific serology test kits. Herpes 2002; 9:38–45. [PubMed] [Google Scholar]
- 45. Bu XL, Yao XQ, Jiao SS, et al. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol 2015; 22:1519–25. [DOI] [PubMed] [Google Scholar]
- 46. Chio M, Aminah S, Osiecki J, Lewinski M, Low L. Performance characteristics of an automated assay on the COBAS 4800 system to detect herpes simplex virus from genital lesions specimens with the COBAS HSV 1 and 2 test. Sex Trans Infect 2015; 91(suppl 2):A1–258. [Google Scholar]
- 47. Kawana T, Kawagoe K, Takizawa K, Chen JT, Kawaguchi T, Sakamoto S. Clinical and virologic studies on female genital herpes. Obstet Gynecol 1982; 60:456–61. [PubMed] [Google Scholar]
- 48. Sun Y, Pei W, Wu Y, Yang Y. An association of herpes simplex virus type 1 infection with type 2 diabetes. Diabetes Care 2005; 28:435–6. [DOI] [PubMed] [Google Scholar]
- 49. Bogaerts J, Ahmed J, Akhter N, et al. Sexually transmitted infections among married women in Dhaka, Bangladesh: unexpected high prevalence of herpes simplex type 2 infection. Sex Transm Infect 2001; 77:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chang RX. The distribution of herpes simplex and cytomegalovirus antibody in differential age groups in Guangzhou. Zhonghua Liu Xing Bing Xue Za Zhi 1986; 7:257–60. [PubMed] [Google Scholar]
- 51. Chen CY, Shen JH, Huang YC. Seroepidemiology of Epstein-Barr virus and herpes simplex virus-1 in Taiwan. Int J Antimicrob Agents 2013; 42(suppl 2):S135. [Google Scholar]
- 52. Li YY, Hidaka Y, Kino Y, Mori R. Seroepidemiology of herpes simplex virus type 1 in Yanji, Jilin, China. Microbiol Immunol 1990; 34:551–5. [DOI] [PubMed] [Google Scholar]
- 53. Lin H, He N, Su M, Feng J, Chen L, Gao M. Herpes simplex virus infections among rural residents in eastern China. BMC Infect Dis 2011; 11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Armelia L, Marbun MBH, Susalit E. Serologic profiles in renal transplant candidates in Jakarta. Nephrology 2012; 17:83–4. [Google Scholar]
- 55.Ashley-Morrow R, Nollkamper J, Robinson NJ, Bishop N, Smith J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect 2004; 10:530–6. [DOI] [PubMed] [Google Scholar]
- 56. Cowan FM, French RS, Mayaud P, et al. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex Transm Infect 2003; 79:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Doi Y, Ninomiya T, Hata J, et al. Seroprevalence of herpes simplex virus 1 and 2 in a population-based cohort in Japan. J Epidemiol 2009; 19:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hashido M, Lee FK, Nahmias AJ, et al. An epidemiologic study of herpes simplex virus type 1 and 2 infection in Japan based on type-specific serological assays. Epidemiol Infect 1998; 120:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hashido M, Kawana T, Matsunaga Y, Inouye S. Changes in prevalence of herpes simplex virus type 1 and 2 antibodies from 1973 to 1993 in the rural districts of Japan. Microbiol Immunol 1999; 43:177–80. [DOI] [PubMed] [Google Scholar]
- 60. Kaur R, Gupta N, Nair D, Kakkar M, Mathur MD. Screening for TORCH infections in pregnant women: a report from Delhi. Southeast Asian J Trop Med Public Health 1999; 30:284–6. [PubMed] [Google Scholar]
- 61. Kaur R, Gupta N, Baveja UK. Seroprevalence of HSV1 and HSV2 infections in family planning clinic attenders. J Commun Dis 2005; 37:307–9. [PubMed] [Google Scholar]
- 62. Patnaik P, Herrero R, Morrow RA, et al. Type-specific seroprevalence of herpes simplex virus type 2 and associated risk factors in middle-aged women from 6 countries: the IARC multicentric study. Sex Transm Dis 2007; 34:1019–24. [PubMed] [Google Scholar]
- 63. Schmid DS, Brown DR, Nisenbaum R, et al. Limits in reliability of glycoprotein G-based type-specific serologic assays for herpes simplex virus types 1 and 2. J Clin Microbiol 1999; 37:376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shivaswamy KN, Thappa DM, Jaisankar TJ, Sujatha S. High seroprevalence of HSV-1 and HSV-2 in STD clinic attendees and non-high risk controls: a case control study at a referral hospital in south India. Indian J Dermatol Venereol Leprol 2005; 71:26–30. [DOI] [PubMed] [Google Scholar]
- 65. Yue J. Sero-epidemiological survey of virus infections in pregnant women of the Changchun district. Zhonghua Fu Chan Ke Za Zhi 1990; 25:269–71, 315. [PubMed] [Google Scholar]
- 66. Zegans ME, Srinivasan M, McHugh T, et al. Mooren ulcer in South India: serology and clinical risk factors. Am J Ophthalmol 1999; 128:205–10. [DOI] [PubMed] [Google Scholar]
- 67. Shen JH, Huang KY, Chao-Yu C, Chen CJ, Lin TY, Huang YC. Seroprevalence of herpes simplex virus type 1 and 2 in Taiwan and risk factor analysis, 2007. PLoS One 2015; 10:e0134178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shyamala G, Sowmya P, Madhavan HN, Malathi J. Relative efficiency of polymerase chain reaction and enzyme-linked immunosorbant assay in determination of viral etiology in congenital cataract in infants. J Postgrad Med 2008; 54:17–20. [DOI] [PubMed] [Google Scholar]
- 69. Kaur R, Mittal N, Bhalla P, Reddy BN, Baveja UK. Risk factors of herpes simplex virus type 2 among STD clinic attenders in Delhi, India. J Commun Dis 2006; 38:339–43. [PubMed] [Google Scholar]
- 70. Patwardhan V, Bhalla P. Role of type-specific herpes simplex virus-1 and 2 serology as a diagnostic modality in patients with clinically suspected genital herpes: a comparative study in Indian population from a tertiary care hospital. Indian J Pathol Microbiol 2016; 59:318–21. [DOI] [PubMed] [Google Scholar]
- 71. Theng CT, Sen PR, Chio TW, Tan HH, Wong ML, Chan RK. Seroprevalence of herpes simplex virus-1 and -2 in attendees of a sexually transmitted infection clinic in Singapore. Sex Health 2006; 3:269–74. [DOI] [PubMed] [Google Scholar]
- 72. Lee A, Lee K. Type-specific herpes simplex virus-1 and herpes simplex virus-2 seroprevalence in Korea. International Journal of Antimicrobial Agents 2015; 45:S138. [Google Scholar]
- 73. Chu K, Jiamton S, Pepin J, et al. Association between HSV-2 and HIV-1 viral load in semen, cervico-vaginal secretions and genital ulcers of Thai men and women. Int J STD AIDS 2006; 17:681–6. [DOI] [PubMed] [Google Scholar]
- 74. Limpakarnjanarat K, Mastro TD, Saisorn S, et al. HIV-1 and other sexually transmitted infections in a cohort of female sex workers in Chiang Rai, Thailand. Sex Transm Infect 1999; 75:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Neal JD, Tobian AA, Laeyendecker O, et al. Performance of the Euroline Western blot assay in the detection of herpes simplex virus type 2 antibody in Uganda, China and the USA. Int J STD AIDS 2011; 22:342–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qutub M, Akhter J. Epidemiology of genital herpes (HSV-2) among brothel based female sex workers in Bangladesh. Eur J Epidemiol 2003; 18:903–5. [DOI] [PubMed] [Google Scholar]
- 77. Theng TS, Sen PR, Tan HH, Wong ML, Chan KW. Seroprevalence of HSV-1 and 2 among sex workers attending a sexually transmitted infection clinic in Singapore. Int J STD AIDS 2006; 17:395–9. [DOI] [PubMed] [Google Scholar]
- 78. van Griensven F, Thienkrua W, McNicholl J, et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS 2013; 27:825–32. [DOI] [PubMed] [Google Scholar]
- 79. Yap SH, Abdullah NK, McStea M, et al. HIV/Human herpesvirus co-infections: impact on tryptophan-kynurenine pathway and immune reconstitution. PLoS One 2017; 12:e0186000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chua SH, Cheong WK. Genital ulcer disease in patients attending a public sexually transmitted disease clinic in Singapore: an epidemiologic study. Ann Acad Med Singapore 1995; 24:510–4. [PubMed] [Google Scholar]
- 81. Hooi PS, Chua BH, Karunakaran R, Lam SK, Chua KB. A retrospective review of mucocutaneous infections by human herpesvirus 1 and 2 in an urban population in Malaysia. Med J Malaysia 2002; 57:80–7. [PubMed] [Google Scholar]
- 82. Thirumoorthy T, Sng EH, Doraisingham S, Ling AE, Lim KB, Lee CT. Purulent penile ulcers of patients in Singapore. Genitourin Med 1986; 62:253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cheong WK, Thirumoorthy T, Doraisingham S, Ling AE. Clinical and laboratory study of first episode genital herpes in Singapore. Int J STD AIDS 1990; 1:195–8. [DOI] [PubMed] [Google Scholar]
- 84. Chiam CW, Chan YF, Sam IC. Changing trends of genital herpes in Kuala Lumpur, Malaysia, 1982-2008. Int J STD AIDS 2010; 21:450–1. [DOI] [PubMed] [Google Scholar]
- 85. Doraisingham S, Thirumoorthy T, Ling AE, Lee CT, Lim KB. Genital herpes in Singapore. Ann Acad Med Singapore 1987; 16:627–30. [PubMed] [Google Scholar]
- 86. Ishiguro T, Ozaki Y, Matsunami M, Funakoshi S. Clinical and virological features of herpes genitalis in Japanese women. Acta Obstet Gynecol Scand 1982; 61:173–6. [DOI] [PubMed] [Google Scholar]
- 87. Jacob M, Rao PS, Sridharan G, John TJ. Epidemiology & clinical profile of genital herpes. Indian J Med Res 1989; 89:4–11. [PubMed] [Google Scholar]
- 88. Kao CL, Lee CN, Lee WL, Hsieh MT, Shih HM. Isolation and typing of herpes simplex virus from clinical specimens collected at National Taiwan University Hospital, 1981–1990. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 1991; 24:255–63. [PubMed] [Google Scholar]
- 89. Puthavathana P, Kanyok R, Horthongkham N, Roongpisuthipong A. Prevalence of herpes simplex virus infection in patients suspected of genital herpes; and virus typing by type specific fluorescent monoclonal antibodies. J Med Assoc Thai 1998; 81:260–4. [PubMed] [Google Scholar]
- 90. Sen P, Sun YJ, Tan HH, Tan SH, Chan R. Comparison of nested-polymerase chain reaction and virus culture for the diagnosis of genital herpes simplex virus infection. Singapore Med J 2008; 49:466–9. [PubMed] [Google Scholar]
- 91. Theng TS, Chan RK. Genital herpes in a sexually-transmitted infection clinic in Singapore: a 1-year retrospective study. Ann Acad Med Singapore 2004; 33:200–3. [PubMed] [Google Scholar]
- 92. Nasrallah GK, Dargham SR, Mohammed LI, Abu-Raddad LJ. Estimating seroprevalence of herpes simplex virus type 1 among different Middle East and North African male populations residing in Qatar. J Med Virol 2018; 90:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ashley R, Cent A, Maggs V, Nahmias A, Corey L. Inability of enzyme immunoassays to discriminate between infections with herpes simplex virus types 1 and 2. Ann Intern Med 1991; 115:520–6. [DOI] [PubMed] [Google Scholar]
- 94. Ashley RL, Dalessio J, Dragavon J, et al. Underestimation of HSV-2 seroprevalence in a high-risk population by microneutralization assay. Sex Transm Dis 1993; 20:230–5. [DOI] [PubMed] [Google Scholar]
- 95. Sherlock CH, Ashley RL, Shurtleff ML, Mack KD, Corey L. Type specificity of complement-fixing antibody against herpes simplex virus type 2 AG-4 early antigen in patients with asymptomatic infection. J Clin Microbiol 1986; 24:1093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Moseley RC, Corey L, Benjamin D, Winter C, Remington ML. Comparison of viral isolation, direct immunofluorescence, and indirect immunoperoxidase techniques for detection of genital herpes simplex virus infection. J Clin Microbiol 1981; 13:913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.