Mucosal CD4+ T memory responses after typhoid immunization
Keywords: blood, CD4+ T, lamina propria mononuclear cells, mucosal responses, typhoid fever
Abstract
Our current understanding of CD4+ T-cell-mediated immunity (CMI) elicited by the oral live attenuated typhoid vaccine Ty21a is primarily derived from studies using peripheral blood. Very limited data are available in humans regarding mucosal immunity (especially CD4+ T) at the site of infection (e.g. terminal ileum; TI). Here using multiparametric flow cytometry, we examined the effect of Ty21a immunization on TI-lamina propria mononuclear cells (LPMC) and peripheral blood CD4+ T memory (TM) subsets in volunteers undergoing routine colonoscopy. Interestingly, we observed significant increases in the frequencies of LPMC CD4+ T cells following Ty21a immunization, restricted to the T effector/memory (TEM)-CD45RA+ (TEMRA) subset. Importantly, Ty21a immunization elicited Salmonella Typhi-responsive LPMC CD4+ T cells in all major TM subsets [interferon (IFN)γ and interleukin (IL)-17A in TEM; IFNγ and macrophage inflammatory protein (MIP)1β in T central/memory (TCM); and IL-2 in TEMRA]. Subsequently, we analyzed LPMC S. Typhi-responsive CD4+ T cells in depth for multifunctional (MF) effectors. We found that LPMC CD4+ TEM responses were mostly MF, except for those cells exhibiting the characteristics associated with IL-17A responses. Finally, we compared mucosal to systemic responses and observed that LPMC CD4+S. Typhi-specific responses were unique and distinct from their systemic counterparts. This study provides the first demonstration of S. Typhi-specific CD4+ TM responses in the human TI mucosa and provides valuable information about the generation of mucosal immune responses following oral Ty21a immunization.
Introduction
Salmonella enterica serovar Typhi (S. Typhi) is a human-restricted pathogen that causes typhoid fever and constitutes a major global health threat. The burden of S. Typhi infection is an estimated 26.9 million cases of typhoid fever annually resulting in ~217000 deaths worldwide (1–4). Following ingestion by the oral route, S. Typhi infects and invades the host ‘M’ cells and epithelial cells and subsequently translocates to the submucosa where it encounters intestinal lymphoid tissues, before entering draining mesenteric lymph nodes, and disseminating to the liver, spleen and other secondary lymphoid tissues, resulting in systemic illness (4). The most serious complication of typhoid fever is intestinal perforation which occurs mainly at the terminal ileum (TI) (in ~78% of perforation cases) suggesting that it is the favored intestinal active invasion site for S. Typhi (5, 6). Only very limited information is available regarding the generation of CD4+ T cells to S. Typhi in the human intestinal mucosa (7, 8). Recently, we have reported that oral Ty21a immunization elicits CD8+ T memory (TM) S. Typhi-specific responses in human TI specimens which differs in some key aspects from their systemic counterparts (9). To our knowledge, there are no data on the induction of CD4+ TM responses to S. Typhi in the TI mucosa following wild-type (wt) S. Typhi infection or immunization with the live attenuated oral vaccine Ty21a (Ty21a). Given that the gastrointestinal tract is a major reservoir of CD4+ TM cells, understanding the host mucosal immune responses against S. Typhi and other enteric pathogens at their preferred site of natural infection is required to provide novel insights for the development of oral vaccines.
Two licensed typhoid vaccines, namely the oral live attenuated Ty21a and the parenteral Vi polysaccharide vaccine, are available in the USA for use in humans (4) but they both have their limitations. The licensed attenuated Ty21a typhoid vaccine is typically administered in four spaced doses and confers a moderate level of long-lived protection (60–80%, 5–7 years) (4, 10–12). Hence, there is a need to develop effective new vaccines that will provide durable, long-lasting protection. We and others have extensively studied the induction of humoral and cell-mediated immunity (CMI) responses in peripheral blood mononuclear cells (PBMC) obtained from healthy volunteers following immunization with four doses of Ty21a (12–16). These studies showed that live oral S. Typhi vaccines induced both CD4+ and CD8+ T-cell responses, including interferon (IFN)γ, cytotoxic T cells (CTL), proliferation and multifunctional (MF) antigen-specific cytokine-producing cells (12, 15, 17–19), which might play a key role in long-term immunity. We have also reported that Ty21a elicits S. Typhi-specific CD4+ T-cell responses in PBMC by various CD4+ TM cell subsets, including T central/memory (TCM), T effector/memory (TEM) and RA+ TEM (TEMRA) (20, 21). These responses were predominantly in the TEM and TEMRA subsets with a low magnitude of responses observed in CD4+ TCM subsets (12, 21, 22). Furthermore, S. Typhi-specific MF cells were increased in CD4+ TEM and TEMRA subsets post-vaccination predominantly producing IFNγ and/or TNFα, while IL-2, MIP1β, IL-17A and CD107a expression (a marker associated with cytotoxicity) were observed in a small proportion of MF. In addition, it appears that CD4+ T- and CD8+ T-cell responses against S. Typhi depend on the nature of the stimulant. For example, CD4+ cells were more susceptible to respond to S. Typhi soluble antigens than S. Typhi-infected targets (14, 23). The function of CD4+ T cells in protection against typhoid fever in humans is still not fully understood. Salmonella Typhi-specific CD4+ T-cell responses have been detected in individuals with typhoid fever (24, 25) and very recently, using a human infection model with wt S. Typhi, clonotypes of CD4+ T cells recognizing distinct immunodominant antigens were identified (26). Furthermore, using genome-wide association studies, it was also shown that the expression of specific major histocompatibility complex class II alleles confers resistance to typhoid fever (27). Taken together, these data suggest that CD4+ T cells might play a protective function in the control of S. Typhi in humans. However, all of these detailed CMI responses were evaluated in peripheral blood; CD4+ TM responses in the human TI have never been directly evaluated. Therefore, we hypothesized that S. Typhi-specific responses by various CD4+ TM subsets elicited in the TI following Ty21a immunization would differ in magnitude and characteristics to their systemic counterparts. In this study, we have characterized TI-lamina propria mononuclear cells (LPMC) CD4+ TM in Ty21a-vaccinated and unvaccinated volunteers. We then determined and compared CD4+ TMS. Typhi-specific responses from the two groups following stimulation with (i) autologous target cells infected with or without wt S. Typhi and (ii) S. Typhi antigens [e.g. Ty21a homogenate, flagella (FliC)]. Finally, we assessed these responses in depth by analyzing their multifunctionality and directly compared peripheral and mucosal CD4+ TEM MF responses. These comparisons provide a unique insight into the similarities and differences between mucosal and peripheral immunity.
Methods
Volunteers, immunization and sample collection
The human experimentation guidelines of the US Department of Health and Human Services and those of the University of Maryland, Baltimore, were followed in the conduct of this study. All TI biopsies and blood specimens were collected from volunteers who participated in the University of Maryland Institutional Review Board approved protocol number HP-0005632. Volunteers undergoing routine colonoscopy who had no history of typhoid fever were recruited from the Baltimore–Washington metropolitan area and University of Maryland, Baltimore campus. Written informed consent was obtained from volunteers and all procedures were approved by the University of Maryland, Baltimore Institutional Review Board (IRB). Volunteers (demographics shown in Supplementary Table S1) were assigned into two groups. The first group (n = 16) were immunized with four spaced doses of 2–6 × 109 CFU of oral live attenuated Ty21a at an interval of 48 h between doses (Vivotif enteric-coated capsules; Crucell, Bern, Switzerland) while volunteers assigned to the second group were not vaccinated (control group) (n = 30) as shown in the study design (Supplementary Figure S1). Blood samples were collected at least 21 days before immunization (pre-immunization) and on colonoscopy day (day 0) together with TI biopsies using large capacity forceps (Supplementary Figure S1). PBMC were isolated immediately after blood draws by density gradient centrifugation and cryopreserved in liquid nitrogen following standard techniques (22).
Isolation of LPMC from TI biopsies
TI-LPMC were freshly isolated using an optimized procedure as previously described (9, 28, 29). Briefly, after collection of biopsies from routine colonoscopy volunteers, tissues were treated with HBSS (without CaCl2, MgCl2, MgSO4) (Gibco, Carlsbad, CA, USA) and EDTA (1 mM; Ambion, Grand Island, NY, USA) to remove intra-epithelial cells (IEL). LPMC were then isolated following enzymatic digestion of the biopsies with Collagenase D (100 μg ml−1; Roche, Indianapolis, IN, USA) and DNase I (10 μg ml−1; Affymetrix, Cleveland, OH, USA) and homogenization using the Bullet Blender homogenizer (Next Advance Inc., Averill, NY, USA). Cells were then washed and re-suspended in complete medium (cRPMI) [RPMI 1640 (Gibco Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (BioWhittaker, Walkersville, MD, USA), 2 mM l-glutamine (HyClone, Logan, UT, USA), 2.5 mM sodium pyruvate (Gibco), and 10 mM HEPES (Gibco), 100 U ml−1 penicillin (Sigma-Aldrich, St Louis, MO, USA), 100 μg ml−1 streptomycin (Sigma-Aldrich), and 50 μg ml−1 gentamicin (Gibco)] and counted using Kova Glastic Slides (Hycor Biomedical, CA, USA). Isolated LPMC were either stained immediately for immune phenotyping by flow cytometry or stimulated overnight with either S. Typhi-infected targets or soluble antigens [Ty21a homogenate, FliC, or tetanus toxoid (TT)] or controls before staining with a 14-color flow cytometry panel and analyzed using a customized LSR-II instrument (BD, Franklin Lakes, NJ, USA).
Target cell preparation
Autologous Epstein–Barr virus (EBV)-transformed lymphoblastoid cell lines (EBV-B cells) were generated from each participant’s pre-immunization PBMC, which were isolated at least 21 days before colonoscopy [Supplementary Figure S1 as previously described (9, 22, 30)]. Briefly, EBV-B cells were obtained by incubation of PBMC with EBV-containing supernatant from the B95-8 cell line (ATCC CRL1612) and cyclosporine (0.5 μg ml−1; Sigma-Aldrich) at 37°C with 5% CO2. After transformation, EBV-B were maintained in culture in RPMI 1640 (Gibco, Grand Island, NY, USA) supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 50 μg ml−1 gentamicin, 2 mM l-glutamine, 2.5 mM sodium pyruvate, 10 mM HEPES buffer and 10% heat-inactivated fetal bovine serum (R10) until used in the experiments.
Salmonella Typhi infection of target cells
Autologous target cells (EBV-B) generated as described above were infected with wt S. Typhi strain (ISP1820, Vi+, a clinical isolate from Chile) (16) at a multiplicity of infection of 7:1 as previously described (9, 22, 30). Briefly, the targets and bacteria were incubated for 3 h at 37°C in RPMI without antibiotics, washed three times with cRMPI and incubated overnight with cRPMI containing 150 μg ml−1 gentamicin. Salmonella Typhi-infected and uninfected cells were gamma-irradiated (6000rad) for 6 min before being used as ‘targets’ for ex vivo TI-LPMC and PBMC stimulation. Cells were washed and the efficiency of the infection with S. Typhi-infected EBV-B was confirmed by staining with anti-Salmonella common structural Ag (CSA-1)-FITC (Kierkegaard and Perry, Gaithersburg, MD, USA) and analysis by flow cytometry using a customized LSR-II instrument (BD) as previously described (18). The percentage of cells infected with S. Typhi was recorded for each experiment. Infected targets were only used if the infection was detected (CSA-1 positive) in 30–60% of viable cells.
Soluble proteins
The Ty21a bacteria strain was obtained from the Center for Vaccine Development, University of Maryland, USA (CVD) reference stocks and was grown for 14–16 h in Luria-Bertani (LB) supplemented with 0.1% galactose as described previously (31). The bacteria were then homogenized using a French press (1 cycle at 20000 psi) and the homogenate centrifuged at 17700 × g for 10 min. The pellet was discarded and the supernatant filtered through a 0.8 μm filter and aliquots were then kept at −20°C. The protein concentration of the Ty21a homogenate was then measured with a BCA protein kit (Fisher) and using a protein standard (BSA, Fisher). The recombinant and purified S. Typhi FliC was prepared as described previously (16). Briefly, the FliC encoding region (residues 53–450) was subcloned from S. Typhi ISP1820 into pET15b. The plasmid was transformed into Escherichia coli Tuner (DE3) and the protein over-expressed following induction with 100 μM of isopropyl β-d-1-thiogalactopyranoside (IPTG). The over-expressed protein was then purified by standard immobilized metal affinity column chromatography (IMAC) methods.
Stimulation of PBMC and TI-LPMC
Freshly isolated TI-LPMC and PBMC were used as effector cells as previously described (9, 29). Briefly, LPMC and PBMC (1 × 106 cells per ml) effectors were co-cultured with either non-infected or S. Typhi-infected autologous EBV-B at an effector to stimulator cell ratio of 7:1. For soluble antigen stimulation, LPMC and PBMC effectors were induced with Ty21a homogenate (10 μg ml−1), FliC (10 μg ml−1) or TT (10 μg ml−1) (Sigma-Aldrich). LPMC and PBMC cultured with media only or in the presence of α-CD3/CD28 beads (Life Technologies, Grand Island, NY, USA) were used as negative and positive controls, respectively. At the time of stimulation, anti-human CD107a-FITC (5 μl; H4A3, BD, San Jose, CA, USA) was added. The CD107a antibody was used to evaluate degranulation as a measure of cytotoxicity, a mechanism essential for the killing of S. Typhi-infected targets by T cells (32). After 2 h, 0.5 μl of Golgi Stop (Monensin, BD) and Golgi Plug (Brefeldin A, BD) were added and cultures continued overnight at 37°C in 5% CO2. After overnight (16–18 h) incubation, cells were harvested and prepared for flow cytometry analysis.
Surface and intracellular staining
Following stimulation, PBMC and TI-LPMC were stained for flow cytometry analysis as previously described (9, 28). Following stimulation in the presence of CD107a [LAMP-1, a molecule expressed on the cell membrane which is widely accepted to be associated with cytotoxic T-cell activity (32)], PBMC and LPMC were stained for live/dead discrimination (YEVID) (Invitrogen). Blocking of Fc receptors was performed using human immunoglobulin (3 µg ml−1; Sigma) and was followed by surface staining. Briefly, cells were stained with fluorescently labeled monoclonal antibodies (mAbs) directed to CD13-Pacific Orange (conjugated in-house), CD19-BV570 (HIB19, BioLegend), CD3-BV650 (OKT3, BioLegend), CD4-PE-Cy5 (RPA-T4, BD), CD8-PerCP-Cy5.5 (SK1, BD), CD45RA-biotin (HI100, BD), CD62L-APC-A780 (DREG-56, eBioscience) and integrin α4β7-A647 (ACT1; conjugated in-house) at 4°C for 30 min. Cells were washed with wash buffer and stained with streptavidin (SAV)-Qdot800 (Invitrogen) at 4°C for 30 min. Cells were then fixed and permeabilized using IC fixation and permeabilization buffers (8222/8333, eBioscience) according to the manufacturer’s recommendations. This was followed by staining (4°C overnight) with mAbs directed to IL-17A-BV421 (BL168, BioLegend), IFNγ-PE-Cy7 (B27, BD), TNFα-Alexa 700 (MAb11, BD), and CD69-ECD (TP1.55.3, Beckman Coulter, Danvers, MA, USA), IL-2-BV605 (MQ1-17H12, BioLegend), and MIP1β-PE (IC271P, R&D Systems). After staining, cells were stored in 1% paraformaldehyde at 4°C until data collection. Data were collected using a customized LSR-II flow cytometer (BD) and then analyzed using the WinList version 7 (Verity Software House, Topsham, ME, USA) software package. Salmonella Typhi-specific responses were expressed as net percentage of positive cells (backgrounds after stimulation with uninfected cells were subtracted from values obtained with S. Typhi-infected stimulators). A response was considered specific if the differential in the number of positive events between experimental (S. Typhi-infected targets) and negative control (uninfected targets) cultures was significantly increased by z-tests. Salmonella Typhi-specific responses were expressed as net percentage of positive cells (backgrounds after stimulation with uninfected cells or with media control were subtracted from values obtained with S. Typhi-infected stimulators or with soluble antigens, respectively).
Surface and intracellular staining for homing markers
Freshly isolated TI-LPMC and PBMC were characterized for homing markers. Briefly, PBMC and LPMC were stained for live/dead discrimination (YEVID) (Invitrogen). Blocking of Fc receptors was performed using human immunoglobulin (3 µg ml−1; Sigma) and was followed by surface staining. Briefly, cells were stained with fluorescently labeled mAbs directed to CD13-Pacific Orange (conjugated in-house), CD19-BV570 (HIB19, BioLegend), CD3-BV650 (OKT3, BioLegend), CD4-V450 (RPA-T4, BD), CD8-APC-H7 (SK1, BD), CCR9-APC (FAB1791A, R&D Systems), CCR6-biotin (11A9, BD), and integrin α4β7-A647 (ACT1; conjugated in-house), Ki67-A700 (B56, BD) at 4°C for 30 min. Cells were washed with wash buffer and stained with streptavidin (SAV)-Qdot800 (Invitrogen) at 4°C for 30 min. Cells were then fixed and permeabilized using IC fixation and permeabilization buffers (8222/8333, eBioscience) according to the manufacturer’s recommendations. This was followed by staining (4°C overnight) with mAbs directed to IL-17A-PerCP-Cy5.5 (N49-653, BD), IFNγ-PE-Cy7 (B27, BD), and CD69-ECD (TP1.55.3, Beckman Coulter, Danvers, MA). After staining, cells were stored in 1% paraformaldehyde at 4°C until data collection. Data were collected using a customized LSR-II flow cytometer (BD) and then analyzed using the WinList version 7 (Verity Software House) software package. This package includes the FCOM function, a subroutine that enables the analysis of the multifunctionality of the responses on a single cell basis, enabling the classification of events on the basis of combinations of selected gates. This function informs whether particular cells are single producing cells or produced two or more cytokines and/or express surface markers simultaneously.
Statistical analysis
Data were analyzed using the statistical software GraphPad Prism™ version 5.03 (Graphpad, San Diego, CA, USA). Statistical differences in median values between two groups were determined using Mann–Whitney tests. Wilcoxon matched pair tests were used to assess statistical differences between LPMC and PBMC paired responses. On the basis of a recent recommendation by the American Statistical Association (ASA), particularly when analyzing data sets with relatively low numbers of volunteers (33, 34), we also indicated trends in expression of markers or cytokine responses where appropriate using a P ≤ 0.15.
Results
Oral Ty21a immunization alters mucosal CD4+ T-cell frequencies
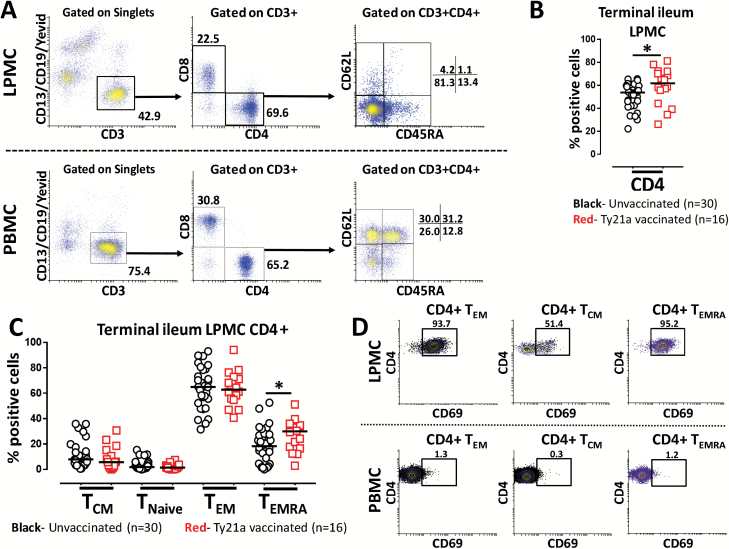
The consequence of oral Ty21a immunization on human TI-LPMC CD4+ T cells in healthy volunteers has not yet been explored. To determine whether Ty21a immunization may influence (i) frequencies of CD4+ T cells, (ii) frequencies of CD4+ TM subsets, (iii) expression of the activation/retention marker CD69 and (iv) expression of homing molecules on CD4+ T cells, we isolated TI-LPMC and PBMC from volunteers who either received four doses of Ty21a or were unvaccinated. We then characterized CD4+ T and TM subsets from freshly isolated TI-LPMC obtained from biopsies of Ty21a-vaccinated and unvaccinated volunteers using CD62L and CD45RA markers as shown by the gating strategy depicted in Fig. 1(A). Significantly increased frequencies of total LPMC CD4+ T cells were observed in Ty21a vaccinees (n = 16) compared to those present in unvaccinated (n = 30) volunteers (Fig. 1B). We also observed that LPMC CD4+ TEM (CD62L−CD45RA−) cells were the predominant TM population (~70%) in TI-LPMC while CD4+ TEMRA (CD62L−CD45RA+) (~20%) and CD4+ TCM (CD62L+CD45RA−) (~10%) represented, as expected, relatively minor populations (Fig. 1A). Cumulative data showed that the percentage of CD4+ TEM, Tnaive and TCM subsets was not significantly different between Ty21a-vaccinated (n = 16) and unvaccinated (n = 30) volunteers (Fig. 1C). In contrast, the frequency of CD4+ TEMRA was significantly increased following Ty21a immunization (Fig. 1C). Examination of the levels of CD69 expression in LPMC CD4+ TM (TEM, TCM, TEMRA) showed that almost all TEM and TEMRA cells, but only approximately half of TCM were CD69+ (Fig. 1D). In contrast, CD69 was expressed in a very small proportion of CD4+ TM subsets in PBMC, indicating that the vast majority of these were, as expected, not activated (Fig. 1D). Note that, largely because of the limited numbers of freshly isolated TI-LPMC available, the number of volunteers studied varies throughout in the manuscript depending on the experimental conditions being evaluated.
Fig. 1.
Gating strategy and CD4+ T-cell subset frequencies in TI-LPMC isolated from Ty21a-vaccinated and non-vaccinated volunteers. (A) Freshly isolated TI-LPMC and blood (PBMC) obtained from a Ty21a-vaccinated individual were characterized for memory CD4+ T (CD4+ TM) cell subsets using CD62L and CD45RA markers on gated CD4+ T cells as shown. (B) Frequencies of CD4+ T cells were measured and compared between TI-LPMC obtained from Ty21a-vaccinated (n = 16; red symbols) and unvaccinated volunteers (n = 30; black symbols). (C) Comparison of the frequencies of CD4+ TM subsets [TCM (CD62L+CD45RA−), TEM (CD62L−CD45RA−), TEMRA (CD62L−CD45RA+) and Tnaive (CD62L+CD45RA+)] in TI-LPMC obtained from Ty21a-vaccinated (n = 16; red symbols) and unvaccinated (n = 30; black symbols) volunteers. (D) The frequency of CD4+ TM subsets (TEM, TCM and TEMRA) obtained from TI-LPMC (top panel) and PBMC (bottom panel) co-expressing the activation marker CD69 was determined using flow cytometry. The percentages of CD69 expression are denoted above the corresponding gating boxes in each cytogram. Significant differences between Ty21a-vaccinated and unvaccinated volunteers are denoted as *P < 0.05. Median values for each group are represented as horizontal black bars.
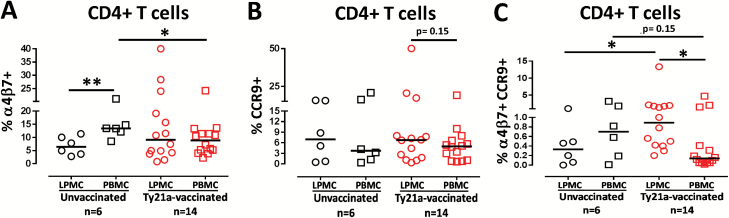
Our group and others have demonstrated that significant proportions of S. Typhi-specific T cells generated following Ty21a immunization are primed for mucosal homing by expressing the intestine-homing molecule integrin α4β7 (8, 21, 35). However, a comparison of the expression of homing markers between blood and TI-LPMC CD4+ T cells to evaluate the potential accumulation and retention of these incoming cells was not previously reported. To directly address this key issue, we determined the ex vivo frequencies of CD4+ T cells obtained concurrently from TI biopsies and their corresponding PBMC expressing the homing markers integrin α4β7, CCR9 and CCR6. Interestingly, we observed that the frequencies of CD4+ T cells expressing integrin α4β7 in TI-LPMC were significantly (P < 0.05) lower than those in PBMC in unvaccinated volunteers (Fig. 2A). Our results also show that the frequencies of CD4+ T cells expressing integrin α4β7 in blood (PBMC) decrease significantly (P < 0.05) following Ty21a immunization (Fig. 2A). However, no significant differences in the frequencies of integrin α4β7+ CD4+ T cells were detected between TI-LPMC and PBMC following Ty21a immunization. To further understand the homing patterns on TI-LPMC and PBMC CD4+ T cells, we evaluated the frequency of CCR9+ CD4+ T cells in volunteers in both groups. No significant differences in the frequencies of CCR9+ CD4+ T cells in blood and TI-LPMC following Ty21a immunization were noted (Fig. 2B). However, we found that the frequencies of CCR9+ CD4+ T cells in LPMC showed a trend (P = 0.15) to be higher than their PBMC counterparts following Ty21a immunization (Fig. 2B). Throughout the manuscript we indicated P ≤ 0.15 as a trend in expression of markers or cytokine responses on the basis of a recent recommendation by the ASA stating that the P-value depends on the degree of association and the sample size and thus, by itself, a P-value does not provide a good measure of evidence regarding a model or hypothesis (33, 34). In this study, we observed that the homing patterns and net CMI responses were markedly increased in some individuals, but as a group did not reach statistical significance, likely because of the relatively limited number of participants evaluated. Thus, we indicated trends when appropriate.
Fig. 2.
Ex vivo mucosal and systemic homing phenotypes of CD4+ T cells following Ty21a oral vaccination. Ex vivo percentages of (A) integrin α4β7+, (B) CCR9+ and (C) integrin α4β7+ CCR9+ were evaluated in LPMC and PBMC CD4+ T cells isolated from TI biopsies and blood of Ty21a-vaccinated (red symbols; n = 14) and unvaccinated (black symbols; n = 6) volunteers using flow cytometry. Significant differences between TI-LPMC and PBMC in vaccinated and unvaccinated volunteers are denoted as *P < 0.05 and **P < 0.005. P-value for trends was also denoted. Median values for each group are represented as horizontal black bars.
We next determined the frequencies of integrin α4β7+ CCR9+ CD4+ T cells in LPMC and PBMC in both groups of volunteers. No differences were observed in the frequencies of integrin α4β7+ CCR9+ CD4+ T cells between LPMC and PBMC in unvaccinated volunteers (Fig. 2C). Moreover, the frequencies of integrin α4β7+ CCR9+ CD4+ T cells showed a trend (P = 0.15) to be lower in PBMC following Ty21a immunization (Fig. 2C). In contrast, following Ty21a immunization we found significant increases (P < 0.05) in integrin α4β7+ CCR9+ CD4+ T cells in TI-LPMC following Ty21a immunization (Fig. 2C). Furthermore, we observed that following Ty21a immunization, the frequencies of integrin α4β7+ CCR9+ CD4+ T cells in TI-LPMC were significantly higher than those in PBMC (Fig. 2C). Finally, we also characterized the expression of the homing marker CCR6 on CD4+ T cells in PBMC and LPMC obtained from both groups. No significant differences were observed in the frequencies of either LPMC or PBMC CCR6+ CD4+ T cells following Ty21a immunization. These data suggest that CD4+ T cells co-expressing integrin α4β7 and CCR9 may accumulate in the local TI mucosa following Ty21a immunization.
Activation of TI-LPMC CD4+ T cells
Most of our knowledge of CD4+ T responses elicited by S. Typhi infection or Ty21a immunization in humans is based solely on data derived from blood (8, 12, 13). Virtually, no information is available on TI CD4+ T immune responses following wt S. Typhi infection or immunization with the Ty21a vaccine. In addition, the observation that oral Ty21a immunization significantly increases CD4+ TM subsets (especially TEMRA) (Fig. 1) suggests that TI CD4+ T cells might respond differently in magnitude and characteristics following stimulation with S. Typhi-infected targets. Furthermore, we hypothesized that Ty21a immunization might also influence the baseline responses or the capacity to respond to stimulation.
To address this hypothesis, we first evaluated whether S. Typhi-specific mucosal responses were due to baseline responses or the capacity of TM to be activated following Ty21a immunization. Thus, we assessed the levels of CD4+ TEM cytokine-producing cells and their cytotoxic potential following an overnight culture either alone (unstimulated) or following stimulation with α-CD3/CD28 beads. Cumulative data show that neither the background levels (LPMC alone) nor their activation with α-CD3/CD28 beads were significantly different between LPMC isolated from Ty21a-vaccinated (n = 16) and unvaccinated (n = 30) individuals (Supplementary Figure S2A and B). These results indicate that ex vivo LPMC CD4+ T cells did not exhibit intrinsic differences between the Ty21a-vaccinated and unvaccinated groups in background and non-specific stimulatory characteristics.
TI-LPMC CD4+ TM subsets have unique S. Typhi-specific response profiles
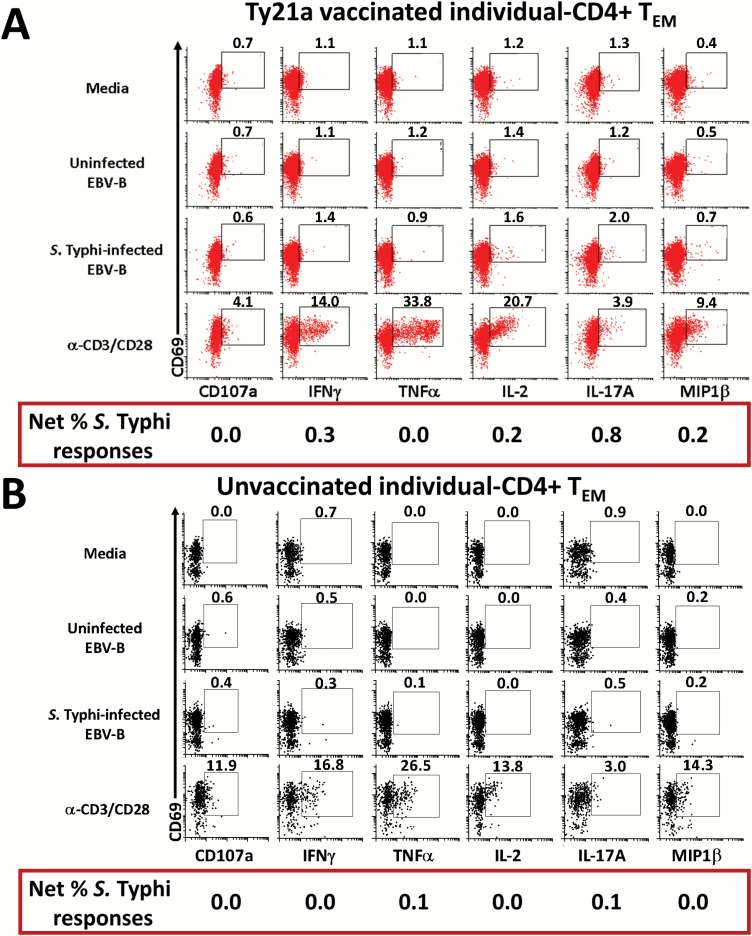
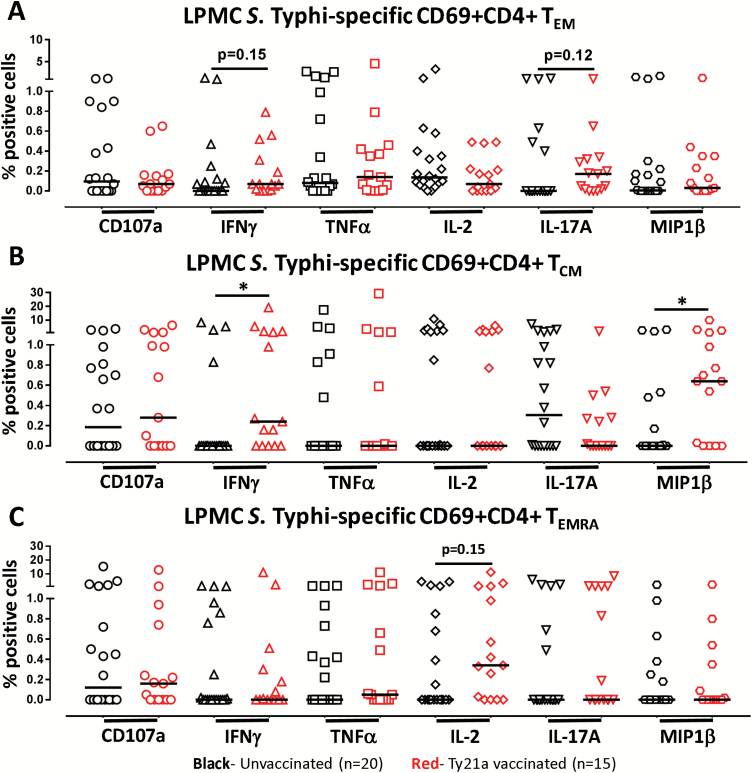
Next, we determined the ability of LPMC CD4+ TM cell subsets obtained from Ty21a-vaccinated (n = 15) and unvaccinated (n = 20) volunteers to be activated following co-culture with autologous S. Typhi-infected or uninfected EBV-B cells by assessing their cytokines/cytotoxic responses following stimulation. Responses of representative subjects are presented in Fig. 3. Following stimulation with S. Typhi-infected EBV-B, we observed in Ty21a vaccinees substantial net increases (% of S. Typhi-infected EBV-B responses − % of uninfected EBV-B responses) in the frequencies of CD4+ TEM cells producing cytokines/chemokines (e.g. INFγ, IL-2, IL-17A and MIP1β) (Fig. 3A and B). Interestingly, the levels of individual cytokines varied following oral Ty21a immunization. Since LPMC CD4+ TEM is the predominant memory subset in the TI, representing over 70% of total CD4+ T cells, we first assessed their S. Typhi-specific response profile (Fig. 4A). Cumulative data of S. Typhi-specific responses were expressed as net percentages of TEM positive cells. Interestingly, LPMC CD4+ TEM producing IFNγ and IL-17A show trends to exhibit higher levels (P = 0.15 and 0.12, respectively) in Ty21a-vaccinated than in unvaccinated volunteers (Fig. 4A). We next assessed S. Typhi-specific responses by TI-LPMC CD4+ TCM and TEMRA subsets. Remarkably, we observed significantly (P < 0.05) higher frequencies of CD4+ TCM producing cytokines (IFNγ and MIP1β) in LPMC obtained from Ty21a-vaccinated than in unvaccinated volunteers (Fig. 4B). No significant differences in CD4+ TCM TNFα, IL-2, and IL-17A-producing cells and CD107a-expressing cells were detected between Ty21a-vaccinated and unvaccinated volunteers (Fig. 4B). A similar assessment performed in CD4+ TEMRA revealed that this cell subset exhibited trends (P = 0.15) to show higher frequencies of IL-2-producing cells in LPMC obtained from Ty21a vaccinees than in their unvaccinated counterparts (Fig. 4C).
Fig. 3.
Salmonella Typhi-specific responses by LPMC CD4+ T cells isolated from the TI of a Ty21a-vaccinated and an unvaccinated representative volunteer. Using a 14-color flow cytometry panel we studied the induction of cytokine/chemokine production (IFNγ, TNFα, IL-2, IL-17A and MIP1β) and up-regulation of CD107a expression in CD69+ CD4+ TEM cells obtained from (A) Ty21a-vaccinated and (B) unvaccinated representative volunteers following an overnight stimulation with non-infected or S. Typhi-infected autologous EBV-B cells at 37°C, 5% CO2. Anti (α)-CD3/CD28 beads (1 μl) and unstimulated cells were used as a positive and negative controls, respectively, in both volunteers. The percentages of positive cells in the gated regions are shown above their corresponding black boxes.
Fig. 4.
Effect of oral Ty21a immunization on TI-LPMC CD4+ TM (TEM, TCM and TEMRA) Salmonella Typhi-specific responses in healthy adults. The net percentages of S. Typhi-specific responses (IFNγ, TNFα, IL-2, IL-17A, and MIP1β production and CD107a expression) in (A) CD69+ CD4+ TEM, (B) CD69+ CD4+ TCM and (C) CD69+ CD4+ TEMRA subsets were compared between Ty21a-vaccinated (n = 15; red symbols) and unvaccinated volunteers (n = 20; black symbols) following stimulation of LPMC with autologous S. Typhi-infected and uninfected EBV-B. The net percentages were calculated as % responses in S. Typhi EBV-B stimulated cultures − % responses in uninfected EBV stimulated cultures. Significant differences (*P < 0.05) and P-values for trends are indicated. Horizontal black bars represent median values.
Multifunctional TI-LPMC CD4+ TEM responses following oral Ty21a immunization
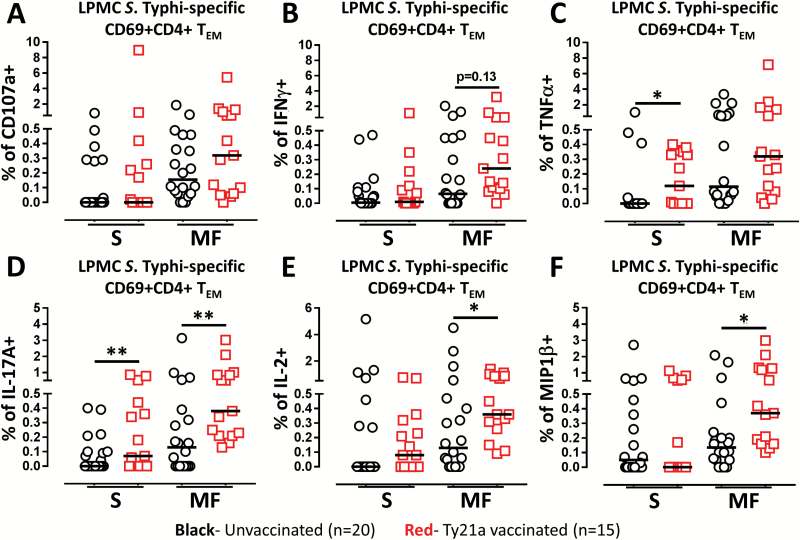
Our group has previously shown that peripheral blood CD4+ T cells respond to S. Typhi by secreting single or multiple cytokines simultaneously (12–16). However, it is unknown whether TI S. Typhi-specific CD4+ T-cell responses exhibit multifunctionality, i.e. the ability to express more than one function (e.g. IFNγ) concomitantly. Thus, we next investigated the multifunctionality of LPMC S. Typhi-specific CD4+ TEM responses in Ty21a-vaccinated and unvaccinated volunteers. Using WinList’s FCOM function, TI-LPMC CD4+ TEM responses were analyzed for multiple cytokines/chemokines (IFNγ, TNFα, IL-2, IL-17A and MIP1β) and/or CD107a expression (64 possible combinations) and characterized S. Typhi-specific responding cells as either single cytokine producers/CD107a expressors (S) or multifunctional (sum of double, triple, quadruple, quintuple or sextuple cytokine producers/CD107a expressors) (MF). First, we analyzed LPMC CD4+ TEM responses associated with expression of CD107a, a cytotoxic marker (32) (Fig. 5A). Interestingly, in unvaccinated volunteers, responses had a tendency to be mostly CD107a+ CD4+ TEM MF rather than CD107a+ CD4+ TEM S (Fig. 5A). However, no significant differences were observed between the levels of CD107a+ CD4+ TEM S and MF cells following Ty21a immunization (Fig. 5A).
Fig. 5.
Single and multifunctional net Salmonella Typhi-specific LPMC CD69+ CD4+ TEM cells following oral Ty21a immunization. Following stimulation with autologous S. Typhi-infected and uninfected EBV-B targets and using the FCOM function of WinList, net S. Typhi-specific CD4+ TEM responses were segregated into single positive effectors (S; producing only one cytokine or expressing just CD107a) and multifunctional (MF; simultaneously producing two, three, four or five cytokines and/or expressing CD107a). Comparison of TI-LPMC CD4+ TEMS. Typhi-specific responses as measured by (A) CD107a+, (B) INFγ+, (C) TNFα+, (D) IL-17A+, (E) IL-2+ and (F) MIP1β+ MF and S were examined in Ty21a-vaccinated (n = 15; red symbols) and unvaccinated volunteers (n = 20; black symbols) with significant differences shown (*P < 0.05; **P < 0.005). Horizontal black bars represent median values.
Next, we examined the IFNγ responses for multifunctionality in Ty21a vaccinees and controls. Similar to the CD107a response, IFNγ responses were mostly MF in both volunteer groups but the levels of IFNγ+ CD4+ TEM MF in Ty21a-vaccinated volunteers displayed a trend (P < 0.13) to show higher frequencies than in unvaccinated volunteers (Fig. 5B). Similar observations were made for IL-2 (Fig. 5E) and MIP1β (Fig. 5F), where the frequencies of IL-2+ and MIP1β+ CD4+ TEM MF were significantly (P < 0.05) higher in Ty21a vaccinees than in unvaccinated volunteers (Fig. 5E and F). In contrast, CD4+ TEM TNFα+ responses were significantly higher as single (S) effectors following Ty21a immunization (Fig. 5C). Remarkably, the responses associated with IL-17A production showed significant increases in S. Typhi-specific IL-17A+ MF and S in Ty21a-vaccinated than in unvaccinated volunteers (Fig. 5D).
In addition, we analyzed individual IL-17A-associated MF subsets and observed significantly higher percentages of responders in LPMC CD69+ CD4+ TEM double (IL-2+ IL-17A+) (P < 0.05), and quadruple (CD107a+ IL-2+ IL-17A+ MIP1β+) (P < 0.0005) positive subsets following Ty21a immunization (Supplementary Figure S3A). We also analyzed individual CD107a-associated responses and found significantly higher level of quadruple (CD107a+ IL-2+ IL-17A+ MIP1β+) (P < 0.0005) following Ty21a immunization (Supplementary Figure S3B).
Multifunctional TI-LPMC CD4+-TEM responses following stimulation with soluble antigens
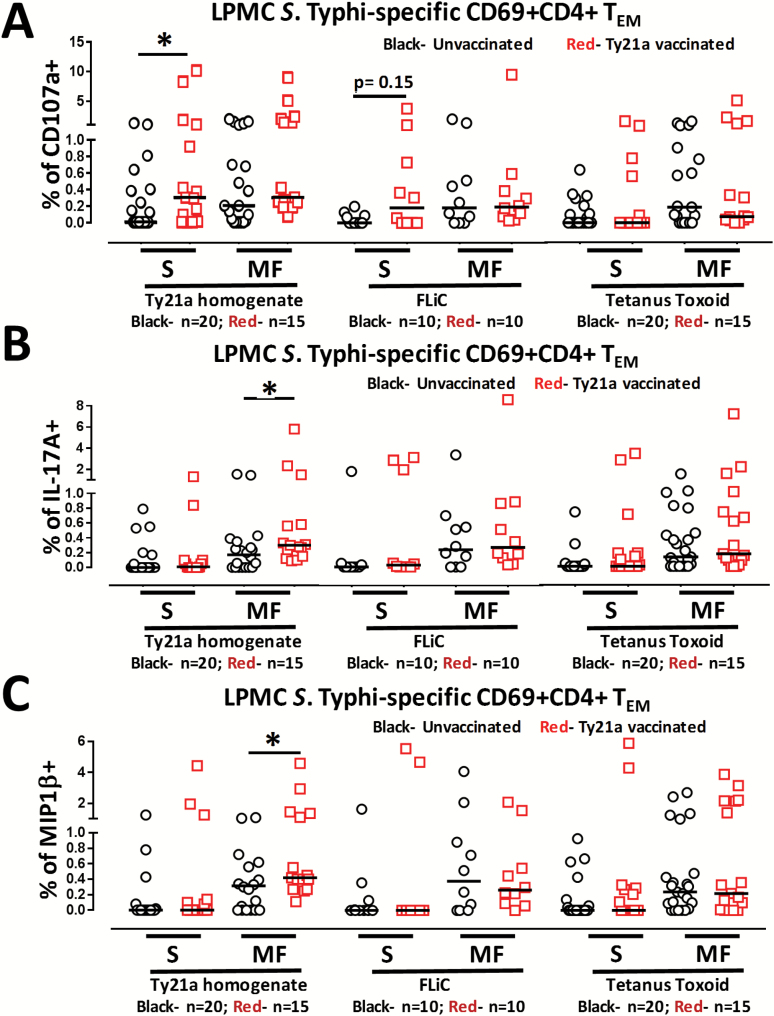
Our group and others have observed that CMI against S. Typhi mediated by CD4+ T cells appears to depend on the nature of the stimulant (12–16). For example, CD4+ T cells seem more prone to respond to S. Typhi soluble antigens than to infected targets (23). We therefore examined whether LPMC CD4+ TEM obtained from Ty21a-vaccinated and unvaccinated volunteers will respond differently following stimulation with S. Typhi soluble antigens (Ty21a homogenate or FliC) or to an unrelated antigen, TT. Using the FCOM function of WinList, we analyzed and stratified the data into S and MF effectors as described above. First, we analyzed LPMC CD4+ TEM responses associated with expression of CD107a, a cytotoxic marker (32) (Fig. 6A). Interestingly, stimulation of LPMC CD4+ TEM with Ty21a homogenate (10 μg ml−1) resulted in significant (P < 0.05) expression of CD107a+ S in Ty21a-vaccinated than in unvaccinated volunteers (Fig. 6A). A similar trend (P = 0.15) to show increases in the expression of CD107a+ S in LPMC CD4+ TEM obtained from Ty21a-vaccinated than unvaccinated volunteers was noted following stimulation with FliC but not with TT (Fig. 6A). Next, we examined the IL-17A responses for multifunctionality in Ty21a vaccinees and controls following stimulation with soluble antigens. In contrast to the CD107a responses, IL-17A responses were mostly MF in both groups of volunteers, but the levels of IL-17A+ CD4+ TEM MF in Ty21a-vaccinated volunteers were significantly higher than in unvaccinated volunteers following stimulation with the Ty21a homogenate, but not with FliC or TT (Fig. 6B). Similar observations were made for MIP1β (Fig. 6C), where the frequencies of MIP1β+ CD4+ TEM MF were significantly (P < 0.05) higher in Ty21a vaccinees than in unvaccinated volunteers following stimulation with Ty21a homogenate, but not with FliC or TT (Fig. 6C). In addition, we examined IFNγ, TNFα and IL-2 multifunctional responses following stimulation with soluble antigens. We observed significant increase in only CD69+ CD4+ TEM IFNγ+ MF (Supplementary Figure S4A) obtained from Ty21a-vaccinated than in unvaccinated volunteers following stimulation with Ty21a homogenate but not with FliC or TT. Of note, no significant differences were observed in the levels of IL-2+ and TNFα+ CD4+ TEM S and MF cells between Ty21a vaccinees and unvaccinated volunteers following stimulation with soluble antigens (Supplementary Figure S4B and C).
Fig. 6.
Single and multifunctional Salmonella Typhi-specific LPMC CD69+ CD4+ TEM responses to soluble antigens following oral Ty21a immunization. LPMC CD4+ TEM were stimulated with a Ty21a homogenate (10 μg ml−1), FliC (10 μg ml−1) or TT (10 μg ml−1) and the net S. Typhi-specific CD4+ TEM responses were calculated using the FCOM function of WinList segregating them into single positive effectors (S; producing only one cytokine or expressing just CD107a) and multifunctional (MF; simultaneously producing two, three, four or five cytokines and/or expressing CD107a). Comparison of TI-LPMC CD4+ TEMS. Typhi-specific responses as measured by (A) CD107a+, (B) IL-17A+ and (C) MIP1β+ MF and S were evaluated in Ty21a-vaccinated (red symbols) and unvaccinated volunteers (black symbols) with significant differences (*P < 0.05) and P-value for trends shown. Horizontal black bars represent median values.
Mucosal S. Typhi-specific CD4+ TM subsets (TEM, TCM, TEMRA) responses are different from their systemic counterparts
Recent findings have indicated that the immune responses at the site of infection are distinct from those in peripheral blood (28, 29, 36, 37). We have previously reported that TI CD8+ TM cells respond differently than their systemic counterparts (9). Based on these observations, we hypothesized that the specific immune responses elicited in the TI for S. Typhi CD4+ TM would differ in magnitude and characteristics from their blood counterparts. To directly address this hypothesis, we sampled simultaneously blood and TI biopsies from each individual and used the exact stimulation protocol (same infected and uninfected EBV-B cells) to determine LPMC and PBMC CD4+ TMS. Typhi-specific responses. Mucosal and systemic net S. Typhi-specific CD69+ CD4+ TM subset (TEM, TCM and TEMRA) responses were compared in Ty21a-vaccinated and in unvaccinated volunteers (Supplementary Table S2). In unvaccinated individuals, LPMC CD69+ CD4+ TEM showed trends to exhibit higher expression of CD107a and produced higher levels of IL-2 than their blood counterparts (Supplementary Table S2). Following Ty21a immunization, LPMC CD69+ CD4+ TEM exhibited trends (P < 0.1) to produce higher levels of IL-17A and to express higher levels of CD107a than their PBMC counterparts (Supplementary Table S2). Interestingly, LPMC CD69+ CD4+ TCM produced significantly more IL-17A (P < 0.05) and expressed significantly (P < 0.005) higher frequencies of CD107a than their blood counterparts in unvaccinated volunteers (Supplementary Table S2). In contrast, following Ty21a vaccination, LPMC CD69+ CD4+ TCM exhibited a trend to produce higher levels of IFNγ and MIP1β and expressed significantly more CD107a (P < 0.005) than their PBMC counterparts (Supplementary Table S2). Finally, we compared the responses of LPMC and PBMC CD69+ CD4+ TEMRA in unvaccinated and Ty21a-vaccinated individuals. We observed no significant differences in cytokine/chemokine production (IFNγ, TNFα, IL-17A, IL-2 and MIP1β) between LPMC and PBMC but significantly higher expression of CD107a (P < 0.05) in LPMC than PBMC in both Ty21a-vaccinated and unvaccinated volunteers (Supplementary Table S2).
Mucosal S. Typhi-specific multifunctional CD4+ TEM responses are different from their systemic counterparts
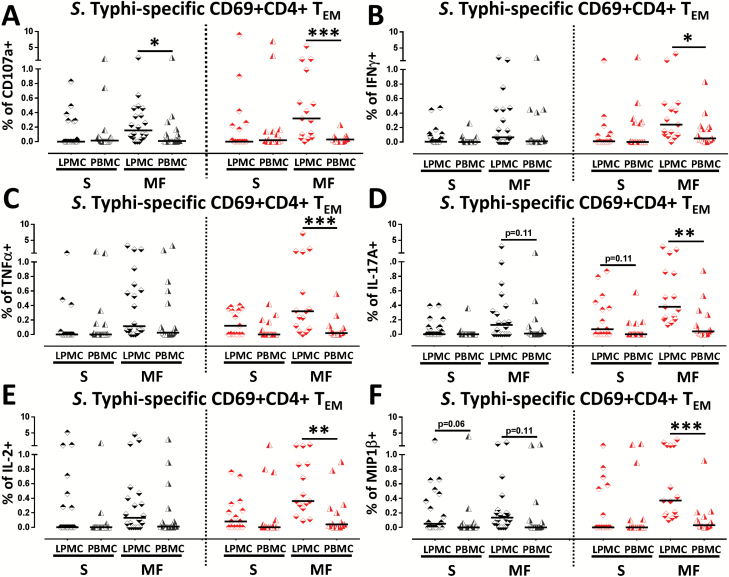
We have showed above (Result section showing differences between LPMC and PBMC CD4+ TMS. Typhi responses) that TI CD69+ CD4+ TEM cells obtained from unvaccinated and Ty21a vaccinees exhibited trends (P < 0.1) toward exhibiting higher levels of IL-17A or IL-2 production and cytotoxic potential (CD107a) than their corresponding blood counterparts. In addition, we have observed that CD4+ TEM is the predominant TM subset in the TI mucosa. Thus, we deemed it important to further stratify these responses into those exhibiting a single function and those that were multifunctional. We hypothesized that CD4+ TEM responses will differ between the two tissues in terms of quality and quantity. Hence, we analyzed the differences in CD4+ TEM responses between LPMC and PBMC by detailing their multifunctionality. Using the multifunctionality strategy described above, we stratified net S. Typhi-specific responses into multifunctional (MF) or single (S) responses for both LPMC and PBMC in Ty21a-vaccinated and unvaccinated volunteers. Interestingly, the frequencies of CD4+ CD107a+ TEM MF were significantly higher (P < 0.05) in LPMC than in PBMC in both Ty21a-vaccinated and unvaccinated volunteers (Fig. 7A). Of note, no significant differences in CD4+ CD107a+ TEM S were observed between LPMC and PBMC in unvaccinated or following Ty21a immunization (Fig. 7A). Thus, the magnitude of CD107a responses in LPMC CD4+ TEM is different from PBMC. In contrast, the frequencies of CD4+ TEM IFNγ+ (S or MF) were similar between TI-LPMC and PBMC obtained from unvaccinated volunteers (Fig. 7B), while in Ty21a-vaccinated volunteers, significantly higher frequencies of CD4+ TEM IFNγ+ MF were observed in LPMC than in PBMC (Fig. 7B). In contrast, no differences were noted in CD4+ TEM IFNγ+ S between LPMC and PBMC following Ty21a immunization (Fig. 7B). A similar analysis was performed for S. Typhi-specific TNFα (Fig. 7C) and IL-2 (Fig. 7E) associated responses whereby no significant differences were noted in CD69+ CD4+ TEM TNFα+ and IL-2+ MF and S between LPMC and PBMC in unvaccinated volunteers. However, following Ty21a immunization, a significant increase in CD69+ CD4+ TEM TNFα+ (P < 0.0005) (Fig. 7C) and IL-2+ MF (P < 0.005) (Fig. 7E) was observed in LPMC compared to PBMC. Interestingly, for IL-17A responses, we observed that the frequencies of CD4+ TEM IL-17A+ MF, but not S exhibited a trend (P = 0.11) to show higher responses in LPMC than in PBMC obtained from unvaccinated volunteers (Fig. 7D). However, following Ty21a immunization, we found that both CD4+ TEM IL-17A+ MF (P < 0.005) and CD4+ TEM IL-17A+ S (P = 0.1) were higher in LPMC compared to PBMC (Fig. 7D). Thus, these results suggest that IL-17A responses in LPMC CD4+ TEM differ from their PBMC counterparts in both magnitude and characteristics. Remarkably, in unvaccinated volunteers, the frequencies of CD4+ TEM MIP1β+ MF (P = 0.1) and S (P = 0.06) showed trends to exhibit higher responses in LPMC than in PBMC (Fig. 7F). However, following Ty21a immunization, we observed that significantly (P < 0.0005) higher frequencies of CD4+ TEM MIP1β+ MF were present in LPMC than PBMC (Fig. 7F). No significant differences were noted in CD4+ TEM MIP1β+ S following Ty21a vaccination (Fig. 7F). These data suggest that CD4+ TM responses at the TI mucosa are different from peripheral blood.
Fig. 7.
Mucosal and systemic single and multifunctional Salmonella Typhi-specific CD69+ CD4+ TEM responses following oral Ty21a immunization. TI-LPMC and PBMC were stimulated in parallel with autologous S. Typhi-infected and uninfected EBV-B overnight. Net S. Typhi-specific CD4+ TEM multifunctional (MF; simultaneously producing two, three, four or five cytokines and/or expressing CD107a) and single positive (S; producing only one cytokine or expressing just CD107a) effectors were evaluated and compared between TI-LPMC and PBMC obtained from Ty21a-vaccinated (n = 15; red symbols) and unvaccinated volunteers (n = 20; black symbols). Responses measured by (A) CD107a+, (B) INFγ+, (C) TNFα+, (D) IL-17A+, (E) IL-2+ and (F) MIP1β+ are shown. Significant differences (*P < 0.05; **P < 0.005; ***P < 0.0005) and P-value for trends are indicated. Horizontal black bars represent median values.
Discussion
CD4 T cells are crucial for the generation of vaccine-mediated immune responses which are likely to contribute to effective protective immunity against a multitude of pathogens. Furthermore, it is becoming clear that the immune responses in tissues differ from those in peripheral blood. Thus, it is imperative to evaluate T-cell-mediated (T-CMI) responses at the site of infection as part of vaccine development efforts. Here, we determined the effect of oral immunization with the attenuated oral typhoid vaccine Ty21a on CD4+ TMS. Typhi-specific responses in human TI-LPMC and PBMC. We showed that the effect of oral Ty21a immunization at the terminal ileum mucosa, the preferred site of infection for S. Typhi (5, 38) resulted in: (i) increased of CD4+ T-cell frequencies, (ii) influence the homing and accumulation of effectors and (iii) induces LPMC S. Typhi-responsive CD4+ T multifunctional (IL-17A, IL-2 and/or MIP1β) cells. Specifically, we observed that all major CD4+ TM subsets (TEM, TCM and TEMRA) are activated and appear to display distinct response profiles (TEM: trends to show increases in IFNγ and IL-17A production; TCM: significant increases in IFNγ and MIP1β production; TEMRA: trends to show increases in IL-2 production) in the TI mucosa following Ty21a immunization. Moreover, we demonstrated that TI-LPMC CD4+ TMS. Typhi-specific responses were multifunctional and different to those present in their systemic counterparts following Ty21a immunization. Taken together, these results contribute important novel information to the immune responses elicited following oral Ty21a immunization in TI mucosal responses in humans, which could have significant implications in future vaccine design and development.
Herein we provide the first direct evidence that oral Ty21a immunization elicits S. Typhi-specific LPMC CD4+ T multifunctional responses (IL-17A, IL-2 and/or MIP1β) in the TI. Interestingly, it was recently shown that oral Ty21a immunization generates multifunctional S. Typhi-responsive CD4+ T cells obtained from human duodenum biopsies but not from CD4+ T cells isolated from human colon biopsies (7). Taken together, these data indicate that human intestinal responses are compartmentalized following oral Ty21a immunization. However, the magnitude and characteristics of S. Typhi-responsive CD4+ T cells appear to be different between duodenum and TI. Based on the frequencies of CD4+ T cells producing IFNγ, TNFα and IL-2 from both studies, TI CD4+ T responses appear to be lower in magnitude, but exhibited marked differences in their characteristics (e.g. S versus MF), when compared to those in the duodenum. However, it is unclear whether this and other inconsistencies are the result of differences in the antigens used to stimulate cells isolated from duodenal and TI biopsies. For example, it is possible that the use of Ty21a-killed bacteria, instead of the S. Typhi-infected autologous targets or Ty21a homogenate employed in the present study, favored the detection of CD4+ responses in the duodenum (7). Of importance, following immunization we observed only trends in the induction of CD4+ TEM subsets while we observed significant responses when analyzing the data for S and MF CD4+ TEM effectors. This suggests that studying heterogeneous CD4+ T populations may not reveal the ‘true’ impact of immunization because of the ‘averaging effect’ inherent to the analyses of whole populations/subsets composed of responding and non-responding cells, each with their own characteristics. Thus, when analyzing immunity elicited by vaccination it is essential to focus on the fine granularity (e.g. S. Typhi-specific TM subsets, whether the responses are S versus MF, which combinations of cytokines are produced following antigenic stimulation) to better characterize the responses and properly study differences between immune compartments. We here demonstrated that oral Ty21a immunization elicited local TI-LPMC CD4+ T cells that respond specifically to S. Typhi antigens through effector mechanisms (e.g. IL-17) that might be well suited for protection against intracellular pathogens.
Remarkably, we also noted that several of the unvaccinated volunteers showed relatively high level of baseline S. Typhi-specific CD4+ T responses. These differences in baseline responses could be due to cross-reactive in memory responses elicited by previous exposure to other Salmonella serovars (39–41) or other Enterobacteriaceae, including those present in the normal gut microbiota (12, 42–44). The importance of the gut microbiota in modulating host immune responses to pathogens or to vaccination has been demonstrated previously (42–44). Furthermore, genetic determinants like HLA molecules can also be important in defining the variation in immune responses. For example, the presence of the HLA-DRB1*04:05 allele was recently associated with protection against S. Typhi (27). Interestingly, higher baseline levels of multifunctional S. Typhi-specific CD4+ T cells might well play a role in the protection of disease similar to what was observed with baseline CD8+ T cells in an oral challenge model with wt S. Typhi in humans (45, 46).
Our observations that oral Ty21a elicited higher frequencies of TI CD4+ T cells mainly in the TEMRA subsets are intriguing. However, increases in the frequencies of these subsets were not necessarily accompanied by increases in their functional properties. For example, increases in the frequencies of TEMRA did not result in increases in the cytokines they produced (except for IL-2 which exhibited a trend to show higher levels in Ty21a vaccinees). However, the observation that TEMRA increased in number suggests that they are induced by Ty21a immunization and might play a role in protection.
We speculate that the most likely scenario is that Ty21a immunization induces localized inflammation, thereby triggering proliferation and differentiation of S. Typhi-specific CD4+ TM and enhanced recruitment of effector cells to the site of infection. We hypothesize that the increased frequencies in the TI-LPMC CD4+ TEMRA subset may be a result of recruitment of these TEMRA cells from circulation or that CD4+ TEM were elicited to proliferate and differentiate into TEMRA in the local microenvironment. Remarkably, the patterns of homing markers (integrin α4β7 and CCR9) displayed simultaneously in isolated TI-LPMC and PBMC CD4+ T cells were distinct. We observed that integrin α4β7+ CD4+ T cells are significantly decreased in PBMC but not in LPMC following Ty21a immunization. Furthermore, CCR9+ CD4+ or integrin α4β7+ CCR9+ CD4+ LPMC T cells were increased in the TI, suggesting that S. Typhi-specific cells are recruited and accumulate in the mucosa. These observations argue in favor of integrin α4β7-driven recruitment and retention of specific CD4+ TM in the mucosa, likely associated with a potential down-regulation of integrin α4β7+ CD4+ T cells in the local microenvironment (28, 47). Our observations together with data supporting the induction of S. Typhi-specific responses in the duodenum but not the colon following Ty21a immunization suggest that integrin α4β7+/α4β7+ CCR9+ and CCR9+ CD4+ T cells may play a crucial role in anti-S. Typhi immunity in the human small intestine.
We have demonstrated that all major CD4+ TM subsets (TEM, TCM and TEMRA) are elicited in the TI mucosa following Ty21a immunization. However, each CD4+ TM subset appears to display unique response profiles (e.g. trends by CD4+ TEM to exhibit increases in the production of IFNγ and IL-17A) and CD4+ TEM MF cells predominate, as we have previously shown in peripheral blood (20). Of note, the frequencies of LPMC CD4+ TCM and TEMRA (10–30% and 20–40%, respectively) are significantly lower than CD4+ TEM (45–80%) in the TI mucosa. Taken together, these data suggest that LPMC CD4+ TEM and TCM (by producing increased levels of IFNγ and MIP1β) may contribute to the induction of Th1 and Th17 effectors while CD4+ TEMRA which exhibited a trend to produce higher levels of IL-2 may be required for expansion of antigen-specific CD4+ T-cell populations and maintenance of regulatory T cells (Treg) following Ty21a immunization or challenge with wt S. Typhi (48).
Generation of CD4+ TMS. Typhi-specific responses is likely to be dependent on the type of antigenic stimulation, as shown by the exposure of LPMC CD4+ TEM to either soluble antigens (e.g. Ty21a homogenate or flagella) or autologous infected targets (e.g. S. Typhi-EBV-B) in our studies. We noted that CD4+ TEMS. Typhi-specific IFNγ MF, TNFα S, MIP1β MF and IL-17A MF were similarly elicited by stimulation with either targets or soluble antigens following Ty21a immunization. In contrast, the potential to be cytotoxic (CD107 expression; which showed significant increases with soluble antigen) and production of IL-2 (which exhibited significant increases with infected targets) seems to be dependent on the type of stimulation. Therefore, CD4+ TEM responses against S. Typhi seem to display a core response in terms of Th1 (IFNγ, TNFα and/or MIP1β) and Th17 (IL-17A) but may be dependent on the type of stimulation for other effector responses (IL-2 and cytotoxic).
A recent study using a human infection model with wt S. Typhi has identified important components of the CD4+ T-cell responses selectively targeting S. Typhi (26). This study suggests that only the bacterial antigens expressed in the infected tissues are targeted during the CD4+ T-cell responses to Salmonella. Thus, it appears that antigens present in the tissues during Salmonella infection dictate the antigen-specific repertoire of CD4+ T cells consisting of cross-reactive and serovar-specific T-cell clonotypes. Interestingly, these authors also showed that the identified circulating CD4+ effector cells (CD4+CD38+CCR7−) expressed gut homing markers CD49d and integrin β7 (26), suggesting that these cells migrate to the site of infection in the intestine. These data are concordant with our observations that circulating CD4+ effectors are recruited from blood and accumulate in the gut mucosa. These cells, together with resident CD4+ T cells, might play an important role in protection against S. Typhi infection. Future studies are required to examine whether wt infection or immunization influences the antigen-specific repertoire as well as the frequency of Salmonella-responsive tissue-resident CD4+ T cells.
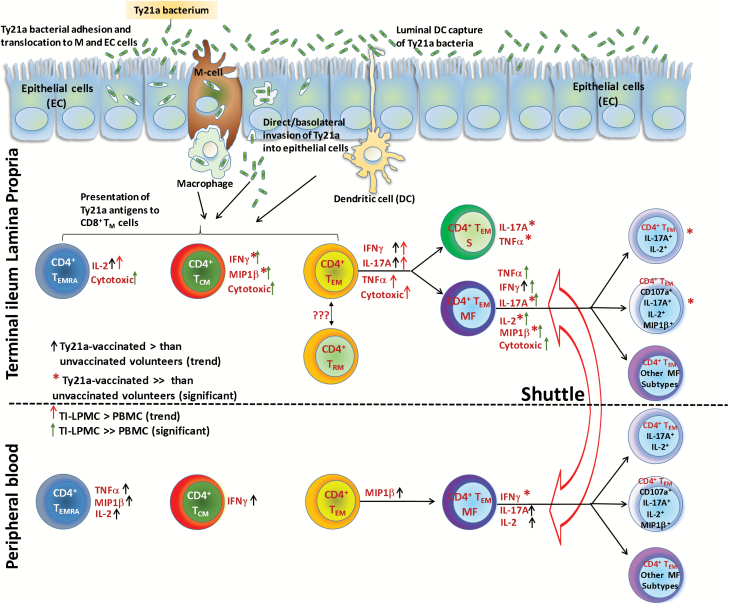
Salmonella Typhi-specific multifunctional (MF) T cells have been observed in blood in response to various vaccines, including Ty21a (12, 13, 15). Of importance, the induction of MF cells has been associated with controlling a variety of viral and bacterial pathogens (49, 50). We now show that CD4+ TEM MF cells are induced in the TI mucosa following Ty21a immunization. For all cytokines/chemokine and cytotoxic responses evaluated (IFNγ, TNFα, IL-17A, IL-2, MIP1β and/or CD107a), MF CD4+ TEM cells are dominant in the TI mucosa and are of higher magnitude than their PBMC counterparts regardless of Ty21a immunization. These observations argue that the TI is a major reservoir of S. Typhi-responsive multifunctional CD4+ TM cells, and that the frequencies of these cells increase further following Ty21a vaccination. In addition, these data confirm and extend our previous findings that some volunteers respond specifically to S. Typhi before Ty21a vaccination (9, 45, 46). Finally, another notable observation is that following Ty21a immunization, LPMC CD4+ TEM S responses (TNFα and IL-17A) are preferentially induced in the local microenvironment as opposed to the responses observed in peripheral blood. These and other differences noted in the present studies suggest that TI-LPMC CD4+ TEM exhibit unique patterns of S. Typhi-specific responses compared to their systemic counterparts. This is an important observation as most human studies depend on data acquired from the systemic compartment and largely assumed that these peripheral blood responses impact directly those present in the mucosal compartment (9, 36). Consequently, interpretation of immunological response data obtained from blood may not fully be representative of responses at the site of infection and may be different at various times following immunization. In Fig. 8, we include a cartoon summarizing the multitude of responses observed in these studies, both in peripheral blood and TI.
Fig. 8.
Cartoon depicting Salmonella Typhi-specific CD4+ TM responses elicited in the TI mucosa and peripheral blood (PBMC) following Ty21a immunization in humans. Following oral immunization with the attenuated vaccine strain Ty21a, these bacteria enter the host by various mechanisms (e.g. M-cell adhesion, epithelial invasion and capture by luminal dendritic cells) and are presented by antigen-presenting cells (APC, i.e. macrophages, dendritic cells) to immune cells (i.e. CD4+ TM) in the lamina propria (LP) compartment. CD4+ TM are subsequently activated to produce higher levels of cytokines/chemokines (IFNγ, TNFα, IL-2, IL-17A and MIP1β) and increased cytotoxicity (up-regulation of CD107a). Following Ty21a immunization each major CD4+ TM (TEM, TCM and TEMRA) subset acquires unique characteristics in the TI-LP. CD4+ TEM responses include single producing effector cells (S) and multifunctional cells (MF). LPMC CD4+ TEM TNFα and IL-17A responses were observed both as S and MF cells while the other responses were observed only as MF cells. The relationship between mucosal and systemic immunity focused on CD4+ TEM responses is also depicted in this cartoon. Following Ty21a immunization, PBMC CD4+ TEM are modulated to produce cytokine responses (IFNγ, IL-17A and IL-2). These responses are almost exclusively as MF cells rather than single producing (S) cells. Of importance, the magnitude of PBMC CD4+ TEM responses is significantly lower than that of LPMC CD4+ TEM as denoted by the green arrows in the LPMC compartment (↑). These results suggest that only CD4+ TEM IL-17A+ S effectors elicited by Ty21a immunization might have the capacity to shuttle between the TI mucosa and peripheral blood or that these cells become MF once they are in the gut mucosa. CD4+ TEM in the TI can be composed of various subsets including tissue-resident memory T cells (TRM) and other CD4+ T-cell subsets. Additionally, CD4+ TEM MF represent 64 different combinations of effector subtypes defined by the expression of CD107a, IFNγ, IL-17A, TNFα, IL-2 and/or MIP1β, including doublet to sextuplet subtypes. This adds another layer of complexity in defining effector responses. This is illustrated in the figure by showing, for example, CD4+ TEM MF quadruplets (subtype CD107a+, IL-17A+, IL-2+ and MIP1β+) which exhibited significantly higher responses in Ty21a-vaccinated than unvaccinated volunteers. Significantly higher responses in Ty21a vaccinees than in unvaccinated volunteers are denoted with red asterisks (*). Trends toward Ty21a vaccinees exhibiting higher responses than unvaccinated volunteers are denoted with black arrows (↑). Significantly higher responses in TI-LPMC compared to PBMC are denoted with green arrows (↑). Trends toward TI-LPMC showing higher responses than PBMC are denoted with red arrows (↑).
In conclusion, we have demonstrated that oral Ty21a immunization elicits S. Typhi-specific CD4+ TM responses in the TI mucosa with distinct effector functions and characteristics that are unique, overlapping only partially with those observed in the systemic compartment (Fig. 8). Additionally, our data offer major insights into the S. Typhi-specific CD4+ TM responses elicited in the TI mucosa and suggest that these responses are the result of local immunomodulatory mechanisms capable of influencing T cell activation, expansion and differentiation, resulting in unique phenotypes and perhaps specificities than those in the systemic compartment.
Funding
This work was supported by NIAID, NIH, DHHS grants R01-AI036525, U19-AI082655 [Cooperative Center for Human Immunology (CCHI)] and U19-AI109776 [Center of Excellence for Translational Research (CETR)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Supplementary Material
Acknowledgements
We are indebted to the volunteers who allowed us to perform this study. We thank the staff from the Clinical Studies Group of the Center for Vaccine Development for their help in collecting TI biopsies and blood specimens; Mr Jeffery Floyd for isolating PBMC and Ms Regina Harley and Catherine Storrer for excellent technical assistance in the performance of the flow cytometric determinations. J.S.B. performed the experiments, contributed to study design, acquisition of data, analysis and drafting of the manuscript; R.S.B. contributed to patient recruitment, collection of PBMC and TI biopsies and reviewed the manuscript; E.G. and S.A.P. performed endoscopies, obtained TI biopsies and reviewed the manuscript; B.D.G., performed endoscopies, obtained TI biopsies and reviewed the manuscript; M.B.S. designed the study, supervised the work and drafted the manuscript.
Conflicts of interest statement: The authors declared no conflicts of interest.
References
- 1. Bhutta Z. A. and Threlfall J. 2009. Addressing the global disease burden of typhoid fever. JAMA 302:898. [DOI] [PubMed] [Google Scholar]
- 2. Crump J. A. Luby S. P. and Mintz E. D. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346. [PMC free article] [PubMed] [Google Scholar]
- 3. Crump J. A. and Mintz E. D. 2010. Global trends in typhoid and paratyphoid Fever. Clin. Infect. Dis. 50:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine M. M. 2018. Typhoid fever vaccines. In Plotkin S. A., Orenstein W. A., Offit P. A. and Edwards K. M, eds., Plokin’s vaccines, p. 1114 Elsevier, Inc, Philadelphia, PA. [Google Scholar]
- 5. Parry C. M. Hien T. T. Dougan G. White N. J. and Farrar J. J. 2002. Typhoid fever. N. Engl. J. Med. 347:1770. [DOI] [PubMed] [Google Scholar]
- 6. Ukwenya A. Y. Ahmed A. and Garba E. S. 2011. Progress in management of typhoid perforation. Ann. Afr. Med. 10:259. [DOI] [PubMed] [Google Scholar]
- 7. Pennington S. H., Thompson A. L., Wright A. K., et al. 2016. Oral typhoid vaccination with live-attenuated Salmonella Typhi strain Ty21a generates Ty21a-responsive and heterologous influenza virus-responsive CD4+ and CD8+ T cells at the human intestinal mucosa. J. Infect. Dis. 213:1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lundin B. S. Johansson C. and Svennerholm A. M. 2002. Oral immunization with a Salmonella enterica serovar Typhi vaccine induces specific circulating mucosa-homing CD4(+) and CD8(+) T cells in humans. Infect. Immun. 70:5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Booth J. S., Patil S. A., Ghazi L., et al. 2017. Systemic and terminal ileum mucosal immunity elicited by oral immunization with the Ty21a typhoid vaccine in humans. Cell. Mol. Gastroenterol. Hepatol. 4:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferreccio C. Levine M. M. Rodriguez H. and Contreras R. 1989. Comparative efficacy of two, three, or four doses of TY21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. J. Infect. Dis. 159:766. [DOI] [PubMed] [Google Scholar]
- 11. Guzman C. A., Borsutzky S., Griot-Wenk M. et al. 2006. Vaccines against typhoid fever. Vaccine 24:3804. [DOI] [PubMed] [Google Scholar]
- 12. Sztein M. B. Salerno-Goncalves R. and McArthur M. A. 2014. Complex adaptive immunity to enteric fevers in humans: lessons learned and the path forward. Front. Immunol. 5:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salerno-Gonçalves R., Wyant T. L., Pasetti M. F., et al. 2003. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain CVD 908-htrA. J. Immunol. 170:2734. [DOI] [PubMed] [Google Scholar]
- 14. Sztein M. B. 2007. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica serovar Typhi strains used as live oral vaccines in humans. Clin. Infect. Dis. 45(Suppl. 1):S15. [DOI] [PubMed] [Google Scholar]
- 15. Wahid R. Fresnay S. Levine M. M. and Sztein M. B. 2016. Cross-reactive multifunctional CD4+ T cell responses against Salmonella enterica serovars Typhi, Paratyphi A and Paratyphi B in humans following immunization with live oral typhoid vaccine Ty21a. Clin. Immunol. 173:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salerno-Gonçalves R., Tettelin H., Lou D., et al. 2017. Use of a novel antigen expressing system to study the Salmonella enterica serovar Typhi protein recognition by T cells. PLoS Negl. Trop. Dis. 11:e0005912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wahid R. Salerno-Gonçalves R. Tacket C. O. Levine M. M. and Sztein M. B. 2008. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue- homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol. 1:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salerno-Goncalves R. Pasetti M. F. and Sztein M. B. 2002. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J. Immunol. 169:2196. [DOI] [PubMed] [Google Scholar]
- 19. Salerno-Gonçalves R. Wahid R. and Sztein M. B. 2005. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect. Immun. 73:3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salerno-Goncalves R. Wahid R. and Sztein M. B. 2010. Ex vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin. Vaccine Immunol. 17:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wahid R. Fresnay S. Levine M. M. and Sztein M. B. 2015. Immunization with Ty21a live oral typhoid vaccine elicits crossreactive multifunctional CD8+ T-cell responses against Salmonella enterica serovar Typhi, S. Paratyphi A, and S. Paratyphi B in humans. Mucosal Immunol. 8:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sztein M. B. Tanner M. K. Polotsky Y. Orenstein J. M. and Levine M. M. 1995. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella Typhi in humans. J. Immunol. 155:3987. [PubMed] [Google Scholar]
- 23. Wahid R. Salerno-Gonçalves R. Tacket C. O. Levine M. M. and Sztein M. B. 2007. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine 25:1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reynolds C. J., Jones C., Blohmke C. J., et al. 2014. The serodominant secreted effector protein of Salmonella, SseB, is a strong CD4 antigen containing an immunodominant epitope presented by diverse HLA class II alleles. Immunology 143:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheikh A., Khanam F., Sayeed M. A., et al. 2011. Interferon-γ and proliferation responses to Salmonella enterica serotype Typhi proteins in patients with S. Typhi bacteremia in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 5:e1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Napolitani G., Kurupati P., Teng K. W. W. et al. 2018. Clonal analysis of Salmonella-specific effector T cells reveals serovar-specific and cross-reactive T cell responses. Nat. Immunol. 19:742. [DOI] [PubMed] [Google Scholar]
- 27. Dunstan S. J., Hue N. T., Han B., et al. 2014. Variation at HLA-DRB1 is associated with resistance to enteric fever. Nat. Genet. 46:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Booth J. S., Toapanta F. R., Salerno-Goncalves R., et al. 2014. Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Front. Immunol. 5:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Booth J. S., Salerno-Goncalves R., Blanchard T. G., et al. 2015. Mucosal-associated invariant T cells in the human gastric mucosa and blood: role in Helicobacter pylori infection. Front. Immunol. 6:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salerno-Gonçalves R. Fernandez-Viña M. Lewinsohn D. M. and Sztein M. B. 2004. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J. Immunol. 173:5852. [DOI] [PubMed] [Google Scholar]
- 31. Van Dissel J. T. Kwappenberg K. and Van Furth R. 1995. S. Typhi vaccine strain Ty21a can cause a generalized infection in whole body-irradiated but not in hydrocortisone-treated mice. Scand. J. Immunol. 41:457. [DOI] [PubMed] [Google Scholar]
- 32. Alter G. Malenfant J. M. and Altfeld M. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15. [DOI] [PubMed] [Google Scholar]
- 33. Wasserstein R. L. L., Lazer N. A. 2016. The ASA’s statement on p-values: context, process, and purpose. Am. Stat. 70:129. [Google Scholar]
- 34. Yaddanapudi L. N. 2016. The American Statistical Association statement on P-values explained. J. Anaesthesiol. Clin. Pharmacol. 32:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kantele A. Zivny J. Häkkinen M. Elson C. O. and Mestecky J. 1999. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 162:5173. [PubMed] [Google Scholar]
- 36. Jozwik A., Habibi M. S., Paras A., et al. 2015. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 6:10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang O. O., Ibarrondo F. J., Price C., et al. 2014. Differential blood and mucosal immune responses against an HIV-1 vaccine administered via inguinal or deltoid injection. PLoS One 9:e88621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma A. Sharma R. Sharma S. Sharma A. and Soni D. 2013. Typhoid intestinal perforation: 24 perforations in one patient. Ann. Med. Health Sci. Res. 3(Suppl. 1):S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McClelland M., Sanderson K. E., Spieth J., et al. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852. [DOI] [PubMed] [Google Scholar]
- 40. Wahid R. Simon R. Zafar S. J. Levine M. M. and Sztein M. B. 2012. Live oral typhoid vaccine Ty21a induces cross-reactive humoral immune responses against Salmonella enterica serovar Paratyphi A and S. Paratyphi B in humans. Clin. Vaccine Immunol. 19:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pakkanen S. H. Kantele J. M. Herzog C. and Kantele A. 2014. Cross-reactive immune response elicited by parenteral Vi polysaccharide typhoid vaccine against non-typhoid Salmonellae. Vaccine 32:544. [DOI] [PubMed] [Google Scholar]
- 42. Belkaid Y. and Hand T. W. 2014. Role of the microbiota in immunity and inflammation. Cell 157:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferreira R. B. Antunes L. C. and Finlay B. B. 2010. Should the human microbiome be considered when developing vaccines?PLoS Pathog. 6:e1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eloe-Fadrosh E. A., McArthur M. A., Seekatz A. M., et al. 2013. Impact of oral typhoid vaccination on the human gut microbiota and correlations with S. Typhi-specific immunological responses. PLoS One 8:e62026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fresnay S., McArthur M. A., Magder L., et al. 2016. Salmonella Typhi-specific multifunctional CD8+ T cells play a dominant role in protection from typhoid fever in humans. J. Transl. Med. 14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fresnay S., McArthur M. A., Magder L. S., et al. 2017. Importance of Salmonella Typhi-responsive CD8+ T cell immunity in a human typhoid fever challenge model. Front. Immunol. 8:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Masopust D., Choo D., Vezys V., et al. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McArthur M. A., Fresnay S., Magder L. S., et al. 2015. Activation of Salmonella Typhi-specific regulatory T cells in typhoid disease in a wild-type S. Typhi challenge model. PLoS Pathog. 11:e1004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Rosa S. C., Lu F. X., Yu J., et al. 2004. Vaccination in humans generates broad T cell cytokine responses. J. Immunol. 173:5372. [DOI] [PubMed] [Google Scholar]
- 50. Tan A. C., Eriksson E. M., Kedzierska K., et al. 2012. Polyfunctional CD8(+) T cells are associated with the vaccination-induced control of a novel recombinant influenza virus expressing an HCV epitope. Antiviral Res. 94:168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.