Abstract
Background
Cross-sectional studies suggest that trunk muscle morphology in the lumbar spine is an important determinant of kyphosis severity in older adults. The contribution of age-related changes in muscle morphology in the thoracic and lumbar spine to progression of kyphosis is not known. Our objective was to determine cross-sectional and longitudinal associations of thoracic and lumbar muscle size and density with kyphosis.
Methods
Participants were 1,087 women and men (mean age: 61 years) of the Framingham Heart Study who underwent baseline and follow-up quantitative computed tomography (QCT) scanning 6 years apart. We used QCT scans to measure trunk muscle cross-sectional area (CSA, cm2) and density (HU) at the thoracic and lumbar spine and Cobb angle (degrees) from T4 to T12. Linear regression models estimated the association between muscle morphology and kyphosis.
Results
At baseline, smaller muscle CSA and lower density of thoracic (but not lumbar) spine muscles were associated with a larger (worse) Cobb angle in women and men. For example, each standard deviation decrease in baseline thoracic paraspinal muscle CSA was associated with a larger baseline Cobb angle in women (3.7 degrees, 95% CI: 2.9, 4.5) and men (2.5 degrees, 95% CI: 1.6, 3.3). Longitudinal analyses showed that loss of muscle CSA and density at the thoracic and lumbar spine was not associated with progression of kyphosis.
Conclusions
Our findings suggest that kyphosis severity is related to smaller and lower density trunk muscles at the thoracic spine. Future studies are needed to determine how strengthening mid-back musculature alters muscle properties and contributes to preventing kyphosis progression.
Keywords: Posture, Spinal muscle, Fat infiltration, Epidemiology, Longitudinal study
Hyperkyphosis is an exaggerated anterior curvature of the thoracic spine that is evidenced as a hunched posture. Increasing severity of thoracic kyphosis is associated with declines in lung function, impaired physical function, and increased risk of falls, fractures, and mortality (1–4). Risk factors for hyperkyphosis include older age, low bone density, vertebral fracture, degenerative disc disease, and genetic predisposition (5,6). Some evidence suggests that postmenopausal women with weaker back extensor muscles have greater risk of hyperkyphosis (7–9).
The musculature that stabilizes and extends the spine is an attractive target for resistance training interventions, since exercise can improve trunk strength and may thereby reduce the progression of hyperkyphosis (10). However, muscle (eg back extensor) strength may be limited as a valid functional measure in at-risk populations. Back extension is a biomechanically complex movement that requires adequate neuromuscular coordination to contract several muscles and is influenced by an individual’s motivation levels (11). Omnibus measures of back extensor strength are unable to distinguish between the level of strength in specific muscles or muscle groups. Moreover, measuring back extensor strength requires spinal movements that may be limited or painful for older adults with poor spine health, including vertebral fracture, intervertebral disc degeneration, or facet joint osteoarthritis. Measures of muscle size and density may be more easily obtained and have demonstrated reasonable utility as proxies for muscle strength. Low density trunk muscles (ie those with a greater amount of intramuscular fat content), as indicated by low attenuation of x-rays by muscle tissue in computed tomography (CT) scans, relate to reduced trunk extension strength, independent of muscle size (12). Low density of the paraspinal muscles in healthy adults aged 70–79 years is associated with increased likelihood of hyperkyphosis (13). In men older than 65 years, those with the smallest paraspinal muscle volume had the largest Cobb angle (40.0 degrees, 95% CI: 37.8, 42.1) compared with those with the largest paraspinal muscles (36.3 degrees, 95% CI: 34.2, 38.4) (14).
Although imaging-based properties of muscle size and density appear to be important determinants of kyphosis severity, prior work (13,14) has focused exclusively on the lumbar region. The properties of trunk muscles in the thoracic region, the site of kyphosis angulation, have not been investigated with respect to age-related kyphosis severity. Furthermore, although some studies of the contribution of trunk muscles to kyphosis have taken into account vertebral fractures (7,14), investigations have not considered disc height narrowing and facet joint osteoarthritis. These features of spinal degeneration are associated with increased back pain and reduced spinal flexibility (15,16), which may result in inactivity that reduces trunk muscle size and density. Finally, studies to-date have been cross-sectional and unable to discern whether age-related changes in muscle size and density contribute to the progression of kyphosis.
The purpose of our study was to determine the cross-sectional association between thoracic and lumbar muscle size and density and kyphosis severity, as well as the longitudinal association between 6 year change in thoracic and lumbar muscle size and density and change in kyphosis progression. We hypothesized as follows: (i) in cross-sectional analysis, smaller and lower density muscles in both the thoracic and lumbar spine would be positively associated with kyphosis severity, and (ii) in longitudinal analysis, loss of thoracic and lumbar muscle size and density would be associated with worsening of kyphosis.
Methods
Participants and Study Design
Participants were members of the Framingham Heart Study (FHS) Offspring and Third Generation Cohorts, who participated in the Multi-Detector Computed Tomography (MDCT) Study. The MDCT Study is an ancillary study of the FHS that preferentially enrolled participants if they lived in close proximity to Framingham, MA, or had a large number of family members in the FHS. Eligibility for the MDCT Study was age 35 years and older for men, 40 years and older and nonpregnant for women, and weight less than 352 lbs. Participants signed informed consent before enrollment, and the study was approved by institutional review boards at Boston University Medical Center, Beth Israel Deaconess Medical Center, and Hebrew SeniorLife Institute for Aging Research.
Participants in our study included women and men aged 50 years and older, who underwent CT scanning at baseline (2002–2005) and follow-up (2008–2011). We excluded 62 individuals with missing or unreadable scans. The remaining participants were 1,087 cohort members (592 women, 495 men) with readable scans at both baseline and follow-up; the median time between measurements was 6.1 years.
CT Scan Acquisition
At baseline, participants underwent volumetric CT scanning using an 8-section multidetector CT (LightSpeed Ultra, General Electric Medical Systems, Milwaukee, WI, USA) at a tube voltage of 120 kVp, tube current 320–400 mA (≤220/>220 lbs body weight), and gantry rotation of 500 milliseconds. Contiguous CT images (slice thickness = 2.5 mm) were acquired in the chest region from the carina of the trachea to the diaphragm, and in the abdominal region superior from the L5/S1 junction. At follow-up, CT scanning was performed using a 64-section multidetector CT (Discovery VCT, General Electric Medical Systems, Milwaukee, WI) operating at tube voltage 120 kVp, tube current 300–350 mA (≤220/>220 lbs body weight), and gantry rotation of 350 milliseconds. Contiguous images of the entire chest (slice thickness = 0.625 mm) were acquired from the base of the lungs to apices, and abdominal region images (slice thickness = 5 mm) were acquired approximately 2 cm superior from the S1 vertebra. Prior to analysis, follow-up CT image stacks were reconstructed to correspond to the same field of view and slice thickness as baseline CT images.
Muscle Morphology
Muscle cross-sectional area (CSA, mm2) and density (x-ray attenuation coefficient in Hounsfield Units, HU) in both the thoracic and lumbar regions of the trunk were measured using CT images. Each muscle in the CT image was individually contoured using Analyze image processing software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) (17). In the thoracic region, we analyzed one transverse midvertebral slice (located at the mid-height of the vertebra) from each of T7 and T8. In the lumbar region, one transverse midvertebral slice (located at the midheight of the vertebra) was analyzed from each of L3 and L4. Using the muscle contour, we calculated muscle size as the CSA (mm2) by averaging the T7 (or L3) left-right muscle CSA average and the T8 (or L4) left-right muscle CSA average. At each level, overall measures of muscle density (HU) of muscle groups were computed as a weighted average of the relevant muscles; these means were then averaged across levels to obtain a density measure for each muscle group. Muscle density calculations excluded voxel attenuation values outside the range of −50 to 150 HU. In cases where one vertebral level was missing or unable to be analyzed, we used a single-imputation regression approach to impute the T7-T8 or L3-L4 average muscle CSA or density. To determine reliability, two trained readers evaluated scans for 20 individuals on two occasions 2 weeks apart. Inter-rater and intrarater reliability for muscle CSA and density was high [intraclass correlation coefficients (ICCs) > 0.85].
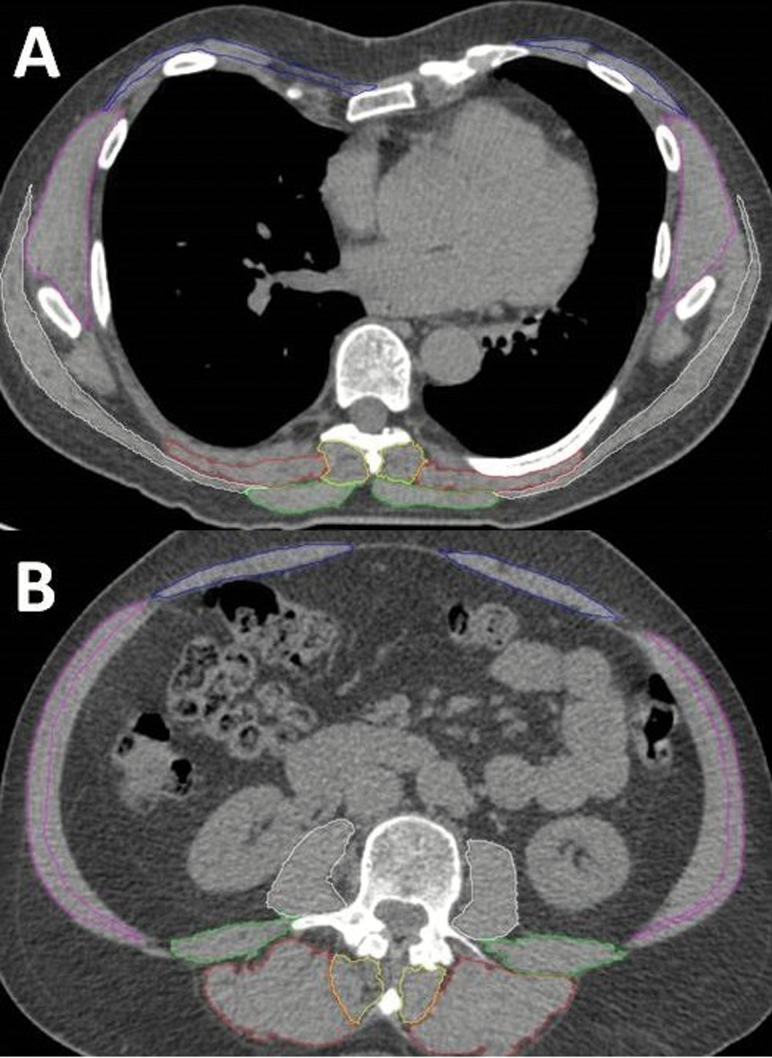
We assessed the following thoracic muscles: erector spinae, transversospinalis, and trapezius (Figure 1A). For analysis, we grouped individual thoracic muscles into two groups: paraspinal (erector spinae and transversospinalis) and posterior (erector spinae, transversospinalis, and trapezius). In the lumbar region, we assessed the following: erector spinae, transversospinalis, psoas major, quadratus lumborum, external oblique, internal oblique, and rectus abdominis. Lumbar muscles were grouped for analysis: extensors (erector spinae and transversospinalis), paraspinal muscles (erector spinae, transversospinalis, psoas major, and quadratus lumborum), and peripheral muscles (external oblique, internal oblique, and rectus abdominis) (Figure 1B). The thoracic transversospinalis muscle represents the semispinalis muscle, whereas the lumbar region transversospinalis represents multifidus. As part of the current study, we measured thoracic muscle properties in the full sample of 1,087 participants. However, lumbar region muscle properties were previously evaluated as part of an ancillary study in a convenience sample of 827 participants.
Figure 1.
Axial computed tomography images of the thoracic (A) and lumbar (B) muscles segmented using SpineAnalyzer. Thoracic muscles (A) measured at the midvertebral slice of T8 were the trapezius, erector spinae, and transversospinalis. Lumbar muscles (B) measured at the midvertebral slice of L3 were the erector spinae, transversospinalis, psoas major, quadratus lumborum internal, external obliques, and rectus abdominis.
Kyphosis
We evaluated thoracic kyphosis from lateral CT scout images at baseline and follow-up using the Cobb angle method. Using previously published methods, two operators used a semiautomated program (SpineAnalyzer, Opstasia Medical, Cheadle, UK) to analyze the baseline and follow-up images in pairs (18). The algorithm places six morphometry points on each vertebral body between T4 and L4. The Cobb angle (degrees) was measured as the angle between superior endplate of T4 and inferior endplate of T12. A larger Cobb angle indicates a greater magnitude, or severity, of thoracic kyphosis. To evaluate reliability, the same two readers evaluated scans for 20 participants on two occasions 2 weeks apart. Intrarater and inter-rater reliability was excellent (ICC > 0.97).
Other Variables
We used standardized methods to measure integral volumetric bone mineral density (vBMD; g/cm3) at L3 from baseline CT scans (19,20). The presence and severity of vertebral fracture, disc height narrowing, and facet joint osteoarthritis at baseline were assessed by a musculoskeletal radiologist using standardized methods (21–24). The radiologist, blinded to clinical information, scored vertebral fracture, disc height narrowing, and facet joint osteoarthritis at every vertebral level from T4-L4 using a semiquantitative (SQ) scoring: 0 = none, 1 = mild, 2 = moderate, and 3 = severe. We defined prevalent vertebral fracture as SQ score of ≥1 at baseline and incident vertebral fracture as a SQ score of 0 at baseline and ≥1 at follow-up. We created a summary index for facet joint osteoarthritis by summing the SQ scores across all 26 levels (T4-L4, bilaterally) to provide an overall measure of facet joint osteoarthritis severity (range, 0–78). The summary index for disc height narrowing was calculated by summing the SQ scores across all 13 levels from T4-L4 (range, 0–39). Intrareader reliability was high for all spinal features (ICCs ranged from 0.73 to 1.00).
Information on covariates was obtained from examinations conducted closest in time and prior to the baseline CT. Height (cm) and weight (kg) were measured using a stadiometer and balance beam scale. Standardized questionnaires were used to ascertain current smoking (yes/no), postmenopausal status (absence of menstrual periods for ≥1 year), and current use of hormone replacement therapy (yes/no). Physical activity was assessed using the Framingham physical activity index that sums the product of hours per day spent at doing slight, moderate, and heavy activity, as well as sitting and sleeping, by a weight based on the oxygen consumption required for that activity (25).
Statistical Analysis
We calculated change in muscle CSA, muscle density, and Cobb angle by subtracting the baseline values from the follow-up values. Decreases in CSA (ie negative change scores) represented loss of muscle density, whereas increases (ie positive change scores) in Cobb angle denoted kyphosis progression. We conducted regression analyses separately for women and men.
We used linear regression models to estimate the association, as quantified by linear slopes, between participant characteristics and (i) baseline Cobb angle and (ii) 6 year change in Cobb angle, with and without adjustment for covariates. Muscle size and density values were scaled to unit variance such that slope estimates represent the mean difference in Cobb angle associated with a one standard deviation (SD) difference in muscle morphology.
Prespecified models were adjusted for age (years), height (cm), and weight (kg). We evaluated as potential confounder variables with significant bivariate associations with Cobb angle in our data (Supplementary Table 1) or in previous research (5). These included current smoking (yes/no), vBMD (g/cm3), prevalent vertebral fracture (yes/no), facet joint osteoarthritis and disc height narrowing summary indices, physical activity (unitless), osteoporosis medications (yes/no), postmenopausal status (yes/no), and hormone replacement therapy (current vs former/never) in women. Because results did not change with additional adjustment for these factors, we employed the more parsimonious model.
In sensitivity analyses, we additionally adjusted for incident vertebral fracture (n = 12 in women, n = 10 in men), and because results did not change it was not retained in the model. Finally, we conducted age-stratified analysis, using the median age (60 years) as a threshold, but we found no evidence of interaction.
We used SAS version 9.4 (SAS Institute, Cary, NC) software for analyses. Emphasis was placed on estimation, but in limited hypothesis testing, we employed two-sided type-I error rate of 0.05.
Results
Participants included 592 women and 495 men. Mean baseline age was 61 years and ranged from 50 to 85 years (Table 1). Mean BMI was 28 kg/m2 for women and 29 kg/m2 for men. Eight per cent of women and 9 per cent of men were current smokers. The Framingham physical activity index ranged from 26 to 78, and the mean was 38 in women and 37 in men. Prevalence of vertebral fracture was 11 per cent in women and 19 per cent in men. Baseline Cobb angle was mean 33.9 degrees (±9.6) for women and 32.6 degrees (±8.7) in men; 26 per cent of women and 20 per cent of men had a baseline Cobb angle larger than 40 degrees. Over a mean 6.0 (±0.9) years follow-up, Cobb angle increased (worsened) a mean of 3.2 degrees (±5.0) for women and 2.0 degrees (±3.7) for men.
Table 1.
Baseline Characteristics (2002–2005) and 6 Year Longitudinal Change in Cobb Angle in Participants, Framingham Study
| Characteristics | Women (N = 592) | Men (N = 495) | ||
|---|---|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | |||
| Age (y) | 61 | (8) | 61 | (8) |
| Height (cm) | 162 | (6) | 176 | (7) |
| Weight (kg) | 72 | (15) | 88 | (14) |
| Body mass index (kg/m2) | 28 | (5) | 29 | (4) |
| Current smoker (%) | 47 | (8%) | 43 | (9%) |
| Postmenopausal (%) | 449 | (76%) | — | |
| Current hormone replacement therapy (%) | 36 | (6%) | — | |
| Current osteoporosis medication (%) | 92 | (16%) | 2 | (<1%) |
| Physical activity index (range, 26–78) | 38 | (7) | 37 | (6) |
| SQ ≥ 1 Vertebral fracture (%) | 66 | (11%) | 92 | (19%) |
| Facet joint osteoarthritis summary index (range, 0–78) | 24 | (12) | 23 | (13) |
| Disc height narrowing summary index (range, 0–39) | 7 | (5) | 8 | (6) |
| Volumetric bone mineral density (g/cm3) | 0.17 | (0.04) | 0.18 | (0.04) |
| Cobb angle (degrees) | 34 | (10) | 33 | (9) |
| Cobb angle > 40 degrees (%) | 153 | (26%) | 100 | (20%) |
| Change in Cobb angle, degrees | 3.2 degrees | (5.0) | 2.0 degrees | (3.7) |
Older age, lower weight, current smoking, lower vBMD, prevalent vertebral fractures, and more severe facet joint osteoarthritis and disc height narrowing were associated with larger Cobb angle at baseline (Supplementary Table 1). Women who used hormone replacement therapy had larger Cobb angle. Height and BMI were not associated with baseline Cobb angle in women or men.
In cross-sectional analysis, women and men with worse baseline muscle properties (smaller CSA, lower density) for the thoracic spine had a larger Cobb angle at baseline (Table 2). For example, one SD lower baseline posterior muscle CSA at the thoracic spine was associated with a 3.4 degrees (95% CI: 2.6, 4.2) larger Cobb angle (at baseline) in women and 2.9 degrees (95% CI: 2.1, 3.7) larger Cobb angle in men, and lower baseline posterior muscle density at the thoracic spine was associated with a 1.4 degrees (95% CI: 0.4, 2.4) larger Cobb angle in women and 2.9 degrees (95% CI: 2.1, 3.7) larger Cobb angle in men. In contrast, baseline muscle properties at the lumbar spine were not associated with baseline Cobb angle in women or men.
Table 2.
Cross-Sectional Association Between Baseline Muscle Properties and Baseline Cobb Angle†, Adjusted For Age, Height, and Weight
| Baseline muscle properties | Association with baseline Cobb angle (degrees) | |||||||
|---|---|---|---|---|---|---|---|---|
| Women (N = 592) | Men (N = 495) | Women (N = 592) | Men (N = 495) | |||||
| Mean | (SD) | Mean | (SD) | β‡ | (95% CI) | β‡ | (95% CI) | |
| Baseline thoracic muscle | ||||||||
| Paraspinal CSA | 616 | (122) mm2 | 891 | (174) mm2 | 3.73 | (2.93, 4.53) | 2.46 | (1.61, 3.30) |
| Paraspinal density | 31 | (9) HU | 35 | (10) HU | 1.37 | (0.46, 2.29) | 2.83 | (2.01, 3.65) |
| Posterior CSA | 915 | (169) mm2 | 1348 | (254) mm2 | 3.41 | (2.62, 4.20) | 2.90 | (2.07, 3.73) |
| Posterior density | 33 | (8) HU | 37 | (8) HU | 1.38 | (0.41, 2.35) | 2.93 | (2.12, 3.74) |
| Baseline lumbar muscle | ||||||||
| Extensor CSA | 1872 | (473) mm2 | 2250 | (441) mm2 | 0.35 | (−0.68, 1.38) | 0.76 | (−0.10, 1.62) |
| Extensor density | 38 | (10) HU | 43 | (10) HU | 0.28 | (−0.68, 1.23) | 0.45 | (−0.44, 1.34) |
| Parspinal CSA | 3237 | (877) mm2 | 4118 | (789) mm2 | 0.34 | (−0.70, 1.38) | 0.50 | (−0.35, 1.35) |
| Paraspinal density | 41 | (8) HU | 45 | (7) HU | 0.07 | (−1.05, 1.19) | −0.04 | (−0.92, 0.84) |
| Peripheral CSA | 2064 | (558) mm2 | 2799 | (667) mm2 | 0.14 | (−0.87, 1.15) | 0.30 | (−0.65, 1.25) |
| Peripheral density | 35 | (10) HU | 39 | (8) HU | 0.77 | (−0.33, 1.87) | −0.10 | (−1.05, 0.84) |
Notes: Estimates in bold are significant (p < .05).
†Mean (±SD) baseline Cobb angle (degrees) was 33.9 (±9.6) for women and 32.6 (±8.7) for men.
‡Standardized slope estimates represent the mean cross-sectional difference in baseline Cobb angle per standard deviation lesser muscle CSA or density; positive values, indicating worse kyphosis associated with lower muscle density, are expected.
CSA = Cross-sectional area; HU = Hounsfield unit.
Thoracic and lumbar muscle CSA and density declined over the 6 years follow-up in women and men (Table 3). For example, mean (SD) change in thoracic paraspinal muscle CSA was −9 (±73) mm2 in women and −35 (±94) mm2 in men, and mean change in lumbar paraspinal muscle CSA was −45 (±237) mm2 in women and −105 (±267) mm2 in men. However, change in thoracic and lumbar muscle CSA and density was largely unrelated to change in Cobb angle. Specifically, longitudinal analyses showed no association for change in thoracic posterior muscle properties or lumbar extensor, paraspinal, and peripheral muscle properties with change in Cobb angle. In contrast, change in Cobb angle was positively associated with change in thoracic paraspinal muscle CSA. On average, those with greater magnitude decreases in muscle density had lesser increases in Cobb angle; per SD greater loss of thoracic paraspinal muscle CSA, women and men had reductions of change in Cobb angle of −0.95 degrees (95% CI: −1.35, −0.55) and −0.36 degrees (95% CI: −0.69, −0.03), respectively.
Table 3.
Longitudinal Association Between Change in Muscle Properties and Change in Cobb Angle†, Adjusted For Age, Height, and Weight
| Change in muscle properties† | Association with change in Cobb angle (degrees) | |||||||
|---|---|---|---|---|---|---|---|---|
| Women (N = 592) | Men (N = 495) | Women (N = 592) | Men (N = 495) | |||||
| Mean | (SD) | Mean | (SD) | β‡ | (95% CI) | β‡ | (95% CI) | |
| Thoracic muscle change | ||||||||
| Paraspinal CSA | −9 | (73) mm2 | −35 | (94) mm2 | −0.95 | (−1.35, −0.55) | −0.36 | (−0.69, −0.03) |
| Paraspinal density | −7 | (7) HU | −6 | (8) HU | 0.01 | (−0.41, 0.42) | 0.21 | (−0.13, 0.55) |
| Posterior CSA | −32 | (102) mm2 | −71 | (132) mm2 | −0.17 | (−0.57, 0.23) | 0.11 | (−0.22, 0.44) |
| Posterior density | −6 | (7) HU | −6 | (7) HU | 0.02 | (−0.36, 0.39) | 0.19 | (−0.14, 0.51) |
| Lumbar muscle change | ||||||||
| Extensor CSA | −13 | (147) mm2 | −28 | (164) mm2 | 0.48 | (−0.01, 0.97) | −0.08 | (−0.48, 0.32) |
| Extensor density | −10 | (6) HU | −9 | (7) HU | −0.15 | (−0.63, 0.33) | −0.29 | (−0.71, 0.14) |
| Parspinal CSA | −45 | (237) mm2 | −105 | (267) mm2 | 0.34 | (−0.15, 0.83) | 0.03 | (−0.37, 0.43) |
| Paraspinal density | −9 | (6) HU | −9 | (6) HU | −0.21 | (−0.72, 0.30) | −0.25 | (−0.62, 0.12) |
| Peripheral CSA | −40 | (220) mm2 | −73 | (284) mm2 | 0.06 | (−0.46, 0.58) | −0.13 | (−0.57, 0.30) |
| Peripheral density | −12 | (5) HU | −11 | (5) HU | −0.48 | (−1.00, 0.03) | −0.22 | (−0.62, 0.19) |
Notes: Estimates in bold are significant (p < .05).
†Mean (±SD) change in Cobb angle (degrees) was 3.2 (±5.0) for women and 2.0 (±3.7) for men.
‡Standardized slope estimates represent the mean between-person difference in change in Cobb angle per SD larger decrease in muscle CSA or density. Positive values, indicating greater magnitude of kyphosis progression associated with greater amount of muscle loss, are expected.
CSA = Cross-sectional area; HU = Hounsfield unit.
Discussion
We conducted the first longitudinal study in a large, community-based population of women and men to determine the association between changes in lumbar and thoracic spinal muscle properties and change in thoracic kyphosis. Cross-sectional analyses showed that women and men with larger muscle CSA and higher muscle density at the thoracic spine, but not the lumbar spine, had less severe kyphosis. Longitudinal analyses showed that muscle CSA and density declined over 6 years follow-up in both the thoracic and lumbar regions; however, the magnitude of loss of muscle CSA and density was not associated with the magnitude of kyphosis progression in women or men.
The cross-sectional findings in the present study suggest that muscles in the thoracic region may be related to kyphosis to a greater extent than lumbar muscles. Because no previous studies of kyphosis have evaluated muscle properties at the thoracic spine, we are not able to directly compare our findings with others. However, cross-sectional studies in the Health Aging, and Body Composition (Health ABC) and Osteoporosis in Men (MrOS) cohorts evaluated associations between lumbar muscle morphology and thoracic kyphosis. The Health ABC Study found that women and men had increased odds of hyperkyphosis, defined as a Cobb angle larger than 40 degrees, with lower lumbar spinal muscle density (OR = 0.71, 95% CI: 0.58, 0.87, per SD in HU) (13). The MrOS study reported that men with the lowest lumbar paraspinal muscle volume had a larger Cobb angle: 40.0 degrees, 95% CI: 37.8, 42.1 in the lowest tertile versus 36.3 degrees, 95% CI: 34.2, 38.4 in the highest tertile (p-trend = 0.02) (14). Similar to our methods, these studies measured Cobb angle from images acquired in supine position. However, variations in lumbar muscle group definitions, vertebral levels assessed, and CT image analyses may contribute to the discordance in findings between our studies (13,14).
Our definition of the lumbar paraspinal muscle group, averaged at midvertebral L3 and L4, included the psoas major, quadratus lumborum, and tranversospinalis (or multifidi) muscles. In comparison, the Health ABC and MrOS cohorts evaluated lumbar paraspinal muscles (erector spinae and multifidi) at the L4-L5 disc space (13,14). The density of spinal muscles varies by vertebral level such that muscles at levels located inferiorly have a lower density compared with those located nearer to the thoracolumbar junction (26). Thus, our measurement site may have underestimated the amount of fatty infiltration of lumbar region trunk muscles and the association with kyphosis. Similar to the MrOS methods, our muscle contours included lean tissue and adipose tissue within the fascial plane of the specific muscle. In contrast, the Health ABC Study assessed muscle CSA with contours that excluded intermuscular and visible intramuscular adipose tissue that may have contributed to a higher muscle density estimate. Variation in the number of slices used to measure muscle properties may also help us to explain differences across studies. We used one slice per vertebral level which is subject to greater level-to-level variability in muscle properties than approaches using more than one slice per vertebral level. The absence of standardized CT image analyses protocols for quantifying muscle CSA and density poses challenges for quantifying muscle CSA and density poses challenges for comparing results across studies (13). Developing a standardized approach to quantify muscle morphology may help us to improve our understanding of the role of muscle properties and risk of hyperkyphosis.
Participants in our study (mean: 61 years) were on average 13 years younger than cohort members of Health ABC and MrOS (mean age: 74 years) (13,14). Accordingly, our participants had less severe kyphosis and higher levels of physical function compared with other cohorts, including MrOS (27). The relatively younger age of participants in the current investigation may have reduced the ability to detect associations between lumbar muscle properties and Cobb angle. However, we did find cross-sectional associations between thoracic spine CSA and density and Cobb angle, despite the relatively younger age of participants.
Longitudinal analyses showed no relation between the magnitude of loss of muscle density and CSA and progression of kyphosis in women or men. It is possible that the follow-up time may have been too short to detect associations. Although we did observe declines in muscle CSA and density in both the thoracic and lumbar regions over 6 years follow-up, small effect sizes and imperfect measurements may have also contributed to our null results from the longitudinal analyses. There are no observational longitudinal studies that have assessed spinal muscle attributes using CT imaging in healthy adults, so we cannot readily compare our findings with others.
The Cobb angle change over 6 years observed in our sample, 3.2 degrees in women and 2.0 degrees in men, appears to be consistent with findings for women in the Study of Osteoporotic Fractures (SOF), 2.6 degrees over 3.4 years (5). Similar to our study, SOF assessed Cobb angle change from supine lateral spine images. Few studies have measured change in Cobb angle (28). A 6 month muscle strengthening and postural training intervention, including women mean 70 years old with Cobb angle at least 40 degrees, improved Cobb angle by −3.0 degrees (95% CI: −5.2, −0.8), but had no effect on change in muscle strength, whether measured as the density of lumbar spinal muscles on quantitative computed tomography (QCT) images or by clinical assessment of physical function (29). Together, these findings suggest that other muscle properties, such as muscle activation or neuromuscular coordination, may play an important role for determining kyphosis progression.
In contrast, other interventions to increase back extensor muscle strength and improve posture through resistance training, stretching, and yoga, ranging from 2 to 6 months, showed improvements in thoracic kyphosis from 3 to 6 degrees, as measured by various measures of kyphosis (28). It is worth noting that these interventions measured kyphosis in standing position and it is unknown how muscle morphology associations with kyphosis differ by kyphosis measurement method.
We unexpectedly found that decline in thoracic paraspinal muscle CSA was associated with a greater magnitude of decrease in Cobb angle (−0.9 degrees in women and −0.3 degrees in men, per SD decrease in CSA). We suggest that this result may be a spurious finding. The effect of muscle loss on Cobb angle change was small, and this was an isolated significant finding. Although we hypothesized that muscle loss would be associated with increasing kyphosis severity, it is also plausible that in some individuals, increases in kyphosis over time could provoke increases in muscular strength to compensate. Thus, causal relationships between changes in muscle size and kyphosis progression remain unclear.
Low muscle density, a marker for increased muscle fat infiltration or myosteatosis, has emerged as an important predictor of negative health outcomes and is known to increase with advancing age (30). However, the literature to-date has predominantly evaluated thigh muscles due to their important influence on mobility and the feasibility for CT scanning without increased radiation to central body organs (31). Studies have found that smaller muscles with greater amount of intramuscular fat content are weaker and demonstrate impaired function (32,33). Although thoracic muscles have not been evaluated by prior studies, lower density of lumbar muscles is related to increased low back pain, decreased lumbar lordosis, increased postural sway, and poor physical function (34–36). Furthermore, it might be possible to prevent fatty infiltration of muscles and improve posture by reducing visceral fat depots. Strategies for preventing, managing, and treating hyperkyphosis require further research to assess the types and intensities of exercise that could reduce fatty infiltration of muscles and improve posture.
The etiology of age-related hyperkyphosis is multifactorial and is likely attributable to the degenerative changes of multiple tissues. Our results suggest a role for thoracic muscle in kyphosis severity, independent of vertebral fractures and spinal degeneration. Vertebral fractures are often considered the primary risk factors responsible for the onset and perpetuation of hyperkyphosis; however, as many as two thirds of older women with hyperkyphosis do not have prevalent vertebral fractures (37). Since the spine is supported by a complex framework of joints, discs, ligaments, and muscles, our study accounted for structural deterioration of the facet joints and intervertebral discs, which are known to occur with advancing age. Despite the high prevalence of facet joint osteoarthritis and disc height narrowing in participants, adjusting for the severity of these features did not appreciably influence the relation between thoracic muscle size and density and thoracic kyphosis.
This study included a well characterized, community-based cohort of women and men. However, participants were predominantly Caucasian, because the original cohort was selected at the time of enrollment to represent the sociodemographic characteristics of the residents living in the town of Framingham, MA. Thus, we may not be able to fully generalize our findings to other ethnic and race groups. Although we adjusted for important potential confounders, we acknowledge the potential for residual confounding in an observational study. We did not adjust for conditions that can affect bone and muscle health including cervical osteoarthritis, cardiopulmonary disease, or thyroid or parathyroid disease.
High-resolution CT imaging allows quantification of muscle size and muscular fat infiltration in specific muscles. However, muscle CSA and density incompletely reflect muscle strength. Correlations between paraspinal muscle CSA and back extensor strength in adults (age 78 years) were moderate (r = .34) and summary measures of trunk muscle density explained up to 11 per cent of the unique variance in back extensor strength after accounting for muscle CSA (12). Nevertheless, a large proportion of muscle strength remains unexplained by imaging-based measures. Image-based measures of muscle do not capture muscle tightness in the chest region which may contribute to muscular imbalances between flexors and extensors of the spine, and thereby contribute to the progression of thoracic kyphosis. In addition, supine positioning for CT acquisitions in our study and lack of gravitational effects on the spine may vary in relation to assessments of muscle properties and kyphosis severity (38). Although Kado showed reasonable agreement between standing assessments of kyphosis by Debrunner kyphometer and supine measures of Cobb angle (ICC = 0.68), a recent study found that only standing measures of thoracic kyphosis were related to trunk lean mass, but not supine measures (39). Thus, it is possible that supine imaging in our study may have obscured potential associations between muscle properties and kyphosis.
In conclusion, we found that smaller and lower density thoracic (but not lumbar) spinal muscles are associated with increased severity of thoracic kyphosis in community-dwelling women and men. We did not find evidence that, over a 6 year period, individuals with more muscle loss (at either the thoracic or lumbar spine) have greater magnitude of kyphosis progression. Our findings support the notion that smaller and lower density spinal muscles of the mid-back, those situated nearest to the kyphosis curvature, are associated with a forward hunched posture. Future studies are needed to determine how strengthening the midback musculature could alter muscle properties and whether these changes contribute to preventing age-related changes in spinal curvature.
Funding
This work was supported by the National Institute on Aging of the National Institutes of Health (R01 AG041658, R01 AR053986, R01 AR041398, T32-AG023480) and the National Heart, Lung, and Blood Institute’s Framingham Heart Study (HHSN268201500001I).
Conflict of Interest
None declared.
Supplementary Material
References
- 1. Lorbergs AL, O’Connor GT, Zhou Y, et al. Severity of kyphosis and decline in lung function: the Framingham Study. J Gerontol A Biol Sci Med Sci. 2016;72:689–694. doi: 10.1093/gerona/glw124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katzman WB, Harrison SL, Fink HA, et al. Osteoporotic Fractures in Men (MrOS) Study Research Group. Physical function in older men with hyperkyphosis. J Gerontol A Biol Sci Med Sci. 2015;70:635–640. doi: 10.1093/gerona/glu213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kado DM, Miller-Martinez D, Lui LY, et al. Hyperkyphosis, kyphosis progression, and risk of non-spine fractures in older community dwelling women: the study of osteoporotic fractures (SOF). J Bone Miner Res. 2014;29:2210–2216. doi: 10.1002/jbmr.2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kado DM, Huang MH, Karlamangla AS, Barrett-Connor E, Greendale GA. Hyperkyphotic posture predicts mortality in older community-dwelling men and women: a prospective study. J Am Geriatr Soc. 2004;52:1662–1667. doi: 10.1111/j.1532-5415.2004.52458.x [DOI] [PubMed] [Google Scholar]
- 5. Kado DM, Huang MH, Karlamangla AS, et al. Factors associated with kyphosis progression in older women: 15 years’ experience in the study of osteoporotic fractures. J Bone Miner Res. 2013;28:179–187. doi: 10.1002/jbmr.1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yau MS, Demissie S, Zhou Y, et al. Heritability of thoracic spine curvature and genetic correlations with other spine traits: the Framingham Study. J Bone Miner Res. 2016;31:2077–2084. doi: 10.1002/jbmr.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinaki M, Itoi E, Rogers JW, Bergstralh EJ, Wahner HW. Correlation of back extensor strength with thoracic kyphosis and lumbar lordosis in estrogen-deficient women. Am J Phys Med Rehabil. 1996;75:370–374. [DOI] [PubMed] [Google Scholar]
- 8. Mika A, Unnithan VB, Mika P. Differences in thoracic kyphosis and in back muscle strength in women with bone loss due to osteoporosis. Spine (Phila Pa 1976). 2005;30:241–246. [DOI] [PubMed] [Google Scholar]
- 9. Hongo M, Miyakoshi N, Shimada Y, Sinaki M. Association of spinal curve deformity and back extensor strength in elderly women with osteoporosis in Japan and the United States. Osteoporos Int. 2012;23:1029–1034. doi: 10.1007/s00198-011-1624-z [DOI] [PubMed] [Google Scholar]
- 10. Bautmans I, Van Arken J, Van Mackelenberg M, Mets T. Rehabilitation using manual mobilization for thoracic kyphosis in elderly postmenopausal patients with osteoporosis. J Rehabil Med. 2010;42:129–135. doi: 10.2340/16501977-0486 [DOI] [PubMed] [Google Scholar]
- 11. Granata KP, Lee PE, Franklin TC. Co-contraction recruitment and spinal load during isometric trunk flexion and extension. Clin Biomech (Bristol, Avon). 2005;20:1029–1037. doi: http://dx.doi.org/10.1016/j.clinbiomech.2005.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson DE, Bean JF, Holt NE, Keel JC, Bouxsein ML. Computed tomography-based muscle attenuation and electrical impedance myography as indicators of trunk muscle strength independent of muscle size in older adults. Am J Phys Med Rehabil. 2014;93:553–561. doi: 10.1097/PHM.0000000000000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katzman W, Cawthon P, Hicks GE, et al. Association of spinal muscle composition and prevalence of hyperkyphosis in healthy community-dwelling older men and women. J Gerontol A Biol Sci Med Sci. 2012;67:191–195. doi: 10.1093/gerona/glr160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katzman WB, Miller-Martinez D, Marshall LM, Lane NE, Kado DM. Kyphosis and paraspinal muscle composition in older men: a cross-sectional study for the Osteoporotic Fractures in Men (MrOS) research group. BMC Musculoskelet Disord. 2014;15:19. doi: 10.1186/1471-2474-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suri P, Hunter DJ, Rainville J, Guermazi A, Katz JN. Presence and extent of severe facet joint osteoarthritis are associated with back pain in older adults. Osteoarthritis Cartilage. 2013;21:1199–1206. doi: 10.1016/j.joca.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujiwara A, Lim TH, An HS, et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976). 2000;25:3036–3044. [DOI] [PubMed] [Google Scholar]
- 17. Robb RA, Hanson DP. A software system for interactive and quantitative visualization of multidimensional biomedical images. Australas Phys Eng Sci Med. 1991;14:9–30. [PubMed] [Google Scholar]
- 18. Lorbergs AL, Murabito JM, Jarraya M, et al. Thoracic kyphosis and physical function: the Framingham Study. J Am Geriatr Soc. 2017;65:2257–2264. doi: 10.1111/jgs.15038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samelson EJ, Christiansen BA, Demissie S, et al. QCT measures of bone strength at the thoracic and lumbar spine: the Framingham Study. J Bone Miner Res. 2012;27:654–663. doi: 10.1002/jbmr.1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307 [DOI] [PubMed] [Google Scholar]
- 21. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915 [DOI] [PubMed] [Google Scholar]
- 22. Videman T, Battié MC, Ripatti S, Gill K, Manninen H, Kaprio J. Determinants of the progression in lumbar degeneration: a 5-year follow-up study of adult male monozygotic twins. Spine (Phila Pa 1976). 2006;31:671–678. doi: 10.1097/01.brs.0000202558.86309.ea [DOI] [PubMed] [Google Scholar]
- 23. Pathria M, Sartoris DJ, Resnick D. Osteoarthritis of the facet joints: accuracy of oblique radiographic assessment. Radiology. 1987;164:227–230. doi: 10.1148/radiology.164.1.3588910 [DOI] [PubMed] [Google Scholar]
- 24. Weishaupt D, Zanetti M, Boos N, Hodler J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol. 1999;28:215–219. [DOI] [PubMed] [Google Scholar]
- 25. Shortreed SM, Peeters A, Forbes AB. Estimating the effect of long-term physical activity on cardiovascular disease and mortality: evidence from the Framingham Heart Study. Heart. 2013;99:649–654. doi: 10.1136/heartjnl-2012-303461 [DOI] [PubMed] [Google Scholar]
- 26. Lee SH, Park SW, Kim YB, Nam TK, Lee YS. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J. 2017;17:81–87. doi: 10.1016/j.spinee.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 27. Allaire BT, DePaolis Kaluza MC, Bruno AG, et al. Evaluation of a new approach to compute intervertebral disc height measurements from lateral radiographic views of the spine. Eur Spine J. 2017;26:167–172. doi: 10.1007/s00586-016-4817-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bansal S, Katzman WB, Giangregorio LM. Exercise for improving age-related hyperkyphotic posture: a systematic review. Arch Phys Med Rehabil. 2014;95:129–140. doi: 10.1016/j.apmr.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katzman WB, Vittinghoff E, Lin F, et al. Targeted spine strengthening exercise and posture training program to reduce hyperkyphosis in older adults: results from the study of hyperkyphosis, exercise, and function (SHEAF) randomized controlled trial. Osteoporos Int. 2017;28:2831–2841. doi: 10.1007/s00198-017-4109-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Delmonico MJ, Harris TB, Visser M, et al. Health, Aging, and Body Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson DE, D’Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J Gerontol A Biol Sci Med Sci. 2013;68:317–323. doi: 10.1093/gerona/gls168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J Appl Physiol (1985). 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 33. Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. [DOI] [PubMed] [Google Scholar]
- 34. Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–887. [DOI] [PubMed] [Google Scholar]
- 35. Kalichman L, Li L, Hunter DJ, Been E. Association between computed tomography-evaluated lumbar lordosis and features of spinal degeneration, evaluated in supine position. Spine J. 2011;11:308–315. doi: 10.1016/j.spinee.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson DE, Quinn E, Parker E, et al. Associations of computed tomography-based trunk muscle size and density with balance and falls in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:811–816. doi: 10.1093/gerona/glv185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider DL, von Mühlen D, Barrett-Connor E, Sartoris DJ. Kyphosis does not equal vertebral fractures: the Rancho Bernardo study. J Rheumatol. 2004;31:747–752. [PubMed] [Google Scholar]
- 38. Kado DM, Christianson L, Palermo L, Smith-Bindman R, Cummings SR, Greendale GA. Comparing a supine radiologic versus standing clinical measurement of kyphosis in older women: the Fracture Intervention Trial. Spine (Phila Pa 1976). 2006;31:463–467. doi: 10.1097/01.brs.0000200131.01313.a9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamamoto J, Bergstrom J, Davis A, et al. Trunk lean mass and its association with 4 different measures of thoracic kyphosis in older community dwelling persons. PLoS One. 2017;12:e0174710. doi: 10.1371/journal.pone.0174710 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.