Abstract
Nuclear nanomedicine, with its targeting ability and heavily loading capacity, along with its enhanced retention to avoid rapid clearance as faced with molecular radiopharmaceuticals, provides unique opportunities to treat tumors and metastasis. Despite these promises, this field has seen limited activities, primarily because of a lack of suitable nanocarriers, which are safe, excretable and have favorable pharmacokinetics to efficiently deliver and retain radionuclides in a tumor. Here, we introduce biodegradable laser-synthesized Si nanoparticles having round shape, controllable low-dispersion size, and being free of any toxic impurities, as highly suitable carriers of therapeutic 188Re radionuclide. The conjugation of the polyethylene glycol-coated Si nanoparticles with radioactive 188Re takes merely 1 hour, compared to its half-life of 17 hours. When intravenously administered in a Wistar rat model, the conjugates demonstrate free circulation in the blood stream to reach all organs and target tumors, which is radically in contrast with that of the 188Re salt that mostly accumulates in the thyroid gland. We also show that the nanoparticles ensure excellent retention of 188Re in tumor, not possible with the salt, which enables one to maximize the therapeutic effect, as well as exhibit a complete time-delayed conjugate bioelimination. Finally, our tests on rat survival demonstrate excellent therapeutic effect (72% survival compared to 0% of the control group). Combined with a series of imaging and therapeutic functionalities based on unique intrinsic properties of Si nanoparticles, the proposed biodegradable complex promises a major advancement in nuclear nanomedicine.
Introduction
Сancer therapy using radiopharmaceutical products has become increasingly important over the last decades, promising an attractive and powerful alternative to conventional chemotherapy1. This nuclear medicine modality implies an injection of short decay time radionuclides in vivo (systemically or intratumorally), while their ionizing radiation (α, β, γ) is used to damage the DNAs of actively proliferating cancer cells, thus causing their selective death while keeping normal cells weakly affected1. The radionuclide therapy becomes especially efficient when one can achieve a high tumor/non-tumor radionuclide contrast, which enables to minimize side effects related to the irradiation of healthy issues. In a conventional approach, one employs vectoring molecules (specific antibodies, etc.) to target radionuclides to the tumor, but these molecules are typically small (less than 60–65 kDa) and can carry only a few chelates linked to radionuclide atoms2,3. Consequently, one has to deliver very high concentrations of radionuclide-carrying molecules to achieve any sufficient therapeutic effect, but this leads to severe side effects, taking into account that the efficiency of molecular targeting typically does not exceed 10–12%. In addition, the size of most targeting molecules appears to be within the renal glomerular filtration range (<7 nm)4, which leads to too fast accumulation of radionuclide complexes in the kidney, causing consequent interstitial nephritis or renal failure problems5,6.
Recently, there has been a great deal of interest in developing nuclear nanomedicine which utilizes nanoparticles (NPs) as carriers of radionuclides7,8. When functionalized by biopolymers such as polyethylene glycol (PEG), NPs promise safe and controllable transport of radionuclides in the blood stream, as well as offer a passive vectoring mechanism for targeting tumors based on their selective size accumulation (enhanced permeability and retention (EPR) effect)2. In addition, NPs can be more heavily loaded with radionuclides to ensure an enhanced therapeutic outcome in the tumor region7,8. However, some stringent requirements to make nuclear medicine safe and effective, have been challenging. The challenges to be met are: (1) NPs-based carrier should be large enough (>20–30 nm) to avoid immediate renal filtration and ensure efficient delivery of radionuclides to the intended site; (II) the NP –radiopharmaceutical conjugate should be safe and excretable from the organism to minimize toxicity and residual accumulation risks4,9; (III) the NP –conjugate should have targeting ability to effectively localize in high concentrations in the tumor; (IV) the coupling to the radioactive nuclei should be fast compared to their half life in order to maximize radiation therapy. Despite the presence of several classes of highly biocompatible nanomaterials, these challenges are very difficult to meet, as the required large size of NPs beyond the renal filtration range drastically complicates their further bioelimination4,10.
In this article, we propose a pathway to meet these challenges by introducing silicon (Si) NPs (Si*NPs), synthesized by pulsed laser ablation in liquids11–13, as a nearly ideal carrier of radionuclides for nuclear nanomedicine. The uniqueness of such Si*NPs is based on their biodegradability, which makes possible elimination of these structures from the organism within several days, even if their initial size is large (30–80 nm)12,13 under absence of any toxic effects, which was earlier confirmed in a mice model12. In addition, in contrast to Si nanostructures prepared by conventional electrochemical14 or chemical15 routes, laser-synthesized Si*NPs have ideal round shape, controllable size with a small size dispersion, and are free of any toxic impurities11, which promises a better transport in vivo and no side effects. Here, we demonstrate the possibility for coating of laser-synthesized Si*NPs by PEG and a fast conjugation of the Si*NPs-PEG complex with the Rhenium-188 (188Re) radionuclide, which is one of most promising generator-type therapeutic beta-emitters with the energy of positron emission of 1.96 MeV (16.7%) and 2.18 MeV (80%) and half-decay time of 17 hours1. We show that such Si*NPs-PEG-188Re conjugates can efficiently deliver the radionuclide through the blood stream and retain it in the tumor region. We also demonstrate strong therapeutic effect under intratumoral administration of the conjugate.
Results and Discussion
Fabrication, characterization and functionalization of Si*NPs
Bare (ligand-free) Si*NPs were fabricated by femtosecond laser ablation in deionized water11–13, as shown schematically in Fig. 1a and described in details in the Methods section. Being composed of crystalline Si covered by a 1–2 nm thick oxide shell13, laser-synthesized Si*NPs have an ideal spherical shape and are relatively monodispersed, with their mean size being about 25 nm (Fig. 1b). The Si*NPs were coated with PEG according to our newly developed protocol (see methods section), in order to minimize the immune response of the biological system. Due to high hydrophilicity, PEG is known to form a water cloud around the NPs, which protects them from the interaction with antibodies and opsonic proteins, and dramatically increases the circulation of nanomaterials in the blood streem16. Finally, we conjugated Si NPs-PEG complex with 188Re ions using coordination with the carboxyl group available on the PEG surface, as described in the Methods section.
Figure 1.
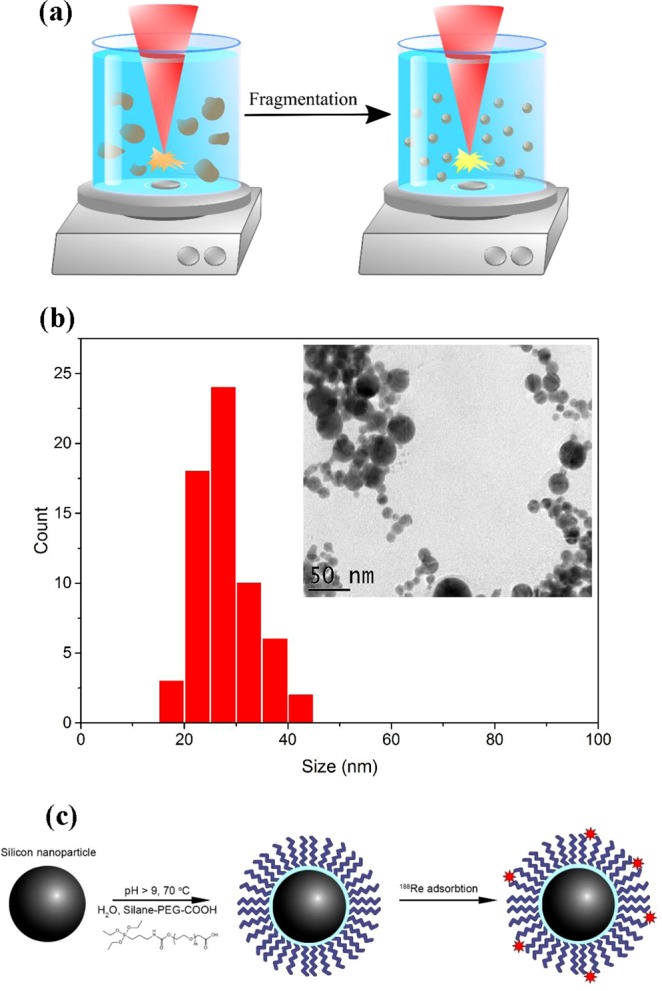
Synthesis and functionalization of Si nanoparticles for nuclear medicine tasks. (a) Schematic of laser synthesis of Si*NPs. Crystalline Si microcolloids (~0.5 μm in size), preliminarily prepared by mechanical milling of a Si wafer, are dispersed in deionized water and illuminated by focused radiation from fs laser. The laser-ablative process leads to the formation of spherical, small size-dispersed Si*NPs exempt of any toxic impurity; (b) Typical transmission electron microscopy image (inset) and corresponding size distribution of Si*NPs prepared by fs laser ablation; (c) Schematic presentation of functionalization protocol for the coating of Si*NPs by polyethylene glycol (PEG) and subsequent decoration by radioactive 188Re atoms. All images were designed and drawn by authors of this manuscript.
Biodistribution of Si*NPs-PEG-188Re conjugates under systemic administration
In our tests, biodistribution of the nanoparticle carrier-based Si*NPs-PEG-188Re conjugate was compared with that of freely circulating radioactive rhenium using its salt sodium perrhenate form, Na188ReO4. Five sub-groups of 4 Wistar female rats from the “signal” group, implanted with liver cholangioma RS-1, were intravenously administered with a single dose of 56.8–62.5 µg/kg of animal weight of Si*NPs-PEG-188Re conjugates. Similar number of animals from the “control” group were injected with water-dissolved Na188ReO4, containing radioactive rhenium atoms at the same concentration.
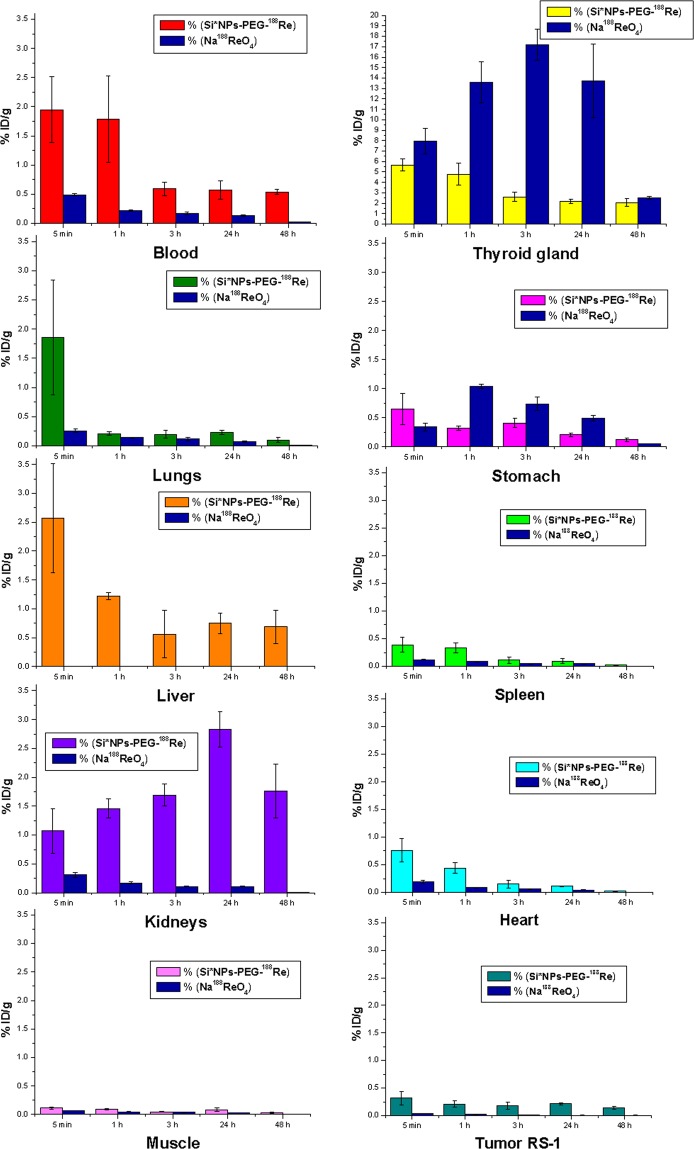
As shown in Fig. 2, the maximal level of radioactivity in blood was recorded after 5 minutes of injection of both Si*NPs-PEG-188Re and Na188ReO4 solutions which then gradually decreased. For free 188Re (in 188ReO4-), the level of radioactivity in blood was much lower (<0.5%, after 5 min; <0.2% after 1 hour, <0.1% after 24 hours, and finally not detectable after 48 hours). At the same time, the injection of free Na188ReO4 was accompanied by an immediate increase of the 188Re concentration mainly in the thyroid gland, reaching its maximum values of 17% 3 hours after the radionuclide injection. The accumulation of 188Re in other organs was much lower (Fig. 2), although we recorded a certain concentration of 188Re in the stomach just after the injection (1.2% after one hour), and its smaller concentrations in lungs and kidneys (less than 0.25% and 0.3%, respectively, after five minutes). Notice that the recorded biodistribution and pharmcokinetics with much preferable accumulation of the product in thyroid gland and stomach is typical for free 188Re and other radionuclides1.
Figure 2.
Biodistribution of 188Re under its systemic administration in Wistar rats with the nanocarrier-based Si*NPs-PEG-188Re conjugate. Different colors show relative amounts of radioactivity for different organs and tissues (blood, thyroid gland, lungs, stomach, liver, spleen, kidneys, heart, muscle, tumor) of Wistar rats with implanted liver cholangioma RS-1 after 5 min, 1 hour, 3 hours, 24 hours and 48 hours of intravenous administration of Si*NPs-PEG-188Re complexes. The blue color shows the relative amount of radioactivity in organs and tissues for control group, in which 188Re was systemically administered in the free state (with dissolved sodium perrhenate Na188ReO4 molecules).
However, the biodistribution and pharmacokinetics were quite different for nanoparticle carrier-based Si*NPs-PEG-188Re conjugates. First, the maximal level of 188Re in the blood was much higher (1.95% and 1.8% after 5 min and 1 hour, respectively) and easily detectable, even 48 hours after the injection (0.5%). In contrast to the free 188Re case, there was no preferential accumulation of radionuclide in any particular organ or tissue. Here, we also recorded certain radionuclide signal in the thyroid gland and stomach (5.5% and 0.65% after 5 min, with a further rapid decrease), but we attributed this signal to washing out of some 188Re atoms from the Si*NPs-PEG-188Re complexes due to possible non-optimized protocol of their conjugation. Surprisingly, the accumulation of radionuclide in liver and spleen was very weak (less than 2.5% and 0.3% after 5 minutes and then rapidly decreased down to 0.5–0.7% and 0.05% after 24 hours, respectively). The absence of any significant accumulation of Si*NPs-PEG-188Re conjugates in organs of reticuloendothelial system (liver, spleen) can only be explained by their invisibility to the immune system, which was obviously due to the PEG-based coating of Si*NPs. As follows from Fig. 2, such a coating led to prolonged circulation of Si*NPs-PEG-188Re conjugates in the blood stream and their efficient delivery to most organs. It is also important that the concentration of 188Re gradually increased in the kidneys, reaching its maximal value 24 hours after the injection (almost 3%), which is consistent with gradual dissolution of nanoformulations and their time-delayed elimination via renal clearance12,13. For comparison, in the case of free rhenium (injection of Na188ReO4 solutions) its concentration in the kidney was maximal just after the injection (5 min), which can lead to undesirable kidneys damage.
Biodistribution of Si*NPs-PEG-188Re conjugates under intratumoral administration
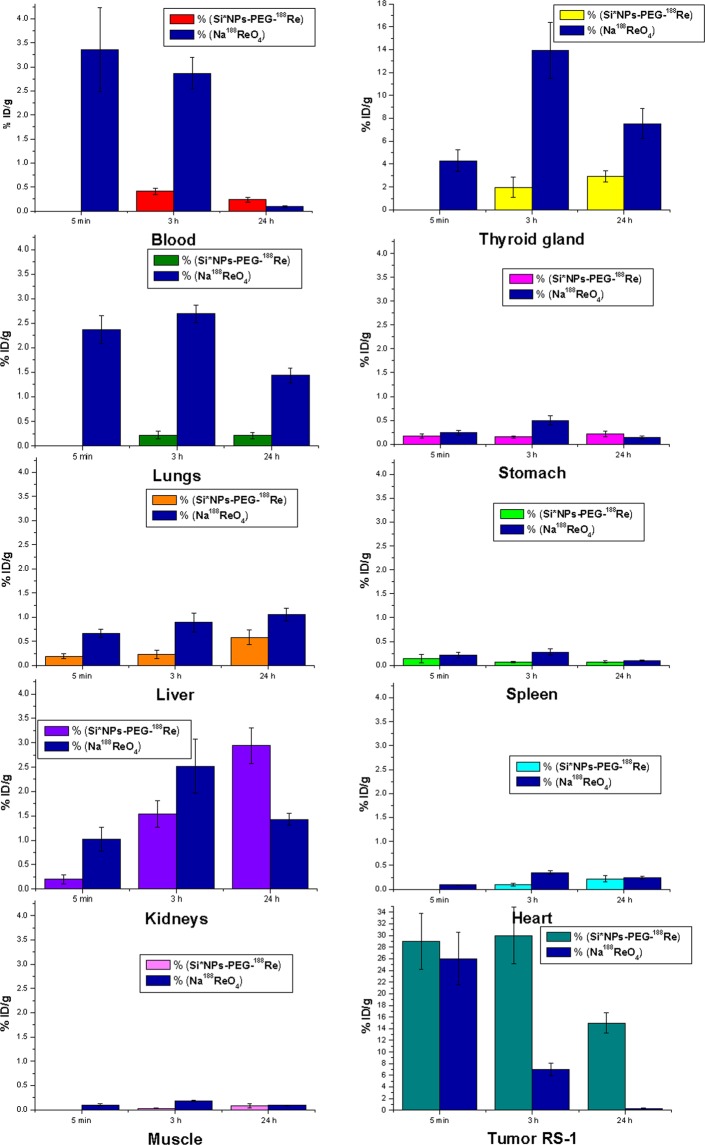
Three sub-groups of 4 Wistar rats with implanted cholangioma RS-1 from the “signal” group were intratumorally administered with a single dose of 56.8–62.5 µg/kg of nanoparticle carrier-based Si*NPs-PEG-188Re complexes, while the same number of animals from the “control” group were intratumorally administered Na188ReO4 solutions having a similar concentration of 188Re atoms. Different sub-groups of animals from the “signal” and the “control” groups were sacrificed 5 minutes, 3 hours and 24 hours after the injection and examined for 188Re distribution in different organs. As shown in Fig. 3, in the case of free 188Re atoms, we recorded a drastic (4-fold) decrease of 188Re concentration in the tumor during the first 3 hours (from 25% to 6%), while after 24 hours, it was not detectable in this area, suggesting a fast washing out of the radionuclide. At the same time, we recorded a fast increase of 188Re concentration in blood (3.75% after 5 min),with a further slow decrease down to 2.75% after 3 hours and 0.05% after 24 hours. After 3 hours, 188Re mostly migrated into the thyroid gland (14%) that looks consistent with typical biodistribution for this radionuclide. Significant concentrations of 188Re were also recorded in lungs, kidneys and liver (2.75%, 2.5% and 1% after 3 hours, respectively), while its concentration in the stomach was much lower compared with intravenous injection (0.4% after 3 hours). In general, our data on intratumoral injection of free 188Re showed immediate washing out of the radionuclide from the tumor area and its further accumulation preferably in the thyroid gland. As shown in Fig. 3, nanoparticle carrier-based Si*NPs-PEG-188Re conjugate demonstrated a radically different biodistribution and pharmacokinetics. Here, we did not observe any decrease of the 188Re concentration in the tumor during the first 3 hours (its value was always higher than 30%) and the concentration of the radionuclide in this area was very high (>15%) even after 24 hours. Thus, due to the employment of Si*NPs-based carrier, we had very good retention of 188Re over its half-decay time, enabling maximal therapeutic effect. On the other hand, the migration of 188Re to other organs was very weak, although we recorded certain accumulation of the radionuclude in the thyroid gland (less than 2.8%), blood (less than 0.3%), lungs (less than 0.2%), liver (less than 0.6%), stomach (less than 0.15%) and spleen (less than 0.1%). We believe that a relatively strong signal in the thyroid gland could arise from washing out of some 188Re atoms from Si*NPs-PEG-188Re conjugates, similarly to what happened after intravenous injection, while the increase of 188Re concentration in other organs can be due to the interjection of certain number of Si*NPs-PEG-188Re conjugates from the tumor to the blood stream. Of particular attention, we can mention a gradual increase of 188Re concentration in the kidneys, with a maximal value of 3% reached 24 hours after the injection, which contrasts the data for free rhenium atoms. To summarize, our tests established a very good retention of 188Re in the tumor, which shows promise for successful use of Si*NPs as carriers of radionuclides.
Figure 3.
Biodistribution of 188Re under its intratumoral administration with the nanocarrier-based Si*NPs-PEG-188Re conjugate. Different colors show relative amount of radioactivity in the organs and tissues of Wistar rats (blood, thyroid gland, lungs, stomach, liver, spleen, kidneys, heart, muscle, tumor) with implanted liver cholangioma RS-1 after 5 min, 3 hours and 24 hours of intratumoral administration of the Si*NPs-PEG-188Re complexes.The blue color shows relative amount of radioactivity in organs and tissues for control group subjected to intratumoral injection of Na188ReO4.
Therapeutic efficiency using Si*NPs-188Re conjugates
The therapeutic efficiency of Si*NPs-188Re conjugates was assessed by using Wistar rats with cholangioma RS-1 implanted in the right femoral muscle. We used 30 rats divided into three sub-groups of 10 animals: the 1st and 2nd “signal” groups were intratumorally administered with a single dose of 37 and 74 MBq of NPs carrier-based Si*NPs-PEG-188Re conjugates, respectively, while the 3rd “control” group were intratumorally injected by 0.1 mL of physiological solutions. Figure 4 shows results of survival tests for these 3 groups. One can see that after 20 days, only 40% of rats from the control group survived, while the survival rate for the 1st and 2nd “signal” groups was 100%. After 30 days, all animals from the control group were dead, while the survival rate for the 1st and 2nd groups was 50% and 72%, respectively. Thus, our experiments clearly demonstrate a remarkable therapeutic effect under intratumoral injection of the Si*NPs-PEG-188Re conjugates. It should be noted that the accomplished injection protocol was not optimized to maximize the therapeutic effect. We believe that the efficiency of the treatment can still be improved, e.g., by using 2 and more injections and further optimization of dose radioactivity.
Figure 4.
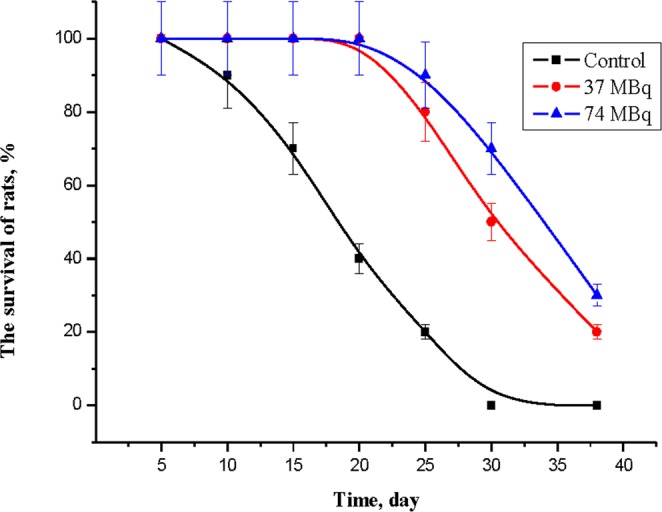
Assessment of therapeutic effect. Survival curves for Wistar rats with implanted cholangioma RS-1 after intratumoral injection of the Si*NPs-PEG-188Re conjugates providing different doses of radioactivity (37 and 74 MBq) and for control group injected with physiological solutions. Each group was composed of 10 animals.
Biodegradation, bioelimination and safety of conjugates
As shown in Fig. 2, intravenous administration with the Si*NPs-PEG-188Re conjugates leads to at least a 10-fold higher concentration of 188Re in the kidneys compared to the case of free 188Re atoms (injection of Na188ReO4 solutions). This unambiguously indicates that the radionuclide comes to the kidneys in the conjugated state. The pharmacokinetics of 188Re is also completely different in the case of the Si*NPs-PEG-188Re conjugates, as the radionuclide comes not immediately, but after some delay (24 hours).
It should be noted that by themselves, the Si*NPs prepared by laser ablation present a highly safe product for biomedical use, as follows from the results of our recent tests in a mouse model12,17. Here, we considered the worst “stress” scenario, when the NPs are bare (non-PEGylated) and should be immediately sequestrated by the reticuloendothelial system. Indeed, after systemic administration, almost 100% of Si*NPs immediately accumulated in the liver and the spleen, but in contrast to silica (SiO2) and many other nanomaterials whose accumulation in the liver causes a series of damaging effects (hyperplasia of Kupffer cells, hepatic inflammation, oxidative stress etc.18), we observed only minor inflammation effects which completely disappeared 48 h after the injection, as evidenced by a histopathological investigation of tissues12. At the same time, we recorded stability of blood parameters (aminotrasferases, alkaline phosphatase, bilirubin, cholesterol, etc.)17 and absence of any liver or kidney toxicity, as was confirmed by ALAT, ASAT and the serum creatinine levels, and negligible changes of oxidative stress parameters including catalase, SOD, GPx activities, Vit A and E7212. Furthermore, Si*NPs started to decompose into orthosilicic acid Si(OH)4 soon after the administration and then migrated to kidneys where the decomposition process continued to reduce the NPs size down to a renal glomerular filtration range (<7 nm), rendering possible their excretion with the urine. The complete bioelimination process took 5–7 days, as was controlled by monitoring the Si content in the urine12. In the presented study, Si*NPs were additionally PEGylated, which prolonged the circulation time in the organism and radically changed biodistribution, giving access to most organs. Nevertheless, the Si*NPs-PEG-188Re conjugates similarly migrated to kidneys where they were supposed to decompose and finally excrete via renal filtration. It is important that such filtration starts only after some delay (typically, after 24 hours), which should minimize damaging effects in the kidney as the radioactivity of 188Re is already much lower after the half-decay time.
To summarize, we established the merits of the Si*NPs as safe and effective carriers of 188Re radionuclide for nuclear therapy. Our study has revealed a quite different biodistribution and pharmacokinetics of the Si*NPs-PEG-188Re conjugates compared to the free 188Re atoms (water-dissolved sodium perrhenate Na188ReO4) in a Wistar rat model. Our tests on intravenous administration showed that the NPs-based carrier conjugate can freely circulate in the blood stream and target tumors, while the free 188Re atoms mostly accumulate in the thyroid gland. In addition, intratumoral administration tests evidenced very good retention of the radionuclide in the tumor for more than 24 hours, while the free 188Re rapidly washed out form the tumor under similar conditions. Under both administrations, we recorded at least a 24-hour delayed delivery of 188Re to kidney for the Si*NPs-PEG-188Re conjugates, which is consistent with gradual decomposition of these complexes; it promises much reduced side effects in the kidneys. Finally, our tests evidenced a considerable increase of the rat survival rate for the groups of animals intratumorally administered with the Si*NPs-PEG-188Re conjugates; the radioactive doses of the Si*NPs-PEG-188Re conjugates compared to the control group were: (i) 100% for both groups compared to 40% after 20 days; (ii) 50% and 72% compared to 0% after 30 days.
We believe that the Si*NPs-based transport vehicle complex can be considered as a general biodegradable platform for targeted delivery of radionuclides for nuclear therapy. We demonstrated its successful conjugation with 188Re, which is one of the very efficient beta-emitters that can be synthesized in portable 188W/188Re generators, making possible its low-cost fabrication and worldwide distribution19. Nevertheless, other promising diagnostic (64Cu, 68Ga) or therapy (e.g., 90Y) radionuclides can equally be conjugated with biodegradable Si*NPs carriers to maximize the efficacy of imaging or therapy. It is also important that crystalline nano-Si is a IV group semiconductorwhose intrinsic properties make possible a series of unique imaging and therapy functionalities, including room temperature photoluminescence for bioimaging20–22, light-induced generation of singlet oxygen for photodynamic therapy of cancer23, and infrared24, radio frequency17, and ultrasound-induced25 cancer hyperthermia. In fact, the choice of Si*NPs as radionuclide carrier means that all these imaging and therapy modalities can be utilized in parallel with the main nuclear medicine modality to produce image guided therapy, and thus maximize the final therapeutic outcome. One of the most promising tandem therapeutic approaches, we see, is radio frequency-induced hyperthermia using Si*NPs as sensitizers of local heating17 which can be used even for the treatment of deep tissues due to good transparency of the body to the RF radiation. As another additional functionality, one can imagine the use of fluorescence properties of Si*NPs to track the localization of therapeutic agents in the tumor, although this modality is limited by superficial tissues due to low transmission of light even in the spectral range of relative tissue transparency (750–900 nm).
Methods
Synthesis and characterization of Si*NPs
Si*NPs were prepared by ultra-short (fs) laser fragmentation in water ambience, as described in our recent publications11,13. Briefly, a powder of 0.5 µm Si microparticles, preliminarily prepared by mechanical milling of a Si wafer, was introduced into a glass cuvette at 0.35 g/L and dispersed in deionized water by a sonication bath step for 30 minutes. The dispersed Si microparticles were then fragmented under laser irradiation for one hour using a Yb:KGW (fs) laser (Avesta Inc., Russia, 1030 nm, 270 fs, 1–30 kHz). The laser beam was focused at 1 cm below the water level, while the solution was continually homogenized by a magnetic stirrer. In addition, the initial concentration was varied in the range 0.15 g/L to 0.5 g/L in order to control the mean size of the NPs according to the protocol proposed in ref.11. To determine the size characteristics of nanoparticles, a high-resolution transmission electron microscopy (HR-TEM) system (JEOL JEM 3010) was employed in the imaging and diffraction modes. A droplet of solution containing laser-synthesized nanoparticles was deposited onto the surface of a carbon-coated TEM copper grid, dried and finally examined by the TEM system.
Chemical modification and functionalization of Si*NPs
Materials
Silane-PEG-COOH (average Mw 5000) were purchased from Biochempeg Scientific Inc. Ethanol and 30% ammonium hydroxide were obtained from Sigma-Aldrich. MilliQ-grade water was used in the preparation of buffers and aqueous solutions.
PEG-based coating of Si*NPs
The functionalization of laser-synthesized Si*NPs with polyethylene glycol was performed as follows. The Si*NPs were dispersed in 10 mL of 96% ethanol to a final concentration of 2 g/L. Then, 200 mg of the silane-PEG-COOH solution in 20 mL of ethanol was added to the NPs under continuous stirring at room temperature. Since only dense coating of the NPs by PEG is able to provide stealth properties, we used a large molar excess of the silane-PEG chains in this reaction. The resulting mixture was ultra-sonicated for 1 min, and 1 mL of 3% ammonium hydroxide was quickly drop added into the mixture under vigorous stirring to catalyze the hydrolysis and condensation of the silane groups on the surface of the Si*NPs. Then we tested if the pH of the mixture had reached 9–10, and heated this solution for 2.5 h at 70 °C. To prevent further hydrolysis and self-aggregation of unreacted silanes, the mixture was cooled down to the room temperature and the NPs were washed by centrifugation (15 min, 5000 g), firstly with pure ethanol and further with water 3 times. After washing, the product was redispersed in 20 mL of PBS (pH 7.4). The obtained NPs suspension did not contain any aggregates and had long-term colloidal stability at physiological conditions, as was confirmed by Dynamic Light Scattering (Zetasizer Nano ZS, Malvern Instruments, UK). Also, we observed a strong negative zeta-potential after coupling of the PEG-COOH chains with the silicon surface. All the measurements were conducted in MilliQ water.
Preparation of the Si*NPs-PEG-188Re conjugates
Preparation of radioactive 188Re solutions
188Re was obtained in the form of a Na188ReO4 solution by elution with saline from a column of a 188Re/188W generator (A. I. Leipunsky Institute of Physics and Power Engineering, Joint Stock Company, Obninsk, Russia). The radioactive purity of the 188Re eluate exceeded 99%. Volume activity of eluate 188Re was 185 MBq·ml−1 (5,0 mCi·ml−1).
Conjugation of 188Re with Si*NPs carrier and assessment of its efficiency
The conjugation was performed according to the protocol developed in ref.26. Briefly, 5 ml of the Si*NPs-PEG complexes having a concentration of 1 mg/ml was added to 1.5 ml of distilled water and 7 mg of ascorbic acid. The ingredients were then mixed for 5 min via ultrasound, to which was added 9.5 mg of SnCl2 2H2O (5.0 mg for Sn2+) in 0.1 ml 0.1 M HCl. After 5-minutes of mixing by ultrasound for 5 min, the 74 MBq 188Re eluate in 0.2 ml of physiological solution was added to it and then mixed once again for 1 hour. Then, a centrifugation step was applied to wash out the unconjugated 188Re elute. Then, the superficial layer was removed, while the precipitate was re-suspended in 4 ml of physiological solution. The centrifugation procedure was repeated once again to remove the superficial layer. The precipitate was once again re-suspended in 2 ml physiological solution and examined for radiochemical purity using chromatography methods. The efficiency of conjugation of 188Re with the Si*NPs-PEG complex was determined by paper chromatography using a Whatman 1 paper (Germany). 3.0 µl samples of the reaction mixture were applied with a micropipette onto chromatographic paper stripes (10*110 mm). The stripes were placed vertically in a beaker, and elution was performed with acetone buffer. The Si*NPs-PEG-188Re conjugate stayed at the bottom start region of the stripes (Rf = 0.1–0.15), while the free 188ReO4− ions ascended with the eluent front, until Rf = 0.8–0.9. The amounts of the Si*NPs-PEG-188Re and free 188Re were determined by radiometry of chromatographic paper stripes using an automatic gamma counter, “Wizard 2480” (Perkin Elmer/Wallac, Finland). Our tests showed that the radiometric outcome of Si*NPs-PEG-188Re was 59.2 MBq in 2 ml (29.6 MBq/ml), which corresponds to 80% of the initial radioactivity of the 188Re eluate (Supplementary Fig. 1Sa). We also found that the 188Re-based nanoconjugates were stable for more than 48 hours, while the impact of radionuclide impurities after the washing procedure was less than 5% (Supplementary Fig. 1Sb).
Methodology of animal tests
Implantation of RS-1 tumor
All experiments were carried out using female Wistar rats with a body weight of 120–140 g (branch of “Stolbovaya” of the Scientific Center of Biomedical Technologies of Federal Medical-Biological Agency (FMBA)). As a tumor model, we used cholangioma RS-1 (Tumor strain depository of N.N. Blokhin National Medical Research Center of Oncology of the Ministry of Health of the Russian Federation). To obtain an initial sample of the solid tumor, a donor rat with the tumor was sacrificed and the tumor tissue was extracted. Then, the tumor tissue was fragmented, diluted in physiological solution in the proportion of 1:3, and implanted into the right femoral muscle. Each injection was about 100 µg of the tissue in 0.1 ml per animal, which was optimal for cholangioma RS-1, as it ensured 100% implantation of the tumor and its good growth, as well as provided maximal lifetime for the animals. Every three days, the tumor volume of every animal was examined and its volume was assessed. 8–10 days after the implantation, when the tumor volume was about 0.7–0.8 cm3, all animals were subdivided into “signal” and control groups, according to the planned experiments.
Intravenous administration of Si*NPs-PEG-188Re and free 188Re
40 Wistar rats were divided into 2 groups (20 rats in each). Rats from the first group were intravenously injected in the jugular vein (under isofluorane anesthesia) with a single dose of 0.74–1.11 MBq of Si*NPs-PEG-188Re in 0.1 ml of physiological solution (12.5 µg of Si*NPs-PEG-188Re), which provided 56.8–62.5 µg per kg of animal weight. Rats from the second group were injected in the jugular vein with a single dose of 0.74–1.11 MBq of free Na188ReO4) in 0.1 ml of physiological solution, which provided 3.7–5.55 MBq per kg of animal weight.
Intratumoral administration of Si*NPs-PEG-188Re and free 188Re
24 Wistar rats were divided into 2 groups (12 rats in each). Rats from the first group were intratumorally (in the tumor center) injected with a single dose of 0.74–1.11 MBq of Si*NPs-PEG-188Re in 0.1 ml of physiological solution (12.5 µg of Si*NPs-PEG-188Re), which provided 56.8–62.5 µg per kg of animal weight. Rats from the second group were intratumorally injected in a similar way with a single dose of 0.74–1.11 MBq of free Na188ReO4) in 0.1 ml of physiological solution, which provided 3.7–5.55 MBq per kg of animal weight.
Methodology of 188Re biodistribution experiments
Different sub-groups of animals (4 of each) were sacrificed 5 minutes, 1 hour, 3 hours, 24 hours and 48 hours after the injection of both solutions. Samples of key organs and tissues were collected, weighted in electronic balance (“Sartorius”, Germany) and placed in plastic boxes to assess the intensity of ionizing emission from 188Re by a gamma counter (2480 Wizard, Perkin Elmer-Wallac, Finland). 0.1 mL of the Si*NPs-PEG-188Re solution was collected at the moment of NPs administration in mice, and placed in a separate cuvette to serve as a calibration standard. Based on the radiometric data for every observation point, we calculated the relative radioactivity per 1 g of organs or tissues as well as the total radioactivity of all organs or tissues.
All experimental procedures and animal care were carried out in accordance with the legislation of Russian Federation, directive 2010/63/EU of European Parliament and EU Council from 22 September 2010, as well as with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publ. No. 80–23, revised 1996). Experiments and animal care were performed at the National Medical Research Radiological Center (NMRRC) of the Ministry of Health of the Russian Federation, Obninsk, Russia. All experimental protocols were approved by the Scientific Council and Committee of Ethics on Animal experiments of NMRRC.
Supplementary information
Acknowledgements
The authors are grateful to V. Shipunova for her help during the implementation of PEGylization protocol. The authors acknowledge support from the MEPhI Academic Excellence Project (Contract No. 02.a03.21.0005). A.V.K. acknowledges the contribution from the ITMO Cancer AVIESAN (National Alliance for Life Sciences & Health) within the framework of the Cancer Plan (GRAVITY Project). V.Y.T. acknowledges the support from the Russian Science Foundation (project No. 16-13-10145). S.M.D. acknowledges the support from the Russian Foundation for Basic Research (project No. 17-00-00121).
Author Contributions
A.V.K. conceived and designed the research. A.A.P., G.T., V.Y.T. and A.V.K. designed laser fabrication setup, fabricated and characterized Si NPs. I.Z. and S.M.D. chemically modified and functionalized Si NPs. V.M.P., V.K.T. and A.A.M. performed all animal tests. V.M.P., V.K.T., A.A.M., A.A.P., G.T., I.Z., S.M.D., A.D.K., S.I., V.Y.T., P.N.P., I.N.Z., A.V.K. analyzed and discussed obtained data. A.V.K. and P.N.P. prepared the manuscript using data from co-authors. A.V.K., I.N.Z. and P.N.P. guided the project. All authors have given approval to the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
P. N. Prasad, Email: pnprasad@buffalo.edu
A. V. Kabashin, Email: kabashin@lp3.univ-mrs.fr
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38474-7.
References
- 1.Volkert WA, Hoffman TJ. Therapeutic Radiopharmaceuticals. Chem. Rev. 1999;99:2269–2292. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 3.Deyev SM, Waibel R, Lebedenko EN, Schubiger AP, Plückthun A. Design of multivalent complexes using the barnase-barstar module. Nature Biotech. 2003;21:1486–1492. doi: 10.1038/nbt916. [DOI] [PubMed] [Google Scholar]
- 4.Yu M, Zheng J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano. 2015;9:6655–6674. doi: 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamaly N, He JC, Ausiello DA, Farokhzad OC. Nanomedicines for renal disease: current status and future applications. Nature Rev. Nephrol. 2016;12:738–753. doi: 10.1038/nrneph.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AT. Radionuclides in Nephrourology, Part 1: Radiopharmaceuticals, Quality Control, and Quantitative Indices. J. Nucl. Med. 2014;55:608–615. doi: 10.2967/jnumed.113.133447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamoudeh M, Kamleh MA, Diab R, Fessi H. Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer,”. Adv. Drug Delivery Rev. 2008;60:1329–1346. doi: 10.1016/j.addr.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Mitra A, Nan A, Line BR, Ghandehari H. Nanocarriers for nuclear imaging and radiotherapy of cancer. Cur. Pharm. Des. 2006;12:4729–4749. doi: 10.2174/138161206779026317. [DOI] [PubMed] [Google Scholar]
- 9.Auffan M, et al. Toward a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotech. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 10.James WD, Hirsch LR, West JL, O’Neal PD, Payne JD. Application of INAA to the build-up and clearance of gold nanoshells in clinical studies in mice. J. Radioanal. Nucl. Chem. 2007;271:455–459. doi: 10.1007/s10967-007-0230-1. [DOI] [Google Scholar]
- 11.Blandin P, et al. Femtosecond laser fragmentation from water-dispersed microcolloids: toward fast controllable growth of ultrapure Si-based nanomaterials for biological applications. J. Mater. Chem. B. 2013;1:2489–2495. doi: 10.1039/c3tb20285b. [DOI] [PubMed] [Google Scholar]
- 12.Baati T, et al. Ultrapure laser-synthesized Si-based nanomaterials for biomedical applications: in vivo assessment of safety and biodistribution. Sci. Rep. 2016;6:25400. doi: 10.1038/srep25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Kattan, et al. Ultrapure laser-synthesized Si nanoparticles with variable oxidation state for biomedical applications. J. Mater. Chem. B. 2016;4:7852. doi: 10.1039/C6TB02623K. [DOI] [PubMed] [Google Scholar]
- 14.Santos, H. A. Porous silicon for biomedical applications (Elsevier Cambridge, 2014).
- 15.English DS, Pell LE, Yu Z, Barbara PF, Korgel BA. Size Tunable Visible Luminescence from Individual Organic Monolayer Stabilized Silicon Nanocrystal Quantum Dots. Nano Lett. 2002;2:681–685. doi: 10.1021/nl025538c. [DOI] [Google Scholar]
- 16.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamarov KP, et al. Radio Frequency Radiation-Induced Hyperthermia Using Si Nanoparticle-Based Sensitizers for Mild Cancer Therapy. Sci. Rep. 2014;4:7034. doi: 10.1038/srep07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie G, et al. Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch. Toxicol. 2010;84:183–190. doi: 10.1007/s00204-009-0488-x. [DOI] [PubMed] [Google Scholar]
- 19.Pillai MR, Dash A, Knapp FF. Rhenium-188: availability from the (188)W/(188)Re generator and status of current applications. Current Radiopharm. 2012;5:228–43. doi: 10.2174/1874471011205030228. [DOI] [PubMed] [Google Scholar]
- 20.Erogbogbo F, et al. Biocompatible Luminescent Silicon Quantum Dots for Imaging of Cancer Cells. ACS Nano. 2008;2:873–878. doi: 10.1021/nn700319z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu L, et al. In Vivo Time-Gated Fluorescence Imaging with Biodegradable Luminescent Porous Silicon Nanoparticles. Nat. Commun. 2013;4:2326. doi: 10.1038/ncomms3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gongalsky MB. Laser-synthesized oxide-passivated bright Si quantum dots for bioimaging. Sci. Rep. 2016;6:24732. doi: 10.1038/srep24732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timoshenko VY, et al. Silicon nanocrystals as photosensitizers of active oxygen for biomedical applications. JETP Lett. 2006;83:423–426. doi: 10.1134/S0021364006090128. [DOI] [Google Scholar]
- 24.Lee C, et al. Porous Silicon as an Agent for Cancer Thermotherapy Based on near-Infrared Light Irradiation. J. Mater. Chem. 2008;18:4790–4795. doi: 10.1039/b808500e. [DOI] [Google Scholar]
- 25.Sviridov AP, et al. Porous Silicon Nanoparticles as Sensitizers for Ultrasonic Hyperthermia. Appl. Phys. Lett. 2013;103:193110. doi: 10.1063/1.4829148. [DOI] [Google Scholar]
- 26.Petriev VM. Influence of reactant concentrations and solution acidity on the complexation of 188Re with 1-hydroxyethane-1,1-diphosphonic acid. Radiochemistry. 2008;50:203–207. doi: 10.1134/S1066362208020227. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.