Abstract
Chemotherapy of East Coast fever, a lymphoproliferative cancer-like disease of cattle causing significant economic losses in Africa, is largely dependent on the use of buparvaquone, a drug that was developed in the late 1980's. The disease is caused by the tick-borne protozoan pathogen Theileria parva. Buparvaquone can be used prophylactically and it is also active against tropical theileriosis, caused by the related parasite Theileria annulata. Recently, drug resistance was reported in T. annulata, and could occur in T. parva. Using a 3H-thymidine incorporation assay we screened 796 open source compounds from the Medicines for Malaria Venture (MMV) to discover novel chemicals with potential inhibitory activity to T. parva. We identified nine malaria box compounds and eight pathogen box compounds that inhibited the proliferation of F100TpM, a T. parva infected lymphocyte cell line. However, only two compounds, MMV008212 and MMV688372 represent promising leads with IC50 values of 0.78 and 0.61 μM, respectively, and CC50 values > 5 μM. The remaining compounds exhibited a high degree of toxicity (CC50 values < 1.09 μM) on the proliferation of bovine peripheral blood mononuclear cells stimulated with concanavalin A. We also tested the anti-cancer drug, dasatinib, used in the chemotherapy of some leukemias. Dasatinib was as active and safe as buparvaquone in vitro, with an IC50 of 5 and 4.2 nM, respectively, and CC50 > 10 μM. Our preliminary data suggest that it may be possible to repurpose compounds from the cancer field as well as MMV as novel anti-T. parva molecules.
Keywords: Medicines for malaria venture, Malaria box, East coast fever, Dasatinib, Theileria parva
Graphical abstract

Highlights
-
•
17 of 796 MMV compounds inhibited proliferation of T parva infected lymphocytes.
-
•
15 of the 17 hit compounds also inhibited proliferation of control cells.
-
•
MMV008212 and MMV688372 represent promising leads, therapeutic index >8.
-
•
Dasatinib was as active and safe as buparvaquone in vitro, therapeutic index >2000.
1. Introduction
East Coast fever (ECF), a lymphoproliferative disease caused by Theileria parva is the most important tick-borne disease in sub-Saharan Africa, killing cattle and causing economic losses (Norval et al., 1992). Theileria belongs to the phylum Apicomplexa, which contains other parasites responsible for major human and animal diseases, such as malaria, babesiosis, toxoplasmosis and cryptosporidiosis (Morrison, 2009). Considerable progress has been made in developing vaccines for the control of some of these diseases. For example, a live infection and treatment method of immunization is commercially available for the control of ECF (reviewed in Perry, 2016) and tissue-culture vaccines are available for the control of tropical theileriosis caused by T. annulata (Brown, 1990). Advanced clinical trials are in progress with a malaria vaccine (Clinical Trials Partnership, 2015). However, drug treatment often remains a frontline method of disease control.
Derivatives of naphthoquinones exhibit significant pharmacological properties and have given rise to development of anti-parasitic drugs, including commercial products to control malaria, e.g., atavaquone, a hydroxy-napthoquinone (Nixon et al., 2013). One of the byproducts of this effort gave rise to development of anti-theilerial drugs, e.g., parvaquone and the improved current drug of choice for treatment of theileriosis, buparvaquone (McHardy et al., 1985). Members of this class of compounds also display activity against other human and animal pathogens, e.g., trypanosomes (Salas et al., 2011). Unfortunately, resistance to atavaquone, an analog of ubiquinone, emerged quite rapidly and drug resistance to atavaquone in Plasmodium is associated with mutations in the mitochondrial gene encoding apo-cytochrome b (Vaidya and Mather, 2000). Resistance to other classes of anti-malarial drugs has also emerged, limiting the efficacy of several frontline drugs (Cui et al., 2015). The Medicines for Malaria Venture (MMV) was inaugurated in 1999, with a mandate of developing new anti-malarial drugs, as it was recognized that the pipeline for developing such drugs was small and not an attractive venture for the pharmaceutical industry.
Resistance to buparvaquone in T. parva has not been described. However, the recent identification of drug resistance in T. annulata (Mhadhbi et al., 2010; Sharifiyazdi et al., 2012) is a cause for concern as it could occur in T. parva. As for many tropical diseases, there is no program to develop new anti-theilerial drugs. Although focused on developing novel anti-malarial drugs, MMV has catalyzed neglected disease drug discovery by making available drug-like and probe-like compounds to the scientific community through the malaria box (Van Voorhis et al., 2016) and followed by the pathogen box. A unique aspect of the biology of T. parva and T. annulata is that schizont-infected leukocytes behave and proliferate like cancer cells in vitro, a property that is dependent on parasite viability (reviewed in Dobbelaere and Rottenberg, 2003). These infected cells are the principal cause of disease. We have taken advantage of this phenomenon and the availability of MMV compounds to screen for molecules that inhibit the growth the T. parva infected cells.
We screened compounds in both the malaria and pathogen boxes against F100TpM, a bovine lymphocyte cell line infected with the schizont stage of T. parva and against bovine peripheral blood mononuclear cells (PBMC) stimulated by concanavalin A (ConA), the latter to determine toxicity on uninfected but proliferating bovine lymphocytes. We identified two compounds with an in vitro therapeutic index >5, which could act as starting points for discovery of novel anti-theilerial drugs. In addition, we screened an anti-cancer drug, dasatinib, which is used for treatment of chronic myelogenous leukemia and acute lymphoblastic leukemia as an inhibitor of protein-tyrosine kinases (Steinberg, 2007; Talpaz et al., 2006) as these enzymes are modulated in T. parva infected cells (Fich et al., 1998). Dasatinib was found to selectively inhibit F100TpM cells with an in vitro therapeutic index >2000.
2. Methods
2.1. Experimental compounds
The MMV malaria box and pathogen box were obtained from the Medicines for Malaria Venture (MMV, Geneva, Switzerland). Plate mapping and full data on the compounds in the malaria box were accessed at (http://www.mmv.org/research-development/malaria-box-supporting information) and (http://www.pathogenbox.org/) for the pathogen box. All compounds were received in plates at 10 mM stock concentrations and were diluted in 100% DMSO to form copies of each plate containing each compound at 1 mM concentration. From the 1 mM stock plates serial dilutions were made in RPMI 1640 culture media to a 10 μM working stock plates. Buparvaquone (Butalex®) was used as a positive control drug and dasatinib was purchased from Selleckchem, USA (cat no. S1021). The molecular weight (MW) and lipophilicity index (ALogP) of active compounds were provided with the data sheets from MMV and are listed in Table 1 and Table 2.
Table 1.
Malaria box compound inhibitory activity.
| Compound | Structurea | MWb g/mol | ALogPc | IC50 (μM) F100TpM |
CC50 (μM) Con A blasts | TId (CC50/IC50) | Activity on other organisms (reference) |
|---|---|---|---|---|---|---|---|
| MMV008212 | 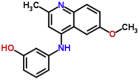 |
280.32 | 3.59 | 0.78 | >10 | >12 | Trypanosoma cruzi, P. berghei ookinete, P. falciparum gametocyte NF54-late stage (Van Voorhis et al., 2016) |
| MMV498479 | 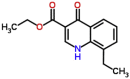 |
245.27 | 2.32 | 0.05 | 0.13 | 2.6 | T. cruzi (Van Voorhis et al., 2016) |
| MMV006455 | 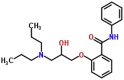 |
370.49 | 3.97 | 0.84 | 0.90 | 1.07 | P. falciparum respiratory target in early ring stage and gametocyte (Van Voorhis et al., 2016) |
| MMV665820 | 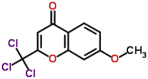 |
293.53 | 3.13 | 0.15 | 0.25 | 1.67 | Cryptosporidium parvum, Leishmania donovani axenic amastigotes (Van Voorhis et al., 2016), T. annulata (Hostettler et al., 2016) |
| MMV665841 | 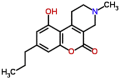 |
273.33 | 3.13 | 0.36 | 0.66 | 1.83 | T. cruzi and Wolbachia (Van Voorhis et al., 2016) |
| MMV665800 | 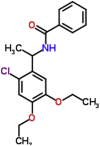 |
347.84 | 4.33 | 0.49 | 0.53 | 1.08 | P. falciparum (inhibitor of ATP4 activity) and P.berghei (Van Voorhis et al., 2016) |
| MMV000356 | 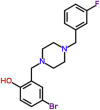 |
379.27 | 4.16 | 0.87 | 0.86 | 0.99 | Leishmania donovani amastigotes axenic (extracellular) (Van Voorhis et al., 2016) |
| MMV007363 | 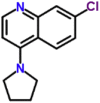 |
232.71 | 3.3 | 1.93 | 1.09 | 0.56 | T. cruzi, Toxoplasma gondii (Van Voorhis et al., 2016) |
| MMV007273 | 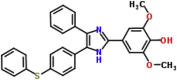 |
480.58 | 7.24 | 0.04 | 0.06 | 1.5 | P. falciparum (Van Voorhis et al., 2016) |
| Buparvaquone (control drug) | 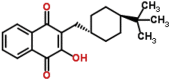 |
326.435 | 6.45 | 0.0042 | >10 | >2380 | Theileria annulata (Mhadhbi et al., 2010) |
Structure of compound from ChemSpider (http://www.chemspider.com/) or DrugBank (https://www.drugbank.ca/).
Molecular weight of the compound.
Lipophilicity index of the compound.
Therapeutic index (TI) of the compound.
Table 2.
Pathogen box compounds inhibitory activity.
| Compound | Structurea | MWb (g/mol) | ALogPc | IC50 (μM) F100TpM |
CC50 (μM) Con A blasts | T.Id (CC50/IC50) | Activity on other organisms (reference) |
|---|---|---|---|---|---|---|---|
| MMV688372 | 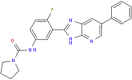 |
401.44 | 3.83 | 0.61 | 5.3 | 8.69 | Anti-Trypanosoma (Duffy et al., 2017) |
| MMV676600 | 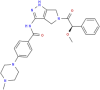 |
474.55 | 2.06 | 0.55 | <0.001 | <0.001 | Anti-Trypanosoma (Duffy et al., 2017) |
| MMV676602 | 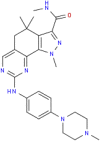 |
460.57 | 3.83 | 0.35 | 0.30 | 0.86 | Anti-Trypanosoma (Duffy et al., 2017) |
| MMV688180 |  |
495.43 | 3.22 | 0.97 | <0.001 | <0.001 | Anti-Trypanosoma (Duffy et al., 2017) |
| MMV688271 | 403.27 | 3.81 | 0.59 | 0.56 | 0.95 | Anti-Trypanosoma (Duffy et al., 2017) | |
| MMV023985 | 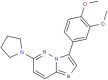 |
324.38 | 3.18 | 0.52 | <0.001 | <0.001 | Anti-malarial, CDPK1 or PK7 proposed targets (Duffy et al., 2017) |
| MMV003152 (Mebendazole) | 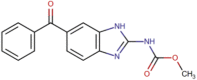 |
295.29 | 3.06 | 0.36 | <0.001 | <0.001 | Anti-helminthic (Oxberry et al., 2001) |
| MMV688978 (Auranofin) | 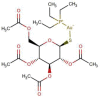 |
678.484 | - | 0.54 | 0.40 | 0.74 | Rheumatoid arthritis drug in human (Jeon et al., 2003) |
Structure of compound from ChemSpider (http://www.chemspider.com/) or DrugBank (https://www.drugbank.ca/).
Molecular weight of the compound.
Lipophilicity index of the compound.
Therapeutic index (TI) of the compound.
2.2. In vitro cell growth assays
All the MMV compounds were initially tested for inhibitory activity on the growth of F100TpM, a bovine lymphocyte cell line infected by T. parva. Cells were seeded in 100 μl RPMI 1640 media at 1 × 104 cells per well in 96 well plates and incubated overnight in 5% CO2 at 37 °C before addition of compounds at 1 μM final concentration for 48 h prior to pulsing with 3H-thymidine. The IC50 of inhibitory compounds was determined by titration of the compound from 0 to 2 μM final concentration. Compounds found to inhibit growth of F100TpM were further screened for non-specific toxicity on the proliferation of uninfected bovine cells stimulated with ConA. Briefly, 1 × 104 PBMCs isolated from uninfected bovine blood by Ficoll-Paque density gradient centrifugation were seeded in 100 μl RPMI 1640 media per well in 96 well plates and cultured overnight in 5% CO2 at 37 °C in the presence of ConA (final concentration of 5μg/ml) before addition of compounds from 0 to 10 μM final concentration for 48 h prior to pulsing with 3H-thymidine. Due to their high efficacy, lower drug concentrations of buparvaquone and dasatinib were used, 0–0.08 μM final concentration, to determine their IC50 and CC50 values. All compounds were tested in triplicate.
2.3. Thymidine incorporation as a marker for inhibitory activity
The radiolabeled precursor 3H-thymidine is incorporated into DNA strands during cell replication and radioisotope incorporation is often used as a measure of cell proliferation. All cells were pulsed with 20 μl of RPMI 1640 media containing 3H-thymidine (0.5 μCi) per well and incubated in 5% CO2 at 37 °C for a minimum of 8 h s before harvesting. The cells were then harvested by a Filtermat Harvester (Perkin Elmer) onto Glass fiber matt filters (part No. 6005422) that captures DNA. The filters were then air-dried and placed in Omni Filter cassette and 30 μl of MicroScintTM (Perkin Elmer Cat No 6013611) liquid added, then sealed with TopSeal (Perkin Elmer, Part No 6050195) and loaded on TopCount NXT reader (Perkin Elmer) for determination of incorporation of 3H-thymidine.
2.4. Calculation of IC50 and CC50 for bioactive compounds
IC50 values were calculated using the Graph Pad Prism 7 software (GraphPad Software, Inc., La Jolla, CA), and average values of radio-isotope incorporation in wells with and without compound on the growth of F100TpM cells. The CC50 values were calculated using 3H-thymidine incorporation values determined on the growth of PBMC stimulated with ConA. The therapeutic index of a compound was calculated by dividing the CC50 value with the IC50 value.
3. Results and discussion
In a search for new lead anti-theilerial compounds, we screened the open source MMV malaria box and pathogen box using a multi-step process. First, we used compound-induced inhibition of incorporation of 3H-thymidine by a T. parva infected cell line (F100TpM) at a final concentration of 1 μM as a marker of anti-theilerial activity. Proliferation of infected cells is parasite-dependent and appears to occur through complex manipulation of several host-cell signaling and metabolic pathways (Metheni et al., 2015; Shiels et al., 2006). Only 17 of the 796 compounds were found to exhibit inhibitory activity on F100TpM cell growth. There was little difference in the incorporation of radiolabel in the control and non-inhibitory compound wells indicating that residual levels of DMSO in these wells were not toxic to the cells (data not shown). Second, the IC50 of compounds that were active on F100TpM was determined as an indication of in vitro potency. Third, to rule out non-specific inhibitory activity on proliferating cells, these compounds were also tested on bovine PBMCs stimulated with ConA and used to calculate CC50 values, as an indicator of non-specific in vitro cell toxicity. Most T. parva infected cells lines are derived by “transformation” of T-lymphocytes by the parasite (Baldwin et al., 1988). Since ConA drives the proliferation of T-lymphocytes (Palacios, 1982) we reasoned that ConA blasts represent a more suitable and stringent cell line control for the in vitro assays.
Nine compounds from the malaria box, with IC50 values ranging from 0.04 to 1.93 μM (Table 1), and eight compounds from the pathogen box, with IC50 values ranging from 0.35 to 0.97 μM (Table 2) inhibited F100TpM proliferation, representing a 2% hit rate. However, the majority of these compounds were highly toxic on the control cell line. Only two compounds, one from each box, MMV008212 and MMV688372, exhibited an in vitro therapeutic index of 8 and 10, respectively. An index >5 has been used to determine parasite selectivity of MMV compounds (Duffy et al., 2017). MMV008212 and MMV688372 exhibit an ALogP lipophilicity index of 3.59 and 3.83, respectively. This index is one of several indicators used in compound design of drugs destined for oral or systemic delivery. It describes the partition coefficient between an organic, e.g., octanol, and aqueous phase and, hence, its potential bioavailability as a drug (Comer and Tam, 2001). The structure of the MMV compounds is depicted in Table 1, Table 2. For many compounds the therapeutic index was close to or less than one. In our hands, the positive control drug, buparvaquone had an IC50 of 4.2 nM and a CC50 > 10 μM, with a therapeutic index of more than 2000. Interestingly, four pathogen box compounds were more inhibitory on the control cell line, than on F100TpM, indicating that there could be differences in the molecular and cellular biology of the two cell lines.
The malaria box compound identified with anti-theilerial activity, MMV008212, also has activities against Trypanosoma cruzi (100% growth inhibition at 5 μM), Plasmodium berghei ookinete (76% growth inhibition at 10 μM), P. falciparum gametocyte NF54-late stage (91% growth inhibition at 5 μM) and P. falciparum 3D7 asexual blood stage (98% growth inhibition at 5 μM) (reviewed in Van Voorhis et al., 2016). Cytotoxicity of this compound was observed only in one cell line (U87, 76% cell death at 5 μM) of the nine cell lines tested (Van Voorhis et al., 2016). However, the mode of action of this compound is still unknown. The unsuitable remaining eight malaria box compounds are reported to exhibit varied inhibitory activities on other organisms including Plasmodium, Toxoplasma, Leishmania, Cryptosporidium and Wolbachia (Table 1). In contrast to our finding, these compounds exhibited less cytotoxicity on the mammalian cell lines and zebrafish embryo that were tested (Van Voorhis et al., 2016).
MV688372, the pathogen box compound identified here with a high therapeutic index against F100TpM cells has anti-trypanosomal activity. This compound is a substituted 2-phenylimidazopyridine with activities against Trypanosoma brucei brucei and T. cruzi with therapeutic indexes of 230 and 24, respectively, in relation to HEK293 cells (Duffy et al., 2017). The mode of action of MMV688372 is still unclear, but a structurally related molecule, azabenzoxazole, has been identified as a proteasome inhibitor in kinetoplastid parasites (Duffy et al., 2017). Of the remaining seven compounds with a low therapeutic index, five exhibit anti-trypanosomal activity, one has anti-malarial activity and two are reference compounds (Table 2). The latter are interesting components of the pathogen box as mebendazole (MMV003152) is a known anti-helminthic drug that binds tubulin, inhibiting its assembly into microtubules and consequently perturbing the motility, division and the secretion processes of cells (Wampande et al., 2007). In helminths, binding of the drug leads to loss of cytoplasmic microtubules which perturbs glucose intake leading to exhaustion of glycogen reserves and consequent death (Oxberry et al., 2001). The second reference compound, auranofin (MMV688978), is a drug indicated for rheumatoid arthritis and thought to act through inhibition of nuclear factor kappa-B kinase subunit beta (Jeon et al., 2003) and thioredoxin reductase (Rigobello et al., 2005) leading to reduced inflammatory responses and reduced free radical production, respectively. Although both reference compounds demonstrated inhibitory activities on F100TpM with IC50s < 1 μM, their low therapeutic index questions a role for them as anti-theilerial lead compounds.
The malaria box has been screened before for inhibitory activity on Theileria annulata infected cell lines and only one inhibitory compound in our assays, MMV665820, was also inhibitory to T. annulata cell lines (Hostettler et al., 2016). Although this compound did not impair viability of the human foreskin fibroblast control cells in the T. annulata assay, it was eliminated as a lead compound since it failed to lower the relative expression of T. annulata gene TaSP by 50%, suggesting that its activity was not parasite specific (Hostettler et al., 2016). In our assay, the therapeutic index for this compound was 1.67 leading to its elimination as a lead compound. The different profile of inhibitory compound activities on these two highly related parasite cell lines illustrates the differences in biology between these parasites and underscores the difficulty associated with discovery and development of anti-theilerial drug(s). We are not aware of a screen of compounds from the pathogen box on T. annulata cell lines. Hence, MV688372, should be tested against T. annulata cell lines as this might lead to discovery of compound(s) effective in both organisms.
Dasatinib exhibited very potent inhibitory activity on F100TpM cells with IC50 (5 nM) and CC50 (>10 μM), values similar to buparvaquone, the current drug of choice for chemotherapy of ECF. It will be interesting to test dasatinib activity on T. annulata and other transforming Theileria pathogen cell lines. Dasatinib is a commercial drug approved and indicated for chronic myelogenous leukemia and acute lymphoblastic leukemia (Steinberg, 2007; Talpaz et al., 2006). It is being considered for treatment of other cancers (Finn et al., 2007; Hochhaus et al., 2008; Johnson et al., 2010). In humans, the drug has multiple targets including tyrosine-protein kinases (Bcr-Abl, Lck and Src family) (Quintas-Cardama et al., 2006), platelet-derived growth factor receptor beta (Chen et al., 2006) and signal transducer and activator of transcription 5B (Nam et al., 2007). Therefore, dasatinib's mode of action on T. parva infected lymphocytes could be through one or a combination of the targets, as these cells exhibit cancer-like properties with an up-regulation of growth factor receptors, NF-kB and tyrosine protein kinase cell-signaling pathways, among other cellular changes (Shiels et al., 2006). This raises the possibility that the inhibitory activity of dasatinib on T. parva infected cells is via inhibiting a host cellular pathway, rather than directly targeting the pathogen, and opens an additional avenue for drug discovery for the control of ECF.
In conclusion, we have identified two lead MMV compounds one from the malaria box and the other from the pathogen box with a therapeutic index >5 that could form starting points for development of alternative drugs to control ECF, and, possibly other forms of theileriosis. In addition, we have demonstrated a very high therapeutic index of dasatinib on a T. parva infected lymphocyte cell line. A large number of related tyrosine kinase inhibitors are available and suggests that these molecules could be explored for their use as repurposed drugs, provided cost is not an inhibitory factor.
Conflicts of interest
Authors declare no conflict of interest.
Acknowledgements
We are grateful to Mike Witty for scientific discussions, Robert Mugambi and Elizabeth Kibwana (International Livestock Research Institute, Kenya) for providing cells. We thank the CGIAR Research Program on Livestock and Fish, Kenya, the Norman Borlaug Commemorative Research Initiative, an initiative between the Feed the Future program of United States Agency for International Development, USA and United States Department of Agriculture-Agricultural Research Service, USA (58-5348-2-117F) and the Department for International Development of the United Kingdom and the Bill and Melinda Gates Foundation, USA (OPP1078791) for financial support. We would also like to thank MMV for provision of the Malaria and Pathogen box compounds.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2019.01.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Baldwin C.L., Black S.J., Brown W.C., Conrad P.A., Goddeeris B.M., Kinuthia S.W., Lalor P.A., MacHugh N.D., Morrison W.I., Morzaria S.P. Bovine T cells, B cells, and null cells are transformed by the protozoan parasite Theileria parva. Infect. Immun. 1988;56:462–467. doi: 10.1128/iai.56.2.462-467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.G. Control of tropical theileriosis (Theileria annulata infection) of cattle. Parassitologia. 1990;32:23–31. [PubMed] [Google Scholar]
- Chen Z., Lee F.Y., Bhalla K.N., Wu J. Potent inhibition of platelet-derived growth factor-induced responses in vascular smooth muscle cells by BMS-354825 (dasatinib) Mol. Pharmacol. 2006;69:1527–1533. doi: 10.1124/mol.105.020172. [DOI] [PubMed] [Google Scholar]
- Clinical Trials Partnership R. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet (London, England) 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer J., Tam K. Wiley-VCH; Zürich, Switzerland: 2001. Lipophilicity Profiles: Theory and Measurement; pp. 275–304. [Google Scholar]
- Cui L., Mharakurwa S., Ndiaye D., Rathod P.K., Rosenthal P.J. Antimalarial drug resistance: literature review and activities and findings of the ICEMR network. Am. J. Trop. Med. Hyg. 2015;93:57–68. doi: 10.4269/ajtmh.15-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere D.A.E., Rottenberg S. Theileria-induced leukocyte transformation. Curr. Opin. Microbiol. 2003;6:377–382. doi: 10.1016/s1369-5274(03)00085-7. [DOI] [PubMed] [Google Scholar]
- Duffy S., Sykes M.L., Jones A.J., Shelper T.B., Simpson M., Lang R., Poulsen S.-A., Sleebs B.E., Avery V.M. Screening the Medicines for malaria venture pathogen box across multiple pathogens reclassifies starting points for open-source drug discovery. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fich C., Klauenberg U., Fleischer B., BrÖKer B.M. Modulation of enzymatic activity of Src-family kinases in bovine T cells transformed by Theileria parva. Parasitology. 1998;117:107–115. doi: 10.1017/s0031182098002832. [DOI] [PubMed] [Google Scholar]
- Finn R.S., Dering J., Ginther C., Wilson C.A., Glaspy P., Tchekmedyian N., Slamon D.J. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Canc. Res. Treat. 2007;105:319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- Hochhaus A., Baccarani M., Deininger M., Apperley J.F., Lipton J.H., Goldberg S.L., Corm S., Shah N.P., Cervantes F., Silver R.T., Niederwieser D., Stone R.M., Dombret H., Larson R.A., Roy L., Hughes T., Müller M.C., Ezzeddine R., Countouriotis A.M., Kantarjian H.M. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- Hostettler I., Müller J., Hemphill A. In vitro screening of the open source MMV malaria box reveals novel compounds with profound activities against Theileria annulata schizonts. Antimicrob. Agents Chemother. 2016;60:3301–3308. doi: 10.1128/AAC.02801-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon K.I., Byun M.S., Jue D.M. Gold compound auranofin inhibits IkappaB kinase (IKK) by modifying Cys-179 of IKKbeta subunit. Exp. Mol. Med. 2003;35:61–66. doi: 10.1038/emm.2003.9. [DOI] [PubMed] [Google Scholar]
- Johnson F.M., Bekele B.N., Feng L., Wistuba I., Tang X.M., Tran H.T., Erasmus J.J., Hwang L.-L., Takebe N., Blumenschein G.R., Lippman S.M., Stewart D.J. Phase II study of dasatinib in patients with advanced non–small-cell lung cancer. J. Clin. Oncol. 2010;28:4609–4615. doi: 10.1200/JCO.2010.30.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHardy N., Wekesa L.S., Hudson A.T., Randall A.W. Antitheilerial activity of BW720C (buparvaquone): a comparison with parvaquone. Res. Vet. Sci. 1985;39:29–33. [PubMed] [Google Scholar]
- Metheni M., Lombès A., Bouillaud F., Batteux F., Langsley G. HIF-1α induction, proliferation and glycolysis of Theileria-infected leukocytes. Cell Microbiol. 2015;17:467–472. doi: 10.1111/cmi.12421. [DOI] [PubMed] [Google Scholar]
- Mhadhbi M., Naouach A., Boumiza A., Chaabani M.F., BenAbderazzak S., Darghouth M.A. In vivo evidence for the resistance of Theileria annulata to buparvaquone. Vet. Parasitol. 2010;169:241–247. doi: 10.1016/j.vetpar.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Morrison D.A. Evolution of the Apicomplexa: where are we now? Trends Parasitol. 2009;25:375–382. doi: 10.1016/j.pt.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Nam S., Williams A., Vultur A., List A., Bhalla K., Smith D., Lee F.Y., Jove R. Dasatinib (BMS-354825) inhibits Stat5 signaling associated with apoptosis in chronic myelogenous leukemia cells. Mol. Canc. Therapeut. 2007;6:1400–1405. doi: 10.1158/1535-7163.MCT-06-0446. [DOI] [PubMed] [Google Scholar]
- Nixon G.L., Moss D.M., Shone A.E., Lalloo D.G., Fisher N., O'Neill P.M., Ward S.A., Biagini G.A. Antimalarial pharmacology and therapeutics of atovaquone. J. Antimicrob. Chemother. 2013;68:977–985. doi: 10.1093/jac/dks504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norval R.A.I., Perry B.D., Young A. Academic press; London: 1992. The Epidemiology of Theileriosis in Africa. [Google Scholar]
- Oxberry M.E., Gear T.G., Prichard R.K. Assessment of benzimidazole binding to individual recombinant tubulin isotypes from Haemonchus contortus. Parasitology. 2001;122:683–687. doi: 10.1017/s0031182001007788. [DOI] [PubMed] [Google Scholar]
- Palacios R. Concanavalin A triggers T lymphocytes by directly interacting with their receptors for activation. J. Immunol. 1982;128:337–342. [PubMed] [Google Scholar]
- Perry B.D. The control of East Coast fever of cattle by live parasite vaccination: a science-to-impact narrative. One Health. 2016;2:103–114. doi: 10.1016/j.onehlt.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintas-Cardama A., Kantarjian H., Cortes J. Targeting ABL and SRC kinases in chronic myeloid leukemia: experience with dasatinib. Future Oncol. (London, England) 2006;2:655–665. doi: 10.2217/14796694.2.6.655. [DOI] [PubMed] [Google Scholar]
- Rigobello M.P., Folda A., Baldoin M.C., Scutari G., Bindoli A. Effect of auranofin on the mitochondrial generation of hydrogen peroxide. Role of thioredoxin reductase. Free Radic. Res. 2005;39:687–695. doi: 10.1080/10715760500135391. [DOI] [PubMed] [Google Scholar]
- Salas C.O., Faundez M., Morello A., Diego Maya J., A. Tapia R. Natural and synthetic naphthoquinones active against trypanosoma cruzi: an initial step towards new drugs for chagas disease. Curr. Med. Chem. 2011;18:144–161. doi: 10.2174/092986711793979779. [DOI] [PubMed] [Google Scholar]
- Sharifiyazdi H., Namazi F., Oryan A., Shahriari R., Razavi M. Point mutations in the Theileria annulata cytochrome b gene is associated with buparvaquone treatment failure. Vet. Parasitol. 2012;187:431–435. doi: 10.1016/j.vetpar.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Shiels B., Langsley G., Weir W., Pain A., McKellar S., Dobbelaere D. Alteration of host cell phenotype by Theileria annulata and Theileria parva: mining for manipulators in the parasite genomes. Int. J. Parasitol. 2006;36:9–21. doi: 10.1016/j.ijpara.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clin. Therapeut. 2007;29:2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Talpaz M., Shah N.P., Kantarjian H., Donato N., Nicoll J., Paquette R., Cortes J., O'Brien S., Nicaise C., Bleickardt E., Blackwood-Chirchir M.A., Iyer V., Chen T.T., Huang F., Decillis A.P., Sawyers C.L. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- Vaidya A.B., Mather M.W. Atovaquone resistance in malaria parasites. Drug Resist. Updates. 2000;3:283–287. doi: 10.1054/drup.2000.0157. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W.C., Adams J.H., Adelfio R., Ahyong V., Akabas M.H., Alano P., Alday A., Alemán Resto Y., Alsibaee A., Alzualde A., Andrews K.T., Avery S.V., Avery V.M., Ayong L., Baker M., Baker S., Ben Mamoun C., Bhatia S., Bickle Q., Bounaadja L., Bowling T., Bosch J., Boucher L.E., Boyom F.F., Brea J., Brennan M., Burton A., Caffrey C.R., Camarda G., Carrasquilla M., Carter D., Belen Cassera M., Chih-Chien Cheng K., Chindaudomsate W., Chubb A., Colon B.L., Colón-López D.D., Corbett Y., Crowther G.J., Cowan N., D'Alessandro S., Le Dang N., Delves M., DeRisi J.L., Du A.Y., Duffy S., Abd El-Salam El-Sayed S., Ferdig M.T., Fernández Robledo J.A., Fidock D.A., Florent I., Fokou P.V.T., Galstian A., Gamo F.J., Gokool S., Gold B., Golub T., Goldgof G.M., Guha R., Guiguemde W.A., Gural N., Guy R.K., Hansen M.A.E., Hanson K.K., Hemphill A., Hooft van Huijsduijnen R., Horii T., Horrocks P., Hughes T.B., Huston C., Igarashi I., Ingram-Sieber K., Itoe M.A., Jadhav A., Naranuntarat Jensen A., Jensen L.T., Jiang R.H.Y., Kaiser A., Keiser J., Ketas T., Kicka S., Kim S., Kirk K., Kumar V.P., Kyle D.E., Lafuente M.J., Landfear S., Lee N., Lee S., Lehane A.M., Li F., Little D., Liu L., Llinás M., Loza M.I., Lubar A., Lucantoni L., Lucet I., Maes L., Mancama D., Mansour N.R., March S., McGowan S., Medina Vera I., Meister S., Mercer L., Mestres J., Mfopa A.N., Misra R.N., Moon S., Moore J.P., Morais Rodrigues da Costa F., Müller J., Muriana A., Nakazawa Hewitt S., Nare B., Nathan C., Narraidoo N., Nawaratna S., Ojo K.K., Ortiz D., Panic G., Papadatos G., Parapini S., Patra K., Pham N., Prats S., Plouffe D.M., Poulsen S.-A., Pradhan A., Quevedo C., Quinn R.J., Rice C.A., Abdo Rizk M., Ruecker A., St. Onge R., Salgado Ferreira R., Samra J., Robinett N.G., Schlecht U., Schmitt M., Silva Villela F., Silvestrini F., Sinden R., Smith D.A., Soldati T., Spitzmüller A., Stamm S.M., Sullivan D.J., Sullivan W., Suresh S., Suzuki B.M., Suzuki Y., Swamidass S.J., Taramelli D., Tchokouaha L.R.Y., Theron A., Thomas D., Tonissen K.F., Townson S., Tripathi A.K., Trofimov V., Udenze K.O., Ullah I., Vallieres C., Vigil E., Vinetz J.M., Voong Vinh P., Vu H., Watanabe N.-a., Weatherby K., White P.M., Wilks A.F., Winzeler E.A., Wojcik E., Wree M., Wu W., Yokoyama N., Zollo P.H.A., Abla N., Blasco B., Burrows J., Laleu B., Leroy D., Spangenberg T., Wells T., Willis P.A. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog. 2016;12:e1005763. doi: 10.1371/journal.ppat.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampande E.M., Richard McIntosh J., Lubega G.W. Classical ligands interact with native and recombinant tubulin from Onchocerca volvulus with similar rank order of magnitude. Protein Expr. Purif. 2007;55:236–245. doi: 10.1016/j.pep.2007.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


