Abstract
Introduction
Amyloid pathology in cognitively normal adults is associated with subjective cognitive decline, potentially reflecting awareness of Alzheimer's-related memory deficits. To clarify the mechanism underlying this relationship, we used mediational analyses to determine the role of depression, anxiety, and actual memory performance.
Methods
To assess amyloid deposition, we imaged 85 cognitively normal adults with florbetapir positron emission tomography imaging. Subjective cognitive decline was measured using a multidimensional instrument that assessed seven subjective memory domains. Mediational measures included assessments of actual memory performance (current and retrospective longitudinal change), depression, and anxiety.
Results
The relationship between amyloid and subjective cognitive decline was mediated by poorer memory performance and greater retrospective memory decline, not depression or anxiety. The mediational roles were significant for domains associated with memory function and memory-related anxiety.
Discussion
In individuals harboring amyloid, self-reported beliefs of declining memory likely indicate early self-awareness of actual worsening function rather than depression or anxiety.
Keywords: Amyloid, Subjective cognitive decline, Subjective cognitive concerns, Memory complaints, Metamemory, Preclinical AD, Retrospective decline, Longitudinal change, Anxiety, Depression, Mediation
1. Background
Growing evidence indicates that amyloid deposition in cognitively normal adults signals a prolonged asymptomatic period of subtle neuropathology, lasting a decade or more, that precedes the clinical symptoms of Alzheimer's disease (AD) [1], [2]. The high prevalence of AD combined with considerable media attention to the disorder has resulted in many older adults having concerns that their declining memory is symptomatic of AD [3]. The mechanism underlying this relationship between amyloid and subjective cognitive decline (SCD) is thought to be actual memory performance but is in fact poorly understood [4], [5], [6]. An issue of growing importance is whether increased SCD in cognitively normal individuals with elevated amyloid reflects actual declines related to early AD pathology [7], [8] or perhaps results from anxiety or depression.
Among existing studies that found a relationship between amyloid and SCD (see a comprehensive summary in Appendix A), five included both measures of actual cognition and depression or anxiety but yielded inconsistent findings [9], [10], [11], [12], [13]. To address this issue, we used mediational analysis [14] to assess the mechanism underlying the relationship between amyloid and SCD. This approach provides stronger evidence than traditional models that correlate depression, anxiety, or actual cognition to SCD and directly investigates whether a mediational model including potential mediators (e.g., actual memory, depression, anxiety) explains the amyloid/SCD relationship.
SCD presumably reflects an individual's belief of past memory change, so it is conceptually more closely related to retrospective memory change rather than just present performance. Thus, the present study included two measures of actual memory—current memory performance measured at the time of SCD assessment and retrospective memory change over the previous 4 years—allowing us to verify that not just level of performance, but actual decline, mediated increased SCD in people harboring amyloid. In addition, concerns of SCD are multidimensional [15], but there has been little investigation of different categories of memory complaints.
The present study clarifies these issues by (1) simultaneously assessing the mediational effects of depression, anxiety, and actual memory as mediators of the amyloid/SCD relationship, (2) including both current performance and retrospective decline as measures of actual memory, and (3) using a multidimensional SCD questionnaire, the “Metamemory in Adulthood” [16] that differentiates seven subjective memory domains, allowing us to isolate the specific features of SCD that are most sensitive to amyloid deposition.
In addition, we studied a broader age spectrum than previous research by including middle-aged adults as there is some evidence that SCD is more predictive of amyloid pathology [17] and memory decline [18] in younger-older adults compared with the oldest adults. We also included measures of other cognitive abilities, including processing speed, inductive reasoning, and vocabulary, and examined whether deficits or declines in any of these domains mediated the amyloid/SCD relationship. We expected to see a specific association between actual memory and subjective complaints in memory.
2. Methods
2.1. Participants
This study included a total of 85 cognitively normal adults (aged 34-93 years) from the Dallas Lifespan Brain Study who completed the Metamemory in Adulthood questionnaire, and the assessment in memory, depression, and anxiety, as well as 18F florbetapir positron emission tomography (PET) imaging. Detailed information about participant characteristics is included in Table 1.
Table 1.
Demographic information and characteristics of participants
| Variable | Total (N = 85) | Liberal threshold SUVR = 1.09 |
Conservative threshold SUVR = 1.12 |
||||
|---|---|---|---|---|---|---|---|
| Negative (N = 53) | Positive (N = 32) | P | Negative (N = 61) | Positive (N = 24) | P | ||
| Age (SD) | 66.97 (15.11) | 61.25 (14.86) | 76.46 (9.96) | <.001∗ | 62.80 (14.82) | 77.59 (9.85) | <.001∗ |
| Female, n (%) | 56 (65.9) | 32 (60.4) | 24 (75.0) | .171 | 39 (63.9) | 17 (70.8) | .548 |
| APOE ε3/ε4 or ε4/ε4 (%) | 17 (20) | 8 (15.1) | 9 (28.1) | .189 | 10 (16.4) | 7 (29.2) | .228 |
| Amyloid, SUVR (SD) | 1.13 (0.19) | 1.02 (0.36) | 1.30 (0.21) | <.001∗ | 1.03 (0.42) | 1.37 (0.20) | <.001∗ |
| Years of education (SD) | 15.99 (2.34) | 15.76 (2.24) | 16.38 (2.47) | .238 | 15.89 (2.30) | 16.25 (2.45) | .520 |
| MMSE (SD) | 29.12 (1.19) | 29.40 (1.08) | 28.66 (1.23) | .005∗ | 29.41 (1.07) | 28.38 (1.17) | <.001∗ |
| CES-D (SD) | 4.62 (4.94) | 4.48 (5.16) | 4.83 (4.65) | .760 | 4.70 (5.01) | 4.41 (4.87) | .819 |
| NIHTB fear survey (SD) | 9.04 (2.68) | 9.44 (3.04) | 8.35 (1.76) | .042∗ | 9.22 (2.76) | 8.57 (1.90) | .324 |
| Wave 1 and wave 2 interval | 3.99 (0.23) | 4.02 (0.25) | 3.95 (0.17) | .155 | 4.00 (0.24) | 3.97 (0.18) | .533 |
| Wave 1 Memory Performance | |||||||
| VRM immediate recall | 7.41 (2.01) | 7.75 (2.09) | 6.84 (1.76) | .043∗ | 7.67 (2.13) | 6.75 (1.51) | .029∗ |
| VRM delayed recognition | 22.47 (1.71) | 22.83 (1.59) | 21.88 (1.76) | .012∗ | 22.66 (1.66) | 22.00 (1.77) | .112 |
| Hopkins immediate recall | 6.95 (1.88) | 7.17 (1.81) | 6.59 (1.97) | .172 | 7.16 (1.73) | 6.42 (2.15) | .099 |
| Hopkins delayed recognition | 20.82 (2.26) | 21.04 (2.34) | 20.47 (2.12) | .264 | 20.97 (2.45) | 20.46 (1.69) | .354 |
| Hopkins delayed recall | 5.34 (2.55) | 5.55 (2.55) | 5.00 (2.55) | .341 | 5.59 (2.42) | 4.71 (2.81) | .153 |
| Wave 2 Memory Performance | |||||||
| VRM immediate recall | 6.99 (2.10) | 7.36 (2.04) | 6.38 (2.09) | .036∗ | 7.34 (1.97) | 6.08 (2.19) | .012∗ |
| Hopkins immediate recall | 7.00 (1.89) | 7.34 (1.89) | 6.42 (1.78) | .031∗ | 7.31 (1.84) | 6.17 (1.83) | .047∗ |
| Hopkins delayed recognition | 20.40 (2.39) | 20.65 (2.28) | 20.00 (2.54) | .076 | 20.58 (2.21) | 19.96 (2.79) | .013∗ |
| Hopkins delayed recall | 5.52 (2.63) | 5.92 (2.63) | 4.88 (2.55) | .226 | 5.88 (2.54) | 4.63 (2.70) | .282 |
| Logical memory immediate | 29.52 (6.99) | 31.32 (6.49) | 26.53 (6.87) | .002∗ | 31.25 (6.28) | 25.13 (6.92) | <.001∗ |
| Logical memory delayed | 25.54 (8.86) | 27.49 (7.97) | 22.31 (9.44) | .008∗ | 27.28 (7.70) | 21.13 (10.18) | .003∗ |
| Memory for names immediate | 52.71 (12.07) | 56.30 (10.67) | 46.58 (12.01) | <.001∗ | 55.46 (10.61) | 45.43 (12.90) | <.001∗ |
| Memory for names delayed | 21.23 (9.65) | 24.33 (8.63) | 16.03 (9.12) | <.001∗ | 23.43 (8.85) | 15.48 (9.42) | .001∗ |
Abbreviations: CES-D, Center for Epidemiologic Studies-Depression; MMSE, Mini-Mental State Examination; NIHTB, NIH Toolbox Emotion Battery; SUVR, standard uptake value ratio; VRM, Verbal Recognition Memory.
NOTE. Results are presented as means (SDs) or percents. Values in bold indicate a significant higher group mean compared with the other gorup.
Indicates significance level of P < .05.
2.2. Behavioral measures
SCD was measured using the Metamemory in Adulthood questionnaire, an instrument that assesses specific domains of subjective memory, including participants' perception of memory capacity, stability, control, anxiety of poor memory performance, memory strategy use, knowledge about memory processes, and importance of memory achievements. Each domain is assessed with 10-18 statements (Appendix B). Participants rated how much they agree with each statement on a five-point Likert scale with lower scores reflecting greater complaints, except for memory-related anxiety where a high value reflects greater complaints.
To measure actual memory, eight measures from four episodic memory tasks were standardized and then averaged to form a single composite that represents the level of participants' current performance. The eight measures included immediate and delayed scores from the WMS Logical Memory [19]; immediate and delayed scores from the Woodcock-Johnson Memory for Names Test [20]; immediate free-recall, delayed free-recall, and delayed recognition scores from the Hopkins Verbal Learning Test [21]; and immediate free-recall from the CANTAB Verbal Recognition Memory [22]. This composite score of current performance had a very high internal consistency, Cronbach's α = .915. In addition, we obtained a retrospective memory score from data collected from the same participants 4 years earlier, using five episodic memory measures (immediate free-recall, delayed free-recall, and delayed recognition from the Hopkins Verbal Learning Test and immediate free-recall and delayed recognition from the CANTAB Verbal Recognition Memory). Four of the measures were assessed at both waves of testing, and the retrospective composite score also yielded a high internal consistency, Cronbach's α = .809. Retrospective decline was computed as the difference score between the current score and the retrospective score 4 years prior (wave 2 − wave 1).
In addition to memory, we also had measures of processing speed, inductive reasoning, and vocabulary (see Appendix C for details of the assessments used). We constructed current performance measures and retrospective change measures for each cognitive domain, using the same method described previously for actual memory.
To measure depression, participants completed the Center for Epidemiologic Studies–Depression (CES-D) Scale [23]. Anxiety was measured by the Fear-Affect Survey, taken from the NIH Toolbox Emotion Battery [24].
2.3. Magnetic resonance imaging protocol
Participants were scanned using a 3T Philips Achieva scanner with an eight-channel head coil. High-resolution anatomical images were collected with a T1-weighted magnetization-prepared rapid gradient-echo sequence with 160 sagittal slices (field of view: 204 × 256 × 160 mm; voxel size: 1 × 1 × 1 mm3; time to repetition: 8.1 milliseconds; echo time: 3.7 milliseconds; flip angle: 12°).
Anatomical images were processed using FreeSurfer 5.3 (FreeSurfer, http://surfer.nmr.mgh.harvard.edu/) with thorough manual editing, as described previously [25]. Specifically, the imaging data for each individual were visually inspected, edited for inaccuracies (with rechecking and reediting, as needed). Particular attention was paid to accurate skull stripping, white matter segmentation, gray and white matter boundaries, and surface reconstruction. FreeSurfer volumetric segmentation was used to obtain cortical parcellations according to the Desikan-Killiany atlas [26].
2.4. PET protocol
Participants were injected with a 370-MBq (10 mCi) bolus of AV45-florbetapir. Fifty minutes after the injection, a 2-frame by 5-minute each dynamic emission acquisition was started using an ECAT HR PET scanner (Siemens Healthineers). The PET scan was coregistered to the magnetic resonance imaging using FMRIB's Linear Image Registration Tool (FLIRT, https://fsl.fmrib.ox.ac.uk/fsl) with a mutual-information cost function.
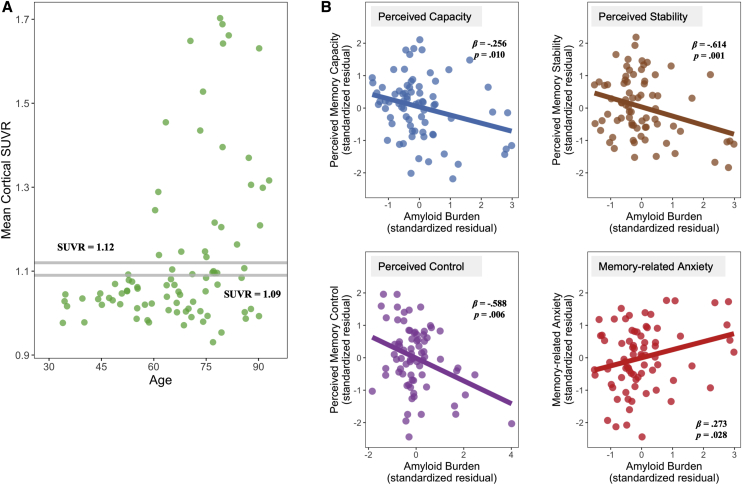
We used a continuous measure of the mean cortical standard uptake value ratio (SUVR) in all analyses. It was computed from seven bilateral FreeSurfer-derived regions of interest (dorsolateral prefrontal, orbitofrontal, lateral parietal, lateral temporal, precuneus, isthmus cingulate, and rostral anterior cingulate cortices), using the whole cerebellum as a reference region [24]. For descriptive purposes, we reported sample characteristics based on their amyloid positivity, using two SUVR thresholds, a liberal one of 1.09 and a conservative one of 1.12, separately set two or three SDs above the mean SUVR for a young reference group in the Dallas Lifespan Brain Study sample at baseline [8]. We had 37.6% participants (N = 32) categorized as amyloid-positive based on the liberal threshold and 28.2% amyloid-positive participants (N = 24) based on the conservative one, similar to the ratio in other samples [2], [27]. Fig. 1A presents the distribution of amyloid burden across age in our sample.
Fig. 1.
(A) Distribution of amyloid across age. (B) Partial regression plot of the relationship between amyloid burden and SCD, controlling for age, sex, and education. Abbreviation: SCD, subjective cognitive decline.
2.5. Statistical analyses
First, we assessed whether there was a relationship between amyloid and SCD in different domains (perceived capacity, perceived stability, perceived control, memory-related anxiety, memory strategy use, knowledge about memory processes, and importance of memory achievements). We used multiple regression to model the effects of age, amyloid, and age × amyloid interaction on each of the seven SCD domains, controlling for sex and education. Both age and amyloid were treated as continuous variables. The significance of effects was estimated using a bootstrap procedure with the bias-corrected and accelerated 95% confidence interval (BCa 95% CI) with 5000 iterations. A false discovery rate procedure was used to adjust P values within models exploring the same family of hypotheses [28], correcting for multiple comparisons. Nonsignificant interactions were removed to conserve power.
Next, we investigated the relationship between the proposed mediators (current performance, retrospective decline, depression, anxiety) and measures of SCD. We conducted a series of multiple regressions that examined the individual and joint effects of potential mediators on each domain of SCD, while controlling for age, sex, and education. When examining the effect of retrospective decline, we also controlled for the baseline memory 4 years earlier.
We then subjected those SCD domains that showed significant relationships with amyloid to a mediational analysis using PROCESS [14]. Although traditionally mediation requires a significant association between the mediator and the dependent variable, the most recent studies suggest that it is more appropriate to consider all a priori potential mediators, even if they do not have a significant individual effect [29], [30]. We simultaneously assessed the role of actual memory, depression, and anxiety in explaining the amyloid/SCD relationship, while controlling for age, sex, and education. We then also considered age as a moderator of the mediation.
Finally, we conducted supplementary analyses that examined (1) reverse causality [31] by modeling possible mediation of SCD in the relationship between amyloid and actual memory, (2) possible influences of hippocampal and entorhinal volume measures (based on the volumetric measures from FreeSurfer segmentation), race, and APOE status in any observed mediation effects, and (3) whether the mediation was limited to memory or would also be found with measures in other cognitive domains. All analyses were performed in SPSS v25.
3. Results
3.1. Relationship between amyloid and different domains of SCD
The first set of regression assessed the relationship between amyloid and SCD. Amyloid burden was associated with four of the seven domains (Fig. 1B; Appendix D): higher amyloid was related to lower perceived capacity (β = −.256, P = .010, Padj = .023), lower perceived stability (β = −.614, P = .001, Padj = .007), lower perceived control (β = −.588, P = .006, Padj = .049), and greater memory-related anxiety (β = .273, P = .027, Padj = .021). Although there was no age effect, we found a significant age × amyloid integration for perceived stability (P = .025) and control (P = .027). Both occurred because higher amyloid burden was related to greater SCD at younger ages1 (Appendix E). The other three SCD domains that involved attributes of memory rather than actual function—use of memory strategy, knowledge about memory processes, and importance of memory achievement—were not related to amyloid (P's > .1; Appendix D).
3.2. Relationship between different domains of SCD and actual memory, depression, and anxiety
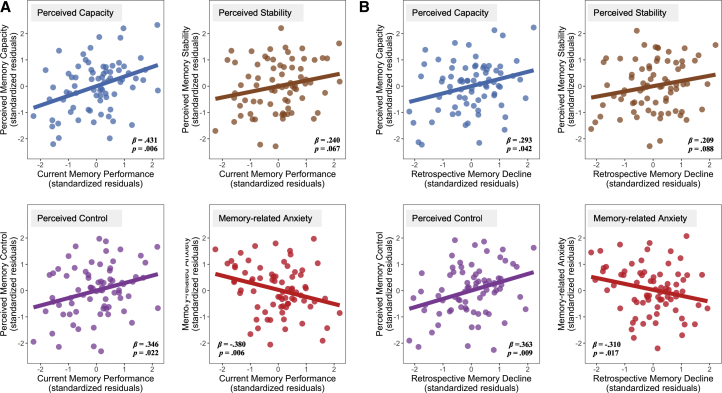
The next set of analyses address the relationships between potential mediators (current performance/retrospective decline, depression, and anxiety) and SCD. Using multiple regression, we found that current memory performance was related to lower perceived capacity (β = .431, P = .006, Padj = .021), lower perceived control (β = .346, P = .022, Padj = .051), and memory-related anxiety (β = −.380, P = .006, Padj = .021) and marginally related to lower perceived stability (β = .240, P = .067, Padj = .117; Fig. 2A; Appendix D). Depression was associated with lower perceived stability (β = −.303, P = .021, Padj = .081) and memory-related anxiety (β = .334, P = .023, Padj = .081) and marginally with lower perceived capacity (β = .334, P = .073, Padj = .128). Anxiety, however, was not related to any SCD domains (P's > .1). Models for the other three SCD domains were not significant (P's > .1; Appendix D).
Fig. 2.
(A) Partial relationships between actual current memory performance and SCD, controlling for age, sex, education, depression, and anxiety. (B) Partial relationships between actual retrospective memory declines and SCD, controlling for age, sex, education, depression, anxiety, and baseline memory. Abbreviation: SCD, subjective cognitive decline.
Similarly, regressions with retrospective decline, depression, and anxiety showed that greater retrospective decline was significantly associated with lower perceived capacity (β = .293, P = .042, Padj = .098), lower perceived control (β = .363, P = .009, Padj = .060), and greater memory-related anxiety (β = -.310, P = .060) and marginally associated with lower perceived stability (β = .209, P = .088, Padj = .154; Fig. 2B). Depression was related to lower perceived stability (β = −.297, P = .028, Padj = .098) and greater memory-related anxiety (β = .330, P = .028, Padj = .098) and marginally related to lower perceived capacity (β = −.218, P = .058, Padj = .135). Anxiety did not show any significant relationships (P's > .1). None of the effects of potential mediators for the other three SCD domains were significant (P's > .1; Appendix D).
In addition, to better interpret the effects of retrospective decline, we examined the effects of testing wave on memory scores to verify the direction of longitudinal change. We used a mixed-design analysis of variance including wave, age, and amyloid as factors and found a significant main effect of wave, suggesting that the overall memory score declined from wave 1 to wave 2. We also found a significant interaction between amyloid and wave indicating that the overall decline was driven by the individuals with greater amyloid (Appendix F).
3.3. Mediational mechanism underlying the relationship between amyloid and SCD
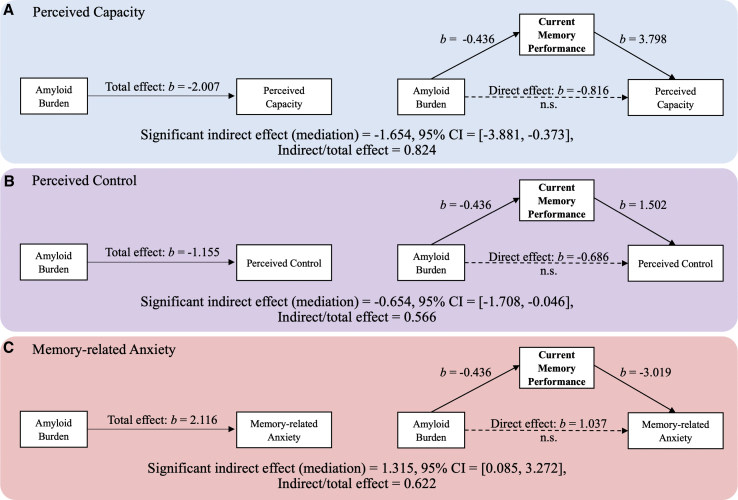
We first simultaneously examined whether current performance, depression, and/or anxiety mediated the amyloid/SCD relationship, while controlling for age, sex, and education. We found that only current performance, not depression or anxiety, had significant mediational effects: the significant mediation models (perceived capacity, perceived control, memory-related anxiety) are shown in Fig. 3. In each model, the total effect refers to the initial relationship of amyloid on SCD, excluding the mediator. The direct effect represents the direct amyloid effect that is independent of the mediator. The indirect effect represents the magnitude of the effect accounted for by the mediator, implying a possible causal relationship of amyloid on SCD through the mediator [32]. Models A, B, and C show that the effect of amyloid on perceived capacity, perceived control, and memory-related anxiety is greatly reduced when current memory performance is included as a mediator (Fig. 3; Appendix G). Depression or anxiety did not show significant mediation for any SCD domain (Appendix G).
Fig. 3.
Mediation of current memory performance for the relationship between amyloid and SCD on perceived capacity (A), perceived control (B), and memory-related anxiety (C). Depression and anxiety were simultaneously estimated as potential mediators but did not show any significant mediation. Age, sex, and education were controlled. Age × amyloid interaction was also controlled for perceived control. Abbreviations: CI, confidence interval; SCD, subjective cognitive decline.
Then, using the same approach, we examined the mediational effects of retrospective decline, depression, and anxiety, controlling for age, sex, education, and baseline memory. For the same SCD domains previously noted, the retrospective memory decline, but not depression or anxiety, mediated the relationships (Fig. 4; Appendix G), suggesting a consistent finding that SCD in individuals harboring amyloid reflects actual memory deficits that are related to early amyloid pathology, rather than depressive or anxious symptoms (Appendix G). Excluding anxiety did not change the results (Appendix H).
Fig. 4.
Mediation of retrospective memory decline for the relationship between amyloid and SCD on perceived capacity (A), perceived control (B), and memory-related anxiety (C). Depression and anxiety were simultaneously estimated as potential mediators but did not show any significant mediation. Age, sex, education, and baseline memory were controlled. Age × amyloid interaction was also controlled for perceived control. Abbreviations: CI, confidence interval; SCD, subjective cognitive decline.
We also tested whether the mediation differed as a function of age. For perceived capacity, we found a significant moderated mediation of current performance (Table 2), such that poorer memory mediated the amyloid/SCD relationship at the middle (age = 53) and younger-old age (age = 68), but not the oldest age (age = 83). Despite that both amyloid/SCD relationship and amyloid/memory relationship were significant at all ages, poorer memory did not mediate SCD at the oldest age, suggesting that younger individuals' SCD of perceived capacity more accurately reflected their current memory difficulties related to amyloid, compared with the oldest individuals. We did not find any age-related differences in any other mediational effect.
Table 2.
Age-moderated mediation of current memory performance
| Perceived memory capacity | Effect coefficient | 95% confidence interval |
|---|---|---|
| Current performance × Age | −3.003 | [−5.240, −0.766] |
| Indirect effect at age = 53 | −3.142 | [−6.074, −1.068] |
| Indirect effect at age = 68 | −1.834 | [−3.985, −0.471] |
| Indirect effect at age = 83 | −0.526 | [−2.636, 0.522] |
NOTE. Confidence interval in bold indicates that zero is outside the confidence interval, suggesting a significant coefficient at α = .05 level for bootstrap estimates.
Finally, we conducted a series of supplementary analyses to verify the significant mediations. First, to confirm the directionality of the mediation, we considered reverse causality [31] and examined whether SCD may mediate the relationship between amyloid and actual performance. We did not find any significant effect of SCD as a mediator (Appendix I), confirming the legitimacy of the mediation models and the directionality of the relationship. Then, we considered possible influences of hippocampal and entorhinal volume differences, race, and APOE status (ε4 carrier or non-carrier) and repeated the mediation analyses including them as additional covariates. All observed effects remained significant or marginally significant (Appendix J). Finally, we examined whether the mediation of actual performance was limited to memory function, or declines in other cognitive domains may also be mediating the amyloid/SCD relationship. Measures of current performance and retrospective decline in three additional cognitive domains (processing speed, inductive reasoning, and vocabulary) were examined as mediators individually. We found no evidence of mediation for any SCD domain (Appendix K).
4. Discussion
The goal of the present study was to examine the mechanism underlying the increased SCD related to amyloid deposition in cognitively normal individuals. The recent NIA-AA framework of AD recognized SCD as one of the earliest behavioral markers that precede the clinical symptoms of AD [33]. Understanding this marker is of critical importance because there is increasing evidence that effective interventions to slow or prevent AD will need to be administered before individuals are clinically impaired [34]. Our results show that poorer memory performance and greater decline accounted for a significant proportion of the amyloid/SCD relationships for the complaints on perceived capacity, perceived control, and memory-related anxiety, suggesting that individuals with greater amyloid burden report more SCD probably because they actually experience greater memory difficulties due to their amyloid pathology.
4.1. Actual performance mediates the Amyloid/SCD relationship
The main finding was that the relationship between amyloid pathology and greater complaints was mediated by actual memory function, both at the time of test and the magnitude of decline over the past 4 years. This highlights the validity of SCD as an indicator of pathological and cognitive changes in the very early stage of AD. We found little evidence that depression and anxiety contributed to the amyloid-related SCD. The use of mediational analyses provides convincing evidence that actual deficits and declines are important basis for SCD in individuals harboring amyloid, suggesting an early and accurate awareness of worsening cognition related to pathology.
The correspondence between subjective and objective memory in cognitively normal individuals has been controversial [35], [36], [37], and most studies have only focused on the cross-sectional associations (for a meta-analysis, see [38]). Thus, particularly notable was the finding that the longitudinal measure of memory decline mediates the amyloid/complaint relationship, validating the sensitivity of SCD in reflecting actual retrospective declines, likely resulted from AD pathology, which may be too subtle to detect with clinical testing in cognitively normal adults.
We also note that depression is also related to SCD and may be an important predictor of SCD in individuals low in amyloid, but amyloid-related SCD, which is more predictive of future declining [9], [39], is more likely due to actual deficits and declines. This also suggests the complexity of SCD and the importance of considering the validity of SCD in the context of AD biomarkers [33].
Finally, it is also important to recognize that the oldest individuals' complaints about low memory capacity may not as accurately reflect actual memory deficits compared with younger individuals and are likely related to other sources of memory dysfunction that increase with age [40], such as declines in cerebrovascular health [41], [42] and white matter integrity [43], [44].
4.2. The relationship between amyloid and SCD is multidimensional
We expected some but not all SCD domains to be associated with amyloid deposition. Indeed, three domains, use of memory strategy, knowledge about memory processes, and importance of memory achievement, were not significant. SCD domains that reflected perceptions of actual performance (perceived capacity, perceived stability, and perceived control) and reaction to poor performance (memory-related anxiety) are particularly sensitive in reflecting amyloid pathology. The inclusion of a comprehensive questionnaire for SCD is an important feature of the present study, as it allowed us to capture its multidimensionality and to discriminate the domains that are sensitive to amyloid pathology and reflect objective declines.
4.3. Limitations
The findings reported may be limited to the typical profile of participants in cognitive aging studies as they were highly educated and had a low minority presence. Such participants may be more likely to subjectively sense subtle cognitive change [38], [45], [46]. Nevertheless, these variables were controlled in the analyses and did not have any significant effect in our sample. We also note that our sample only included normal individuals and that cognitive deficits and mood-related symptoms observed were all subclinical. It is possible that the impact of mental health on SCD may be larger and even overtakes the mediational role of actual decline in a clinically depressed or anxious population. Finally, it would be ideal to also have longitudinal measures of SCD. Future longitudinal studies may examine SCD changes in the same individual along with the accumulation of amyloid burden, as well as the regional distribution. This will further validate the reliability of SCD in identifying individuals at risk for AD and potentially identify the domains of SCD particularly related to very early amyloid pathology.
5. Conclusion
In conclusion, the present study indicates that concerns about lower perceived capacity, perceived control, and memory-related anxiety in individuals with higher amyloid burden were due to current memory difficulties and greater retrospective declines, rather than depression or anxiety. Moreover, SCD is multidimensional, and specific concerns are more reflective of amyloid pathology and objective declines. We argue that, with considerable media attention to the disorder, individuals' perceived memory self-efficacy reflects valid concerns of their declining functioning and may be useful to identify individuals at risk for AD.
Research in Context.
-
1.
Systematic review: A thorough literature search was performed using “subjective cognitive decline/subjective memory complaints + amyloid/preclinical AD” in Google Scholar. Authors went through articles and identified 31 studies examining the amyloid-SCD relationship in cognitively normal individuals. The systematic review (Table A) showed discrepancy across studies and a lack of understanding on the mechanism underlying SCD related to amyloid pathology.
-
2.
Interpretation: Our findings that the amyloid-SCD relationship is mediated by poorer memory performance and greater decline, not depression/anxiety, suggest that actual deficits related to amyloid pathology, although subtle, are likely the basis of increased SCD in individuals harboring amyloid. We also considered the multidimensionality of SCD and identified that SCD domains on memory functioning have the potential utility as markers of AD pathology.
-
3.
Future directions: Future study with longitudinal measures of SCD and AD biomarkers is needed to further valid its reliability and use in identifying individuals at higher risk for AD.
Acknowledgment
This study was supported by funding from the National Institute on Aging 5R37AG-006265 (D.C.P.) and RC1AG036199 (D.C.P.), as well as the Alzheimer's Association. The tracer was provided at no cost by Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly, Inc. Avid also provided financial support for PET imaging costs.
The authors thank Neil Savalia, BS, and Micaela Chan, PhD, for FreeSurfer data processing and quality control. Finally, the authors thank Melissa Rundle for administrative and scientific management of the Dallas Lifespan Brain Study.
Footnotes
The authors have no conflicts of interest to report. Denise C. Park had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
We repeated the analyses for perceived stability and perceived control treating amyloid as a dichotomized variable (threshold SUVR = 1.09) and found that amyloid-positive individuals had a trend of reporting more SCD (perceived stability: P = .06; perceived control: P = .094), suggesting that dichotomizing amyloid leads to similar but weaker effects and that slightly elevated, but below-threshold, amyloid deposition has a predictive value for SCD.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.12.007.
Supplementary data
References
- 1.Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavin M.J., Brodaty H., Kochan N.A., Crawford J.D., Trollor J.N., Draper B. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18:701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 4.Avila-Villanueva M., Fernandez-Blazquez M.A. Subjective cognitive decline as a preclinical marker for Alzheimer's disease: The challenge of stability over time. Front Aging Neurosci. 2017;9:377. doi: 10.3389/fnagi.2017.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ossenkoppele R., Jagust W.J. The complexity of subjective cognitive decline. JAMA Neurol. 2017;74:1400–1402. doi: 10.1001/jamaneurol.2017.2224. [DOI] [PubMed] [Google Scholar]
- 6.Rabin L.A., Smart C.M., Amariglio R.E. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13:369–396. doi: 10.1146/annurev-clinpsy-032816-045136. [DOI] [PubMed] [Google Scholar]
- 7.Hedden T., Oh H., Younger A.P., Patel T.A. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell M.E., Kennedy K.M., Rodrigue K.M., Wig G., Bischof G.N., Rieck J.R. Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: Evidence for a dose-response relationship. JAMA Neurol. 2017;74:830–838. doi: 10.1001/jamaneurol.2017.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amariglio R.E., Buckley R.F., Mormino E.C., Marshall G.A., Johnson K.A., Rentz D.M. Amyloid-associated increases in longitudinal report of subjective cognitive complaints. Alzheimer's & Dementia: Translational Research & Clinical Interventions. 2018;4:444–449. doi: 10.1016/j.trci.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amariglio R.E., Mormino E.C., Pietras A.C., Marshall G.A., Vannini P., Johnson K.A. Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology. 2015;85:56–62. doi: 10.1212/WNL.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Joie R., Perrotin A., Egret S., Pasquier F., Tomadesso C., Mézenge F. Qualitative and quantitative assessment of self-reported cognitive difficulties in nondemented elders: Association with medical help seeking, cognitive deficits, and β-amyloid imaging. Alzheimers Dement (Amst) 2016;5:23–34. doi: 10.1016/j.dadm.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrotin A., La Joie R., de La Sayette V., Barre L., Mezenge F., Mutlu J. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimers Dement. 2017;13:550–560. doi: 10.1016/j.jalz.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Snitz B.E., Weissfeld L.A., Cohen A.D., Lopez O.L., Nebes R.D., Aizenstein H.J. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry. 2015;23:985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes A.F. Guilford Press; New York, NY: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- 15.Buckley R.F., Saling M.M., Frommann I., Wolfsgruber S., Wagner M. Subjective cognitive decline from a phenomenological perspective: A review of the qualitative literature. J Alzheimers Dis. 2015;48:S125–S140. doi: 10.3233/JAD-150095. [DOI] [PubMed] [Google Scholar]
- 16.Dixon R.A., Hultsch D.F., Hertzog C. The Metamemory in Adulthood (Mia) questionnaire. Psychopharmacol Bull. 1988;24:671–688. [PubMed] [Google Scholar]
- 17.Zwan M.D., Villemagne V.L., Dore V., Buckley R., Bourgeat P., Veljanoski R. Subjective memory complaints in APOEvarepsilon4 carriers are associated with high amyloid-beta burden. J Alzheimers Dis. 2016;49:1115–1122. doi: 10.3233/JAD-150446. [DOI] [PubMed] [Google Scholar]
- 18.Wang L., van Belle G., Crane P.K., Kukull W.A., Bowen J.D., McCormick W.C. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Psychological corporation San Antonio; TX: 1997. Wechsler memory scale (WMS-III) [Google Scholar]
- 20.Woodcock R.W., Johnson M.B. DLM Teaching Resources; Allen, TX: 1989. Woodcock-Johnson tests of cognitive ability. [Google Scholar]
- 21.Brandt J. The Hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clin neuropsychologist. 1991;5:125–142. [Google Scholar]
- 22.Robbins T.W., James M., Owen A.M., Sahakian B.J., McInnes L., Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 23.Radloff L.S. The CES-D Scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 24.Salsman J.M., Butt Z., Pilkonis P.A., Cyranowski J.M., Zill N., Hendrie H.C. Emotion assessment using the NIH Toolbox. Neurology. 2013;80:S76–S86. doi: 10.1212/WNL.0b013e3182872e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savalia N.K., Agres P.F., Chan M.Y., Feczko E.J., Kennedy K.M., Wig G.S. Motion-related artifacts in structural brain images revealed with independent estimates of in-scanner head motion. Hum Brain Mapp. 2017;38:472–492. doi: 10.1002/hbm.23397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Aizenstein H.J., Nebes R.D., Saxton J.A., Price J.C., Mathis C.A., Tsopelas N.D. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995:289–300. [Google Scholar]
- 29.Darlington R., Hayes A. Guilford Press; New York, NY: 2017. Regression analysis and linear models: Concepts, application, and implementation. [Google Scholar]
- 30.Hayes A.F., Rockwood N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther. 2017;98:39–57. doi: 10.1016/j.brat.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Fairchild A.J., MacKinnon D.P. A general model for testing mediation and moderation effects. Prev Sci. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preacher K.J., Hayes A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 33.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperling R.A., Jack C.R., Jr., Aisen P.S. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crumley J.J., Stetler C.A., Horhota M. Examining the relationship between subjective and objective memory performance in older adults: a meta-analysis. Psychol Aging. 2014;29:250–263. doi: 10.1037/a0035908. [DOI] [PubMed] [Google Scholar]
- 36.Horn M.M., Kennedy K.M., Rodrigue K.M. Association between subjective memory assessment and associative memory performance: Role of ad risk factors. Psychol Aging. 2018;33:109–118. doi: 10.1037/pag0000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell A.J., Beaumont H., Ferguson D., Yadegarfar M., Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr Scand. 2014;130:439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 38.Burmester B., Leathem J., Merrick P. Subjective cognitive complaints and objective cognitive function in aging: a systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol Rev. 2016;26:376–393. doi: 10.1007/s11065-016-9332-2. [DOI] [PubMed] [Google Scholar]
- 39.Vogel J.W., Doležalová M.V., La Joie R., Marks S.M., Schwimmer H.D., Landau S.M. Subjective cognitive decline and β-amyloid burden predict cognitive change in healthy elderly. Neurology. 2017 doi: 10.1212/WNL.0000000000004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fjell A.M., McEvoy L., Holland D., Dale A.M., Walhovd K.B., Alzheimer's Disease Neuroimaging Initative What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Vis J.B., Peng S.L., Chen X., Li Y., Liu P., Sur S. Arterial-spin-labeling (ASL) perfusion MRI predicts cognitive function in elderly individuals: A 4-year longitudinal study. J Magn Reson Imaging. 2018;48:449–458. doi: 10.1002/jmri.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng S.-L., Chen X., Li Y., Rodrigue K.M., Park D.C., Lu H. Age-related changes in cerebrovascular reactivity and their relationship to cognition: A four-year longitudinal study. NeuroImage. 2018;174:257–262. doi: 10.1016/j.neuroimage.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohlhauser L., Parker A., Smart C., Gawryluk J.R. 2018. White matter and its relationship with cognition in subjective cognitive decline. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minett T.S., Dean J.L., Firbank M., English P., O'Brien J.T. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry. 2005;13:665–671. doi: 10.1176/appi.ajgp.13.8.665. [DOI] [PubMed] [Google Scholar]
- 45.Aghjayan S.L., Buckley R.F., Vannini P., Rentz D.M., Jackson J.D., Sperling R.A. The influence of demographic factors on subjective cognitive concerns and beta-amyloid. Int Psychogeriatr. 2017;29:645–652. doi: 10.1017/S1041610216001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blazer D.G., Hays J.C., Fillenbaum G.G., Gold D.T. Memory complaint as a predictor of cognitive decline: a comparison of African American and White elders. J Aging Health. 1997;9:171–184. doi: 10.1177/089826439700900202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.