Abstract
The balance between pro‐ and anti‐angiogenic signalling is tightly regulated in normal tissues to maintain the functions of the vasculature. In contrast, the overproduction of angiogenic factors and enhanced angiogenesis are frequently observed in several types of tumours. Although there have been many reports on the correlation between tumour progression and angiogenesis in humans, little is known about tumour angiogenesis in canines. Hence, we attempted to clarify whether angiogenesis contributes to tumour progression in canines as well as humans. In this study, we investigated the expression of several angiogenesis‐related genes, including CD34,VEGF‐A,VEGFR‐1,VEGFR‐2, Ang‐1, Ang‐2, Tie1, and Tie2, in 66 canine tumour tissues and in the normal tissues surrounding the tumours by quantitative real‐time PCR analysis. Our comparative analysis between canine tumour tissues and normal tissues revealed that several angiogenesis‐related genes, such as vascular endothelial growth factor (VEGF) and VEGF‐receptor genes, were significantly upregulated in canine tumour tissues when compared to the normal tissues. We also found that the angiopoietin (Ang)‐1/Ang‐2 gene expression ratio was lower in canine tumour tissues than in the normal tissues, suggesting less association between vascular endothelial cells and perivascular cells in the canine tumour tissues. Taken together, our results suggest that several angiogenesis‐related genes may contribute to the malignant progression of canine tumours via tumour angiogenesis.
Keywords: angiogenesis, angiopoietin, canine, tumour, VEGF‐A, VEGFR
Introduction
Angiogenesis, which enables the adequate supply of oxygen and nutrients throughout the body, is critical for normal embryonic development; however, it is also involved in the development of pathological conditions, such as cancer (Folkman 1990; Carmeliet 2005). Angiogenesis differs between normal tissues and rapidly growing solid tumour tissues (Bergers & Benjamin 2003; Carmeliet 2005). A variety of angiogenic factors that regulate angiogenesis, including vascular endothelial growth factor (VEGF) and angiopoietin (Ang), are secreted from vascular endothelial cells and tumour cells (Shibuya & Claesson‐Welsh 2006; Augustin et al. 2009; Goel & Mercurio 2013). A recent trend for cancer therapy is the suppression of tumour angiogenesis by inhibiting the function of angiogenic factors (Wedam et al. 2006; Ellis & Hicklin 2008).
VEGF‐A, which belongs to the VEGF family, has important roles in the growth and survival of vascular endothelial cells (Holash et al. 1999; Meeson et al. 1999; Lobov et al. 2002). The production of VEGF‐A also results as a response to tumour growth under hypoxic conditions, when it is upregulated by hypoxia‐inducible factor (Pichiule et al. 2004; Goel & Mercurio 2013). VEGF‐A signalling is mediated by the receptor tyrosine kinases VEGF receptor‐1 (VEGFR‐1, also known as Flt‐1) and VEGF receptor‐2 (VEGFR‐2, also known as KDR or Flk‐1) (Takahashi et al. 2001; Ferrara et al. 2003; Goel & Mercurio 2013). VEGFRs are expressed not only in vascular endothelial cells, but also in many tumour types, and they mediate VEGF signalling in tumour cells (Goel & Mercurio 2013; Dang et al. 2017). Signal transduction of VEGFR‐1 and VEGFR‐2 promotes survival and growth of vascular endothelial cells (Ferrara et al. 2003; Shibuya & Claesson‐Welsh 2006). Interestingly, it has been reported that although VEGF‐A associates more strongly with VEGFR‐1 than with VEGFR‐2, the signal intensity of VEGFR‐1 is weaker than that of VEGFR‐2 (Waltenberger et al. 1994; Ferrara et al. 2003; Goel & Mercurio 2013). Therefore, VEGFR‐1 is considered as negative regulator of angiogenesis, especially in embryogenesis (Shibuya & Claesson‐Welsh 2006).
Ang and its receptor, Tie, are reported to have important roles in vascular remodelling and maturation (Thurston et al. 1999; Augustin et al. 2009; Thomas & Augustin 2009). Ang‐1 is mainly secreted by perivascular cells, and it binds to Tie2 receptors on vascular endothelial cells (Suri et al. 1996, 1998; Augustin et al. 2009). After binding, the ligand induces the auto‐phosphorylation of Tie2, stimulates intracellular signalling, and then promotes the survival of endothelial cells and suppresses apoptosis (Maisonpierre et al. 1997; Bogdanovic et al. 2006; Augustin et al. 2009; Thomas & Augustin 2009). Although Tie1 is also expressed on vascular endothelial cells, the function of Tie1 in angiogenesis remains mostly unknown (Augustin et al. 2009; Thomas & Augustin 2009). In contrast, Ang‐2 is secreted by endothelial cells, and it binds to Tie2 in an autocrine manner Augustin et al. 2009. However, since the association between Ang‐2 and Tie2 does not significantly induce the auto‐phosphorylation of Tie2 (Maisonpierre et al. 1997; Bogdanovic et al. 2006; Reiss et al. 2007), Ang‐2 likely interrupts Tie2‐mediated signalling via an antagonistic action and destabilises endothelial cells (Falcón et al. 2009). Thus, the balance between Ang‐1 and Ang‐2 is tightly regulated in normal adult tissues to maintain vascular stability.
In the field of veterinary oncology, the importance of angiogenesis in tumour progression has been recognised (Restucci et al. 2003; Millanta et al. 2006; Yonemaru et al. 2006; Camacho et al. 2014), and several therapies targeting tumour angiogenesis are being used for some tumour types (Scharf et al. 2013; Li et al. 2016). However, although several studies have reported on the expression of angiogenesis‐related genes in canine tumours, few studies have compared the expression profiles of angiogenesis‐related genes in normal and tumour tissues. It was reported that the expression of VEGF‐A was correlated with malignant progression of canine squamous cell carcinomas (SCC) and seminomas (Al‐Dissi et al. 2007; Sleeckx et al. 2014). In addition, the upregulation of VEGFR‐2 was also observed in canine SCC Al‐Dissi et al. 2007. Kool et al. (2014) reported that the relative expression of Ang‐2 was higher in adrenocortical tumours compared with normal adrenal tissues and the change in balance between Ang‐1 and Ang‐2 could be enhanced tumour progression.
In this study, we investigated the expression of several angiogenesis‐related genes, including CD34, VEGF‐A, VEGFR‐1, VEGFR‐2, Ang‐1, Ang‐2, Tie1, and Tie2, in canine tumours and the normal tissues surrounding the tumours. The gene expression of CD34 gene was evaluated for amounts of vascular endothelial precursor cells, VEGF‐A, VEGFR‐1 and VEGFR‐2 gene were evaluated for proliferation character of vascular endothelial cells, and Ang‐1, Ang‐2, Tie1 and Tie2 gene were evaluated for the structural stability of vascular endothelial cells. We found that several angiogenesis‐related genes were upregulated in tumour tissues as compared to the normal tissues. Furthermore, the Ang‐1/Ang‐2 gene expression ratio was significantly lower in benign and malignant tumours than in the normal tissues. Taken together, canine tumours have a high angiogenic potency, and the characteristic features of canine angiogenesis may provide an effective target for canine cancer therapy.
Materials and Methods
Canine tumour and normal tissue samples
Canine tumour tissues and the normal tissues surrounding the tumours were collected from 66 canine cancer patients undergoing surgery at Taguchi Animal Hospital (Saitama, Japan) between 2010 and 2011. Written informed consent was obtained from all dog owners. The sampling of all tumour tissues and normal tissues surrounding each tumour was performed according to standard surgical resection procedures. Consequently, we always excised normal tissues close to the tumour, and both the tumour and this adjacent normal tissue were used for the study. The final diagnosis of that tumour or normal tissue was determined by histopathological examination, and the classification of the tumour was also based on the pathological diagnosis. Consequently, 28 of 66 cases were benign tumours and 38 were malignant tumours. The types of each tumour are shown in Tables 1 and 2.
Table 1.
Sample list (benign tumour)
| Histopathological diagnosis | Number of cases |
|---|---|
| Breast adenoma | 5 |
| Benign mixed adenoma of the breast | 4 |
| Skin adnexal neoplasms | 4 |
| Leydig cell adenoma | 3 |
| Trichoepithelioma | 3 |
| Perianal gland adenoma | 2 |
| Trichoblastoma | 2 |
| Apocrine adenoma | 1 |
| Apocrine cystadenoma | 1 |
| Hemangioma | 1 |
| Inflammatory colorectal polyp | 1 |
| Mixed apocrine adenoma | 1 |
| Total | 28 |
Table 2.
Sample list (malignant tumour)
| Histopathological diagnosis | Number of cases |
|---|---|
| Mastocytoma | 9 |
| Breast carcinoma | 6 |
| Hemangiosarcoma | 3 |
| Malignant mixed tumour of the breast | 2 |
| Sertoli cell tumour | 2 |
| Soft tissue sarcoma | 2 |
| Adrenal carcinoma | 1 |
| Colorectal carcinoma | 1 |
| Hemangiopericytoma | 1 |
| Hepatocellular carcinoma | 1 |
| Leiomyoma | 1 |
| Lymphoma | 1 |
| Mixed apocrine carcinoma | 1 |
| Nictitating membrane gland tumour | 1 |
| Osteosarcoma | 1 |
| Peripheral nerve tumour | 1 |
| Squamous cell carcinoma | 1 |
| Thyroid tumour | 1 |
| Transitional cell carcinoma | 1 |
| Undifferentiated sarcoma | 1 |
| Total | 38 |
RNA extraction and quantitative real‐time PCR
Resected tissues were immediately immersed in RNA later (QIAGEN Inc., Hilden, Germany) and stored at 4°C. Total RNA was prepared using an RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions and the total RNA samples were treated with DNase I to degradation and contamination of genomic DNA. cDNA was reverse‐transcribed from 1 μg of total RNA using Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Mnnheim, Germany). The measurement of gene expression by quantitative analysis was performed using the LightCycler system (Roche Applied Science). Quantitative real‐time PCR analysis of the GAPDH, CD34, VEGF‐A, VEGFR‐1, VEGFR‐2 Ang‐1, Ang‐2, Tie1, and Tie2 genes was performed using the LightCycler FastStart DNA MasterPLUS SYBR Green I system (Roche Applied Science). Primers used for PCR were listed in Table 3. PCR amplification of the housekeeping gene, GAPDH, was performed for each sample as control for sample loading and to allow normalisation among samples. To determine the absolute copy number of the target transcripts, the fragments of target gene amplified by PCR using the primer set were constructed with pGEM‐T‐easy cloning vector (Promega, Madison, WI). The concentrations of these purified plasmids were measured by absorbance at 260 nm and copy numbers were calculated from concentration of samples. A standard curve was created by plotting the threshold cycle (Ct) versus the known copy number for each plasmid template in the dilutions. The copy numbers for all unknown samples were determined according to the standard curve using LightCycler version 3.5.3 (Roche Applied Science). To correct for differences in both RNA quality and quantity between samples, each target gene was first normalised by dividing the copy number of the target by the copy number of GAPDH.
Table 3.
Primers used for quantitative real‐time PCR
| Gene | Sequence (5′ → 3′) |
|---|---|
| GAPDH | F: AACGGGAAGCTCACTGGCAT |
| R: CTTGACAAAGTGGTCATTGAGGG | |
| CD34 | F: AGTCTGAGGTGAGGCCTCACT |
| R: TGCGGCGGTTCATCAGGAAGT | |
| VEGF‐A | F: CCCACTGAGGAGTTCCAACATCAC |
| R: GGGTTTATACCGGGATTTCTTG | |
| VEGFR‐1 | F: GATGCACAGTGAAATACCCGAAA |
| R: CAGGTTATTCGCTTCCCATCA | |
| VEGFR‐2 | F: TAGTAGGCACGGCAGTGATTG |
| R: GTCGATTCCAAAGGCATCTGC | |
| Ang‐1 | F: AAAGTGTCACACTGGGACAG |
| R: CAGCAGTGTAGAACATTCCA | |
| Ang‐2 | F: TAAAGGACTTACAGGGACGG |
| R: GATCATCATGGTTGTGCCCT | |
| Tie1 | F: CTTGTGAGAACCGAGGTTAC |
| R: GTCTCTGTGGATGAACTGCT | |
| Tie2 | F: AGCTTCTCCATTTCGCAGCGG |
| R: ACTCGATTGCCATCCAGCGCAC |
F, Forward primer.
R, Reverse primer.
Statistical analyses
The Statcel 3 add‐in (OMS Publishing, Saitama, Japan) for Microsoft Excel was used for the statistical analysis. The relative expression of each of angiogenesis‐related genes was compared between tumour tissues and the normal tissues surrounding the tumours using the Wilcoxon rank‐sum test. The Ang‐1 to Ang‐2 gene expression ratio was also compared between tumour tissues and the normal tissues surrounding the tumours using the Wilcoxon rank‐sum test. A correlation between the gene expression of VEGF‐A and VEGFR‐2 calculated by Spearman rank correlation coefficient (Rs). Values of P < 0.05 were considered statistically significant.
Results
Several angiogenesis‐related genes are upregulated in canine tumour tissues
To investigate whether angiogenesis‐related genes are upregulated in canine tumours, we measured the mRNA expression levels of the genes in 66 canine tumour tissues (28 benign, 38 malignant) as well as the normal tissues surrounding the tumours by quantitative real‐time PCR. As shown in Fig. 1, the gene expression of each of the VEGF‐A, VEGFR‐1, VEGFR‐2, Ang‐1, Tie1, and Tie2 genes was significantly upregulated in the benign tumour tissues when compared to the normal tissues surrounding the tumours (Fig. 1b–e,g,h). In contrast, in malignant tumour tissues, significant upregulation was only observed for the expression of the VEGFR‐2 gene (Fig. 2d). Because VEGFR‐2 mediates the angiogenic signalling by VEGF‐A, we investigated a correlation between the gene expression of VEGFR‐2 and VEGF‐A. Consequently, a positive correlation was observed in both benign (Rs = 0.57, P = 0.003) and malignant (Rs = 0.52, P = 0.002) tumour tissues. These results suggested that several angiogenesis‐related gene expressions may be upregulated in tumour tissues. Especially, the upregulation of VEGFR‐2 gene was observed in common with benign and malignant tumours, but it remains unknown whether the VEGFR‐2 gene is required for tumour progression.
Figure 1.
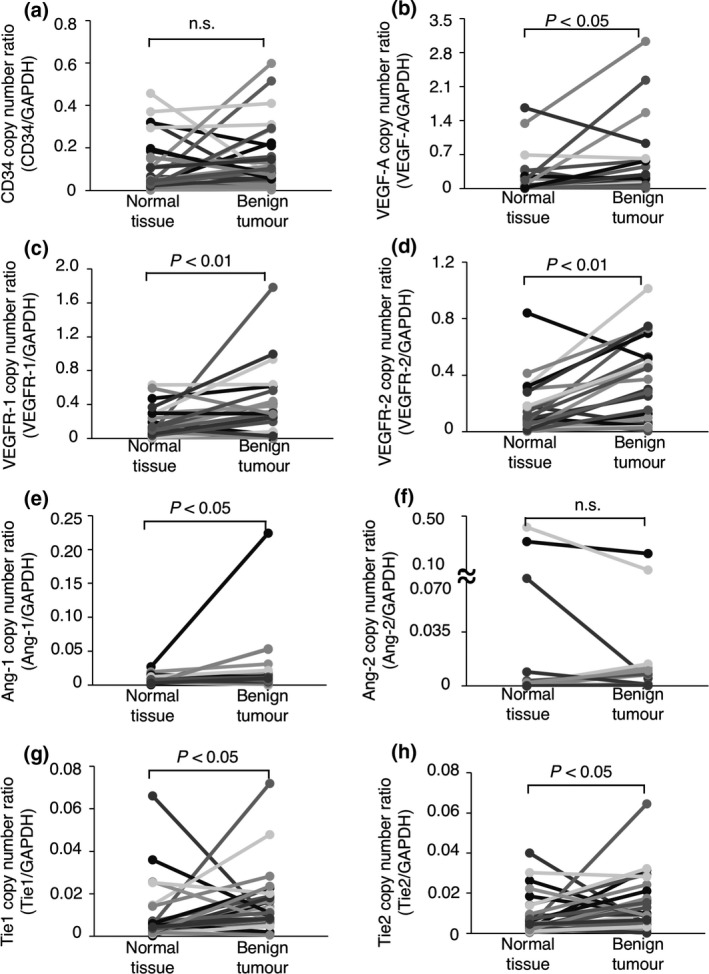
The expression of angiogenesis‐related genes in canine benign tumour tissues and the normal tissues surrounding each tumours. (a) The expression of the CD34 gene. (b) The expression of the VEGF‐A gene. (c) The expression of the VEGFR‐1 gene. (d) The expression of the VEGFR‐2 gene. (e) The expression of the Ang‐1 gene. (f) The expression of the Ang‐2 gene. (g) The expression of the Tie1 gene. (h) The expression of the Tie2 gene. The expression of each gene was compared between tumour tissues and the normal tissues surrounding the tumours using the Wilcoxon rank‐sum test. n. s. = not significant.
Figure 2.
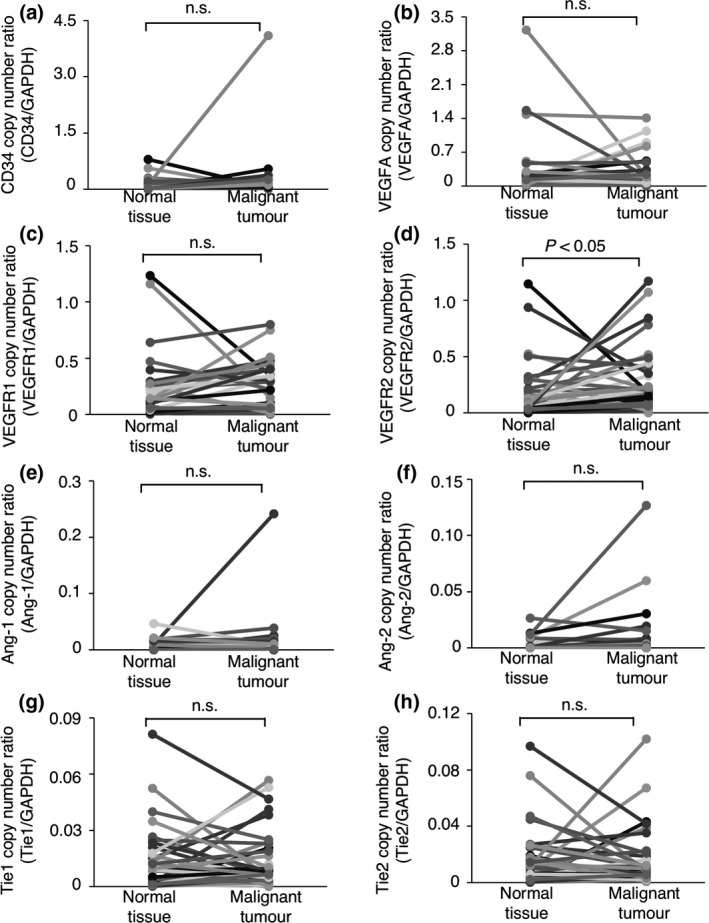
The expression of angiogenesis‐related genes in canine malignant tumour tissues and the normal tissues surrounding each tumours. (a) The mRNA expression of the CD34 gene. (b) The expression of the VEGF‐A gene. (c) The expression of the VEGFR‐1 gene. (d) The expression of the VEGFR‐2 gene. (e) The expression of the Ang‐1 gene. (f) The expression of the Ang‐2 gene. (g) The expression of the Tie1 gene. (h) The expression of the Tie2 gene. The expression of each gene was compared between tumour tissues and the normal tissues surrounding the tumours using the Wilcoxon rank‐sum test. n. s. = not significant.
The expression of angiogenesis‐related receptor genes on vascular endothelial precursor cells
The CD34 gene is broadly accepted as a marker of vascular endothelial precursor cells (Fina et al. 1990; Asahara et al. 1997; Sideny et al. 2008). As shown in Figs 1a and 2a, the expression of the CD34 gene did not differ significantly between the benign or malignant tumour tissues and the normal tissues surrounding the tumours; this suggested that the number of vascular endothelial precursor cells did not differ between the tumour tissues and the normal tissues. We further investigated whether the expression of angiogenesis‐related receptor genes per vascular endothelial precursor cell differed between the tumour tissues and the normal tissues surrounding the tumours. To quantify the expression of angiogenesis‐related receptor genes per vascular endothelial precursor cell, the mRNA copy numbers were normalised to that of the CD34 gene [(mRNA copy number of target gene/mRNA copy number of the GAPDH gene)/(mRNA copy number of CD34 gene/mRNA copy number of the GAPDH gene)] (Mori et al. 2008). As shown in Figs 3a,c,d and 4a,c,d, the expression of the VEGFR‐1, Tie1 or Tie2 gene per vascular endothelial precursor cell did not differ significantly between the benign or malignant tumour tissues and the normal tissues surrounding the tumours. In contrast, the expression of the VEGFR‐2 gene per vascular endothelial precursor cell was significantly upregulated in the benign and malignant tumour tissues when compared to that in the normal tissues surrounding the tumours (Figs 3b,4b). These results suggested that the upregulation of the VEGFR‐2 gene on tumour‐associated vascular endothelial precursor cells may play an important role in tumour angiogenesis regardless of the benign or malignant status of the tumour.
Figure 3.
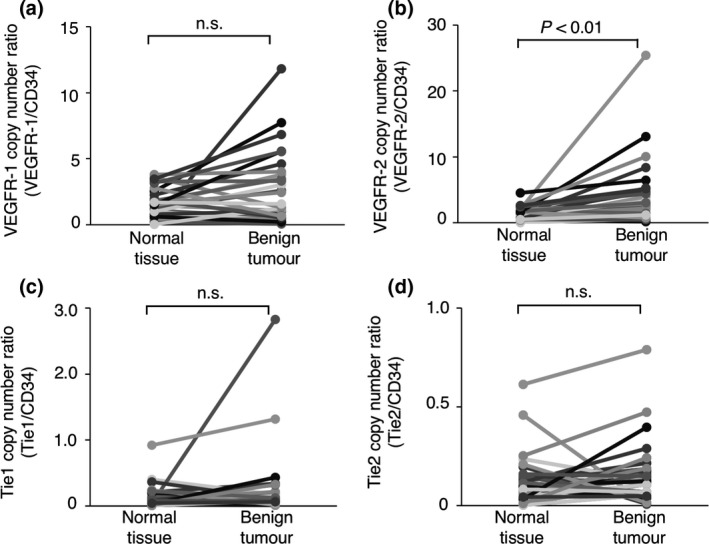
The expression of angiogenesis‐related receptor genes on vascular endothelial precursor cells in canine benign tumour tissues and the normal tissues surrounding each tumours. (a) The expression of the VEGFR‐1 gene. (b) The expression of the VEGFR‐2 gene. (c) The expression of the Tie1 gene. (d) The expression of the Tie2 gene. The expression of each gene was compared between tumour tissues and the normal tissues surrounding the tumours using the Wilcoxon rank‐sum test. n. s. = not significant.
Figure 4.
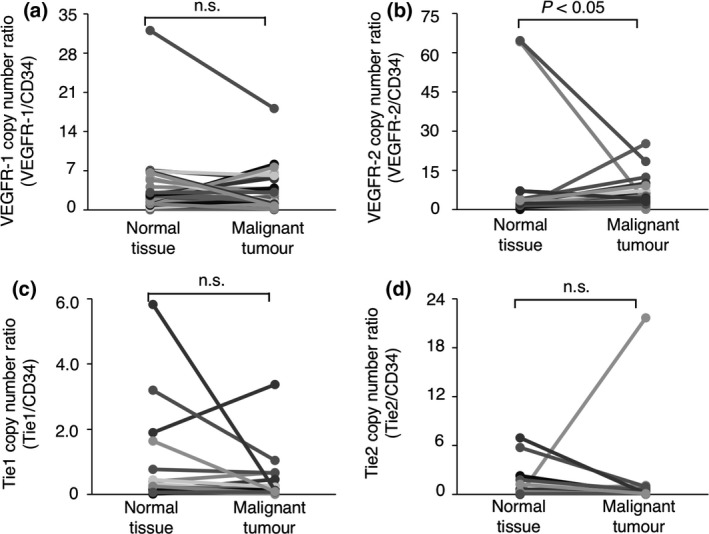
The expression of angiogenesis‐related receptor genes on vascular endothelial precursor cells in canine malignant tumour tissues and the normal tissues surrounding each tumours. (a) The expression of the VEGFR‐1 gene. (b) The expression of the VEGFR‐2 gene. (c) The expression of the Tie1 gene. (d) The expression of the Tie2 gene. The expression of each gene was compared between tumour tissues and the normal tissues surrounding the tumours using the Wilcoxon rank‐sum test. n. s. = not significant.
The Ang‐1/Ang‐2 gene expression ratio in canine tumour tissues
The Ang‐1/Tie2 receptor signalling pathway plays important roles for the structural stability of the vasculature, such as interaction between vascular endothelial cell and perivascular cell (Augustin et al. 2009; Thomas & Augustin 2009). In contrast, Ang‐2 inhibits the Tie2 signalling pathway and decreases the interaction between vascular endothelial cell and perivascular cell (Maisonpierre et al. 1997; Falcón et al. 2009). To evaluate whether the balance of Ang‐1 and Ang‐2 gene expression is changed between the tumour tissues and the normal tissues surrounding the tumours, the Ang‐1/Ang‐2 gene expression ratio was calculated [(mRNA copy number of Ang‐1 gene/mRNA copy number of the GAPDH gene) / (mRNA copy number of Ang‐2 gene/mRNA copy number of the GAPDH gene)]. The Ang‐1/Ang‐2 gene expression ratio was significantly lower in both the benign and malignant tumour tissues than in the normal tissues surrounding the tumours (Fig. 5a,b). These results suggested that Ang‐2 may be dominant over Ang‐1 in canine tumour tissues, resulting that may induce the decrease in structure stability in vascular formations.
Figure 5.
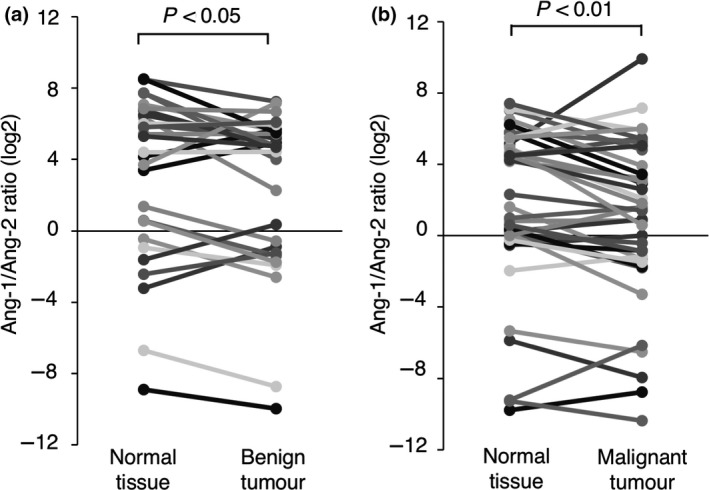
The expression ratio of Ang‐1 to Ang‐2 in canine tumour tissues and the normal tissues surrounding each tumours. (a) The Ang‐1 to Ang‐2 gene expression ratio in benign tumour tissues and the normal tissues surrounding each tumours. (b)The Ang‐1 to Ang‐2 gene expression ratio in malignant tumour tissues and the normal tissues surrounding each tumours. The gene expression ratio in each tissue is represented by log2. The gene expression ratio was compared between tumour tissues and the normal tissues surrounding each tumours using the Wilcoxon rank‐sum test.
Discussion
The vasculature is generally quiescent and stable in normal adult tissues, with the exception of injured or developing tissues (Bergers & Benjamin 2003; Carmeliet 2005). To maintain normal vasculature, the production of angiogenic factors is tightly regulated in normal tissues (Shibuya & Claesson‐Welsh 2006; Augustin et al. 2009; Thomas & Augustin 2009; Goel & Mercurio 2013). In contrast, angiogenic factors are frequently upregulated in tumour tissues, and the tumour vasculature is often architecturally distorted (Ellis & Hicklin 2008; Falcón et al. 2009). Physiological stimuli resulting from the rapid growth of a tumour, including hypoxia and nutrient starvation, activate the expression of angiogenesis‐related genes in tumour and vascular endothelial cells (Zhang et al. 2003; Pichiule et al. 2004).
In this study, we investigated mRNA expression levels of the angiogenesis‐related genes in 66 canine tumour tissues as well as the normal tissues surrounding the tumours. Those canine tissues contained various tumour types and lacked homogeneity. However, since the main purpose of this study was to see the common tendency of angiogenesis‐related gene expression in various kinds of canine tumours, we did not focus on the heterogeneity of tumour types in this study. Also, we used GAPDH gene as an internal control for the quantitative real‐time PCR. GAPDH gene has been commonly considered as a housekeeping gene that be expressed stably regardless of experimental conditions. However, the expression of GAPDH gene may change greatly under certain conditions, such as hypoxia (Zhong & Simons 1999). Therefore, we cannot rule out the possibility that parts of our quantitative real‐time PCR data might be influenced by the change in the expression of GAPDH gene. For more precisely experiment, it would be necessary to measure several housekeeping genes and choose adequate internal controls in each cell and tissue type.
As a result of analysis, we observed that several angiogenesis‐related genes were upregulated in canine tumour tissues and tumour‐associated vascular endothelial precursor cells. The expression of the VEGFR‐2 gene was significantly upregulated in both canine tumour tissues and tumour‐associated vascular endothelial precursor cells as compared to normal tissues surrounding the tumours. In particular, when the gene expression of VEGFR‐2 was examined in nine breast adenomas (five adenomas and four mixed adenomas of the breast) and eight breast carcinomas (six carcinomas and two mixed carcinomas of the breast), these tumours were also observed the upregulation as compared to normal tissues as well as other types of tumour, but there was no difference in adenoma and carcinoma in its gene expression (data not shown). VEGFR‐2 is a key receptor that mediates VEGF‐A signalling; it is expressed not only in vascular endothelial cells but also in many tumour types, and it promotes the proliferation of vascular endothelial cells and tumour cells (Waltenberger et al. 1994; Shibuya & Claesson‐Welsh 2006; Goel & Mercurio 2013). Several studies have reported that VEGFR‐2 is associated with the progression of some canine tumour types (Yonemaru et al. 2006; Al‐Dissi et al. 2007). A positive correlation between the gene expression of VEGFR‐2 and VEGF‐A was observed in benign and malignant tumours, suggesting that these gene expressions might be regulated by a similar control mechanism and work in cooperation with each other on tumour angiogenesis.
Even if the vascular endothelial precursor cells in tumour tissue are derived from these cells in normal tissues, the gene expressions would be influenced by various tumour‐derived factors, such as angiogenic factors and hypoxic condition (Bergers & Benjamin 2003). Therefore, we speculated that there was difference in gene expression profile in vascular endothelial precursor cells between the niches of normal and tumour tissue. The gene expression of receptor genes, such as VEGFR‐1, VEGFR ‐2, Tie1 and Tie 2, was normalised to that of CD34 gene that is well known as a marker of vascular endothelial precursor cells, and examined amount of these gene expression (Fina et al. 1990; Asahara et al. 1997; Sideny et al. 2008). Consequently, VEGFR‐2 gene expression per vascular endothelial precursor cell in the tumour niche was upregulated as compared to normal. These data raise the possibility that gene expression profiles in vascular endothelial precursor cells might be different in depending on the niche.
In addition, we showed that the expression of the VEGFR‐1, Tie1, and Tie2 genes was also upregulated in benign tumour tissues when compared to the normal tissues surrounding the tumours. However, because the upregulation of these receptor genes of vascular endothelial precursor cells that normalised to CD34 gene was not observed in a tumour niche, the upregulation of these receptor genes might occur mainly in tumour cells. Although these genes are mainly expressed in vascular endothelial cells and perivascular cells, they were occasionally observed to be upregulated in several types of tumours (Plate et al. 1992; Brown et al. 2000; Dang et al. 2017). Taken together, these results indicated that the upregulation of angiogenesis‐related genes may be important for canine tumourigenesis, and VEGFR‐2 may be a candidate target gene for cancer therapy regardless of the benign or malignant status of the tumour.
We also demonstrated that the Ang‐1/Ang‐2 gene expression ratio was lower in benign and malignant tumour tissues than in the normal tissues surrounding the tumours. These data suggested that Ang‐2 gene expression could be dominant as compared to Ang‐1 in tumour tissues. It is widely accepted that the Ang/Tie system plays important roles in the maturation and structural stability of blood vessels and the maintenance of the association between endothelial cells and perivascular cells (Augustin et al. 2009; Thomas & Augustin 2009). The binding of Ang‐1 to the Tie2 receptor enhances the stability of endothelial cells by inducing auto‐phosphorylation of the receptor and activating downstream signalling (Maisonpierre et al. 1997; Bogdanovic et al. 2006; Reiss et al. 2007). In contrast, Ang‐2 would decrease the stability of endothelial cells by antagonising Ang‐1 signalling, and would promote the invasion of endothelial cells and the extravasation of endothelial precursor cells from blood vessels (Ahmad et al. 2001; Lobov et al. 2002; Ellis & Hicklin 2008). It has been reported that the expression of Ang‐2 in tumour cells is upregulated by various growth factors and physiological stimuli, such as VEGF‐A and hypoxia (Zhang et al. 2003; Pichiule et al. 2004). Taken together, our analysis that is the Ang‐1/Ang‐2 gene expression ratio was lower in tumour than normal tissues would be in agreement with these reports, suggesting that vascular instability in tumour is responsible for the balance of Ang‐1/Ang‐2.
In conclusion, our results provide useful information on the expression profiles of angiogenesis‐related genes in canine tumours, suggesting that tumour angiogenesis is potentially an attractive target for canine cancer therapy.
Source of funding
This work was supported by JSPS KAKENHI Grant Number JP18K06006 and MEXT*‐Supproted Program for the Private University Resesarch Branding Project, 2016‐2020. (*Ministry of Education, Culture, Sports, Science and Technology of JAPAN).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethics statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee of Azabu University Veterinary Teaching Hospital approval has been received.
Contributions
Hiroeki Sahara designed the research; Daisuke Kobayashi and Koki Maeda carried out the research; Atsushi Tanabe performed the data analysis; Masayuki Taguchi collected canine tissue samples; Atsushi Tanabe and Hiroeki Sahara drafted the manuscript.
Acknowledgements
We would like to thank the owners who cooperated in providing the surgical sample used in this study.
References
- Ahmad S.A., Liu W., Jung Y.D., Fan F., Wilson M., Wilson M. et al (2001) The effects of angiopoietin‐1 and ‐2 on tumor growth and angiogenesis in human colon cancer. Cancer Research 61, 1255–1259. [PubMed] [Google Scholar]
- Al‐Dissi A.N., Haines D.M., Singh B. & Kidney B.A. (2007) Immunohistochemical expression of vascular endothelial growth factor and vascular endothelial growth factor receptor associated with tumor cell proliferation in canine cutaneous squamous cell carcinomas and trichoepitheliomas. Veterinary Pathology 44, 823–830. [DOI] [PubMed] [Google Scholar]
- Asahara T., Murohara T., Sullivan A. et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–966. [DOI] [PubMed] [Google Scholar]
- Augustin H.G., Young Koh G., Thurston G. et al (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nature Reviews Molecular Cell Biology 10, 165–177. [DOI] [PubMed] [Google Scholar]
- Bergers G. & Benjamin L.E. (2003) Angiogenesis: tumorigenesis and the angiogenic switch. Nature Reviews Cancer 3, 401–410. [DOI] [PubMed] [Google Scholar]
- Bogdanovic E., Nguyen V.P.K.H. & Dumont D.J. (2006) Activation of Tie2 by angiopoietin‐1 and angiopoietin‐2 results in their release and receptor internalization. Journal of Cell Science 119, 3551–3560. [DOI] [PubMed] [Google Scholar]
- Brown L.F., Dezube B.J., Tognazzi K. et al (2000) Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in Kaposi's sarcoma and cutaneous angiosarcoma. American Journal of Pathology 156, 2179–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L., Peña L., Gil A.G. et al (2014) Immunohistochemical vascular factor expression in canine inflammatory mammary carcinoma. Veterinary Pathology 51, 737–748. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. (2005) Angiogenesis in life, disease and medicine. Nature 438, 932–936. [DOI] [PubMed] [Google Scholar]
- Dang Y., Zhang Y., Li J. et al (2017) High VEGFR1/2 expression levels are predictors of poor survival in patients with cervical cancer. Medicine (Baltimore) 96, e5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L.M. & Hicklin D.J. (2008) VEGF‐targeted therapy: mechanisms of anti‐tumor activity. Nature Reviews Cancer 8, 579–591. [DOI] [PubMed] [Google Scholar]
- Falcón B.L., Hashizume H., Koumoutsakos P. et al (2009) Contrasting actions of selective inhibitors of angiopoietin‐1 and angiopoietin‐2 on the normalization of tumor blood vessels. American Journal of Pathology 175, 2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Gerber H.‐P. & LeCouter J. (2003) The biology of VEGF and its receptors. Nature Medicine 9, 669–676. [DOI] [PubMed] [Google Scholar]
- Fina L., Molgaard H.V., Robertson D. et al (1990) Expression of the CD34 gene in vascular endothelial cells. Blood 75, 2417–2426. [PubMed] [Google Scholar]
- Folkman J. (1990) What is the evidence that tumor are angiogenesis dependent? Journal of the National Cancer Institute 82, 4–6. [DOI] [PubMed] [Google Scholar]
- Goel H.L. & Mercurio A.M. (2013) VEGF targets the tumor cell. Nature Reviews Cancer 13, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J., Misonpierre P.C., Compton D. et al (1999) Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998. [DOI] [PubMed] [Google Scholar]
- Kool M.M.J., Galac S., Kooistra H.S. et al (2014) Expression of angiogenesis‐related genes in canine cortisol‐secreting adrenocortical tumors. Domestic Animal Endocrinology 47, 73–82. [DOI] [PubMed] [Google Scholar]
- Li W., Guo M., Liu Y. et al (2016) Selenium induces an anti‐tumor effect via inhibiting intratumoral angiogenesis in a mouse model of transplanted canine mammary tumor cells. Biological Trace Element Research 171, 371–379. [DOI] [PubMed] [Google Scholar]
- Lobov I.B., Brooks P.C. & Lang R.A. (2002) Angiopoietin‐2 displays VEGF‐dependent modulation of capillary structure and endothelial cell survival in vivo. Proceedings of the National Academy of Sciences 99, 11205–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P.C., Suri C., Jones P.F. et al (1997) Angiopoietin‐2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60. [DOI] [PubMed] [Google Scholar]
- Meeson A.P., Argilla M., Ko K. et al (1999) VEGF deprivation‐induced apoptosis is a component of programmed capillary regression. Development 126, 1407–1415. [DOI] [PubMed] [Google Scholar]
- Millanta F., Silvestri G., Vaselli C. et al (2006) The role of vascular endothelial growth factor and its receptor Flk‐1/KDR in promoting tumor angiogenesis in feline and canine mammary carcinomas: a preliminary study of autocrine and paracrine loops. Research in Veterinary Science 81, 350–357. [DOI] [PubMed] [Google Scholar]
- Mori Y., Sahara H., Matsumoto K. et al (2008) Downregulation of Tie2 gene by a novel antitumor sulfolipid, 3′‐sulfoquinovosyl‐1′‐monoacylglycerol, targeting angiogenesis. Cancer Science 99, 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichiule P., Chavez J.C. & LaManna J.C. (2004) Hypoxic regulation of angiopoietin‐2 expression in endothelial ells. Journal of Biological Chemistry 279, 12171–12180. [DOI] [PubMed] [Google Scholar]
- Plate K.H., Breier G., Weich H.A. et al (1992) Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature 355, 242–244. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Droste J., Heil M. et al (2007) Angiopoietin‐2 impairs revascularization after limb ischemia. Circulation Research 101, 88–96. [DOI] [PubMed] [Google Scholar]
- Restucci B., Maiolino P., Paciello O. et al (2003) Evaluation of angiogenesis in canine seminomas by quantitative immunohistochemistry. Journal of Comparative Pathology 128, 252–259. [DOI] [PubMed] [Google Scholar]
- Scharf V.F., Farese J.P., Coomer A. et al (2013) Effect of bevacizumab on angiogenesis and growth of canine osteosarcoma cells xenografted in athymic mice. American Journal of Veterinary Research 74, 771–778. [DOI] [PubMed] [Google Scholar]
- Shibuya M. & Claesson‐Welsh L. (2006) Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Experimental Cell Research 312, 549–560. [DOI] [PubMed] [Google Scholar]
- Sideny L.E., Branch M.J., Dua H.S. et al (2008) Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 26, 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeckx N., Van Brantegem L., Van den Eynden G. et al (2014) Lymphangiogenesis in canine mammary tumors: a morphometric and prognostic study. Journal of Comparative Pathology 150, 184–193. [DOI] [PubMed] [Google Scholar]
- Suri C., Jones P.F., Patan S. et al (1996) Requisite role of angiopoietin‐1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180. [DOI] [PubMed] [Google Scholar]
- Suri C., McClain J., Thurston G. et al (1998) Increased vascularization in mice overexpressing angiopoietin‐1. Science 282, 468–471. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Yamaguchi S., Chida K. et al (2001) A single autophosphorylation site on KDR / Flk‐1 is essential for VEGF‐A‐dependent activation of PLC‐g and DNA synthesis in vascular endothelial cells. EMBO Journal 20, 2678–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. & Augustin H.G. (2009) The role of the angiopoietins in vascular morphogenesis. Angiogenesis 12, 125–137. [DOI] [PubMed] [Google Scholar]
- Thurston G., Suri C., Smith K. et al (1999) Leakage‐resistant blood vessels in mice transgenically overexpressing angiopoietin‐1. Science 286, 2511–2514. [DOI] [PubMed] [Google Scholar]
- Waltenberger J., Claesson‐Welsh L., Siegbahn A. et al (1994) Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. Journal of Biological Chemistry 269, 26988–26995. [PubMed] [Google Scholar]
- Wedam S.B., Low J.A., Yang S.X. et al (2006) Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. Journal of Clinical Oncology 24, 769–777. [DOI] [PubMed] [Google Scholar]
- Yonemaru K., Sakai H., Murakami M. et al (2006) Expression of vascular endothelial growth factor, basic fibroblast growth factor, and their receptors (Flt‐1, Flk‐1, and Flg‐1) in canine vascular tumors. Veterinary Pathology 43, 971–980. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yang N., Park J. et al (2003) Tumor‐derived vascular endothelial growth factor up‐regulates angiopoietin‐2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Research 63, 3403–3412. [PubMed] [Google Scholar]
- Zhong H. & Simons J.W. (1999) Direct comparison of GAPDH, β‐Actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochemical and Biophysical Research Communications 259, 523–526. [DOI] [PubMed] [Google Scholar]


