Abstract
AIM
To explore the correlation between several blood cell-associated inflammatory indices including mean platelet volume (MPV), platelet distribution width (PDW), neutrophil to lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), and the presence and severity of diabetic retinopathy (DR).
METHODS
We searched for eligible studies from PubMed, EMBASE, Web of Science and CNKI up to December 13, 2017. Standardized mean difference (SMD) calculated with confidence interval (CI) of 95% was used to estimate the values of those indices.
RESULTS
A total of 31 studies were included in the present Meta-analysis. As compared with type 2 diabetes mellitus (T2DM) patients without DR, the values of MPV, PDW, NLR, and PLR were higher in patients with DR (SMD=0.67; 95%CI: 0.36 to 0.98; SMD=0.51; 95%CI: 0.27 to 0.75; SMD=0.77; 95%CI: 0.49 to 1.05 and SMD=1.18; 95%CI: 0.07 to 2.28). Additionally, it was also observed that MPV was closely correlated with the severity of DR.
CONCLUSION
MPV, PDW, NLR, and PLR could be recommended as diagnostic biomarkers for DR, and MPV could be applied to assess the severity of DR.
Keywords: mean platelet volume, platelet distribution width, neutrophil to lymphocyte ratio, platelet-lymphocyte ratio, diabetic retinopathy
INTRODUCTION
Diabetes mellitus is a heavy burden worldwide, the morbidity and mortality of which keep growing in recent years[1]–[2]. Diabetes, as a system metabolic disorder disease, is always involved in the injury of many organs and tissues, leading to various micro- and macrovascular complications. Diabetic retinopathy (DR) is one of the most common microangiopathies in patients with type 2 diabetes mellitus (T2DM) and can be divided into the “no-proliferative diabetic retinopathy” (NPDR) and the “proliferative diabetic retinopathy” (PDR) according to its severity[3]. According to the World Health Organization (WHO), DR accounts for 4.8% of the number of cases of blindness (37 million) globally[4]. It is widely accepted that screening, early detection and prompt treatment of vision-threatening DR largely contribute to preventing diabetes-associated visual impairment or loss[5]–[7]. Nevertheless, until now DR screening services are still at uneven levels between developing and developed countries, and there are no definite guidelines regarding the optimal screen method, which makes it urgent and imperative to develop cost-effective comprehensive screening programs based on DR epidemiology and economical condition of community[5]–[7]. It has been proposed that diabetes duration, the duration of hyperglycemia, gene polymorphism, aberrant blood lipid levels, obesity, hypertension, and smoking may all contribute to the development and progression of DR[7]–[8]. Besides, functional and structural changes in retinal arterioles was also been considered as a risk factor for DR as well[9].
Accumulating evidence implicates that several blood cell-associated indices, including mean platelet volume (MPV), platelet distribution width (PDW), neutrophil to lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) are potential novel biomarkers of systemic inflammatory responses[10]–[13]. MPV is a parameter reflecting the average size of platelets and high MPV indicates that platelets have large size. Larger platelets usually display more metabolic and enzymatic activities and release more thromboxan-A2, b-thromboglobulin, and adhesion molecules as compared to the smaller size[14]–[15]. It has been reported that high MPV might be the risk for some vascular conditions, including peripheral artery disease, coronary artery disease, myocardial infarction and cerebral ischemia[16]. PDW is an indicator of the distribution of platelet size, and its high value indicates the increased production of larger reticulated platelet. Moreover, PDW may also play a considerable role in some vascular diseases, such as atherosclerosis and thrombosis[17]. Additionally, numerous studies has shown that the NLR and PLR are potential inflammatory biomarkers in tumors[18]–[23], cardiovascular diseases[24]–[25]. More importantly, in recent years, many studies have also investigated the association of MPV, PDW, NLR and PLR with DR[26]–[56]. However, the results of those studies in this regard were inconsistent. Considering that the small sample size in a single study might challenge the statistical power, we herein performed the first Meta-analysis and systematic review to further the relationship of MPV, PDW, NLR and PLR to the presence of DR and its severity.
MATERIALS AND METHODS
Search Strategy
This Meta-analysis was performed according to Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) guidelines[57]. The database of PubMed, EMBASE, Web of Science and CNKI were systematically search for potential eligible studies up to December 13, 2017. The search terms included: “neutrophil to lymphocyte ratio or NLR”, “platelet-lymphocyte ratio or PLR”, “platelet distribution width or PDW”, “mean platelet volume or MPV”, “hematological or hematologic” and “marker or indices” and “diabetic retinopathy”. The limitations of language and region were not applied in this Meta-analysis.
Selection Criteria
The criteria for eligible studies included the following points: 1) Enrolled patients of the studies were diagnosed with T2DM; 2) Observational or retrospective study design; 3) The data of hematologic inflammatory markers were available including MPV, PDW, NLR and PLR. Exclusion criteria of selection process were as follows: 1) Reviews, letters, editorials, meeting abstracts and case reports; 2) Data were unavailable; 3) If there are overlapping patients in different studies, the earlier published one was excluded.
Data Collection and Quality Assessment
Two independent investigators extracted all the data from the eligible literatures, and divergences in data extraction were resolved by discussion between the authors. The collected data included: name of first author, publication year, study design, country, the number of patients, mean age and sex in each group, values of MPV, PDW, NLR, and PLR. The quality of eligible studies was evaluated according to the Newcastle-Ottawa Scale (NOS)[58], which comprises eight points with three aspects: selection, comparability, and exposure. The scores of NOS system range from 0 to 9, and studies with 6 scores or more are considered as high quality[58].
Data Analysis
STATA version 12.0 (Stata Corporation, College Station, TX, USA) was used to conducted the statistical analysis. Standardized mean difference (SMD) and its 95% confidence interval (CI) was used to describe the synthesized continuous variables. If the data of hematologic inflammatory markers were both presented in NPDR and PDR patients, the data in NPDR was used in the comparison with DM group. With 95%CI not crossing 0, SMD>0 indicated that the specific hematologic inflammatory marker increased in patients with DR. In addition, Cochrane Q test (χ2) and I2 statistic were used to assess the statistical heterogeneity among the included studies. If P<0.01 for Q test and/or I2>50% for I2 statistic, the heterogeneity of the synthesized SMD was considered statistically significant, then a random effects model was used to pool the data, otherwise, a fixed effects model was employed for analysis. In order to testify the stabilization of our results, sensitivity analysis was conducted by sequentially dropping single study to investigate the effect of each individual article on the synthesized SMD. Publication bias was detected by Begg's test and Egger's test[59]–[60]. P<0.01 for Begg's test or Egger's test indicates that significant publication bias might exist. Furthermore, when there was significant publication bias, Duval's nonparametric trim-and-fill method was carried out to assess the potential influence of publication bias on the pooled results in this Meta-analysis[61].
RESULTS
Search Results
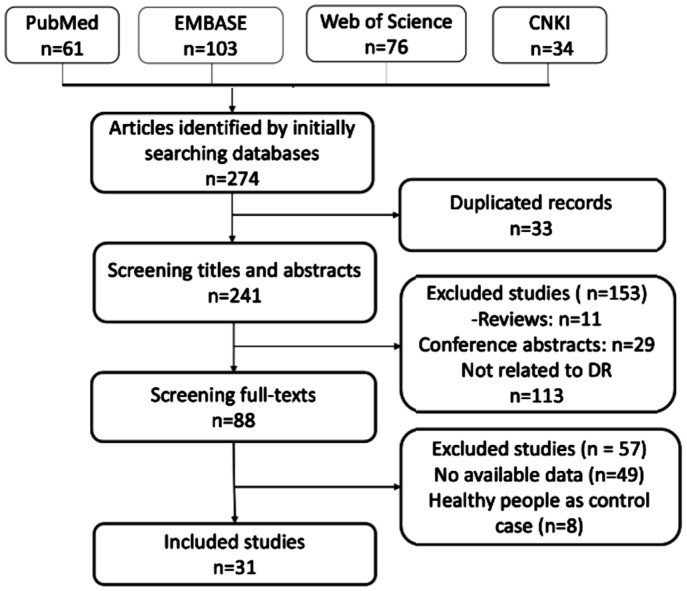
A total of 668 potential articles were yielded after primary literatures searching for PubMed, EMBASE, Web of Science and CNKI. After removing 156 duplicated studies, 560 were left for title and abstract screening. After omitting reviews, letters, editorials, meeting abstracts, case reports and articles not pertinent T2DM, 234 articles were excluded. Subsequently, full-text review was performed for remaining 60 articles, 56 articles were removed according to our selection criteria. Eventually, 31 articles with 5126 patients were included in this Meta-analysis[26]–[56]. All the details of literatures screening process were shown in Figure 1.
Figure 1. Flow chart of study selection process.
Study Characteristics and Quality Assessment
All the eligible studies were published from 2000 to 2017. The sample size of the included studies in DM group and in DR group ranged from 20 to 328 and 20 to 192, respectively. Only 3 studies were prospectively designed[30]–[31],[44], and the other studies were retrospectively designed. A total of 16 articles came from China[34]–[37],[40],[42]–[43],[45]–[49],[51]–[52],[54]–[55], 11 articles were conducted in Turkey[26]–[33],[41],[44],[56], 2 papers were from India, and 2 articles were performed in Greece[38]and Kazakhstan[50], respectively. Of the 31 included articles, 23 reported MPV[26]–[34],[35]–[44],[46]–[49], 12 reported PDW[26],[29]–[30],[35]–[37],[40]–[44],[48], 10 reported NLR[26],[45]–[46],[50]–[56], and 3 reported PLR[26],[42],[45]. All the basic characteristics and the values of these hematological indices of the included studies were summarized in Tables 1–4. According to the NOS, we evaluated the quality of 31 included studies. Only 4 articles scored 5[27],[36],[39],[49], and the scores of the rest of studies varied from 6 to 7, indicating that most of the eligible studies were high-quality.
Table 1. The main characteristics of the included studies on DR.
| First author | Year | Study design | Country | No. of patients |
Age mean/median | Male/female | NOS | ||
| Non-DR | NPDR | PDR | |||||||
| Akdoğan[26] | 2016 | Retrospective | Turkey | 158 | 120 | Non-DR: 57.3±12.2 | Non-DR: 59/99 | 7 | |
| DR: 59.8±9.2 | DR: 47/73 | ||||||||
| Ateş[27] | 2009 | Retrospective | Turkey | 30 | 30 | 30 | NR | NR | 5 |
| Buch[29] | 2017 | Retrospective | India | 220 | 80 | NR | NR | 6 | |
| Citirik[30] | 2015 | Prospective | Turkey | 43 | 45 | 52 | Non-DR: 60.4±8.5 | Non-DR: 22/21 | 6 |
| NPDR: 61.4±9.3 | NPDR: 17/28 | ||||||||
| PDR: 59.4±7.2 | PDR: 20/32 | ||||||||
| Demirtas[31] | 2015 | Prospective | Turkey | 240 | 67 | NR | NR | 6 | |
| Dindar[32] | 2013 | Retrospective | Turkey | 36 | 24 | NR | NR | 6 | |
| Güngör[33] | 2016 | Retrospective | Turkey | 50 | 52 | NR | Non-DR: 19/31 | 6 | |
| DR: 18/34 | |||||||||
| Li[34] | 2014 | Retrospective | China | 72 | 67 | 70 | Non-DR: 54.2±9.2 | Non-DR: 35/37 | 7 |
| NPDR: 57.7± 10.0 | NPDR: 34/33 | ||||||||
| PDR: 58.3±9.4 | PDR: 33/37 | ||||||||
| Li[47] | 2013 | Retrospective | China | 103 | 132 | NR | NR | 6 | |
| Li[35] | 2016 | Retrospective | China | 52 | 47 | Non-DR: 55.1±15.2 | Non-DR: 31/21 | 6 | |
| DR: 54.1±10.8 | DR: 26/21 | ||||||||
| Ma[36] | 2017 | Retrospective | China | 20 | 20 | 20 | Non-DR: 57.3±6.5 | Non-DR: 12/8 | 5 |
| NPDR: 60.8±7.3 | NPDR: 8/12 | ||||||||
| PDR: 57.6±7.3 | PDR: 12/8 | ||||||||
| Niu[37] | 2013 | Retrospective | China | 20 | 25 | Non-DR: 46.5±8.3 | Non-DR: 12/8 | 6 | |
| DR: 51.2±8.3 | DR: 13/12 | ||||||||
| Papanas[38] | 2004 | Retrospective | Greece | 89 | 167 | NR | NR | 6 | |
| Radha[39] | 2016 | Retrospective | India | 30 | 14 | NR | NR | 5 | |
| Sheng[40] | 2017 | Retrospective | China | 102 | 102 | NR | NR | 7 | |
| Tetikoğlu[41] | 2016 | Retrospective | Turkey | 63 | 56 | 80 | NR | NR | 6 |
| Tuzcu[28] | 2014 | Retrospective | Turkey | 70 | 64 | 58 | Non-DR: 55.8±10.5 | Non-DR: 38/32 | 6 |
| NPDR: 60.1±8.6 | NPDR: 32/32 | ||||||||
| PDR: 57.5±9.3 | PDR: 31/27 | ||||||||
| Wei[42] | 2017 | Retrospective | China | 94 | 52 | 40 | Non-DR: 58.14±11.93 | Non-DR: 50/44 | 7 |
| DR: 58.42±12.09 | DR: 49/43 | ||||||||
| Xu[43] | 2012 | Retrospective | China | 45 | 40 | NR | Non-DR: 23/22 | 5 | |
| DR: 26/14 | |||||||||
| Yilmaz[44] | 2016 | Prospective | Turkey | 89 | 88 | 86 | Non-DR: 60.9±6.3 | Non-DR: 49/40 | 7 |
| NPDR: 62.7±7.2 | NPDR: 48/40 | ||||||||
| PDR: 61.7±7.9 | PDR: 49/37 | ||||||||
| Yu[48] | 2000 | Retrospective | China | 60 | 40 | NR | NR | 6 | |
| Yue[45] | 2015 | Retrospective | China | 125 | 62 | 59 | Non-DR: 56.00±3.75 | Non-DR: 73/52 | 6 |
| NPDR: 53.50±3.56 | NPDR: 34/28 | ||||||||
| PDR: 56.0±3 | PDR: 28/3 | ||||||||
| Zhang[49] | 2002 | Retrospective | China | 20 | 20 | Non-DR: 58.0±9.0 | Non-DR: 9/11 | 5 | |
| DR: 60.0±1.3 | DR: 8/12 | ||||||||
| Zhou[46] | 2016 | Retrospective | China | 328 | 51 | Non-DR: 57±16 | Non-DR: 198/130 | 6 | |
| DR: 63±15 | DR: 34/17 | ||||||||
DR: Diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; NR: Not reported; NOS: Newcastle-Ottawa Scale.
Table 4. Values of MPV and PDW in T2DM subjects with and without DR.
| First author | Year | MPV (fL) |
PDW (%) |
||||||||||
| T2DM patients Without DR |
Patients with NPDR |
Patients with PDR |
T2DM patients Without DR |
Patients with NPDR |
Patients with PDR |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Akdoğan[26] | 2016 | 9.7 | 1.2 | 9.6±1.0 | 16.2 | 0.8 | 16.2±0.5 | ||||||
| Ateş[27] | 2009 | 7.76 | 0.72 | 7.94 | 0.61 | 8.18 | 0.89 | NR | NR | NR | NR | NR | NR |
| Buch[29] | 2017 | 10.24 | 2.04 | 11.40±1.96 | 13.94 | 3.33 | 14.92±4.14 | ||||||
| Citirik[30] | 2015 | 7.94 | 0.63 | 8.05 | 0.76 | 8.10 | 0.68 | 14.85 | 1.27 | 15.15 | 1.19 | 14.92 | 1.15 |
| Demirtas[31] | 2015 | 9.20 | 0.92 | 9.54±0.88 | NR | NR | NR | NR | NR | NR | |||
| Dindar[32] | 2013 | 10.68 | 1.68 | 11.26±1.08 | NR | NR | NR | NR | NR | NR | |||
| Güngör[33] | 2016 | 8.8 | 1.1 | 9.3±1.0 | NR | NR | NR | NR | NR | NR | |||
| Li[34] | 2014 | 7.8 | 1.1 | 8.3 | 1.38 | 8.9 | 1.65 | NR | NR | NR | NR | NR | NR |
| Li[47] | 2013 | 9.05 | 0.44 | 9.73±0.53 | NR | NR | NR | NR | NR | NR | |||
| Li[35] | 2016 | 10.39 | 0.90 | 10.72±1.57 | 13.80 | 3.32 | 16.17±1.66 | ||||||
| Ma[36] | 2017 | 8.12 | 0.82 | 8.96 | 0.86 | 10.76 | 1.12 | 15.66 | 2.37 | 17.85 | 2.26 | 17.90 | 2.41 |
| Niu[37] | 2013 | 10.25 | 2.04 | 14.21±2.35 | 16.05 | 1.56 | 18.12±1.25 | ||||||
| Papanas[38] | 2004 | 10.9 | 1.1 | 15.8±1.3 | NR | NR | NR | NR | NR | NR | |||
| Radha[39] | 2016 | 8.39 | 0.67 | 9.2±0.61 | NR | NR | NR | NR | NR | NR | |||
| Sheng[40] | 2017 | 9.76 | 0.86 | 10.17±0.92 | 11.31 | 1.67 | 12.04±1.88 | ||||||
| Tetikoğlu[41] | 2016 | 8.51 | 1.0 | 8.42 | 0.9 | 8.91 | 0.7 | 16.9 | 0.7 | 16.8 | 0.7 | 17.3 | 3.1 |
| Tuzcu[28] | 2014 | 7.90 | 1.26 | 8.20 | 1.55 | 8.78 | 1.73 | NR | NR | NR | NR | NR | NR |
| Wei[42] | 2017 | 11.12 | 1.3 | 11.50 | 1.39 | 11.56 | 1.06 | 13.70 | 2.90 | 14.40 | 2.88 | 14.20 | 1.99 |
| Xu[43] | 2012 | 11.21 | 1.71 | 13.44±2.01 | 15.98 | 1.23 | 17.41±1.42 | ||||||
| Yilmaz[44] | 2016 | 7.84 | 0.76 | 7.90 | 0.85 | 8.31 | 0.76 | 13.02 | 1.29 | 13.49 | 1.18 | 13.77 | 1.26 |
| Yu[48] | 2000 | 10.93 | 2.35 | 13.08±2.04 | 17.77 | 1.97 | 21.48±5.94 | ||||||
| Yue[45] | 2015 | NR | NR | NR | NR | NR | NR | ||||||
| Zhang[49] | 2002 | 9.82 | 1.53 | 10.19±2.06 | NR | NR | NR | NR | NR | NR | |||
| Zhou[46] | 2016 | 10.0 | 1.1 | 10.4±1.1 | NR | NR | NR | NR | NR | NR | |||
DR: Diabetic retinopathy; NR: Not reported; T2DM: Type 2 diabetes mellitus; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; MPV: Mean platelet volume; PDW: Platelet distribution width.
Table 2. The main characteristics of the included studies on the relationship of NLR and PLR to DR.
| First author | Year | Study design | Country | No. of patients |
Age mean/median | Male/female | NOS | ||
| Non-DR | NPDR | PDR | |||||||
| Akdoğan[26] | 2016 | Retrospective | Turkey | 158 | 120 | Non-DR: 57.3±12.2 | Non-DR: 59/99 | 7 | |
| DR: 59.8±9.2 | DR: 47/73 | ||||||||
| Ciray[50] | 2015 | Retrospective | Kazakhstan | 59 | 55 | Non-DR: 57.8±11.5 | NR | 6 | |
| DR: 61.8±10.8 | |||||||||
| Kuang[51] | 2015 | Retrospective | China | 62 | 44 | 22 | Non-DR: 60.73±11.24 | Non-DR: 29/33 | 6 |
| NPDR: 60.50±8.45 | NPDR: 17/27 | ||||||||
| PDR: 55.18±13.05 | PDR: 11/11 | ||||||||
| Öztürk[56] | 2013 | Retrospective | Turkey | 97 | 79 | NR | NR | NR | 5 |
| Shen[52] | 2016 | Retrospective | China | 118 | 134 | 58 | Non-DR: 55.19±5.51 | Non-DR: 63/55 | 6 |
| NPDR: 58.04±7.53 | NPDR: 73/61 | ||||||||
| PDR: 59.84±8.76 | PDR: 34/24 | ||||||||
| Ulu[53] | 2013 | Retrospective | Turkey | 34 | 24 | NR | NR | 5 | |
| Wei[42] | 2017 | Retrospective | China | 94 | 52 | 40 | Non-DR: 58.14±11.93 | Non-DR: 50/44 | 6 |
| DR: 58.42±12.09 | DR: 49/43 | ||||||||
| Wang[54] | 2015 | Retrospective | China | 138 | 131 | Non-DR: 60.3±6.0 | Non-DR: 65/73 | 7 | |
| DR: 66.6±5.8 | DR: 53/78 | ||||||||
| Yin[55] | 2015 | Retrospective | China | 64 | 28 | 36 | Non-DR: 56.83±9.01 | Non-DR: 35/29 | 6 |
| NPDR: 53.09±8.82 | NPDR:13/15 | ||||||||
| PDR: 53.16±10.64 | PDR:19/17 | ||||||||
| Yue[45] | 2015 | Retrospective | China | 125 | 62 | 59 | Non-DR: 56.00±3.75 | Non-DR: 73/52 | 6 |
| NPDR: 53.50±3.56 | NPDR: 34/28 | ||||||||
| PDR: 56.0±3 | PDR: 28/31 | ||||||||
| Zhou[46] | 2016 | Retrospective | China | 328 | 51 | Non-DR: 57±16 | Non-DR: 198/130 | 6 | |
| DR: 63±15 | DR: 34/17 | ||||||||
DR: Diabetic retinopathy; NR: Not reported; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; NLR: Neutrophil to lymphocyte ratio; PLR: Platelet-lymphocyte ratio.
Table 3. Values of NLR in T2DM subjects with and without DR.
| First author | Year | Neutrophil to lymphocyte ratio (%) |
Platelet to lymphocyte ratio (%) |
||||||||||
| T2DM patients without DR |
Patients with NPDR |
Patients with PDR |
T2DM patients without DR |
Patients with NPDR |
Patients withPDR |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Akdoğan[26] | 2016 | 2.4 | 1.9 | 3.0±4.4 | 116 | 66 | 140±87 | ||||||
| Ciray[50] | 2015 | 1.99 | 1.03 | 2.10±1.02 | NR | NR | NR | NR | |||||
| Kuang[51] | 2015 | 1.68 | 0.48 | 2.20 | 0.40 | 2.58 | 0.41 | NR | NR | NR | NR | ||
| Öztürk[56] | 2013 | 2.04 | 0.72 | 2.58±1.34 | NR | NR | NR | NR | |||||
| Shen[52] | 2016 | 1.52 | 0.26 | 1.68 | 0.21 | 1.95 | 0.17 | NR | NR | NR | NR | ||
| Ulu[53] | 2013 | 1.96 | 0.86 | 3.59±2.07 | NR | NR | NR | NR | |||||
| Wei[42] | 2017 | NR | NR | NR | NR | NR | 98.46 | 10.63 | 127.25±12.98 | ||||
| Wang[54] | 2015 | 2.1 | 1.3 | 3.7±1.4 | NR | NR | NR | NR | |||||
| Yin[55] | 2015 | 1.54 | 0.55 | 1.83 | 0.59 | 2.15 | 0.77 | NR | NR | NR | NR | ||
| Yue[45] | 2015 | 1.74 | 0.245 | 2.05 | 0.3 | 1.91 | 0.28 | 94.04 | 12.365 | 105.07 | 17.47 | 115.73 | 14.54 |
| Zhou[46] | 2016 | 2.4 | 1.5 | 4.4±2.7 | NR | R | NR | NR | |||||
DR: Diabetic retinopathy; NR: Not reported; T2DM: Type 2 diabetes mellitus; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; NLR: Neutrophil to lymphocyte ratio.
Meta-analysis Results
The relationship between mean platelet volume and the presence of diabetic retinopathy
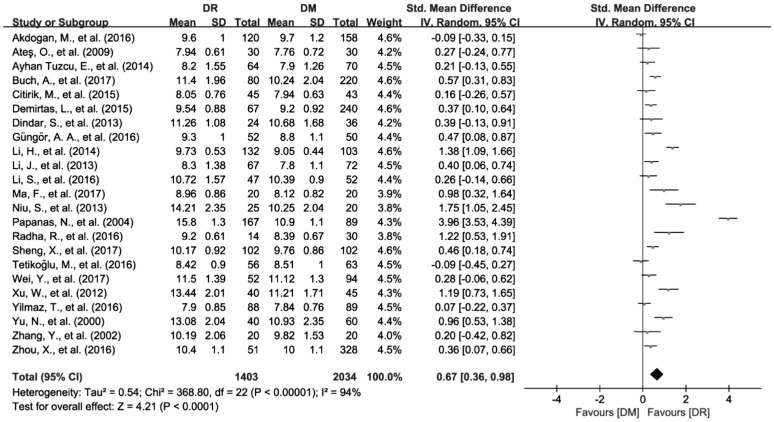
A total of 23 studies with 3437 patients reporting the data of MPV were included in this Meta-analysis[26]–[44],[46]–[49]. When synthesizing the data, the significant heterogeneity among the included studies was found (I2=94.1%, P<0.01). Therefore, a random effects model was used to pool the data and the results showed that MPV was significantly increased in patient with DR compared to DM group (SMD=0.67; 95%CI: 0.36 to 0.98; Figure 2).
Figure 2. Increased MPV values in DR patients compared with DM patients.
The relationship between mean platelet volume and the presence of diabetic retinopathy
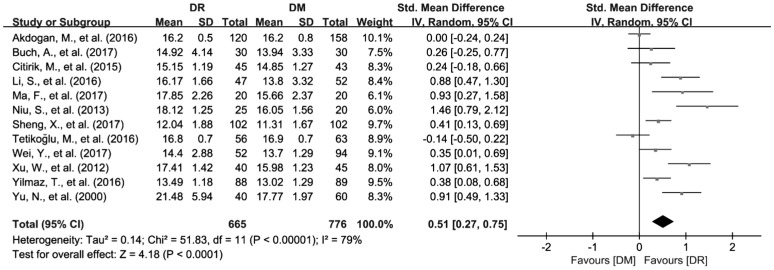
The data of PDW from 12 articles with 1681 patients were synthesized in this Meta-analysis[26],[29]–[30],[35]–[37],[40]–[44],[48]. Considering the significant heterogeneity among the selected studies (I2=79.3%, P<0.01), we used random effects model to pool the data. The results showed that PDW was higher in patients with DR compared to DM group (SMD=0.51; 95%CI: 0.27 to 0.75; Figure 3).
Figure 3. Increased PDW values in DR patients compared with DM patients.
The relationship between neutrophil to lymphocyte ratio and the presence of diabetic retinopathy
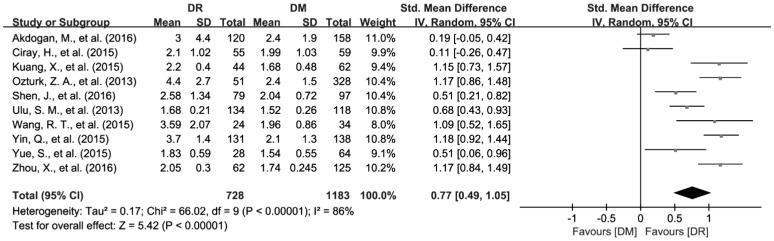
A total of 10 articles involving 1911 patients reported the data of NLR[26],[45]–[46],[50]–[56]. Randomized effects model was applied to synthesize the data since the significant heterogeneity was found among the included studies (I2=86.5%, P<0.01). The pooled results showed that NLR was substantially increased in DR patients compared to DM patients (SMD=0.77; 95%CI: 0.49 to 1.05; Figure 4).
Figure 4. Increased NLR values in DR patients compared with DM patients.
The relationship between platelet-lymphocyte ratio and the presence of diabetic retinopathy
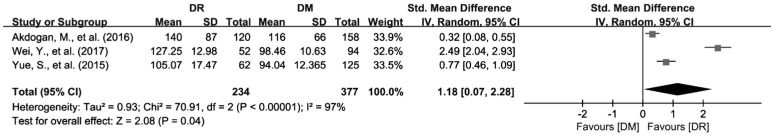
Three articles with 611 patients referring to PLR were included in this Meta-analysis[26],[42],[45]. Heterogeneity test showed I2=97.2% with P<0.01, so random effects model was applied to synthesize the data. As the results showed, PLR was elevated in patients with DR than in DM patients (SMD=1.18; 95%CI: 0.07 to 2.28; Figure 5).
Figure 5. Increased PLR values in DR patients compared with DM patients.
The relationship of mean platelet volume, platelet distribution width and neutrophil to lymphocyte ratio the severity of diabetic retinopathy
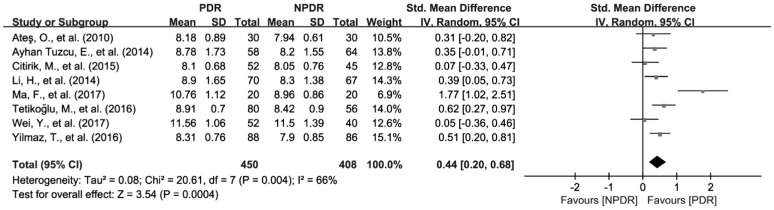
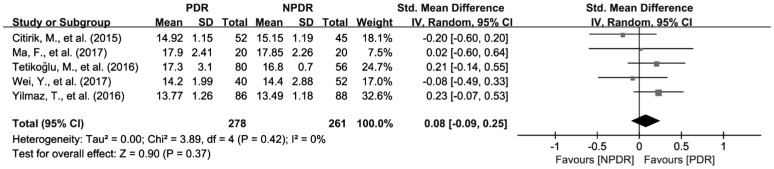
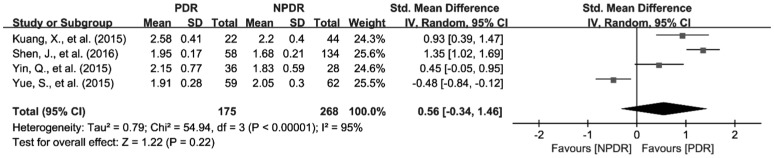
To investigate the relationship of MPV, PDW and NLR the severity of DR, we further analyzed the differences of MPV, PDW and NLR in patients between NPDR and PDR. There were 8 studies with 858 patients reporting the association of MPV with the severity of DR[27]–[28],[30],[34],[36],[41]–[42],[44]. Because of the significant heterogeneity among the included studies (I2=66%, P<0.01), random effects model was applied to pool the data, and the results revealed that MPV was significantly increased in patients with PDR compared to NPDR group (SMD=0.44; 95%CI: 0.20 to 0.68; Figure 6). In addition, 5 articles with 539 patients provide available data for the pooled analysis of the association of PDW with the severity of DR[30],[36],[41]–[42],[44]. Fixed effects Meta-analysis was performed since the significant heterogeneity was not found and (I2=0, and P=0.42). However, the results showed that the difference of PDW was not observed between NPDR and PDR patients (SMD=0.08; 95%CI: −0.09 to 0.25; Figure 7). As for NLR, 4 studies involving 443 patients were included for the Meta-analysis of its association with NLR[45],[51]–[52],[55]. Similar to PDW, the difference of NLR was not found between NPDR and PDR patients (SMD=0.56; 95%CI: −0.34 to 1.46) either (Figure 8).
Figure 6. Increased MPV values in PDR patients compared with NPDR patients.
Figure 7. Increased PDW values in PDR patients compared with NPDR patients.
Figure 8. Increased NLR values in PDR patients compared with NPDR patients.
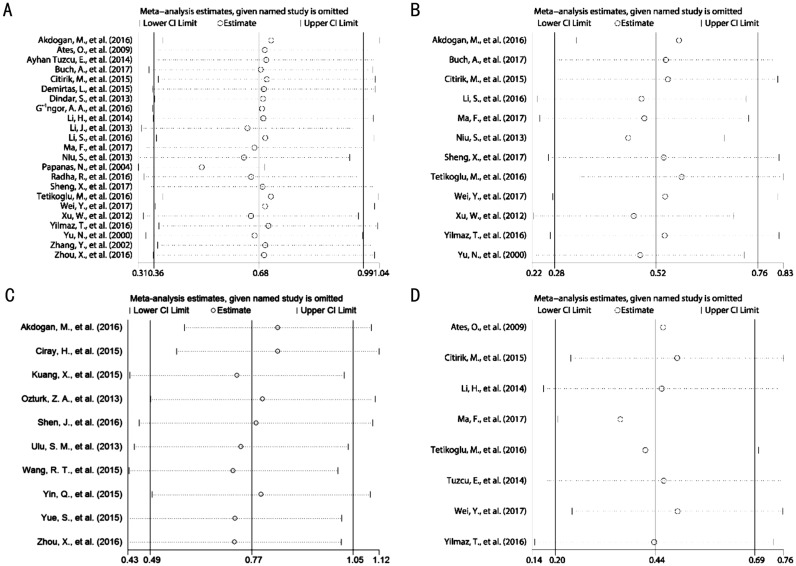
Sensitivity Analysis
Sensitivity analyses were conducted by omitting single study in each step to assess the effect of each individual study on the pooled SMDs for MPV, PDW and NLR in DR patients as compared with DM patients. The results showed that the pooled SMDs for MPV (Figure 9A), PDW (Figure 9B) and NLR (Figure 9C) did not alter significantly when any individual study was excluded, indicating that the results of this Meta-analysis were vigorous. Additionally, we also applied the sensitivity analysis to test the stability of the pooled SMD for MPV in patients with PDR as compared with NPDR. Similarly, the result also indicated that the pooled SMD for MPV in patients with PDR as compared with NPDR was robust (Figure 9D). Due to the limited number of studies, sensitivity analyses were not applicable to verify the robustness of the pooled SMD for PLR in DR patients as compared with DM patients, as well as of the pooled SMDs for NLR and PDW in patients with PDR as compared with NPDR.
Figure 9. Sensitivity analysis on SMD by removing each study in each model.
A: SMD on the relationship between MPV value and the risk of DR; B: SMD on the relationship between PDW value and the risk of DR; C: SMD on the relationship between NLR value and the risk of DR; D: SMD on the relationship between MPV value and the severity of DR.
Publication Bias
We used Begg's and Egger's test to evaluate the publication bias among included studies. The result showed that there was no significant bias for the synthesized SMDs for NLR (Begg's tests, P=0.929; Egger's tests, P=0.588) in DR patients as compared with DM patients. However, the publication bias might exist among the included studies for the synthesized SMDs for MPV (Begg's test, P=0.039; Egger's test, P=0.148) and PDW (Begg's tests, P=0.06; Egger's tests, P=0.02) in DR patients as compared with DM patients. Therefore, Meta-trim method was conducted to investigate the influence of publication bias on the reliability of pooled SMDs for MPV and PDW. From the results of meta-trim method, we observed that the adjusted SMDs of MPV (0.68; 95%CI: 0.36–0.99) and PDW (0.68; 95%CI: 0.36–0.99) did not change substantially, which implicated that the pooled results of the MPV and PDW for DR were still reliable, although the publication bias existed in this Meta-analysis. Besides, the publication bias test was not available for the pooled SMD of PLR due to the limitation of the number of the eligible studies.
DISCUSSION
In our Meta-analysis, a total of 31 eligible studies were included for pooling analysis. As compared with T2DM patients without DR, the values of MPV (SMD=0.67; 95%CI: 0.36 to 0.98), PDW (SMD=0.51; 95%CI: 0.27 to 0.75), NLR (SMD=0.77; 95%CI: 0.49 to 1.05), and PLR (SMD=1.18; 95%CI: 0.07 to 2.28) were much higher in patients with DR. Additionally, patients with PDR had much higher MPV value than NPDR patients (SMD=0.44; 95%CI: 0.20 to 0.68), indicating that MPV might be closely correlated with the severity of DR. Furthermore, our sensitivity analysis showed that the pooled SMDs mentioned above did not change significantly when sequentially omitting any of the included studies, which demonstrated that our pooled results were stable and reliable. Meanwhile, the results of publication bias assessment indicated that publication bias did not substantially affect our pooled results either.
Various factors including systematic inflammation, elevated phosphorylation and glycosylation of cellular proteins, oxidative stress, abnormal calcium metabolism, reduced bioavailability of nitric oxide may promote the release of prothrombotic and proinflammatory substances and contribute to the platelet activation in diabetic patients[62]. Furthermore, it was proposed that elevated activation of platelets play an important role in the development of coagulation abnormalities and thromboembolic events in diabetic patients[63]. MPV is a parameter evaluating the average size of platelets and high MPV indicates that platelets have large size. Larger platelets usually display more metabolic and enzymatic activities and release more thromboxan-A2, b-thromboglobulin, and adhesion molecules as compared to the smaller size[14]–[15]. Considering that microthrombosis plays a key role in the vascular complications, many studies were conducted to explore the association of MPV with vascular complications including DR[26]–[44],[46]–[49]. However, the results of those studies were inconsistent. Hence, we conducted the present Meta-analysis to further determine the association of MPV with DR. From the results of this Meta-analysis, we found that MPV was significantly higher in diabetic patients with DR than in those without DR, as well as higher in diabetic patients with PDR as compared to those with NPDR. As with MPV, PDW has also been thought as a marker of platelet activation[64]. Conflicting results have been reported for the association of PDW with the presence of DR and the degree of DR[26],[29]–[30],[35]–[37],[40]–[44],[48]. Herein, our Meta-analysis indicated that higher PDW was closely associated with the presence of DR, but not with the severity of DR. Based on our results, we may speculate that PDW was only linked with the formation of DR, but not with the progression of DR. However, considering that only 5 studies provide available data for our Meta-analysis of the correlation of PDW to the severity of DR, the small sample size may partly be responsible for the negative association of PDW with the severity of DR. Therefore, further studies are in need to investigate the association between PDW and the severity of DR.
The counts of WBCs and its subtypes are important inflammation response biomarkers. In addition, the NLR and PLR have also been considered as potential markers that reflect the status of inflammation and immune responses[13]. More importantly, the stability of NLR and PLR is superior to independent blood neutrophils, monocyte and lymphocytes due to their less susceptibility to various physiological and pathological condition, which indicates that the alteration of NLR and PLR can reflect the status of inflammation and immune responses better. Furthermore, a body of literature suggested that NLR and PLR had diagnostic and prognostic values in various diseases, including DM, acute coronary syndromes, and various malignancies[11]–[12],[18],[24]–[25],[56],[65]–[67]. In particular, it has been reported that neutrophils could promote the development and progression of microangiopathy and inflammation, when adhering to the endothelial cell wall[68]–[69]. For instance, a study by Woo et al[70], showed that neutrophil count in circulation increased in patients with DR, and was significantly correlated with the severity of DR, suggesting the key role of neutrophil-mediated inflammation in the development and progression of DR. Moreover, several recent studies reported that NLR, as a novel inflammation marker, NLR was found to be elevated in patients with DR and associated with the severity of DR[13],[56],[70]–[72]. Nevertheless, in a study by Ciray et al[73], NLR was not found to be correlated with DR. Additionally, there were also several studies focusing on investigating the relationship of PLR to DR and its severity[26],[42],[45]. However, the reliability of conclusions on the association of NLR, and PLR with DR were challenged by the limitation of small sample size in an individual study. Thus, we herein conducted a Meta-analysis to further determine the link of NLR and PLR with DR. In the present study, we found that the levels of NLR, and PLR were higher in patients with DR than in patients with DM and without DR, but there were no differences in NLR levels between patients with NPDR and PDR. These results implicated that NLR may only play a key role in the initiation of DR, but not in the progression of DR. Similar to PDW, only 4 studies with a small sample size had available data for the Meta-analysis of the correlation of NLR to the severity of DR, suggesting that a weak statistical power might also be responsible for the no associations of NLR with the severity of DR. Hence, further studies are needed to investigate the association between NLR and the severity of DR.
When we interpreted the results of our study, some limitations should be taken into account. First, although random effect model was used to conduct this Meta-analysis, substantial heterogeneities among the included studies still existed. The potential sources of the heterogeneity might come from the differences in some characteristics of the included studies, such as age, ethnicity, diseases duration and body mass index, etc. Second, the definition of the DR severity was not uniform, which may introduce bias. Third, the number of included studies was limited for a reliable Meta-analysis of the correlation of NLR and PDW with the DR severity. At last but not least, our study only focused on systematically analyzing the link between blood cell-related inflammatory indices and DR. Actually, in addition to blood cell-related inflammatory indices, there were many other serum biomarkers that are also closely correlated with inflammation, and these serum biomarkers have been considered to play key roles in DR development as well, such as pentosidine[74]–[81], C-reactive protein[82]–[83], interleukin-6[84]–[86] and TNF-alpha[87]. However, because of lacking available data, our Meta-analysis failed to systematically assess the association of blood cell-related inflammatory indices with other serum inflammatory biomarkers. Therefore, in future more studies should be conducted to analyze the associations of MPV, PDW, NLR, and PLR with other serum inflammatory biomarkers, which will further confirm our findings in this present Meta-analysis.
In conclusion, our study suggested that MPV, PDW, NLR and PLR may be associated with the presence of DR and MPV is also correlated with the severity of DR, but NLR and PDW might not be linked with the severity of DR. Overall, MPV, PDW, NLR and PLR could be recommended as inexpensive diagnostic markers for DR. However, considering several limitations in our study, further high-quality studies are needed to investigate the association of these hematologic inflammatory indices with DR, especially the correlation of NLR and PDW with the severity of DR.
Acknowledgments
Conflicts of Interest: Luo WJ, None; Zhang WF, None.
REFERENCES
- 1.Pan S, Liu ZW, Shi S, Ma X, Song WQ, Guan GC, Zhang Y, Zhu SM, Liu FQ, Liu B, Tang ZG, Wang JK, Lv Y. Hamilton rating scale for depression-24 (HAM-D24) as a novel predictor for diabetic microvascular complications in type 2 diabetes mellitus patients. Psychiatry Res. 2017;258:177–183. doi: 10.1016/j.psychres.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 2.Niu WQ, Qi Y. An updated meta-analysis of methylenetetrahydrofolate reductase gene 677C/T polymorphism with diabetic nephropathy and diabetic retinopathy. Diabetes Res Clin Pract. 2012;95(1):110–118. doi: 10.1016/j.diabres.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Shi R, Zhao L, Wang F, Liu F, Chen Z, Li R, Liu Y, Lin R. Effects of lipid-lowering agents on diabetic retinopathy: a Meta-analysis and systematic review. Int J Ophthalmol. 2018;11(2):287–295. doi: 10.18240/ijo.2018.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 5.Chua J, Lim CXY, Wong TY, Sabanayagam C. Diabetic retinopathy in the asia-pacific. Asia Pac J Ophthalmol (Phila) 2018;7(1):3–16. doi: 10.22608/APO.2017511. [DOI] [PubMed] [Google Scholar]
- 6.Lin S, Ramulu P, Lamoureux EL, Sabanayagam C. Addressing risk factors, screening, and preventative treatment for diabetic retinopathy in developing countries: a review. Clin Exp Ophthalmol. 2016;44(4):300–320. doi: 10.1111/ceo.12745. [DOI] [PubMed] [Google Scholar]
- 7.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–277. doi: 10.1111/ceo.12696. [DOI] [PubMed] [Google Scholar]
- 8.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. The Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 9.Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009;23(7):1496–1508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
- 10.Okyay GU, İnal S, Öneç K, Er RE, Paşaoğlu Ö, Paşaoğlu H, Derici Ü, Erten Y. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Renal Failure. 2013;35(1):29–36. doi: 10.3109/0886022X.2012.734429. [DOI] [PubMed] [Google Scholar]
- 11.Kahraman C, Kahraman NK, Aras B, Coşgun S, Gülcan E. The relationship between neutrophil-to-lymphocyte ratio and albuminuria in type 2 diabetic patients: a pilot study. Arch Med Sci. 2016;12(3):571–575. doi: 10.5114/aoms.2016.59931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin BD, Ma N, Tang QQ, Wei TT, Yang M, Fu HT, Hu ZD, Liang Y, Yang ZX, Zhong RQ. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372–376. doi: 10.3109/14397595.2015.1091136. [DOI] [PubMed] [Google Scholar]
- 13.Şenel E, Acar B, Demir E. Mean platelet volume: a reliable marker of inflammation in recurrent apthous stomatitis and Behçet disease? Indian Dermatol Online J. 2017;8(6):468–470. doi: 10.4103/idoj.IDOJ_405_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J. 2001;22(17):1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- 15.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bozlu G, Karpuz D, Hallioglu O, Unal S, Kuyucu N. Relationship between mean platelet volume-to-lymphocyte ratio and coronary artery abnormalities in Kawasaki disease. Cardiol Young. 2018;28(6):832–836. doi: 10.1017/S1047951118000422. [DOI] [PubMed] [Google Scholar]
- 17.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 18.Cannon NA, Meyer J, Iyengar P, Ahn C, Westover KD, Choy H, Timmerman R. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10(2):280–285. doi: 10.1097/JTO.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 19.Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunduz S, Mutlu H, Tural D, Yıldız Ö, Uysal M, Coskun HS, Bozcuk H. Platelet to lymphocyte ratio as a new prognostic for patients with metastatic renal cell cancer. Asia Pac J Clin Oncol. 2015;11(4):288–292. doi: 10.1111/ajco.12358. [DOI] [PubMed] [Google Scholar]
- 21.Gunduz S, Mutlu H, Uysal M, Coskun HS, Bozcuk H. Prognostic value of hematologic parameters in patients with metastatic renal cell carcinoma using tyrosine kinase inhibitors. Asian Pac J Cancer Prev. 2014;15(8):3801–3804. doi: 10.7314/apjcp.2014.15.8.3801. [DOI] [PubMed] [Google Scholar]
- 22.Ulas A, Avci N, Kos T, Cubukcu E, Olmez OF, Bulut N, Degirmenci M. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? J BUON. 2015;20(3):714–722. [PubMed] [Google Scholar]
- 23.Wang YQ, Xu F, Pan JH, Zhu YJ, Shao XG, Sha JJ, Wang ZZ, Cai Y, Liu Q, Dong BJ, Xue W, Huang YR. Platelet to lymphocyte ratio as an independent prognostic indicator for prostate cancer patients receiving androgen deprivation therapy. BMC Cancer. 2016;16:329. doi: 10.1186/s12885-016-2363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akyel A, Yayla Ç, Erat M, Çimen T, Doğan M, Açıkel S, Aydoğdu S, Yeter E. Neutrophil-to-lymphocyte ratio predicts hemodynamic significance of coronary artery stenosis. Anatol J Cardiol. 2015;15(12):1002–1007. doi: 10.5152/akd.2015.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang GY, Chen M, Yu ZM, Wang XD, Wang ZQ. Relation between neutrophil-to-lymphocyte ratio and severity of coronary artery stenosis. Genet Mol Res. 2014;13(4):9382–9389. doi: 10.4238/2014.November.11.4. [DOI] [PubMed] [Google Scholar]
- 26.Akdoğan M, Ustundag-Budak Y, Huysal K. The association of hematologic inflammatory markers with atherogenic index in type 2 diabetic retinopathy patients. Clin Ophthalmol. 2016;10:1797–1801. doi: 10.2147/OPTH.S110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ateş O, Kiki İ, Bilen H, Keleş M, Koçer İ, Kulaçoğlu D, Baykal O. Association of mean platelet volume with the degree of retinopathy in patients with diabetes mellitus. European Journal of General Medicine. 2009;6(2):99–102. [Google Scholar]
- 28.Ayhan Tuzcu E, Arıca S, Ilhan N, Daglioglu M, Coskun M, Ilhan O, Ustun I. Relationship between mean platelet volume and retinopathy in patients with type 2 diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2014;252(2):237–240. doi: 10.1007/s00417-013-2444-y. [DOI] [PubMed] [Google Scholar]
- 29.Buch A, Kaur S, Nair R, Jain A. Platelet volume indices as predictive biomarkers for diabetic complications in Type 2 diabetic patients. J Lab Physicians. 2017;9(2):84–88. doi: 10.4103/0974-2727.199625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Citirik M, Beyazyildiz E, Simsek M, Beyazyildiz O, Haznedaroglu IC. MPV may reflect subcinical platelet activation in diabetic patients with and without diabetic retinopathy. Eye (Lond) 2015;29(3):376–379. doi: 10.1038/eye.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demirtas L, Degirmenci H, Akbas EM, Ozcicek A, Timuroglu A, Gurel A, Ozcicek F. Association of hematological indicies with diabetes, impaired glucose regulation and microvascular complications of diabetes. Int J Clin Exp Med. 2015;8(7):11420–11427. eCollection 2015. [PMC free article] [PubMed] [Google Scholar]
- 32.Dindar S, Cinemre H, Sengul E, Annakkaya AN. Mean platelet volume is associated with glycaemic control and retinopathy in patients with type 2 diabetes mellitus. West Indian Med J. 2013;62(6):519–523. doi: 10.7727/wimj.2012.284. [DOI] [PubMed] [Google Scholar]
- 33.Güngör AA, Gürsoy G, Güngör F, Bayram SM, Atalay E. The relationship of mean platelet volume with retinopathy in type 2 diabetes mellitus. Turk J Med Sci. 2016;46(5):1292–1299. doi: 10.3906/sag-1410-95. [DOI] [PubMed] [Google Scholar]
- 34.Li HY, Bi CC, Qu CY. Relationship between mean platelet volume and development of diabetic retinopathy: a cross-sectional study. Rec Adv Ophthalmol. 2014;34(8):766–768. [Google Scholar]
- 35.Li SJ, Cao WJ, Sun XH. Role of platelet parameters on neovascular glaucoma: a retrospective case-control study in China. PLoS One. 2016;11(12):e0166893. doi: 10.1371/journal.pone.0166893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma FF, Zhang YX. Changes and clinical significance of platelet activation and four platelet parameters in diabetic retinopathy. Rec Adv Ophthalmol. 2017;37(2):164–166. [Google Scholar]
- 37.Niu SL. Changes of HbAlc, FPG and platelet parameters in patients with diabetic retinopathy and risk factors. Rec Adv Ophthalmol. 2013;33(7):655–657. [Google Scholar]
- 38.Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, Lakasas G. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15(8):475–478. doi: 10.1080/0953710042000267707. [DOI] [PubMed] [Google Scholar]
- 39.Radha RK, Selvam D. MPV in uncontrolled & controlled diabetics- its role as an indicator of vascular complication. J Clin Diagn Res. 2016;10(8):EC22–EC26. doi: 10.7860/JCDR/2016/21499.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng X, Xie Q, Liu C, Liu W, Fu L. Changes of blood glucose and coagulation parameters in patients with diabetic retinopathy. China Journal of Modern Medicine. 2017;27(12):133–136. [Google Scholar]
- 41.Tetikoğlu M, Aktas S, Sagdık HM, Tasdemir Yigitoglu S, Özcura F. Mean platelet volume is associated with diabetic macular edema in patients with type-2 diabetes mellitus. Semin Ophthalmol. 2017;32(5):651–654. doi: 10.3109/08820538.2016.1157612. [DOI] [PubMed] [Google Scholar]
- 42.Wei Y, Guan S, Zhou Q, Yang K. Platelet-to-lymphocyte ratio as potential biomarker in the diagnosis of diabetic retinopathy. Acta Universitatis Medicinalis Anhui. 2017;52(3):409–411. [Google Scholar]
- 43.Xu W, Cai YM, Wang CX. Study on clinical significance of changes in MPV and HbAlc in patients with diabetic retinopathy. Journal of Clinical and Experimental Medicine. 2012;11(8):584–585. [Google Scholar]
- 44.Yilmaz T, Yilmaz A. Relationship between altered platelet morphological parameters and retinopathy in patients with type 2 diabetes mellitus. J Ophthalmol. 2016;2016:9213623. doi: 10.1155/2016/9213623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yue S, Zhang JH, Wu JY, Teng WP, Liu L, Chen L. Use of the monocyte-to-lymphocyte ratio to predict diabetic retinopathy. International Journal of Environmental Research and Public Health. 2015;12(8):10009–10019. doi: 10.3390/ijerph120810009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou X, Liu Q, Bau H, Liang L. Clinical significance of mean platelet volume and neutrophil-to-lymphocyte ratio in patients with diabetic retinopathy. Guoji Yanke Zazhi (Int Eye Sci) 2016;16(5):981–983. [Google Scholar]
- 47.Li J, Chen K, Chen W, Zhang K, Deng S. Changes and significance of mean platelet volume in patients with type 2 diabetes mellitus. Chongqing Med. 2013;42(15):1742–1744. [Google Scholar]
- 48.Yu N, Qiu W, Jiang L. Relationship between diabetic retinopathy and platelet volume. Journal of Practical Diabetology. 2000;2(2):33–34. [Google Scholar]
- 49.Zhang Y, Liu J, Shang G. Clinical analysis for mean platelet volume, mean red cells volume in diabetic retinopathy patients. Journal of Clinical Ophthalmology. 2000;10(1):52–53. [Google Scholar]
- 50.Ciray H, Aksoy A, Ulu N, Cizmecioglu A, Gaipov A, Solak Y. Nephropathy, but not angiographically proven retinopathy, is associated with neutrophil to lymphocyte ratio in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2015;123(5):267–271. doi: 10.1055/s-0035-1547257. [DOI] [PubMed] [Google Scholar]
- 51.Kuang X, Wang SL. Relationship of serum neutrophil-to-lymphocyte ratio with diabetic retinopathy. Chin J Diabetes. 2015;(5):438–440. [Google Scholar]
- 52.Shen J, Zhang Q, Li M, Xie S, Niu M, Wang Y. Association between neutrophil-to-lymphocyte ratio and diabetic retinopathy. Chin J Diabetes. 2016;24(7):617–621. [Google Scholar]
- 53.Ulu SM, Dogan M, Ahsen A, Altug A, Demir K, Acartürk G, Inan S. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther. 2013;15(11):942–947. doi: 10.1089/dia.2013.0097. [DOI] [PubMed] [Google Scholar]
- 54.Wang RT, Zhang JR, Li Y, Liu TM, Yu KJ. Neutrophil-lymphocyte ratio is associated with arterial stiffness in diabetic retinopathy in type 2 diabetes. J Diabetes Complicat. 2015;29(2):245–249. doi: 10.1016/j.jdiacomp.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Yin Q, Guo SQ, Zhang YL, Wang J, Ma ZP, Ti SF, Kang JP, Meng LR. Changes and significance of neutrophil-lymphocyte ratio in patients with type 2 diabetes and retinopathy. Chinese Journal of Mrcrocirculation. 2013;(4):46–49. [Google Scholar]
- 56.Öztürk ZA, Kuyumcu ME, Yesil Y, Savas E, Yıldız H, Kepekçi Y, Arıoğul S. Is there a link between neutrophil-lymphocyte ratio and microvascular complications in geriatric diabetic patients? J Endocrinol Invest. 2013;36(8):593–599. doi: 10.3275/8894. [DOI] [PubMed] [Google Scholar]
- 57.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 59.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 60.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 62.Suslova TE, Sitozhevskii AV, Ogurkova ON, Kravchenko ES, Kologrivova IV, Anfinogenova Y, Karpov RS. Platelet hemostasis in patients with metabolic syndrome and type 2 diabetes mellitus: cGMP- and NO-dependent mechanisms in the insulin-mediated platelet aggregation. Front Physiol. 2014;5:501. doi: 10.3389/fphys.2014.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El Haouari M, Rosado JA. Platelet signalling abnormalities in patients with type 2 diabetes mellitus: a review. Blood Cells Mol Dis. 2008;41(1):119–123. doi: 10.1016/j.bcmd.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K, Singh S. Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011;16(2):86–89. doi: 10.1179/102453311X12902908412110. [DOI] [PubMed] [Google Scholar]
- 65.Hudzik B, Szkodzinski J, Gorol J, Niedziela J, Lekston A, Gasior M, Polonski L. Platelet-to-lymphocyte ratio is a marker of poor prognosis in patients with diabetes mellitus and ST-elevation myocardial infarction. Biomark Med. 2015;9(3):199–207. doi: 10.2217/bmm.14.100. [DOI] [PubMed] [Google Scholar]
- 66.Lee YS, Nam HS, Lim JH, Kim JS, Moon Y, Cho JH, Ryu JS, Kwak SM, Lee HL. Prognostic impact of a new score using neutrophil-to-lymphocyte ratios in the serum and malignant pleural effusion in lung cancer patients. BMC Cancer. 2017;17(1):557. doi: 10.1186/s12885-017-3550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang HW, Lu WP, Li BM, Li CH, Xu YZ, Dong JH. Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(22):36857–36868. doi: 10.18632/oncotarget.16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujita T, Hemmi S, Kajiwara M, Yabuki M, Fuke Y, Satomura A, Soma M. Complement-mediated chronic inflammation is associated with diabetic microvascular complication. Metabolism Research and Reviews. 2013;29(3):220–226. doi: 10.1002/dmrr.2380. [DOI] [PubMed] [Google Scholar]
- 69.Sala A, Folco G. Neutrophils, endothelial cells, and cysteinyl leukotrienes: a new approach to neutrophil-dependent inflammation? Biochem Biophys Res Commun. 2001;283(5):1003–1006. doi: 10.1006/bbrc.2001.4865. [DOI] [PubMed] [Google Scholar]
- 70.Woo SJ, Ahn SJ, Ahn J, Park KH, Lee K. Elevated systemic neutrophil count in diabetic retinopathy and diabetes: a hospital-based cross-sectional study of 30,793 Korean subjects. Invest Ophthalmol Vis Sci. 2011;52(10):7697–7703. doi: 10.1167/iovs.11-7784. [DOI] [PubMed] [Google Scholar]
- 71.Ulu SM, Dogan M, Ahsen A, Altug A, Demir K, Acarturk G, Inan S. Neutrophil-to-lymphocyte ratio as a quick and reliable predictive marker to diagnose the severity of diabetic retinopathy. Diabetes Technol Ther. 2013;15(11):942–947. doi: 10.1089/dia.2013.0097. [DOI] [PubMed] [Google Scholar]
- 72.Wang RT, Zhang JR, Li Y, Liu TM, Yu KJ. Neutrophil-Lymphocyte ratio is associated with arterial stiffness in diabetic retinopathy in type 2 diabetes. J Diabetes Complicat. 2015;29(2):245–249. doi: 10.1016/j.jdiacomp.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Ciray H, Aksoy AH, Ulu N, Cizmecioglu A, Gaipov A, Solak Y. Nephropathy, but not angiographically proven retinopathy, is associated with neutrophil to lymphocyte ratio in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2015;123(5):267–271. doi: 10.1055/s-0035-1547257. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura N, Hasegawa G, Obayashi H, Yamazaki M, Ogata M, Nakano K, Yoshikawa T, Watanabe A, Kinoshita S, Fujinami A, Ohta M, Imamura Y, Ikeda T. Increased concentration of pentosidine, an advanced glycation end product, and interleukin-6 in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Research and Clinical Practice. 2003;61(2):93–101. doi: 10.1016/s0168-8227(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 75.Ghanem AA, Elewa A, Arafa LF. Pentosidine and N-carboxymethyl-lysine: biomarkers for type 2 diabetic retinopathy. Eur J Ophthalmol. 2011;21(1):48–54. doi: 10.5301/ejo.2010.4447. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto K, Kunikata H, Yasuda M, Ito A, Aizawa N, Sawada S, Kondo K, Satake C, Takano Y, Nishiguchi KM, Katagiri H, Nakazawa T. The relationship between advanced glycation end products and ocular circulation in type 2 diabetes. J Diabetes Complicat. 2016;30(7):1371–1377. doi: 10.1016/j.jdiacomp.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 77.Hirata K, Kubo K. Relationship between blood levels of N-carboxymethyl- lysine and pentosidine and the severity of microangiopathy in type 2 diabetes. Endocr J. 2004;51(6):537–544. doi: 10.1507/endocrj.51.537. [DOI] [PubMed] [Google Scholar]
- 78.Kerkeni M, Saïdi A, Bouzidi H, Yahya SB, Hammami M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvascular Research. 2012;84(3):378–383. doi: 10.1016/j.mvr.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 79.Lim XL, Teo BW, Tai BC, Wong TY, Ng DP. Pentosidine levels in nonproteinuric diabetes associated with both low estimated glomerular filtration rate and cataract. Diabetes Metab Syndr Obes. 2012;5:155–164. doi: 10.2147/DMSO.S32283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salman AG, Mansour DE, Swelem AH, Al-Zawahary WM, Radwan AA. Pentosidine - a new biochemical marker in diabetic retinopathy. Ophthalmic Res. 2009;42(2):96–98. doi: 10.1159/000225661. [DOI] [PubMed] [Google Scholar]
- 81.Sato E, Nagaoka T, Yokota H, Takahashi A, Yoshida A. Correlation between plasma pentosidine concentrations and retinal hemodynamics in patients with type 2 diabetes. Am J Ophthalmol. 2012;153(5):903–909. e901. doi: 10.1016/j.ajo.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Song J, Chen S, Liu X, Duan H, Kong J, Li Z. Relationship between c-reactive protein level and diabetic retinopathy: a systematic review and meta-analysis. PLoS One. 2015;10(12):e0144406. doi: 10.1371/journal.pone.0144406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang XF, Deng Y, Gu H, Lim A, Snellingen T, Liu XP, Wang NL, Domalpally A, Danis R, Liu NP. C-reactive protein and diabetic retinopathy in Chinese patients with type 2 diabetes mellitus. Int J Ophthalmol. 2016;9(1):111–118. doi: 10.18240/ijo.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gustavsson C, Agardh CD, Agardh E. Profile of intraocular tumour necrosis factor-α and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmol. 2013;91(5):445–452. doi: 10.1111/j.1755-3768.2012.02430.x. [DOI] [PubMed] [Google Scholar]
- 85.Mocan MC, Kadayifcilar S, Eldem B. Elevated intravitreal interleukin-6 levels in patients with proliferative diabetic retinopathy. Can J Ophthalmol. 2006;41(6):747–752. doi: 10.3129/i06-070. [DOI] [PubMed] [Google Scholar]
- 86.Myśliwiec M, Balcerska A, Zorena K, Myśliwska J, Lipowski P, Raczyńska K. The role of vascular endothelial growth factor, tumor necrosis factor alpha and interleukin-6 in pathogenesis of diabetic retinopathy. Diabetes Res Clin Pract. 2008;79(1):141–146. doi: 10.1016/j.diabres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 87.Yao Y, Li R, Du JH, Li XN, Zhao L, Long LH, Li DM, Lu SM. Tumor necrosis factor-α and diabetic retinopathy: review and meta-analysis. Clinica Chimica Acta. 2018;485:210–217. doi: 10.1016/j.cca.2018.06.028. [DOI] [PubMed] [Google Scholar]