Abstract
AIM
To investigate the expression of visual system homeobox 1 (VSX1) and myofibroblast marker alpha smooth muscle actin (α-SMA) in keratoconus (KC).
METHODS
Thirty corneal tissue were collected from KC patients after corneal transplantation and 15 normal donor corneas were obtained. All corneal tissues divided into 4 parts for different detections. Scanning electron microscopy was used to observe the ultrastructure of the specimens. VSX1 and α-SMA localization in cornea tissues was detected using immunofluorescence histochemistry. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and Western blot were performed to analyze the expression level of VSX1 and α-SMA.
RESULTS
Compared to normal cornea tissue, the collagen fibers in KC stroma were distortional and attenuated and keratocytes were abnormally changed. VSX1 and α-SMA located in the corneal stroma. The mRNA and protein expression level of VSX1 in KC were about 3 times as high as that of normal tissue (P<0.001). α-SMA was hardly expressed in the normal corneas, however, its expression in the KC was about 1.5 times higher than that of the normal corneas (P<0.0001).
CONCLUSION
Compared with normal corneal the expression of VSX1 and α-SMA in KC both increased. VSX1 is related to the activation of keratocytes and involved in the pathogenesis of keratoconus.
Keywords: visual system homeobox 1, keratoconus, alpha smooth muscle actin, keratocytes
INTRODUCTION
Keratoconus (KC) is a degenerative disorder associated with stromal thinning and conically shaped protrusion of the cornea, resulting in loss of visual function[1]. The reported incidence ranges from 5 to 54 per 100 000 per year across different populations. It is reported a significantly higher incidence and prevalence in Asians compared with whites, suggesting the influence of ethnicity on the disease[2]. The histopathology of KC presents changes in the gross organization of the lamellae and an uneven distribution of collagen fibrillar mass, especially around the apex of the cone. However, the pathogenesis of KC is not fully understood[3]–[4].
Genes with mutations were found to be responsible for human diseases. Single nucleotide polymorphisms (SNPs) of visual system homeobox 1 (VSX1) were previously reported to be associated with KC. Genetic analysis of KC patients from different ethnic backgrounds has revealed several coding variations in the VSX1 gene[5]–[9]. Further, our previous study found that three SNPs (rs56157240, rs12480307 and rs6050307) in VSX1 were associated with risk of KC in Chinese Han population[10].
VSX1 belongs to the CVC domain containing paired-like class of homeoprotein. It was first found in the goldfish retina and then in the human retina. VSX1 gene has been localized to human chromosome 20p11-q11and its five exons are distributed across about 6.2 kb of coding sequence[11]. The VSX1 gene expression in humans was detected in embryonic craniofacial, adult retinal, and adult corneal tissue. It is considered to play an important role in ocular development and is particularly involved in the cornea development[12]. Several pathogenic mutations in VSX1 associated with KC have been validated by multiple studies[13]. In fact, VSX1 may be a pleiotropic action factor among the cornea tissue leading to KC.
Although there were many researches about the polymorphism and gene expression of VSX1, the expression of VSX1 protein in human KC has not been investigated to date. As a first step in assessing the role of VSX1 protein in KC, we used corneal tissue from patients with KC and control human corneas to compare and examine the distribution and expression of this protein. Alpha-smooth muscle actin (α-SMA), a marker of myofibroblasts and presents in fibrotic stroma, also had been detected.
MATERIALS AND METHODS
Ethical Approval
KC tissue and donor corneas were obtained with consent and approval from the institutional research ethics committee of Xi'an No.1 Hospital (2017/06). All procedures were in accordance with the Declaration of Helsinki. Informed consents were obtained from all participants prior to collection of corneal tissue. Normal donor corneas were obtained from Xi'an Eye Bank following appropriate consent.
Corneal Specimens
Thirty corneal tissue were collected from KC patients (age range from 10 to 35y) undergoing corneal transplantation at Xi'an No.1 Hospital. All KC patients were diagnosed on the basis of clinical signs and corneal topography. Patients with SimK readings higher than 53 D and corneal thickness thicker than 400 µm were included according to the Amsler-Krumeich new classification system[14]. Fifteen normal donor corneas without other eye diseases (age range 3 to 68y) were obtained from the Xi'an Eye Bank (Table 1). The cornea were divided into four parts. Part A and Part B were stored at -80°C used for RNA and Western blot analysis, respectively. Part C was stored in Karnovsky Improvement Fixative Solution for scanning electron microscope (SEM) observation and Part D was fixed by formalin for immunofluorescence.
Table 1. Demographic information of the study participants.
| Demography | Normal cornea (n=15) | KC (n=30) |
| Sex (M/F) | 11/4 | 28/2 |
| Age, y (range) | 44.0±5.7 (3-68) | 22.3±6.4 (10-35) |
| SimK (D) | ||
| OD | - (n=6) | 61.3±3.8 (n=18) |
| OS | - (n=9) | 58.6±7.6 (n=12) |
| Corneal thinkness (µm) | ||
| OD | 531.7±36.2 (n=6) | 369.4±45.6 (n=18)a |
| OS | 522.2±52.3 (n=9) | 337.8±55.8 (n=12)a |
aP<0.05 vs normal cornea using independent-samples t-test.
mean±SD
Scanning Electron Microscope
The specimens subsequently fixed with Karnovsky Improvement Fixative Solution at 4°C for 2h (Huayueyang Biotechnology Co., Ltd., Beijing, China). For 4wk decalcification, the specimens were cut into small cubes and then treated with 1% thiocarbohydrazide. For enblocstaining, the specimens were immersed in 4% uranyl acetate solution overnight and washed with distilled water. The specimens were stained with Walton's lead aspartate solution. They were then dehydrated with a graded ethanol series and infiltrated with an epoxy resin mixture. Subsequently, they were polymerized at 60°C for 72h. The resin blocks were trimmed and placed on an appropriate holder. The ultrastructure of specimens was observed by SEM.
Immunofluorescence Histochemistry
Briefly, the KC and normal corneal tissues were formalin-fixed and paraffin-embedded. Then the specimens were cut into 4 µm sections. After being baked at 55°C for 1.5h, the samples were deparaffinized in xylene and rehydrated using a series of graded alcohols. And then, the antigens were retrieved in 0.01 mol/L sodium citrate buffer (pH 6.0) using microwave oven and the tissue slides were treated with 3% hydrogen peroxide in methanol for 10min to exhaust endogenous peroxidase activity, and then preincubated in 10% normal goat serum for 30min to prevent nonspecific staining. The samples were incubated overnight using a primary antibody VSX1 (1:500, Santa Cruz Biotechnology, Inc., California, USA), α-SMA (1:1000, Sigma-Aldrich, Inc., St. Louis, Mo, USA) in a humidified container at 4°C. On the second day, after washing with PBS three times, fluorescein isothiocyanate (FITC)-coupled mouse secondary antibody (1:100, Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) were added to the tissue slides for 30min at room temperature. Then washing three times with PBS, the tissue slides were observed and taken photos under a fluorescence microscope.
RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction Analysis
Total RNA was isolated according to the manufacturer's protocol (RNAprep pure Tissue Kit, TianGen Biotech Co., Ltd., Beijing, China) and was used for reverse transcription at 37°C for 60min in a 20-µL reaction mixture by using a first-strand cDNA synthesis kit (QuantScript RT Kit, TianGen Biotech Co., Ltd., Beijing, China). The RealMaster Mix SYBR Green reagents (TianGen Biotech Co., Ltd., Beijing, China) were utilized in real-time PCR. The primers and the fluorogenic probe were designed and synthesized by Sangon Biotech (Shanghai, China). Vsx1, NM_014588, Forward: 5′-GCCTCCTCTGTGGCTTCG-3′, Reverse: 5′-CTCATCGGACGTGGAGACG-3′, 182 bp; Homo sapiens actin, NM_001141945.2, Forward: 5′-GAGCGTGGCTATTCCTTCGT-3′, Reverse: 5′-GCCCATCAGGCAACTCGTAA-3′, 154 bp; Gapdh, NM_008084.2, Forward: 5′-CATGGCCTCCAAG GAGTAAGA-3′, Reverse: 5′-GA GGGAGATGCTCAGTG TTGG-3′, 104 bp. PCR reaction was performed as follows: 1min at 95°C followed by 40 cycles of amplification for 15s at 95°C and 1min at 60°C. The Relative expression of Vsx1 was normalized against the endogenous control, Gapdh, using the 2−ΔΔCq (Livak and Schmittgen) method[15]. Each sample was assayed in triplicate and the average results were calculated.
Western Blotting
Cellular total proteins were extracted according to the manufacturer's protocol by One Step Animal Tissue Active Protein Extraction Kit (Sangon Biotech, Shanghai, China). The protein concentration was determined by using a Modified Bradford Protein Assay Kit (Sangon Biotech, Shanghai, China). Equal amounts of protein (15 µg) were loaded and separated by 12% SDS-PAGE gel and then transferred onto a PVDF membrane. The membrane was soaked with 5% milk for 2h. After washing with PBS-Tween-20 (PBST; 5min/wash), the membrane was incubated with the primary antibodies including VSX1 (1:500, Santa Cruz Biotechnology, Inc., California, USA), α-SMA (1:1000, Sigma-Aldrich, Inc., St. Louis, Mo, USA) and GAPDH (1:1000, Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) at 4°C overnight. The membranes then were washed with PBST three times and incubated at room temperature for 2h with the HRP-conjugated secondary antibody (1:5000, Thermofisher Scientific,Massachusetts, USA). After that, it was washed three times with PBST and detected using the High sensitive ECL luminescence reagent (Sangon Biotech, Shanghai, China). Then, the relative band density was quantified using odyssey FC 5.25 (LI-COR Biotechnology, Nebraska, USA). GAPDH was used as the internal control.
Statistical Analysis
The data were expressed as the mean± standard error of the mean. Independent-samples t-test was used for comparing the differences between normal and KC groups. IBM SPSS Statistics 19 was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
RESULTS
Ultramicroscopic Structure
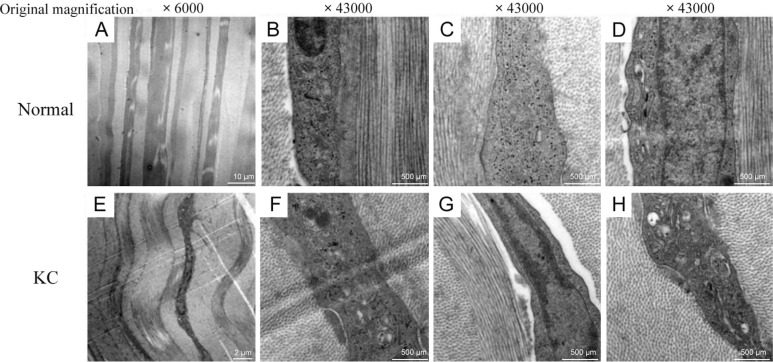
The ultramicroscopic structure of normal and KC stroma were observed by SEM. Compared with normal corneal stroma, KC stroma showed several distinguishing properties. First of all, the collagen fibers were distortional and attenuate (Figure 1A, 1E). At the same time, the nucleus gap widened. Additionally, mitochondrion swells and mitochondrial cristae disappear. Golgi apparatus presents significant expansion and endoplasmic reticulum displays severe swelling (Figure 1B-1D, 1F-1H).
Figure 1. Ultramicroscopic structures of corneal stroma.
A-D: Normal corneal stroma; E-H: KC stroma.
Expression of VSX1 in Keratoconus Corneas
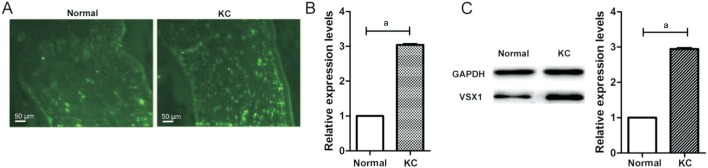
Immunoflurescence showed that VSX1 signal was clearly localized in the stroma of normal and KC cornea. Compared with normal tissue, increased VSX1 staining was detected in the KC (Figure 2A). These data were confirmed by RT-qPCR and Western blot respectively. Results in Figure 2B demonstrated that VSX1 mRNA level in KC was about 3 times higher than normal corneal tissues (P<0.001). Western blotting confirmed significantly VSX1 protein up-regulation in KC tissues (Figure 2C, P<0.001). Together, these results demonstrate VSX1 up-expression in KC tissues.
Figure 2. VSX1 up-expression in KC tissues compared to normal cornea tissues.
A: Immunoflurescence showed the expression level of VSX1. Scale bars: 50 µm; B: RT-qPCR analysis of VSX1 mRNA expression levels; C: Western blot analysis of VSX1 protein expression levels. aP<0.0001, independent-samples t-test.
Expression of α-SMA in Keratoconus Corneas
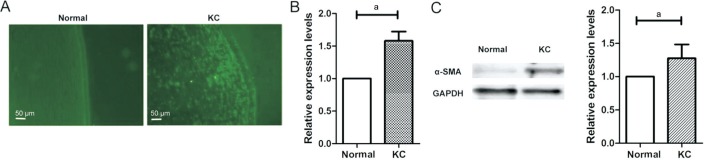
Immunoflurescence showed that α-SMA also localised within the stroma. There is almost no immunostaining was observed in normal cornea, however, for KC, stronger immunostaining of α-SMA compared to normal cornea (Figure 3A). RT-qPCR assay results in Figure 3B showed that α-SMA mRNA level was about 1.5 times higher in the KC tissues than that in normal cornea tissues (Figure 3B; P<0.0001). Western blotting also showed that α-SMA protein expression was increased in KC tissues (Figure 3C; P=0.0002).
Figure 3. α-SMA up-expression in KC tissues compared to normal cornea tissues.
A: Immunoflurescence showed the expression level of VSX1; B: RT-qPCR analysis of α-SMA mRNA expression levels; C: Western blot analysis of α-SMA protein expression levels. aP<0.001, independent-samples t-test.
DISCUSSION
KC is a corneal thinning disease whose etiology remains unknown. The pathophysiology of KC is characterized by the presence of a thin corneal stroma, altered extracellular matrix assembly and myofibroblast differentiation[16]. The etiology of KC, which is multifactorial with genetic and environmental influences, remains elusive[17]. While many studies examine how environmental factors influence disease development, finding that the genetic triggers has been a major emphasis of KC research[18]. A large number of candidate genes have been studied in relation to KC pathogenesis. The link between VSX1 variants and KC susceptibility was first reported in Canada by Heon et al[19] and then many other studies had examined the potential mutations of VSX1 in KC patients. It is reported that the susceptibility of mutations in VSX1 can vary in different ethnic groups[6]–[7],[20]–[28]. Our team also reported 3 SNPs (rs56157240, rs12480307 and rs6050307) in VSX1 associated with the susceptibility of Chinese KC patients, however, the actual function of VSX1 is unclear[10]. As a first step to understand the possible role of VSX1, we examined patterns of VSX1 expression in both human normal and KC. Different from previous research that VSX1 was not detected in mice cornea[29], in our study, VSX1 was expressed slightly in the stromal of normal human corneas but strongly in KC.
The loss of structural integrity in the KC was caused by the presence of abnormal keratocytes and matrix proteins. Keratocytes are activated into myofibroblast, causing abnormal secretion of extracellular matrix[3]. Our SEM results confirmed that the collagen fibers of KC stromal were distortional and attenuate and keratocytes were abnormal changed, revealing that the reduction of the interfibrillar distance of collagen sheets and the increase of proteoglycans with abnormalities in their configuration as the condition evolves. Barbaro et al[30] had analyzed VSX1 expression and its involvements in wound-healing processes, in which differentiation of corneal keratocytes into myofibroblasts occurs and myofibroblast-specific marker (α-SMA) expresses. They considered that VSX1 sharply high expressed in the corneal stroma may elucidate the pathophysiological origin of KC. Xu et al[31] investigated the effects of bovine pituitary extract on the proliferation of keratocytes and confirmed that the expression of VSX1 reached a high level when many keratocytes underwent fibroblastic transformation. In this study, we also have analyzed α-SMA expression in normal human and KC, which is absent in the stromal of normal human cornea but intense expression in KC. The results indicate that the upgrade expression of VSX1 might cause the intense expression of α-SMA in the keratocytes, which may lead to differentiation of corneal keratocytes into myofibroblasts. The presence of abnormal keratocytes could alter the secretion of extracellular matrix and the structure of corneal stroma, which is the causation of stroma thinning and keratoconus occurrence.
In summary, compared with normal corneal stroma, we found that the expression level of VSX1 and α-SMA in KC increased. Taken together previous SNP studies, these observations indicate that VSX1 is related to the activation of keratocytes and involved in the pathogenesis of keratoconus. However, it remains to be determined whether the increased expression of VSX1 observed is directly involved in KC pathogenesis or is secondary responses. The specific role of VSX1 in KC pathogenesis needs further study.
Acknowledgments
We are grateful to all subjects who kindly agreed to participate in this study. We also appreciate the support and cooperation of the staff of Xi'an Eye Bank.
Foundations: Supported by Natural Science Foundation of Shaanxi Province (No. 2017JM8040); Xi'an Science and Technology Project [No.2017116SF/YX010(7)].
Conflicts of Interest: Wang YN, None; Liu XN, None; Wang XD, None; Yin Y, None; Chen Y, None; Xiao XH, None; Xu K, None; Zhu XP, None.
REFERENCES
- 1.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 2.Barsam A, Petrushkin H, Brennan N, Bunce C, Xing W, Foot B, Tuft S. Acute corneal hydrops in keratoconus: a national prospective study of incidence and management. Eye (Lond) 2015;29(4):469–474. doi: 10.1038/eye.2014.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, Bron AJ. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46(6):1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 4.Galvis V, Sherwin T, Tello A, Merayo J, Barrera R, Acera A. Keratoconus: an inflammatory disorder? Eye (Lond) 2015;29(7):843–859. doi: 10.1038/eye.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardak H, Gunay M, Yildiz E, Bardak Y, Gunay B, Ozbas H, Bagci O. Novel visual system homeobox 1 gene mutations in Turkish patients with keratoconus. Genet Mol Res. 2016;15(4) doi: 10.4238/gmr15049024. [DOI] [PubMed] [Google Scholar]
- 6.Guan T, Wang X, Zheng LB, Wu HJ, Yao YF. Analysis of the VSX1 gene in sporadic keratoconus patients from China. BMC Ophthalmol. 2017;17(1):173. doi: 10.1186/s12886-017-0567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shetty R, Nuijts RM, Nanaiah SG, Anandula VR, Ghosh A, Jayadev C, Pahuja N, Kumaramanickavel G, Nallathambi J. Two novel missense substitutions in the VSX1 gene: clinical and genetic analysis of families with Keratoconus from India. BMC Med Genet. 2015;16:33. doi: 10.1186/s12881-015-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moussa S, Grabner G, Ruckhofer J, Dietrich M, Reitsamer H. Genetics in keratoconus-what is new? Open Ophthalmol J. 2017;11:201–210. doi: 10.2174/1874364101711010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent AL, Jordan C, Sheck L, Niederer R, Patel DV, McGhee CN. Screening the visual system homeobox 1 gene in keratoconus and posterior polymorphous dystrophy cohorts identifies a novel variant. Mol Vis. 2013;19:852–860. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Jin T, Zhang X, Wei W, Cui Y, Geng T, Liu Q, Gao J, Liu M, Chen C, Zhang C, Zhu X. Common single nucleotide polymorphisms and keratoconus in the Han Chinese population. Ophthalmic Genet. 2013;34(3):160–166. doi: 10.3109/13816810.2012.743569. [DOI] [PubMed] [Google Scholar]
- 11.Levine EM, Hitchcock PF, Glasgow E, Schechter N. Restricted expression of a new paired-class homeobox gene in normal and regenerating adult goldfish retina. J Comp Neurol. 1994;348(4):596–606. doi: 10.1002/cne.903480409. [DOI] [PubMed] [Google Scholar]
- 12.Semina EV, Mintz-Hittner HA, Murray JC. Isolation and characterization of a novel human paired-like homeodomain-containing transcription factor gene, VSX1, expressed in ocular tissues. Genomics. 2000;63(2):289–293. doi: 10.1006/geno.1999.6093. [DOI] [PubMed] [Google Scholar]
- 13.Valgaeren H, Koppen C, Van Camp G. A new perspective on the genetics of keratoconus: why have we not been more successful? Ophthalmic Genet. 2018;39(2):158–174. doi: 10.1080/13816810.2017.1393831. [DOI] [PubMed] [Google Scholar]
- 14.Belin MW, Duncan JK. Keratoconus: The ABCD Grading System. Klin Monbl Augenheilkd. 2016;233(6):701–707. doi: 10.1055/s-0042-100626. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Gokul A, Vellara HR, Patel DV. Advanced anterior segment imaging in keratoconus: a review. Clin Exp Ophthalmol. 2018;46(2):122–132. doi: 10.1111/ceo.13108. [DOI] [PubMed] [Google Scholar]
- 17.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karolak JA, Gajecka M. Genomic strategies to understand causes of keratoconus. Mol Genet Genomics. 2017;292(2):251–269. doi: 10.1007/s00438-016-1283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Héon E, Greenberg A, Kopp KK, Rootman D, Vincent AL, Billingsley G, Priston M, Dorval KM, Chow RL, McInnes RR, Heathcote G, Westall C, Sutphin JE, Semina E, Bremner R, Stone EM. VSX1: a gene for posterior polymorphous dystrophy and keratoconus. Hum Mol Genet. 2002;11(9):1029–1036. doi: 10.1093/hmg/11.9.1029. [DOI] [PubMed] [Google Scholar]
- 20.Bisceglia L, Ciaschetti M, De Bonis P, Campo PA, Pizzicoli C, Scala C, Grifa M, Ciavarella P, Delle Noci N, Vaira F, Macaluso C, Zelante L. VSX1 mutational analysis in a series of Italian patients affected by keratoconus: detection of a novel mutation. Invest Ophthalmol Vis Sci. 2005;46(1):39–45. doi: 10.1167/iovs.04-0533. [DOI] [PubMed] [Google Scholar]
- 21.Dash DP, George S, O'Prey D, Burns D, Nabili S, Donnelly U, Hughes AE, Silvestri G, Jackson J, Frazer D, Héon E, Willoughby CE. Mutational screening of VSX1 in keratoconus patients from the European population. Eye (Lond) 2010;24(6):1085–1092. doi: 10.1038/eye.2009.217. [DOI] [PubMed] [Google Scholar]
- 22.De Bonis P, Laborante A, Pizzicoli C, Stallone R, Barbano R, Longo C, Mazzilli E, Zelante L, Bisceglia L. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol Vis. 2011;17:2482–2494. [PMC free article] [PubMed] [Google Scholar]
- 23.Eran P, Almogit A, David Z, Wolf HR, Hana G, Yaniv B, Elon P, Isaac A. The D144E substitution in the VSX1 gene: a non-pathogenic variant or a disease causing mutation? Ophthalmic Genet. 2008;29(2):53–59. doi: 10.1080/13816810802008242. [DOI] [PubMed] [Google Scholar]
- 24.Mintz-Hittner HA, Semina EV, Frishman LJ, Prager TC, Murray JC. VSX1 (RINX) mutation with craniofacial anomalies, empty sella, corneal endothelial changes, and abnormal retinal and auditory bipolar cells. Ophthalmology. 2004;111(4):828–836. doi: 10.1016/j.ophtha.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Mok JW, Baek SJ, Joo CK. VSX1 gene variants are associated with keratoconus in unrelated Korean patients. J Hum Genet. 2008;53(9):842–849. doi: 10.1007/s10038-008-0319-6. [DOI] [PubMed] [Google Scholar]
- 26.Paliwal P, Singh A, Tandon R, Titiyal JS, Sharma A. A novel VSX1 mutation identified in an individual with keratoconus in India. Mol Vis. 2009;15:2475–2479. [PMC free article] [PubMed] [Google Scholar]
- 27.Saee-Rad S, Hashemi H, Miraftab M, Noori-Daloii MR, Chaleshtori MH, Raoofian R, Jafari F, Greene W, Fakhraie G, Rezvan F, Heidari M. Mutation analysis of VSX1 and SOD1 in Iranian patients with keratoconus. Mol Vis. 2011;17:3128–3136. [PMC free article] [PubMed] [Google Scholar]
- 28.Hao XD, Chen P, Chen ZL, Li SX, Wang Y. Evaluating the association between keratoconus and reported genetic loci in a Han Chinese population. Ophthalmic Genet. 2015;36(2):132–136. doi: 10.3109/13816810.2015.1005317. [DOI] [PubMed] [Google Scholar]
- 29.Watson T, Chow RL. Absence of Vsx1 expression in the normal and damaged mouse cornea. Mol Vis. 2011;17:737–744. [PMC free article] [PubMed] [Google Scholar]
- 30.Barbaro V, Di Iorio E, Ferrari S, Bisceglia L, Ruzza A, De Luca M, Pellegrini G. Expression of VSX1 in human corneal keratocytes during differentiation into myofibroblasts in response to wound healing. Invest Ophthalmol Vis Sci. 2006;47(12):5243–5250. doi: 10.1167/iovs.06-0185. [DOI] [PubMed] [Google Scholar]
- 31.Xu ZZ, Li ZJ, Du LX, Li J, Wang LY. Using bovine pituitary extract to increase proliferation of keratocytes and maintain their phenotype in vitro. Int J Ophthalmol. 2013;6(6):758–765. doi: 10.3980/j.issn.2222-3959.2013.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]