Abstract
AIM
To compare the clinical and microstructural changes induced by different transepithelial iontophoresis-assisted corneal cross-linking (I-CXL) methods for keratoconus.
METHODS
A total of 42 eyes of 42 patients with progressive keratoconus were divided into two groups. Group A received I-CXL for 5min, while group B received I-CXL for 10min. Visual acuity, optical coherence tomography (OCT), specular microscopy and confocal microscopy were evaluated preoperatively and at 1, 3, 6, and 12mo postoperatively.
RESULTS
Twelve months after the operation, uncorrected visual acuity (UCVA) and corrected distance visual acuity (CDVA) were improved in both groups, with a better outcome in the I-CXL 10min group (P=0.025, 0.021, respectively). Kmax values decreased by 0.94±3.00 D in the I-CXL 10min group (P=0.033) but increased by 1.87±3.29 D in the I-CXL 5min group (P=0.012). OCT scans showed that the demarcation line was most visible and substantially deeper in the I-CXL 10min group. Confocal microscopy showed greater anterior stromal keratocyte decreases in the I-CXL 10min group than in the I-CXL 5min group at 3 and 6mo postoperatively (P<0.001); however, anterior stromal keratocytes and subbasal nerve density were not significantly different between the two groups at 12mo postoperatively.
CONCLUSION
I-CXL for 10min more effectively halts the progression of keratoconus than I-CXL for 5min after 12mo of follow-up. However, long-term studies are needed to evaluate the efficacy and safety of I-CXL.
Keywords: keratoconus, transepithelial, corneal cross-linking, iontophoresis, anterior stromal keratocyte, subbasal nerve density
INTRODUCTION
Corneal cross-linking (CXL) can strengthen the biomechanical properties of the cornea and halt the progression of ectatic diseases[1]–[2]. Thus, this surgical approach has become one of the most important treatment options for keratoconus in recent years[3]–[4].
The standard CXL technique works by removing the epithelial layer to allow riboflavin to penetrate the corneal stroma[5]–[6]. Epithelium-off CXL can effectively manage the progression of keratoconus and improve visual acuity[3]–[6]. However, some complications, such as postoperative pain[7], worse visual acuity after correction, infectious keratitis, and haze formations in the stroma, have been reported[8]–[10]. Transepithelial corneal cross-linking (TE-CXL) was developed to avoid these complications and has become increasingly popular over the past 10y[11]–[13]. However, some studies have shown that the stromal riboflavin level of TE-CXL is insufficient and that the efficacy of TE-CXL cannot match that of standard epithelium-off CXL[14]–[15].
Iontophoresis-assisted corneal cross-linking (I-CXL), a modified TE-CXL technique, has been applied in recent years. This approach is based on the theory that a small electric current can non-invasively enhance the penetration of an ionized drug. In some ex-vivo studies, the I-CXL protocol improved transepithelial riboflavin penetration in the corneal stroma[16]–[17]. In addition, an animal study showed that I-CXL could significantly increase mechanical strength and collagenase resistance[18]. Some initial clinical results have shown that compared with traditional TE-CXL, I-CXL can increase the levels of riboflavin in the stroma and that the effect of I-CXL is stronger than that of traditional TE-CXL[14],[19]–[20]. However, there are no consensus guidelines for the application of the iontophoresis protocol. Currently, there are two clinical iontophoresis protocols that differ in terms of the procedure duration; the duration of iontophoresis treatment is 5min in one protocol and 10min in the other. Regarding the riboflavin concentration in the stroma, an in vivo study showed that the riboflavin concentration in rabbit corneas treated by iontophoresis was 2-fold greater after treatment for 10min than after treatment for 5min[21]. Although some initial clinical results have shown that this CXL protocol could effectively halt the progression of keratoconus[20],[22]–[24], there is no comparative clinical study of I-CXL for 5 and 10min, and studies of microstructural changes due to I-CXL are limited.
In this study, we compared microstructural changes and evaluated the efficacy and safety of these two I-CXL protocols for the treatment of keratoconus over a one-year follow-up period.
SUBJECTS AND METHODS
Ethical Approval
The study was approved by Hankou Aier Eye Hospital Ethics Committee under the principles of the Declaration of Helsinki and registered at www.chictr.org.cn (identification number ChiCTR1800019878). All patients signed a medical informed consent document before surgery.
Subjects
This study was designed as a prospective, continuous, non-randomized clinical evaluation of patients diagnosed with progressive keratoconus between December 2015 and February 2016. Eighteen eyes of 18 patients were treated with I-CXL using the 5min protocol, and 24 eyes of 24 patients were treated with I-CXL using the 10min protocol.
Inclusion criteria included an increase of at least 1 diopter (D) in the Kmax value derived from computerized corneal topography over the preceding 12mo. Exclusion criteria included a minimum corneal thickness less than 400 µm, central corneal scarring, previous corneal surgery, a history of chemical burns, severe infections, and other corneal or ocular surface disorders. At 12mo postoperatively, the clinical stabilization of keratoconus was defined as a Kmax increase no more than 1 D over the preoperative value.
Assessments
During the baseline and postoperative visits at 1, 3, 6, and 12mo, the following assessments were performed: uncorrected visual acuity [UCVA; expressed as the logarithm of the minimum angle of resolution (logMAR)], corrected distance visual acuity (CDVA; expressed as the logMAR), spherical and cylindrical error on subjective refraction, spherical equivalent, Kmax, Ksteep, Kflat, minimum corneal thickness (Pentacam, Oculus, Germany), and demarcation line evaluation using optical coherence tomography (OCT; Topcon, Japan). Confocal microscopy was performed using an HRT-3 (Heidelberg, Germany). Stromal demarcation line depth was measured by anterior segment OCT 1mo postoperatively by 2 separate examiners.
All confocal microscopy images measured 384×384 pixels over a 400×400 mm2 field of view. The first clear images of the nerves at the level of Bowman's layer were used to evaluate subbasal nerve density using Neuron J software (US National Institutes of Health, Bethesda, MD, USA). The first clear images at the level of 120 µm deep in the anterior stroma were used to evaluate keratocyte density using the HRT-3 cell counting module (Heidelberg Engineering GmBH, Dossenheim, Germany).
Surgical Procedures
All patients received 0.5% levofloxacin drops 4 times a day for 3d before surgery. Thirty minutes prior to surgery, 2% pilocarpine (Sigma-Aldrich, USA) and 0.4% oxybuprocaine hydrochloride (Bausch & LombPty Ltd., NSW, Australia) drops were administered 3 times, with 5min between each administration. After a return electrode was adhered to the patient's forehead, a suction ring connected to a current generator with a 1.0 mA current was placed on the cornea. The whole metal net of the iontophoresis device was submerged under 0.1% dextran-free riboflavin (Ricrolin+; SOOFT, Montegiorgio, Italy). The patients were divided into the following subgroups according to the conduction duration: 5 and 10min (the total current intensities were 5 and 10 mA, respectively). After conduction, the cornea was exposed to 370 nm UVA light (Vega 10 mW, CSO, Firenze, Italy) for 9min (5.4 J/cm2 surface dose) at an irradiance of 10 mW/cm2.
Statistical Analysis
Statistical analyses were performed using SPSS 18.0 software. The difference in each parameter between baseline and each time point (1, 3, 6, and 12mo) was calculated for each eye. Differences within each group were compared using one-way analysis of variance. Differences between results at 1, 3, 6, and 12mo postoperatively and baseline were compared using paired t-tests. Baseline characteristics were analyzed by independent samples t-test. P value <0.05 was considered statistically significant.
RESULTS
A total of 42 eyes of 42 patients with a mean age of 19.4±3.3y (range: 15-29y) were included. Twenty-six patients were men (61.9%), and 16 were women (38.1%). Each patient received treatment in one eye only. Baseline characteristics are listed in Table 1.
Table 1. Baseline parameters and clinical characteristics of the eyes in the two treatment groups.
| Parameters | I-CXL 5min | I-CXL 10min | P |
| Median age (y) | 19.13±2.70 | 19.31±3.80 | 0.877 |
| Male (%) | 55.6 | 66.7 | 0.787 |
| UCVA (logMAR) | 0.93±0.41 | 0.79±0.29 | 0.188 |
| CDVA (logMAR) | 0.31±0.22 | 0.32±0.25 | 0.893 |
| Spherical equivalent (D) | -11.0±7.4 | -10.8±5.0 | 0.357 |
| Pachymetry thinnest point (µm) | 437±24 | 452±33 | 0.117 |
| Maximal keratometry (D) | 61.33±10.56 | 56.89±8.95 | 0.160 |
| Intraocular pressure (mm Hg) | 11.11±3.86 | 12.41±3.26 | 0.257 |
| Endothelium (cells/mm2) | 3019±284 | 2925±298 | 0.329 |
I-CXL: Iontophoresis-assisted corneal cross-linking; UCVA: Uncorrected visual acuity; CDVA: Corrected distance visual acuity.
Visual Acuity and Refractive Outcomes
The visual acuity outcomes after surgery for the two groups are shown in Table 2. UCVA and CDVA showed improvement in both groups 12mo postoperatively, with a better outcome in the I-CXL 10min group (P=0.025 and 0.021, respectively). Additionally, all the results showed that the CDVA was significantly improved during the entire follow-up period in the I-CXL 10min group, while it was improved only at 1mo after surgery in the I-CXL 5min group (Table 2).
Table 2. Clinical characteristics of the eyes in the two groups after 1, 3, 6 and 12mo compared with the baseline measurements.
| Groups | 1mo | 3mo | 6mo | 12mo | bP |
| ΔUCVA | |||||
| I-CXL 5min | -0.07±0.32 | -0.11±0.29 | -0.05±0.32 | -0.07±0.24 | 0.302 |
| I-CXL 10min | -0.19±0.26 | -0.16±0.28 | -0.15±0.32 | -0.13±0.27 | 0.025 |
| aP | 0.230 | 0.649 | 0.370 | 0.491 | |
| ΔCDVA | |||||
| I-CXL 5min | -0.01±0.15 | -0.03±0.18 | -0.01±0.18 | -0.03±0.24 | 0.674 |
| I-CXL 10min | -0.11±0.20 | -0.12±0.23 | -0.14±0.22 | -0.11±0.23 | 0.021 |
| aP | 0.101 | 0.229 | 0.053 | 0.264 | |
| ΔSphere | |||||
| I-CXL 5min | 0.55±1.58 | 1.15±2.37 | 0.58±3.51 | 0.77±2.46 | 0.004 |
| I-CXL 10min | 0.32±1.52 | 0.41±2.43 | 0.39±1.74 | 0.22±2.14 | 0.604 |
| aP | 0.644 | 0.351 | 0.818 | 0.462 | |
| ΔCylinder | |||||
| I-CXL 5min | 0.13±2.26 | 0.48±2.88 | 1.15±2.39 | 0.58±2.35 | 0.352 |
| I-CXL 10min | -0.04±1.42 | -0.32±1.6 | -0.16±1.52 | -0.47±1.82 | 0.772 |
| aP | 0.766 | 0.355 | 0.037 | 0.182 | |
| ΔSE | |||||
| I-CXL 5min | 0.62±1.46 | 1.39±2.63 | 1.16±3.87 | 1.06±2.76 | 0.160 |
| I-CXL 10min | 0.30±1.26 | 0.43±2.07 | 0.31±1.33 | 0.17±1.86 | 0.639 |
| aP | 0.467 | 0.204 | 0.313 | 0.227 | |
| ΔKsteep | |||||
| I-CXL 5min | 1.17±1.65 | 0.82±1.56 | 1.12±1.71 | 0.93±1.89 | 0.078 |
| I-CXL 10min | 0.29±0.92 | 0.09±1.13 | 0.29±2.23 | -0.37±1.1 | 0.097 |
| aP | 0.034 | 0.165 | 0.234 | 0.008 | |
| ΔKflat | |||||
| I-CXL 5min | 0.71±1.19 | 0.53±2.16 | 0.77±2.2 | 1.32±3.32 | 0.146 |
| I-CXL 10min | -0.07±0.99 | -0.23±1.06 | -0.03±0.94 | -0.19±0.9 | 0.287 |
| aP | 0.032 | 0.140 | 0.108 | 0.034 | |
| ΔKmax | |||||
| I-CXL 5min | 2.87±5.17 | 2.22±4.47 | 2.27±5.66 | 1.87±3.29 | 0.012 |
| I-CXL 10min | -0.25±3.69 | -0.94±3.95 | -0.92±3.65 | -0.94±3.00 | 0.033 |
| aP | 0.030 | 0.024 | 0.034 | 0.028 | |
| ΔCCT | |||||
| I-CXL 5min | -7.93±20.14 | -10.4±16.08 | -7±17.49 | -11.13±17.3 | 0.026 |
| I-CXL 10min | -11.65±11.02 | -6.62±12.23 | -9.08±22.96 | -3.92±13.19 | 0.142 |
| aP | 0.447 | 0.400 | 0.764 | 0.141 | |
| ΔECD | |||||
| I-CXL 5min | -15±31 | 12±31 | -18±39 | -11±27 | 0.149 |
| I-CXL 10min | 26±35 | 11±48 | 28±34 | -12±17 | 0.252 |
| aP | 0.237 | 0.624 | 0.538 | 0.169 |
I-CXL: Iontophoresis-assisted corneal cross-linking; UCVA: Uncorrected visual acuity; CDVA: Corrected distance visual acuity; SE: Spherical equivalent; CCT: Corneal thickness at thinnest point; ECD: Endothelial cell density. aComparison between two groups at the same point; bComparison with baseline at 1, 3, 6 and 12mo postoperatively in each group.
Corneal Topography
At 12mo after surgery, Kmax values decreased by 0.94±3.00 D in the I-CXL 10min group (P=0.033) but increased by 1.87±3.29 D in the I-CXL 5min group (P=0.012; Table 2), with a significant difference between the two groups (P=0.028). Kmax steepened by more than 1.00 D in 5 eyes in the I-CXL 5min group (27.8%) and 4 eyes in the I-CXL 10min group (16.7%). The corneal thickness at the thinnest point decreased by 11.13±17.3 µm in the I-CXL 5min group at 12mo postoperatively compared with that at baseline (P=0.026). Although corneal thickness was decreased at 1, 3 and 6mo postoperatively in the I-CXL 10min group, there was no significant difference at 12mo postoperatively (P=0.142).
Demarcation Line
Over time, visualization of the demarcation line becomes increasingly difficult. The demarcation line was visualized most clearly at 1mo postoperatively. The demarcation line was detected in 47.1% of eyes in the I-CXL 10min group, with an average depth of 260±36 µm at 1mo postoperatively. In the I-CXL 5min group, the demarcation line was visible in 22.2% of patients with a depth of 246±44 µm.
Confocal Microscopy
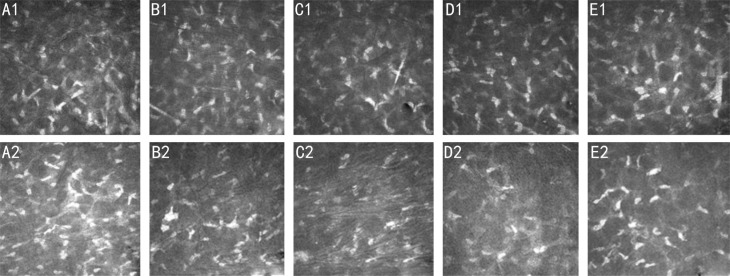
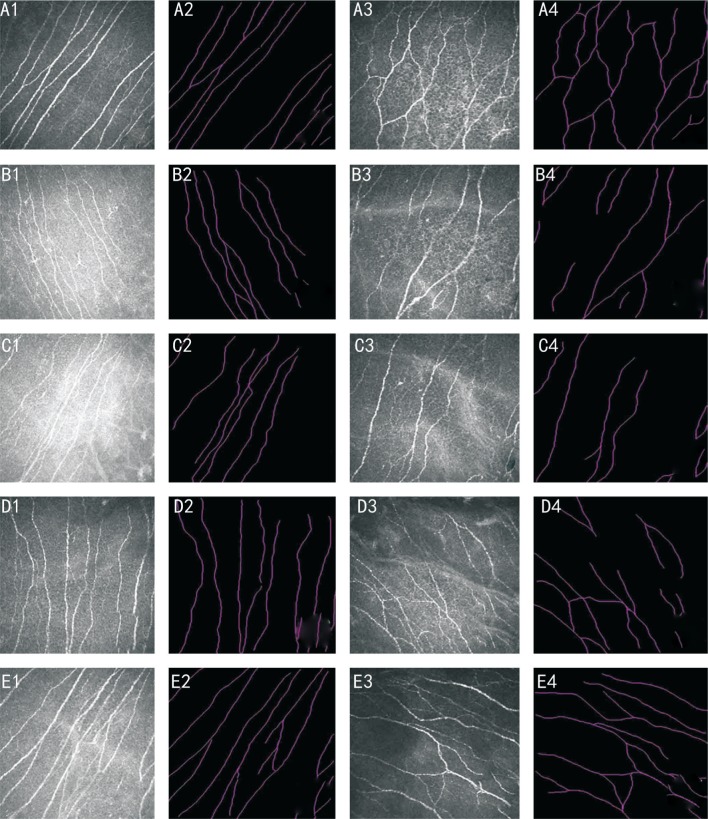
Morphologic changes in anterior stromal keratocytes and subbasal nerve are shown in Figures 1 and 2. Confocal microscopy images of anterior stromal keratocytes (depth of 120 µm) in the two groups were selected for quantitative analysis preoperatively and at 1, 3, 6, and 12mo postoperatively (Table 3). The results showed greater anterior stromal keratocyte decreases in the I-CXL 10min group than in the I-CXL 5min group at 3 and 6mo postoperatively (P<0.001). However, there was no significant difference between the two groups at 12mo postoperatively (P=0.206).
Figure 1. Anterior keratocytes are shown preoperatively and at 1, 3, 6, and 12mo postoperatively at a corneal depth of 120 µm.
A1, B1, C1, D1, E1: I-CXL 5min group; A2, B2, C2, D2, E2: I-CXL 10min group. The corneal confocal microscopy images are 400×400 mm2 (scale bar: 50 mm).
Figure 2. Subbasal nerve plexuses are shown preoperatively and at 1, 3, 6 and 12mo postoperatively.
A1, A2, B1, B2, C1, C2, D1, D2, E1, E2: I-CXL 5min group; A3, A4, B3, B4, C3, C4, D3, D4, E3, E4: I-CXL 10min group. The corneal confocal microscopy images are 400×400 mm2 (scale bar: 50 mm).
Table 3. Mean subbasal nerve and anterior stromal keratocyte densities preoperatively and 1, 3, 6, and 12mo postoperatively in the two groups.
| Parameters | Mean subbasal nerve density (nerve/mm2) | bP | Anterior stromal keratocyte density (cells/mm2) | bP |
| Preoperative | ||||
| I-CXL 5min | 13.27±3.01 | 247.13±35.10 | ||
| I-CXL 10min | 12.03±2.23 | 232.08±39.70 | ||
| aP | 0.348 | 0.897 | ||
| 1mo | ||||
| I-CXL 5min | 8.31±1.64 | <0.001 | 215.00±33.60 | 0.001 |
| I-CXL 10min | 7.57±1.35 | <0.001 | 186.19±60.41 | <0.001 |
| aP | 0.13 | 0.098 | ||
| 3mo | ||||
| I-CXL 5min | 9.81±1.53 | 0.001 | 201.40±29.41 | <0.001 |
| I-CXL 10min | 8.89±1.24 | <0.001 | 154.15±38.79 | <0.001 |
| aP | 0.041 | <0.001 | ||
| 6mo | ||||
| I-CXL 5min | 12.06±2.52 | 0.012 | 215.13±35.28 | 0.011 |
| I-CXL 10min | 10.63±2.00 | 0.032 | 156.35±49.80 | <0.001 |
| aP | 0.052 | <0.001 | ||
| 12mo | ||||
| I-CXL 5min | 12.78±2.32 | 0.391 | 177.80±46.35 | <0.001 |
| I-CXL 10min | 11.44±1.97 | 0.104 | 160.73±37.62 | <0.001 |
| aP | 0.057 | 0.206 |
I-CXL: Iontophoresis-assisted corneal cross-linking. aComparison between two groups at the same point; bComparison with baseline at 1, 3, 6 and 12mo postoperatively in each group.
In addition, the subbasal nerve densities in both groups showed the greatest decreases at 1mo postoperatively (P<0.001). The densities gradually increased and stabilized in both groups at 12mo postoperatively (Table 3). Subbasal nerve density showed no significant differences between the two groups at 1, 6 and 12mo postoperatively, although there was a significant difference at 3mo postoperatively (P=0.041). Endothelial cell density counts showed no significant differences between the two groups at 12mo postoperatively (P=0.169).
DISCUSSION
Iontophoresis has been developed as a novel drug delivery approach for riboflavin and I-CXL effectively halts the progression of keratoconus[20],[23]. A randomized controlled clinical trial between I-CXL and standard CXL (Dresden protocol) demonstrated that I-CXL could achieve the same clinical result as standard CXL[25]. However, this previous study did not reach a conclusion regarding the duration of the iontophoresis procedure during surgery. The current study was the first to compare the clinical effects of two different I-CXL treatment durations. Our study showed that I-CXL for 10min could halt the progression of keratoconus more effectively than I-CXL for 5min when assessed at one year postoperatively.
In terms of efficacy, compared with UCVA and CDVA at baseline, UCVA and CDVA at 12mo postoperatively were improved in both groups. The corneal topography results showed that Kmax values were decreased after 12mo only in the I-CXL 10min group, while the I-CXL 5min group showed an increase. Based on these visual acuity and refractive outcomes, we concluded that both I-CXL protocols could improve morphologic parameters and visual quality, although 10min of I-CXL demonstrated more significant effects. We defined CXL failure as an increase of more than 1 D, indicating keratoconus progression[26]–[27]. Our results showed that CXL failure occurred in 5 eyes in the I-CXL 5min group (27.8%) and 4 eyes in the I-CXL 10min group (16.7%). Several factors contribute to CXL failure, including a preoperative Kmax greater than 58.00 D[8]. In this study, a preoperative Kmax greater than 58.00 D was found in 10 eyes in the I-CXL 5min group (55.6%) and 14 eyes in the I-CXL 10min group (58.3%). CXL failure happened only in 3 eyes and 1 eye with a preoperative Kmax greater than 58.00 D in the I-CXL 5min and I-CXL 10min groups, respectively. Thus, we concluded that there was no correlation between a high preoperative Kmax and keratoconus progression.
In addition to keratometry values, another important corneal topography observation index is corneal thickness at its thinnest point. Our results showed that the thinnest corneal thickness in both groups declined compared with the baseline value, especially in the I-CXL 10min group. Corneal thickness declined at 1mo postoperatively in the I-CXL 10min group; however, it increased slightly and then stabilized at 12mo postoperatively. Other studies have also shown decreases in postoperative corneal thickness compared with preoperative corneal thickness[27]–[28]. However, O'Brart et al[29] reported that corneal thickness was unchanged in an epithelium-off CXL group in their seven-year follow-up study. Therefore, additional time is needed to observe changes in corneal thickness after I-CXL. Moreover, whether there is a correlation between microstructural alterations and the biomechanical response of the cornea after the I-CXL procedure is unclear. Thus, future studies should focus on biomechanical changes in the cornea.
The demarcation line has been associated with the effectiveness of CXL treatment because deeper CXL treatment produces additional photochemical reactions and causes the cornea to become stiffer[30]–[32]. We observed that the demarcation line was visualized more frequently and clearly and appeared to be deeper in the I-CXL 10min group at 1mo postoperatively. However, we could not evaluate stromal fluorescence after riboflavin application during surgery; this evaluation would have objectively provided information on the riboflavin concentration.
In terms of safety, we did not observe any complications in our patients after I-CXL, including haze or infection. Confocal microscopy showed that subbasal nerve density was not significantly different between the two groups (Table 3), and subbasal nerve density stabilized at 12mo postoperatively. Although the results showed that anterior stromal keratocytes decreased after the operation, this value was not significantly different between the two groups at 12mo postoperatively (P=0.206). We initially hypothesized that the subbasal nerve density and anterior stromal keratocytes in the I-CXL 10min group would be lower than those in the I-CXL 5min group[33]. According to our results, 10min of iontophoresis is safe in regard to subbasal nerve density. However, considering that our assessment of subbasal nerve density was limited, future studies should focus greater attention on subbasal nerve density as an indicator of the safety of CXL.
Additionally, according to some recently obtained data after 2y of follow-up, anterior stromal keratocytes and subbasal nerve density were not significantly different between I-CXL 5min and I-CXL 10min group (data not shown). Another indicator of safety is endothelial cell density, and our results showed that endothelial cell density remained stable after both I-CXL protocols (Table 2), which is consistent with the results of many other studies[24]–[26]. Thus, we concluded that 10min of I-CXL was safe in regard to the corneal microstructure.
In conclusion, our results showed that I-CXL 10min could more effectively halt the progression of keratoconus than I-CXL 5min after 12mo of follow-up. Nevertheless, more long-term follow-up studies are needed to evaluate the efficacy and safety of I-CXL. Moreover, future studies should compare the efficacy of I-CXL with that of standard epithelium-off CXL as well as other types of accelerated CXL.
Acknowledgments
Foundations: Supported by Natural Science Foundation of Hunan Province (No.2016JJ2163); Natural Science Foundation of Hubei Province (No.2015CFC837); Health and Family Planning Committee Science Foundation of Hubei Province (No.WJ2015MB259); Health and Family Planning Committee Science Foundation of Wuhan Municipality (No.WX17A13).
Conflicts of Interest: Liao K, None; Hu M, None; Chen F, None; Li P, None; Song P, None; Zeng QY, None.
REFERENCES
- 1.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34(5):796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 2.Yildirim A, Cakir H, Kara N, Uslu H, Gurler B, Ozgurhan EB, Colak HN. Corneal collagen crosslinking for ectasia after laser in situ keratomileusis: long-term results. J Cataract Refract Surg. 2014;40(10):1591–1596. doi: 10.1016/j.jcrs.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Chan E, Snibson GR. Current status of corneal collagen cross-linking for keratoconus: a review. Clin Exp Optom. 2013;96(2):155–164. doi: 10.1111/cxo.12020. [DOI] [PubMed] [Google Scholar]
- 4.Chunyu T, Xiujun P, Zhengjun F, Xia Z, Feihu Z. Corneal collagen cross-linking in keratoconus: a systematic review and meta-analysis. Sci Rep. 2014;4:5652. doi: 10.1038/srep05652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randleman JB, Khandelwal SS, Hafezi F. Corneal cross-linking. Surv Ophthalmol. 2015;60(6):509–523. doi: 10.1016/j.survophthal.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Meiri Z, Keren S, Rosenblatt A, Sarig T, Shenhav L, Varssano D. Efficacy of corneal collagen cross-linking for the treatment of keratoconus: a systematic review and meta-Analysis. Cornea. 2016;35(3):417–428. doi: 10.1097/ICO.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 7.Ghanem VC, Ghanem RC, de Oliveira R. Postoperative pain after corneal collagen cross-linking. Cornea. 2013;32(1):20–24. doi: 10.1097/ICO.0b013e31824d6fe3. [DOI] [PubMed] [Google Scholar]
- 8.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35(8):1358–1362. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Dhawan S, Rao K, Natrajan S. Complications of corneal collagen cross-linking. J Ophthalmol. 2011;2011:869015. doi: 10.1155/2011/869015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzotta C, Balestrazzi A, Baiocchi S, Traversi C, Caporossi A. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clin Exp Ophthalmol. 2007;35(6):580–582. doi: 10.1111/j.1442-9071.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 11.Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. J Refract Surg. 2010;26(12):942–948. doi: 10.3928/1081597X-20100212-09. [DOI] [PubMed] [Google Scholar]
- 12.Filippello M, Stagni E, O'Brart D. Transepithelial corneal collagen crosslinking: bilateral study. J Cataract Refract Surg. 2012;38(2):283–291. doi: 10.1016/j.jcrs.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Buzzonetti L, Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: early results. J Refract Surg. 2012;28(11):763–767. doi: 10.3928/1081597X-20121011-03. [DOI] [PubMed] [Google Scholar]
- 14.Taneri S, Oehler S, Lytle G, Dick HB. Evaluation of epithelial integrity with various transepithelial corneal cross-linking protocols for treatment of keratoconus. J Ophthalmol. 2014;2014:614380. doi: 10.1155/2014/614380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acar BT, Utine CA, Ozturk V, Acar S, Ciftci F. Can the effect of transepithelial corneal collagen cross-linking be improved by increasing the duration of topical riboflavin application? An in vivo confocal microscopy study. Eye Contact Lens. 2014;40(4):207–212. doi: 10.1097/ICL.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 16.Hayes S, Morgan SR, O'Brart DP, O'Brart N, Meek KM. A study of stromal riboflavin absorption in ex vivo porcine corneas using new and existing delivery protocols for corneal cross-linking. Acta Ophthalmol. 2016;94(2):e109–e117. doi: 10.1111/aos.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gore DM, O'Brart DP, French P, Dunsby C, Allan BD. A comparison of different corneal iontophoresis protocols for promoting transepithelial riboflavin penetration. Invest Ophthalmol Vis Sci. 2015;56(13):7908–7914. doi: 10.1167/iovs.15-17569. [DOI] [PubMed] [Google Scholar]
- 18.Cassagne M, Laurent C, Rodrigues M, Galinier A, Spoerl E, Galiacy SD, Soler V, Fournié P, Malecaze F. Iontophoresis transcorneal delivery technique for transepithelial corneal collagen crosslinking with riboflavin in a rabbit model. Invest Ophthalmol Vis Sci. 2016;57(2):594–603. doi: 10.1167/iovs.13-12595. [DOI] [PubMed] [Google Scholar]
- 19.Mastropasqua L, Nubile M, Calienno R, Mattei PA, Pedrotti E, Salgari N, Mastropasqua R, Lanzini M. Corneal cross-linking: intrastromal riboflavin concentration in iontophoresis-assisted imbibition versus traditional and transepithelial techniques. Am J Ophthalmol. 2014;157(3):623–630.e1. doi: 10.1016/j.ajo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Vinciguerra P, Randleman JB, Romano V, Legrottaglie EF, Rosetta P, Camesasca FI, Piscopo R, Azzolini C, Vinciguerra R. Transepithelial iontophoresis corneal collagen cross-linking for progressive keratoconus: initial clinical outcomes. J Refract Surg. 2014;30(11):746–753. doi: 10.3928/1081597X-20141021-06. [DOI] [PubMed] [Google Scholar]
- 21.Jia Hz, Peng XJ. Efficacy of iontophoresis-assisted epithelium-on corneal cross-linking for keratoconus. Int J Ophthalmol. 2018;11(4):687–694. doi: 10.18240/ijo.2018.04.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buzzonetti L, Petrocelli G, Valente P, Iarossi G, Ardia R, Petroni S. Iontophoretic transepithelial corneal cross-linking to halt keratoconus in pediatric cases: 15-month follow-up. Cornea. 2015;34(5):512–515. doi: 10.1097/ICO.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 23.Bikbova G, Bikbov M. Transepithelial corneal collagen cross-linking by iontophoresis of riboflavin. Acta Ophthalmol. 2014;92(1):e30–e34. doi: 10.1111/aos.12235. [DOI] [PubMed] [Google Scholar]
- 24.Vinciguerra P, Romano V, Rosetta P, Legrottaglie EF, Piscopo R, Fabiani C, Azzolini C, Vinciguerra R. Transepithelial iontophoresis versus standard corneal collagen cross-linking: 1-year results of a prospective clinical study. J Refract Surg. 2016;32(10):672–678. doi: 10.3928/1081597X-20160629-02. [DOI] [PubMed] [Google Scholar]
- 25.Lombardo M, Giannini D, Lombardo G, Serrao S. Randomized controlled trial comparing transepithelial corneal cross-linking using iontophoresis with the dresden protocol in progressive keratoconus. Ophthalmology. 2017;124(6):804–812. doi: 10.1016/j.ophtha.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Bikbova G, Bikbov M. Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: randomized control trial. Acta Ophthalmol. 2016;94(7):e600–e606. doi: 10.1111/aos.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittig-Silva C, Chan E, Islam FM, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121(4):812–821. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Fan Z, Peng X, Pang X, Tian C. Clinical observation of transepithelial corneal collagen cross-linking by lontophoresis of riboflavin in treatment of keratoconus. Eye Sci. 2014;29(3):160–164. [PubMed] [Google Scholar]
- 29.O'Brart DP, Patel P, Lascaratos G, Wagh VK, Tam C, Lee J, O'Brart NA. Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: seven-year follow-up. Am J Ophthalmol. 2015;160(6):1154–1163. doi: 10.1016/j.ajo.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Mazzotta C, Balestrazzi A, Traversi C, Baiocchi S, Caporossi T, Tommasi C, Caporossi A. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26(4):390–397. doi: 10.1097/ICO.0b013e318030df5a. [DOI] [PubMed] [Google Scholar]
- 31.Doors M, Tahzib NG, Eggink FA, Berendschot TT, Webers CA, Nuijts RM. Use of anterior segment optical coherence tomography to study corneal changes after collagen cross-linking. Am J Ophthalmol. 2009;148(6):844–851. doi: 10.1016/j.ajo.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 32.Yam JC, Chan CW, Cheng AC. Corneal collagen cross-linking demarcation line depth assessed by Visante OCT after CXL for keratoconus and corneal ectasia. J Refract Surg. 2012;28(7):475–481. doi: 10.3928/1081597X-20120615-03. [DOI] [PubMed] [Google Scholar]
- 33.Jouve L, Borderie V, Sandali O, Temstet C, Basli E, Laroche L, Bouheraoua N. Conventional and iontophoresis corneal cross-linking for keratoconus: efficacy and assessment by optical coherence tomography and confocal microscopy. Cornea. 2017;36(2):153–162. doi: 10.1097/ICO.0000000000001062. [DOI] [PubMed] [Google Scholar]