Abstract
AIM
To investigate the regulation and mechanisms of periostin expression in retinal Müller glia, and to explore the relevance to retinal neovascularization.
METHODS
The oxygen-induced retinopathy (OIR) mouse model and the human Moorfield/Institute of Ophthalmology-Müller 1 (MIO-M1) cell line were used in the study. Immunofluorescence staining was used to determine the distribution and expression of periostin and a Müller glial cell marker glutamine synthetase (GS). Cytokines TNF-α and IFN-γ were added to stimulate the MIO-M1 cells. ShRNA was used to knockdown periostin expression in MIO-M1 cells. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was conducted to assess the mRNA expression of periostin.
RESULTS
Immunofluorescence staining showed that periostin was expressed by MIO-M1 Müller glia. GS-positive Müller glia and periostin increased in OIR retinas, and were partially overlaid. The stimulation of TNF-α and IFN-γ reduced the mRNA expression of periostin significantly and dose-dependently in MIO-M1 cells. Knockdown of periostin reduced mRNA expression of vascular endothelial growth factor A (VEGFA) in MIO-M1 cells, while VEGFA expression was not changed in periostin knock-out OIR retinas.
CONCLUSION
Müller glia could be one of the main sources of periostin in the retina, and might contribute to the pathogenesis of retinal neovascularization. Proinflammatory cytokines TNF-α and IFN-γ attenuate the periostin expression in retinal Müller glia, which provides a potential and novel method in treating retinal neovascular diseases.
Keywords: TNF-α, IFN-γ, periostin, Müller glia, retinal neovascularization
INTRODUCTION
Retinal neovascular diseases induced by hypoxia, such as proliferative diabetic retinopathy (PDR), retinopathy of prematurity (ROP) and retinal vein occlusion, often cause severe vision impairment worldwide[1]. Thus, targeting pathological retinal neovascularization can be a major therapeutic strategy for treating these disorders. It has been clarified that anti-vascular endothelial growth factor (VEGF) treatment is effective to inhibit retinal neovascularization, and has been widely used in clinical applications in the eye[2]. However, anti-VEGF therapies were weakly responsive in some patients and might cause many complications[2]–[3].
Macrophages can be polarized to M1/M2 phenotype by T helper (Th) 1 cytokines and Th2 cytokines respectively[4]–[6]. We recently reported that the role of macrophage M1/M2 polarization in oxygen-induced retinopathy (OIR) mouse model[7]. The polarization of macrophages, as well as macrophage-related cytokines, could be considered as novel therapeutic targets to treat retinal neovascularization[4]. Therefore, it is necessary to further clarify the molecular mechanism of the formation of retinal neovascularization, this may lead to the identification of the new target of blocking the formation of retinal neovascularization safely and effectively.
Periostin, as a fasciclin family member, is a matricellular protein with functions in cell motility through interacting with integrins in the development as well as remodeling of tissue[8]–[9]. Recent studies showed that periostin plays essential roles in proliferative vitreoretinal diseases[9]. Periostin overexpressed in vitreous and fibrovascular membranes, and had a strong positive correlation with M2 macrophages[10]–[12]. In an in vivo study, periostin highly expressed accompanied with the pathogenesis of retinal neovascularization of OIR, and the knockdown of periostin resulted in the blockade of pathological neovascularization in vivo[13]. It has been reported that glioblastoma stem cells (GSC)-produced periostin recruits M2 tumor-associated macrophages and enhances malignant growth[14].
Moreover, it has been indicated that periostin plays a key role in promoting chronic allergic inflammation in response to Th2 cytokines[15]. Interleukin (IL)-13, as a Th2 cytokine, enhanced periostin production in macrophages, and human retinal microvascular endothelial cells[11],[13]. Th2 cytokines IL-4 and IL-13 were demonstrated to play essential roles in producing periostin in gingival fibroblasts and periodontal ligament cells, whereas pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) inhibited periostin expressions in these cells[16]. Thus, Th2 cytokines and M2 polarization of macrophages may be involved in periostin production, and targeting periostin might be an efficient way in inhibiting retinal neovascularization.
Müller glia is a type of retinal glial cells, which is important to the development of the retina[17]. Retinal Müller cells are a major source of many growth factors, such as VEGF and nerve growth factor[18]. Periostin could be produced by other kinds of glial cells, such as Schwann cells and astroglia[19]–[20]. However, the relationship between periostin and Müller glia still remains unclear.
The purpose of this study was to investigate whether Müller glia are involved in the periostin production in retinal neovascularization. The possible relevance between periostin and VEGF were also discussed.
MATERIALS AND METHODS
Ethical Approval
All experiments with animals were conducted following the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The experimental procedures have been approved by the Kyushu University Institutional Animal Care and Use Committee.
Animals
In the study, we used wild type C57BL/6J mice and periostin knock-out (KO) mice for the animal experiments as previously described[13].
Oxygen-Induced Retinopathy Mouse Model
The OIR mouse model was induced as described[21]–[23]. Briefly, we exposed pups to 75% oxygen at postnatal day 7 (P7), and returned them to room air at P12. Pups kept in room air continuously were used as controls.
Culture of the Human Moorfield/Institute of Ophthalmology-Müller 1 Cell Line Cells
The human Moorfield/Institute of Ophthalmology-Müller 1 (MIO-M1) cell line, which was established and characterized previously in Limbs lab[24], were maintained with Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F12) medium (Gibco, Grand Island, NY, USA) in 5% CO2 at 37°C. MIO-M1 cells suspensions (started at 50 000 cells/3 mL/well) were seeded in 6 wells plates, cultured in DMEM/F12 medium with 10% fetal bovine serum (FBS) for attachment. After starvation in DMEM/F12 medium (serum-free) for 24h, the medium was replaced with (or without) human recombinant TNF-α and interferon-gamma (IFN-γ; R&D System, Minneapolis, MN, USA) for 8h and/or 24h. Then the collected cells were examined for the mRNA expressions.
Lentivirus Transduction for Gene Silencing of Periostin
Lentiviral vectors encoding shRNA against human periostin or scrambled shRNA was designed and manufactured by Obio Technology Co., Ltd (Shanghai, China). The target sequences for human periostin-shRNA is 5′-GCACCAAAAAG AAAUACUU-3′. The lentivirus infection was performed according to the manufacturer's instructions.
Quantitative Real-time Reverse Transcription Polymerase Chain Reaction
Total RNA of Müller cells was extracted using RNeasy Mini Kit (Qiagen, Redwood City, CA, USA) and the total RNA from the homogenized retinas was extracted using Trizol. After removing DNA traces using TURBO DNase (Ambiom, Thermo Fisher Scientific, Waltham, MA, USA), the concentrations of the RNAs were quantified by NanoVue plus (Biochrom, Cambridge, UK), and complementary DNAs were synthesized by a RevertAid® First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). Quantitative Real-time reverse transcription polymerase chain reaction (qRT-PCR) was conducted and analyzed by Taqman Gene Expression Master Mix with Taqman® gene expression assays (Applied Biosystems, Foster City, CA, USA) in a StepOne® Real-time PCR System (Applied Biosystems). GAPDH was used as the endogenous control for normalization. The following assays were used in the study: Mm99999915_g1 (GAPDH), Mm00450111_m1 (periostin), Mm01281449_m1 (VEGFA), Hs02758991_g1 (GAPDH), Hs00900055_m1 (VEGFA), Hs01566750_m1 (periostin).
Immunofluorescent Staining
Cryostat sections were prepared as described[7] with some modifications. In brief, the eye sections (20 µm) were cut by a cryostat (Leica CM 1800, Bannockburn, IL, USA). PBS with 0.1% Tween was used for rinsing. Sections were blocked by 3% skim milk, and the cells were blocked by 5% bovine serum albumin. After blocking, the sections or cells were incubated with the primary antibodies overnight at 4°C, and the secondary antibodies were used for incubation on the next day. Then, the cryostat sections or the cells were covered with aqueous mounting medium (Sigma-Aldrich, St. Louis, MO, USA).
The primary antibodies used for the study were: human/mouse periostin antibodies (5 µg/mL, R&D System, MN, USA) and anti-glutamine synthetase (GS) antibody (1:250 dilution, Abcam, Cambridge, MA, USA). Alexa Fluor 488 and 546 (1:100 dilution; Molecular Probes) were used as second antibodies. Hoechst 33342 (1:1000 dilution, Molecular Probes) was used to counterstain the nuclei. The stained sections and cells were photographed by the fluorescent microscope DMI4000B (Leica, Wetzlar, Germany).
Statistical Analysis
The results in figures were presented as means±SEMs. The statistical analyses were performed by Student's t test or one-way ANOVA which followed by Dunnett's tests. A P-value of less than 0.05 was considered significant. The statistical software JMP 10.0.2 (SAS Institute, Cary, NC, USA) was used for all analyses.
RESULTS
Periostin Co-localized with Retinal Müller Glia
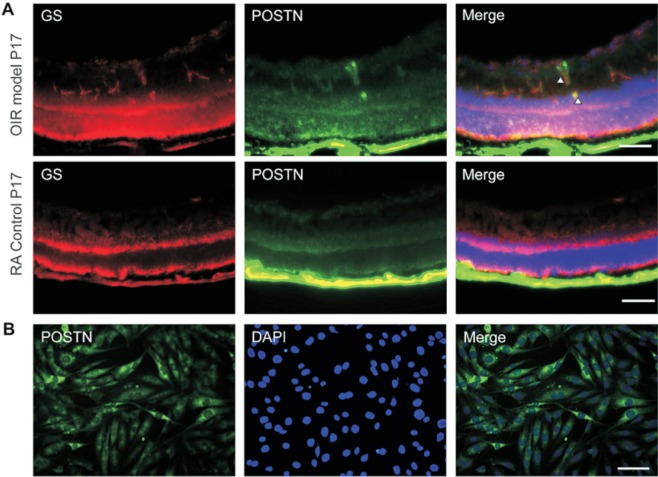
To determine the involvement of Müller glia in periostin production during the development of retinal neovascularization, we co-immunostained cryostat sections of OIR and room air control with antibodies against GS-periostin at P17. Immunofluorescence staining of the sections showed that both periostin and Müller glia marker GS were highly expressed in the OIR retina compared to the room air control (Figure 1), and periostin was partially overlayed with GS-positive Müller glia in retinal neovascularization. Moreover, periostin was immunostained in MIO-M1 cells, and the results showed that periostin was expressed by Müller glia in vitro (Figure 1B). These results indicated that periostin might be produced by retinal Müller glia.
Figure 1. Immunofluorescence staining revealed high expression of periostin and GS in the OIR retina, and periostin expressed in MIO-M1 cells.
Double staining for GS (red) and periostin (green) in cryostat sections of OIR and room air control eyes at P17 (A). Immunostaining of periostin in MIO-M1 cells (B). Hoechst 33342 and DAPI were used to counterstain the nuclei. Scale bars: 100 µm.
IFN-γ and TNF-α Attenuate Periostin Expression in MIO-M1 Cell Line
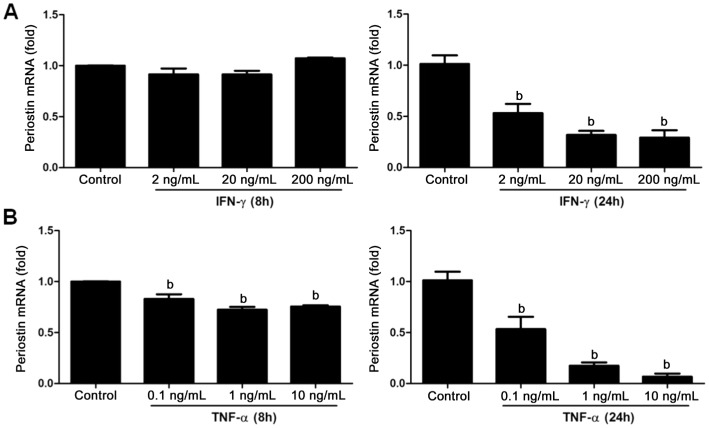
To further investigate the mechanism of periostin expression in retinal Müller glia, we stimulate the MIO-M1 cell line with TNF-α and IFN-γ, and qRT-PCR was performed. The results showed that IFN-γ inhibited periostin expression at 24h after stimulation at the concentration of 2, 20, 200 ng/mL (Figure 2A, P<0.01). Moreover, TNF-α with the concentration of 0.1, 1, 10 ng/mL attenuated periostin expression at both time points of 8h and 24h after stimulation (Figure 2B, P<0.01). The effects of both cytokines were dose-dependent.
Figure 2. IFN-γ and TNF-α down-regulated periostin expression in Müller glia in vitro.
Fold change in mRNA expression for periostin in Müller cells after stimulation with IFN-γ and TNF-α by different concentrations, 2, 20, 200 ng/mL for IFN-γ (A) and 0.1, 1, 10 ng/mL for TNF-α (B) respectively at 8h and 24h time points. Human GAPDH mRNA was used as an endogenous control in all qRT-PCR analysis. Data represent the mean±SEM (n=4). bP<0.01 compared to the controls.
IFN-γ and TNF-α Down-regulate the VEGFA mRNA Level in MIO-M1 Cell Line
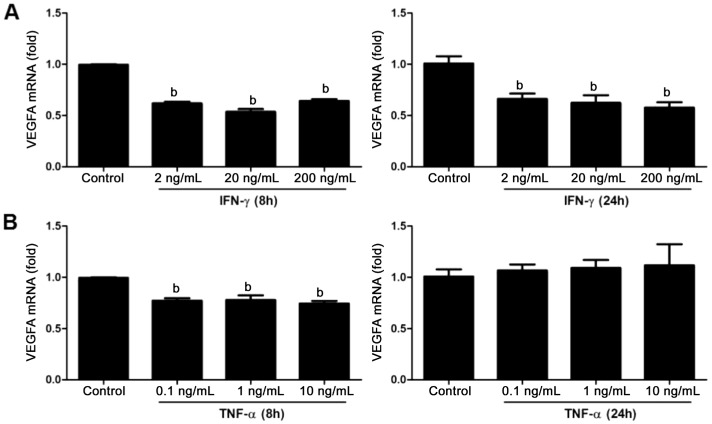
To clarify whether IFN-γ and TNF-α affect the mRNA expression level of VEGFA in retinal Müller glia in vitro, we stimulated the MIO-M1 cell line with IFN-γ and TNF-α, and qRT-PCR was performed to examine the mRNA expression of VEGFA. The results showed that both IFN-γ and TNF-α down-regulated the mRNA levels of VEGFA in MIO-M1 cells (Figure 3A, 3B; P>0.05). After 8h stimulation of IFN-γ at the concentration of 2, 20, 200 ng/mL, the VEGFA mRNA expression level decreased, and this change continued until 24h after stimulation. However, another cytokine TNF-α only attenuated the mRNA expression level of VEGFA after 8h stimulation. While the results stimulated by TNF-α at the concentration of 0.1, 1, 10 ng/mL showed no statistically significant difference.
Figure 3. The effect of IFN-γ and TNF-α stimulation to the mRNA levels of VEGFA in MIO-M1 cells.
Fold change showed in bar plots in the expression for VEGFA in Müller cells after stimulation by IFN-γ and TNF-α by different concentration, 2, 20, 200 ng/mL for IFN-γ (A) and 0.1, 1, 10 ng/mL for TNF-α (B) respectively after 8h and 24h stimulation. GAPDH was used as normalization in all qRT-PCR results. Data were represented the mean±SEM (n=4). bP<0.01 compared to the controls.
Knockdown of Periostin Inhibit mRNA Expression of VEGFA in MIO-M1 Cell Line
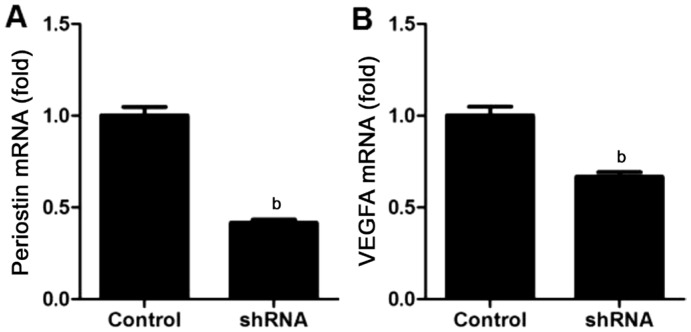
Knockdown effect induced by shRNA on mRNA expression of periostin in MIO-M1 cells was assessed. The results of qRT-PCR showed that mRNA expression of periostin was significantly inhibited following the transfection compared to the control (P<0.01, n=4; Figure 4A).
Figure 4. Periostin-knockdown attenuated VEGFA mRNA expression levels in MIO-M1 cells.
qRT-PCR results showed in bar graph revealed downregulation in MIO-M1 cells of periostin (A) and VEGFA (B) at mRNA expression levels after periostin knockdown. Results were normalized to human GAPDH mRNA expression levels correspondingly, and data were represented the mean±SEM (n=4). bP<0.01 compared to the controls.
To confirm the effect of periostin knockdown on VEGFA expression, qRT-PCR was also performed. The results showed that mRNA expression of VEGFA was also decreased after the knockdown of periostin (P<0.01, n=4; Figure 4B). These results indicated that knockdown of periostin inhibited mRNA expression of VEGFA by retinal glial cells.
Deficiency of Periostin does not Affect the mRNA Expression of VEGFA in OIR Retinas
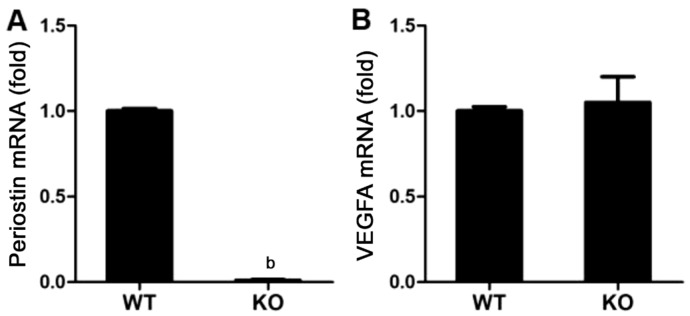
To determine the relationship between periostin and VEGFA, wild-type C57/B6J (WT) mice and periostin KO mice were exposed to 75% oxygen from P7 to P12, and we quantified mRNA expressions of periostin and VEGFA in OIR retinas at P17. The mRNA expression of periostin was remarkably attenuated in KO mice, which shows a well-deficiency of the gene (P<0.01; Figure 5A). However, there was no significant difference of VEGFA expression in the retinas between WT and KO mice (P>0.05; Figure 5B). These data indicate that the deficiency of periostin does not affect the mRNA expression of VEGFA in the whole retina of OIR.
Figure 5. mRNA expression of VEGFA had no relevance with deficiency of periostin (periostin KO mice) in OIR retinas.
qRT-PCR results showed in bar plots for mRNA expression levels of periostin was remarkably downregulated in KO mice compared to WT control mice (A). No change was detected for VEGFA expression both in periostin KO mice and WT control mice (B). Results were normalized to mouse GAPDH mRNA expression levels correspondingly, and data were represented the mean±SEM (n=4). bP<0.01 compared to the controls.
DISCUSSION
Periostin is considered to be involved in promoting tissue regeneration, wound healing and fibrosis by interacting with integrins[8]. Numerous studies of periostin has been conducted in tumor[25]–[27], asthma[28]–[29], cardiac diseases[30]–[31] and kidney fibrosis[32]–[33]. Recently, periostin has emerged as a player in the field of vitreoretinal diseases[9], which has been found to be upregulated in tissues of PDR, proliferative vitreoretinopathy as well as age-related macular degeneration. Müller glia are essential source cells of cytokines and inflammatory factors in the gliotic retinas with proliferative vitreoretinopathy[34]. In the present study, the results showed that periostin overlaid with Müller glia marker GS, and MIO-M1 Müller glial cells expressed periostin in vitro (Figure 1). Thus, Müller glia might be one major source for periostin. TNF-α and IFN-γ attenuated periostin mRNA expression in MIO-M1 cells (Figure 2). These results indicated that proinflammatory cytokines TNF-α and IFN-γ might be important in the regulation of periostin produced by Müller glia.
The mechanism why these two cytokines could reduce periostin in vitro still remained unclear. Previous studies showed that M2-polarized macrophages produced periostin[11], and periostin could be induced by Th2 cytokines such as IL-4 and IL-13[16]. Periostin should have strong relevance to Th2 response with an anti-inflammatory effect. Moreover, both periostin and anti-inflammatory M2 macrophages enhanced ocular neovascularization[7],[13],[35]–[36]. Thus, pro-inflammation might possess an inhibitory effect on periostin production.
The relationship between periostin and VEGF still remains controversial. Liu et al[37] reported that periostin enhanced tumor angiogenesis through activation of Erk/VEGF signaling in pancreatic cancer, and VEGF in the cancer tissues was significantly correlated with the expression of periostin. Periostin was also considered to have a significant positive correlation with VEGF in osteosarcoma patients[38] and in patients with esophageal squamous cell carcinoma[39], inducing and/or promoting tumor angiogenesis. Periostin also promoted VEGF secretion in keloid fibroblasts[40]. Another study showed that periostin shRNA-infection reduced VEGFA expression in GSC. Moreover, periostin and VEGFA were strongly associated with the progression of GSC[26]. However, the concentration of periostin in vitreous was not significantly correlated with those of VEGF in the vitreous of PDR patients[12].
In the present study, the in vitro and in vivo studies showed controversial results. IFN-γ and TNF-α down-regulate the VEGFA mRNA level in MIO-M1 cell line after 8h stimulation, while the VEGFA mRNA level has not been affected at 24h after the stimulation of TNF-α (Figure 3). Moreover, although the knockdown of periostin attenuated mRNA expression of VEGFA in MIO-M1 cell line, the deficiency of periostin does not affect the expression of VEGFA in the whole retina of OIR (Figures 4 and 5). A possible reason of this phenomenon might be that under hypoxia, VEGF can be expressed by many kinds of source cells besides Müller glia, such as macrophages[41], retinal pigment epithelial (RPE) cells[42], etc. The attenuation of VEGFA in glial cells could be masked by the increase from those cells.
Further studies are needed to determine whether experimental inhibition of periostin can attenuate retinal fibrovascular membrane formation ex-vivo and in vivo. Moreover, the mechanisms by which TNF-α and IFN-γ can induce down-regulation of periostin in mice is worth investigation. Periostin might be a novel target for PDR and it might have a synergistic effect with anti-VEGF treatment, and targeting these molecules by state-of-the-art strategies such as gene-editing using CRSPR-Cas9 system could be considered for the future investigations and clinical applications[43].
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81800855; No.81800856; No.81700837); Natural Science Foundation of Hunan Province (No.2018JJ3765); Department of Science and Technology, Hunan (No.2015TP2007); Japan Society for the Promotion of Science KAKENHI Grants (No.26293374; No.16K15734).
Conflicts of Interest: Peng YQ, None; Cao MJ, None; Yoshida S, None; Zhang LS, None; Zeng HL, None; Zou JL, None; Kobayashi Y, None; Nakama T, None; Shi JM, None; Jia SB, None; Zhou YD, None.
REFERENCES
- 1.Yoshida A, Yoshida S, Ishibashi T, Inomata H. Intraocular neovascularization. Histol Histopathol. 1999;14(4):1287–1294. doi: 10.14670/HH-14.1287. [DOI] [PubMed] [Google Scholar]
- 2.Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 2014;28(5):510–520. doi: 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salam A, Mathew R, Sivaprasad S. Treatment of proliferative diabetic retinopathy with anti-VEGF agents. Acta Ophthalmologica. 2011;89(5):405–411. doi: 10.1111/j.1755-3768.2010.02079.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhou YD, Yoshida S, Peng YQ, Kobayashi Y, Zhang LS, Tang LS. Diverse roles of macrophages in intraocular neovascular diseases: a review. Int J Ophthalmol. 2017;10(12):1902–1908. doi: 10.18240/ijo.2017.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 7.Zhou YD, Yoshida S, Nakao S, Yoshimura T, Kobayashi Y, Nakama T, Kubo Y, Miyawaki K, Yamaguchi M, Ishikawa K, Oshima Y, Akashi K, Ishibashi T. M2 macrophages enhance pathological neovascularization in the mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2015;56(8):4767–4777. doi: 10.1167/iovs.14-16012. [DOI] [PubMed] [Google Scholar]
- 8.Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang GL, Arron JR, Holweg CTJ, Kudo A. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014;71(7):1279–1288. doi: 10.1007/s00018-013-1494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida S, Nakama T, Ishikawa K, Nakao S, Sonoda KH, Ishibashi T. Periostin in vitreoretinal diseases. Cell Mol Life Sci. 2017;74(23):4329–4337. doi: 10.1007/s00018-017-2651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi Y, Yoshida S, Nakama T, Zhou YD, Ishikawa K, Arita R, Nakao S, Miyazaki M, Sassa Y, Oshima Y, Izuhara K, Kono T, Ishibashi T. Overexpression of CD163 in vitreous and fibrovascular membranes of patients with proliferative diabetic retinopathy: possible involvement of periostin. Br J Ophthalmol. 2015;99(4):451–456. doi: 10.1136/bjophthalmol-2014-305321. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida S, Kobayashi Y, Nakama T, Zhou YD, Ishikawa K, Arita R, Nakao S, Miyazaki M, Sassa Y, Oshima Y, Izuhara K, Kono T, Ishibashi T. Increased expression of M-CSF and IL-13 in vitreous of patients with proliferative diabetic retinopathy: implications for M2 macrophage-involving fibrovascular membrane formation. Br J Ophthalmol. 2015;99(5):629–634. doi: 10.1136/bjophthalmol-2014-305860. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S, Ishikawa K, Asato R, Arima M, Sassa Y, Yoshida A, Yoshikawa H, Narukawa K, Obika S, Ono J, Ohta S, Izuhara K, Kono T, Ishibashi T. Increased expression of periostin in vitreous and fibrovascular membranes obtained from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(8):5670–5678. doi: 10.1167/iovs.10-6625. [DOI] [PubMed] [Google Scholar]
- 13.Nakama T, Yoshida S, Ishikawa K, Kubo Y, Kobayashi Y, Zhou YD, Nakao S, Hisatomi T, Ikeda Y, Takao K, Yoshikawa K, Matsuda A, Ono J, Ohta S, Izuhara K, Kudo A, Sonoda KH, Ishibashi T. Therapeutic effect of novel single-stranded RNAi agent targeting periostin in eyes with retinal neovascularization. Mol Ther Nucleic Acid. 2017;6:279–289. doi: 10.1016/j.omtn.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou WC, Ke SQ, Huang Z, Flavahan W, Fang XG, Paul J, Wu L, Sloan AE, McLendon RE, Li XX, Rich JN, Bao SD. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17(2):170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izuhara K. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122(7):2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima M, Honda T, Miyauchi S, Yamazaki K. Th2 cytokines efficiently stimulate periostin production in gingival fibroblasts but periostin does not induce an inflammatory response in gingival epithelial cells. Arch Oral Biol. 2014;59(2):93–101. doi: 10.1016/j.archoralbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Müller glial cells. J Comp Neurol. 2008;509(2):225–238. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, He C, Zhou T, Huang ZJ, Zhou LL, Liu XL. NGF increases VEGF expression and promotes cell proliferation via ERK1/2 and AKT signaling in Müller cells. Mol Vis. 2016;22:254–263. [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnenberg-Riethmacher E, Miehe M, Riethmacher D. Promotion of periostin expression contributes to the migration of Schwann cells. J Cell Sci. 2015;128(17):3345–3355. doi: 10.1242/jcs.174177. [DOI] [PubMed] [Google Scholar]
- 20.Shimamura M, Taniyama Y, Katsuragi N, Koibuchi N, Kyutoku M, Sato N, Allahtavakoli M, Wakayama K, Nakagami H, Morishita R. Role of central nervous system periostin in cerebral ischemia. Stroke. 2012;43(4):1108–1114. doi: 10.1161/STROKEAHA.111.636662. [DOI] [PubMed] [Google Scholar]
- 21.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LEH. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4(11):1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaji Y, Yoshida S, Ishikawa K, Sengoku A, Sato K, Yoshida A, Kuwahara R, Ohuchida K, Oki E, Enaida H, Fujisawa K, Kono T, Ishibashi T. TEM7 (PLXDC1) in neovascular endothelial cells of fibrovascular membranes from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(7):3151–3157. doi: 10.1167/iovs.07-1249. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa K, Yoshida S, Nakao S, Sassa Y, Asato R, Kohno R, Arima M, Kita T, Yoshida A, Ohuchida K, Ishibashi T. Bone marrow-derived monocyte lineage cells recruited by MIP-1β promote physiological revascularization in mouse model of oxygen-induced retinopathy. Lab Invest. 2012;92(1):91–101. doi: 10.1038/labinvest.2011.141. [DOI] [PubMed] [Google Scholar]
- 24.Limb GA, Salt TE, Munro PM, Moss SE, Khaw PT. In vitro characterization of a spontaneously immortalized human Müller cell line (MIO-M1) Invest Ophthalmol Vis Sci. 2002;43(3):864–869. [PubMed] [Google Scholar]
- 25.Cui D, Huang ZJ, Liu YF, Ouyang GL. The multifaceted role of periostin in priming the tumor microenvironments for tumor progression. Cell Mol Life Sci. 2017;74(23):4287–4291. doi: 10.1007/s00018-017-2646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SY, Piao Y, Jeong KJ, Dong J, de Groot JF. Periostin (POSTN) regulates tumor resistance to antiangiogenic therapy in glioma models. Mol Cancer Ther. 2016;15(9):2187–2197. doi: 10.1158/1535-7163.MCT-15-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JI. Role of periostin in hepatocellular carcinoma: the importance of tumor microenvironment. Gut Liver. 2016;10(6):871–872. doi: 10.5009/gnl16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asano T, Kanemitsu Y, Takemura M, et al. Serum periostin as a biomarker for comorbid chronic rhinosinusitis in patients with asthma. Ann Am Thorac Soc. 2017;14(5):667–675. doi: 10.1513/AnnalsATS.201609-720OC. [DOI] [PubMed] [Google Scholar]
- 29.James A, Janson C, Malinovschi A, Holweg C, Alving K, Ono J, Ohta S, Ek A, Middelveld R, Dahlén B, Forsberg B, Izuhara K, Dahlén SE. Serum periostin relates to type-2 inflammation and lung function in asthma: data from the large population-based cohort Swedish GA(2)LEN. Allergy. 2017;72(11):1753–1760. doi: 10.1111/all.13181. [DOI] [PubMed] [Google Scholar]
- 30.Conway SJ, Doetschman T, Azhar M. The inter-relationship of periostin, TGFβ, and BMP in heart valve development and valvular heart diseases. ScientificWorldJournal. 2011;11:1509–1524. doi: 10.1100/tsw.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devrim AK, Sozmen M, Devrim T, Sudagidan M, Cinar M, Kabak YB. Periostin normalizes levels of cardiac markers in rats with experimental isoproterenol cardiotoxicity. Bratisl Lek Listy. 2017;118(11):705–709. doi: 10.4149/BLL_2017_133. [DOI] [PubMed] [Google Scholar]
- 32.Hwang JH, Yang SH, Kim YC, Kim JH, An JN, Moon KC, Oh YK, Park JY, Kim DK, Kim YS, Lim CS, Lee JP. Experimental inhibition of periostin attenuates kidney fibrosis. Am J Nephrol. 2018;46(6):501–517. doi: 10.1159/000485325. [DOI] [PubMed] [Google Scholar]
- 33.François H, Chatziantoniou C. Renal fibrosis: recent translational aspects. Matrix Biol. 2018;68:318–332. doi: 10.1016/j.matbio.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Eastlake K, Banerjee PJ, Angbohang A, Charteris DG, Khaw PT, Limb GA. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia. 2016;64(4):495–506. doi: 10.1002/glia.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakama T, Yoshida S, Ishikawa K, Kobayashi Y, Zhou Y, Nakao S, Sassa Y, Oshima Y, Takao K, Shimahara A, Yoshikawa K, Hamasaki T, Ohgi T, Hayashi H, Matsuda A, Kudo A, Nozaki M, Ogura Y, Kuroda M, Ishibashi T. Inhibition of choroidal fibrovascular membrane formation by new class of RNA interference therapeutic agent targeting periostin. Gene Therapy. 2015;22(2):127–137. doi: 10.1038/gt.2014.112. [DOI] [PubMed] [Google Scholar]
- 36.Zandi S, Nakao S, Chun KH, Fiorina P, Sun D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, Taher M, Melhorn MI, Schering A, Gatti F, Tezza S, Xie F, Vergani A, Yoshida S, Ishikawa K, Yamaguchi M, Sasaki F, Schmidt-Ullrich R, Hata Y, Enaida H, Yuzawa M, Yokomizo T, Kim YB, Sweetnam P, Ishibashi T, Hafezi-Moghadam A. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep. 2015;10(7):1173–1186. doi: 10.1016/j.celrep.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Li F, Gao F, Xing LX, Qin P, Liang XX, Zhang JJ, Qiao XH, Lin LZ, Zhao Q, Du LF. Periostin promotes tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling. Oncotarget. 2016;7(26):40148–40159. doi: 10.18632/oncotarget.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu F, Shang XF, Wang W, Jiang W, Fang C, Tan D, Zhou HC. High-level expression of periostin is significantly correlated with tumour angiogenesis and poor prognosis in osteosarcoma. Int J Exp Pathol. 2016;97(1):86–92. doi: 10.1111/iep.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu M, Tian H, Yue WM, Li L, Li SH, Qi L, Hu WS, Gao C, Si LB. Overexpression of TFIIB-related factor 2 is significantly correlated with tumor angiogenesis and poor survival in patients with esophageal squamous cell cancer. Med Oncol. 2013;30(2):553. doi: 10.1007/s12032-013-0553-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Nie FF, Chen XL, Qin ZL, Kang CF, Chen B, Ma JX, Pan BL, Ma YG. Upregulated periostin promotes angiogenesis in keloids through activation of the ERK 1/2 and focal adhesion kinase pathways, as well as the upregulated expression of VEGF and angiopoietin-1. Mol Med Report. 2015;11(2):857–864. doi: 10.3892/mmr.2014.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naug HL, Browning J, Gole GA, Gobe G. Vitreal macrophages express vascular endothelial growth factor in oxygen-induced retinopathy. Clin Exp Ophthalmol. 2000;28(1):48–52. doi: 10.1046/j.1442-9071.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 42.Nagineni CN, Kommineni VK, William A, Detrick B, Hooks JJ. Regulation of VEGF expression in human retinal cells by cytokines: implications for the role of inflammation in age-related macular degeneration. J Cell Physiol. 2012;227(1):116–126. doi: 10.1002/jcp.22708. [DOI] [PubMed] [Google Scholar]
- 43.Peng YQ, Tang LS, Yoshida S, Zhou YD. Applications of CRISPR/Cas9 in retinal degenerative diseases. Int J Ophthalmol. 2017;10(4):646–651. doi: 10.18240/ijo.2017.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]