Abstract
In the last decade, the generation and maintenance of organotypic structures has been propelled to the center stage of biomedical research. In the lung, a variety of protocols has been devised to generate organoids mimicking lung structures, but most methods with human cells have complicated lengthy protocols or a progressive decline in differentiation potential and physiological function with increasing passaging. A new study from Sachs et al (2019) seeks to solve these issues, providing a versatile methodology to efficiently isolate, indefinitely culture, and manipulate human airway organoids, potentially allowing the in vitro modeling of a plethora of lung diseases.
Subject Categories: Development & Differentiation, Methods & Resources, Molecular Biology of Disease
Recently, the establishment and manipulation of organotypic cultures, termed organoids, from cells representing a variety of epithelial tissues has gained enormous attention. The appeal is clear: Organoids are self‐organizing units that mimic the physiological state of the organ of origin at the morphological and molecular levels, offering a convenient means to study development, organogenesis, and stem niches. Organoids also allow the modeling of a variety of pathological states of environmental or genetic origin that can be easily manipulated in vitro for the exploration and validation of new therapeutic approaches. In an article published in this issue of The EMBO Journal, Sachs and colleagues (Sachs et al, 2019) go beyond current lung cell culturing limitations, introducing a new methodology to indefinitely propagate human airway epithelial cells as three‐dimensional organoids, named airway organoids (AOs, see Fig 1).
Figure 1. Long‐term cultivation of human airway organoids for disease modeling.
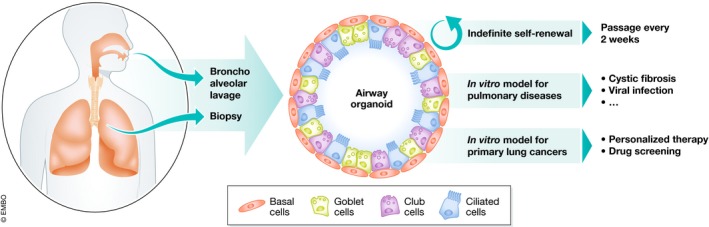
A standardized and feasible method to indefinitely propagate and differentiate lung epithelium from human samples allows for in‐depth investigation of the biology of the human lung in health and disease states.
Airway organoids provide an opportunity to model the diversity of the airway, a relatively simple epithelium that serves as the main port of entry for a number of environmental challenges and the origin of a wide range of pathologies. The pseudo‐stratified epithelium that lines the conducting airways is characterized by a basal layer of stem cells, basal cells, that constantly maintain the more specialized luminal cells (Rock et al, 2009). Multiple classes of secretory cells, including goblet and club cells, produce a complex mix of mucins and glycoproteins that, combined with the motility provided by multi‐ciliated cells, guarantees an anti‐microbial environment that continuously filters and clears large particles from the inhaled air. Airway health is abrogated in lung diseases including chronic bronchitis, COPD, and asthma. Furthermore, airways are the primary site of interaction with numerous bacterial and viral pathogens. Finally, the airway epithelium has been implicated as the site of origin of squamous cell carcinoma (SCC), the second‐most common and deadly non‐small cell cancer of the lung (Chen et al, 2014).
Great advances have been made in organoid culturing of human and murine lung cells in the last decade. However, important differences between patients and mouse models of some lung diseases—e.g., lack of lung disease of CFTR knockout mice to model cystic fibrosis—have made it an important priority to improve animal models and to advance our ability to culture human lung cells.
Three‐dimensional organoids of human airways have been generated using two main approaches: by precisely directing the differentiation of iPS cells or by cultivating human primary airway cells. Protocols to drive human iPS into lung epithelial cells are based on knowledge of the stimuli that orchestrate lung development. Three‐dimensional hiPS‐derived organoids constituted of both mesenchyme and epithelium mimic human fetal development and can also model virus infections and features of lung fibrosis (Huang et al, 2014; Chen et al, 2017). hiPS‐derived airway organoids have been shown to efficiently reproduce CF‐related phenotypes (McCauley et al, 2017, 2018). Despite their unequivocal successes, these hiPS‐based protocols are technically challenging and time‐consuming. On the other hand, primary airway cells can be isolated, propagated in vitro as a monolayer, and eventually induced to differentiate (both in 2D and 3D) to generate airway‐like structures (Rock et al, 2009; Mou et al, 2016). Despite less labor‐intense protocols for isolation and cultivation, the ability to passage primary lung epithelial cells while maintaining differentiation potential and functional properties has been so far limited to a few passages, greatly restricting their use for translational applications. Prior to the work of Sachs et al, it was therefore of vital importance to establish human‐based systems to better understand the mechanism underlying the homeostasis and the response to insult of the airway epithelium and to study a number of human pathologies.
As other studies before, Sachs et al report that primary human airway cells obtained by biopsies or bronchoalveolar lavages (fresh or cryopreserved) give rise to hollow spherical organoids closely resembling the tissue organization of the human airways when embedded in a drop of basement membrane extract and submerged in medium. However, instead of progressively losing their proliferative and differentiation potential, these airway organoids (AOs) can be passaged for at least one year, maintaining their features. The retained abilities may be attributable to the specific cocktail of small molecules in the medium that activate or inhibit the major signaling pathways involved in lung differentiation, yet more future studies will be needed to define the precise mechanisms that regulate AO formation and function. Even though no particular lung cell populations were pre‐selected in Sachs’ method, it is intriguing that only airway organoids emerge from this protocol; it also remains to be determined why alveolar organoids are not supported by the cocktail. AO also demonstrates interesting differences in functional properties compared with rectal or intestinal organoids, demonstrating the specificity and utility of the new AO method.
Sachs et al provide proof of concept for multiple methods to manipulate lung organoids and effectively model multiple lung pathologies. AOs derived from cystic fibrosis patients show an attenuated swelling when challenged with cAMP‐inducing agents like forskolin. Cells from AOs can also be grown in 2D air–liquid interface setting, allowing for more conventional testing of CFTR function using the Ussing chamber assay, a gold standard in the field of CFTR function. To date, it has been challenging to use iPS‐derived cells in the Ussing assay. The dynamics of respiratory infections of the upper airways can also be modeled: Infection of AOs with respiratory syncytial virus (RSV) showed virus‐host specificity and induced alterations of the epithelial layers that resemble RSV pathology. Finally, the authors also modeled lung cancers using AOs. Surprisingly, even though AO represents airway epithelia instead of alveolar cells, which are likely the origin of adenocarcinomas, the cancer‐derived AOs maintained histological features of the original tumor, regardless of the type of lung cancer from which they were derived.
In summary, Sachs et al provide the field with a standardized and feasible method to indefinitely cultivate and differentiate the airway epithelium from human samples, allowing for a deeper understanding of the biology of the human lung in health and disease states. The main advantage of the AO method resides in its relative simplicity and the substantially shorter time required from isolating patient‐derived cells to the generation of organoids faithfully resembling human airway. The versatility of potential applications adds to the importance of these findings. This includes a promising trajectory toward drug discovery efforts for the treatment of genetic disorders of the lung or pathogenic infections, as well as potentially quick testing of patient‐derived cancer AOs for personalized therapy strategies. To further evolve this system, next steps may involve the investigation of the role of mesenchyme in AO, to generate even more refined models for complex lung pathologies such as idiopathic pulmonary fibrosis, asthma, and cancer, and to perform gene correction analyses of AO. Future challenges for the field in a broader sense will involve further developing lung organoids to demonstrate functional interactions with nerves, blood vessels, and the matrix, as well as establishing the potential of organoid‐derived cells for cell‐based therapy. For now, the promise of a forever airway organoid will sustain many to strive to meet these challenges.
The EMBO Journal (2019) 38: e101526
See also: N Sachs et al (February 2019)
References
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong K‐K (2014) Non‐small‐cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14: 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y‐W, Huang SX, de Carvalho ALRT, Ho S‐H, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova J‐L, Bhattacharya J, Liang AF, Palermo LM, Porotto M, Moscona A, Snoeck H‐W (2017) A three‐dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SXL, Islam MN, O'Neill J, Hu Z, Yang Y‐G, Chen Y‐W, Mumau M, Green MD, Vunjak‐Novakovic G, Bhattacharya J, Snoeck H‐W (2014) Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32: 84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN (2017) Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 20: 844–857.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley KB, Alysandratos K‐D, Jacob A, Hawkins F, Caballero IS, Vedaie M, Yang W, Slovik KJ, Morley M, Carraro G, Kook S, Guttentag SH, Stripp BR, Morrisey EE, Kotton DN (2018) Single‐cell transcriptomic profiling of pluripotent stem cell‐derived SCGB3A2 + airway epithelium. Stem Cell Rep 10: 1579–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, Zhang B, Solomon GM, Turner B, Bihler H, Harrington J, Lapey A, Channick C, Keyes C, Freund A, Artandi S, Mense M, Rowe S, Engelhardt JF, Hsu Y‐C et al (2016) Dual SMAD signaling inhibition enables long‐term expansion of diverse epithelial basal cells. Cell Stem Cell 19: 217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106: 12771–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, Papaspyropoulos A, Zomer‐van Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz‐Prince G, Iakobachvili N, Amatngalim GD, de Ligt J, van Hoeck A, Proost N, Viveen MC, Lyubimova A, Teeven L, Derakhshan S, Korving J, Begthel H, Dekkers JF et al (2019) Long‐term expanding human airway organoids for disease modeling. EMBO J 38: e100300 [DOI] [PMC free article] [PubMed] [Google Scholar]


