Abstract
Objectives
This study examined a more effective pain management method, without sucrose, on heel lance in preterm infants using the Premature Infant Pain Profile (PIPP).
Design
In a nonblinded, randomized controlled, two-period, two-sequence crossover trial, 25 infants were randomly allocated to intervention (a Brahms lullaby with non-nutritive sucking, facilitated tucking and holding) or standard care (facilitated tucking and holding).
Setting
Local Perinatal Medical Centre’s NICU in Japan, July 2014 until June 2015.
Outcome measures
The primary outcome variable was PIPP, and secondary outcomes were heart rate (HR), oxygen saturation, and abnormal HR (> baseline mean plus 2 SDs, or <120 minus 2 SDs).
Results
The infants were 33.8 weeks gestational age at birth, 1,983.7 g birth weight, and 32 to 35 weeks postconceptual age. At all 10 measurement points, constructed of every 30 seconds postheel lance, mean PIPP of infants during the intervention (3.6 to 2.4) was significantly lower than during the standard care (8.0 to 4.6) (range, P=0.0039 to P<0.0001). All PIPP reduction rates from the 30 seconds point were similar between the two groups. The HR of preterm infants at the 120 seconds points were significantly lower (P=0.0151), and the HRs of 6 points were considerably lower during the intervention than during the standard care (range, P≤0.0879 to P≥0.049). The abnormal HR total number was significantly lower during the intervention (2) than the standard care (23) (frequency ratio=0.087, P<0.0001).
Conclusion
This method demonstrated stronger analgesia, early pain relief, and maintenance of homeostasis on heel lance in preterm infants.
Keywords: Facilitated tucking, Heel lance, Music (Lullaby: Brahms), Non-nutritive sucking, Pain, Preterm infant
Preterm infants are at increased risk of impaired neurodevelopmental outcomes including cognitive abnormalities or motor deficits, and the risk of impairment increases with decreasing gestational age (1,2). Preterm infants are exposed to a large number of painful procedures (3), which have been linked to delayed postnatal growth, poor early neurodevelopment, and altered brain development (4).
Oral sucrose solutions are commonly administered to infants in the neonatal intensive care unit (NICU) as a nonpharmacologic intervention for managing acute procedural pain (5). However, the long-term effects of repeated oral sucrose usage have not been systematically studied (5,6). Non-nutritive sucking (NNS), facilitated tucking, and swaddling are also effective for immediate pain control in preterm infants (7), but yield a Premature Infant Pain Profile (PIPP) score higher than 6 points (8–13). PIPP scores of 6 or less generally indicate minimal or no pain, and scores greater than 12 reflect moderate to severe pain (14). In previous studies of preterm infants during heel lance, mean PIPP scores were as follows; with NNS slight pain (6.3 to 8.4) (9,10), with swaddling slight pain (7 to 10.2) (12,13), with Kangaroo mother care slight pain (8.9) (13), with facilitated tucking slight – severe pain (7.2 to 14.4) (8,15), with sucrose no to slight pain (3.0 to 9.8) (16,17), and with both sucrose and NNS no to slight pain (4.6 to 8.2) (9,16). All studies confirmed that preterm infants experience significant pain from heel lance (18).
Music for preterm infants is a noninvasive, nonpharmaceutical intervention (15,19–21). Although the mean PIPP score of 21 preterm infants with music and facilitated tucking during heel lance indicated no pain (5.1), the standard deviation (SD) was 1.9, which indicates that some still had pain (15). Also, a reduction in the heart rate (HR), behavioural state and facial expression of pain during heel lance with a Brahms lullaby recording (seven heard the piano version and seven a capella version) appeared to only occur in infants at a minimum 32 weeks’ postconceptual age (PCA) (22). During heel lance in infants at minimum 32 weeks PCA, 20 infants with pacifier-activated female traditional lullabies had significantly lower behaviour states and stress levels than 20 infants in the control group (23). Although music (22) and NNS and music (23) have a facilitating effect on returning to homeostasis, the sample size of the two studies was small. To develop a more effective pain management method than oral sucrose, this study evaluated the pain alleviation effect and the time to return to homeostasis facilitation effect of a recorded Brahms lullaby combined with NNS for heel lance in preterm infants using a more standardized pain scale (the PIPP) (5).
METHODS
Design
This nonblinded, randomized controlled, two-period, two-sequence crossover trial was approved by the institutional review board at the Takamatsu Red Cross Hospital in Japan (approval number 14-008). A crossover design was used to reduce the impact of confounding variables outside the control of the study itself (24,25). This trial was registered at UMIN Clinical Trials Registry (UMIN-CTR) (UMIN 000024876). The study followed the CONSORT guidelines for reporting randomized controlled trials.
Sample and setting
Inclusion criteria of infants were as follows: (a) 28 to 35 weeks PCA at birth (infants born at < 36 weeks PCA receive heel lance), (b) 32 to 35 weeks PCA at the time of the intervention, based on the evidence that infants at 32 weeks PCA have fully coordinated sucking (8,26), and are able to listen to the voice version of lullabies (19), (c) Apgar score of 6 or more at 5 minutes after birth, (d) intraventricular hemorrhage grade of 2 or less, (e) 48 hours or older in the case of birth by caesarean operation, and f) permission of the attending physician. Exclusion criteria included: (a) a congenital anomaly or a serious condition, and (b) sedative or analgesic drug usage within 48 hours prior to the heel lance.
To calculate study power, we first determined that the effect size was 0.63 (8). Thus, using the Wilcoxon signed-rank test in G*power 3.1.9.2, we estimated that 25 preterm infants would be needed to detect the effect size of 0.6 with an alpha level of 0.05 and a power of 80%.
Standard care or pain-relief intervention was performed when preterm infants met the following conditions established by the NICU for performing heel lance: 1 hour or more after suckling milk, quiet rest condition in a face up position, and not crying.
Measures
Outcome variables of PIPP selected as primary outcome included preterm infants’ behavioural responses and physiological responses (HR and oxygen saturation [O2 Sat]) (8,22,23). The PIPP is a reliable, valid, feasible measure of acute pain as an effective outcome measure in pain intervention studies in infants (18,27), and a previous study demonstrated the reliability and validity of the Japanese versions (28).
HR and O2 Sat were used to determine return to homeostasis as a secondary outcome (13,22,23). Abnormal HR was defined as 2 SDs above the baseline, or <120 beats/minute minus 2 SDs (8). The frequency of abnormal HR was calculated (total number of abnormal HRs for each observation). Potential stress O2 Sat was considered more than 2 SDs below the baseline mean; abnormal O2 Sat was defined as <87% (29). The sampling points of PIPP indicators, HR and O2 Sat were constituted from the baseline and 10 points that were constructed at every 30 seconds after heel lance.
Adverse events recorded included choking, vomiting, oxygen desaturation, apnea, and self-limiting bradycardia (5).
Procedures
The study was from July 2014 through June 2015. Following parental consent, each infant was assigned using a random table format to two sequences: sequence one with pain-relief intervention first (period 1), followed by standard care (period 2), sequence two with standard care first (period 1), followed by pain-relief intervention (period 2). Based on the random table, a research assistant sealed an envelope containing the written randomized method (the order of the two interventions). The practitioner of the heel lance and the researcher did not know the order until opening the envelope. The washout period was set for at least 8 hours between the two periods.
To analyze the recovery response from pain of heel lance, and to ascertain whether adjustment of database scores between the two groups was requisite or not, the researcher measured the baseline scores of preterm infants in two groups. HR and O2 Sat were measured using pulse oximeters (MAsimoSET radical, IMI). The preterm infant’s facial expressions and the monitor screen displayed HR and O2 Sat were recorded by two video cameras (Panasonic, HC-V550M) from before baseline (before intervention) until 5 minutes postheel lance, and stored on DVD. The PIPP was derived from videos by a blinded research assistant or by an investigator who was not blinded. Prior to the study, to quantify the reliability of the PIPP provided by the two coders, an assessment of inter-rater reliability was completed. The inter-rater reliability of the PIPP of six preterm infants ranged from 0.851 to 1.0, which was considered satisfactory (30,31).
The time of blood collection was defined as the time from pricking the heel to putting an adhesive plaster on the wound.
The pain relief intervention included the addition of the Brahms lullaby and a pacifier. After the baseline score was measured for 1 minute, a pacifier (Soothie21307, ATOM) was placed in the mouth, the infant was held and facilitated tucking was used. Then a Brahms lullaby by a Japanese female vocalist with instrumental music (World lullaby, A collection of famous children’s songs, Nippon Crown) was played (this version was selected because preterm infants are sensitive to native language speech based on exposure to the native language in utero (32,33). The lullaby volume was below 65 to 75 dB, scale C (34) played from a CD player (CD ZABADY Orange AV-J165OR, TWINBIRD) set 20 to 25 cm away from the head of the infant. NNS use was coded as an infant sucking on or holding a pacifier in his/her mouth without being fed breast milk or formula.
One minute after the lullaby was started, the practitioner (paediatrician or nurse) disinfected the heel of the preterm infant with alcohol raw cotton. Fifteen seconds after disinfecting, the practitioner performed the heel lance to the preterm infants using the BD Quikheel lancet (Japan Becton, Dickinson, BD Microtainer Quikheel TM Lancet 368102). The pain-relief intervention was continued until 5 minutes postheel lance.
When infants were in the standard care group, they received only facilitated tucking and holding. After a 1-minute baseline check by the researcher, the practitioner performed the heel lance. The practitioner continued the standard care until infants’ calmness resumed, such as the disappearance of crying and agitation after blood collection. All the infants in the standard care group became calm within 5 minutes of blood collection.
Statistical analysis
Differences in PIPPs, HRs, and O2 Sats were tested using a two-sided type 3 F test of the intervention effect in a general linear mixed model, where the final model included fixed-effects for intervention, sequence, period, and with random effects for participants (35–37). The model was fit using the MIXED procedure in SAS. The protocol-defined model included evaluation of carry-over effect, period effects, and intervention effect. The difference-in-differences model was selected as the appropriate strategy comparing change from baseline or 30 seconds postheel lance between the two groups (38,39). The Mantel-Haenszel Test was used to compare the frequencies of abnormal HR, potential stress O2 Sat and abnormal O2 Sat between the two groups. The PIPP reduction rate was calculated by dividing the value of subtracting the PIPP at each point from the PIPP at 30 seconds by the PIPP at 30 seconds. SAS version 9.4 for Windows was used for statistical analysis.
RESULTS
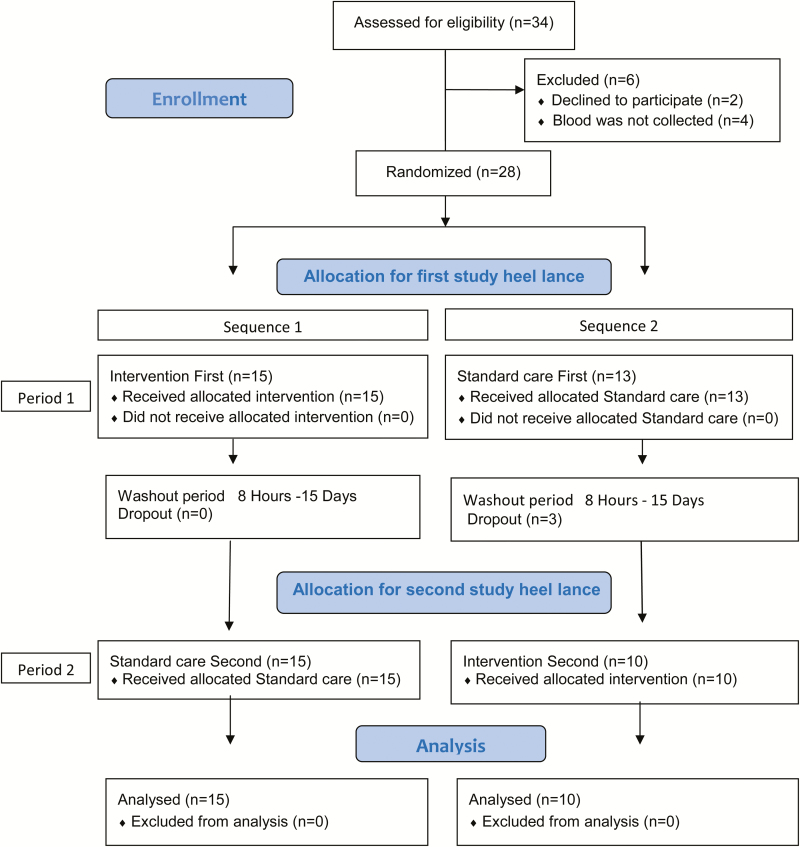
The parents of 34 infants were approached and 32 parents consented (Figure 1). Four infants were eventually excluded because blood collection was not performed.
Figure 1.
Flowchart of participant recruitment according to CONSORT 2010 guidelines.
Comparison of infants between the two groups showed no differences in baseline characteristics (Table 1). No carry-over effect or period effects were found in PIPP, HRs, and O2 Sats for all points in a general linear mixed model.
Table 1.
Demographic variables and confounding variables (N=25)
| Variable | During intervention | During standard care | P-value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Gestational age at birth (weeks) | 33.8 | 1.5 | |||||
| Birth weight (gram) | 1,983.7 | 383.9 | |||||
| Apgar score (1 min) | 6.9 | 2.3 | |||||
| Apgar score (5 min) | 8.5 | 1.1 | |||||
| Male (n) | 15 | ||||||
| Female (n) | 10 | ||||||
| Postconceptual age (weeks) | 34.6 | 0.8 | 34.5 | 1.0 | 0.11 | ||
| Postnatal age (days) | 5.2 | 6.5 | 5.0 | 5.3 | 0.36 | ||
| Weight on day of study (gram) | 1818.5 | 339.6 | 1810.3 | 352.8 | 0.38 | ||
| Duration from last feeding (minutes) | 154.6 | 31.5 | 149.1 | 26.6 | 0.78 | ||
| Blood glucose level (mg/dL) | 75.8 | 13.6 | 84.9 | 20.7 | 0.13 | ||
| Blood collection time (seconds) | 74.9 | 64.6 | 55.5 | 32.3 | 0.69 | ||
| Baseline: the Premature Infant Pain Profile | 2.1 | 1.7 | 2.3 | 1.4 | 0.46 | ||
| Baseline: Heart rate (/minutes) | 150.9 | 18.3 | 152.8 | 19.0 | 0.67 | ||
| Baseline: Oxygen saturation (%) | 96.6 | 2.6 | 96.8 | 2.3 | 0.66 | ||
Unpaired t-test or Wilcoxon rank sum test P<0.05.
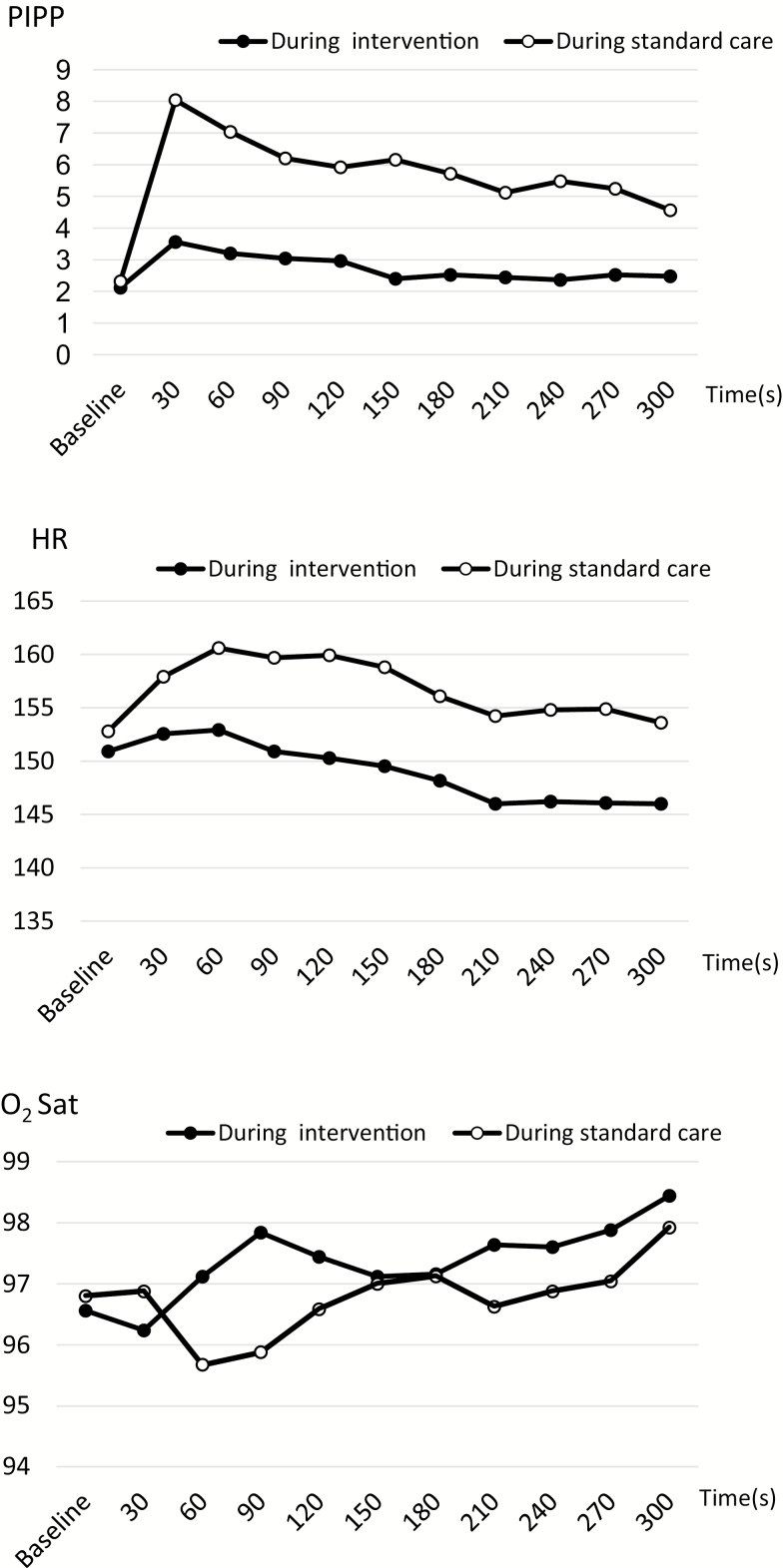
At all measurement points postheel lance, the PIPP of the preterm infants in the intervention group was significantly lower than the PIPP in the standard care group (range, P=0.0039 to P<0.0001) (Figure 2, Table 2). The odds ratios of pain (PIPP>6) for preterm infants in the intervention group versus standard care group ranged from 0.1497 to 0.0212, where all differences were statistically significant with the corresponding P-values ranging from P=0.0072 to P<0.0001. At the 120 seconds point, the HR of preterm infants was significantly lower in the intervention group than in the standard care group (P=0.0151). The HRs of 6 points were considerably lower in the intervention group than in the standard care group (range, P≤0.0879 to P≥0.049). The abnormal HR total number was significantly lower in the intervention group (2) than in the standard care group (23) (frequency ratio=0.087, P<0.0001). O2 Sats of point postheel lance were similar between the two groups except the 90 seconds point.
Figure 2.
Comparison of PIPP, HR, and O2 Sat between the intervention group and the standard care group. HR Heart rate; PIPP Premature Infant Pain Profile; O2Sat Oxygen saturation.
Table 2.
Comparison of outcomes between the intervention group and the standard care group (N=25)
| Outcome | Measurement time | During intervention | During standard care | Intervention effect | Difference in differences | Difference in differences | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P-value* | P-value† | P-value‡ | ||
| PIPP | Baseline | 2.1 | 1.7 | 2.3 | 1.4 | 0.595 | - | - |
| 30 s | 3.6 | 2.0 | 8.0 | 3.2 | <0.0001 | <0.0001 | - | |
| 60 s | 3.2 | 2.1 | 7.0 | 3.7 | 0.0001 | <0.0001 | 0.7144 | |
| 90 s | 3.0 | 2.2 | 6.2 | 3.3 | 0.0006 | 0.0008 | 0.3478 | |
| 120 s | 3.0 | 1.7 | 5.9 | 3.4 | 0.0009 | 0.001 | 0.4151 | |
| 150 s | 2.4 | 1.2 | 6.2 | 3.4 | <0.0001 | <0.0001 | 0.6636 | |
| 180 s | 2.5 | 1.5 | 5.7 | 3.5 | 0.0005 | 0.0004 | 0.9376 | |
| 210 s | 2.4 | 1.3 | 5.1 | 3.2 | 0.0007 | 0.0008 | 0.9376 | |
| 240 s | 2.4 | 1.3 | 5.5 | 3.3 | <0.0001 | 0.0003 | 0.6017 | |
| 270 s | 2.5 | 1.5 | 5.2 | 3.1 | 0.001 | 0.0013 | 0.5819 | |
| 300 s | 2.5 | 1.2 | 4.6 | 3.0 | 0.0039 | 0.0118 | 0.7579 | |
| HR | Baseline | 150.9 | 18.3 | 152.8 | 19.0 | 0.6429 | - | |
| 30 s | 152.6 | 15.8 | 157.9 | 18.7 | 0.188 | 0.5259 | ||
| 60 s | 152.9 | 16.0 | 160.6 | 22.3 | 0.0879 | 0.3162 | ||
| 90 s | 150.9 | 15.3 | 159.7 | 20.1 | 0.049 | 0.2121 | ||
| 120 s | 150.3 | 15.6 | 159.9 | 21.6 | 0.0151 | 0.1521 | ||
| 150 s | 149.5 | 13.6 | 158.8 | 23.0 | 0.0567 | 0.1888 | ||
| 180 s | 148.2 | 13.8 | 156.1 | 23.1 | 0.0804 | 0.2752 | ||
| 210 s | 146.0 | 12.8 | 154.2 | 22.7 | 0.112 | 0.2726 | ||
| 240 s | 146.2 | 12.1 | 154.8 | 21.4 | 0.0652 | 0.2226 | ||
| 270 s | 146.1 | 12.4 | 154.9 | 21.3 | 0.0641 | 0.2113 | ||
| 300 s | 146.0 | 13.0 | 153.6 | 19.6 | 0.1019 | 0.3116 | ||
| O2 Sat | Baseline | 96.6 | 2.6 | 96.8 | 2.3 | 0.5578 | ||
| 30 s | 96.2 | 5.0 | 96.9 | 2.5 | 0.625 | |||
| 60 s | 97.1 | 4.8 | 95.7 | 4.1 | 0.1145 | |||
| 90 s | 97.8 | 2.5 | 95.9 | 4.5 | 0.0293 | |||
| 120 s | 97.4 | 3.3 | 96.6 | 3.6 | 0.1642 | |||
| 150 s | 97.1 | 3.6 | 97.0 | 2.9 | 0.938 | |||
| 180 s | 97.2 | 3.5 | 97.1 | 2.9 | 0.9832 | |||
| 210 s | 97.6 | 3.4 | 96.6 | 2.7 | 0.3041 | |||
| 240 s | 97.6 | 3.8 | 96.9 | 2.6 | 0.5218 | |||
| 270 s | 97.9 | 3.8 | 97.0 | 2.8 | 0.3993 | |||
| 300 s | 98.4 | 2.1 | 97.9 | 1.8 | 0.3246 | |||
HR Heart rate; PIPP Premature Infant Pain Profile; SD Standard deviation.
*A general linear mixed model included fixed-effects for intervention, sequence, period, and with random effects for participants, using the MIXED procedure in SAS. †Difference-in-differences analysis estimated the difference in baseline-postheel lance changes in an outcome between an intervention and a standard care group, where the analysis included fixed-effects for intervention, sequence, period, and with random effects for participants, using the MIXED procedure in SAS. Difference-in-differences analysis of O2 Sat could not be performed because there were no parallel trends between the two groups. ‡The PIPP reduction rate was calculated by dividing the value of subtracting the PIPP at each point from the PIPP at 30 s by the PIPP at 30 s. A general linear mixed model for the reduction rate of PIPP included fixed-effects for intervention, sequence, period, and with random effects for participants, using the MIXED procedure in SAS. P<0.05.
Difference-in-differences estimated that all changes of PIPP between baseline and each point postheel lance were significantly higher in the intervention group than in the standard care group. However, all PIPP reduction rates were similar between the two groups. All changes in HR between baseline and each point postheel lance were similar between the two groups. The O2 Sat could not be subjected to difference-in-differences analysis because there were no parallel trends between the two groups.
As the washout periods were not done at regular intervals due to participants’ treatment, the washout periods included outliers in both groups. Data were analyzed according to intention to treat, and all results including washout were the same as all results that did not include washout. No adverse events from the intervention were detected.
DISCUSSION
The addition of the recorded Brahms lullaby to NNS with facilitated tucking and holding, resulted in decreased pain levels during heel lance in preterm infants. In the intervention group, all mean PIPP scores postheel lance were less than six points, which suggests minimal or no pain. The number of preterm infants who felt slight pain in the intervention group was about 15% lower than that of the preterm infants in the standard care group.
The mean PIPP score during intervention at 30 seconds postheel lance in the current study (3.6, SD 2.0) was lower than the mean PIPP score over 1 minute after the end of the heel lance in the previous music study (5.1, SD 1.9) (15), and also indicates that about 80% of preterm infants had no pain. The mean PIPP scores in the current study were lower than those of previous interventions, such as NNS, swaddling, Kangaroo mother care, and facilitated tucking (8–13,15). Although the PIPP scores of the preterm infants receiving sucrose with or without NNS were lower than 6 (9,17), sucrose usage has some problems such as a risk of poorer neurobehavioral development due to repeated oral sucrose usage (40) and oxidative stress (9,41). The alleviation effect of the current study shows a possible combination effect from using many methods (Brahms lullaby, pacifier, facilitated tucking and holding). Also, the similarity in the PIPP reduction rate between the two groups indicated that a lower value of PIPP postheel lance may induce shorter pain duration (PIPP<6).
The HRs were considerably lower in the intervention group than in the standard care group. The incidence of abnormal HR was less than 10% of that with standard care. The current study demonstrated stronger pain relief and the maintenance of homeostasis for heel lance in preterm infants.
These results are supported by the findings of many non-pharmacological interventions on heel lance in preterm infants: kangaroo care (13), facilitated tucking (8,42), swaddling (12), music (22), and NNS and lullaby (23).
The results showing no adverse events detected from the intervention in the current study indicate the safety of this intervention.
One limitation is that the sample size of this study was small. The sample size in previous studies using music was also small: 28 preterm infants (22), 42 preterm infants (15), and 60 preterm infants (23). Second, the washout periods were nonuniform, ranging from 8 hours to 15 days. It is necessary to consider setting the washout period in postmenstrual ages as uniformly as possible because postmenstrual age is the dominant predictor regarding maturation of NNS patterns (43). Finally, this intervention was carried out for a limited set of participants (32 to 35 weeks PCA) and procedures (heel lance). However, preterm infants and term infants with disease in the NICU frequently suffer a variety of procedural pains from the pain of routine care to severe pain associated with an examination for retinopathy at prematurity (3). Therefore, further research is necessary to determine whether implementing this intervention during a variety of procedures significantly reduces the pain of preterm infants.
CONCLUSION
A new pain management method, the addition of a recorded Brahms lullaby to non-nutritive sucking, facilitated tucking and holding, demonstrated stronger analgesia and maintenance of homeostasis on heel lance in preterm infants.
Acknowledgements
The investigators wish to express their deepest appreciation to all of the participants and their parents in this study. Also, the investigators are sincerely grateful to Yuriko Ohnishi who cooperated in the data analysis, and all the nurses and doctors in the NICU and newborn nursery in Takamatsu Red Cross Hospital. We express our sincere appreciation to Prof. Masayuki Kakehashi whose comments and suggestions for the statistical analyses were of great value. The investigators presented a part of this study at the 25th Congress of the Japan Academy of Neonatal Nursing.
Funding: Nursing grant in aid of Japan Red Cross Society (2014).
JSPS KAKENHI Grant Number JP17K198180A.
Conflict of Interest
None declared.
Ethical Approval
This study received approval from the ethical boards of Takamatsu Red Cross Hospital (No. 14-08).
This trial has been registered at http://www.umin.ac.jp/ (UMIN 000024876).
References
- 1. Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet 2012;379(9814):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson-Costello D. Is there evidence that long-term outcomes have improved with intensive care?Seminars in Fetal & Neonatal Medicine 2007;12(5):e344–54. [DOI] [PubMed] [Google Scholar]
- 3. Carbajal R, Rousset A, Danan C, et al. . Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300(1):60–70. [DOI] [PubMed] [Google Scholar]
- 4. Valeri BO, Holsti L, Linhares MB. Neonatal pain and developmental outcomes in children born preterm: A systematic review. Clin J Pain 2015;31(4):355–62. [DOI] [PubMed] [Google Scholar]
- 5. Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2016;7:CD001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Academy of Pediatrics, Committee on fetus and newborn and section on anesthesiology and pain medicine. Prevention and management of procedural pain in the neonate: An update. Pediatrics 2016;137(2):1–13. [DOI] [PubMed] [Google Scholar]
- 7. Cignacco E, Hamers JP, Stoffel L, et al. . The efficacy of non-pharmacological interventions in the management of procedural pain in preterm and term neonates. A systematic literature review. Eur J Pain 2007;11(2):139–52. [DOI] [PubMed] [Google Scholar]
- 8. Liaw JJ, Yang L, Katherine Wang KW, Chen CM, Chang YC, Yin T. Non-nutritive sucking and facilitated tucking relieve preterm infant pain during heel-stick procedures: A prospective, randomised controlled crossover trial. Int J Nurs Stud 2012;49(3):300–9. [DOI] [PubMed] [Google Scholar]
- 9. Asmerom Y, Slater L, Boskovic DS, et al. . Oral sucrose for heel lance increases adenosine triphosphate use and oxidative stress in preterm neonates. J Pediatr 2013;163(1):29–35.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens B, Johnston C, Franck L, Petryshen P, Jack A, Foster G. The efficacy of developmentally sensitive interventions and sucrose for relieving procedural pain in very low birth weight neonates. Nurs Res 1999;48(1):35–43. [DOI] [PubMed] [Google Scholar]
- 11. Sundaram B, Shrivastava S, Pandian JS, Singh VP. Facilitated tucking on pain in pre-term newborns during neonatal intensive care: A single blinded randomized controlled cross-over pilot trial. J Pediatr Rehabil Med 2013;6(1):19–27. [DOI] [PubMed] [Google Scholar]
- 12. Ho LP, Ho SS, Leung DY, So WK, Chan CW. A feasibility and efficacy randomised controlled trial of swaddling for controlling procedural pain in preterm infants. J Clin Nurs 2016;25(3-4):472–82. [DOI] [PubMed] [Google Scholar]
- 13. Johnston CC, Filion F, Campbell-Yeo M, et al. . Kangaroo mother care diminishes pain from heel lance in very preterm neonates: A crossover trial. BMC Pediatr 2008;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: Development and initial validation. Clin J Pain 1996;12(1):13–22. [DOI] [PubMed] [Google Scholar]
- 15. Cavaiuolo C, Casani A, Di Manso G, Orfeo L. Effect of Mozart music on heel prick pain in preterm infants: A pilot randomized controlled trial. J Pediatr Neonat Individual Med 2015;4(1):e040109. [Google Scholar]
- 16. Gibbins S, Stevens B, Hodnett E, Pinelli J, Ohlsson A, Darlington G. Efficacy and safety of sucrose for procedural pain relief in preterm and term neonates. Nurs Res 2002;51(6):375–82. [DOI] [PubMed] [Google Scholar]
- 17. Kumari S, Datta V, Rehan H. Comparison of the efficacy of oral 25% glucose with oral 24% sucrose for pain relief during heel lance in preterm neonates: A double blind randomized controlled trial. J Trop Pediatr 2017;63(1):30–5. [DOI] [PubMed] [Google Scholar]
- 18. Ballantyne M, Stevens B, McAllister M, Dionne K, Jack A. Validation of the premature infant pain profile in the clinical setting. Clin J Pain 1999;15(4):297–303. [DOI] [PubMed] [Google Scholar]
- 19. Standley JM. A meta-analysis of the efficacy of music therapy for premature infants. J Pediatr Nurs 2002;17(2):107–13. [DOI] [PubMed] [Google Scholar]
- 20. Yildiz A, Arikan D. The effects of giving pacifiers to premature infants and making them listen to lullabies on their transition period for total oral feeding and sucking success. J Clin Nurs 2012;21(5-6):644–56. [DOI] [PubMed] [Google Scholar]
- 21. Hodges AL, Wilson LL. Effects of music therapy on preterm infants in the neonatal intensive care unit. Altern Ther Health Med 2010;16(5):72–3. [PubMed] [Google Scholar]
- 22. Butt ML, Kisilevsky BS. Music modulates behaviour of premature infants following heel lance. Can J Nurs Res 2000;31(4):17–39. [PubMed] [Google Scholar]
- 23. Whipple J. The effect of music-reinforced nonnutritive sucking on state of preterm, low birthweight infants experiencing heelstick. J Music Ther 2008;45(3):227–72. [DOI] [PubMed] [Google Scholar]
- 24. Senn S. Cross-over Trials in Clinical Research, 2nd edn. Chichester: John Wiley & Sons, Ltd, 2002. [Google Scholar]
- 25. Hilderley AJ, Fehlings D, Lee GW, Wright FV. Comparison of a robotic-assisted gait training program with a program of functional gait training for children with cerebral palsy: Design and methods of a two group randomized controlled cross-over trial. Springerplus 2016;5(1):1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldson E. Nonnutritive sucking in the sick infant. J Perinatol 1987;7(1):30–4. [PubMed] [Google Scholar]
- 27. Stevens B, Johnston C, Taddio A, Gibbins S, Yamada J. The premature infant pain profile: Evaluation 13 years after development. Clin J Pain 2010;26(9):813–30. [DOI] [PubMed] [Google Scholar]
- 28. Ozawa M, Kanda K, Hirata M, Kusakawa I, Suzuki C. Utiliy of a Japanese version of the Premature Infant Pain Profile. J Jap Acad Neonat Nurs 2010;16(1): 28–33. (in Japanese). [Google Scholar]
- 29. Bradshaw WT, Tanaka DT. Physiologic monitoring. In: Merenstein GB, Gardner SL, eds. Handbook of Neonatal Intensive Care. St. Louis: Mosby, 2011:134–52. [Google Scholar]
- 30. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994;6(4):284–90. [Google Scholar]
- 31. Hallgren KA. Computing inter-rater reliability for observational data: An overview and tutorial. Tutor Quant Methods Psychol 2012;8(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kisilevsky BS, Hains SM, Brown CA, et al. . Fetal sensitivity to properties of maternal speech and language. Infant Behav Dev 2009;32(1):59–71. [DOI] [PubMed] [Google Scholar]
- 33. Benavides-Varela S, Hochmann JR, Macagno F, Nespor M, Mehler J. Newborn’s brain activity signals the origin of word memories. Proc Natl Acad Sci USA 2012;109(44):17908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Standley J. Music therapy research in the nicu: An updated meta-analysis. Neonatal Netw 2012;31(5):311–6. [DOI] [PubMed] [Google Scholar]
- 35. Zhou L. Application of linear mixed-effects models to crossover designs. 2012. Electronic Theses and Dissertations. Paper 1646. https://doi.org/10.18297/etd/1646 (cited July 13, 2018). http://ir.library.louisville.edu/cgi/viewcontent.cgi?article=2645&context=etd
- 36. Mueller-Cohrs J, GmbH A. Analysis of incomplete two-period crossover trials with SAS PROC MIXED (cited July 13, 2018). http://www.phusewiki.org/docs/2006/ST07.pdf
- 37. Putt M, Chinchilli VM. A mixed effects model for the analysis of repeated measures cross-over studies. Stat Med 1999;18(22):3037–58. [DOI] [PubMed] [Google Scholar]
- 38. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: The difference-in-differences approach. JAMA 2014;312(22):2401–2. [DOI] [PubMed] [Google Scholar]
- 39. Warton EM, Parker MM, Karter AJ. How D-I-D you do that? Basic Difference-in-Differences Models in SAS® (cited July 13, 2018). http://www.lexjansen.com/wuss/2016/49_Final_Paper_PDF.pdf
- 40. Johnston CC, Filion F, Snider L, et al. . Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks’ postconceptional age. Pediatrics 2002;110(3):523–8. [DOI] [PubMed] [Google Scholar]
- 41. Angeles DM, Asmerom Y, Boskovic DS, et al. . Oral sucrose for heel lance enhances adenosine triphosphate use in preterm neonates with respiratory distress. SAGE Open Med 2015;3:2050312115611431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leslie A, Marlow N. Non-pharmacological pain relief. Semin Fetal Neonatal Med 2006;11(4):246–50. [DOI] [PubMed] [Google Scholar]
- 43. Hafström M, Kjellmer I. Non-nutritive sucking in the healthy pre-term infant. Early Hum Dev 2000;60(1):13–24. [DOI] [PubMed] [Google Scholar]