Abstract
Recently, electrospun polymeric nanofibers have proven to be an interesting strategy for drug delivery systems application. The high surface-to-volume ratio of the fibers can improve some processes, such as cell binding and proliferation, drug loading, and mass transfer processes. One of the most im-portant and studied areas of electrospinning is in the drug delivery field, for the controlled release of active substances ranging from antibiotics and anticancer agents to macromolecules such as proteins and DNA. The advantage of this method is that a wide variety of low solubility drugs can be loaded into the fibers to improve their bioavailability or to attain controlled release. This review presents an overview of the re-ported drugs loaded into polymeric nanofibers, to be used as drug delivery systems. For instance, it pre-sents the reports on drugs with different bioactivities such as anti-inflammatory, anti-microbial, anticancer, cardiovascular, anti-histamine, gastrointestinal, palliative and contraceptive drugs, etc. It also analyzes the electrospinning techniques used in each system, as well as the polymers used as matrices for the prepara-tion of the nanofibers; unfolding the advantages of electrospun polymeric nanofibers over other drug delivery systems. This review intends to enlist and summarize the reported literature concerning this topic. In addition, it proposes future research in the field
Keywords: Biopolymers, electrospinning, nanofibers, drug release, fast release, controlled release
1. Introduction
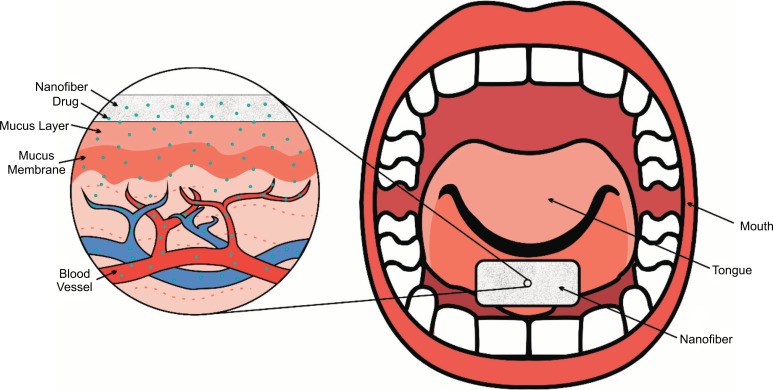
Rapid dissolution or controlled release have become important for developing novel strategies for drug delivery applications; this is because of their advantages: increasing the drug solubility and bioavailability, or controlling the rate and site of delivery. Most of the known drug delivery systems are administered by enteral routes, in the form of tablets, capsules, granules, etc., while some are administered by parenteral routes, such as intravenous, intra-arterial, intramuscular, or subcutaneous. The routs and forms of administration have some disadvantages, such as first-pass metabolism, discomfort or pain. These problems can be resolved by administrating the drugs directly in the buccal cavity. For this purpose, it is convenient to incorporate the active substances into nanofibers. Nanofibers prepared by electrospinning are designed to be wetted instantly by saliva, dissolved or disintegrated in the patient's mouth without the need to drink or chew, releasing drugs almost instantaneously into the buccal mucosa for immediate absorption (Fig. 1). This can be achieved using water-soluble polymers and a large surface exposed to the dissolution medium. In the case of controlled release, the drug delivery system is required to dissolve or disintegrate in a set period of time. Both oral or transcutaneous controlled-release allow the administration of pharmaceutical drugs once or twice a day, improving the patient compliance and reducing the toxic plasma peak concentrations that can be produced by multiple administration of immediate release formulations [1, 2].
Fig. (1).
Nanofibers scaffolds instantly disintegrated by saliva, in the patient's mouth without the need to drink or chew, releasing drugs almost instantaneously into the buccal mucosa for fast absorption. Scheme based on [1, 2].
An alternative method for these types of release is loading active pharmaceutical ingredients by the electrospinning technique, since this is a method that produces ultra-fine fibers (from micro- to nanometers of diameter), with controlled surface morphology. These fibers are fabricated by generating a strong electrical field on the desired polymer solution or, if the polymer lacks a good solvent, by melting the polymer and exposing it to the electrical field [1, 3].
When the diameters of the polymer fiber materials are reduced to micrometers or nanometers, interesting features may appear, such as an increased surface area to volume ratio, flexibility in surface functionalities, and superior mechanical performance (e.g., stiffness and resistance to traction); compared to any other known form of the material. These outstanding properties make polymer nanofibers the optimum candidates for many important applications in the biomedical areas [1, 2].
Because of the nanofibers characteristics mentioned above, the purpose of this review is to highlight a great variety of drugs with different bioactivities, such as: anti-inflammatory, anti-microbial, anticancer, cardiovascular, miscellaneous, anti-histamine, gastrointestinal, palliative and contraceptive drugs: as well as the polymers used for this application such as poly(vinyl alcohol) (PVAL), poly(ethylene oxide) (PEO), poly(ε-caprolactone) (PCL), chitosan (CHS), poly(acrylic acid) (PAA), ethyl cellulose (EC), cellulose acetate (CA), hydroxypropylmethyl cellulose (HPMC), poly(L- lactic acid) (PLLA), poly(lactic-co-glycolic acid) (PLGA), poly(acrylonitrile) (PAN), cellulose acetate phthalate (CAP), and poly(urethane) (PU), among others, unfolding the advantages of electrospun polymeric nanofibers over other drug delivery systems. Finally, this review intends to summarize the reported literature regarding this subject and guide scientist in this field, including a critical review of publications on drugs currently on the market, administered in conventional formulations, with the aim of improving their bioavailability [4].
2. Electrospinning and application on drug delivery systems
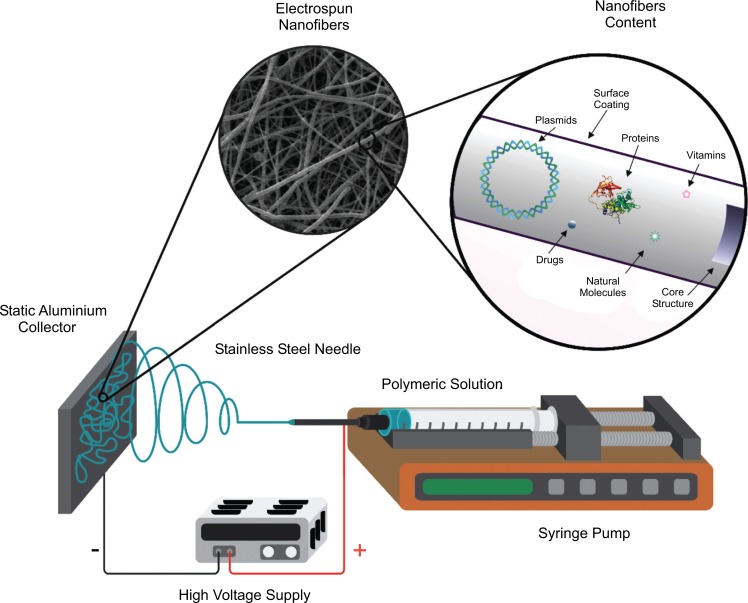
The electrospinning term is derived from “Electrostatic Yarn” which is a dry spinning process that utilizes electrostatic forces to form small fibers (10-100 μm to 10-100 nm) from a polymer solution (or in its case, a melted polymer). In Fig. (2), it is illustrated that a basic configuration for the electrospinning device, as well as what type of chemical and biological components may be inside of the nanofibers. This technique consists of three main components: a high voltage source, a syringe pump and a conductive collector [3, 4].
Fig. (2).
Basic diagram of the electrospinning device. Scheme based on [3].
The polymer solution (dissolved or molten) is filled in a syringe and placed over the syringe pump. When a droplet of the polymer solution appears on the tip of the needle (this should be made of a conductive metal), and a high voltage is applied (usually from 5 kV to 50 kV) to the same droplet, this will become highly electrified and the induced charges will be evenly distributed over its surface. Then the droplet will deform into a conical object (also known as the “Taylor cone”). This cone appears when the space between the electrical conducting liquid and insulator exceeds a critical voltage, making the liquid drop unstable and transforming from a rounded shape to a conical shape [5, 6]. When the voltage exceeds a threshold value, the electric force exceeds the surface tension of the drop and then one or more jets are expelled from the tip of the drop depending on the electric field strength. As the jet travels to a metal collector (usually a conductive one, like aluminum), the solvent evaporates and a nonwoven scaffold is formed on the collector surface [7-9].
Various drug delivery systems have been developed or are currently being investigated: Nanoscale formulations (like liposomes), polymer micelles, some complexes and nanofibers. These have attracted special attention during these last decades because they have the potential to improve the therapeutic effects and reduce the toxicity of conventional dosage forms. Some of the attractive features for an ideal drug delivery system would be high loading capacity, high encapsulation efficiency, simultaneous delivery of various therapies, ease of operation and cost-effectiveness, either for immediate or extended release, as well as wound dressing and local chemotherapy. In comparison to other drug delivery systems, the electrospinning technique is very versatile in the selection of its materials and Active Pharmaceutical Ingredients (APIs) for their release. In contrast with other systems, in the electrospinning strategy, the researcher can have manipulated the rate of degradation of the fibers, hence the delivering rate of the drug. On the other hand, the administration of the drug to a patient is easier than other methods, since it is easy to place the electrospun mats in the tongue of a patient. For all the above, the electrospinning is an attractive technique for the area of drug delivery systems [1].
3. Components of nanofibers (polymers + drugs)
Nanofibers can be fabricated with a great variety of polymers. In drug delivery applications, a polymer solution (polymer + specific solvent) is prepared, then, a defined proportion of the drug is mixed into the polymeric solution, creating a homogeneous solution or a suspension (depending on drug´s solubility into polymeric solution). This mixture is electrospun to produce nanofiber composed with a solid complex of polymer-drug. The solvent is evaporated in the process. Different types of nanofibers can be synthesized, depending of the electrospinning strategy used. In the other hand, nanofibers are composed by a polymeric backbone or base, which represent most of the fiber composition and a bioactive molecule (drugs, proteins, hormones, etc.), or other polymer but in smaller proportion than the base polymer [6].
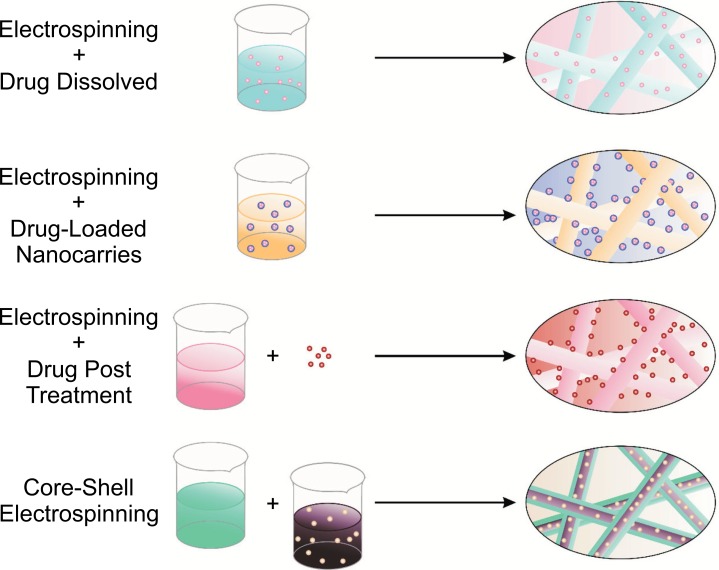
Fig. (3), explains how nanofibers are obtained from different strategies of the electrospinning method: with the (a) electrospinning + drug dissolved; it can obtain embedded molecules into nanofibers, (b) Electrospinning + drug loaded nanocarriers; it can prepare nanocarriers that are attached outside the fibers, (c) Electrospinning + drug post treatment; the drug or a biomolecule are attached outside the fibers. Finally, (d) Core-shell electrospinning; molecules are inside a bulk into nanofibers.
Fig. (3).
Graphic representation of several strategies for the preparation of drug-loaded nanofibers. (a) Electrospinning + drug dissolved (b) Electrospinning + drug loaded nanocarriers. (c) Electrospinning + drug post treatment. (d) Core-shell electrospinning. Scheme based on [6].
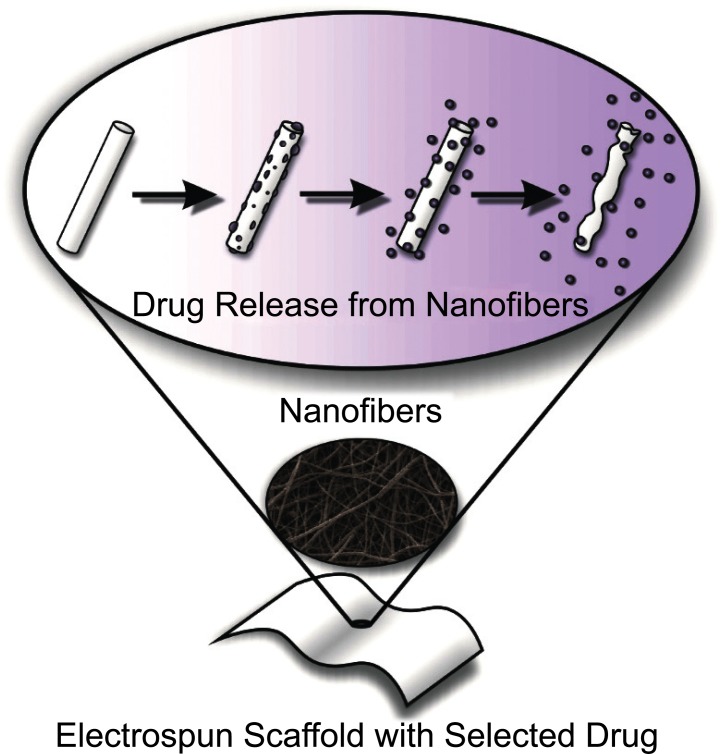
When the resulting nanofiber mats are placed in aqueous media (e.g., the mouth), the system delivers continuously the drug meanwhile the nanofibers are degradated (Fig. 4) [1].
Fig. (4).
Release of drug components from an electrospun nanofiber. Scheme based on [1, 4].
4. Polymers
Various carrier materials, including natural and synthetic polymers and/or a mixture of both (biodegradable and non-degradable) have been an object of experimentation and research with the electrospinning technique, as well as with many active pharmaceutical components. As for the drug delivery behavior, this is determined by the diffusion of the drug and/or degradation of the carrier. In a few words, the fibers formed by this technique can be designed to control the dispersion of a drug and thus improving the release kinetics of APIs [10].
The use of polymers -especially biodegradable ones- has drawn a special attention in electrospinning investigations because polymers have different advantages: For example, no second surgery is required to remove an implanted support since the polymer is absorbed and degraded by the body. In rapid dissolution drug delivery systems, the polymers are degraded, while the loaded drugs are absorbed. Polymers such as poly(ε-caprolactone) (PCL), poly(vinyl alcohol) (PVAL), poly(vinylpyrrolidone) (PVP) and chitosan (CHS) have been extensively investigated to make fibers with desired properties for tissue engineering and drug delivery applications [9, 11].
One of the great benefits in the area of drug delivery it is the ability to transport drugs and release them as the polymer degrades. As the polymer slowly breaks down into smaller, non-toxic fragments, releases the drug wherever is necessary. It is also noted that the body can degrade polymers that are being placed in it, by the metabolic pathways that take effect for the natural elimination of these compounds, into much simpler products. In tissue regeneration, these polymers are of interest because they reduce the risk of the body rejecting the implant or response of the immune system. Polymers that are biocompatible have been extensively investigated for the manufacture of scaffolds with properties desired for tissue regeneration [9], or delivery of APIs (Table 1) [9, 11, 12].
Table 1.
Biocompatible polymers used as drug delivery carriers.
| Polymer | Chemical Structure | Loaded API | Refs. |
|---|---|---|---|
| PVP |
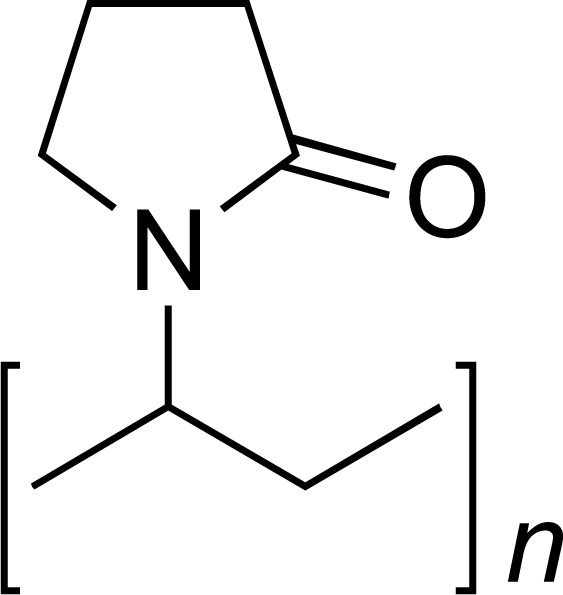
|
IBU, NP, INDO, MEL, KETO, APAP, FOG, LOR, SPIRO, GRIS, AMOX, SDS | [12-22] |
| PVAL |
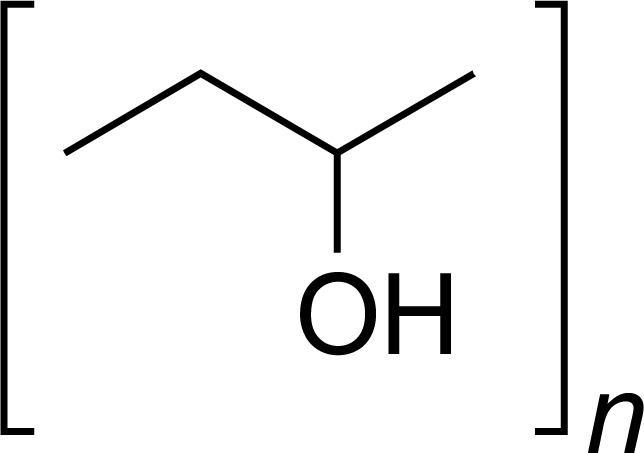
|
SUM, AC, CA, RFN, DOC, CIPRO, DONEPEZIL | [10, 22-24] |
| PEO |
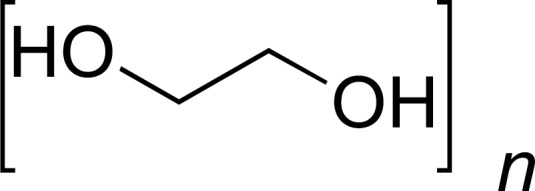
|
GML, DOX, PTX | [25-27] |
| PCL |
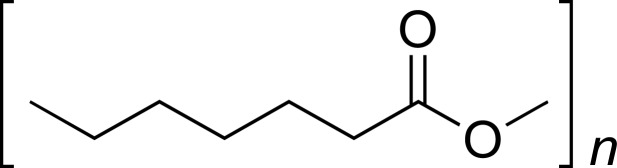
|
IBU, SUM, NP, CVD, TCN, AMB |
[11, 28-30] |
| CHS |
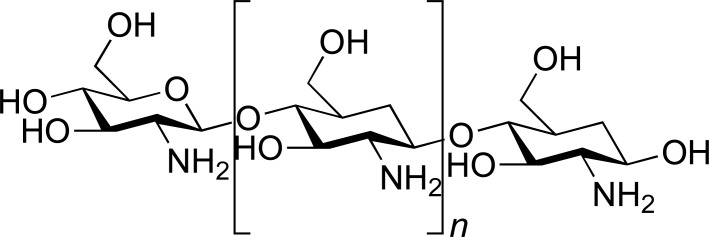
|
SUM | [28] |
| PAA |
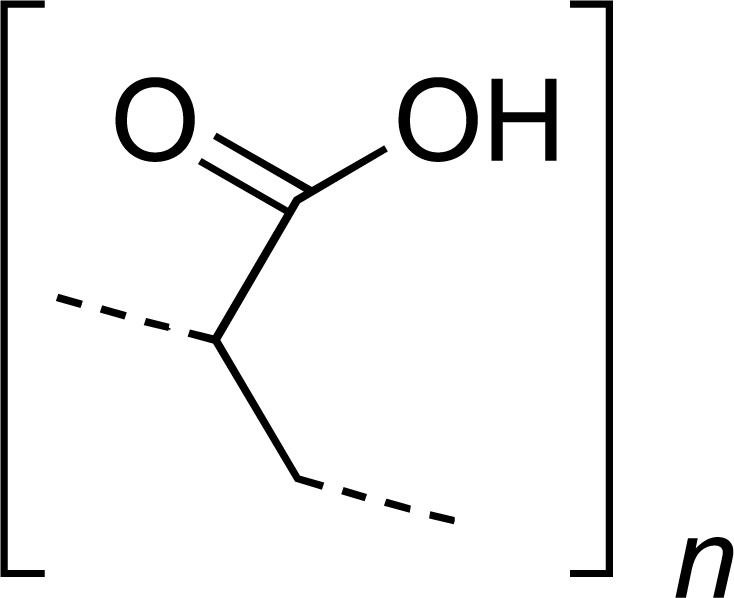
|
SUM | [28] |
| EC |
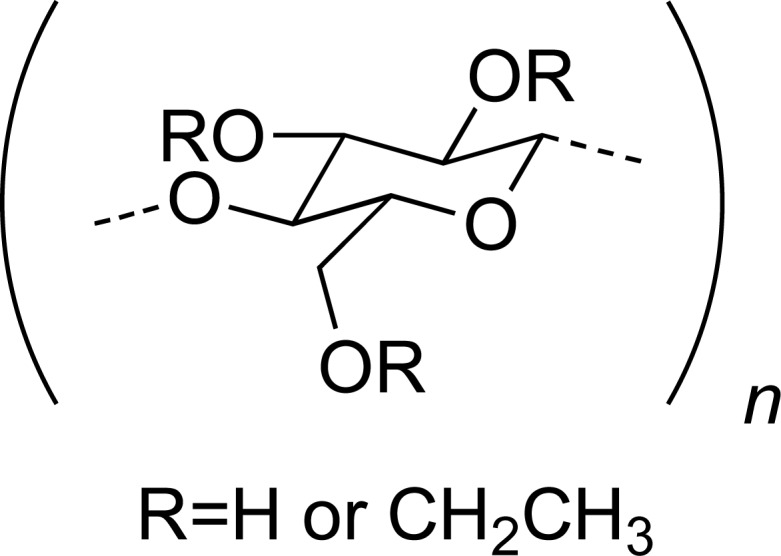
|
KETO | [16] |
| CA |
|
KETO | [16] |
| Eudragit S100 |
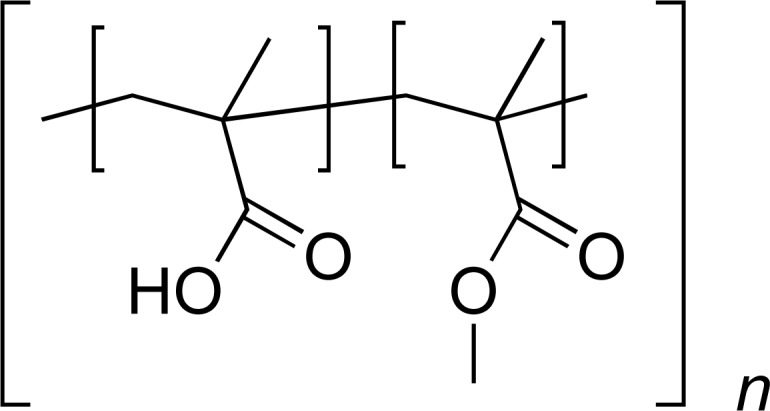
|
AC | [31] |
| HPMC |
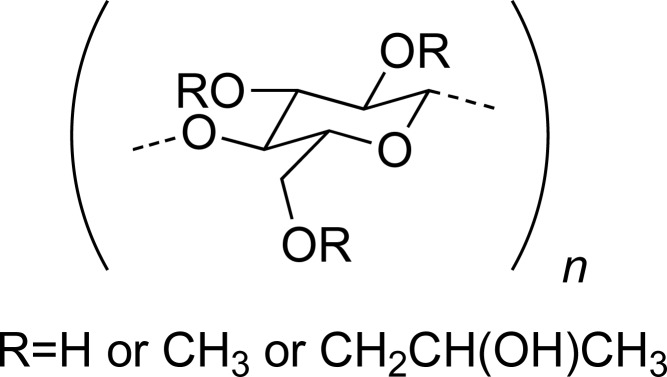
|
DPH | [32] |
| Polymer | Chemical Structure | Loaded API | Refs. |
| PLLA |
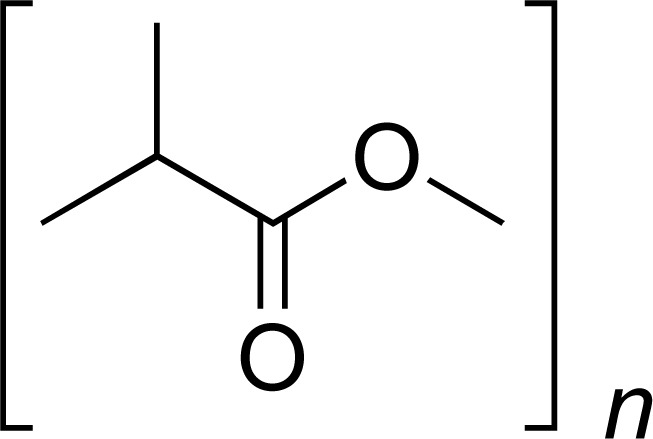
|
TCH DOX |
[26, 33] |
| PLGA |
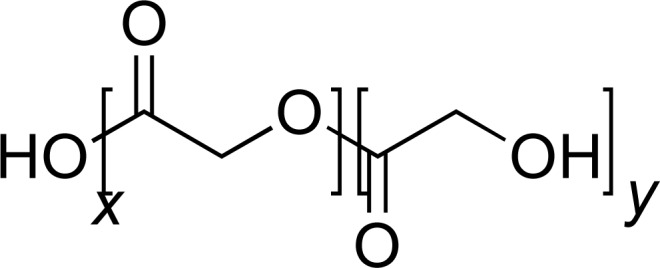
|
MET-HCl PTX |
[30, 34] |
| PAN |
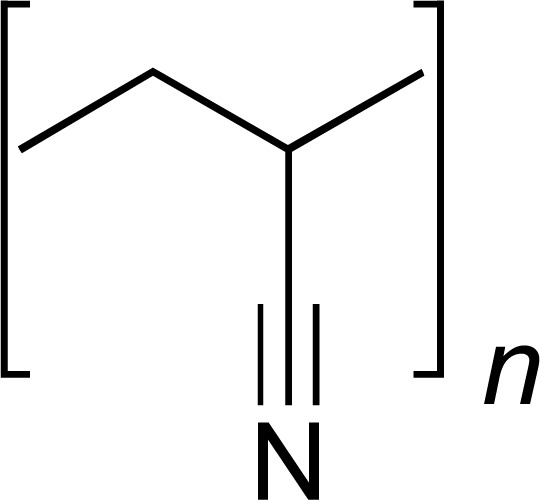
|
ACV | [35] |
| Kollidon VA64 |
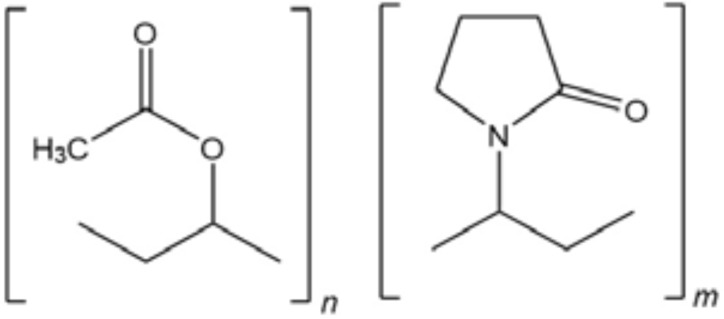
|
ITR | [36] |
| CAP |
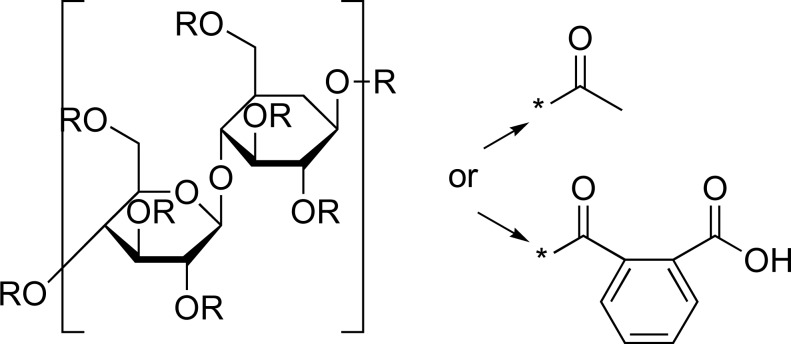
|
TDF | [37] |
| PU |
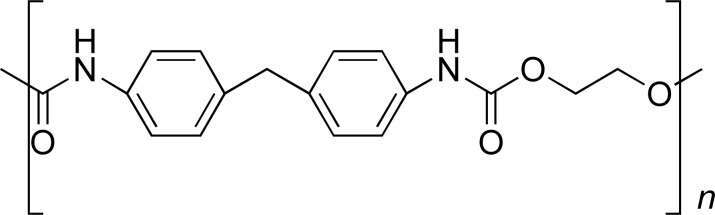
|
ITR | [38] |
Polymers: PVP: poly(vinyl pyrrolidone); PVAL: poly(vinyl alcohol); PEO: poly(ethylene oxide); PCL: poly(ε-caprolactone); CHS: Chitosan; PAA: poly(acrylic acid); EC: Ethyl cellulose; CA: Cellulose acetate; HPMC: Hydroxypropylmethyl cellulose; PLLA: poly(L- lactic acid); PLGA: poly(lactic-co-glycolic acid); PAN: poly(acrylonitrile); CAP: Cellulose acetate phthalate; PU: Poly(urethane). Active pharmaceutical ingredient: IBU: Ibuprofen; NP: Naproxen; INDO: Indomethacin; MEL: Meloxicam; KETO: Ketoprofen; APA: Acetaminophen; LOR: Loratadine; SPIRO: Spirolactone; GRIS: Griseofulvin; AMOX: Amocycilin; SDS: Sodium dodecylsulfate; SUM: Sumatriptan; AC: Aceclofenac; CAF: Caffeine; RFN: Riboflavin; DOC: Docetaxel; CIPRO: Ciprofloxacin; DONEPEZIL: Donepezil hydrochloride; GML: Glycerol Monolaurate; DOX: Doxorubicin; PTX: Paclitaxel; CVD: Carvedilol; TCN: Tetracycline; TCH: Tetracycline hydrochloride; AMB: Amphotericin B; DPH: Diphenhydramine; MET-HCl: Metoclopramide hydrochloride; ACV: Acyclovir; ITR: Itraconazole; TDF: Tenofovir disoproxil fumarate.
5. Drugs loaded into electrospun nanofibers
The aforementioned properties of the electrospun fibers, such as a large surface area, a possibility of loading large amounts of drug, simultaneous administration of various therapies, ease of operation and cost-effectiveness has led the scientific community to expand, in the past few years, in the area of improving the current drug delivery systems. The last-mentioned properties about the fibers make them good prospects for the administration of a poorly water-soluble or a low-bioavailability active pharmaceutical components. Among the most investigated areas, drug delivery (either rapid dissolution or controlled release), tissue regeneration and local cancer treatments are of the most interest. The following are some of the drugs used in the electrospinning area.
5.1. Anti-inflammatory Drugs
Anti-inflammatory drugs are substances that reduce inflammation and swelling symptoms and may possess analgesic and antipyretic effects [39]. It is noted that these drugs have been previously used in electrospinning to formulate new delivery systems because many pharmaceutical components of this kind are poorly water-soluble and the desired effect of an almost immediate relief is of a great interest for many. It is shown below that with the electrospinning technique, the electrospun nanofibers that have been studied are usually fabricated with an anti-inflammatory and another pharmaceutical component (mostly with analgesics), to have an improved treatment [4, 13-17].
A drug widely studied is IBU, which is a therapeutic agent in the class of nonsteroidal used to treat pain, fever and inflammation. This includes painful menstrual periods, migraines and rheumatoid arthritis [40]. IBU has been studied using the electrospinning method because it is poorly water soluble, but with a high bioavailability, been a candidate for an immediate release system in various articles [11, 12, 41].
Yu et al. (2009), reported a PVP K30 electrospun mesh loaded with IBU. The polymer´s solutions used were from solid dispersions or as some amorphous physical form. In the first paper, Diffraction Scattering Calorimetry (DSC) and X-ray Diffraction (XRD) results, and morphological observations showed that IBU was distributed as nanosolid dispersions in the fibers. Fourier Transform Infrared Spectroscopy (FTIR) results showed that the major interactions between PVP and IBU were mediated by hydrogen bonds. It was shown that the drug delivery membranes, with different drug concentrations, had almost the same wetting and disintegration times (about 15 and 8 seconds, respectively), but these had differences that were significant in the dissolution rate, this because of the drug presents different physical states. IBU was released at 84.9% and 58.7% respectively, in the first 20 seconds, for the membranes with drug to PVP ratios of 1:4 and 1:2, respectively [12].
In a second paper by the same authors, it was shown that the drug had good compatibility with the polymer, and was well distributed in an amorphous physical state. This was confirmed with the results from Scanning Electron Microscopy (SEM), DSC and FTIR. In vitro dissolution tests showed that the electrospun fiber mats were capable of dissolving in 10 s. The authors concluded that the rapid dissolution of drug-loaded fibers may lead to applications that improve the dissolution rates of drugs that are poorly soluble in water [41].
The objective of a similar study was to explore electrospun nanofibers of PCL loaded with IBU, as a model for the oromucosal administration of drugs that were poorly soluble in water. It was seen that incorporating IBU in the PCL reduces its crystallinity. DSC analysis demonstrated that the components that were incorporated in the nanofibers were partially dispersed in the polymer matrix, and partly in the form of dispersed nanocrystals. The incorporation of IBU into PCL nanofibers significantly improved their dissolution
rates. PCL nanofibers released nearly 100% of the incorporated IBU in 4 h, demonstrating the influence of drug properties, such as molecular weight and solubility on the release from the polymer matrix [11].
Vrbata et al. (2013), incorporated NP in fibers. This API is a non-steroidal anti-inflammatory drug that relieves pain, fever, swelling and stiffness. It is indicated for juvenile and rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, pain, primary dysmenorrhea, tendonitis, bursitis and acute gout [28, 40].
NP and its salt were incorporated into membranes through the electrospinning process. As mentioned above, anti-inflammatory drugs have been studied in combination with other drugs for a more effective treatment; in this case, NP was combined with SUM succinate. They were studied alone and in combination. The release of NP and SUM with three hydrophilic polymers was tested: CHS, PVAL and PAA; and one hydrophobic: PCL. The later showed a very rapid release of the two drugs used. All polymers used in the work had a release of more than 90% of the APIs, and the fiber were dissolved in the acceptor phase within 10 min. SEM images showed that the fibers had the homogeneity, flexibility, mechanical resistance and drug loading capacity (up to a 40% of the membrane mass) that the authors were aiming for. These investigations are very advantageous for the formulation of sublingual drug delivery systems, as well as controlled release systems [28].
Canbolat et al. (2014), selected PCL as the polymer matrix to create a drug delivery system for NP. In this case, the drug was complexed with beta-cyclodextrin (BCD) to form an inclusion complex and then electrospun. The diameter of the fibers was around 300 nm, according to SEM. The complexed drug in the fibers showed a better release than from fibers with uncomplexed drug; this because the complex improved the solubility of the drug [29].
Wu et al. (2014), reported a successful coaxial electrospinning to fabricate NP-loaded PVP nanofibers with only one solvent: ethanol; as part of the PVP solution, and as a solvent for the shell. Field Emission Scanning Electron Microscopy (FESEM) showed that higher quality nanofibers could be obtained by this method, with a diameter of 270 ± 60 nm, also with linear morphologies, with no beads or spindles, and a smooth surface. The authors analyzed XRD patterns and verified that the nanofibers were amorphous nanocomposites with the drug distributed homogeneously in the polymer. The in vitro dissolution tests showed that the medicated nanofibers could release the contained drug rapidly, at once, while in contact with the dissolution medium, which is considerably faster than for the commercially available, dispersible tablets [13].
López et al. (2014), studied another anti-inflammatory drug in fiber-loading, INDO, which is a non-steroidal anti-inflammatory drug used as an antipiretic, against pain and stiffness. It is estimated that INDO is about 20 times more potent than acetylsalicylic acid (AA). The authors of the article state that over a long term of administration, there is a chance that a high rate of intolerance limits drug use. This drug is not usually administered as an analgesic or antipyretic of first choice, unless the fever has been resistant to other treatments. A series of experiments were performed for the optimization of a release system, involving two sets of electrospun PVP fibers loaded with INDO produced with a mixture of solvents (acetone/dimethylacetamide). The authors stated that was possible to obtain loadings of the API up to a 33% w/w. Their SEM analysis showed that the fibers were smooth and uniform and, it was also found by XRD and DSC that the drug exists in amorphous physical state in the fibers. Interesting enough, the authors found that the amorphous form of the drug was stable after storing the fibers for 8 months in a desiccator with relative humidity <25%. This means that the fibers could be stored for long periods of time and still be stable if administered after months of being fabricated. And finally the functional performance of the fibers was also studied in the article; the fibers released their components very rapidly, offering accelerated dissolution compared to the drug formulated in conventional ways [14].
MEL is a drug that has also been studied as an immediate release by the electrospinning technique. This drug is an oxycodone derivative, which are enolic acids that inhibit cyclooxygenases 1 and 2 (COX-1 and COX-2) with anti-inflammatory, analgesic and antipyretic activity; also, show moderate selectivity against COX-2. It was approved as an NSAID selective for COX-2 in some countries [40]. This drug is commercially produced in suspensions and tablets. However, these forms of dosage have received complains and have poor acceptance in the community because of their bitter taste and difficulty in swallowing, especially for children. In addition, MEL has a poor solubility in oral administration, so its absorption is incomplete. So the main point of the article was to improve these limitations and thereby prepare formulations for rapid release, even for the masking of its bad taste. So, electrospun PVP fibers were fabricated, loaded with MEL. The incorporation of BCD and sweeteners were carried out to improve solubility, disintegration time, and release of MEL, as well as to improve the taste of the dosage forms. The authors found that disintegration time, MEL release characteristics, cytotoxicity and stability the drug improved, achieving masking of its bad taste [15].
KETO is another non-steroidal anti-inflammatory drug that is derived from the propionic acid and has analgesic and antipyretic effects. In addition to the inhibition of COX, it can stabilize the lysosomal membranes and antagonize the actions of bradykinin. Nanofibers containing KETO have been developed using partially and fully hydrolyzed PVAL as a drug delivery system. Something interesting to note is that the electrospun PVAL fibers were stabilized against disintegration in water by treatment with methanol. Fibers were analyzed by UV spectrophotometer at body temperature (37°C) and at room temperature (20°C), the results showed that fibers treated with methanol do not present accelerated release, but a controlled release fashion. This may be beneficial for certain treatments that need a more controlled release and still use a well-known and studied polymer for these applications [23].
Moreover, Um-I-Zahra S and Zhu L (2015), also studied the formation of nanofibers loaded with KETO by the electrospinning technique and their in vitro drug release profiles. The difference is that three polymers were selected for this method: EC, CA and PVP. They showed that the fibers had a clear formation and that they are affected by the type of solvent and amount of drug used, this by SEM analysis. The in vitro drug release studies indicate that nanofibers that are hydrophilic have a higher drug loading percentages and also showed a faster drug release profiles compared to those nanofibers that are hydrophobic, so, as expected, hydrophilic polymers are more attractive for rapid release formulations [16].
One well-known drug today is APAP, which is used as antipyretic and analgesic, usually for mild to moderate pain. APAP increases the threshold to painful stimuli and therefore exerts an analgesic effect against pain due to various causes. This is often sold in combination with other ingredients (e.g., opioid analgesics). APAP is also used for more intense pain, such as cancer pain (chemotherapy) and after surgery [40].
APAP is an electrospinning candidate because of its low water solubility. Solid dispersions, involving an electrospinning process, with PVP as the base polymer and APAP as the drug were produced and showed successful results. Solid dispersions of electrospun fibers were compared to three traditional dispersion system processes: freeze drying, vacuum drying and heating drying. By using SEM, DSC, XRD, and FTIR techniques, the authors analyzed the surface morphologies, drug physical status, drug-polymer interactions and in vitro dissolution, demonstrating that the electrospun nanofibers released 93.8% of the pharmaceutical compound content within the first 2 min and that the APAP dissolution rates of the different solid dispersions were as follows: Electrospun membranes> vacuum-dried membrane ≈ freeze dried membrane > heat-dried membrane. Electrospun solid dispersions showed a better dissolution performance in comparison to the other methods described above, this is because fibers showed larger surface area, high porosity (resulting from their web structure) and that had a more homogeneous distribution of APAP in the fibers [17].
Quan et al. (2011), studied another compound that was studied was the feruloyl-glycerol-oleyl (FOG), which is a mixture of a (3)-feruloyl-monooleoyl-glycerol (FMOG) and a (3)-feruloyl-dioleyl-glycerol (FDOG) (these last two being less studied as anti-inflammatory compounds) which are derivatives of the ferulic acid (FA). The authors explained that this mixture has the biological properties of FA (antioxidant, anti-inflammatory, antiviral and UV filter properties). This means that this drug can be used to prevent infection or bacteria growth. The downside of the compound is poor water solubility which may limit its use. The authors choose to use FOG in conjunction with PVP (as the base polymer matrix) to fabricate fast dissolving drug release membranes. The two materials were dissolved in a mixture of chloroform/ethanol (4:1 v/v). The results showed that the FOG had a good compatibility with the polymer, as well as a good FOG distribution within the fibers. They observed that the diameter of the fibers was influenced by the concentration of PVP and voltage, in this case, fibers with a diameter of 700-800 nm were obtained with PVP concentration of 5% (w/v) and a voltage of 14 kV. Release assays confirmed that the fibers could be used for rapid dissolution system (dissolution time of 2.0 ± 1.5 s). These results demonstrated the potential of using solid dispersion with the electrospinning technique to improve the dissolution profile of poorly water soluble compounds [42].
Goodman et al. (2011), reported that another drug that has been electrospun has been diclofenac (DF), which has analgesic, antipyretic and anti-inflammatory activities. Its potency is much greater than that of INDO, NP, or other traditional NSAIDs. In addition, the drug appears to reduce intracellular concentrations of free amino acids in leukocytes and modifies their release or uptake [40].
Karmoker et al. (2016), evaluated three drugs that were electrospun with PVAL as the drug carrier. These drugs were non-steroidal anti-inflammatories with different water solubility: diclofenac sodium (DFS) (sparingly soluble in water), NP and INDO (both water insoluble). The composition of the drugs give the drug-loaded fibers different morphological appearances. The authors emphasize that the chemical composition of the drugs was not affected by the electrospinning process, this backed by the fact that the 1H NMR spectra showed favorable results. It was noted by the authors that the molecular weight of the drugs used in their investigation played an important role on the rate and the total amount of the drugs release, both decreasing when the increasing of the molecular weight of the drugs. Also, the authors compared the characteristics of release between fibers and films that were drug-loaded, and they concluded that the films did not exceed the release characteristics of the fibers [43].
Another pharmaceutical compound that has been studied is AC, which is a Non-Steroidal Anti-Inflammatory Drug (NSAID), an aryl derivative of acetic acid, structurally related to DF, with an intermediate half-life indicated for the relief of pain and inflammation associated with rheumatic disorders, such as rheumatoid arthritis, osteoarthritis and ankylosing spondylitis [43].
AC has previously been electrospun with Pantoprazole (PP). The drug combinations were used for restricting and compensating adverse effects of the Non-Steroidal Anti-Inflammatory Drug (NSAID), so co-administration with a proton pump inhibitors may be beneficial to patients that consume Nonsteroidal Anti-Inflammatory Drugs (NSAID) for the treatment of chronic diseases such as arthritis; avoiding, in this way, to struggle with the irritation of the gastric mucosa [31].
The drugs were simultaneously electrospun by using a composite solution of zein/Eudragit S100. The physical-thermal characterization assays as thermogravimetric analysis (TGA) and Differential Scanning Calorimetry (DSC) of the nanofibers showed that both active pharmaceutical compounds were evenly distributed in the nanofibers in an amorphous state. They also noted that the in vitro release studies maintained the release of both drugs until 8 h, making it a good candidate for a controlled release treatment. Also, the co-administration of the active pharmaceutical compounds (PP with AC) reduced the gastrointestinal toxicity that NSAID induce. This was confirmed by animal experiments done in vivo. The researchers successfully developed a dual drug delivery system comprising polymers with different release characteristics and achieved oral administration of AC with reduced side effects [31].
5.2. Antimicrobial Drugs
Antimicrobials constitute a large group of compounds with diverse structures and mechanisms of action against bacteria (antibiotics), viruses (antiviral), fungi (antifungals) and parasites (antiparasitics); however, some generalizations can be made regarding important issues regarding the use of such drugs [4, 44].
GRIS is fungistatic against various species of the Dermatophytes microsporum, Epidermophyton and Trichophyton. The drug has no effect on bacteria or other fungi. Although there is a rare lack of improvement in ringworm lesions, the strains of these patients are generally still susceptible to GRIS in vitro. In a previous study, the same authors that electrospun INDO also electrospun grisefulvin for the optimization of release, producing PVP fibers with an acetone/dimethylacetamide solvent system. The results were similar to the results with INDO, this is: drug loading up to a 33% w/w, smooth and uniform nanofibers were the drug was in amorphous physical state. This form was stable after being stored 8 months with a relative humidity <25%, and it had a very fast release of the pharmaceutical compounds compared to the conventional formulations [14].
A bactericidal that is well known in the market is CIPRO, which inhibits Deoxy Ribonucleic Acids (DNA) replication by binding to DNA gyrase and topoisomerase IV. It is usually used against urinary tract infections, gastroenteritis, osteomyelitis and anthrax [45]. Modgill et al. (2016) studied the permeability of CIPRO incorporated to electrospun fibers. The research focused on investigating the permeability of CIPRO from PVAL nanofibers through different biological membranes because of the afforded mentioned properties of the electrospun fibers. The researchers found that the fibers generated greater drug permeability than the pure bactericidal. Permeability following the order: Intestinal tissue> eye> trachea> sublingual> rectal> skin. The authors also found that these studies showed there was a steady-state release of the drug, which has not been seen with the current presentations of the drug, which also have a high degree of fluctuation on the release. The authors concluded that the fibers provide numerous advantages for use as a support system for encapsulating pharmaceutical compounds and thus enhancing current therapies [46].
Moreover, GML has been proposed as a microbicide component acting by blocking transmission-facilitating innate immune response to vaginal exposure. It was shown in an especially rigorous test of protection in the SIV (Simian immunodeficiency viruses)-rhesus macaque model of HIV-1 transmission to women, that GML used daily and before vaginal challenge protects against repeated high doses of SIV, by criteria that include virological and immunological assays to detect occult infection. It has also been evidenced for indirect mechanisms of action in GML-mediated protection. This compound has been tested for the electrospinning process and has been successfully electrospuned. The authors discussed and suggested the use as prevention technologies (technologies that simultaneously prevents unwanted pregnancy and Sexually Transmitted Infections (STIs) and emphasize that this is a global health priority). It was demonstrated that GML fibers designed for topical administration can function as a combination of chemical and physical barrier as prevention technology. The polymers that were used in the work were PLLA and PEO (both are FDA approved materials) which would act as controlled-release of drugs and may facilitate the release of multiple agents simultaneously, against sperm and both viruses: HIV-1 and HSV-2. It was observed by the authors that the infection of HIV-1 in vitro was inhibited and the physical obstruction of the spermatozoa was successful by the drug-loaded fibers. It was also noted the activity by GML to inhibit the motility and viability of spermatozoa. The application of drug-loaded fibers for preventing STI’s (such as HIV-1) and unwanted pregnancies may be an innovative platform for drug delivery for future researches [25].
Buschle-Diller et al. (2007), reported another compound that has antimicrobial properties is TCN, which was electrospun with chlortetracycline hydrochloride and AMB, these studies were carried out to investigate release properties and antimicrobial efficacies of the model drugs. They were electrospun by a coaxial electrospinning. TCN was discharged from PCL at a higher rate, while AMB was slower. In dissolution tests, PCL drug loaded was almost completely released over time, while PLA released only about 10% of the total loaded drugs. By forming PCL-PLA fibers, the authors stated that the surface and release characteristics could be modified to conform a sensitive drug delivery system. The authors talk about the importance of biocompatible nanofibers, that they can adapt to the physiological conditions of the human body and how they have become increasingly important for clinical applications in recent years. Electrospun fibers offer special advantages because of their large surface area and their absorption/release properties and if loaded with pharmaceutical compounds, the administration properties can be adapted to a specific release rate, so it is a versatile technique for the loading of these compounds [33].
Coaxial electrospinning was used to fabricate core-shell fibers for a drug delivery application, PLLA was used as the shell structure and TCH as a core material. The authors performed SEM, TEM, DSC and a tensile test to characterize and investigate the viability of the resulting nanofibers for use as drug delivery vehicles. In vitro drug release behavior was also examined by UV-VIS spectroscopy. The results from the investigation indicated that the drug delivery device can be conveniently obtained for encapsulation of TCH in PLLA fibers, which generates the sustained release of TCH. They concluded that drug-containing hydrophilic fibers can be used as drug delivery vehicles or transformed into biomedical devices such as sutures and wound dressings [47].
Amoxicillin (AMOX) is also an antibiotic that has been previously studied in electrospinning techniques. Authors found the optimum conditions for the preparation of a composite of CA and PVP fibers, containing AMOX within the fibers, whereby the structure would be: CA/PVP-amoxycillin/CA. The geometric, physicochemical and thermal properties of the membranes were characterized by FTIR, DSC, SEM and TEM. In addition, the mechanical characterization of the fibers showed that the tensile strength of the membrane is not affected by the presence of AMOX inside the fibers. The effect of pH on the release rate of AMOX was also studied. The amount of antibiotic release increased with increasing pH, from 61% at pH 3 to 79% at pH 7.2. The authors assume that the release of amoxicillin is due to a diffusion mechanism. These composites are recommended to resolve the problem of possible drug loss during the matrix entrapment process, with potential local application to treat dental or cutaneous infections [20].
The high temperature electrospinning process was used for the preparation of drug-loaded PAN fibers with acyclovir (ACV), an antiviral. The fibers were prepared from a dimethyl sulfoxide solution of PAN and ACV at 80°C. As the temperature increased, the viscosities and surface tensions of the co-dissolution decreased while the conductivities increased, which may explain the improved electrospinning ability of the solutions. 1H-NMR analysis showed that the chemical integrity of ACV was maintained during the high temperature electrospinning process. The ultra-fine fibers had a smooth surface and a uniform structure with no bead configurations on the strings. 94% of the fiber diameters fell within the range of 400-700 nm. X-ray diffractograms and DSC showed that almost all the ACV was distributed in the PAN fiber matrix in the amorphous state, and the FTIR spectra demonstrated that PAN and ACV had sufficient compatibility due to hydrogen bonding. The fibers produced sustained drug release (16 h), in vitro. This study demonstrates that an elevated temperature electrospinning process can extend the processing window and allow the preparation of new types of high-quality functional fibers for drugs which are insoluble in most solvents [35, 36].
Huang et al. (2006), argues that despite advances in modern medicine, the human immunodeficiency virus (HIV) still affects the health of millions of people around the world and a lot of effort is being put into developing methods to prevent infection or eradicate the virus after infection. Authors describe the potential use of CAP electrospun fibers with TDF as a tool to prevent transmission of HIV. It was noted in an article that because of the pH-dependent solubility of the CAP, the fibers were stable in the vaginal fluid (healthy vaginal flora has a pH below 4.5), while the addition of small amounts of human semen (pH between 7.4 and 8.4) immediately dissolves the fibers, producing the release of encapsulated drugs. The pH-dependent release properties were carefully studied by the researchers and they showed that the antiviral drugs released, along with CAP dissolution have an intrinsic antimicrobial activity, efficiently neutralize HIV in vitro [37].
Yu et al. (2011), described a new type of solid dispersion electrospinning was developed, in the form of a core-shell using coaxial electrospinning for poorly water-soluble drugs. The authors selected ACV as a model drug, PVP as a hydrophilic filament-forming polymeric matrix, sodium dodecyl sulfate as a transmembrane enhancer and sucralose as a sweetener. The authors successfully prepared core-shell nanofibers, with the shell consisting of PVP, SDS and sucralose, and the core part composed of PVP and ACV. It was reported by the authors that the fibers had an average diameter of 410 ± 94 nm, with a uniform structure, as well as a smooth surface. The authors stated that the ACV, SDS and sucralose were well distributed in the amorphous matrix of PVP. This was determined by DSC and XRD, showing that the interactions were of second-order. In vitro dissolution and permeation studies demonstrated that solid dispersions of core-shell nanofibers could rapidly release ACV in one minute, with an increased permeation rate six-fold across the sublingual mucosa compared to that of the crude ACV. The authors concluded that the study provides an example of the systematic design, preparation, characterization and application of a new type of solid dispersion consisting of multiple components and structural characteristics [21].
ITR was also studied for the electrospinning process, however due to its low solubility in water, four different methods were tested: casting films, spray-drying, single syringe electrospinning and high-speed electrospinning, using copovidone (Kollidon ® VA64) as the polymer matrix, which is a vinylpyrrolidone/vinyl acetate copolymer (with a 6:4 ratio). The high-speed electrospinning process was used by the authors to demonstrate the feasibility of this electrospinning process compared to the single-needle electrospinning process. The formulations were evaluated in terms of improvement in the dissolution rate of ITR and were analyzed by SEM, DSC and XRPD. Despite the significant increase in productivity of the high-speed electrospinning, it was demonstrated that the morphology obtained was very similar to the fibrous material of simple electrospinning. The results of the DSC and XRPD showed that the drug was transformed into an amorphous form in most cases, except for the film casting samples. The authors conclude that using the high-speed electrospinning system to the amorphous solid dispersions produce fibers proved to be a good alternative from the single-needle electrospinning, this because the fibers were very flexible, the process was scalable and easy to be set in a continuous manufacturing line, with good cost-effectiveness ratio, since is a fast process [36].
Electrospinning was applied to the preparation of non-biodegradable drug-loaded nanofibers, in order to create topical drug delivery and wound healing. The specific objective of these studies was to assess if these systems could be of interest as water-poor delivery systems. ITR and ketanserin were selected as model compounds while polyurethane (PUR) was selected as the polymeric material. For both these drugs, the authors obtained an amorphous nanodispersion for ITR with dimethylformamide (DMF) and ketanserin with dimethylacetamide (DMAc). It was demonstrated that the collected fibers release the drugs at various speeds and profiles based on the morphology of the nanofibers and the content of the drugs. The data were generated using a specially designed release apparatus around a rotating cylinder. The authors found that loading a small amount of ITR, the release is a linear function of the square root of time (Fick kinetics), and that the drug did not have an initial burst release, being suitable as sustained release system [38].
5.3. Anticancer Drugs
Cancer is a group of diseases that affect abnormal cell growth with the potential to invade or spread to other parts of the body. DOC is a well-known mitotic inhibitor for oral cancer. It is available only as an intravenous formulation on the market. It faces problems of extravasation, inflammation of the veins and other side effects of chemotherapy. The aim of the authors was to design a mucoadhesive nano-carrier system that is maintained at the site of application and maximizes the therapeutic potential of the anticancer drug as well as attenuates its systemic side effects. In the study, DOC-PVAL fibers were prepared using the electrospinning method. The resulting fibers were characterized by various parameters such as surface morphology, drug loading, and in vitro drug release, tensile strength, mucoadhesivity, and drug permeability, degree of swelling and anticancer activities against selective cell lines to establish their therapeutic potential. The results were positive and it was concluded that the current approach comprising polymeric nanofibers can be successfully used for local administration of anticancer drugs [8].
Zhang et al. (2014), reported another compound that is used in the treatment of cancer is cisplatin (CP). CP is used in the treatment of liver cancer, but it has the drawbacks that the drug tends to accumulate in this organ and it has a poor intake after intravenous administration. Research focuses on making the chemotherapy less aggressive for the patient's liver by using an electrospun system. The system consisted of five layers, the first, third and fifth layer being the polymer (PLA) and the second and fourth layer being the drug. The rationale for that conformation was to have a prolonged release of cisplatin and prevent local cancer recurrence after a surgical resection. The in vivo studies at 24 h showed that the multilayer fiber mat had a prolonged release and the retention in the tissue was more stable. The authors observed in studies with mice that liver cancer had been retarded, mice had a prolonged survival time, and there was a reduced toxicity compared with other groups with different treatments. This study shows us the potential that electrospun fibers may have over the aggressiveness of some APIs. On the other hand, DOX is a chemotherapy medication used in a numerous of types of cancer. This includes leukemia, lymphoma and many types of carcinomas (solid tumors), and soft tissue sarcomas. It is usually given by injection into a vein, a treatment that has never been well received [48].
The electrospinning of poly(ethylene glycol) (PEG) fibers loaded with DOX has been extensively studied. In one report, the authors developed fibers using a water-in-oil emulsion. The polymers used in the article (PEG and PLLA) were dissolved in chloroform which constitute de oily phase while DOX was contained in the aqueous phase. The purpose of this was to encapsulate the drug in the fibers within the oil phase. The diameter of the electrospun fibers was in the range of 300 nm to 1 μm (these being ultrafine). The content of DOX in the fibers was 1-5% by weight, and it was fully encapsulated within the fibers. The release was controlled by diffusion and the mechanism of enzymatic degradation. The antitumor activity of DOX incorporated into PEG-PLLA fibers versus mouse glioma cells (C6 cell lines) was assessed by the MTT method. The results showed that DOX could be released from the fibers without losing cytotoxicity, thus making the system object of interest for further studies [26].
Vrbata et al. (2013), evaluated the drug release behavior of the loaded fibers prepared by an emulsion was the object of study, also with DOX as the model drug. Confocal microscopic images of laser scanning indicated that the drug was incorporated in the PEG-PLLA copolymer nanofibers, forming drug-loaded structural fibers. Drug release behavior of this system showed a three-stage diffusion controlled mechanism in which the rate of release of the first stage was slower than that of the second stage, but both obeyed the second law of Fick. Based on these results, it is concluded that the DOX-loaded fibers prepared by emulsion electrospinning may be used as a reservoir-type delivery system in which the rate of DOX release decreases when it is found at a higher concentration in the fibers [27].
Nanofibers sensitive to the stimuli were developed through the electrospinning method. Poly(N-isopropylacrylamide-co-acrylamide-co-vinylpyrrolidone) (poly(NIPAAM-AAm-VP)) was used as the material to prepare the electrospun fibers. DOX-loaded fibers were prepared and characterized by XRD, SEM and FTIR. The cytotoxicity of DOX-loaded fibers was evaluated by MTT test on A549 lung cancer cell lines. The in vitro cytotoxicity assay showed that pristine fibers did not affect the growth of A549 cells. The antitumor activity of DOX-laden fibers against cells was maintained throughout the experimental process, whereas the effect of dissolved DOX disappeared within 48 h. The drug release pattern of these systems is zero order and the rate of drug release does not depend on the drug/polymer ratio in different implant formulations. The researchers found that these new fibers were stable and retained their morphology even after incubation in release medium (pH 7.4, 37°C), while they collapse and disperse rapidly in an aqueous solution of acid medium at room temperature [49].
PTX has also been electrospun. PTX is a drug that acts as a toxic substance for the mitotic spindle through its binding of high affinity to the microtubules with the intensification of tubulin polymerization. It has a remarkable activity in a wide range of solid tumors, such as ovarian, breast, non-small cell lung cancer, head and neck cancer, esophageal, prostatic and bladder cancer, as well as AIDS-related Kaposi's sarcoma [45].
Vrbata et al. (2013), also studied emulsion electrospinning of DOX in combination with PTX. These drugs were successfully loaded onto the PEG-PLA fibers for multiple drug delivery. The release behaviors of both drugs from the same fiber mats were attributed to their solubility and distribution status in the fibers. They explained that due to its high hydrophilicity, DOX was easy to diffuse from the fibers, and its release rate was always faster than the hydrophobic PTX. In addition, the rate of release of PTX was accelerated by the release of DOX, so the both antitumoral compounds had a better effect in conjunction. The in vitro cytotoxicity against rat C6 glioma cells was performed by the authors and they indicated that the dual drug combination showed greater inhibition and apoptosis compared to single-drug system. The authors suggest multiple drug delivery that combines antitumoral components can be reached in the future [27].
As for the treatment of C6 glioma in vitro, implants for a sustain delivery of PTX have been studied, this by electrospinning the drug with PLGA as a matrix to obtain fibers with diameters ranging from 10-30 nm (ultra-fine nanofibers) by adding organic salts. The encapsulation efficiency was over 90% and was found in a solid solution state in the fibers (DSC measurements). The in vitro release study shows that the antitumoral achieved a sustained release of the drug for 60 days, this is important because sustained release of this type leads to effectiveness of the treatment. The authors stated that PTX-loaded PLGA fibers (36 mg/ml) are comparable to the commercial formulation of PTX-taxol and concluded that electrospun fibers are promising for the treatment of tumors as an alternative drug delivery device [34].
5.4. Cardiovascular Drugs
Cardiovascular Diseases (CVD) are a group of disorders of the heart and blood vessels, including: coronary heart disease, cerebrovascular diseases, peripheral arteriopathies, rheumatic heart disease, congenital heart defects, and pulmonary emboli, which can detach and lodge in the vessels of the heart and lungs. Nicorandil (NICO) is a vasodilator medication used to treat angina pectoris, it is the principal therapeutic agent that has a capability to hyperpolarize muscle tissues, and is an effective coronary vasodilator, it seems to be active in all types of angina pectoris, including advanced coronary artery lesion [50]. Angina pectoris is a chest pain resulting from episodes of transient myocardial ischemia. This can be caused by diseases such as arteriosclerosis, coronary arteriosclerosis and aortic stenosis. Angina commonly arises from vasospasm of the coronary arteries [51]. This drug has been electrospun for sublingual administration in an attempt to reduce mucosal ulceration and improve bioavailability of the drug. Polymeric nanofibers were obtained using vitamin B12, and a mixture of hyaluronic acid and PVAL. The authors reported that the prepared nanofibers were found to be uniform by SEM (with a diameter of 200-450 nm). Histopathological studies showed no evidence of mucosal ulceration at the site of application and a preclinical safety study was performed to evaluate the maintenance of an effective therapeutic level over an extended period. The authors demonstrated that the biocompatible nanofibers work as perfect carrier system for the sublingual administration of antianginal drugs, since one of the problems of NICO is its low bioavailability [50].
Spironolactone (SPIRO) is a drug mainly used to treat fluid accumulation due to heart failure, liver scarring, or kidney disease, and for the treatment of high blood pressure. The lipophilic SPIRO was electrospun with PVP K90. However, instead of an immediate release of the drug, a temporary precipitation was observed. A small addition of BCD provided a dramatic increase in the rate of release, even at high concentrations of the drug. This approach ensured the near-total release of the drug in one minute, making the system a suitable formulation for rapid release [19].
CVD is a drug used for the treatment of congestive heart failure. It is indicated in the treatment of hypertension and to reduce the risk of mortality and hospitalizations in a subset of people after a heart attack. It may be used alone or with other antihypertensive agents. It was observed that the incorporation of this drug into electrospun PCL fibers reduced its crystallinity. Drug incorporated in the nanofibers was partially molecularly dispersed in the polymer matrix and partly in the form of dispersed nanocrystals. The incorporation of the drug into PCL nanofibers significantly improved their dissolution rate. PCL loaded nanofibers released nearly 77% of the incorporated CVD in 4 h, indicating the influence of drug properties, such as molecular weight and solubility, on their release from the polymeric matrix [11].
5.5. Antihistamine Drugs
Antihistamine drugs have also been loaded into nanofibers. One study focused on the administration of chlorpheniramine maleate (CPM). CPM was incorporated into the glutinous rice starch (GRS) with PVA electrospun fibers to prove a drug delivery carrier concept and drug release control from the nanofibers [52].
LOR is indicated for the symptomatic relief of allergies, such as hay fever (allergic rhinitis), urticaria, chronic idiopathic urticaria and other skin allergies. For allergic rhinitis, LOR is effective for both nasal and ocular symptoms: sneezing, runny nose, itching or burning eyes. LOR has been previously electrospun [40]. The authors observed that a low polymer concentration, low feed rate with the injection pump, and a high applied voltage (30% PVP concentration in ethanol, drug-to-polymer ratio of 1:4, 10 kV of voltage, and a feed rate of 1 ml/h), nanofibers with a smaller diameter and greater uniformity were created. The study concluded that the smaller the diameter of the fiber and the amount of drug, the faster its dissolution and lower the time of the release of this pharmaceutical component [18].
Another antihistamine is DPH, which decreases or prevents the histamine effects on smooth muscle and immune cells, as well as antagonizes muscarinic receptors and α-adrenergic receptors. This drug is normally used for insomnia, common cold symptoms and nausea [53, 54]. DPH has been electrospun directly onto a polymeric backing film of HPMC and glycerol to improve a mucoadhesive system. The performance variables were evaluated: disintegration time, adhesion work, adhesion strength, the area under the curve (at 1 min) and the area under the curve of the permeation (at 3 min); these last two were used to calculate the independent variables of the process which are the filling volume, HPMC and the glycerol concentration. The authors studied the physicochemical and physicomechanical properties with the following methods: Rheology, FTIR, determination of tenacity, mucoadhesion and nanotensile tests. The data obtained from the physical-mechanical characterization confirmed the suitability of the systems for the application in the delivery of drugs. The authors reach their goal to optimize the system and obtained a drug entrapment of 2.3 mg/1.5 cm2 with disintegration time of 12.8 s, this by the way, a fast release of the compound. In the case of antihistaminic, this is important since the goal of the administration of antihistamines is to obtain immediate relief of the annoying effects of an allergic response [32].
5.6. Gastrointestinal Drugs
Gastrointestinal disease is the name given to all of those diseases that damage the digestive system (esophagus, stomach, small intestine, large intestine and rectum, and the accessory organs of digestion: liver, gallbladder, and pancreas) [55].
Metoclopramide is a prokinetic drugs, which has an in vitro effects on contractility of colonic smooth muscle. It has been reported that MET.HCl was loaded into PVA-PCL (core-shell) fibers, which exhibited an initial burst of about 55% of the total release. It was suggested that the burst effect is most likely due to the presence of pores (either micron or nano-sized) in the PCL shell [56].
Core-Shell electrospinning of MET-HC has also been performed using various polymers (PCL, PLLA and PLGA 80/20). The strategy to control the release was to have fibers consisted of two layers (polymer on the outside and the drug inside). It was shown that the rate of release of this hydrophilic drug was controllable by varying the physical and chemical properties of the core and shell solutions. The objectives of this study were to discuss and point the importance of the controlled release of hydrophilic entities, such as peptides, proteins and even pDNAs (plasmid DNA), and the difficulties in being administered; as well as the advantages of using core-shell structures, to protect sensitive materials (Fig. 2). The results showed a clear difference in the release pattern between monolithic fibers made from hydrophilic and hydrophobic polymers, and various core-shell fibers with PCL, PLLA and PLGA 80/20 as shell polymers. The study gives us an overview of how much can be achieved by controlling release using core-shell fibers, and thereby, options for controlled release systems for hydrophilic drugs, peptides and pDNAs [30].
5.7. Palliative Drugs
Palliative medicine is a multidisciplinary approach to specialized medical care for people with serious illness. It focuses on providing patients with relief from symptoms, pain, physical stress and mental stress of a serious illness, whatever the diagnosis. The aim of this therapy is to improve the quality of life of both the patient and the family [57].
Nanofibers loaded with donepezil.HCl were prepared as a dosage form so it could be dissolved orally. The authors in this research aimed to create a removable scaffold with an ultrafast release electrospinning, using PVAL as the matrix polymer and donepezil. HCl as the drug to be loaded. The authors found that the PVAL at a lower molecular weight had the best results. They also found diameters between 100 nm and 300 nm by SEM. The in vitro tests performed showed an immediate release of the drug release (less than 30 s) regardless of the drug content in the fibers, due to the large surface area the nanofibers. The authors compared the scaffolds and the commercial tablets and conclude that the electrospinning technology is a promising method for the production of alternative effective dosage forms, especially with patients, children and elderly people with dysphagia [22].
5.8. Contraceptive Drugs
Oral contraceptives are used to prevent pregnancy. Estrogen and progestin are two female sex hormones that in combinations avoid ovulation. They also modify the lining of the uterus (womb) to prevent the development of pregnancy, and modify the mucosa of the cervix to prevent sperm from entering [40].
Blakney et al. (2014), produced electrospun PVAL nanofibers with different microscale geometries for the administration of the progestin levonorgestrel. The authors examined composite materials stacked on tissue paper and studied the release kinetics of the drug and measured its cytotoxicity. In their results, they reported on the PVAL/ levonorgestrel solution and processing parameters for the electrospinning of free surfaces of medical tissues with controlled microarchitecture and high drug loading (up to 20% by weight). It was observed that the in vitro release of levonorgestrel was affected by the composite microarchitecture, fabric thickness and drug content, so the authors reached their goal and the electrospinning process was optimized [58].
5.9. Miscellaneous
Among the most widely used substances in the world is CA, which antagonizes adenosine receptors, thus, CA temporarily prevents or alleviates drowsiness, and therefore maintains or restores alertness. Some of the uses are: bronchopulmonary dysplasia in preterm infants for both prevention and treatment, apnea of prematurity as primary treatment (but not as a preventive treatment), treatment of orthostatic hypotension and general vasodilation, usually used to relieve headaches or even migraine [24, 59, 60].
On the other hand, RFN (vitamin B2) is used in individuals with protein depletion and infections that complicate it, in other words: In the treatment of individuals with generalized macroscopic malnutrition. Electrospinning of the CA and RFN has also been studied, for example, rapid dissolution drug release systems were prepared by electrospinning using PVAL as the base polymer. These systems were evaluated by the authors using SEM, FTIR and XRD, to investigate the physicochemical properties of the electrospun nanofibers. The SEM images showed that nanofibers prepared from aqueous PVAL/drug solutions by electrospinning had a diameter in the range of 260-370 nm (ultrafine morphology). In pharmaceutical tests, the authors showed that PVAL/CA and PVAL/RFN nanofibers had almost the same dissolution time (approximately 1.5 s) and wetting time (approximately 4.5 s). Measurements of the release indicated that the drugs could be released in an accelerated manner (CA in an extent of 100% and RFN up to 40%, in 60 s). This study demonstrates that nanofibers can also carry molecules that are not considered drugs, but vitamins or substances that have another uses [61].
Borbás et al. (2015), reported that the number of drugs poorly soluble in water is increasing continuously, so improving the aqueous dissolutions of novel APIs has become one of the central challenges of pharmaceutical studies. Emphasizes that up to now, preclinical studies have mainly focused on formulation methods to enhance the dissolution of active compounds, in many cases without considering that the formulation matrix affects not only dissolution but also influences the transport of the molecules of the drug through membranes. The objective of a study was to test an electrospinning formulation based on cyclodextrin (CD) with aripiprazole (ARI) (an antipsychotic), using the μFlux apparatus to monitors the permeation along with the dissolution, and by this means to have a better in vitro-in vivo correlation. It has been demonstrated by the authors that the electrospinning formulation based on CD-ARI has the potential to ensure rapid delivery of drug through the oral mucosa due to the rapid dissolution of the drug in the formulation and the improved flow through the membranes [60].
Conclusion
Electrospinning is an old technology for the manufacture of continuous nanofibers with a relatively simple configuration. However, in recent years it attracted much attention because of its potential in biomedical and other nanotechnical applications. Its inherent high surface-volume ratio, ease of operation and cost-effectiveness are all attractive features for its biomedical application. Electrospinning for drug loading into hydrophilic fibers is especially important to increase the dissolution and for instance biodisponibility, of poorly water-soluble drugs. Immediate dissolution formulations for buccal absorption of drug are produced with this technique for fast drug absorption and to avoid first pass metabolism, or degradation in gastric fluids. Systems for local delivery of antineoplastics, antimicrobials, etc. can also be developed by electrospinning. With the development of electrospinning techniques, such as coaxial electrospinning, and the availability of a rich variety of materials (including natural, synthetic and semisynthetic polymers), several drugs have been electrospun into ultrafine fibers with controllable diameters and morphologies. Advanced electrospinning arrangements allow the production of delivery systems for hydrophilic drugs including macromolecules such as proteins and DNA.
This paper summarizes the modification of the electrospinning system configurations and the effect of the process parameters on the fibers, their application in drug delivery, including carrier materials, loaded drugs and their release kinetics, and illustrates their application for local chemotherapy. To date, most studies on the release of antibacterial agents, drugs (psychoactive, antineoplasic, etc.), are carried out in vitro. In vivo, in-depth systemic studies are necessary before any clinical marketing is contemplated, especially those on the kinetics and dynamics of drug release in vivo; the effects of drug dosage and release kinetics on therapeutic efficacy and the biodistribution of the liberated drugs. Complete studies are required on the toxic effect, as well as distribution and elimination process, of the polymeric carriers.
Until now, many pharmaceutical drugs have been loaded into nanofibers, but these studies are limited in just the loading and characterization of nanofibers. It is observed that the lack of the correct dosage is a common issue in most articles. It can be concluded, that this disadvantage is the stronger weakness of electrospinning: it is difficult to load a desired concentration into nanofibers with the end purpose of applied in clinical studies in humans. Also, it is important to incorporate flavors and sweeteners in the drug-loaded nanofibers, particularly for applications on fast dissolving systems intended for buccal administration of drugs, considering that most drugs have an unpleasant taste. However, with the exception of reports on the loading of sugar in core-shell nanofibers, the efficacy of this process is not widely reported.
This review proposes to continue the investigation to optimize the incorporation of interesting drugs into nanofibers, but further clinical studies, considering patient acceptance of the administration form. Scaling to mass productions of drug loaded electrospun mats is also an issue that has to be considered.
Finally, it can be said, that electrospinning had demonstrated been effective in a great diversity of biomedical application and studies will continue on its different uses, because its versatility, cost-effectivity, easy to use and easy to fabricate in any research facilities, even with low economic support.
Acknowledgements
The authors are grateful to the Sectorial Fund for Research in Health and Social Security (FOSSIS) of CONACYT for funding through project number 272310.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Huang Z-M., Zhang Y-Z., Kotaki M., Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003;63(15):2223–2253. [Google Scholar]
- 2.Villarreal-Gómez L.J., Vera-Graziano R., Vega-Ríos M.R., Pineda-Camacho J.L., Almanza-Reyes H., Mier-Maldonado P.A., Cornejo-Bravo J.M. Biocompatibility evaluation of electrospun scaffolds of poly (L-Lactide) with pure and grafted hydroxyapatite. J. Mex. Chem. Soc. 2014;58(4):435–443. [Google Scholar]
- 3.Villarreal-Gómez L.J., Cornejo-Bravo J.M., Vera-Graziano R., Grande D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. Polym. Ed. 2016;27(2):157–176. doi: 10.1080/09205063.2015.1116885. [DOI] [PubMed] [Google Scholar]
- 4.Pires L.R. 9-Electrospun fibers for drug and molecular delivery. In: Guarino V., Ambrosio L., editors. Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices, Woodhead Publishing Series in Biomaterials. Protugal; 2018. pp. 157–177. [Google Scholar]
- 5.Chou S-F., Carson D., Woodrow K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release. 2015;220:584–591. doi: 10.1016/j.jconrel.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández de la Mora J. The fluid dynamics of Taylor cones. Annu. Rev. Fluid Mech. 2007;39(1):217–243. [Google Scholar]
- 7.Tucker N., Stanger J., Staiger M., Razzaq H., Hofman K. The history of the science and technology of electrospinning from 1600 to 1995. J. Eng. Fibers Fabrics. 2012;7(Special Issue):63–73. [Google Scholar]
- 8.Singh H., Sharma R., Joshi M., Garg T., Goyal A.K., Rath G. Transmucosal delivery of docetaxel by mucoadhesive polymeric nanofibers. Artif. Cells Nanomed. Biotechnol. 2015;43(4):263–269. doi: 10.3109/21691401.2014.885442. [DOI] [PubMed] [Google Scholar]
- 9.Velasco Barraza R., Alvarez Suarez A.S., Villarreal Gómez L.J., Paz González J.A., Iglesias A.L., Vera Graziano R. Designing a low-cost electrospinning device for practical learning in a bioengineering biomaterials course. Rev. Mex. Ing. Biomed. 2016;37(1):7–16. [Google Scholar]
- 10.Manuel C.B.J., Jesus V.G.L., Aracely S.M. 2016. [Google Scholar]
- 11.Potrč T., Baumgartner S., Roškar R., Planinšek O., Lavrič Z., Kristl J., Kocbek P. Electrospun polycaprolactone nanofibers as a potential oromucosal delivery system for poorly water-soluble drugs. Eur. J. Pharm. Sci. 2015;75:101–113. doi: 10.1016/j.ejps.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Yu D.-G., Shen X.-X., Branford-White C., White K., Zhu L.-M., Annie Bligh S.W. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. 2009. [DOI] [PubMed]
- 13.Wu Y.H., Yu D.G., Li H.C., Feng D.N. Electrospun nanofibers for fast dissolution of naproxen prepared using a coaxial process with ethanol as a shell fluid. Appl. Mech. Mater. 2014;662:29–32. [Google Scholar]
- 14.Lopez F.L., Shearman G.C., Gaisford S., Williams G.R. Amorphous formulations of indomethacin and griseofulvin prepared by electrospinning. Mol. Pharm. 2014;11(12):4327–4338. doi: 10.1021/mp500391y. [DOI] [PubMed] [Google Scholar]
- 15.Samprasit W., Akkaramongkolporn P., Ngawhirunpat T., Rojanarata T., Kaomongkolgit R., Opanasopit P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int. J. Pharm. 2015;487(1-2):213–222. doi: 10.1016/j.ijpharm.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Um-I-Zahra S., Zhu L. Novel drug loaded duplicate nanofibers and their in-vitro drug release profiles. Am. Res. Thoughts. 2015;1(6):1683–1698. [Google Scholar]
- 17.Yu D-G., Branford-White C., White K., Li X-L., Zhu L-M. Dissolution improvement of electrospun nanofiber-based solid dispersions for acetaminophen. Am. Assoc. Pharm. Sci. 2010;11(2):809–817. doi: 10.1208/s12249-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akhgari A., Ghalambor Dezfuli A., Rezaei M., Kiarsi M., Abbaspour M. The design and evaluation of a fast-dissolving drug delivery system for loratadine using the electrospinning method. Jundishapur J. Nat. Pharm. Prod. 2016;11(2) doi: 10.17795/jjnpp-33613. [DOI] [Google Scholar]
- 19.Vigh T., Horváthová T., Balogh A., Sóti P.L., Drávavölgyi G., Nagy Z.K., Marosi G. Polymer-free and polyvinylpirrolidone-based electrospun solid dosage forms for drug dissolution enhancement. Eur. J. Pharm. Sci. 2013;49(4):595–602. doi: 10.1016/j.ejps.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Castillo-Ortega M.M., Montaño-Figueroa A.G., Rodríguez-Félix D.E., Munive G.T., Herrera-Franco P.J. Amoxicillin embedded in cellulose acetate-poly (vinyl pyrrolidone) fibers prepared by coaxial electrospinning: Preparation and characterization. Mater. Lett. 2012;76:250–254. [Google Scholar]
- 21.Yu D-G., Zhu L-M., Branford-White C-J., Yang J-H., Wang X., Li Y., Qian W. Solid dispersions in the form of electrospun core-sheath nanofibers. Int. J. Nanomedicine. 2011;6:3271–3280. doi: 10.2147/IJN.S27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy Z.K., Nyul K., Wagner I., Molnar K., Marosi G. Electrospun water soluble polymer mat for ultrafast release of Donepezil HCl. Express Polym. Lett. 2010;4(12):763–772. [Google Scholar]
- 23.Kenawy E-R., Abdel-Hay F.I., El-Newehy M.H., Wnek G.E. Controlled release of ketoprofen from electrospun poly(vinyl alcohol) nanofibers. Mater. Sci. Eng. A. 2007;459(1-2):390–396. [Google Scholar]
- 24.Li X., Kanjwal M.A., Lin L., Chronakis I.S. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B Biointerfaces. 2013;103:182–188. doi: 10.1016/j.colsurfb.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Ball C., Krogstad E., Chaowanachan T., Woodrow K.A. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS One. 2012;7(11):e49792. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X., Yang L., Xu X., Wang X., Chen X., Liang Q., Zeng J., Jing X. Ultrafine medicated fibers electrospun from W/O emulsions. J. Control. Release. 2005;108(1):33–42. doi: 10.1016/j.jconrel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Chen X., Wang Z., Jing X. Ultrafine PEG-PLA fibers loaded with both paclitaxel and doxorubicin hydrochloride and their in vitro cytotoxicity. Eur. J. Pharm. Biopharm. 2009;72(1):18–25. doi: 10.1016/j.ejpb.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Vrbata P., Berka P., Stránská D., Doležal P., Musilová M., Čižinská L. Electrospun drug loaded membranes for sublingual administration of sumatriptan and naproxen. Int. J. Pharm. 2013;457(1):168–176. doi: 10.1016/j.ijpharm.2013.08.085. [DOI] [PubMed] [Google Scholar]
- 29.Canbolat M.F., Celebioglu A., Uyar T. Drug delivery system based on cyclodextrin-naproxen inclusion complex incorporated in electrospun polycaprolactone nanofibers. Colloids Surf. B Biointerfaces. 2014;115:15–21. doi: 10.1016/j.colsurfb.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Tiwari S.K., Tzezana R., Zussman E., Venkatraman S.S. Optimizing partition-controlled drug release from electrospun core-shell fibers. Int. J. Pharm. 2010;392(1-2):209–217. doi: 10.1016/j.ijpharm.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Karthikeyan K., Guhathakarta S., Rajaram R., Korrapati P.S. Electrospun zein/eudragit nanofibers based dual drug delivery system for the simultaneous delivery of aceclofenac and pantoprazole. Int. J. Pharm. 2012;438(1-2):117–122. doi: 10.1016/j.ijpharm.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 32.Dott C., Tyagi C., Tomar L.K., Choonara Y.E., Kumar P., du Toit L.C., Pillay V. A Mucoadhesive Electrospun nanofibrous matrix for rapid oramucosal drug delivery. J. Nanomater. 2013;2013:1–19. [Google Scholar]
- 33.Buschle-Diller G., Cooper J., Xie Z., Wu Y., Waldrup J., Ren X. Release of antibiotics from electrospun bicomponent fibers. Cellulose. 2007;14(6):553–562. [Google Scholar]
- 34.Xie J., Wang C-H. Electrospun micro- and nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm. Res. 2006;23(8):1817–1826. doi: 10.1007/s11095-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 35.Chen H-M., Yu D-G. An elevated temperature electrospinning process for preparing acyclovir-loaded PAN ultrafine fibers. J. Mater. Process. Technol. 2010;210(12):1551–1555. [Google Scholar]
- 36.Nagy Z.K., Balogh A., Démuth B., Pataki H., Vigh T., Szabó B., Molnár K., Schmidt B.T., Horák P., Marosi G., Verreck G., Van Assche I., Brewster M.E. High speed electrospinning for scaled-up production of amorphous solid dispersion of itraconazole. Int. J. Pharm. 2015;480(1-2):137–142. doi: 10.1016/j.ijpharm.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Huang Z-M., He C-L., Yang A., Zhang Y., Han X-J., Yin J., Wu Q. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J. Biomed. Mater. Res. A. 2006;77A(1):169–179. doi: 10.1002/jbm.a.30564. [DOI] [PubMed] [Google Scholar]
- 38.Verreck G., Chun I., Rosenblatt J., Peeters J., Van Dijck A., Mensch J., Noppe M., Brewster M.E. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release. 2003;92(3):349–360. doi: 10.1016/s0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 39.Buer J.K. Origins and impact of the term “NSAID.”. Inflammopharmacology. 2014;22(5):263–267. doi: 10.1007/s10787-014-0211-2. [DOI] [PubMed] [Google Scholar]
- 40.Goodman L.S., Brunton L.L., Chabner B., Knollmann B.C. Goodman & Gilman’s pharmacological basis of therapeutics. New York: McGraw-Hill; 2011. [Google Scholar]
- 41.Yu D-G., Zhang X-F., Shen X-X., Brandford-White C., Zhu L-M. Ultrafine ibuprofen-loaded polyvinylpyrrolidone fiber mats using electrospinning. Polym. Int. 2009;58(9):1010–1013. [Google Scholar]
- 42.Quan J., Yu Y., Branford-White C., Williams G.R., Yu D-G., Nie W., Zhu L-M. Preparation of ultrafine fast-dissolving feruloyl-oleyl-glycerol-loaded polyvinylpyrrolidone fiber mats via electrospinning. Colloids Surf. B Biointerfaces. 2011;88(1):304–309. doi: 10.1016/j.colsurfb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Karmoker J.R., Sarkar S., Joydhar P., Chowdhury S.F. Comparative in vitro equivalence evaluation of some aceclofenac generic tablets marketed in Bangladesh. Pharma Innov. J. TPI. 2016;5(3):3–7. [Google Scholar]
- 44.Collignon P., Powers J.H., Chiller T.M., Aidara‐Kane A., Aarestrup F.M. World health organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin. Infect. Dis. 2009;49(1):132–141. doi: 10.1086/599374. [DOI] [PubMed] [Google Scholar]
- 45.Katzung B.G., Masters S.B., Trevor A.J. Basic & clinical pharmacology. 2012. [Google Scholar]
- 46.Modgill V., Garg T., Goyal A.K., Rath G. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif. Cells Nanomed. Biotechnol. 2016;44(1):122–127. doi: 10.3109/21691401.2014.924007. [DOI] [PubMed] [Google Scholar]
- 47.He C.L., Huang Z-M., Han X-J., Liu L., Zhang H-S., Chen L-S. Coaxial electrospun poly(L‐lactic acid) ultrafine fibers for sustained drug delivery. J. Macromol. Sci. Part B. 2006;45(4):515–524. [Google Scholar]
- 48.Zhang Y., Liu S., Wang X., Zhang Z., Jing X.b., Zhang P., Xie Z.G. Prevention of local liver cancer recurrence after surgery using multilayered cisplatin-loaded polylactide electrospun nanofibers. Chin. J. Polym. Sci. 2014;32(8):1111–1118. [Google Scholar]
- 49.Salehi R., Irani M., Rashidi M-R., Aroujalian A., Raisi A., Eskandani M., Haririan I., Davaran S. Stimuli-responsive nanofibers prepared from poly(N-isopropylacrylamide-acrylamide-vinylpyrrolidone) by electrospinning as an anticancer drug delivery. Des. Monomers Polym. 2013;16(6):515–527. [Google Scholar]
- 50.Singh B., Garg T., Goyal A.K., Rath G. Development, optimization, and characterization of polymeric electrospun nanofiber: A new attempt in sublingual delivery of nicorandil for the management of angina pectoris. Artif. Cells Nanomed. Biotechnol. 2016;44:1498–1507. doi: 10.3109/21691401.2015.1052472. [DOI] [PubMed] [Google Scholar]
- 51.Maton A. Human Biology and Health. 1997.
- 52.Jaiturong P., Sirithunyalug B., Eitsayeam S., Asawahame C., Tipduangta P., Sirithunyalug J. Preparation of glutinous rice starch/polyvinyl alcohol copolymer electrospun fibers for using as a drug delivery carrier. Asian J. Pharm. Sci. 2018;13(3):293–247. doi: 10.1016/j.ajps.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyagi C., Tomar L., Choonara Y.E., Du Toit L.C., Kumar P., Pillay V. Electrospun nanofiber matrix with a mucoadhesive backing film for oramucosal drug delivery. Int. J. Mater. Mech. Manuf. 2014;2(1):81–85. [Google Scholar]
- 54.Zitek T., Gates M., Pitotti C., Bartlett A., Patel J., Rahbar A., Forred W., Sontgerath J.S., Clark J.M. A Comparison of headache treatment in the emergency department: Prochlorperazine versus ketamine. Ann. Emerg. Med. 2017;•••:1–10. doi: 10.1016/j.annemergmed.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann E.M., Breitenbach A., Breitkreutz J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011;8:299–316. doi: 10.1517/17425247.2011.553217. [DOI] [PubMed] [Google Scholar]
- 56.Jaber B.M., Petroianu G.A., Rizvi S.A., Borai A., Saleh N.A., Hala S.M., Saleh A.M. Protective effect of metoclopramide against organophosphate-induced apoptosis in the murine skin fibroblast L929. J. Appl. Toxicol. 2018;38(3):329–340. doi: 10.1002/jat.3543. [DOI] [PubMed] [Google Scholar]
- 57.Fallowfield L.J., Jenkins V.A., Beveridge H.A. Truth may hurt but deceit hurts more: Communication in palliative care. Palliat. Med. 2002;16(4):297–303. doi: 10.1191/0269216302pm575oa. [DOI] [PubMed] [Google Scholar]
- 58.Blakney A.K., Krogstad E.A., Jiang Y.H., Woodrow K.A. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. Int. J. Nanomedicine. 2014;9:2967–2978. doi: 10.2147/IJN.S61664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Illangakoon U.E., Gill H., Shearman G.C., Parhizkar M., Mahalingam S., Chatterton N.P., Williams G.R. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int. J. Pharm. 2014;477(1-2):369–379. doi: 10.1016/j.ijpharm.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 60.Borbás E., Balogh A., Bocz K., Müller J., Kiserdei É., Vigh T., Sinkó B., Marosi A., Halász A., Dohányos Z., Szente L., Balogh G.T., Nagy Z.K. In vitro dissolution-permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using μFluxTM. Int. J. Pharm. 2015;491(1-2):180–189. doi: 10.1016/j.ijpharm.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 61.Li X., Kanjwal M.A., Lin L., Chronakis I.S. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf. B Biointerfaces. 2013;103:182–188. doi: 10.1016/j.colsurfb.2012.10.016. [DOI] [PubMed] [Google Scholar]