Abstract
Background
Surgical applications using breast implants are individualized operations to fill and shape the breast. Physical properties beyond shape, size, and surface texture are important considerations during implant selection.
Objectives
Compare form stability, gel material properties, and shell thickness of textured shaped, textured round, and smooth round breast implants from 4 manufacturers: Allergan, Mentor, Sientra, and Establishment Labs, through bench testing.
Methods
Using a mandrel height gauge, form stability was measured by retention of dimensions on device movement from a horizontal to vertical supported orientation. Dynamic response of the gel material (gel cohesivity, resistance to gel deformation, energy absorption) was measured using a synchronized target laser following application of graded negative pressure. Shell thickness was measured using digital thickness gauge calipers.
Results
Form stability, gel material properties, and shell thickness differed across breast implants. Of textured shaped devices, Allergan Natrelle 410 exhibited greater form stability than Mentor MemoryShape and Sientra Shaped implants. Allergan Inspira round implants containing TruForm 3 gel had greater form stability, higher gel cohesivity, greater resistance to gel deformation, and lower energy absorption than those containing TruForm 2 gel and in turn, implants containing TruForm 1 gel. Shell thickness was greater for textured vs smooth devices, and differed across styles.
Conclusions
Gel cohesivity, resistance to gel deformation, and energy absorption are directly related to form stability, which in turn determines shape retention. These characteristics provide information to aid surgeons choosing an implant based on surgical application, patient tissue characteristics, and desired outcome.
Numerous breast implant options are available for various surgical applications, such as augmentation, revision-augmentation, and reconstruction surgery. Available implants differ in multiple fundamental ways, including shape (round vs anatomical), size, gel material and characteristics, fill ratio, and shell surface texture, which may influence aesthetic outcome and device performance.1,2 The decision on which implant to use may be based on a variety of factors, including breast anatomy and tissue measurements, surgeon experience, specific surgical application, and patient and surgeon preference.3-5 Current practice focuses on the shape and fill material of breast implants as important factors in selection.1 However, other physical properties also help differentiate among available implants and aid in the selection process.1 In the present report, we describe physical testing of 3 textured shaped, 6 textured round, and 8 smooth round silicone gel breast implants manufactured by Allergan (Allergan plc, Dublin, Ireland), Mentor (Santa Barbara, CA), Sientra (Santa Barbara, CA), and Establishment Labs (Alajuela, Costa Rica) in a laboratory setting. This is the first time that similar implants from different manufacturers have been compared with respect to key characteristics that can be objectively and quantitatively measured, including form stability, gel material properties, and shell thickness.
METHODS
Implants of moderate profile with similar base width and height measurements were selected for the study. All implants were analyzed in an identical fashion to determine their individual properties. The implants and their manufacturers evaluated are identified in Table 1, and the physical properties assessed are defined in Table 2. The gel material for the silicone implants evaluated in this study was supplied by 2 vendors, NuSil Technology (Carpinteria, CA) and Applied Silicone Corporation (Santa Paula, CA), according to individual manufacturer’s specifications and formulated according to ISO 9001 standards. Analyses were conducted from March 2015 to October 2016.
Table 1.
Implants Measured for Form Stability, Gel Material Properties, and Shell Thickness
| Manufacturer | Stylea | Volume |
|---|---|---|
| Smooth round | ||
| Allergan SRM-310 | Natrelle Inspira Smooth Round Moderate Profile TruForm 1 | 310 cc |
| Allergan SSM-310 | Natrelle Inspira Smooth Round Moderate Profile TruForm 2 | 310 cc |
| Allergan SCM-310 | Natrelle Inspira Smooth Round Moderate Profile TruForm 3 | 310 cc |
| Mentor 350-3001BC | MemoryGel Smooth Round Moderate Plus Profile Style 1000 (Cohesive I) | 300 cc |
| Sientra10621-355MP | Smooth Round Moderate Plus Profile (High-Strength Cohesive) | 355 cc |
| Sientra 10721-355MP | Smooth Round Moderate Profile (High-Strength Cohesive Plus) | 355 cc |
| Establishment Labs ERSD-340Q | Motiva Ergonomix Round SILKSURFACE Demi with Qid (ProgressiveGel Ultima) | 340 cc |
| Establishment Labs RSD-340+ | Motiva Round SILKSURFACE Plus Demi (ProgressiveGel Plus) | 340 cc |
| Textured round | ||
| Allergan TRM-310 | Natrelle Inspira Moderate Profile, Biocell TruForm 1 | 310 cc |
| Allergan TSM-310 | Natrelle Inspira Moderate Profile, Biocell TruForm 2 | 310 cc |
| Allergan TCM-310 | Natrelle Inspira Moderate profile, Biocell TruForm 3 | 310 cc |
| Mentor 354–3001 | MemoryGel Round Moderate Plus Profile Style 1000, SILTEX (Cohesive I) | 300 cc |
| Mentor 324–5300 | MemoryGel Round Moderate Plus Profile, SILTEX (Cohesive II) | 300 cc |
| Sientra 20621-355MP | Textured Round Moderate Profile (High-Strength Cohesive) | 355 cc |
| Textured shapedb | ||
| Allergan MM-410280 | Natrelle 410 Moderate Height/Moderate Projection, Biocell TruForm 3 | 280 cc |
| Mentor 354–1208 | MemoryShape Medium Height/Moderate Profile, SILTEX (Cohesive III) | 280 cc |
| Sientra 20645-250MP | Textured Shaped Oval Base Moderate Profile (High-Strength Cohesive Plus) | 250 cc |
aCohesivity of silicone gel increases with higher numbers (ie, Truform 3, Cohesive III, High-Strength Cohesive Plus, and Progressive Gel Plus represent the highest cohesive gel from the respective manufacturers).
bTextured shaped implants are only available with one gel type per manufacturer.
Table 2.
Definitions of Physical Properties of Breast Implants
| Term | Definition |
|---|---|
| Form stability | Shape retention of the breast implant |
| Gel cohesivity | Elastic response of the gel to maximum applied pressure (15 mmHg); a less cohesive gel has higher elastic deformation |
| Resistance to gel deformation | Characterizes response of the gel to resisting deformation; higher resistance indicates greater stiffness |
| Energy absorption | Measures the entire deformation process, reflecting the overall softness of the gel; greater energy absorption indicates a softer gel |
| Shell thickness | Thickness of the material covering the gel that fills the implant |
Form Stability Testing
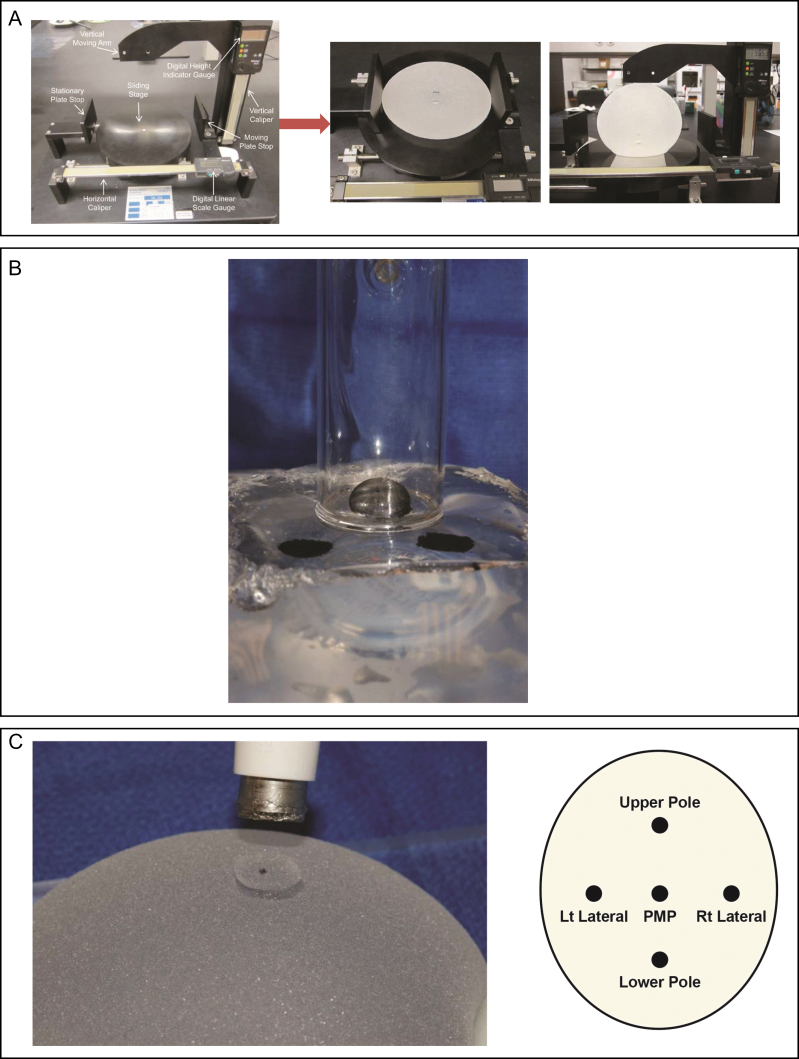
Form stability is a measure of shape retention when the breast implant moves between a horizontal and vertical orientation.6,7 This property was tested using a mandrel height gauge consisting of a platform with horizontal and vertical absolute digimatic calipers (Figure 1A). Six devices of each implant type were tested. The horizontal orientation refers to the implant positioned with the back or posterior device surface placed downward on a horizontal surface, whereas the supported vertical orientation refers to the implant positioned on a vertical support with the lower pole of the device placed downward on a horizontal surface. Parameters measured included the width, height, lower pole depth, and upper pole depth for shaped implants, and height, projection, and upper pole depth for round implants. Each parameter was measured 3 times for each device, with the device removed from the testing instrument and repositioned between measurements. Maximum projection of shaped implants was defined as the lower pole depth when in a horizontal position and maximum projection for round implants was defined as the apex at the center of the device. Upper pole depth is the thickness of the upper pole, which is defined as 17% of the mean horizontal height as measured from the top of a shaped implant, or 25% of the mean horizontal height as measured from the top of a round implant.
Figure 1.
Methodology for measuring physical properties of silicone gel breast implants. (A) Mandrel height gauge used to evaluate form stability. (B) BTC-200 system used to measure silicone gel material properties. (C) Measurement of shell thickness including sample sites for such measurements.
Less change in implant dimensions when moved from the horizontal to vertical orientation is indicative of better form stability (shape retention). The mean values determined from the 3 measurements of each parameter (width, height, lower pole depth, and upper pole depth) were used to calculate retention and net per cent change in dimension. Retention is an index of the amount of change in dimension, with 100% indicating no change. Mean per cent retention of dimensions was calculated for each parameter as (vertical value/horizontal value) × 100. Net per cent change in dimension is a measure of the change in retention, where 0% represents no change in dimension and the farther away from 0%, the greater the change (ie, higher percentages represent greater changes). Net per cent change in dimension was calculated as ([vertical value – horizontal value]/[horizontal value]) × 100.
Gel Material Property Testing
The breast implant gel cohesivity (elastic deformation), resistance to gel deformation (stiffness), and energy absorption (softness) were tested using a BTC-2000 system (SRLI Technologies, Franklin, TN) in 8 devices of each implant type (Figure 1B). This instrument applies negative pressure to an elastic material while measuring the dynamic response of material deformation using a synchronized target laser. A test site (≈1 cm in diameter) was prepared by cutting an opening in the anterior shell of the implant, removing the shell and dusting the exposed gel with toner for laser target detection. The BTC test chamber was lowered onto the sample surface with a maximum of 5 grams of force applied to create a vacuum seal, and then negative pressure was applied at ≈1 mmHg per second, up to a maximum pressure differential from ambient of 15 mmHg. The gel in each implant was tested at 3 sites, all at or near the apex of the anterior side of the device.
Gel cohesivity is the amount of gel deformation obtained up to the point of maximum negative pressure applied (ie, 15 mmHg), and was measured as distance in mm. Higher elastic deformation depicts a less cohesive gel. Resistance to gel deformation was calculated from the slope of the linear region (0-8 mmHg) in the pressure-deformation curve and measured in mmHg/mm. Higher values indicate greater gel resistance to deformation. Energy absorption reflects the entire deformation response to the applied negative pressure, and indicates the overall softness of the gel expressed as mmHg•mm. Higher values indicate a softer gel (ie, softer gels absorb more energy).
Shell Thickness Testing
The thickness of the shell surrounding the silicone-filled breast implant was measured at 5 regions around the anterior shell of each breast implant: left lateral, right lateral, lower pole, upper pole, and point of maximum projection (Figure 1C). Eight devices of each implant type were tested. The shell samples were collected using a 12-mm biopsy punch, the gel was removed from the shell by wiping with isopropyl alcohol, and the thickness of the shell samples were measured using a digital thickness gauge caliper.
Statistical Analysis
The form stability of textured shaped implants was compared using the Wilcoxon rank-sum test for two group comparisons, whereas the form stability of smooth round implants was compared using an analysis of variance (ANOVA) with a Tukey’s post hoc test. The gel material properties and shell thickness were compared using ANOVA. For each comparison, P < 0.05 was required for statistical significance.
RESULTS
Form Stability
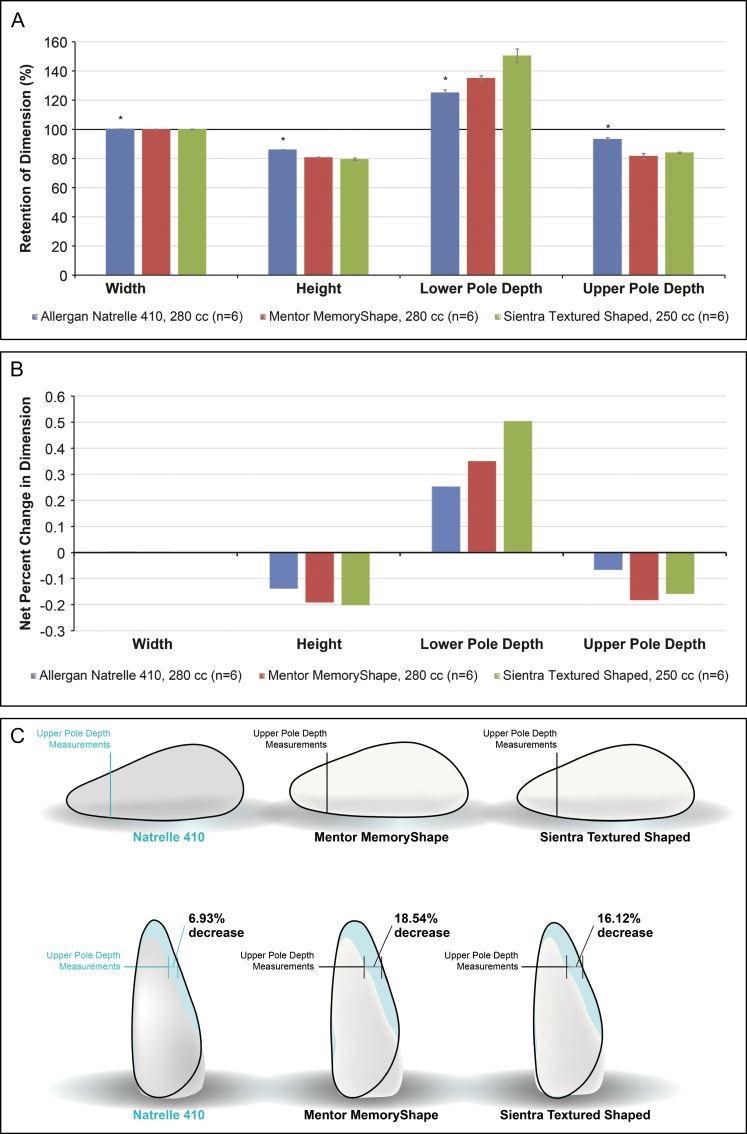
Of the textured shaped devices tested, Allergan Natrelle 410 implants containing TruForm 3 gel retained significantly more of the original dimensions (ie, closer to 100%) in height, lower pole depth, and upper pole depth when moved from a horizontal orientation to a vertical supported orientation compared with Mentor MemoryShape and Sientra Textured Shaped implants, indicating greater form stability for these parameters. Mean per cent retention of height, lower pole depth, and upper pole depth was 85.8%, 125.1%, and 93.1%, respectively, for the Allergan Natrelle 410 implants, 80.5%, 134.9%, and 81.5%, respectively, for the Mentor MemoryShape implants, and 79.4%, 150.5%, and 83.9%, respectively, for the Sientra implants (P < 0.05 for all comparisons of Allergan Natrelle 410 vs other implants [Figure 2A]). Mean per cent retention of width ranged from 99.9% to 100.2% for the 3 implants. The net per cent changes in height, lower pole depth, and upper pole depth were smaller (ie, closer to 0%) with the Allergan Natrelle 410 implants compared with the other textured shaped devices (Figure 2B). The absolute net per cent change in the upper pole depth measurement when moving from a horizontal to vertical supported orientation is shown in Figure 2C, ranging from a decrease of 6.9% with the Allergan Natrelle 410 implant to a decrease of 18.5% with the Mentor MemoryShape implant.
Figure 2.
Form stability of textured shaped breast implants. (A) Mean per cent retention of dimensions; 100% indicates no change in dimensions when implant is moved from the horizontal to vertical orientation. *P < 0.05 for comparisons between Allergan Natrelle 410 vs Mentor MemoryShape and Sientra Textured Shaped implants. (B) Net per cent change in dimensions when the breast implant was moved from a horizontal to a vertical supported orientation. When the implant moves from horizontal to vertical, height will decrease but lower pole and projection will increase giving a value >100% for retention for lower pole/projection. (C) Change in upper pole depth measurements when the implant was moved from a horizontal to a vertical supported orientation.
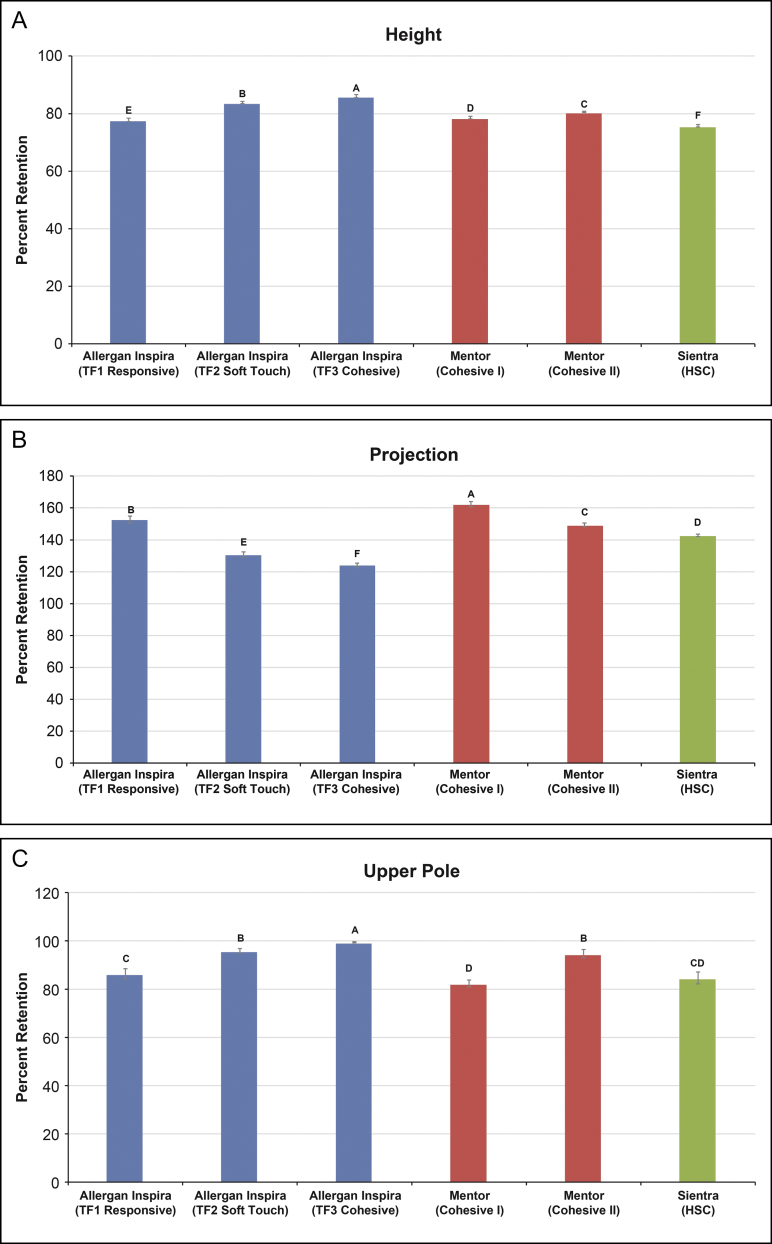
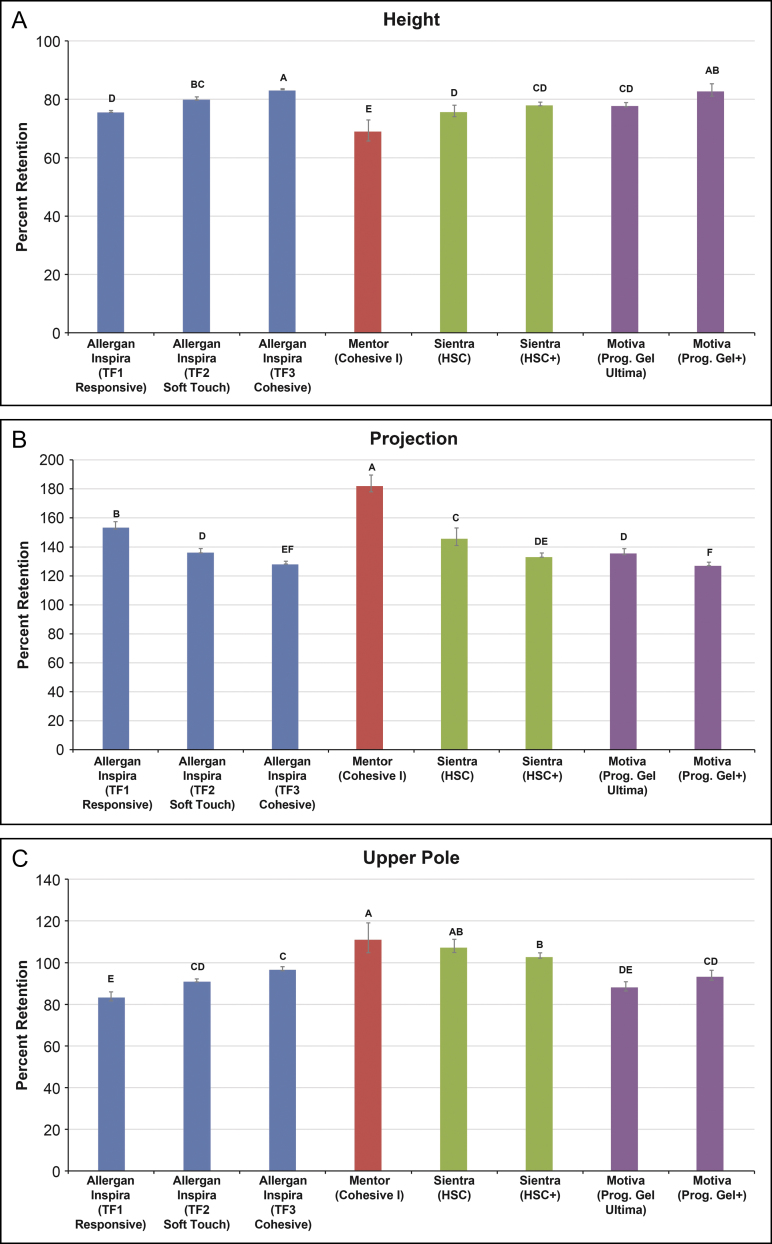
Form stability differed among the textured and smooth round implants depending on the silicone gel type. Among the Allergan textured round devices, Inspira implants containing TruForm 3 gel were significantly more form stable with respect to height, projection, and upper pole depth than those containing TruForm 2 gel (P ≤ 0.012), which in turn had greater form stability in the same dimensions compared with implants containing TruForm 1 gel (P ≤ 0.001) (Figure 3). For the Allergan smooth round devices, Inspira implants containing TruForm 3 gel were significantly more form stable with respect to height and projection than those containing TruForm 2 gel (P ≤ 0.04), which in turn had greater form stability in height and projection compared with implants containing TruForm 1 gel (P ≤ 0.002) (Figure 4A,B). Retention of upper pole depth was also significantly greater with Inspira smooth round implants containing TruForm 2 gel compared with TruForm 1 gel (P = 0.003) (Figure 4C).
Figure 3.
Form stability of textured round breast implants. Mean per cent retention of height (A), projection (B), and upper pole depth (C) when moving from a horizontal to a vertical supported orientation. When the implant moves from horizontal to vertical, height will decrease but lower pole and projection will increase giving a value >100% for retention for lower pole/projection. Retention values that do not share a letter are significantly different (P < 0.05). Error bars represent the standard deviation. HSC, high-strength cohesive; TF, TruForm.
Figure 4.
Form stability of smooth round breast implants. Mean per cent retention of height (A), projection (B), and upper pole depth (C) when moving from a horizontal to a vertical supported orientation. Retention values that do not share a letter are significantly different (P < 0.05). Error bars represent the standard deviation. In panel C, values for upper pole depth above 100% reflect collapsing of the implant in the vertical orientation. HSC, high-strength cohesive; Prog., progressive; TF, TruForm.
The greatest form stability among textured round devices was found with Inspira implants containing TruForm 3 gel followed by Inspira implants containing TruForm 2 gel (Figure 3). The devices containing TruForm 3 gel had significantly greater form stability in height, projection, and upper pole depth compared with each of the other textured round devices (P < 0.001 for all comparisons, except P = 0.012 for comparison of upper pole depth vs TruForm 2). In contrast, the Sientra device containing High-Strength Cohesive gel had the lowest form stability in height, and the Mentor device containing Cohesive I gel had the lowest form stability in projection. Both of these devices as well as the Inspira implant containing TruForm 1 gel had lower form stability in upper pole depth compared with the other tested textured round devices. The net per cent change in dimensions when moving from horizontal to vertical supported orientation was smallest for Inspira implants containing TruForm 3 gel (−14.0% in height, 24.3% in projection, and −0.6% in upper pole depth) or TruForm 2 gel (−16.2%, 30.8%, and −4.1%, respectively), and greatest for the Sientra device in terms of height (−24.2%) and for the Mentor device containing Cohesive I gel in terms of projection (62.3%) and upper pole depth (−17.6%).
Among the smooth round devices, form stability of the Inspira implants containing TruForm 3 gel were generally comparable to Motiva implants containing ProgressiveGel Plus, whereas form stability of Inspira implants containing TruForm 2 gel were generally comparable to Motiva implants containing ProgressiveGel Ultima (Figure 4). However, other comparisons between smooth round implants showed a significant difference in retention of at least 1 dimension when moving from a horizontal to vertical supported orientation. Of the devices tested, the Mentor implant containing Cohesive I gel exhibited the lowest form stability in each dimension. The net per cent change in dimensions when moving from horizontal to vertical supported orientation were smallest for Inspira implants containing TruForm 3 gel (−16.5% in height, 29.2% in projection, and −2.9% in upper pole depth), Motiva implants containing ProgressiveGel Plus (−16.8%, 28.3%, −6.1%, respectively), and Sientra implants containing High-Strength Cohesive Plus gel (−21.5%, 34.4%, and 3.5%, respectively), and greatest for the Mentor implant containing Cohesive I gel (−30.6%, 83.7%, and 11.9%, respectively).
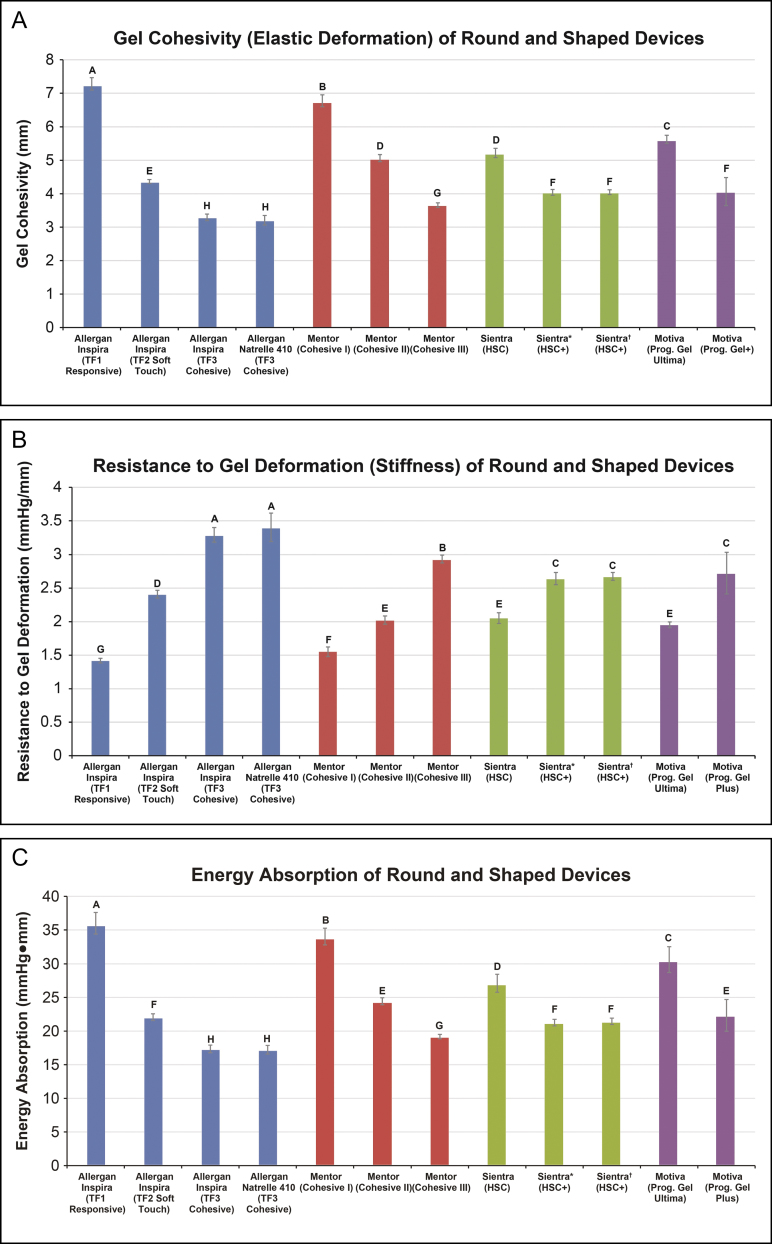
Gel Material Properties
Among the shaped devices, Allergan Natrelle 410 implants containing TruForm 3 gel had significantly higher gel cohesivity (P < 0.0001), greater resistance to gel deformation (P < 0.0001), and lower energy absorption (P ≤ 0.002) compared with Mentor MemoryShape and Sientra Textured Shaped implants (Figure 5). Similarly, of the Allergan textured and smooth round devices, Inspira implants containing TruForm 3 gel had higher gel cohesivity, greater resistance to gel deformation, and lower energy absorption (all P < 0.0001) than implants containing TruForm 2 gel, which in turn had higher gel cohesivity, greater resistance to gel deformation, and lower energy absorption than implants containing TruForm 1 gel (all P < 0.0001).
Figure 5.
Gel material properties of round and textured shaped breast implants: mean gel cohesivity (A), resistance to gel deformation (B), and energy absorption (C). Textured shaped implants are Allergan Natrelle 410, Mentor Cohesive III, and Sientra High-Strength Cohesive Plus (250MP). Values that do not share a letter are significantly different (P < 0.05). Error bars represent the standard deviation. *355MP. †250MP. HSC, high-strength cohesive; Prog., progressive; TF, TruForm.
In general, the gel material properties of the breast implants we studied differed significantly across devices. The rank order from highest to lowest gel cohesivity was Allergan Natrelle 410 and Inspira implant containing TruForm 3 gel, followed by Mentor textured shaped implant containing Cohesive III gel, Sientra implant containing High-Strength Cohesive-Plus gel, and Motiva implant containing ProgressiveGel Plus. This was followed by the Inspira implant containing TruForm 2 gel, Mentor implant containing Cohesive II gel, Sientra implant containing High-Strength Cohesive gel, Motiva implant containing ProgressiveGel Ultima, Mentor implant containing Cohesive I gel, then the Inspira implant containing TruForm 1 gel (Figure 5A). The rank order was generally similar for greater resistance to gel deformation and lower energy absorption (Figure 5B,C).
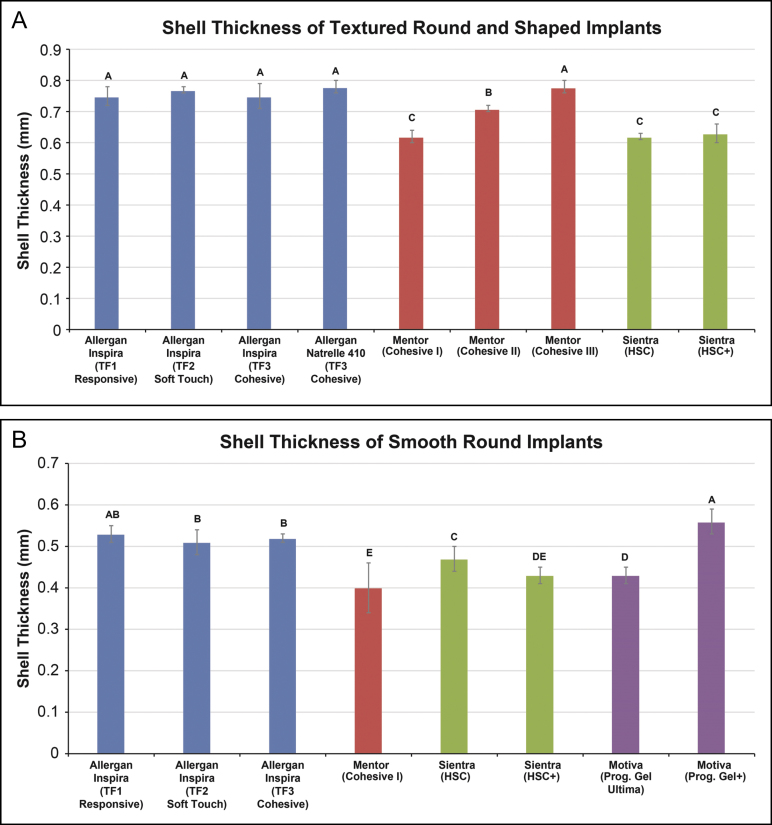
Shell Thickness
Shell thickness was consistent across all Allergan textured implants, regardless of shape. Of the textured shaped devices, Allergan Natrelle 410 implants and Mentor MemoryShape implants had the same mean shell thickness (0.78 mm), both of which were significantly greater than that of the Sientra Textured Shaped implants (0.63 mm; P < 0.0001) (Figure 6A). Of the textured round devices, the mean thickness of the Inspira implants containing TruForm 1, TruForm 2, or TruForm 3 gel ranged from 0.75 to 0.77 mm (P ≥ 0.06), which was significantly greater than the mean shell thickness of the Mentor implants containing Cohesive Gel II (0.71 mm) or Cohesive Gel I (0.62 mm) and the Sientra implant containing High-Strength Cohesive gel (0.62 mm; P < 0.0001). Of the smooth round devices, the mean shell thickness of the Allergan Inspira TruForm 1, TruForm 2, and TruForm 3 implants ranged from 0.51 to 0.53 mm (P ≥ 0.12) (Figure 6B). Except for the Motiva implant containing ProgressiveGel Plus with a mean shell thickness of 0.56 mm, the other smooth round implants had a mean shell thickness of ≤0.47 mm.
Figure 6.
Mean shell thickness of breast implants. Statistical comparisons were made separately among textured (A) and smooth (B) implants. Textured shaped implants are Allergan Natrelle 410, Mentor Cohesive III, and Sientra High-Strength Cohesive Plus. Values that do not share a letter are significantly different (P < 0.05). Error bars represent the standard deviation. HSC, high-strength cohesive; Prog., progressive; TF, TruForm.
DISCUSSION
This is the first study to provide a comprehensive assessment of the physical properties of similar breast implants across manufacturers using material science testing protocols. Our findings provide unique comparative and differentiating information on a range of silicone breast implants beyond shape, size, and surface texture. The differences in form stability and gel material properties among breast implant styles and brands evaluated in this study may contribute to differences in performance of the devices in varying clinical settings. Form stability, as defined herein, is a measure of shape retention of the breast implant. Greater form stability is associated with less implant and shell collapse and apex tilt, and as a result, the upper pole is less likely to bend in an upright position.7 Form stable implants have increased shape retention when moved between the horizontal and vertical planes. Higher gel cohesivity, greater resistance to gel deformation, and less energy absorption contribute to greater form stability. Our data show that gel cohesivity, resistance to gel deformation, and energy absorption are directly related to form stability, which in turn determines shape retention. Other factors, such as shell thickness, may also impact form stability.
Increasing the complexity of the silicone cross-linking influences the physical properties of the gel and form stability of the implant.6 The results in this study showed differing form stability based on the silicone gel type. Comparisons were made among 3 textured shaped devices with highly cohesive gels. Allergan Natrelle 410 implants containing TruForm 3 gel, had greater gel cohesivity and, in turn, provided greater form stability, compared with the Mentor MemoryShape implant containing Cohesive III gel and Sientra Textured Shaped implant containing High-Strength Cohesive Plus gel. From a clinical perspective, devices with highly cohesive gels have enough firmness to help shape the breast while devices with less cohesive gels may be shaped by the breast.
Our evaluation of round implants allowed assessments across a range of gels within and across manufacturers. Comparisons of implants within manufacturers demonstrated that more cohesive gels were associated with increasingly greater form stability. Between manufacturers, however, there were differences in form stability among round implants with similar gel material properties. For example, there was greater upper pole collapse with smooth round Mentor Cohesive I implants and Sientra High-Strength Cohesive implants compared with Allergan TruForm 1 implants when moved from a horizontal to vertical supported orientation. Some similarities did exist in that smooth round Inspira implants with TruForm 3 gel and TruForm 2 gel were generally comparable to Motiva implants with ProgressiveGel Plus and ProgressiveGel Ultima, respectively, in regard to form stability. Gel material properties varied across implants and manufacturers with greater resistance to gel deformation and lower energy absorption observed in the more cohesive gels compared with the less cohesive gels. Further, a hierarchy was observed within implants containing gels of similar cohesivity (eg, Allergan Inspira TruForm 3 exhibited greater resistance to gel deformation and less energy absorption compared with the Mentor Cohesive III, Sientra High-Strength Cohesive Plus, and Motiva ProgressiveGel Plus implants). Although comparisons of physical properties between higher fill and lower fill implants are not reported here, clinical experience has shown that fill volume should be considered as one factor in choosing the appropriate implant based on its contribution to implant firmness.8
The shell thickness of the textured shaped implants was significantly greater than that of the smooth round implants. This observation was not unexpected in that the layers of texture added to smooth devices to create a textured surface would naturally result in a thicker shell.
We developed a novel technique for quantifying form stability using the mandrel height gauge. Other studies have used manual or imaging methods to determine changes in breast implants when moved from a horizontal to vertical orientation.9-11 The “tilt” test is a qualitative method of evaluating form stability that involves holding an implant in one hand and tilting it vertically while observing the upper pole for changes in dimension. Tebbetts used the “tilt” test to ascertain adequate fill volume of round or anatomic shaped saline-filled implants prior to breast augmentation, and found a very low rate of wrinkling or rippling that required reoperation.9 Imaging was used in a morphological analysis comparing shaped and round breast implants manufactured by Allergan, Mentor, and Sientra, and demonstrated changes in upper pole dimension when moving the implants from a horizontal to a vertical orientation.10 An in vivo magnetic resonance imaging study conducted at least 12 months after implantation of the Allergan Natrelle 410 breast implant in 9 patients found changes in dimension on movement from the supine to prone position, including a mean 29.5% increase in maximum projection.11
Other investigators have examined the physical properties of the filler gel.10,12 Kinney and colleagues used the BTC-2000 method to evaluate the gel material properties of round and anatomic shaped implants.10 Of the shaped implants, the Allergan Natrelle 410 exhibited less gel elasticity compared with the Sientra implants containing High-Strength Cohesive Plus gel,10 a finding confirmed in the present study. The round implants tested differed from those evaluated in the current study. Atlan and coworkers evaluated gel stiffness of 5 shaped implants by measuring resistance to penetration.12 The Allergan Natrelle 410 implant containing TruForm 3 gel exhibited less resistance to gel penetration (ie, were firmer) compared with an implant containing TruForm 2 gel,12 which is consistent with findings reported in the current study for round implants with these respective gels. The other shaped implants evaluated in that study were not included in the present study.
Several limitations in the present study should be noted. First, fill volume and fill ratio are additional factors contributing to the softness of breast implants,3,6 but were not measurable. Second, there are no universally accepted methods for quantifying the physical properties examined in this study. Third, although the differences observed among implants are likely to affect the outcome of breast surgery, the clinical significance of experimental testing has not been established (eg, resistance to gel deformation defines one physical property of a gel and not necessarily the clinical feel of the implant). As noted above, individual patient factors, such as skin elasticity, breast volume, breast tissue firmness, and pocket selection, as well as surgeon and technical factors, also affect the final outcome of breast shaping with implants.3,4,13
The information reported in this study may be useful to surgeons when choosing among the specific styles available. The optimal degree of shape retention in a breast implant is a matter of patient and surgeon preference and depends upon many patient-specific factors, including soft tissue coverage, skin elasticity, and individual breast anatomy.13 Implant pocket location is also important in shape retention and may differ based on implant shape and surgeon preference.14,15 Form stability and shape retention in gel implants offer surgeons the option of managing volume distribution within the breast envelope. Depending on the degree of form stability, the implant may either hold its shape and position or, if less cohesive, the gel may redistribute into the lower portion of the implant or breast pocket. If an anatomically shaped implant does not retain its shape or hold its position secondary to decreased gel cohesivity, the relative benefits of shaped implants vs round implants may be negated. Round implant cohesivity is also critically important in implant selection. In patients with an extremely thin skin envelope undergoing primary augmentation, revision-augmentation or reconstruction it is often preferable to use an implant with the highest level of cohesivity because it shows the least amount of clinical rippling and wrinkling. In patients with adequate soft tissue coverage, it may be preferable to match a patient’s parenchymal feel with the implant cohesivity. Ongoing clinical studies are utilizing high resolution ultrasound to evaluate implants with varying degrees of cohesivity in vivo, looking at shell collapse and wrinkling with patients in the upright position (BP Bengtson, unpublished data). Early results from this study show that in vitro form stability is also translated into clinical outcomes with the devices having the greatest form stability showing the least visible deformity both in vitro and in vivo.
CONCLUSION
The breast implants tested in this study showed a range of differences in form stability, gel material properties, and shell thickness that may be associated with differences in clinical performance. The selection of implant types (ie, shaped vs round) and available gel formulations based on level of cohesivity and other physical properties varied among manufacturers. Taken together, the physical properties of the implants evaluated in this study may provide surgeons with increasing options for achieving the best aesthetic results based on individualized preoperative planning. Using this information, plastic surgeons will be better equipped to select a specific style of implant that matches the patient breast characteristics, surgical application, and desired outcome.
Disclosures
Dr Jewell is a consultant for Allergan and NewBeauty magazine. Dr Bengtson is a consultant for Allergan and LifeCell (acquired by Allergan in 2017). Ms Smither and Dr Perry were employees of Allergan at the time of study conduct and manuscript preparation. Ms Nuti is an employee of Allergan plc.
Funding
This study was funded by Allergan plc (Dublin, Ireland). Writing and editorial support was provided to the authors by Barry Weichman, PhD, of Peloton Advantage (Parsippany, NJ) and was funded by Allergan plc. Allergan plc participated in the development of the study design and in the collection, analysis and interpretation of data. The authors received no honorarium or other form of financial support related to the development of this article.
REFERENCES
- 1. Hedén P, Montemurro P, Adams WP Jr, Germann G, Scheflan M, Maxwell GP. Anatomical and round breast implants: how to select and indications for use. Plast Reconstr Surg. 2015;136(2):263-272. [DOI] [PubMed] [Google Scholar]
- 2. Maxwell GP, Scheflan M, Spear S, Nava MB, Hedén P. Benefits and limitations of macrotextured breast implants and consensus recommendations for optimizing their effectiveness. Aesthet Surg J. 2014;34(6):876-881. [DOI] [PubMed] [Google Scholar]
- 3. Adams WP Jr, Small KH. The process of breast augmentation with special focus on patient education, patient selection and implant selection. Clin Plast Surg. 2015;42(4):413-426. [DOI] [PubMed] [Google Scholar]
- 4. Hedén P, Brown MH, Luan J, Maxwell GP, Munhoz AM, Carter M. Delphi study consensus recommendations: patient selection and preoperative planning measurements for Natrelle 410. Plast Reconstr Surg Glob Open. 2015;3(11):e556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mallucci P, Branford OA. Design for natural breast augmentation: the ICE principle. Plast Reconstr Surg. 2016;137(6):1728-1737. [DOI] [PubMed] [Google Scholar]
- 6. Calobrace MB, Capizzi PJ. The biology and evolution of cohesive gel and shaped implants. Plast Reconstr Surg. 2014;134:6S-11S. [DOI] [PubMed] [Google Scholar]
- 7. Bengtson BP. The highly cohesive, style 410 form-stable gel implant for primary breast augmentation. In: Spear SL, ed. Surgery of the Breast. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011:1346-1365. [Google Scholar]
- 8. Jewell ML, Jewell JL. A comparison of outcomes involving highly cohesive, form-stable breast implants from two manufacturers in patients undergoing primary breast augmentation. Aesthet Surg J. 2010;30(1):51-65. [DOI] [PubMed] [Google Scholar]
- 9. Tebbetts JB. Patient acceptance of adequately filled breast implants using the tilt test. Plast Reconstr Surg. 2000;106(1):139-147; discussion 148. [DOI] [PubMed] [Google Scholar]
- 10. Kinney BM, Jeffers LL, Ratliff GE, Carlisle DA. Silicone gel breast implants: science and testing. Plast Reconstr Surg. 2014;134:47S-56S. [DOI] [PubMed] [Google Scholar]
- 11. Weum S, de Weerd L, Kristiansen B. Form stability of the Style 410 anatomically shaped cohesive silicone gel-filled breast implant in subglandular breast augmentation evaluated with magnetic resonance imaging. Plast Reconstr Surg. 2011;127(1):409-413. [DOI] [PubMed] [Google Scholar]
- 12. Atlan M, Bigerelle M, Larreta-garde V, Hindié M, Hedén P. Characterization of breast implant surfaces, shapes, and biomechanics: a comparison of high cohesive anatomically shaped textured silicone, breast implants from three different manufacturers. Aesthetic Plast Surg. 2016;40(1):89-97. [DOI] [PubMed] [Google Scholar]
- 13. Vegas MR, Martin del Yerro JL. Stiffness, compliance, resilience, and creep deformation: understanding implant-soft tissue dynamics in the augmented breast: fundamentals based on materials science. Aesthetic Plast Surg. 2013;37(5):922-930. [DOI] [PubMed] [Google Scholar]
- 14. Calobrace MB. Teaching breast augmentation: a focus on critical intraoperative techniques and decision making to maximize results and minimize revisions. Clin Plast Surg. 2015;42(4):493-504. [DOI] [PubMed] [Google Scholar]
- 15. Maxwell GP, Brown MH, Hedén P, Luan J, Munhoz AM, Carter M. Delphi consensus recommendations: intraoperative technique and postoperative management of patients with Natrelle 410 implants. Plast Reconstr Surg Glob Open. 2015;3(11):e557. [DOI] [PMC free article] [PubMed] [Google Scholar]