Abstract
The objective was to develop a stable and non-compliance coated solid–lipid nanoparticles (coated SLN) using polymer (Eudragit L100) and lipoid (glycerol monostearate: soya lecithin) for partial dose reduction of isradipine [ISR; 2.5 mg by combination of bioenhancing agent (rutin; Ru) in equivalent ratio]. The physicochemical characterizations were performed by FT-IR and DSC of elected model drug (ISR), drug mixer with Ru/polymer and coated SLN with Ru (ONbp); the resulted distinctive peaks demonstrated that no chemical interaction and incompatibility found between them. The plasma samples of formulation (ONbp) were analyzed by liquid chromatography (HPLC) using UV-spectrometer. Data were integrated and analyzed with the help of a computer-designed program “Kinetica Software” (Thermo Scientific Kinetica, PK/PD Analysis, version 5.0, Philadelphia, PA). The pharmacokinetic study showed 3.2- to 4.7-folds enhancement in oral bioavailability of coated SLN of ISR with Ru (ONbp) when compared to a coated formulation of ISR without Ru (ONps) and conventional drug suspension. In vivo studies were revealed significantly at greater extent in (drug stability and solubility) oral absorption, which has shown potential entrapment efficiency (97.85% ± 1.02%) to improve biological activity against hypertension. Hence, nano-system of ISR against hypertension is achieved with consequent dose reduction with enhanced systemic bioavailability.
Keywords: nano-colloidal, bioenhancer, coated solid–lipid nanoparticles, hypertension, drug delivery
Introduction
In the beginning of 1990, lipid-based nano-drug delivery system (LBDDS) technology brought innovatory in the pharmaceutical sciences drug delivery, treatment, and management. This advancement further leads to developing newfangled nano-carrier or vehicles that are useful for preparing new nano-formulations for management of one of non-infectious cardiovascular diseases, i.e., hypertension. Various LBDDS approaches for successful tool for hypertension management are microemulsions, nanoemulsion, self-emulsifying drug delivery system, solid–lipid nanoparticles (SLN), vesicular system, such as transfersomes, liposomes, etc. LBDDS presents an opportunity for formulation scientists to overcome several challenges associated with antihypertensive drug therapy, thereby improving the management of patient with hypertension. Most of these antihypertensive drugs bear some significant drawbacks, such as relatively short biological half-life, low permeability, and undesirable side effects. LBDDS is most important tool to overcome the drawbacks, minimize the problem of conventional therapy like poor solubility and dissolution, poor penetration, hepatic first-pass metabolism, etc., and provide a delivery system, which is stable and encapsulates both hydrophilic and lipophilic drugs, which release the drug in controlled and targeted way to minimize the side effects and provide a means of non-invasive delivery [1–4]. There are number of antihypertensive drugs, which are encapsulated in polymer as well as lipid-based formulations to improve their bioavailability, such as nisoldipine, lercandipine, valsartan, nimodipine, isradipine (ISR) etc. [5–12]. This point was a key to begin an aim to develop a polymeric-lipid carrier as antihypertensive system, which should be superior in activity, tolerability, or both. Additionally, herbal flavonoid component, i.e., Rutin (Ru, quercetin disaccharide conjugate) is used as bioenhancing agent. The specific goals of the research were to develop a nano-colloidal carrier, i.e., coated SLN containing ISR using homogenization ultrasonic probe-techno with enhancing agent (Ru) and performed its characterization in vitro as well as in vivo studies. To investigate the potential of ISR along with Ru lipid drug delivery system, which can be further considered as oral drug delivery system against hypertension.
Materials and Methods
A free sample of ISR was obtained from Orchid Chemicals & Pharmaceuticals Ltd., Tamil Nadu, India. Lipoid [glycerol monostearate (GMS)], soya lecithin, and polysorbate (co-surfactant Tween 80) were received from Central Drug House Pvt. Ltd. (India). The Eudragit (EL100) and Rutin (Ru) were procured from Sigma-Aldrich Chemicals Pvt. Ltd. (India) for the preparation of hydrochloric acid (HCl; pH 1.2), phosphate buffer (pH 7.4 solutions), potassium phosphate monobasic, and solvents were purchased from Sigma Aldrich Chemicals Pvt. Ltd. (India), respectively.
Formulation of ISR via Ru-loaded coated SLN
The drug (ISR) loaded coated SLN with bioenhancer (ONbp) and without bioenhancer (ONps) was prepared using lipid to surfactant ratio [GMS: soya lecithin ratio 2.218, polymer concentration (Eudragit L100, 1.212 %w/w and sonication time, 23.79 (≃24 min) by modification the homogenization followed by ultrasonication method [11, 12]. The obtained nanosuspension was kept at −2 to 3 °C for 10 min. Finally, the converted coated nanoparticles were stored at 4 °C in a glass container [13, 14].
Characterization
The physicochemical compatibility between formulation with final excipient used in the study for drug-excipient interaction by differential scanning calorimetry (DSC; Pyris 6 DSC, Perkin Elmer, USA) and Fourier transform infrared (FT-IR; Agilent Cary 630, India) spectroscopy.
Morphology, particle size, and entrapment efficiency
The surface morphology of coated SLN with Ru nanoparticles was studied by high-resolution transmission electron microscopy operating at 300 KV and indicated magnification of 15 k×. The analysis of particles size was performed using fresh centrifugation, and resulted suspension droplet was placed on dried air surrounding glass slide. The diluted nanoparticles size was determined by dynamic light-scattering method, using a computerized inspection system (Zetasizer Nano ZS®, Malvern Instruments, UK) in triplicate for the clarity. The entrapment capacity of formulation was determined by an indirect method [11, 12].
Zeta potential
The charge on drug with bioenhancer-loaded particles surface was determined using Zetasizer Nano ZS, Malvern Instruments, Worcesterhire, UK. The measurements were made in triplicate.
In vitro simulated gastrointestinal fluid stability
During oral drug delivery of SLN, there was a barrier that is gastrointestinal tract environment, which can be an obstacle for absorption and create negative effect on the stability of SLN formulations [15]. Therefore, in vitro stability of formulation of ISR-loaded coated SLN with Ru (ONbp) and without Ru (ONps) was compared with conventional drug-loaded nano-suspension. All formulations were studied in simulated gastrointestinal fluid solutions [pH 1.2 simulated gastric fluid – SGF solutions with pepsin and pH 6.8 simulated intestinal fluid – SIF solutions] [16].
Preparation of SGF and SIF
The SGF prepared by sodium chloride (2.0 g) and purified pepsin (3.2 g with an activity of 800–2,500 units per mg protein) was dissolved in hydrochloric acid (7.0 ml) and volume was made up to 1 L with purified water and set to pH 1.2. Intestinal solution of pH 6.8 was prepared by dissolving monobasic potassium phosphate (6.8 g), sodium hydroxide (77 ml) having normality 0.2 N and pancreatin (10 g) in purified water, and volume was adjusted to 500 ml. The obtained resulting solution was adjusted to pH 6.8 by adding sodium hydroxide (0.25 N) or HCl (0.2 N) before diluted with water to make 1 L [United States Pharmacopeia (USP), XXVII, 2007] [17]. The formulation particle sizes were studied for 0, 1, and 2 h in SGF after 2 h in SIF up to 6 h.
Ex vivo permeation studies
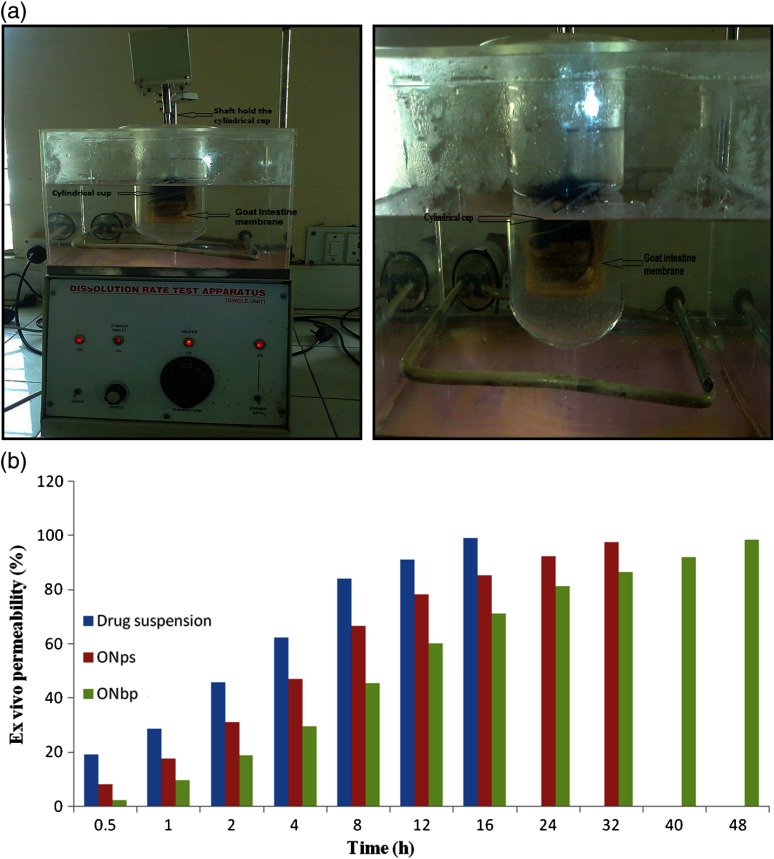
The procedure used was a modification of Barr and Riegelman’s method [18] with some adjustments. Goat intestine of scarified goat collected from slaughter house and a section of intestine (about 7 cm) was removed from goat. Intestine was cleaned with simple saline using pressure pump and washed with Krebs–Ringer bicarbonate solution, pH 7.4. The formulations equivalent to 1 day dose of drug encapsulated into capsule cell and placed in the cylindrical cup, which was connected to shaft, and stabilized membrane was tied at the lower end of cylindrical cup. The cup of tied membrane was immersed in USP dissolution jar containing 250 ml of intestinal fluid (pH 7.4 at 37 ± 2 °C). The medium was stirred at 45 rpm and the samples were withdrawn at predetermined time intervals and same quantity of sample was replaced with fresh medium. The analysis was carried out by UV-spectrophotometer (UV Jasco spectrophotometer) at λmax (326 nm) of ISR.
Release studies and kinetics
To determine the formulation release in gastrointestinal fluid using dialysis membrane method and to ascertain the release kinetics of the formulations, the release data were applied to zero order, first order, Higuchi kinetics models, and Korsmeyer–Peppas equations used to evaluate the release mechanism.
In vivo studies
Animals
Wistar rats weighing 200–250 g [Approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals of PDM College of Pharmacy, Bahadurgarh, Protocol approval No. PDM/CPCESA/RES/IAEC-2017-II (2)] was used. The rats were kept under standardized conditions in clean cages with free access to food and water. The animals were acclimatized to laboratory conditions over the week before the experiment.
Hypertension-induced model
Hypertension was induced by injecting methyl prednisolone acetate (MPA; 20 mg/kg/week) subcutaneously for 2 weeks. According to the initial blood pressure (BP), the rats were divided into five groups (Groups I–V), each group having six rats (5 × 6 = 30 rats) (Table I). Group I was selected as control (no treatment). Hypertension will be induced in the remaining groups (Groups II–V) by subcutaneous injection of MPA (20 mg/kg/week for 2 weeks) as per the method [14, 19]. The blood sampling was carried out for 48 h and was performed at the decided time periods (0.5, 1, 2, 4, 6, 8, 12, 16, 24, 32, 40, and 48 h).
Table I.
Group and dose calculation for rats based on the body surface area
| Groups | Name of groups | No. of animals | Treatment and dose | Route of administration |
|---|---|---|---|---|
| I | Normal control | 6 | Untreated | No treatment |
| II | Hypertensive (diseases) control | 6 | Methyl prednisolone acetate (20 mg/kg/week for 2 weeks + distilled water orally; 2 ml/kg body weight) | Subcutaneously |
| III | Standard control | 6 | Isradipine drug suspension (0.26 mg/kg) by orally | Oral |
| IV | Test group | 6 | Isradipine-loaded coated solid lipid nanoparticles without bioenhancer, Ru (ONps; 0.13 mg/kg) | Oral |
| V | Test group | 6 | Isradipine-loaded coated solid lipid nanoparticles with bioenhancer, Ru (ONbp; 0.13 mg/kg) | Oral |
Pharmacodynamic and pharmacokinetic evaluation
After 30 min of last MPA injection, the drug was administered once in a dose (the selected dose of ISR is clinically approved dose by Food and Drug Administration) of 0.26 mg/kg, conventional suspension and formulations were administered in a dose of 0.13 mg/kg. This dose was calculated on the basis of surface area and then converted to rat dose [20]. A BP-measuring instrument with a non-invasive tail cuff is a common and convenient method to measure systolic pressure in rats. The rats were trained in order to stay in the rat holder and warming the tail required in a calm and non-aggressive method for BP measurement. Once the “pulse level ready” signal appeared, the BP-recording button will be pressed and the systolic BP will be recorded at different time intervals, i.e., 0, 0.5, 1, 2, 4, 6, 8, 12, 24, 32, 40, and 48 h. All group’s BP was measured from 0 to 48 h after administration. For each rat at each timepoint, three readings were taken and mean was calculated [21, 22]. The pharmacokinetic parameters were measured by taking 0.5 ml of blood from tail (dorsal) vein of 20- to 25-week-old disease-free Wistar rats (weight: 200–250 g) at different time intervals 0.5, 1, 2, 4, 6, 8, 12, 16, 24, 32, 40, and 48 h, post-dose in Eppendorf tubes having ethylene diamine tetra acetic acid and centrifuged at 4,000 rpm for 20 min. The obtained samples stored at −20 °C and their analyses were carried out by reversed-phase high-performance liquid chromatography method [23, 24].
Statistical analysis
The parameters Cmax (peak serum concentration), Tmax (time for peak serum concentration), AUCo-t; AUCo-∞, half-life, mean residence time were studied using Kinetica software (version 5.0). The data from BP experiments and pharmacokinetic data were analyzed by Student’s t-test. p ≤ 0.05 and p ≤ 0.001 were considered statistically significant for data wherever relevant and presented as mean ± standard deviation.
Stability studies
The aim of this study was to understand, design, and develop formulation to get the desired stable pharmaceutical product as per ICH guidelines [25]. The formulation was tested for entrapment and drug content stability by storing them at 4.0 ± 2.0 °C in refrigerator in stability-testing chamber for 3 months. The formulation was collected at prearranged intervals (0, 30, 60, and 90 days) and analysis was carried out according to guidelines.
Results and Discussion
Physicochemical characterizations
During FT-IR (Agilent Cary 630) of elected model drug (ISR), drug mixer with Ru and coated SLN with Ru (ONbp) spectrums were demonstrated to be the most distinctive wave number peaks at 3,322, 3,131, and 3,187 cm−1 (N–H stretching); 2,954, 2,935, and 2,971 cm−1 (O–H stretching); 1,676, 1,710, and 1,735 cm−1 (C=O stretching); and 1,430, 1,443, and 1,449 cm−1 (C=C stretching), respectively, indicated that no chemical interaction and incompatibility found between them. All noteworthy peaks have been appeared in FT-IR overlapping spectrum (3,329 cm−1 stretching of N–H; 2,971 cm−1 stretching of C–H; 2,824 cm−1 stretching of OH; 1,711 cm−1 stretching of C=O; 1,458 cm−1 stretching of carbon–carbon double bond of ONbp) and ISR indicating that there is no interaction and instability between the drug, bioenhancer, and other excipient. Finally, it is concluded that FT-IR spectra showed the characteristic peaks as obtained in the drug spectrum, which demonstrated that there were no remarkable change, indicated that no chemical interaction between the drug, bioenhancer, and other excipients. It is also confirmed by thermal scanning (DSC) analysis, which showed lipid (GMS), polymer (Eudragit L100), drug (ISR), and bioenhancer (Ru) characterized peaks at 65.25, 216.5, 168, and 242 °C, respectively. On the other hand, presence of endothermic peak at 173 °C (onset 167.3–176.8 °C end via ∆H 100.59 J/kg) indicates transformation into nano-bioparticles during the preparation of coated SLN with Ru (ONbp). During the transformation, all peaks of drug, bioenhancer, and excipients were disappeared and confirmed the complete conversion into formulation without showing any interaction. The DSC analysis of formulation (Fig. 1a) showed no detectable peak was observed and showed absence of any interaction after complete transformation into coated SLN with bioenhancer (termed as coated SLN with Ru). FT-IR and DSC analysis results concluded that the formulation of coated SLN with bioenhancer (Ru) showed no interaction and incompatibility during its preparation.
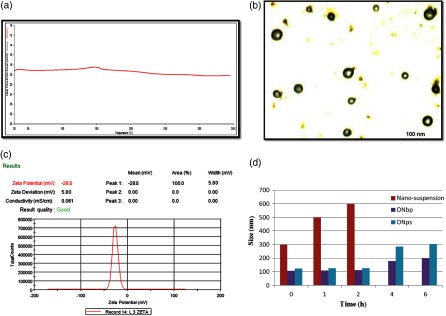
Fig. 1.
(a) The DSC thermogram of formulation (ONbp). (b) TEM image of coated SLN with Ru (ONbp) formulation with average size distribution was 106 ± 3.21 nm. (c) The formulation (ONbp) zeta potential. (d) Particle size of nano-susension, ONbp, and ONbp in gastric fluid (SGF) upto 2 h; after 2 h in intestinal fluid (SIF)
Morphology, particle size, and entrapment efficiency
The electronic image of coated SLN exposed smooth morphology by regular spherical in shape without aggregation and rough surfaces along with fine particle size (Fig. 1b). The percentage entrapment efficiency of drug (ISR) and bioenhancer (Ru) of formulation of coated SLN with Ru (ONbp) was determined by measuring the absorbance of the collected supernatants using UV-VIS spectrophotometer (Jasco V-600, JASCO International Co., Ltd., Japan). The higher entrapment efficiency of ISR (97.85%±1.02%) and Ru (97.58% ± 1.12%) was obtained in coated SLN (ONbp).
Zeta potential
The zeta potential (Fig. 1c) of ONbp formulation was found to be −28.6 mV with good quality (usually it is accepted that a zeta potential having values higher than +30 mV or lower than −30mV is assumed to be stable and good [26]).
In vitro stability
The in vitro stability of formulation of ISR-loaded coated SLN with Ru (ONbp), without Ru (ONps), and drug nano-suspension were studied and compared the particle sizes of all formulation (Fig. 1d). Generally, the study of particle size was significant, because it plays a critical role in their gastrointestinal uptake and their reticuloendothelial clearance [27]. All formulation subjected to suspend into SGF fluid with pepsin 0, 1 and 2 h, after 2 h up to 6 h in SIF. During the study, it was observed that the particle size of the nano-suspension grew rapidly, mean diameter 500–600 nm after only 10–20 min of exposition due to acid environment. The pH of gastric fluid created agglomeration of the particles due to strong reduction of particle surface charges [16]. Whereas in case of coated formulations (ONbp and ONps), there is no significant change in size of formulations. It was observed that the particle size of ONbp and ONps was 106–110 and 120–124 nm, respectively, until the end of the study. The reason was no significant change in the size of formulations because of polymeric coating done by Eudrgait L 100 (EL100) and showed a stabilizing effect against phenomena of agglomeration. The polymer stabilizing effect may be due to enteric coating in the form of film; solubility of this film was above pH 6.0. It is concluded that in the presence of polymer (EL 100) coating with lipid core; both were effective to stabilize the SLN formulations in acid by preventing the aggregation phenomena. Instead of this, during exposition (6 h) of formulation of ONbp and ONps in the SIF conditions, there were minute changes observed in particle size, whose range holds stable between 200 and 300 nm (Fig. 1d) due to salt formation, which was slowly degraded in basic pH. Literature [27], for intestinal transport showed that particle size less than 300–400 nm is suitable for drug transport through intestinal system. In vitro stability in SGF and SIF confirmed that particle size of formulations were found in nano-ranges in gastric fluid due to effectiveness of polymeric coating (EL100) and almost maintained in the basic environment of the intestinal fluid till their arrival and maintained positivity in terms of particle size. In the literature, it was described that SLN stability can be improved by polymer coating [28–30].
Ex vivo studies
The formulation of ISR-loaded coated SLN with Ru (ONbp) and without Ru (ONps), and drug suspension were selected for ex vivo study by assembling the instrument as shown in Fig. 2a. During the study, the obtained results (Fig. 2b) showed that drug from suspension permeable fast from the intestinal membrane as compared with ONbp and ONps nanoparticle formulations. This is because the drug in suspension was simply available for diffusion through membrane as compared to nanoparticle formulations where the drug was entrapped in lipid matrix with polymeric coating [18, 31]. This phenomenon was happened because film coating was soluble by the mechanism of salt formation of coating material in phosphate buffer above pH 6. In case of ONbp, the permeability was slow but prolonged as compared to ONps and drug suspension. This is due to less solubility of Ru in equal amount of drug that caused significant effect on solubility of drug, which results in prolonging permeability.
Fig. 2.
(a) The ex vivo study using dissolution assembly. (b) Ex vivo profile of optimized formulations and drug suspension
Release studies
First, in vitro release study of formulation of coated SLN with Ru (ONbp) and without Ru (ONps) was done in gastrofluid (pH 1.2 for 2 h because, it would follow simulating from gastro to intestine during orally administration). Then, the medium was replaced with intestinal fluid (phosphate buffer pH 7.4) and release pattern was studied. In gastrofluid, there was slow release shown by ONbp and ONps due to polymer (EL100) coating. As shown below, the release of drug was more in basic pH due to pH-based degradation of EL-100 polymer. The EL-100 polymer has been used as a coating material to prevent the degradation of acidic pH prone therapeutic agents and has been successfully used for sustained release of various drugs. In vitro stability in SGF and SIF confirmed in vitro cumulative drug releases of ONbp (%CDR approx. 50% release in 7–8 h that means drug exhibits higher stability in acidic condition) showed sustained behavior as compared to drug suspension in phosphate buffer. The results are shown in Table II. Moreover, formulation has prolonged the release as a result of higher amount of drug encapsulating due to better solubility in lipid–lecithin core, which also enhanced its absorption, polymeric coating prevented drug release in gastric fluid.
Table II.
Cumulative drug release (%) of formulation (ONbp)
| Time (h) | Drug suspension | ONps | ONbp |
|---|---|---|---|
| 0.5 | 12.09 | 9.86 | 5.59 |
| 1 | 30.61 | 19.98 | 12.96 |
| 2 | 48.71 | 33.72 | 20.54 |
| 4 | 67.25 | 48.66 | 30.61 |
| 8 | 84.76 | 68.77 | 48.49 |
| 12 | 92.25 | 80.43 | 62.16 |
| 16 | 96.21 | 86.08 | 73.78 |
| 24 | 99.98 | 94.98 | 80.73 |
| 32 | – | 99.89 | 87.29 |
| 40 | – | – | 93.61 |
| 48 | – | – | 99.91 |
Kinetic release model studies
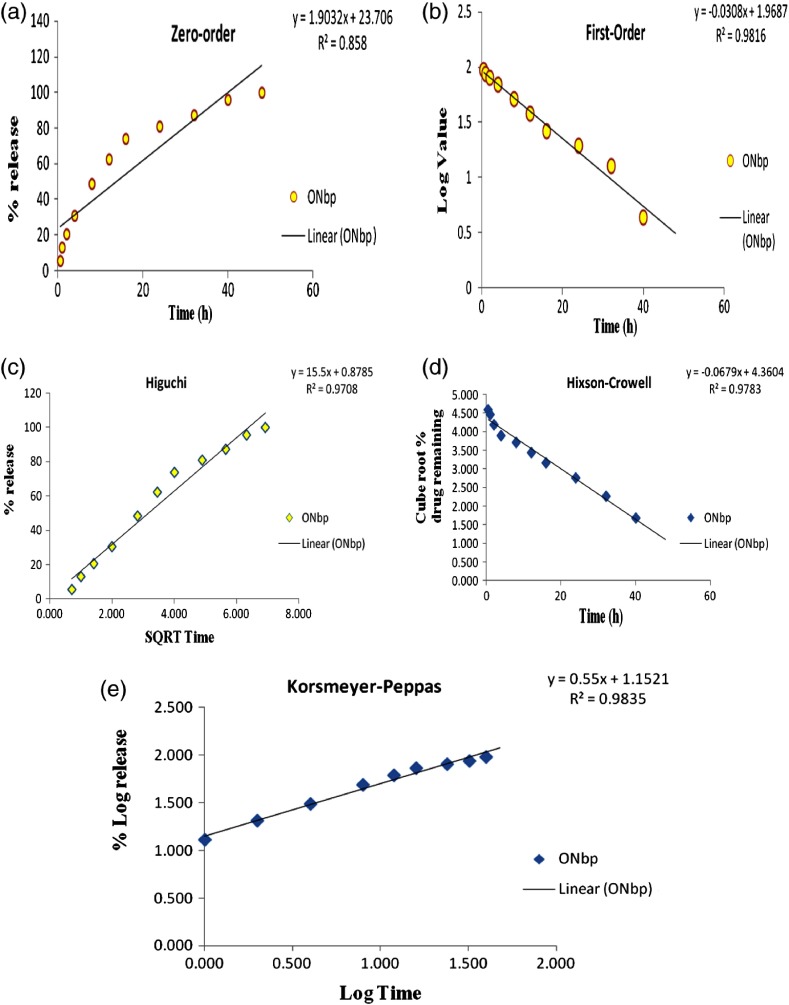
The dissolution profile obtained for formulation (ONbp) was evaluated for model-dependent release kinetics. The model-dependent approaches were zero order, first order, Higuchi, Hixson–Crowell, and Korsmeyer–Peppas. To determine release mechanism, data were applied to various kinetics models and obtained R2 0.858, 0.981, 0.971, and 0.978 for zero, first, Higuchi, and Hixson–Crowell (Fig. 3). The Korsmeyer–Peppas n value 0.5 < n < 1.0 with higher R2 value (0.983) as compared to other model was obtained. Therefore, the higher R2 value of model that is Peppas suggested that drug-release formulation (ONbp) followed “Non-Fickian Diffusion Transport” mechanism. The resulted mechanism support prolongs drug-release behavior through diffusion and relaxation.
Fig. 3.
Various kinetic models and their R2 value; (a) zero-order plot, (b) first order, (c) Higuchi, (d) Hixon–Crowell, and (e) Peppas model of formulation (ONbp)
In vivo study
Pharmacokinetic studies
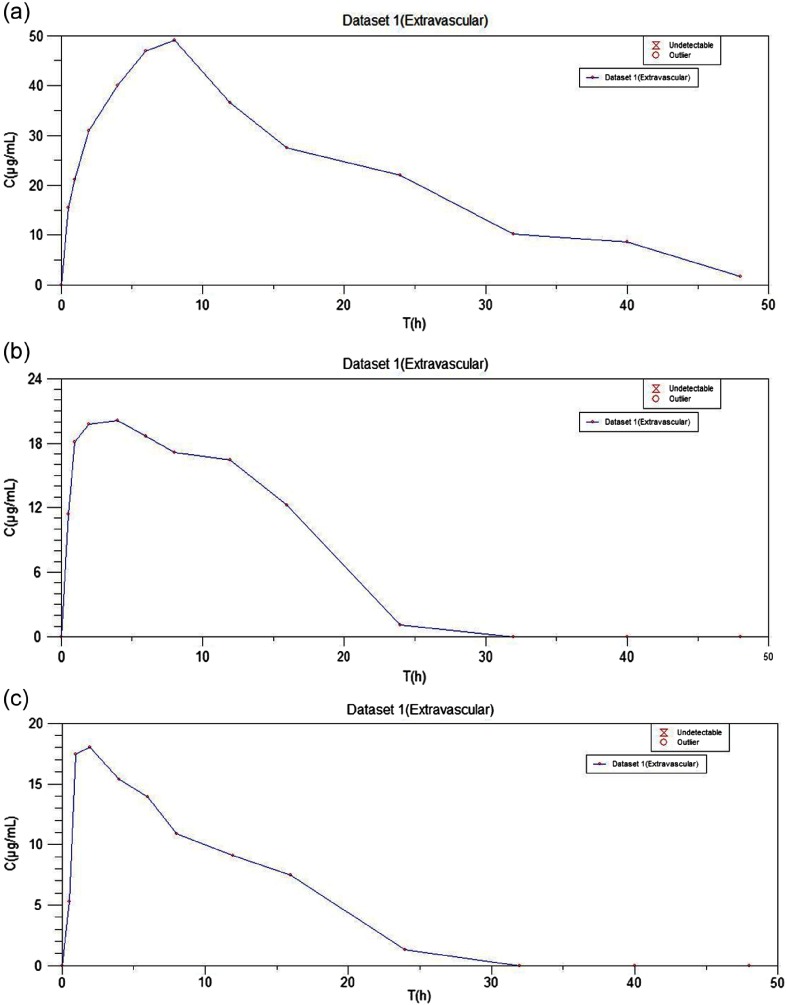
To quantify bioavailability of ISR after oral application of formulations, in vivo study was performed and the pharmacokinetic parameters were calculated with the help of a computer-designed program “Kinetica Software” (Thermo Scientific Kinetica, PK/PD Analysis, version 5.0, Philadelphia, PA). The plasma profiles of ISR following application of oral-coated SLN with Ru (ONbp), without Ru (ONps), and conventional oral drug (ISR) suspension to adult Wistar rats were compared (Table III). The plasma time concentration curve of ONbp, ONps, and drug suspension are illustrated in Fig. 4. The Cmax of ISR in formulation with bioenhancer (ONbp) was 49.17 ± 1.57 μg/ml and significant (p < 0.01) as compared to the Cmax of formulation without Ru (ONps) and suspension, which was found to be 20.12 ± 1.17 μg/ml and 18.01 ± 1.08 μg/ml, respectively. The ONbp Cmax was 2.44- and 2.73-folds, respectively, more than ONps and oral suspension, respectively, whereas ONps was 1.11-folds more as compared to drug suspension. The Tmax of ISR in formulation (ONbp) was attained in 8 h, whereas Tmax of ONps (4.0 h) and oral suspension (2.0 h) was attained. The elimination half-life (t1/2) was also high for formulation (ONbp), i.e., 14.42 h as compared to ONps (8.98 h) and suspension (8.59 h). The Tmax, half-life, and mean residence values were also higher (Table III) for formulation (ONbp) with Ru as compared to ONps and conventional suspension. The AUCo→48h of ONbp was found to be 1,035.03 ± 2.10 μg/ml h in comparison of ONps and suspension, which was 320.94 ± 4.36 and 220.26 ± 5.24 μg/ml h, respectively, and found significant (p < 0.001) on comparison. The AUCo→48h of ONbp 3 was 3.22-folds more than ONps, whereas ONbp showed 4.70-folds more bioavailability than oral suspension of ISR. In case of ONps, bioavailability of ISR is onefold more as compared to suspension. The high AUCo→∞ 1,091.63 ± 22.26 μg/ml h of ONbp showed the extent of absorption as compared to ONps (339.66 ± 4.60 μg/ml h) and conventional suspension (245.00 ± 8.50 μg/ml h) and found statistically significant (p < 0.0001). The oral bioavailability of ISR in ONbp was 3.21- and 4.45-folds as compared to ONps and drug suspension, respectively. The pharmacokinetic study showed 3.2- to 4.7-folds enhancement in oral bioavailability of coated SLN with Ru when compared to a coated formulation without Ru and conventional drug suspension. Whereas the coated SLN without Ru was shown to be 1.00- to 1.11-folds more bioavailability than drug suspension. The obtained results were also confirmed by the literature that bioavailability enhancement can be 1.0–2.0 times by reduction of particle size. This can be happened due to oral formulations nano meter size, which improved the contact time of the formulation, consequently the effective surface to improve the 2.17-folds bioavailability [24, 32]. The study results clearly indicated that formulation had sustained release of the drug over a period of 48 h as compared to conventional suspension. Because of nano formulation with polymeric coating consequently, improved sustained time of the coated nanoparticles and bioenhancer (Ru) inhibitory enzymatic activity (CYP3A) helped in reduction of the first-pass metabolism, which enhances the drug bioavailability. Therefore, the enhanced oral bioavailability of ONbp was probably due to the contribution of individual and combined mechanisms. Hence, it could be concluded that the developed formulation (ONbp) enhanced oral bioavailability of ISR, which can be considered as nano-therapeutic system.
Table III.
The pharmacokinetic profile of isradipine loaded coated SLN with Ru (ONbp) and without Ru (ONps) formulation and conventional suspension
| Parameter studied | Drug suspension | ONps | ONbp |
|---|---|---|---|
| Tmax (h) | 2.0 | 4.0 | 8.0 |
| t½ (h) | 8.59 | 8.98 | 14.42 |
| Cmax (μg.h/ml) | 18.01 ± 1.08 | 20.12 ± 1.17 | 49.17 ± 1.57 |
| AUCo→48h (μg.h/ml) | 220.26 ± 5.24 | 320.94 ± 4.36 | 1,035.03 ± 2.10 |
| AUCo→∞ (μg.h/ml) | 245.00 ± 8.50 | 339.66 ± 4.60 | 1,091.63 ± 22.26 |
| MRTo→48h (h) | 8.61 | 9.14 | 15.9 |
| MRTo→∞h (h) | 9.04 | 9.45 | 17.5 |
Tmax: time of peak plasma concentration; Cmax: peak plasma concentration; AUCo→48h: area under the plasma concentration versus time curve until the last observation; AUCo→∞: area under the plasma concentration versus time curve extrapolated to infinity; MRT: mean residence time; MRTo→∞h: mean residence time when the drug concentration profile is extrapolated to infinity, t½: half-life; SLN: solid–lipid nanoparticles
Fig. 4.
The plasma time concentration curve; (a) ONbp, (b) ONps, and (c) conventional suspension
Pharmacodynamic studies
The formulation of coated SLN with bioenhancer (ONbp) was studied and compared with coated SLN without bioenhancer (ONps) and drug suspension in the rat model by measured systolic BP at fixed intervals (0.5, 1, 2, 4, 6, 8, 12, 24, 32, 40, and 48 h) and evaluated using BIO-Pac Instrument (Table IV). The conventional suspension was obtained after administration through orally; the maximum effect was observed initially and the hypertension was controlled significantly up to 2 h with the maximum effect. Whereas, ONps controlled the BP at 4 h (% reduction 23.74 ± 1.49) gradually decrease up to 8 h (% reduction 20.65 ± 1.1). The ONbp 3 oral administration with bioenhancer showed the maximum effect observed at 8 h resulted in a gradual decrease of BP up to 24 h and further continued to 48 h. In the hypertensive group, there was considerable decrease observed at 24 h in the systolic BP but no significant decrease up to 48 h due to methylprednisolone hypertension induction effect. The formulation (ONbp) reduced the systolic BP 21.55% ± 1.41%, 20.54% ± 1.30%, and 20.14% ± 1.14% in 8, 12, and 24 h, respectively. The coated SLN with Ru (ONbp) minimized the limitation of oral delivery of ISR by progressively controlling the hypertension for prolonged period and enhanced bioavailability due to sustain pattern and reduction of first-pass metabolism (because of polymeric coating and bioenhancer respectively) when compared with formulation without Ru and drug suspension.
Table IV.
Antihypertensive effect of coated SLN with Ru (ONbp) and without Ru (ONps) formulation and conventional suspension
| Group I | Group II | Group III | Group IV | Group V | |
|---|---|---|---|---|---|
| Time (h) | Normal | Hypertension control | ISR suspension | ONps | ONbp |
| Initial | 121.37 ± 0.23 | 154.45 ± 0.31 | 154.32 ± 0.22 | 152.12 ± 0.11 | 154.12 ± 0.10 |
| 0.5 | 124.25 ± 0.11 | 154.92 ± 0.91 | 146.55 ± 0.31 | 140.72 ± 0.22 | 151.45 ± 0.31 |
| 1 | 121.65 ± 0.54 | 154.91 ± 0.43 | 130.78 ± 0.63 | 132.39 ± 0.35 | 147.54 ± 0.42 |
| 2 | 118.67 ± 0.61 | 158.61 ± 0.38 | 121.35 ± 0.19 | 128.17 ± 0.12 | 140.36 ± 0.23 |
| 4 | 118.04 ± 0.33 | 156.37 ± 0.32 | 124.59 ± 0.23 | 119.24 ± 0.21 | 134.21 ± 0.16 |
| 6 | 122.13 ± 0.82 | 155.73 ± 0.62 | 130.98 ± 0.64 | 123.57 ± 0.43 | 127.16 ± 0.12 |
| 8 | 121.74 ± 0.14 | 154.69 ± 0.28 | 137.87 ± 0.21 | 133.45 ± 0.33 | 121.35 ± 0.27 |
| 12 | 120.78 ± 0.71 | 153.88 ± 0.48 | 140.74 ± 0.55 | 140.87 ± 0.71 | 122.27 ± 0.19 |
| 24 | 121.93 ± 0.96 | 154.38 ± 0.19 | 151.29 ± 0.18 | 151.89 ± 0.64 | 123.25 ± 0.24 |
| 32 | 122.61 ± 0.45 | 156.47 ± 0.32 | 155.41 ± 0.36 | 155.46 ± 0.35 | 128.41 ± 0.38 |
| 40 | 122.26 ± 0.61 | 154.11 ± 0.10 | 153.67 ± 0.46 | 153.79 ± 0.64 | 139.67 ± 0.61 |
| 48 | 120.98 ± 0.48 | 154.21 ± 0.19 | 154.16 ± 0.13 | 154.12 ± 0.11 | 149.56 ± 0.50 |
Presented data p < 0.05 and as mean ± SD, n = 6, compared to conventional formulation. SLN: solid–lipid nanoparticles
Accelerated stability study
During this study, there were no significant changes in entrapment efficiency and drug content, recorded till 90 days period in the storage conditions. The results are presented in Table V. The obtained results had shown that formulation had good stability at storage condition according to the ICH guidelines.
Table V.
Accelerated stability study of formulation of coated SLN with Ru (ONbp)
| Time (days) | Entrapment efficiency (%) | Drug content |
|---|---|---|
| 0 | 97.85 ± 1.02 | 97.54 ± 2.04 |
| 15 | 97.75 ± 1.11 | 97.52 ± 1.94 |
| 30 | 97.71 ± 1.10 | 97.52 ± 2.11 |
| 60 | 97.68 ± 1.02 | 97.48 ± 2.07 |
| 90 | 97.65 ± 1.04 | 97.46 ± 1.88 |
SLN: solid–lipid nanoparticles
Conclusions
The study involved the concept of development and evaluation of the coated SLN to overcome the difficulties of metabolic degradation of CYP3A4 substrate ISR (a calcium channel blocker antihypertensive BCS II drug having oral absolute bioavailability of 15%–34% due to presystemic metabolism) by inhibiting the enzyme using bioenhancer like Ru, thereby improving the oral bioavailability. The ONbp formulation was considered best due to small particle size, provided a large surface area and achieved desired sustained effect, which may get the desired bioavailability and higher encapsulating efficiency into nano-lipid carrier. In vitro studies revealed significant sustain release up to 48 h, as compared to drug suspension, respectively. In addition, in vitro stability study of particle size showed coated SLN formulation's effectiveness in gastric fluid till they reached in the basic environment of the intestinal fluid. The permeability was slow but prolonged as compared to drug suspension. The in vivo study revealed 3.21- to 4.70-folds more bioavailability than suspension of ISR. Moreover, the sustained effect can help maintained optimum release rate supported to a day continual dose reduction when using drug–bioenhancer combinations. Furthermore, there are no changes in physical parameters (appearances, color, uniformity, and separation), entrapment efficiency, and drug content till 90 days period at the mentioned storage conditions, which indicated better stability of the formulation. This approach has shown promising results (effective solubilization, enhance absorption, prolong sustain release rate, and inhibition of enzymatic metabolism), which help attain better oral availability of ISR nano-therapeutic-system for controlled antihypertensive therapy.
Acknowledgements
The authors are grateful and thankful to I. K. Gujral Punjab Technical University, Kapurthala, Punjab and PD Memorial Group of Institutions, College of Pharmacy, Bahadurgarh, India for support and provided facilities to carried out the research work.
Funding Statement
Funding sources: None.
Conflict of interest
None.
Authors’ contribution
The presented work was performed in collaboration with all authors. AK and HC participated in the study. VK developed the formulation and carried out their evaluation experimentally. VK, HC, and AK performed statistical analysis and interpretation of data along with manuscript preparation and editing. On the behalf of all authors, VK read and approved the final version of the manuscript.
References
- 1.Porter CJ, Charman WN: In-vitro assessment of oral lipid based formulation. Adv Drug Deliv Rev 50, S127–147 (2001) [DOI] [PubMed] [Google Scholar]
- 2.Humberstone AJ, Charman WN: Lipid based vehicles for the oral delivery of poorly soluble drugs. Adv Drug Deliv Rev 25, 103–128 (1997) [Google Scholar]
- 3.Nekkanti V, Sandeep K: Development of novel lipid based drug delivery system for raloxifene hydrochloride. Int Res J Pharma 3, 166–173 (2012) [Google Scholar]
- 4.Pouton CW, Porter CJH: Formulation of lipid based delivery system for oral administration: Material, methods and strategies. Adv Drug Deliv Rev 60, 625–637 (2008) [DOI] [PubMed] [Google Scholar]
- 5.Le Verger ML, Fluckiger L, Kim Y, Hoffman M, Maincent P: Preparation and characterization of nanoparticles containing antihypertensive agent. Eur J Pharm Biopharm 46, 137–143 (1998) [DOI] [PubMed] [Google Scholar]
- 6.Bobbala SKR, Veerareddy PR: Enhanced oral bioavailability of isradipine via proniosomal systems. Drug Dev Ind Pharm 39, 909–917 (2013) [DOI] [PubMed] [Google Scholar]
- 7.Bobbala SKR, Veerareddy PR: Formulation, evaluation, and pharmacokinetics of isradipine proliposomes for oral delivery. J Liposome Res 22, 285–294 (2012) [DOI] [PubMed] [Google Scholar]
- 8.Kiran VKP, Nair R, Raju PY, Chakrapani M, Dhanalakshmi P: Preparation and characterization of lercanidipine loaded solid lipid nanoparticles. Int J Biopharm 3, 82–88 (2012) [Google Scholar]
- 9.Kumara VV, Chandrasekar D, Ramakrishna S, Kishan V, Rao YM, Diwan VP: Development and evaluation of nitrendipine loaded solid lipid nanoparticles: Influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm 335, 167–175 (2007) [DOI] [PubMed] [Google Scholar]
- 10.Rathee P, Kamboj A, Sidhu S: Optimization and development of nisoldipine nanobioenhancers by novel orthogonal array (L27 array). Int J Biol Macromol 86, 556–561 (2016) [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Kharb R, Chaudhary H: Optimization & design of isradipine loaded solid lipid nanobioparticles using rutin by Taguchi methodology. Int J Biol Macromol 92, 338–346 (2016) [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Chaudhary H, Kamboj A: Nano-colloidal carrier via polymeric coating for oral delivery of isradipine. Interv Med Appl Sci 9, 1–13 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardouh AR, Gad S, Ghonaim HM, Ghorab MM: Design and characterization of glyceryl monostearate solid lipid nanoparticles prepared by high shear homogenization. Br J Pharm Res 3, 326–346 (2013) [Google Scholar]
- 14.Havanoor SM, Manjunath K, Bhagawati ST, Veerapur VP: Isradipine loaded solid lipid nanoparticles for better treatment of hypertension-preparation, characterization and in vivo evaluation. Int J Biopharm 5, 218–224 (2014) [Google Scholar]
- 15.Roger E, Lagarce F, Benoit JP: The gastrointestinal stability of lipid nanocapsules. Int J Pharm 379, 260–265 (2009) [DOI] [PubMed] [Google Scholar]
- 16.Gonçalves LMD, Maestrelli F, Cesare Mannelli LD, Ghelardini C, Almeida AJ, Mura P: Development of solid lipid nanoparticles as carriers for improving oral bioavailability of glibenclamide. Eur J Pharm Biopharm 102, 41–50 (2016) [DOI] [PubMed] [Google Scholar]
- 17.Untied State Pharmacopoeia National Formulary (2004): The official compendia of standards (Asian ed). Untied States Pharmacopeial Convention, Rockville [Google Scholar]
- 18.Barr WH, Riegelmanm S: Intestinal drug absorption and metabolism comparison of methods and models to study physiological factors of in vitro and in vivo intestinal absorption. J Pharm Sci, 59, 154–163 (1970) [DOI] [PubMed] [Google Scholar]
- 19.Krakoff LR, Selvadurai R: Sutter E effect of methylprednisolone upon arterial pressure and the renin angiotensin system in the rat. Am J Physiol 228, 613 (1975) [DOI] [PubMed] [Google Scholar]
- 20.Shin JW, Seol IC, Son CG: Interpretation of animal dose and human equivalent dose for drug development. J Korean Oriental Med 31, 17 (2010) [Google Scholar]
- 21.Daugherty A, Rateri D, Hong L, Balakrishnan A: Measuring blood pressure in mice using volume pressure recording, a tail cuff method. J Visual Exp 27, 12 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venugopal V, Kumar KJ, Muralidharan S, Parasuraman S, Raj PV, Kumar KV: Optimization and in-vivo evaluation of isradipine nanoparticles using Box-Behnken design surface response methodology. Open Nano 1, 1–15 (2016) [Google Scholar]
- 23.Muralidharan S, Ling KB, Dhanyasri, Kaur G, Kiat KW, Santhi K, Parasuraman S, Kumar J: Pharmacokinetic evaluation of newly developed isradipine sustained release formulation. Int J Drug Deliv 7, 126–140 (2015) [Google Scholar]
- 24.Dudhipala N, Veerabrahma K: Pharmacokinetic and pharmacodynamic studies of nisoldipine loaded solid lipid nanoparticles developed by central composite design. Drug Dev Ind Pharm 41, 1968–1977 (2015) [DOI] [PubMed] [Google Scholar]
- 25.ICH Tripartite Guideline Q1A (R2) (2013): Stability testing of new drug substances and products. In: International conference on harmonization of technical requirements for registration of pharmaceuticals for human use, European Union, Japan, and USA, pp. 1–18 [Google Scholar]
- 26.Bhattacharjee S: DLS and zeta potential–What they are and what they are no. J Control Rel 235, 337–351 (2016) [DOI] [PubMed] [Google Scholar]
- 27.Mehnert W, Mäder K: Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 64, 83–101 (2012) [DOI] [PubMed] [Google Scholar]
- 28.Lopes R, Eleutério CV, Gonçalves LMD, Cruz MEM, Almeida AJ: Lipid nanoparticles containing oryzalin for the treatment of leishmaniasis. Eur J Pharm Sci 45, 442–450 (2012) [DOI] [PubMed] [Google Scholar]
- 29.Mancini G, Lopes R, Clemente P, Raposo S, Gonçalves L, Bica A, Ribeiro HM, Almeida AJ: Lecithin and parabens play a crucial role in tripalmitinbased lipid nanoparticle stabilization throughout moist heat sterilization and freeze drying: Physical stability of tripalmitin solid lipid nanoparticles. Eur J Lipid Sci Technol 117, 1947–1959 (2015) [Google Scholar]
- 30.Qi C, Chen Y, Jing QZ, Wang XG: Preparation and characterization of catalase loaded solid lipid nanoparticles protecting enzyme against proteolysis. Int J Mol Sci 12, 4282–4293 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satish S, Harikumar SL. (2012): In-vitro and in-vivo studies of Celecoxib non effervescent floating microparticulates. Inventi Journals (P) Ltd. Pharm Tech, Bhopal, India, p. 2 [Google Scholar]
- 32.Krishnamoorthy B, Rahman SMH, Selvan NT, Prasad RH, Rajkumar M, Selvakumar MS, Vamshikrishna K, Gregory M, Vijayaraghavan C: Design, formulation, in vitro, in vivo and pharmacokinetic evaluation of nisoldipine-loaded self-nanoemulsifying drug delivery system. J Nanopart Res 17, 34 (2015) [Google Scholar]