Abstract
We have previously reported that the mouse gut microbiome contributes to pulmonary responses to ozone, a common asthma trigger, and that short-chain fatty acids, end products of bacterial fermentation, likely contribute to this role of the microbiome. A growing body of evidence indicates that there are sex-related differences in gut microbiota and these differences can have important functional consequences. The purpose of this study was to determine whether there are sex-related differences in the impact of the gut microbiota on pulmonary responses to ozone. After acute exposure to ozone, male mice developed greater airway hyperresponsiveness than female mice. This difference was abolished after antibiotic ablation of the gut microbiome. Moreover, weanling female pups housed in cages conditioned by adult male mice developed greater ozone-induced airway hyperresponsiveness than weanling female pups raised in cages conditioned by adult females. Finally, ad libitum oral administration via drinking water of the short-chain fatty acid propionate resulted in augmented ozone-induced airway hyperresponsiveness in male, but not female, mice. Overall, these data are consistent with the hypothesis that the microbiome contributes to sex differences in ozone-induced airway hyperresponsiveness, likely as a result of sex differences in the response to short-chain fatty acids.
Keywords: airway responsiveness, antibiotics, neutrophil, 16S rRNA gene sequencing, short-chain fatty acids
Clinical Relevance
The data presented in this work are consistent with the hypothesis that the microbiome contributes to sex differences in ozone-induced airway hyperresponsiveness, likely as a result of sex differences in the response to short-chain fatty acids. A better understanding of the relationship between male and female microbiota and sex hormones could lead to more precise biomarkers or sex-specific therapeutics to address asthma in male and female patients, especially asthma triggered by air pollution.
Ozone (O3), a common air pollutant, is a trigger for asthma. Exposure to O3 causes asthma symptoms such as cough, wheeze, shortness of breath, and chest tightness, as well as decreases in lung function (1, 2). O3 also induces airway hyperresponsiveness (AHR) (3, 4), a canonical feature of asthma. Furthermore, hospital admissions for asthma increase on days after high ambient O3 concentrations (5).
Sex differences in responses of human subjects to O3 have been reported (4, 6–8), though the direction of the difference appears to depend on the age of the subjects. For example, O3-induced decrements in forced expiratory volume in 1 second (FEV1) tend to be greater in female young adults than in male young adults, whereas in older adults, males tend to have greater decrements than females (8). Sex differences in pulmonary responses to O3 are also observed in mice. After acute O3 exposure, female mice have more BAL neutrophils and acute-phase cytokines/chemokines than males (9, 10), although the effect depends on the mouse strain used (11). Females exposed to O3 also have greater infection-induced mortality than males (12). In contrast, there are no data on sex differences in O3-induced AHR, although innate airway responsiveness is greater in males than in females (13). To determine whether there are also sex differences in O3-induced AHR, we exposed male and female C57BL/6 mice to room air or to O3 (2 ppm for 3 h). Our data indicate that male mice have substantially greater O3-induced AHR than females.
In male mice, the microbiome contributes to O3-induced AHR (14), and both antibiotics and germ-free (GF) conditions reduce O3-induced AHR. The role of the microbiome in pulmonary responses to O3 in female mice has not been examined, but it could differ from that in males, as several groups have reported sex differences in the composition of the gut microbiome (15–18). Moreover, these sex differences can have important functional consequences. In nonobese diabetic (NOD) mice, which spontaneously develop type 1 diabetes (T1D), the incidence of T1D is almost doubled in females compared with males (19). In contrast, sex differences in the incidence of T1D are not observed in GF NOD mice. Furthermore, weanling females given male microbiota by gavage displayed elevated testosterone levels and a lower incidence of T1D (19). Microbiota also contribute to lupus, a sex-specific disease that affects more women than men (20).
The purpose of this study was to examine the hypothesis that the gut microbiome contributes to sex differences in pulmonary responses to O3. Therefore, we perturbed the gut microbiomes of male and female mice using antibiotics administered via the drinking water. The mice were subsequently exposed to air or O3. Antibiotic treatment abolished sex differences in O3-induced AHR. We also perturbed the microbiome by placing female mouse pups at weaning into cages that had previously been occupied by either adult males or females and still contained their bedding and fecal matter. Once the mice reached adulthood, females that had been in cages conditioned by adult males developed greater O3-induced AHR than females in cages conditioned by adult females. Thus, exposing female mice to a male microbiome reproduced the male pattern of O3-induced AHR. Together, our data indicate that the microbiome contributes to sex differences in the magnitude of O3-induced AHR.
The short-chain fatty acids (SCFAs) acetate, propionate, and butyrate are end products of bacterial fermentation of dietary fiber. SCFAs likely play a role in the effects of the microbiome on pulmonary responses to O3 in male mice (14). Serum propionate is reduced by antibiotics that also reduce O3-induced AHR (14). In addition, diets that increase serum SCFAs and propionate supplementation of drinking water both augment O3-induced AHR (14). To determine whether there were sex differences in the synthesis or response to SCFAs, we measured serum SCFAs and examined the impact of ad libitum oral administration via drinking water of the SCFA propionate in male and female mice. Our data indicate that there are no sex differences in circulating SCFAs, but males are more sensitive to the effects of propionate on O3-induced AHR than females.
Methods
Animals
All protocols were approved by the Harvard Medical Area Standing Committee on Animals. Experiments were performed in male and female C57BL/6 mice that were purchased from The Jackson Laboratory or bred in the Harvard T. H. Chan School of Public Health specific-pathogen-free animal facility. See the data supplement for details.
Protocol
Four cohorts of mice were examined. In the first cohort, to determine whether there were sex differences in O3-induced AHR, male and female mice from the same litters were exposed to room air or to O3 (2 ppm for 3 h). Twenty-four hours later, the mice were anesthetized for measurement of airway responsiveness to aerosolized methacholine and then killed. Blood was collected for the preparation of serum and BAL was performed.
In the second cohort, to examine the impact of antibiotic treatment on sex differences in O3-induced AHR, a cocktail of antibiotics (ampicillin, metronidazole, neomycin, and vancomycin) was added to the drinking water of male and female mice as previously described (14). Sucralose was added for taste. Control mice were given regular drinking water with sucralose. After 2 weeks of treatment, the mice were exposed to room air or to O3 and evaluated as described above. Fecal samples were collected from each mouse 1 day before exposure.
In the third cohort, we examined the impact of male microbiota on female recipients by adapting a cage-conditioning protocol described in several cohousing studies (21–23). Eight-week-old male and female mice (donors) were housed individually in cages for 3 days (Figure E1 in the data supplement). After 3 days, the donors were moved to new cages. Three weanling female pups (receivers) were placed into these male- or female-conditioned cages. This process was repeated every 3 days for 4 weeks. The receiver mice were always placed into cages conditioned by the same male or female donor. At 7 weeks of age, the receivers were exposed to room air or to O3 and evaluated as described above. Microbial transfer by cohousing or by placing donor feces in receiver cages has been previously described (24, 25) and likely occurs because of coprophagic behavior and grooming.
In the fourth cohort, we examined sex-related differences in the impact of SCFAs on O3-induced AHR. Male and female 9-week-old C57BL/6J mice were given sodium propionate (200 mM) in the drinking water for 3 days as described previously (26). Control mice were given saline (50 mM) in the drinking water (26). After 3 days, the mice were exposed to room air or to O3 and evaluated as described above. In addition, lungs, livers, and colons were collected and frozen in liquid nitrogen for subsequent preparation of RNA to evaluate expression Ffar2 and Ffar3, the receptors for SCFAs.
The methodologic procedures used for O3 exposure, measurement of airway responsiveness, BAL, 16S rRNA gene sequencing and analysis, measurement of SCFAs, and statistical analysis are described in the data supplement.
Results
Sex Differences in O3-induced AHR
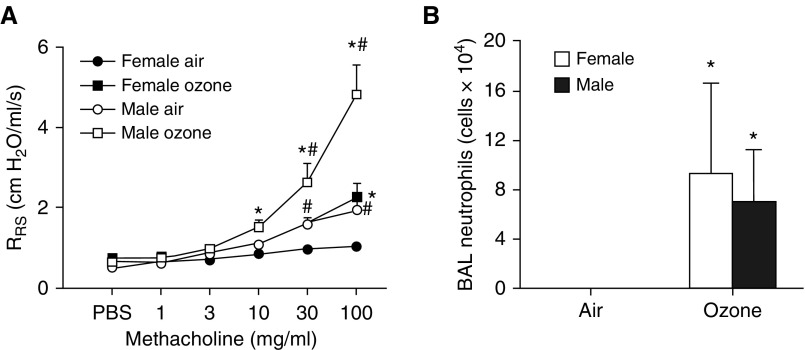
In air-exposed mice, airway responsiveness was greater in male mice than in female mice, consistent with previous reports (13). Acute exposure to O3 increased airway responsiveness in both male and female mice (Figure 1A). However, the magnitude of this increase in responsiveness was significantly greater in male mice than in female mice. In contrast, although exposure to O3 increased BAL neutrophils in both males and females, there was no sex difference in this response (Figure 1B). Similarly, BAL macrophages and BAL protein increased with O3 exposure, but there were no sex differences in these responses (Figure E2).
Figure 1.
Sex differences in ozone-induced airway hyperresponsiveness (AHR). Male and female mice were exposed to room air or to ozone (2 ppm) for 3 hours and studied 24 hours after exposure. (A) Airway responsiveness to inhaled aerosolized methacholine. (B) BAL neutrophils. Results are mean ± SE of data from n = 7–10 mice per group. *P < 0.05 compared with air; #P < 0.05 compared with female mice with the same exposure. RRS = respiratory system resistance.
Sex Differences in O3-induced AHR Are Abolished with Antibiotic Treatment
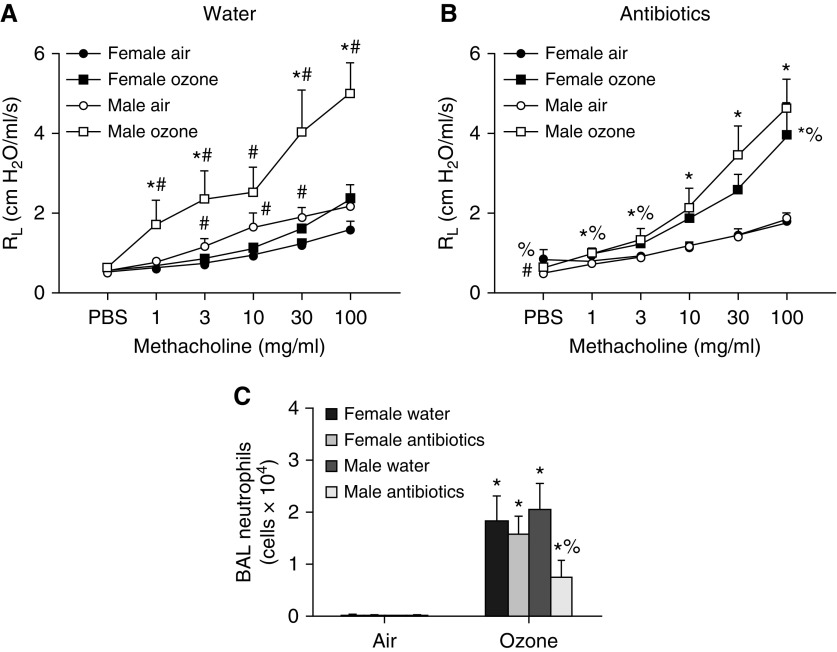
To determine whether the microbiome contributed to the observed sex differences in O3-induced AHR (Figure 1A), a separate cohort of males and females was administered antibiotics via the drinking water, or given control drinking water, for 2 weeks. At the time of exposure, male mice weighed more than females, but antibiotic treatment did not affect body mass in either males or females (Figure E3A). In air-exposed mice treated with control water, airway responsiveness was greater in males than in females (Figure 2A), as described above (Figure 1A). However, in air-exposed mice treated with antibiotics, this sex difference in airway responsiveness was no longer observed (Figure 2B). The magnitude of O3-induced AHR was greater in males treated with control water than in females treated with control water (Figure 2A), as described above (Figure 1A). Interestingly, antibiotic treatment reduced O3-induced AHR at the lower doses of methacholine in male mice, whereas in female mice the magnitude of O3-induced AHR was increased after antibiotic treatment (Figure 2B). The net effect of these sex differences in the impact of antibiotics was such that sex differences in O3-induced AHR observed in control mice (Figure 2A) were abolished in antibiotic-treated mice (Figure 2B). Together, these findings implicate the microbiota in sex differences in O3-induced AHR.
Figure 2.
An antibiotic cocktail abolishes male/female differences in O3-induced AHR. Mice were treated with a cocktail of antibiotics (ampicillin 1 g/L, metronidazole 1 g/L, neomycin 1 g/L, vancomycin 0.5 g/L) via their drinking water for 2 weeks. Sucralose (8 g/L) was added to the water for taste. Control mice were treated with drinking water containing sucralose only. The mice were then exposed to room air or to ozone. (A) Airway responsiveness of mice treated with water. (B) Airway responsiveness of mice treated with antibiotics. (C) BAL neutrophils. Results are mean ± SE of data from n = 7–11 mice per group. *P < 0.05 compared with air. #P < 0.05 compared with female mice with same exposure; %P < 0.05 compared with water-treated mice of the same sex and with the same exposure. RL = resistance of the lung.
In control mice, there were no sex-related differences in O3-induced neutrophil recruitment (Figure 2C), consistent with data described above (Figure 1B). Treatment with antibiotics reduced BAL neutrophils in male mice, consistent with our previous observations (14), but had no effect in females (Figure 2C). BAL macrophages and BAL protein also increased with O3 exposure (Figures E2B and E2C). There were no significant sex differences in these outcomes in water-treated mice, but both BAL macrophages and BAL protein were greater in male than female mice treated with antibiotics.
The observed effects of oral antibiotics (Figure 2) likely reflect changes in the gut rather than the lung microbiome: when male mice were treated with vancomycin alone, the gut microbiome was significantly affected, as assessed by 16S rRNA sequencing (14). In contrast, orally administered vancomycin does not reach the systemic circulation (27) and does not alter lung microbial community structure (28). Nevertheless, vancomycin does cause a significant reduction in O3-induced AHR similar to that observed with a full cocktail of antibiotics (14). Consequently, fecal samples from male and female littermates treated with antibiotics or control drinking water were collected before O3 or air exposure, and 16S rRNA sequencing was performed.
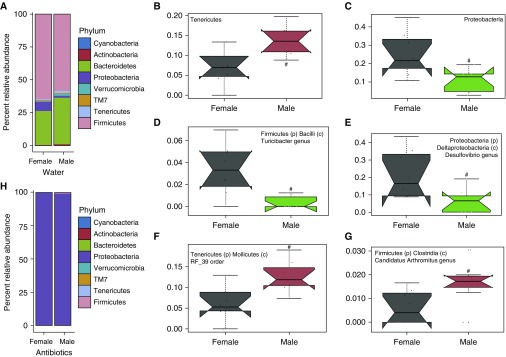
In both female and male water-treated mice, fecal microbial communities were dominated by members of the Bacteroidetes and Firmicutes phyla (Figure 3A), consistent with other reports (23, 29). Females harbored significantly fewer Tenericutes and more Proteobacteria (Deltaproteobacteria class) than males (Figures 3B and 3C). Further examination of microbial communities indicated additional sex differences: four taxonomic clades were significantly differentially abundant. Compared with females, males carried less Turicibacter (Figure 3D) and Desulfovibrio (Figure 3E) genera, and were enriched for RF_39 (Figure 3F) order and Candidatus Arthromitus (Figure 3G) genus (morphologically characterized as segmented filamentous bacteria). Antibiotic treatment had a profound effect on the fecal microbiome. Consistent with other reports (30) and our previous data (14), in both males and females many members of the Firmicutes, Bacteroidetes, Actinobacteria, Deferribacteres, and Tenericutes phyla were depleted by the antibiotic treatments, with concurrent enrichment for the Proteobacteria phylum (specifically the Gammaproteobacteria class) (Figure 3H).
Figure 3.
Sex differences in the microbiome. (A) Relative abundance of bacteria at the phylum level in feces from water-treated male and female mice. (B–G) Relative abundance of (B) Tenericutes and (C) Proteobacteria phyla, and (D) Turicibacter, (E) Desulfovibrio, (F) RF_39, and (G) Candidatus Arthromitus subphyla as assessed by multivariate association with linear models (MaAsLin) (35). (H) Relative abundance of bacterial phyla in antibiotic-treated male and female mice. Sequence reads were assigned to each phylum at 97% sequence similarity cutoff. The trapezoid boxes indicate the 25th and 75th percentiles. The whiskers indicate the minimum and maximum values, and each dot denotes one mouse. The Tukey’s notches on either side of the median line indicate within-sample variance. Red means higher abundance compared with females, and green means lower relative abundance compared with females. n = 7–8 per group; #q < 0.25 as significant.
Cage Conditioning Transfers Male-like O3-induced AHR to Female Mice
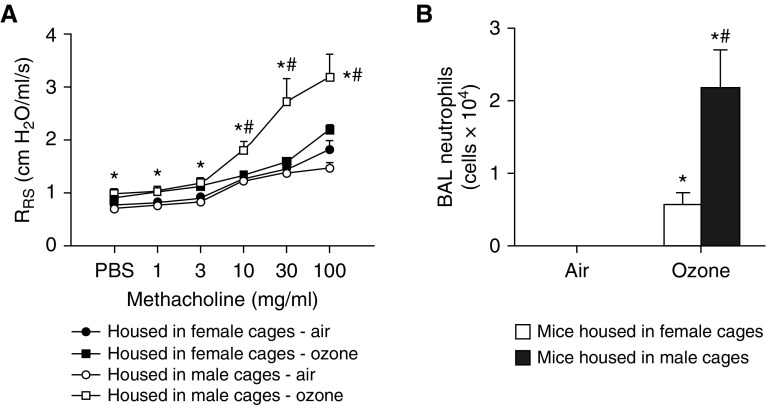
To further evaluate the hypothesis that the microbiome contributes to sex differences in O3-induced AHR, we placed weanling female mice in cages conditioned by (and containing feces of) adult males or females (see Figure E1). Cage conditioning did not affect body mass (17.8 ± 0.5 g vs. 17.7 ± 0.5 g in mice living in cages conditioned by females vs. males). O3-induced AHR was greater in mice living in cages conditioned by males versus females (Figure 4A). In contrast, cage conditioning did not impact airway responsiveness in mice exposed to room air (Figure 4A). BAL neutrophils were also greater in O3-exposed mice living in cages conditioned by males versus females (Figure 4B), but there was no effect of cage conditioning on BAL macrophages or BAL protein (Figures E4A and E4B).
Figure 4.
Females housed in cages conditioned by males develop increased ozone-induced AHR. Female weanlings were housed in cages conditioned by adult male or adult female mice as described in Figure E1 and subsequently exposed to air or ozone. (A) Airway responsiveness. (B) BAL neutrophils. Results are mean ± SE of data from n = 7–8 per group. *P < 0.05 compared with air; #P < 0.05 compared with mice housed in female-conditioned cages.
Placement of weanling female mice into cages occupied by males accelerates sexual maturation in the females (31–34). We considered the possibility that placement of females into male-conditioned cages may have had similar effects and that these effects may have been responsible for the observed changes in the response to O3 (Figure 4). For example, earlier sexual maturation may have impacted lung growth in such a manner as to affect airway responsiveness. However, measurement of lung volumes from pressure-volume curves indicated no effect of cage conditioning (Figure E4C). We also measured serum estradiol in these mice. In air-exposed mice, there was no difference in serum estradiol in the females living in female-conditioned versus male-conditioned cages (5.4 ± 0.5 vs. 5.3 ± 0.6 pg/ml, respectively). Remarkably, in ozone-exposed mice, serum estradiol levels were below the limit of detection (<3 pg/ml) in all but one mouse in each group.
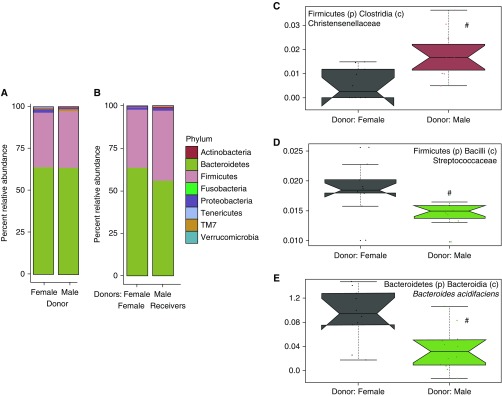
To evaluate the impact of cage conditioning on the recipients’ fecal microbiota, we performed 16S rRNA gene sequencing on fecal samples of female weanlings that were housed in cages conditioned by male and female donors. At the phylum level, microbial community taxonomic composition was similar between the two groups of mice (Figures 5A and 5B). However, using the Multivariate Association with Linear Models (MaAsLin) per-feature multivariable association analysis pipeline (35), we identified several taxa that differed in the mice living in male- versus female-conditioned cages. There was a greater abundance of the Christensenellaceae family but a smaller abundance of the Streptococcaceae family and Bacteroides acidifaciens species (Figures 5C–5E) in mice living in cages conditioned by males than females. When we examined these taxa in males and females from the same colony that conditioned the cages (Figure 3), no significant sex differences in Christensenellaceae, Streptococcaceae, or Bacteroides acidifaciens were observed.
Figure 5.
Phylum-level microbial abundance of cage-conditioned mice. (A) Relative abundance of bacteria at the phylum level in male and female donors used in cage-conditioning experiments. (B) Relative abundance of various phyla in females housed in male-conditioned cages and females housed in female-conditioned cages. (C–E) Relative abundance of Christensenellaceae (C), Streptococcaceae (D), and Bacteroides acidifaciens (E) in females housed in male-conditioned cages and females housed in female-conditioned cages. Sequence reads assigned to each phylum at 97% sequence similarity cutoff. Relative abundance was assessed using MaAsLin (35), with q < 0.25 considered significant (#); n = 11–12.
Mechanistic Basis for the Role of the Microbiome in Sex Differences in O3-induced AHR
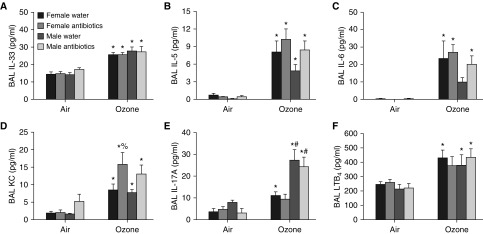
To determine how gut microbiota contribute to sex differences in O3-induced AHR, we measured BAL concentrations of cytokines and chemokines previously implicated in responses to O3. For example, IL-33 is released in the lung after O3 exposure and contributes to O3-induced AHR (36, 37). Consequently, either sex differences or microbiome-dependent effects on the release of IL-33 might explain the observed microbiome-dependent sex differences in O3-induced AHR (Figures 2A and 2B). IL-33 exerts its effects at least in part through its ability to cause type 2 cytokine release from ILC2s (38) and to evoke the release of IL-6 and KC (CXCL1) (37). O3 did increase the BAL concentrations of each of these cytokines (Figures 6A–6D), but sex differences were observed only for IL-6 and indicated greater responses in the females, but not the males, consistent with other reports (10). BAL IL-33, IL-5, IL-6, and KC were not affected by antibiotic treatment in either sex, except for a significant increase in BAL KC after antibiotics in female mice (Figures 6A–6D). IL-17A is also released in the lung after O3 exposure (39) and can contribute to O3-induced AHR (39, 40). BAL IL-17A was greater in O3-exposed males versus females that had been treated with water or antibiotics (Figure 6E). However, there was no effect of antibiotic treatment on BAL IL-17A in either males or females, even though antibiotics did abolish sex differences in the magnitude of O3-induced AHR (Figures 2A and 2B). These data suggest that neither IL-17A nor IL-33 accounts for the microbiome-dependent sex differences in O3-induced AHR.
Figure 6.
BAL cytokines and chemokines in mice treated with antibiotics or control water. (A–F) BAL concentrations of IL-33 (A), IL-5 (B), IL-6 (C), KC (D), IL-17A (E), and leukotriene B4 (LTB4) (F) in male and female mice treated with an antibiotic cocktail or control drinking water and exposed to air or ozone. Data for IL-5, IL-6, and KC for male mice were previously reported (14). Results are mean ± SE of data from n = 6–8 mice per group. *P < 0.05 compared with air; #P < 0.05 compared with female mice with same exposure; %P < 0.05 compared with water-treated mice of the same sex and with the same exposure.
In male mice, SCFAs likely contribute to microbiome-dependent effects on O3-induced AHR (14): antibiotics that reduce O3-induced AHR also reduce serum SCFAs. Furthermore, both exogenous administration of SCFAs via the drinking water and diets that promote bacterial production of SCFAs augment O3-induced AHR (14). Consequently, we measured serum SCFAs in male and female mice. No sex differences in serum acetate, propionate, or butyrate were observed (Figure E5), nor were there differences in serum SCFAs in female mice housed in cages conditioned by male versus female mice (Figure E6).
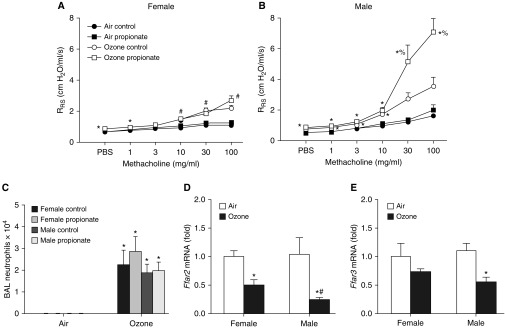
To determine whether instead of sex differences in SCFA production there might be sex differences in the response to SCFA, we administered propionate to male and female mice for 3 days (via their drinking water) before O3 exposure. Propionate treatment did not affect body weight in either sex (Figure E7). In control mice, O3-induced AHR was greater in male than in female mice (compare Figures 7A and 7B), as described above (Figures 1A and 2A). Propionate had no effect on airway responsiveness in males or females exposed to air (Figures 7A and 7B). However, in male mice exposed to O3, propionate increased airway responsiveness (Figure 7B), as previously described (14). In contrast, propionate had no effect on airway responsiveness in female mice exposed to O3 (Figure 7A), indicating that there are indeed sex-related differences in the response to ingested exogenous SCFAs, at least as regards SCFA effects on O3-induced AHR. Propionate did not affect O3-induced neutrophil recruitment in either sex (Figure 7C).
Figure 7.
Propionate treatment augments ozone-induced AHR in male, but not female, mice. (A–C) Mice were treated with sodium propionate (50 mM) or control (PBS, 50 mM) via their drinking water and exposed to room air or ozone. (A) Airway responsiveness of female mice. (B) Airway responsiveness of male mice. (C) BAL neutrophils. The male mice were part of a larger group (14), but were exposed and studied at the same time as the female mice. (D and E) Ffar2 and Ffar3 mRNA abundances in colons of male and female mice exposed to air or ozone. Results are expressed relative to the female air-exposed mice. Results are mean ± SE of data from four air-exposed and eight ozone-exposed mice per group. *P < 0.05 compared with air-exposed mice of the same sex; #P < 0.05 compared with male mice; %P < 0.05 compared with control water-treated mice.
Propionate administered via the gastrointestinal tract is largely cleared by enterocytes and hepatocytes before reaching the systemic circulation (41). These data suggest that observed sex differences in the response to propionate added to the drinking water (Figures 7A and 7B) may be mediated via effects on enterocytes or hepatocytes, perhaps via effects on enterocyte-derived hormone production or hepatocyte-mediated changes in sex hormone metabolism. The role of SCFAs in mediating other physiological or pathophysiological effects is attributed in part to their ability to bind to G protein–coupled receptors, including FFAR2 and FFAR3 (mouse homologs of human GPR43 and GPR41) (42). To determine whether there were differences in the intestinal or hepatic expression of these SCFA receptors that might explain the observed sex differences in the response to propionate, we measured Ffar2 and Ffar3 mRNA abundance in colons (Figures 7D and 7E) and livers (Figures E8C and E8D) of males and females exposed to air or O3. In the colon, there were no sex differences in Ffar2 or Ffar3 expression in air-exposed mice. Ffar2 and Ffar3 mRNA abundances were both reduced in male mice after O3 exposure (Figures 7D and 7E). Ffar2 was also reduced in female mice after O3 exposure, but the effect of O3 on Ffar2 mRNA abundance was significantly greater in males than in females (Figure 7D). Ffar3 mRNA abundance was not changed in female mice in response to O3. To our knowledge, this is the first report of O3 exposure affecting the expression of any gene in colonic tissue. In the liver, mRNA abundances of Ffar2 and Ffar3 were not significantly affected by either sex or by O3 exposure (Figures E8C and E8D), nor was there any significant effect on the mRNA expression of Ffar2 and Ffar3 in lung tissue (Figures E8A and E8B), though there was a trend toward greater Ffar3 expression in lungs of male mice.
Mechanistic Basis for Sex Differences in the Impact of Antibiotics on O3-induced Neutrophil Recruitment
The neutrophil chemotactic factor LTB4 may account for reductions in O3-induced neutrophil recruitment that occur in male mice after antibiotic treatment or under GF conditions, as BAL LTB4 is reduced in both conditions (14). Because there were sex differences in the impact of antibiotics on O3-induced increases in BAL neutrophils, we also measured BAL LTB4 in these mice (Figure 6F). Compared with air, O3 caused a significant increase in BAL LTB4, but neither sex nor antibiotics had any significant effect, nor was there any difference in BAL LTB4 in mice conditioned in male versus female cages (Figure E4D). As discussed above, other cytokines and chemokines with neutrophil chemotactic activity were released in response to O3 (IL-17A, IL-6, and KC) (Figures 6C–6E), but none were reduced after antibiotic treatment. The data indicate that other microbiome-dependent factors must account for the antibiotic-induced reductions in BAL neutrophils observed in male mice (Figure 3C).
Discussion
We report that the magnitude of O3-induced AHR was substantially greater in male mice than in female mice. Importantly, this sex difference was abrogated with antibiotic treatment, and the greater O3-induced AHR of male mice could be transferred to female weanling mice by placing them in cages conditioned by adult males. Overall, these data are consistent with the hypothesis that the microbiome contributes to sex differences in O3-induced AHR.
The magnitude of O3-induced AHR was greater in male mice than in female mice. This is the first report of such sex differences in O3-induced AHR, although the greater airway responsiveness observed in male versus female air-exposed mice (Figures 1 and 2) has been previously reported (13). Several studies using human subjects have reported sex differences in the response to O3, but only one, by Que and colleagues (4), examined airway responsiveness as an outcome indicator. The authors observed no difference in O3-induced AHR between male and female subjects, but only a minority of the subjects actually exhibited AHR after O3 exposure. Given the role of the microbiome in sex differences reported here, the much greater intersubject variability in gut microbial community structure extant in humans versus inbred mice may have obscured any sex differences.
Sex differences in the magnitude of O3-induced AHR were abolished in antibiotic-treated mice (Figure 2). Although it is possible that this effect was due to an off-target effect of the antibiotics rather than depletion of gut microbes, we think this explanation is unlikely. In male mice, effects of antibiotics on responses to O3 are mimicked by GF conditions, and in GF mice, antibiotics have no impact on responses to O3 (14). Thus, the data are consistent with an important role for the microbiome in mediating sex-related differences in O3-induced AHR. The observation that placing weanling female mice in cages conditioned by male mice was sufficient to impart male-like responses to O3 to the female mice also supports a role for the microbiome in these events. Transfer of fecal microbiota by cohousing or by placing donor feces in receiver cages has been previously described (24, 25) and occurs via coprophagic and grooming behavior, allowing the microbiota from fecal pellets in cage nesting material to be transferred.
Microbial production of SCFAs likely accounts for the ability of the microbiome to promote O3-induced AHR in male mice (14). However, we noted no difference in serum SCFAs in males versus females, nor was there any difference in serum SCFAs in female mice placed in cages conditioned by males versus females. Moreover, although there were sex differences in the abundance of certain bacterial taxa in the fecal material of mice housed in cages conditioned by male versus female mice (Figure 5), these differences did not include taxa that are prominent SFCA producers. Thus, sex differences in the ability of the gut microbiota to produce SCFAs do not appear to account for sex differences in the response to O3. Instead, sex differences in the response to SCFAs appear to be sufficient to account for the role of the microbiome in the observed sex differences in the response to O3.
We do not know the precise actions or cellular targets of propionate that resulted in augmented responses to O3 in male mice (Figure 7). Propionate administered via the gastrointestinal tract is largely cleared before it reaches the systemic circulation (41), suggesting that propionate acts on gut epithelial cells, on sensory afferents within the intestines, or on hepatocytes. Although we did observe sex differences in the colonic expression of the SCFA receptor Ffar3 in mice exposed to O3, the direction of the effect (lower expression in the male mice) is not consistent with the ability of propionate to affect male but not female mice. Instead, the observed sex differences in the response to propionate may be mediated either downstream of SCFA receptor activation or by effects of SCFAs on histone deacetylases (42) and histone crotonylation (43).
As described above, it is not necessary to evoke sex differences in the gut microbial community structure to explain the role of the microbiome in the observed sex differences in response to O3. Given the sex difference in the response to SCFAs (Figure 7), only the fact that microbiota account for the majority of the production of SCFAs (42) is necessary. However, our data do confirm reports of others (15, 44) indicating that the gut microbial community structures of male and female mice are different, even when the mice are littermates and exposed to the same microbiota at birth. For example, Org and colleagues (15) and Liang and colleagues (44) reported a greater abundance of Tenericutes in male versus female mice, consistent with our data. Liang and colleagues (44) observed a greater prevalence of Proteobacteria in female versus male mice, just as we did; however, Org and colleagues (15) observed greater numbers of Proteobacteria in males.
Our observations supporting a role for the microbiome in sex differences in O3-induced AHR do not rule out the possibility that sex hormones also play a role. Indeed, sex hormones may account for observed sex differences in the gut microbiome, as these differences are abolished by male castration (15, 45) and only develop after puberty (19). Indeed, sex hormones are known to affect microbiota (15, 19, 45), and some bacteria have receptors for mammalian sex hormones (46). Sex hormones also impact the intestinal mucosa in a way that permits greater survival of certain intestinal taxa over others (47). However, other microbiome-independent effects of sex hormones, for example, on immune or inflammatory cells, may also contribute to sex differences in the response to O3. It is also interesting to note that even after room-air exposure, males had greater airway responsiveness than females, consistent with other reports (13), whereas this sex difference was no longer apparent in antibiotic-treated mice. Thus, sex differences in innate airway responsiveness are also impacted by the gut microbiome. Sex hormones, particularly testosterone, contribute to sex differences in innate airway responsiveness (13) and may also be driving the microbiome-dependent sex differences in O3-induced AHR observed here.
Although there were marked sex differences in O3-induced AHR, no sex differences in O3-induced neutrophil recruitment were observed in the absence of antibiotics. In contrast, Cabello and colleagues reported greater neutrophilic inflammation in female versus male mice exposed to O3 (9). They used the same O3-exposure regimen as we did and also used C57BL/6J mice. The reason for these disparate results likely lies in the environmental conditions extant in the two mouse facilities and the impact of those conditions on the gut microbiomes of the mice (48). Indeed, there was a reduction in neutrophil recruitment after antibiotic treatment in the male mice, consistent with our previous observations (14), but not in female mice. There was also an increase in BAL neutrophils in O3-exposed female mice that had been housed in cages conditioned by males versus females. These data indicate that the male microbiome does affect O3-induced neutrophil influx. However, whereas microbiota-dependent changes in propionate likely account for sex differences in O3-induced AHR, SCFAs do not appear to account for the role of the microbiome in neutrophil responses, as propionate had no effect on BAL neutrophils in either male or female mice.
The cage conditioning strategy we employed resulted in the female receivers ingesting the feces of male donors via grooming or coprophagic behavior, and also resulted in the female receivers developing O3-induced AHR similar in magnitude to that observed in the males (Figure 4). We do not know whether reductions in O3-induced AHR could also be achieved by rearing males in cages conditioned by females. The cage conditioning strategy also exposed the receivers to urine and other secretions from the donors. Even just the odor of male mice is sufficient to accelerate sexual maturation in young females (31–34). Thus, we cannot rule out the possibility that this effect, rather than changes in the microbiota, contributed to the observed effects of cage conditioning (Figure 4). We did not observe any differences in serum estradiol in the male- versus female-conditioned mice, but our measurements were performed on serum harvested at the time of exposure, well after the initial exposure of the mice to the male cages. Earlier sexual maturation could have altered O3-induced AHR by inducing dysanaptic growth of lungs versus airways, but such changes should have also affected airway responsiveness in air-exposed mice, which was not observed (Figure 4A), nor was there any effect on absolute lung volumes (Figure E4C).
One additional technical issue regarding the cage conditioning experiments requires consideration. We were able to transfer the augmented male O3-induced AHR response to female receivers via cage conditioning, and there were differences in gut microbiota in the mice in cages conditioned by males versus females. However, the bacterial taxa that differed in the mice reared in male- versus female-conditioned cages (Figure 5) were not the same as those that differed in males versus females (Figure 3). Many types of bacteria can perform the same metabolic functions, and it is possible that the functional capabilities of the bacteria affected by cage conditioning (Figure 5) are more important in transfer of the male phenotype than the specific bacteria affected. That the cage conditioning protocol did not reproduce the microbiomes of male and female mice with great fidelity is not terribly surprising. The heritability of microbiota differs from microbe to microbe (49), and microbiota with high heritability are unlikely to be substantially influenced by external factors such as cage conditioning. Thus, cage conditioning should result in the transfer of certain bacteria but not others, resulting in a microbial community in the recipient that is not identical to that in the donor or to its original self, as was observed in this work (Figures 3 and 5) and previous studies (19, 50). Additionally, each donor-conditioned cage was occupied by three receivers whose feces could also be passed from mouse to mouse. Furthermore, the receivers in the cage conditioning experiments were born and initially reared at The Jackson Laboratory, whereas the donors were born and reared in the Harvard T. H. Chan School of Public Health vivarium from a colony that had been in this facility for several generations. Indeed, even the female mice from both groups had different gut microbial community structures (compare the female mice in Figure 3A with the female mice in female-conditioned cages in Figure 5A), consistent with other reports of differences in the microbiomes of mice with the same genetic background but reared in different facilities (48).
In summary, our study indicates a role for the microbiome in sex differences in O3-induced AHR. A better understanding of the relationship between male and female microbiota and sex hormones could lead to more precise biomarkers or sex-specific therapeutics to address asthma in male and female patients, especially asthma triggered by air pollution.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Lester Kobzik and Dr. Jeffrey Drazen for their insightful comments.
Footnotes
Supported by National Institutes of Health grants ES-013307, ES-024032, HL-007118, ES-000002, and P50-HD28934.
Author Contributions: Y.C., H.T., T.A.B., D.I.K., C.H., and S.A.S. conceived and designed the experiments. Y.C., G.A.-A., H.T., T.A.B., R.S.O., and D.I.K. performed the experiments and analyzed the data. Y.C. wrote the paper. Y.C., G.A.-A., H.T., T.A.B., R.S.O., D.I.K., C.H., and S.A.S. reviewed, revised, and approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0099OC on September 21, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290:1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- 2.Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure and lung function: effect modified by obesity and airways hyperresponsiveness in the VA normative aging study. Chest. 2007;132:1890–1897. doi: 10.1378/chest.07-1126. [DOI] [PubMed] [Google Scholar]
- 3.Foster WM, Brown RH, Macri K, Mitchell CS. Bronchial reactivity of healthy subjects: 18-20 h postexposure to ozone. J Appl Physiol (1985) 2000;89:1804–1810. doi: 10.1152/jappl.2000.89.5.1804. [DOI] [PubMed] [Google Scholar]
- 4.Que LG, Stiles JV, Sundy JS, Foster WM. Pulmonary function, bronchial reactivity, and epithelial permeability are response phenotypes to ozone and develop differentially in healthy humans. J Appl Physiol (1985) 2011:679–687. doi: 10.1152/japplphysiol.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fauroux B, Sampil M, Quénel P, Lemoullec Y. Ozone: a trigger for hospital pediatric asthma emergency room visits. Pediatr Pulmonol. 2000;30:41–46. doi: 10.1002/1099-0496(200007)30:1<41::aid-ppul7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Sheffield PE, Zhou J, Shmool JL, Clougherty JE. Ambient ozone exposure and children’s acute asthma in New York City: a case-crossover analysis. Environ Health. 2015;14:25. doi: 10.1186/s12940-015-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang BF, Chen YH, Lin YT, Wu XT, Leo Lee Y. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ Res. 2015;137:382–390. doi: 10.1016/j.envres.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Hazucha MJ, Folinsbee LJ, Bromberg PA. Distribution and reproducibility of spirometric response to ozone by gender and age. J Appl Physiol (1985) 2003;95:1917–1925. doi: 10.1152/japplphysiol.00490.2003. [DOI] [PubMed] [Google Scholar]
- 9.Cabello N, Mishra V, Sinha U, DiAngelo SL, Chroneos ZC, Ekpa NA, et al. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1150–L1163. doi: 10.1152/ajplung.00018.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra V, DiAngelo SL, Silveyra P. Sex-specific IL-6-associated signaling activation in ozone-induced lung inflammation. Biol Sex Differ. 2016;7:16. doi: 10.1186/s13293-016-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vancza EM, Galdanes K, Gunnison A, Hatch G, Gordon T. Age, strain, and gender as factors for increased sensitivity of the mouse lung to inhaled ozone. Toxicol Sci. 2009;107:535–543. doi: 10.1093/toxsci/kfn253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikerov AN, Gan X, Umstead TM, Miller L, Chinchilli VM, Phelps DS, et al. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after Klebsiella pneumoniae infection. Respir Res. 2008;9:24. doi: 10.1186/1465-9921-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho Y, Abu-Ali G, Tashiro H, Kasahara DI, Brown TA, Brand JD, et al. The microbiome regulates pulmonary responses to ozone in mice. Am J Respir Cell Mol Biol. 2018;59:346–354. doi: 10.1165/rcmb.2017-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, Parks B, et al. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun. 2014;5:4500. doi: 10.1038/ncomms5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amato KR, Leigh SR, Kent A, Mackie RI, Yeoman CJ, Stumpf RM, et al. The role of gut microbes in satisfying the nutritional demands of adult and juvenile wild, black howler monkeys (Alouatta pigra) Am J Phys Anthropol. 2014;155:652–664. doi: 10.1002/ajpa.22621. [DOI] [PubMed] [Google Scholar]
- 18.Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11:e0154090. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Liao X, Sparks JB, Luo XM. Dynamics of gut microbiota in autoimmune lupus. Appl Environ Microbiol. 2014;80:7551–7560. doi: 10.1128/AEM.02676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Org E, Parks BW, Joo JW, Emert B, Schwartzman W, Kang EY, et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 2015;25:1558–1569. doi: 10.1101/gr.194118.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deloris Alexander A, Orcutt RP, Henry JC, Baker J, Jr, Bissahoyo AC, Threadgill DW. Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. Mamm Genome. 2006;17:1093–1104. doi: 10.1007/s00335-006-0063-1. [DOI] [PubMed] [Google Scholar]
- 25.Bel S, Elkis Y, Elifantz H, Koren O, Ben-Hamo R, Lerer-Goldshtein T, et al. Reprogrammed and transmissible intestinal microbiota confer diminished susceptibility to induced colitis in TMF-/- mice. Proc Natl Acad Sci USA. 2014;111:4964–4969. doi: 10.1073/pnas.1319114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 27.Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun. 2009;77:2741–2753. doi: 10.1128/IAI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barfod KK, Vrankx K, Mirsepasi-Lauridsen HC, Hansen JS, Hougaard KS, Larsen ST, et al. The murine lung microbiome changes during lung inflammation and intranasal vancomycin treatment. Open Microbiol J. 2015;9:167–179. doi: 10.2174/1874285801509010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenbergh JG. Effect of the presence of a male on the sexual maturation of female mice. Endocrinology. 1967;81:345–349. doi: 10.1210/endo-81-2-345. [DOI] [PubMed] [Google Scholar]
- 32.Vandenbergh JG. Male odor accelerates female sexual maturation in mice. Endocrinology. 1969;84:658–660. doi: 10.1210/endo-84-3-658. [DOI] [PubMed] [Google Scholar]
- 33.Jouhanneau M, Cornilleau F, Keller M. Peripubertal exposure to male odors influences female puberty and adult expression of male-directed odor preference in mice. Horm Behav. 2014;65:128–133. doi: 10.1016/j.yhbeh.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Szymanski LA, Keller M. Activation of the olfactory system in response to male odors in female prepubertal mice. Behav Brain Res. 2014;271:30–38. doi: 10.1016/j.bbr.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 35.Mallick H, Ma S, Franzosa EA, Vatanen T, Morgan XC, Huttenhower C. Experimental design and quantitative analysis of microbial community multiomics. Genome Biol. 2017;18:228. doi: 10.1186/s13059-017-1359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaudel C, Mackowiak C, Maillet I, Fauconnier L, Akdis CA, Sokolowska M, et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J Allergy Clin Immunol. 2018;142:942–958. doi: 10.1016/j.jaci.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 37.Mathews JA, Krishnamoorthy N, Kasahara DI, Cho Y, Wurmbrand AP, Ribeiro L, et al. IL-33 drives augmented responses to ozone in obese mice. Environ Health Perspect. 2017;125:246–253. doi: 10.1289/EHP272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumagai K, Lewandowski R, Jackson-Humbles DN, Li N, Van Dyken SJ, Wagner JG, et al. Ozone-induced nasal type 2 immunity in mice is dependent on innate lymphoid cells. Am J Respir Cell Mol Biol. 2016;54:782–791. doi: 10.1165/rcmb.2015-0118OC. [DOI] [PubMed] [Google Scholar]
- 39.Mathews JA, Krishnamoorthy N, Kasahara DI, Hutchinson J, Cho Y, Brand JD, et al. Augmented responses to ozone in obese mice require IL-17A and gastrin-releasing peptide. Am J Respir Cell Mol Biol. 2018;58:341–351. doi: 10.1165/rcmb.2017-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 43.Fellows R, Denizot J, Stellato C, Cuomo A, Jain P, Stoyanova E, et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat Commun. 2018;9:105. doi: 10.1038/s41467-017-02651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci USA. 2015;112:10479–10484. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kendall MM, Sperandio V. What a dinner party! Mechanisms and functions of interkingdom signaling in host-pathogen associations. MBio. 2016;7:e01748. doi: 10.1128/mBio.01748-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menon R, Watson SE, Thomas LN, Allred CD, Dabney A, Azcarate-Peril MA, et al. Diet complexity and estrogen receptor β status affect the composition of the murine intestinal microbiota. Appl Environ Microbiol. 2013;79:5763–5773. doi: 10.1128/AEM.01182-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, et al. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22:516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray A, Basu S, Gharaibeh RZ, Cook LC, Kumar R, Lefkowitz EJ, et al. Gut microbial dysbiosis due to helicobacter drives an increase in marginal zone B Cells in the absence of IL-10 signaling in macrophages. J Immunol. 2015;195:3071–3085. doi: 10.4049/jimmunol.1500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.