Abstract
The complement system provides host defense against pathogens and environmental stress. C3, the central component of complement, is present in the blood and increases in BAL fluid after injury. We recently discovered that C3 is taken up by certain cell types and cleaved intracellularly to C3a and C3b. C3a is required for CD4+ T-cell survival. These observations made us question whether complement operates at environmental interfaces, particularly in the respiratory tract. We found that airway epithelial cells (AECs, represented by both primary human tracheobronchial cells and BEAS-2B [cell line]) cultured in C3-free media were unique from other cell types in that they contained large intracellular stores of de novo synthesized C3. A fraction of this protein reduced (“storage form”) but the remainder did not, consistent with it being pro-C3 (“precursor form”). These two forms of intracellular C3 were absent in CRISPR knockout-induced C3-deficient AECs and decreased with the use of C3 siRNA, indicating endogenous generation. Proinflammatory cytokine exposure increased both stored and secreted forms of C3. Furthermore, AECs took up C3 from exogenous sources, which mitigated stress-associated cell death (e.g., from oxidative stress or starvation). C3 stores were notably increased within AECs in lung tissues from individuals with different end-stage lung diseases. Thus, at-risk cells furnish C3 through biosynthesis and/or uptake to increase locally available C3 during inflammation, while intracellularly, these stores protect against certain inducers of cell death. These results establish the relevance of intracellular C3 to airway epithelial biology and suggest novel pathways for complement-mediated host protection in the airway.
Keywords: anaphylatoxins, chronic obstructive pulmonary disease, cystic fibrosis, interstitial lung disease, oxidants
Clinical Relevance
Historically, complement has been viewed as a liver-derived, fluid-phase system that is important for host defense, but recent work has shown that it is produced in extrahepatic sites and has multiple roles intracellularly affecting cell development, polarity, and metabolism. In this work, we show that airway epithelial cells synthesize and store large amounts of complement protein C3, which can be mobilized in the setting of proinflammatory cytokine exposure. Additionally, these stores can be augmented to mitigate death due to different stressors. This creates a precedence to modulate intracellular C3 in airway epithelial cells to assist with their function.
The complement system has evolved as a first line of innate immunity against pathogens (1, 2). C3, its central component, is the most abundant complement protein in the blood and is protective against bacterial infections, especially Streptococcus pneumoniae and Pseudomonas aeruginosa (3, 4). C3 is a 190-kD heterodimer that is made up of an α-chain and a β-chain, which are linked by a disulfide bond (Figure 1). Upon activation of the complement cascade by the classical, alternative, or lectin pathway, C3 is cleaved to C3a (a proinflammatory mediator with chemotactic and vasodilatory activities) and C3b (an opsonin). The liver is the predominant source of circulating C3 (5, 6). However, C3 can also be synthesized by immune and nonimmune cells such as lymphocytes, neutrophils, and epithelial, endothelial, and mesenchymal cells (7–10). Among these cells, neutrophils and monocytes are the primary human cells known to contain biosynthetically derived C3 stores, as detected by radiolabeling (11, 12).
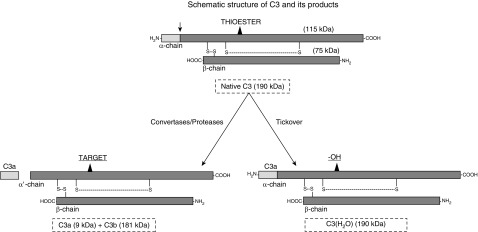
Figure 1.
Schematic representation of native C3 and C3(H2O). C3 is a two-chain protein consisting of an α-chain and a β-chain linked by a disulfide bond. The thioester bond on the α-chain allows C3 to covalently attach to a target. Upon activation via a protease or a specific C3 convertase, C3a is released (the arrow shows the cleavage site) and C3b attaches to a nearby target via an ester or amide bond. Constitutively, there is a low-grade spontaneous tickover in the blood where the hydroxyl group (−OH) from H2O reacts with the thioester, forming C3(H2O). In this case, C3a remains attached. Adapted from Reference 15.
Other investigators and we have previously shown that in addition to being a source of opsonins and anaphylatoxins at the site of inflammation, intracellular C3 activation affects human CD4+ T-cell differentiation and metabolism (13, 14). Activation of CD4+ T cells by engaging CD3 and CD46 increases intracellular C3 and skews naive CD4+ T cells toward a T-helper cell type 1 phenotype. Moreover, the constitutive generation of C3a by intracellular proteases (such as cathepsin-L) was shown to be crucial for CD4+ T-cell survival through the mTOR pathway (13). We subsequently showed that CD4+ T cells also internalize C3, which modulates cytokine expression, increasing IL-6 production (15). Furthermore, intracellular C3 activation aggravated tissue damage in a murine model of gut ischemia-reperfusion injury (16, 17). However, intracellular C3 was protective against cytokine-induced death in rodent and human pancreatic β-cells (18, 19). These findings indicate that intracellular C3 functions beyond its role as a guardian of the intravascular space against pathogen invasion by providing previously unrecognized tissue-specific protection against distinct stimuli such as injury and infection (20–22).
Complement may also have a broader function in the lung, where direct communication with the environment requires rapid responses to airspace insults. Complement proteins are present in BAL fluid from humans and increase after LPS administration (23, 24). Airway epithelial cells (AECs) are known to secrete complement proteins (including C3), but whether AECs store C3, and how modulating these stores affects their phenotype, has not been systematically studied (10, 25, 26). We proposed that AECs have high levels of intracellular C3 that may be mobilized as a stress response (10). However, it is unknown how intracellular C3 stores in AECs are modulated and whether altering these stores is deleterious (such as in the gut) or protective (such as in pancreatic β-cells). Here, we show that human AECs synthesize and secrete large amounts of C3, but are unique in their ability to contain such substantial stores, because, until now, most of the C3 that is synthesized by cells from a solid organ system was believed to be destined for secretion (5, 6). Further, AECs can “load” exogenous C3, which rescues cell death induced by factors such as H2O2 and growth factor deprivation. These results reveal the importance of intracellular complement—in particular, C3—for host protection in the airway. Some of these results have been previously reported in abstract form (27, 28).
Methods
Human Samples
The Washington University School of Medicine Institutional Review Board approved the human studies. All subjects provided consent to donate their lung tissues for research. Lung samples from patients with end-stage lung disease were obtained from tissues resected for transplant surgery. Control lung tissues were obtained through a biomaterial transfer agreement with the International Institute for the Advancement of Medicine. Blood was collected from healthy donors after they provided written informed consent.
AEC Culture
Primary human tracheobronchial epithelial cells (hTECs) were isolated from tracheae and proximal bronchi of lungs donated for transplantation (29). BEAS-2B cells, an SV-40 transformed human hTEC line, were obtained from ATCC (CRL-9609) (8, 30).
Complement Proteins
Purified human C3 was generated in-house (15) or purchased from Complement Technologies (A113), as were purified human C3b (A114), C3a (A118), and C3-dpl serum (A314). To prepare C3-methylamine (C3-MA), purified C3 was incubated with 0.4 M methylamine hydrochloride (Sigma Aldrich) and 0.1 M Tris (pH 8.0) for 1 hour at 37°C (15). C3-MA was denatured by heating at 85°C for 3 minutes.
Cell Treatments
Cytokine exposure experiments used BEAS-2B cells (at 70% confluence) and hTECs (at 100% confluence and a transepihtelial electrical resistance of > 180 milliohnms × cm2). hTECs (at 100% confluence), and a transepithelial electrical resistance of >180 milliohms × cm2. Cells were incubated in media lacking FBS for 24 hours and then exposed to recombinant human TNF-α, IFN-γ, or IL-1β (Peprotech), either alone or as a combination (“cytomix”) applied to the apical and/or basal chamber of Transwells.
Hydrogen peroxide (H2O2; Sigma-Aldrich) treatment was performed in confluent parent and C3-deficient BEAS-2B cells. Cells were incubated with 500–1000 μM H2O2 for 60 minutes and then washed with PBS. The cells were then incubated in the presence or absence of an exogenous source of C3 for 3 hours. The cells were also treated with serum-starved media, Earle’s basic salt solution (EBSS; no calcium, magnesium, or phenol red; Thermo Fisher Scientific), or 250 nM staurosporine (Sigma-Aldrich) for 4 hours in the presence or absence of an exogenous source of C3, as detailed above.
Details regarding the methods used for CRISPR-induced C3 deletion, immunoblot analysis, immunostaining, imaging, flow cytometry, siRNA treatment, RT-PCR, and mass spectrometry are provided in the data supplement.
Statistics
Statistical analysis was performed using Prism Software version 7 (GraphPad). Comparisons between two groups were performed using a two-tailed Student’s t test (parametric). Comparisons between multiple groups were performed using a one-way ANOVA with Bonferroni’s multiple comparison test (parametric) or Dunn’s multiple comparison test (nonparametric). A P value < 0.05 was considered significant.
Results
AECs Contain Different Forms of Intracellular C3 Stores
To investigate intracellular C3 in AECs, we initially used the human bronchial epithelial cell line BEAS-2B and primary-culture hTECs. To determine whether C3 was constitutively present in AECs, we performed confocal microscopy in cells cultured and passaged in media free of human serum for 2–3 weeks (to preclude C3 uptake). Intracellular C3 was detected in both the BEAS-2B cells (Figure 2A, upper panel) and, to a greater extent, the hTECs (Figure 2A, lower panel). This suggested that the intracellular C3 was derived from biosynthesis. To assess whether C3 was present on the cell surface, we performed flow cytometry with and without permeabilization using both C3-sufficient and -deficient AECs. A majority of the C3 was intracellular—only a trace amount, if any, was present on the surface (Figure 2B). A control C3-deficient line of BEAS-2B cells lacked C3 (Figures 2C and E1A). Constitutive C3 production was further established by demonstrating C3 mRNA in BEAS-2B cell extracts (Figures E1B–E1D). C3 protein also decreased when protein synthesis was inhibited by using siRNA against C3, further indicating that this C3 was primarily a precursor form derived through biosynthesis (Figure E1E in the data supplement).
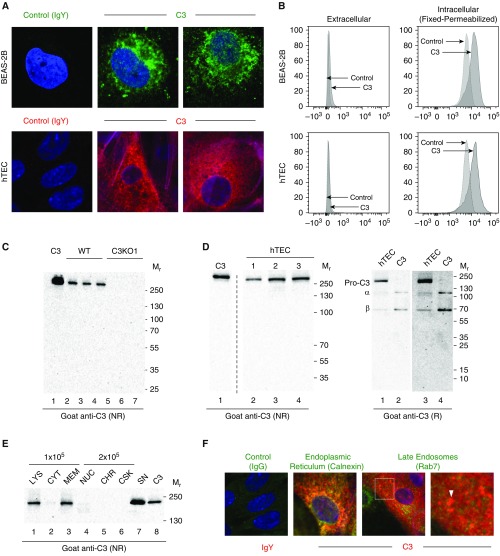
Figure 2.
Human airway epithelial cells contain stores of intracellular C3. (A) Representative confocal-microscopic images of permeabilized BEAS-2B (upper panel) and primary human tracheal epithelial cells (hTECs, lower panel) cultured in C3-free media. Control: chicken IgY. Primary antibody: chicken anti-C3. Representative of five independent experiments for each cell type. A different secondary antibody (Alexa 647, red) was used in the lower panel to address any concerns associated with autofluorescence in the upper panel with Alexa 488 (green). DAPI (blue), ×400. (B) C3 is predominantly intracellular in both the BEAS-2B cells (upper panel) and hTECs (lower panel) by flow cytometry. Antibody: chicken anti-human C3. Representative of three independent experiments for each cell type. CRISPR-induced C3KO cells were used as a control for both BEAS-2B cells and hTECs. The x-axis is the geometric mean fluorescent intensity of C3. The y-axis is modal, which scales all channels as a percentage of the maximum count. (C) C3 analysis of lysates from BEAS-2B cell lines—wild-type (WT) and a C3-deficient clone (C3KO1; see Figure E2A)—under nonreducing (NR) conditions using Western blotting (WB). Equivalent amounts of cell lysates (Bradford assay) were used for WB. The blot shows that C3 is absent in the CRISPR-induced C3KO clones. Positive control: C3, 50 ng (lane 1). The numbers represent lysates prepared from cells growing in three different wells for each condition. (D) C3 analysis of lysates from hTECs under NR (left) and reducing (R, right) conditions by WB. C3, 40 ng (left), 20 ng (right). Lanes 3 and 4 are longer exposures (10 min) of lanes 1 and 2 (5 min). Representative of three independent experiments. This antibody binds more strongly to the NR form of C3 than to its α- or β-chains, as demonstrated in this image. (E) C3 analysis of hTEC subcellular fractions using WB. Cytoplasmic (CYT), membrane (MEM), nuclear (NUC), chromatin (CHR), and cytoskeletal (CSK) fractions were obtained; 1 × 105 cell equivalents/lane were loaded for the CYT and MEM fractions, and 2 × 105 cell equivalents/lane were loaded for the others. Serum-free supernatant (SN) was collected after 24 hours and concentrated five-fold. Lysate (LYS) was loaded to serve as a comparator on the left. C3, 60 ng. Representative of three independent experiments. (F) Colocalization of intracellular C3 (green) and markers of the endoplasmic reticulum (ER; red, calnexin) and late endosomes (red, Rab7, arrows, magnified inset) in hTECs cultured in C3-free conditions. DAPI (blue), original magnification, ×630. Mr = relative molecular mass.
C3 is cleaved by the C3 convertases or certain other proteases to release C3a and C3b (Figure 1). C3a is attached to the single-chain precursor form (pro-C3) via its α-chain. To identify the nature of the C3 present in AECs, lysates were analyzed by immunoblot before and after DTT treatment. Before DTT treatment, a band was detected migrating at 200–250 kD, suggesting that the AECs contained full-length C3 (Figure 2D, left). After DTT treatment, this protein partly degraded to the α-chain and β-chain (Figure 2D, right). However, a fraction of this protein was resistant to cleavage by DTT. We interpret these results to indicate that AECs contain at least two forms of C3: a precursor form that is resistant to disulfide cleavage, and a mature form that can be reduced into its two chains.
To better determine the intracellular storage and potential functions of C3 in AECs, we performed subcellular fractionation and confocal microscopy. Subcellular fractionation showed that a majority of the precursor form localized to the membrane fraction (plasma, mitochondria, endoplasmic reticulum [ER], and endosomal and Golgi membranes), whereas a minor component was present in the nonmembrane cytoplasmic fraction (Figure 2E). Consistent with these findings, intracellular C3 colocalized with calnexin, an ER protein, and a minor fraction colocalized with Rab7, a marker for late endosomes (Figures 2F and E4C–E4F). These results indicate that a majority of the C3 that is synthesized by AECs localizes to the ER, and a smaller amount is stored in late endosomes.
Intracellular C3 Stores Are Upregulated by Stress Signals and Proinflammatory Mediators
To evaluate whether cytokines associated with airway inflammation altered intracellular C3 stores, we treated AECs with a combination of TNF-α, IL-1β and IFN-γ (i.e., “cytomix”) or each individually for 24 hours. C3 protein was upregulated and detectable by 4 hours (Figure 3A). Higher cytokine doses above 25 ng/ml did not increase the amount of C3 protein. A similar response was observed in undifferentiated hTECs growing at an air–liquid interface cultured on Transwell membranes: each cytokine increased C3 individually, but the combination increased it the most (Figures 3B and E2A). Using confocal microscopy, we were able to identify that the increase in C3 protein was not accompanied by binding of C3 to the surface, suggesting that the increase was in intracellular stores (Figure 3C). Subcellular fractionation showed that a majority of the intracellular increase occurred in the cytoplasmic and membrane fractions (Figure 3D), consistent with de novo synthesis. This finding was supported by the observation that C3 colocalized with the ER (calnexin) and the late endosomes (Rab7) (Figures 3E and E3C–E3H). In the membrane fraction, cytomix upregulated both the C3 precursor protein and the form that reduced to the α- and β-chains (Figure E2B).
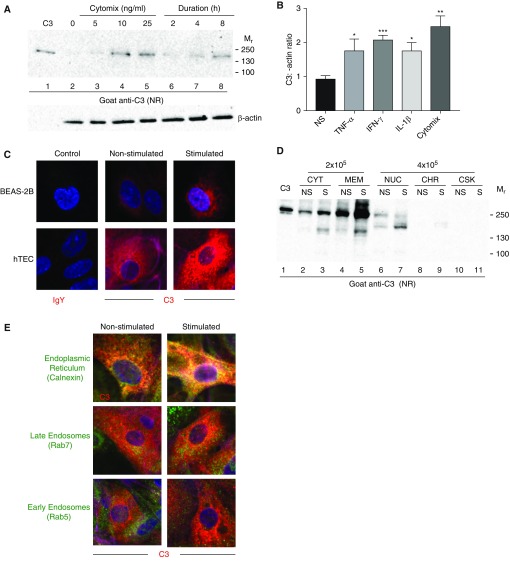
Figure 3.
Intracellular C3 is upregulated by proinflammatory stimuli. (A) C3 analysis of lysates from BEAS-2B cells under NR conditions by WB. Cells were incubated in the presence of serum-free media with increasing doses of cytomix (a combination of TNF-α, IFN-γ, and IL-1β; lanes 2–5), and with 25 ng/ml for increasing durations of time (2, 4, and 8 h). C3, 60 ng. Representative of three independent experiments. (B) C3 analysis of lysates from hTECs under NR conditions by WB. Cells were treated in a manner similar to that described in A, with or without 25 ng/ml of cytomix for 24 hours (or each individual cytokine at the same dose). Graphical representation based on densitometric scanning from WB (representative blot; Figure E2A). Shown is the mean ± SEM of two independent experiments. All three cytokines increased C3 significantly over nonstimulated (NS) cells (*P < 0.05, ***P < 0.0001 by t test), but the highest increase occurred with the cytomix (**P < 0.01). (C) Confocal-microscopic images showing an increase in intracellular C3 (red) in BEAS-2B cells (upper panel) and hTECs (lower panel) after stimulation with 50 ng/ml of cytomix for 24 hours. DAPI (blue), original magnification, ×630. Representative of three independent experiments. C3 did not colocalize with the nucleus (Figure E3B). (D) C3 analysis of hTEC subcellular fractions (prepared in a manner similar to that described in Figure 2E) at rest (NS) and after exposure to 50 ng/ml of cytomix (S) for 24 hours. C3, 50 ng. CYT, MEM: 2 × 105 cell equivalents/lane loaded; other fractions: 4 × 105 cell equivalents/lane loaded. Bands running at 130–150k likely represent proteolytic fragments. Representative of two independent experiments. (E) Representative confocal microscopy of hTECs demonstrating that upon exposure to 50 ng/ml of cytomix for 24 hours, intracellular C3 (red) increases and colocalizes primarily with the ER (calnexin) and the late endosomes (Rab7). C3 in early endosomes (Rab5) is unchanged. DAPI (blue). Original magnification, ×630.
C3 Is Secreted by AECs at Rest and Increases upon Cytokine Exposure
Effective innate immunity likely requires that C3 be secreted from the luminal surface of the airway epithelium to interact directly with pathogens that invade the airway (3, 4). Thus, we asked whether the C3 that is upregulated after proinflammatory cytokine exposure is secreted, and if so, what are the structural forms, as they may have distinct functions. C3 was constitutively secreted into the AEC supernatant and was upregulated after cytomix exposure (Figure 4A). At 70% confluence, the baseline secretion of C3 in BEAS-2B cells was 16–18 ng/105 cells/24 hours in serum-free media. This increased to 26–28 ng/105cells/24 hours upon exposure to 25 ng/ml cytomix (Figure 4B). No C3 was detected in the supernatant from C3-deficient BEAS-2B cells (Figure E9A).
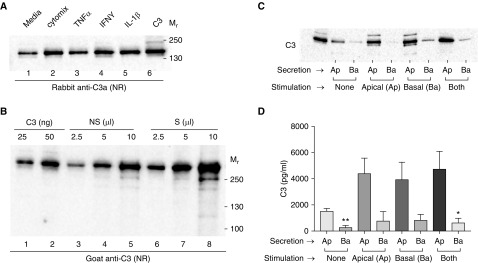
Figure 4.
C3 is secreted by airway epithelial cells and increases upon cytokine exposure. (A) C3 analysis of SN from BEAS-2B cells were incubated with serum-free media alone, media plus cytomix (25 ng/ml), or media with each of the individual components of the cytomix at 25 ng/ml. SN was collected after 24 hours, concentrated 10-fold, and analyzed by WB. Antibody: rabbit anti-C3a (to identify that the C3a fragment is attached to the secreted protein). C3, 50 ng. (B) C3 analysis of serum-free SN from the BEAS-2B cells before (NS) and after stimulation (S) with 25 ng/ml cytomix. SN was concentrated 20-fold and analyzed by WB. Increasing amounts of SN were used to estimate the quantity of C3 per 100,000 cells secreted over a 24-hour period. (C) Undifferentiated hTECs cultured on Transwells in serum-free media were treated on the apical (Ap) or basal (Ba) surface with 25 ng/ml of a cytomix combination for 24 hours. The media from each chamber was then collected, concentrated 10-fold, and analyzed for C3 under NR conditions using WB (goat anti-C3). Purified C3, 50 ng. Left: representative immunoblot. (D) Graphical representation of C based on densitometric scanning of WB. Shown is the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01 when comparing apical versus basal for each condition.
We considered the possibility that a part of the intracellular C3 was being continually produced and secreted into the air space as a source of local, extracellular C3. To determine whether secretion was polarized, hTECs were cultured on supported Transwell membranes, treated with cytomix in the apical or basal compartments, and then analyzed for the site of secretion. Immunoblot analysis indicated that C3 secretion was apically polarized, and this polarity was maintained upon cytomix exposure (Figure 4C) without impairing the transepithelial resistance. These findings agree with our hypothesis that a higher C3 concentration is present on the apical surface, which could increase when necessary to assist in host defense.
C3 Is Taken Up by AECs as C3(H2O)
To further evaluate the source of intracellular C3 stores in AECs, we incubated BEAS-2B cells with C3-deficient and -sufficient 10% normal human serum (NHS). We had previously determined that certain cell types internalize C3 as C3(H2O), a form of C3 that has a -OH group attached to the thioester bond (Figure 1). AECs rapidly took up C3(H2O) from the serum (Figure 5A) in a dose-dependent manner (Figure E4A). It reduced to the α- and β-chains upon DTT treatment, suggesting that this form is present intracellularly in AECs in addition to the precursor form derived from biosynthesis (Figure 5B). C3-MA is commonly used to mimic C3(H2O) in in vitro studies (methylamine is attached to the thioester bond instead of H2O). Both C3-MA and C3(H2O) reduce to the α- and β-chains when treated with DTT, unlike the precursor form. When exogenous C3 was provided to cells, in the form of either NHS or C3-MA, it predominantly localized to the cytoplasmic and membrane fractions, suggesting that it supplemented the endogenously produced C3 (Figures 5C, E4A, and E4B). This was confirmed when C3(H2O) was also internalized from NHS by BEAS-2B cells rendered C3 deficient by CRISPR (Figure 5D). No C3 was seen in the subcellular fractions of C3-deficient cells, and C3 increased in the cytoplasmic and membrane fractions after incubation with NHS containing C3(H2O). Additionally, C3 that was internalized by C3KO cells primarily localized to the early endosomes (Rab5) and not the late endosomes (Rab7), suggesting that biosynthesis and uptake contribute to distinct stores in AECs (Figures E4C–E4F).
Figure 5.
C3 is taken up by airway epithelial cells. (A) C3 analysis using WB of lysate from a CRISPR-induced C3KO clone. C3KO2 cells were incubated without (lanes 2–4) and with 10% normal human serum (NHS) for 15 minutes (lanes 5–7), after which the cells were washed with PBS and lysed. Equivalent amounts of cell lysates (Bradford assay) were used. The blot shows that C3 is absent in the CRISPR-induced C3KO clones. Positive control: C3, 50 ng (lane 1). The numbers represent lysates prepared from cells growing in three different wells for each condition. See Figure 2C for C3KO1. (B) hTEC lysates (L) incubated with either 10% C3-depleted (C3 dp) serum or 10% NHS for 15 minutes were analyzed for C3 under both NR and R conditions using WB. C3, 50 ng (lane 1) and 100 ng (lane 6), C3b, 100 ng (lane 7). C3b has an α′-chain that runs at a lower Mr (106k) than the C3α-chain (115k). The figure is from a single blot in which lanes relevant to the experiment have been selected. Lanes 4–7 represent a longer exposure (20 min) of the same blot, as those were done under R conditions. The antibody binds the α′ fragment of C3b better than the α-chain of C3. (C) C3 analysis of subcellular fractions (prepared in a manner similar to that described in Figure 2E). hTECs were incubated with either serum-free media alone (−) or media + 10% NHS (+) for 15 minutes. CYT, MEM: 1.25 × 105 cell equivalents/lane loaded; other fractions: 2.5 × 105 cell equivalents/lane loaded. C3, 50 ng. Lanes 5 and 8 contain only PBS to avoid the possibility of spillover. Bands running at 130–150k likely represent proteolytic fragments. Representative of two independent experiments. (D) CRISPR-induced C3KO clones that were incubated with either serum-free media alone (−) or media + 10% NHS (+) for 15 minutes. CYT, MEM: 2.5 × 105 cell equivalents/lane loaded; other fractions: 5 × 105 cell equivalents/lane loaded. C3, 50 ng. Representative of two experiments using distinct clones (C3KO1 and C3KO2).
C3(H2O) Taken Up by AECs Mitigates Certain Forms of Stress-associated Cell Death
Although AECs constitutively produce and secrete C3 for host defense (25, 26, 31), it was unclear why they additionally took up C3(H2O). Based on observations that intracellular C3a maintains CD4+ T-cell survival, we considered that C3 in AECs may have a function to enhance cell survival in the setting of lung inflammation. We used two model systems to induce AEC death: oxidant stress (32) and growth factor deprivation (starvation) (33, 34). We found that C3(H2O) internalized by BEAS-2B cells rescued them from H2O2-induced cell death, as indicated by Annexin V-propidium iodide staining (Figures 6A–6C). We tested the role of exogenous C3 in rescuing cell death, using multiple sources of C3(H2O) compared with serum depleted of C3. After cell death was induced with a high-oxidant dose (500 μM H2O2 for 60 min), a 3-hour rescue with purified C3, C3-MA, or NHS (containing C3[H2O]) reduced the number of dead cells, whereas C3-depleted serum failed to do so. This effect was also seen in C3KO cells, although the differences were smaller and not statistically significant (Figures E9B and E9C).
Figure 6.
C3 taken up by airway epithelial cells mitigates stress-induced cell death. (A–C) BEAS-2B cells were grown to confluence in a 12-well plate and treated with either serum-free media or H2O2 (500 μM) for 60 minutes, washed, and incubated with 10% C3 dp serum, 10% NHS, C3 dp serum + C3 (15 μg/ml), or C3 dp serum + C3-methylamine (C3-MA, 15 μg/ml) for 3 hours. The proportion of live cells (Q4) and dead cells (Q2 + Q3) was determined using annexin (x-axis) and propidium iodide (PI, y-axis) staining by flow cytometry. (A and B) Graphs represent the mean ± SEM of three to five replicates for each condition. (C) Representative flow-cytometry plots for each condition. (D–F) To evaluate whether the cytoprotective effect of C3 extends beyond H2O2-induced cell death, a growth factor deprivation model was used. BEAS-2B cells were grown to confluence in a 12-well plate and incubated with Earle’s balanced salt solution (EBSS) for 4 hours in the absence or presence of 10% C3 dp serum, 10% NHS, or C3 (15 μg/ml). (D and E) Graphs represent the mean ± SEM of two or three replicates for each condition. (F) Representative flow-cytometry plots for each condition. *P < 0.05, **P < 0.01. Representative of three independent experiments.
C3 also reduced cell death due to growth factor deprivation (starvation). After BEAS-2B cells were incubated with EBSS for 4 hours, 68.6% ± 3.8% of the cells were alive (Figures 6D–6F). When EBSS was supplemented with C3 (15 μg/ml) for the same duration, the proportion of alive cells increased to 83.5% ± 1.2% (P = 0.019). This difference was also seen when EBSS was supplemented with 10% C3-depleted serum (79.5% ± 0.2% alive cells) versus 10% NHS (which contains ∼100–150 μg of C3 [98.2% alive cells, P < 0.0001]). Together, these observations suggest that C3 may mitigate cell death in conditions of cell stress initiated by several factors.
These results led us to hypothesize that AECs internalize C3 in the setting of an acute injury to increase intracellular C3 stores, until C3 reaches a critical threshold to protect the cell. To increase endogenously synthesized C3 stores, BEAS-2B cells were treated with proinflammatory cytokines (cytomix) for 24 hours before oxidative stress was induced. Cytomix treatment reduced H2O2-induced cell death, even after the H2O2 dose was doubled (Figures E5A–E5C). However, this effect was not specific to increasing endogenous C3, because the cytoprotective effect of the cytomix also occurred in CRISPR-induced C3KO cells (Figures E5D–E5F). Our results suggest that the cytoprotective effect of C3 is specific to stores that are derived from uptake, and is facilitated by other proteins (likely including but not restricted to C3) that increase in response to proinflammatory stimuli.
To further elucidate the potential mechanisms for this observation, we explored how C3 modulates various cell death pathways in the setting of stress. We first evaluated whether C3 directly quenches H2O2, and observed that this did not occur at the doses of C3 and H2O2 used in our study (Figure E8). We also considered that C3 plays a role in modulating cell death driven by apoptosis and/or secondary necrosis, as cells taking up C3(H2O) exhibited fewer Annexin V-propidium iodide double-positive cells (Figures 6A–6C). To test this, we examined whether C3 rescued cell death induced by staurosporine, a potent inducer of apoptosis. C3 uptake failed to rescue staurosporine-induced apoptosis at various time points (Figures E6 and E7). We obtained similar results by examining the protein profiles of apoptotic protein markers and comparing staurosporine-induced cell death with H2O2-induced oxidative stress. Using PARP and caspase 3 cleavage as markers of apoptosis, we noted that C3 uptake failed to reduce cleavage of these proteins in staurosporine-treated cells (Figures E7A–E7C). Notably, although C3 effectively mitigated H2O2-induced cell death, it did not significantly affect PARP or caspase 3 cleavage in this condition (Figures E7B and E7C), We also examined the cleavage of the pyroptosis marker GSDMD-1 to evaluate whether the effect of C3 on mitigating H2O2-induced cell death was achieved through this form of programmed cell death; it was not (Figure E7A) (35). These results suggest that C3 mitigates cell death in some conditions by affecting pathways that are independent of programmed cell death mechanisms such as apoptosis and pyroptosis.
Increased Expression of Intracellular C3 Is Found in End-Stage Lung Diseases
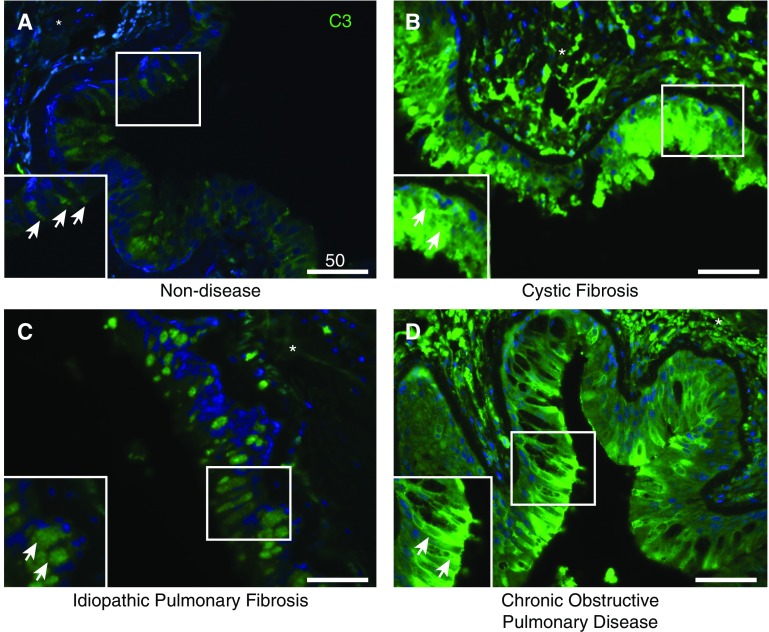
To evaluate C3 involvement in lung disease, we immunostained airways in human lung tissues that were removed during lung transplantation. We first chose to examine lungs from individuals with cystic fibrosis (CF), because they have a history of ongoing infections and acute-on-chronic exacerbations associated with cellular stress. We compared these samples with lung tissues from individuals with chronic obstructive pulmonary disease (COPD) or idiopathic pulmonary fibrosis (IPF). In nondiseased lungs, intracellular C3 was present primarily at low levels in AECs (Figure 7A). Under the same imaging parameters, intracellular C3 was present at comparatively higher levels in AECs of lungs from individuals with end-stage CF (Figure 7B), IPF (Figure 7C), or COPD (Figure 7D). In all disease processes (n = 3 each; Figure E10), C3 was patchy and predominantly localized in luminal cells. Increased submucosal deposition was also identified in samples from individuals with these diseases. Intracellular C3 appeared to be most abundant in individuals with CF. However, we could not exclude the possibility that secreted C3 was being deposited along the apical cell surface.
Figure 7.
Intracellular complement protein C3 is upregulated in end-stage lung disease. (A–D) Representative confocal-microscopic images of formalin-fixed paraffin-embedded sections of lung tissue from control subjects without disease (A) and patients with end-stage cystic fibrosis (CF, B), idiopathic pulmonary fibrosis (IPF, C), or chronic obstructive pulmonary disease (COPD, D) that were stained for C3 (green). Control samples were obtained from donors without CF, IPF, or COPD using lung explants that were otherwise not usable for transplantation and excess lung tissue that was resected for downsizing. Representative images of three different individuals for each disease (Figure E5). Original magnification, ×400; scale bars: 50 μM for each figure. Images from the three sections are comparable because they were obtained in identical conditions (at the same time and the same exposure, and including an isotype control). Arrows denote the epithelium in the magnified inset, and the asterisk denotes the subepithelial/submucosal space. DAPI (blue) intensity was globally adjusted for all images.
In all samples examined, intracellular C3 stores were primarily located in club cells, identified with SCGB1A1 (Figures E11C and E11D), and in goblet cells, identified with MUC5AC immunostaining (Figures E11E and E11F). Localization of C3 in club and goblet cells was most common in regions where cells containing C3 stores were most abundant, as observed in tissues from individuals with CF (Figures E11D and E11F). This suggests that in end-stage lung diseases, especially those associated with ongoing infection and high levels of cellular stress in the airways, intracellular C3 stores may be elevated for host defense but may affect the local environment in the setting of ongoing injury.
Discussion
Complement proteins are produced by a number of immune and nonimmune cells (7–10). The role of the complement system has expanded beyond host defense to include metabolic reprogramming, maturation, migration, and regeneration (1, 2, 20, 36). Many of these functions are governed by an intracellular system (recently termed the “complosome”) (20). Complement proteins have also been implicated in several lung diseases, but their source has not been systematically investigated (37–39). Primary AECs synthesize and secrete C3 (25, 40), but whether they contain intracellular stores was unknown. Although other investigators and we have recently shown that many cell types contain intracellular C3 (13, 15–17), most of these cells do not contain substantial C3 stores. A majority of the C3 that is synthesized is secreted, and the only cells that were previously known to synthesize and store large amounts of C3 were monocytes (11) and neutrophils (12). Here, we demonstrate that human AECs contain intracellular C3 stores, which are predominantly derived by biosynthesis, and augmented by either stimulation or internalization of C3 (i.e., C3[H2O]). Additionally, we show that internalized C3 protects AECs from stress-induced death (32, 41).
We observed that C3 was increased in the intracellular compartment of AECs in the lungs of subjects with end-stage lung disease due to CF, COPD, or IPF. This is consistent with data from investigators who have found C3 upregulation in the transcriptome and secretome of patients with CF (26, 39, 42). Our findings also align with studies that found upregulated C3 transcripts in lungs from patients with IPF compared with normal lungs (43–45), and increased complement activation in patients with COPD (38, 46). There was also an increase in the C3 staining intensity in the submucosal region in these individuals. This likely represents the deposition of cleaved C3 proteins (such as C3d) originating from infiltrating inflammatory cells, with the cleaved proteins presumably being generated by proteases in the inflammatory environment (9, 38). In vitro, a combination of immunoblotting, flow cytometry, and immunofluorescence led us to conclude that AECs contain intracellular C3 stores derived from both biosynthesis and uptake. Thus, a key finding of this work is the demonstration of C3 stores in human AECs and how C3 is protective against environmental stress. This creates a precedent for studying expanded roles of complement proteins at other environmental surfaces that concurrently need to maintain microbicidal activities, such as the cornea and the genitourinary tract.
We demonstrate that the majority of the C3 in AECs is stored intracellularly, with only trace amounts on the surface, regardless of proinflammatory cytokine exposure. This is in contrast to CD4+ T cells, wherein upon activation, C3 redistributed toward the cell surface, engaging with its receptor, C3aR, and polarizing naive CD4+ T cells toward a T-helper cell type 1 phenotype (13). In addition to the precursor form that has been detected in cells such as hepatocytes and is destined for secretion (5, 6), AECs store the mature form that reduces to the α- and β-chains, and has not been reported in nonimmune cells. The majority of these stores that are synthesized by AECs are detected in the ER and the late endosomes. This is in contrast to C3(H2O), which is internalized by cells and primarily localizes to the early endosomes (15). Hence, our data indicate that there are at least two sources of intracellular C3 stores in AECs: 1) biosynthesis, stored in the ER and late endosomes, and 2) uptake, stored in the early endosomes. These contribute to C3 stores in distinct subcellular compartments.
Multiple other investigators have found that exposing BEAS-2B and A549 cells to proinflammatory cytokines increases the synthesis of C3, which then gets secreted (30, 31, 47). What distinguishes our observations is that we show that such stimulation concurrently increases intracellular C3 stores, which creates a precedent for evaluating the roles of both the stored and secreted forms. This increase primarily occurred through biosynthesis. Although we found that stimulating cells did not affect uptake (unpublished data), it increased C3 secretion in the supernatants, and the secretion was predominantly polarized apically. This is in line with proteomic data from individuals with CF, which showed that C3 was significantly increased in apical secretions from air–liquid interface epithelial cell cultures (26).
Together with these observations, we propose that the human airway epithelium provides a locally available source of C3 at the luminal surface that can be recruited in the setting of an infection for host defense, before the arrival of other components of the immune system. This is also consistent with data from Sass and colleagues showing that incubating the sputum of patients with CF with P. aeruginosa or Staphylococcus aureus increased C5a generation, which is downstream of C3 activation (48). Although we did not evaluate C5 or C5a in this study, we were intrigued by their follow-up study, which showed that sputum C5a levels were a marker of increased inflammation and overall disease severity, whereas C3a levels were associated with lower levels of inflammatory markers and fewer acute exacerbations (42). This suggests that low levels of constitutive C3 generation and activation may be protective to cells, whereas long-term activation, such as with chronic infections, results in activation of the complement cascade, promoting ongoing epithelial injury and lung damage.
While we were dissecting the nature of the intracellular C3 stores, we identified that CD4+ T cells also internalize C3 rapidly from the blood in the form of C3(H2O), which has a hydroxyl group (derived from H2O) attached at the site of the thioester bond, and still has C3a attached to it (Figure 1) (15). In CD4+ T cells, the uptake of C3(H2O) serves as a source of intracellular C3a and plays a critical role in cell maturation and survival, and proinflammatory cytokine production (13, 15). However, most cells are not believed to contain substantial C3 stores unless they are exposed to an exogenous source (15). Because this was in contrast to our observation that AECs store C3 without exposure to an exogenous source, we asked whether AECs also take up C3. Consistent with our previous findings, we found that AECs took up C3 rapidly in the form of C3(H2O), and this contributed to the native (reducible) form. However, we wanted to determine why AECs would internalize C3 despite having biosynthetically derived stores. Exogenously provided C3 reduced H2O2-induced death compared with when only C3-depleted serum was provided, suggesting that C3 uptake was cytoprotective in the setting of oxidant-induced damage. This effect was also observed during growth factor deprivation using EBSS. We also observed that increasing intracellular stores generated after cytokine exposure could mitigate stress-induced death. Hence, we speculate that at early time points, C3 internalized by cells reduces death; however, a proinflammatory environment increases other endogenous proteins that are cytoprotective. Internalized C3 also mitigated cell death in certain C3KO cell clones, albeit to a lesser degree. Hence, we are currently investigating whether the internalized C3 eventually contributes to a common pool of C3 derived from biosynthesis over longer time points or continues to remain distinct, and whether the protective effect of internalized C3 (as C3[H2O]) is dependent on the ability of cells to produce C3 endogenously.
The cytoprotective nature of C3 has been recognized in certain other organ systems, especially the pancreas. Dos Santos and colleagues recently reported that apoptosis increased after knockdown of C3 using siRNA in pancreatic islet β cells at baseline and after treatment with IL-1β plus IFN-γ (19). This cytokine-induced β-cell apoptosis was mitigated in both rat and human cells treated with C3 (0.5–1 μg/ml) before the induction of apoptosis. Interestingly, we did not see a similar rescue effect when we induced apoptosis using staurosporine, a broad-spectrum protein kinase inhibitor. This suggests that C3(H2O) might modulate certain components upstream of programmed cell death pathways (e.g., directly by affecting intracellular reactive oxygen species or indirectly by upregulating cellular antioxidant mechanisms), or that the effect of C3 on mitigating cell death is specific to the stimuli. Further studies need to be conducted to evaluate whether C3 affects redox changes in viable cells, which might underlie its protective effect. However, the protective effect of C3 was not restricted to H2O2 and extended to growth factor deprivation–induced death. This suggests that there may be common pathways between multiple stressors that can be altered by C3.
To assess whether C3 modulated programmed cell death in the setting of oxidative stress, we examined the two caspase-dependent pathways, apoptosis and pyroptosis. Although this was an initial analysis of these pathways, we found that C3 was less likely to rescue cell death by mitigating these pathways in the setting of oxidative stress. Given the short time course of the H2O2 treatment, we did not anticipate necroptosis (a caspase-independent programmed cell death pathway) to be activated. Preliminary experiments suggest that phospho-MLKL, the protein marker of necroptosis, was not turned on in any of the conditions we used in this study (data not shown). Studies to identify the specific mechanisms by which C3 mitigates cell death in the setting of specific inducers of cell death have been initiated.
Our studies also indicate that an exogenous source of C3 was required after incubation with H2O2. This may reflect differences in how cells process C3(H2O). For example, our laboratory has previously shown that nearly 40% of C3(H2O) that was internalized by Farage cells (human B lymphocyte line) was lost within 5 hours after the exogenous source was removed (15). In Jurkat (human T lymphocyte line) and ARPE-19 (human retinal pigment epithelial cell line) cells, this loss occurred even more quickly, with over 70% being lost in 2 hours. Our findings thus suggest that the C3(H2O) recycling pathway is likely cell-type specific.
AECs are crucial for host defense and produce multiple cytokines that maintain epithelial integrity as well as protect the airspace (49). However, they can be damaged by noxious stimuli such as oxidant stress and ozone, and cytokines such as TNF-α (50). Hence, one can speculate that if the levels of certain proteins are locally augmented, this could reduce damage to the epithelium and eventually allow it to survive an acute injury. Such effects have been identified for proteins such as α-Klotho (which was shown to protect against oxidative stress in a rat model of hyperoxia-induced lung injury by increasing the endogenous antioxidative capacity of pulmonary epithelial cells) (51), and vascular endothelial growth factor (which was found to be a critical paracrine factor, secreted by intratracheally administered mesenchymal stem cells, in mitigating hyperoxia-induced lung injury in rats) (52). We speculate that AECs need to have a certain threshold of intracellular C3 that protects them. This can be derived either by augmenting biosynthesis or through uptake. Regardless of how this increase occurs, intracellular C3 is cleaved to C3a. In CD4+ T cells, C3 is constitutively cleaved by cathepsin-L to C3a, which binds to its receptor, C3aR, and activates mTORC1 and the mTOR pathway, promoting cell survival (13). We are actively investigating whether it is the identical pathway or a different one by which C3 mitigates stress-associated AEC death.
The translation of certain aspects of our in vitro studies has limitations. First, we used relatively large doses of H2O2 as a model system to induce AEC death. Although such doses have been used by other investigators to evaluate the cytoprotective effects of proteins (e.g., Kruppel-like factor) (53–55), this model’s biological relevance in terms of oxidative stress occurring in vivo is limited, as the respiratory burst induced by neutrophil influx is a highly localized event that is dissimilar to the experimental conditions provided by globally high H2O2 levels. Thus, this in vitro model may not be as relevant to the development of end-stage lung disease. Second, we focused on short time points (3–4 h) to minimize the effect of protein synthesis by the cells, thus emphasizing uptake. C3 may modulate epithelial cell responses to stress differently over a longer time period. Moreover, in end-stage lung disease, C3 derived from endothelial cells, stromal cells, and/or immune cells may be playing additional roles.
Using a combination of primary cells and CRISPR-induced, C3-deficient BEAS-2B cell lines, we demonstrate how intracellular C3 in AECs is derived, stored, and processed, and provide an example of how it is protective in the setting of acute stress. We have previously proposed that throughout evolution, the intracellular complement system has expanded from protecting a single cell to being secreted and protecting the cell membrane and interstitium, and subsequently the intravascular space (1). We now hypothesize that AEC-derived C3 is inherently protective both intracellularly and at the luminal surface to handle ongoing injury and infection (Figure E12). For example, C3 is activated by P. aeruginosa (56) and is important in protecting against infection (57). However, it may be inadequate in the setting of a large inflammatory response to protect the cell from stress-induced damage (58, 59). In such a scenario, we speculate that cells internalize locally available C3 from plasma or cells (e.g., neutrophils) that die and release C3 (Figure E12). A body of literature suggests that the generation of C3(H2O) from C3 may be greatly accelerated when C3 interacts with different biological surfaces (60, 61). Hence, this could form a system in which cells could internalize C3(H2O) in the setting of an injury to protect themselves until other endogenously derived cytoprotective proteins are sufficiently upregulated. However, the synthesis and deposition of C3 in the setting of persistent inflammation could also promote fibrosis and irreversible tissue injury (62, 63). Using models of lung injury, our future directions involve dissecting the mechanisms by which C3 differentially modulates acute and chronic epithelial injury, with the hope of therapeutically targeting the cascade to mitigate end-stage lung disease.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Monika Minner, Xiaohua Jin, Steve Austin, Ella Katz, Paula Bertram, Lynne Mitchell, the Pulmonary Morphology Core (Teresa Tolley and Darlene Stewart), and members of the Brody laboratory (Jian Xu, Sean Gunsten, Tao Huang, Andrew Schaffer, and JieHong Pan) for their technical assistance. They also thank Drs. Devesha Kulkarni, Michael Holtzman, Robert Senior, Dennis Hourcade, Xiaobo Wu, Anuja Java, Andrew Gelman, James Fitzpatrick (and the Center for Cellular Imaging), Monika Minner and Monica Sentmanat (and the Genome Engineering and iPSC Center), Sjoerd Van Der Post, Jennifer Alexander-Brett, and Amjad Horani for their intellectual insights regarding this work.
Footnotes
Supported by the National Institutes of Health (NIH) grants R01 GM099111 and R01 AI041592 (J.P.A.), and an NIH training grant in the Principles of Pulmonary Research (5T32 HL007317, H.S.K.), in Cancer Biology (5T32 CA009547, Y.-C.P.), and in the Immunobiology of Rheumatic Diseases (5T32-AR007279, H.S.K. and M.L.E.). Research reported in this publication is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases/NIH (P30AR048335), by the National Center for Advancing Translational Sciences of the NIH under award number KL2 TR002346 (H.S.K.), an NIH grant for the Washington University Institute of Clinical and Translational Sciences (3UL1 TR000448), and a Hubert C. and Dorothy R. Moog Professorship (S.L.B.) supported by the Barnes-Jewish Hospital Foundation. Experiments were performed in part through the use of Washington University Center for Cellular Imaging supported by the Washington University School of Medicine, the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CDI-CORE-2015-505), the National Institute for Neurological Disorders and Stroke (NS086741), and the Foundation for Barnes Jewish Hospital. Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences of the NIH.
Author Contributions: Conception and design: H.S.K., M.L.E., M.K.L., D.E.B., S.L.B., and J.P.A. Analysis and interpretation: H.S.K., M.L.E., Y.-C.P., M.K.L., D.E.B., C.F., D.J.L., S.L.B., and J.P.A. Drafting the manuscript for important intellectual content: H.S.K., M.L.E., Y.-C.P., M.K.L., R.D.Y., D.J.L., S.L.B., and J.P.A.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0405OC on August 29, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Elvington M, Liszewski MK, Atkinson JP. Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol Rev. 2016;274:9–15. doi: 10.1111/imr.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3—the “Swiss Army Knife” of innate immunity and host defense. Immunol Rev. 2016;274:33–58. doi: 10.1111/imr.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect. 2011;13:1133–1145. doi: 10.1016/j.micinf.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Circolo A, Garnier G, Fukuda W, Wang X, Hidvegi T, Szalai AJ, et al. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 6.Morris KM, Goldberger G, Colten HR, Aden DP, Knowles BB. Biosynthesis and processing of a human precursor complement protein, pro-C3, in a hepatoma-derived cell line. Science. 1982;215:399–400. doi: 10.1126/science.7199205. [DOI] [PubMed] [Google Scholar]
- 7.Morgan BP, Gasque P. Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol. 1997;107:1–7. doi: 10.1046/j.1365-2249.1997.d01-890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandermeer J, Sha Q, Lane AP, Schleimer RP. Innate immunity of the sinonasal cavity: expression of messenger RNA for complement cascade components and toll-like receptors. Arch Otolaryngol Head Neck Surg. 2004;130:1374–1380. doi: 10.1001/archotol.130.12.1374. [DOI] [PubMed] [Google Scholar]
- 9.Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188:183–194. doi: 10.1111/cei.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni HS, Liszewski MK, Brody SL, Atkinson JP. The complement system in the airway epithelium: An overlooked host defense mechanism and therapeutic target? J Allergy Clin Immunol. 2018;141:1582–1586.e1. doi: 10.1016/j.jaci.2017.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einstein LP, Hansen PJ, Ballow M, Davis AE, III, Davis JS, IV, Alper CA, et al. Biosynthesis of the third component of complement (C3) in vitro by monocytes from both normal and homozygous C3-deficient humans. J Clin Invest. 1977;60:963–969. doi: 10.1172/JCI108876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botto M, Lissandrini D, Sorio C, Walport MJ. Biosynthesis and secretion of complement component (C3) by activated human polymorphonuclear leukocytes. J Immunol. 1992;149:1348–1355. [PubMed] [Google Scholar]
- 13.Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolev M, Dimeloe S, Le Friec G, Navarini A, Arbore G, Povoleri GA, et al. Complement regulates nutrient influx and metabolic reprogramming during Th1 cell responses. Immunity. 2015;42:1033–1047. doi: 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elvington M, Liszewski MK, Bertram P, Kulkarni HS, Atkinson JPA. A C3(H20) recycling pathway is a component of the intracellular complement system. J Clin Invest. 2017;127:970–981. doi: 10.1172/JCI89412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satyam A, Kannan L, Matsumoto N, Geha M, Lapchak PH, Bosse R, et al. Intracellular activation of complement 3 is responsible for intestinal tissue damage during mesenteric ischemia. J Immunol. 2017;198:788–797. doi: 10.4049/jimmunol.1502287. [DOI] [PubMed] [Google Scholar]
- 17.Sünderhauf A, Skibbe K, Preisker S, Ebbert K, Verschoor A, Karsten CM, et al. Regulation of epithelial cell expressed C3 in the intestine—relevance for the pathophysiology of inflammatory bowel disease? Mol Immunol. 2017;90:227–238. doi: 10.1016/j.molimm.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Atanes P, Ruz-Maldonado I, Pingitore A, Hawkes R, Liu B, Zhao M, et al. C3aR and C5aR1 act as key regulators of human and mouse β-cell function. Cell Mol Life Sci. 2018;75:715–726. doi: 10.1007/s00018-017-2655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dos Santos RS, Marroqui L, Grieco FA, Marselli L, Suleiman M, Henz SR, et al. Protective role of complement C3 against cytokine-mediated β-cell apoptosis. Endocrinology. 2017;158:2503–2521. doi: 10.1210/en.2017-00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbore G, Kemper C, Kolev M. Intracellular complement—the complosome—in immune cell regulation. Mol Immunol. 2017;89:2–9. doi: 10.1016/j.molimm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess C, Kemper C. Complement-mediated regulation of metabolism and basic cellular processes. Immunity. 2016;45:240–254. doi: 10.1016/j.immuni.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liszewski MK, Elvington M, Kulkarni HS, Atkinson JP. Complement’s hidden arsenal: new insights and novel functions inside the cell. Mol Immunol. 2017;84:2–9. doi: 10.1016/j.molimm.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watford WT, Ghio AJ, Wright JR. Complement-mediated host defense in the lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L790–L798. doi: 10.1152/ajplung.2000.279.5.L790. [DOI] [PubMed] [Google Scholar]
- 24.Bolger MS, Ross DS, Jiang H, Frank MM, Ghio AJ, Schwartz DA, et al. Complement levels and activity in the normal and LPS-injured lung. Am J Physiol Lung Cell Mol Physiol. 2007;292:L748–L759. doi: 10.1152/ajplung.00127.2006. [DOI] [PubMed] [Google Scholar]
- 25.Pillai DK, Sankoorikal B-JV, Johnson E, Seneviratne AN, Zurko J, Brown KJ, et al. Directional secretomes reflect polarity-specific functions in an in vitro model of human bronchial epithelium. Am J Respir Cell Mol Biol. 2014;50:292–300. doi: 10.1165/rcmb.2013-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters-Hall JR, Brown KJ, Pillai DK, Tomney A, Garvin LM, Wu X, et al. Quantitative proteomics reveals an altered cystic fibrosis in vitro bronchial epithelial secretome. Am J Respir Cell Mol Biol. 2015;53:22–32. doi: 10.1165/rcmb.2014-0256RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulkarni HS, Elvington ML, Liszewski MK, Brody SL, Atkinson JP. Presence of an intracellular C3-C3aR system in the human lung epithelium. Immunobiology. 2016;221:1148–1149. [Google Scholar]
- 28.Kulkarni HS, Elvington ML, Liszewski MK, Brody SL, Atkinson JP. Human airway epithelial cells have multiple sources and stores of the intracellular complement protein C3. Am J Respir Crit Care Med. 2017;195:A5206. [Google Scholar]
- 29.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol. 2013;49:341–347. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varsano S, Kaminsky M, Kaiser M, Rashkovsky L. Generation of complement C3 and expression of cell membrane complement inhibitory proteins by human bronchial epithelium cell line. Thorax. 2000;55:364–369. doi: 10.1136/thorax.55.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman BL, Merrow M, Bamba M, Kennedy T, Kreutzer DL. Biosynthesis of the third and fifth complement components by isolated human lung cells. Am Rev Respir Dis. 1989;139:212–220. doi: 10.1164/ajrccm/139.1.212. [DOI] [PubMed] [Google Scholar]
- 32.Faner R, Rojas M, Macnee W, Agustí A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 33.Dokladny K, Zuhl MN, Mandell M, Bhattacharya D, Schneider S, Deretic V, et al. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J Biol Chem. 2013;288:14959–14972. doi: 10.1074/jbc.M113.462408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan X, Weng Y, Bai Y, Wang Z, Wang S, Zhu J, et al. Lipin-1 determines lung cancer cell survival and chemotherapy sensitivity by regulation of endoplasmic reticulum homeostasis and autophagy. Cancer Med. 2018;7:2541–2554. doi: 10.1002/cam4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–514.e4. doi: 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Presumey J, Bialas AR, Carroll MC. Complement system in neural synapse elimination in development and disease. Adv Immunol. 2017;135:53–79. doi: 10.1016/bs.ai.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Pandya PH, Fisher AJ, Mickler EA, Temm CJ, Lipking KP, Gracon A, et al. Hypoxia-inducible factor-1α regulates CD55 in airway epithelium. Am J Respir Cell Mol Biol. 2016;55:889–898. doi: 10.1165/rcmb.2015-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan X, Shan M, You R, Frazier MV, Hong MJ, Wetsel RA, et al. Activation of C3a receptor is required in cigarette smoke-mediated emphysema. Mucosal Immunol. 2015;8:874–885. doi: 10.1038/mi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polineni D, Dang H, Gallins PJ, Jones LC, Pace RG, Stonebraker JR, et al. Airway mucosal host defense is key to genomic regulation of cystic fibrosis lung disease severity. Am J Respir Crit Care Med. 2018;197:79–93. doi: 10.1164/rccm.201701-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali M, Lillehoj EP, Park Y, Kyo Y, Kim KC. Analysis of the proteome of human airway epithelial secretions. Proteome Sci. 2011;9:4. doi: 10.1186/1477-5956-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mossman BT, Lounsbury KM, Reddy SP. Oxidants and signaling by mitogen-activated protein kinases in lung epithelium. Am J Respir Cell Mol Biol. 2006;34:666–669. doi: 10.1165/rcmb.2006-0047SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hair PS, Sass LA, Vazifedan T, Shah TA, Krishna NK, Cunnion KM. Complement effectors, C5a and C3a, in cystic fibrosis lung fluid correlate with disease severity. PLoS One. 2017;12:e0173257. doi: 10.1371/journal.pone.0173257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang IV, Burch LH, Steele MP, Savov JD, Hollingsworth JW, McElvania-Tekippe E, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med. 2007;175:45–54. doi: 10.1164/rccm.200601-062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu H, Mickler EA, Cummings OW, Sandusky GE, Weber DJ, Gracon A, et al. Crosstalk between TGF-β1 and complement activation augments epithelial injury in pulmonary fibrosis. FASEB J. 2014;28:4223–4234. doi: 10.1096/fj.13-247650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto T, Mathai SK, Hennessy CE, Hancock LA, Walts AD, Stefanski AL, et al. The relationship between complement C3 expression and the MUC5B genotype in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315:L1–L10. doi: 10.1152/ajplung.00395.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westwood J-P, Mackay AJ, Donaldson G, Machin SJ, Wedzicha JA, Scully M. The role of complement activation in COPD exacerbation recovery. ERJ Open Res. 2016;2 doi: 10.1183/23120541.00027-2016. pii: 00027-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao YX, Andoh A, Shimada M, Takaya H, Hata K, Fujiyama Y, et al. Secretion of complement components of the alternative pathway (C3 and factor B) by the human alveolar type II epithelial cell line A549. Int J Mol Med. 2000;5:415–419. doi: 10.3892/ijmm.5.4.415. [DOI] [PubMed] [Google Scholar]
- 48.Sass LA, Hair PS, Perkins AM, Shah TA, Krishna NK, Cunnion KM. Complement effectors of inflammation in cystic fibrosis lung fluid correlate with clinical measures of disease. PLoS One. 2015;10:e0144723. doi: 10.1371/journal.pone.0144723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiemstra PS. Parallel activities and interactions between antimicrobial peptides and complement in host defense at the airway epithelial surface. Mol Immunol. 2015;68:28–30. doi: 10.1016/j.molimm.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Ahmad S, Ahmad A, Dremina ES, Sharov VS, Guo X, Jones TN, et al. Bcl-2 suppresses sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression in cystic fibrosis airways: role in oxidant-mediated cell death. Am J Respir Crit Care Med. 2009;179:816–826. doi: 10.1164/rccm.200807-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravikumar P, Ye J, Zhang J, Pinch SN, Hu MC, Kuro-o M, et al. α-Klotho protects against oxidative damage in pulmonary epithelia. Am J Physiol Lung Cell Mol Physiol. 2014;307:L566–L575. doi: 10.1152/ajplung.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang YS, Ahn SY, Jeon HB, Sung DK, Kim ES, Sung SI, et al. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014;51:391–399. doi: 10.1165/rcmb.2013-0385OC. [DOI] [PubMed] [Google Scholar]
- 53.Chapman KE, Waters CM, Miller WM. Continuous exposure of airway epithelial cells to hydrogen peroxide: protection by KGF. J Cell Physiol. 2002;192:71–80. doi: 10.1002/jcp.10115. [DOI] [PubMed] [Google Scholar]
- 54.Saito Y, Nishio K, Ogawa Y, Kimata J, Kinumi T, Yoshida Y, et al. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res. 2006;40:619–630. doi: 10.1080/10715760600632552. [DOI] [PubMed] [Google Scholar]
- 55.Smit-de Vries MP, van der Toorn M, Bischoff R, Kauffman HF. Resistance of quiescent and proliferating airway epithelial cells to H2O2 challenge. Eur Respir J. 2007;29:633–642. doi: 10.1183/09031936.00093906. [DOI] [PubMed] [Google Scholar]
- 56.Jensen ET, Kharazmi A, Garred P, Kronborg G, Fomsgaard A, Mollnes TE, et al. Complement activation by Pseudomonas aeruginosa biofilms. Microb Pathog. 1993;15:377–388. doi: 10.1006/mpat.1993.1087. [DOI] [PubMed] [Google Scholar]
- 57.Mueller-Ortiz SL, Morales JE, Wetsel RA. The receptor for the complement C3a anaphylatoxin (C3aR) provides host protection against Listeria monocytogenes-induced apoptosis. J Immunol. 2014;193:1278–1289. doi: 10.4049/jimmunol.1302787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aoshiba K, Nagai A. Oxidative stress, cell death, and other damage to alveolar epithelial cells induced by cigarette smoke. Tob Induc Dis. 2003;1:219–226. doi: 10.1186/1617-9625-1-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim HJ, Park Y-D, Moon UY, Kim J-H, Jeon JH, Lee J-G, et al. The role of Nox4 in oxidative stress-induced MUC5AC overexpression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2008;39:598–609. doi: 10.1165/rcmb.2007-0262OC. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson B, Nilsson Ekdahl K. The tick-over theory revisited: is C3 a contact-activated protein? Immunobiology. 2012;217:1106–1110. doi: 10.1016/j.imbio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Lachmann PJ, Lay E, Seilly DJ. Experimental confirmation of the C3 tickover hypothesis by studies with an Ab (S77) that inhibits tickover in whole serum. FASEB J. 2018;32:123–129. doi: 10.1096/fj.201700734. [DOI] [PubMed] [Google Scholar]
- 62.Schraufstatter IU, Khaldoyanidi SK, DiScipio RG. Complement activation in the context of stem cells and tissue repair. World J Stem Cells. 2015;7:1090–1108. doi: 10.4252/wjsc.v7.i8.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho MS, Rupaimoole R, Choi H-J, Noh K, Chen J, Hu Q, et al. Complement component 3 is regulated by TWIST1 and mediates epithelial-mesenchymal transition. J Immunol. 2016;196:1412–1418. doi: 10.4049/jimmunol.1501886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.