Abstract
Low–tidal volume (Vt) ventilation might protect healthy lungs from volutrauma but lead to inflammation resulting from other mechanisms, namely alveolar derecruitment and the ensuing alveolar collapse and tidal reexpansion. We hypothesized that the different mechanisms of low- and high-volume injury would be reflected in different mechanical properties being associated with development of pulmonary inflammation and mortality: an increase of hysteresis, reflecting progressive alveolar derecruitment, at low Vt; an increase of elastance, as a result of overdistension, at higher Vt. Mice were allocated to “protective” (6 ml/kg) or “injurious” (15–20 ml/kg) Vt groups and ventilated for 16 hours or until death. We measured elastance and hysteresis; pulmonary IL-6, IL-1β, and MIP-2 (macrophage inflammatory protein 2); wet-to-dry ratio; and blood gases. Survival was greater in the protective group (60%) than in the injurious group (25%). Nonsurvivors showed increased pulmonary cytokines, particularly in the injurious group, with the increase of elastance reflecting IL-6 concentration. Survivors instead showed only modest increases of cytokines, independent of Vt and unrelated to the increase of elastance. No single lung strain threshold could discriminate survivors from nonsurvivors. Hysteresis increased faster in the protective group, but, contrary to our hypothesis, its change was inversely related to the concentration of cytokines. In this model, significant mortality associated with pulmonary inflammation occurred even for strain values as low as about 0.8. Low Vt improved survival. The accompanying increase of hysteresis was not associated with greater inflammation.
Keywords: artificial respiration, ventilator-induced lung injury, acute lung injury, respiratory mechanics
Clinical Relevance
Low–tidal volume (Vt) ventilation is standard of care for acute respiratory distress syndrome and may also be beneficial in healthy lungs. However, it can lead to alveolar derecruitment and repeated end-expiratory alveolar collapse and reexpansion, which might promote inflammation. Consequently, there is a need to understand the interplay among ventilation strategy, respiratory mechanics, inflammation, and outcome in animal models over time scales and Vt values comparable to those of patients undergoing general anesthesia or receiving ventilation for days even if they do not have acute respiratory distress syndrome. In a 16-hour murine mechanical ventilation model, mortality was 1) associated with development of pulmonary inflammation even for lung strain values of approximately 0.8, much lower than the threshold of 2 above which mortality has been attributed to ventilator-induced lung injury in a variety of species; and 2) reduced by lower Vt. Survivors developed only modest pulmonary inflammation, regardless of Vt and despite an increase of hysteresis consistent with progressive alveolar derecruitment. The relative magnitude of the hysteresis increase was inversely related to the expression of pulmonary cytokines.
Low–tidal volume (Vt) ventilation reduces mortality of acute respiratory distress syndrome (ARDS) (1) and may also be beneficial for patients with initially healthy lungs (2–4). This has significant importance, considering that more than 230 million patients per year require mechanical ventilation for major surgery under general anesthesia (5) and that 35% of all patients in intensive care units (ICUs) worldwide receive mechanical ventilation even if they do not have ARDS (6). However, it is still uncertain whether low-Vt mechanical ventilation in healthy lungs has detrimental effects of its own. This “low-volume” injury (7) would originate from two main mechanisms. One mechanism consists of increased mechanical stress at the boundary of derecruited lung regions (8, 9). This mechanism does not require intratidal recruitment, because it originates from local amplification of distending pressures in parenchyma abutting derecruited regions, even in purely static conditions. A second mechanism results from the shear forces that develop during reopening of derecruited airspaces with tidal breathing (10–12). Consequently, for both these mechanisms of low-volume injury to be at play, part of the lung must be in a derecruited state. A consequence of alveolar derecruitment is an increase of hysteresis. In fact, magnetic resonance imaging has shown that hysteresis during mechanical ventilation of uninjured rodent lungs predominantly reflects alveolar derecruitment–recruitment (i.e., reaeration [“opening”] of previously nonaerated airspaces) (13).
Part of the uncertainty concerning the significance of low-volume injury stems from the fact that most of the knowledge of ventilator-induced lung injury (VILI) has been obtained from experimental models employing high Vts (“high-volume” injury [14–16]). Because the pathophysiologic mechanisms of low-volume injury are expected to exert their detrimental effects in vivo on time scales much longer (17) than the volutrauma induced by marked overdistension, there is a need for animal models of ventilation over time scales and Vt ranges comparable to those of patients undergoing general anesthesia or patients in the ICU who require mechanical ventilation for days even if they do not have ARDS (18–20). Although large-animal models of ARDS and VILI have the advantage of replicating gravitational forces on a scale similar to the human, murine models allow for more convenient and mechanistically advanced assessment of molecular and genetic pathways. In addition, because the biologic time scale is compressed in mice relative to larger mammals with longer life-spans, it should be possible to study processes that are expected to unfold over several days in the latter within more manageable experimental durations in the former. Consequently, we developed a long-term murine model of mechanical ventilation that specifically avoids marked overdistension.
The aim of this study was to compare the alterations of respiratory mechanics, their time course, and their relationship to the expression of pulmonary cytokines in a group exposed to the mechanisms of low-volume VILI (Vt 6 ml/kg; “protective” group) and a group with mild to moderate overdistension (Vt 15–20 ml/kg; “injurious” group). We hypothesized the following:
The main mechanical alteration in the protective group would be an increase of hysteresis, reflecting progressive alveolar derecruitment at lower Vt (13). In the injurious group, we would expect an increase of elastance as a result of injury induced by tidal overdistension, but not of hysteresis, because the higher Vt would keep the initially healthy lung in a recruited state, thus minimizing the amount of cyclic recruitment–derecruitment.
These mechanical alterations would become manifest only over periods longer than the experimental durations common to murine VILI, given the lower Vt range employed.
Given the different pathophysiologic mechanisms of high- and low-volume injury, the pulmonary expression of cytokines would mainly parallel the increase of elastance in the injurious group and that of hysteresis in the protective group.
Some results of this study were previously reported in the form of abstracts (21, 22).
Methods
The methods are described in detail in the data supplement.
Experimental Design
C57BL/6 mice were anesthetized with intraperitoneal ketamine (120 mg/kg bolus followed by 30 mg/kg/h infusion) and fentanyl (0.05 mg/kg). After tracheotomy, the animals were mechanically ventilated with Vt 8 ml/kg, respiratory rate 160/min, and positive end-expiratory pressure (PEEP) 2 cm H2O. The carotid artery was cannulated to monitor blood pressure and administer muscle relaxant (vecuronium, 1.5–2 mg/kg/h) and heparin (4 U/kg/h) in lactated Ringer’s with 5% dextrose solution. After surgical preparation, mice were connected to a flexiVent ventilator (SCIREQ) and assigned to two main groups according to ventilator settings: the low-Vt (“protective”) group (Vt, 6 ml/kg; respiratory rate, 180/min; n = 10) and the “injurious” group. The latter was composed of two subgroups: inj-15 (Vt, 15 ml/kg; respiratory rate, 80/min; n = 7) and inj-20 (Vt, 20 ml/kg; respiratory rate, 52/min; n = 5). All groups had fraction of inspired oxygen of 50%, PEEP of 2 cm H2O, and inspiratory-to-expiratory time ratio of 1:2. Lung volume history was standardized with two recruitment maneuvers (30 cm H2O, 5-s ramp and 1-s plateau) upon connection to the flexiVent. One maneuver was performed every 5 minutes during the experiment to recruit atelectatic lung. Frequency and pressure of the maneuver were based on studies showing that these settings maintained respiratory mechanics stable in mice ventilated for 2 to 6 hours with Vt and PEEP similar to those in our study (23, 24). Ventilation was continued for 16 hours or until the mouse died. Mice that died within the first 3 hours were excluded from the study because, at the Vt employed, death within this time window is not expected to result from VILI (Figure 1B in Reference 25). Seven mice served as sham control animals.
In Vivo Measurements
Respiratory input impedance was measured every hour using the broadband forced oscillation technique with the flexiVent ventilator. Tissue elastance was calculated by fitting the constant phase model to the respiratory impedance (26). Inspiratory capacity was determined from the recruitment maneuver, which is expected to reach total lung capacity (27). Pressure–volume curves to 30 cm H2O were generated every hour to compute hysteresis. An upper pressure limit of 30 cm H2O was chosen, both because it is considered to correspond to total lung capacity (28) and because it corresponded to the pressure of the recruitment maneuver, which the curve replaced at hourly intervals (see flowchart in Figure E1 in the data supplement). Elastance, inspiratory capacity, and hysteresis were normalized by the first (baseline) value of each subject. Lung strain and weighted lung strain (i.e., the average of the strain applied to the lung during inspiration and expiration, weighted by the inspiratory-to-expiratory time ratio) were computed as previously described (29). Arterial blood gas samples were collected from the carotid cannula when possible (n = 13) (Table 1).
Table 1.
Arterial Blood Gases
| pH | PaCO2 (mm Hg) | PaO2 (mm Hg) | Bicarbonate (mM) | Lactate (mM) | |
|---|---|---|---|---|---|
| Reference* | 7.39 ± 0.02 | 30 ± 2 | 18.4 ± 0.8 | 4.6 ± 0.7 | |
| Protective (n = 7) | 7.11 ± 0.05 | 43 ± 3 | 99 ± 27 | 13.9 ± 1.4 | 2.8 ± 0.7 |
| Injurious (n = 6) | 7.17 ± 0.09 | 33 ± 4 | 102 ± 24 | 13.5 ± 2.9 | 5.0 ± 2.1 |
| P value | 0.541 | 0.062 | 0.929 | 0.894 | 0.534 |
| Survivors (n = 6) | 7.23 ± 0.04 | 38 ± 2 | 123 ± 24 | 16 ± 1.2 | 1.6 ± 0.4 |
| Nonsurvivors (n = 7) | 7.06 ± 0.07 | 39 ± 5 | 81 ± 23 | 11.7 ± 2.4 | 5.7 ± 1.6 |
| P value | 0.035 | 0.945 | 0.241 | 0.149 | 0.008 |
Definition of abbreviations: PaCO2 = arterial carbon dioxide tension; PaO2 = arterial oxygen tension; pH = arterial pH.
Data are mean ± SEM.
Reference values from Reference 73.
Ex Vivo Measurements
Protein concentrations of IL-1β and IL-6 in lung homogenate were measured by ELISA (protective group, n = 10; injurious group, n = 7). The mRNA concentrations of IL-1β, IL-6, and MIP-2 (macrophage inflammatory protein 2) were measured by real-time qRT-PCR. The right upper lobe was harvested to measure wet-to-dry ratio.
Human Data
We compared plasma concentrations of IL-6 on Day 3 between the lower- and higher-Vt groups for the patients who did or did not survive to hospital discharge in the ARMA study (Lower Tidal Volume Trial) (1).
Statistical Analysis
Changes in elastance, inspiratory capacity, and hysteresis relative to baseline, and their differences between the protective and injurious groups, were analyzed with two-way repeated measures analysis of variance and the Holm-Sidak method for pairwise comparisons. Hourly values were fit to a mixed-random and fixed-effects longitudinal model (30) to assess the temporal behavior of these variables regardless of differences in timing of the final value (defined as the last hourly value before the animal died or at 16 h). Cytokine concentrations were logarithmically transformed for normality and compared by one-way analysis of variance and the Holm-Sidak method. Lung strain and blood gases, wet-to-dry ratio, and human IL-6 were analyzed with Student’s t, Kruskal-Wallis, and Mann-Whitney-Wilcoxon rank-sum tests, respectively. P < 0.05 was considered significant. Data are reported as mean ± SEM.
Results
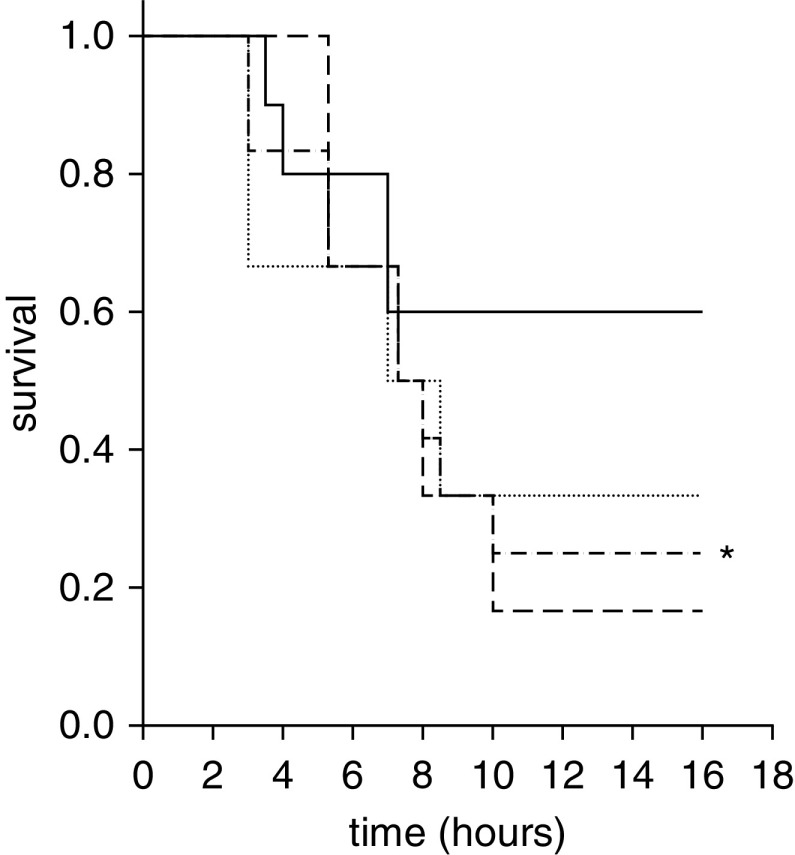
Blood pressure was not significantly different between the two groups (Figure E2). Survival rate at 16 hours was higher in the protective (60%) group than in the injurious (25%) group (P < 0.05) (Figure 1).
Figure 1.
A significant difference was found between the survival rates of the “injurious” (inj) and “protective” groups at the end of the study (*P < 0.05). Solid line = protective group (tidal volume [Vt], 6 ml/kg; respiratory rate [RR], 180/min; n = 10); dotted line = inj-15 subgroup (Vt, 15 ml/kg; RR, 80/min; n = 7); dashed line = inj-20 subgroup (Vt, 20 ml/kg; RR, 52/min; n = 5); dashed-dotted line = inj-15 and inj-20 subgroups pooled.
Respiratory Mechanics
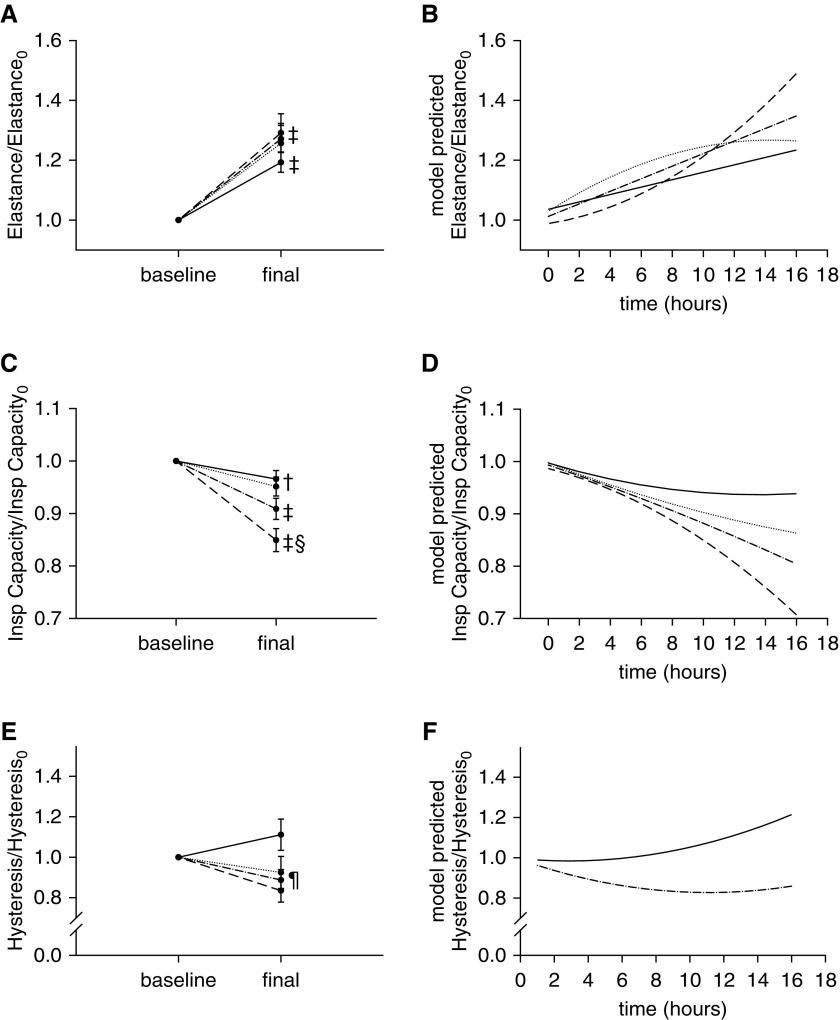
Elastance increased during the study in all groups (protective, +19.3%; inj-15, +25.7%; inj-20, +29.1%; P < 0.001 for all) (Figure 2A), but at a significantly faster rate in the injurious group, particularly the inj-20 subgroup (Figure 2B). Inspiratory capacity decreased in all groups (protective, −3.4%; P < 0.05; inj-15, −4.9%; P < 0.05; inj-20, −15.1%; P < 0.001), and the relative magnitude of this decrease was significantly higher in the inj-20 subgroup (P < 0.001 vs. both inj-15 and protective groups) (Figure 2C). In fact, the model revealed that the rate of decrease became progressively faster with increasing Vt, such that 16 hours were necessary for inspiratory capacity of the protective group to decrease by the same fraction as in the inj-20 subgroup at 5 hours (Figure 2D). Taken together, these results indicate that several hours are necessary for respiratory mechanics to deteriorate in models of VILI using Vt values closer to physiologic values.
Figure 2.
(A) Elastance increased significantly in all groups from the beginning (Elastance0) to the end of the study (‡P < 0.001 vs. baseline). (B) The longitudinal model indicated a faster rise of elastance in the injurious subgroups (P < 0.001 for interaction between time and group), with the inj-20 subgroup showing a marked increase after 8 hours of mechanical ventilation. (C) Inspiratory (Insp) capacity decreased in all groups (‡P < 0.001 and †P < 0.05 vs. baseline), and the relative magnitude of this decrease was significantly larger in the inj-20 subgroup (§P < 0.001 vs. protective and inj-15). †Refers to both protective and inj-15 subgroups. (D) The longitudinal model indicated a progressively faster rate of decline of inspiratory capacity with increasing Vt (P < 0.001). (E) Final hysteresis normalized by its initial value (hysteresis0) was significantly lower in the injurious than in the protective group (¶P < 0.01). (F) Whereas hysteresis increased progressively in the protective group, it decreased until approximately 11 hours of ventilation in the injurious group and then started to increase (P < 0.05 for interaction between time and group). The longitudinal model did not detect significant differences in the time course of hysteresis between the two injurious subgroups. Solid line = protective group (Vt, 6 ml/kg; RR, 180/min; n = 10); dotted line = inj-15 subgroup (Vt, 15 ml/kg; RR, 80/min; n = 7); dashed line = inj-20 subgroup (Vt, 20 ml/kg; RR, 52/min; n = 5); dashed-dotted line = inj-15 and inj-20 subgroups pooled. Data are mean ± SEM.
The relative change of hysteresis was significantly higher in the protective group than in the injurious group (P < 0.01) (Figure 2E). In contrast to elastance, hysteresis rose at a faster rate in the protective group than in the injurious group, in which hysteresis declined initially, reached a minimum at approximately 11 hours, and then started to increase (Figure 2F).
Relationship between Inflammation, Mechanics, and Survival
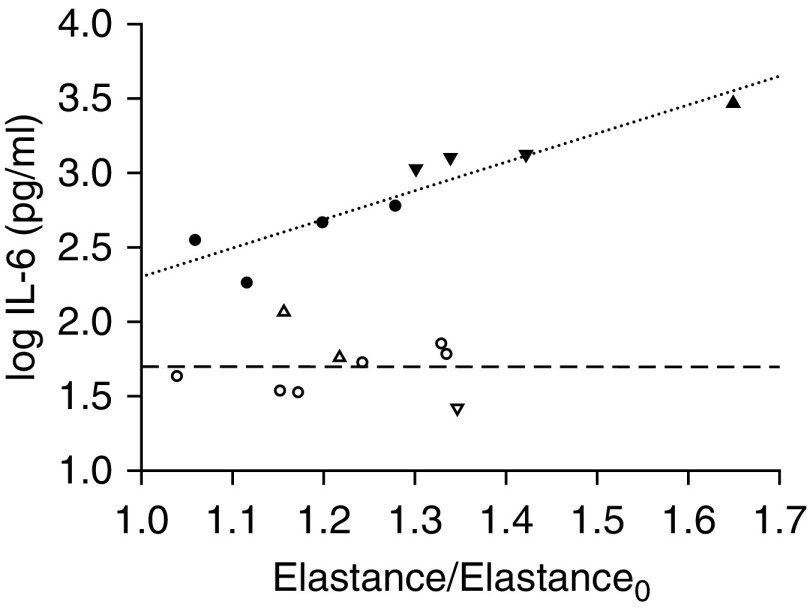
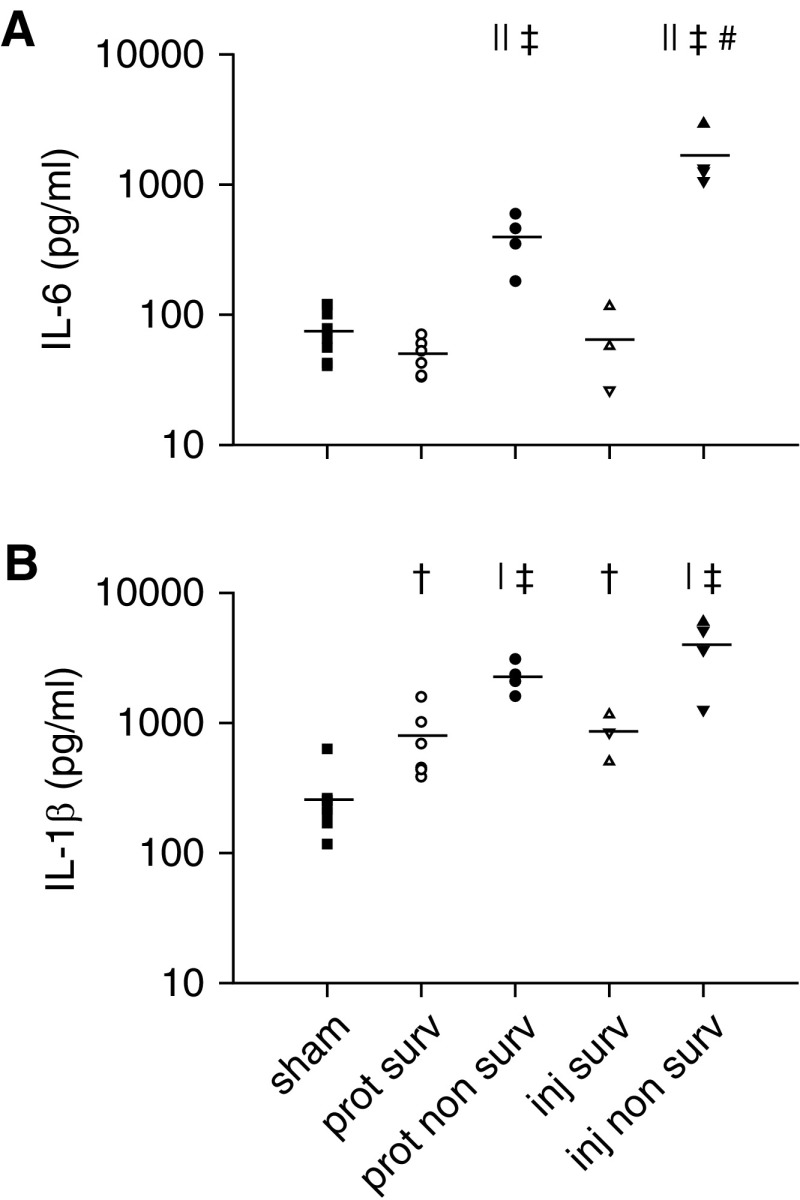
IL-6 protein concentration correlated with the relative increase in elastance by the end of the study among all ventilated animals (r = 0.53; P < 0.05). However, inspection of the individual data points (Figure 3) revealed two distinct clusters: In one cluster, IL-6 was invariant, whereas in the other cluster, IL-6 showed a strong, direct relationship with the change in elastance. Individual data points could not be segregated into these two clusters on the basis of Vt (i.e., injurious vs. protective). Instead, what clearly clustered them was survival status. For animals that survived the 16 hours, there was no relationship between IL-6 and the change in elastance. In contrast, a strong correlation was observed in nonsurvivors (r = 0.93; P < 0.001; dotted line in Figure 3). Within this nonsurviving cluster, protectively ventilated animals corresponded to data points with lower IL-6 and smaller increases of elastance than injuriously ventilated animals. In fact, IL-6 was higher in the injurious group nonsurvivors than in the protective group nonsurvivors (1,651 ± 432 pg/ml vs. 397 ± 88 pg/ml; P < 0.01) and higher in both these groups than in the survivors and sham control group (P < 0.001). IL-6 was not increased in the survivors of either group compared with the sham group (Figure 4A).
Figure 3.
Lung IL-6 protein concentration as a function of the relative increase in elastance from the beginning to the end of the study in mice that survived (dashed line) or did not survive (dotted line) (r = 0.93; P < 0.001) 16 hours of mechanical ventilation. Open symbols = survivors; solid symbols = nonsurvivors; circles = protective group (Vt, 6 ml/kg; RR, 180/min; n = 10); triangles = inj-15 subgroup (Vt, 15 ml/kg; RR, 80/min; n = 7); inverted triangles = inj-20 subgroup (Vt, 20 ml/kg; RR, 52/min; n = 5).
Figure 4.
(A) Lung IL-6 protein concentration at the end of the study. (B) Lung IL-1β protein concentration at the end of the study. ‡P < 0.001 and †P < 0.01 versus sham; ||P < 0.001 and |P < 0.01 versus corresponding survivor group; #P < 0.01 versus protective nonsurvivors. Squares = sham; open symbols = survivors (surv); solid symbols = nonsurvivors; circles = protective (prot) group (Vt, 6 ml/kg; RR, 180/min; n = 10); triangles = inj-15 subgroup (Vt, 15 ml/kg; RR, 80/min; n = 7); inverted triangles = inj-20 subgroup (Vt, 20 ml/kg; RR, 52/min; n = 5); lines = mean.
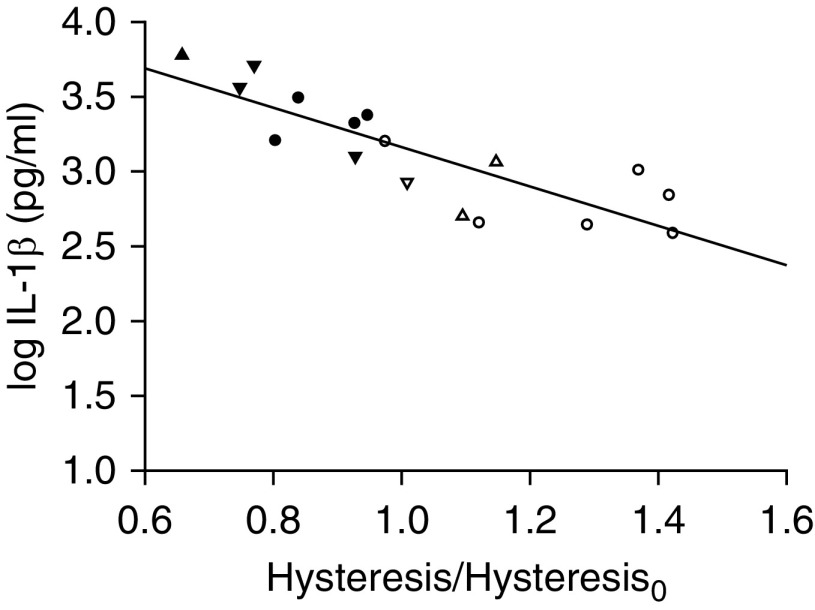
Similarly, IL-1β was lower in the survivors than in the nonsurvivors (protective, 761 ± 190 pg/ml vs. 2,287 ± 312 pg/ml, respectively; injurious, 836 ± 189 pg/ml vs. 4,004 ± 1,033 pg/ml, respectively; P < 0.01 for both). In contrast to IL-6, IL-1β was mildly elevated also in the survivors of both groups compared with the sham group (264 ± 55 pg/ml; P < 0.01), similar to previous findings (31), but again it did not differ between the two survivor groups (Figure 4B). Moreover, IL-1β was negatively correlated with the relative change in hysteresis by the end of the study (r = −0.84; P < 0.001) (Figure 5). In contrast to the relationship shown in Figure 3, all data points followed a single line. However, the survivors clustered on the portion of this relationship corresponding to an increase in hysteresis and lower IL-1β, whereas the nonsurvivors showed a decrease in hysteresis and higher IL-1β, particularly in the injurious group. A similar inverse relationship with the relative change in hysteresis was found for IL-6 (r = −0.79; P < 0.001). Both these relationships remained statistically significant after normalizing the cytokine measurement by the wet-to-dry ratio of the individual animal (for IL-1β, r = −0.60; P < 0.05; for IL-6, r = −0.77; P < 0.001).
Figure 5.
Lung IL-1β as a function of the relative change in hysteresis by the end of the study (r = −0.84; P < 0.001). Open symbols = survivors; solid symbols = nonsurvivors; circles = protective group (Vt, 6 ml/kg; RR, 180/min; n = 10); triangles = inj-15 subgroup (Vt, 15 ml/kg; RR, 80/min; n = 7); inverted triangles = inj-20 subgroup (Vt, 20 ml/kg; RR, 52/min; n = 5).
For both IL-1β and IL-6, their protein concentrations correlated with those of mRNA (r = 0.94; P < 0.001; and r = 0.59; P < 0.05, respectively) (Figure E3), indicating transcriptional control of these cytokines. MIP-2 mRNA was highest in the nonsurvivors and tended to be higher in the survivors than in sham control animals (Figure E4), similar to IL-1β. In fact, MIP-2 mRNA was strongly correlated with IL-1β mRNA (r = 0.94; P < 0.001), as expected on the basis of previous studies that showed induction of MIP-2 mRNA by IL-1β (32). Hysteresis changes showed the same inverse relationship with mRNA concentrations of IL-1β (r = −0.76; P < 0.001), IL-6 (r = −0.56; P < 0.05), and MIP-2 (r = −0.67; P < 0.01) as with IL-1β and IL-6 protein concentrations. Taken together, these results show concordant responses of various pulmonary cytokines and consistent relationships with changes in hysteresis.
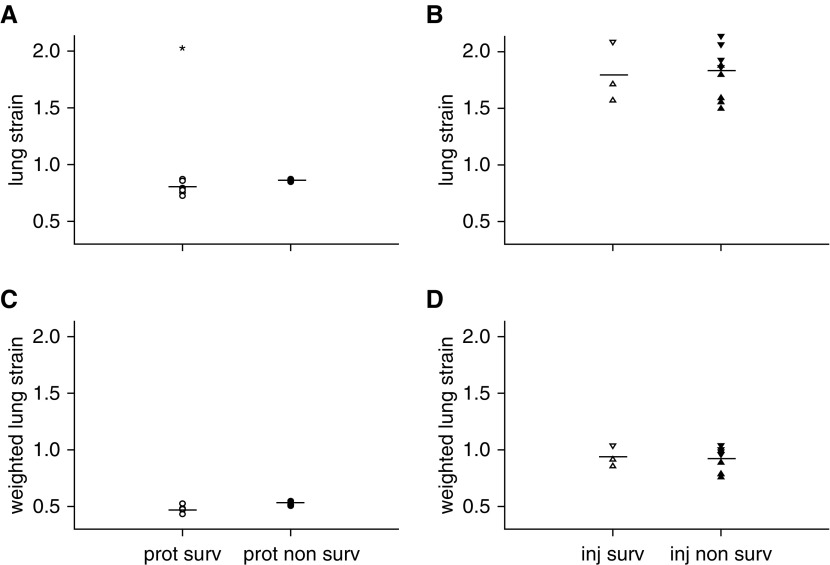
There was a small difference in lung strain at the onset of mechanical ventilation between the protective group survivors and nonsurvivors (0.796 ± 0.024 vs. 0.860 ± 0.006, respectively; P < 0.05), but not between the injurious group survivors and nonsurvivors (1.790 ± 0.154 and 1.812 ± 0.075, respectively). When the applied lung strain was weighted by the inspiratory-to-expiratory time ratio, there was no significant difference between survivors and nonsurvivors of either the protective (0.486 ± 0.015 and 0.521 ± 0.010, respectively) or injurious (0.936 ± 0.054 and 0.911 ± 0.036) group. No threshold could discriminate survivors from nonsurvivors, regardless of how strain was calculated (Figure 6).
Figure 6.
Lung strain in (A) protective and (B) injurious groups at the beginning of the study. Lung strain weighted by the inspiratory-to-expiratory time ratio in (C) protective and (D) injurious groups. *P < 0.05 survivors versus nonsurvivors. Open symbols = survivors (surv); solid symbols = nonsurvivors; circles = prot group (Vt, 6 ml/kg; RR, 180/min; n = 10); triangles = inj-15 subgroup (Vt, 15 ml/kg; RR, 80/min; n = 7); inverted triangles = inj-20 subgroup (Vt, 20 ml/kg; RR, 52/min; n = 5); lines = mean.
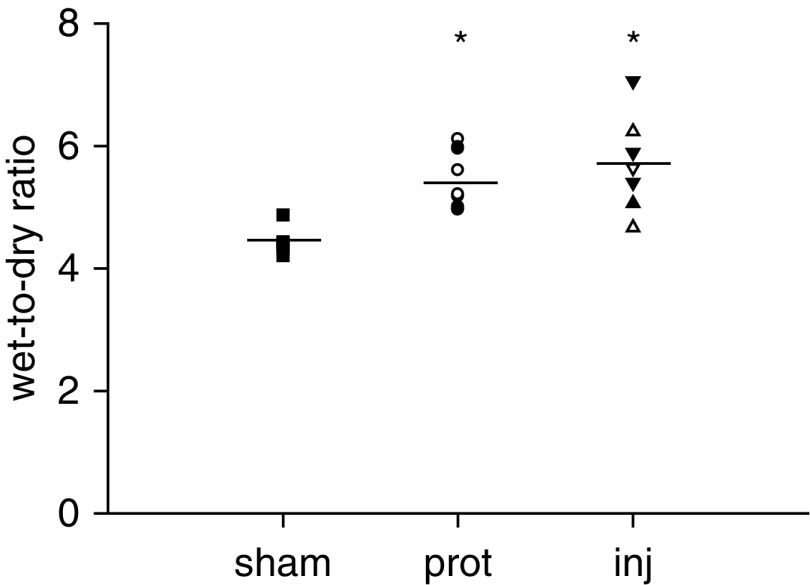
Prolonged mechanical ventilation caused a mild increase in lung wet-to-dry weight ratio (sham, 4.4 ± 0.1; protective, 5.4 ± 0.1; injurious, 5.7 ± 0.3; P < 0.05) (Figure 7). There was no significant difference between the protective and injurious groups or between survivors and nonsurvivors (5.5 ± 0.2 and 5.5 ± 0.3, respectively).
Figure 7.
Lung wet-to-dry weight ratio at the end of the study. *P < 0.05 versus sham. Squares = sham; open symbols = survivors; solid symbols = nonsurvivors; circles = prot group (Vt, 6 ml/kg; RR, 180/min; n = 10); triangles = inj-15 subgroup (Vt, 15 ml/kg; RR, 80/min; n = 7); inverted triangles = inj-20 subgroup (Vt, 20 ml/kg; RR, 52/min; n = 5); lines = mean.
Arterial carbon dioxide tension tended to be lower in the injurious group. When analyzed according to survival status, the nonsurvivors had lower pH (P < 0.05) and higher lactate concentration (P < 0.01) than the survivors (Table 1).
Plasma IL-6 in the ARMA Trial
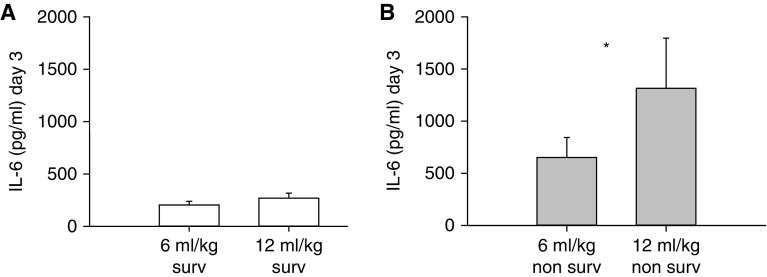
Plasma IL-6 on Day 3 was similarly low in the 6 and 12 ml/kg Vt groups for patients who survived to hospital discharge in the ARMA trial, whereas the 12 ml/kg nonsurvivors had significantly higher plasma IL-6 than the 6 ml/kg nonsurvivors (P < 0.05) (Figure 8).
Figure 8.
Plasma IL-6 on Day 3 in (A) survivors and (B) nonsurvivors of the 6 and 12 ml/kg Vt groups in the ARMA trial (Lower Tidal Volume Trial) (1). *P < 0.05 for 12 ml/kg versus 6 ml/kg. Data are mean ± SEM.
Discussion
Thanks to its extended duration, this study separated the survival animals, who tolerated mechanical ventilation well regardless of the Vt, from the nonsurvivors. In the survivors, pulmonary expression of cytokines was not substantially increased and was unaffected by Vt. In contrast, nonsurvivors showed marked elevation of cytokines even in the low-Vt group, in which lung strain was as low as about 0.8. In the nonsurvivors, but not in the survivors, the increase of elastance reflected the concentration of IL-6 (Figure 3). The concentration of all measured cytokines was instead negatively correlated with the relative change of hysteresis during the study.
Time Course of Respiratory Mechanics: Why Longer-Duration Models of VILI Are Needed
All groups showed an increase of elastance. This differs from shorter studies that did not report any deterioration in respiratory mechanics when Vt and PEEP similar to the protective group of our study were applied for 3 to 6 hours (23, 33). Furthermore, within the range of Vts employed (6–20 ml/kg), the detrimental effect of higher Vt became apparent only after 6–8 hours of ventilation, when elastance started to deteriorate rapidly in the inj-20 subgroup (Figures 2B and 2D). This time interval is much longer than the 1.5 hours required to develop VILI with Vt of 45 ml/kg (34). Consequently, the first message of this study is that several hours are necessary to model VILI with Vt values closer to those encountered clinically. In fact, PEEP and Vt combinations used in this study were deemed noninjurious on the basis of lung injury cost function models validated on data from shorter studies (35, 36).
The measurement of hysteresis allowed insights into the cause of the increase of elastance. Lung hysteresis increased in the protective group, suggesting that progressive alveolar derecruitment leading to less open lung was the cause of the increase in elastance (13, 37). Derecruitment occurred despite a recruitment maneuver performed every 5 minutes, a frequency that maintained respiratory mechanics stable in shorter studies (23, 24, 37). In contrast, hysteresis decreased in the injurious group, at least up to 11 hours (Figure 2F), suggesting that 1) up to this time, the larger Vt maintained the lung in a recruited state, possibly by stimulating surfactant function, which can also independently decrease hysteresis, and by generating higher mean airway pressures that prevented derecruitment; and consequently, 2) the increase of elastance was predominantly due to the prolonged effect of moderate overstretching, which indeed resulted in mild pulmonary edema, rather than to derecruitment. In fact, Vt of the injurious group was two to three times that of spontaneously breathing mice (38). These results thus temper conclusions of shorter studies that Vt values up to 20 ml/kg lead only to “atelectrauma” in mice (25) and instead suggest that mechanisms of both high- and low-volume injury can be modeled over the same Vt spectrum for which we would expect them in humans (i.e., 6 to 15–20 ml/kg), provided that ventilation is extended for a sufficiently long period (39–41).
Relationship between Respiratory Mechanics, Cytokines, and Survival
The protective group had better survival than the injurious group, resembling what was reported previously with a larger Vt difference (42). Although it has recently been shown that 20 ml/kg Vt for 4 hours leads to activation of the integrated stress response pathway and release of cytokines (43), there are conflicting data on whether low-Vt ventilation of initially uninjured lungs leads to increased pulmonary expression of cytokines in vivo (44–47). In our study, there was no effect of Vt magnitude on cytokine concentrations in the survivors. In contrast, cytokines were markedly elevated in the nonsurvivors, particularly of the injurious group. Because an increase of elastance is expected to result from both volutrauma (14, 16, 48) and derecruitment (24), we expected that such increase would correlate with the concentration of cytokines as it would be causally related to the pathogenetic mechanisms. Our results revealed that such correlation was present only in nonsurvivors, despite the fact that elastance and wet-to-dry ratio increased to a comparable extent also in survivors (Figures 3 and 7). The second message of this study is thus that the increase of elastance does not necessarily reflect the degree of underlying pulmonary inflammation due to VILI.
To identify the reason why some mice developed pulmonary inflammation and died whereas others showed only minimal inflammation and tolerated mechanical ventilation well, we computed lung strain. Although strain was slightly higher in the nonsurvivors than in the survivors of the protective group, there was no significant difference in the injurious group. Furthermore, strain less lower than 2 in most animals of both groups (Figure 6), and no threshold could be identified, in contrast to previous studies (49, 50). Studies that demonstrated that VILI would develop and be lethal above a strain threshold of 1.5–2 in various species were based on experiments employing high Vt (29, 50). In contrast, values of weighted lung strain such as those of this study, particularly in the protective group (Figure 6C), have been speculated to be associated with VILI due to alveolar derecruitment and intratidal cyclic alveolar opening and closing, rather than to overdistension (29). The finding that protective nonsurvivors presented consistent and significant elevation of pulmonary cytokines (Figures 4 and E4) proves that, even with strain below 1.5–2, VILI developed and was the likely cause of death (51, 52). Metabolic acidosis and cardiovascular collapse are indeed consistent with the systemic inflammatory response that characterizes the terminal stage of VILI (16, 48, 51). In fact, IL-6, for example, has been associated with mortality attributable to VILI in patients with ARDS. Interestingly, IL-6 plasma concentrations in the ARMA trial revealed a pattern similar to that of pulmonary IL-6 in mice (Figures 4A and 8). Consequently, we propose that VILI results from an interaction between the magnitude of the mechanical insult and an intrinsic susceptibility to pulmonary inflammation induced by mechanical ventilation. If each subject had an individual strain threshold above which VILI ensued, then tidal strain below this threshold would not cause significant inflammation, which would instead develop in a Vt-dependent fashion above this threshold. Accordingly, ventilator parameters that are protective in one subject might be injurious in another. Although caution must be exercised in extrapolating to humans, these results offer a rationale for personalized mechanical ventilation. Interindividual differences in the ratio of elastin to collagen, cross-linking of collagen, and magnitude of the cytokine response could represent potential determinants of this threshold, similarly to what has been hypothesized to explain age-related variation in susceptibility to VILI (53, 54).
Possible explanations for the observation that VILI occurred for Vt and strain values lower than in prior experimental studies are: 1) the same Vt per kilogram results in greater strain in smaller than in larger species because functional residual capacity is proportional to body weight to a power of approximately 1.2 (29, 55). In addition, the murine chest wall is more compliant than the human (56, 57), and hence a given PEEP is expected to cause greater distension in the former. We accounted for these factors by limiting PEEP to 2 cm H2O and Vt of the protective group to slightly less than that of spontaneously breathing mice (38). 2) Subtle bloodstream infection may have ensued and increased susceptibility to VILI (58). If so, however, we would have expected the mortality rate to increase over the course of the study because the cumulative probability of contamination should increase with time. Furthermore, this risk should have been present also in other long-term studies that identified a strain threshold (49, 50).
Role of Derecruitment in VILI
Because several ex vivo studies demonstrated that low-Vt ventilation of a derecruited lung can induce injury (10, 11, 59), we hypothesized that the increase of hysteresis in the protective group would predict pulmonary cytokine concentrations. In fact, a direct relationship between hysteresis and BAL fluid protein content, which is an index of inflammation, was reported in other models of murine lung injury characterized by alveolar derecruitment (60). Instead, we found that the higher the increase of hysteresis, the lower the expression of cytokines. One interpretation of this result is that derecruited airspaces are protected from injury (61, 62). An alternative interpretation is that, in this study, derecruitment was simply an epiphenomenon of resilience to VILI: Animals that did not mount a substantial pulmonary inflammatory response to mechanical ventilation survived longer and, as a result of being ventilated for longer times, developed greater derecruitment, particularly in the protective group. Regardless of the interpretation, the third message of this study, supported also by others (63, 64), is that derecruitment per se does not appear to be a potent inflammatory trigger in vivo, even during low-Vt ventilation. However, recent evidence suggests that derecruitment may act predominantly indirectly in the intact respiratory system: Because of alveolar interdependence, derecruitment may induce overdistension of the airspaces that remain aerated (65, 66). This could constitute an additional mechanism of low-volume injury. Furthermore, atelectasis can be injurious by mechanisms other than VILI, such as right ventricular dysfunction and increased pulmonary microvascular permeability (67).
Limitations and Critique of the Study
Although a recruitment maneuver every 5 minutes may sound excessive for larger mammals, it is important to properly scale the murine variables. Because the spontaneous respiratory rate of C57/BL6 mice is approximately 350 breaths per minute (68), one maneuver every 5 minutes would correspond to one every 2.5 hours at a respiratory rate of 12 per minute. This 30× scaling is consistent with that reported in the literature (69). According to this scaling, the 16 hours of ventilation in mice should correspond to approximately 20 days for a human.
We measured a limited number of cytokines, selected on the basis of the consistency with which they were reported to be elevated in VILI studies in mice (24, 45–47, 70) and humans (1, 71, 72), as well as on the basis of their kinetic profile. Other cytokines, such as TNF-α, also play a role in VILI, but because TNF-α expression is transient and peaks in the early stage of VILI (33), it did not seem the best mediator to assess prolonged VILI.
Conclusions
In a 16-hour model of murine mechanical ventilation, mortality was 1) associated with development of pulmonary inflammation despite lung strain as low as 0.8, and 2) lower with Vt 6 ml/kg than with Vt 15–20 ml/kg. Survivors developed only modest pulmonary inflammation, regardless of Vt and despite an increase of hysteresis whose magnitude was, in fact, inversely related to the concentration of pulmonary cytokines. These results suggest that there is no single strain threshold for developing VILI and that alveolar derecruitment during low-Vt ventilation does not necessarily lead to pulmonary inflammation.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Boyd Taylor Thompson, M.D., and Nancy J. Ringwood, R.N., for their assistance in the process of obtaining the human data. The authors also thank Tilo Winkler, Ph.D., and Luiz Fernando dos Reis Falcão, M.D., Ph.D., who provided insight and expertise that greatly aided this research. This manuscript was prepared using NHLBI ARDS Network (ARDSNet) research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinion or views of ARDSNet or the NHLBI.
Footnotes
Supported by National Institutes of Health grant R01HL094639 (G.M.).
Author Contributions: M.V.S.: was involved in the conception and design of the study, execution of experiments, acquisition, analysis, and interpretation of the data, and writing of the manuscript; K.T. and Y.F.: were involved in the execution of experiments, acquisition and analysis of the biochemical data, and revision of the manuscript; J.J.L.: was involved in the analysis and interpretation of the data, especially regarding statistical modeling, and revision of the manuscript; W.C. and E.A.C.: were involved in the analysis and interpretation of the molecular biology and biochemical data and in revision of the manuscript; M.F.V.M.: contributed to interpretation of the data and revision of the manuscript; and G.M.: was involved in the conception and design of the study, interpretation of the data, and writing and revision of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0100OC on September 10, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 3.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Espósito DC, de Oliveira Prado Pasqualucci M, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 4.Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 5.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 6.Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 7.De Prost N, Dreyfuss D. How to prevent ventilator-induced lung injury? Minerva Anestesiol. 2012;78:1054–1066. [PubMed] [Google Scholar]
- 8.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 9.Makiyama AM, Gibson LJ, Harris RS, Venegas JG. Stress concentration around an atelectatic region: a finite element model. Respir Physiol Neurobiol. 2014;201:101–110. doi: 10.1016/j.resp.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 11.Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med. 2004;32:168–174. doi: 10.1097/01.CCM.0000104203.20830.AE. [DOI] [PubMed] [Google Scholar]
- 12.Bilek AM, Dee KC, Gaver DP., III Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol (1985) 2003;94:770–783. doi: 10.1152/japplphysiol.00764.2002. [DOI] [PubMed] [Google Scholar]
- 13.Cereda M, Xin Y, Emami K, Huang J, Rajaei J, Profka H, et al. Positive end-expiratory pressure increments during anesthesia in normal lung result in hysteresis and greater numbers of smaller aerated airspaces. Anesthesiology. 2013;119:1402–1409. doi: 10.1097/ALN.0b013e3182a9b0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures: protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 15.Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis. 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- 16.Kolobow T, Moretti MP, Fumagalli R, Mascheroni D, Prato P, Chen V, et al. Severe impairment in lung function induced by high peak airway pressure during mechanical ventilation: an experimental study. Am Rev Respir Dis. 1987;135:312–315. doi: 10.1164/arrd.1987.135.2.312. [DOI] [PubMed] [Google Scholar]
- 17.Tucci MR, Costa EL, Wellman TJ, Musch G, Winkler T, Harris RS, et al. Regional lung derecruitment and inflammation during 16 hours of mechanical ventilation in supine healthy sheep. Anesthesiology. 2013;119:156–165. doi: 10.1097/ALN.0b013e31829083b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 20.Simonis FD, Binnekade JM, Braber A, Gelissen HP, Heidt J, Horn J, et al. PReVENT - protective ventilation in patients without ARDS at start of ventilation: study protocol for a randomized controlled trial. Trials. 2015;16:226. doi: 10.1186/s13063-015-0759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabari MV, Takahashi K, Carter EA, Feng Y, Chao W, Musch G. Inflammatory effect of protective long-term mechanical ventilation [abstract] Am J Respir Crit Care Med. 2015;191:A3121. [Google Scholar]
- 22.Szabari MV, Falcao LFR, Musch G. Long-term clinically relevant ventilator-induced lung injury in mice [abstract] Am J Respir Crit Care Med. 2014;189:A1182. [Google Scholar]
- 23.Reiss LK, Kowallik A, Uhlig S. Recurrent recruitment manoeuvres improve lung mechanics and minimize lung injury during mechanical ventilation of healthy mice. PLoS One. 2011;6:e24527. doi: 10.1371/journal.pone.0024527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JH. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L710–L717. doi: 10.1152/ajplung.00532.2005. [DOI] [PubMed] [Google Scholar]
- 25.Wilson MR, Patel BV, Takata M. Ventilation with “clinically relevant” high tidal volumes does not promote stretch-induced injury in the lungs of healthy mice. Crit Care Med. 2012;40:2850–2857. doi: 10.1097/CCM.0b013e31825b91ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hantos Z, Daróczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol (1985) 1992;72:168–178. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- 27.Struhar D, Harbeck RJ. An apparatus for the measurement of lung volume and compliance in mice. Lab Anim. 1990;24:328–331. doi: 10.1258/002367790780865930. [DOI] [PubMed] [Google Scholar]
- 28.Tankersley CG, Rabold R, Mitzner W. Differential lung mechanics are genetically determined in inbred murine strains. J Appl Physiol (1985) 1999;86:1764–1769. doi: 10.1152/jappl.1999.86.6.1764. [DOI] [PubMed] [Google Scholar]
- 29.Caironi P, Langer T, Carlesso E, Protti A, Gattinoni L. Time to generate ventilator-induced lung injury among mammals with healthy lungs: a unifying hypothesis. Intensive Care Med. 2011;37:1913–1920. doi: 10.1007/s00134-011-2388-9. [DOI] [PubMed] [Google Scholar]
- 30.Locascio JJ, Atri A. An overview of longitudinal data analysis methods for neurological research. Dement Geriatr Cogn Disord Extra. 2011;1:330–357. doi: 10.1159/000330228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaneker M, Joosten LA, Heunks LM, Snijdelaar DG, Halbertsma FJ, van Egmond J, et al. Low-tidal-volume mechanical ventilation induces a Toll-like receptor 4-dependent inflammatory response in healthy mice. Anesthesiology. 2008;109:465–472. doi: 10.1097/ALN.0b013e318182aef1. [DOI] [PubMed] [Google Scholar]
- 32.Xu WB, Haddad EB, Tsukagoshi H, Adcock I, Barnes PJ, Chung KF. Induction of macrophage inflammatory protein 2 gene expression by interleukin 1 beta in rat lung. Thorax. 1995;50:1136–1140. doi: 10.1136/thx.50.11.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson MR, Choudhury S, Goddard ME, O’Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol (1985) 2003;95:1385–1393. doi: 10.1152/japplphysiol.00213.2003. [DOI] [PubMed] [Google Scholar]
- 34.Smith BJ, Grant KA, Bates JH. Linking the development of ventilator-induced injury to mechanical function in the lung. Ann Biomed Eng. 2013;41:527–536. doi: 10.1007/s10439-012-0693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamlington KL, Smith BJ, Allen GB, Bates JH. Predicting ventilator-induced lung injury using a lung injury cost function. J Appl Physiol (1985) 2016;121:106–114. doi: 10.1152/japplphysiol.00096.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seah AS, Grant KA, Aliyeva M, Allen GB, Bates JH. Quantifying the roles of tidal volume and PEEP in the pathogenesis of ventilator-induced lung injury. Ann Biomed Eng. 2011;39:1505–1516. doi: 10.1007/s10439-010-0237-6. [DOI] [PubMed] [Google Scholar]
- 37.Allen G, Lundblad LK, Parsons P, Bates JH. Transient mechanical benefits of a deep inflation in the injured mouse lung. J Appl Physiol (1985) 2002;93:1709–1715. doi: 10.1152/japplphysiol.00473.2002. [DOI] [PubMed] [Google Scholar]
- 38.Tankersley CG, Fitzgerald RS, Levitt RC, Mitzner WA, Ewart SL, Kleeberger SR. Genetic control of differential baseline breathing pattern. J Appl Physiol (1985) 1997;82:874–881. doi: 10.1152/jappl.1997.82.3.874. [DOI] [PubMed] [Google Scholar]
- 39.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolobow T. The (ir)relevance of short term studies. Int J Artif Organs. 1990;13:1–2. [PubMed] [Google Scholar]
- 41.Wilson MR, Takata M. Inflammatory mechanisms of ventilator-induced lung injury: a time to stop and think? Anaesthesia. 2013;68:175–178. doi: 10.1111/anae.12085. [DOI] [PubMed] [Google Scholar]
- 42.Caironi P, Ichinose F, Liu R, Jones RC, Bloch KD, Zapol WM. 5-Lipoxygenase deficiency prevents respiratory failure during ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172:334–343. doi: 10.1164/rccm.200501-034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolinay T, Himes BE, Shumyatcher M, Lawrence GG, Margulies SS. Integrated stress response mediates epithelial injury in mechanical ventilation. Am J Respir Cell Mol Biol. 2017;57:193–203. doi: 10.1165/rcmb.2016-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurkan OU, O’Donnell C, Brower R, Ruckdeschel E, Becker PM. Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2003;285:L710–L718. doi: 10.1152/ajplung.00044.2003. [DOI] [PubMed] [Google Scholar]
- 45.Vaneker M, Halbertsma FJ, van Egmond J, Netea MG, Dijkman HB, Snijdelaar DG, et al. Mechanical ventilation in healthy mice induces reversible pulmonary and systemic cytokine elevation with preserved alveolar integrity: an in vivo model using clinical relevant ventilation settings. Anesthesiology. 2007;107:419–426. doi: 10.1097/01.anes.0000278908.22686.01. [DOI] [PubMed] [Google Scholar]
- 46.Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Juffermans NP, Schultz MJ. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care. 2009;13:R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hegeman MA, Hemmes SN, Kuipers MT, Bos LD, Jongsma G, Roelofs JJ, et al. The extent of ventilator-induced lung injury in mice partly depends on duration of mechanical ventilation. Crit Care Res Pract. 2013;2013:435236. doi: 10.1155/2013/435236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuno K, Prato P, Kolobow T. Acute lung injury from mechanical ventilation at moderately high airway pressures. J Appl Physiol (1985) 1990;69:956–961. doi: 10.1152/jappl.1990.69.3.956. [DOI] [PubMed] [Google Scholar]
- 49.Lex D, Uhlig S. One-hit models of ventilator-induced lung injury: benign inflammation versus inflammation as a by-product. Anesthesiology. 2017;126:909–922. doi: 10.1097/ALN.0000000000001605. [DOI] [PubMed] [Google Scholar]
- 50.Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, et al. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med. 2011;183:1354–1362. doi: 10.1164/rccm.201010-1757OC. [DOI] [PubMed] [Google Scholar]
- 51.Slutsky AS, Tremblay LN. Multiple system organ failure: is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 52.Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:109–116. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- 53.Copland IB, Martinez F, Kavanagh BP, Engelberts D, McKerlie C, Belik J, et al. High tidal volume ventilation causes different inflammatory responses in newborn versus adult lung. Am J Respir Crit Care Med. 2004;169:739–748. doi: 10.1164/rccm.200310-1417OC. [DOI] [PubMed] [Google Scholar]
- 54.Kornecki A, Tsuchida S, Ondiveeran HK, Engelberts D, Frndova H, Tanswell AK, et al. Lung development and susceptibility to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;171:743–752. doi: 10.1164/rccm.200408-1053OC. [DOI] [PubMed] [Google Scholar]
- 55.Stahl WR. Scaling of respiratory variables in mammals. J Appl Physiol. 1967;22:453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- 56.Lai YL, Chou H. Respiratory mechanics and maximal expiratory flow in the anesthetized mouse. J Appl Physiol (1985) 2000;88:939–943. doi: 10.1152/jappl.2000.88.3.939. [DOI] [PubMed] [Google Scholar]
- 57.Crosfill ML, Widdicombe JG. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol. 1961;158:1–14. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Zheng H, et al. Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep. Crit Care Med. 2014;42:e491–e500. doi: 10.1097/CCM.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wakabayashi K, Wilson MR, Tatham KC, O’Dea KP, Takata M. Volutrauma, but not atelectrauma, induces systemic cytokine production by lung-marginated monocytes. Crit Care Med. 2014;42:e49–e57. doi: 10.1097/CCM.0b013e31829a822a. [DOI] [PubMed] [Google Scholar]
- 60.Allen G, Bates JH. Dynamic mechanical consequences of deep inflation in mice depend on type and degree of lung injury. J Appl Physiol (1985) 2004;96:293–300. doi: 10.1152/japplphysiol.00270.2003. [DOI] [PubMed] [Google Scholar]
- 61.Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, et al. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med. 2006;174:279–289. doi: 10.1164/rccm.200506-1006OC. [DOI] [PubMed] [Google Scholar]
- 62.Cereda M, Kavanagh BP. Compartmentalization of lung injury—atelectasis versus overstretch. Crit Care Med. 2014;42:223–224. doi: 10.1097/CCM.0b013e3182a264ed. [DOI] [PubMed] [Google Scholar]
- 63.Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, et al. Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med. 2011;183:1193–1199. doi: 10.1164/rccm.201008-1318OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Güldner A, Braune A, Ball L, Silva PL, Samary C, Insorsi A, et al. Comparative effects of volutrauma and atelectrauma on lung inflammation in experimental acute respiratory distress syndrome. Crit Care Med. 2016;44:e854–e865. doi: 10.1097/CCM.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cereda M, Emami K, Kadlecek S, Xin Y, Mongkolwisetwara P, Profka H, et al. Quantitative imaging of alveolar recruitment with hyperpolarized gas MRI during mechanical ventilation. J Appl Physiol (1985) 2011;110:499–511. doi: 10.1152/japplphysiol.00841.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cereda M, Xin Y, Hamedani H, Clapp J, Kadlecek S, Meeder N, et al. Mild loss of lung aeration augments stretch in healthy lung regions. J Appl Physiol (1985) 2016;120:444–454. doi: 10.1152/japplphysiol.00734.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duggan M, McCaul CL, McNamara PJ, Engelberts D, Ackerley C, Kavanagh BP. Atelectasis causes vascular leak and lethal right ventricular failure in uninjured rat lungs. Am J Respir Crit Care Med. 2003;167:1633–1640. doi: 10.1164/rccm.200210-1215OC. [DOI] [PubMed] [Google Scholar]
- 68.Milton PL, Dickinson H, Jenkin G, Lim R. Assessment of respiratory physiology of C57BL/6 mice following bleomycin administration using barometric plethysmography. Respiration. 2012;83:253–266. doi: 10.1159/000330586. [DOI] [PubMed] [Google Scholar]
- 69.Demetrius L. Aging in mouse and human systems: a comparative study. Ann N Y Acad Sci. 2006;1067:66–82. doi: 10.1196/annals.1354.010. [DOI] [PubMed] [Google Scholar]
- 70.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002;110:1703–1716. doi: 10.1172/JCI15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, et al. NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 72.Stüber F, Wrigge H, Schroeder S, Wetegrove S, Zinserling J, Hoeft A, et al. Kinetic and reversibility of mechanical ventilation-associated pulmonary and systemic inflammatory response in patients with acute lung injury. Intensive Care Med. 2002;28:834–841. doi: 10.1007/s00134-002-1321-7. [DOI] [PubMed] [Google Scholar]
- 73.Iversen NK, Malte H, Baatrup E, Wang T. The normal acid-base status of mice. Respir Physiol Neurobiol. 2012;180:252–257. doi: 10.1016/j.resp.2011.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.