Abstract
Purpose
The aims of present study are to clarify the follow questions: 1) what constitutes paediatric chondrosarcoma?; 2) what are the effects of the demographic and tumour characteristics on survival in patients with paediatric chondrosarcoma?; 3) which prognostic factors of paediatric chondrosarcoma differ from those of the adult population, which have been reported previously?
Methods
Paediatric patients who were diagnosed with chondrosarcoma were searched for using the case listing session protocol of the National Cancer Institute’s Surveillance, Epidemiology, and End Results 18 databases (1973 to 2014). The extracted demographic information includes: age, race, gender, year of diagnosis, tumour sites, tumour histological subtype, grade, stage and treatment.
Results
A total of 247 paediatric chondrosarcoma patients were extracted and included in our present study. We find that the paediatric patients have significantly better survival rates than the adult patients. The year of diagnosis, tumour sites, tumour histological subtype, grade, stage and surgery received are independent prognostic factors for the survival rate of paediatric chondrosarcoma patients, but race, gender and age are not.
Conclusion
The paediatric chondrosarcoma patients have better survival rates than the adults. Paediatric patients with a diagnosis at an early age, tumour site at the vertebral column and pelvis/sacrococcyx, myxoid variants, high grade, distant stage and who did not have surgery have a poorer prognosis than patients with a diagnosis at a later age, tumour site at limbs, head and base, chondrosarcoma not otherwise specified, lower grade, localized stage and who received surgery.
Level of Evidence
II -Prognostic Study
Keywords: chondrosarcoma, paediatric, prognosis, survival
Introduction
Chondrosarcoma is a slow-growing malignant tumour that is comprised of transformed cells that produce a cartilaginous matrix.1,2 It is the second-most common type of bone tumour and has a high rate of recurrence and risk of death.3-6 The incidence of paediatric chondrosarcoma is very rare and comprises < 10% of total chondrosarcoma cases.7 Paediatric chondrosarcoma has mostly been reported as a case series8,9 or as a case report.10-17
The Surveillance, Epidemiology, and End Results (SEER) database represents approximately 28% of the US population, and this registry is the largest source of information on cancer incidence and survival data in United States. Since it provides data on patient demographics and survival formation, it is a very useful resource for studying cancer.18,19 In the present study, we use the SEER database to study chondrosarcoma in the 0-18-year-old population and in particular to investigate the associations between prognostic factors and survival outcomes.
Our questions include the following: 1) what constitutes paediatric chondrosarcoma?; 2) what is the effect of demographics and tumour characteristics on survival in paediatric chondrosarcoma?; 3) what are the prognostic factors of paediatric chondrosarcoma that differ from those of the adult population, which have been reported previously?
Methods and materials
This is a retrospective study of the National Cancer Institute’s SEER 18 databases (1973 to 2014).20 We submitted a data agreement form to the SEER administration and the agreement was accepted. The database uses publicly available information without personal identifiers; therefore, no additional Institutional Review Board approval is required in this study.
Our study is compliant with the checklist of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement21,22 (supplemental material).
Patients with ages from 0 to 18 years that were diagnosed with chondrosarcoma (including histological Type International Classification of Diseases (ICD)-O-323: “9220: Chondrosarcoma not otherwise specified’,’ “9221: Juxtacortical chondrosarcoma’,’ “9230: Chondroblastoma, malignant”, “9231: Myxoid chondrosarcoma”, “9240: Mesenchymal chondrosarcoma”, “9242: Clear cell chondrosarcoma” and “9243: Dedifferentiated chondrosarcoma”) were searched using the case-listing session protocol of the National Cancer Institute’s SEER 18 databases.24
The demographic information includes the patients’ age, gender, race, year of diagnosis, tumour sites, tumour histological subtypes (chondrosarcoma not otherwise specified (chondrosarcoma NOS), juxtacortical chondrosarcoma, chondroblastoma malignant, myxoid chondrosarcoma, mesenchymal chondrosarcoma, clear cell chondrosarcoma and dedifferentiated chondrosarcoma), grade, stage (localized, regional and distant) and treatment (surgery or non-surgery).
Statistical analysis
We first attempted to convert the patient ages to categorical variables of 0 to four, five to nine, ten to 14 and 15 to 18 years old, but the category of 0 to four only included five cases; therefore, we combined the 0 to four and five to nine categories into one category of 0 to nine years old. The year of diagnosis was converted into categorical variables of 2004 to 2014, 1994 to 2003, 1984 to 1993 and 1973 to 1983. Tumour sites are also categorized into the following five locations: vertebral column, pelvis/sacrococcyx, limbs, other bones (such as skull, rib, sternum, clavicle and mandible) and extraskeletal sites (outside of the bone). The tumour grades were also reclassified into two grades (low grade: ICD-O-3 Grades 1 and 2; high grade: ICDO-3 Grades 3 and 4).5,25,26
Survival curves were generated using the Kaplan-Meier method and log rank test. Both bivariable and multivariable Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) with 95% confidence intervals (CIs) for chondrosarcoma cancer-specific survival (CCSS) and overall survival (OS).
Results
A total of 247 paediatric chondrosarcoma patients were extracted and included in our present study; 166/247 (67.21%) were male and 81/247 (32.79%) were female. Furthermore, 190/247 (76.92%) cases were white. Chondrosarcoma (NOS) is the most common histological subtype (approximately 61.13%). Additionally, most patients undergo surgery (86.23%); only 13.77% patients did not undergo surgery. The constituents and characteristics of the included patients are summarized in Table 1.
Table 1.
Characteristics of the paediatric chondrosarcoma patients from the Surveillance, Epidemiology, and End Results 18 Registries Research Database, 1973 to 2014
| Category | Value (%) |
|---|---|
| Total number | 247 (100) |
| Gender | |
| Female | 81 (32.79) |
| Male | 166 (67.21) |
| Race | |
| White | 190 (76.92) |
| Black | 31 (12.55) |
| Other/Unr | 26 (10.53) |
| Year of diagnosis | |
| 1973 to 1983 | 41 (16.60) |
| 1984 to 1993 | 32 (12.96) |
| 1994 to 2003 | 64 (25.91) |
| 2004 to 2014 | 110 (44.53) |
| Tumour sites | |
| Vertebral column | 8 (3.24) |
| Pelvis/sacrococcyx | 20 (8.10) |
| Limbs | 120 (48.58) |
| Head and base | 38 (15.38) |
| Other bones | 20 (8.10) |
| Extraskeletal sites | 41 (16.60) |
| Histological subtype | |
| Chondrosarcoma, not otherwise specified | 151 (61.13) |
| Juxtacortical | 7 (2.83) |
| Chondroblastoma, malignant | 25 (10.12) |
| Myxoid | 21 (8.50) |
| Mesenchymal | 39 (15.79) |
| Clear cell | 2 (0.81) |
| Dedifferentiated | 2 (0.81) |
| Grade | |
| Low grade | 135 (47.04) |
| High grade | 46 (16.03) |
| Grade X | 106 (36.93) |
| Stage | |
| Localized | 131 (53.04) |
| Regional | 58 (23.48) |
| Distant | 29 (11.74) |
| Stage X | 29 (11.74) |
| Treatment | |
| Surgery | 213 (86.23) |
| Non-surgery | 34 (13.77) |
Unr, unreported; Grade X, unknown grade; Stage X, unknown stage
Paediatric versus adult patients
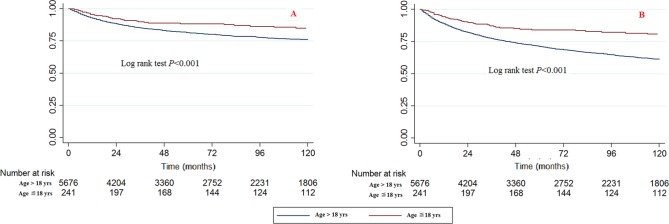
The paediatric patients had significantly better survival rates than the adult patients (Fig. 1). The bivariable and multivariable Cox proportional hazard regression models of CCSS and OS patients also showed the same results. When the age > 18 years category was used as a reference, the HRs (95% CI) of death caused by chondrosarcoma cancer in the 0 to 18 age category were 0.540 (0.378 to 0.772, p = 0.001) in the bivariable analysis and 0.449 (0.311 to 0.647, p < 0.001) in the multivariable analysis (Table 2). The HRs (95% CI) of death caused by all reasons in the 0 to 18 age category were 0.379 (0.285 to 0.503, p < 0.001) in the bivariable analysis and 0.345 (0.259 to 0.461, p < 0.001) in the multivariable analysis, respectively (Table 3).
Fig. 1.
Kaplan-Meier survival curves illustrating the chondrosarcoma cancer-specific survival (a) and overall survival (b) between paediatric and adult patients.
Table 2.
Bivariable and multivariable Cox analyses of chondrosarcoma cancer-specific survival
| Bivariable analysis | Multivariable analysis* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yrs) | ||||||
| > 18 | Reference | Reference | ||||
| 0-18 | 0.540 | 0.378 to 0.772 | 0.001 | 0.449 | 0.311 to 0.647 | < 0.001 |
| Gender | ||||||
| Female | Reference | Reference | ||||
| Male | 0.583 | 0.287 to 1.183 | 0.135 | 0.640 | 0.277 to 1.480 | 0.297 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 1.078 | 0.374 to 3.100 | 0.890 | 0.949 | 0.262 to 3.439 | 0.937 |
| Other/Unr | 0.566 | 0.134 to 2.389 | 0.438 | 0.664 | 0.134 to 3.296 | 0.616 |
| Year of diagnosis | ||||||
| 1973 to 1983 | Reference | Reference | ||||
| 1984 to 1993 | 0.473 | 0.167 to 1.344 | 0.160 | 0.363 | 0.091 to 1.451 | 0.152 |
| 1994 to 2003 | 0.390 | 0.160 to 0.955 | 0.039 | 0.304 | 0.089 to 1.039 | 0.058 |
| 2004 to 2014 | 0.246 | 0.091 to 0.661 | 0.005 | 0.219 | 0.054 to 0.882 | 0.033 |
| Tumour sites | ||||||
| Vertebral column | Reference | Reference | ||||
| Pelvis/sacrococcyx | 0.684 | 0.163 to 2.867 | 0.604 | 0.456 | 0 078 to 2.677 | 0.385 |
| Limbs | 0.129 | 0.034 to 0.479 | 0.002 | 0.170 | 0.035 to 0.833 | 0.029 |
| Head and base | 0.206 | 0.046 to 0.923 | 0.039 | 0.134 | 0.022 to 0.827 | 0.030 |
| Other bones | 0.492 | 0.117 to 2.065 | 0.333 | 0.192 | 0.032 to 1.145 | 0.070 |
| Extraskeletal sites | 0.251 | 0.060 to 1.053 | 0.059 | 0.139 | 0.028 to 0.696 | 0.016 |
| Histological subtype | ||||||
| Chondrosarcoma, not otherwise specified | Reference | Reference | ||||
| Juxtacortical | - | - | - | - | - | - |
| Chondroblastoma malignant | - | - | - | - | - | - |
| Myxoid | 2.543 | 1.013 to 6.382 | 0.047 | 4.023 | 1.136 to 14.252 | 0.031 |
| Mesenchymal | 1.033 | 0.385 to 2.770 | 0.948 | 1.130 | 0.283 to 4.510 | 0.863 |
| Clear cell | - | - | - | - | - | - |
| Dedifferentiated | 5.849 | 0.768 to 44.562 | 0.088 | 0.450 | 0.024 to 8.469 | 0.594 |
| Grade | ||||||
| Low grade | Reference | Reference | ||||
| High grade | 7.289 | 2.728 to 17.471 | < 0.001 | 4.333 | 1.274 to 14.743 | 0.019 |
| Grade X | 2.614 | 0.993 to 6.879 | 0.052 | 3.601 | 1.067 to 12.157 | 0.039 |
| Stage | ||||||
| Localized | Reference | Reference | ||||
| Regional | 3.485 | 1.326 to 9.156 | 0.011 | 2.955 | 0.967 to 9.031 | 0.057 |
| Distant | 10.920 | 4.294 to 27.767 | < 0.001 | 11.127 | 3.545 to 34.924 | < 0.001 |
| Stage X | 1.288 | 0.268 to 6.201 | 0.752 | 0.560 | 0.094 to 3.321 | 0.523 |
| Treatment | ||||||
| Non-surgery | Reference | Reference | ||||
| Surgery | 0.206 | 0.099 to 0.431 | < 0.001 | 0.180 | 0.067 to 0.484 | 0.001 |
adjusted by Cox regression model including the factors: gender, race, year of diagnosis, tumour sites, histological subtype, grade, stage and treatment
HR, hazard ratio; CI, confidence interval; Unr, unreported; Grade X, unknown grade; Stage X, unknown stage
Table 3.
Bivariable and multivariable Cox analyses of chondrosarcoma overall survival
| Bivariable analysis | Multivariable analysis* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yrs) | ||||||
| > 18 | Reference | Reference | ||||
| 0 to 18 | 0.379 | 0.285 to 0.503 | < 0.001 | 0.345 | 0.259 to 0.461 | < 0.001 |
| Gender | ||||||
| Female | Reference | Reference | ||||
| Male | 0.568 | 0.324 to 0.998 | 0.049 | 0.809 | 0.421 to 1.555 | 0.525 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 1.225 | 0.547 to 2.744 | 0.622 | 1.043 | 0.384 to 2.834 | 0.933 |
| Other/Unr | 0.522 | 0.161 to 1.690 | 0.278 | 0.663 | 0.189 to 2.322 | 0.521 |
| Year of diagnosis | ||||||
| 1973 to 1983 | Reference | Reference | ||||
| 1984 to 1993 | 0.436 | 0.183 to 1.038 | 0.061 | 0.375 | 0.127 to 1.106 | 0.076 |
| 1994 to 2003 | 0.407 | 0.190 to 0.873 | 0.021 | 0.407 | 0.149 to 1.109 | 0.079 |
| 2004 to 2014 | 0.317 | 0.143 to 0.703 | 0.005 | 0.331 | 0.120 to 0.917 | 0.033 |
| Tumour sites | ||||||
| Vertebral column | Reference | Reference | ||||
| Pelvis/sacrococcyx | 0.941 | 0.289 to 3.072 | 0.920 | 0.808 | 0.196 to 3.329 | 0.768 |
| Limbs | 0.143 | 0.047 to 0.438 | 0.001 | 0.146 | 0.039 to 0.552 | 0.005 |
| Head and base | 0.280 | 0.082 to 0.960 | 0.043 | 0.179 | 0.041 to 0.777 | 0.022 |
| Other bones | 0.572 | 0.171 to 1.910 | 0.364 | 0.310 | 0.073 to 1.322 | 0.114 |
| Extraskeletal sites | 0.287 | 0.084 to 0.982 | 0.047 | 0.164 | 0.042 to 0.631 | 0.009 |
| Histological subtype | ||||||
| Chondrosarcoma, not otherwise specified | Reference | Reference | ||||
| Juxtacortical | - | - | - | - | - | - |
| Chondroblastoma malignant | 0.447 | 0.107 to 1.872 | 0.270 | 0.508 | 0.094 to 2.737 | 0.430 |
| Myxoid | 2.408 | 1.095 to 5.298 | 0.029 | 3.053 | 1.078 to 8.645 | 0.036 |
| Mesenchymal | 1.056 | 0.460 to 2.425 | 0.897 | 1.258 | 0.392 to 4.040 | 0.699 |
| Clear cell | - | - | - | - | - | - |
| Dedifferentiated | 9.547 | 2.211 to 41.228 | 0.003 | 1.257 | 0.135 to 11.687 | 0.840 |
| Grade | ||||||
| Low grade | Reference | Reference | ||||
| High grade | 4.816 | 2.272 to 10.211 | < 0.001 | 3.244 | 1.289 to 8.165 | 0.012 |
| Grade X | 2.025 | 0.995 to 4.120 | 0.052 | 2.526 | 1.013 to 6.299 | 0.047 |
| Stage | ||||||
| Localized | Reference | Reference | ||||
| Regional | 3.202 | 1.514 to 6.773 | 0.002 | 3.135 | 1.329 to 7.396 | 0.009 |
| Distant | 8.683 | 4.048 to 18.627 | < 0.001 | 7.628 | 3.104 to 18.745 | < 0.001 |
| Stage X | 2.131 | 0.799 to 5.683 | 0.131 | 1.498 | 0.474 to 4.734 | 0.491 |
| Treatment | ||||||
| Non-surgery | Reference | Reference | ||||
| Surgery | 0.273 | 0.147 to 0.509 | < 0.001 | 0.343 | 0.153 to 0.769 | 0.009 |
adjusted by Cox regression model including the factors: gender, race, year of diagnosis, tumour sites, histological subtype, grade, stage and treatment
HR, hazard ratio; CI, confidence interval; Unr, unreported; Grade X, unknown grade; Stage X, unknown stage
Gender
No significant differences were observed between the male and female paediatric CCSS population when using the Kaplan-Meier method or the log rank test, but the log rank test for OS showed that p = 0.046 (Supplemental Fig. 1). The bivariable Cox proportional hazard regression models for CCSS found p = 0.049 between male and female. No significant differences were found with the multivariable Cox proportional hazard regression models of CCSS or the bivariable and multivariable Cox proportional hazard regression models of OS (Table 2 and Table 3).
Race
No significant differences were observed among races for CCSS and OS when using the Kaplan-Meier method and log rank test (Supplemental Fig. 2). The bivariable and multivariable Cox proportional hazard regression models for CCSS and OS also found no significant differences among races (Table 2 and Table 3).
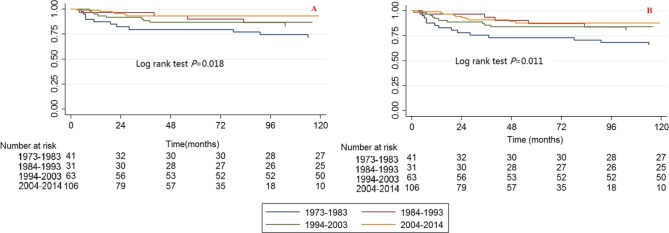
Year of diagnosis
Significant differences were observed among the year of diagnosis categories for CCSS and OS when using the Kaplan-Meier method and log rank test (Fig. 2). The 2004 to 2014 category had the best survival and the 1973 to 1983 category had the worst survival.
Fig. 2.
Kaplan-Meier survival curves illustrating the chondrosarcoma cancer-specific survival (a) and overall survival (b) among different categories of year of diagnosis.
The bivariable and multivariable Cox proportional hazard regression models for CCSS and OS found that the survival trends have improved throughout the years. When the 1973 to 1983 category was used as a reference, the HRs (95% CI) of death caused by chondrosarcoma cancer in the 1984 to 1993, 1994 to 2003 and 2004 to 2014 categories were 0.473 (0.167 to 1.344, p = 0.160), 0.390 (0.159 to 0.955, p = 0.039) and 0. 246 (0.091 to 0.661, p = 0.005), respectively, for the bivariable analysis, and they were 0.363 (0.091 to 1.451, p = 0.152), 0.304 (0.089 to 1.039, p = 0.058) and 0.219 (0.054 to 0.882, p = 0.033), respectively, for the multivariable analysis (Table 2). The HRs (95% CI) of death caused by all reasons in the 1984 to 1993, 1994 to 2003 and 2004 to 2014 categories were 0.436 (0.183 to 1.038, p = 0.061), 0.407 (0.190 to 0.873, p = 0.021) and 0.317 (0.143 to 0.703, p = 0.005), respectively, for the bivariable analysis, and they were 0.375 (0.127 to 1.106, p = 0.076), 0.407 (0.149 to 1.109, p = 0.079) and 0.331 (0.120 to 0.917, p = 0.033), respectively, for the multivariable analysis (Table 3).
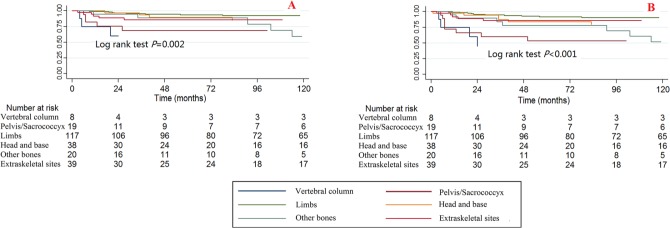
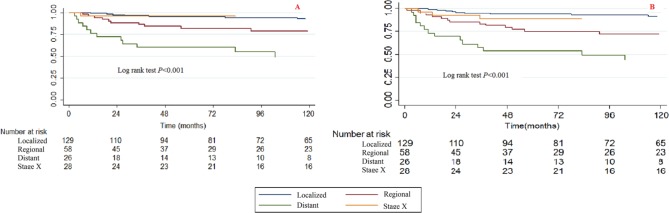
Tumour sites
Significant differences were observed among different tumour sites for CCSS and OS when using the Kaplan-Meier method and log rank test (Fig. 3). The tumour sites at the vertebral column and the pelvis/sacrococcyx had poorer survival rates, while those at the limbs, head and base had better survival rates.
Fig. 3.
Kaplan-Meier survival curves illustrating the chondrosarcoma cancer-specific survival (a) and overall survival (b) among different tumour sites.
The bivariable and multivariable Cox proportional hazard regression models for CCSS and OS found no significant differences in survival for patients with tumour sites at the vertebral column and pelvis/sacrococcyx, while the multivariable Cox proportional hazard regression models for OS found that patients with tumour sites at the limbs, head and base had significantly lower death risks than those with tumour sites at the vertebral column (Tables 2 and 3).
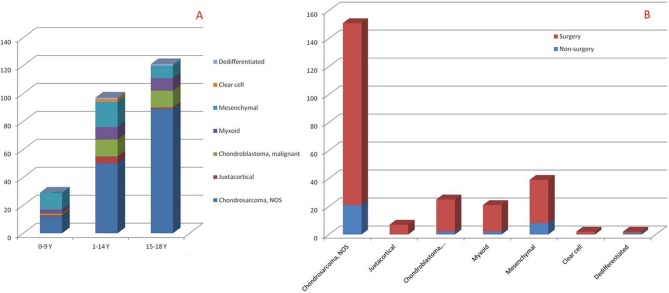
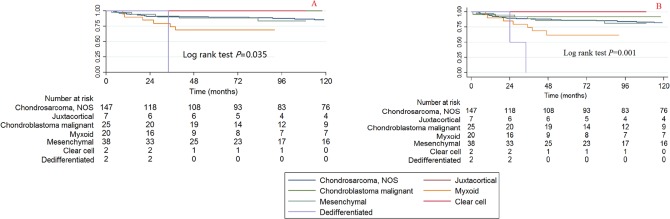
Histological subtypes
Since some of the histological subtypes had very small simple sizes, the HRs of some of the histological subtypes cannot be calculated (Tables 2 and 3). Therefore, we provide the summarized information of the histological subtypes. The number of patients with each histological subtype in each age category is summarized in Figure 4a and the surgical ratio of each histological subtype is summarized in Figure 4b. The Kaplan-Meier curve and log rank test was also performed; the log rank P value for CCSS is 0.035 and for OS is 0.001 (Fig. 5).
Fig. 4.
The summarized number of histological subtypes in each age categories and the surgical ratio of each histological subtype (NOS, not otherwise specified).
Fig. 5.
Kaplan-Meier survival curves illustrating the chondrosarcoma cancer-specific survival (a) and overall survival (b) among different histological subtypes (NOS, not otherwise specified).
The bivariable and multivariable Cox proportional hazard regression models for CCSS and OS found that the histological subtype of myxoid has the poorest survival rate (Tables 2 and 3) and the bivariable HRs (95% CI) of all reasons causing death in patients with the dedifferentiated histological subtype is 9.547 (2.211 to 41.228, p = 0.003). No significant difference was observed for the other histological subtypes.
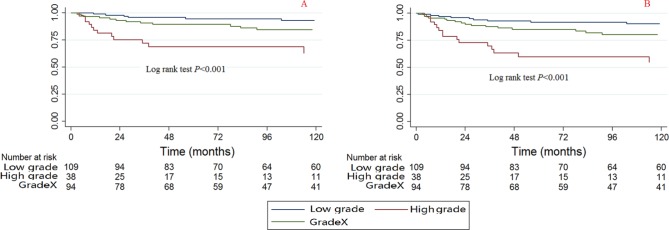
Grade
A significant difference was observed among the grades of CCSS and OS when using the Kaplan-Meier method and log rank test (Fig. 6). The high-grade tumours were associated with poorer survival compared with the low-grade tumours.
Fig. 6.
Kaplan-Meier survival curves illustrating the chondrosarcoma cancer-specific survival (a) and overall survival (b) among different grades (GradeX, unknown grade).
The bivariable and multivariable Cox proportional hazard regression models for CCSS and OS found that patients with high-grade tumours also have poorer survival (Tables 2 and 3); the HRs (95% CI) for bivariable CCSS, multivariable CCSS, bivariable OS and multivariable OS are 7.289 (2.728 to 17.471, p < 0.001), 4.333 (1.274 to 14.743, p = 0.019), 4.816 (2.272 to 10.211, p < 0.001) and 3.244 (1.289 to 8.165, p = 0.012), respectively.
Stage
A significant difference was observed among stages in CCSS and OS when using the Kaplan-Meier method and the log rank test (Fig. 7). Patients with a localized stage tumour had the best survival and those with a distant category had the worst survival.
Fig. 7.
Kaplan-Meier survival curves illustrating the chondrosarcoma cancer-specific survival (a) and overall survival (b) among different stages (StageX, unknown stage).
The bivariable and multivariable Cox proportional hazard regression models for CCSS and OS found that patients with higher stage tumours have poorer survival rates. When we used the localized stage as a reference, the regional stage HRs (95% CI) for bivariable CCSS, multivariable CCSS, bivariable OS and multivariable OS were 3.485 (1.326 to 9.156, p = 0.011), 2.955 (0.967 to 9.031, p = 0.057), 3.202 (1.514 to 6.773, p = 0.002) and 3.135 (1.329 to 7.396, p = 0.009), respectively. The distant stage HRs (95% CI) of bivariable CCSS, multivariable CCSS, bivariable OS and multivariable OS were 10.920 (4.294 to 27.767, p < 0.001), 11.127 (3.545 to 34.924, p < 0.001), 8.683 (4.048 to 18.627, p < 0.001) and 7.628 (3.104 to 18.745, p < 0.001), respectively.
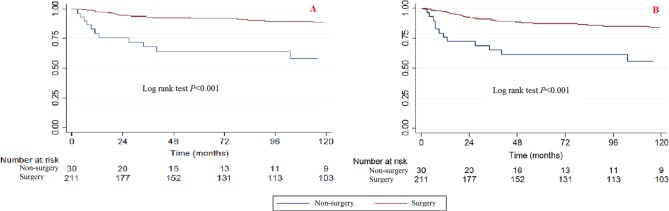
Treatment
Significant differences were observed between surgery and non-surgery patients when using the Kaplan-Meier method and log rank test (Fig. 8). Patients who had received surgery had better survival rates than those without surgery.
Fig. 8.
Kaplan-Meier survival curves illustrating the chondrosarcoma cancer-specific survival (a) and overall survival (b) between patients with or without surgery.
The bivariable and multivariable Cox proportional hazard regression models of CCSS and OS found that surgery can also improve the survival rate (Tables 2 and 3). When the non-surgery category was used as a reference, the surgery HRs (95% CI) for bivariable CCSS, multivariable CCSS, bivariable OS and multivariable OS were 0.206 (0.099 to 0.4311, p < 0.001), 0.180 (0.067 to 0.484, p = 0.001), 0.273 (0.147 to 0.509, p < 0.001) and 0.343 (0.153 to 0.769, p = 0.009), respectively.
Sub-analysis of the chondrosarcoma NOS subtype
There were 151 cases of patients with the chondrosarcoma NOS subtype, and the sub-analysis found that most of the results of the analysis of this chondrosarcoma NOS subtype (Supplemental Tables 1 and 2) were similar to the results of total chondrosarcoma (Tables 2 and 3); however, some of the multivariable analyses of “treatment” and “year of diagnosis” still did not show a significant difference.
Discussion
Chondrosarcoma is a rare histological entity arising both in the bones and in the soft tissues of the paediatric population.27,28 Bishop et al9 retrospectively reviewed 12 paediatric chondrosarcoma patients (aged from 1.3 to 19.7 years) that were diagnosed at their medical centre from 01 January 1990 to 30 May 2014. With a median follow-up of 4.8 years, they found that the five-year disease-free survival and OS rates were 68.2% and 88.9%, respectively. Dantonello et al29 reported that in 15 chondrosarcoma patients with a median age of 16.6 years (1 to 25), the median follow-up at 9.6 years found that the ten-year event-free and OS rates were 53% and 67%, respectively. Most of the other studies on paediatric chondrosarcoma are case reports.11-17 The limited number of patients means that the prognostic factors and survival outcomes cannot be measured.
Giuffrida et al5 reported that stage was an independent prognostic factor for survival outcomes of adult patients with chondrosarcoma. In our present study, we also found that the year of diagnosis is an independent prognostic factor: the survival rate increases with the years, the 2004 to 2014 category had the best survival and the 1973 to 1983 category had the worst survival. Several reasons may contribute to this outcome, including the advancement of therapeutic techniques (surgical strategy, adjuvant treatments of radiotherapy and chemotherapy and comprehensive care) that have been developed from 1973 to the present,30 and it is possible that the time of follow-up also influences the results.
Additionally, we found that patients with tumours in the vertebral column and pelvis/sacrococcyx had poorer survival outcomes than those with tumours located in the limbs and other bones. We propose that it is more difficult to surgically remove tumours at the spine and pelvis, which may decrease the survival rates in these regions.3 Recently, particle beam therapy has been developed and used for pelvic chondrosarcoma, and it may be an acceptable alternative treatment,31 especially for patients with non-resectable or incompletely resected tumours.32
There are seven histological subtypes of chondrosarcoma, including chondrosarcoma (NOS), juxtacortical, chondroblastoma malignant, myxoid chondrosarcoma and mesenchymal chondrosarcoma. Some subtypes of chondrosarcoma may respond to the different adjuvant treatments (for example, mesenchymal chondrosarcoma is sensitive to chemotherapy), but the SEER database of 1973 to 2014 did not provide any information about adjuvant treatments; therefore, we cannot provide information on whether adjuvant treatments may improve the survival rates of patients with chondrosarcoma. Since the chondrosarcoma (NOS) subtype consisted of 151 cases, we conducted a sub-analysis of chondrosarcoma (NOS) patients. While most results from this subtype were similar to the results of total chondrosarcoma, some multivariable analyses of ‘treatment’ and ‘year of diagnosis’ still did not show any significant differences, so it may be that the sample size of 151 cases is still not large enough for the analysis of seven variables. Some other histological subtypes also have very small sample sizes in our study (the juxtacortical, chondroblastoma, malignant, myxoid, mesenchymal, clear cell and dedifferentiated subtypes only had seven, 25, 21, 39, two and two cases, respectively); therefore, the HRs of some histological subtypes cannot be calculated.
The different histological subtypes may have variable biological and clinical behaviours. We found that patients with the myxoid chondrosarcoma histological subtype have poorer survival than those with the chondrosarcoma (NOS) subtype. Bumpass et al33 conducted a review of the literature in 2011 and found from the follow-up data of 26 patients (after a mean period of 49 months) that the rate of local recurrence was 42% and that of metastases was 31%. Mesenchymal chondrosarcoma is similar to Ewing’s tumour,34 and it has historically been considered to be associated with a poor prognosis; however, recent modern therapies have significantly improved patient survival.29,35
The grade in the SEER database is in accordance with the ICD-O-3 edition, which included four grades: well-differentiated (Grade I), moderately differentiated (Grade II), poorly-differentiated (Grade III) and undifferentiated (anaplastic, Grade IV). Referring to Giuffrida et al5 and Chen et al,25 we reclassified tumours into two grades (low grade: ICD-O-3 Grades I and II and high grade: ICDO-3 Grades III and III) in the present study. The high-grade patients had poorer outcomes than the low-grade patients, and the results are consistent with those of the adults.5,26
Surgical resection is the mainstream therapeutic method for chondrosarcoma, and in non-paediatric populations, surgery can significantly improve the survival rate for chondrosarcoma.3 The paediatric outcomes of our present study are consistent with the non-paediatric ones; both suggest a survival benefit from surgery.
The strengths of our present study are as follows: 1) this is the largest paediatric chondrosarcoma study that includes both the demographic and survival information;36,37 2) we performed analyses of both CCSS and OS as well as bivariable analyses and multivariable analyses, and most of the outcomes of the prognostic factors consistently show significant differences at all of these four analyses; and 3) the multivariable Cox hazard regression model includes age at diagnosis, race, gender, year of diagnosis, tumour sites, histological subtypes, grade stages and treatments.
There are some limitations to our present study. This is a retrospective study, and the SEER database has its natural drawbacks in that it does not contain some of the detailed information on patient comorbidities and adjuvant treatments, it does not include surgical information of intralesional, marginal and wide margin tumours and a central pathology review is not available. The study is an observational study, but it is not a clinical controlled study, so it may have some cofounders that cannot be included; the present update of the SEER database did not have information on radiotherapy, although patients with the chondrosarcoma NOS histological subtype are resistant to radiation.3,38 The mesenchymal chondrosarcoma and chondrosarcoma tumours located at the base of the skull may show favourable results after radiotherapy,39,40 but this cannot be proven by an analysis from the present SEER database.
Conclusion
Paediatric chondrosarcoma patients have better survival rates than the adults. The year of diagnosis, tumour sites, tumour histological subtype, grade, stage and whether patients received surgery are independent prognostic factors for the survival rates of paediatric chondrosarcoma patients. Patients with diagnoses at a younger age, tumour site at the vertebral column and pelvis/sacrococcyx, myxoid variants, high grade, distant stage, and who did not receive surgery have poorer prognoses than those who were diagnosed at an older age or who had tumour site at the limbs, head and base, chondrosarcoma not otherwise specified, localized stage and who had received surgery.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Compliance with ethical standards
Funding statement
This work was funded by the Wenzhou Municipal Science and Technology Bureau (Y20170389), Zhejiang Provincial Natural Science Foundation of China (LY14H060008), Zhejiang Provincial Medical Technology Foundation of China (2018KY129), Wenzhou leading talent innovative project (RX2016004) and National Natural Science Foundation of China (81501933, 81572214). The funders had no role in the design, execution or writing of the study.
Ethical statement
Ethical approval: We submitted the data agreement form to the Surveillance, Epidemiology, and End Results administration and received the acceptance of the agreement. The database uses publicly available information without personal identifiers; therefore, no additional Institutional Review Board approval is required in this study.
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent: Not required for this work.
ICMJE Conflict of interest statement
There are no conflicts of interest to declare for any of the authors.
Author Contributions
AMW, GL and HX designed the study.
AMW, GL, JWZ, CHC, and DC developed and tested the data collection forms.
AMW, GL, SRS, YSW, NFT and ZKL acquired the data.
AMW, GL, ZGQ, JGZ, BW, and WLF conducted the analysis and interpreted the data.
AMW, GL and HX drafted the manuscript.
All authors critically revised the manuscript.
References
- 1.Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. . The clinical approach towards chondrosarcoma. Oncologist 2008;13:320-329. [DOI] [PubMed] [Google Scholar]
- 2.Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am 2009;40:21-36. [DOI] [PubMed] [Google Scholar]
- 3.Arshi A, Sharim J, Park DY, et al. . Chondrosarcoma of the osseous spine: an analysis of epidemiology, patient outcomes, and prognostic factors using the SEER Registry from 1973 to 2012. Spine 2017;42:644-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strike SA, McCarthy EF. Chondrosarcoma of the spine: a series of 16 cases and a review of the literature. Iowa Orthop J 2011;31:154-159. [PMC free article] [PubMed] [Google Scholar]
- 5.Giuffrida AY, Burgueno JE, Koniaris LG, et al. . Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg [Am] 2009;91-A:1063-1072. [DOI] [PubMed] [Google Scholar]
- 6.Duchman KR, Lynch CF, Buckwalter JA, Miller BJ. Estimated cause-specific survival continues to improve over time in patients with chondrosarcoma. Clin Orthop Relat Res 2014;472:2516-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosier SM, Patel T, Strenge K, Mosier AD. Chondrosarcoma in childhood: the radiologic and clinical conundrum. J Radiol Case Rep 2012;6-12:32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young CL, Sim FH, Unni KK, McLeod RA. Chondrosarcoma of bone in children. Cancer 1990;66:1641-1648. [DOI] [PubMed] [Google Scholar]
- 9.Bishop MW, Somerville JM, Bahrami A, et al. . Mesenchymal chondrosarcoma in children and young adults: a single institution retrospective review. Sarcoma 2015;2015:608279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sardi I, Massimino M, Genitori L, et al. . Intracranial mesenchymal chondrosarcoma: report of two pediatric cases. Pediatr Blood Cancer 2011;56:685-686. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara N, Tomita K, Murakami H, et al. . Total excision of a recurrent chondrosarcoma of the thoracic spine: a case report of a seven-year-old boy with fifteen years follow-up. Spine 2010;35:E481-E487. [DOI] [PubMed] [Google Scholar]
- 12.Li Y-H Yao X-H. Primary intradural mesenchymal chondrosarcoma of the spine in a child. Pediatr Radiol 2007;37:1155-1158. [DOI] [PubMed] [Google Scholar]
- 13.Nimonkar P, Bhola N, Jadhav A, et al. . Myxoid chondrosarcoma of maxilla in a pediatric patient: a rare case report. Case Rep Oncol Med 2016;2016:5419737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zibis AH, Shrader MW, Segal LS. Case report: Mesenchymal chondrosarcoma of the lumbar spine in a child. Clin Orthop Relat Res 2010;468:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bawa HS, Moore DD, Pelayo JC, et al. . Pediatric chondrosarcoma of the sternum resected with thorascopic assistance. Open Orthop J 2017;11:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Küpeli S, Varan A, Gedikoğlu G, Büyükpamukçu M. Sacral mesenchymal chondrosarcoma in childhood: a case report and review of the literature. Pediatr Hematol Oncol 2010;27:564-573. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurthy A, Gowthaman S, Majhi U. Paediatric chondrosarcoma of the sinonasal region. J Cancer Res Ther 2013;9:163-164. [DOI] [PubMed] [Google Scholar]
- 18.Davenport JR, Vo KT, Goldsby R, West DC, DuBois SG. Conditional survival and predictors of late death in patients with Ewing sarcoma. Pediatr Blood Cancer 2016;63:1091-1095. [DOI] [PubMed] [Google Scholar]
- 19.Karski EE, McIlvaine E, Segal MR, et al. . Identification of discrete prognostic groups in Ewing sarcoma. Pediatr Blood Cancer 2016;63:47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.No authors listed. SEER. https://seer.cancer.gov (date last accessed 27November2018).
- 21.von Elm E, Altman DG, Egger M, et al. . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-1457. [DOI] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.No authors listed. SEER. https://seer.cancer.gov/icd-o-3/ (date last accessed 28November2018).
- 24.Fritz AG. Has there been a real drop in the number of expected cancer cases in the United States? Top Health Inf Manage 1997;17:15-28. [PubMed] [Google Scholar]
- 25.Chen D, Chen CH, Zhang LL, et al. . Chondrosarcoma of the osseous spine treated by surgery with or without radiotherapy: a propensity score matched and grade/stage-stratified study. Clin Spine Surg 2018;31:E310-E316. [DOI] [PubMed] [Google Scholar]
- 26.Chen C-H, Chen D, Lin Z-K, et al. . Prognostic factors and outcomes of osseous chondrosarcoma after surgery: the 2004–2014 Surveillance, Epidemiology, and End Results database study. Transl Cancer Res 2018;7:341-346. [Google Scholar]
- 27.Davis EJ, Wu YM, Robinson D, et al. . Next generation sequencing of extraskeletal myxoid chondrosarcoma. Oncotarget 2017;8:21770-21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krejci P. Putting the brakes on chondrosarcoma. Oncotarget 2015;6:24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dantonello TM, Int‐Veen C, Leuschner I, et al. . Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 2008;112:2424-2431. [DOI] [PubMed] [Google Scholar]
- 30.Zeng C, Wen W, Morgans AK, et al. . Disparities by race, age, and sex in the improvement of survival for major cancers: results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA oncology 2015;1:88-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Outani H, Hamada K, Imura Y, et al. . Comparison of clinical and functional outcome between surgical treatment and carbon ion radiotherapy for pelvic chondrosarcoma. Int J Clin Oncol 2016;21-1:186-193. [DOI] [PubMed] [Google Scholar]
- 32.Demizu Y, Jin D, Sulaiman NS, et al. . Particle therapy using protons or carbon ions for unresectable or incompletely resected bone and soft tissue sarcomas of the pelvis. Int J Radiat Oncol Biol Phys 2017;98:367-374. [DOI] [PubMed] [Google Scholar]
- 33.Bumpass DB, Kyriakos M, Rubin DA, Manske PR, Goldfarb CA. Myxoid chondrosarcoma of the phalanx with an EWS translocation: a case report and review of the literature. J Bone Joint Surg [Am] 2011;93-A:e23. [DOI] [PubMed] [Google Scholar]
- 34.Lee AF, Hayes MM, Lebrun D, et al. . FLI-1 distinguishes Ewing sarcoma from small cell osteosarcoma and mesenchymal chondrosarcoma. Appl Immunohistochem Mol Morphol 2011;19:233-238. [DOI] [PubMed] [Google Scholar]
- 35.Riedel RF, Larrier N, Dodd L, et al. . The clinical management of chondrosarcoma. Curr Treat Options Oncol 2009;10:94-106. [DOI] [PubMed] [Google Scholar]
- 36.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care 2002;40:19-25. [DOI] [PubMed] [Google Scholar]
- 37.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:3-18. [DOI] [PubMed] [Google Scholar]
- 38.Moussavi-Harami F, Mollano A, Martin JA, et al. . Intrinsic radiation resistance in human chondrosarcoma cells. Biochem Biophys Res Commun 2006;346:379-385. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi S, Weiss I, Lin PP, Huh WW, Lewis VO. Radiation therapy is associated with fewer recurrences in mesenchymal chondrosarcoma. Clin Orthop Relat Res 2014;472:856-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahgal A, Chan MW, Atenafu EG, et al. . Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: preliminary outcomes. Neuro Oncol 2015;17:889-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.