Abstract
Purpose
The paediatric sickle cell disease (SCD) osteomyelitis (OM) incidence is 0.3% to 12%. Differentiating vaso-occlusive crises (VOC) from OM is a diagnostic challenge, with limited evidence guiding management. We present a 15-year review of a paediatric sickle cell cohort. We aim to identify OM incidence and provide a management protocol for these children presenting with bone pain.
Methods
A prospective database of children with haemoglobinopathies (2002 to 2017) was analyzed for temperature, C-reactive protein (CRP) and white cell count (WCC) on admission as well as imaging, treatment and cultures. OM diagnosis was supported by imaging and blood cultures. VOC was defined as bone pain that improved without antibiotics.
Results
Over 15 years, 96 children with SCD presented 358 times to hospital. Empirical antibiotics were given in 308 presentations. There were five cases of OM (1.4%); two acute and three chronic. In all, 50 presentations of VOC were identified. No significant differences in age were noted between the OM and VOC group. Temperature and CRP were significantly elevated in the OM group with no significant difference in WCC. Cultures were only positive in the chronic OM admissions. There were no cases of septic arthritis. No surgical intervention was required.
Conclusion
In children with SCD presenting with persistent bone pain, fever, elevated CRP and WCC, OM should be suspected and prompt antibiotic treatment started. Our treatment pathway was successful avoiding OM in 98.6% and septic arthritis in 100%. Further research on novel biological markers distinguishing OM from VOC should be investigated.
Level of Evidence
III
Keywords: sickle cell disease, vaso-occlusive crisis, osteomyelitis
Introduction
Sickle cell disease (SCD) is an autosomal recessive haemoglobinopathy causing a chronic haemolytic anaemia. It is one of the most common severe monogenic disorders in the world.1-3 The prevalence of SCD is highest in sub-Saharan Africa, where it is estimated to affect almost 1% of children born each year.1,4 Although the incidence in Europe is significantly lower, prevalence of SCD has been steadily increasing due to migration. Subsequently, new-born screening was introduced in the United Kingdom in 2006.5-7
In SCD, red blood cells form abnormal, rigid, crescent-shaped sickle cells. This causes occlusion of the microvascular circulation and subsequent tissue hypoxia. Hypoxia of tissues causes a secondary inflammatory reaction, resulting in increased intramedullary pressure and bone pain.1,8
Individuals with SCD are susceptible to a variety of osteoarticular complications throughout their life. These include painful vaso-occlusive crises (VOC), dactylitis, osteomyelitis (OM), avascular necrosis and septic arthritis.9,10 Due to the combination of ischaemia and impaired immunity, children with SCD are more susceptible to bacterial infections affecting bones and joints, causing OM and septic arthritis.11-14 Salmonella and Staphylococcus aureus species are reported as the most common causative organisms of OM in SCD.9,12,13,15 There is limited published data on the incidence and prevalence of OM within the paediatric SCD population in Europe and North America. The reported incidence of OM ranges from 0.36% to 3% across adults and children with SCD and other haemoglobinopathies.10,11,16 The incidence of septic arthritis is reported to be 0.2% in children with SCD.10
Children with SCD frequently experience bone and joint pain and VOCs have been shown to be 50-times more common than OM in children with SCD.17 However, the two disease processes may initially present with similar symptoms and signs, including bone pain, fever and restriction in joint movement.18,19
Early recognition and prompt treatment of OM is essential to minimize serious complications. However, differentiating between VOC and early OM is a diagnostic challenge and there are limited validated diagnostic tools to help make this distinction. Unwarranted antibiotic treatment may result in morbidity, antibiotic resistance and increased cost.18 However, delayed antibiotic treatment in OM has been shown to be a risk factor for relapse, slow recovery and poor outcome.20-22
Multiple diagnostic algorithms have been proposed to guide practice. Some include clinical findings and biochemical parameters alone18,21 whilst others incorporate imaging such as ultrasound19 or magnetic resonance imaging (MRI),23,24 to improve decision-making.
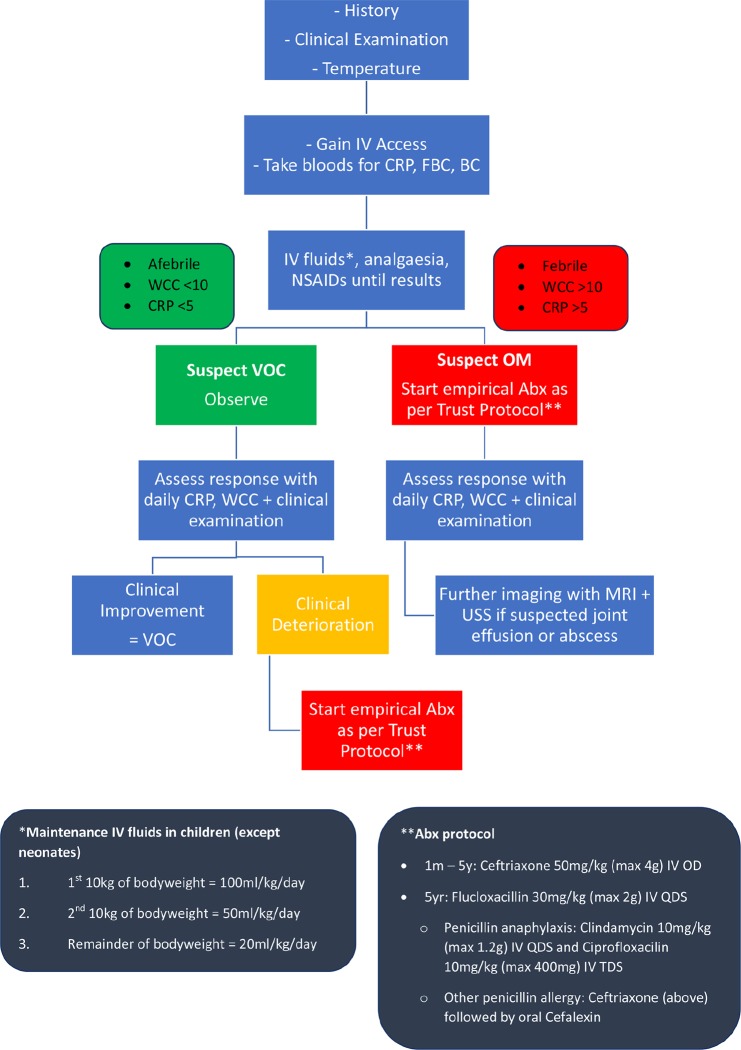
The modern approach to children with SCD, presenting with bone pain, fever and raised inflammatory markers, is a low threshold for empirical antibiotic treatment whilst awaiting confirmation with positive culture results or findings on imaging.10,25 This has led the pathway adopted at our institution since 2002. In this paper, we aim to provide a pragmatic protocol of assessment and management of children with SCD presenting with bone pain combining clinical, biochemical, microbiological and radiological findings (Fig. 1).
Fig. 1.
Hospital protocol of assessment and management of children with sickle cell disease (SCD) presenting with bone pain (FBC, full blood count; BC, blood culture; WCC, white cell count; NSAIDS, non-steroidal anti-inflammatory drugs; OM, osteomyelitis; VOC, vaso-occlusive crisis; Abx, antibiotics; USS, ultrasound scan; IV, intravenous; TDS, three times a day; OD, oral dose; QDS, once a day).
This study aims to identify the incidence of OM in a large cohort of children with SCD that were treated according to our hospital protocol. In addition, the study aims to provide a clinical pathway for managing children with SCD and bone pain.
Materials and methods
A prospective database of paediatric patients with haemoglobinopathies under the care of our regional referral centre was created in 2002. This is a referral centre for all sickle cell patients in the South West London region. Study inclusion criteria were all patients with a confirmed diagnosis of homozygous SCD up to and including the age of 18 years at the time of presentation. Individuals with other haemoglobinopathies were excluded.
The medical notes of all patients meeting the inclusion criteria were reviewed to identify each acute presentation to hospital with bone pain. The data extracted included: 1) gender and age at presentation; 2) temperature, C-reactive protein (CRP) and white cell count (WCC) at presentation; and 3) blood culture results, radiographic imaging and any surgical interventions.
Cases of OM were confirmed radiologically with MRI and or with positive blood cultures.
The VOC cohort was defined as: bone pain in the absence of trauma that improved with conservative treatment as an inpatient according to our Trust protocol without administration of antibiotics (Fig. 1).
Our hospital protocol of empirical antibiotic treatment for suspected OM consisted of intravenous administration of antibiotics for two weeks followed by an oral course of four weeks pending clinical improvement. In chronic OM, treatment began with six weeks of intravenous antibiotics. Children under five years old received ceftriaxone and children over five years old received flucloxacillin (Fig. 1). IV treatment can be continued in the community with aid of the outpatient parenteral antimicrobial therapy service with on-going clinical monitoring and treatment course dependent on clinical improvement.
The primary outcome was the rate of identified cases of OM and septic arthritis under the current treatment protocol in children presenting with bone pain. The secondary outcomes were the difference in WCC, temperature and CRP between the two groups at presentation.
Local audit approval was gained for the purpose of the study.
Statistical analysis
Non-parametric statistics were suitable for analysis. Normality was assessed by the Kolmogorov-Smirnov test. Descriptive statistics were used for calculating of frequencies, sd and the mean in the normally distributed cohort. For skewed variables the interquartile range and the median were utilized. The Mann-Whitney U test was used to compare the continuous variables between the two groups and the Pearson chi-square was used for the non-continuous variable. Significance was set at p < 0.05. Analyses were performed using the IBM SPSS Statistics for Windows, Version 23.0., IBM Corp., Armonk, New York.
Results
The 15-year database from October 2002 to December 2017 comprised of 177 children with haemoglobinopathies. In all, 96 patients had homozygous SCD and met the inclusion criteria. Children with SCD presented a total of 358 times with bone pain during the study period. In all, 97% of children with SCD presented at least once during the study period, with a mean of 3.8 presentations per patient.
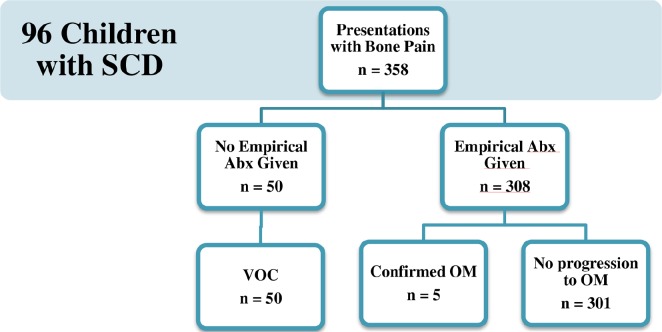
Of the 358 presentations with bone pain, empirical antibiotics were given in 308 cases. There were 50 cases that improved without administration of empirical antibiotics and five cases of OM confirmed by cultures or imaging (1.4% of total presentations) (Fig. 2). There were two acute cases of OM that involved the humerus and pelvis, respectively, and three presentations of a flare up of a known chronic tibial OM. There were no cases of septic arthritis. Neither of the two acute cases went on to require surgical intervention whereas the chronic case had been operated on in a different country before data collection began. Blood cultures were positive in two presentations in the child with chronic OM (Pseudomonas stutzeri and Staphylococcus aureus).
Fig. 2.
Flow diagram depicting the vaso-occlusive crisis (VOC) and osteomyelitis (OM) cohorts (SCD, sickle cell disease; Abx, antibiotics).
The two acute cases responded well to antibiotic treatment with no complications. The chronic case also improved with no immediate complications. All cases completed six weeks of antibiotic treatment.
The comparison of WCC, CRP and temperature of the OM and VOC groups is presented in Table 1. Pyrexia occurred significantly more frequently in the OM group, presenting with a median temperature of 37.8°C compared with 36.9°C in the VOC group. CRP was significantly higher in the OM group (51.4 mg/dL versus 7.8 mg/dL). White blood cell count was also higher in the OM group (15.8 versus 14.4), however, the difference did not reach statistical significance.
Table 1.
Comparison of white cell count, C-reactive protein and temperature between osteomyelitis and vaso-occlusive crises groups. Data presented as median (interquartile range)
| Variable | Osteomyelitis group (n = 5) | Vaso-occlusive crisis group (n = 50) | p-value |
|---|---|---|---|
| Age (yrs) | 10 | 8 | 0.559 |
| Gender (M:F) | 0:5 | 25:25 | 0.032 |
| White cell count (109/L) | 15.8 (3.25) | 14.4 (7.10) | 0.45 |
| C-reactive protein (mg/L) | 51.4 (52.40) | 7.8 (22.63) | 0.007 |
| Temperature (°C) | 37.8 (1) | 36.9 sd 0.5 | 0.010 |
There was no statistically significant difference in patient age at presentation between the OM and VOC cohorts (median age ten years versus eight years). All five OM cases occurred in female patients, and 25 of the 50 VOC cases were female.
Discussion
In this study, early administration of empirical antibiotics to children with SCD who presented with bone pain resulted in a very low incidence of OM and no cases of septic arthritis. The results support the current protocol of treatment developed in our hospital based on years of experience in treating SCD that was successful in prevention of septic arthritis in 100% and OM in 98.6% of presentations with bone pain.
There is limited published data on the incidence and prevalence of OM and septic arthritis within the paediatric SCD population in Europe and northern America. Chambers et al10 have focused specifically on osteoarticular infections in children with SCD and reported a 0.5% incidence of OM and 0.2% incidence of septic arthritis across 22 years in their population in Georgia, North America. Piehl et al11 studied the incidence of OM in both adults and children with SCD and reported a 0.36% annual incidence across 13 years in South Carolina, North America. Telfer et al16 analyzed clinical outcomes in a cohort of 250 children with a variety of haemoglobinopathies, identifying eight episodes of OM in seven children across 2158 patient years of observation.16 This last study was based in East London, making it likely to be an equivalent population to our study.
In this study, there were five cases of confirmed OM in three children among a cohort of 96 children with SCD during a 15-year period (1.4% of presentations with bone pain). Of these five cases, there were two acute cases affecting the pelvis and humerus which were diagnosed on clinical findings and MRI. No positive blood cultures were recorded in these cases. The three presentations of one child with chronic tibial OM were confirmed with positive cultures (Pseudomonas stutzeri and Staphylococcus aureus) and MRI imaging.
The low diagnostic yield of blood cultures among our cohort is unsurprising given it has been reported that a causative pathogen may not be identified in 33% to 55% of cases of OM.26-28 Given the small cohort size and only two positive cultures in one child, it is unsurprising that there were no cases of Salmonella species, which has been the most prevalent causative organism of OM in SCD reported in the literature.9,12,13 No bone biopsies were taken in our cohort. Although bone biopsies were once heralded as the ‘benchmark’ diagnostic tool, they have recently been shown to be similarly low-yield to blood cultures and not without procedural risks.29,30
In this study, there were 50 cases of VOC which were defined as bone pain that improved without empirical antibiotics. Comparing the VOC cases with OM cases, a non-significant increase in WCC was seen on admission in the OM group (median 15.8 versus 14.4 × 109/L). In contrast, CRP and temperature were both significantly elevated in the OM group (median CRP 51.4 mg/dL versus 7.8 mg/dL, median temperature 37.8°C versus 36.9°C).
In clinical practice, a VOC is a diagnosis of exclusion in children with SCD who present with localized bone pain that improves with fluids, analgesia and anti-inflammatory drugs as per the treatment protocol in Figure 1.4,17-19,31 From this protocol one can conclude that an afebrile patient with a low CRP is treated as a VOC with continued close assessment for any change. Differentiating between VOC and early OM is a diagnostic challenge and imaging techniques are often used to aid clinical diagnosis. MRI is a useful adjunct to clinical findings and has been shown to be highly sensitive in diagnosing OM.23,24,32,33 However, differentiation between bony infarction, and acute and chronic OM on MRI is extremely difficult.18,31,34 Although MRI is readily available at our tertiary centre, its specificity is low and must be interpreted alongside clinical and biochemical findings. The cost of MRI use should also be weighed against the potential diagnostic yield in these cases.
Combining imaging and biological markers can aid in differentiating a VOC from OM. Although CRP was significantly elevated in the OM group in our study, it is limited as it is a non-specific marker of inflammation and/or infection.
There is emerging evidence that pro- and anti-inflammatory cytokines may provide novel biological markers to aid diagnosis. Altered cytokine expression in OM in SCD has been suggested and the role of interleukin (IL)-10, a Th2 cytokine demonstrating anti-inflammatory and immunomodulatory properties,35,36 in the pathogenesis of OM has further been studied. Decreased IL-10 values have been associated with an increased risk of OM since lower levels of the anti-inflammatory cytokine were reportedly noted in affected patients.37 Future prospective studies could elaborate further on the link between IL-10 and OM in SCD and explore its role in OM diagnosis and follow-up.
This study gives some insight into the morbidity associated with SCD in children. In all, 97% of children with SCD presented at least once during the 15-year study period, with 3.8 presentations per patient on average. The musculoskeletal complications affecting children with SCD contribute to significant morbidity throughout childhood and adulthood.9,10,31 Painful crises result in missed school days, disruption to social activities and may affect mood and quality of life.38-40 Further research in this area is needed.
Strengths of this study include the large patient cohort over a 15-year period. Patients were from a broad geographic area of South West London, and hence likely representative of the Greater London paediatric SCD population. Electronic patient records meant availability and accuracy of historical blood test results, cultures and imaging results.
The study is limited by the small number of cases of OM identified. Although it is reassuring that our current protocol results in a low infection rate, it limits the comparison between the OM and VOC group. Children who presented with bone pain and received early antibiotics may have had VOC or indeed an emerging OM that was halted by the early administration of empirical antibiotics. We can only confirm that the known combined clinical, biochemical and radiological parameters may be suggestive of OM rather than suggest any novel individual diagnostic tool.
Although the potential impact of bone and joint infections seem to outweigh the impact of over treatment with antibiotics, future prospective studies could shed a light on the adverse outcomes of over-use of empirical antibiotics – leading to increased morbidity, antibiotic resistance and healthcare cost. Such future studies could provide a clearer insight to clinical parameters that differentiate between VOC and OM and aid healthcare professionals in distinguishing between these two clinical entities.
Conclusion
This study reaffirms that clinicians should suspect OM in SCD children presenting with bone pain that is persistent and accompanied by fever and raised inflammatory markers. Early empirical antibiotics resulted in a low rate of OM and no septic arthritis.
The musculoskeletal manifestations of SCD continue to be a cause of significant morbidity for children, which may affect their education attainment, physical and mental health. Prospective work is needed to explore this area and to identify predicative factors to aid clinicians in making the challenging differentiation between OM and VOC.
Open access
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Acknowledgements
We would like to thank Dr Alison Thomas for contributing the database to this study.
Compliance with ethical standards
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Ethical statement
Ethical approval: Local hospital audit approval was gained for the purpose of the study. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent: Not required for this work.
ICMJE Conflict of interest statement
The authors declare no conflict of interest.
Author Contributions
AF: study design, data collection, data analysis and interpretation of results, wrote initial manuscript.
YG: conceptualised and designed study, wrote initial manuscript, reviewed and revised manuscript, interpretation of results.
KH, MPN, MW: designed data collection instruments, conducted, coordinated or supervised data collection, critically reviewed manuscript.
AY and DL: study design, reviewed and revised manuscript.
All authors approved the final manuscript as submitted.
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–2031. [DOI] [PubMed] [Google Scholar]
- 2.Schnog JB, Duits AJ, Muskiet FAJ, et al. Sickle cell disease; a general overview. Neth J Med 2004;62:364–374. [PubMed] [Google Scholar]
- 3.Ballas SK. Sickle cell disease: clinical management. Baillieres Clin Haematol 1998;11:185–214. [DOI] [PubMed] [Google Scholar]
- 4.Aliyu SU, Rufa’I AA, Saidu IA, Jajere AM. Musculoskeletal complications in sickle cell anemia patients: a ten-year retrospective review of hospital-based records (1991-2000) in two Nigerian hospitals. Int J Contemp Pediatr 2015:2;329–334. [Google Scholar]
- 5.Howard J, Davies SC. Sickle cell disease in North Europe. Scand J Clin Lab Invest 2007;67:27–38. [DOI] [PubMed] [Google Scholar]
- 6.Coppinger C. We’ve helped thousands of babies during 10 years of newborn screening for sickle cell disease - PHE screening. Public Health England Screening. 2016. https://phescreening.blog.gov.uk/2016/09/01/weve-helped-thousands-of-babies-during-10-years-of-newborn-screening-for-sickle-cell-disease/ (date last accessed 26 November 2018).
- 7.Bain BJ. Neonatal/newborn haemoglobinopathy screening in Europe and Africa. J Clin Pathol 2009;62:53–56. [DOI] [PubMed] [Google Scholar]
- 8.Quirolo K, Vichinsky E. Hemoglobin disorders. In Nelson Text Book of Paediatrics 17th ed. Philadephia: Saunders Company, 2004:1623–1634. [Google Scholar]
- 9.da Silva Junior GB, Daher Ede F, da Rocha FA. Osteoarticular involvement in sickle cell disease. Rev Bras Hematol Hemoter 2012;34:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers JB, Forsythe DA, Bertrand SL, Iwinski HJ, Steflik DE. Retrospective review of osteoarticular infections in a pediatric sickle cell age group. J Pediatr Orthop 2000;20:682–685. [DOI] [PubMed] [Google Scholar]
- 11.Piehl FC, Davis RJ, Prugh SI. Osteomyelitis in sickle cell disease. J Pediatr Orthop 1993;13:225–227. [PubMed] [Google Scholar]
- 12.Burnett MW, Bass JW, Cook BA. Etiology of osteomyelitis complicating sickle cell disease. Pediatrics 1998;101:296–297. [DOI] [PubMed] [Google Scholar]
- 13.Onwubalili JK. Sickle cell disease and infection. J Infect 1983;7:2–20. [DOI] [PubMed] [Google Scholar]
- 14.Mallouh A, Talab Y. Bone and joint infection in patients with sickle cell disease. J Pediatr Orthop 1985;5:158–162. [PubMed] [Google Scholar]
- 15.Yeo A, Ramachandran M. Acute haematogenous osteomyelitis in children. BMJ 2014;348:g66. [DOI] [PubMed] [Google Scholar]
- 16.Telfer P, Coen P, Chakravorty S, et al. Clinical outcomes in children with sickle cell disease living in England: a neonatal cohort in East London. Haematologica 2007;92:905–912. [DOI] [PubMed] [Google Scholar]
- 17.Keeley K, Buchanan GR. Acute infarction of long bones in children with sickle cell anemia. J Pediatr 1982;101:170–175. [DOI] [PubMed] [Google Scholar]
- 18.Wong AL, Sakamoto KM, Johnson EE. Differentiating osteomyelitis from bone infarction in sickle cell disease. Pediatr Emerg Care 2001;17:60–63. [DOI] [PubMed] [Google Scholar]
- 19.Inusa BP, Oyewo A, Brokke F, Santhikumaran G, Jogeesvaran KH. Dilemma in Differentiating between acute osteomyelitis and bone infarction in children with sickle cell disease: the role of ultrasound. PLoS One 2013;8:e65001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris NH. Some problems in the diagnosis and treatment of acute osteomyelitis. J Bone Joint Surg [Br] 1960;42-B:535–541. [DOI] [PubMed] [Google Scholar]
- 21.Peltola H, Pääkkönen M. Acute osteomyelitis in children. N Engl J Med 2014;370:352–360. [DOI] [PubMed] [Google Scholar]
- 22.Peltola H, Pääkkönen M, Kallio P, et al. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 2010;29:1123–1128. [DOI] [PubMed] [Google Scholar]
- 23.Bouden AK, Kaïs C, Abdallah NB, Kraiem NH, Jamoussi MM. MRI contribution in diagnosis of acute bone infarcts in children with sickle cell disease. Tunis Med 2005;83:344–348. [PubMed] [Google Scholar]
- 24.Jain R, Sawhney S, Rizvi SG. Acute bone crises in sickle cell disease: the T1 fat-saturated sequence in differentiation of acute bone infarcts from acute osteomyelitis. Clin Radiol 2008;63:59–70. [DOI] [PubMed] [Google Scholar]
- 25.Berger E, Saunders N, Wang L, Friedman JN. Sickle cell disease in children: differentiating osteomyelitis from vaso-occlusive crisis. Arch Pediatr Adolesc Med 2009;163:251–255. [DOI] [PubMed] [Google Scholar]
- 26.Chen W-L, Chang W-N, Chen Y-S, et al. Acute community-acquired osteoarticular infections in children: high incidence of concomitant bone and joint involvement. J Microbiol Immunol Infect 2010;43:332–338. [DOI] [PubMed] [Google Scholar]
- 27.Chometon S, Benito Y, Chaker M, et al. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J 2007;26:377–381. [DOI] [PubMed] [Google Scholar]
- 28.Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr 2013;25:58–63. [DOI] [PubMed] [Google Scholar]
- 29.Fritz JM, McDonald JR. Osteomyelitis: approach to diagnosis and treatment. Phys Sportsmed 2008;36:nihpa116823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikus JR, Worsham J, Aung H, Clifton D, Walser EM. The role of bone biopsy in osteomyelitis—utility or futility? J Vasc Interv Radiol 2013;24:S31–S32. [Google Scholar]
- 31.Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol 2005;129:482–490. [DOI] [PubMed] [Google Scholar]
- 32.Lee YJ, Sadigh S, Mankad K, Kapse N, Rajeswaran G. The imaging of osteomyelitis. Quant Imaging Med Surg 2016;6:184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pugmire BS, Shailam R, Gee MS. Role of MRI in the diagnosis and treatment of osteomyelitis in pediatric patients. World J Radiol 2014;6:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonnerot V, Sebag G, de Montalembert M, et al. Gadolinium-DOTA enhanced MRI of painful osseous crises in children with sickle cell anemia. Pediatr Radiol 1994;24:92–95. [DOI] [PubMed] [Google Scholar]
- 35.Rowbottom AW, Lepper MA, Garland RJ, et al. Interleukin-10-induced CD8 cell proliferation. Immunology 1999;98:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santin AD, Hermonat PL, Ravaggi A, et al. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J Virol 2000;74:4729–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarray S, Almawi WY. Contribution of reduced interleukin-10 levels to the pathogenesis of osteomyelitis in children with sickle cell disease. Clin Vaccine Immunol 2015;22:1020–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro BS, Dinges DF, Orne EC, et al. Home management of sickle cell-related pain in children and adolescents: natural history and impact on school attendance. Pain 1995;61:139–144. [DOI] [PubMed] [Google Scholar]
- 39.Fuggle P, Shand PA, Gill LJ, Pain Davies SC., quality of life, and coping in sickle cell disease. Arch Dis Child 1996;75:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gil KM, Carson JW, Porter LS, et al. Daily stress and mood and their association with pain, health-care use, and school activity in adolescents with sickle cell disease. J Pediatr Psychol 2003;28:363–373. [DOI] [PubMed] [Google Scholar]