Abstract
Antibody deficiency or hypogammaglobulinemia can have primary or secondary etiologies. Primary antibody deficiency (PAD) is the result of intrinsic genetic defects, whereas secondary antibody deficiency may arise as a consequence of underlying conditions or medication use. On a global level, malnutrition, HIV, and malaria are major causes of secondary immunodeficiency. In this review we consider secondary antibody deficiency, for which common causes include hematological malignancies, such as chronic lymphocytic leukemia or multiple myeloma, and their treatment, protein-losing states, and side effects of a number of immunosuppressive agents and procedures involved in solid organ transplantation. Secondary antibody deficiency is not only much more common than PAD, but is also being increasingly recognized with the wider and more prolonged use of a growing list of agents targeting B cells. SAD may thus present to a broad range of specialties and is associated with an increased risk of infection. Early diagnosis and intervention is key to avoiding morbidity and mortality. Optimizing treatment requires careful clinical and laboratory assessment and may involve close monitoring of risk parameters, vaccination, antibiotic strategies, and in some patients, immunoglobulin replacement therapy (IgRT). This review discusses the rapidly evolving list of underlying causes of secondary antibody deficiency, specifically focusing on therapies targeting B cells, alongside recent advances in screening, biomarkers of risk for the development of secondary antibody deficiency, diagnosis, monitoring, and management.
Keywords: secondary antibody deficiency, chronic lymphocytic leukemia, lymphoma, multiple myeloma, solid organ transplant, immunoglobulin replacement (IgRT)
Introduction
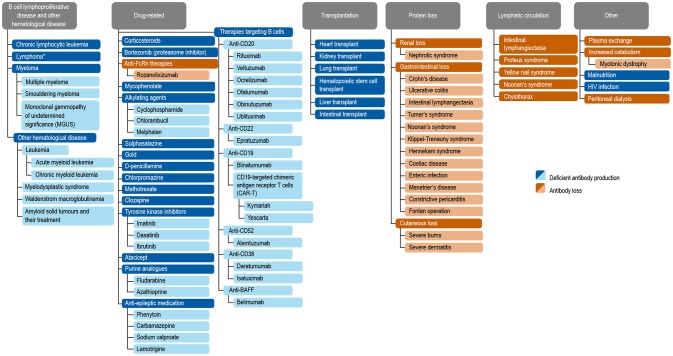
Antibody deficiencies, a subset of immunodeficiencies, are classified as primary or secondary in etiology. Primary antibody deficiency (PAD) refers to a heterogeneous group of genetic disorders characterized by an intrinsic impairment in antibody production or function (1, 2). The prevalence of PAD has been estimated to be around 1 in 2,000 children, 1 in 1,200 individuals of any age, and 1 in 600 households in the United States (~150,000–360,000 patients) (3). Secondary immunodeficiencies (SID) on a worldwide scale occur as a consequence of an extrinsic influences, such as malnutrition, human immunodeficiency virus (HIV) infection, malaria, neutropenia, or as a side effect of certain medications (4). Secondary antibody deficiency, a type of SID, is often multifactorial in etiology, related to both the underlying condition and its treatment, including a growing range of treatments targeting B cells. Secondary antibody deficiency occurs across a broad wide disease spectrum, and is therefore of importance to clinicians in both primary and secondary care. Secondary antibody deficiencies are can be estimated to be 30-fold more common than PADs, but unlike PADs are sometimes reversible if the underlying cause is resolved (4). There are several types of secondary antibody deficiency, the most common being disease-related secondary antibody deficiency, caused by hematologic malignancies such as chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma (MM). Other types of secondary antibody deficiency include iatrogenic secondary antibody deficiency as a side effect of specific therapies designed to target B cells directly as well as non-B cell specific therapies or processes which nonetheless impact on B cells or antibodies including conventional immunosuppressive agents (e.g., cyclophosphamide, methotrexate, mycophenolate mofetil) steroids, radiation therapy, solid organ transplantation (SOT), and secondary antibody deficiency related to protein-losing states due to renal, gastrointestinal, or cutaneous loss (Figure 1) (2, 4–6). Patients with secondary antibody deficiency due to renal or gastrointestinal loss of IgG often have retained specific antibody production and hence may have a lower infection risk when compared to a failure to produce antibodies (2). The removal of antibodies by plasma exchange or blockade of the neonatal Fc receptor (FcRn) is also likely to confer a lower risk of infection than deficiencies in antibody production (7–10).
Figure 1.
Common causes of secondary antibody deficiency (26), (5), (251), (255), (256), (242), (6), (144), (7), (165), (70), (242), (18), (168), (244), (134), (141), (135), (139), (133), (155), (245), (147), (138), (38), (162), (124), (163), (246), (174), (233), (253), (257), (247), (252), (249), (250), (10), (254). Reproduced with the permission of the copyright holder John Wiley & Sons Inc (5). *Including non-Hodgkin's lymphoma, Hodgkin's Lymphoma, diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma, and Burkitt's lymphoma (5).
The spectrum of clinical impact of secondary antibody deficiency may range from a rather limited infection susceptibility to a more significant burden characterized by predominantly sinopulmonary recurrent, chronic, systemic, or complicated infections or even including opportunistic infections, such as cytomegalovirus (CMV) in transplantation (5). Although secondary antibody deficiency is a recognized phenomenon, there are only a limited number of studies addressing the incidence and clinical importance of this disorder (11) and treatment outcomes have not been fully explored.
The prevalence of secondary antibody deficiency, which is estimated to be 30 times more common than PAD (12–15), is increasing for a number of reasons. Growth in new therapies for autoimmune, inflammatory, and malignant disease, many targeting B cells, is rapid. It is being increasingly appreciated that the use of such therapies, such as rituximab, alongside high-dose, and long-term steroid treatment, either in combination with other treatments, sequentially over time or as maintenance therapies may contribute to the increased development of iatrogenic secondary antibody deficiency (5, 6). Studies report an increased risk of infection with B cell-targeting drugs such as rituximab or ibrutinib, and with long-term steroid treatment (16, 17). It is becoming increasingly important to address the unmet needs in this growing patient population, including risk factors for the development of secondary antibody deficiency, improved screening, monitoring, and treatment strategies.
The clinical management of secondary antibody deficiency involves a range of potential interventions based on a careful clinical and laboratory assessment of risk and may include patient education, prompt access to emergency antibiotics, prophylactic antibiotic treatment, vaccination, and reducing immunosuppression or treating the underlying cause in the small proportion where this is possible. A trial of immunoglobulin replacement therapy (IgRT) may be warranted for selected patients with low immunoglobulin G (IgG; <4 g/L) who continue to suffer recurrent infections despite prophylactic antibiotics and who fail to respond to vaccination. Available studies report IgRT as an effective treatment for reducing the rate of serious infections in patients with CLL or MM (17–19). Recent market research of secondary specialty pharmacy data from the United States, France, the United Kingdom, Germany, Spain, Australia and Canada reported that the major secondary antibody deficiency indications leading to IgRT usage were CLL and MM, which represent 39.2–54.9% of all patients with secondary antibody deficiency receiving IgRT (20).
In this review, we discuss the causes, diagnosis, and management of secondary antibody deficiency, specifically focusing on new developments in agents targeting B cells (Figures 1, 2).
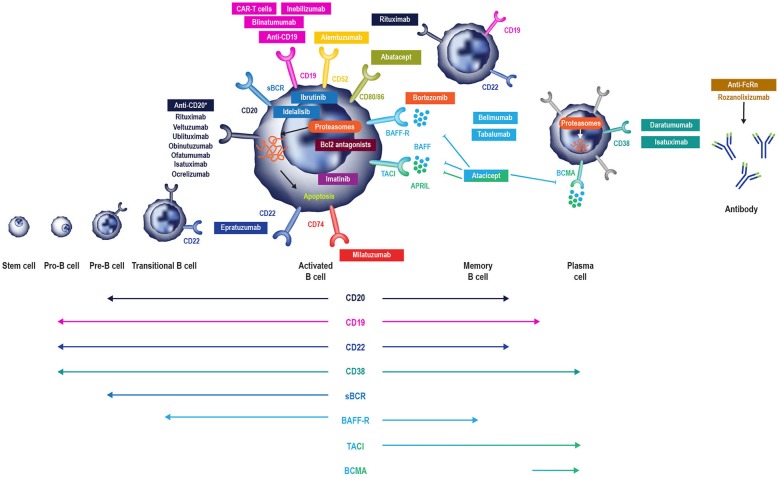
Figure 2.
B cell-specific chemotherapeutic causes of secondary antibody deficiency. Reproduced with the permission of the copyright holder The Royal College of Physicians (2). *Anti-CD20 compounds conjugated to other drugs are also in development. APRIL, a proliferation inducing ligand; BAFF(-R), B-cell activating factor (receptor); Bcl2, B cell lymphoma 2; BCMA, B-cell maturation antigen; (s)BCR, (surface) B cell receptor; TACI, transmembrane activator, and calcium modulator.
Causes of Secondary Antibody Deficiency
CLL, MM, and Lymphoma
Hematological malignancies such as CLL, MM, and lymphoma are commonly associated with hypogammaglobulinemia. CLL is one of the most common leukemias, with an annual incidence of 4.7 in 100,000 (15). Infection frequency in CLL correlates positively with hypogammaglobulinemia, which is present in up to 85% of these patients and contributes substantially to morbidity and mortality, with infection-related deaths in 25–50% of patients (13). The prevalence of hypogammaglobulinemia in CLL is more pronounced with prolonged disease duration and advanced-stage disease and correlates with patient age and comorbidities (13).
Multiple myeloma has an annual incidence of 6.6 cases per 100,000 persons in the United States (14). Secondary antibody deficiency is also common in MM, occurring in 45–83% of patients with smoldering MM at some point during the disease course (21). Infections in such patients are predominantly caused by encapsulated bacteria, such as certain strains of Haemophilus influenzae, however overall susceptibility is wide and includes Clostridium difficile and Escherichia coli, Staphylococcus aureus, and fungal and viral infections, such as Varicella Zoster virus (VZV) (6, 22). One study of more than 3,000 patients with MM demonstrated that infection was responsible for 45% of deaths within 6 months of diagnosis. Respiratory tract infections (RTIs) are noted most frequently, with pneumonia, septicemia, and urinary tract infections (UTIs) also occurring commonly in this patient population (6, 23). The hazard ratios of developing pneumonia, septicemia, or meningitis have been shown to be 7.7-, 15.6-, and 16.6-fold, respectively, in patients with MM, compared with population-based age-matched controls (23).
The mechanisms of antibody deficiency and hence infection susceptibility in CLL are multifactorial. Defective function of the non-clonal CD5-negative B cells and direct suppression of CD95+ bone marrow plasma cells through CD95L/CD95 interactions between plasma cells and CLL-B cells are postulated to cause a B cell defect (13). Regulatory abnormalities in T cells (e.g., decreased helper T cell or increased T suppressor cell activity) (24) and dysfunctional dendritic cells or natural killer cells may also contribute to the infection burden associated with hypogammaglobulinemia in CLL and MM (2, 6, 13). There is also evidence that CLL-B cells replace normal B cells (25), thereby inhibiting the function of non-malignant B cells by subverting T cell help in the pseudofollicle (26), and may also directly suppress IgG production by bone marrow plasma cells (27). Additional B cell–independent risk factors, such as neutropenia, and significant renal dysfunction can be both disease related and a consequence of treatment. Furthermore, renal disease can act as a cofactor in increasing infection burden not only in CLL and MM but in other settings where there is significant renal impairment (28, 29).
Therapeutic Agents That Can Cause Secondary Antibody Deficiency
Although CLL and MM can themselves result in secondary antibody deficiency, there is also an additional risk of iatrogenic secondary antibody deficiency posed by the therapies used to treat these, and other conditions (Table 1). Therapies for CLL and MM often suppress immune function, increasing the likelihood of clinically significant infection primarily depending on the actions of the drug, its dose, the duration of treatment, and the stage of CLL (123). According to the market research survey mentioned above, iatrogenic secondary antibody deficiency accounted for 12.8–22.1% of all secondary antibody deficiency cases worldwide (20).
Table 1.
Reported outcomes of therapeutic agents with the potential to cause iatrogenic secondary antibody deficiency.
| Drug | Total number of patients in cited studies | Secondary antibody deficiency | Infection incidence | Vaccine responses | B cell subsets | References |
|---|---|---|---|---|---|---|
| Atacicept | 697 | Decreased IgA, IgG | Increased infection incidence | No effect on tetanus or diphtheria vaccine immunization status | Transiently increased mature and total B cells, followed by decreased mature and total B cells. Transiently increased memory and naïve B cells | (30–37) |
| Belimumab | 4552 | Rare minor hypogammaglobulinemia | No change in infection incidence | No effect on pneumococcal, tetanus or influenza vaccine immunization status | Decreased total, transitional, naïve and activated B cells. Possibly transiently increased memory B cells | (38–52) |
| Bendamustine | 396 | Hypogammaglobulinemia (IgG) | Increased infection incidence | – | – | (53–55) |
| Blinatumomab | 332 | Hypogammaglobulinemia (predominantly IgA) | Possibly increased serious infection incidence | – | Decreased total and peripheral blood cells | (56–61) |
| Bortezomib | 741 | Decreased IgG, IgA, and IgM (but not hypogammaglobulinemia) | Increased incidence of HZ infections and VZV reactivation | No effect on measles, mumps or tetanus vaccine immunization status | No effect on B cells | (62–64) |
| Chlorambucil | 24 | Decreased IgM (but not hypogammaglobulinemia) | No change in infection incidence | – | – | (65, 66) |
| Cladribine | 205 | No effect on immunoglobulins | Increased infection incidence | – | Decreased total B cells | (67–69) |
| Clozapine | 119 | Hypogammaglobulinemia (IgG, IgA, and IgM) | – | – | – | (70–72) |
| Corticosteroids | 274 | Hypogammaglobulinemia | Increased infection incidence | No effect on influenza vaccine immunization status | Decreased naïve and transitional B cells. No effect on memory B cells | (73–75) |
| Daratumumab | 319 | – | Increased infection incidence | – | No effect on B cells | (76, 77) |
| Epratuzumab | 1468 | Decreased IgM (no effect/possibly increased IgA or IgG) | – | – | Decreased total B cells | (78–84) |
| Fludarabine | 27 | Hypogammaglobulinemia | Increased infection incidence | – | – | (85) |
| Ibrutinib | 894 | Hypogammaglobulinemia (IgG, IgA, and IgM) | Increased infection incidence | No effect on influenza vaccine immunization status | – | (16, 86–91) |
| Idelalisib | 267 | No effect on immunoglobulins | Increased infection incidence | – | – | (92–95) |
| Inebilizumab | 51 | Decreased IgG, IgE, IgG, and IgM (but not hypogammaglobulinemia) | Increased infection incidence | – | Decreased total B cells | (96, 97) |
| Mycophenolate mofetil | 669 | Decreased IgG and IgM | Increased CMV and bacterial infection incidence | Suppressed response to influenza vaccine | Decreased plasmablasts | (98–102) |
| Ocrelizumab | 2830 | Hypogammaglobulinemia reported (mostly IgM) | Increased infection incidence | No effect on mumps, rubella, varicella, or pneumococcal immunization status | Decreased total and peripheral B cells | (103–110) |
| Rituximab | 500 | Hypogammaglobulinemia (IgA, IgG, IgM) | Increased infection incidence | Suppressed response to pneumococcal and haemophilus influenzae immunization status. No effect on tetanus or diphtheria immunization status | Decreased total B cells | (17, 111–118) |
| Rozanolixizumab | 36 | Decreased IgG (no effect on IgA, IgD, IgE or IgM) | No change in infection incidence | No effect on tetanus or influenza immunization status | No effect on B cells | (7) |
| Thiopurines | 102 | Decreased IgA, IgG, and IgM | No change in infection incidence | No effect on pneumococcal, tetanus or haemophilus influenzae type B vaccine immunization status | – | (119–122) |
Drugs given as chemotherapy include alkylating agents (cyclophosphamide, chlorambucil, bendamustine), corticosteroids, and purine analogs (fludarabine, cladribine, and thiopurines) (6). Treatment with alkylating agents is known to be associated with the development of myelosuppression, during which common infections include pneumonia and bacteremia, caused predominantly by S. aureus, Streptococcus pneumoniae, H. influenzae, and Klebsiella pneumoniae (6). Purine analogs and purine synthesis inhibitors (such as mycophenolate mofetil) inhibit DNA synthesis, thereby reducing T and B cell proliferation. Use of these therapies, therefore, is more commonly associated with opportunistic infections (e.g., VZV, Listeria monocytogenes, and Candida spp.) in patients with hematological malignancies (6).
It is well-known that high-dose and long-term treatment with systemic steroids exerts immunosuppressive effects on cellular immunity; however, there is a growing appreciation of the impact on antibody production. A study of the prevalence of hypogammaglobulinemia in 36 patients with giant cell arteritis and polymyalgia rheumatica on glucocorticoid therapy reported that approximately half of the patients developed IgG deficiency with less impact on IgA and IgM and a reduction in naïve B cells with relative preservation of class switched memory B cells (73).
Importantly, diagnostic findings such as this relatively IgG-specific effect of glucocorticoid therapy, can be used clinically to help determine the etiology of antibody deficiency (primary or secondary), a distinction which is diagnostically challenging (73). It is particularly difficult to determine causality of antibody deficiency following administration of therapies known to potentially cause secondary antibody deficiency, especially in situations where both the disease and the treatment used can cause secondary antibody deficiency (e.g., CLL), as well as when multiple lines of immunosuppressive therapy have been used over time (5, 73). In the case of heart transplantation, single center, multi-center, and metanalysis studies have demonstrated the role of immunological monitoring of humoral immunity using similar tools and cut-offs as those used in the diagnosis of PAD (124–126).
There are also diseases other than hematological malignancies that are associated with an increased risk of secondary antibody deficiency following treatment. For example, in antineutrophil cytoplasmic antibody-associated vasculitis (AAV), secondary antibody deficiency of IgG <4 g/L occurs commonly, presenting in 9% of patients after rituximab induction and in a further 4.6% of patients with maintenance therapy in those with low initial IgG levels (127). This degree of secondary antibody deficiency is higher than observed for the long-term combination of rituximab and methotrexate in rheumatoid arthritis, where reduced levels of IgG, IgA, and IgM were observed in 3.5, 1.1, and 22.4% of patients, respectively (128). It is of course difficult to determining whether this increase in the incidence of secondary antibody deficiency is clearly disease specific, but the findings do suggest that a high index of suspicion is needed in this condition in particular.
In neurology, B cell ablation is being increasingly used, for conditions including neuromyelitis optica spectrum disorders, relapsing-remitting multiple sclerosis, myasthenia gravis, autoimmune encephalitis, immune mediated peripheral neuropathies and primary angiitis of the central nervous system, with reports of secondary antibody deficiency consequent upon the use of these agents (129, 130). Clozapine, a drug used in treatment-resistant schizophrenia, has been associated with an increased incidence of pneumonia and death from infection. It has also recently been found to be associated with secondary antibody deficiency, as demonstrated by levels of IgG, IgA, and IgM below the reference range in 8.5%, 13.8%, and 34% of patients, respectively (70).
Newer Therapeutic Agents That Can Cause Secondary Antibody Deficiency
While the risk of secondary antibody deficiency in patients with CLL and MM and those treated with traditional chemotherapeutic agents has been relatively well-described in older studies, there have been major therapeutic advances which have significantly influenced the risk of iatrogenic secondary antibody deficiency. Amongst these advances, of particular relevance are the many new B cell targets shown in Figure 2 (2, 6). These treatments either deplete B cells (anti-CD20, anti-CD52, anti-CD74, anti CD19, anti-CD22) and plasma cells (anti-CD38) or inhibit B cell survival (anti-B-cell activating factor [BAFF]), impair activation (proteasome inhibitors, tyrosine kinase inhibitors) or interaction with T cells (anti-CD80/86) (2) and all have the potential to cause iatrogenic secondary antibody deficiency. Bruton's tyrosine kinase (BTK) inhibitors, such as ibrutinib, target BTK to inhibit B cell survival and proliferation (Figure 2), and are increasingly used in CLL (16, 131, 132), with severe infections of Grade 3 or higher reported in up to 35% of patients (132). Blinatumomab, a bispecific T cell engager (BiTE) antibody, crosslinks CD3 on T cells to CD19 on B cells activating endogenous T cells to proliferate and become cytotoxic to CD19-positive B cell targets (133) and, as such, is also expected to have the potential to cause secondary antibody deficiency. The phosphoinositide 3-kinase δ inhibitor idelalisib, approved for the treatment of CLL, follicular lymphoma, and small lymphocytic lymphoma, has been associated with increased risk of infections, including pneumonia (92). In addition to these, there is a rapidly increasing number of other new anti-B cell agents, including those targeting molecules expressed by B cells throughout different parts of their developmental pathway such as CD19, CD20 CD22, CD38, CD74, and proteasomes (Figure 2) (38, 96, 134–148).
Given the relatively recent development of some of these therapies, evidence for secondary antibody deficiency during their use is limited by study size, disease setting, and duration of therapy.
In the case of anti-CD19 agents, reductions in all immunoglobulin classes have been reported (although still falling within the reference range) during a small study of inebilizumab in multiple sclerosis (96) and hypogammaglobulinemia was reported in 6% of patients treated with blinatumomab (149). There is more limited information on the incidence of secondary antibody deficiency for the anti-CD20 antibodies ofatumumab and ocrelizumab, however an increase in infection incidence has reported for ocrelizumab (150).
Results thus far have shown a reduction in IgM but not IgG for the anti-CD22 antibody epratuzumab (78), and a potential increase in the incidence of VZV infection with the anti-CD38 agent daratumumab which targets plasma cells (151). The proteasome inhibitor bortezomib has been associated with increased incidence of VZV infection, in addition to herpes simplex virus infection (29). For the anti-CD74 agent milatuzumab, currently in development, there are as yet no published data related to secondary antibody deficiency or risk of infection, and for all of these more recently-developed agents, longer-term studies will be needed to define the degree of impact on immunoglobulins in different disease settings.
Interestingly, anti-BAFF therapy with belimumab has not been associated with a reduction in IgG, pneumococcal antibodies or an increase in infection, and IgM memory B cells have been shown to be reduced while class switched memory B cells were preserved (38, 39). For the transmembrane activator and calcium-modulating cyclophilin ligand interactor-Ig fusion protein atacicept, used in systemic lupus erythematous and rheumatoid arthritis, a reduction in all immunoglobulins has been reported, although this was not linked to infection (152–154).
A good example of increased potency of some of the new therapies are chimeric antigen receptor (CAR)-T cells with synthetic, engineered receptors targeting B cells (e.g., anti-CD19) which are used for the treatment of hematological malignancies (155–157). These engineered T cells can proliferate and retain effector functions of the activated T cells. The likelihood of iatrogenic secondary antibody deficiency with CAR-T therapy is so high that B cell depletion is acknowledged as an “expected on-target result” which may require the use of IgRT (155).
Future Therapeutic Agents That Could Cause Iatrogenic Secondary Antibody Deficiency
Novel therapeutic strategies continue to be identified. The tetravalent bispecific anti-CD19/CD3 T and Ab tandem diabody AFM11, which consists only of Fv domains, with two binding sites for CD19 on B cells and two for CD3 on T cells, was designed to exhibit higher potency than blinatumomab owing to the bivalent binding of the Fv domains to both B and T cells, resulting in enhanced B cell lysis (158). These immune cell-recruiting bispecific antibodies harness the cytotoxic potency of endogenous T cells (or in some cases NK cells) to kill both malignant and normal B cells (158).
Yet other treatments are becoming available that may be able to treat their target diseases not by interfering with antibody production but by removing pathogenic autoantibodies by reduction of their half-life. Rozanolixizumab, for example, is an anti- FcRn monoclonal antibody (Figure 2) (7). FcRn salvages and recycles plasma IgG autoantibodies, thus extending their lifespan (7, 159). Selective inhibition of FcRn, and hence the salvage pathway, offers a new approach for the removal of pathogenic IgG autoantibodies in the treatment of autoantibody-mediated disease. While pre-clinical and clinical studies of rozanolixizumab have not shown an increase in the incidence of infection (7), long-term studies and close monitoring will be needed. As mentioned above, the risk of infection appears lower where there is removal (plasma exchange, anti-FcRn mAb) or loss (protein-losing enteropathy or renal loss) of functionally normal antibody rather than a production failure. This may translate into a lower infection risk with anti-FcRn therapies; however, long-term data will be required and it would seem prudent to optimize protection from infection by ensuring appropriate vaccination prior to commencement of therapy.
Solid Organ Transplantation (SOT)
In addition to the risks associated with hematological malignancy and immunosuppressive drugs, there are also other, perhaps less well-recognized situations in which patients are at risk of developing secondary antibody deficiency. Solid organ transplantations (SOTs) are now routine surgical procedures for treating organ dysfunction, with an estimated 119,873 SOTs performed worldwide in 2014, and 30,970 in the US alone in 2015 (160). Patients receiving SOT are given immunosuppressive drugs before, during, and after transplantation to prevent organ rejection (161). While immunosuppressive drugs are recognized as a common cause of secondary antibody deficiency, there are additional risks in SOT including the transplantation procedures themselves as well as other interventions, such as the use of ventricular assist devices in heart recipients or extracorporeal membrane oxygenation in heart and lung recipients, which are also associated with the occurrence of SOT-related secondary antibody deficiency (162, 163). Several studies have shown a high prevalence of hypogammaglobulinemia after SOT, particularly after heart, lung, and kidney transplantation, with an associated increased risk for infections (124, 164). Infections, most frequently non-CMV infections, are reported to be the leading cause of death during the first year after heart or lung transplantation (Figure 3) (163). In one meta-analysis of 669 SOT patients, 15% developed severe hypogammaglobulinemia (IgG < 4 g/L) during their first year post transplantation (124). This study also reported a 4.8-fold increase in respiratory infections, a 2.4-fold increase in CMV infections, and an 8-fold increase in aspergillus infections (3.7-fold increase in other fungal infections) when severe hypogammaglobulinemia was present (124). Furthermore, two other groups have also reported an increased incidence of opportunistic infections in heart transplantation with hypogammaglobulinemia, particularly CMV viremia (126, 162). In addition, lower levels of anti-pneumococcal antibodies are a risk factor for development of severe bacterial infections in heart recipients (165).
Figure 3.

Relative incidence of leading causes of death (31 days to 1 year) in adults receiving heart transplants Jan 1994–June 2016. Developed using data from The International Society for Heart & Lung Transplantation, (2017) (163); CMV, cytomegalovirus.
Diagnosis
The prompt diagnosis of secondary antibody deficiency is key in reducing infection burden and is dependent on appropriate screening and an appreciation of risk factors for secondary antibody deficiency development. Monitoring of patients at risk of developing secondary antibody deficiency, such as patients receiving conventional immunosuppressive drugs or newer, more targeted therapies, could help in identifying these patients before they develop a serious infection. For rituximab, which is known to be associated with the development of secondary antibody deficiency, the risks for an individual are increased with low baseline IgG prior to rituximab initiation; exposure to prior therapies, such as cyclophosphamide, steroids, and purine analogs, purine synthesis inhibitors (mycophenolate mofetil), or the use of combination therapy or granulocyte-colony stimulating factor (166). Furthermore, the single use of rituximab rarely causes hypogammaglobulinemia, yet there is an increased risk for patients undergoing maintenance or multiple cycles of treatment. Finally, patients with co-morbidities, such as chronic lung or heart disease and extra-articular rheumatoid arthritis, have higher instances of hypogammaglobulinemia, with more infections occurring when IgG is low for longer than 6 months (166). Although there may also be particular non-hematological diseases which have higher rates of secondary antibody deficiency, such as AAV and neuromyelitis optica spectrum disease, the caveat remains that it is difficult to separate the effect of treatment from disease.
The evaluation of patients at high risk or with suspected hypogammaglobulinemia should include quantitative serum Ig concentrations and analysis of vaccination responses if initial specific antibody levels are found to be low. High-risk patients may merit annual immunoglobulin measurements to screen for the development of secondary antibody deficiency. It is likely that in some cases it will be helpful to determine levels and reconstitution of B cell numbers, including the preservation of class-switched memory B cell and plasma cell compartments. For example, in some settings the measurement of the return of class-switched memory B cells is already being used to determine the timing of retreatment with rituximab to reduce the cumulative rituximab dose without apparent loss of efficacy (167). In addition, complete blood count will assist in identifying additional risk factors such as neutropenia, lymphopenia, or lymphocytosis (2, 168).
Some treatment-specific screening or monitoring programs have been introduced to mitigate infection-related risk; for example, the Pharmacovigilance Risk Assessment Committee of the EMA recommends that patients receiving idelalisib should be informed about the risk of serious and/or fatal infections, given prophylaxis for Pneumocystis jirovecii pneumonia, and monitored for signs and symptoms of respiratory infections throughout the idelalisib treatment. Regular clinical and laboratory screening for CMV infection should also be conducted and absolute neutrophil counts monitored in all patients at least every 2 weeks for the first 6 months of treatment. In the case of clozapine, the Clozapine Risk Evaluation and Mitigation Strategy (REMS) monitoring scheme has been established to reduce the risks associated with neutropenia, however antibody monitoring is not currently part of the scheme (169).
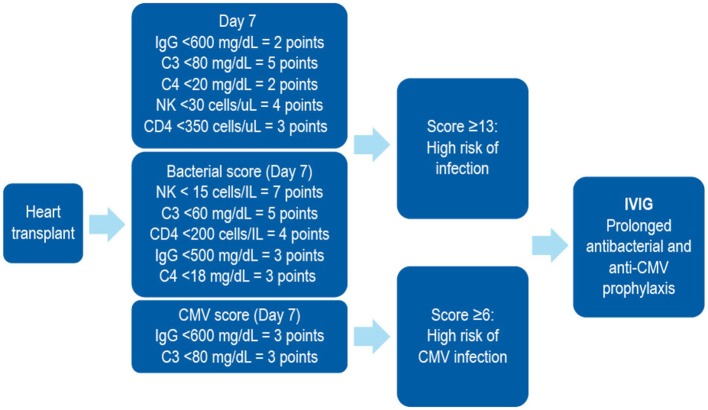
In patients receiving SOT, several biomarkers have been shown to be useful in stratifying the risk of developing SOT-related secondary antibody deficiency and associated infections. For example, in patients receiving a heart transplantation, IgG levels of < 10 g/L prior to transplantation may predict high frequency of bacterial infections, therefore suggesting that monitoring of IgG levels prior to transplantation may be important in predicting secondary antibody deficiency and subsequent infection. Similarly, low levels of class-switched memory B cells may also act as pre-heart transplantation biomarkers. Several post-heart transplantation biomarkers, such as low peripheral lymphocyte populations, may also help in predicting infections. For example, low anti-CD8 response to CMV antigens assessed by enzyme-linked immune absorbent spot (ELISPOT) or flow cytometry can be used as a biomarker for the risk of developing CMV infection and disease (170). Other prospective post-SOT biomarkers include complement components (e.g., C3 and C4), owing to their effector functions in the innate and adaptive humoral immune responses (171). The classic in vitro hemolytic assays utilized for assessing classical and alternative complement pathway function are complex, susceptible to pre-analytical sample handling issues, and time consuming. Nephelometry represents a convenient option for the measurement of certain complement components in serum (172). As complement activation is responsible for the clearance of encapsulated bacteria, hypocomplementemia may result in increased susceptibility to infection (171). It has been shown that hypocomplementemia is an indicator of bacterial infection risk in patients following kidney transplantation (173), with similar findings reported for liver (174) and heart (175) recipients. The combination of low IgG and low C3 in serum can be particularly detrimental in SOT patients. One multivariate analysis demonstrated that hypogammaglobulinemia (IgG <6 g/L) or C3 hypocomplementemia (C3 <80 mg/dL) on Day 7 post heart transplantation were independent risk factors for infection (especially bacterial infections) and CMV disease (125). However, the combination of both was more strongly associated with the risk of infections than either hypogammaglobulinemia or C3 hypocomplementemia alone. Furthermore, on Day 7, low anti-CMV antibody titers and low anti-pneumococcal polysaccharide antibody concentrations as biomarkers of impaired specific antibody responses were independent predictors of CMV disease and bacterial infections, respectively (125). These biomarkers can be used to identify patients at high risk who may benefit from IgRT post heart transplantation (Table 2, Figure 4). The evaluation of CMV IgG serology of donor and recipient is a classic evaluation to identify the risk of CMV disease after SOT (170). CMV seronegative recipients receiving an allograft from a CMV seropositive donor are considered to be at higher risk of having this complication than other serological combinations. More recently, the assessment of CD8 responses to CMV antigens has also been introduced to evaluate the risk of CMV disease in SOT (170).
Table 2.
Biomarkers used following heart transplantation.
| Organ | Biomarker (post HT) | Outcome | References |
|---|---|---|---|
| Heart | IgG <3.5 g/L | Opportunistic infection | (162) |
| Heart | IgG <3.1 g/L | Opportunistic infection | (176) |
| Heart | IgG <6 g/L | Bacterial, CMV infection | (126) |
| Heart | IgG <7 g/L | Bacterial, CMV infection | (165) |
| Heart | IgG <4 g/L | Clostridium difficile | (177) |
| Heart | IgG <5 g/L | CMV disease | (178) |
| Heart | Low IgG2 | Overall infection | (175) |
| Heart | anti-PPS <5 mg/dL | Bacterial infection | (165) |
| Paed heart | Low anti-PPS | Not done | (179, 180) |
| Heart | Low anti-CMV titres | CMV infection | (178, 181, 182) |
| Heart | C3 <80 mg/dL | Overall infection | (183) First proposal of an immunological score |
| Heart | NK <30 cells/uL | ||
| Heart | CD4 <350 cells/uL | ||
| Heart | Low anti-CD8 response to CMV | CMV infection | (183–185) |
C3, complement component 3; CD, cluster of differentiation; CMV, cytomegalovirus; HT, heart transplant; IgG, immunoglobulin; NK, natural killer; PPS, pneumococcal polysaccharide.
Figure 4.
A combined immunodeficiency profile identifies risk of severe infection in heart transplant recipients. Developed using data from Sarmiento et al. (186). C, complement; CD, cluster of differentiation; CMV, cytomegalovirus; IVIG, intravenous immunoglobulin; NK, natural killer.
An interesting aspect of the management of heart and other SOT recipients is that vaccination is not common practice during the first months after transplantation, when the majority of severe infections occur, because the specific antibody response after immunization in this period has been demonstrated to be low (187). This should be taken into account at the time of diagnosing secondary antibody deficiency in this setting. Moreover, the titers of anti-pneumococcal or anti-CMV antibodies gradually decline during the first year after transplantation as a result of the immunosuppressive therapy, leaving the patients exposed to infection (165, 178, 188).
Treatment
While removal of the underlying causative factor would be the preferred option for treating secondary antibody deficiency, (e.g., as a consequence of the removal of an abnormal section of bowel in protein-losing enteropathies or reduction of immunosuppression post hematopoietic stem cell transplantation [HSCT]) this is overall rarely possible, particularly when treatment cannot be easily avoided as is the case with SOT, CLL, MM, or lymphoma. Indeed, in the case of HSCT, this may also itself lead to secondary antibody deficiency and subsequent infection, the risks of which are increased by the presence of graft vs. host disease (GvHD) in these patients (189, 190). While the mechanism of GvHD-mediated secondary antibody deficiency is not fully understood, it is thought to potentially delay B cell engraftment, leading to B cell lymphopenia, and to delay/prevent B cell maturation and memory development, markedly decreasing memory and switched memory B cells (191, 192).
In hematological malignancy, supportive treatments including prophylactic vaccination (with non-live vaccines), antibiotics and/or IgRT may be considered for patients with secondary antibody deficiency. A careful review of the patient's history, assessment of the risk factors for developing secondary antibody deficiency and evaluation of serum IgG levels and specific antibody levels is key to the diagnosis and treatment. Immunologists often have greater experience in managing PAD than secondary antibody deficiency, and it is likely that the clinical and laboratory assessments used in this setting would also be of utility in secondary antibody deficiency. However, there is limited evidence in the literature, particularly relating to the increasing number of newer therapies, to guide clinical management of secondary antibody deficiency.
Prophylactic Vaccination
Prophylactic non-live vaccinations such as influenza are recommended for patients with secondary antibody deficiency, as is the case in PAD. Although the antibody response may not be optimal, some helpful protection via antibodies and T cell immunity may still be achieved; thus, influenza vaccination is advised. Live vaccination is generally not recommended in patients with secondary antibody deficiency (although individual assessment and decisions are warranted). Instead, component or inactivated vaccines should be used. Studies suggest that vaccination at an early stage (before initiation of chemotherapy and the onset of hypogammaglobulinemia) may be more helpful in generating immunological memory when specific antibody levels are low (123). In addition, evaluation of post-vaccination–specific antibody levels can be beneficial both therapeutically (if protective levels are achieved) and in stratification of risk before and following treatment. Vaccination against S. pneumoniae and H. influenza is recommended in patients with CLL; however, the protective effect of these vaccines may be variable, emphasizing the importance of ongoing immunological assessment and individualization of care (6). In a UK-based study of patients with MM, 61% (26/43) showed a suboptimal response to S. pneumoniae vaccination, whereas 75% (33/46) showed a response to H. influenzae type B vaccination comparable with those in the healthy adult UK population. The European Myeloma Network guidelines (193) suggest that “vaccination against influenza virus is appropriate and is recommended for both patients and their contacts. Moreover, vaccination against Streptococcus pneumoniae and Haemophilus influenzae is recommended, but efficacy for all vaccines is not guaranteed, due to suboptimal immune response (grade 1C). In general, live vaccines should be avoided in myeloma patients (grade 2C)” (193).
The Infectious Diseases Society of America has recommended the routine use of inactivated vaccines in patients with MM unless they are actively receiving chemotherapy or monoclonal antibody (194). The Advisory Committee for Immunization Practices recommends that patients with MM and SOT should receive vaccination with pneumococcal conjugate vaccine at diagnosis followed by revaccination more than 8 weeks later (195). Recent MM guidelines from the National Comprehensive Cancer Network do not include specific recommendations for vaccination, but do note to “consider Pneumovax and influenza vaccine” (196).
In terms of pre-transplantation vaccination schedules, it is suggested that primary immunizations should be given as soon as possible before transplantation (197). Data from a study of pediatric patients showed that vaccination prior to transplantation was effective in reducing the incidence of Varicella Zoster virus infection from 45–12% (198). Guidelines for vaccination of SOT recipients recommend vaccination against S. pneumoniae and H. influenzae before transplantation (197, 199). As mentioned, post-transplantation, anti-pneumococcal antibody titers have been shown to significantly decrease in kidney (188) and lung transplant recipients (200). Questions remain regarding the utility and timing of vaccination in secondary antibody deficiency and further studies are needed (6).
While it is generally accepted that inactivated vaccines can be administered in patients with secondary antibody deficiency, many vaccinations are not required for those patients receiving IgRT, as protective levels of antibodies are already present in the IgRT preparations and should offer some protection against a range of infectious diseases (201, 202). Influenza vaccination is an exception, as these vaccines are reformulated annually to reflect changes in the antigenic composition of the influenza virus and protection from IgRT is therefore less likely. However, even if the antibody response post vaccination is suboptimal, patients with PAD and also secondary antibody deficiency may benefit from the T cell-mediated responses (112, 203). Live vaccines are generally not recommended in the context of significant immunodeficiency and in patients receiving IgRT may also be less effective (204).
Prophylactic Antibiotics
Treatment of secondary antibody deficiency with prophylactic antibiotics is the recommended first-line therapy in CLL (205) and during periods of neutropenia in patients undergoing chemotherapy or other immunocompromising treatments (11). Antibiotic therapy should take into account the previous culture and sensitivity results as well as any allergies, tolerance, and the likelihood of Pseudomonas or macrolide-resistant H. influenzae infection. In the event of a breakthrough infection, if there has been no or limited response to a back-up course of antibiotics and a second course of different antibiotics, then intravenous antibiotic (IVAB) treatment should be considered. Prophylactic and back-up antibiotics should be of different classes (e.g., macrolide and penicillin class), rather than simply increasing the dose of the existing prophylactic regimen. Monitoring (e.g., electrocardiogram) and additional patient information (e.g., regarding the development of tinnitus) may be needed for those on long-term macrolides. There are many potential antibiotic options and the examples shown in Table 3 are illustrative, with individual decisions being made on clinical grounds and local prescribing policy. Nebulized antibiotics and intermittent IVAB are options that are used mainly for severe bronchiectasis and pseudomonal colonization (Table 3).
Table 3.
Graded antibiotic regimens*.
| Antibiotic regimen | Dosing schedule | Additional options | Emergency plan | Example |
|---|---|---|---|---|
| Intermittent antibiotics | None | Attend GP with symptoms | N/A | |
| None | Early use of home back-up antibiotics | Co-amoxyclav 625 mg tds for 2 weeks; held at home | ||
| Prophylactic antibiotics during the winter months with home rescue during the summer | Low-dose and full-dose options, e.g., azithromycin 250 or 500 mg 3 days/week | Early use of home back-up antibiotics | Azithromycin 500 mg 3 days/week plus back-up Co-amoxyclav for 2 weeks; held at home | |
| Ongoing prophylaxis | Prophylactic antibiotics | Low-dose and full-dose options, e.g., azithromycin 250 or 500 mg 3 days/week | Early use of home back-up antibiotics | Azithromycin 500 mg 3 days/week plus back-up Co-amoxyclav 625 mg tds for 2 weeks; held at home |
| Rotating prophylactic antibiotics | Early use of home back-up antibiotics | |||
| Prophylactic antibiotics | Nebulized antibiotics | Early use of home back-up antibiotics | Nebulized Colomycin 1–2 mega units bd | |
| Prophylactic antibiotics | Intermittent planned IVAB | Early use of home back-up antibiotics | Meropenem 2g IV tds and Ceftazidime Co-amoxyclav for 2 weeks; held at home |
GP, general practitioner; IV, intravenous; IVAB, intravenous antibiotic; N/A, not available; bd, twice daily; tds, three times daily.
If there has been an inadequate response to back-up antibiotics and an additional antibiotic in another class then intravenous antibiotics (IVAB) should be considered. The Table shows examples of antibiotic regimens and the antibiotic choice will depend on individual clinical circumstances.
Current Use of IgG
Many studies have demonstrated decreased infection incidence in patients with CLL treated with intravenous IgG (IVIG; Table 4). The beneficial effect of IVIG was demonstrated in a randomized, controlled, double-blind clinical trial conducted by the Cooperative Group for the Study of Immunoglobulin, where use of IVIG was associated with lower incidence of bacterial infections and longer time during which patients were free of serious bacterial infections compared with patients not receiving IVIG (206). A subsequent double-blind crossover follow-up study confirmed that maintenance on IgG results in reduction of the infection incidence (210). Later, another double-blind crossover study compared two doses of IVIG (250 and 500 mg/kg) over a year, suggesting a significant difference in the incidence of bacterial infections between doses (208). These promising results prompted a consensus statement supporting the use of IVIG in patients with CLL with hypogammaglobulinemia and associated infections (213). However, as these studies are well over a decade old, there is a clear need for newer randomized trials in this area, particularly given the vast advances in treatment and improved survival.
Table 4.
Summary of evidence for the use of immunoglobulin replacement therapy in CLL.
| Reference | Number of patients | Number of patients in advanced stage* | Type of study | Dose IVIG/schedule | Study duration (months) | Infection rate during IVIG administration |
|---|---|---|---|---|---|---|
| Cooperative group (206) | 81 | 32 (39.5%) | Controlled, randomized double-blind | 400 mg/kg/ 21 days | 12 | Decreased |
| Jurlander et al. (207) | 15 | 8 (53.3%) | Not controlled, pilot | 10 g/28 days | 12 (mean time) | Decreased |
| Chapel et al. (208) | 34 | 15 (44.1%) | Controlled, randomized double-blind | 250 mg/kg vs. 500 mg/kg/28 days | 12 | Decreased |
| Sklenar et al. (209) | 31 | 2 (6.4%) | Dose-finding | 100–800 mg/kg/ 21 days | 4.5 | Decreased |
| Griffiths et al. (210) | 10 | 3 (30%) | Controlled, randomized double-blind | 400 mg/kg 21 days | 12 | Decreased |
| Broughton et al. (211) | 42 | 15 (35.7%) | Randomized | 18 g/21 days | 12 | Decreased |
| Molica et al. (212) | 30 | 25 (83.3%) | Randomized, crossover | 300 mg/kg/28 days | 6 or 12 | Decreased |
Binet stage C or Rai III-IV.
Protocols for IgRT in secondary antibody deficiency vary. In clinics based in Oxford and Cardiff, UK, very similar approaches are employed where the use of IgRT and the dose administered are individualized per patient (5). In patients referred to the immunology department for recurrent and/or serious bacterial infections, immunization responses are measured 4–6 weeks after administration of protein and polysaccharide vaccines (if initial levels are low) and during the waiting period, for up to 3 months, prophylactic antibiotic use is considered. A 12-month trial of IgRT (with infection monitoring) is considered in case of antibody failure and a lack of an adequate response to prophylactic antibiotics in association with a significant ongoing infection burden. Neutrophil count, serum IgG trough levels, and infection burden should be monitored regularly, and IgG use, including dosage, should be reviewed after 6 and 12 months to maintain plasma IgG trough levels sufficient for an optimal reduction in infection rate. Levels of IgG should be reviewed if chemotherapy is introduced during IgRT. Because patients react differently to IgRT, individualization of the treatment regimen is required (5).
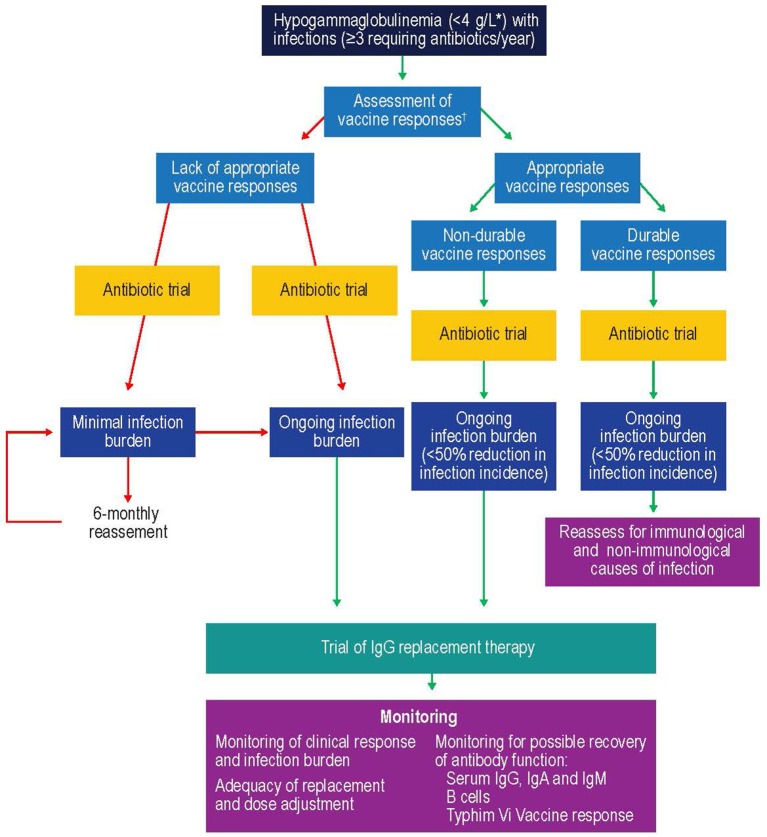
One of the key factors to consider when initiating IgRT is selection of patients most likely to benefit (Figure 5). Studies suggest that patients with IgG levels <4 g/L and/or low levels of antibodies against encapsulated organisms with an ongoing history of recurrent bacterial infections that have not responded adequately to prophylactic antibiotics could especially benefit from IVIG (211, 215). Furthermore, selection of the patients should include a wider assessment of comorbidities and innate immunological abnormalities, such as neutropenia, as well as demonstrating antibody failure (exposure/test immunization). If IgRT is to be administered, a careful risk-benefit assessment should be made. This should take into consideration, for example, the reported adverse effects of IgRT, such as the rare complications of thromboembolism and hemolysis in patients with hematological malignancies (216, 217). Once it is established that IgRT should be offered to a patient with secondary antibody deficiency, the route [subcutaneous IgG [SCIG] or IVIG] and location (home vs. clinic) of infusions should be considered. SCIG has similar efficacy to IVIG (218, 219) but offers several potential benefits, including more stable serum IgG levels (219, 220), improved patient health-related quality of life (221), and time and cost efficiencies for both patients and healthcare providers. It has also previously been shown that the feasibility and safety of home IVIG therapy in selected CLL patients may improve patient compliance and considerably decrease costs (222). It is also recommended that patients complete at least 12 months (encompassing all 4 seasons) of IgRT in order to optimally assess the response to therapy (11). This will require ongoing review to reflect any clinical changes in the patient's underlying condition and therapy. Treatment discontinuation may be considered in stable patients with reduced incidence of infections (i.e., once treatment has restored function) to determine if IgRT is still required, or in patients where treatment does not seem to be effective in preventing infection (i.e., treatment failure) (11).
Figure 5.
Suggested protocol for the investigation, monitoring, and management of secondary antibody deficiency. Reproduced with the permission of the copyright holder John Wiley & Sons Inc (5). *See (57, 58, 214); †Only >6 month after solid organ transplant. CSMB, class-switched memory B cells; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; SOT, solid organ transplant.
Prophylactic use of IgRT in patients post SOT has been shown to decrease the incidence of severe infection in patients with hypogammaglobulinemia (162, 223). It has also been shown to decrease the incidence of CMV infection in patients with hypogammaglobulinemia (224). Retrospective studies have demonstrated that addition of IVIG to antimicrobial therapy in heart transplantation recipients with secondary antibody deficiency and severe infections is associated with reconstitution of specific antibodies (anti-CMV, anti-tetanus toxoid) and lower rates of death (183, 225). A case series performed in heart transplantation recipients with difficult-to-control CMV infection in whom secondary antibody deficiency was found demonstrated that IVIG was associated with reduction in detectable CMV viremia and with remission of clinical symptoms of CMV disease (226).
Current Evidence-Based Guidelines for IgRT in Secondary Antibody Deficiency
The majority of guidelines recommend prophylactic antibiotics as first-line therapy for patients with CLL susceptible to serious infections (2). However, an individualized dose and duration of IgRT is also suggested where there is lack of responsiveness or failure of the antibiotics to sufficiently reduce the infection burden in such patients. Looking at specific recommendations, according to European Medicines Agency (EMA) 2018 (valid from January 2019); IVIG can be used in patients with secondary immunodeficiencies “who suffer from severe or recurrent infections, ineffective antimicrobial treatment and either proven specific antibody failure (PSAF) or serum IgG level of <4 g/l,” where PSAF is defined as “failure to mount at least a 2-fold rise in IgG antibody titer to pneumococcal polysaccharide and polypeptide antigen vaccines” (214). The dose required is stated to be probably patient dependent, but is likely to be 0.2–0.4 g/kg every 3–4 weeks (214). With regard to SCIG, EMA guidelines state that it is indicated in hypogammaglobulinemia and recurrent bacterial infections in patients with MM and in patients with CLL in whom prophylactic antibiotics have failed or are contraindicated (227). SCIG is also indicated for hypogammaglobulinemia in patients pre- and post-allogeneic HSCT. However, the EMA states that “the above indications would be granted as long as efficacy has been proven in primary immunodeficiency syndromes” (227).
The European Society for Medical Oncology (ESMO) guidelines, published in 2015 (228), state that “the use of prophylactic systemic IgG [in patients with CLL] does not have an impact on overall survival and is only recommended in patients with severe hypogammaglobulinemia and repeated infections [Level of evidence I, A].” They also state that “Antibiotic and antiviral prophylaxis should be used in patients with recurrent infections and/or very high risk of developing infections (e.g., pneumocystis prophylaxis with co-trimoxazole during treatment with chemoimmunotherapies based on purine analogs or bendamustine) [IV, B]” and finally, that “pneumococcal vaccination as well as seasonal influenza vaccination is recommended in early-stage CLL [IV, B]” (228).
According to The British Committee for Standards in Hematology, 2012 (205), ” in the absence of recent randomized studies, recommendations for the use of immunoglobulin replacement in CLL are largely based on clinical experience and data from use in primary immunodeficiencies.” Their recommended indication for the use of IgRT is “recurrent/severe infection with encapsulated bacteria despite prophylactic oral antibiotic therapy in patients with a serum IgG <5 g/L (excluding paraprotein)” (205).
Guidelines published in 2011 by the UK Department of Health (229) recommend that “Immunoglobulin replacement therapy is recommended in secondary antibody deficiency if the underlying cause of hypogammaglobulinaemia cannot be reversed or reversal is contraindicated, or is associated with B-cell malignancy where severe infections with encapsulated bacteria are persistent despite prophylactic antibiotic therapy (grade C recommendation, level III evidence)” (229).
The Criteria for Clinical Use of Immunoglobulin from the National Blood Authority in Australia provide a variety of recommendations for IgRT, including: “Prevention of recurrent bacterial infections due to hypogammaglobulinaemia associated with hematological malignancies or post haemopoietic stem cell transplant” (230), and “replacement therapy for recurrent or severe bacterial infections or disseminated enterovirus infection associated with hypogammaglobulinaemia caused by a recognized disease process or B cell depletion therapy and/or immunosuppressant therapy” (231). Finally, in Canada, guidelines published in 2018 state that “Immunoglobulin replacement is recommended for preventing recurrent, severe infection due to hypogammaglobulinemia (excluding paraprotein) related to other diseases or medical therapy” (232). Specifically, secondary antibody deficiency is defined as “Hypogammaglobulinemia secondary to underlying disease or medical therapy (including HSCT) with all of the following: Serum IgG less than the lower limit of the reference range on two separate occasions AND at least one of the following: a) one invasive or life-threatening bacterial infection (e.g., pneumonia, meningitis, sepsis) in the previous year; b) recurrent, severe bacterial infections; c) Clinically active bronchiectasis confirmed by radiology or d) assessment by a physician specializing in immunodeficiency indicating a significant antibody defect that would benefit from immunoglobulin replacement” (232).
In the field of SOT, there are currently no published guidelines regarding the use of IgRT in patients with recurrent bacterial infections and hypogammaglobulinemia. In the updated International Consensus Guidelines on the management of CMV in SOT, published by the Transplantation Society International CMV Consensus Group, there is a recommendation that, when CMV disease is difficult to control, IgG testing is advisable (170). According to expert opinion, CMV-specific IgRT is recommended for prophylaxis of CMV disease in high risk CMV seronegative recipients in specific settings, such as thoracic, intestinal, and pediatric transplantation (170). The European Society of Clinical Microbiology and Infectious Diseases guidelines recommend antiviral prophylaxis treatment for patients at high risk of CMV disease, such as lung and intestinal transplant recipients (233).
Overall, most guidelines are in support of considering IgRT initiation in selected patients with secondary antibody deficiency. In patients with risk factors (e.g., cardiovascular disease, diabetes, renal impairment, and thrombosis risk), a careful risk-benefit assessment should determine whether initiating IgRT is advisable. Also, the dose, route, and administration frequency should be optimized for each patient individually to maintain acceptable plasma IgG levels and a substantial reduction of infection rates (234). However, further clinical studies are required to improve and/or validate our current practice in prescribing IgG to patients with secondary antibody deficiency especially given the growth in newer therapies. It is also clear that individual assessment remains paramount, as diagnostic testing remains imperfect and not all patients will fit neatly into guidelines.
Future Directions
As the number and variety of immunosuppressive therapies increases, so will the incidence of secondary antibody deficiency (5). Accordingly, the EMA are currently in the process of updating their guidelines on the use of IVIG to specifically address secondary antibody deficiency, recommending its use for “secondary immunodeficiencies in patients who suffer from severe or recurrent bacterial infections, ineffective antibiotic treatment and either proven specific antibody failure (failure to mount at least a 2-fold rise in IgG antibody titer to pneumococcal polysaccharide and polypeptide antigen vaccines) or serum IgG level of <4 g/L” (235). Their recommended dose is 0.2–0.4 g/kg every 3 to 4 weeks. Indeed, there is also increasing evidence that SCIG offers an efficacious and efficient treatment option for secondary antibody deficiency (236).
It should be emphasized that currently, evidence regarding the newer agents is limited by study size, duration, and the number of disease settings, and longer-term prospective follow-up studies will be needed to clearly define the long term cumulative effect on antibody production and thus inform future guidelines.
In PAD, there are screening and treatment guidelines and protocols in place to identify and treat hypogammaglobulinemia (237–239). However, even in these newer, updated guidelines, there remains a paucity of recommendations related to the diagnosis and screening for secondary antibody deficiency or those addressing the treatment of secondary antibody deficiency specifically. As discussed above, vaccine-specific antibody response is a key tool in the diagnosis of secondary antibody deficiency that will benefit from new assays to assess responses to polysaccharide vaccination, such as Typhim Vi (typhoid polysaccharide vaccine, Sanofi Pasteur, UK), which are being increasingly introduced (240, 241). It will also be important to harness pharmacogenetics in patient selection and risk stratification. Future guidelines will need to focus on these under-represented and rapidly growing patient populations to harmonize and optimize screening, diagnostic, monitoring, and management approaches.
Avoiding the Consequences of Hypogammaglobulinemia
Ongoing advances in testing, earlier screening, reducing diagnostic delay, and optimizing treatment regimens are all ways to reduce the burden of secondary antibody deficiency. Determining the concentration of the serum globulin fraction (calculated globulin), of which immunoglobulins are a major component, is a useful tool for identifying undiagnosed patients with secondary antibody deficiency (242, 243). However, given the variety of causes and settings in which secondary antibody deficiency may present, raising awareness of conditions, and treatments that increase the risk of secondary antibody deficiency is also vital (Figure 5). The identification of biomarkers, such as those following heart transplantation (e.g., low anti-CD8 response to CMV, reduced complement components), will help to stratify patients by risk and improve recognition. In a wider context, clinical biomarkers that may assist in patient identification may include infection burden (particularly sinopulmonary infection), chronic sinusitis, chronic daily sputum production suggestive of bronchiectasis (5), or IgG level (<4 g/L). With regard to infection burden, factors such as infection frequency, duration, severity, and site should be considered, along with rates of antibiotic (particularly intravenous) use and hospitalization. Alongside vaccine responses, it is advisable to consider other factors, such as neutropenia, significant renal impairment, multiple lines of treatments, and ongoing therapy requirement and timing of vaccination (e.g., in relation to HSCT). In addition, performing measurements of potential biomarkers at baseline will enable objective measurement of the outcome of subsequent interventions (5).
Conclusions
The prevalence and burden of secondary antibody deficiency are substantial and increasing. Secondary antibody deficiency occurs in the majority of patients with hematological malignancies, such as CLL and MM, either as a result of disease-related effects on the immune system or as a side effect of the treatment. Secondary antibody deficiency can also emerge in less well-known at-risk patient populations, such as patients undergoing SOT (due to post-transplantation immunosuppressive therapy) (6), patients with neurological conditions (129, 130) and patients with psychiatric conditions (70). With the growth in new and more effective improved therapies for malignancy and organ transplantation, the incidence of secondary antibody deficiency is likely to increase further, in particular when targeting different and multiple components of B cell development and survival (Figure 2). As the use of these newer, more effective immunosuppressive medications becomes more prevalent, the resultant increased survival in malignancy will be accompanied by a concomitant increase in the prevalence of secondary antibody deficiency. Given the diverse etiologies of secondary antibody deficiency, healthcare practitioners from a wide range of specialties will need to anticipate the possible immunological adverse effects of the new therapeutic agents targeting the immune system. Not surprisingly, secondary antibody deficiency is often missed despite recurrent infections, resulting in delayed diagnosis and intervention (168). Therefore, there is a real need for increased awareness, screening, and improved monitoring of patients at risk of developing secondary antibody deficiency to aid early identification of these patients to avoid infection-related morbidity and mortality.
Improved screening, such as by measuring calculated globulin, is an important goal in the quest for identifying secondary antibody deficiency as early as possible. In addition, improved risk stratification and the use of biomarkers to identify the patients most likely to require interventions, such as IgRT, within each patient population will be key. secondary antibody deficiency patients will be no less individual than PAD patients and should have the same access to treatments once antibody failure has been demonstrated. The limited evidence available in secondary antibody deficiency suggests that there are significant similarities in management approaches with those used for PAD. However, cohort and prospective studies in secondary antibody deficiency are needed to support decisions regarding patient selection and treatment with targeted vaccination, prophylactic antibiotics, or IgRT. These studies should also help to identify any distinctive clinical features that may differ depending on the etiology of secondary antibody deficiency (e.g., drug-induced vs. malignancy vs. specific disease vs. transplantation setting) and how to optimize management of this under-appreciated condition.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
SJ has participated in advisory boards, trials, projects, and has been a speaker with Baxalta, CSL Behring, Shire, Thermofisher, Swedish Orphan Biovitrum, Biotest, Binding Site, Grifols, BPL, Octapharma, LFB, GSK, Weatherden, Zarodex, Sanofi, and UCB Pharma. SP has been a speaker for CSL and Biotest. JC has participated in advisory boards and projects, and has been a speaker for Grifols, Biotest, Shire, LFB, CSL Behring, Octapharma. He has a grant from the Instituto de Salud Carlos III FIS 1501472 with participation of FEDER funds, a way of making Europe.
Acknowledgments
Editorial and graphical support was provided by Vibhuti Singh and Heather Shawcross, Fishawack Communications GmbH, Basel, Switzerland, a member of the Fishawack Group of Companies, supported by CSL Behring.
References
- 1.Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. (2015) 35:696–726. 10.1007/s10875-015-0201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava S, Wood P. Secondary antibody deficiency - causes and approach to diagnosis. Clin Med. (2016) 16:571–6. 10.7861/clinmedicine.16-6-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. (2007) 27:497–502. 10.1007/s10875-007-9103-1 [DOI] [PubMed] [Google Scholar]
- 4.Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. (2010) 125(2 Suppl.):S195–203. 10.1016/j.jaci.2009.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolles S, Chapel H, Litzman J. When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: a practical approach. Clin Exp Immunol. (2017) 188:333–41. 10.1111/cei.12915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friman V, Winqvist O, Blimark C, Langerbeins P, Chapel H, Dhalla F. Secondary immunodeficiency in lymphoproliferative malignancies. Hematol Oncol. (2016) 34:121–32. 10.1002/hon.2323 [DOI] [PubMed] [Google Scholar]
- 7.Kiessling P, Lledo-Garcia R, Watanabe S, Langdon G, Tran D, Bari M, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med. (2017) 9: eaan1208. 10.1126/scitranslmed.aan1208 [DOI] [PubMed] [Google Scholar]
- 8.Ulrichts P, Guglietta A, Dreier T, van Bragt T, Hanssens V, Hofman E, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Investig. (2018) 128:4372–86. 10.1172/JCI97911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guptill JT, Juel VC, Massey JM, Anderson AC, Chopra M, Yi JS, et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity (2016) 49:472–9. 10.1080/08916934.2016.1214823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pohl MA, Lan S, Berl T, Lupus Nephritis Collaborative Study Group PLasmapheresis does not increase the risk for infection in immunosuppressed patients with severe lupus nephritis. Ann Intern Med. (1991) 114:924–9. 10.7326/0003-4819-114-11-924 [DOI] [PubMed] [Google Scholar]
- 11.Agostini C, Blau IW, Kimby E, Plesner T. Prophylactic immunoglobulin therapy in secondary immune deficiency - an expert opinion. Expert Rev Clin Immunol. (2016) 12:921–6. 10.1080/1744666X.2016.1208085 [DOI] [PubMed] [Google Scholar]
- 12.ADIVO . Historical Audit Analysis (2017). 24204681 [Google Scholar]
- 13.Dhalla F, Lucas M, Schuh A, Bhole M, Jain R, Patel SY, et al. Antibody deficiency secondary to chronic lymphocytic leukemia: should patients be treated with prophylactic replacement immunoglobulin? J Clin Immunol. (2014) 34:277–82. 10.1007/s10875-014-9995-5 [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute Cancer Stat Facts: Myeloma. Available online at: https://seer.cancer.gov/statfacts/html/mulmy.html (Accessed January 15, 2019).
- 15.National, Cancer Institute Cancer Stat Facts: LeumQ14kemia - Chronic Lymphocytic Leukemia.Available online at: https://seer.cancer.gov/statfacts/html/clyl.html (Accessed January 15, 2019).
- 16.Byrd JC, O'Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. (2013) 369:1278–9. 10.1056/NEJMoa1215637 [DOI] [PubMed] [Google Scholar]
- 17.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. (2013) 13:106–11. 10.1016/j.clml.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compagno N, Cinetto F, Semenzato G, Agostini C. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: a single-center experience in 61 patients. Haematologica (2014) 99:1101–6. 10.3324/haematol.2013.101261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunther G, Dreger B. Post-marketing observational study on 5% intravenous immunoglobulin therapy in patients with secondary immunodeficiency and recurrent serious bacterial infections. Microbiol Immunol. (2013) 57:527–35. 10.1111/1348-0421.12060 [DOI] [PubMed] [Google Scholar]
- 20.Adivo Associates. Market Research: immunoglobulin Usage in SID (2016). [Google Scholar]
- 21.Seppänen M. Immunoglobulin G treatment of secondary immunodeficiencies in the era of novel therapies. Clin Exp Immunol. (2014) 178(Suppl. 1):10–3. 10.1111/cei.12493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tete SM, Bijl M, Sahota SS, Bos NA. Immune defects in the risk of infection and response to vaccination in monoclonal gammopathy of undetermined significance and multiple myeloma. Front Immunol. (2014) 5:257. 10.3389/fimmu.2014.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blimark C, Holmberg E, Mellqvist UH, Landgren O, Bjorkholm M, Hultcrantz M, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica (2015) 100:107–13. 10.3324/haematol.2014.107714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kay NE. Abnormal T-cell subpopulation function in CLL: excessive suppressor (T gamma) and deficient helper (T mu) activity with respect to B-cell proliferation. Blood (1981) 57:418–20. [PubMed] [Google Scholar]
- 25.Dighiero G. An attempt to explain disordered immunity and hypogammaglobulinemia in B-CLL. Nouv Rev Fr Hematol. (1988) 30:283–8. [PubMed] [Google Scholar]
- 26.Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. (2008) 87:49–62. 10.1093/bmb/ldn034 [DOI] [PubMed] [Google Scholar]
- 27.Sampalo A, Navas G, Medina F, Segundo C, Camara C, Brieva JA. Chronic lymphocytic leukemia B cells inhibit spontaneous Ig production by autologous bone marrow cells: role of CD95-CD95L interaction. Blood (2000) 96:3168–74. Available online at: http://www.bloodjournal.org/content/96/9/3168 [PubMed] [Google Scholar]
- 28.Anaissie EJ, Kontoyiannis DP, O'Brien S, Kantarjian H, Robertson L, Lerner S, et al. INfections in patients with chronic lymphocytic leukemia treated with fludarabine. Ann Intern Med. (1998) 129:559–66. 10.7326/0003-4819-129-7-199810010-00010 [DOI] [PubMed] [Google Scholar]
- 29.Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. (2009) 49:1211–25. 10.1086/605664 [DOI] [PubMed] [Google Scholar]
- 30.Dall'Era M, Chakravarty E, Wallace D, Genovese M, Weisman M, Kavanaugh A, et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. (2007) 56:4142–50. 10.1002/art.23047 [DOI] [PubMed] [Google Scholar]
- 31.Ansell SM, Witzig TE, Inwards DJ, Porrata LF, Ythier A, Ferrande L, et al. Phase I clinical study of atacicept in patients with relapsed and refractory B-cell non-Hodgkin's lymphoma. Clin Cancer Res. (2008) 14:1105–10. 10.1158/1078-0432.CCR-07-4435 [DOI] [PubMed] [Google Scholar]
- 32.Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. (2008) 58:61–72. 10.1002/art.23178 [DOI] [PubMed] [Google Scholar]
- 33.Pena-Rossi C, Nasonov E, Stanislav M, Yakusevich V, Ershova O, Lomareva N, et al. An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus. Lupus (2009) 18:547–55. 10.1177/0961203309102803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi JF, Moreaux J, Hose D, Requirand G, Rose M, Rouillé V, et al. Atacicept in relapsed/refractory multiple myeloma or active Waldenström's macroglobulinemia: a phase I study. Br J Cancer (2009) 101:1051–8. 10.1038/sj.bjc.6605241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese MC, Kinnman N, de La Bourdonnaye G, Pena Rossi C, Tak PP. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum. (2011) 63:1793–803. 10.1002/art.30373 [DOI] [PubMed] [Google Scholar]
- 36.van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. (2011) 63:1782–92. 10.1002/art.30372 [DOI] [PubMed] [Google Scholar]
- 37.Isenberg D, Gordon C, Licu D, Copt S, Rossi CP, Wofsy D. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis. (2015) 74:2006–15. 10.1136/annrheumdis-2013-205067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two–week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. (2017) 69:1016–27. 10.1002/art.40049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagel J, Saxne T, Geborek P, Bengtsson AA, Jacobsen S, Svaerke Joergensen C, et al. Treatment with belimumab in systemic lupus erythematosus does not impair antibody response to 13-valent pneumococcal conjugate vaccine. Lupus (2017) 26:1072–81. 10.1177/0961203317695465 [DOI] [PubMed] [Google Scholar]
- 40.Furie R, Stohl W, Ginzler EM, Becker M, Mishra N, Chatham W, et al. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther. (2008) 10:R109. 10.1186/ar2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallace DJ. Thrombovascular events in systemic lupus erythematosus: comment on the article by Jung et al. Arthritis Rheum. (2010) 62:2824–5. 10.1002/art.27621 [DOI] [PubMed] [Google Scholar]
- 42.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3918–30. 10.1002/art.30613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. (2010) 62:201–10. 10.1002/art.27189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatham WW, Wallace DJ, Stohl W, Latinis KM, Manzi S, McCune WJ, et al. Effect of belimumab on vaccine antigen antibodies to influenza, pneumococcal, and tetanus vaccines in patients with systemic lupus erythematosus in the BLISS-76 trial. J Rheumatol. (2012) 39:1632–40. 10.3899/jrheum.111587 [DOI] [PubMed] [Google Scholar]
- 45.Merrill JT, Ginzler EM, Wallace DJ, McKay JD, Lisse JR, Aranow C, et al. Long-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosus. Arthritis Rheum. (2012) 64:3364–73. 10.1002/art.34564 [DOI] [PubMed] [Google Scholar]
- 46.van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. (2012) 71:1343–9. 10.1136/annrheumdis-2011-200937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stohl W, Merrill JT, McKay JD, Lisse JR, Zhong ZJ, Freimuth WW, et al. Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled, dose-ranging Study. J Rheumatol. (2013) 40:579–89. 10.3899/jrheum.120886 [DOI] [PubMed] [Google Scholar]
- 48.Ginzler EM, Wallace DJ, Merrill JT, Furie RA, Stohl W, Chatham WW, et al. Disease control and safety of belimumab plus standard therapy over 7 years in patients with systemic lupus erythematosus. J Rheumatol. (2014) 41:300–9. 10.3899/jrheum.121368 [DOI] [PubMed] [Google Scholar]
- 49.Pontarini E, Fabris M, Quartuccio L, Cappeletti M, Calcaterra F, Roberto A, et al. Treatment with belimumab restores B cell subsets and their expression of B cell activating factor receptor in patients with primary Sjogren's syndrome. Rheumatology (2015) 54:1429–34. 10.1093/rheumatology/kev005 [DOI] [PubMed] [Google Scholar]
- 50.Seror R, Nocturne G, Lazure T, Hendel-Chavez H, Desmoulins F, Belkhir R, et al. Low numbers of blood and salivary natural killer cells are associated with a better response to belimumab in primary Sjogren's syndrome: results of the BELISS study. Arthritis Res Ther. (2015) 17:241. 10.1186/s13075-015-0750-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quartuccio L, Salvin S, Corazza L, Gandolfo S, Fabris M, De Vita S. Efficacy of belimumab and targeting of rheumatoid factor-positive B-cell expansion in Sjogren's syndrome: follow-up after the end of the phase II open-label BELISS study. Clin Exp Rheumatol. (2016) 34:311–4. [PubMed] [Google Scholar]
- 52.Chatham W, Chadha A, Fettiplace J, Kleoudis C, Bass D, Roth D, et al. A randomized, open-label study to investigate the effect of belimumab on pneumococcal vaccination in patients with active, autoantibody-positive systemic lupus erythematosus. Lupus (2017) 26:1483–90. 10.1177/0961203317703495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knauf WU, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. (2009) 27:4378–84. 10.1200/JCO.2008.20.8389 [DOI] [PubMed] [Google Scholar]
- 54.Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma (2016) 57:512–9. 10.3109/10428194.2015.1110748 [DOI] [PubMed] [Google Scholar]
- 55.Gafter-Gvili A, Ribakovsky E, Mizrahi N, Avigdor A, Aviv A, Vidal L, et al. Infections associated with bendamustine containing regimens in hematological patients: a retrospective multi-center study. Leuk Lymphoma (2016) 57:63–9. 10.3109/10428194.2015.1046862 [DOI] [PubMed] [Google Scholar]
- 56.Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell–engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. (2011) 29:2493–8. 10.1200/JCO.2010.32.7270 [DOI] [PubMed] [Google Scholar]
- 57.Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood (2012) 119:6226–33. 10.1182/blood-2012-01-400515 [DOI] [PubMed] [Google Scholar]
- 58.Zugmaier G, Topp MS, Alekar S, Viardot A, Horst HA, Neumann S, et al. Long-term follow-up of serum immunoglobulin levels in blinatumomab-treated patients with minimal residual disease-positive B-precursor acute lymphoblastic leukemia. Blood Cancer J. (2014) 4:244. 10.1038/bcj.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. (2015) 16:57–66. 10.1016/S1470-2045(14)71170-2 [DOI] [PubMed] [Google Scholar]
- 60.Goebeler M-E, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-Cell Engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-hodgkin lymphoma: final results from a phase I study. J Clin Oncol. (2016) 34:1104–11. 10.1200/JCO.2014.59.1586 [DOI] [PubMed] [Google Scholar]
- 61.Zhu M, Wu B, Brandl C, Johnson J, Wolf A, Chow A, et al. Blinatumomab, a Bispecific T-cell Engager (BiTE®) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. (2016) 55:1271–88. 10.1007/s40262-016-0405-4 [DOI] [PubMed] [Google Scholar]
- 62.Chanan-Khan A, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. (2008) 26:4784–90. 10.1200/JCO.2007.14.9641 [DOI] [PubMed] [Google Scholar]
- 63.Yi YS, Chung JS, Song MK, Shin HJ, Seol YM, Choi YJ, et al. The risk factors for herpes zoster in bortezomib treatment in patients with multiple myeloma. Korean J Hematol. (2010) 45:188–92. 10.5045/kjh.2010.45.3.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexander T, Sarfert R, Klotsche J, Kühl AA, Rubbert-Roth A, Lorenz H-M, et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis. (2015) 74:1474–8. 10.1136/annrheumdis-2014-206016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoofnagle JH, Davis GL, Schafer DF, Peters M, Avigan MI, Pappas SC, et al. Randomized trial of chlorambucil for primary biliary cirrhosis. Gastroenterology (1986) 91:1327–34. 10.1016/0016-5085(86)90183-6 [DOI] [PubMed] [Google Scholar]
- 66.Montserrat E, Villamor N, Urbano-Ispizua A, Ribera JM, Lozano M, Vives-Corrons JL, et al. Treatment of early stage-B chronic lymphocytic leukemia with alpha-2b interferon after chlorambucil reduction of the tumoral mass. Ann Hematol. (1991) 63:15–9. 10.1007/BF01714955 [DOI] [PubMed] [Google Scholar]
- 67.Robak T, Błonski JZ, Urbanska-Ryś H, Błasinska-Morawiec M, Skotnicki AB. 2-Chlorodeoxyadenosine (Cladribine) in the treatment of patients with chronic lymphocytic leukemia 55 years old and younger. Leukemia (1999) 13:518–23. [DOI] [PubMed] [Google Scholar]
- 68.Davis JC, Austin H, Boumpas D, Fleisher TA, Yarboro C, Larson A, et al. A pilot study of 2-chloro-2′-deoxyadenosine in the treatment of systemic lupus erythematosus-associated glomerulonephritis. Arthritis Rheum. (2004) 41:335–43. [DOI] [PubMed] [Google Scholar]
- 69.Inwards DJ, Fishkin PAS, Hillman DW, Brown DW, Ansell SM, Kurtin PJ, et al. Long-term results of the treatment of patients with mantle cell lymphoma with cladribine (2-CDA) alone (95-80-53) or 2-CDA and rituximab (N0189) in the North Central Cancer Treatment Group. Cancer (2008) 113:108–16. 10.1002/cncr.23537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ponsford M, Castle D, Tahir T, Robinson R, Wade W, Steven R, et al. Clozapine is associated with secondary antibody deficiency. Br J Psychiatry (2018):1–7. [Epub ahead of print]. 10.1192/bjp.2018.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hinze-Selch D, Becker EW, Stein GM, Berg PA, Mullington J, Holsboer F, et al. Effects of clozapine on in vitro immune parameters: a longitudinal study in clozapine-treated schizophrenic patients. Neuropsychopharmacology (1998) 19:114–22. 10.1016/S0893-133X(98)00006-2 [DOI] [PubMed] [Google Scholar]
- 72.Lozano R, Marin R, Santacruz MJ, Pascual A. Effect of clozapine on immunoglobulin M plasma levels. Ther Adv Psychopharmacol. (2016) 6:58–60. 10.1177/2045125315591925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wirsum C, Glaser C, Gutenberger S, Keller B, Unger S, Voll RE, et al. Secondary antibody deficiency in glucocorticoid therapy clearly differs from primary antibody deficiency. J Clin Immunol. (2016) 36:406–12. 10.1007/s10875-016-0264-7 [DOI] [PubMed] [Google Scholar]
- 74.Kubiet MA, Gonzalez-Rothi RJ, Cottey R, Bender BS. Serum antibody response to influenza vaccine in pulmonary patients receiving corticosteroids. CHEST (1996) 110:367–70. 10.1378/chest.110.2.367 [DOI] [PubMed] [Google Scholar]
- 75.Teh BW, Harrison SJ, Worth LJ, Spelman T, Thursky KA, Slavin MA. Risks, severity and timing of infections in patients with multiple myeloma: a longitudinal cohort study in the era of immunomodulatory drug therapy. Br J Haematol. (2015) 171:100–8. 10.1111/bjh.13532 [DOI] [PubMed] [Google Scholar]
- 76.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood (2016) 128:384–94. 10.1182/blood-2015-12-687749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnsrud JJ, Susanibar S, Jo-Kamimoto J, Johnsrud AJ, Kothari A, Burgess MJ, et al. Infection risk associated with daratumumab. Open Forum Infect Dis. (2017) 4(Suppl. 1):S702–S3. 10.1093/ofid/ofx163.1884 [DOI] [Google Scholar]
- 78.Gottenberg JE, Dörner T, Bootsma H, Devauchelle-Pensec V, Bowman SJ, Mariette X, et al. Efficacy of Epratuzumab, an Anti-CD22 monoclonal IgG antibody, in systemic lupus erythematosus patients with associated Sjögren's Syndrome: post hoc analyses from the EMBODY trials. Arthritis Rheumatol. (2018) 70:763–73. 10.1002/art.40425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leonard JP, Coleman M, Ketas JC, Chadburn A, Furman R, Schuster MW, et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma. Phase I/II Clin Trial Results (2004) 10:5327–34. 10.1158/1078-0432.CCR-04-0294 [DOI] [PubMed] [Google Scholar]
- 80.Steinfeld SD, Tant L, Burmester GR, Teoh NKW, Wegener WA, Goldenberg DM, et al. Epratuzumab (humanised anti-CD22 antibody) in primary Sjögren's syndrome: an open-label phase I/II study. Arthritis Res Ther. (2006) 8:R129. 10.1186/ar2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dörner T, Kaufmann J, Wegener WA, Teoh N, Goldenberg DM, Burmester GR. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res. Ther. (2006) 8:R74. 10.1186/ar1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wallace DJ, Kalunian K, Petri MA, Strand V, Houssiau FA, Pike M, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. (2014) 73:183–90. 10.1136/annrheumdis-2012-202760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsuru T, Tanaka Y, Kishimoto M, Saito K, Yoshizawa S, Takasaki Y, et al. Safety, pharmacokinetics, and pharmacodynamics of epratuzumab in Japanese patients with moderate-to-severe systemic lupus erythematosus: results from a phase 1/2 randomized study. Modern Rheumatol. (2016) 26:87–93. 10.3109/14397595.2015.1079292 [DOI] [PubMed] [Google Scholar]
- 84.Clowse MEB, Wallace DJ, Furie RA, Petri MA, Pike MC, Leszczynski P, et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase III randomized, double-blind, placebo-controlled trials. Arthritis Rheumatol. (2017) 69:362–75. 10.1002/art.39856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer (2012) 12:121–32. 10.1038/nrc3204 [DOI] [PubMed] [Google Scholar]
- 86.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. (2013) 31:88–94. 10.1200/JCO.2012.42.7906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. (2014) 371:213–23. 10.1056/NEJMoa1400376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O'Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. (2014) 15:48–58. 10.1016/S1470-2045(13)70513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun C, Tian X, Lee YS, Gunti S, Lipsky A, Herman SE, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood (2015) 126:2213–9. 10.1182/blood-2015-04-639203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun C, Gao J, Couzens L, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol. (2016) 2:1656–7. 10.1001/jamaoncol.2016.2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.UK CLL Forum Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica (2016) 101:1563–72. 10.3324/haematol.2016.147900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood (2016) 128:195–203. 10.1182/blood-2016-03-707133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood (2014) 123:3390–7. 10.1182/blood-2013-11-535047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flinn IW, Kahl BS, Leonard JP, Furman RR, Brown JR, Byrd JC, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood (2014) 123:3406–13. 10.1182/blood-2013-11-538546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. (2014) 370:1008–18. 10.1056/NEJMoa1314583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agius MA, Klodowska-Duda G, Maciejowski M, Potemkowski A, Li J, Patra K, et al. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler J. (2017):1352458517740641. [Epub ahead of print]. 10.1177/1352458517740641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schiopu E, Chatterjee S, Hsu V, Flor A, Cimbora D, Patra K, et al. Safety and tolerability of an anti-CD19 monoclonal antibody, MEDI-551, in subjects with systemic sclerosis: a phase I, randomized, placebo-controlled, escalating single-dose study. Arthritis Res Ther. (2016) 18:131. 10.1186/s13075-016-1021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith KGC, Isbel NM, Catton MG, Leydon JA, Becker GJ, Walker RG. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dialys Transplant. (1998) 13:160–4. 10.1093/ndt/13.1.160 [DOI] [PubMed] [Google Scholar]
- 99.Munoz MA, Andres A, Gallego R, Morales E, Morales JM, Aguado JM, et al. Mycophenolate mofetil immunosuppressive therapies increase the incidence of cytomegalovirus infection in renal transplantation. Transplant Proc. (2002) 34:97. 10.1016/S0041-1345(01)02683-5 [DOI] [PubMed] [Google Scholar]
- 100.Yip NH, Lederer DJ, Kawut SM, Wilt JS, D'Ovidio F, Wang Y, et al. Immunoglobulin G levels before and after lung transplantation. Am J Respir Crit Care Med. (2006) 173:917–21. 10.1164/rccm.200510-1609OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fassbinder T, Saunders U, Mickholz E, Jung E, Becker H, Schluter B, et al. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther. (2015) 17:92. 10.1186/s13075-015-0603-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Subedi A, Magder LS, Petri M. Effect of mycophenolate mofetil on the white blood cell count and the frequency of infection in systemic lupus erythematosus. Rheumatol Int. (2015) 35:1687–92. 10.1007/s00296-015-3265-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Genovese MC, Kaine JL, Lowenstein MB, Del Giudice J, Baldassare A, Schechtman J, et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. (2008) 58:2652–61. 10.1002/art.23732 [DOI] [PubMed] [Google Scholar]
- 104.Kappos L, Li D, Calabresi PA, O'Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet (2011) 378:1779–87. 10.1016/S0140-6736(11)61649-8 [DOI] [PubMed] [Google Scholar]
- 105.Harigai M, Tanaka Y, Maisawa S. Safety and efficacy of various dosages of ocrelizumab in Japanese patients with rheumatoid arthritis with an inadequate response to methotrexate therapy: a Placebo-Controlled Double-Blind Parallel-group Study. J Rheumatol. (2012) 39:486–95. 10.3899/jrheum.110994 [DOI] [PubMed] [Google Scholar]
- 106.Stohl W, Gomez-Reino J, Olech E, Dudler J, Fleischmann RM, Zerbini CAF, et al. Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial. Ann Rheum Dis. (2012) 71:1289–96. 10.1136/annrheumdis-2011-200706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tak PP, Mease PJ, Genovese MC, Kremer J, Haraoui B, Tanaka Y, et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to at least one tumor necrosis factor inhibitor: results of a forty-eight–week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum. (2012) 64:360–70. 10.1002/art.33353 [DOI] [PubMed] [Google Scholar]
- 108.Mysler EF, Spindler AJ, Guzman R, Bijl M, Jayne D, Furie RA, et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. (2013) 65:2368–79. 10.1002/art.38037 [DOI] [PubMed] [Google Scholar]
- 109.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. (2017) 376:221–34. 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 110.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. (2017) 376:209–20. 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
- 111.Lim SH, Zhang Y, Wang Z, Esler WV, Beggs D, Pruitt B, et al. Maintenance rituximab after autologous stem cell transplant for high-risk B-cell lymphoma induces prolonged and severe hypogammaglobulinemia. Bone Marrow Transplant. (2004) 35:207–8. 10.1038/sj.bmt.1704742 [DOI] [PubMed] [Google Scholar]
- 112.Bingham CO, III, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. (2009) 62:64–74. 10.1002/art.25034 [DOI] [PubMed] [Google Scholar]
- 113.Tobinai K, Igarashi T, Itoh K, Kurosawa M, Nagai H, Hiraoka A, et al. Rituximab monotherapy with eight weekly infusions for relapsed or refractory patients with indolent B cell non-Hodgkin lymphoma mostly pretreated with rituximab: a multicenter phase II study. Cancer Sci. (2011) 102:1698–705. 10.1111/j.1349-7006.2011.02001.x [DOI] [PubMed] [Google Scholar]
- 114.Nazi I, Kelton JG, Larché M, Snider DP, Heddle NM, Crowther MA, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood (2013) 122:1946–53. 10.1182/blood-2013-04-494096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Besada E, Koldingsnes W, Nossent JC. Serum immunoglobulin levels and risk factors for hypogammaglobulinaemia during long-term maintenance therapy with rituximab in patients with granulomatosis with polyangiitis. Rheumatology (2014) 53:1818–24. 10.1093/rheumatology/keu194 [DOI] [PubMed] [Google Scholar]
- 116.Makatsori M, Kiani-Alikhan S, Manson AL, Verma N, Leandro M, Gurugama NP, et al. Hypogammaglobulinaemia after rituximab treatment—incidence and outcomes. QJM Int J Med. (2014) 107:821–8. 10.1093/qjmed/hcu094 [DOI] [PubMed] [Google Scholar]
- 117.Aguiar R, Araújo C, Martins-Coelho G, Isenberg D. Use of rituximab in systemic lupus erythematosus: a single center experience over 14 years. Arthritis Care Res. (2016) 69:257–62. 10.1002/acr.22921 [DOI] [PubMed] [Google Scholar]
- 118.Shah S, Jaggi K, Greenberg K, Geetha D. Immunoglobulin levels and infection risk with rituximab induction for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin Kidney J. (2017) 10:470–4. 10.1093/ckj/sfx014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levy J, Barnett EV, MacDonald NS, Klinenberg JR, Pearson CM. The effect of azathioprine on gammaglobulin synthesis in man. J Clin Investig. (1972) 51:2233–8. 10.1172/JCI107031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jeurissen MEC, Th. Boerbooms AM, Van De Putte LBA, Doesburg WH, Mulder J, Rasker JJ, et al. Methotrexate versus azathioprine in the treatment of rheumatoid arthritis. A forty-eight–week randomized, double-blind trial. Arthritis Rheum. (1991) 34:961–72. 10.1002/art.1780340805 [DOI] [PubMed] [Google Scholar]
- 121.Keven K, Sahin M, Kutlay S, Sengul S, Erturk S, Ersoz S, et al. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine. Transplant Infect Dis. (2003) 5:181–6. 10.1111/j.1399-3062.2003.00035.x [DOI] [PubMed] [Google Scholar]
- 122.Dotan I, Werner L, Vigodman S, Agarwal S, Pfeffer J, Horowitz N, et al. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis. (2012) 18:261–8. 10.1002/ibd.21688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dhalla F, Misbah SA. Secondary antibody deficiencies. Curr Opin Allergy Clin Immunol. (2015) 15:505–13. 10.1097/ACI.0000000000000215 [DOI] [PubMed] [Google Scholar]
- 124.Florescu DF, Kalil AC, Qiu F, Schmidt CM, Sandkovsky U. What is the impact of hypogammaglobulinemia on the rate of infections and survival in solid organ transplantation? A meta-analysis. Am J Transplant. (2013) 13:2601–10. 10.1111/ajt.12401 [DOI] [PubMed] [Google Scholar]
- 125.Sarmiento E, Jaramillo M, Calahorra L, Fernandez-Yanez J, Gomez-Sanchez M, Crespo-Leiro MG, et al. Evaluation of humoral immunity profiles to identify heart recipients at risk for development of severe infections: a multicenter prospective study. J Heart Lung Transplant. (2017) 36:529–39. 10.1016/j.healun.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 126.Sarmiento E, Rodriguez-Molina J, Munoz P, Fernandez-Yanez J, Palomo J, Fogueda M, et al. Decreased levels of serum immunoglobulins as a risk factor for infection after heart transplantation. Transplant Proc. (2005) 37:4046–9. 10.1016/j.transproceed.2005.09.153 [DOI] [PubMed] [Google Scholar]
- 127.Cortazar FB, Pendergraft WF, Wenger J, Owens CT, Laliberte K, Niles JL. The effect of continuous B cell depletion with rituximab on pathogenic autoantibodies and total IgG levels in ANCA vasculitis. Arthritis Rheumatol. (2017) 69:1045–53. 10.1002/art.40032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Vollenhoven RF, Emery P, Bingham CO, Keystone EC, Fleischmann RM, Furst DE, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. (2013) 72:1496–502. 10.1136/annrheumdis-2012-201956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tallantyre EC, Robertson NP, Jolles S. Secondary antibody deficiency in neurology. Curr Opin Allergy Clin Immunol. (2018) 18:481–8. 10.1097/ACI.0000000000000485 [DOI] [PubMed] [Google Scholar]
- 130.Tallantyre EC, Whittam DH, Jolles S, Paling D, Constantinesecu C, Robertson NP, et al. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J Neurol. (2018) 265:1115–22. 10.1007/s00415-018-8812-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. (2017) 127:3052–64. 10.1172/JCI89756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parmar S, Patel K, Pinilla-Ibarz J. Ibrutinib (imbruvica): a novel targeted therapy for chronic lymphocytic leukemia. P T. (2014) 39:483–519. [PMC free article] [PubMed] [Google Scholar]
- 133.Golay J, D'Amico A, Borleri G, Bonzi M, Valgardsdottir R, Alzani R, et al. A novel method using blinatumomab for efficient, clinical-grade expansion of polyclonal T cells for adoptive immunotherapy. J Immunol. (2014) 193:4739–47. 10.4049/jimmunol.1401550 [DOI] [PubMed] [Google Scholar]
- 134.Kalaycio ME, George Negrea O, Allen SL, Rai KR, Abbasi RM, Horne H, et al. Subcutaneous injections of low doses of humanized anti-CD20 veltuzumab: a phase I study in chronic lymphocytic leukemia*. Leuk Lymphoma (2016) 57:803–11. 10.3109/10428194.2015.1085531 [DOI] [PubMed] [Google Scholar]
- 135.Babiker HM, Glode AE, Cooke LS, Mahadevan D. Ublituximab for the treatment of CD20 positive B-cell malignancies. Expert Opin Investig Drugs (2018) 27:407–12. 10.1080/13543784.2018.1459560 [DOI] [PubMed] [Google Scholar]
- 136.Said R, Tsimberidou AM. Obinutuzumab for the treatment of chronic lymphocytic leukemia and other B-cell lymphoproliferative disorders. Expert Opin Biol Ther. (2017) 17:1463–70. 10.1080/14712598.2017.1377178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Masoud S, McAdoo SP, Bedi R, Cairns TD, Lightstone L. Ofatumumab for B cell depletion in patients with systemic lupus erythematosus who are allergic to rituximab. Rheumatology (2018) 57:1156–61. 10.1093/rheumatology/key042 [DOI] [PubMed] [Google Scholar]
- 138.van de Donk NWCJ. Immunomodulatory effects of CD38-targeting antibodies. Immunol Lett. (2018) 199:16–22. 10.1016/j.imlet.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 139.Geh D, Gordon C. Epratuzumab for the treatment of systemic lupus erythematosus. Expert Rev Clin Immunol. (2018) 14:245–58. 10.1080/1744666X.2018.1450141 [DOI] [PubMed] [Google Scholar]
- 140.Martin P, Furman RR, Rutherford S, Ruan J, Ely S, Greenberg J, et al. Phase I study of the anti-CD74 monoclonal antibody milatuzumab (hLL1) in patients with previously treated B-cell lymphomas. Leuk Lymphoma (2015) 56:3065–70. 10.3109/10428194.2015.1028052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Syed YY. Ocrelizumab: a review in multiple sclerosis. CNS Drugs (2018) 32:883–90. 10.1007/s40263-018-0568-7 [DOI] [PubMed] [Google Scholar]
- 142.Jain P, Kantarjian H, Alattar ML, Jabbour E, Sasaki K, Gonzalez GN, et al. Analysis of long term responses and their impact on outcomes in patients with chronic phase CML treated with four different TKI modalities – analysis of 5 prospective clinical trials. Lancet Haematol. (2015) 2:e118–28. 10.1016/S2352-3026(15)00021-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ursini F, Russo E, De Giorgio R, De Sarro G, D'Angelo S. Current treatment options for psoriatic arthritis: spotlight on abatacept. Ther Clin Risk Manag. (2018) 14:1053–9. 10.2147/TCRM.S148586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pellom ST, Dudimah DF, Thounaojam MC, Sayers TJ, Shanker A. Modulatory effects of bortezomib on host immune cell functions. Immunotherapy (2015) 7:1011–22. 10.2217/imt.15.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Witcher J, Fleischmann R, Chindalore VL, Hansen RJ, Hu L, Radtke D, et al. Pharmacokinetics and safety of single doses of tabalumab in subjects with rheumatoid arthritis or systemic lupus erythematosus. Br J Clin Pharmacol. (2016) 81:908–17. 10.1111/bcp.12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cogollo E, Silva MA, Isenberg D. Profile of atacicept and its potential in the treatment of systemic lupus erythematosus. Drug Design Dev Ther. (2015) 9:1331–9. 10.2147/DDDT.S71276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sanchez L, Wang Y, Siegel DS, Wang ML. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol. (2016) 9:51. 10.1186/s13045-016-0283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martin T, Baz R, Benson DM, Lendvai N, Wolf J, Munster P, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood (2017) 129:3294–303. 10.1182/blood-2016-09-740787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mikulska M, Lanini S, Gudiol C, Drgona L, Ippolito G, Fernández-Ruiz M, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect. (2018) 24:S71–82. 10.1016/j.cmi.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 150.Emery P, Rigby W, Tak PP, Dörner T, Olech E, Martin C, et al. Safety with ocrelizumab in rheumatoid arthritis: results from the ocrelizumab phase III program. PLoS ONE (2014) 9:e87379. 10.1371/journal.pone.0087379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Drgona L, Gudiol C, Lanini S, Salzberger B, Ippolito G, Mikulska M. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid or myeloid cells surface antigens [II]: CD22, CD30, CD33, CD38, CD40, SLAMF-7 and CCR4). Clin Microbiol Infect. (2018) 24:S83–94. 10.1016/j.cmi.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 152.Gordon C, Wofsy D, Wax S, Li Y, Pena Rossi C, Isenberg D. Post hoc Analysis of the Phase II/III APRIL-SLE study: association between response to atacicept and serum biomarkers including BLyS and APRIL. Arthritis Rheumatol. (2016) 69:122–30. 10.1002/art.39809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Merrill JT, Wallace DJ, Wax S, Kao A, Fraser PA, Chang P, et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus. Arthritis Rheumatol. (2017) 70:266–76. 10.1002/art.40360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van Vollenhoven RF, Wax S, Li Y, Tak PP. Safety and efficacy of atacicept in combination with rituximab for reducing the signs and symptoms of rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled pilot trial. Arthritis Rheumatol. (2015) 67:2828–36. 10.1002/art.39262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood (2014) 123:2625–35. 10.1182/blood-2013-11-492231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Enblad G, Karlsson H, Gammelgard G, Wenthe J, Lovgren T, Amini RM, et al. A phase I/IIa trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin Cancer Res. (2018) 24:6185–94. 10.1158/1078-0432.CCR-18-0426 [DOI] [PubMed] [Google Scholar]
- 157.Ramos CA, Rouce R, Robertson CS, Reyna A, Narala N, Vyas G, et al. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin's lymphomas. Mol Ther. (2018) 26:2727–37. 10.1016/j.ymthe.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reusch U, Duell J, Ellwanger K, Herbrecht C, Knackmuss SH, Fucek I, et al. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. MAbs (2015) 7:584–604. 10.1080/19420862.2015.1029216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. (2007) 7:715–25. 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- 160.Organdonor.gov. Organ Donation Statistics (2016). Available online at: https://www.organdonor.gov/statistics-stories/statistics.html (Accessed January 15, 2019).
- 161.Reske A, Reske A, Metze M. Complications of immunosuppressive agents therapy in transplant patients. Minerva Anestesiol. (2015) 81:1244–61. 10.1016/j.rcl.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 162.Yamani MH, Avery R, Mawhorter S, Young JB, McNeill A, Cook DJ, et al. Hypogammaglobulinemia after heart transplantation: impact of pre-emptive use of immunoglobulin replacement (CytoGam) on infection and rejection outcomes. Transpl Infect Dis. (2001) 3(Suppl. 2):40–3. 10.1034/j.1399-3062.2001.00008.x [DOI] [PubMed] [Google Scholar]
- 163.ISHLT International Registry for Heart and Lung Transplantation Heart/Lung Registries (2017) Available online at: https://ishltregistries.org/registries/slides.asp?year=2017 (Accessed January 15, 2019).
- 164.Compagno N, Malipiero G, Cinetto F, Agostini C. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. (2014) 5:626. 10.3389/fimmu.2014.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sarmiento E, Rodriguez-Hernandez C, Rodriguez-Molina J, Fernandez-Yanez J, Palomo J, Anguita J, et al. Impaired anti-pneumococcal polysaccharide antibody production and invasive pneumococcal infection following heart transplantation. Int Immunopharmacol. (2006) 6:2027–30. 10.1016/j.intimp.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 166.Christou EAA, Giardino G, Worth A, Ladomenou F. Risk factors predisposing to the development of hypogammaglobulinemia and infections post-Rituximab. Int Rev Immunol. (2017) 36:352–9. 10.1080/08830185.2017.1346092 [DOI] [PubMed] [Google Scholar]
- 167.Cohen M, Romero G, Bas J, Ticchioni M, Rosenthal M, Lacroix R, et al. Monitoring CD27+ memory B-cells in neuromyelitis optica spectrum disorders patients treated with rituximab: Results from a bicentric study. J Neurol Sci. (2017) 373:335–8. 10.1016/j.jns.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 168.Duraisingham SS, Buckland MS, Grigoriadou S, Longhurst HJ. Secondary antibody deficiency. Expert Rev Clin Immunol. (2014) 10:583–91. 10.1586/1744666X.2014.902314 [DOI] [PubMed] [Google Scholar]
- 169.Curry B, Palmer E, Mounce C, Smith G, Shah V. Assessing prescribing practices of clozapine before and after the implementation of an updated risk evaluation and mitigation strategy. Mental Health Clin. (2018) 8:63–7. 10.9740/mhc.2018.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation (2018) 102:900–31. 10.1097/TP.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 171.Asgari E, Zhou W, Sacks S. Complement in organ transplantation. Curr Opin Organ Transplant. (2010) 15:486–91. 10.1097/MOT.0b013e32833b9cb7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fernandez-Ruiz M, Kumar D, Humar A. Clinical immune-monitoring strategies for predicting infection risk in solid organ transplantation. Clin Transl Immunol. (2014) 3:e12. 10.1038/cti.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fernandez-Ruiz M, Lopez-Medrano F, Varela-Pena P, Morales JM, Garcia-Reyne A, San Juan R, et al. Hypocomplementemia in kidney transplant recipients: impact on the risk of infectious complications. Am J Transplant. (2013) 13:685–94. 10.1111/ajt.12055 [DOI] [PubMed] [Google Scholar]
- 174.Carbone J, Micheloud D, Salcedo M, Rincon D, Banares R, Clemente G, et al. Humoral and cellular immune monitoring might be useful to identify liver transplant recipients at risk for development of infection. Transpl Infect Dis. (2008) 10:396–402. 10.1111/j.1399-3062.2008.00329.x [DOI] [PubMed] [Google Scholar]
- 175.Sarmiento E, del Pozo N, Gallego A, Fernandez-Yanez J, Palomo J, Villa A, et al. Decreased levels of serum complement C3 and natural killer cells add to the predictive value of total immunoglobulin G for severe infection in heart transplant recipients. Transpl Infect Dis. (2012) 14:526–39. 10.1111/j.1399-3062.2012.00757.x [DOI] [PubMed] [Google Scholar]
- 176.Corales R, Chua J, Mawhorter S, Young JB, Starling R, Tomford JW, et al. Significant post-transplant hypogammaglobulinemia in six heart transplant recipients: an emerging clinical phenomenon? Transpl Infect Dis. (2000) 2:133–9. 10.1034/j.1399-3062.2000.020306.x [DOI] [PubMed] [Google Scholar]
- 177.Munoz P, Giannella M, Alcala L, Sarmiento E, Fernandez Yanez J, Palomo J, et al. Clostridium difficile-associated diarrhea in heart transplant recipients: is hypogammaglobulinemia the answer? J Heart Lung Transplant. (2007) 26:907–14. 10.1016/j.healun.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 178.Sarmiento E, Lanio N, Gallego A, Rodriguez-Molina J, Navarro J, Fernandez-Yanez J, et al. Immune monitoring of anti cytomegalovirus antibodies and risk of cytomegalovirus disease in heart transplantation. Int Immunopharmacol. (2009) 9:649–52. 10.1016/j.intimp.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 179.Gennery AR, Cant AJ, Spickett GP, Walshaw D, Hunter S, Hasan A, et al. Effect of immunosuppression after cardiac transplantation in early childhood on antibody response to polysaccharide antigen. Lancet (1998) 351:1778–81. 10.1016/S0140-6736(97)08486-9 [DOI] [PubMed] [Google Scholar]
- 180.Gennery AR, Barge D, Spickett GP, Cant AJ. Lymphocyte subset populations in children with polysaccharide antibody deficiency following cardiac transplantation. J Clin Immunol. (2001) 21:37–42. 10.1023/A:1006741015452 [DOI] [PubMed] [Google Scholar]
- 181.Lazzarotto T, Varani S, Spezzacatena P, Pradelli P, Potena L, Lombardi A, et al. Delayed acquisition of high-avidity anti-cytomegalovirus antibody is correlated with prolonged antigenemia in solid organ transplant recipients. J Infect Dis. (1998) 178:1145–9. 10.1086/515671 [DOI] [PubMed] [Google Scholar]
- 182.Iberer F, Halwachs-Baumann G, Rodl S, Pleisnitzer A, Wasler A, Auer T, et al. Monitoring of cytomegalovirus disease after heart transplantation: persistence of anti-cytomegalovirus IgM antibodies. J Heart Lung Transplant. (1994) 13:405–11. [PubMed] [Google Scholar]
- 183.Carbone J, Sarmiento E, Del Pozo N, Rodriguez-Molina JJ, Navarro J, Fernandez-Yanez J, et al. Restoration of humoral immunity after intravenous immunoglobulin replacement therapy in heart recipients with post-transplant antibody deficiency and severe infections. Clin Transplant. (2012) 26:E277–83. 10.1111/j.1399-0012.2012.01653.x [DOI] [PubMed] [Google Scholar]
- 184.Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. (2005) 201:1031–6. 10.1084/jem.20042384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tu W, Potena L, Stepick-Biek P, Liu L, Dionis KY, Luikart H, et al. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation (2006) 114:1608–15. 10.1161/CIRCULATIONAHA.105.607549 [DOI] [PubMed] [Google Scholar]
- 186.Sarmiento E, Navarro J, Fernandez-Yanez J, Palomo J, Munoz P, Carbone J. Evaluation of an immunological score to assess the risk of severe infection in heart recipients. Transpl Infect Dis. (2014) 16:802–12. 10.1111/tid.12284 [DOI] [PubMed] [Google Scholar]
- 187.Chong PP, Avery RK. A comprehensive review of immunization practices in solid organ transplant and hematopoietic stem cell transplant recipients. Clin Ther. (2017) 39:1581–98. 10.1016/j.clinthera.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 188.Broeders EN, Wissing KM, Ghisdal L, Lemy A, Hoang AD, Vereerstraeten P, et al. Large decrease of anti-tetanus anatoxin and anti-pneumococcal antibodies at one year after renal transplantation. Clin Nephrol. (2013) 79:313–7. 10.5414/CN107779 [DOI] [PubMed] [Google Scholar]
- 189.Frangoul H, Min E, Wang W, Chandrasekhar R, Calder C, Evans M, et al. Incidence and risk factors for hypogammaglobulinemia in pediatric patients following allo-SCT. Bone Marrow Transplant. (2013) 48:1456–9. 10.1038/bmt.2013.76 [DOI] [PubMed] [Google Scholar]
- 190.Witherspoon RP, Storb R, Ochs HD, Fluornoy N, Kopecky KJ, Sullivan KM, et al. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood (1981) 58:360–8. [PubMed] [Google Scholar]
- 191.Kuzmina Z, Greinix HT, Weigl R, Kormoczi U, Rottal A, Frantal S, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood (2011) 117:2265–74. 10.1182/blood-2010-07-295766 [DOI] [PubMed] [Google Scholar]
- 192.Podgorny PJ, Liu Y, Dharmani-Khan P, Pratt LM, Jamani K, Luider J, et al. Immune cell subset counts associated with graft-versus-host disease. Biol Blood Marrow Transplant. (2014) 20:450–62. 10.1016/j.bbmt.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 193.Terpos E, Kleber M, Engelhardt M, Zweegman S, Gay F, Kastritis E, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica (2015) 100:1254–66. 10.3324/haematol.2014.117176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. (2014) 58:e44–100. 10.1093/cid/cit684 [DOI] [PubMed] [Google Scholar]
- 195.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. (2015) 372:1114–25. 10.1056/NEJMoa1408544 [DOI] [PubMed] [Google Scholar]
- 196.Anderson KC, Alsina M, Atanackovic D, Biermann JS, Chandler JC, Costello C, et al. Multiple myeloma, version 2.2016: clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2015) 13:1398–435. 10.6004/jnccn.2015.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Duchini A, Goss JA, Karpen S, Pockros PJ. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. (2003) 16:357–64. 10.1128/CMR.16.3.357-364.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Broyer M, Tete MJ, Guest G, Gagnadoux MF, Rouzioux C. Varicella and zoster in children after kidney transplantation: long-term results of vaccination. Pediatrics (1997) 99:35–9. 10.1542/peds.99.1.35 [DOI] [PubMed] [Google Scholar]
- 199.Danzinger-Isakov L, Kumar D, Practice ASTIDCo. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. (2009) 9(Suppl. 4):S258–62. 10.1111/j.1600-6143.2009.02917.x [DOI] [PubMed] [Google Scholar]
- 200.van Kessel DA, Hoffman TW, Kwakkel-van Erp JM, Oudijk ED, Zanen P, Rijkers GT, et al. Long-term follow-up of humoral immune status in adult lung transplant recipients. Transplantation (2017) 101:2477–83. 10.1097/TP.0000000000001685 [DOI] [PubMed] [Google Scholar]
- 201.Lee S, Kim HW, Kim K-H. Antibodies against Hepatitis A and Hepatitis B virus in intravenous immunoglobulin products. J Korean Med Sci. (2016) 31:1937–42. 10.3346/jkms.2016.31.12.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nobre FA, Gonzalez IGdS, Simão RM, de Moraes Pinto MI, Costa-Carvalho BT. Antibody levels to tetanus, diphtheria, measles and varicella in patients with primary immunodeficiency undergoing intravenous immunoglobulin therapy: a prospective study. BMC Immunol. (2014) 15:26. 10.1186/1471-2172-15-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gilbert SC. T-cell-inducing vaccines – what's the future. Immunology (2012) 135:19–26. 10.1111/j.1365-2567.2011.03517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.National Center for Immunization and Respiratory Diseases General recommendations on immunization — recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. (2011) 60:1–64. Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm [PubMed] [Google Scholar]
- 205.Oscier D, Dearden C, Eren E, Fegan C, Follows G, Hillmen P, et al. Guidelines on the diagnosis, investigation and management of chronic lymphocytic leukaemia. Br J Haematol. (2012) 159:541–64. 10.1111/bjh.12067 [DOI] [PubMed] [Google Scholar]
- 206.Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. Gale RP, Chapel HM, Bunch C, Rai KR, Foon K, et al. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled clinical trial. N Engl J Med. (1988) 319:902–7. 10.1056/NEJM198810063191403 [DOI] [PubMed] [Google Scholar]
- 207.Jurlander J, Geisler CH, Hansen MM. Treatment of hypogammaglobulinaemia in chronic lymphocytic leukaemia by low-dose intravenous gammaglobulin. Eur J Haematol. (1994) 53:114–8. 10.1111/j.1600-0609.1994.tb01874.x [DOI] [PubMed] [Google Scholar]
- 208.Chapel H, Dicato M, Gamm H, Brennan V, Ries F, Bunch C, et al. Immunoglobulin replacement in patients with chronic lymphocytic leukaemia: a comparison of two dose regimes. Br J Haematol. (1994) 88:209–12. 10.1111/j.1365-2141.1994.tb05002.x [DOI] [PubMed] [Google Scholar]
- 209.Sklenar I, Schiffman G, Jonsson V, Verhoef G, Birgens H, Boogaerts M, et al. Effect of various doses of intravenous polyclonal IgG on in vivo levels of 12 pneumococcal antibodies in patients with chronic lymphocytic leukaemia and multiple myeloma. Oncology (1993) 50:466–77. 10.1159/000227231 [DOI] [PubMed] [Google Scholar]
- 210.Griffiths H, Brennan V, Lea J, Bunch C, Lee M, Chapel H. Crossover study of immunoglobulin replacement therapy in patients with low-grade B-cell tumors. Blood (1989) 73:366–8. [PubMed] [Google Scholar]
- 211.Boughton BJ, Jackson N, Lim S, Smith N. Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clin Lab Haematol. (1995) 17:75–80. 10.1111/j.1365-2257.1995.tb00322.x [DOI] [PubMed] [Google Scholar]
- 212.Molica S, Musto P, Chiurazzi F, Specchia G, Brugiatelli M, Cicoira L, et al. Prophylaxis against infections with low-dose intravenous immunoglobulins (IVIG) in chronic lymphocytic leukemia. Results of a crossover study. Haematologica (1996) 81:121–6. [PubMed] [Google Scholar]
- 213.National Institutes on Health Consensus on IVIG. Lancet (1990) 336:470–2. 10.1016/0140-6736(90)92016-B1974993 [DOI] [Google Scholar]
- 214.European Medicines Agency Guideline on Core SmPC for Human Normal Immunoglobulin for Intravenous Administration (IVIg): EMA (2018). Available online at: https://www.ema.europa.eu/documents/scientific-guideline/guideline-core-smpc-human-normal-immunoglobulin-intravenous-administration-ivig-rev-5_en.pdf (October 2018).
- 215.Besa EC, Klumpe D. Prophylactic immune globulin in chronic lymphocytic leukemia. N Engl J Med. (1992) 326:139. 10.1056/NEJM199201093260216 [DOI] [PubMed] [Google Scholar]
- 216.Ammann EM, Jones MP, Link BK, Carnahan RM, Winiecki SK, Torner JC, et al. Intravenous immune globulin and thromboembolic adverse events in patients with hematologic malignancy. Blood (2016) 127:200–7. 10.1182/blood-2015-05-647552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. (2013) 27:171–8. 10.1016/j.tmrv.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 218.Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M, Subcutaneous Ig GSG. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. (2006) 26:265–73. 10.1007/s10875-006-9021-7 [DOI] [PubMed] [Google Scholar]
- 219.Wasserman RL, Melamed I, Nelson RP, Jr, Knutsen AP, Fasano MB, Stein MR, et al. Pharmacokinetics of subcutaneous IgPro20 in patients with primary immunodeficiency. Clin Pharmacokinet. (2011) 50:405–14. 10.2165/11587030-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 220.Jolles S. Subcutaneous and Intramuscular Immune Globulin Therapy (2018). Available online at: https://www.uptodate.com/contents/subcutaneous-and-intramuscular-immune-globulin-therapy (Accessed January 15, 2019).
- 221.Gardulf A, Nicolay U, Math D, Asensio O, Bernatowska E, Bock A, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. (2004) 114:936–42. 10.1016/j.jaci.2004.06.053 [DOI] [PubMed] [Google Scholar]
- 222.Chapel H, Brennan V, Delson E. Immunoglobulin replacement therapy by self-infusion at home. Clin Exp Immunol. (1988) 73:160–2. [PMC free article] [PubMed] [Google Scholar]
- 223.Sarmiento E, Diez P, Arraya M, Jaramillo M, Calahorra L, Fernandez-Yanez J, et al. Early intravenous immunoglobulin replacement in hypogammaglobulinemic heart transplant recipients: results of a clinical trial. Transpl Infect Dis. (2016) 18:832–43. 10.1111/tid.12610 [DOI] [PubMed] [Google Scholar]
- 224.Yamani MH, Avery R, Mawhorter SD, McNeill A, Cook D, Ratliff NB, et al. The impact of CytoGam on cardiac transplant recipients with moderate hypogammaglobulinemia: a randomized single-center study. J Heart Lung Transplant. (2005) 24:1766–9. 10.1016/j.healun.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 225.Carbone J, Sarmiento E, Palomo J, Fernandez-Yanez J, Munoz P, Bouza E, et al. The potential impact of substitutive therapy with intravenous immunoglobulin on the outcome of heart transplant recipients with infections. Transplant Proc. (2007) 39:2385–8. 10.1016/j.transproceed.2007.06.050 [DOI] [PubMed] [Google Scholar]
- 226.Sarmiento E, Fernandez-Yanez J, Munoz P, Palomo J, Rodriguez-Molina JJ, Bermejo J, et al. Hypogammaglobulinemia after heart transplantation: use of intravenous immunoglobulin replacement therapy in relapsing CMV disease. Int Immunopharmacol. (2005) 5:97–101. 10.1016/j.intimp.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 227.European Medicines Agency Guideline on the Clinical Investigation of Human Normal Immunoglobulin for Subcutaneous and/or Intramuscular Administration (SCIg/IMIg): EMA (2015). Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/07/WC500190211.pdf (Accessed January 15, 2019).
- 228.Eichhorst B, Robak T, Montserrat E, Ghia P, Hillmen P, Hallek M, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2015) 26(Suppl. 5):v78–84. 10.1093/annonc/mdv303 [DOI] [PubMed] [Google Scholar]
- 229.Department of Health Clinical Guidelines for Immunoglobulin Use (2011). Available online at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216671/dh_131107.pdf (Accessed January 15, 2019).
- 230.Australian National Blood Authority Acquired Hypogammaglobulinaemia Secondary to Haematological Malignancies, or Post-Haemopoietic Stem Cell Transplantation (HSCT): BloodStar (2018). Available online at: https://www.criteria.blood.gov.au/MedicalCondition/View/2570 (Accessed January 15, 2019).
- 231.Australian National Blood Authority Secondary Hypogammaglobulinaemia Unrelated to Haematological Malignancy or Haemopoeitic Stem Cell Transplant (HSCT): BloodStar (2018). Available online at: https://www.criteria.blood.gov.au/MedicalCondition/View/2577 (Accessed January 15, 2019).
- 232.Alberta Ministry of Health, Shared Health Manitoba, Saskatchewan Ministry of Health Criteria for the Clinical Use of Immune Globulin (2018). Available online at: https://www.ihe.ca/download/criteria_for_the_clinical_use_of_immune_globulin_first_edition.pdf (Accessed January 15, 2019).
- 233.Lumbreras C, Manuel O, Len O, ten Berge IJ, Sgarabotto D, Hirsch HH. Cytomegalovirus infection in solid organ transplant recipients. Clin Microbiol Infect. (2014) 20(Suppl. 7):19–26. 10.1111/1469-0691.12594 [DOI] [PubMed] [Google Scholar]
- 234.Jolles S, Orange JS, Gardulf A, Stein MR, Shapiro R, Borte M, et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. (2015) 179:146–60. 10.1111/cei.12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.European Medicines Agency Guideline on the Clinical Investigation of Human Normal Immunoglobulin for Intravenous Administration (IVIg): EMA (2018). Available online at: https://www.ema.europa.eu/documents/scientific-guideline/guideline-clinical-investigation-human-normal-immunoglobulin-intravenous-administration-ivig-rev-3_en.pdf (Accessed January 15, 2019).
- 236.Streu E, Banerji V, Dhaliwal DHS. The efficacy and cost effectiveness of Subcutaneous Immunoglobulin (SCIG) replacement in patients with immune deficiency secondary to chronic lymphocytic leukemia. Blood (2016) 128:4778 Available online at: http://www.bloodjournal.org/content/128/22/4778?sso-checked=true [Google Scholar]
- 237.Abolhassani H, Sagvand BT, Shokuhfar T, Mirminachi B, Rezaei N, Aghamohammadi A. A review on guidelines for management and treatment of common variable immunodeficiency. Expert Rev Clin Immunol. (2013) 9:561–75. 10.1586/eci.13.30 [DOI] [PubMed] [Google Scholar]
- 238.Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. (2015) 136:1186–205.e78. 10.1016/j.jaci.2015.04.049 [DOI] [PubMed] [Google Scholar]
- 239.Immune Deficiency Foundation Diagnostic & Clinical Care Guidelines for Primary Immunodeficiency Diseases (2009). Available online at: https://primaryimmune.org/wp-content/uploads/2011/04/IDF-Diagnostic-Clinical-Care-Guidelines-for-Primary-Immunodeficiency-Diseases-2nd-Edition.pdf (Accessed January 15, 2019).
- 240.Evans C, Bateman E, Steven R, Ponsford M, Cullinane A, Shenton C, et al. Measurement of Typhim Vi antibodies can be used to assess adaptive immunity in patients with immunodeficiency. Clin Exp Immunol. (2018) 192:292–301. 10.1111/cei.13105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Parker AR, Bradley C, Harding S, Sánchez-Ramón S, Jolles S, Kiani-Alikhan S. Measurement and interpretation of Salmonella typhi Vi IgG antibodies for the assessment of adaptive immunity. J Immunol Methods (2018) 459:1–10. 10.1016/j.jim.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 242.Jolles S, Borrell R, Zouwail S, Heaps A, Sharp H, Moody M, et al. Calculated globulin (CG) as a screening test for antibody deficiency. Clin Exp Immunol. (2014) 177:671–8. 10.1111/cei.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Holding S, Jolles S. Current screening approaches for antibody deficiency. Curr Opin Allergy Clin Immunol. (2015) 15:547–55. 10.1097/ACI.0000000000000222 [DOI] [PubMed] [Google Scholar]
- 244.Smith J, Fernando T, McGrath N, Ameratunga R. Lamotrigine-induced common variable immune deficiency. Neurology (2004) 62:833–4. 10.1212/01.WNL.0000113754.29225.5D [DOI] [PubMed] [Google Scholar]
- 245.Yu KK, Dasanu CA. Rapidly fatal dissemination of merkel cell carcinoma in a patient treated with alemtuzumab for chronic lymphocytic leukemia. Conn Med. (2016) 80:353–8. [PubMed] [Google Scholar]
- 246.Uhm J, Hamad N, Gupta V, Kuruvilla J, Messner HA, Seftel M, et al. The risk factors for IgG hypogammaglobulinemia after allogeneic hematopoietic stem cell transplantation and its impact on transplant outcomes. Blood (2014) 124:3928 Available online at: http://www.bloodjournal.org/content/124/21/3928?sso-checked=true [Google Scholar]
- 247.Hlavackova E, Liska M, Jicinska H, Navratil J, Litzman J. Secondary combined immunodeficiency in pediatric patients after the fontan operation: three case reports Int Arch Allergy Immunol. 2016; 170: 251–6 10.1159/000449163 [DOI] [PubMed] [Google Scholar]
- 248.Norlin AC, Sairafi D, Mattsson J, Ljungman P, Ringdén O, Remberger M. Allogeneic stem cell transplantation: low immunoglobulin levels associated with decreased survival. Bone Marrow Transplant. (2008) 41:267–73. 10.1038/sj.bmt.1705892 [DOI] [PubMed] [Google Scholar]
- 249.Stiehm ER. Use of immunoglobulin therapy in secondary antibody deficiencies. In: Imbach P, Nydegger UE, Morell A, et al., editors. Immunotherapy With Intravenous Immunoglobulins. London: Academic Press; (1991). p. 115–26. 10.1016/B978-0-12-370725-3.50015-4 [DOI] [Google Scholar]
- 250.Hodge D, Misbah SA, Mueller RF, Glass EJ, Chetcuti PA. Proteus syndrome and immunodeficiency. Arch Dis Child. (2000) 82:234–5. 10.1136/adc.82.3.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hargreaves RM, Lea JR, Griffiths H, Faux JA, Holt JM, Reid C, et al. Immunological factors and risk of infection in plateau phase myeloma. J Clin Pathol. (1995) 48:260–6. 10.1136/jcp.48.3.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chakrabarti S, Keeton BR, Salmon AP, Vettukatti JJ. Acquired combined immunodeficiency associated with protein losing enteropathy complicating Fontan operation. Heart (2003) 89:1130–1. 10.1136/heart.89.10.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dominguez-Pinilla N, Benitez EM, Gonzalez-Tome MI, Ruiz-Contreras J, Gonzalez-Granado LI. Invasive pneumococcal infection secondary to hypogammaglobulinemia due to Menetrier disease. Pediatr Infect Dis J. (2013) 32:578. 10.1097/INF.0b013e3182815064 [DOI] [PubMed] [Google Scholar]
- 254.Kaminsky P, Lesesve JF, Jonveaux P, Pruna L. IgG deficiency and expansion of CTG repeats in myotonic dystrophy. Clin Neurol Neurosurg. (2011) 113:464–8. 10.1016/j.clineuro.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 255.Santachiara R, Maffei R, Martinelli S, Arcari A, Piacentini F, Trabacchi E, et al. Development of hypogammaglobulinemia in patients treated with imatinib for chronic myeloid leukemia or gastrointestinal stromal tumor. Haematologica (2008) 93:1252–5. 10.3324/haematol.12642 [DOI] [PubMed] [Google Scholar]
- 256.Srivannaboon K, Conley ME, Coustan-Smith E, Wang WC. Hypogammaglobulinemia and reduced numbers of B-cells in children with myelodysplastic syndrome. J Pediatr Hematol Oncol. (2001) 23:122–5. 10.1097/00043426-200102000-00011 [DOI] [PubMed] [Google Scholar]
- 257.Braamskamp MJAM, Dolman KM, Tabbers MM. Clinical practice: protein-losing enteropathy in children. Eur J Pediatrics (2010) 169:1179–85. 10.1007/s00431-010-1235-2 [DOI] [PMC free article] [PubMed] [Google Scholar]