Abstract
Tetrazole derivatives are a prime class of heterocycles, very important to medicinal chemistry and drug design due to not only their bioisosterism to carboxylic acid and amide moieties but also to their metabolic stability and other beneficial physicochemical properties. Although more than 20 FDA-approved drugs contain 1H- or 2H-tetrazole substituents, their exact binding mode, structural biology, 3D conformations, and in general their chemical behavior is not fully understood. Importantly, multicomponent reaction (MCR) chemistry offers convergent access to multiple tetrazole scaffolds providing the three important elements of novelty, diversity, and complexity, yet MCR pathways to tetrazoles are far from completely explored. Here, we review the use of multicomponent reactions for the preparation of substituted tetrazole derivatives. We highlight specific applications and general trends holding therein and discuss synthetic approaches and their value by analyzing scope and limitations, and also enlighten their receptor binding mode. Finally, we estimated the prospects of further research in this field.
1. Introduction
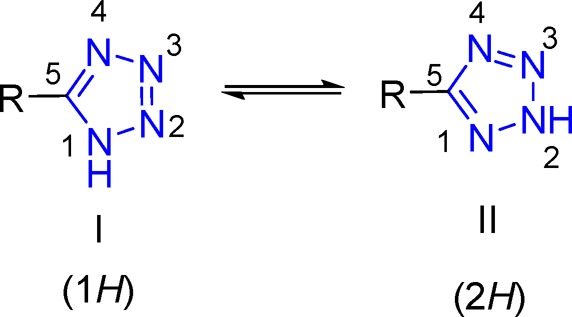
Tetrazoles belong to the class of twice unsaturated five-membered ring aromatic heterocycles, consisting of one carbon and four nitrogen atoms. They do not exist in nature. Interestingly, they have the highest number of nitrogen atoms among the stable heterocycles because pentazoles are highly explosive compounds even at low temperature.1 The first report of the synthesis of a tetrazole derivative was obtained by the Swedish chemist J. A. Bladin in 1885 at the University of Upsala.2,3 He observed that the reaction of dicyanophenylhydrazine and nitrous acid led to the formation of a compound with the chemical formula of C8H5N5 which he later proposed the name “tetrazole” for the new ring structure. On the basis of the number of the substituents, tetrazoles can be classified as un-, mono-, di-, and trisubstituted. 5-Substituted tetrazoles with 6π electrons may exist in tautomeric forms as either I or II (Scheme 1). In solution, the 1H tautomer is the predominant form, but in the gas phase the 2H-tautomer is more stable.1
Scheme 1. Tautomerism of Tetrazole Derivatives.
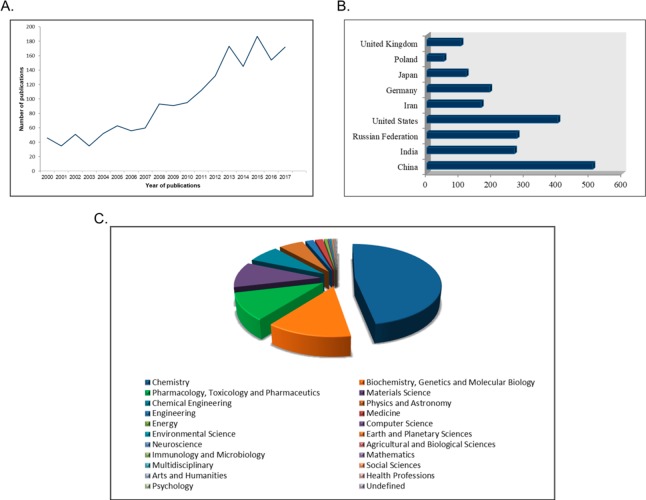
The tetrazole motif is an important synthetic scaffold that found broad applications in numerous fields such as in medicine, biochemistry, pharmacology, and in industry as materials, e.g., in photography, imaging chemicals, and military.4−9 Indicatively, tetrazole derivatives are investigated both as a potential explosives and as rocket propellant components based on their high energy properties.10−14 Moreover, tetrazoles, due to their high number of nitrogen atoms, could serve as an environmentally benign component of gas generators with a high burn rate and relative stability.15 However, the most important and fruitful application of tetrazoles with many future prospects is their utility in medicinal chemistry.16−32 Not surprisingly, the number of publications on new drugs and promising biologically active compounds containing the tetrazole moiety increased dramatically the last seven years, 2010–2017 (Scopus, SciFinder, Figure 1).
Figure 1.
(A) Number of publications containing the keyword “tetrazole(s)” in the title of the articles plotted against the publication date as analyzed by Scopus (December 2018, 2707 articles). (B) Documents by country/territory of most publications contain the keyword “tetrazole(s)” in the title of the articles as analyzed by Scopus (December 2018, 2707 articles). (C) Documents by subject area as analyzed by Scopus.
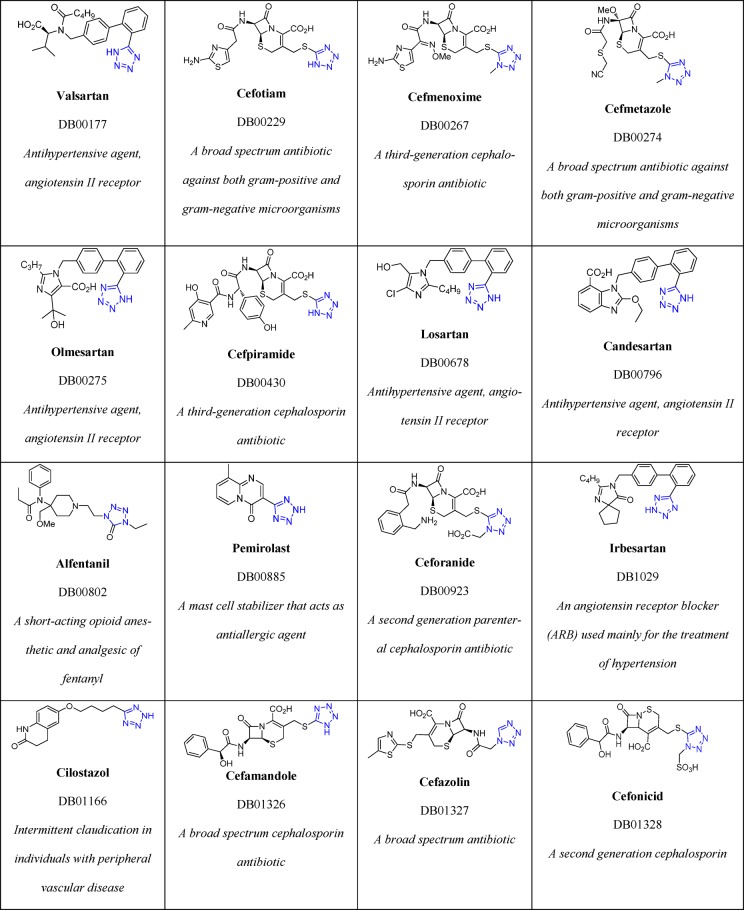
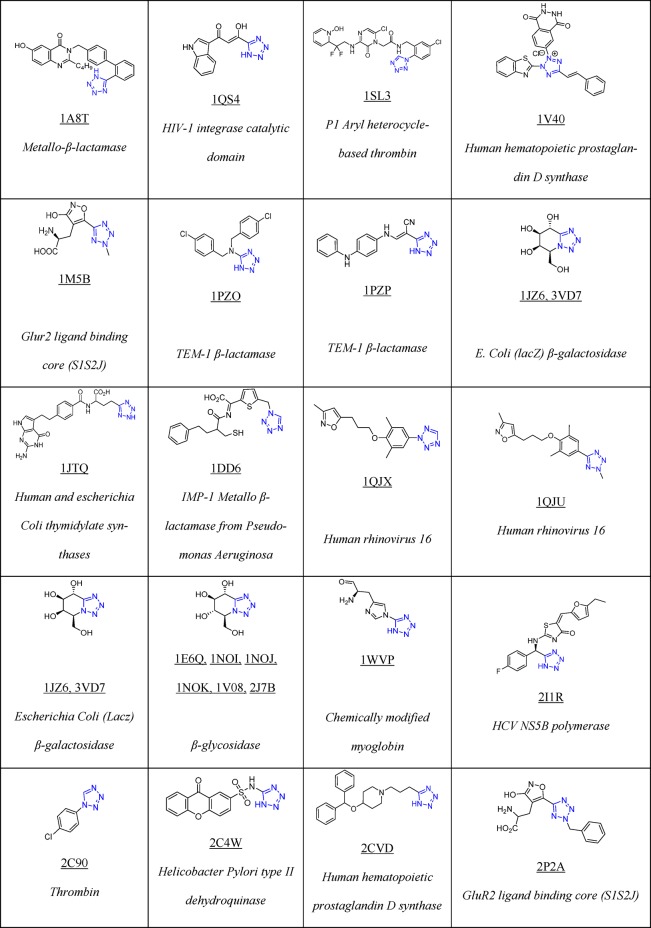
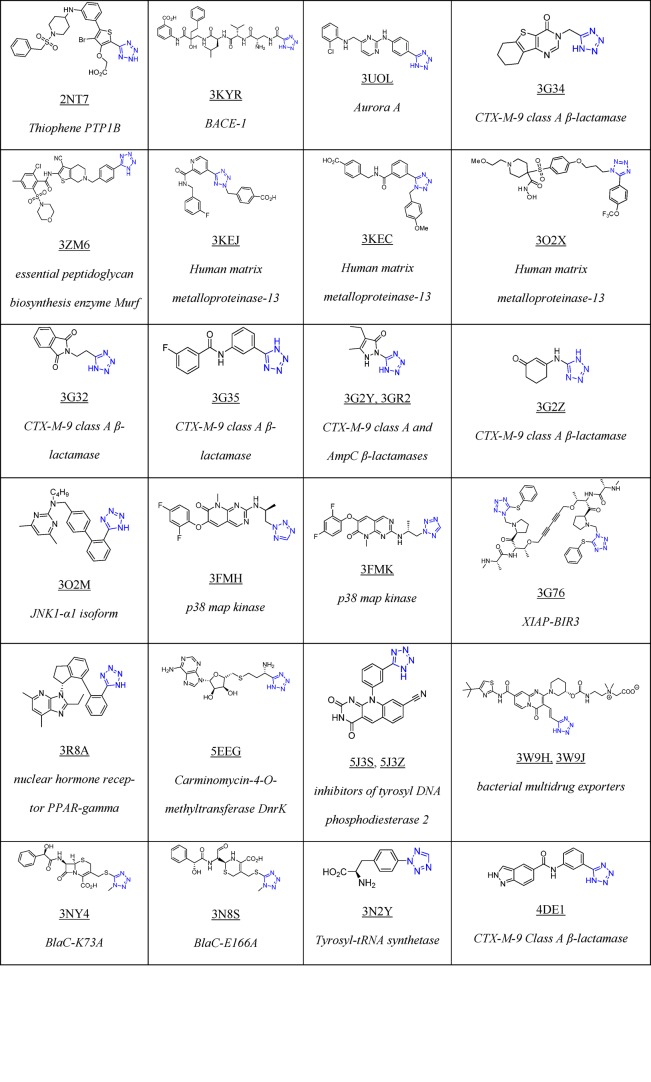
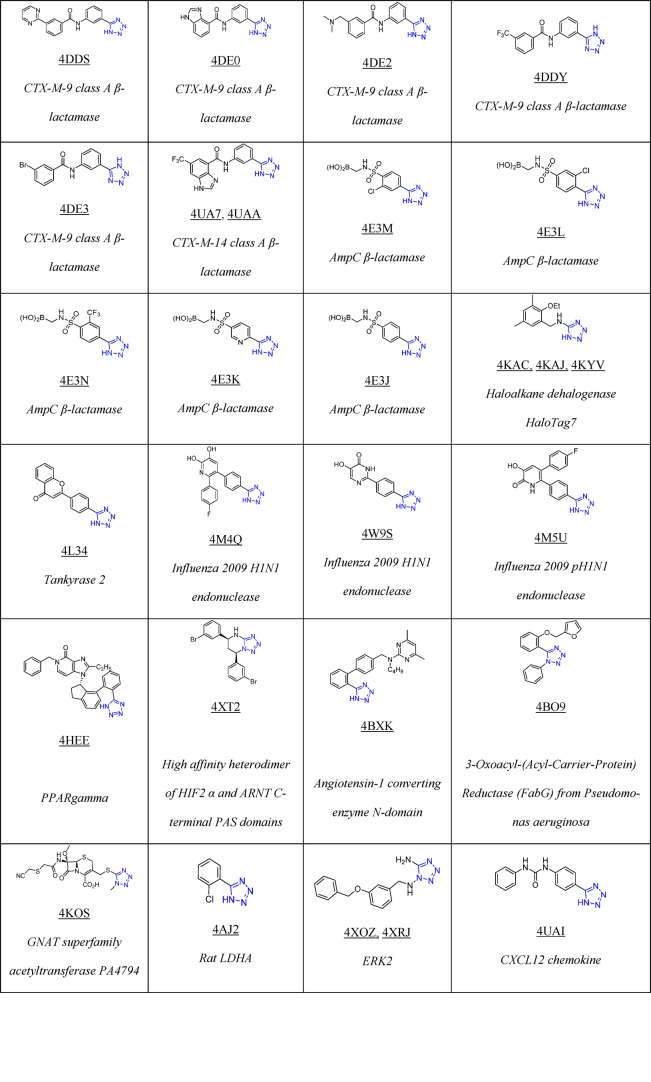
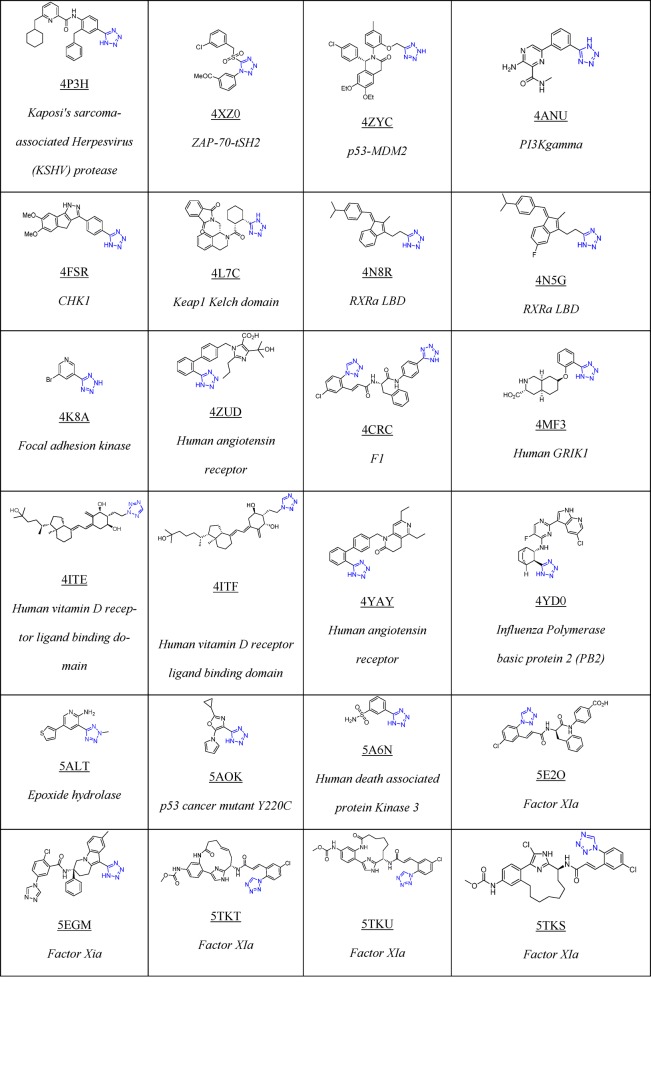
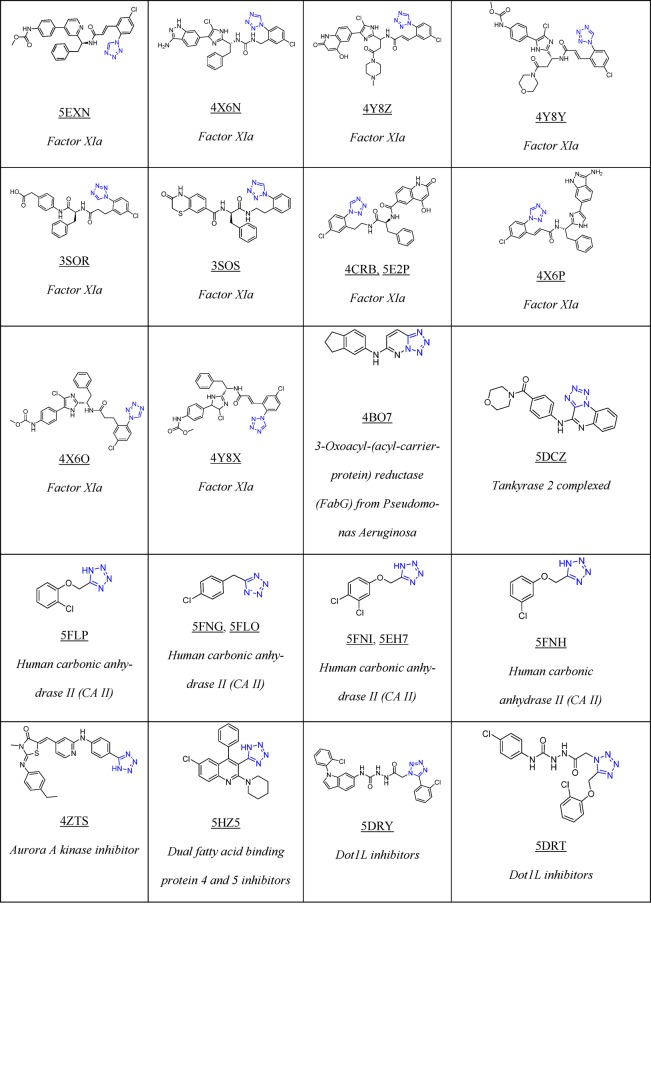
To date, Drug Bank33 mentions 43 drugs that contain 1H- or 2H-tetrazole substituents, 23 of them FDA approved; these compounds possess hypertensive, antimicrobial, antiviral, antiallergic, cytostatic, nootropic, and other biological activities (Table 1).
Table 1. 23 FDA Approved and Selected Experimental Drugs Containing the Tetrazole Moiety.
Bioisosterism,34 defined as classical or nonclassical, is a useful strategy for rational lead modification and drug design and prevail in medicinal chemistry to alter unfavorable ADME properties and/or to access free patent space. Among −CO2H isosteres,35 5-substituted tetrazole, which has a mobile hydrogen (on the contrary 1- or 2-substituted tetrazole have no mobile hydrogen), is of special interest because it has a comparable pKa (tetrazole 4.5–4.9 vs carboxylic acid 4.2–4.4), a similar size, spatial arrangement of the heteroatom lone pairs, and a similar molecular electrostatic potential (Figure 2A).36 Therefore, it often undergoes very similar receptor–ligand interactions.37,38 However, the tetrazole group often exhibits a prolonged half-life because of the enhanced metabolic stability,39,40 enhanced spatial delocalization of the negative charge, and better membrane penetration resulting from increased lipophilicity (tetrazoles with a mobile H are ionized at physiological pH (∼7.4), but are almost 10 times more lipophilic than the corresponding carboxylates).41,42 In addition, the high density of nitrogens in tetrazoles could provide more opportunities to form hydrogen bonds or π-stacking with the receptor recognition sites, explaining the sometimes-increased binding affinity.43 A thorough analysis on Isostar from the Cambridge Structural Database (CSD)44 showed the probability of occurrence and spatial characteristics of interactions between the 5-substituted tetrazole and different functional groups as −NH (aliphatic and aromatic), −OH (aliphatic, phenol, aromatic), carbonyl (ester, amide, ketones, etc.), and sp2-N (aromatic N included). This analysis clearly demonstrates a few things: First of all, the similarity with carboxylic acids with the mobile N–H as hydrogen bond donor (Figure 2B–E). The negative charge delocalization among N2–N3–N4 of the tetrazole is obvious (Figure 2B,C), and moreover, the hydrogen bonds via the σ-lone pairs of nitrogens are almost coplanar with the tetrazole plane.45 Finally, data mining in CSD revealed π–π interactions of the tetrazole ring with phenyl rings;46 for the interactions between these two π systems, the T-shaped edge-to-face and the parallel-displaced stacking arrangement are predominant (Figure 2F).
Figure 2.
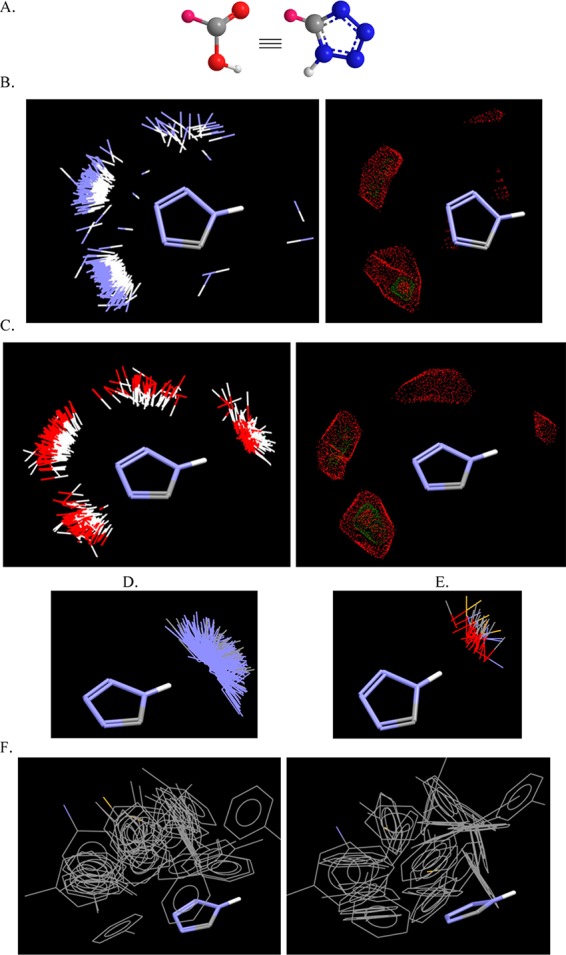
(A) Tetrazolic acids (5-substituted 1H-tetrazole or 2H-tetrazole) are bioisosteres of carboxylic acids. (B) The interactions of the 5-substituted 1H-tetrazoles with any N–H in CSD (655 different plotted compounds, left). The majority of these interactions exist around the two sp2 3- and 4-nitrogens of the tetrazole ring as shown also by the contour surface (right). (C) The interactions of the 5-substituted 1H-tetrazoles with any O–H in CSD (696 different plotted compounds, left). The majority of these interactions is distributed among the sp2 nitrogens of the tetrazole ring and the N–H, respectively, as shown also by the contour surface (right). (D) The interactions of the 5-substituted 1H-tetrazoles with aromatic or sp2 N in CSD (1315 different plotted compounds), which demonstrate the acidic character of the N–H of the tetrazole. (E) Likewise, the interactions of the 5-substituted 1H-tetrazoles with terminal oxygen (carbonyl, amides, esters, acids, etc.) in CSD (159 different plotted compounds) depict the hydrogen bond formation of N–H···O=C. (F) π–π Interactions of the 5-substituted 1H-tetrazoles with phenyl rings (different poses in left and right picture) in T-shaped edge-to-face and parallel-displaced stacking arrangement in CSD (50 different plotted compounds).
In general, 5-substituted-1H-tetrazolic acids exhibit physical characteristics similar to carboxylic acids and are strongly influenced by the effect of substituents at the C5-position. Finally, tetrazoles are more resistant to biological metabolic degradation pathways, for example, β-oxidation or amino acid conjugation.
The same analysis on Isostar for the 1,5-disubstituted tetrazoles showed that most of the aforementioned interactions with different functional groups, due to the absence of the free NH, are focused on the electronegative sp2 nitrogens of the tetrazole (characteristic examples are given with the −NH and −OH groups, Figure 3A,B). Furthermore, it seems that there is mostly a parallel-displaced stacking arrangement in the π–π interactions with phenyl groups (Figure 3C).
Figure 3.
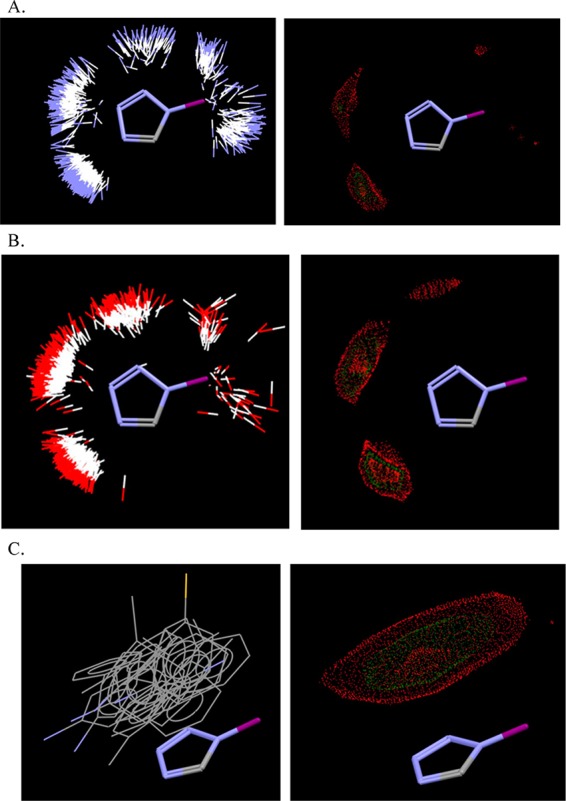
(A) The interactions of the 1,5-disubstituted 1H-tetrazoles with any N–H in CSD (2567 different plotted compounds, left). The majority of these interactions exists again around the two sp2 3- and 4-nitrogens of the tetrazole ring as shown also by the contour surface (right). (B) The interactions of the 1,5-disubstituted 1H-tetrazoles with any O–H in CSD (2180 different plotted compounds, left). The majority of these interactions is distributed among the sp2 nitrogens of the tetrazole ring and the N–H, respectively, as shown also by the contour surface (right). (C) π–π Interactions of the 1,5-disubstituted 1H-tetrazoles with phenyl rings, mostly in parallel-displaced stacking arrangement (left) as shown also by the contour surface (right) in CSD (946 different plotted compounds).
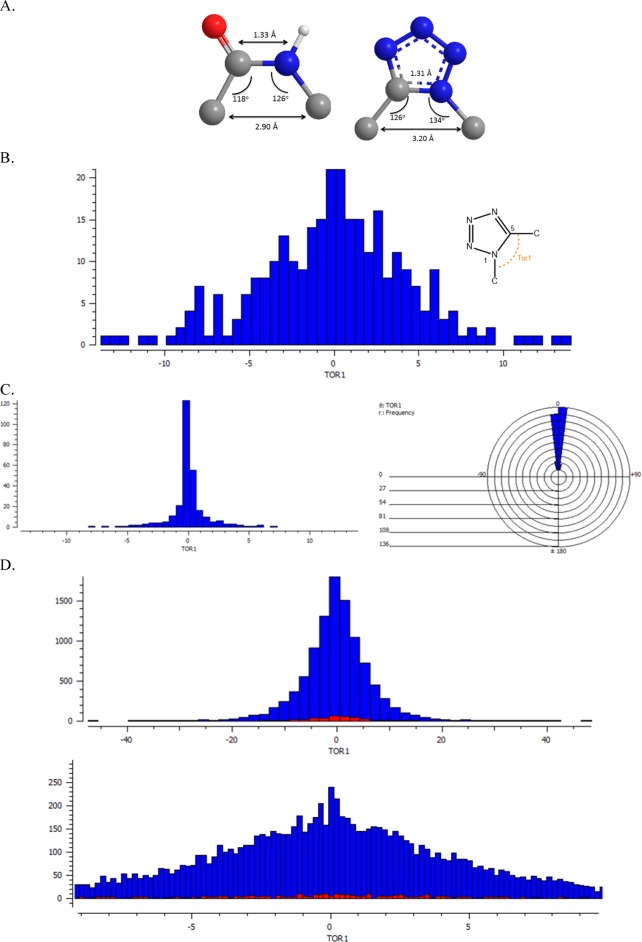
The most important feature of 1,5-disubstituted tetrazoles, though, is that they are effective bioisosteres for the cis-amide bonds in peptidomimetics, whereas the 5-substituted tetrazoles are mostly used as surrogates for carboxylic acids.37,47−49 In CSD, there are 20272 different crystal structures of amide-surrogates. An analysis of their torsion angle is shown in the histogram below (Figure 4A). It clearly shows that the majority of these amides are in a trans conformation (blue, torsion angle ±180°), and 6961 of the aforementioned structures have a cis conformation (red, −30° to +30°). A more close analysis on the cis-amide surrogates (Figure 4B) shows a normal distribution with a mean value of 0.007 o.
Figure 4.
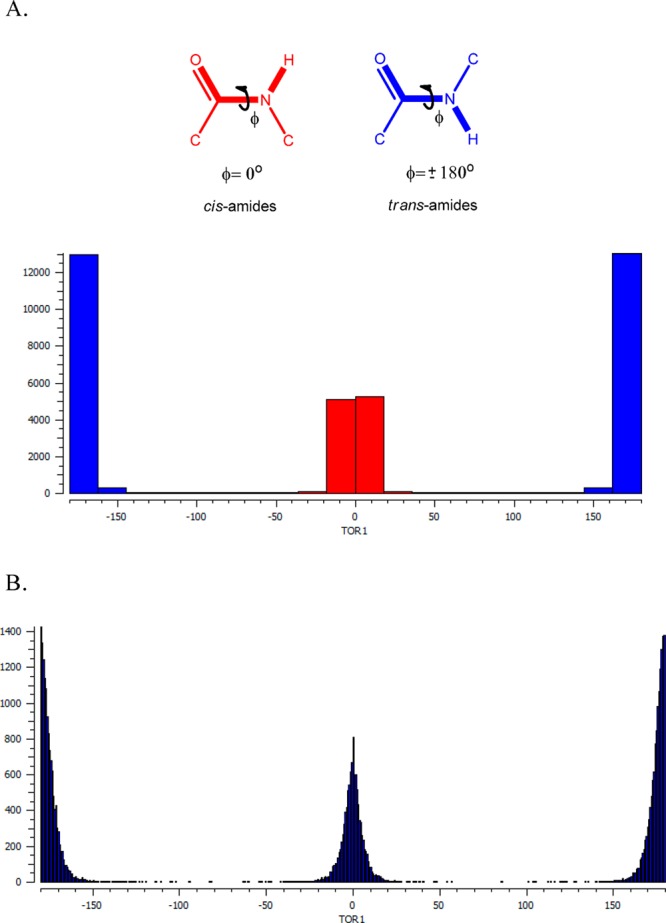
Geometrical features of cis and trans amides. (A) A histogram of the torsion angle analysis. (B) A close-up histogram of the torsion angle analysis.
The average geometrical features, that derived from the inspection of 241 available crystal structures of 1,5-disubstituted tetrazoles compared with the cis-amide surrogates, demonstrating the similarity, are shown in Figure 5A. In Figure 5B,C, the plot of the torsion angle (C6–C5–N1-C7) of 1,5-disubstituted tetrazoles is depicted, clearly showing the favorable synperiplanar conformation. However, the distribution is not normal (Figure 5,C, mean value 0.050°). A comparison of the corresponding torsion angles of both cis-amide surrogates (blue) and 1,5-disubstituted tetrazoles (red) showed that the latter are more constrained as expected (Figure 5D).
Figure 5.
(A) Geometrical features of 1,5-disubstituted tetrazoles as cis-amides surrogates. (B) Plot of the torsion angle of 1,5-disubstituted tetrazoles. (C) Corresponding cone angle correlation (left) and the polar histogram (right) revealing the favorable synperiplanar conformation. (D) A comparison of the torsion angle (picture in bottom in zoom pose) between the cis-amides (blue) and 1,5-disubstituted tetrazoles (red), showing a more constrained conformation for the latter.
For the reader to have a conclusive and spherical perspective, we made a query in CSD for 2-substituted and 2,5-disubstituted tetrazole derivatives (Figure 6A). We found 14 crystal structures of 2-substituted and 152 crystal structures of 2,5-disubstituted tetrazoles, with the average geometrical characteristics depicted in Figure 6B,C.
Figure 6.
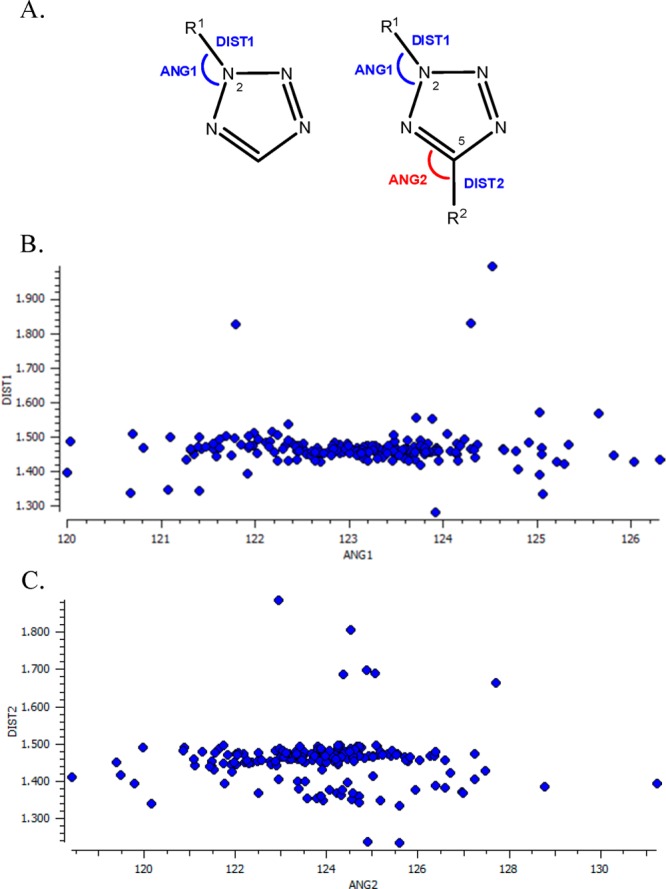
(A) Geometrical features of 2-substituted and 2,5-disubstituted tetrazoles. (B) Scatterplot of the distance R1–N (DIST1, blue color) with the angle R1–N-N (ANG1, blue color) of the 2,5-disubstituted tetrazoles with average values of 1.47 Å and 123.1°, respectively. (C) Scatterplot of the distance R2–N (DIST2, red color) with the angle R2–N-N (ANG2, red color) of the 2,5-disubstituted tetrazoles with average values of 1.46 Å and 123.9°, respectively.
For all these reasons, 5-substituted tetrazole represents a first-choice bioisosteric group if the corresponding −CO2H has issues in medicinal chemistry projects. Thus, effective and time-saving synthetic methods are important to build up libraries of tetrazoles for high-throughput screening or other low-throughput pharmaceutical research applications.
Multicomponent reactions (MCRs) are chemical reactions where more than two compounds react to form a single product with several descriptive features, such as atom economy, efficiency, and convergence.50,51 In 1961, Ugi et al.52,53 first reported the use of HN3 to replace carboxylic acid in the Passerini reaction54−56 and in the Ugi reaction to form tetrazole derivatives, and since then, numerous advancements were published on the synthesis of tetrazoles via MCRs. In this review, we shortly summarize the currently mostly used synthetic routes for the preparation of tetrazole derivatives through nonmulticomponent reaction, however, our focus is on the use of multicomponent reactions for the preparation of substituted tetrazole derivatives. We wish to reveal specific applications and general trends holding therein and discuss synthetic approaches and their value by analyzing scope and limitations and estimated prospects of further research in this field. Moreover, we believe that the structural understanding of this scaffold class and its 3D conformations are of uttermost importance for the process of understanding and predicting binding properties of compounds toward its receptor, e.g., in structure-based drug design and in a wider sense to predict properties of specific molecules. Therefore, in addition to synthetic accessibility, we will discuss both the 3D solid state conformations of tetrazole derivatives as well as some cocrystal structures with their protein receptors. Thus, this review covers the literature in this area reported to date as exhaustive as possible. Other published reviews on tetrazoles are more specialized on specific aspects.57−63
1.1. Structural Biology of Tetrazoles
As of March 2018, there are 155 tetrazole cocrystal structures present in the Protein Data Bank (PDB, Table 2).64 Their classification according to their structures showed that the majority of them belongs to the 5-monosubstituted tetrazole derivatives (58%), followed by 1-monosubstituted (18%) and 1,5-disubstituted tetrazoles (14%, Figure 7). The PDB files can serve as excellent resource to study preferential binding poses and interactions of the tetrazole moiety toward the receptors.65−90 These can be used to understand their bioisosteric character toward the carboxylic acids, elaborate similarities and differences, and develop guiding rules for the use of tetrazole scaffolds in medicinal chemistry (1 and 2, Figure 8). Understanding typical binding poses of tetrazoles in certain receptor pockets can help in the structure-based design of novel inhibitors, thus a few selected examples will be discussed.
Table 2. Structure of Selected Tetrazoles with Their Protein Receptors and Its PDB ID.
Figure 7.
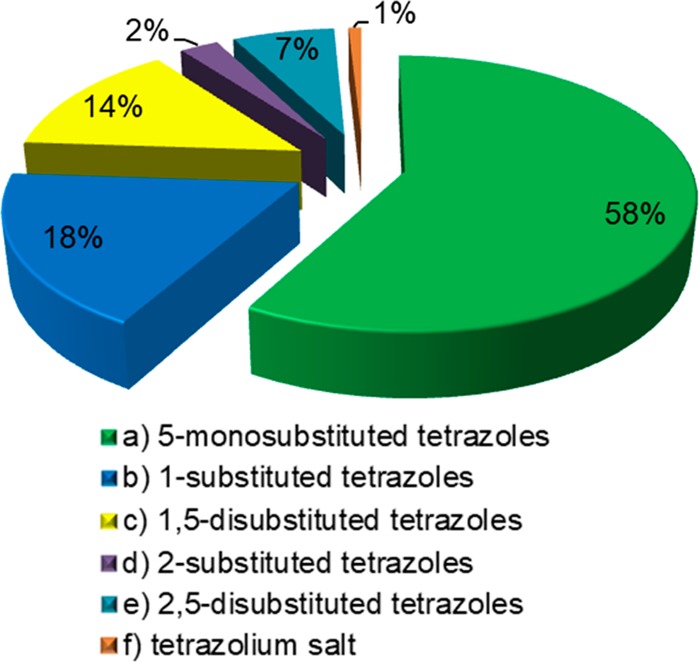
Classification of the selected PDB cocrystal structures of tetrazole derivatives into the categories of 5-monosubstituted tetrazoles (green), 1-substituted tetrazoles (blue), 1,5-disubstituted tetrazoles (yellow), 2-substituted tetrazoles (magenta), 2,5-disubstituted tetrazoles (cyan), and tetrazolium salt (orange).
Figure 8.
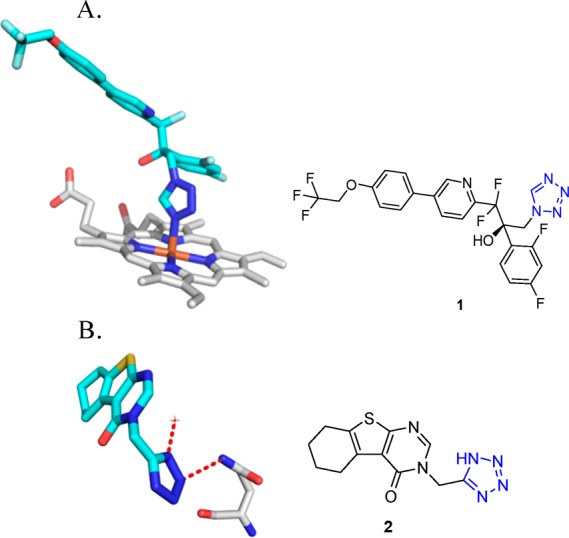
Examples of characteristic receptor–tetrazole binding modes found in the PDB. (A) Sterol 14α-demethylase (CYP51) from Trypanosoma cruzi in complex with the 1-monosubstituted-tetrazole derivative VT-1161 (1) (PDB 5AJR) exhibiting the metal ligand character of tetrazoles. (B) CTX-M-9 class A β-lactamase complexed with 1H-tetrazole 2 (PDB 3G34), exhibiting a hydrogen contact to water and one hydrogen contact to Gln188 side chain amide.
1.1.1. Tetrazole Undergoes up to Four Hydrogen Bindings with Its Four Nitrogen σ-Lone Pairs
This is exemplified in Figure 9 of a β-lactamase inhibitor complex, where the central tetrazole moiety 3 is embedded between two serines, one threonine, and one water molecule, forming an extended hydrogen bonding network with distances between 2.7 and 2.8 Å.91 Remarkably, the four receptor heavy atoms involved in the hydrogen bonds are almost coplanar with the tetrazole plane underlining the involvement of the σ-lone pairs of the four nitrogens. This structure also reveals the key difference between the two isosteres, carboxylic acid and tetrazole, based on their lone pairs both which can form in principle four hydrogen bonds, however with differential spatial orientation: The tetrazolyl forms four orthogonal hydrogen bonds in the plane of the five-membered ring, whereas the carboxylate forms four hydrogen bonds along the O-lone pairs in the plane spanned by the three atoms O–C–O.
Figure 9.
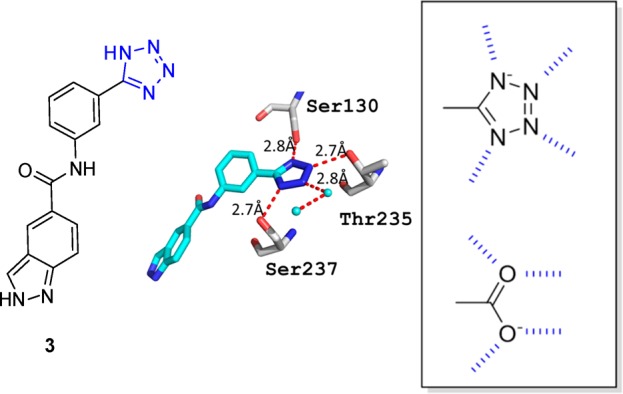
Comparison of the hydrogen bonding pattern of tetrazolyl and carboxyl. Example of a tetrazolyl (3) forming four hydrogen bonds (PDB 4DE1).6 Ser130 and Ser237 form each a hydrogen bond to the tetrazole −N2 and −N5 via their side chain −OH at 2.8 and 2.7 Å, respectively. N-3 is in a 2.7 Å contact to the side chain −OH of Thr.235 The fourth N-4 forms a close hydrogen bonding contact of 2.8 Å to a water molecule, which itself is further involved into hydrogen bonding contacts.
1.1.2. The Tetrazole Moiety Is an Efficient Metal Chelator Similar to Carboxylate92
The X-ray crystal structure of the enzyme bound to the biphenyl tetrazole L-159,061 (4) (Figure 10) shows that the tetrazole moiety of the inhibitor interacts directly with one of the two zinc atoms in the active site, replacing a metal-bound water molecule. Two N–N polar interactions and two C–N interactions are presented in Figure 10.
Figure 10.
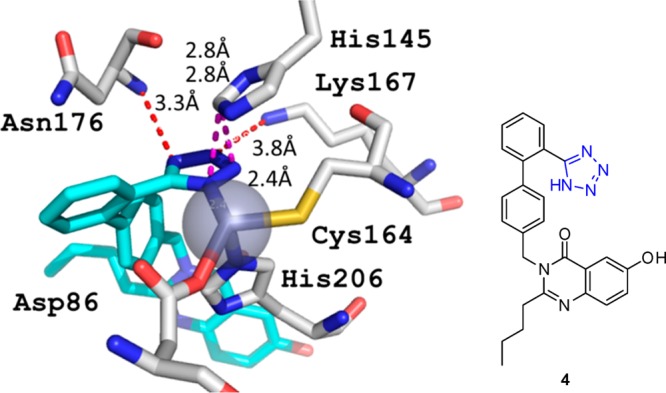
Tetrazole compound 4 as a ligand for the metallo-β-lactamase (PDB 1A8T).92 The central Zn2+ is tetrahedrally coordinated by the ligands tetrazole-N1, the His206 side chain N3, Asp86 carboxyl-O, and Cys164 side chain-S. The tetrazoloyl not only forms a bond to Zn2+ but forms several hydrogen bonds to the receptor, including Asn176 backbone NH (3.3 Å), His145 side chain NH (2.8 Å), and Lys187 side chain NH2 (3.8 Å). Moreover, the His145 imidazole moiety is on top of the tetrazolyl moiety, forming an electrostatic interaction with an interplane angle of ∼30°.
1.1.3. The Tetrazolyl Unit Is Forming an Arg Sandwich93
The protein–protein interaction of the Keap1 with Nef2 recently became a hot target in drug discovery for neuro-inflammatory diseases.94 The tetrazole molecule 5 was described binding to the Kelch domaine (Figure 11). Interestingly, the bioisostere carboxylic acid compound 6 (PDB 4l7B, Figure 12) is also available together with structural biology information, thus providing the opportunity for a direct comparative analysis.95 The alignment of the two structures is very good, and only small differences in the two ligand and receptor side chain orientations can be observed (RMSD 0.142). Both acid units of 5 and 6 are sandwiched between Arg415 and Arg380. However, tetrazole 5 is able to bury a water molecule underneath the tetrazole moiety that makes possible several close contacts to the receptor which cannot be seen with the carboxylic acid 6. Therefore, the highly buried water molecule can be considered as part of the receptor. Moreover, the conformation of Arg415 is slightly different in 5 and 6, placing Arg415 closer to the two carboxylic acid oxygens by a ∼80° turn around the C2–C3–Arg415 bond. Taken together, carboxylic acid 6 binds with an IC50 of 2.4 μM, slightly better than the tetrazole 5 with 7.4 μM.
Figure 11.
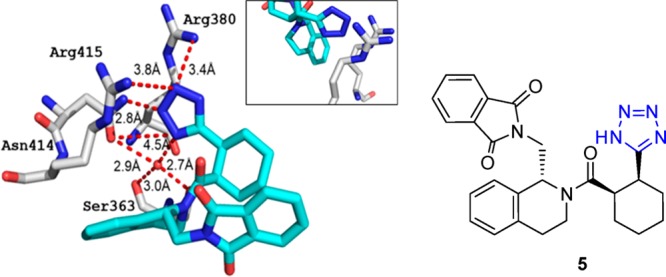
Kelch domain interaction of Keap1 with tetrazole 5 (PDB 4L7C). A dense network of electrostatic and hydrogen bindings contributes to the tight small molecule receptor interaction. It features an interesting sandwich charge–charge interaction driven motive between two positively charged arginines and the tetrazole moiety. The boxed figure shows the Arg sandwich from a different orientation.
Figure 12.
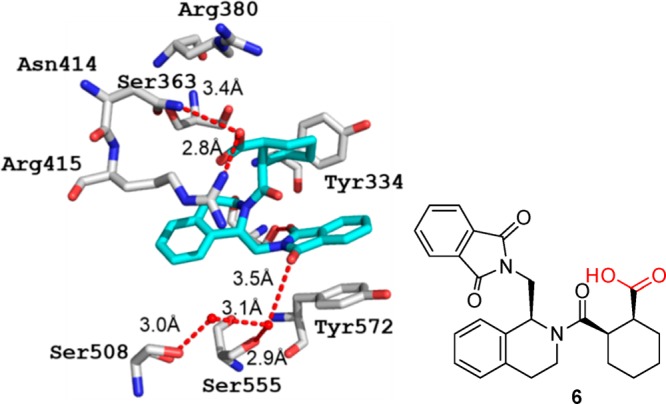
Kelch domain interaction of Keap1 with compound 6 (PDB 4L7B). Same as its bioisostere tetrazole 5, a dense network of electrostatic and hydrogen bindings also contributes to the tight small molecule receptor interaction. The difference is the weaker interaction between residue Arg380 and the carboxylic ligand, which is caused by the special orientation of carboxylic group.
In addition, the in vivo brain exposure was tested for both compounds and several physicochemical and DMPK properties are summarized in Table 3. None of the two compounds showed sufficient brain penetration, likely due to being substrates for efflux pumps phosphoglyco proteins (PGP).
Table 3. Physicochemical and DMPK Properties of Compounds 5 and 6.
| compd | log Da | polar surface area (PSA) [Å2]b | efflux ratio (ER)c | unbound brain-to-plasma (Bu/Pu)d | Cu [μM]e |
|---|---|---|---|---|---|
| 5 (tetrazole) | 0.69 | 107 | NT | <0.01 | <0.01 |
| 6 (carboxylic acid) | 1.36 | 95 | 20 | <0.01 | <0.01 |
| 0.4a | 0.18a |
Measured at pH 7.4.
Polar surface area.
Efflux ratio in MDCK-MDR1 cells (10 mm incubated up to 120 min).
Unbound brain-to-plasma ratio measured in mice.
Unbound brain concentration measured in mice at Cmax.
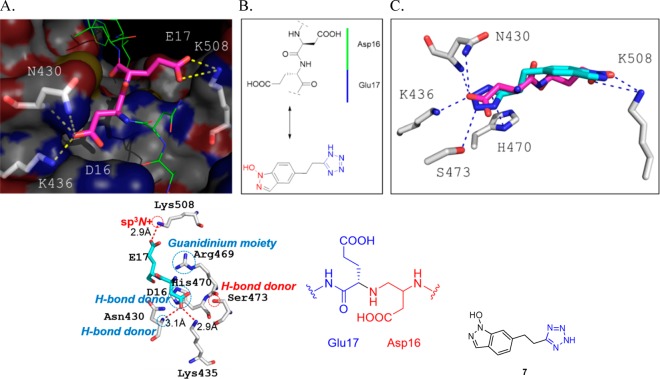
Yu et al.96 designed inhibitors of the β-catenin/T-cell factor protein–protein interaction by pursuing a bioisosteric replacement approach. The available crystal structures revealed a very large protein–protein contacting surface between β-catenin and Tcf4 of ≥2800 Å2 (PDB 2GL7). Moreover, biochemical analyses indicate that the dissociation constant (Kd) value of β-catenin/Tcf PPIs is in the 7–10 nM range. To disrupt such a large and tightly binding complex, it requires an extraordinarily high ligand efficiency of the small molecule. Biochemical analysis of truncated and mutated Tcf peptide epitopes revealed several potential hot spots for small molecule design. The Asp16 (D16) and Glu17 (E17) of human Tcf was chosen as a critical binding element and converted into small molecules mimicking this key element (Figure 13).96 The tetrazole ring (pKa= 4.5–4.95) was used to replace the carboxyl group of Asp16 (D16) and mimic the charge–charge and H-bond interactions with Lys435 (K435) and Asn430 (N430) of β-catenin. The four lone pairs of the deprotonated tetrazole ring are evenly distributed on the five-membered ring and can form two additional H-bonds with the side chains of His470 (H470) and Ser473 (S473). These two H-bonds do not exist in the β-catenin/Tcf complex. Tetrazole derivative 7 with a molecular weight of 230 and a ligand efficiency of 0.512 has a Kd of 0.531 μM for binding to β-catenin and a Ki of 3.14 μM to completely disrupt β-catenin/Tcf interactions. Replacement of the tetrazole moiety with other carboxyl bioisosteres such as 5-oxo-1,2,4-oxadiazole and 5-thioxo-1,2,4-oxadiazole (pKa = 6.1–6.7) decreased binding affinity dramatically. According to modeling studies, the tetrazole and the indazole-1-ol moiety mimic the Asp16 carboxylic acid and the carboxyl group of Glu17, respectively (Figure 13).
Figure 13.
Bioisosteric replacement strategy for the design of β-catenin/Tcf protein protein interaction. (A) Hot spot of β-catenin/Tcf interaction showing key electrostatic interactions (PBD 2GL7).97 Tcf peptide is shown in pink and green, and the hot spot Asp16-Glu17 is highlighted as pink sticks. β-Catenin is shown as surface representation, and interacting amino acids are shown as gray sticks. (B) Bioisosteric replacement step. (C) Close-up analysis of the aligned 7 and Asp16-Glu17 of Tcf with the β-catenin receptor. The indazole-1-ol forms H-bond and charge–charge interactions with β-catenin Lys508. The tetrazole ring was used to replace the carboxyl group of Asp16 and mimics the charge–charge and H-bond interactions with Lys435 and Asn430 of β-catenin. The deprotonated tetrazole ring with two more Lewis bases can form two additional H-bonds with the side chains of His470 and Ser473. These two H-bonds do not exist in the β-catenin/Tcf complex.
2. Tetrazoles through Non-Multicomponent Reaction Routes
To date, the multitude of synthetic methods of 1,5-disubstituted tetrazoles and monosubstituted tetrazoles have been reviewed several times58,98−103 and thus will only be briefly mentioned here.
The most common used synthesis of tetrazole derivatives is the 1,3-dipolar cycloaddition reaction between nitriles and azides (azide ion or hydrazoic acid, Scheme 2).104−114 It was first described by Hantzsch and Vagt115 in 1901 through a [2 + 3] cycloaddition of an azide to a nitrile (Scheme 2). Electron withdrawing groups lower the LUMO of the nitriles and thus enhance the interaction opportunities with the HOMO of the azide, leading to a smooth reaction.116,117 However, the requirement of the strong electron withdrawing groups in the nitrile substrate somehow limits the scope of the reaction, needing, in general, high reaction temperature and catalysts. The synthesis of several ω-chloroalkyl tetrazoles and their subsequent attachment to a solid support was also described.118 Recently, selenium-containing triazole carbonitriles were used as precursors for the corresponding tetrazole derivatives with antioxidant activity based on the aforementioned reaction.119
Scheme 2. Different Synthetic Routes to Tetrazoles Using Non-Multicomponent Reactions.
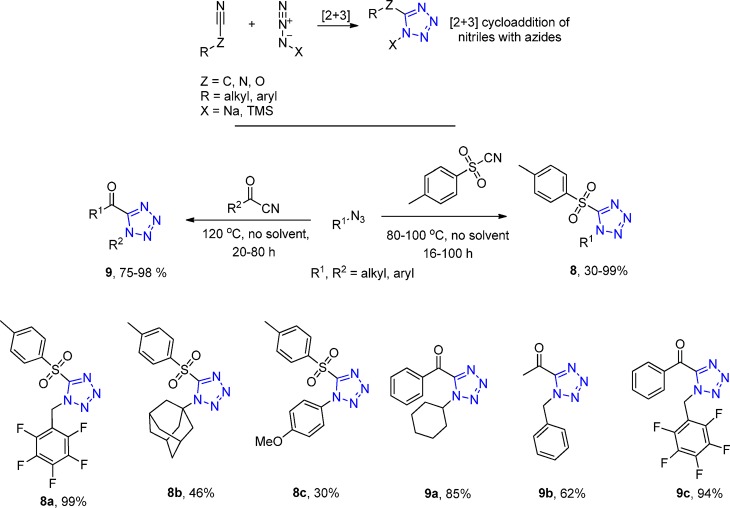
Sharpless et al.,120−122 among the many existing methods, reported the [2 + 3] cycloaddition of an azide to the p-toluenesulfonyl cyanide (TsCN) with a nice substrate scope of aromatic and aliphatic azides under solvent-free conditions followed by simple isolation in good yields (8a–c, Scheme 2). Later, they extended this methodology to produce acyltetrazoles 9 in high yields with readily available acyl cyanides and aliphatic azides with simple purification.123
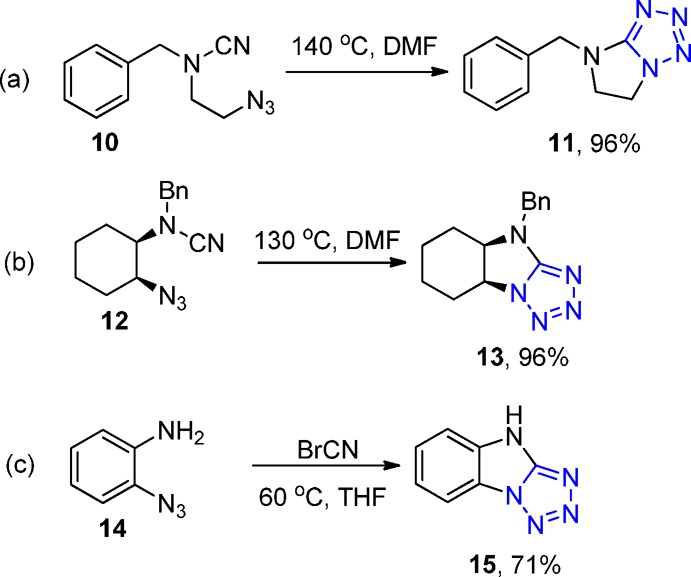
Moreover, fused 5-heterotetrazole ring systems 11, 13, and 15 were synthesized in high yields via intramolecular [2 + 3] cycloadditions of organic azides and heteroatom substituted nitriles 10, 12, and 14, respectively (Scheme 3). Cyanates, thiocyanates, and cyanamides were employed, yielding various five- and six-membered heterocyclic systems fused to a tetrazole ring.124
Scheme 3. Intramolecular Cycloaddition of Azidonitriles: (a) Heterocyclic Nitrile, (b) Aliphatic Nitrile, (c) Aromatic Nitrile.
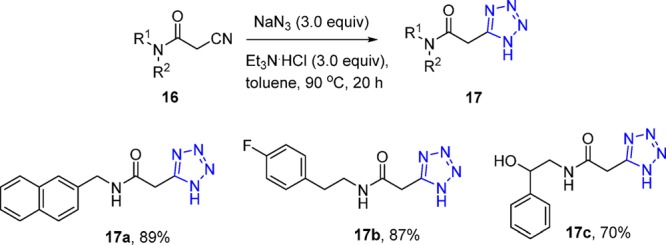
In addition, the synthesis of more than 20 5-substituted 1H-tetrazoles (17) was described by Dömling et al.125 from various, readily available cyanoacetamides 16.126 The combination of sodium azide, trimethylamine hydrochloride in toluene at 90 °C afforded the corresponding library in excellent yields with broad reaction scope (Scheme 4).
Scheme 4. Synthesis of 5-Substituted 1H-Tetrazoles 17 via N-Substituted Cyanoacetamides.
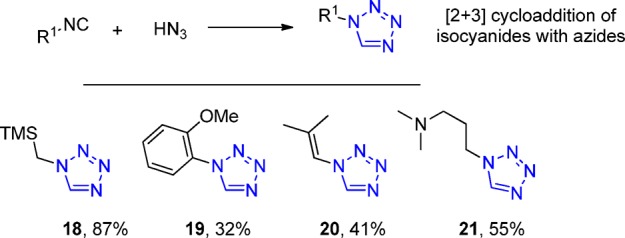
The 1,3-dipolar cycloaddition reaction between nitriles and azides (azide ion or hydrazoic acid) toward 1,5-disubstituted tetrazoles is well established (Schemes 2 and 3). The [2 + 3] cycloaddition of isocyanides and hydrazoic acid or trimethylsilyl azide leading to 1-monosubstituted tetrazole derivatives by Oliveri and Mandala,127 at the beginning of 20th century, is also notable. This reaction is less known, however, it is quite general and works both with aliphatic and aromatic substrates having a broader scope than the corresponding nitrile cycloaddition (Scheme 5). Because of the in situ access to a much greater diversity of isocyanides from their formamides,128 this method offers an alternative pathway for the synthesis of many 1-N-monosusbtituted tetrazoles, 18–21. Considering the importance of this heterocycle, synthetic routes toward labeled tetrazoles have also been described.129 Very recently, the catalytic visible-light reaction of aliphatic, aromatic, and heterocyclic aldehydes with sodium azide via 1,3-dipolar cycloaddition has been described. The azide not only behaves as three-nitrogen donor of tetrazole ring but also it converts the aldehyde into isocyanide.130
Scheme 5. Synthesis of 1-Substituted Tetrazoles by Click Reaction of Azides and Isocyanides.
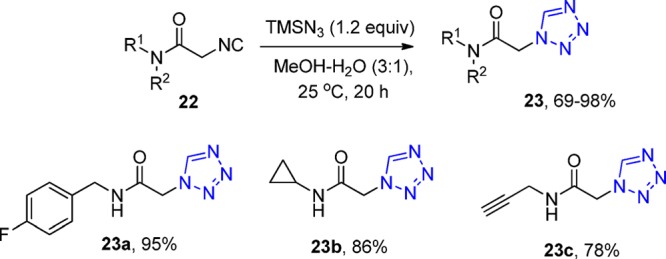
Elaborating the above-mentioned reaction, Dömling et al.125 treated the N-substituted 2-isocyanoacetamides13122 with trimethylsilyl azide with 25% cosolvent water in methanol at rt. A library of 18 1-substituted-1H-tetrazoles 23 was efficiently synthesized as most of the final products were precipitated during workup (Scheme 6).
Scheme 6. Synthesis of 1-Substituted 1H-Tetrazoles 23 via N-Substituted Cyanoacetamides.
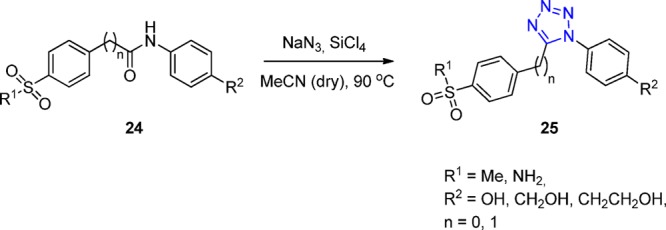
The synthesis of 1,5-diaryl-substituted tetrazoles 25 was reported by the treatment of amides 24 with tetrachlorosilane/sodium azide using a high wall (HW) pressure vessel at 90 °C in a dry MeCN (Scheme 7). The corresponding derivatives were evaluated as COX-2 inhibitors.132,133
Scheme 7. Synthesis of 1,5-Diaryl-Substituted Tetrazoles 25 via Amides 24.
3. Multicomponent Reactions for the Synthesis of Tetrazoles
A main focus of our review is the description of the applications of the MCR synthetic routes toward the tetrazole motif in terms of their utility in medicinal chemistry, understanding the structural behavior on specific examples and their binding properties. Thus, in the following chapter, due to the diversity of tetrazole derivatives, the MCR-based tetrazole syntheses will be classified according to the number of the overall rings, e.g., monocyclic, bicyclic, tricyclic, or polycyclic (Figure 14). Scope and limitations of its scaffold along with the 3D conformations, where available, will be given with special focus on their medicinal and pharmaceutical application.
Figure 14.
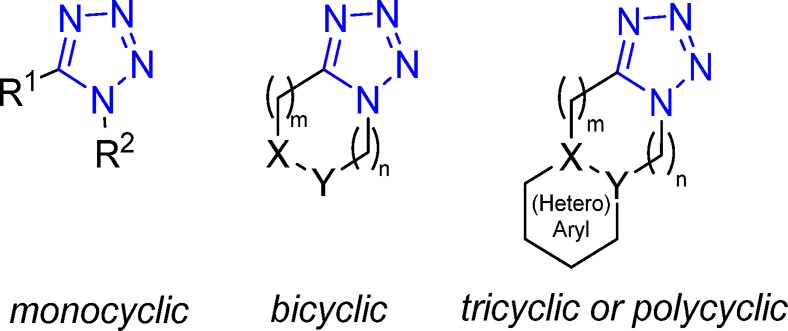
Classification of the MCR-based synthesis of tetrazole derivatives according to the number of cycles.
3.1. Monocyclic Tetrazoles Derivatives
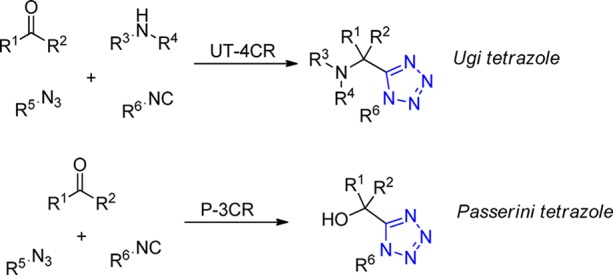
The most important approach to aminomethyl tetrazoles using MCR by far is the Ugi-4CR. Ivar Ugi described the aforementioned reaction in his seminal publication from 1959, where he introduced most of the today’s important variation of his MCR (Scheme 8).134 Some years later, again, Ugi was the first who introduced a Passerini MCR variation leading to α-hydroxymethyl tetrazoles,52,53 a reaction mechanistically related to the Passerini reaction described 30 years earlier (Scheme 8). Furthermore, some other less known MCRs will be discussed. These include reactions involving for example acetylenedicarboxylates and three component reaction of isocyanides, azides, and other nucleophiles, leading to interesting 1,5-disubstituted building blocks.
Scheme 8. Tetrazole MCRs Overview.
3.1.1. Ugi Tetrazole Four-Component Reaction (UT-4CR)
α-Aminomethyl tetrazoles are of great importance due to isosterism to α-amino acids. The classical Ugi tetrazole (UT-4CR) synthesis presents a broad scope regarding to the starting materials, i.e., isocyanides, oxo components, and amines (Figure 15). A representative set of UT-4CR adducts (26–38) that have been cited in this review is presented in Figure 16. In parallel synthesis of UT adducts, among others, in 96-well plates have also been described enabling the production of 5000–10000 compound range.135 This also demonstrates one very attractive feature of MCRs, the relative ease of its automation. The UT-4CR differs from the classical Ugi-4CR in that the azide traps out the intermediate nitrilium ion (replacing the carboxylic acid seen in the classical Ugi variation), leading to the formation of the final 1,5-disubstituted tetrazole. The reaction is often performed in methanol, however, 2,2,2-trifluoroethanol or biphasic water chloroform mixtures were also reported.136−139 Recently, an ultrasound accelerated UT-4CR was described without solvent based on a water-triggered formation of hydrazoic acid via single-proton exchange with TMS azide.140 The reaction is generally fast at room temperature; only some special educt combinations require heating, for example, the reaction of bulky trityl amine.141,142 The UT-4CR is considerably more exothermic than the classical Ugi four-component condensation of isocyanides, oxo components, primary amines, and carboxylic acids, yielding the α-aminoacylamides. Therefore, the addition of the components, especially on a larger scale, should proceed carefully under cooling. The order of addition of the components in the Ugi reaction in most cases does not really matter and the yields are comparable. Often the components are added to the reaction’s vessel in the order of oxo component, amine, isocyanide, and finally the azide source. In the past, Ugi was using isolated hydrazoic acid in a benzene stock solution.143 Nowadays the safer substitute trimethylsilylazide (TMS azide, TMSN3) is utilized, which forms in situ the hydrazoic acid in the typically used protic alcoholic solvent. Alternatively, especially if ammonium salts of the primary or secondary amines are used, the hydrazoic acid source should be sodium azide. Both aromatic and aliphatic isocyanides work well, whereas the functional groups of the isocyanide side chain are often well tolerated, e.g., the amino acid derived isocyano esters work nicely (Figures 15 and 16). However, α- and β-amino acid derived isocyano methyl esters can cyclize with the primary or secondary amine of the tetrazole side chain, forming δ-lactams. This has been advantageously used to create tetrazoloketopiperazines and will be discussed below. Oxo components can be aldehydes, ketones, and substituted variants thereof. Substituted benzaldehydes, heteroaromatic aldehydes, including formyl-ferrocene and substituted aliphatic aldehydes, glyoxals, formaldehyde, cyclic and acyclic aliphatic ketones, and monosubstituted arylketones work efficiently (Figure 15, 16).144 In the UT-4CR, both primary and secondary amines react well, comparing with the classical U-4CR where normally only primary amines are involved.145−151 The amines can be both aliphatic and aromatic and widely substituted. Even the super bulky trityl amine can react with aliphatic aldehydes to give compound 31, however, only under microwave conditions due to the slow Schiff base formation (Figures 15 and 16).141,142 Notably, ammonia, which causes often problems in other Ugi variations, reacts reasonably well with ketones in the UT-4CR (see compound 32).136,152−155 In 2007, Marcaccini and Torroba156 described a detailed protocol for the UT-4CR, including the general mechanism and the effects of the nature of the components as well as the reaction conditions on the Ugi reaction.
Figure 15.
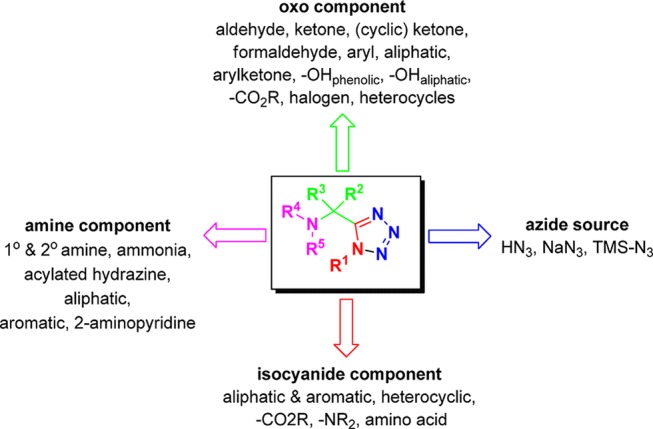
Scope and limitations of the UT reaction.
Figure 16.
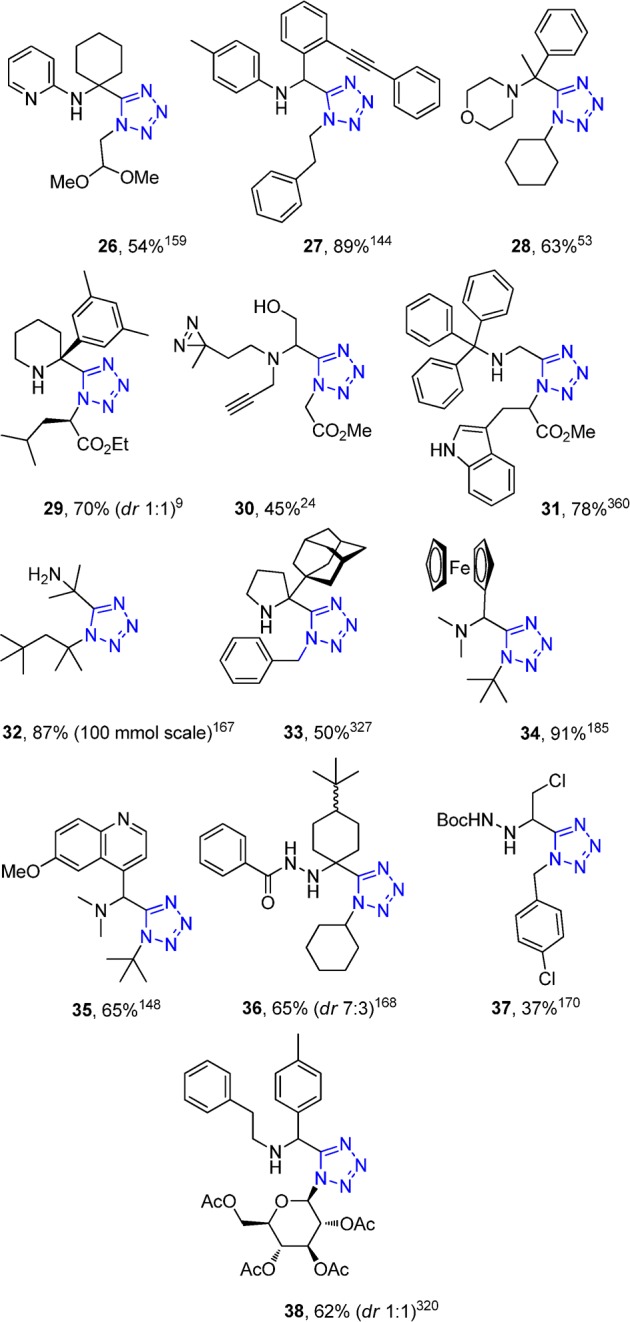
SAR of the UT-4CR and typical reaction products (26–38) which are cited in the current review underlining the scope of the reaction.9,24,53,144,148,159,167,168,170,185,320,327,360
Recently, Nenajdenko et al.9,157 studied the diastereoselectivity of the UT-4CR with cyclic amines 39, yielding the derivatives 40 and 41 (Scheme 9). They found that the reaction with α-substituted five- to seven-membered cyclic amines provided high control of diastereoselectivity (≤100% de, ≤98% yields) under mild conditions. As a matter of fact, the diastereoselectivity of the reaction depends on the ring size of the starting cyclic amines. More rigid piperidines provided the highest selectivity of the reaction.
Scheme 9. Stereoselective Synthesis of Tetrazole Derivatives 40 and 41 via a Diasteroselective UT-4CR with Secondary Cyclic Amines.

Interestingly, the 2-aminopyridine, prone to undergo the Groebke–Blackburn–Bienaymé multicomponent reaction (GBB-3CR) with isocyanides and aldehydes in a competing reaction, reacts in the UT-4CR selectively as an amine component.158−160 Apparently, the GBB-3CR (42) has slower kinetics than the UT-4CR (43) (Scheme 10).
Scheme 10. UT-4CR vs GBB-3CR of the 2-Aminopyridine.
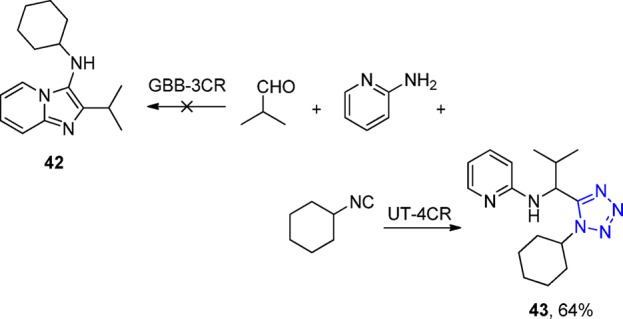
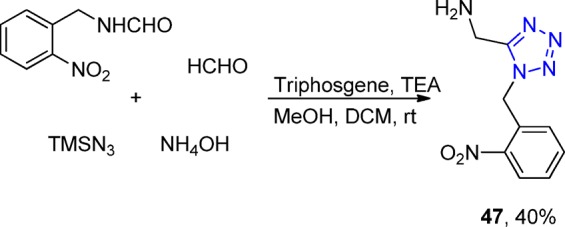
Taken together, the UT-4CR is very easy to perform156,161 and has an amazingly great scope in all three classes of variable starting materials, especially combined with the in situ generation of the isocyanides.128 The substrate scope includes diverse substituted aldehydes and ketones, substituted formamides, and a multitude of primary and secondary amines, yielding the 1,5-disubstituted tetrazoles, e.g., 44–46 in yields of 39–64% (Scheme 11). Another application of this in situ method is the access without the need of protecting group to photoinducible probe 47, a bioisostere of the important neurotransmitter glycine. Photocleavable tetrazole was synthesized, via an UT-4CR, using the Leuckart–Wallach accessible o-nitrobenzyl formamide (Scheme 12). Since its first description in 1959, many researchers have used the UT-4CR, and some applications are highlighted in the following.
Scheme 11. Isocyanide-less Ugi 4-CR Tetrazole Variation (UT-4CR).
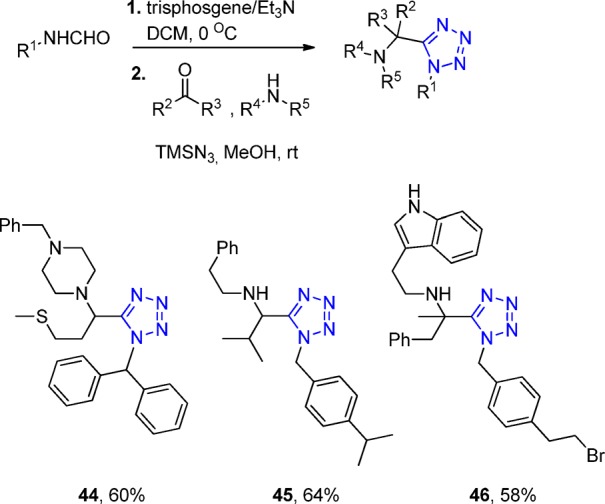
Scheme 12. Example of an Application of the Isocyanide-less UT-4CR to Synthesize the Photocleavable Tetrazole Derivative 47.
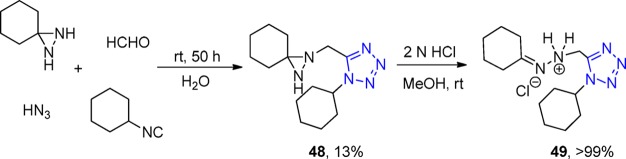
In 1972, Zinner et al.162 started the early studies of UT-4CR using amine variations. In this approach, the corresponding diaziridine reacted with formaldehyde, cyclohexyl isocyanide, and HN3 to generate diaziridine tetrazole derivatives 48, however, in low yields. The subsequent acidic treatment opens up the diaziridine ring, giving, unexpectedly, quantitative yield of the hydrazone derivative 49 (Scheme 13).
Scheme 13. UT-4CR to Diaziridine Tetrazole Derivative 48.
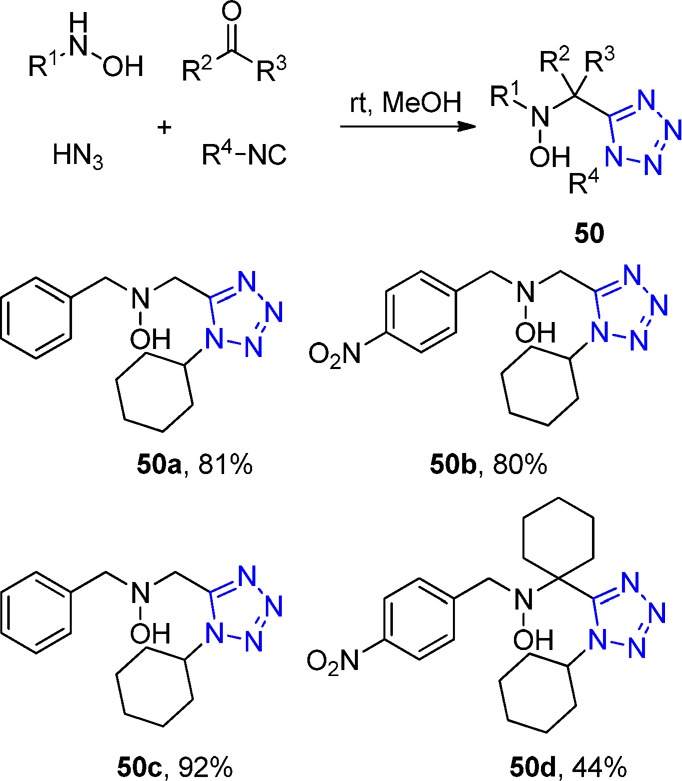
Continuing their studies, in 1974, Zinner et al.163 described an UT-4CR approach to 1,5-disubstituted tetrazoles using hydroxylamines as amine components. Reaction with formaldehyde in the presence of cyclohexyl isocyanide and hydrazoic acid (HN3) afforded the corresponding 1,5-disubstituted tetrazole methylene hydroxylamines 50. Sterically hindered cyclic ketones and different substituted benzylhydroxylamines led to the expected products at mild reaction conditions though with lower yields (Scheme 14).
Scheme 14. Hydroxylamines as Amine Equivalents in UT-4CR.
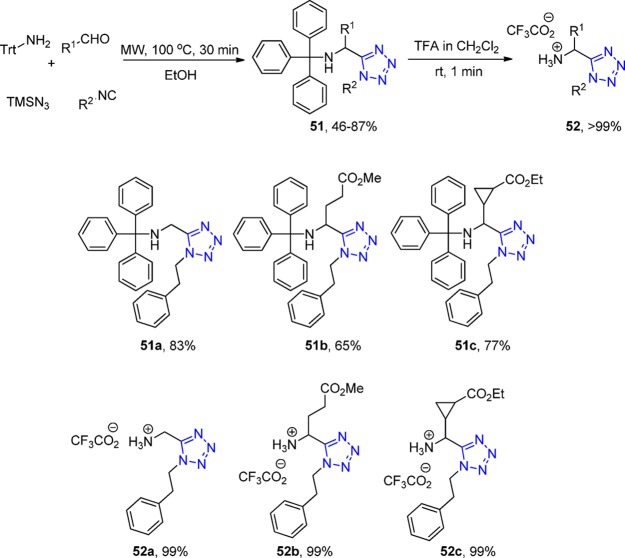
The basic amino group is highly hydrophilic and also a good hydrogen bond acceptor which is of use for potential drug candidates. Ammonia and other amine-like components have been reported sporadically in Ugi reactions, however, they often afford mixed or poor yields, e.g., hydroxylamine, N-acylated hydrazine, N-sulfonated hydrazine, and unprotected hydrazine. Dömling et al.141 introduced tritylamine as a convenient ammonia substitute in the Ugi tetrazole synthesis, synthesizing 15 trityl protected 1,5-disubstituted tetrazole derivatives 51 in satisfactory to good yields. The trityl deprotecting reaction went through a mild acidic condition, with quantitative yields affording tetrazoles 52. Ammonia, as it was expected, was found to lead to a mixture of multiple products caused by its high reactivity (Scheme 15, Figure 17); HPLC-MS analysis of the reaction of tert-butyl isocyanide with formaldehyde, ammonia, and TMS-azide revealed such a mixture of mono-, di-, and tri-Ugi products.
Scheme 15. A Synthetic Pathway to N-Unsubstituted Primary α-Aminotetrazoles 52 Using an Ugi-4CR Employing Tritylamine As an Ammonia Surrogate.
Figure 17.

Crystal structures of tetrazole derivatives 50d,e. They are dominated not only by π-stacking and hydrophobic interactions between the trityl group, the alkyl group, and the phenylethyl groups but also the tetrazole ring makes short intermolecular contacts (CCDC 903083 and 903084).
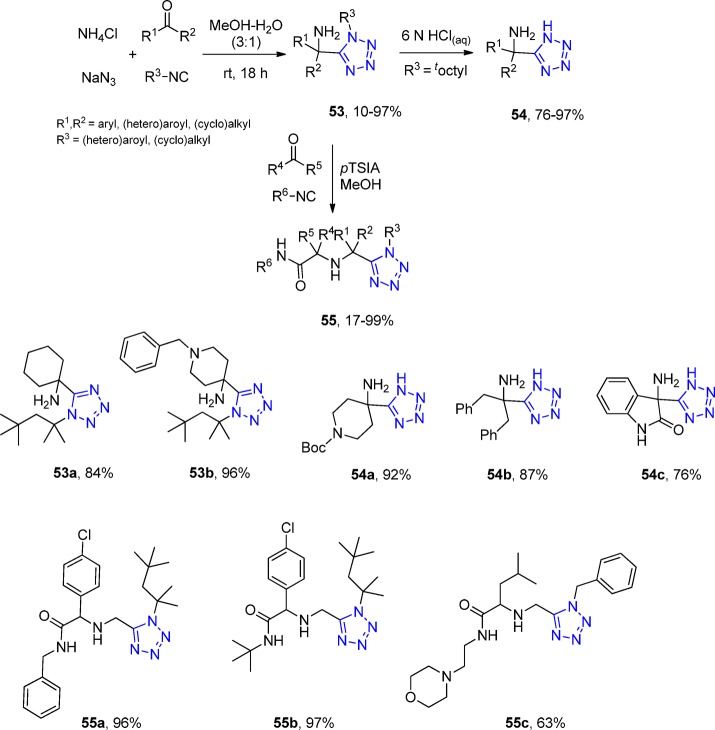
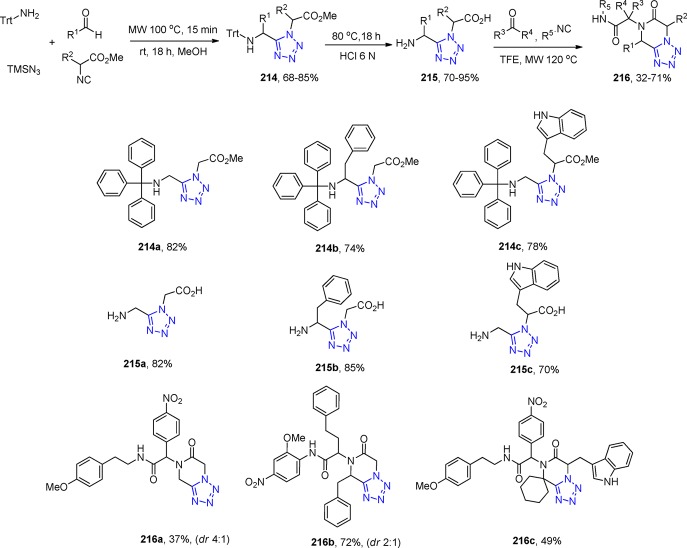
However, this problem was overcome by using ammonium chloride as the ammonia source.164 With in-depth scope and limitation study with more than 70 oxocomponents and 15 isocyanides, it was shown that the UT with ketones, isocyanides, sodium azide, and ammonium chloride afforded the free-amino tetrazoles 53 (Scheme 16). The primary amine component of the α-amino tetrazole is a versatile starting material for further reactions because it can be converted to the tetrazole deprotected α-amino tetrazole compound16554 by choosing the 1,1,3,3-tetramethylbutyl isocyanide (Walborsky’s reagent).166 As a matter of fact, Dömling et al.167 utilized this α-amino tetrazole as the primary amine component in an U-3CR (a so-called “truncated” Ugi reaction, not involving a carboxylic acid) toward the synthesis of the compounds 55 (with more than 50 derivatives), expanding even more the chemical space, establishing a library-to-library approach (Scheme 16, Figure 18).
Scheme 16. A Synthetic Pathway to α,α-Disubstituted α-Aminotetrazoles 53 and 54 Using an UT-4CR Employing Ammonium Chloride as an Ammonia Surrogate and the Post-Modification Towards Tetrazoles 55.
Figure 18.
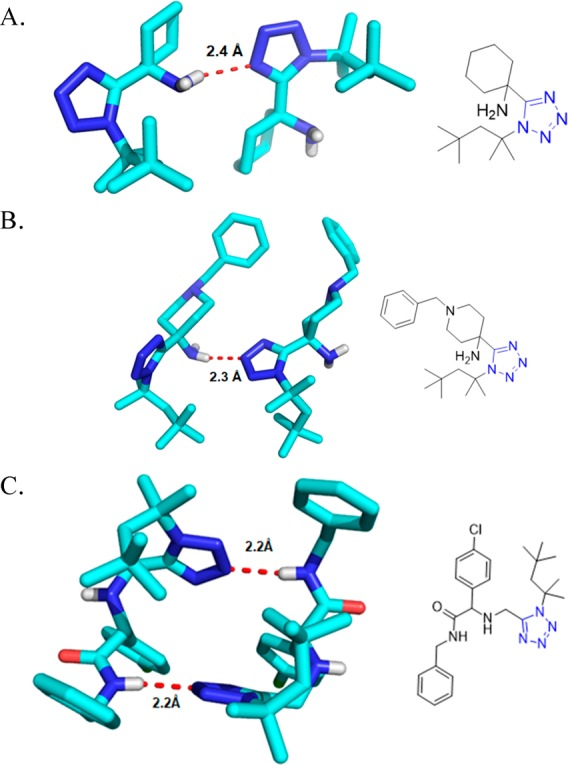
Structures of tetrazoles as seen in the solid-state by X-ray structure analysis. (A) Compound 53a (CCDC 1441248) forms a hydrogen bridge of 2.4 Å length between the amine NH and the N4 of an adjacent molecule; moreover, the benzyl side chains undergo parallel and T-shaped π–π interactions. (B) Compound 53b (CCDC 1441249) forms a hydrogen bridge of 2.3 Å length between the amine NH and the N3 of an adjacent molecule. (C) Compound 55a (CCDC 1484778) forms a hydrogen bridge of 2.2 Å length between the amine NH and the N3 of an adjacent molecule.
Balalaie et al.168,169 reported a novel and efficient method for the diastereoselective synthesis of α-hydrazine tetrazoles 56 using cyclic ketones, TMS azide, hydrazides, and the corresponding isocyanides without any catalyst via an UT-4CR in mostly good yields (Scheme 17). Two diastereomers were observed during the Ugi reaction with dr up to 4:1. On the basis of a solved X-ray structure, the major diastereomer was found to have trans configuration (Figure 19).
Scheme 17. Diastereoselective Synthesis of α-Hydrazine Tetrazoles 56 via a Facile UT-4CR.
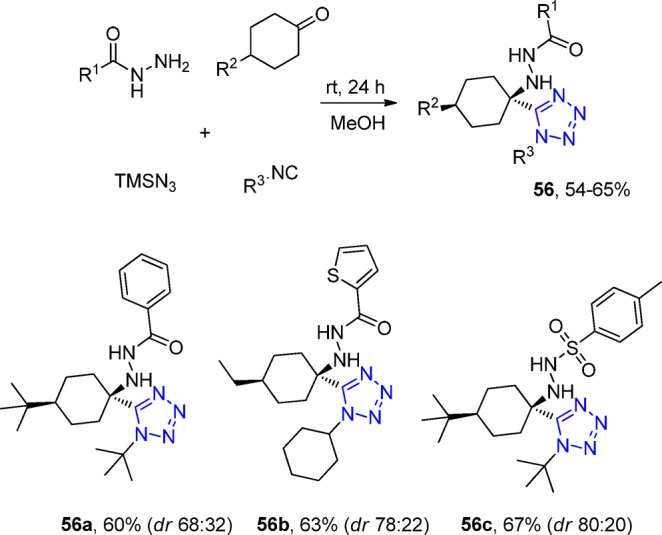
Figure 19.

Crystal structures of α-hydrazine tetrazole 56a and 56d. (A) Hydrophobic interactions between the C of phenyl group and N(2), N(3) of tetrazole, hydrophilic interactions between N(3) of tetrazole, and the N close to C=O (CCDC 950021). (B) Hydrophobic interactions between the C of oxo component cyclohexyl groups, and hydrophilic interactions between N(3), N(4) of tetrazole, and N close to C=O (CCDC 950022).
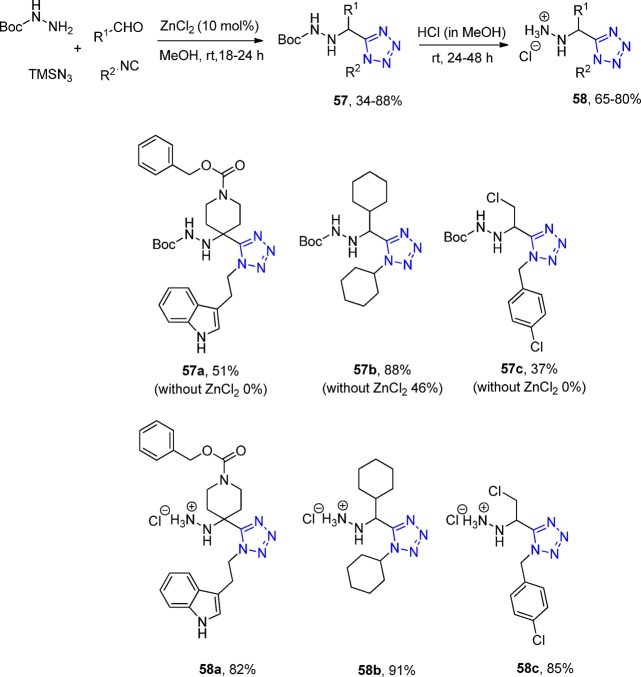
Dömling et al.170 synthesized via a two-step procedure a series of 1-substituted 5-(hydrazinylmethyl)-1-methyl-1H-tetrazoles 58 by an UT-4CR using Boc-protected hydrazine, various aldehydes or ketones, isocyanides, and TMS azide with a subsequent deprotection (57, Scheme 18, Figure 20). To further improve the yield of the Ugi reaction, ZnCl2 was used as a catalyst increasing the Schiff base formation. The straightforward access to highly substituted hydrazines is of interest because hydrazines can act as aspartic protease inhibitors interacting through charge–charge interactions with the active side aspartate residues.
Scheme 18. Typical Two-Step Procedure Synthesis of N-Deprotected Tetrazole Derivatives 58.
Figure 20.
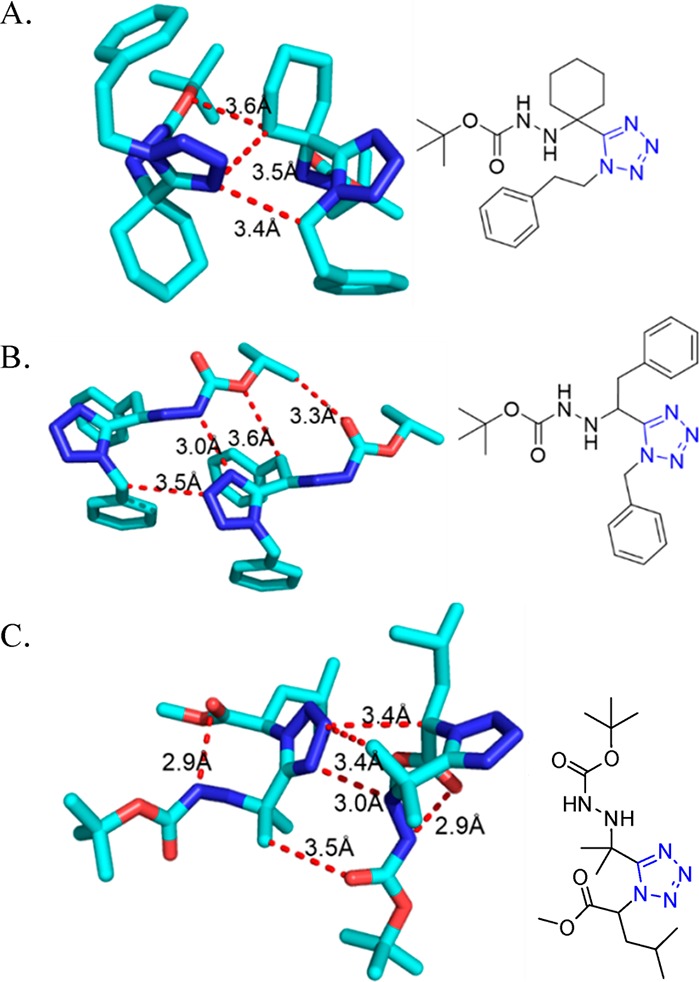
Crystal structures of the highly substituted 5-(Boc-hydrazinylmethyl)-1-methyl-1H-tetrazoles 57. (A) Three hydrophobic interactions between carbon atom of cyclohexanyl and oxygen atom of Boc group, carbon atom of cyclohexanyl and N(4) of tetrazole, and C(1) of benzylethyl and N(4) of tetrazole (57d, CCDC 1438137). (B) Three hydrophobic interactions between carbon atom of methyl of isopropyl and oxygen (C=O) of Boc group, carbon atom of methylene of benzyl and oxygen of Boc group, and carbon atom of benzyl and N(3) of tetrazole, and one hydrophilic interaction between N(4) of tetrazole and N of hydrozine close to Boc group (57e, CCDC 1438135). (C) Four hydrophobic interactions between C(α) of isocyanide and N(3) of tetrazole, carbon atom of methyl of isopropyl and N(3) of tetrazole, and O(C=O) of Boc group and methyl of isopropyl and one hydrophilic interaction between N(4) of tetrazole and N of hydrazine close to C(α) (57f, CCDC 1438136).
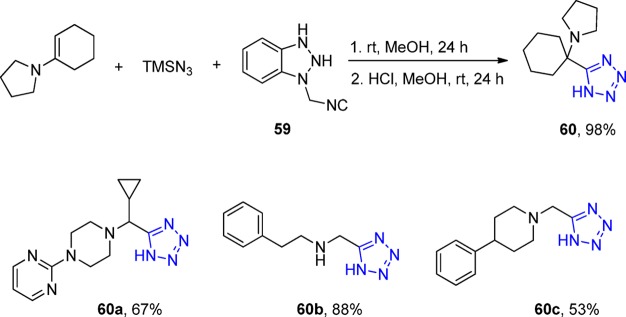
An application of secondary amines in UT-4CR was reported by Dömling et al.165 by investigating a versatile and commercially available isocyanide, the 1-isocyanomethylbenzotriazole 59 (BetMIC). Initially, BetMIC was reacted with an enamine and TMS azide in methanol to form the expected tetrazole in good yields. Moreover, in the following cleavable step, they observed the almost quantitative and mild cleavage of the Ugi product to give the expected α-aminomethyl tetrazole 60 (Scheme 19).
Scheme 19. UT-4CR of BetMIC and Subsequent Acid Hydrolysis Yielding α-Aminomethyl Tetrazoles 60.
The concept of convertible isocyanides was introduced as early as 1963 by Ugi with cyclohexenyl isocyanide, which can be cleaved in the Ugi reaction product using acidic conditions.171 This concept was later extended by many others.166,172−175 Convertible isocyanides are highly useful in that they can be transformed into other functional groups during a multistep synthesis of complex molecules, e.g., natural products.176 However, the majority of the work performed concerns the transformation of the secondary amide formed during the Ugi and Passerini reactions into esters, thioesters, ketones, carboxylic acids, and other groups. Despite the increasing popularity of using convertible isocyanides for further molecular modification, these isocyanides suffer from major disadvantages such as lengthy synthesis procedures, instability, incompatibility with delicate substrates, laborious workup, and multistep cleavage. Furthermore, these isocyanides are only applicable in one type of reactions either U-4CR or UT reactions.
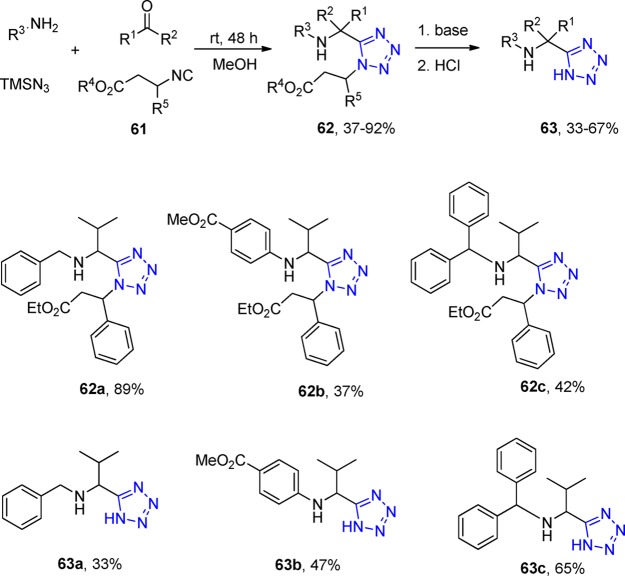
Mayer et al.177 chose two new cleavable isocyanides, the 3-isocyano-3-phenyl-ethylpropionate (61a) and the 2-isocyano succinic acid dimethyl ester (61b), in order to react with aldehydes, amines, and TMS azide synthesizing a library of UT adducts (62) bearing three points of diversity in good yields. These isocyanides could be later cleaved with an alkoxide base (NaOEt, or KOtBu), affording the desired 5-substituted 1H-tetrazoles 63. The two new cleavable isocyanides were both synthesized from β-amino acids (Scheme 20).
Scheme 20. Synthesis of α-Aminoalkyltetrazoles 63.
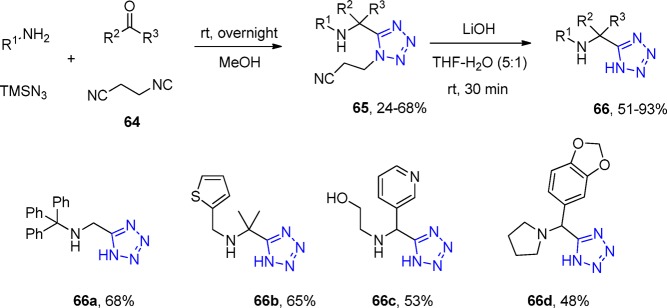
β-Cyanoethyl isocyanide (64) was introduced as a cleavable isocyanide in the UT-4CR, giving rise to the tetrazole derivatives 65 (Scheme 21).178 After the UT reaction, the β-cyanoethyl moiety was cleaved under very mild basic hydrolysis conditions in only 30 min, yielding the free tetrazoles 66.
Scheme 21. Synthesis of α-Aminoalkyltetrazoles 66.
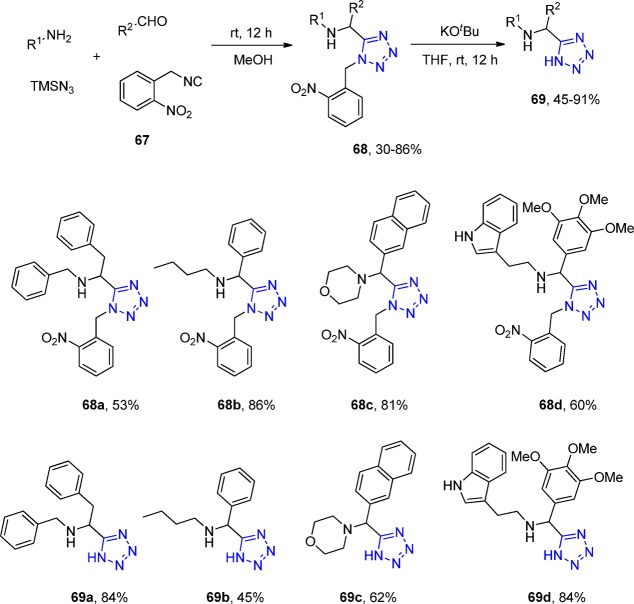
Dömling et al.179 employed successfully the isocyanide 67, which bears a cleavable 2-nitrobenzyl group in both U-4CR and UT reactions using acidic and basic conditions, respectively. They demonstrated its use as a truly convertible isocyanide which performed moderately to good in the UT-4CR, affording tetrazoles 68 and compatible with diverse substrates. The cleavage was performed under basic conditions, by KOtBu, giving the adducts 69 (Scheme 22).
Scheme 22. Synthesis of the UT-4CR Adducts and Their Corresponding Deprotected 5-Substituted 1H-Tetrazoles 69.
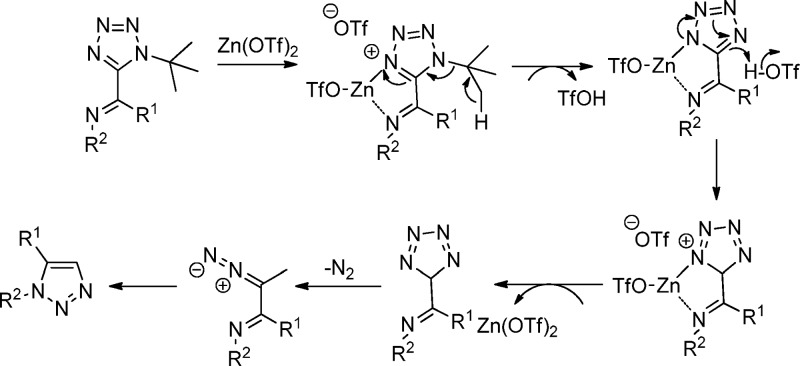
Tetrazoles are not only widely recognized for their pharmacological activities but also for their high chemical and thermal stabilities.100,180 The decomposition of substituted tetrazoles normally occurs above 250 °C, and the fragmentation at lower temperatures mainly was only found during acylation of monosubstituted tetrazoles (Huisgen fragmentation).181,182 El Kaïm et al.183 described a Lewis acid triggered fragmentation of tetrazoles synthesized through an UT-4CR (Scheme 23). The Ugi tetrazole undergoes copper-catalyzed oxidative Schiff base formation (70), and then it is converted into triazoles through Zn(OTf)2 catalyzed fragmentation of the tetrazole under microwave conditions toward the 1,5-disubstituted triazoles 71. The mechanism, as proposed by the authors, is based on an electrocyclization of an intermediate α-diazo imine as the final step. Initial formation of a zinc chelate is triggering tert-butyl E1 elimination, which leads to the liberation of a small amount of triflic acid in the medium. This acid protonates the ring, which leads to a dearomatization of the tetrazole (Scheme 24).
Scheme 23. Synthesis of 1,5-Disubstituted Tetrazoles 70 through Tetrazole Imine Intermediates and Their Subsequent Oxidation.
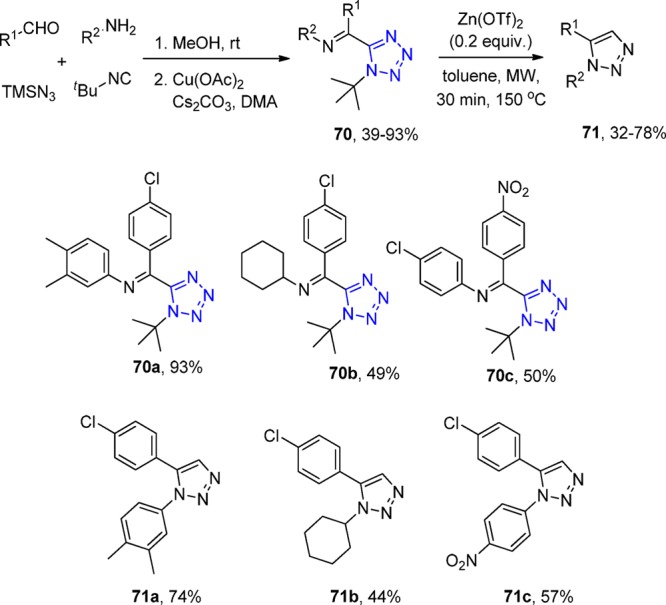
Scheme 24. Plausible Mechanism of the Synthesized Triazoles through the Tetrazole Formation.
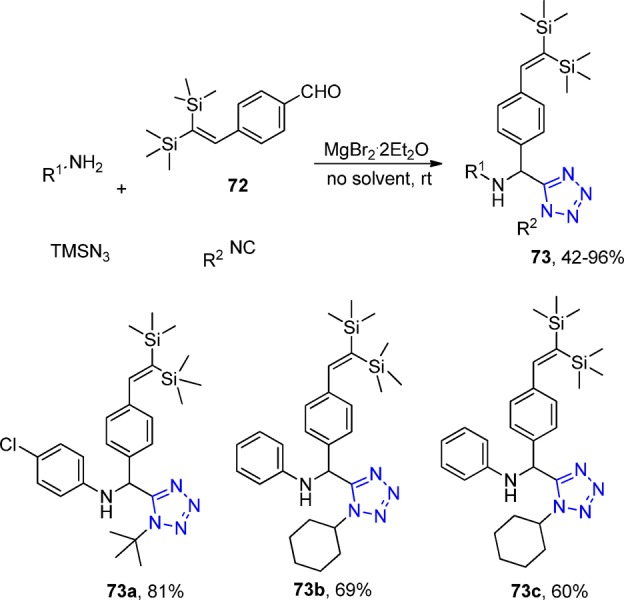
Due to the fact that the C(sp2)–Si bonds in organosilicon compounds undergo numerous transformations, Safa et al.184 developed a library of tetrazole derivatives bearing 2,2-bis(trimethylsilyl)ethenyl groups (73), from the corresponding benzaldehyde (72), via a simple one-pot UT-4CR in the presence of catalytic amounts of MgBr2·2Et2O (Scheme 25). Noteworthy, primary aromatic amines with electron-donating groups such as methoxy and methyl afforded the tetrazole derivatives in slightly higher yields than amines with electron withdrawing groups such as nitro, whereas the cyclohexyl isocyanide instead of tert-butyl isocyanide required longer reaction times to afford similar products.
Scheme 25. Synthesis of a Series of Tetrazoles 73 Containing the 2,2-Bis(trimethylsilyl)ethenyl Group.
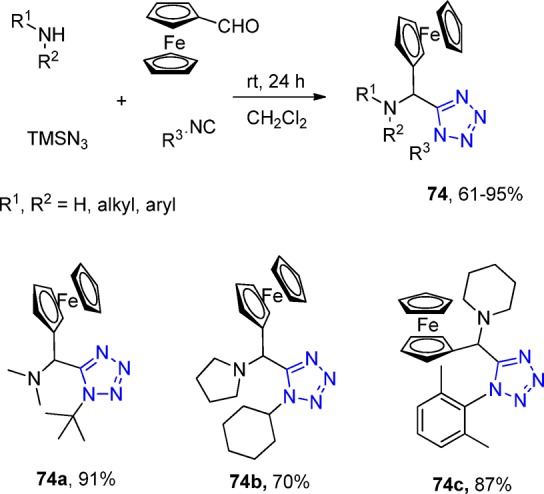
In 2012, Bazgir et al.185 synthesized a series of ferrocenyl dialkylamino tetrazoles and ferrocenyl arylamino tetrazoles 74 via an UT-4CR without any catalyst in dichloromethane (Scheme 26). This is the first example of an efficient synthesis of ferrocenyl-fused tetrazoles. To explore the scope and limitations of the reaction, both aliphatic secondary amines and aromatic primary amines were employed, which afforded the final ferrocenyl tetrazoles in good yields. Because α-ferrocenyl-alkyl amines are important ligands in asymmetric catalysis reaction, such tetrazole derivatives could be further evaluated.186
Scheme 26. Synthesis of Ferrocenyl Substituted Amino Tetrazoles 74.
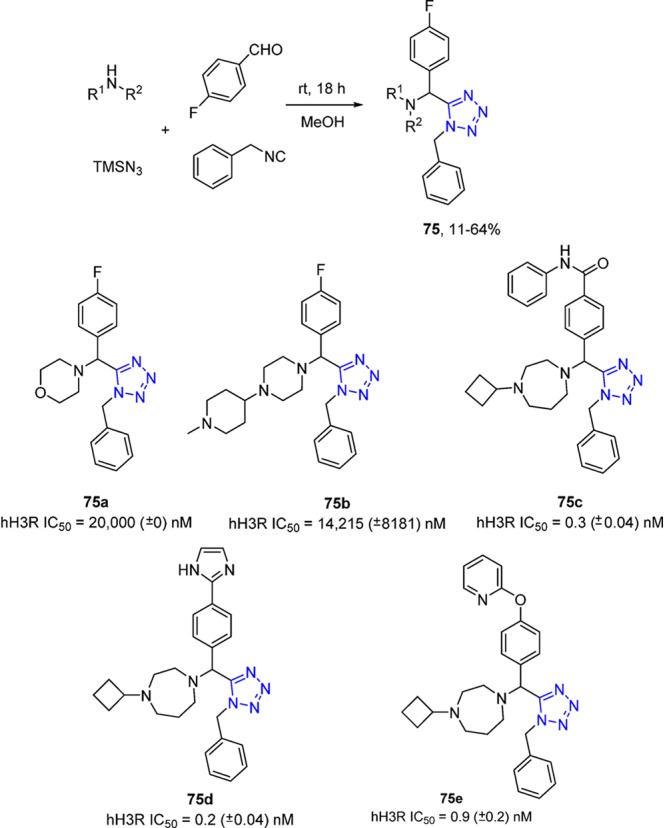
The UT-4CR has found profound application in the field of medicinal chemistry. Histamine H3 receptor (H3R) acts both as an auto receptor in presynaptic histaminergic neurons and also controls histamine turnover by feedback inhibition of histamine synthesis and release.187 Attracted by the potential of the H3R as a drug target, Davenport et al.145 described a series of potent and subtype selective H3 receptor antagonists containing a novel tetrazole core and diamine motif. A one-pot UT-4CR was utilized to rapidly develop the structure–activity relationships (SARs) of these compounds. According to the biological screening results, the piperazine ring with small alkyl groups should be maintained. Shielding around the nitrogen, however, did not afford an improvement in metabolic stability. After modifications of the aromatic substituents and further optimization, potent derivatives (75) were the result (Scheme 27).
Scheme 27. Synthesis of Substituted Benzyl Tetrazoles As Histamine H3 Receptor Antagonists 75.
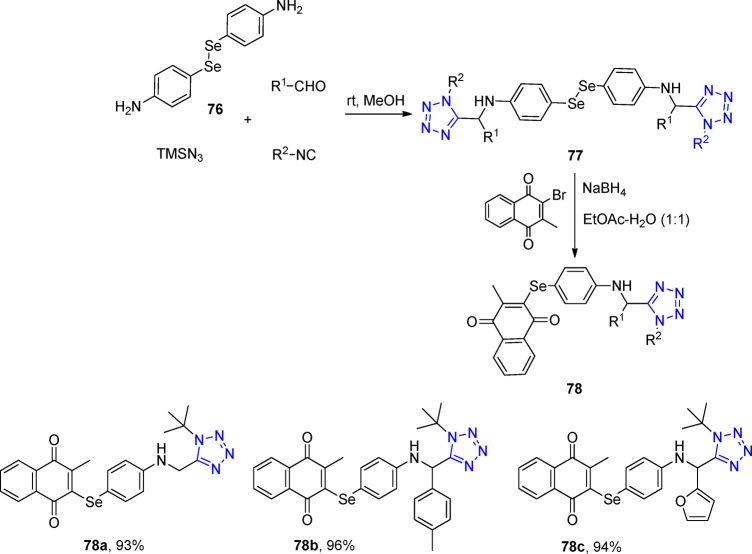
A library of tetrazole-based diselenides and selenoquinones 77 and 78, respectively, were synthesized via UT-4CR and a sequential nucleophilic substitution, which was evaluated against hepatocellular carcinoma.188 Employing the corresponding diamines 76, 18 tetrazole/naphthoquinone-based organoselenium derivatives were synthesized in good yields and their cytotoxic activity was evaluated using hepatocellular carcinoma (HepG2) and breast adenocarcinoma (MCF-7) cancer cells and compared with their cytotoxicity in fibroblast (WI-38) cells. It was found that the selenoquinones 78 downregulated the apoptosis regulator Bcl-2 and Ki-67 expression levels and activated the expression of proapoptotic caspase-8 in HepG2 cells compared to untreated cells (Scheme 28).
Scheme 28. Synthesis of Tetrazole/Naphthoquinone-Based Organoselenium Derivatives 78.
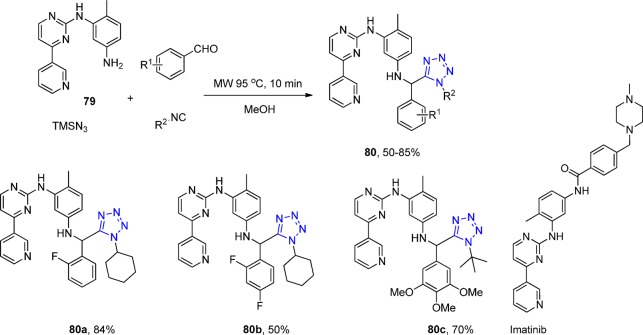
The UT-4CR was also utilized in order to derivatize the anticancer drug Imatinib.189 Under microwave irradiation, 30 adducts (80) with 10 different aldehydes and two isocyanides were synthesized bearing the amine 79, which is the precursor of Imatinib (Scheme 29). Unfortunately no biological results were reported.
Scheme 29. Representative Scheme for the Preparation of 1,5-Disubstituted Tetrazoles 80 Containing a Fragment of the Anticancer Drug Imatinib.
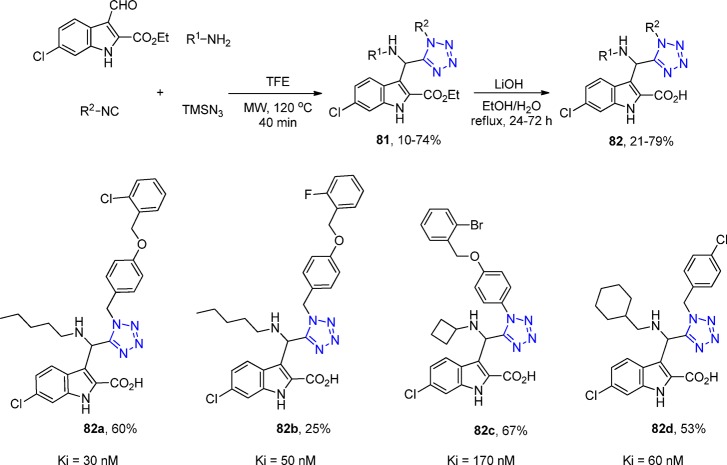
The tumor-suppressor protein p53 is the principal regulator of cell division and growth,190,191 as it is able to control genes that are implicated in cell-cycle control, apoptosis, angiogenesis, senescence, and autophagia. Mutations in this protein are present in ∼50% of human cancers. Inhibiting the binding between wild-type (WT) p53 and its negative regulators MDM2 and/or MDMX has become an important target in oncology to restore the antitumor activity of p53.192 In 2017, a rational design and synthesis of 1,5-disubstituted tetrazoles 81 and 82 as potent inhibitors of the MDM2-p53 interaction was reported (Scheme 30, Figure 21). An extensive SAR study was performed based on the established four-point pharmacophore model, yielding derivatives with affinity to MDM2 in the nanomolar range. Their binding affinity with MDM2 was evaluated using both fluorescence polarization (FP) assay and 2D-NMR-HSQC experiments.193
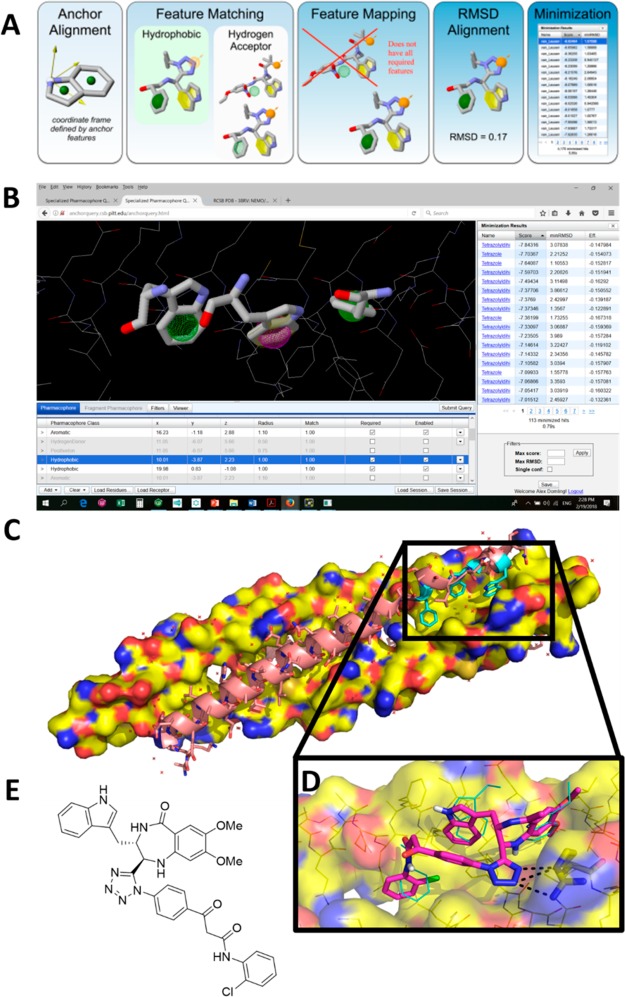
Scheme 30. Synthesis of the Potent 1,5-Disubstituted Tetrazoles 81 and 82 as p53-MDM2 Inhibitors.
Figure 21.
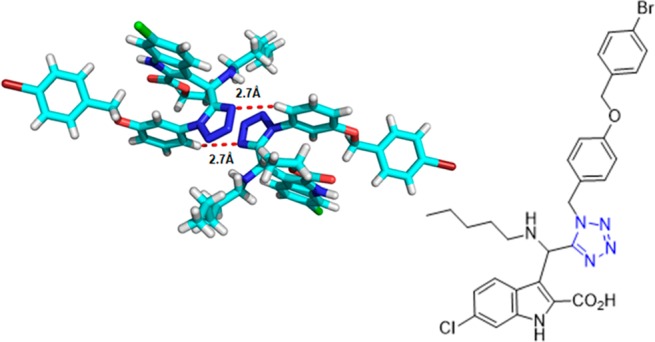
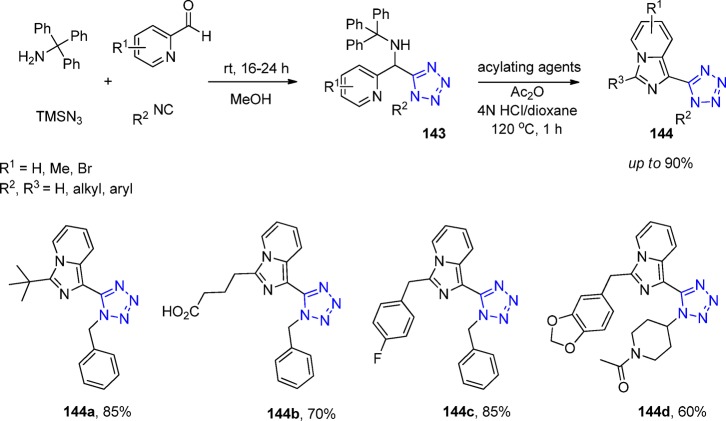
Crystal structure of the 1,5-disubstituted tetrazole 82e (CCDC 1449789). The ring planes of substituents at positions 1 and 5 are almost coplanar, being constrained by tetrazole geometry and are oriented vertically to the plane of the tetrazole ring.
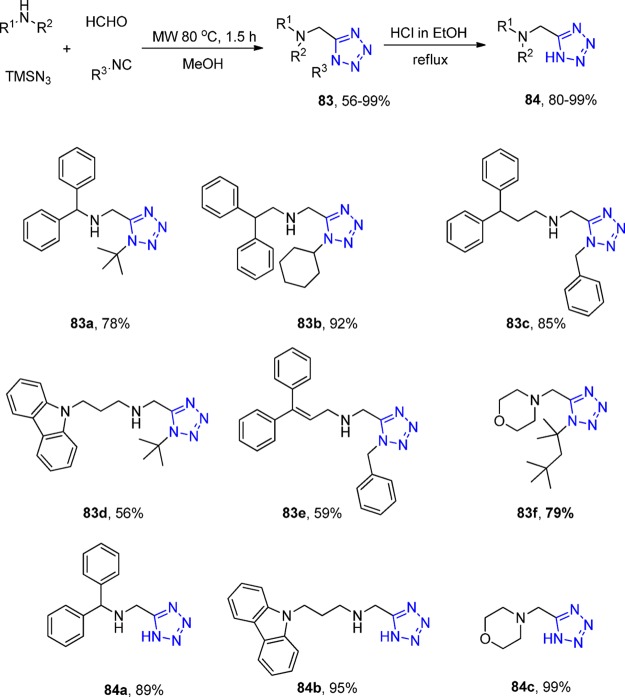
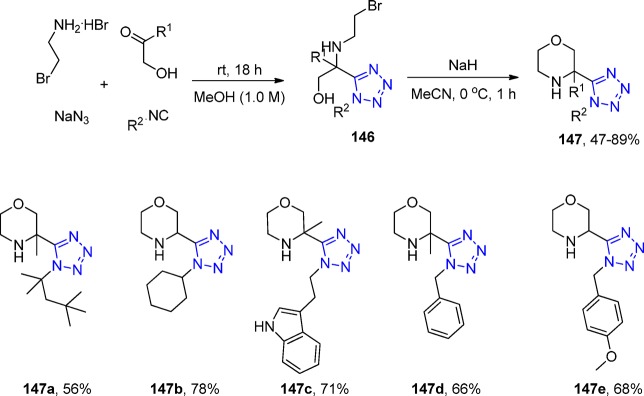
Considering that all receptors, metabolic enzymes, and transporters involved in GABAergic neurotransmission can be considered as valid drug targets, Wanner et al.146 employed an UT-4CR as a key step to synthesize 1,5-disubstituted and 5-monosubstituted aminomethyltetrazole derivatives 83 and 84, respectively, derived from glycine. All products were evaluated regarding their inhibitory potency and subtype selectivity at the four murine GABA transporter subtypes mGAT1-mGAT4. The results showed that none of the 5-monosubstituted tetrazoles has a potential for inhibition of GABA uptake, however, the 1,5-disubstituted tetrazole derivatives displayed a distinct activity, especially at the GABA transport proteins mGAT2–mGAT4. A reasonable potent and selective inhibitor of mGAT3 was found. Additionally, two more compounds were identified as potent inhibitors of mGAT2. Interestingly, up to now, only a few potent and selective inhibitors of mGAT2 that do not affect mGAT1 are known (Scheme 31).
Scheme 31. Synthesis of Aminomethyltetrazoles 83 and 84.
Dysfunction of excitatory amino acid transporters (EAATs) has been implicated in the pathogenesis of various neurological disorders such as stroke, brain trauma, epilepsy, and neurodegenerative diseases among others.194,195 EAAT2 is the main subtype responsible for glutamate clearance in the brain, having a key role in regulating transmission and preventing excitotoxicity. Therefore, compounds that increase the expression or activity of EAAT2 have therapeutic potential for neuroprotection. After a virtual screening of a library of small molecules, 10 hit molecules that interact at the proposed domain were identified as UT-4CR adducts.196 The reaction was performed with a catalytic amount of trifluoroacetic acid in 2-propanol at 95 °C for 24 h. Further characterization of the two best ranking EAAT2 activators 85 and 86 (Figure 22) for efficacy, potency, and selectivity for glutamate over monoamine transporters subtypes and NMDA receptors and efficacy in cultured astrocytes was demonstrated. Authors also found that the EAAT2 activators interact with residues forming the interface between the trimerization and the transport domains; these compounds enhance the glutamate translocation rate, with no effect on substrate interaction, suggesting an allosteric mechanism.
Figure 22.
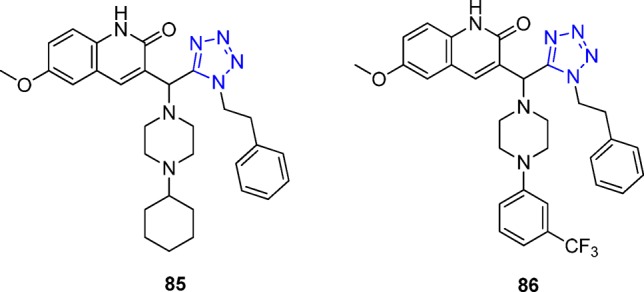
Two of the most potent compounds as positive allosteric modulators of EAATs.
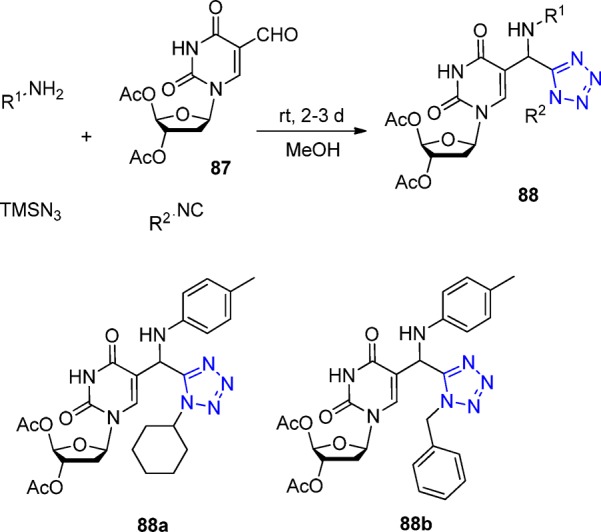
Torrence et al.197 examined the use of the Ugi reaction in the generation of new nucleosides as potential antiviral and antileishmanial agents. In that direction, starting from aldehyde 87, they designed a series of nucleosides using the UT-4CR, which were evaluated for their activity against vaccinia virus, cowpox virus, and the parasite Leishmania donovani. They obtained some novel tetrazole derivatives 88 in good yields, unfortunately, without possessing any significant antiviral activity (Scheme 32).
Scheme 32. Antiviral Tetrazole Desoxyribose Derivatives 88.
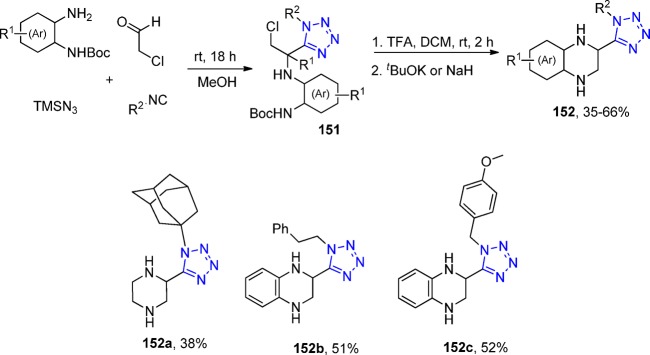
Heterocycle hybrid derivatives 90 bearing both a thiadiazole (89) and a tetrazole ring were designed and synthesized in 2012 by Fan et al.198 These derivatives were formed via an UT-4CR and exhibited both broad-spectrum activity against several fungi and excellent antiviral activity (Scheme 33). A crystal structure of 90d was reported (Figure 23).
Scheme 33. Synthesis of the Thiadiazolo Tetrazole Derivatives 90.

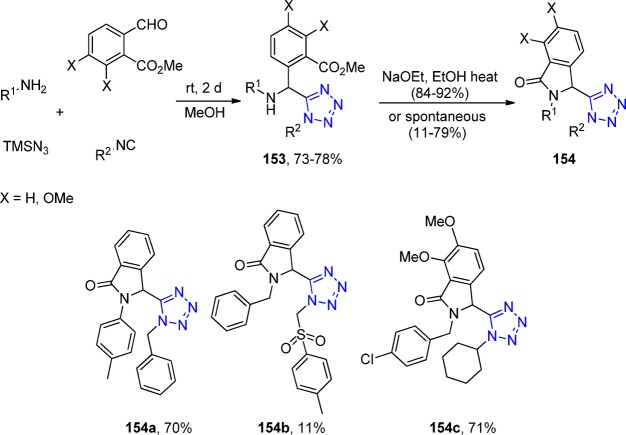
Figure 23.
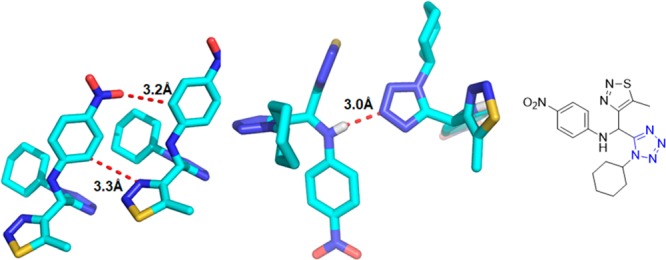
Crystal structure of N-((1-cyclohexyl-1H-tetrazol-5-yl)(5-methyl-1H-1,2,3-triazol-4-yl)methyl)-4-nitroaniline (90d). It shows that the dihedral angles formed between the thiadiazole and tetrazole rings, the benzene and tetrazole rings, and the thiadiazole and benzene rings are 62.59°, 86.73°, and 70.07°, respectively. Three intermolecular hydrogen bonds N(1)–H(2)···N(6), C(4)–H(4B)···O(2), and C(17)–H(17)···N(3) are identified (CCDC 859295).
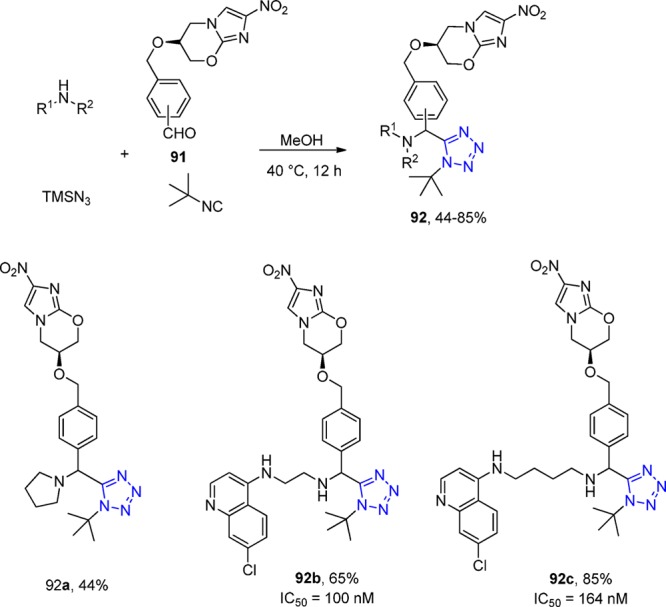
Parasitic diseases are a global problem, affecting 30% of the world’s population and much of the world’s lifestock. Among parasitic diseases, malaria is one of the most devastating infectious diseases claiming many lives. There were at least 216 million cases of acute malaria reported in 2010, and about 655000 people died from malaria, 86% of which were children under 5 years of age.199 Chibale et al.200,201 designed new quinoline-based compounds bearing the tetrazole moiety and protonatable nitrogen(s) that have potential application in malaria. Thus, utilizing the aldehyde 91, he synthesized in a diastereoselective way two new series of nitroimidazole and nitroimidazooxazine derivatives 92 in moderate to excellent yields using the UT-4CR. Three of these compounds appeared to be rapidly metabolized in both human and rat liver microsomes, and they had high metabolic clearance that was comparable to that of amodiaquine (Scheme 34). All synthesized tetrazole derivatives were evaluated in vitro for their antiplasmodial (against the multidrug-resistant K1 strain) and antimycobacterial activity (against the drug-sensitive H37Rv Mtb strain). Two of these compounds exhibited potent activity against the K1 strain of Plasmodium falciparum, with IC50 values in the low micromolar range.
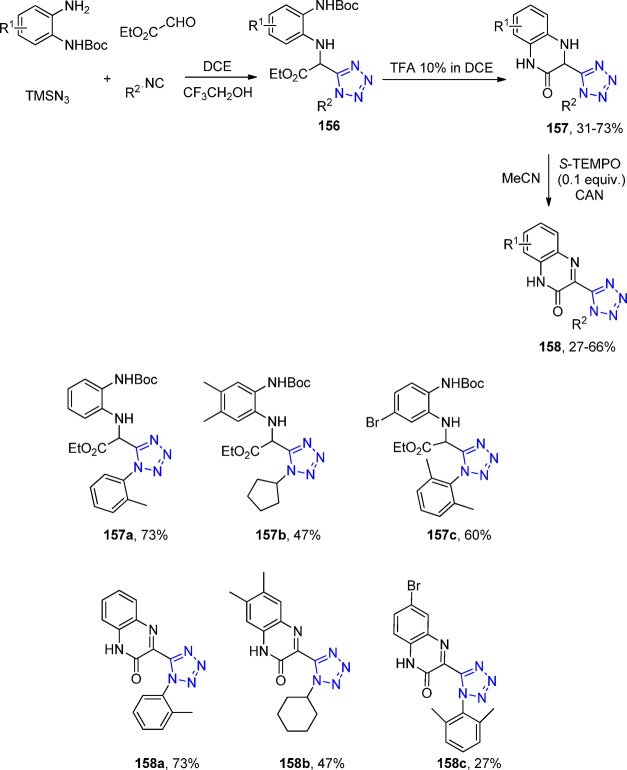
Scheme 34. Synthesis of New Nitroimidazole and Nitroimidazooxazine Derivatives 92.
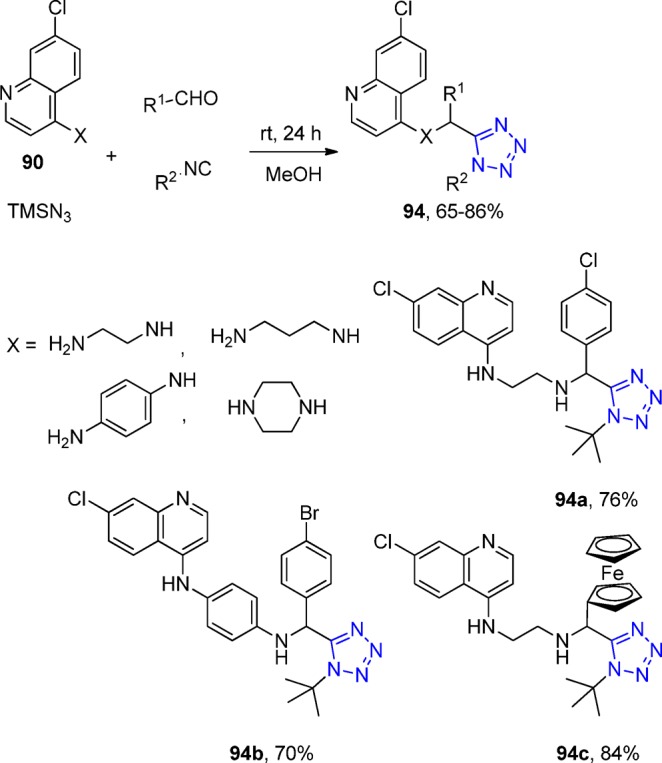
In 2013, Chauhan et al.202 synthesized a series of novel tetrazole derivatives 91 of 4-aminoquinolines (93) via an UT-4CR of primary and secondary amines, aliphatic, aromatic and ferrocene containing aldehydes, TMS azide, and isocyanides (Scheme 35). All the products were screened for their antimalarial activities against both chloroquine-sensitive (3D7) and chloroquine-resistant (K1) strains of Plasmodium falciparum as well as for cytotoxicity against VERO cell lines. Most of the synthesized compounds exhibited potent antimalarial activity as compared to chloroquine against the K1 strain. Some of the compounds with significant in vitro antimalarial activity were then evaluated for their in vivo efficacy in swiss mice against Plasmodium yoelii following both intraperitoneal (ip) and oral administration. Compounds 94a and 94b each showed in vivo suppression of 99.99% parasitaemia on day 4.
Scheme 35. Synthesis of 4-Aminoquinoline-Tetrazole Derivatives 94.
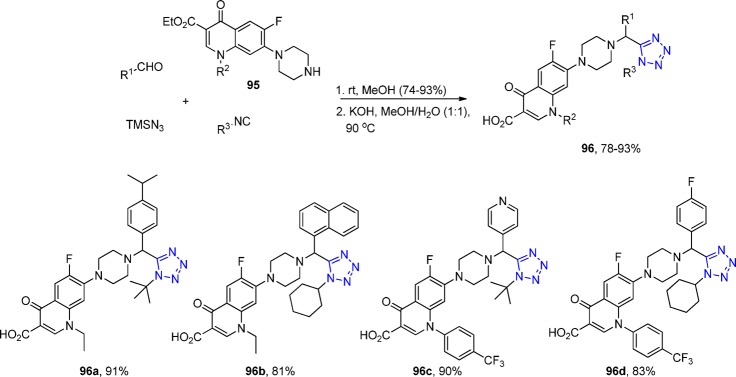
In addition, they introduced a novel series of 7-piperazinylquinolones 95 with tetrazole derivatives 96 and evaluated their antibacterial activity against various strains of Staphylococcus aureus.151 All the compounds showed significant in vitro antibacterial activity against Gram-positive bacteria, whereas some displayed moderate activity in vivo (Scheme 36).
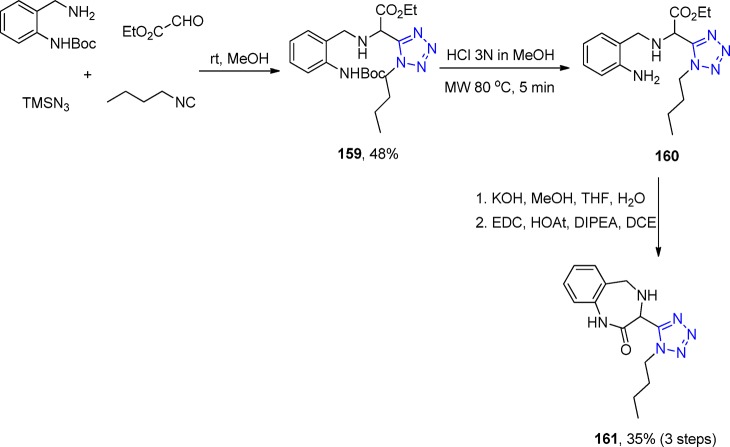
Scheme 36. Representative Scheme for the Preparation of 1H-Tetrazol-5-yl-(aryl)methyl Piperazinyl-6-fluoro-quinolones 96.
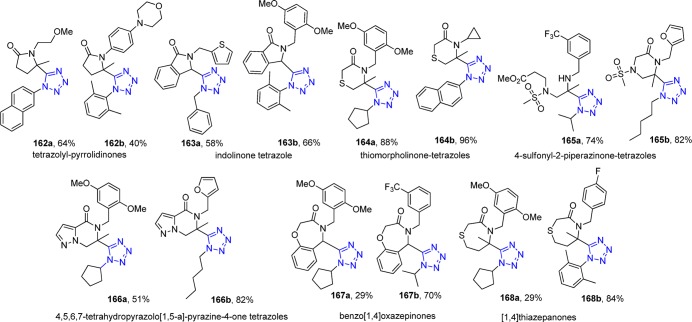
Sharada et al.203 developed a facile one-pot, four-component domino reaction involving the 2-(2-bromoethyl)benzaldehyde, isocyanide, amine, and NaN3 for the synthesis of tetrazolyl-tetrahydroisoquinoline derivatives 97 without the use of any catalyst or additive, under ambient conditions with short reaction times (Scheme 37, Figure 24). The first step is the imine formation, followed by substitution of the bromine and reaction of the resulting cyclic iminium ion with the isocyanide and the azide source. To test the generality of this methodology, various amines with both electron donating and withdrawing aromatic groups as well as aliphatic isocyanides were employed and afforded good to excellent yields. However, nitro-substituted anilines failed to give the expected products due to amine deactivation through the strong electron withdrawing features. Only one aliphatic amine, cyclohexylamine, was tested and also successfully resulted in the final ring-closed compound 97.
Scheme 37. Synthesis of a Variety of Tetrazole Substituted Tetrahydroisoquinolines 97.
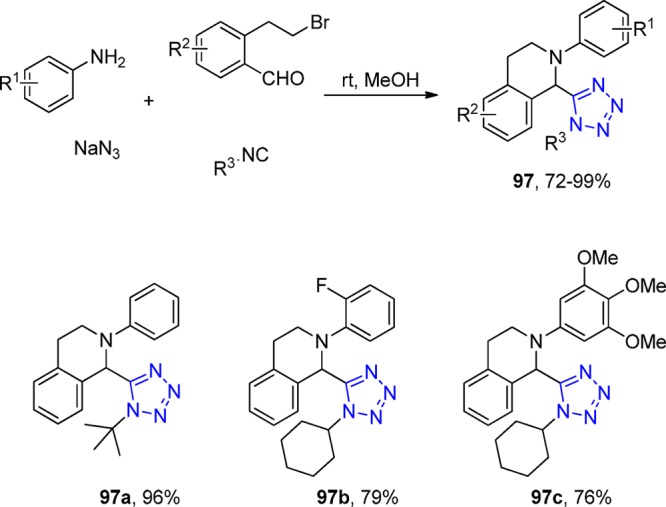
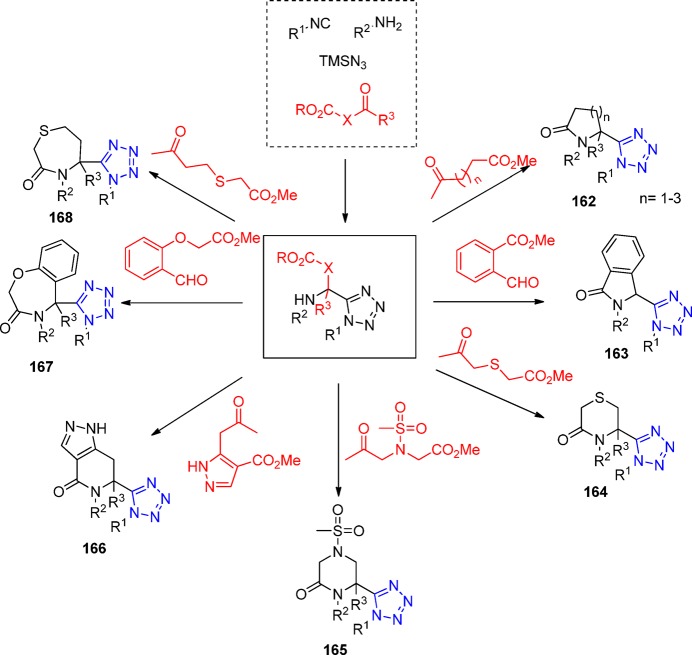
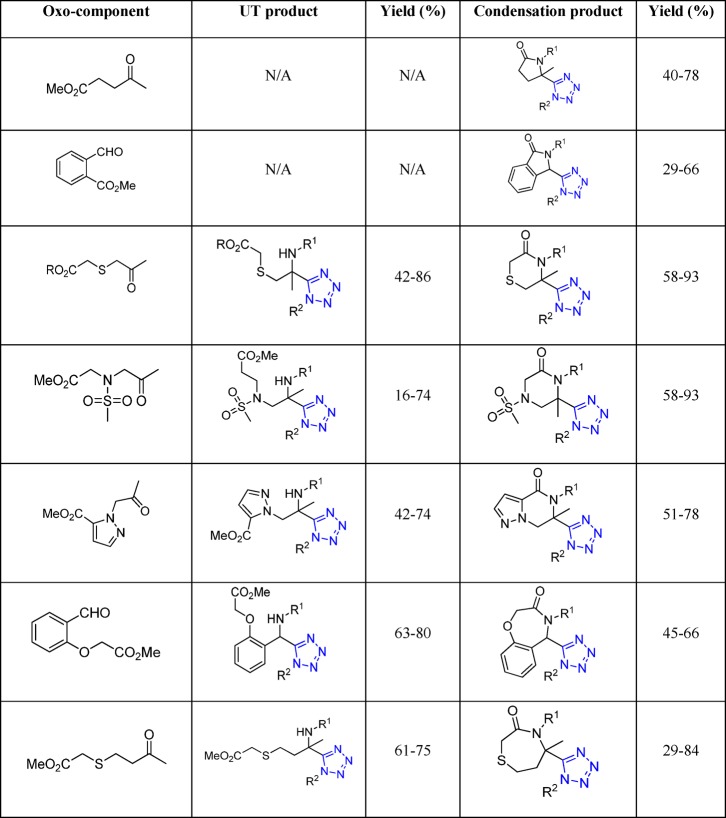
Figure 24.
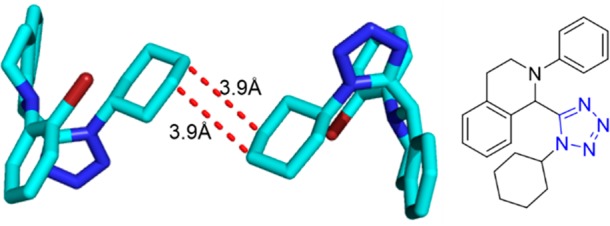
X-ray crystal structure of tetrahydroisoquinoline 97d (CCDC 1012826). Two intermolecular hydrophobic interactions between the two cyclohexyl groups are observed
In a similar fashion, the one-pot synthesis of tetrazole substituted tetrahydro-β-carbolines 98 was reported by Mukkanti et al.204 The UT reaction of the indole-carboxaldehyde with mostly anilines (in some cases benzyl amine was utilized) and various isocyanides afforded the targeted tetrazole substituted β-carbolines in excellent yields (Scheme 38). The process involves the previous formation of a cyclic iminium ion, followed by reaction with the isocyanide and the azide.
Scheme 38. Synthesis of Tetrahydro-β-carbolines 98 Bearing a Tetrazole Moiety through an UT-4CR-5C.
3.1.1.1. Repetitive UT-4CR
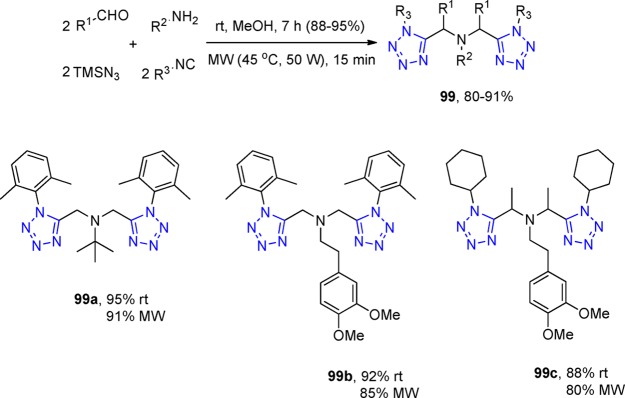
Many proteins in nature exist as symmetrical homodimers, e.g., the HIV-protease. For that reason, symmetrical dimeric MCR reaction products might be useful to interact with the interface of symmetrical protein homodimers to stabilize such complexes.205 Gámez-Montaño et al.206 developed a catalyst-free UT repetitive process to quickly prepare a series of five novel bis-1,5-disubstituted-1H-tetrazoles 99 in excellent yields. They simply mixed one equivalent of the corresponding primary amine and two equivalents of the corresponding aldehyde, isocyanide, and TMS azide in MeOH at room temperature. After several hours, they afforded first the mono Ugi product and then, upon further microwave heating, the repetitive Ugi products in excellent yields as a mixture of two diastereomers (in the case that R1 is not hydrogen, Scheme 39).
Scheme 39. Synthesis of Bis-1,5-disubstituted-1H-tetrazoles 99.
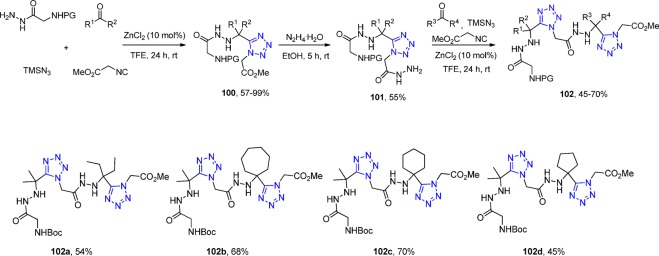
Similarly to the work of Dömling et al.170 in employing hydrazine in UT-4CR, Andrade et al.207 reported two consecutive hydrazine UT-4CR incorporating acylhydrazines within 1,5-disubstituted tetrazoles 102. Their strategy was based on a one-pot hydrazino UT-4CR (100) using protected acyl hydrazines (Boc or Cbz) followed by hydrazinolysis (101) by aqueous hydrazine and finally an additional hydrazino UT-4CR (Scheme 40).
Scheme 40. Synthesis of the Acylhydrazines with 1,5-Disubstituted Tetrazoles 97 via a Two Consecutive Hydrazine UT-4CR.
Another example of a molecule with multiple tetrazole units was described by Dömling et al.208 Reaction of cyclen 103 with formaldehyde, TMS azide, and β-cyanoethylisocyanide 64 quantitatively yielded compound 104 (Scheme 41). The β-cyanoethyl protecting group was used due to its mild deprotection conditions (LiOH in water at rt). The deprotected ligand 105 (TEMDO) was successfully metalated and crystal structures were obtained with Gd, Ln, and Eu. Moreover, the authors utilized the novel Gd-TEMDO complexes 106 in magnetic resonance imaging (MRI) in a left ventricular occlusion (LVO) mouse model (Figure 25). The overall complex and magnetic properties were compared and proved to be equivalent to most of the used Gd-DOTA complexes in the MRI field. The TEMDO synthesis is short, experimentally simple, and high yielding. In addition, in a similar fashion, many more oligo amino tetrazoles could be synthesized accordingly with interesting material properties.
Scheme 41. Synthesis of the MRI Agent Gd-TEMDO 106 Involving a Key UT-MCR.
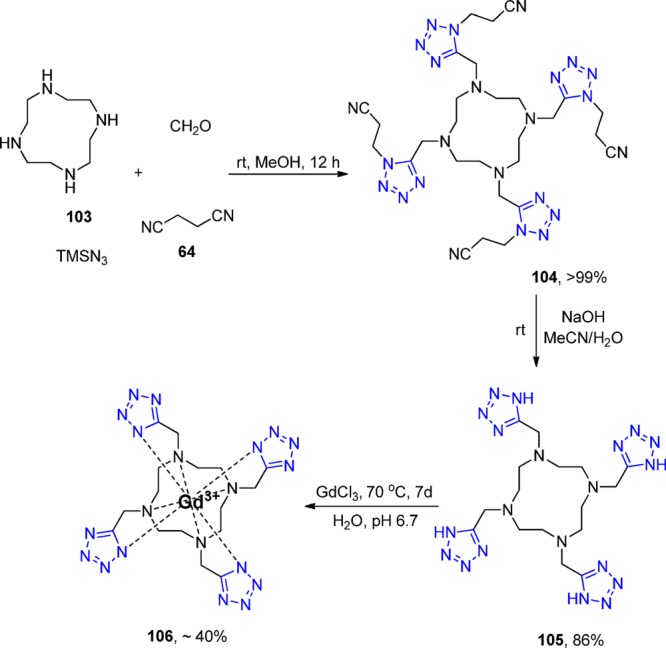
Figure 25.
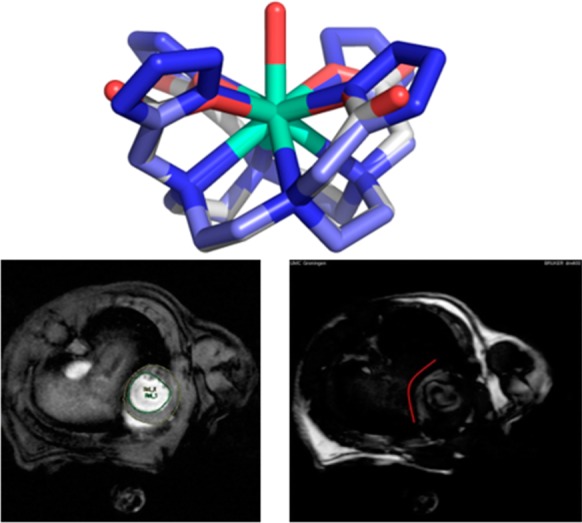
Left: Crystal structure of Gd-TEMDO 106. Middle and right: LVO mouse model showing the MRI properties of Gd-TEMDO. MRI obtained from isoflurane-anaesthetized mice (middle) taken 30 min after IP administration of Gd-TEMDO (0.6 mmol/kg). Middle: the heart fully visible. Right: heart with reduced brightness; the damaged tissue remains visible due to absorbed Gd-TEMDO following the red line. Reproduced with permission from ref (208). Copyright 2016 John Wiley and Sons.
3.1.1.2. UT-4CR on Solid Phase (UT-4CR on SP)
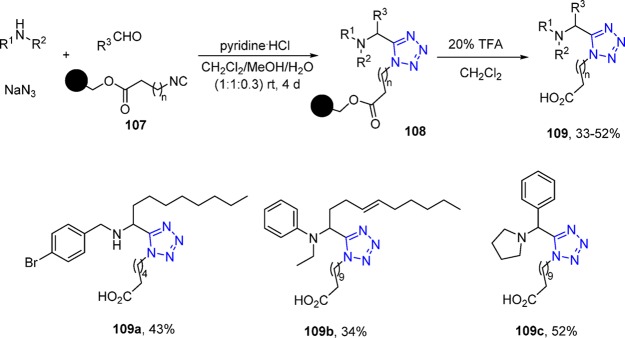
Solid-phase synthesis (SPS) is a method in which a starting material is bound on solid support and reacts with the other reactants in solution. SPS, which has been explored by chemists for many years,209−213 is often performed in sequential syntheses to automate synthesis and intermediate purification, e.g., in oligo-DNA or peptide synthesis. The synthetic application of solid phase in tetrazole synthesis using MCR started in 1997 when Mjalli et al.214 first produced a small library of 1,5-disubstuted tetrazole derivatives encouraged by their success on solid phase to obtain small-ring lactams, α-(dialkylamino)amides, hydantoin 4-imides, and 2-thiohydantoin 4-imides. In their synthetic process, amines, aldehydes, NaN3, and the supported isocyanides 107 were simply stirred for 4 days in a solvent mixture containing methanol, dichloromethane, and water (1:1:0.3) along with pyridine hydrochloride to afford the corresponding tetrazole-resin derivatives 108. The subsequent cleavable step was accomplished by stirring the Ugi products 109 with 20% trifluoroacetic acid in dichloromethane after washing with methanol and dichloromethane (Scheme 42). Various amines and aldehydes could lead to the target tetrazoles by this methodology. Probably caused by poor activity of ketones in this reaction, they did not afford the corresponding tetrazoles under these conditions, but after stirring for long time, only the formamides could be detected.
Scheme 42. Synthesis of 5-(1′-Aminoalkyl)tetrazoles 109 on Solid Phase.
Ugi et al.215 also prepared a variety of hydantoinimide and tetrazole derivatives by the combination of two distinguished Ugi reactions in solid and liquid phases separately. Although many types of the combinations of U-4CRs and further reactions have been developed, this was the first time to employ two different types of U-4CRs with the primary amines supported by the polystyrene AM RAM or the TentaGel S Ram. In the first U-4CR, Fmoc protected amino acid 110 reacted as a carboxylic acid with aldehydes, isocyanides, and the solid supported primary amines to form the corresponding amides 111. Subsequently, after the cleavage of Fmoc group with 20% piperidine in DMF (112), the second U-4CR was carried out with TMS azide as an acid component (113) and the removal of the resin with trifluoroacetic acid treatment led to the final tetrazole derivatives 114 formation (Scheme 43). Interestingly, the aromatic aldehydes were tolerated in the second U-4CR to form tetrazoles with good yields compared with rather low yields of the hydantoinimides. Moreover, they also compared the liquid phase combinational MCRs with that of the solid–liquid method. The results demonstrated that the former one could give higher yields.
Scheme 43. Repetitive Ugi Reaction on the Polystyrene AM RAM.
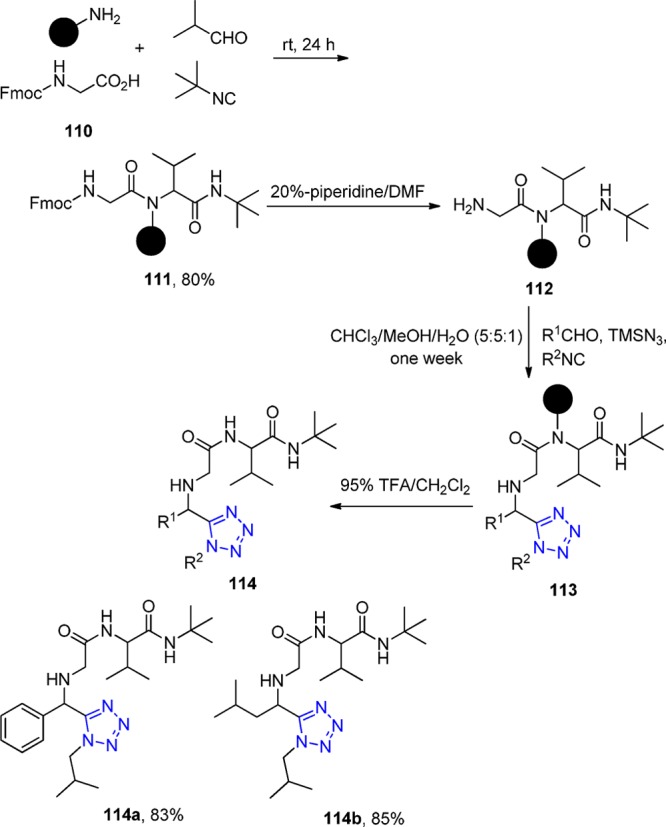
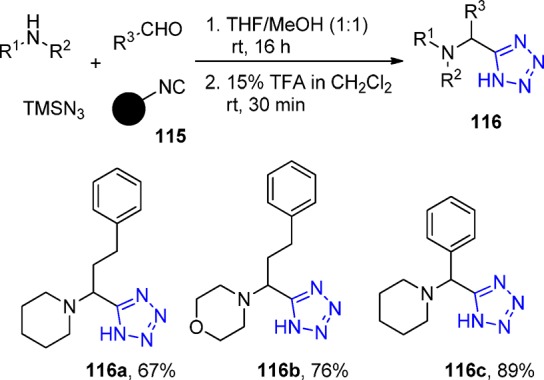
Chen et al.216 employed a Rink-isocyanide resin 115 as a universal platform for classical Ugi reactions to prepare a small library of five 5-substituted 1H-tetrazoles 116. The cleavage of the resin was performed with 15% trifluoroacetic acid in dichloromethane (Scheme 44).
Scheme 44. Synthesis of 5-Substituted Tetrazoles 116 on the Universal Rink-Isocyanide Resin.
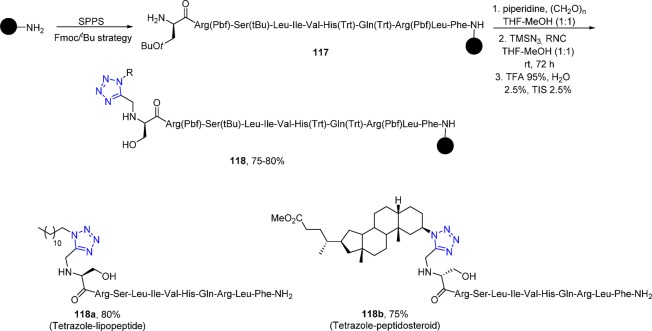
Rivera et al.217,218 reported an efficient and reproducible method implementing on-resin Ugi reactions with peptides (117) and its utilization in combination with peptide couplings for the solid phase synthesis of N-substituted and tetrazolo peptides 118 (Scheme 45).
Scheme 45. On-Resin UT Reactions for the N-Terminal Derivatization of Peptide with Lipids and Steroids.
3.1.1.3. UT-4CR Followed by Subsequent Post Cyclizations
Multicomponent reactions combine two major principles in organic synthesis, convergence, and atom economy. The combination of multicomponent reaction and post-transformation reactions is another tremendously useful tool to increment the complexity and diversity of the molecular scaffolds. An important subgroup of MCRs is the so-called unions of MCRs as coined by Dömling and Ugi,219 where an MCR is combined with a secondary MCR.220 The union of MCRs is the strategy for the rational design of novel MCRs combining two (or more) different types of MCRs in a one-pot process. The presence of orthogonal reactive groups in the product of the primary MCR, which is either formed during the primary MCR or present in one of the inputs, allows the union with the secondary MCR.221
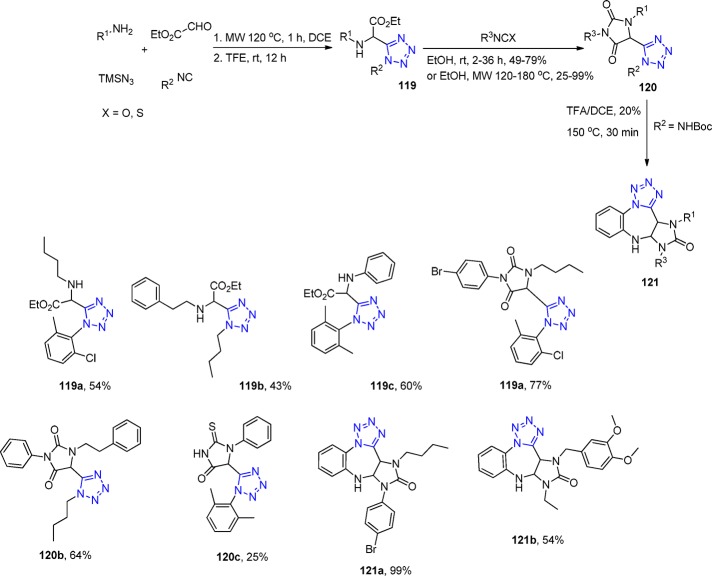
There are many classical documented post-transformation reactions, i.e., Pictet–Spengler cyclization, intramolecular Diels–Alder reaction, Mitsunobu reaction and acyl migration, Knovenagel condensation, amide reduction, metathesis reaction, Ugi–Ugi, and Ugi–Petasis etc.52,120,127,222−236 The strategies entailing intramolecular variants of the Ugi reaction and post condensation modifications of the Ugi product inspire the development of methodology that enables concise access to diverse pharmacologically relevant scaffolds. These Ugi variants indeed afforded enticing structures for further diversification. The hydantoin (imidazoline-2,4-dione) scaffold is a reoccurring motif in many biologically relevant compounds with anticonvulsant, antimuscarinic, antiulcer, antiviral, and antidiabetic activities and showing strong BACE binding for potential anti-Alzheimer application.237−242 Hulme et al.243 described a novel methodology to elegantly obtaining new and biologically appealing 1,5-substituted tetrazole-hydantoins and thiohydantoins 120 with three points of variation (Scheme 46, Figure 26). The UT-4CR is based on the glyoxale ethylester, as a not variable oxo input, followed by the treatment of the Ugi intermediate 119 with an excess of isocyanate or isothiocyanate to generate the final scaffold in moderate to good yields. Various amines, isocyanides and isocyanates, or isothiocyanates were used to test the generality of this methodology. Because of the general availability of a large number of isocyanides, aldehydes, ketones, and iso(thio)cyanates, this reaction sequence is of high combinatorial value representing a large chemical space (Scheme 46). Furthermore, a one-step extension (but still one pot) of this methodology using a functionalized hydantoin with an internal-masked amino nucleophile previously introduced by the isocyanide input has also been reported giving imidazotetrazolodiazepinones 121 in good yields.244 A crystal structure of the hydantoin 120c was reported featuring an interesting intermolecular halogen bonding involving a Br and two nitrogens of the tetrazole (Figure 26).
Scheme 46. Synthesis of 1,5-Substituted Tetrazole Hydantoins and Thiohydantoins 120 and Imidazotetrazolodiazepinones 121.
Figure 26.
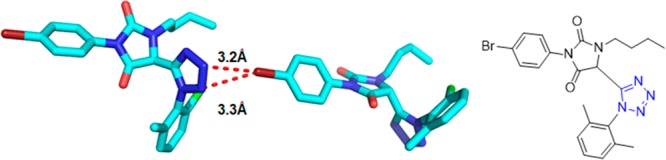
Crystal structure of a 4-bromophenyltetrazolohydantoin 120d featuring two short contacts (3.2 and 3.3 Å) between the p-Br and N2 and N3 of an adjacent tetrazole moiety exhibiting halogen bonding character (CCDC 922820).
Benzodiazepines are important drugs with a wide spectrum of biological and medicinal activities and marketed applications as anxiolytics, anticonvulsants, hypnotics, etc.245,246 Besides these classical applications, the benzodiazepine scaffold is also of interest in numerous other areas as antagonizing the protein–protein interaction p53-MDM2,247 GPIIbIIIa antagonists,248 antioxidants,249,250 and inhibitors of farnesyltransferase.251 Multiple synthetic pathways are described toward benzodiazepines, which also include routes involving MCRs.147,252−263 Because of the privileged scaffold character of tetrazoles and benzodiazepines, several researchers designed synthetic strategies to combine the two heterocycles.264
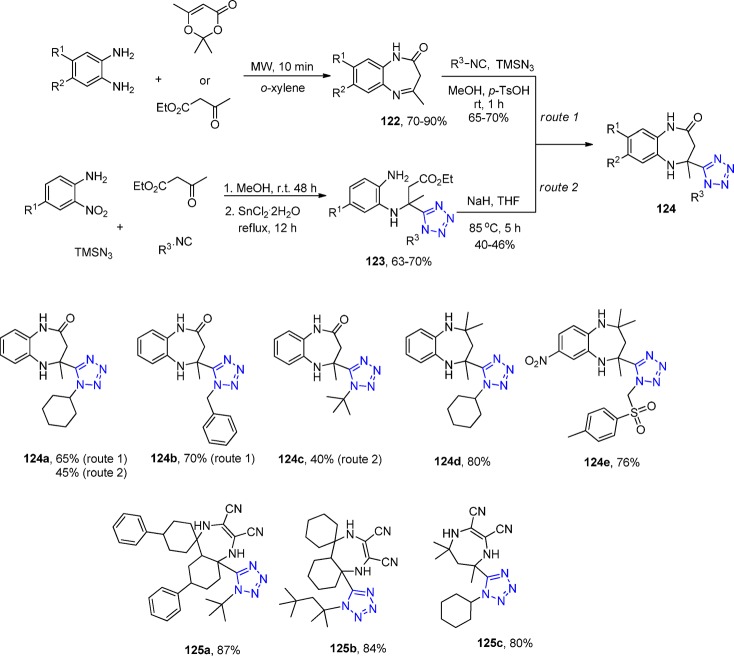
Shaabani et al.265 reported a new class of benzodiazepine-containing tetrazole scaffold, 1H-tetrazol-5-yl-4-methyl-1H-benzo[b][1,4]diazepines 124, via a two-step condensation reaction of o-phenylenediamines (oPDM), ethyl 3-oxobutanoate, or 2,2,6-trimethyl-4H-1,3-dioxin-4-one, an isocyanide, and TMS azide (Scheme 47, route 1). The first reaction involves the cyclocondensation of o-phenylenediamine with a β-ketoester to yield benzodiazepinone Schiff base 122, which reacts in a second step in an UT reaction. Monosubstituted (NO2 and CH3) phenylenediamines reacted highly regioselectively as indicated by NMR and crystal structure (Figure 27). Moreover, they also disclosed two IMCRs,266,267 employing 2,3-diaminomaleonitrile, ketones, isocyanides, and either sodium azide or trimethylsilyl azide in the presence of pTsOH·H2O in various organic solvents and water at room temperature to afford 1H-tetrazolyl-1H-1,4-diazepine-2,3-dicarbonitriles 125 in high yields (Scheme 47, Figure 28).
Scheme 47. Synthesis of 1H-Tetrazol-5-yl-4-methyl-1H-benzo[b][1,4]diazepines 124 and 1H-Tetrazolyl-1H-1,4-diazepine-2,3-dicarbonitriles 125.
Figure 27.
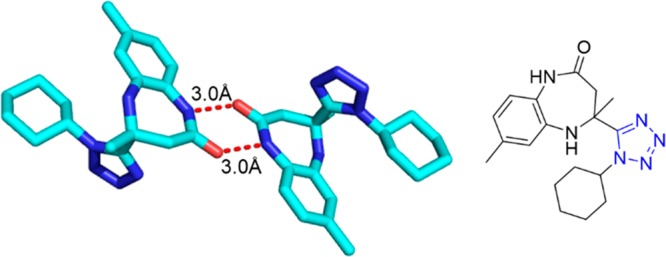
Crystal structure of the benzodiazepin-2-one 124f (CCDC 900744). The symmetrical hydrogen bonding interaction between O and N was measured 3.0 Å
Figure 28.
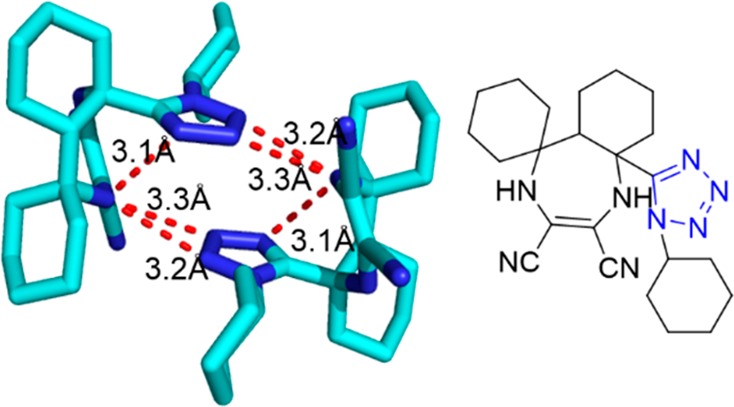
Crystal structure of compound 125d (CCDC 814967). A network of intramolecular hydrogen bonds of N–H can be observed among the NH and CN groups and the tetrazole moieties varying from 3.1 to 3.3 Å
o-Phenylenediamines are a limiting component in this otherwise interesting scaffold because only a few are commercially available. Therefore, Shabaani et al.268 elaborated a second variation to this scaffold by first reacting 2-nitroanilines in the UT reaction, affording the tetrazole intermediate 123 followed by reduction of the o-nitro group and NaH promoted cyclization to yield compounds 124 (Scheme 47, route 2). While the second synthetic access is much more versatile in the o-nitroaniline component, it also involves a longer synthetic route. The overall yields are higher for the first route and also leading to short reaction times.
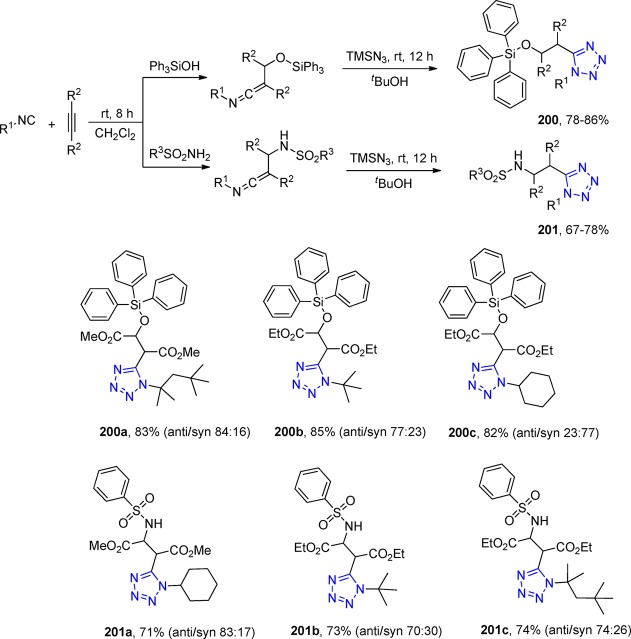
Isoindoline is a heterocyclic organic compound with a bicyclic structure, not found itself in nature although many of its derivatives have, with a broad structural diversity and broad-spectrum biological activities. Thus, many biologically active compounds have been discovered, i.e., endothelin-A receptor antagonists, PPARd agonists, NMDA receptor antagonists, herbicidal, anti-inflammatory, antileukemic agents, etc.269−272 Yet, various synthetic procedures have been reported for the preparation of isoindoline core structural skeletons.
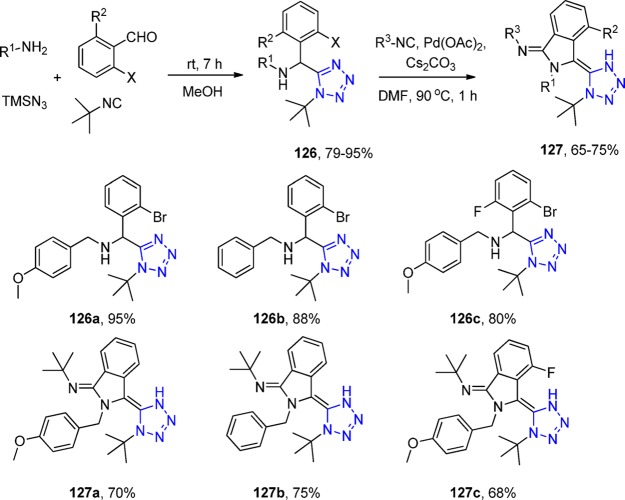
Chauhan et al.273 first employed a two-step combination of an UT reaction (126) and palladium-catalyzed cyclization with isocyanide insertion for the synthesis of tetrazole isoindolines. They constructed a series of 1,5-disubstituted-1H tetrazoles 127 with reaction conditions that could well tolerate a wide range of functional groups in excellent overall yields (Scheme 48).
Scheme 48. General Strategy for the Synthesis of the Tetrazole-isoindolines 127.
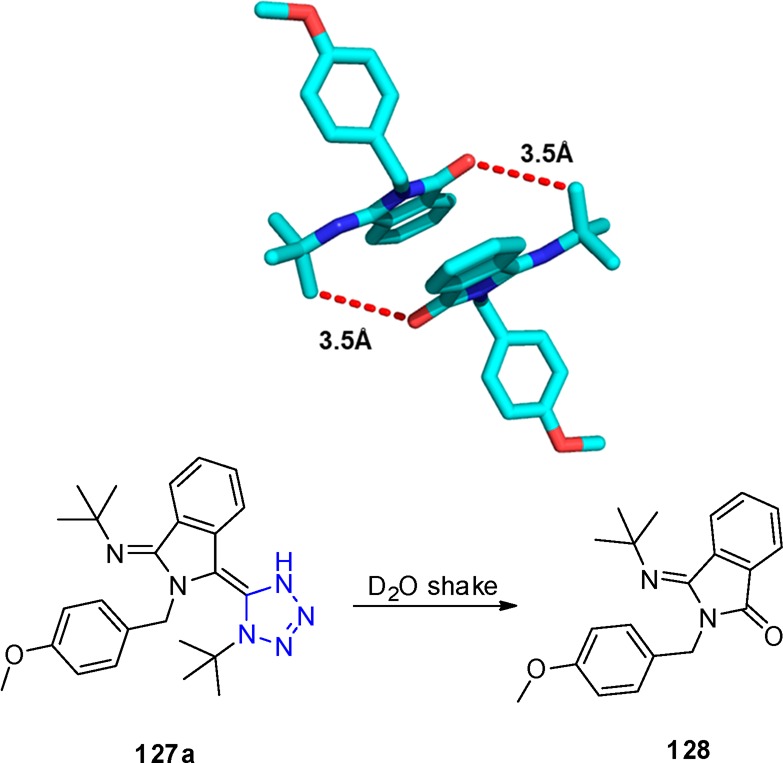
The presence of a tetrazole N–H proton in compound 127a was verified by D2O exchange experiment in which an unexpected change in 1H NMR spectrum was observed as proven by X-ray structure analysis (Scheme 49). Degradation occurred, most probably provoked by water giving the isoindole-1-one 128.
Scheme 49. Compound Degradation after D2O Shake during NMR Experiment and ORTEP Diagram Drawn of the Crystal Structure of (E)-3-(tert-Butylimino)-2-(4-methoxybenzyl)isoindolin-1-one (128) Determined at 293 K (CCDC 959960) (The Interaction between O of Lactam and Methyl of tert-Butyl Was Measured as 3.5 Å.
β-Carbolines are heterocyclic systems which are the key structural motif of a variety of biologically important alkaloids of natural and synthetic origin.274,275 Tetrahydro-β-carbolines are often key intermediates in natural product syntheses.276,277 Because of their structural similarity with a number of neurotransmitters, they are also incorporated in numerous compounds with biological activity. The intramolecular Mannich reaction of electron rich aromatic rings with oxo components and 1° or 2° amines, also called the Pictet–Spengler reaction, is an often used post modification in MCR.278−285
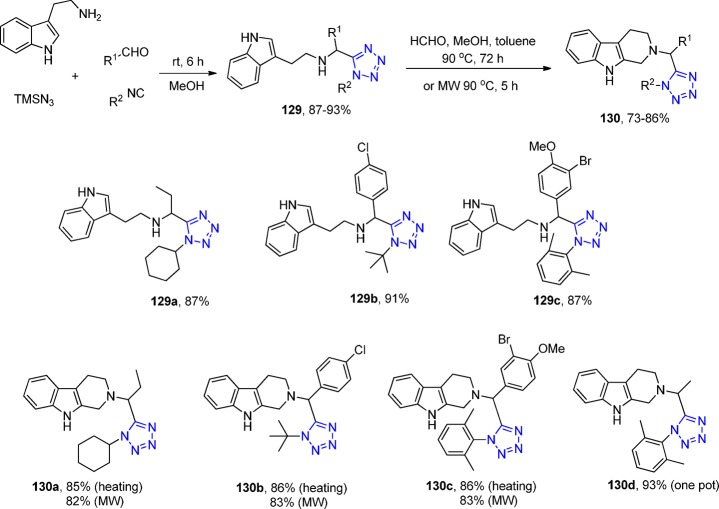
El Kaïm et al.286 first prepared an array of tetrahydro-1H-β-carboline-tetrazoles in excellent overall yields using the UT/Pictet–Spengler reaction sequence. Tryptamine was used as a common starting material in the UT reaction (129), and the subsequent Pictet–Spengler reaction was performed with formaldehyde to form a series of 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines 130 either under refluxing conditions in methanol/toluene or under microwave conditions in the same reaction solvent with generally good to excellent yields (Scheme 50). A direct comparison of these two methods of Pictet–Spengler ring closure reveals that the yields are similar; however, the microwave variation was generally slightly less yielding.
Scheme 50. Synthesis of 2-Tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines 130.
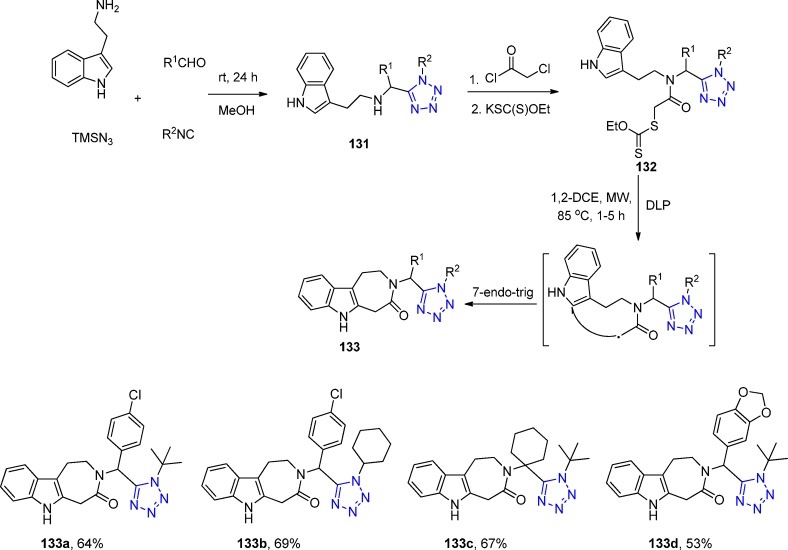
In 2013, R. Gámez-Montaño287 reported the synthesis of nine novel tris-heterocyclic-type 3-tetrazolyl-azepino[4,5-b]indol-4-ones via a sequential combination of a one-pot process (UT-4CR/N-acylation/SN2)/xanthate free-radical-mediated cyclization. Thus, tryptamine was combined sequentially with the corresponding aldehydes, TMS-azide and isocyanides in MeOH as the solvent at room temperature for 24 h to give the corresponding indole-tetrazoles 131, which underwent a N-acylation with chloroacetyl chloride to give the corresponding chlorides. These latter compounds, after a SN2 reaction with potassium ethyl xanthogenate salt, afforded the bis heterocyclic xanthates 132 in 47–71% yield. Then, DLP (dilauroyl peroxide) was added portionwise in 1,2-dichloroethane at 85 °C (using conventional or MW) to generate the azepino[4,5-b]indol-4-one heterocycles 133 in 45–82% yields after a favored 7-endo-trig cyclization (Scheme 51, Figure 29).
Scheme 51. Synthesis of the 3-Tetrazolyl-azepino[4,5-b]indol-4-ones 128 via a One-Pot (UT-4CR/N-Acylation/SN2)/Xanthate Free-Radical Mediated Cyclization.
Figure 29.
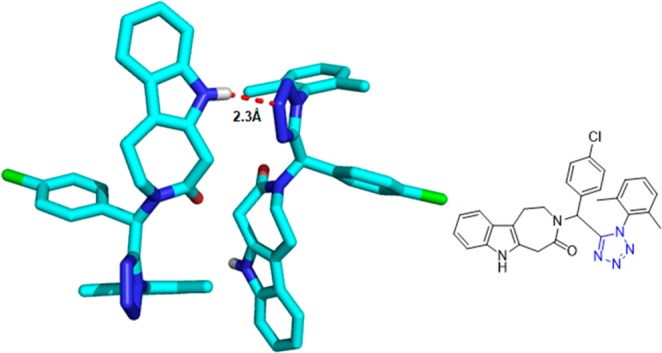
X-ray crystal structure of azepinoindolones 133e (CCDC 948622). An intermolecular hydrogen bond of 2.3 Å is observed between the azepinoindole N–H and the nitrogen of the tetrazole moiety.
Hulme et al.288 described a two-step methodology based on an oxidation/oxidative amidation cyclization strategy toward isatins starting from the o-aminoacetophenone. UT adducts 134 were successfully oxidatively cyclized through a postcondensation process utilizing selenium dioxide, affording valuable peptidomimetic-like isatins 135 (Scheme 52).
Scheme 52. UT Reaction of o-Aminoacetophenone, Aldehydes, Isocyanides, and TMSN3, Followed by an Oxidation/Intramolecular Oxidative Amidation toward the Tetrazole Derivatives 135.
Chalcones is a class of compounds that have a wide range of biological activities289−292 such as antidiabetic, antineoplastic, antihypertensive, antiretroviral, anti-inflammatory, etc. MCR-oxidative deamination approach was employed to access α-ketotetrazoles (with aromatic or aliphatic aldehydes) and α,β-diketotetrazoles (with glyoxals) with two diversity elements (137, Scheme 53, Figure 30).293,294 Dual functionalized α-ketotetrazole compounds were synthesized in two steps in 25–77% yields, accessing also tetrazole chalcones 137a–d via the UT adduct 136. In addition, α,β-diketotetrazoles (137e–h) were formed using various glyoxals as the aldehyde component, providing a route to vicinal tricarbonyl cis-amide bioisosteres.
Scheme 53. Dual α-Ketotetrazoles and α,β-Diketotetrazoles 137 Based on the MCR-Oxidative Deamination Approach.
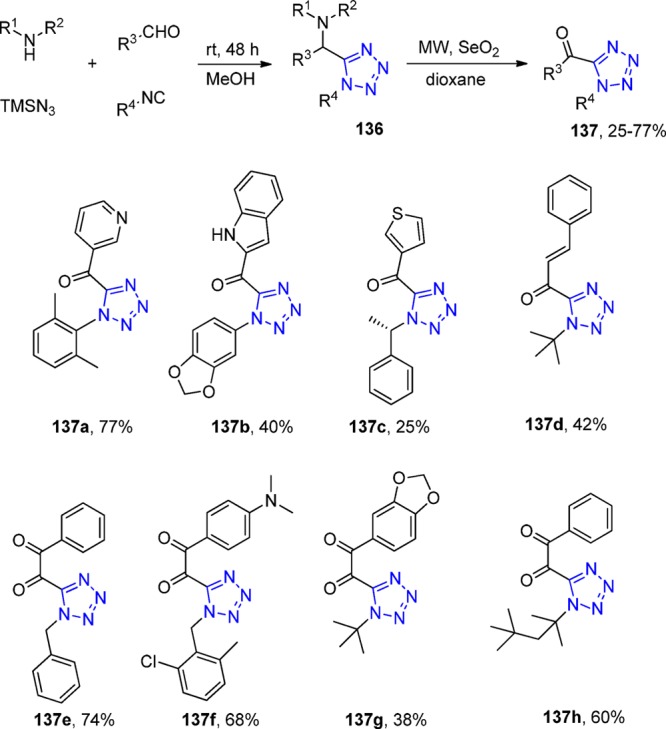
Figure 30.
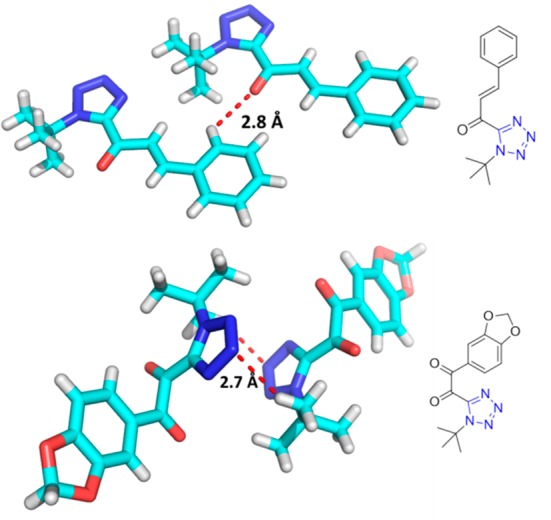
X-ray crystal structure and polar contacts of the tetrazole chalcone 137d (CCDC 1531964) and the α,β-diketotetrazole 137g (CCDC 1554390).
Further functionalization of the aforementioned tetrazole derivatives is presented below, giving rise to derivatives 138–140 (Scheme 54).
Scheme 54. Applications of Tetrazole Chalcones and α,β-Diketotetrazoles to Produce Diverse Tetrazole Chemotypes as the Derivatives 138, 139, and 140.
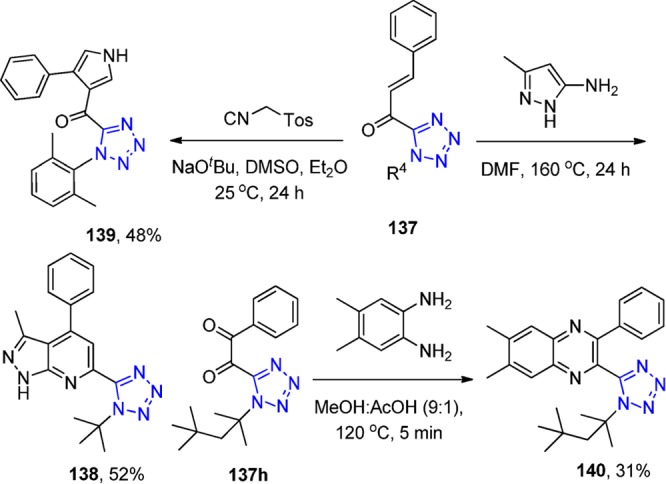
Tron et al.295 discovered an attractive short synthetic approach to 5-aroyl-1-aryltetrazoles 142, a class of compounds hardly accessible by other means. The novel and operationally simple synthetic procedure to obtain elusive 5-aroyl-1-aryltetrazoles in good yields consists of an UT adduct 141, followed by a hydrogenolysis/transamination posttransformation (Scheme 55). This postmodification reaction sequence was based on the Rapoport procedure, which is a simple and mild biomimetic conversion to convert amines to carbonyls in the presence of 4-formyl-1-methylpyridinium benzene-sulfonate as a pyridoxal phosphate (vitamin B6) surrogate.296,297 Different aldehydes and isocyanides with various different electron-withdrawing and electron-donating substituents were employed to demonstrate the functional group tolerance and generality of this new synthetic process. α-Keto (hetero) arynes represent a significant compound class as they have been described as covalent serine protease inhibitors or as tetrazole analogues of chalcones.
Scheme 55. General Procedure for the Synthesis of 5-Aroyl-1-aryltetrazol Analogues 142.
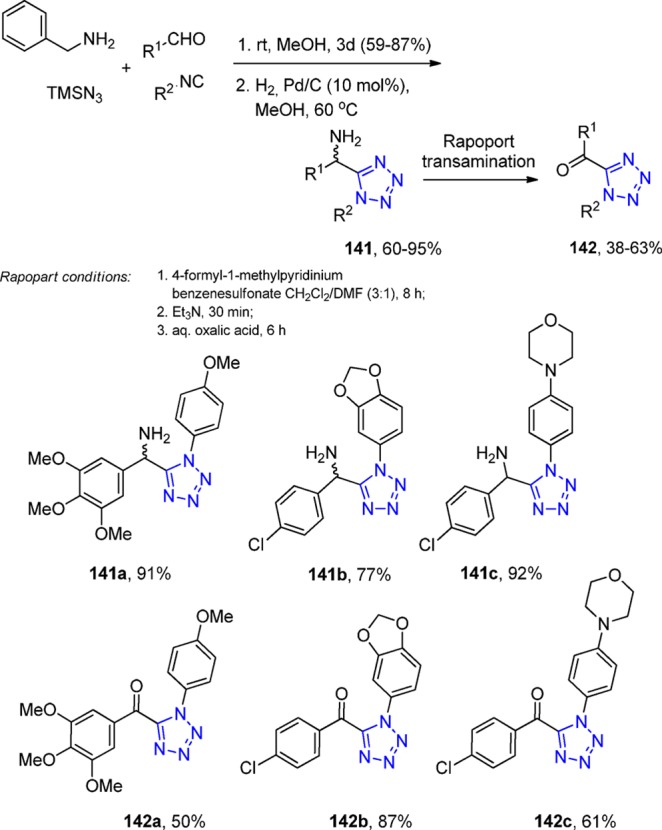
The double bond in the chalcone scaffold is commonly thought to be an important structural linker, but, for example, it is not essential for the interaction with tubulin. Yet, it may be a potential site of metabolic degradation and interaction with biological nucleophiles. To circumvent that, following the same strategy as previously described (an UT-4CR combined with the Rapoport procedure), Tron et al.295,298 investigated the 5-aroyl-1-aryltetrazol analogues 142 for their biological antiproliferative activity. They tested these compounds and their precursors in SH-SY5Y cells, a neuroblastoma cell line. Compound 142g was found active with an IC50 of 4.1 ± 0.3 μΜ, which was confirmed by cell cycle analysis as well by disrupting the mitotic spindle (Scheme 56).
Scheme 56. General Synthesis for Tetrazolic Analogues of Chalcones 142.
A series of tetrazole linked imidazo[1,5-a]pyridines 144 were recently synthesized from simple and readily available building blocks.299 The reaction sequence involves an Ugi tetrazole-deprotection reaction (143), followed by an acetic anhydride mediated N-acylation-cyclization process to afford the target heterocycles. The acylating agents include commercial available acid chlorides, anhydrides, and acids (Scheme 57).
Scheme 57. General Synthesis for Tetrazolyl Imidazo[1,5-a]pyridines 144.
Among the MCRs and postcondensation examples, mostly C–N and C–C bond formations to form monocyclic ring or fused structures were reported,300−302 whereas N–N bond formation were rarely disclosed up to date. El Kaïm et al.303 envisioned that a N–N bond formation as the Ugi postcondensation transformation could lead to unusual scaffolds. They selected as starting materials primary amines, ortho-nitrobenzaldehyde to react with TMS azide and various isocyanides to form the indazole derivatives 145 in good yields via a highly efficient multicomponent condensation process involving an Ugi–Cadogan cascade.304 Indazoles are a highly underused scaffold in drug discovery.305 The UT-4CR reactions are followed by a Cadogan reductive cyclization using triethyl phosphite as the reducing agent. A one-pot synthetic strategy was developed and compared with the two-step procedure. With no significant difference between these two methods, the one-pot sequence gave a slightly lower yield 61% compared with 62% from two-step. A variety of amines was tested, assesing the generality of this reaction. Sterically hindered amines led to the expected products with a slight decreased yield, whereas anilines gave sluggish indazole formation probably caused by the lower nucleophilicity of the nitrogen atom (Scheme 58).
Scheme 58. One-Pot Tetrazolyl Indazole 145 Formation.
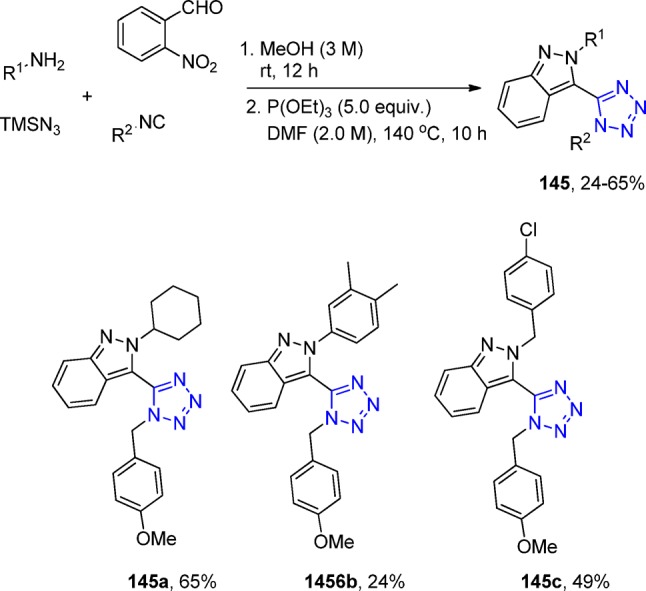
Morpholines and piperazines are privileged structures, which are abundantly used as substituents in medicinal chemistry, improving the pharmacokinetic properties of molecules as water solubility and metabolic stability. These moieties belong to the 25 most frequent nitrogen heterocycles in U.S. FDA approved drugs.306 Dömling et al.307 reported for the first time the successful incorporation of highly substituted morpholines and piperazines in an UT-4CR. After quite a bit of optimization, the reaction of an α-hydroxy oxo-component together with an isocyanide, NaN3, and 2-haloamine yielded the corresponding Ugi-tetrazole adduct 146, which under treatment with NaH gave the corresponding morpholine derivative 147. To facilitate the high throughput process, the aforementioned procedure was also performed in one pot, affording 20 morpholine-tetrazole derivatives (Scheme 59, Figure 31). In addition, underscoring the usefulness of the produced scaffolds, some further transformations of the secondary amine of morpholines and piperazines via sulfonation, acylation (148), urea and thiourea formation (149), and reductive amination (150) were described (Scheme 60). Similarly, the reaction of the mono-Boc protected ethylenediamine or mono-Boc oPDM, 2-chloroacetaldehyde, the corresponding isocyanide, and TMS azide (Scheme 61) afforded the Ugi adducts 151, which were subsequently cyclized to the corresponding piperazine derivatives 152 after basic treatment (tBuOK or NaH).
Scheme 59. Synthesis of the Ugi Adduct 146 and the Morpholines Derivatives 147.
Figure 31.
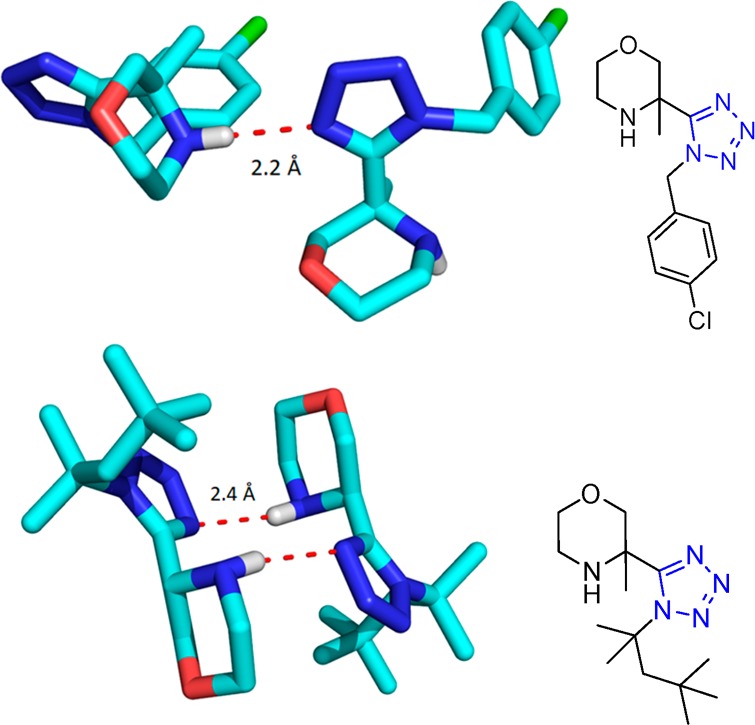
Crystal structures of the morpholine derivatives 138f (CCDC 1507665) and 138a (CCDC 1507068). An intermolecular hydrogen bond of the morpholine N–H to the N of the tetrazole can be identified at 2.2 and 2.4 Å, respectively.
Scheme 60. Further Derivatization of the Morpholine and Piperazine Scaffolds via Acylation, Thiourea Formation, and Reductive Amination, Respectively.
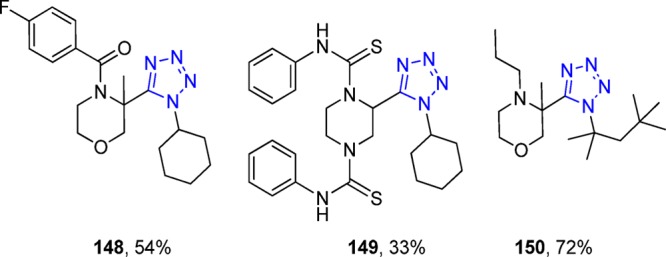
Scheme 61. Synthesis of the Ugi Adduct 151 along with the Piperazine and Tetrahydroquinoxalines Derivatives 152.
3.1.1.3.1. UT-4CR Followed by Cyclizations toward Tetrazole-Lactam Derivatives. The N-unsubstituted γ- and δ-lactam moieties play a very important and diverse role in medicinal chemistry because they are found in many drugs, for example, in the anti-Parkinson drug Oxotremorin,308 and in the antirhinoviral and enteroviral drug Rupintrivir.309 The substitution on the lactam nitrogen position clearly affects its hydrogen bonding profile in the receptor binging site.
The general strategy of post cyclizations toward tetrazole-lactam derivatives is based on the usage of bifunctional building blocks (Scheme 62).
Scheme 62. General Strategy to Lactam-Tetrazoles.
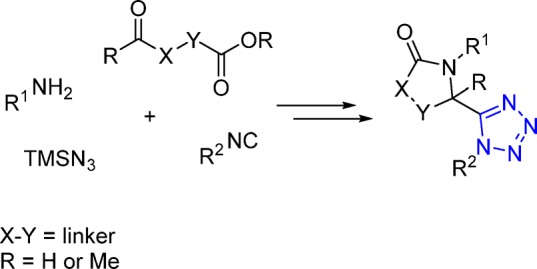
Marcaccini et al.,310 in order to obtain heterocyclic systems by means of postcondensation modifications of the Ugi reaction, employed methyl o-formylbenzoates as bireactive carbonyl components and mixed it with amines, isocyanides, and TMS azide to afford the expected tetrazolyl-isoindolinones 154 with good isolated yields via a tandem Ugi tetrazole (153)/intramolecular amidation. In some cases, the intermediate Ugi tetrazole intermediate cyclized spontaneously, whereas in other cases the cyclization occurred only in ethanolic sodium ethoxide under refluxing conditions. Aliphatic amines generally cyclized spontaneously and also precipitated out, whereas deactivated anilines needed forced conditions for cyclization (Scheme 63).
Scheme 63. Synthesis of Tetrazolyl-Isoindolinones via UT-4CR/Intramolecular Amidation.
Hulme et al.311,312 reported bifunctional building blocks in the UT-4CR, offering an unprecedented significant scope expansion and combinatorial applications toward novel pharmacologically relevant complex bis-heterocyclic lactam-tetrazoles. They reported the reaction of suitable protected and unprotected orthogonal oxo-carboxylic acids, which yielded a great diversity of bis-heterocyclic lactam-tetrazole scaffolds, few of them containing fragments of importance in medicinal chemistry. Clearly, many of these scaffolds can be synthesized in parallel to provide libraries of interesting compounds. He established a postcondensation modification methodology which reacted keto-esters (e.g., methyl levulinate), primary amines, isocyanides, and TMS azide in one pot via the UT reaction followed by the lactam formation under acidic condition to afford a small library of novel peptidomimetic-like bispyrrolidinone tetrazoles 155. It is noteworthy that this is the first example of a trifluoroacetic acid mediated γ-lactam formation. Sterically hindered amines gave no or low yields, such as 2,6-dichlorobenzylamine, 4-morpholinoaniline, 1-benzylpiperidin-4-amine, and cyclohexylamine. A virtual library of 400000 compounds was enumerated and compared to the NIH molecular libraries small-molecule repository (MLSMR) to show uniqueness of occupancy of chemical space by principal component analysis. Moreover, a small library of 84 compounds was obtained in 24-well plates with overall yields ranging from 2 to 84%, with 82 compounds having purity greater than 95% [as judged by UV absorbance at 214 nm, 254 nm, and evaporative light scattering (ELS)] (Scheme 64).
Scheme 64. General Synthetic Route to Access Bis-pyrrolidinone Tetrazole 155.
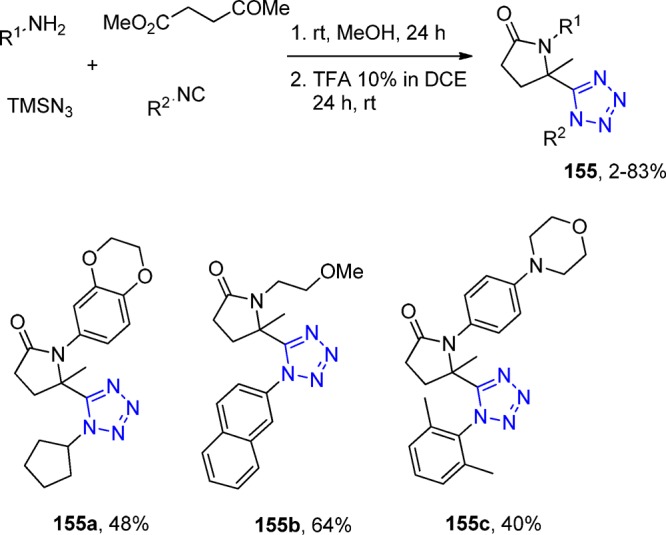
In 2012, Hulme et al.252 utilized the UT to generate unique 1,5-disubstituted tetrazole with ethyl glyoxalate and mono-N-Boc-protected-o-phenylenediamine derivatives (156). The subsequent acid treatment and intramolecular cyclization led to bis-3,4-dihydroquinoxalinone tetrazoles 157 in just two steps but with moderate yields (Scheme 65, Figure 32). Directly catalytic oxidation using a stable solid-phase supported radical catalyst, derived from the 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) with stoichiometric ceric ammonium nitrate (CAN), generated the final targeted bis-quinoxalinone tetrazoles 158. They also extended the research to synthesize the diazepinone derivatives 161 with N-Boc-2-aminobenzylamine via the UT 159 (Scheme 66). Unexpectedly, the similar acidic deprotecting procedure did not further proceed to the cyclized product and the additional aminolysis of the ester by either activating the ester or the amine failed. Therefore, a hydrolysis was performed under basic conditions (160) followed by an EDC-promoted intramolecular amide coupling to obtain the corresponding diazepinones 161 in 27–66% yield (Scheme 66).
Scheme 65. Synthesis of Bis-quinoxalinone Tetrazoles 158.
Figure 32.
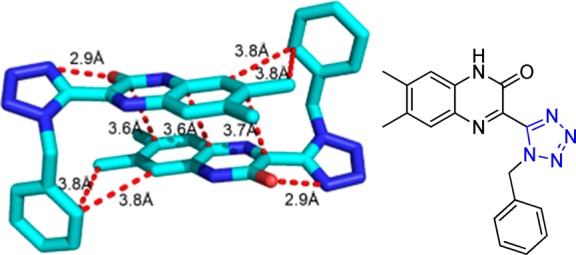
Crystal structure of 3-(1-benzyl-1H-tetrazol-5-yl)-6,7-dimethylquinoxalin-2(1H)-one (158d) exhibiting an antiparallel π stacking alignment of two adjacent quinoxaline moieties, featuring in addition a low energy antiparallel dipole dipole alignment (CCDC 932013)
Scheme 66. Synthesis of Tetrazolobenzodiazepin-2-ones 156.
Dependent on the used oxocarboxylic acid esters, quite different cyclization conditions were used. Seven series of bis-heterocyclic lactam-tetrazoles were synthesized: tetrazolyl-pyrrolidinones 162, indolinonetetrazoles 163, thiomorpholinone-tetrazoles 164, 4-sulfonyl-2-piperazinone-tetrazole derivatives 165, 4,5,6,7-tetrahydropyrazolo[1,5-a]-pyrazine-4-one tetrazole derivatives 166, benzo[1,4]oxazepinone derivatives 167, and [1,4]thiazepanone derivatives 168 (Scheme 67 and Table 4). As it was previously stated,312 in the tetrazolyl-pyrrolidinones 162 series simply trifluoroacetic acid in dichloromethane was added after completion of the Ugi tetrazole reaction. Alternatively, the Ugi intermediate was isolated, purified, and then subjected to methanolic KOH solution to afford the tetrazolyl-pyrrolidinones. The methodology was importantly shown to be compatible with 96-well plate-based production. Yields reported for the eight isolated compounds varied between 40 and 78% (Figure 33).
Scheme 67. Diversity of Bis-heterocyclic Lactam-Tetrazoles.
Table 4. Use of Bifunctional Building Blocks in the UT Reaction Followed by Lactamization.
Figure 33.
Examples of bis-heterocyclic tetrazolo scaffolds.
Concerning the six-membered piperidinone-tetrazoles, cyclization is accomplished by KOH mediated hydrolysis of the UT methylester followed by EDC/DMAP cyclization or alternatively by thionyl chloride mediated cyclization. Interestingly, by using 5-oxo-hexanoic acid the Ugi tetrazole product 169 is formed exclusively, and no trace of the alternatively possible Ugi lactam is formed (Scheme 68).
Scheme 68. Selective Tetrazole Formation over the Intramolecular Ugi Product.
The intermediate and not isolated Ugi tetrazole can then be cyclized in situ using DCC. The authors argue that the small and strongly nucleophilic azide ion leads to a kinetically favorable formation of the four-component Ugi tetrazole product.
Also, several seven-membered lactam motifs were introduced. Four examples of azepinone-tetrazoles were synthesized in two steps comprising consecutive basic hydrolysis and in situ acyl chloride formation. In the case of the tetrazolyl-indolinones 163, 2-acetylbenzoate was found to be a poor substrate in the Ugi reaction, while methyl 2-formylbenzoate worked well in all eight cases in yields between 36 and 66% (Scheme 67). As described previously,310 the cyclization occurred spontaneously at room temperature (Figure 33). The integration of a sulfur atom into the six-member ring to generate tetrazole-thiomorpholinone derivatives 164 was found to be another interesting scaffold. Under optimized conditions, the intermediate UT was hydrolyzed and subsequently the intramolecular amidation using SOCl2 in dichloromethane afforded five isolated products in yields 22–96%. The 4-sulfonyl-2-piperazinone skeleton can be incorporated into the UT reaction sequence by choosing the appropriate starting material (Scheme 67). The 4-sulfonyl-2-piperazinone motif 165 represents an essential structural feature of human factor Xa and gene transcription inhibitors.313,314 A series of six 4-sulfonyl-2-piperazinones were generated with yields between 16 and 74% for the UT reaction and 58–93% for the hydrolysis and cyclization step, respectively (Figure 33).
Intrigued by the potentially pharmaceutical application of unprecedented bifunctional scaffolds, a series of 4,5,6,7-tetrahydropyrazolo[1,5-a]-pyrazine-4-one derivatives 166 were synthesized with moderate to good isolated yields through the combination of UT-4CR and subsequent basic hydrolysis and SOCl2-mediated ring closure step. Five compounds were isolated in yields between 42% and 74% and 51–78% for the UT-4CR and cyclization, respectively.
The benzo[1,4]oxazepinone motif 167 was incorporated into the UT-4CR by employing the appropriate benzaldehyde starting material. Six compounds were isolated with yields between 66% and 80% and 31–84% for the UT-4CR and cyclization, respectively (Figure 33, 34).
Figure 34.
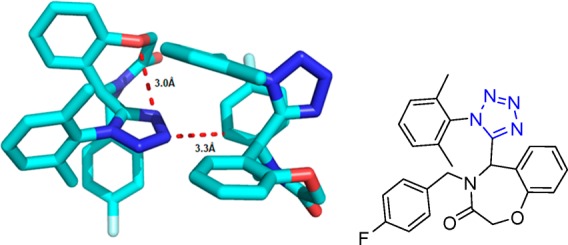
Crystal structure of a benzo[1,4]oxazepinone derivative 167c (CCDC 936637). It is noteworthy that there is an intramolecular hydrogen bond (3.0 Å) between N4 and O9 and a short contact (3.3 Å) between N3 and C10
Last but not least, a small series of five [1,4]thiazepanones 168 was synthesized by UT-4CR, KOH hydrolysis, and SOCl2 mediated cyclization in yields between 61% and 75% and 45–66% for the UT-4CR and cyclization, respectively (Figure 33).
In an analogous fashion, Stolyarenko et al.315 used 1-ethoxycarbonyl-cycloalkane oxo compounds 170, isocyanides and primary amines in the UT-4CR to afford the interesting class of tetrazole-substituted spirocyclic γ-lactams 171. No spontaneous cyclization occurred under the UT-4CR conditions (MeOH, rt), but it was accomplished under acidic conditions in DCE with 10% trifluoroacetic acid under heating conditions for 10 h. A library of 20 compounds was produced with yields between 52% and 72% (Scheme 69). The substrate scope of the reaction is quite broad, including aliphatic, aromatic, and bulky isocyanides and heterocyclic, aliphatic, and aromatic primary amines. Moreover, the straightforward introduction of a spiro tetrahydro-2H-pyran is worth mentioning, which otherwise is very difficult to access. Tetrahydro-2H-pyranes are used in medicinal chemistry to improve pharmacokinetic and CYP inhibition profile of lead compounds.316 In addition, a spirocyclic connection adjacent to an amide carbonyl might protect from spontaneous or enzymatic cleavage. Spirocyclic fragments are present in many biologically active compounds. The γ-lactam moiety is also the common structural unit for a large nootropic class of drugs, called racetames (e.g., piracetam). Racetams are memory enhancers and are hypothesized to interact with cholinergic and glutamate receptors in the central nervous system.
Scheme 69. Synthesis of Tetrazole-Substituted Spirocyclic γ-Lactams 171 by One-Pot UT-Cyclization.
They also described the crystal structures of two compounds which give some ideas on the 3D conformation and intermolecular contacts (Figure 35).
Figure 35.
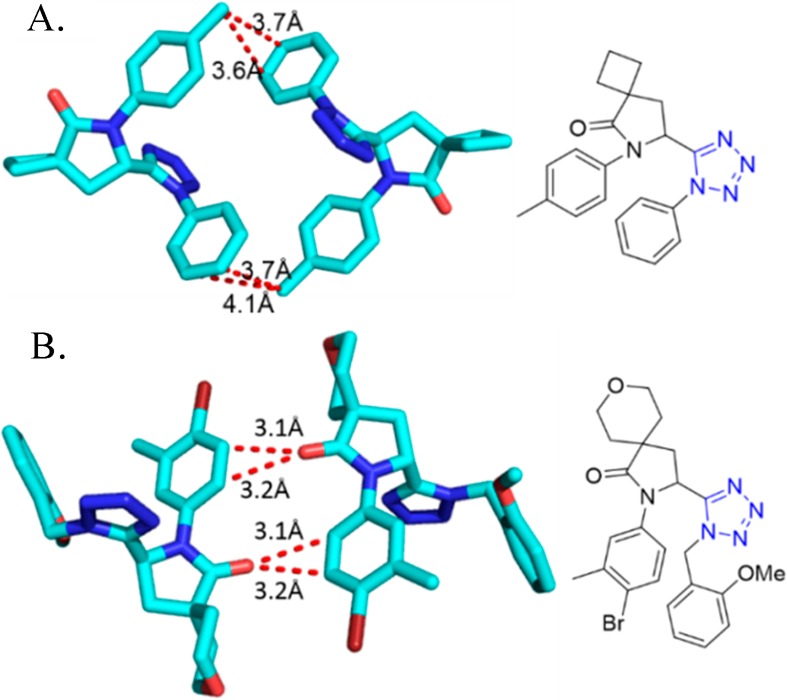
Crystal structure of tetrazole-substituted spirocyclic γ-lactams 171e,f (CCDC 918594 and 918596). It is noteworthythat it is the antiparallel alignment of the phenyl units of two adjacent molecules with short contacts (3.6 Å, 3.7 Å, 4.1 Å) between C (sp3) and C (sp2). Similarly, there is also the semiantiparallel alignment of the phenyl units and lactam ring of two adjacent molecules with short contacts (3.1 Å, 3.2 Å) between O (C=O) and C (sp2).
Dömling et al.317 designed and synthesized a series of N-unsubstituted γ- and δ-lactams 173, which were conveniently accessed in a three-step synthesis involving an UT-4CR followed by cyclization with overall good yields. While ammonia is often troublesome in the Ugi reactions, tritylamine was introduced as a convenient ammonia surrogate.141 However, because of the bulkiness of the trityl group, only aliphatic aldehydes afforded the corresponding products in yields between 40% and 80%. Ketones and aromatic aldehydes did not give the required Schiff base or only traces, respectively. The trityl amine tetrazole intermediate 172 was deprotected in quantitative yields using trifluoroacetic acid in dichloromethane. Optimization of the final cyclization conditions revealed that using sodium hydride is a suitable base to afford γ- and δ-lactams in most cases with reasonable to good yields (Scheme 70). A typical interaction pattern of the γ- and δ-lactam substructures was found by analyzing the PDB crystal structures. A general strong tridirectional hydrogen bond donor–acceptor interaction between the receptor amino acids and the N-unsubstituted γ- and δ-lactam fragment reveals a useful molecular moiety to address corresponding receptor motives (Figure 36). The same motif is generally found in the X-ray structures of small tetrazolo-lactams leading to dimerization via the γ- and δ-lactam NH–CO group.
Scheme 70. Devised Synthetic Pathway to Tetrazolo N-Unsubstituted γ- and δ-Lactams 173.
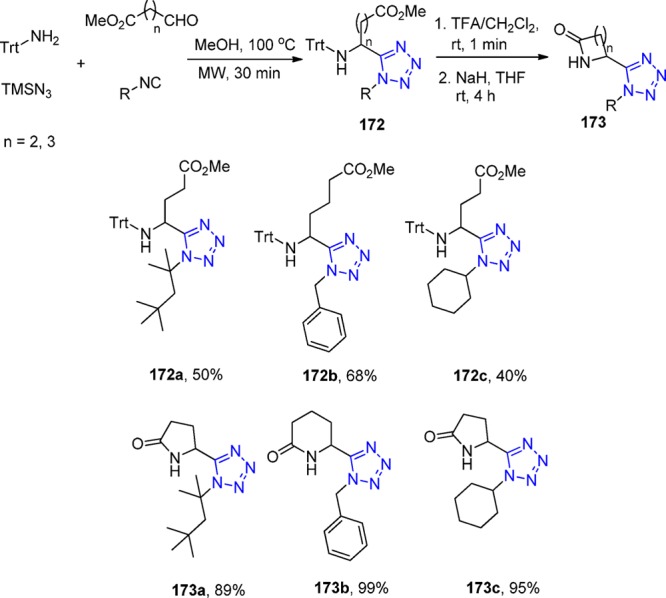
Figure 36.
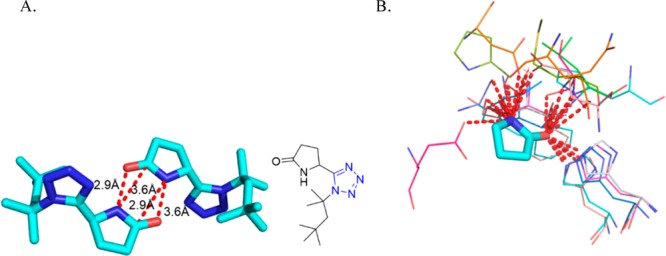
(A) Crystal structure of a tetrazole fused γ-lactam 173a (CCDC 961190). It is worth mentioning that there is a pair wise hydrogen bonding with a neighbor lactam in short contacts (2.9 Å) between N6, O1 and N6′, O1′. (B) Alignment of several PDB structures (3D23, 3EWJ, 3QZR, 3RHK, 3TNT, 3UR9, 3DPM, 1H0V, 3JUC, and 3Q3Y) showing the polar interactions for 10 γ-lactam containing ligands.
3.1.1.4. UT-4CR toward 1,5-Disubstituted Tetrazoles Bearing a Sugar Moiety
Glycosylation is the reaction in which a carbohydrate is attached to a hydroxyl or other functional group of another molecule. Many natural products are glycosylated, and their biological activity is crucially dependent on the glycosylation, which is a form of cotranslational and post-translational modification. In living organisms, glycosylation mainly represents the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules.318
The amino sugar desosamine occurs in diverse natural products with different activities, for example, in the antibiotics tylosin with mycaminose structurally related to desosamine, erythromycin, and methymycin. In 2006, Dömling et al.319 described the employment of desosamine into IMCRs. They prepared desosamine and the corresponding isocyanide (Scheme 71A) in a big scale by acid hydrolysis and subsequent amination from the readily available erythromycin. In addition, two syntheses were accomplished by stirring equimolar amounts of TMS azide, aldehyde, desosamine, and an isocyanide in methanol at rt for 24 h to give the disubstituted α-aminomethyl tetrazoles 174 as a mixture of diastereomers in 37% and 25% yield, respectively (Scheme 71B).
Scheme 71. (A) Acidic Hydrolysis of Erythromycin Yields Desosamine Which Is Subsequently Transforms into 1-Isocyanodesosamine; (B) Synthesis of Disubstituted α-Aminomethyl Tetrazoles 174 Based on Desosamine with UT-4CR.
Sugar moieties in drugs are used for different purposes, e.g., the glycosyl substituent will be recognized by the receptor and contribute directly to the biological activity or it helps to improve transport properties through transporters and increase water solubility. Sugar–organic fragment chimeras are traditionally synthesized by sequential multistep synthesis. To that direction, another successful application of sugar moieties in MCRs was also presented by Dömling et al.320 (Scheme 72). A series of anomeric sugar isocyanides (β-glycosyl and β-arabinosyl), which has been known and sporadically used in IMCRs, were synthesized via the reintroduced Leuckart–Wallach approach321,322 in good overall yields. They also reported the general usage of these two isocyanides in IMCRs to produce 1,5-disubstituted and α-alkylamino tetrazole derivatives 175 among others.
Scheme 72. (A) Leuckart–Wallach Approach to Sugar Isocyanides; (B) Synthesis of 1,5-Disubstituted Tetrazoles 175 Using Glycosyl Isocyanide and Arabinosyl Isocyanide.
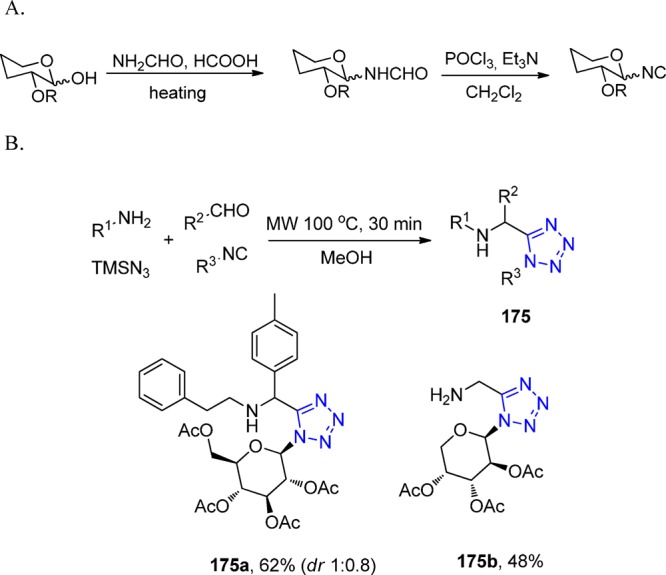
The conjugation of steroids to other biomolecules, like amino acids and proteins, is a common strategy employed both by nature and chemists to modulate the biological and chemical behavior of these molecules. Considering the growing importance of sugar/steroid hybrids in drug discovery and biological chemistry, Rivera et al.218,323 employed multicomponent reactions for the conjugation of carbohydrates to steroidal derivatives 176 with a great level of molecular diversity and complexity generated with the low synthetic cost (Scheme 73).
Scheme 73. Synthesis of Tetrazole-Based Spirostan Saponin Analogues 176.
Calixarenes, are a type of macrocycle or cyclic oligomer produced by the condenseation of p-substituted phenols with aldehydes. They have been widely used in various fields, i.e., the synthesis of multivalent/multifunctional ligands, and they are the ideal candidates for studying noncovalent interactions occurring in many biological processes based on the easy accessibility and functionalization at their wide and narrow rims. Therefore, Zadmard et al.324 synthesized functionalized calixarenes through MCRs. They first prepared the basic precursor calixarene dihydrazide 177 using a previously reported synthetic procedure.325 Then, α-hydrazino tetrazolocalix[4]arene derivatives 178 were synthesized in good yields via an UT-4CR (Scheme 74, Figure 37). Metal ion binding properties of compound 178a as the model compound were also investigated, revealing what exhibits the highest binding affinity toward Ni(II).
Scheme 74. Synthesis of Calixarene Dihydrazide 178 via UT-4CR.
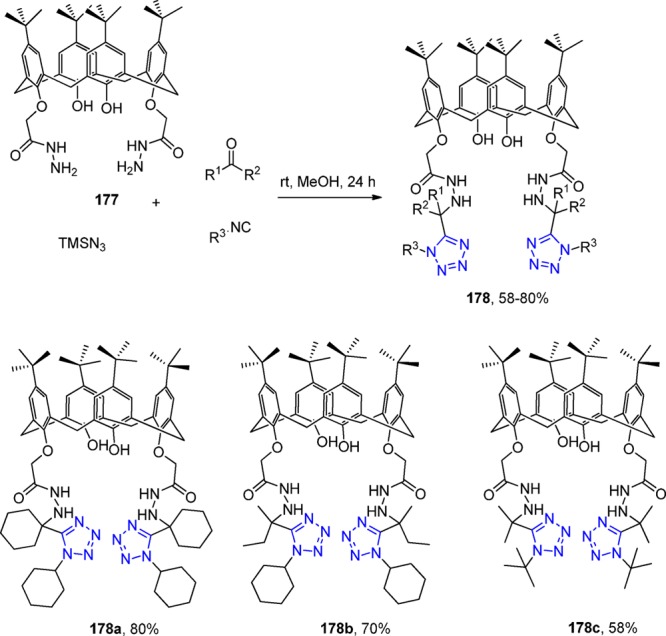
Figure 37.
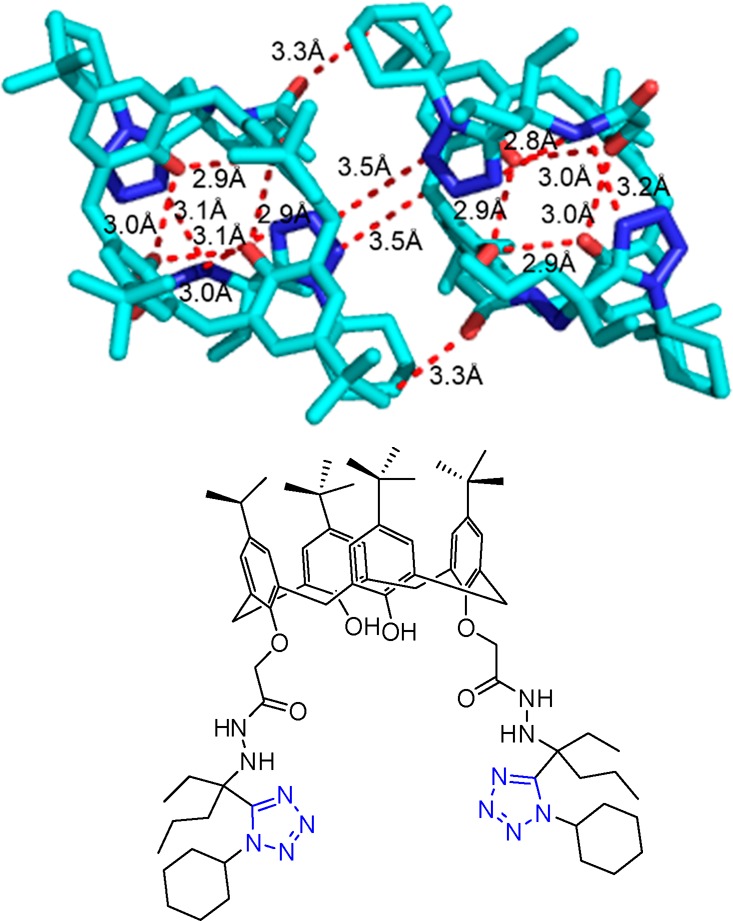
Crystal structure of calixarene dihydrazide 178d (CCDC 1025095). Four hydrophobic interactions of two molecules were observed as O (C=O) and methyl, N(2), and methyl of calixarene ring. Six hydrophilic interactions consist of four interactions between N(4) of tetrazole and N of hydrazine, two interactions between hydroxyls and O of calixarene ring.
3.1.2. Ugi Tetrazole 3-Component Reaction (UT-3CR)
The variation of an Ugi 3-component reaction can be obtained when two of the four reacting functionalities are placed in the same substrate. This is the case when, for example, cyclic imines, oxoacids, and amino acids are employed. In the tetrazole modification, there are fewer possibilities. Essentially these UT-3CR are possible with cyclic imines.
Organofluorine compounds attract more and more interest due to their important properties in pharmaceutical applications and materials science.326 The medicinal chemist often employs bioisosteres to replace the functional group in drugs to improve ADMET properties. The replacement of a hydrogen atom with a fluorine atom at a site of metabolic oxidation in a drug candidate might block metabolism without compromising biological activity and increasing half-life time. Nenajdenko et al.327 studied the application of trifluoroalkylated cyclic imines in UT reactions. They started from different arrays of five-, six-, and seven- membered trifluoroalkylated cyclic amines to form target tetrazole derivatives of saturated nitrogen heterocycles bearing the trifluoroalkyl moieties 179. In addition, the final 1H-tetrazoles 180 could easily be obtained by catalytic hydrogenation in excellent yields (Scheme 75).
Scheme 75. UT-3CR with Trifluoroalkyl Cyclic Imines and Synthesis of N-Unsubstituted Tetrazoles 180.
In 2013, Ukaji et al.328 first synthesized in good yields the novel 1,5-disubstituted tetrazoles 182 containing tetrahydroisoquinoline skeletons based on the UT-3CR. They utilized C,N-cyclic N′-acyl azomethine imines 181, both aliphatic and aromatic isocyanides and in situ TMS azide generated through TMSCl and sodium azide (Scheme 76).
Scheme 76. Synthesis of Tetrahydroisoquinoline Tetrazoles 182.
3.1.3. Passerini Tetrazole 3-Component Reaction (P-3CR)
In 1921, a three-component reaction between carboxylic acids, oxo components, and isocyanides for the synthesis of an α-acyloxy amide was discovered by Passerini (P-3CR).55,56 In 1961, Ugi reported the synthesis of tetrazoles via a Passerini type 3CR (P-3CR) for the first time using HN3 and Al(N3)3.52
Aspartyl proteases which catalyze amide bond hydrolysis found to play a key role in many biological processes, including the development of a variety of diseases and the important therapeutic targets. Hulme et al.225 reported the facile synthesis of analogous cis constrained norstatine mimetics by simply mixing an N-Boc-amino aldehyde 183, an isocyanide, and TMS azide in dichloromethane affording the derivative 184, followed by deprotection with trifluoroacetic acid and N-capping with TFP esters to the desired amides and sulfonamides 185 in good yields. This reaction proved to tolerate a range of functionalities including a variety of isocyanides and N-Boc-α-amino aldehydes (Scheme 77).
Scheme 77. Passerini Reaction Towards Tetrazole Derivatives 185.
Chiral 5-substituted tetrazoles have been recognized as efficient organocatalysts.329−333 Many methods have been developed for the synthesis of 1,5-disubstituted tetrazoles, including the 5-(1-hydroxyalkyl)tetrazoles. Zhu et al.334 first reported to synthesize enantioselective 5-(1-hydroxyalkyl)tetrazole 186 catalyzed by a [(salen)AlIIIMe] (salen = N,N′-bis(salicylidene)ethylenediamine dianion) through Passerini-type reaction of aldehydes, isocyanides, and hydrazoic acid with good-to-excellent enantioselectivity (Scheme 78). Four different catalysts were optimized in several reaction conditions. With the optimized conditions and stoichiometry for the reaction (isobutyraldehyde/1-isocyano-4-methoxybenzene/HN3/catalyst 1.2:1:2.5:0.1), they also examined the generality of this catalytic enantioselective process by varying the structure of the aldehyde and isocyanide. Linear and α-branched aliphatic aldehydes and aliphatic and aromatic isocyanides with electron-donating or electronic-withdrawing groups worked nicely. However, in the case of the sterically encumbered 2,6-dimethylphenylisocyanide, yield and enantioselectivity both diminished. When α-isocyanoester was used, a spontaneous hydrolysis/lactonization sequence proceeded well. Due to the fact that salen-Al complexes catalyze the nucleophilic addition of azide to α,β-unsaturated imides and to α,β-unsaturated ketones, they were tested and found also to perform a tandem Michael addition/enantioselective P-3CR using a α,β-unsaturated aldehyde as the carbonyl substrate. The results showed that 1-(4′-methoxyphenyl)-5-(1′-hydroxy-3-azidopropyl)tetrazole could be detected with good yield and enantioselectivity (Scheme 78).
Scheme 78. Catalytic Enantioselective Synthesis of 5-(1-Hydroxyalkyl)tetrazole 186 by Three-Component Passerini Reaction (P-3CR).

Very often, a synthetic methodology that could lead to a new class of compounds is based on the input of a component with different reactive functionalities in an already established MCR. In 2012, Yanai et al.335 developed a novel four-component reaction of aldehydes, isocyanides, TMS azide, and free aliphatic alcohols without amines catalyzed by the Lewis acid indium(III) triflate to give rise to α-alkoxyamides 187 in good yields (direct O-alkylative tetrazole P-4C reaction, ATP-4CR). Aliphatic and aromatic aldehydes both were well tolerated in this synthetic methodology (Scheme 79, Figure 38).
Scheme 79. Synthesis of Alkoxylated 1H-Tetrazole Derivatives 187.
Figure 38.
Crystal structure of (E)-1-(tert-butyl)-5-(1-(cyclopentyloxy)-3-phenylallyl)-1H-tetrazole 187d (CCDC 862990).
Although MCR proved to be more environmentally benign compared with the classical tetrazole synthetic methods, people still continue to try to employ water as the reaction medium in organic synthesis. To date, its beneficial effects on a variety of organic transformations have been widely recognized.336−338 High cohesion energy density, hydrogen bonding-stabilized transition state, and enhanced hydrophobic effect in the ground vs transition state, could be the reasonable resources to explain the reaction acceleration in aqueous media.336,337,339−344 Meanwhile, there are only a few reports about the influence on the selectivity of organic reactions by adding salt. Vigalok et al.345 demonstrated that simple sodium salts addition in Passerini reaction in aqueous media can completely reverse the product ratios. Furthermore, the use of the “salting-in” effect and a small excess of the nucleophile could lead to significantly higher yields of Passerini tetrazole products 188 instead of the Passerini adducts with different nucleophile than azide (Scheme 80).
Scheme 80. P-3CR under the “In Water” Or “In NaOTs” Conditions.
In that direction, a sonication accelerated, catalyst free, simple, high yielding, and efficient method for the P-3CR has been developed.346 It comprises the reaction of an oxocomponent, an isocyanide, and a TMS azide in methanol–water (1:1) as the solvent system, giving rise to derivatives 189. The use of sonication not only accelerated the rate of the reaction but also provided good to quantitative yields (Scheme 81). The reaction has a high functional group tolerance, applicable to a broad scope of aldehydes/ketones and isocyanides, due to the very mild reaction conditions and in addition the existence of a free hydroxyl group allows various postmodifications; the authors demonstrated the possibility to synthesize fused tetrazoles 191 from the tetrazole precursors 190, which are important scaffolds as they possess a wide spectrum of activity and vast industrial applications (Scheme 82).
Scheme 81. A Green P-3CR under Sonication Conditions.
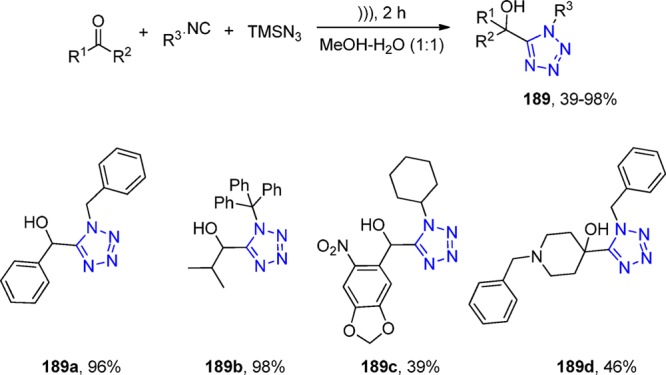
Scheme 82. Post-Modification on the Corresponding Hydroxyl Tetrazole 190 Towards the Fused Tetrazole 191.
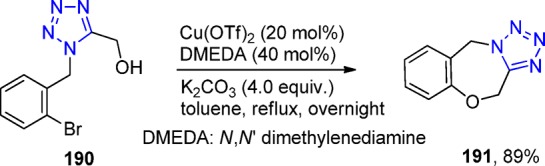
3.1.4. Miscellaneous MCRs toward Monocyclic Tetrazole Derivatives
A microwave-accelerated, simple, efficient, and versatile method for the construction of the 1,5-tetrazole scaffold was developed by Dömling et al.347 Due to the fact that the reported methods for tetrazole formation from amides face major drawbacks, such as harsh conditions, low yields, and missing substrate scope, an in situ amide formation from amines and carboxylic acid derivative followed by imidoyl chloride formation and finally tetrazole formation by azide addition as a one-pot MCR, was proposed.
The majority of the acid chlorides gave complete conversion to the corresponding tetrazoles 192 under the optimized conditions in good to high yields (Scheme 83). Aromatic and aliphatic acid chlorides proved to be equally effective in this reaction. The conversions of aromatic and aliphatic carboxylic acids were as effective as those of the acid chlorides, but these substrates delivered the products in slightly lower yields. Application of this method to esters was also successful; however, a longer reaction time was required (25–30 min) for the total conversion, and moderate to good yields were provided with aliphatic and aryl esters. Bistetrazoles 193 are also accessible and these compounds are highly important in high-energy nitrogen-rich compounds.347
Scheme 83. Synthesis of Tetrazoles 192 and 193 from Carbonyl Compounds, Amines, and TMSN3.
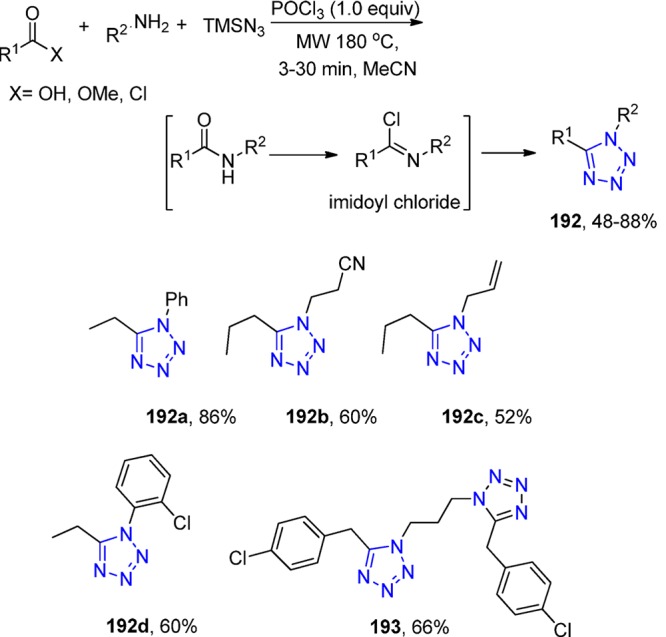
Fused tetrazole scaffolds were also described by this methodology; the use of functionalized carboxylic acids with amines bearing an additional functional group would allow an anticipated domino cyclization process in one step. The reaction of formamide, which works both as an ammonia and formaldehyde surrogate and 2-aminobenzoic acid under the optimized conditions led to the formation of the tetrazolo[1,5-c]quinazoline scaffold 194 in moderate yield (Scheme 84).
Scheme 84. Synthesis of 1,5-Fused Tetrazoles 194 from Carboxylic Acid Derivatives, Amines, and TMSN3.
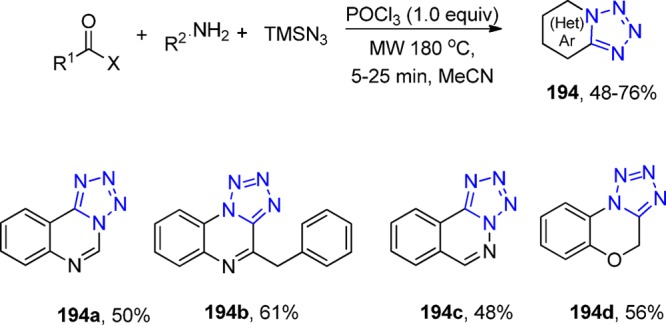
The usefulness of this method was demonstrated in the synthesis of biologically important fused tetrazole scaffolds and the marketed drug cilostazol (196) from the halogenated tetrazole precursor 195 (Scheme 85).
Scheme 85. Two-Step Synthesis of Cilostazol (192) by the MCR Methodology.
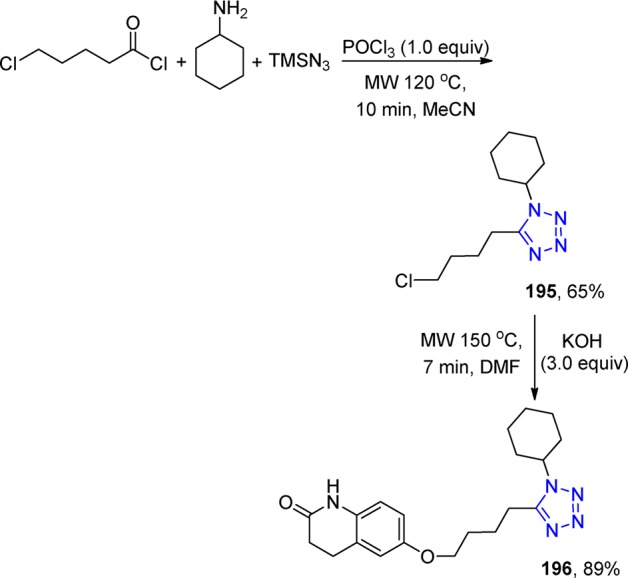
Amino acids were also successfully employed with full stereoretention toward derivatives 197, as shown by HPLC on a chiral stationary phase (Scheme 86).
Scheme 86. Synthesis of the Amino Acid Tetrazoles 197.
Saiprathima et al.348 described the MCR synthesis of 3-tetrazolyl oxindoles 198 from isatines by a facile intermolecular [2 + 3] cycloaddition between an azide and a nitrile group bound to quaternary center of oxindole at C3 position in water. The straightforward preparation of these compounds is the C3 functionalization by nucleophilic addition of isatins (Scheme 87). The use of TMSCN as a nucleophile allows the synthesis of 3-cyano-3-hydroxy oxindoles and their direct conversion into corresponding tetrazole derivatives 198 by facile [2 + 3] cycloaddition of azides (Figure 39). The authors also observed a one-pot four-component tetrazole formation of compound 199 using aniline as an additional component (Figure 40).
Scheme 87. Synthesis of 3-Tetrazolyl Oxindoles 198 by a Facile Intermolecular [2 + 3] Cycloaddition.
Figure 39.
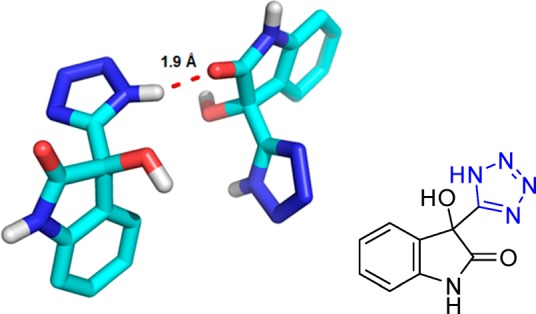
Solid-state structure of 3-hydroxy-3-(1H-tetrazol-5-yl) indolin-2-one 198a. The oxindole-NH acts as a hydrogen bond donor toward N1 of the tetrazole (CCDC 857953).
Figure 40.
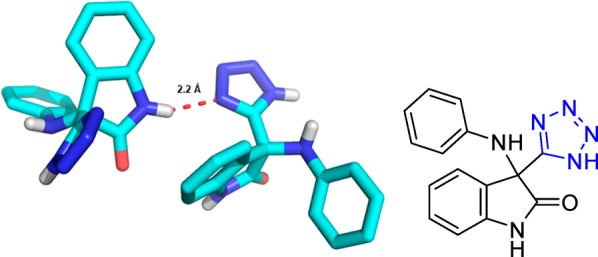
Solid-state structure of 3-(phenylamino)-3-(1H-tetrazol-5-yl)indolin-2-one 199. The oxindole-NH acts as a hydrogen bond donor toward N1 of the tetrazole (CCDC 857954).
In 2011, Shaabani et al.349 reported an efficient and simple two-step strategy for the preparation of 1,5-disubstituted tetrazole derivatives 200 and 201 containing siloxy or sulfonamide groups, respectively, by simply mixing isocyanides, dialkylacetylenedicarboxylates, and triphenylsilanol in fairly good yields. First a formal 1:1:1 addition reaction takes place selectively, yielding ketenimines containing a siloxy group in high yields. Next, an intermolecular cycloaddition reaction of the siloxy ketenimines with TMS azide yields the corresponding 1,5-disubstituted tetrazoles 200 and 201 (Scheme 88, Figure 41).
Scheme 88. Synthesis of the 1,5-Disubstituted Tetrazoles 200 and 201.
Figure 41.
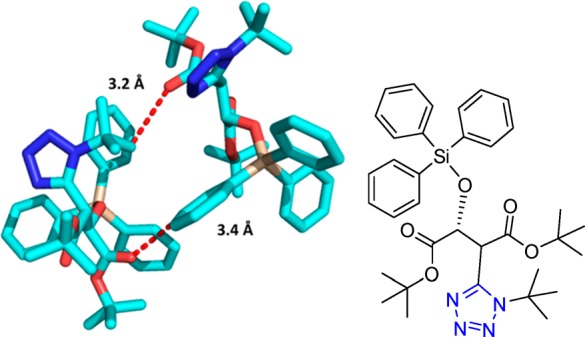
Crystal structure of (3R)-di-tert-butyl-2-(1-(tert-butyl)-1H-tetrazol-5-yl)-3-((triphenylsilyl)oxy)succinate 200d. It shows two short intermolecular interactions, O (C=O) and C (CH3 in tert-butyl group) (CCDC 817391).
The reaction of N-halo succinimide, sodium azide, and phenyl isocyanide in chloroform with a phase transfer catalyst yielded 5-halo-1-phenyltetrazoles 202 in a three-component reaction.350−352 5-Halo-1-substituted tetrazoles might be interested building blocks, e.g., in Pd catalyzed C–C couplings (Scheme 89). For example, the synthesis of tetrazolyl β-lactam systems 205 was described using 5-halo-1-benzyltetrazole 204 and the azetidinone 203 as a coupling building block.353
Scheme 89. 3-CR of N-Halo Succinimide, Sodium Azide, and Phenylisocyanide.
In 2012, Kazemizadeh et al.354 first disclosed a three-component reaction of isocyanides, carbodiimide, and TMS azide in 1:1:1 ratio, leading to 1,5-disubstituted 1H-tetrazole derivatives 206. The reaction proceeded smoothly in methanol, affording the targeted products without the need of any further purification. The mechanism is similar to the classical UT-4CR. Here, carbodiimide reacted similar to a Schiff base and was attacked by the nucleophilic addition of isocyanide. Then, the protonation of the resulting adduct led to the nitrilium intermediate, which subsequently was attacked by the azide anion to form the adduct followed by ring closure (Scheme 90).
Scheme 90. Synthesis of 1,5-Disubstituted 1H-Tetrazole Derivatives.
3.2. Bicyclic Tetrazole Derivatives
3.2.1. UT-4CR toward Fused Tetrazolopyrazine Derivatives
In 1998, Bienaymé et al.355 rigidified the basic UT-4CR scaffold of α-alkylaminotetrazole to result in the 7,8-dihydrotetrazolo[1,5-a]pyrazine scaffold. In this procedure, they mixed an oxo component, a primary amine, methyl-β-(N,N-methylamino)-α-isocyanoacrylate (Schöllkopf’s isocyanide),356 and TMS azide in a ratio of 1:1:1:1.4 at ambient temperature in methanol to give an intermediate UT-4CR adduct. Subsequent treatment with diluted acid catalyzes the secondary amine attack and dimethylamine substitution under ring formation to form the final bicyclic products 207. This constitutes a sequence of an Ugi four-component reaction (U-4CR), forming an α-amino tetrazole containing a secondary amine, followed by a ring closing reaction with the dimethyl amine from the former isocyanide acting as a leaving group with overall yields fair to good (Scheme 91).
Scheme 91. Synthesis of 7,8-Dihydrotetrazolo[1,5-a]pyrazines 207.
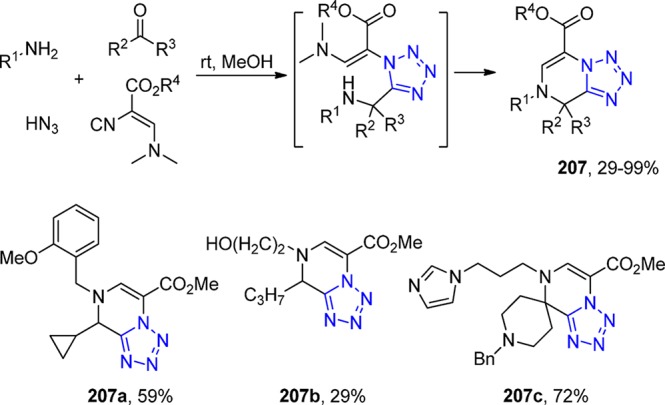
In 2000, Hulme et al.357 disclosed an efficient one-step protocol involving an Ugi reaction followed by a postcondensation reaction to access tetrazolopiperazines 209 with three potential diversity points. α-Amino acid derived isocyano esters 208 react in the UT-4CR, and the secondary amine of the side chain spontaneously undergoes a lactamization. A range of commercially available aldehydes and aliphatic or aromatic substituted primary amines were investigated, and it was shown that more sterically hindered groups in the aldehydes or amines would largely decrease both yields (Scheme 92).
Scheme 92. UT-4CR and Post-Condensation to Form the Tetrazolopiperazines 209.
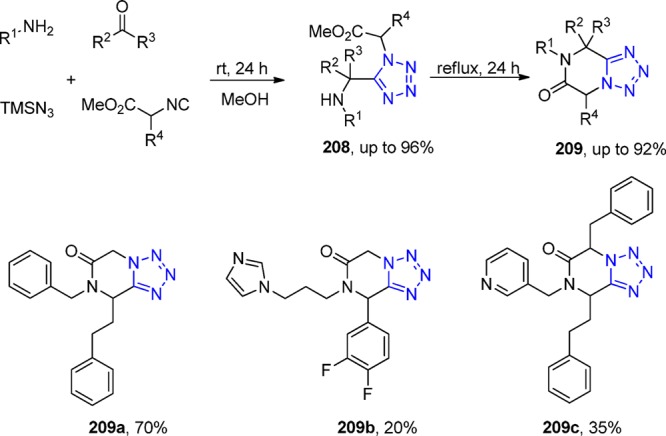
Dömling et al.358 replaced the amine component with ammonia which provided tetrazolopyrazinones 210 in good to high yields in one-pot fashion. After quite some optimization, ammonium chloride proved to be the best ammonia source followed by treatment with catalytic amount (0.1 equiv) of ammonium hydroxide as a base at 50 °C for 18 h, giving the cyclized adducts (Scheme 93, Figure 42).
Scheme 93. Ammonia Promoted One-Pot Tetrazolopiperidinone 210 Synthesis by UT-4CR.
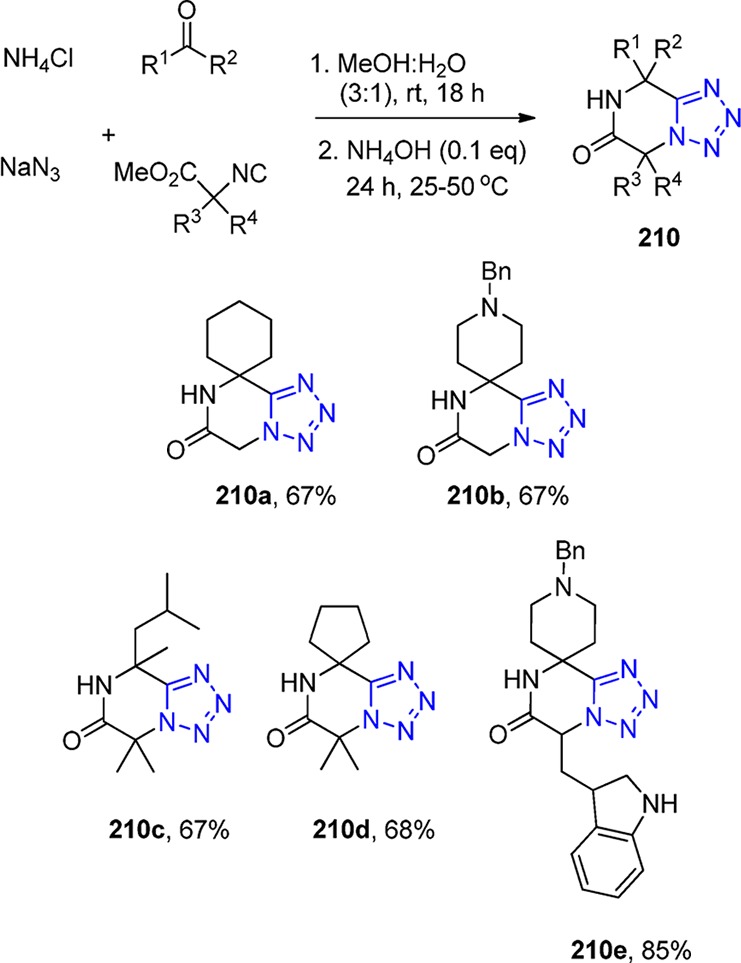
Figure 42.
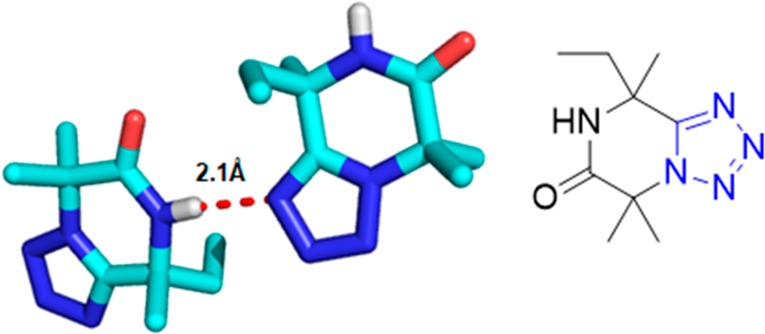
Crystal structure of the tetrazolopiperidinone 210f. A hydrogen bond exhibits between the piperidinone-NH and the N-5 of a tetrazole moiety of an adjacent molecule.
Hiller et al.359 in 2004 employed a synthetic methodology whereby cyclization to the tetrazolopiperazine system occurs in situ via a toluolsulfonate group. It is noteworthy, that the cyclization step could proceed at rt without the addition of acid or refluxing. Simply following a classical UT-4CR procedure mixing aldehydes, primary amines, TMS azide, and 2-isocyanoethyl sulfonate in a ratio of 1:1:1.5:1.5 led to the expected fused tetrazoles 211. The 2-isocyanoethyltoluolsulfonate building block that was employed in this versatile reaction can be synthesized in two steps from ethanolamine via selective N-formylation followed by O-tosylation and dehydration using tosyl chloride. The final products could be synthesized rapidly with two points of potential diversity (Scheme 94).
Scheme 94. Synthesis of Tetrazolopiperazines 211.
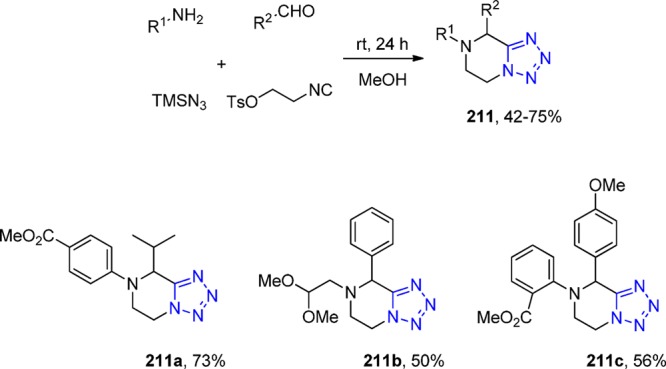
Dömling et al.159 discovered three new different heterocyclic scaffolds easily accessible from isocyanoacetaldehyde dimethylacetal by MCRs. The initial UT-4CR with isocyanoacetaldehyde dimethylacetal yields an intermediate (212), which can undergo a range of condensation reactions, e.g., Pictet–Spengler (see also Schemes 103 and 104). The 7,8-dihydrotetrazolo[1,5-a]pyrazine scaffold 213 is formed from aliphatic or aromatic aldehyde and aliphatic amine components which cannot undergo a subsequent Pictet–Spengler reaction (Scheme 95, Figure 43). The cyclization simply runs in neat methanesulfonic acid, giving generally good to excellent yields of the 7,8-dihydrotetrazolo[1,5-a]pyrazines 213.
Scheme 103. Designed Synthetic Pathway to the Polyfused Tetrazolo Scaffolds 233.
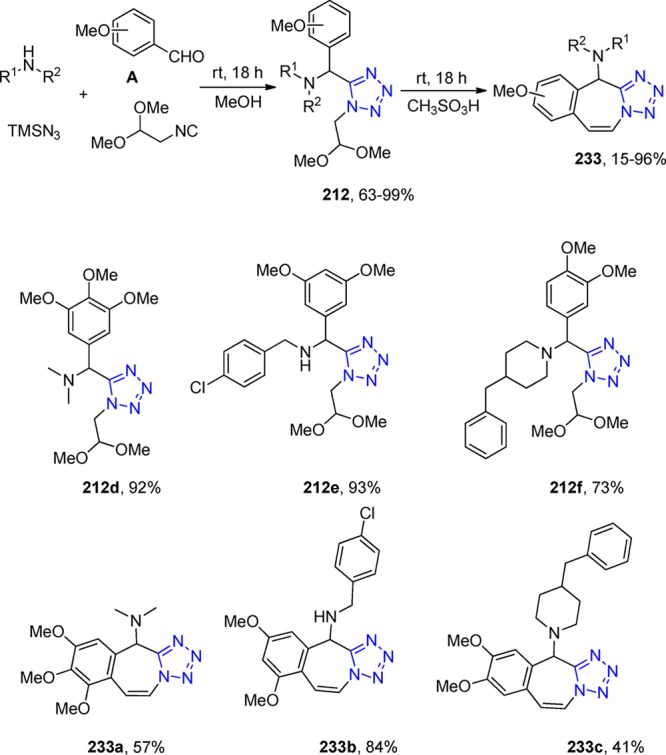
Scheme 104. Synthesis of Tetracyclic Piperazinotetrazoles 234.
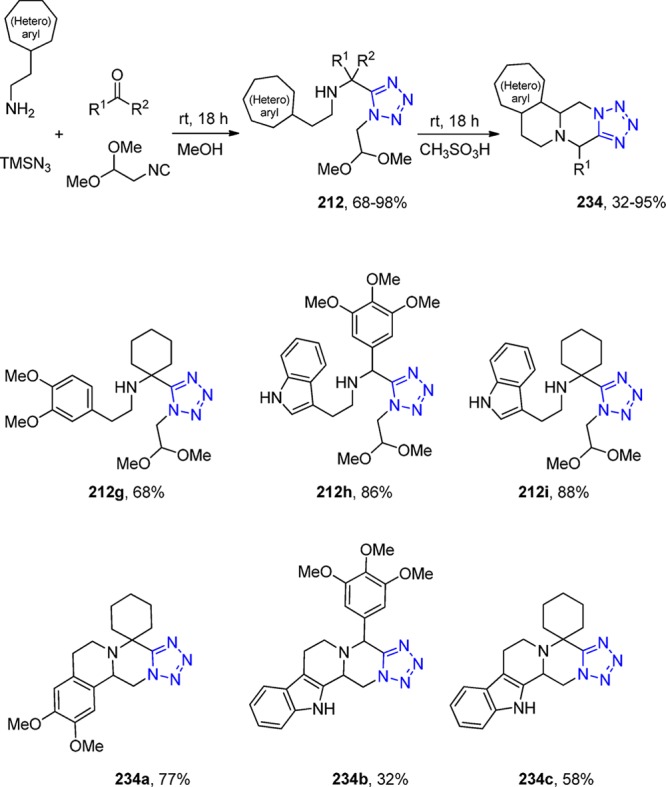
Scheme 95. Designed Synthetic Pathway to Tetrazolo Piperazine Derivatives 213.
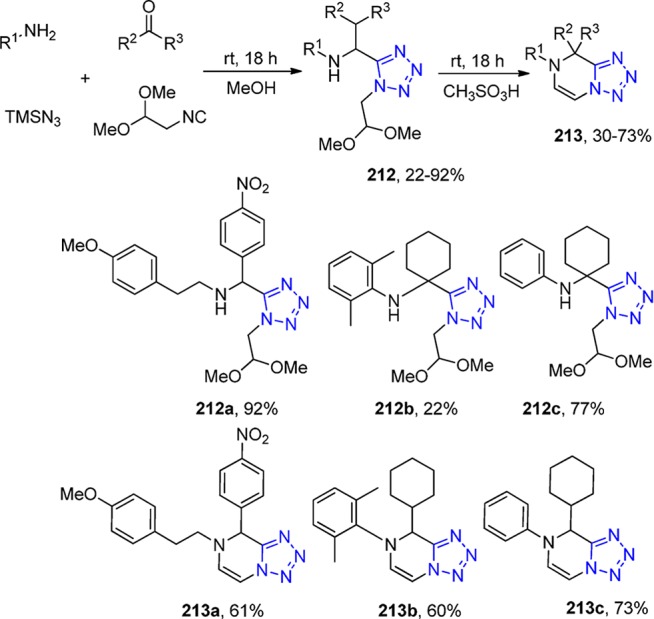
Figure 43.
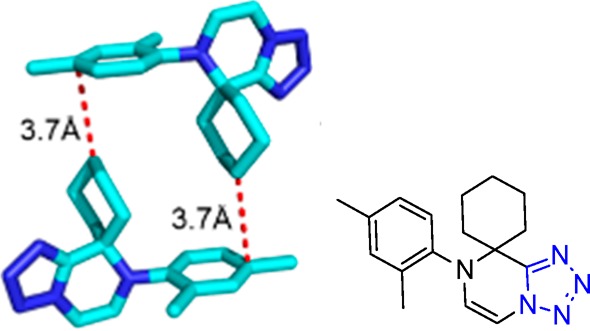
Crystal structures of 208d with the cyclohexyl moiety forming a short T-shaped interaction with the adjacent phenyl group (CCDC 1017121).
Dömling et al.360 also developed an effective procedure for the novel syntheses of highly substituted tetrazole-fused ketopiperazines 216 through UT/deprotection and U-4CR. First, they synthesized the N-unsubstituted α-aminotetrazoles 214 by using an UT-4CR; second, the N-unsubstituted α-aminotetrazoles 215 were then employed in a second intramolecular U-4CR to afford the desired products 216 in moderate to good yields. The UT synthesis was initially performed under Ugi azide conditions with tritylamine (TrtNH2) as the amine component, various aldehydes and isocyanides derived from α-amino acids, and TMS azide (Scheme 96, Figure 44). These scaffolds are structurally related to the clinically investigated oxytocin reactor antagonists Epelsiban and Retosiban.361
Scheme 96. Two-Step Synthesis of N-Unsubstituted ω-Carboxyl α-Aminotetrazoles 216.
Figure 44.
Crystal structures of 214d (CCDC 986844) (top) and 216e (CCDC 986845) (bottom). The tetrazole-fused ketopiperazine undergo three hydrogen-bonds.
The employment of hydrazine in UT-4CR was also reported toward the synthesis of bicyclic fused tetrazole derivatives (Scheme 97).362N-Boc protected hydrazine reacted with α-amino acid derived isocyanides in the UT reaction in a one-pot fashion and it was post cyclized under both acidic and basic conditions, affording 7-aminotetrazolopyrazinone (218) and tetrazolotriazepinone (219) cyclic products. The post cyclization of the isolated UT adduct 217 under basic condition could selectively afford the Boc-protected 7-aminotetrazolopyrazinone derivatives 220 in yields of 38–87%, which can be easily obtained as hydrochloric salt 221 (Scheme 97). Crystal structures of the postcyclized adducts were also obtained (Figure 45).
Scheme 97. Employment of Hydrazine in UT-4CR and Its Post-Cyclization.
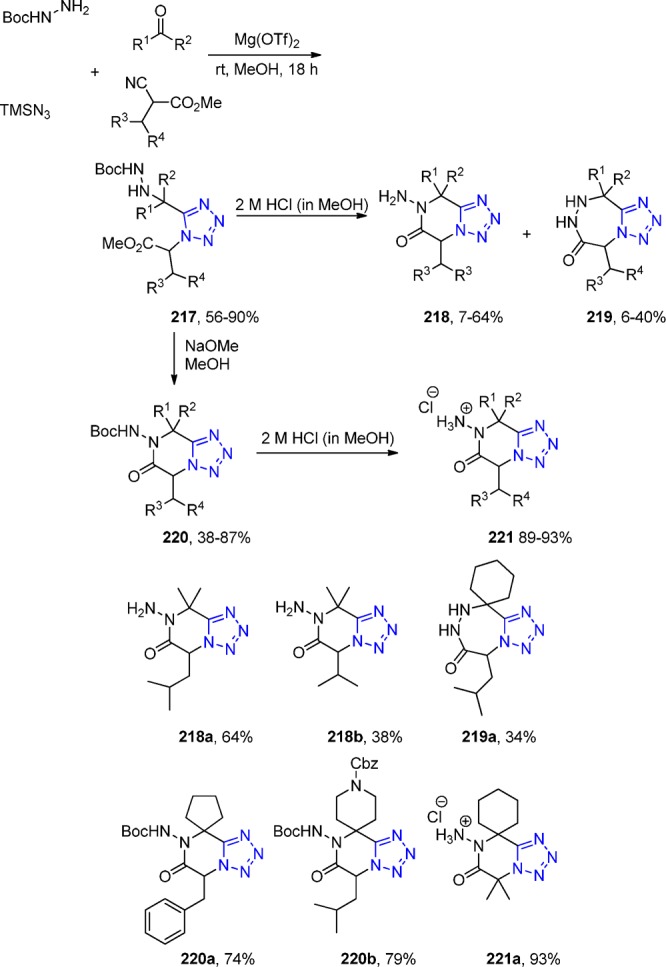
Figure 45.
Crystal structures of 219b (CCDC 1507441) and 221b (CCDC 1507440). (A) Two intermolecular hydrogen bonds of 2.0 Å are observed between the NH and the carbonyl moiety. (B) Hydrogen bond of 2.5 Å is observed between NH2 and the N4 of the tetrazole.
3.2.2. UT-4CR toward Fused Azepine-Tetrazole Derivatives
Hulme et al.363 also described the synthesis of fused azepine-tetrazole libraries 222 in high yields via the UT-4CR (Scheme 98). Compared with their previous work leading to the tetrazolopyrazine system, they employed secondary amines together with Boc protected amino acid derived aldehydes components to enlarge the fused ring by one carbon to form azepine-tetrazoles. The first tetrazole formation was particularly well-suited for the solution phase reaction of methyl-isocyano acetate, N-Boc-aminoaldehydes, TMS azide, and secondary amines and generally proceeded with high yields. The subsequent Boc-deprotection was carried out with 10% trifluoroacetic acid in dichloromethane to free the amine nucleophile for the next cycloamidation step. The lactamization was promoted by proton scavenging with PS-diisopropylethylamine and reflux for 24 h. Final compound purities were substantially improved by removal of the acyclic amine and excess aldehyde, via dissolution in THF–CH3CHCl2 addition of polystyrol bound scavenger resins PS-NCO and PS-TsNHNH2, producing the desired fused product.
Scheme 98. Synthesis of the Azepine-Tetrazoles 222.
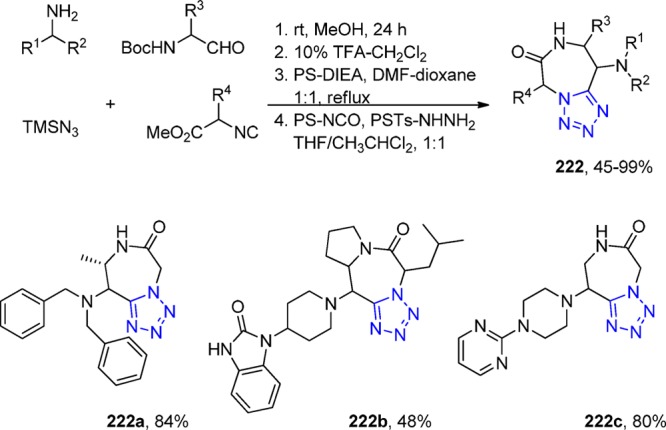
Batra et al.367 first synthesized substituted allyl isocyanides from primary allyl amines using the Baylis–Hillman reaction (Scheme 99). The Baylis–Hillman reaction368−370 occurs between the α-position of an activated alkene and an aldehyde or generally an electrophilic carbon to form a new C–C bond with the help of a nucleophilic catalyst as tertiary amine and phosphine. They employed this E-configured isocyanide in an Ugi/hydrolyze/coupling strategy (223, 224) to obtain tetrazole-fused diazepinones 225 in good yields. After obtaining the expected Ugi adducts at room temperature, they also investigated a one-pot reaction combining Ugi and cyclization process without isolating the intermediate. Two cases were reported successfully with an amine and aldehyde bearing an electron withdrawing group. Noteworthy, they also found that the use of aniline instead of the primary aliphatic amines did not lead to the formation of tetrazoles.
Scheme 99. Synthesis of Tetrazole-Fused Diazepinones 224.
3.2.3. UT-4CR toward 1,5-Disubstituted Tetrazoles in Macrocycles
Macrocycles represent a common motive in natural products, and several of them are marketed as drugs.371,372 Macrocycles are a fascinating and however underrepresented class of compounds in medicinal chemistry, as they do not behave according to drug-likeliness rules and nevertheless can lead to oral bioavailability.373 As a result of their large cycle, from 10 to 25 atoms, they show on the one hand conformational restriction but on the other hand are very flexible and can show multiple conformations.374,375 Because of their large surface area, macrocycles are assumed to be useful to target nontraditional protein–protein interaction targets which often are large, flat, and featureless.371,372 Currently, protein–protein interaction targets, in most cases, belong to the domain of antibodies. Therefore, artificial macrocycles have recently experienced a renaissance as scaffolds in medicinal chemistry. Unfortunately, there are few short, diverse, and general synthetic pathways toward this interesting class of compounds. Multicomponent reactions for accessing macrocycles was first reported by Failli and Immer.376 In 2015 Dömling et al.377 introduced α-isocyano-ω-carboxylic acids in macrocycle synthesis via Ugi reaction (U-4CR). They performed an intramolecular Ugi reaction (U-3CR) using a bifunctional α-isocyano-ω-carboxylic acid, incorporating into macrocycle 229 the other two components (a primary amine and an oxo compound), which can be widely varied. The bifunctional component has been prepared using an Ugi-tetrazole reaction (226–228, Scheme 100).
Scheme 100. UT-4CR/U-4CR/P-3CR Derived Macrocycle Synthesis Strategies.
Adding to the toolkit of macrocyclizations by MCR, Dömling et al.378 utilized for the first time a P-3CR to cyclize macrocycles and thus form artificial macrocyclic depsipeptides 230(379) (15–20 membered). The overall sequence, which again combines two MCRs, has high diversity and broad reaction scope; it introduced different ring sizes and side chain variations in just four steps using readily available starting materials (Scheme 100). Some representative crystal structures are disclosed on Figures 46 and 47.
Figure 46.
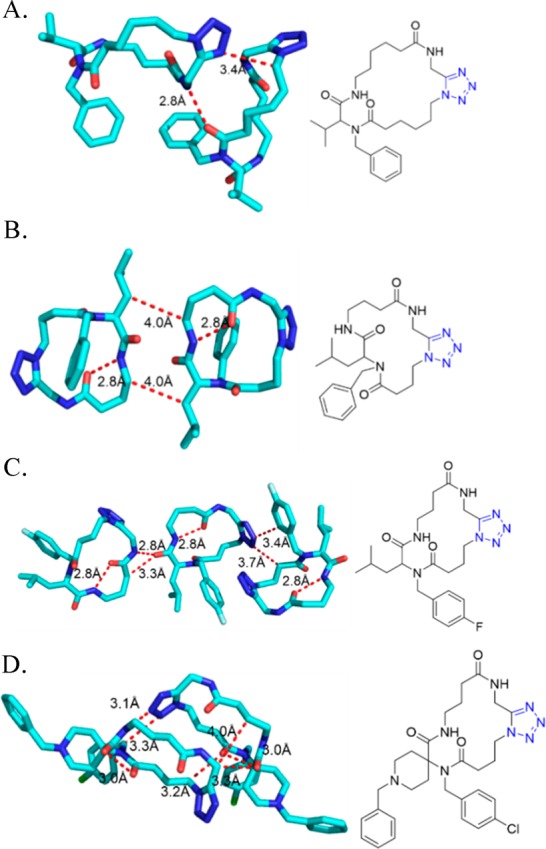
Four X-ray structures of the macrocycles 229 of different size involving different MCR assembly routes and different substituents (CCDC 1408649, 1408650, 1408653, 1408654). The most occupied interactions are included in the interactions between N of tetrazole and C of cycles, O and C of cycles, and C and C of cycles. The intramolecular bindings are mostly between O and N.
Figure 47.
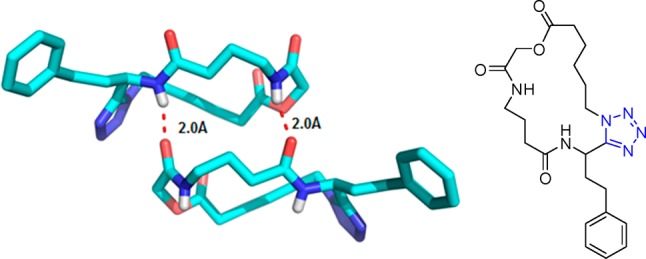
Two secondary amides form intermolecular hydrogen bonds to a neighbor macrocycle, whereas the cis-amide bioisosteric tetrazole moiety is not involved with hydrogen bonding. Looking into the different modules of compound 230b (CCDC 1442896), one can define the two amide groups, the tetrazole, and the lactone group as rigid elements which are separated by flexible sp3 center-based C1, C3, and C5 chain elements. These linker fragments ultimately will determine the flexibility of the overall macrocyclic conformations in aqueous and lipophilic environments, which will be a determinant of the passive diffusion through cell membranes
Moreover, bifunctional α-isocyano-ω-amines 231, derived by a chemoselective amidation of amino acid derived isocyano carboxylic acid esters with unprotected symmetrical diamines, were employed in a concise two-step synthesis of tetrazole containing macrocycles 232.380 A short access to 11–19-membered macrocycles in which substituents can be independently varied at three different positions was allowed (Scheme 101, Figure 48).
Scheme 101. α-Isocyano-ω-amine 231 Synthesis and UT-4CR Derived Macrocycle 232 Synthesis Pathway.
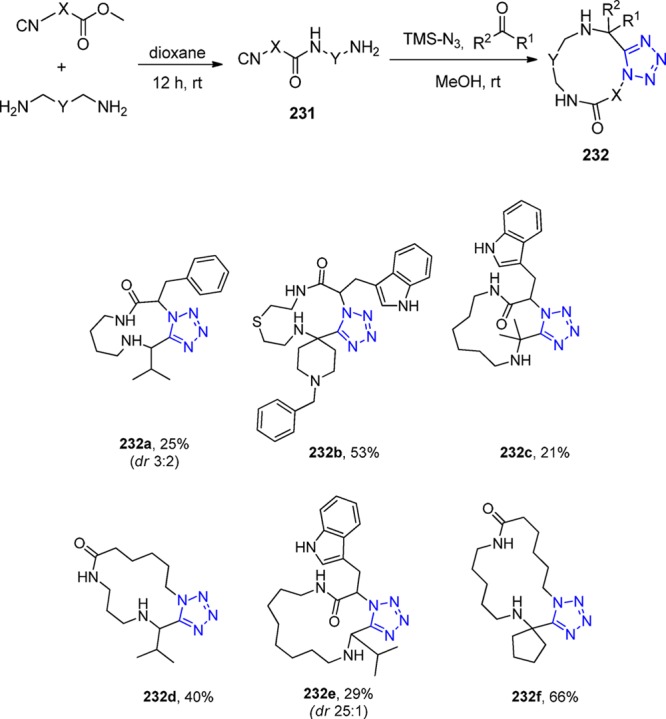
Figure 48.
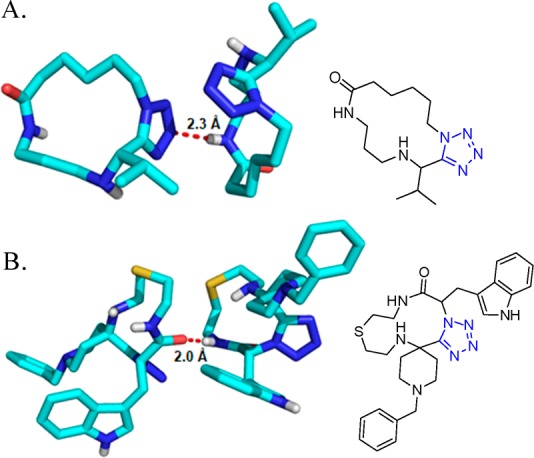
Crystal structures of the MCR-derived 14-membered 232d (CCDC 1548701) and 12-membered 232b (CCDC 1548704) macrocycles in solid state featuring intermolecular hydrogen bonding contacts of 2.3 and 2.0 Å, respectively.
3.3. Tricyclic and Polycyclic Tetrazole Derivatives
Annulated polyheterocyclic structures are interesting to the medicinal chemist due to their rigidity and often good blood–brain barrier penetration to target neurological diseases. Therefore, strategies to reduce the number of synthetic and purification steps to prepare suitably modified compounds are of special interest in medicinal/combinatorial chemistry. As it was previously described (see Scheme 95), the UT-4CR with isocyanoacetaldehyde dimethylacetal yields the intermediate 212, which can undergo a range of condensation reactions toward 233 and 234 (Scheme 102).159
Scheme 102. Diversity of Ring Fused Tetrazole Scaffolds from the Common Precursor Building Block Isocyanoacetaldehyde Dimethylacetal.
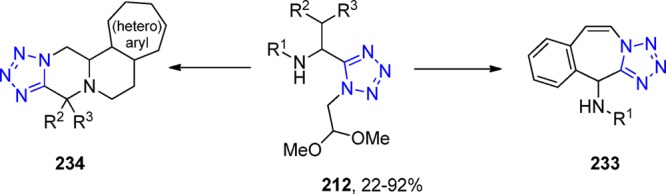
The 11H-benzo[d]tetrazolo[1,5-a]azepin-11-amine scaffold 233 can be accessed from activated electron rich benzaldehydes, primary or secondary amines, and isocyanoacetaldehyde dimethylacetal. The reaction sequence involves an UT-4CR (212d–f) followed by a condensation. The cyclization runs smoothly under methanesulfonic acid (MSA) in neat conditions in good to excellent yields (Scheme 103).
When using electron-rich substituted (hetero)phenylethyl amines, polyfused tetrazoles 234 can be accessed in great diversity (Scheme 104). The intermediate UT-4CR product (212g–i) can undergo a Pictet–Spengler type condensation under MSA room temperature conditions, in decent to excellent yields.279 The reaction involves an acid induced dimethylacetal deprotection, followed by a imine formation and attack onto the nucleophilic (hetero)aromatic ring. Phenylethyl amines and tryptamines lead to the alkaloid-type scaffolds of isoquinolines and ibogaine, respectively. Libraries of >1000 compounds per scaffold have been synthesized and are part of the screening collection of the European Lead Factory.
The 3D structures and other physicochemical properties of each of the aforementioned scaffolds 233 and 234 were also extensively discussed. Unexpectedly, they possess very different characteristics even though these scaffolds are all derived from the same first UT-4CR in terms of their chemical space due to their connectivity, substitution pattern, and ring sizes (Figure 49).
Figure 49.
Crystal structures of 233d and 234d (CCDC 1017123 and 1017122 and some characteristic short contacts of 2.4 and 2.6 Å, respectively).
Kalinski et al.381 described an UT reaction (236) followed by a nucleophilic aromatic substitution for the preparation of a library of polysubstituted fused 4,5-dihydrotetrazolo[1,5-a]quinoxalines 237. The first synthetic step corresponds to a classical UT-4CR, exploring 2-fluorophenylisocyanide as a new bifunctional starting material, yielding tricyclic tetrazoles with two points of diversity (Scheme 105). 2-Fluorophenylisocyanide (235) allows for a subsequent nucleophilic aromatic substitution (SNAr) in a second step, thus forming a ring. They found that the best yield could be reached by mixing the four components amine/aldehyde/TMS azide/isocyanide in a ratio of 1:1:1.5:1.5 in the Ugi reaction. The nucleophilic aromatic substitution–cyclization conditions were optimized by using Cs2CO3 in DMF as the best conditions. They also exploited a range of amines and aldehydes for this strategy, finding that amines and carbonyls can be varied broadly, yielding tricyclic tetrazoles with two potential diversity points.
Scheme 105. Synthesized Fused 4,5-Dihydrotetrazolo[1,5-a]quinoxalines 237.
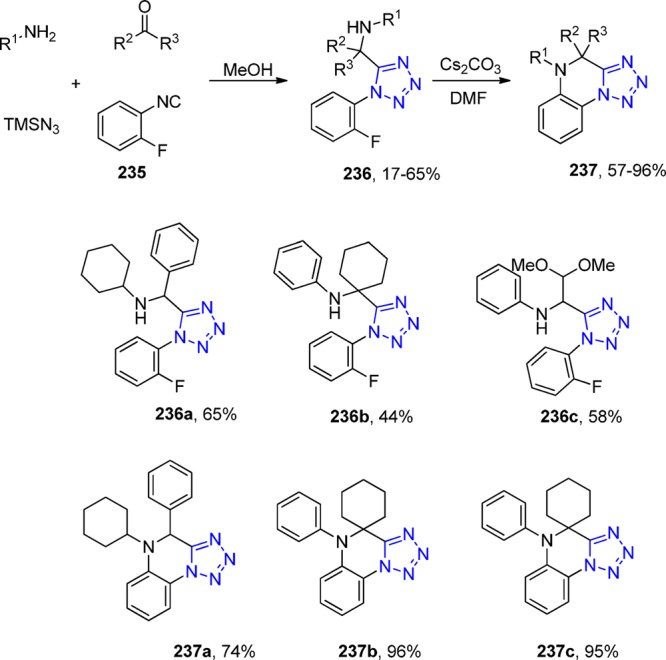
A series of 18 fused tetrazolo-quinolines 238 featuring two tetrazoles were synthesized in 21–90% yields via a novel one-pot UT/SNAr/ring–chain azido-tautomerization process under microwave irradiation or ultrasound and catalyst-free conditions (Scheme 106).382 The overall procedure has a good substrate scope and functional group tolerance.
Scheme 106. Synthesis of the Bis-tetrazolo Quinolones 238.
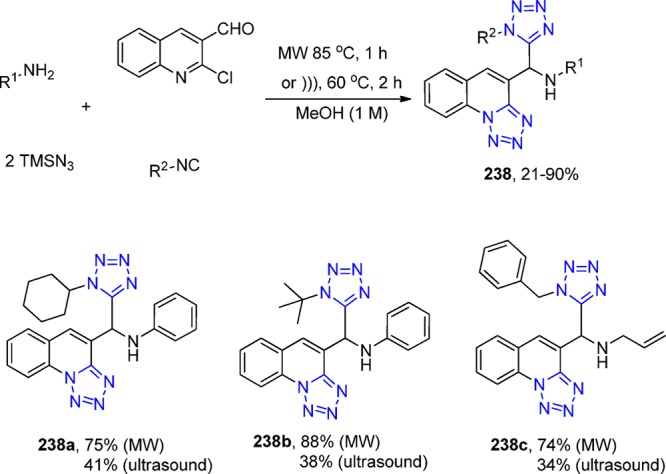
The compound class of 1,4-benzodiazepines are among the most widely used drugs with potent tranquilizer, muscle relaxant, anticonvulsant, antiseizure, and sedative-hypnotic activities.246 In 2010, Voskressensky et al.364 developed an effective procedure for the syntheses of substituted tetrazolo[1,5-a][1,4]benzodiazepines 240 via an UT reaction, followed by an amidocyclization (Scheme 107). The tetrazolodiazepines 240 were synthesized by simply mixing ketone, sodium azide, ammonium chloride, and the corresponding anthranilic acid derived isocyanide 239 in aqueous methanol. After 24–48 h of vigorous stirring at room temperature, the target products precipitated from the reaction mixture. The reaction’s scope was investigated, with symmetrical and unsymmetrical, cyclic and acyclic, and sterically not hindered and very bulky (e.g., adamantyl ketone) ketones being good substrates. Interestingly, all attempts to isolate the corresponding products from aldehydes failed. Moreover, the reaction with methylamine hydrochloride instead of ammonium chloride aiming to yield the N-methyl substituted benzodiazepines stopped at the intermediate Ugi tetrazole stage, and no cyclization was observed under the reaction conditions.
Scheme 107. Fused Tetrazolodiazepines 240 Synthesized by UT.
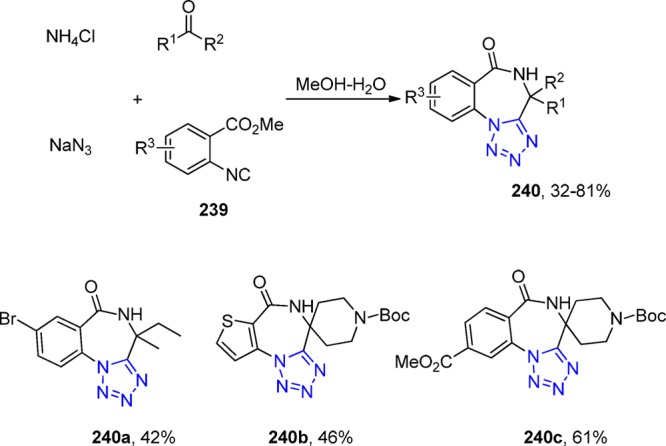
A crystal structure showing the 3D structure of 240d in the solid stage is shown in Figure 50, in which the overall 3D structure comprises a butterfly shape with the cyclohexyl and benzene rings presenting the wings. In general heterocyclic-conjugated benzodiazepines emerged as an important class of epigenetic drugs,364,365 as similar structures are potent inhibitors of the BET family of proteins, e.g., JQ-1.366
Figure 50.
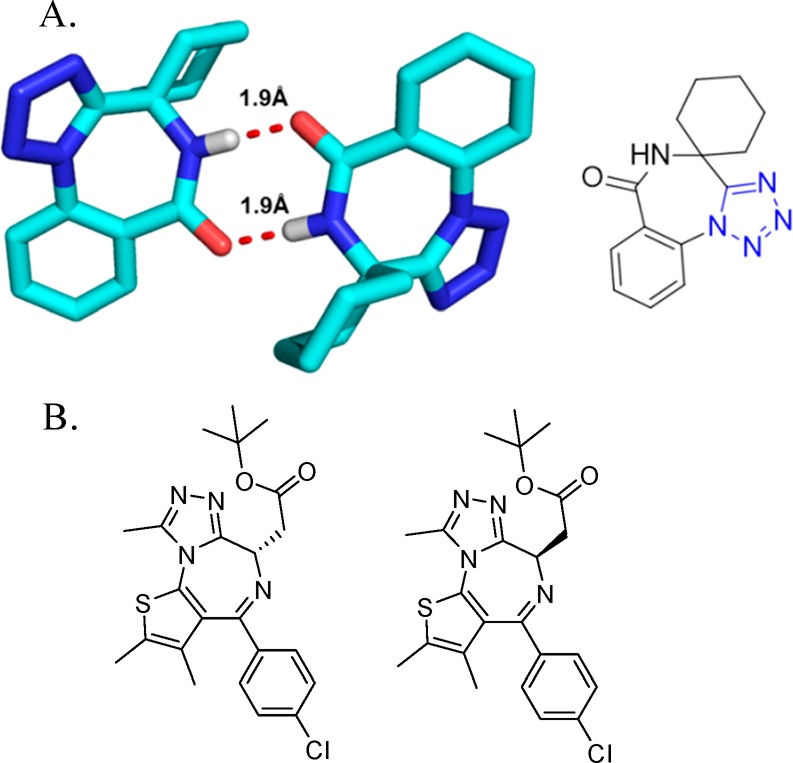
(A) Crystal structure of 240d (CCDC 780553). Two hydrogen bonds of 1.9 Å are shown between the amides of the diazepineone moieties. (B) Structures of the two JQ-1 stereoisomers.
4. Tetrazoles in Virtual Screening
A pharmacophore-based virtual screening platform, ANCHOR.QUERY, was introduced to bring interactive virtual screening of novel protein–protein inhibitors to the desktop (Figure 51).383,384 More than 2 billion 3D conformers of unprecedented compounds based on one-pot MCR can be efficiently and web-based screened against protein targets. A typical project encompasses building of a 3D pharmacophore model based on a PDΒ structure, query against 2 billion conformers, ranking, synthesis of best hits, and biophysical screening. Twenty-three different MCR scaffolds are enumerated, among them two tetrazole backbones (e.g., compound 241, Figure 51). The substituents are chosen based on commercial availability of the corresponding building blocks and on previous experience to yield the products with high confidence. In fact, the success rate of synthesis of the virtual compounds is very high exceeding 90%.
Figure 51.
The ANCHOR.QUERY virtual screening platform. (A) General sequence of steps to interrogate 2 billion MCR derived conformers. (B) Screen shot of ANCHOR.QUERY to search for inhibitors of the protein protein interaction NEMO/IKK-β. (C) View of the protein protein interaction NEMO/IKK-β with NEMO as yellow surface and IKK-β as pink α-helix (PDB 3BRV). (D) Close-up view of the hot spot formed by Trp741, Trp739, and Phe734 and the aligned to hit tetrazole in red sticks. Remarkably, the close alignment with the query amino acids and the hydrogen bonding cluster of the tetrazole with the Arg101. (E) 2D structure of the top hit 241. Reproduced with permission from ref (384). Copyright 2017 John Wiley and Sons.
Multiple successful applications of ANCHOR.QUERY have been recently published.193,385,386 Among them α-amino tetrazoles were found to be potent antagonists of the protein protein interaction of p53-MDM2.193 The virtual screening of very large MCR compound libraries is an interesting, fast, and cost-effective alternative to high throughput screening.
5. Conclusions and Outlook
More than 225 tetrazole-based scaffolds have been presented in this review which can be convergently and easily synthesized by using multicomponent reactions. Especially the Ugi variation UT-4CR of tetrazole synthesis is very fruitful in accessing many different drug-like scaffolds. Thus, among of all organic chemistry methods, clearly MCR stands out and provides the most versatile access to this class of heterocycles. Tetrazole derivatives will continue to be a prime class of heterocycles due to their isosteric character to carboxylic acid and cis-amide moieties and due to their metabolic stability and other physicochemical properties. Efficient synthetic access to a wide variety of derivatives is therefore the key to leverage the potential of tetrazoles to generate lead compounds.
Acknowledgments
This research has been supported to (A.D.) by the National Institute of Health (NIH) (2R01GM097082-05), the European Lead Factory (IMI) under grant agreement no. 115489, and the Qatar National Research Foundation (NPRP6-065-3-012). Moreover, funding was received through ITN “Accelerated Early stage drug dIScovery” (AEGIS, grant agreement no. 675555) and COFUND ALERT (grant agreement no. 665250), Hartstichting (ESCAPE-HF, 2018B012), and KWF Kankerbestrijding grant (grant agreement no. 10504).
Glossary
Abbreviations
- Ac
acetyl
- ADME
absorption, distribution, metabolism, and excretion
- BET
bromodomain and extraterminal domain
- Boc
tert-butyloxycarbonyl protecting group
- CAN
ammonium nitrate
- Cbz
carboxybenzyl
- DCC
N,N′-dicyclohexylcarbodiimide
- DCE
dichloroethane
- DCM
dichloromethane
- DIEA
N,N-diisopropylethylamine
- DMA
dimethylacetamide
- DMF
dimethylformamide
- DMPK
drug metabolism and pharmacokinetic
- oPDM
o-phenylenodiamine
- PDB
Protein Data Bank
- PS
polystyrol
- pTSIA
p-toluenesulfinic acid
- pTsOH
p-toluenesulfonic acid
- TBAF
tetra-n-butylammonium fluoride
- TEMPO
2,2,6,6-tetramethylpiperidin-1-yl)oxyl
- Tf
triflate
- TFA
thrifluoroacetic acid
- TFE
trifluoroethanol
- TFP
tetrafluorophenyl
- THF
tetrahydrofuran
- TMS
trimethylsilyl
- tOctyl
1,1,3,3-tetramethylbutyl
- Trt
trityl
- Ts
tosyl
Biographies
Constantinos G. Neochoritis received his Ph.D. in Organic Chemistry under the guidance of Professors J. Stephanidou Stephanatou and C. Tsoleridis in the Department of Chemistry at Aristotle University of Thessaloniki in 2011. Being fascinated by the applied multicomponent reaction (MCR) chemistry, he joined the research group of Prof. Alexander Dömling in the Drug Design Group at the University of Groningen. He specialized in computational-aided drug design utilizing MCR chemistry. In 2014, he cofounded the biotech company TelesisPharma BV. Very recently, he was appointed as assistant professor in the chemistry department of the University of Crete in Greece. His research interests include bioactive heterocycles, multicomponent reactions, novel materials, and high throughput synthesis. He has published more than 40 peer-reviewed papers and book contributions.
Ting Zhao, received her Bachelor’s degree in 2006 and Master’s degree in 2011 at Lanzhou University. Then she moved to Groningen and joined Prof Dömling’s research group as a Ph.D. candidate under the prestigious CSC fellowship. She focused on discovering concise and rapid routes towards novel drug-like molecules bearing tetrazole moieties, using MCR chemistry. In 2016, she graduated and moved to Norway.
Alexander Dömling studied chemistry and biology at the Technische Universität Munich and obtained his Ph.D. under the guidance of Ivar Ugi. After a postdoc under a Humboldt Fellowship in the group of the Nobel Laureate Barry Sharpless, he founded the biotech company Morphochem, later Carmolex Inc., and most recently, TelesisPharma and SMIO BV. After his habilitation, he worked as full professor at the University of Pittsburgh in the School of Pharmacy. He has held the chair for Drug Design at the University of Groningen since 2011. His interests are centered on MCR chemistry and its application to problems in drug discovery. His special focus is centered on the question of how to leverage the huge MCR space. Thus, he is working on MCR centered pharmacophore methods, structure-based drug design, and MCR-centered fragment-based drug design methods and extreme miniaturization to library synthesis. He is the author of more than 200 scientific articles, reviews, and book contributions. He has applied for more than 30 patents. His long-term vision is to bring a novel drug to patients in an indicated area of unmet medical needs.
The authors declare no competing financial interest.
References
- Bhatt U.Five-Membered Heterocycles with Four Heteroatoms: Tetrazoles. In Modern Heterocyclic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, 2011; pp 1401–1430. [Google Scholar]
- Bladin J. A. Ueber von Dicyanphenylhydrazin Abgeleitete Verbindungen. Ber. Dtsch. Chem. Ges. 1885, 18, 1544–1551. 10.1002/cber.188501801335. [DOI] [Google Scholar]
- Benson F. R. The Chemistry of the Tetrazoles. Chem. Rev. 1947, 41, 1–61. 10.1021/cr60128a001. [DOI] [PubMed] [Google Scholar]
- Wei C.-X.; Bian M.; Gong G.-H. Tetrazolium Compounds: Synthesis and Applications in Medicine. Molecules 2015, 20, 5528–5553. 10.3390/molecules20045528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frija L. M. T.; Ismael A.; Cristiano M. L. S. Photochemical Transformations of Tetrazole Derivatives: Applications in Organic Synthesis. Molecules 2010, 15, 3757–3774. 10.3390/molecules15053757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myznikov L. V.; Hrabalek A.; Koldobskii G. I. Drugs in the Tetrazole Series. (Review). Chem. Heterocycl. Compd. 2007, 43, 1–9. 10.1007/s10593-007-0001-5. [DOI] [Google Scholar]
- Lv F.; Liu Y.; Zou J.; Zhang D.; Yao Z. Synthesis of the Novel Photographic DIAR Couplers. Dyes Pigm. 2006, 68, 211–216. 10.1016/j.dyepig.2004.07.017. [DOI] [Google Scholar]
- Song W.; Wang Y.; Qu J.; Madden M. M.; Lin Q. A Photoinducible 1,3-Dipolar Cycloaddition Reaction for Rapid, Selective Modification of Tetrazole-Containing Proteins. Angew. Chem., Int. Ed. 2008, 47, 2832–2835. 10.1002/anie.200705805. [DOI] [PubMed] [Google Scholar]
- Shmatova O. I.; Nenajdenko V. G. Synthesis of Tetrazole-Derived Organocatalysts via Azido-Ugi Reaction with Cyclic Ketimines. J. Org. Chem. 2013, 78, 9214–9222. 10.1021/jo401428q. [DOI] [PubMed] [Google Scholar]
- Dippold A. A.; Izsák D.; Klapötke T. M.; Pflüger C. Combining the Advantages of Tetrazoles and 1,2,3-Triazoles: 4,5-Bis(Tetrazol-5-Yl)-1,2,3-Triazole, 4,5-Bis(1-Hydroxytetrazol-5-Yl)-1,2,3-Triazole, and Their Energetic Derivatives. Chem. - Eur. J. 2016, 22, 1768–1778. 10.1002/chem.201504624. [DOI] [PubMed] [Google Scholar]
- Fischer D.; Klapötke T. M.; Stierstorfer J. 1,5-Di(Nitramino)Tetrazole: High Sensitivity and Superior Explosive Performance. Angew. Chem., Int. Ed. 2015, 54, 10299–10302. 10.1002/anie.201502919. [DOI] [PubMed] [Google Scholar]
- Klapötke T. M.; Stierstorfer J.. Energetic Tetrazole N-Oxides. In Green Energetic Materials; John Wiley & Sons, Ltd, 2014; pp 133–178. [Google Scholar]
- Hammerl A.; Klapötke T. M.; Nöth H.; Warchhold M.; Holl G. Synthesis, Structure, Molecular Orbital and Valence Bond Calculations for Tetrazole Azide, CHN7. Propellants, Explos., Pyrotech. 2003, 28, 165–173. 10.1002/prep.200300001. [DOI] [Google Scholar]
- Gálvez-Ruiz J. C.; Holl G.; Karaghiosoff K.; Klapötke T. M.; Löhnwitz K.; Mayer P.; Nöth H.; Polborn K.; Rohbogner C. J.; Suter M.; et al. Derivatives of 1,5-Diamino-1 H -Tetrazole: A New Family of Energetic Heterocyclic-Based Salts. Inorg. Chem. 2005, 44, 4237–4253. 10.1021/ic050104g. [DOI] [PubMed] [Google Scholar]
- Fischer N.; Karaghiosoff K.; Klapötke T. M.; Stierstorfer J. New Energetic Materials Featuring Tetrazoles and Nitramines – Synthesis, Characterization and Properties. Z. Anorg. Allg. Chem. 2010, 636, 735–749. 10.1002/zaac.200900521. [DOI] [Google Scholar]
- Quan M. L.; Ellis C. D.; He M. Y.; Liauw A. Y.; Woerner F. J.; Alexander R. S.; Knabb R. M.; Lam P. Y. S.; Luettgen J. M.; Wong P. C.; et al. Nonbenzamidine Tetrazole Derivatives as Factor Xa Inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 369–373. 10.1016/S0960-894X(02)00951-4. [DOI] [PubMed] [Google Scholar]
- Muraglia E.; Kinzel O. D.; Laufer R.; Miller M. D.; Moyer G.; Munshi V.; Orvieto F.; Palumbi M. C.; Pescatore G.; Rowley M.; et al. Tetrazole Thioacetanilides: Potent Non-Nucleoside Inhibitors of WT HIV Reverse Transcriptase and Its K103N Mutant. Bioorg. Med. Chem. Lett. 2006, 16, 2748–2752. 10.1016/j.bmcl.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Upadhayaya R. S.; Jain S.; Sinha N.; Kishore N.; Chandra R.; Arora S. K. Synthesis of Novel Substituted Tetrazoles Having Antifungal Activity. Eur. J. Med. Chem. 2004, 39, 579–592. 10.1016/j.ejmech.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Burke T. R.; Yao Z. J.; Gao Y.; Wu J. X.; Zhu X.; Luo J. H.; Guo R.; Yang D. N-Terminal Carboxyl and Tetrazole-Containing Amides as Adjuvants to Grb2 SH2 Domain Ligand Binding. Bioorg. Med. Chem. 2001, 9, 1439–1445. 10.1016/S0968-0896(01)00014-1. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Pearson A. L.; Pratt R. F. Design, Synthesis, and Evaluation of α-Ketoheterocycles as Class C β-Lactamase Inhibitors. Bioorg. Med. Chem. 2001, 9, 2035–2044. 10.1016/S0968-0896(01)00107-9. [DOI] [PubMed] [Google Scholar]
- Rostom S. A. F.; Ashour H. M. A.; Razik H. A. A. El; Abd El Fattah H.; El-Din N. N. Azole Antimicrobial Pharmacophore-Based Tetrazoles: Synthesis and Biological Evaluation as Potential Antimicrobial and Anticonvulsant Agents. Bioorg. Med. Chem. 2009, 17, 2410–2422. 10.1016/j.bmc.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Zarubaev V. V.; Golod E. L.; Anfimov P. M.; Shtro A. A.; Saraev V. V.; Gavrilov A. S.; Logvinov A. V.; Kiselev O. I. Synthesis and Anti-Viral Activity of Azolo-Adamantanes against Influenza A Virus. Bioorg. Med. Chem. 2010, 18, 839–848. 10.1016/j.bmc.2009.11.047. [DOI] [PubMed] [Google Scholar]
- Li J.; Chen S. Y.; Li J. J.; Wang H.; Hernandez A. S.; Tao S.; Musial C. M.; Qu F.; Swartz S.; Chao S. T.; et al. Discovery of a Tetrazole-Based Growth Hormone Secretagogue: 4-(Hydroxybutyl)Carbamic Acid 2-{5-[1-(2-Amino-2-Methylpropionylamino)-2- Benzyloxyethyl]Tetrazol-1-Yl}ethyl Ester (BMS-317180). J. Med. Chem. 2007, 50, 5890–5893. 10.1021/jm7010595. [DOI] [PubMed] [Google Scholar]
- Kambe T.; Correia B. E.; Niphakis M. J.; Cravatt B. F. Mapping the Protein Interaction Landscape for Fully Functionalized Small-Molecule Probes in Human Cells. J. Am. Chem. Soc. 2014, 136, 10777–10782. 10.1021/ja505517t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.; Fluxe A.; Sheffer J.; Janusz J. M.; Blass B. E.; White R.; Jackson C.; Hedges R.; Murawsky M.; Fang B.; et al. Discovery and in Vitro/in Vivo Studies of Tetrazole Derivatives as Kv1.5 Blockers. Bioorg. Med. Chem. Lett. 2006, 16, 6213–6218. 10.1016/j.bmcl.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Li J.; Chen S. Y.; Tao S.; Wang H.; Li J. J.; Swartz S.; Musial C.; Hernandez A. A.; Flynn N.; Murphy B. J.; et al. Design and Synthesis of Tetrazole-Based Growth Hormone Secretagogue: The SAR Studies of the O-Benzyl Serine Side Chain. Bioorg. Med. Chem. Lett. 2008, 18, 1825–1829. 10.1016/j.bmcl.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Kang S. Y.; Lee S. H.; Seo H. J.; Jung M. E.; Ahn K.; Kim J.; Lee J. Tetrazole-Biarylpyrazole Derivatives as Cannabinoid CB1 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 2385–2389. 10.1016/j.bmcl.2008.02.061. [DOI] [PubMed] [Google Scholar]
- Li J. J.; Wang H.; Li J.; Qu F.; Swartz S. G.; Hernández A. S.; Biller S. A.; Robl J. A.; Tino J. A.; Slusarchyk D.; et al. Tetrazole Based Amides as Growth Hormone Secretagogues. Bioorg. Med. Chem. Lett. 2008, 18, 2536–2539. 10.1016/j.bmcl.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Ortar G.; Schiano Moriello A.; Cascio M. G.; De Petrocellis L.; Ligresti A.; Morera E.; Nalli M.; Di Marzo V. New Tetrazole-Based Selective Anandamide Uptake Inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 2820–2824. 10.1016/j.bmcl.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Boatman P. D.; Schrader T. O.; Kasem M.; Johnson B. R.; Skinner P. J.; Jung J. K.; Xu J.; Cherrier M. C.; Webb P. J.; Semple G.; et al. Potent Tricyclic Pyrazole Tetrazole Agonists of the Nicotinic Acid Receptor (GPR109a). Bioorg. Med. Chem. Lett. 2010, 20, 2797–2800. 10.1016/j.bmcl.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Filichev V. V.; Jasko M. V.; Malin A. A.; Zubarev V. Y.; Ostrovskii V. A. Synthesis of Novel Thymidine Derivatives Containing a Polycyclic Tetrazole Linker. Tetrahedron Lett. 2002, 43, 1901–1903. 10.1016/S0040-4039(02)00134-X. [DOI] [Google Scholar]
- Garipova G.; Gautier A.; Piettre S. R. Stereoselective Synthesis of Tetrazole CB92834, a Potent Retinoid Compound. Tetrahedron 2005, 61, 4755–4759. 10.1016/j.tet.2005.03.023. [DOI] [Google Scholar]
- Wishart D. S. DrugBank: A Comprehensive Resource for in Silico Drug Discovery and Exploration. Nucleic Acids Res. 2006, 34, D668–D672. 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First Symposium on Chemical-Biological Correlation, May 26–27, 1950; National Academy of Sciences, National Research Council, 1050; p 1951.
- Ballatore C.; Huryn D. M.; Smith A. B. Carboxylic Acid (Bio)Isosteres in Drug Design. ChemMedChem 2013, 8, 385–395. 10.1002/cmdc.201200585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta C. F.; Arabi A. A.; Weaver D. F. The Bioisosteric Similarity of the Tetrazole and Carboxylate Anions: Clues from the Topologies of the Electrostatic Potential and of the Electron Density. Eur. J. Med. Chem. 2010, 45, 1868–1872. 10.1016/j.ejmech.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Herr R. J. 5-Substituted-1H-Tetrazoles as Carboxylic Acid Isosteres: Medicinal Chemistry and Synthetic Methods. Bioorg. Med. Chem. 2002, 10, 3379–3393. 10.1016/S0968-0896(02)00239-0. [DOI] [PubMed] [Google Scholar]
- Hansch C.; Leo A.; Hoekman D. H.. Exploring QSAR: Fundamentals and Applications in Chemistry and Biology; American Chemical Society, 1995. [Google Scholar]
- Holland G. F.; Pereira J. N. Heterocyclic Tetrazoles, a New Class of Lipolysis Inhibitors. J. Med. Chem. 1967, 10, 149–154. 10.1021/jm00314a004. [DOI] [PubMed] [Google Scholar]
- Figdor S. K.; von Wittenau M. S. Metabolism of 5-(3-Pyridyl)Tetrazole. J. Med. Chem. 1967, 10, 1158–1159. 10.1021/jm00318a038. [DOI] [PubMed] [Google Scholar]
- Kubo K.; Kohara Y.; Yoshimura Y.; Inada Y.; Shibouta Y.; Furukawa Y.; Kato T.; Nishikawa K.; Naka T. Nonpeptide Angiotensin II Receptor Antagonists. Synthesis and Biological Activity of Potential Prodrugs of Benzimidazole-7-Carboxylic Acids. J. Med. Chem. 1993, 36, 2343–2349. 10.1021/jm00068a011. [DOI] [PubMed] [Google Scholar]
- Kraus J. L. Isosterism and Molecular Modification in Drug Design: Tetrazole Analogue of GABA: Effects on Enzymes of the γ-Aminobutyrate System. Pharmacol. Res. Commun. 1983, 15, 183–189. 10.1016/S0031-6989(83)80060-5. [DOI] [PubMed] [Google Scholar]
- Liljebris C.; Larsen S. D.; Ogg D.; Palazuk B. J.; Bleasdale J. E. Investigation of Potential Bioisosteric Replacements for the Carboxyl Groups of Peptidomimetic Inhibitors of Protein Tyrosine Phosphatase 1B: Identification of a Tetrazole-Containing Inhibitor with Cellular Activity. J. Med. Chem. 2002, 45, 1785–1798. 10.1021/jm011100y. [DOI] [PubMed] [Google Scholar]
- Groom C. R.; Bruno I. J.; Lightfoot M. P.; Ward S. C. The Cambridge Structural Database. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 2016, 72, 171–179. 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissantz C.; Kuhn B.; Stahl M. A Medicinal Chemist’s Guide to Molecular Interactions. J. Med. Chem. 2010, 53, 5061–5084. 10.1021/jm100112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. A.; Castellano R. K.; Diederich F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem., Int. Ed. 2003, 42, 1210–1250. 10.1002/anie.200390319. [DOI] [PubMed] [Google Scholar]
- Tong Y.; Olczak J.; Zabrocki J.; Gershengorn M. C.; Marshall G. R.; Moeller K. D. Constrained Peptidomimetics for TRH: Cis-Peptide Bond Analogs. Tetrahedron 2000, 56, 9791–9800. 10.1016/S0040-4020(00)00886-3. [DOI] [Google Scholar]
- Nachman R. J.; Zabrocki J.; Olczak J.; Williams H. J.; Moyna G.; Ian Scott A.; Coast G. M. Cis-Peptide Bond Mimetic Tetrazole Analogs of the Insect Kinins Identify the Active Conformation. Peptides 2002, 23, 709–716. 10.1016/S0196-9781(01)00651-9. [DOI] [PubMed] [Google Scholar]
- May B. C. H.; Abell A. D. α-Methylene Tetrazole-Based Peptidomimetics: Synthesis and Inhibition of HIV Protease. J. Chem. Soc., Perkin Trans. 1 2002, 1 (8), 172–178. 10.1039/B109128J. [DOI] [Google Scholar]
- Dömling A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- Dömling A.; Wang W.; Wang K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugi I.; Meyr R. Isonitrile, V. Erweiterter Anwendungsbereich Der Passerini-Reaktion. Chem. Ber. 1961, 94, 2229–2233. 10.1002/cber.19610940844. [DOI] [Google Scholar]
- Ugi I.; Steinbrückner C. Isonitrile, II. Reaktion von Isonitrilen Mit Carbonylverbindungen, Aminen Und Stickstoffwasserstoffsäure. Chem. Ber. 1961, 94, 734–742. 10.1002/cber.19610940323. [DOI] [Google Scholar]
- Passerini M.; Ragni G. Isonitrili. XIX: Reazioni Con Aldeidi, Acidi e Chetoni. Gazz. Chim. Ital. 1931, 61, 964–969. [Google Scholar]
- Passerini M. Sopra Gli Isonitrili (I). Composto Del p-Isonitrilazobenzolo Con Acetone Ed Acido Acetico. Gazz. Chim. Ital. 1921, 51, 126–129. [Google Scholar]
- Passerini M. Sopra Gli Isonitrili (VII). Reazione Con Chetoni Ciclici in Presenza Di Acidi Organici. Gazz. Chim. Ital. 1923, 53, 410–417. [Google Scholar]
- Ostrovskii V. A.; Popova E. A.; Trifonov R. E.. Developments in Tetrazole Chemistry (2009–2016), Academic Press, 2017; Vol. 123, pp 1–62. [Google Scholar]
- Sadjadi S.; Heravi M. M.; Nazari N. Isocyanide-Based Multicomponent Reactions in the Synthesis of Heterocycles. RSC Adv. 2016, 6, 53203–53272. 10.1039/C6RA02143C. [DOI] [Google Scholar]
- Bode M. L.; Gravestock D.; Rousseau A. L. Synthesis, Reactions and Uses of Isocyanides in Organic Synthesis. An Update. Org. Prep. Proced. Int. 2016, 48, 89–221. 10.1080/00304948.2016.1138072. [DOI] [Google Scholar]
- Sarvary A.; Maleki A. A Review of Syntheses of 1,5-Disubstituted Tetrazole Derivatives. Mol. Diversity 2015, 19, 189–212. 10.1007/s11030-014-9553-3. [DOI] [PubMed] [Google Scholar]
- Maleki A.; Sarvary A. Synthesis of Tetrazoles via Isocyanide-Based Reactions. RSC Adv. 2015, 5, 60938–60955. 10.1039/C5RA11531K. [DOI] [Google Scholar]
- Ostrovskii V. A.; Trifonov R. E.; Popova E. A. Medicinal Chemistry of Tetrazoles. Russ. Chem. Bull. 2012, 61, 768–780. 10.1007/s11172-012-0108-4. [DOI] [Google Scholar]
- Ibarra I. A.; Islas-Jácome A.; González-Zamora E. Synthesis of Polyheterocycles via Multicomponent Reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. 10.1039/C7OB02305G. [DOI] [PubMed] [Google Scholar]
- Berman H. M.; Westbrook J.; Feng Z.; Gilliland G.; Bhat T. N.; Weissig H.; Shindyalov I. N.; Bourne P. E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N.; Shiro Y.; Iwata T.; Iizuka T.; Iwasaki H. Heme Environmental Structure of a Novel Artificial Myoglobin with a Closed Heme Pocket: Site-Specific Chemical Modification Producing Distal N-Tetrazolylhistidine E7 by Cyanogen Bromide and Azide Ion. J. Am. Chem. Soc. 1991, 113, 1826–1829. 10.1021/ja00005a056. [DOI] [Google Scholar]
- Lewis W. S.; Cody V.; Galitsky N.; Luft J. R.; Pangborn W.; Chunduru S. K.; Spencer H. T.; Appleman J. R.; Blakley R. L. Methotrexate-Resistant Variants of Human Dihydrofolate Reductase with Substitutions of Leucine 22: Kinetics, Crystallography, and Potential as Selectable Markers. J. Biol. Chem. 1995, 270, 5057–5064. 10.1074/jbc.270.10.5057. [DOI] [PubMed] [Google Scholar]
- Zechel D. L.; Boraston A. B.; Gloster T.; Boraston C. M.; Macdonald J. M.; Tilbrook D. M. G.; Stick R. V.; Davies G. J. Iminosugar Glycosidase Inhibitors: Structural and Thermodynamic Dissection of the Binding of Isofagomine and 1-Deoxynojirimycin to β-Glucosidases. J. Am. Chem. Soc. 2003, 125, 14313–14323. 10.1021/ja036833h. [DOI] [PubMed] [Google Scholar]
- Kosmopoulou M. N.; Leonidas D. D.; Chrysina E. D.; Bischler N.; Eisenbrand G.; Sakarellos C. E.; Pauptit R.; Oikonomakos N. G. Binding of the Potential Antitumour Agent Indirubin-5-Sulphonate at the Inhibitor Site of Rabbit Muscle Glycogen Phosphorylase B. Eur. J. Biochem. 2004, 271, 2280–2290. 10.1111/j.1432-1033.2004.04173.x. [DOI] [PubMed] [Google Scholar]
- Verdoucq L.; Morinière J.; Bevan D. R.; Esen A.; Vasella A.; Henrissat B.; Czjze M. Structural Determinants of Substrate Specificity in Family 1 β-Glucosidases: Novel Insights from the Crystal Structure of Sorghum Dhurrinase-1, a Plant β-Glucosidase with Strict Specifity, in Complex with Its Natural Substrate. J. Biol. Chem. 2004, 279, 31796–31803. 10.1074/jbc.M402918200. [DOI] [PubMed] [Google Scholar]
- Howard N.; Abell C.; Blakemore W.; Chessari G.; Congreve M.; Howard S.; Jhoti H.; Murray C. W.; Seavers L. C. A.; van Montfort R. L. M. Application of Fragment Screening and Fragment Linking to the Discovery of Novel Thrombin Inhibitors. J. Med. Chem. 2006, 49, 1346–1355. 10.1021/jm050850v. [DOI] [PubMed] [Google Scholar]
- Gloster T. M.; Madsen R.; Davies G. J. Structural Basis for Cyclophellitol Inhibition of a [Small Beta]-Glucosidase. Org. Biomol. Chem. 2007, 5, 444–446. 10.1039/B616590G. [DOI] [PubMed] [Google Scholar]
- Gloster T. M.; Meloncelli P.; Stick R. V.; Zechel D.; Vasella A.; Davies G. J. Glycosidase Inhibition: An Assessment of the Binding of 18 Putative Transition-State Mimics. J. Am. Chem. Soc. 2007, 129, 2345–2354. 10.1021/ja066961g. [DOI] [PubMed] [Google Scholar]
- Aguilar M.; Gloster T. M.; García-Moreno M. I.; Ortiz Mellet C.; Davies G. J.; Llebaria A.; Casas J.; Egido-Gabás M.; García Fernandez J. M. Molecular Basis for β-Glucosidase Inhibition by Ring-Modified Calystegine Analogues. ChemBioChem 2008, 9, 2612–2618. 10.1002/cbic.200800451. [DOI] [PubMed] [Google Scholar]
- Cossu F.; Milani M.; Mastrangelo E.; Vachette P.; Servida F.; Lecis D.; Canevari G.; Delia D.; Drago C.; Rizzo V.; et al. Structural Basis for Bivalent Smac-Mimetics Recognition in the IAP Protein Family. J. Mol. Biol. 2009, 392, 630–644. 10.1016/j.jmb.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Besle A.; Brazzolotto X.; Tatibouet A.; Cerniauskaite D.; Gallienne E.; Rollin P.; Burmeister W. P. A Micromolar O-Sulfated Thiohydroximate Inhibitor Bound to Plant Myrosinase. Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun. 2010, 66, 152–155. 10.1107/S1744309109052865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnute M. E.; O’Brien P. M.; Nahra J.; Morris M.; Howard Roark W.; Hanau C. E.; Ruminski P. G.; Scholten J. A.; Fletcher T. R.; Hamper B. C.; et al. Discovery of (Pyridin-4-Yl)-2H-Tetrazole as a Novel Scaffold to Identify Highly Selective Matrix Metalloproteinase-13 Inhibitors for the Treatment of Osteoarthritis. Bioorg. Med. Chem. Lett. 2010, 20, 576–580. 10.1016/j.bmcl.2009.11.081. [DOI] [PubMed] [Google Scholar]
- Mitchell E. P.; Withers S. G.; Ermert P.; Vasella A. T.; Garman E. F.; Oikonomakos N. G.; Johnson L. N. Ternary Complex Crystal Structures of Glycogen Phosphorylase with the Transition State Analogue Nojirimycin Tetrazole and Phosphate in the T and R States. Biochemistry 1996, 35, 7341–7355. 10.1021/bi960072w. [DOI] [PubMed] [Google Scholar]
- Fradera X.; Kazemier B.; Carswell E.; Cooke A.; Oubrie A.; Hamilton W.; Dempster M.; Krapp S.; Nagel S.; Jestel A. High-Resolution Crystal Structures of Factor XIa Coagulation Factor in Complex with Nonbasic High-Affinity Synthetic Inhibitors. Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun. 2012, 68, 404–408. 10.1107/S1744309112009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. A.; Brassington C.; Breeze A. L.; Caputo A.; Critchlow S.; Davies G.; Goodwin L.; Hassall G.; Greenwood R.; Holdgate G. A.; et al. Design and Synthesis of Novel Lactate Dehydrogenase A Inhibitors by Fragment-Based Lead Generation. J. Med. Chem. 2012, 55, 3285–3306. 10.1021/jm201734r. [DOI] [PubMed] [Google Scholar]
- Matsuo M.; Hasegawa A.; Takano M.; Saito H.; Kakuda S.; Chida T.; Takagi K.; Ochiai E.; Horie K.; Harada Y.; et al. Synthesis of 2α-Heteroarylalkyl Active Vitamin D3 with Therapeutic Effect on Enhancing Bone Mineral Density in Vivo. ACS Med. Chem. Lett. 2013, 4, 671–674. 10.1021/ml400098w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narwal M.; Koivunen J.; Haikarainen T.; Obaji E.; Legala O. E.; Venkannagari H.; Joensuu P.; Pihlajaniemi T.; Lehtio L. Discovery of Tankyrase Inhibiting Flavones with Increased Potency and Isoenzyme Selectivity. J. Med. Chem. 2013, 56, 7880–7889. 10.1021/jm401463y. [DOI] [PubMed] [Google Scholar]
- Chen L.; Wang Z.-G.; Aleshin A. E.; Chen F.; Chen J.; Jiang F.; Alitongbieke G.; Zeng Z.; Ma Y.; Huang M.; et al. Sulindac-Derived RXRα Modulators Inhibit Cancer Cell Growth by Binding to a Novel Site. Chem. Biol. 2014, 21, 596–607. 10.1016/j.chembiol.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M. J.; Bandarage U. K.; Bennett H.; Byrn R. R.; Davies I.; Gu W.; Jacobs M.; Ledeboer M. W.; Ledford B.; Leeman J. R.; et al. Isosteric Replacements of the Carboxylic Acid of Drug Candidate VX-787: Effect of Charge on Antiviral Potency and Kinase Activity of Azaindole-Based Influenza PB2 Inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 1990–1994. 10.1016/j.bmcl.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Cody V.; Galitsky N.; Luft J. R.; Pangborn W.; Rosowsky A.; Blakley R. L. Comparison of Two Independent Crystal Structures of Human Dihydrofolate Reductase Ternary Complexes Reduced with Nicotinamide Adenine Dinucleotide Phosphate and the Very Tight-Binding Inhibitor PT523. Biochemistry 1997, 36, 13897–13903. 10.1021/bi971711l. [DOI] [PubMed] [Google Scholar]
- Cody V.; Galitsky N.; Luft J. R.; Pangborn W.; Blakley R. L.; A G. Comparison of Ternary Crystal Complexes of F31 Variants of Human Dihydrofolate Reductase with NADPH and a Classical Antitumor Furopyrimidine. Anti-Cancer Drug Des. 1998, 13, 307–315. [PubMed] [Google Scholar]
- Heightman T. D.; Vasella A.; Tsitsanou K. E.; Zographos S. E.; Skamnaki V. T.; Oikonomakos N. G. Cooperative Interactions of the Catalytic Nucleophile and the Catalytic Acid in the Inhibition of β-Glycosidases. Calculations and Their Validation by Comparative Kinetic and Structural Studies of the Inhibition of Glycogen Phosphorylase B. Helv. Chim. Acta 1998, 81, 853–864. 10.1002/hlca.19980810507. [DOI] [Google Scholar]
- Cody V.; Galitsky N.; Rak D.; Luft J. R.; Pangborn W.; Queener S. F. Ligand-Induced Conformational Changes in the Crystal Structures of Pneumocystis Carinii Dihydrofolate Reductase Complexes with Folate and NADP+. Biochemistry 1999, 38, 4303–4312. 10.1021/bi982728m. [DOI] [PubMed] [Google Scholar]
- Burmeister W. P.; Cottaz S.; Rollin P.; Vasella A.; Henrissat B. High Resolution X-Ray Crystallography Shows That Ascorbate Is a Cofactor for Myrosinase and Substitutes for the Function of the Catalytic Base. J. Biol. Chem. 2000, 275, 39385–39393. 10.1074/jbc.M006796200. [DOI] [PubMed] [Google Scholar]
- Juers D. H.; Heightman T. D.; Vasella A.; McCarter J. D.; Mackenzie L.; Withers S. G.; Matthews B. W. A Structural View of the Action of Escherichia Coli (LacZ) β-Galactosidase. Biochemistry 2001, 40, 14781–14794. 10.1021/bi011727i. [DOI] [PubMed] [Google Scholar]
- Oikonomakos N. G.; Zographos S. E.; Skamnaki V. T.; Archontis G. The 1.76 Å Resolution Crystal Structure of Glycogen Phosphorylase b Complexed with Glucose, and CP320626, a Potential Antidiabetic Drug. Bioorg. Med. Chem. 2002, 10, 1313–1319. 10.1016/S0968-0896(01)00394-7. [DOI] [PubMed] [Google Scholar]
- Nichols D. A.; Jaishankar P.; Larson W.; Smith E.; Liu G.; Beyrouthy R.; Bonnet R.; Renslo A. R.; Chen Y. Structure-Based Design of Potent and Ligand-Efficient Inhibitors of CTX-M Class A β-Lactamase. J. Med. Chem. 2012, 55, 2163–2172. 10.1021/jm2014138. [DOI] [PubMed] [Google Scholar]
- Toney J. H.; Fitzgerald P. M. D.; Grover-Sharma N.; Olson S. H.; May W. J.; Sundelof J. G.; Vanderwall D. E.; Cleary K. A.; Grant S. K.; Wu J. K.; et al. Antibiotic Sensitization Using Biphenyl Tetrazoles as Potent Inhibitors of Bacteroides Fragilis Metallo-β-Lactamase. Chem. Biol. 1998, 5, 185–196. 10.1016/S1074-5521(98)90632-9. [DOI] [PubMed] [Google Scholar]
- Jnoff E.; Albrecht C.; Barker J. J.; Barker O.; Beaumont E.; Bromidge S.; Brookfield F.; Brooks M.; Bubert C.; Ceska T.; et al. Binding Mode and Structure-Activity Relationships around Direct Inhibitors of the Nrf2-Keap1 Complex. ChemMedChem 2014, 9, 699–705. 10.1002/cmdc.201300525. [DOI] [PubMed] [Google Scholar]
- Wilson A. J.; Kerns J. K.; Callahan J. F.; Moody C. J. Keap Calm, and Carry on Covalently. J. Med. Chem. 2013, 56, 7463–7476. 10.1021/jm400224q. [DOI] [PubMed] [Google Scholar]
- Stebbins J. L.; Santelli E.; Feng Y.; De S. K.; Purves A.; Motamedchaboki K.; Wu B.; Ronai Z. A.; Liddington R. C.; Pellecchia M. Structure-Based Design of Covalent Siah Inhibitors. Chem. Biol. 2013, 20, 973–982. 10.1016/j.chembiol.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B.; Huang Z.; Zhang M.; Dillard D. R.; Ji H. Rational Design of Small-Molecule Inhibitors for β-Catenin/T-Cell Factor Protein–Protein Interactions by Bioisostere Replacement. ACS Chem. Biol. 2013, 8, 524–529. 10.1021/cb300564v. [DOI] [PubMed] [Google Scholar]
- Sampietro J.; Dahlberg C. L.; Cho U. S.; Hinds T. R.; Kimelman D.; Xu W. Crystal Structure of a β-Catenin/BCL9/Tcf4 Complex. Mol. Cell 2006, 24, 293–300. 10.1016/j.molcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Roh J.; Vavrova K.; Hrabalek A. Synthesis and Functionalization of 5-Substituted Tetrazoles. Eur. J. Org. Chem. 2012, 2012, 6101–6118. 10.1002/ejoc.201200469. [DOI] [Google Scholar]
- Koldobskii G. I. Strategies and Prospects in Functionalization of Tetrazoles. Russ. J. Org. Chem. 2006, 42, 469–486. 10.1134/S1070428006040014. [DOI] [Google Scholar]
- Koldobskii G. I.; Ostrovskii V. A. Tetrazoles. Russ. Chem. Rev. 1994, 63, 797–814. 10.1070/RC1994v063n10ABEH000119. [DOI] [Google Scholar]
- Wittenberger S. J. Recent Developments in Tetrazole Chemistry. A Review. Org. Prep. Proced. Int. 1994, 26, 499–531. 10.1080/00304949409458050. [DOI] [Google Scholar]
- Bode M. L.; Gravestock D.; Rousseau A. L. Synthesis, Reactions and Uses of Isocyanides in Organic Synthesis. An Update. Org. Prep. Proced. Int. 2016, 48, 89–221. 10.1080/00304948.2016.1138072. [DOI] [Google Scholar]
- Ostrovskii V. A.; Popova E. A.; Trifonov R. E.. Developments in Tetrazole Chemistry (2009–2016); Elsevier Ltd, 2017. [Google Scholar]
- Meyee V.; Jacobsen P.. Lehrbuch Der Organischen Chemie, 2nd ed.; Walter De Gruyter and Company: Berlin, Leipzig, 1867; Vol. 3. [Google Scholar]
- Terrett N. K. PlumX. Comb. Chem. Online 2014, 16, 25–28. 10.1016/j.comche.2014.06.001. [DOI] [Google Scholar]
- Gutmann B.; Roduit J.-P.; Roberge D.; Kappe C. O. Synthesis of 5-Substituted 1H-Tetrazoles from Nitriles and Hydrazoic Acid by Using a Safe and Scalable High-Temperature Microreactor Approach. Angew. Chem., Int. Ed. 2010, 49, 7101–7105. 10.1002/anie.201003733. [DOI] [PubMed] [Google Scholar]
- Luther A.; Moehle K.; Chevalier E.; Dale G.; Obrecht D. Protein Epitope Mimetic Macrocycles as Biopharmaceuticals. Curr. Opin. Chem. Biol. 2017, 38, 45–51. 10.1016/j.cbpa.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Misaki Y. Tetrathiapentalene-Based Organic Conductors. Sci. Technol. Adv. Mater. 2009, 10, 024301 10.1088/1468-6996/10/2/024301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanessian S.; Deschênes-Simard B.; Simard D. Exploring the Unique Reactivities of Heterobicyclic Tetrazoles—access to Functionally Diverse and Versatile Heterocyclic Scaffolds. Tetrahedron 2009, 65, 6656–6669. 10.1016/j.tet.2009.06.033. [DOI] [Google Scholar]
- Ek F.; Wistrand L.-G.; Frejd T. Synthesis of Fused Tetrazole- and Imidazole Derivatives via Iodocyclization. Tetrahedron 2003, 59, 6759–6769. 10.1016/S0040-4020(03)00818-4. [DOI] [Google Scholar]
- Hernández A. S.; Cheng P. T. W.; Musial C. M.; Swartz S. G.; George R. J.; Grover G.; Slusarchyk D.; Seethala R. K.; Smith M.; Dickinson K.; et al. Discovery, Synthesis, and Structure–activity Studies of Tetrazole Based Growth Hormone Secretagogues. Bioorg. Med. Chem. Lett. 2007, 17, 5928–5933. 10.1016/j.bmcl.2007.07.099. [DOI] [PubMed] [Google Scholar]
- García Mancheño O.; Bolm C. Synthesis of N-(1H)-Tetrazole Sulfoximines. Org. Lett. 2007, 9, 2951–2954. 10.1021/ol071302+. [DOI] [PubMed] [Google Scholar]
- Burg D.; Hameetman L.; Filippov D. V; Van Der Marel A.; Mulder G. J. Inhibition of Glutathione S-Transferase in Rat Hepatocytes by a Glycine-Tetrazole Modified S-Alkyl–GSH Analogue. Bioorg. Med. Chem. Lett. 2002, 12, 1579–1582. 10.1016/S0960-894X(02)00247-0. [DOI] [PubMed] [Google Scholar]
- Clémençon I. F.; Ganem B. Tandem Multicomponent/Click Reactions: Synthesis of Functionalized Oxazoles and Tetrazoles from Acyl Cyanides. Tetrahedron 2007, 63, 8665–8669. 10.1016/j.tet.2007.03.127. [DOI] [Google Scholar]
- Hantzsch A.; Vagt A. Ueber Das Sogenannte Diazoguanidin. Justus Liebigs Ann. Chem. 1901, 314, 339–369. 10.1002/jlac.19013140307. [DOI] [Google Scholar]
- Schilling C.; Jung N.; Bräse S.. Cycloaddition Reactions with Azides: An Overview. In Organic Azides; John Wiley & Sons, Ltd, 2010; pp 269–284. [Google Scholar]
- Ess D. H.; Jones G. O.; Houk K. N. Conceptual, Qualitative, and Quantitative Theories of 1,3-Dipolar and Diels–Alder Cycloadditions Used in Synthesis. Adv. Synth. Catal. 2006, 348, 2337–2361. 10.1002/adsc.200600431. [DOI] [Google Scholar]
- Matthews D. P.; Green J. E.; Shuker A. J. Parallel Synthesis of Alkyl Tetrazole Derivatives Using Solid Support Chemistry. J. Comb. Chem. 2000, 2, 19–23. 10.1021/cc990035z. [DOI] [PubMed] [Google Scholar]
- Savegnago L.; do Sacramento M.; Brod L. M. P.; Fronza M. G.; Seus N.; Lenardao E. J.; Paixao M. W.; Alves D. Phenylselanyl-1H-1,2,3-Triazole-4-Carbonitriles: Synthesis, Antioxidant Properties and Use as Precursors to Highly Functionalized Tetrazoles. RSC Adv. 2016, 6, 8021–8031. 10.1039/C5RA22445D. [DOI] [Google Scholar]
- Demko Z. P.; Sharpless K. B. A Click Chemistry Approach to Tetrazoles by Huisgen 1,3-Dipolar Cycloaddition: Synthesis of 5-Sulfonyl Tetrazoles from Azides and Sulfonyl Cyanides. Angew. Chem., Int. Ed. 2002, 41, 2110–2113. . [DOI] [PubMed] [Google Scholar]
- Himo F.; Demko Z. P.; Noodleman L.; Sharpless K. B. Why Is Tetrazole Formation by Addition of Azide to Organic Nitriles Catalyzed by Zinc(II) Salts?. J. Am. Chem. Soc. 2003, 125, 9983–9987. 10.1021/ja030204q. [DOI] [PubMed] [Google Scholar]
- Himo F.; Demko Z. P.; Noodleman L.; Sharpless K. B. Mechanisms of Tetrazole Formation by Addition of Azide to Nitriles. J. Am. Chem. Soc. 2002, 124, 12210–12216. 10.1021/ja0206644. [DOI] [PubMed] [Google Scholar]
- Demko Z. P.; Sharpless K. B. A Click Chemistry Approach to Tetrazoles by Huisgen 1,3-Dipolar Cycloaddition: Synthesis of 5-Acyltetrazoles from Azides and Acyl Cyanides. Angew. Chem., Int. Ed. 2002, 41, 2113–2116. . [DOI] [PubMed] [Google Scholar]
- Demko Z. P.; Sharpless K. B. An Intramolecular [2 + 3] Cycloaddition Route to Fused 5-Heterosubstituted Tetrazoles. Org. Lett. 2001, 3, 4091–4094. 10.1021/ol010220x. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Patil P.; Dömling A. Easy Synthesis of Two Positional Isomeric Tetrazole Libraries. Synthesis 2016, 48, 3701–3712. 10.1055/s-0035-1562435. [DOI] [Google Scholar]
- Wang K.; Nguyen K.; Huang Y.; Dömling A. Cyanoacetamide Multicomponent Reaction (I): Parallel Synthesis of Cyanoacetamides. J. Comb. Chem. 2009, 11, 920–927. 10.1021/cc9000778. [DOI] [PubMed] [Google Scholar]
- Oliveri-Mandala E.; Alagna B. Reasioni Con Isonitrili Ezidi. Gazz. Chem. Ital. 1910, 40, 441–448. [Google Scholar]
- Neochoritis C. G.; Stotani S.; Mishra B.; Dömling A. Efficient Isocyanide-Less Isocyanide-Based Multicomponent Reactions. Org. Lett. 2015, 17, 2002–2005. 10.1021/acs.orglett.5b00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduskrasts K. Routes to Labeled Tetrazoles (Microreview). Chem. Heterocycl. Compd. 2016, 52, 533–534. 10.1007/s10593-016-1924-5. [DOI] [Google Scholar]
- Verma F.; Sahu A.; Singh P. K.; Rai A.; Singh M.; Rai V. K. Visible-Light Driven Regioselective Synthesis of 1 H -Tetrazoles from Aldehydes through Isocyanide-Based [3 + 2] Cycloaddition. Green Chem. 2018, 20, 3783–3789. 10.1039/C8GC01321G. [DOI] [Google Scholar]
- Dömling A.; Beck B.; Fuchs T.; Yazbak A. Parallel Synthesis of Arrays of Amino-Acid-Derived Isocyanoamides Useful As Starting Materials in IMCR. J. Comb. Chem. 2006, 8, 872–880. 10.1021/cc060068w. [DOI] [PubMed] [Google Scholar]
- Al-Hourani B. J.; Sharma S. K.; Mane J. Y.; Tuszynski J.; Baracos V.; Kniess T.; Suresh M.; Pietzsch J.; Wuest F. Synthesis and Evaluation of 1,5-Diaryl-Substituted Tetrazoles as Novel Selective Cyclooxygenase-2 (COX-2) Inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 1823–1826. 10.1016/j.bmcl.2011.01.057. [DOI] [PubMed] [Google Scholar]
- Jawabrah Al-Hourani B.; Al-Awaida W.; Matalka K. Z.; El-Barghouthi M. I.; Alsoubani F.; Wuest F. Structure-Activity Relationship of Novel Series of 1,5-Disubstituted Tetrazoles as Cyclooxygenase-2 Inhibitors: Design, Synthesis, Bioassay Screening and Molecular Docking Studies. Bioorg. Med. Chem. Lett. 2016, 26, 4757–4762. 10.1016/j.bmcl.2016.08.034. [DOI] [PubMed] [Google Scholar]
- Badder E. W. Moderne Ergebnisse Der Silikose Forschung. Angew. Chem. 1959, 71, 373–388. [Google Scholar]
- Hulme C.; Bienaymé H.; Nixey T.; Chenera B.; Jones W.; Tempest P.; Smith A. L. Library Generation via Postcondensation Modifications of Isocyanide-Based Multicomponent Reactions. Methods Enzymol. 2003, 369, 469–496. 10.1016/S0076-6879(03)69024-5. [DOI] [PubMed] [Google Scholar]
- Thompson M. J.; Chen B. Ugi Reactions with Ammonia Offer Rapid Access to a Wide Range of 5-Aminothiazole and Oxazole Derivatives. J. Org. Chem. 2009, 74, 7084–7093. 10.1021/jo9014529. [DOI] [PubMed] [Google Scholar]
- Balalaie S.; Motaghedi H.; Tahmassebi D.; Bararjanian M.; Bijanzadeh H. R. A Facile and Efficient Synthesis of 2,2,2-Trifluoroethyl 2-[(E)-N-Phenylcinnamamido]-2-Phenylacetates in Trifluoroethanol via Sequential Ugi Four-Component Reaction/Esterification. Tetrahedron Lett. 2012, 53, 6177–6181. 10.1016/j.tetlet.2012.08.096. [DOI] [Google Scholar]
- Cristau P.; Vors J.-P.; Zhu J. A Rapid Access to Biaryl Ether Containing Macrocycles by Pairwise Use of Ugi 4CR and Intramolecular SNAr-Based Cycloetherification. Org. Lett. 2001, 3, 4079–4082. 10.1021/ol0168420. [DOI] [PubMed] [Google Scholar]
- Hebach C.; Kazmaier U. Via Ugi Reactions to Conformationally Fixed Cyclic Peptides. Chem. Commun. 2003, (5), 596–597. 10.1039/b210952b. [DOI] [PubMed] [Google Scholar]
- Pharande S. G.; Corrales Escobosa A. R.; Gamez-Montano R. Endogenous Water-Triggered and Ultrasound Accelerated Synthesis of 1,5-Disubstituted Tetrazoles via a Solvent and Catalyst-Free Ugi-Azide Reaction. Green Chem. 2017, 19, 1259–1262. 10.1039/C6GC03324E. [DOI] [Google Scholar]
- Zhao T.; Boltjes A.; Herdtweck E.; Dömling A. Tritylamine as an Ammonia Surrogate in the Ugi Tetrazole Synthesis. Org. Lett. 2013, 15, 639–641. 10.1021/ol303348m. [DOI] [PubMed] [Google Scholar]
- Zarganes-Tzitzikas T.; Patil P.; Khoury K.; Herdtweck E.; Dömling A. Concise Synthesis of Tetrazole-Ketopiperazines by Two Consecutive Ugi Reactions. Eur. J. Org. Chem. 2015, 2015, 51–55. 10.1002/ejoc.201403401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivar U.; Rudolf M.. Process for Preparing Certain Tetrazoles. U.S. Patent US3065241A, 1962.
- Wu R.; Gao S.; Chen X.; Yang G.; Pan L.; Hu G.; Jia P.; Zhong W.; Yu C. Synthesis of 1-(1 H -Tetrazol-5-Yl)-2 H -Isoindole Derivatives through Ugi Four-Component and Silver-Catalyzed Reactions. Eur. J. Org. Chem. 2014, 2014, 3379–3386. 10.1002/ejoc.201402098. [DOI] [Google Scholar]
- Davenport A. J.; Stimson C. C.; Corsi M.; Vaidya D.; Glenn E.; Jones T. D.; Bailey S.; Gemkow M. J.; Fritz U.; Hallett D. J. Discovery of Substituted Benzyl Tetrazoles as Histamine H3 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 5165–5169. 10.1016/j.bmcl.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Schaffert E. S.; Höfner G.; Wanner K. T. Aminomethyltetrazoles as Potential Inhibitors of the γ-Aminobutyric Acid Transporters MGAT1–mGAT4: Synthesis and Biological Evaluation. Bioorg. Med. Chem. 2011, 19, 6492–6504. 10.1016/j.bmc.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Gunawan S.; Ayaz M.; De Moliner F.; Frett B.; Kaiser C.; Patrick N.; Xu Z.; Hulme C. Synthesis of Tetrazolo-Fused Benzodiazepines and Benzodiazepinones by a Two-Step Protocol Using an Ugi-Azide Reaction for Initial Diversity Generation. Tetrahedron 2012, 68, 5606–5611. 10.1016/j.tet.2012.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukulula M.; Little S.; Gut J.; Rosenthal P. J.; Wan B.; Franzblau S. G.; Chibale K. The Design, Synthesis, in Silico ADME Profiling, Antiplasmodial and Antimycobacterial Evaluation of New Arylamino Quinoline Derivatives. Eur. J. Med. Chem. 2012, 57, 259–267. 10.1016/j.ejmech.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Tukulula M.; Njoroge M.; Mugumbate G. C.; Gut J.; Rosenthal P. J.; Barteau S.; Streckfuss J.; Heudi O.; Kameni-Tcheudji J.; Chibale K. Tetrazole-Based Deoxyamodiaquines: Synthesis, ADME/PK Profiling and Pharmacological Evaluation as Potential Antimalarial Agents. Bioorg. Med. Chem. 2013, 21, 4904–4913. 10.1016/j.bmc.2013.06.067. [DOI] [PubMed] [Google Scholar]
- Chauhan K.; Sharma M.; Trivedi P.; Chaturvedi V.; Chauhan P. M. S. New Class of Methyl Tetrazole Based Hybrid of (Z)-5-Benzylidene-2-(Piperazin-1-Yl)Thiazol-4(%H)-One as Potent Antitubercular Agents. Bioorg. Med. Chem. Lett. 2014, 24, 4166–4170. 10.1016/j.bmcl.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Chauhan K.; Singh P.; Kumar V.; Shukla P. K.; Siddiqi M. I.; Chauhan P. M. S. Investigation of Ugi-4CC Derived 1H-Tetrazol-5-Yl-(Aryl) Methyl Piperazinyl-6-Fluoro-4-Oxo-1,4-Dihydroquinoline-3-Carboxylic Acid: Synthesis, Biology and 3D-QSAR Analysis. Eur. J. Med. Chem. 2014, 78, 442–454. 10.1016/j.ejmech.2014.03.069. [DOI] [PubMed] [Google Scholar]
- Pick R.; Bauer M.; Kazmaier U.; Hebach C. Ammonia in Ugi Reactions - Four-Component versus Six-Component Couplings. Synlett 2005, (5), 0757–0760. 10.1055/s-2005-863722. [DOI] [Google Scholar]
- Kazmaier U.; Hebach C. Peptide Syntheses via Ugi Reactions with Ammonia. Synlett 2003, (11), 1591–1594. 10.1055/s-2003-40987. [DOI] [Google Scholar]
- Sung K.; Chen F.-L.; Huang P.-C. A Modified U-4CR Reaction with 2-Nitrobenzylamine as an Ammonia Equivalent. Synlett 2006, 2006 (16), 2667–2669. 10.1055/s-2006-951485. [DOI] [Google Scholar]
- Ghosh A. K.; Leshchenko-Yashchuk S.; Anderson D. D.; Baldridge A.; Noetzel M.; Miller H. B.; Tie Y.; Wang Y.-F.; Koh Y.; Weber I. T.; et al. Design of HIV-1 Protease Inhibitors with Pyrrolidinones and Oxazolidinones as Novel P1′-Ligands To Enhance Backbone-Binding Interactions with Protease: Synthesis, Biological Evaluation, and Protein–Ligand X-Ray Studies. J. Med. Chem. 2009, 52, 3902–3914. 10.1021/jm900303m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaccini S.; Torroba T. The Use of the Ugi Four-Component Condensation. Nat. Protoc. 2007, 2, 632–639. 10.1038/nprot.2007.71. [DOI] [PubMed] [Google Scholar]
- Zarezin D. P.; Khrustalev V. N.; Nenajdenko V. G. Diastereoselectivity of Azido-Ugi Reaction with Secondary Amines. Stereoselective Synthesis of Tetrazole Derivatives. J. Org. Chem. 2017, 82, 6100–6107. 10.1021/acs.joc.7b00611. [DOI] [PubMed] [Google Scholar]
- Ugi I. Neuere Methoden Der Präparativen Organischen Chemie IV Mit Sekundär-Reaktionen Gekoppelte α-Additionen von Immonium-Ionen Und Anionen an Isonitrile. Angew. Chem. 1962, 74, 9–22. 10.1002/ange.19620740103. [DOI] [Google Scholar]
- Patil P.; Khoury K.; Herdtweck E.; Dömling A. A Universal Isocyanide for Diverse Heterocycle Syntheses. Org. Lett. 2014, 16, 5736–5739. 10.1021/ol5024882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban S.; Abdel-Wahab B. F. Groebke-Blackburn-Bienayme Multicomponent Reaction: Emerging Chemistry for Drug Discovery. Mol. Diversity 2016, 20, 233–254. 10.1007/s11030-015-9602-6. [DOI] [PubMed] [Google Scholar]
- Pepino R.; Bossio R.; Marcaccini S.; Torroba T. Studies on Isocyanides and Related Compounds. A Facile Synthesis of 1-Substituted 3-Cyano-2-Methoxy-3-Phenylpyrroles. Heterocycles 1999, 50, 463–467. 10.3987/COM-98-S(H)16. [DOI] [Google Scholar]
- Zinner G.; Bock W. Notiz Über Die Ugi-Reaktion Mit Diaziridinen. Arch. Pharm. (Weinheim, Ger.) 1973, 306, 94–96. 10.1002/ardp.19733060204. [DOI] [PubMed] [Google Scholar]
- Zinner G.; Moderhack D.; Hantelmann O.; Bock W. Hydroxylamine in Der Vierkomponenten-Kondensation NachUgi, II. Chem. Ber. 1974, 107, 2947–2955. 10.1002/cber.19741070918. [DOI] [Google Scholar]
- Patil P.; de Haan M.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Versatile Protecting-Group Free Tetrazolomethane Amine Synthesis by Ugi Reaction. ACS Comb. Sci. 2016, 18, 170–175. 10.1021/acscombsci.5b00189. [DOI] [PubMed] [Google Scholar]
- Dömling A.; Beck B.; Magnin-Lachaux M. 1-Isocyanomethylbenzotriazole and 2,2,4,4-Tetramethylbutylisocyanide-Cleavable Isocyanides Useful for the Preparation of α-Aminomethyl Tetrazoles. Tetrahedron Lett. 2006, 47, 4289–4291. 10.1016/j.tetlet.2006.04.026. [DOI] [Google Scholar]
- Walborsky H. M.; Niznik G. E. Synthesis of Isonitriles. J. Org. Chem. 1972, 37, 187–190. 10.1021/jo00967a005. [DOI] [Google Scholar]
- Patil P.; Mishra B.; Sheombarsing G.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Library-to-Library Synthesis of Highly Substituted α-Aminomethyl Tetrazoles via Ugi Reaction. ACS Comb. Sci. 2018, 20, 70–74. 10.1021/acscombsci.7b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezanpour S.; Balalaie S.; Rominger F.; Alavijeh N. S.; Bijanzadeh H. R. Facile, Efficient and Diastereoselective Synthesis of α-Hydrazine Tetrazoles through a Novel One-Pot Four-Component Reaction. Tetrahedron 2013, 69, 10718–10723. 10.1016/j.tet.2013.10.062. [DOI] [Google Scholar]
- Nikbakht A.; Ramezanpour S.; Balalaie S.; Rominger F. Efficient and Stereoselective Synthesis of α-Hydrazino Tetrazoles through a Pseudo Five-Component Domino Reaction. Tetrahedron 2015, 71, 6790–6795. 10.1016/j.tet.2015.07.037. [DOI] [Google Scholar]
- Patil P.; Zhang J.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Hydrazine in the Ugi Tetrazole Reaction. Synthesis 2016, 48, 1122–1130. 10.1055/s-0035-1561353. [DOI] [Google Scholar]
- Ugi I.; Rosendahl F. K. Isonitrile, XV. Δ1-Cyclohexenyl-Isocyanid. Justus Liebigs Ann. Chem. 1963, 666, 65–67. 10.1002/jlac.19636660109. [DOI] [Google Scholar]
- Keating T. A.; Armstrong R. W. Molecular Diversity via a Convertible Isocyanide in the Ugi Four-Component Condensation. J. Am. Chem. Soc. 1995, 117, 7842–7843. 10.1021/ja00134a044. [DOI] [Google Scholar]
- Spallarossa M.; Wang Q.; Riva R.; Zhu J. Synthesis of Vinyl Isocyanides and Development of a Convertible Isonitrile. Org. Lett. 2016, 18, 1622–1625. 10.1021/acs.orglett.6b00483. [DOI] [PubMed] [Google Scholar]
- van der Heijden G.; Sjaak Jong J. A. W.; Ruijter E.; Orru R. V. A. 2-Bromo-6-Isocyanopyridine as a Universal Convertible Isocyanide for Multicomponent Chemistry. Org. Lett. 2016, 18, 984–987. 10.1021/acs.orglett.6b00091. [DOI] [PubMed] [Google Scholar]
- Schöllkopf U.; Porsch P.-H.; Lau H.-H. Synthesen Mit α-Metallierten Isocyaniden, XLIV. Notiz Über β-Dimethylamino-α-Isocyanacrylsäureester Und Ihre Verwendung in Der Heterocyclenchemie. Liebigs Ann. der Chemie 1979, 1979 (9), 1444–1446. 10.1002/jlac.197919790918. [DOI] [Google Scholar]
- Gilley C. B.; Buller M. J.; Kobayashi Y. New Entry to Convertible Isocyanides for the Ugi Reaction and Its Application to the Stereocontrolled Formal Total Synthesis of the Proteasome Inhibitor Omuralide. Org. Lett. 2007, 9, 3631–3634. 10.1021/ol701446y. [DOI] [PubMed] [Google Scholar]
- Mayer J.; Umkehrer M.; Kalinski C.; Ross G.; Kolb J.; Burdack C.; Hiller W. New Cleavable Isocyanides for the Combinatorial Synthesis of α-Amino Acid Analogue Tetrazoles. Tetrahedron Lett. 2005, 46, 7393–7396. 10.1016/j.tetlet.2005.08.101. [DOI] [Google Scholar]
- Kroon E.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Cleavable β-Cyanoethyl Isocyanide in the Ugi Tetrazole Reaction. Org. Lett. 2016, 18, 4762–4765. 10.1021/acs.orglett.6b01826. [DOI] [PubMed] [Google Scholar]
- Chandgude A. L.; Li J.; Dömling A. 2-Nitrobenzyl Isocyanide as a Universal Convertible Isocyanide. Asian J. Org. Chem. 2017, 6, 798–801. 10.1002/ajoc.201700177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnikovich A. I.; Levchik S. V.; Balabanovich A. I.; Ivashkevich O. A.; Gaponik P. N. The Thermal Decomposition of Tetrazoles. Thermochim. Acta 1992, 200, 427–441. 10.1016/0040-6031(92)85135-I. [DOI] [Google Scholar]
- Huisgen R.; Sauer J.; Sturm H. J. Acylierung 5-Substitutierter Tetrazole Zu 1.3.4-Oxdiazolen. Angew. Chem. 1958, 70, 272–273. 10.1002/ange.19580700918. [DOI] [Google Scholar]
- Huisgen R.; Sauer J.; Sturm H. J.; Markgraf J. H. Ringöffnungen Der Azole, II. Die Bildung von 1.3.4-Oxdiazolen Bei Der Acylierung 5-Substituierter Tetrazole. Chem. Ber. 1960, 93, 2106–2124. 10.1002/cber.19600930932. [DOI] [Google Scholar]
- El Kaïm L.; Grimaud L.; Pravin P. Lewis Acid Mediated Fragmentation of Tetrazoles towards Triazoles. Eur. J. Org. Chem. 2013, 2013 (22), 4752–4755. 10.1002/ejoc.201300620. [DOI] [Google Scholar]
- Safa K. D.; Shokri T.; Abbasi H.; Teimuri-Mofrad R. One-Pot Synthesis of New 1,5-Disubstituted Tetrazoles Bearing 2,2-Bis(Trimethylsilyl)Ethenyl Groups via The Ugi Four-Component Condensation Reaction Catalyzed by MgBr2·2Et2O. J. Heterocycl. Chem. 2014, 51, 80–84. 10.1002/jhet.1858. [DOI] [Google Scholar]
- Amanpour T.; Mirzaei P.; Bazgir A. Isocyanide-Based Four-Component Synthesis of Ferrocenyl 1,5-Disubstituted Tetrazoles. Tetrahedron Lett. 2012, 53, 1421–1423. 10.1016/j.tetlet.2012.01.038. [DOI] [Google Scholar]
- Battelle L. F.; Bau R.; Gokel G. W.; Oyakawa R. T.; Ugi I. K. Stereoselective Synthesis. VIII. Absolute Configuration of a 1,2-Disubstituted Ferrocene Derivative with Planar and Central Elements of Chirality and the Mechanism of the Optically Active Alpha-Ferrocenyl Tertiary Amines. J. Am. Chem. Soc. 1973, 95, 482–486. 10.1021/ja00783a030. [DOI] [Google Scholar]
- Passani M. B.; Lin J.-S.; Hancock A.; Crochet S.; Blandina P. The Histamine H3 Receptor as a Novel Therapeutic Target for Cognitive and Sleep Disorders. Trends Pharmacol. Sci. 2004, 25, 618–625. 10.1016/j.tips.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Shaaban S.; Negm A.; Ashmawy A. M.; Ahmed D. M.; Wessjohann L. A. Combinatorial Synthesis, in Silico, Molecular and Biochemical Studies of Tetrazole-Derived Organic Selenides with Increased Selectivity against Hepatocellular Carcinoma. Eur. J. Med. Chem. 2016, 122, 55–71. 10.1016/j.ejmech.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Cortes-García C. J.; Islas-Jácome A.; Rentería-Gómez A.; Gámez-Montaño R. Synthesis of 1,5-Disubstituted Tetrazoles Containing a Fragment of the Anticancer Drug Imatinib via a Microwave-Assisted Ugi-Azide Reaction. Monatsh. Chem. 2016, 147, 1277–1290. 10.1007/s00706-016-1686-x. [DOI] [Google Scholar]
- Lane D. P. P53, Guardian of the Genome. Nature 1992, 358, 15–16. 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J. P53, the Cellular Gatekeeper for Growth and Division. Cell 1997, 88, 323–331. 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Estrada-Ortiz N.; Neochoritis C. G.; Dömling A. How To Design a Successful P53-MDM2/X Interaction Inhibitor: A Thorough Overview Based on Crystal Structures. ChemMedChem 2016, 11, 757–772. 10.1002/cmdc.201500487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmiak E.; Neochoritis C. G.; Musielak B.; Twarda-Clapa A.; Kurpiewska K.; Dubin G.; Camacho C.; Holak T. A.; Dömling A. Rational Design and Synthesis of 1,5-Disubstituted Tetrazoles as Potent Inhibitors of the MDM2-P53 Interaction. Eur. J. Med. Chem. 2017, 126, 384–407. 10.1016/j.ejmech.2016.11.029. [DOI] [PubMed] [Google Scholar]
- McEntee W. J.; Crook T. H. Glutamate: Its Role in Learning, Memory, and the Aging Brain. Psychopharmacology (Berl). 1993, 111, 391–401. 10.1007/BF02253527. [DOI] [PubMed] [Google Scholar]
- Weiler I. J.; Hawrylak N.; Greenough W. T. Morphogenesis in Memory Formation: Synaptic and Cellular Mechanisms. Behav. Brain Res. 1995, 66, 1–6. 10.1016/0166-4328(94)00116-W. [DOI] [PubMed] [Google Scholar]
- Kortagere S.; Mortensen O. V.; Xia J.; Lester W.; Fang Y.; Srikanth Y.; Salvino J. M.; Fontana A. C. K. Identification of Novel Allosteric Modulators of Glutamate Transporter EAAT2. ACS Chem. Neurosci. 2018, 9, 522–534. 10.1021/acschemneuro.7b00308. [DOI] [PubMed] [Google Scholar]
- Fan X.; Zhang X.; Bories C.; Loiseau P. M.; Torrence P. F. The Ugi Reaction in the Generation of New Nucleosides as Potential Antiviral and Antileishmanial Agents. Bioorg. Chem. 2007, 35, 121–136. 10.1016/j.bioorg.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Wang S.-X.; Fang Z.; Fan Z.-J.; Wang D.; Li Y.-D.; Ji X.-T.; Hua X.-W.; Huang Y.; Kalinina T. A.; Bakulev V. A.; et al. Synthesis of Tetrazole Containing 1,2,3-Thiadiazole Derivatives via U-4CR and Their Anti-TMV Activity. Chin. Chem. Lett. 2013, 24, 889–892. 10.1016/j.cclet.2013.05.026. [DOI] [Google Scholar]
- Greenwood B. M.; Bojang K.; Whitty C. J. M.; Targett G. A. T. Malaria. Lancet 2005, 365, 1487–1498. 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- Tukulula M.; Sharma R.-K.; Meurillon M.; Mahajan A.; Naran K.; Warner D.; Huang J.; Mekonnen B.; Chibale K. Synthesis and Antiplasmodial and Antimycobacterial Evaluation of New Nitroimidazole and Nitroimidazooxazine Derivatives. ACS Med. Chem. Lett. 2013, 4, 128–131. 10.1021/ml300362a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukulula M.; Njoroge M.; Abay E. T.; Mugumbate G. C.; Wiesner L.; Taylor D.; Gibhard L.; Norman J.; Swart K. J.; Gut J.; et al. Synthesis and in Vitro and in Vivo Pharmacological Evaluation of New 4-Aminoquinoline-Based Compounds. ACS Med. Chem. Lett. 2013, 4, 1198–1202. 10.1021/ml400311r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S.; Agarwal P.; Srivastava K.; RajaKumar S.; Puri S. K.; Verma P.; Saxena J. K.; Sharma A.; Lal J.; Chauhan P. M. S. Synthesis and Bioevaluation of Novel 4-Aminoquinoline-Tetrazole Derivatives as Potent Antimalarial Agents. Eur. J. Med. Chem. 2013, 66, 69–81. 10.1016/j.ejmech.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Shinde A. H.; Archith N.; Malipatel S.; Sharada D. S. A Facile One-Pot Protocol for the Synthesis of Tetrazolyl-Tetrahydroisoquinolines via Novel Domino Intramolecular Cyclization/Ugi-Azide Sequence. Tetrahedron Lett. 2014, 55, 6821–6826. 10.1016/j.tetlet.2014.10.076. [DOI] [Google Scholar]
- Reddy B. V. S.; Kota K.; Rao B. M.; Sridhar B.; Mukkanti K. Four-Component, Five-Centered, One-Pot Synthesis of 1-(1H-Tetrazol-5-Yl)-2,3,4,9-Tetrahydro-1H-Pyrido[3,4-b]Indole Derivatives. Tetrahedron Lett. 2016, 57, 4529–4532. 10.1016/j.tetlet.2016.08.067. [DOI] [Google Scholar]
- Giordanetto F.; Schäfer A.; Ottmann C. Stabilization of Protein–protein Interactions by Small Molecules. Drug Discovery Today 2014, 19, 1812–1821. 10.1016/j.drudis.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Galindo L.; Islas-Jácome A.; Colmenero-Martínez K.; Martínez-Richa A.; Gámez-Montaño R. Synthesis of Novel Bis-1,5-Disubstituted-1H-Tetrazoles by an Efficient Catalyst-Free Ugi-Azide Repetitive Process. Molecules 2015, 20, 1519–1526. 10.3390/molecules20011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fatima S Barreto A.; Alves dos Santos V.; Kleber Z Andrade C. Consecutive Hydrazino-Ugi-Azide Reactions: Synthesis of Acylhydrazines Bearing 1,5-Disubstituted Tetrazoles. Beilstein J. Org. Chem. 2017, 13, 2596–2602. 10.3762/bjoc.13.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltjes A.; Shrinidhi A.; van de Kolk K.; Herdtweck E.; Dömling A. Gd-TEMDO: Design, Synthesis, and MRI Application. Chem. - Eur. J. 2016, 22, 7352–7356. 10.1002/chem.201600720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermkens P. H. H.; Ottenheijm H. C. J.; Rees D. Solid-Phase Organic Reactions: A Review of the Recent Literature. Tetrahedron 1996, 52, 4527–4554. 10.1016/0040-4020(96)00216-5. [DOI] [Google Scholar]
- Merrifield B. Solid Phase Synthesis. Science (Washington, DC, U. S.) 1986, 232, 341–347. 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- Krchňák V.; Holladay M. W. Solid Phase Heterocyclic Chemistry. Chem. Rev. 2002, 102, 61–92. 10.1021/cr010123h. [DOI] [PubMed] [Google Scholar]
- Guillier F.; Orain D.; Bradley M. Linkers and Cleavage Strategies in Solid-Phase Organic Synthesis and Combinatorial Chemistry. Chem. Rev. 2000, 100, 2091–2158. 10.1021/cr980040+. [DOI] [PubMed] [Google Scholar]
- Seeberger P. H.; Haase W.-C. Solid-Phase Oligosaccharide Synthesis and Combinatorial Carbohydrate Libraries. Chem. Rev. 2000, 100, 4349–4394. 10.1021/cr9903104. [DOI] [PubMed] [Google Scholar]
- Short K. M.; Ching B. W.; Mjalli A. M. M. Exploitation of the Ugi 4CC Reaction: Preparation of Small Molecule Combinatorial Libraries via Solid Phase. Tetrahedron 1997, 53, 6653–6679. 10.1016/S0040-4020(97)00223-8. [DOI] [Google Scholar]
- Constabel F.; Ugi I. Repetitive Ugi Reactions. Tetrahedron 2001, 57, 5785–5789. 10.1016/S0040-4020(01)00516-6. [DOI] [Google Scholar]
- Chen J. J.; Golebiowski A.; Klopfenstein S. R.; West L. The Universal Rink-Isonitrile Resin: Applications in Ugi Reactions. Tetrahedron Lett. 2002, 43, 4083–4085. 10.1016/S0040-4039(02)00700-1. [DOI] [Google Scholar]
- Morales F. E.; Garay H. E.; Muñoz D. F.; Augusto Y. E.; Otero-González A. J.; Reyes Acosta O.; Rivera D. G. Aminocatalysis-Mediated on-Resin Ugi Reactions: Application in the Solid-Phase Synthesis of N-Substituted and Tetrazolo Lipopeptides and Peptidosteroids. Org. Lett. 2015, 17, 2728–2731. 10.1021/acs.orglett.5b01147. [DOI] [PubMed] [Google Scholar]
- Reguera L.; Méndez Y.; Humpierre A. R.; Valdés O.; Rivera D. G. Multicomponent Reactions in Ligation and Bioconjugation Chemistry. Acc. Chem. Res. 2018, 51, 1475–1486. 10.1021/acs.accounts.8b00126. [DOI] [PubMed] [Google Scholar]
- Dömling A.; Ugi I. The Seven-Component Reaction. Angew. Chem., Int. Ed. Engl. 1993, 32, 563–564. 10.1002/anie.199305631. [DOI] [Google Scholar]
- Zarganes-Tzitzikas T.; Chandgude A. L.; Dömling A. Multicomponent Reactions, Union of MCRs and Beyond. Chem. Rec. 2015, 15, 981–996. 10.1002/tcr.201500201. [DOI] [PubMed] [Google Scholar]
- Dömling A. The Discovery of New Isocyanide-Based Multi-Component Reactions. Curr. Opin. Chem. Biol. 2000, 4, 318–323. 10.1016/S1367-5931(00)00095-8. [DOI] [PubMed] [Google Scholar]
- Banfi L.; Riva R.; Basso A. Coupling Isocyanide-Based Multicomponent Reactions with Aliphatic or Acyl Nucleophilic Substitution Processes. Synlett 2010, 2010, 23–41. 10.1055/s-0029-1218527. [DOI] [Google Scholar]
- Pepino R.; Bossio R.; Marcaccini S.; Torroba T. Studies on Isocyanides and Related Compounds. A Facile Synthesis of 1-Substituted 3-Cyano-2-Methoxy-3-Phenylpyrroles. Heterocycles 1999, 50, 463–467. 10.3987/COM-98-S(H)16. [DOI] [Google Scholar]
- Zefirov N. S.; Chapovskaya N. K.; Trach S. S. Z. Umlagerungen Und Cyclisierungen 4. Mitt.Synth. von 5-Halogen- Und 5-Aroyl-Tetrazolen. Org. Khim. 1972, 8, 629. [Google Scholar]
- Nixey T.; Hulme C. Rapid Generation of Cis-Constrained Norstatine Analogs Using a TMSN3-Modified Passerini MCC/N-Capping Strategy. Tetrahedron Lett. 2002, 43, 6833–6835. 10.1016/S0040-4039(02)01505-8. [DOI] [Google Scholar]
- Beck B.; Larbig G.; Mejat B.; Magnin-Lachaux M.; Picard A.; Herdtweck E.; Dömling A. Short and Diverse Route Toward Complex Natural Product-Like Macrocycles. Org. Lett. 2003, 5, 1047–1050. 10.1021/ol034077e. [DOI] [PubMed] [Google Scholar]
- Banfi L.; Riva R.. The Passerini Reaction. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, 2005; Vol. 65, pp 1–140. [Google Scholar]
- Ugi I.; Meyr R. Isonitrile, V. Erweiterter Anwendungsbereich Der Passerini-Reaktion. Chem. Ber. 1961, 94, 2229–2233. 10.1002/cber.19610940844. [DOI] [Google Scholar]
- Ducki S. Antimitotic Chalcones and Related Compounds as Inhibitors of Tubulin Assembly. Anti-Cancer Agents Med. Chem. 2009, 9, 336–347. 10.2174/1871520610909030336. [DOI] [PubMed] [Google Scholar]
- Oikawa M.; Naito S.; Sasaki M. Skeletal Diversity by Ugi Four-Component Coupling Reaction and Post-Ugi Reactions. Heterocycles 2007, 73, 377–392. 10.3987/COM-07-S(U)8. [DOI] [Google Scholar]
- Portlock D. E.; Ostaszewski R.; Naskar D.; West L. A Tandem Petasis–Ugi Multi Component Condensation Reaction: Solution Phase Synthesis of Six Dimensional Libraries. Tetrahedron Lett. 2003, 44, 603–605. 10.1016/S0040-4039(02)02619-9. [DOI] [Google Scholar]
- Moderhack D. Tetrazoles from N-(4-Dimethylaminophenyl)Nitrones and Hydrogen Azide. J. Heterocycl. Chem. 1977, 14, 757–763. 10.1002/jhet.5570140509. [DOI] [Google Scholar]
- Paulvannan K. Preparation of Tricyclic Nitrogen Heterocycles via Tandem Four-Component Condensation/Intramolecular Diels-Alder Reaction. Tetrahedron Lett. 1999, 40, 1851–1854. 10.1016/S0040-4039(99)00072-6. [DOI] [Google Scholar]
- Rivera D. G.; Wessjohann L. A. Architectural Chemistry: Synthesis of Topologically Diverse Macromulticycles by Sequential Multiple Multicomponent Macrocyclizations. J. Am. Chem. Soc. 2009, 131, 3721–3732. 10.1021/ja809005k. [DOI] [PubMed] [Google Scholar]
- Pirali T.; Callipari G.; Ercolano E.; Genazzani A. A.; Giovenzana G. B.; Tron G. C. A Concise Entry into Nonsymmetrical Alkyl Polyamines. Org. Lett. 2008, 10, 4199–4202. 10.1021/ol801612r. [DOI] [PubMed] [Google Scholar]
- Wang W.; Herdtweck E.; Domling A. Polycyclic Indole Alkaloid-Type Compounds by MCR. Chem. Commun. 2010, 46, 770–772. 10.1039/B917660H. [DOI] [PubMed] [Google Scholar]
- Thenmozhiyal J. C.; Wong P. T.-H.; Chui W.-K. Anticonvulsant Activity of Phenylmethylenehydantoins: A Structure–Activity Relationship Study. J. Med. Chem. 2004, 47, 1527–1535. 10.1021/jm030450c. [DOI] [PubMed] [Google Scholar]
- Bazil C. W.; Pedley T. A.. Advances in the Medical Treatment of Epilepsy. Annu. Rev. Med. 1998, 49, 135–162. 10.1146/annurev.med.49.1.135. [DOI] [PubMed] [Google Scholar]
- Matsukura M.; Daiku Y.; Ueda K.; Tanaka S.; Igarashi T.; Minami N. Synthesis and Antiarrhythmic Activity of 2, 2-Dialkyl-1’-(N-Substituted Aminoalkyl)-Spiro-[Chroman-4, 4’-Imidazolidine]-2’, 5′-Diones. Chem. Pharm. Bull. 1992, 40, 1823–1827. 10.1248/cpb.40.1823. [DOI] [PubMed] [Google Scholar]
- Luer M. Fosphenytoin. Neurol. Res. 1998, 20, 178–182. 10.1080/01616412.1998.11740502. [DOI] [PubMed] [Google Scholar]
- Moloney G. P.; Martin G. R.; Mathews N.; Milne A.; Hobbs H.; Dodsworth S.; Sang P. Y.; Knight C.; Williams M.; Maxwell M.; et al. Synthesis and Serotonergic Activity of Substituted 2,N-Benzylcarboxamido-5-(2-Ethyl-1-Dioxoimidazolidinyl)-N,N-Dimethyltrypt- Amine Derivatives: Novel Antagonists for the Vascular 5-HT1B-like Receptor. J. Med. Chem. 1999, 42, 2504–2526. 10.1021/jm9706325. [DOI] [PubMed] [Google Scholar]
- Sutherland A.; Willis C. L. Synthesis of Fluorinated Amino Acids. Nat. Prod. Rep. 2000, 17, 621–631. 10.1039/a707503k. [DOI] [PubMed] [Google Scholar]
- Medda F.; Hulme C. A Facile and Rapid Route for the Synthesis of Novel 1,5-Substituted Tetrazole Hydantoins and Thiohydantoins via a TMSN3-Ugi/RNCX Cyclization. Tetrahedron Lett. 2012, 53, 5593–5596. 10.1016/j.tetlet.2012.07.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda F.; Martinez-Ariza G.; Hulme C. A Facile and Concise Route toward the Synthesis of Novel Imidazo-Tetrazolodiazepinones via Post-Condensation Modifications of the Ugi-Azide Adduct. Tetrahedron Lett. 2015, 56, 5295–5298. 10.1016/j.tetlet.2015.07.083. [DOI] [Google Scholar]
- Calcaterra N. E.; Barrow J. C. Classics in Chemical Neuroscience: Diazepam (Valium). ACS Chem. Neurosci. 2014, 5, 253–260. 10.1021/cn5000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternbach L. H. The Benzodiazepine Story. J. Med. Chem. 1979, 22, 1–7. 10.1021/jm00187a001. [DOI] [PubMed] [Google Scholar]
- Grasberger B. L.; Lu T.; Schubert C.; Parks D. J.; Carver T. E.; Koblish H. K.; Cummings M. D.; LaFrance L. V.; Milkiewicz K. L.; Calvo R. R.; et al. Discovery and Cocrystal Structure of Benzodiazepinedione HDM2 Antagonists That Activate P53 in Cells. J. Med. Chem. 2005, 48, 909–912. 10.1021/jm049137g. [DOI] [PubMed] [Google Scholar]
- Blackburn B. K.; Lee A.; Baier M.; Kohl B.; Olivero A. G.; Matamoros R.; Robarge K. D.; McDowell R. S. From Peptide to Non-Peptide. 3. Atropisomeric GPIIbIIIa Antagonists Containing the 3,4-Dihydro-1H-1,4-Benzodiazepine-2,5-Dione Nucleus. J. Med. Chem. 1997, 40, 717–729. 10.1021/jm960652r. [DOI] [PubMed] [Google Scholar]
- Neochoritis C. G.; Tsoleridis C. a.; Stephanidou-Stephanatou J.; Kontogiorgis C. a.; Hadjipavlou-Litina D. J. 1,5-Benzoxazepines Vs 1,5-Benzodiazepines. One-Pot Microwave-Assisted Synthesis and Evaluation for Antioxidant Activity and Lipid Peroxidation Inhibition. J. Med. Chem. 2010, 53, 8409–8420. 10.1021/jm100739n. [DOI] [PubMed] [Google Scholar]
- Eleftheriadis N.; Neochoritis C. G.; Tsoleridis C. A.; Stephanidou-Stephanatou J.; Iakovidou-Kritsi Z. One-Pot Microwave Assisted Synthesis of New 2-Alkoxycarbonylmethylene-4-Oxo-1,5-Benzo-, Naphtho-, and Pyridodiazepines and Assessment of Their Cytogenetic Activity. Eur. J. Med. Chem. 2013, 67, 302–309. 10.1016/j.ejmech.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Ding C. Z.; Batorsky R.; Bhide R.; Chao H. J.; Cho Y.; Chong S.; Gullo-Brown J.; Guo P.; Kim S. H.; Lee F.; et al. Discovery and Structure–Activity Relationships of Imidazole-Containing Tetrahydrobenzodiazepine Inhibitors of Farnesyltransferase. J. Med. Chem. 1999, 42, 5241–5253. 10.1021/jm990391w. [DOI] [PubMed] [Google Scholar]
- Gunawan S.; Nichol G.; Hulme C. Concise Route to a Series of Novel 3-(Tetrazol-5-Yl)Quinoxalin-2(1H)-Ones. Tetrahedron Lett. 2012, 53, 1664–1667. 10.1016/j.tetlet.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerande S. G.; Newase K. M.; Singh B.; Boltjes A.; Dömling A. Application of Cyclic Ketones in MCR: Ugi/Amide Coupling Based Synthesis of Fused Tetrazolo[1,5-a][1,4]Benzodiazepines. Tetrahedron Lett. 2014, 55, 3263–3266. 10.1016/j.tetlet.2014.04.040. [DOI] [Google Scholar]
- Shen Y.; Han J.; Sun X.; Wang X.; Chen J.; Deng H.; Shao M.; Shi H.; Zhang H.; Cao W. Facile Synthesis of Both Perfluoroalkyl and Phosphonate Groups Substituted Trans-1,5-Benzodiazepine and Its Derivatives via a One-Pot Catalyst-Free Process. Tetrahedron 2015, 71, 4053–4060. 10.1016/j.tet.2015.04.067. [DOI] [Google Scholar]
- Bunin B. A.; Plunkett M. J.; Ellman J. A. The Combinatorial Synthesis and Chemical and Biological Evaluation of a 1,4-Benzodiazepine Library. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 4708–4712. 10.1073/pnas.91.11.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Khoury K.; Chanas T.; Dömling A. Multicomponent Synthesis of Diverse 1,4-Benzodiazepine Scaffolds. Org. Lett. 2012, 14, 5916–5919. 10.1021/ol302837h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa T.; Adachi M.; Toyoda T.; Sasakura K. A New Simple Synthesis of 1,4-Benzodiazepines. J. Heterocycl. Chem. 1979, 16, 445–448. 10.1002/jhet.5570160306. [DOI] [Google Scholar]
- Hulme C.; Peng J.; Morton G.; Salvino J. M.; Herpin T.; Labaudiniere R. Novel Safety-Catch Linker and Its Application with a Ugi/De-BOC/Cyclization (UDC) Strategy to Access Carboxylic Acids, 1,4-Benzodiazepines, Diketopiperazines, Ketopiperazines and Dihydroquinoxalinones. Tetrahedron Lett. 1998, 39, 7227–7230. 10.1016/S0040-4039(98)01593-7. [DOI] [Google Scholar]
- Hulme C.; Cherrier M. P. Novel Applications of Ethyl Glyoxalate with the Ugi MCR. Tetrahedron Lett. 1999, 40, 5295–5299. 10.1016/S0040-4039(99)00960-0. [DOI] [Google Scholar]
- Balakrishna M. S.; Kaboudin B. A Simple and New Method for the Synthesis of 1,5-Benzodiazepine Derivatives on a Solid Surface. Tetrahedron Lett. 2001, 42, 1127–1129. 10.1016/S0040-4039(00)02168-7. [DOI] [Google Scholar]
- Kaoua R.; Bennamane N.; Bakhta S.; Benadji S.; Rabia C.; Nedjar-Kolli B. Synthesis of Substituted 1,4-Diazepines and 1,5-Benzodiazepines Using an Efficient Heteropolyacid-Catalyzed Procedure. Molecules 2011, 16, 92–99. 10.3390/molecules16010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radatz C. S.; Silva R. B.; Perin G.; Lenardão E. J.; Jacob R. G.; Alves D. Catalyst-Free Synthesis of Benzodiazepines and Benzimidazoles Using Glycerol as Recyclable Solvent. Tetrahedron Lett. 2011, 52, 4132–4136. 10.1016/j.tetlet.2011.05.142. [DOI] [Google Scholar]
- Majid S. A.; Khanday W. A.; Tomar R. Synthesis of 1,5-Benzodiazepine and Its Derivatives by Condensation Reaction Using H-MCM-22 as Catalyst. J. Biomed. Biotechnol. 2012, 2012, 1–6. 10.1155/2012/510650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch M. E.; Snyder S. A.; Stockwell B. R. Privileged Scaffolds for Library Design and Drug Discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofakham H.; Shaabani A.; Mousavifaraz S.; Hajishaabanha F.; Shaabani S.; Ng S. W. A Novel One-Pot Pseudo-Five-Component Condensation Reaction towards Bifunctional Diazepine-Tetrazole Containing Compounds: Synthesis of 1H-Tetrazolyl-1H-1,4-Diazepine-2,3-Dicarbonitriles and 1H-Tetrazolyl-Benzo[b][1,4]Diazepines. Mol. Diversity 2012, 16, 351–356. 10.1007/s11030-012-9371-4. [DOI] [PubMed] [Google Scholar]
- Mofakham H.; Shaabani A.; Mousavifaraz S.; Hajishaabanha F.; Shaabani S.; Ng S. W. A Novel One-Pot Pseudo-Five-Component Condensation Reaction towards Bifunctional Diazepine-Tetrazole Containing Compounds: Synthesis of 1H-Tetrazolyl-1H-1,4-Diazepine-2,3-Dicarbonitriles and 1H-Tetrazolyl-Benzo b 1,4 Diazepines. Mol. Diversity 2012, 16, 351–356. 10.1007/s11030-012-9371-4. [DOI] [PubMed] [Google Scholar]
- Shaabani A.; Mofakham H.; Mousavifaraz S. A Two-Step Synthesis of 1H-Tetrazolyl-1H-1,4-Benzonitriles and 1H-Tetrazolyl-Benzo b 1,4 Diazepines. Synlett 2012, 5, 731–736. 10.1055/s-0031-1290603. [DOI] [PubMed] [Google Scholar]
- Shaabani A.; Hezarkhani Z.; Mofakham H.; Ng S. Synthesis of Highly Regioselective Bifunctional Tricyclic Tetrazole-1H-Benzo[b][1,4]Diazepins. Synlett 2013, 24, 1485–1492. 10.1055/s-0033-1338953. [DOI] [Google Scholar]
- Padwa A.; Beall L. S.; Eidell C. K.; Worsencroft K. J. An Approach toward Isoindolobenzazepines Using the Ammonium Ylide/Stevens [1,2]-Rearrangement Sequence. J. Org. Chem. 2001, 66, 2414–2421. 10.1021/jo001684w. [DOI] [PubMed] [Google Scholar]
- Taylor E. C.; Zhou P.; Jennings L. D.; Mao Z.; Hu B.; Jun J.-G. Novel Synthesis of a Conformationally-Constrained Analog of DDATHF. Tetrahedron Lett. 1997, 38, 521–524. 10.1016/S0040-4039(96)02397-0. [DOI] [Google Scholar]
- Portevin B.; Tordjman C.; Pastoureau P.; Bonnet J.; De Nanteuil G. 1,3-Diaryl-4,5,6,7-Tetrahydro-2H-Isoindole Derivatives: A New Series of Potent and Selective COX-2 Inhibitors in Which a Sulfonyl Group Is Not a Structural Requisite. J. Med. Chem. 2000, 43, 4582–4593. 10.1021/jm990965x. [DOI] [PubMed] [Google Scholar]
- Blaskó G.; Gula D. J.; Shamma M. The Phthalideisoquinoline Alkaloids. J. Nat. Prod. 1982, 45, 105–122. 10.1021/np50020a001. [DOI] [Google Scholar]
- Sharma M.; Khan I.; Khan S.; Mahar R.; Shukla S. K.; Kant R.; Chauhan P. M. S. Facile Ligand-Free Pd-Catalyzed Tandem C–C/C–N Coupling Reaction: A Novel Access to Highly Diverse Tetrazole Tag Isoindoline Derivatives. Tetrahedron Lett. 2015, 56, 5401–5408. 10.1016/j.tetlet.2015.08.008. [DOI] [Google Scholar]
- Cao R.; Peng W.; Wang Z.; Xu A. Carboline Alkaloids: Biochemical and Pharmacological Functions. Curr. Med. Chem. 2007, 14, 479–500. 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- Nissen F.; Richard V.; Alayrac C.; Witulski B. Synthesis of [Small Beta]- and [Gamma]-Carbolines via Ruthenium and Rhodium Catalysed [2 + 2+2] Cycloadditions of Yne-Ynamides with Methylcyanoformate. Chem. Commun. 2011, 47, 6656–6658. 10.1039/c1cc11298h. [DOI] [PubMed] [Google Scholar]
- Magnier E.; Langlois Y. Manzamine Alkaloids, Syntheses and Synthetic Approaches. Tetrahedron 1998, 54, 6201–6258. 10.1016/S0040-4020(98)00357-3. [DOI] [Google Scholar]
- Ohmoto T.; Koike K.. Preface. In The Alkaloids; Brossi A., Ed.; Academic Press: San Diego, 1989; Vol. 36, pp 135–170. [Google Scholar]
- Wang W.; Ollio S.; Herdtweck E.; Dömling A. Polycyclic Compounds by Ugi-Pictet-Spengler Sequence. J. Org. Chem. 2011, 76, 637–644. 10.1021/jo102058s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P.; Khoury K.; Herdtweck E.; Dömling A. MCR Synthesis of a Tetracyclic Tetrazole Scaffold. Bioorg. Med. Chem. 2015, 23, 2699–2715. 10.1016/j.bmc.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas-Jácome A.; Cárdenas-Galindo L.; Jerezano A.; Tamariz J.; González-Zamora E.; Gámez-Montaño R. Synthesis of Nuevamine Aza-Analogues by a Sequence: IMCR–Aza Diels–Alder, Pictet–Spengler. Synlett 2012, 23, 2951–2956. 10.1055/s-0032-1317622. [DOI] [Google Scholar]
- Liu H.; William S.; Herdtweck E.; Botros S.; Dömling A. MCR Synthesis of Praziquantel Derivatives. Chem. Biol. Drug Des. 2012, 79, 470–477. 10.1111/j.1747-0285.2011.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi V.; Khan S.; Bajpai V.; Gauniyal H. M.; Kumar B.; Chauhan P. M. S. Skeletal Diverse Synthesis of N-Fused Polycyclic Heterocycles via the Sequence of Ugi-Type MCR and CuI-Catalyzed Coupling/Tandem Pictet–Spengler Reaction. J. Org. Chem. 2012, 77, 1414–1421. 10.1021/jo202255v. [DOI] [PubMed] [Google Scholar]
- Wang H.; Ganesan A. The N-Acyliminium Pictet–Spengler Condensation as a Multicomponent Combinatorial Reaction on Solid Phase and Its Application to the Synthesis of Demethoxyfumitremorgin C Analogues. Org. Lett. 1999, 1, 1647–1649. 10.1021/ol991030d. [DOI] [Google Scholar]
- El Kaim L.; Gageat M.; Gaultier L.; Grimaud L. New Ugi/Pictet-Spengler Multicomponent Formation of Polycyclic Diketopiperazines from Isocyanides and α-Keto Acids. Synlett 2007, 2007, 0500–0502. 10.1055/s-2007-968026. [DOI] [Google Scholar]
- Znabet A.; Zonneveld J.; Janssen E.; De Kanter F. J. J.; Helliwell M.; Turner N. J.; Ruijter E.; Orru R. V. A. Asymmetric Synthesis of Synthetic Alkaloids by a Tandem Biocatalysis/Ugi/Pictet-Spengler-Type Cyclization Sequence. Chem. Commun. 2010, 46, 7706–7708. 10.1039/c0cc02938f. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Galindo L.; Islas-Jácome A.; Alvarez-Rodríguez N.; El Kaim L.; Gámez-Montaño R. Synthesis of 2-Tetrazolylmethyl-2,3,4,9-Tetrahydro-1H-β-Carbolines by a One-Pot Ugi-Azide/Pictet–Spengler Process. Synthesis 2013, 46, 49–56. 10.1055/s-0033-1340051. [DOI] [Google Scholar]
- Gordillo-Cruz R. E.; Rentería-Gómez A.; Islas-Jácome A.; Cortes-García C. J.; Díaz-Cervantes E.; Robles J.; Gámez-Montaño R. Synthesis of 3-Tetrazolylmethyl-Azepino[4,5-b]Indol-4-Ones in Two Reaction Steps: (Ugi-Azide/N-Acylation/SN2)/Free Radical Cyclization and Docking Studies to a 5-Ht6Model. Org. Biomol. Chem. 2013, 11, 6470–6476. 10.1039/c3ob41349g. [DOI] [PubMed] [Google Scholar]
- Foley C.; Shaw A.; Hulme C. Two-Step Route to Diverse N-Functionalized Peptidomimetic-like Isatins through an Oxidation/Intramolecular Oxidative-Amidation Cascade of Ugi Azide and Ugi Three-Component Reaction Products. Org. Lett. 2016, 18, 4904–4907. 10.1021/acs.orglett.6b02383. [DOI] [PubMed] [Google Scholar]
- Mahapatra D. K.; Bharti S. K.; Asati V. Anti-Cancer Chalcones: Structural and Molecular Target Perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. 10.1016/j.ejmech.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Mahapatra D. K.; Bharti S. K.; Asati V. Chalcone Scaffolds as Anti-Infective Agents: Structural and Molecular Target Perspectives. Eur. J. Med. Chem. 2015, 101, 496–524. 10.1016/j.ejmech.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Mahapatra D. K.; Asati V.; Bharti S. K. Chalcones and Their Therapeutic Targets for the Management of Diabetes: Structural and Pharmacological Perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. 10.1016/j.ejmech.2015.01.051. [DOI] [PubMed] [Google Scholar]
- Mahapatra D. K.; Bharti S. K. Therapeutic Potential of Chalcones as Cardiovascular Agents. Life Sci. 2016, 148, 154–172. 10.1016/j.lfs.2016.02.048. [DOI] [PubMed] [Google Scholar]
- Foley C.; Shaw A.; Hulme C. Aza-Riley Oxidation of Ugi-Azide and Ugi-3CR Products toward Vicinal Tricarbonyl Amides: Two-Step MCR-Oxidation Methodology Accessing Functionalized α,β-Diketoamides and α,β-Diketotetrazoles. Org. Lett. 2018, 20, 1275–1278. 10.1021/acs.orglett.7b03977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley C.; Shaw A.; Hulme C. Oxidative Deaminations and Deisatinylations of Ugi-Azide and Ugi-3CR Products: A Two-Step MCR-Oxidation Protocol toward Functionalized α-Ketoamides and α-Ketotetrazoles. Org. Lett. 2017, 19, 2238–2241. 10.1021/acs.orglett.7b00710. [DOI] [PubMed] [Google Scholar]
- Giustiniano M.; Pirali T.; Massarotti A.; Biletta B.; Novellino E.; Campiglia P.; Sorba G.; Tron G. C. A Practical Synthesis of 5-Aroyl-1-Aryltetrazoles Using an Ugi-Like 4-Component Reaction Followed by a Biomimetic Transamination. Synthesis 2010, 2010, 4107–4118. 10.1055/s-0030-1258273. [DOI] [Google Scholar]
- Gilmore J. M.; Scheck R. A.; Esser-Kahn A. P.; Joshi N. S.; Francis M. B. N-Terminal Protein Modification through a Biomimetic Transamination Reaction. Angew. Chem., Int. Ed. 2006, 45, 5307–5311. 10.1002/anie.200600368. [DOI] [PubMed] [Google Scholar]
- Witus L. S.; Netirojjanakul C.; Palla K. S.; Muehl E. M.; Weng C.-H.; Iavarone A. T.; Francis M. B. Site-Specific Protein Transamination Using N-Methylpyridinium-4-Carboxaldehyde. J. Am. Chem. Soc. 2013, 135, 17223–17229. 10.1021/ja408868a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesenzani O.; Massarotti A.; Giustiniano M.; Pirali T.; Bevilacqua V.; Caldarelli A.; Canonico P.; Sorba G.; Novellino E.; Genazzani A. A.; et al. Replacement of the Double Bond of Antitubulin Chalcones with Triazoles and Tetrazoles: Synthesis and Biological Evaluation. Bioorg. Med. Chem. Lett. 2011, 21, 764–768. 10.1016/j.bmcl.2010.11.113. [DOI] [PubMed] [Google Scholar]
- Kurhade S.; Diekstra E.; Sutanto F.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Multicomponent Reaction Based Synthesis of 1-Tetrazolylimidazo[1,5- a ]Pyridines. Org. Lett. 2018, 20, 3871–3874. 10.1021/acs.orglett.8b01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C.; Dietrich J. Emerging Molecular Diversity from the Intra-Molecular Ugi Reaction: Iterative Efficiency in Medicinal Chemistry. Mol. Diversity 2009, 13, 195–207. 10.1007/s11030-009-9111-6. [DOI] [PubMed] [Google Scholar]
- Hulme C.; Gore V. Multi-Component Reactions: Emerging Chemistry in Drug Discovery; From Xylocain to Crixivan. Curr. Med. Chem. 2003, 10, 51–80. 10.2174/0929867033368600. [DOI] [PubMed] [Google Scholar]
- Hulme C.Applications of Multicomponent Reactions in Drug Discovery—Lead Generation to Process Development. In Multicomponent Reactions; Wiley-VCH Verlag GmbH & Co. KGaA, 2005; pp 311–341. [Google Scholar]
- El Kaïm L.; Grimaud L.; Purumandla S. Four-Component Synthesis of Indazole through Ugi-Azide Coupling. Synlett 2012, 2012, 295–297. 10.1055/s-0031-1290075. [DOI] [Google Scholar]
- Cadogan J. I. G. New Series of Nitrene-Induced Aromatic Rearrangements. Acc. Chem. Res. 1972, 5, 303–310. 10.1021/ar50057a003. [DOI] [Google Scholar]
- Gaikwad D. D.; Chapolikar A. D.; Devkate C. G.; Warad K. D.; Tayade A. P.; Pawar R. P.; Domb A. J. Synthesis of Indazole Motifs and Their Medicinal Importance: An Overview. Eur. J. Med. Chem. 2015, 90, 707–731. 10.1016/j.ejmech.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Patil P.; Madhavachary R.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. De Novo Assembly of Highly Substituted Morpholines and Piperazines. Org. Lett. 2017, 19, 642–645. 10.1021/acs.orglett.6b03807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.; Castoldi A. F.; Costa L. G. Effects of the Muscarinic Agonist Oxotremorine on Membrane Fluidity in Rat Lymphocytes. Biochem. Mol. Biol. Int. 1993, 29, 1047–1054. [PubMed] [Google Scholar]
- Zhang X.; Song Z.; Qin B.; Zhang X.; Chen L.; Hu Y.; Yuan Z. Rupintrivir Is a Promising Candidate for Treating Severe Cases of Enterovirus-71 Infection: Evaluation of Antiviral Efficacy in a Murine Infection Model. Antiviral Res. 2013, 97, 264–269. 10.1016/j.antiviral.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Marcos C. F.; Marcaccini S.; Menchi G.; Pepino R.; Torroba T. Studies on Isocyanides: Synthesis of Tetrazolyl-Isoindolinones via Tandem Ugi Four-Component Condensation/Intramolecular Amidation. Tetrahedron Lett. 2008, 49, 149–152. 10.1016/j.tetlet.2007.10.154. [DOI] [Google Scholar]
- Gunawan S.; Hulme C. Bifunctional Building Blocks in the Ugi-Azide Condensation Reaction: A General Strategy toward Exploration of New Molecular Diversity. Org. Biomol. Chem. 2013, 11, 6036–6046. 10.1039/c3ob40900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawan S.; Petit J.; Hulme C. Concise One-Pot Preparation of Unique Bis-Pyrrolidinone Tetrazoles. ACS Comb. Sci. 2012, 14, 160–163. 10.1021/co200209a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Sledeski Y. M.; Kearney R.; Poli G.; Pauls H.; Gardner C.; Gong Y.; Becker M.; Davis R.; Spada A.; Liang G.; et al. Discovery of an Orally Efficacious Inhibitor of Coagulation Factor Xa Which Incorporates a Neutral P1 Ligand. J. Med. Chem. 2003, 46, 681–684. 10.1021/jm020384z. [DOI] [PubMed] [Google Scholar]
- Nishida H.; Miyazaki Y.; Mukaihira T.; Saitoh F.; Fukui M.; Harada K.; Itoh M.; Muraoka A.; Matsusue T.; Okamoto A.; et al. Synthesis and Evaluation of 1-Arylsulfonyl-3-Piperazinone Derivatives as a Factor Xa Inhibitor II. Substituent Effect on Biological Activities. Chem. Pharm. Bull. 2002, 50, 1187–1194. 10.1248/cpb.50.1187. [DOI] [PubMed] [Google Scholar]
- Stolyarenko V. Y.; Evdokimov A. A.; Shishkin V. I. Synthesis of Tetrazole-Substituted Spirocyclic γ-Lactams by One-Pot Azido-Ugi Reaction–cyclization. Mendeleev Commun. 2013, 23, 108–109. 10.1016/j.mencom.2013.03.020. [DOI] [Google Scholar]
- Yang M. G.; Dhar T. G. M.; Xiao Z.; Xiao H.-Y.; Duan J. J. W.; Jiang B.; Galella M. A.; Cunningham M.; Wang J.; Habte S.; et al. Improving the Pharmacokinetic and CYP Inhibition Profiles of Azaxanthene-Based Glucocorticoid Receptor Modulators-Identification of (S)-5-(2-(9-Fluoro-2-(4-(2-Hydroxypropan-2-Yl)Phenyl)-5H-Chromeno[2,3-b]Pyridin-5-Yl)-2-Methylpropanamido)-N-(Tetrahydro-2H. J. Med. Chem. 2015, 58, 4278–4290. 10.1021/acs.jmedchem.5b00257. [DOI] [PubMed] [Google Scholar]
- Boltjes A.; Liao G. P.; Zhao T.; Herdtweck E.; Domling A. Ugi 4-CR Synthesis of [Gamma]- and [Small Delta]-Lactams Providing New Access to Diverse Enzyme Interactions, a PDB Analysis. MedChemComm 2014, 5, 949–952. 10.1039/C4MD00162A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A.; Kinoshita T.; Hart G. W.. Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Press, 2009. [Google Scholar]
- Achatz S.; Dömling A. Desosamine in Multicomponent Reactions. Bioorg. Med. Chem. Lett. 2006, 16, 6360–6362. 10.1016/j.bmcl.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Neochoritis C. G.; Zhang J.; Doemling A. Leuckart-Wallach Approach to Sugar Isocyanides and Its IMCRs. Synthesis 2015, 47, 2407–2413. 10.1055/s-0034-1381046. [DOI] [Google Scholar]
- Neochoritis C. G.; Zarganes-Tzitzikas T.; Stotani S.; Dömling A.; Herdtweck E.; Khoury K.; Dömling A. Leuckart-Wallach Route Toward Isocyanides and Some Applications. ACS Comb. Sci. 2015, 17, 493–499. 10.1021/acscombsci.5b00066. [DOI] [PubMed] [Google Scholar]
- Neochoritis C. G.; Dömling A. Towards a Facile and Convenient Synthesis of Highly Functionalized Indole Derivatives Based on Multi-Component Reactions. Org. Biomol. Chem. 2014, 12, 1649–1651. 10.1039/C4OB00166D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera D. G.; Pérez-Labrada K.; Lambert L.; Dörner S.; Westermann B.; Wessjohann L. A. Carbohydrate–steroid Conjugation by Ugi Reaction: One-Pot Synthesis of Triple Sugar/Pseudo-Peptide/Spirostane Hybrids. Carbohydr. Res. 2012, 359, 102–110. 10.1016/j.carres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Alavijeh N. S.; Zadmard R.; Ramezanpour S.; Balalaie S.; Alavijeh M. S.; Rominger F. Efficient Synthesis of Lower Rim [Small Alpha]-Hydrazino Tetrazolocalix[4]Arenes via an Ugi-Azide Multicomponent Reaction. New J. Chem. 2015, 39, 6578–6584. 10.1039/C5NJ00845J. [DOI] [Google Scholar]
- Quinlan E.; Matthews S. E.; Gunnlaugsson T. Colorimetric Recognition of Anions Using Preorganized Tetra-Amidourea Derived Calix[4]Arene Sensors. J. Org. Chem. 2007, 72, 7497–7503. 10.1021/jo070439a. [DOI] [PubMed] [Google Scholar]
- Hiyama T.Organofluorine Compounds; Yamamoto H., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2000. [Google Scholar]
- Shmatova O. I.; Nenajdenko V. G. Tetrazole-Substituted Five, Six, and Seven-Membered Cyclic Amines Bearing Perfluoroalkyl Groups - Efficient Synthesis by Azido-Ugi Reaction. Eur. J. Org. Chem. 2013, 2013, 6397–6403. 10.1002/ejoc.201300861. [DOI] [Google Scholar]
- Soeta T.; Tamura K.; Fujinami S.; Ukaji Y. A Three-Component Reaction of C,N-Cyclic N ’-Acyl Azomethine Imines, Isocyanides, and Azide Compounds: Effective Synthesis of 1,5-Disubstituted Tetrazoles with Tetrahydroisoquinoline Skeletons. Org. Biomol. Chem. 2013, 11, 2168–2174. 10.1039/c3ob27297d. [DOI] [PubMed] [Google Scholar]
- Torii H.; Nakadai M.; Ishihara K.; Saito S.; Yamamoto H. Asymmetric Direct Aldol Reaction Assisted by Water and a Proline-Derived Tetrazole Catalyst. Angew. Chem. 2004, 116, 2017–2020. 10.1002/ange.200352724. [DOI] [PubMed] [Google Scholar]
- Cobb A. J. A.; Shaw D. M.; Ley S. V. 5-Pyrrolidin-2-Yltetrazole: A New, Catalytic, More Soluble Alternative to Proline in an Organocatalytic Asymmetric Mannich-Type Reaction. Synlett 2004, (3), 558–560. 10.1055/s-2004-817745. [DOI] [Google Scholar]
- Hartikka A.; Arvidsson P. I. Rational Design of Asymmetric Organocatalysts––increased Reactivity and Solvent Scope with a Tetrazolic Acid. Tetrahedron: Asymmetry 2004, 15, 1831–1834. 10.1016/j.tetasy.2004.04.029. [DOI] [Google Scholar]
- Chowdari N. S.; Barbas C. F. Total Synthesis of LFA-1 Antagonist BIRT-377 via Organocatalytic Asymmetric Construction of a Quaternary Stereocenter. Org. Lett. 2005, 7, 867–870. 10.1021/ol047368b. [DOI] [PubMed] [Google Scholar]
- Makarieva T. N.; Denisenko V. A.; Dmitrenok P. S.; Guzii A. G.; Santalova E. A.; Stonik V. A.; MacMillan J. B.; Molinski T. F. Oceanalin A, a Hybrid α,ω-Bifunctionalized Sphingoid Tetrahydroisoquinoline β-Glycoside from the Marine Sponge Oceanapia Sp. Org. Lett. 2005, 7, 2897–2900. 10.1021/ol050796c. [DOI] [PubMed] [Google Scholar]
- Yue T.; Wang M. X.; Wang D. X.; Zhu J. P. Asymmetric Synthesis of 5-(1-Hydroxyalkyl)Tetrazoles by Catalytic Enantioselective Passerini-Type Reactions. Angew. Chem., Int. Ed. 2008, 47, 9454–9457. 10.1002/anie.200804213. [DOI] [PubMed] [Google Scholar]
- Yanai H.; Sakiyama T.; Oguchi T.; Taguchi T. Four Component Reaction of Aldehydes, Isocyanides, Me3SiN3, and Aliphatic Alcohols Catalyzed by Indium Triflate. Tetrahedron Lett. 2012, 53, 3161–3164. 10.1016/j.tetlet.2012.04.046. [DOI] [Google Scholar]
- Chanda A.; Fokin V. V. Organic Synthesis “On Water.. Chem. Rev. 2009, 109, 725–748. 10.1021/cr800448q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R. N.; Coyne A. G. Water: Nature’s Reaction Enforcer—Comparative Effects for Organic Synthesis “In-Water” and “On-Water.. Chem. Rev. 2010, 110, 6302–6337. 10.1021/cr100162c. [DOI] [PubMed] [Google Scholar]
- Simon M.-O.; Li C.-J. Green Chemistry Oriented Organic Synthesis in Water. Chem. Soc. Rev. 2012, 41, 1415–1427. 10.1039/C1CS15222J. [DOI] [PubMed] [Google Scholar]
- Lubineau A. Water-Promoted Organic Reactions: Aldol Reaction under Neutral Conditions. J. Org. Chem. 1986, 51, 2142–2144. 10.1021/jo00361a045. [DOI] [Google Scholar]
- Gajewski J. J. The Claisen Rearrangement. Response to Solvents and Substituents: The Case for Both Hydrophobic and Hydrogen Bond Acceleration in Water and for a Variable Transition State. Acc. Chem. Res. 1997, 30, 219–225. 10.1021/ar9600493. [DOI] [Google Scholar]
- Jung Y.; Marcus R. A. On the Theory of Organic Catalysis “on Water.. J. Am. Chem. Soc. 2007, 129, 5492–5502. 10.1021/ja068120f. [DOI] [PubMed] [Google Scholar]
- Otto S.; Engberts J. B. F. N. Hydrophobic Interactions and Chemical Reactivity. Org. Biomol. Chem. 2003, 1, 2809–2820. 10.1039/b305672d. [DOI] [PubMed] [Google Scholar]
- Engberts J. B. F. N. Diels-Alder Reactions in Water: Enforced Hydrophobic Interaction and Hydrogen Bonding. Pure Appl. Chem. 1995, 67, 823–828. 10.1351/pac199567050823. [DOI] [Google Scholar]
- Pirrung M. C.; Sarma K. D.; Wang J. Hydrophobicity and Mixing Effects on Select Heterogeneous, Water-Accelerated Synthetic Reactions. J. Org. Chem. 2008, 73, 8723–8730. 10.1021/jo801134r. [DOI] [PubMed] [Google Scholar]
- Sela T.; Vigalok A. Salt-Controlled Selectivity in “on Water” and “in Water” Passerini-Type Multicomponent Reactions. Adv. Synth. Catal. 2012, 354, 2407–2411. 10.1002/adsc.201200448. [DOI] [Google Scholar]
- Chandgude A. L.; Dömling A. An Efficient Passerini Tetrazole Reaction (PT-3CR). Green Chem. 2016, 18, 3718–3721. 10.1039/C6GC00910G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandgude A. L.; Dömling A. Convergent Three-Component Tetrazole Synthesis. Eur. J. Org. Chem. 2016, 2016, 2383–2387. 10.1002/ejoc.201600317. [DOI] [Google Scholar]
- Saiprathima P.; Srinivas K.; Sridhar B.; Rao M. M. On Water” One-Pot Synthesis of Quaternary Centered 3-Hydroxy-3-(1H-Tetrazol-5-Yl)Indolin-2-Ones. RSC Adv. 2013, 3, 7708–7712. 10.1039/c3ra00021d. [DOI] [Google Scholar]
- Sarvary A.; Shaabani S.; Shaabani A.; Ng S. A Two-Step Synthesis of 1,5-Disubstituted Tetrazoles Containing a Siloxy or Sulfonamide Group. Tetrahedron Lett. 2011, 52, 5930–5933. 10.1016/j.tetlet.2011.08.114. [DOI] [Google Scholar]
- Collibee W. L.; Nakajima M.; Anselme J.-P. 5-Halo-1-Phenyltetrazoles. J. Org. Chem. 1995, 60, 468–469. 10.1021/jo00107a032. [DOI] [Google Scholar]
- Hutchins R. O.; Hutchins M. K.. Sodium Cyanoborohydride. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd, 2001. [Google Scholar]
- Fowler F. W.; Hassner A.; Levy L. A. Stereospecific Introduction of Azide Functions into Organic Molecules. J. Am. Chem. Soc. 1967, 89, 2077–2082. 10.1021/ja00985a019. [DOI] [Google Scholar]
- Klich M.; Teutsch G. Synthese de N-(Tetrazol-5-Yl) Azetidin-2-Ones1. Tetrahedron 1986, 42, 2677–2684. 10.1016/S0040-4020(01)90553-8. [DOI] [Google Scholar]
- Kazemizadeh A. R.; Hajaliakbari N.; Hajian R.; Shajari N.; Ramazani A. Synthesis of 1,5-Disubstituted 1H-Tetrazole Derivatives via a Three-Component Reaction of Carbodiimides, Isocyanides, and Trimethylsilyl Azide. Helv. Chim. Acta 2012, 95, 594–597. 10.1002/hlca.201100327. [DOI] [Google Scholar]
- Bienaymé H.; Bouzid K. Synthesis of Rigid Hydrophobic Tetrazoles Using an Ugi Multi-Component Heterocyclic Condensation. Tetrahedron Lett. 1998, 39, 2735–2738. 10.1016/S0040-4039(98)00283-4. [DOI] [Google Scholar]
- Dömling A.; Illgen K. 1-Isocyano-2-Dimethylamino-Alkenes: Versatile Reagents in Diversity-Oriented Organic Synthesis. Synthesis 2005, 2005, 662–667. 10.1055/s-2004-831236. [DOI] [Google Scholar]
- Nixey T.; Kelly M.; Hulme C. The One-Pot Solution Phase Preparation of Fused Tetrazole-Ketopiperazines. Tetrahedron Lett. 2000, 41, 8729–8733. 10.1016/S0040-4039(00)01563-X. [DOI] [Google Scholar]
- Patil P.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Ammonia-Promoted One-Pot Tetrazolopiperidinone Synthesis by Ugi Reaction. ACS Comb. Sci. 2017, 19, 343–350. 10.1021/acscombsci.7b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umkehrer M.; Kolb J.; Burdack C.; Ross G.; Hiller W. Synthesis of Tetrazolopiperazine Building Blocks by a Novel Multi-Component Reaction. Tetrahedron Lett. 2004, 45, 6421–6424. 10.1016/j.tetlet.2004.06.133. [DOI] [Google Scholar]
- Zarganes-Tzitzikas T.; Patil P.; Khoury K.; Herdtweck E.; Dömling A. Concise Synthesis of Tetrazole–Ketopiperazines by Two Consecutive Ugi Reactions. Eur. J. Org. Chem. 2015, 2015, 51–55. 10.1002/ejoc.201403401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick A. D.; Liddle J.; Davies D. E.; Exall A. M.; Hamlett C.; Hickey D. M.; Mason A. M.; Smith I. E. D.; Nerozzi F.; Peace S.; et al. Pyridyl-2,5-Diketopiperazines as Potent, Selective, and Orally Bioavailable Oxytocin Antagonists: Synthesis, Pharmacokinetics, and In Vivo Potency. J. Med. Chem. 2012, 55, 783–796. 10.1021/jm201287w. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Patil P.; Kurpiewska K.; Kalinowska-Tluscik J.; Dömling A. Two Cycles with One Catch: Hydrazine in Ugi 4-CR and Its Postcyclizations. ACS Comb. Sci. 2017, 19, 193–198. 10.1021/acscombsci.7b00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixey T.; Kelly M.; Semin D.; Hulme C. Short Solution Phase Preparation of Fused Azepine-Tetrazoles via a UDC (Ugi/de-Boc/Cyclize) Strategy. Tetrahedron Lett. 2002, 43, 3681–3684. 10.1016/S0040-4039(02)00636-6. [DOI] [Google Scholar]
- Borisov R. S.; Polyakov A. I.; Medvedeva L. A.; Khrustalev V. N.; Guranova N. I.; Voskressensky L. G. Concise Approach toward Tetrazolo[1,5-a][1,4]Benzodiazepines via a Novel Multicomponent Isocyanide-Based Condensation. Org. Lett. 2010, 12, 3894–3897. 10.1021/ol101590w. [DOI] [PubMed] [Google Scholar]
- Chung C. W.; Coste H.; White J. H.; Mirguet O.; Wilde J.; Gosmini R. L.; Delves C.; Magny S. M.; Woodward R.; Hughes S. A.; et al. Discovery and Characterization of Small Molecule Inhibitors of the BET Family Bromodomains. J. Med. Chem. 2011, 54, 3827–3838. 10.1021/jm200108t. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P.; Qi J.; Picaud S.; Shen Y.; Smith W. B.; Fedorov O.; Morse E. M.; Keates T.; Hickman T. T.; Felletar I.; et al. Selective Inhibition of BET Bromodomains. Nature 2010, 468, 1067–1073. 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak M.; Batra S. Isonitriles from the Baylis–Hillman Adducts of Acrylates: Viable Precursor to Tetrazolo-Fused Diazepinones via Post-Ugi Cyclization. Tetrahedron Lett. 2010, 51, 510–516. 10.1016/j.tetlet.2009.11.051. [DOI] [Google Scholar]
- Basavaiah D.; Rao A. J.; Satyanarayana T. Recent Advances in the Baylis–Hillman Reaction and Applications. Chem. Rev. 2003, 103, 811–892. 10.1021/cr010043d. [DOI] [PubMed] [Google Scholar]
- Basavaiah D.; Veeraraghavaiah G. The Baylis-Hillman Reaction: A Novel Concept for Creativity in Chemistry. Chem. Soc. Rev. 2012, 41, 68–78. 10.1039/C1CS15174F. [DOI] [PubMed] [Google Scholar]
- Basavaiah D.; Reddy B. S.; Badsara S. S. Recent Contributions from the Baylis–Hillman Reaction to Organic Chemistry. Chem. Rev. 2010, 110, 5447–5674. 10.1021/cr900291g. [DOI] [PubMed] [Google Scholar]
- Raboisson P.; de Kock H.; Rosenquist Å.; Nilsson M.; Salvador-Oden L.; Lin T.-I.; Roue N.; Ivanov V.; Wähling H.; Wickström K.; et al. Structure–activity Relationship Study on a Novel Series of Cyclopentane-Containing Macrocyclic Inhibitors of the Hepatitis C Virus NS3/4A Protease Leading to the Discovery of TMC435350. Bioorg. Med. Chem. Lett. 2008, 18, 4853–4858. 10.1016/j.bmcl.2008.07.088. [DOI] [PubMed] [Google Scholar]
- Giordanetto F.; Kihlberg J. Macrocyclic Drugs and Clinical Candidates: What Can Medicinal Chemists Learn from Their Properties?. J. Med. Chem. 2014, 57, 278–295. 10.1021/jm400887j. [DOI] [PubMed] [Google Scholar]
- Villar E. A.; Beglov D.; Chennamadhavuni S.; Porco J. A.; Kozakov D.; Vajda S.; Whitty A. How Proteins Bind Macrocycles. Nat. Chem. Biol. 2014, 10, 723–731. 10.1038/nchembio.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A. D.; Fleming A.; Kelleher F.; McGinley J.; Prajapati V.; Skovsgaard S. Synthesis and Characterisation of Tetra-Tetrazole Macrocycles. Tetrahedron 2007, 63, 6835–6842. 10.1016/j.tet.2007.04.065. [DOI] [Google Scholar]
- Whitty A.; Zhong M.; Viarengo L.; Beglov D.; Hall D. R.; Vajda S. Quantifying the Chameleonic Properties of Macrocycles and Other High-Molecular-Weight Drugs. Drug Discovery Today 2016, 21, 712–717. 10.1016/j.drudis.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failli A.; Immer H.; Götz M. The Synthesis of Cyclic Peptides by the Four Component Condensation (4 CC). Can. J. Chem. 1979, 57, 3257–3261. 10.1139/v79-533. [DOI] [Google Scholar]
- Liao G. P.; Abdelraheem E. M. M.; Neochoritis C. G.; Kurpiewska K.; Kalinowska-Tłuścik J.; McGowan D. C.; Dömling A. Versatile Multicomponent Reaction Macrocycle Synthesis Using α-Isocyano-ω-Carboxylic Acids. Org. Lett. 2015, 17, 4980–4983. 10.1021/acs.orglett.5b02419. [DOI] [PubMed] [Google Scholar]
- Abdelraheem E. M. M.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Artificial Macrocycles by Ugi Reaction and Passerini Ring Closure. J. Org. Chem. 2016, 81, 8789–8795. 10.1021/acs.joc.6b01430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Jimenez G.-M.; Burgos-Hernandez A.; Ezquerra-Brauer J.-M. Bioactive Peptides and Depsipeptides with Anticancer Potential: Sources from Marine Animals. Mar. Drugs 2012, 10, 963–986. 10.3390/md10050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelraheem E. M. M.; de Haan M. P.; Patil P.; Kurpiewska K.; Kalinowska-Tłuścik J.; Shaabani S.; Dömling A. Concise Synthesis of Tetrazole Macrocycle. Org. Lett. 2017, 19, 5078–5081. 10.1021/acs.orglett.7b02319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski C.; Umkehrer M.; Gonnard S.; Jager N.; Ross G.; Hiller W. A New and Versatile Ugi/SNAr Synthesis of Fused 4,5-Dihydrotetrazolo 1,5-a Quinoxalines. Tetrahedron Lett. 2006, 47, 2041–2044. 10.1016/j.tetlet.2006.01.027. [DOI] [Google Scholar]
- Unnamatla M. V. B.; Islas-Jácome A.; Quezada-Soto A.; Ramírez-López S. C.; Flores-Álamo M.; Gámez-Montaño R. Multicomponent One-Pot Synthesis of 3-Tetrazolyl and 3-Imidazo[1,2- a]Pyridin Tetrazolo[1,5- a]Quinolines. J. Org. Chem. 2016, 81, 10576–10583. 10.1021/acs.joc.6b01576. [DOI] [PubMed] [Google Scholar]
- Koes D.; Khoury K.; Huang Y.; Wang W.; Bista M.; Popowicz G. M.; Wolf S.; Holak T. A.; Dömling A.; Camacho C. J. Enabling Large-Scale Design, Synthesis and Validation of Small Molecule Protein-Protein Antagonists. PLoS One 2012, 7, e32839 10.1371/journal.pone.0032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koes D. R.; Dömling A.; Camacho C. J. AnchorQuery: Rapid Online Virtual Screening for Small-Molecule Protein-Protein Interaction Inhibitors. Protein Sci. 2018, 27, 229–232. 10.1002/pro.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E.; Schulze J. O.; Süß E.; Camacho C. J.; Biondi R. M.; Dömling A. Discovery of a Potent Allosteric Kinase Modulator by Combining Computational and Synthetic Methods. Angew. Chem., Int. Ed. 2015, 54, 13933–13936. 10.1002/anie.201506310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaabani S.; Neochoritis C. G.; Twarda-Clapa A.; Musielak B.; Holak T. A.; Dömling A. Scaffold Hopping via ANCHOR.QUERY: β-Lactams as Potent P53-MDM2 Antagonists. MedChemComm 2017, 8, 1046–1052. 10.1039/C7MD00058H. [DOI] [PMC free article] [PubMed] [Google Scholar]