Abstract
Electronic cigarettes (ECIGs) are battery-powered devices that heat and vaporize solutions containing propylene glycol (PG) and/or vegetable glycerin (VG), nicotine and possible trace flavorants to produce an inhalable aerosol. The heating process can lead to the formation of reactive oxygen species (ROS), which are linked to various oxidative damage-initiated diseases. Several studies in the literature have addressed ROS emissions in ECIG aerosols, but the effects of power, ECIG device design and liquid composition on ROS are relatively unknown. In addition, ROS emissions have not been examined in the emerging high power, sub-Ohm device (SOD) category. In this study, an acellular 2',7'-dichlorofluorescin (DCFH) probe technique was optimized to measure ROS in ECIG aerosols. The technique was deployed to measure ROS emissions in SOD and supra-Ohm ECIGs while varying power, heater coil head design and liquid composition (PG/VG ratio and nicotine concentration). Liquids were made from analytical standards of PG, VG and nicotine and contained no flavorants. At high powers, ROS emissions in ECIGs and combustible cigarettes were similar. Across device designs, ROS emissions were uncorrelated with power (R2 = 0.261) but were highly correlated with power per unit area (R2 = 0.78). It was noticed that an increase in the VG percentage in the liquid yielded higher ROS flux, and nicotine did not affect ROS emissions. ROS emissions are a function of device design and liquid composition at a given power. For a given liquid composition, a promising metric for predicting ROS emissions across device designs and operating conditions is power per unit area of the heating coil. Importantly, ROS formation is significant even when the ECIG liquid consists of pure analytical solutions of PG and VG; it can therefore be viewed as intrinsic to ECIG operation and not solely a by-product of particular flavorants, contaminants or additives.
Introduction
Electronic cigarette (ECIG) use has become an epidemic worldwide, especially among youth (1, 2). ECIG use prevalence among cigarette smokers, former smokers and previously nicotine-naïve groups alike has increased tremendously in the last decade (3, 4). While it is often claimed that ECIGs are good smoking cessation tools (5), the issue is still controversial and empirical data to resolve it is sparse (6). On the other hand, ECIGs may renormalize smoking among users and bystanders (7, 8) and may initiate nicotine dependence among young users, potentially constituting a gateway to cigarette smoking (9–13).
ECIGs are battery-powered devices that heat and vaporize solutions mainly consisting of propylene glycol (PG) and/or vegetable glycerin (VG) and nicotine to generate inhalable aerosols (14) that are not toxicant-free (15). Toxicants detected in ECIG aerosols are either present in the liquid solutions even prior to heating (16, 17) or are produced via the thermal decomposition of the liquid constituents on the hot surface of the heating coil (18–20). The most studied toxicants formed in situ are carbonyl compounds that result from the dehydration and oxidation of the alcohol functional groups on a metal surface (21). Other toxicants include furanic and aromatic compounds, which have been identified when additives such as sugar and fruit flavors are present in the mix (22, 23). In addition to carbonyls, thermal breakdown of chemical bonds in ECIG liquids may lead to the formation of reactive oxygen species (ROS), a class of chemicals, which induce oxidative stress in cells.
It has been well established that oxidative stress from cigarette smoke exposure leads to pulmonary diseases (24–27). A growing number of studies have linked ROS emissions from ECIG to cytotoxicity in pulmonary tissues (28–30). Several studies in the literature have reported ROS emissions in ECIG aerosols using cellular and acellular assays (31–36). In addition, electron paramagnetic resonance (EPR) studies have revealed the presence of radical species in ECIG aerosols (37, 38), and a recent report by Zhao et al. assessed the effect of various parameters, including brand, flavor, power and users’ puffing regimens, on the generation of ROS (38). In this work, we used an optimized 2′,7′-dichlorofluorescin (DCFH) probe solution in order to measure ROS emissions from conventional tank and sub-Ohm ECIG devices (SODs) as a function of power, coil head geometry and ECIG liquid composition (PG/VG ratio, nicotine content). ROS emissions were compared across conditions to ROS emissions from combustible cigarette and plotted vs power per coil surface area, which we have recently shown as the relevant predictor of ECIG toxicant emissions that are formed in situ (39).
Materials and Methods
Materials
PG (99.5%) (CAS № 57-55-6), VG (99–101%) (CAS № 56-81-5), ethanol and deionized (DI) water were procured from Sigma-Aldrich. Pure nicotine (CAS № 54-11-5), horseradish peroxidase (HRP) (52 units/mg) (CAS № 9003-99-0), potassium phosphate monobasic (CAS № 7778-77-0) and dibasic (CAS № 7758-11-4) were purchased from Sigma-Aldrich. 2′,7′-dichlorofluorescin diacetate (DCFH-DA) was purchased from Molecular Probes (product code D399). Quartz filters (ADVENTEC, QR-100.47 mm) were procured from Whatman International.
Preparation of DCFH probe solution
DCFH-DA was dissolved in ethanol in order to prepare a 125 μM solution. The DCFH-DA solution (10 mL) was deacetylated with 40 ml of 0.01 M NaOH aqueous solution. The activated DCFH solution was wrapped in aluminum foil and kept in the dark for 30 min. A phosphate buffer (pH = 7.1), prepared by mixing monobasic and dibasic potassium phosphate to attain a 0.25 mM concentration (200 ml), was added to 50 mL of DCFH solution. Horseradish peroxidase (0.5 units/mL) was added (2.4 mg) to amplify the fluorescence signal. The final 250 mL working solution had a concentration of 5 μM of DCFH. A linear calibration curve (1 × 10−7 to 10−6 M) was constructed using hydrogen peroxide (H2O2) to express ROS equivalents. The limit of detection (LOD) was 0.14 × 10−7 M, and the limit of quantification (LOQ) was 0.48 × 10−7 M of H2O2. LOD and LOQ of the method were calculated according to the equations mentioned in the eighth edition of Quantitative Chemical Analysis by Daniel C. Harris (p. 103–106), which is LOD = 3 SD/m and LOQ = 10 SD/m, where SD stands for standard deviation of replicates of a low concentration (C = 0.5 × 10−7 M of H2O2) and m is the slope of the calibration curve.
Probe solution
The optimal experimental conditions of the DCFH solution were determined so that the photo- and auto-oxidation of the probe solution were minimized (40). Several combinations of DCFH concentration, storage temperature and duration, and mixing time were tested in order to achieve this goal. The final probe was a 5 μM DCFH solution, stored at 4°C and mixed for 30 min with the samples (41, 42). This solution provided a >98% calibration R2 with <6% bias error due to auto-oxidation at the maximum allowed solution storage time of 2.5 h. All storage and reacting samples were wrapped in an aluminum foil to prevent photo-oxidation.
Aerosol generation
The American University of Beirut’s aerosol lab vaping instrument (ALVIN) (43) was used to generate ECIG aerosols. Puff duration, inter-puff interval and flow rate were selected to represent the pattern of an “experienced” ECIG user (4-s puff duration, 10-s inter-puff interval, 1 L/min flow rate) (43). A vaping session constituted from five puffs on a supra-Ohm ECIG and two puffs on a sub-Ohm ECIG, both sessions having a 4-s puff duration, a 10-s puff interval and a volume of 67 mL/puff. In the case of the conventional cigarette, 10 puffs were executed using ISO protocol puffing parameters (2-s puff duration, 60-s inter-puff interval, 35 mL/puff). For both the ECIG and combustible cigarette conditions, the aerosol was drawn through a particulate filter trap as described by Zhao and Hopke (41).
Study design and sampling
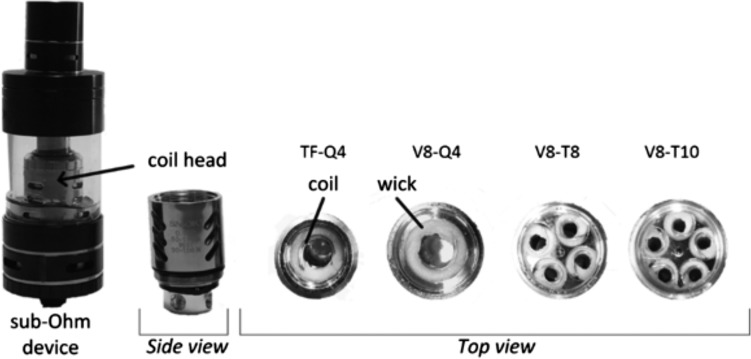
ROS emissions in the total particulate matter (TPM) of ECIG aerosols and the smoke of the tobacco cigarettes were assessed, as it was previously found that ROS concentrations in the particle phase are much greater than in the gas phase (41). TPM was trapped on a 47-mm quartz filter installed at the mouth end of the ECIG and the tobacco cigarettes and then directly immersed in 20 mL of a freshly prepared DCFH probe solution. Fluorescence was read on a SpectraMax M5 microplate reader acting as a fluorimeter. Liquids containing 50/50 PG/VG solution with 12 mg/mL of nicotine were vaped in a VaporFi platinum tank at two different powers (5 and 11 W) and in a sub-Ohm SmokTFV8 device equipped with a V8-T8 coil head (eight coils) at five different powers (50, 75, 100, 150 and 200 W). Keeping the power and liquid constant at 50 W and using a 50/50 PG/VG solution with 12 mg/mL of nicotine, the effect of different coil heads was assessed using the sub-Ohm device (SOD) equipped with V8-Q4 (4 coils), V8-T8 (8 coils), V8-T10 (10 coils) and TF-Q4 (4 coils) (Figure 1). Three PG/VG ratios were prepared from standard liquid PG and VG (100/0, 50/50 and 0/100 PG/VG ratios), and three different nicotine loads in a 50/50 PG/VG solution were tested (0, 6 and 12 mg/mL nicotine concentrations). These solutions were vaped at two different powers for each device (5 and 11 W for supra-Ohm device and 50 and 150 W for SOD). Each condition was repeated in triplicate, and the results are reported as the mean of three measurements after blank subtraction.
Figure 1.
The sub-Ohm SmokTFV8 device with illustration of the different coil heads (retrieved from the study of Talih et al. (39)).
TPM, surface area, and ROS flux
The amount of TPM was determined gravimetrically by weighing the filter pad and its holder before and after each sampling session. The total surface area of the coil was calculated based on the coil wire diameter (measured using calipers), the length of coil wire and the number of coils (39). ROS emissions are reported as the number of moles of H2O2 equivalent per second of vaping/smoking in order to facilitate comparison between different puffing regimens.
Statistical analysis
T-test was used to estimate the statistical significance of the difference between powers relative to the lowest power for each ECIG and relative to the combustible cigarette level. It was also used to assess the effect of liquid composition (PG/VG ratio and nicotine content) on ROS emission.
Results
Effect of power and power per unit coil surface area
ROS emission rates as a function of power are shown in Table I for VaporFi Platinum and SmokTFV8 SOD. In the supra-Ohm device, the ROS flux in the aerosols generated using 11 W was three times higher than that of 5 W (P < 0.1). In the SOD, the ROS flux showed an increase between 50 and 200 W (P < 0.1). ROS emissions at the highest power tested (200 W) in SOD device was comparable to those of conventional cigarettes (Table I).
Table I.
ROS flux as a function of power and coil head in VaporFi Platinum and SmokTFV8 SOD devices in comparison to a conventional cigarette. Statistical significance is shown in comparison with the conventional cigarette
| ECIG | Coil head | Power (W) | ROS flux (nmole/s) |
|---|---|---|---|
| VaporFi | Single coil | 5 | 0.238 ± 0.253** |
| 11 | 0.696 ± 0.096* | ||
| SmokTFV8 SOD | V8-T8 | 50 | 0.114 ± 0.034** |
| 75 | 0.109 ± 0.042** | ||
| 100 | 0.167 ± 0.117** | ||
| 150 | 0.241 ± 0.029* | ||
| 200 | 1.143 ± 0.606 | ||
| V8-Q4 | 50 | 0.066 ± 0.030** | |
| V8-T10 | 50 | 0.045 ± 0.019** | |
| TF-Q4 | 50 | 0.049 ± 0.016** | |
| Tobacco cig | 1.240 ± 0.210 |
Significant difference from tobacco cigarette level: *P < 0.05, **P < 0.01.
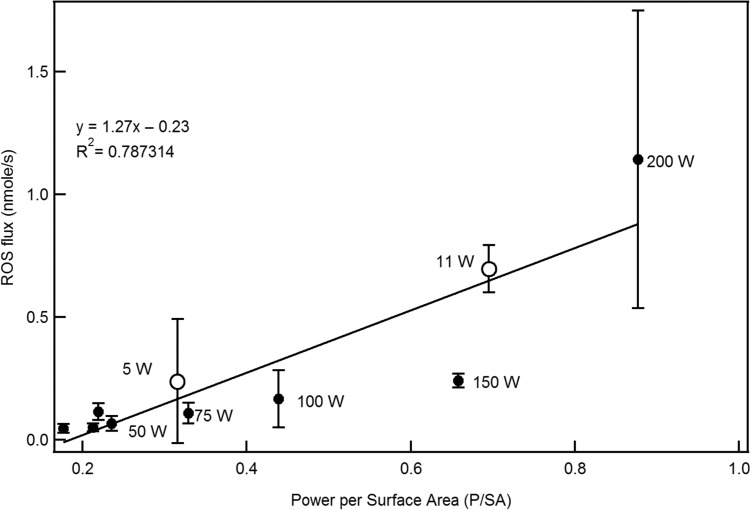
Holding power and liquid composition constant, there was a significant difference only between the coil head V8-T8 and both V8-T10 and TF-Q4 (P < 0.1). ROS emission was weakly correlated with power across devices (R2 = 0.26); however, a significant correlation was found when the ROS flux was plotted as a function of power per surface area, P/SA (R2 = 0.78), as shown in Figure 2. The two data points of the supra-Ohm device seem to fit well within this correlation.
Figure 2.
ROS flux as a function of power per surface area of the coil. Filled circles correspond to SOD, while empty circles correspond to supra-Ohm.
Effect of liquid composition
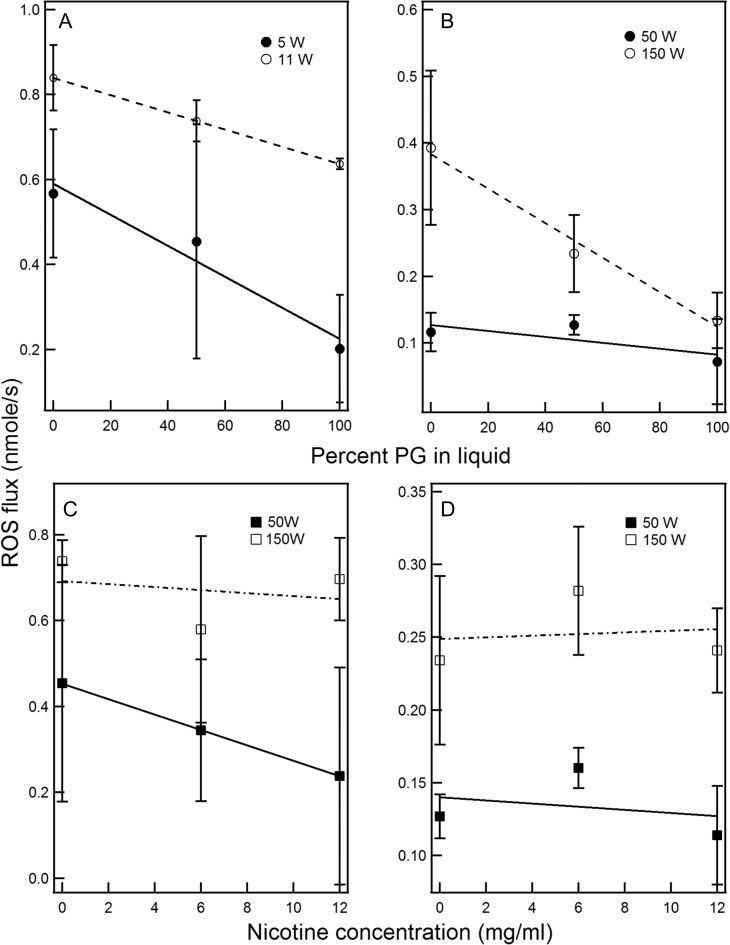
The effect of the PG/VG ratio on ROS emissions from ECIG is shown in Figure 3A and B for the VaporFi and the SmokTFV8 SOD. In both devices, ROS flux trended downward with increasing VG content and attained significant difference between pure PG and VG liquids (P < 0.05). On the other hand, nicotine concentration did not have any effect on ROS emissions (Figures 3C and D).
Figure 3.
ROS flux as a function of the PG/VG ratio in the liquid vaped on the supra-Ohm device (A) and SOD (B). ROS flux vs nicotine content in the vaped liquid on the supra-Ohm device (C) and SOD (D).
Discussion
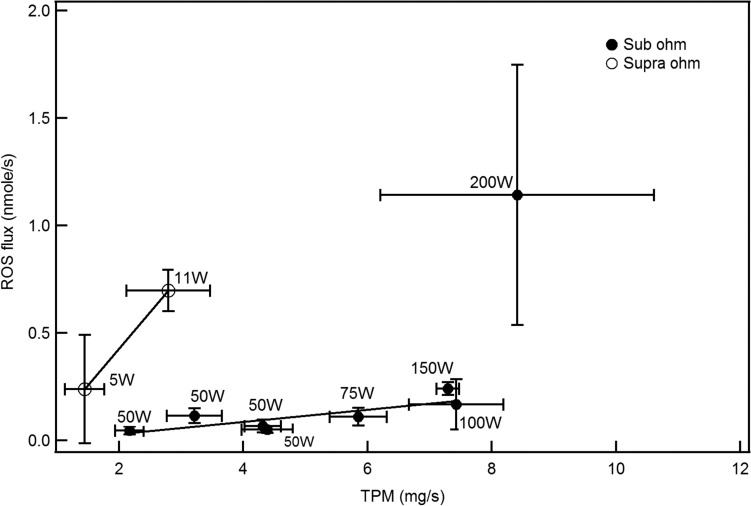
Our results showed that ROS flux in tank and SOD ECIGs increases with power within the same device design. At high powers, ROS emissions from both ECIGs, especially the SOD, can reach levels that are similar to those of tobacco cigarettes. Higher powers have been associated with elevated temperatures on the coil surface causing an increase in the TPM emitted and/or a higher probability of the degradation of the chemical bonds in the molecules of the vaped liquids (38). ROS emission is not always significantly affected by the coil head design. In this study, we showed that the ROS flux is significantly correlated (78%) with P/SA, supporting the theory that P/SA is a better predictor of toxicant emissions in general than the power or the number of coils (39). The surge of the ROS flux level at 200 W is not linked to an increase in TPM (Figure 4), and therefore high ROS flux in this particular case can be ascribed to a spike in temperature caused by the “dry puff” phenomenon.
Figure 4.
ROS flux (nmole/s) vs TPM flux (mg/s) in both devices at different powers and with different coil heads in the case of the SOD.
The chemical degradation of the ECIG liquids—PG, VG and nicotine—are thought to play a determinant role in ROS emissions. In this study, we showed that an increase in the VG percentage in the liquid yielded higher ROS flux, and this may be due to the emission of higher ROS from VG molecules or to a slower wicking and consequently higher probabilities of the “dry puff” phenomenon, particularly at high powers. This is in disagreement with a recent paper in which a higher PG/VG ratio in the liquid was correlated with higher radical emissions from ECIGs (44). Our study also showed that nicotine does not affect ROS emissions, which are mainly a function of the chemical nature of the solvent and the P/SA of the coil.
Conclusion
Our results showed that ECIGs intrinsically emit ROS even in the absence of flavorants. ROS levels from conventional tank ECIGs and SODs at high powers could reach tobacco cigarette-like levels. P/SA is a better predictor of ROS emissions than power. In addition, ROS emission is affected by the chemical constituents of the vaped liquid (PG/VG ratio). Toxicant flux is an easy tool to compare the results across different puffing regimens and among studies.
Funding
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (grant number P50DA036105) and the Center for Tobacco Products of the US Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1. US-Surgeon-General. E-cigarette Use Among Youth and Young Adults: A Report of the Surgeon General 2016. https://e-cigarettes.surgeongeneral.gov/getthefacts.html; https://e-cigarettes.surgeongeneral.gov/documents/2016_SGR_Full_Report_508.pdf (accessed Nov 14, 2017).
- 2. Rennie L.J., Bazillier-Bruneau C., Rouëssé J. (2016) Harm reduction or harm introduction? Prevalence and correlates of e-cigarette use among French adolescents. Journal of Adolescent Health, 58, 440–445. [DOI] [PubMed] [Google Scholar]
- 3. Cho H.-J., Dutra L.M., Glantz S.A. (2017) Differences in adolescent e-cigarette and cigarette prevalence in two policy environments: South Korea and the United States. Nicotine and Tobacco Research, ntx198 10.1093/ntr/ntx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dai H., Hao J. (2017) Direct marketing promotion and electronic cigarette use among US adults, National Adult Tobacco Survey, 2013–2014. Preventing Chronic Disease, 14, 170073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giovenco D.P., Delnevo C.D. (2018) Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers. Addictive Behaviors, 76, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartmann-Boyce J., McRobbie H., Bullen C., Begh R., Stead L.F., Hajek P. (2016) Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews, 9, CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cataldo J.K., Petersen A.B., Hunter M., Wang J., Sheon N. (2015) E-cigarette marketing and older smokers: road to renormalization. American Journal of Health Behavior, 39, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sæbø G., Scheffels J. (2017) Assessing notions of denormalization and renormalization of smoking in light of e-cigarette regulation. International Journal of Drug Policy, 49, 58–64. [DOI] [PubMed] [Google Scholar]
- 9. Etter J.-F. (2017) Gateway effects and electronic cigarettes. Addiction. 10.1111/add.13924n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 10. Akre C., Suris J.-C. (2017) Adolescents and young adults’ perceptions of electronic cigarettes as a gateway to smoking: a qualitative study in Switzerland. Health Education Research, 32, 448–454. [DOI] [PubMed] [Google Scholar]
- 11. Barrington-Trimis J.L., Urman R., Berhane K., Unger J.B., Cruz T.B., Pentz M.A., et al. (2016) E-cigarettes and future cigarette use. Pediatrics, 138, e20160379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stanbrook M.B. (2016) Electronic cigarettes and youth: a gateway that must be shut. Canadian Medical Association Journal, 188, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gautier S., Kinouani S., Raherison C. (2017) Do electronic cigarettes increase the risk of smoking among adolescents and young adults? Santé Publique, 29, 333–340. [PubMed] [Google Scholar]
- 14. Breland A., Soule E., Lopez A., Ramôa C., El-Hellani A., Eissenberg T. (2017) Electronic cigarettes: what are they and what do they do? Annals of the New York Academy of Sciences, 1394, 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goniewicz M.L., Gawron M., Smith D.M., Peng M., Jacob I.I.I.P., Benowitz N.L. (2017) Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine and Tobacco Research, 19, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beauval N., Howsam M., Antherieu S., Allorge D., Soyez M., Garçon G., et al. (2016) Trace elements in e-liquids—development and validation of an ICP-MS method for the analysis of electronic cigarette refills. Regulatory Toxicology and Pharmacology, 79, 144–148. [DOI] [PubMed] [Google Scholar]
- 17. Lisko J.G., Tran H., Stanfill S.B., Blount B.C., Watson C.H. (2015) Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine and Tobacco Research, 17, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khlystov A., Samburova V. (2016) Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environmental Science and Technology, 50, 13080–13085. [DOI] [PubMed] [Google Scholar]
- 19. Margham J., McAdam K., Forster M., Liu C., Wright C., Mariner D., et al. (2016) Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chemical Research in Toxicology, 29, 1662–1678. [DOI] [PubMed] [Google Scholar]
- 20. Talih S., Balhas Z., Salman R., Karaoghlanian N., Shihadeh A. (2016) “Direct dripping”: a high-temperature, high-formaldehyde emission electronic cigarette use method. Nicotine and Tobacco Research, 18, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uchiyama S., Senoo Y., Hayashida H., Inaba Y., Nakagome H., Kunugita N. (2016) Determination of chemical compounds generated from second-generation e-cigarettes using a sorbent cartridge followed by a two-step elution method. Analytical Sciences, 32, 549–555. [DOI] [PubMed] [Google Scholar]
- 22. Soussy S., EL-Hellani A., Baalbaki R., Salman R., Shihadeh A., Saliba N.A. (2016) Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tobacco Control, 25, ii88–ii93. [DOI] [PubMed] [Google Scholar]
- 23. Pankow J.F., Kim K., McWhirter K.J., Luo W., Escobedo J.O., Strongin R.M., et al. (2017) Benzene formation in electronic cigarettes. PLoS One, 12, e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartal M. (2005) COPD and tobacco smoke. Monaldi Archives for Chest Disease, 63, 213–225. [DOI] [PubMed] [Google Scholar]
- 25. Bialas A.J., Sitarek P., Mikowska-Dymanowska J., Piotrowski W.J., Gorski P. (2016) The role of mitochondria and oxidative/antioxidative imbalance in pathobiology of chronic obstructive pulmonary disease. Oxidative Medicine and Cellular Longevity, doi: 10.1155/2016/7808576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. US-Surgeon-General. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. 2010. https://www.ncbi.nlm.nih.gov/books/NBK53021/#ch7.s17 (accessed Nov 14, 2017). [PubMed]
- 27. Sundar I.K., Yao H., Rahman I. (2012) Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxidants and Redox Signaling, 18, 1956–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moses E., Wang T., Corbett S., Jackson G.R., Drizik E., Perdomo C., et al. (2017) Molecular impact of electronic cigarette aerosol exposure in human bronchial epithelium. Toxicological Sciences, 155, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Javed F., Kellesarian S.V., Sundar I.K., Romanos G.E., Rahman I. (2017) Recent updates on electronic cigarette aerosol and inhaled nicotine effects on periodontal and pulmonary tissues. Oral Diseases, 23, 1052–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ganapathy V., Manyanga J., Brame L., McGuire D., Sadhasivam B., Floyd E., et al. (2017) Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One, 12, e0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lerner C.A., Rutagarama P., Ahmad T., Sundar I.K., Elder A., Rahman I. (2016) Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochemical and Biophysical Research Communications, 477, 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lerner C.A., Sundar I.K., Watson R.M., Elder A., Jones R., Done D., et al. (2015) Environmental health hazards of e-cigarettes and their components: Oxidants and copper in e-cigarette aerosols. Environmental Pollution, 198, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lerner C.A., Sundar I.K., Yao H., Gerloff J., Ossip D.J., McIntosh S., et al. (2015) Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One, 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rubenstein D.A., Hom S., Ghebrehiwet B., Yin W. (2015) Tobacco and e-cigarette products initiate Kupffer cell inflammatory responses. Molecular Immunology, 67, 652–660. [DOI] [PubMed] [Google Scholar]
- 35. Shaito A., Saliba J., Husari A., El-Harakeh M., Chhouri H., Hashem Y., et al. (2017) Electronic cigarette smoke impairs normal mesenchymal stem cell differentiation. Scientific Reports, 7, 14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson C., Majeste A., Hanus J., Wang S. (2016) E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicology Science, 154, 332–340. [DOI] [PubMed] [Google Scholar]
- 37. Goel R., Durand E., Trushin N., Prokopczyk B., Foulds J., Elias R.J., et al. (2015) Highly reactive free radicals in electronic cigarette aerosols. Chemical Research in Toxicology, 28, 1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao J., Zhang Y., Sisler J.D., Shaffer J., Leonard S.S., Morris A.M., et al. (2018) Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. Journal of Hazardous Materials, 344, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Talih S., Salman R., Karaoghlanian N., El-Hellani A., Saliba N., Eissenberg T., et al. (2017) “Juice monsters”: sub-Ohm vaping and toxic volatile aldehyde emissions. Chemical Research in Toxicology, 30, 1791–1793. [DOI] [PubMed] [Google Scholar]
- 40. Chen X., Zhong Z., Xu Z., Chen L., Wang Y. (2010) 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: forty years of application and controversy. Free Radical Research, 44, 587–604. [DOI] [PubMed] [Google Scholar]
- 41. Zhao J., Hopke P.K. (2012) Concentration of reactive oxygen species (ROS) in mainstream and sidestream cigarette smoke. Aerosol Science and Technology, 46, 191–197. [Google Scholar]
- 42. Wang Y., Hopke P.K., Sun L., Chalupa D.C., Utell M.J. (2011) Laboratory and field testing of an automated atmospheric particle-bound reactive oxygen species sampling-analysis system. Journal of Toxicology, 2011, 419476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Talih S., Balhas Z., Eissenberg T., Salman R., Karaoghlanian N., El Hellani A., et al. (2015) Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine and Tobacco Research, 17, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bitzer Z.T., Goel R., Reilly S.M., Foulds J., Muscat J., Elias R.J., et al. (2018) Effects of solvent and temperature on free radical formation in electronic cigarette aerosols. Chemical Research in Toxicology, 31, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]