Abstract
Pyrrolizidine alkaloids (PAs) are common constituents of many plant species around the world. PA-containing plants are probably the most common poisonous plants affecting livestock and wildlife. They can inflict harm to humans through contaminated food sources, herbal medicines and dietary supplements. Half of the identified PAs are genotoxic and many of them are tumorigenic. The mutagenicity of PAs has been extensively studied in different biological systems. Upon metabolic activation, PAs produce DNA adducts, DNA cross-linking, DNA breaks, sister chromatid exchange, micronuclei, chromosomal aberrations, gene mutations and chromosome mutations in vivo and in vitro. PAs induced mutations in the cII gene of rat liver and in the p53 and K-ras genes of mouse liver tumors. It has been suggested that all PAs produce a set of (±)-6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine-derived DNA adducts and similar types of gene mutations. The signature types of mutations are G : C → T : A transversion and tandem base substitutions. Overall, PAs are mutagenic in vivo and in vitro and their mutagenicity appears to be responsible for the carcinogenesis of PAs. Published in 2010 by John Wiley & Sons, Ltd.
Keywords: pyrrolizidine alkaloid, genotoxicity, mutation, DNA damage, carcinogenesis, mutational signature
INTRODUCTION
Occurrence and Toxicity
Pyrrolizidine alkaloids (PAs) are constitutively formed in many plant species around the world. More than 660 PAs and PA N-oxides have been identified in over 6000 plants mainly contained in the Boraginaceae, Compositae and Leguminosae families (Roeder, 1995, 2000; Stegelmeier et al., 1999). About half of these PAs formed are toxic. Therefore, The PA-containing plants are poisonous to livestock and wildlife and have caused tremendous livestock loss (Arzt and Mount, 1999; de Lanux-Van Gorder, 2000; Fletcher et al., 2009; Fowler, 1968; Knight et al., 1984; Seaman, 1978, 1987; Sharrock, 1969; van der Watt et al., 1972; Wiltjer and Walker, 1974). PAs are also the leading plant toxins associated with disease in humans through contamination of staple foods, honey, milk, herbal teas and herbal medicines (Arseculeratne et al., 1981, 1985; Bach et al., 1989; Bah et al., 1994; Culvenor et al., 1981; Deinzer et al., 1977; Dickinson et al., 1976; Edgar et al., 1992, 2002; Huxtable et al., 1986; Jago, 1969; Kumana et al., 1983: 1985; Mattocks, 1980; Ridker et al., 1985; Roitman, 1981; Steenkamp et al., 2000; White et al., 1984; Zhao et al., 1989).
Chemical Structure
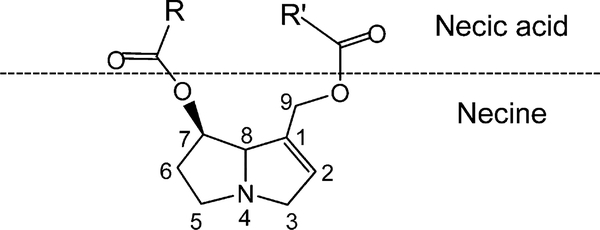
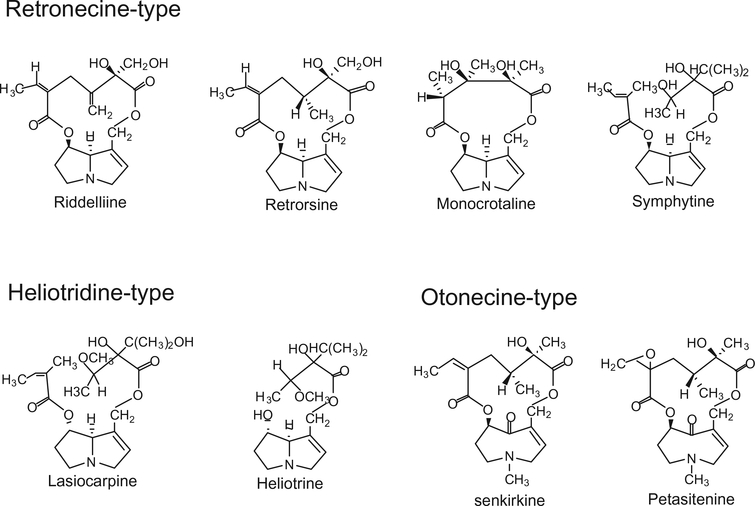
PAs are ester alkaloids composed of a necine (two fused five-membered rings joined by a single nitrogen atom) and a necic acid (one or two carboxylic ester arms) (Fig. 1). Toxic PAs are esters of unsaturated necines having a 1,2 double bond. Structures of several representative carcinogenic PAs are shown in Fig. 2. Riddelliine, retrosine, monocrotaline and symphytine are retronecine-type PAs; lasiocarpine and heliotrine are heliotridine-type PAs; and senkirkine and petasitenine are otonecine-type PAs. In contrast to otonecine-type PAs that contain monocyclic necines, retronecine- and heliotridine-type PAs have bicyclic necine bases and they are enantiomers each other at the C7 position, the retronecine-type PAs possessing an R absolute configuration and the heliotridine-type PAs with an S stereochemistry.
Figure 1.
Schematic structure of pyrrolizidine alkaloid.
Figure 2.
Structures of representative carcinogenic pyrrolizidine alkaloids.
Metabolism
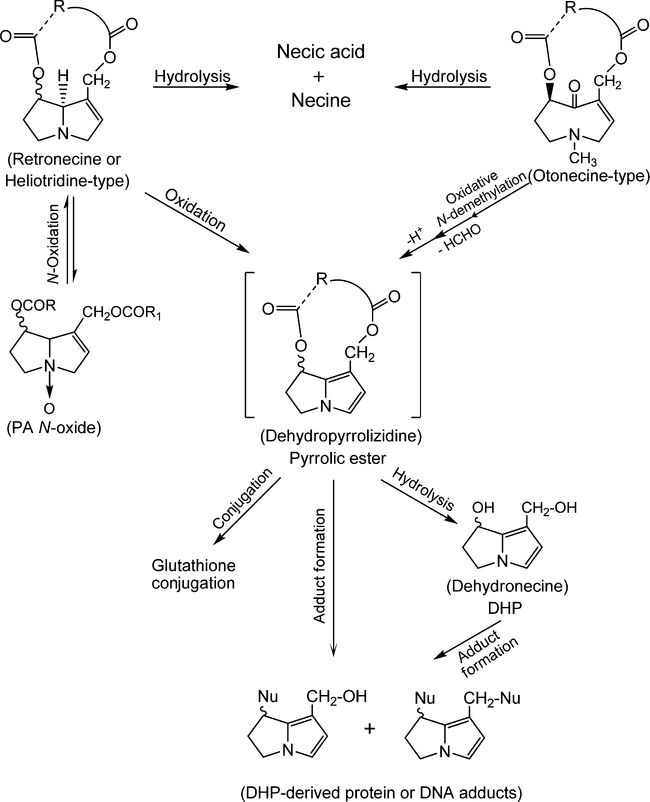
As with many other xenobiotics, PAs require biotransformation to introduce reactive or polar groups so that they can be conjugated to form polar metabolites for excretion out of the body. There are three principal pathways for PA metabolism, including hydrolysis of PAs to release necines and necic acids, N-oxidation to form PA N-oxides and oxidation of PAs to produce dehydropyrrolizidine (pyrrolic ester) derivatives (Fig. 3). The liver is the main organ for PA metabolism, although metabolism in other tissues has also been identified (Lafranconi and Huxtable, 1984).
Figure 3.
Metabolism of pyrrolizidine alkaloids.
When PAs are absorbed into tissues like liver and lung through blood, some of them are cleaved into necines and necic acids by nonspecific esterases. The necines and necic acids are not toxic and the necines may undergo further conjugation to be excreted via the kidneys and urine (Roeder, 1995). This metabolic pathway is an important detoxification route. Rats are very susceptible to toxicity of PAs, partially if not totally, due to lack esterase activity in their livers, whereas guinea pigs possess marked resistance to the toxic effects of PAs because of their particularly high liver esterase activity (Dueker et al., 1992).
Retronecine- and heliotridine-type PAs can become PA N-oxides via N-oxidation of the necine bases while otonecine-type PAs cannot form PA N-oxides because the nitrogen in their necine base is methylated. PA N-oxides are generally regarded as detoxification products because the metabolites can be conjugated for excretion (Williams et al., 1989a, b). However, PA N-oxides can be metabolically converted back to their parent PAs to produce toxic/tumorigenic effects if their parent PAs are toxic/ tumorigenic (Chou et al., 2003b; Mattocks, 1971; Wang et al., 2005c; Yan et al., 2008).
Pyrrolic esters are produced through hydroxylation of the necine base of retronecine- and heliotridine-type PAs at the C3 and C8 position to form 3- or 8-hydroxynecine derivatives followed by spontaneous dehydration. For otonecine-type PA, pyrrolic esters are generated through oxidative N-demethylation of the necine base followed by ring closure and dehydration (Fu et al., 2004). Pyrrolic ester metabolites are very reactive and can bind to one or two molecules of glutathione to form glutathione conjugates for excretion. They also can bind to DNA and proteins to generate DNA adducts, protein adducts and DNA and protein cross-links. Thus, metabolic formation of pyrrolic ester metabolites has been considered as the primary metabolic activation for the genotoxicity and carcinogenicity of PAs. In addition, less reactive longer-lived (±)-6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine (DHP) produced by hydrolysis of pyrrolic esters is a considered secondary toxic metabolite due to its antimitotic, mutagenic and carcinogenic effects (Fu et al., 2004; Prakash et al., 1999).
Carcinogenicity and Hepatotoxicity
Many PA-containing plants and individual PA compounds have been tested in animal models and shown to be carcinogenic in different tissues. The liver is the main carcinogenic target. However, tumors induced by PAs also were found in lung, kidney, skin, bladder, brain and spinal cord, pancreatic islets and adrenal gland (Allen et al., 1975; Brandange et al., 1970; Chan et al., 1994; Cook et al., 1950; Furuya et al., 1976; Harris and Chen, 1970; Hirono et al., 1976, 1977, 1978, 1979, 1983; Johnson et al., 1978; Kuhara et al., 1980; Mattocks and Cabral, 1982; Mori et al., 1984; Peterson et al., 1983; Rao et al., 1983; Rao and Reddy, 1978; Schoental, 1975; Schoental and Cavanagh, 1972; Schoental et al., 1954, 1970, 1971; Schoental and Head, 1957; Shumaker et al., 1976; Svoboda and Reddy, 1972, 1974; Williams, 1970). Despite no clear evidence that PAs induce tumors in humans, the frequent occurrence of primary liver tumors in the natives of Central Africa and South Africa has been associated with the consumption of traditional medicinal PA-containing plants (Pavlica and Samuel, 1970; Schoental, 1968; Schoental and Coady, 1968; Williams et al., 1967). It has been reported that PA-containing plants induce hepatic veno-occlusive disease in humans. This disease is regarded as specific for PA intoxication. The clinical symptoms usually occur suddenly and include vomiting, enlargement of the liver and bleeding diarrhea. Children are more sensitive to PA intoxications than adults (Bach et al., 1989; Bras et al., 1954; Kumana et al., 1985; Mohabbat et al., 1976; Ortiz Cansado et al., 1995; Ridker and McDermott, 1989; Ridker et al., 1985; Schoental and Coady, 1968; Sperl et al., 1995; Tandon et al., 1976; Weston et al., 1987; Yeong et al., 1990).
In this article, we review and update information on the genotoxicity and mutagenicity of PAs, including primary DNA damage, chromosome damage and mutations induced by PAs or PA-containing plants. For detailed information concerning the chemical and books and other review articles on PAs and PA-containing plants (Cheeke, 1988; Fu et al., 2004; IARC, 1976; IPCS, 1988; Mattocks, 1986; Prakash et al., 1999; Robins, 1984; Roeder, 1995, 2000; Stegelmeier et al., 1999).
Primary DNA Damage Induced by PAs
DNA adducts
Metabolism of PAs in vitro and in vivo generates DHP-derived DNA adducts (Table 1). Candrian et al. (1985) treated rats of both sexes with tritiated senecipylline and senecionine and determined covalent binding of the alkaloids to DNA using HPLC/radioactivity analysis of hydrolyzed DNA 6 h or 4–5 days after the treatment. They found PA-derived DNA adducts in rat livers, lungs and kidneys. The DNA damage was induced 6 h after treatment and persisted during the following 4 days. However, the DNA adducts were not isolated and characterized.
Table 1.
Primary DNA damage induced by pyrrolizidine alkaloids
| Agent | Testing system | Result | Reference |
|---|---|---|---|
| DNA adducts Comfrey root extract/comfrey compound oil | In vivo 32P-postlabeling/HPLC analysis | DHP-derived DNA adducts were formed in rat liver | Chou and Fu (2006) |
| Clivorine | In vitro 32P-postlabeling/HPLC analysis with metabolic activation | DHP-derived DNA adducts were formed in vitro | Xia et al. (2004) |
| Coltsfoot root extract | In vivo 32P-postlabeling/HPLC analysis | DHP-derived DNA adducts were formed in rat liver | Chou and Fu (2006) |
| Flos farfara Extract | In vivo 32P-postlabeling/HPLC analysis | DHP-derived DNA adducts were formed in rat liver | Chou and Fu (2006) |
| Heliotrine | In vitro 32P-postlabeling/HPLC analysis with metabolic activation | DHP-derived DNA adducts were formed | Xia et al. (2008) |
| Ligularia hodgsonnii extracts | In vitro 32P-postlabeling/HPLC analysis with metabolic activation | DHP-derived DNA adducts were formed | Xia et al. (2004) |
| Lasiocarpine | In vitro 32P-postlabeling/HPLC analysis with metabolic activation | DHP-derived DNA adducts were formed | Xia et al. (2006) |
| Monocrotaline | In vivo or in vitro with metabolic activation 32P-postlabeling/HPLC analysis | DHP-derived DNA adducts were formed in vitro and in rat liver | Wang et al. (2005b) |
| Retrorsine | In vivo or in vitro with metabolic activation 32P-postlabeling/HPLC analysis | DHP-derived DNA adducts were formed in vitro and in rat liver | Wang et al. (2005a) |
| Riddelliine | In vivo or in vitro with metabolic activation 32P-postlabeling/HPLC analysis | Two enantiomers of DHP-7deoxyguanosin-2N-yl adducts and DHP-modified dinucleotides were formed in vitro and in rat liver | Chou et al. (2003a–c, 2004); Xia et al. (2003); Yang et al. (2001) |
| Senecionine | In vivo covalent binding analysis in rat using 3H labeling | Uncharacterized DNA adducts were identified in rat liver, lung and kidney | Candrian et al. (1985) |
| Seneciphylline | In vivo covalent binding analysis in rat using 3H labeling | Uncharacterized DNA adducts were identified in rat liver, lung and kidney | Candrian et al. (1985) |
| DNA cross-linking Heliosupine | In vitro cells | DNA–DNA crosslinks | Hincks et al. (1991) |
| Jacobine | Alkaline elution in rat liver | DNA–DNA crosslinks DNA–protein crosslinks |
Petry et al. (1986) |
| Latifoline | In vitro cells | DNA–DNA crosslinks | Hincks et al. (1991) |
| Monocrotaline (Dehydromonocrotaline) |
In vitro cross-linking assay of PA-exposed cells or pyrrolic PA-exposed nucleiIn vivo and in vitro alkaline elution assay |
DNA–DNA crosslinks DNA–protein crosslinks |
Coulombe et al. (1999); Kim et al. (1995); Pereira et al. (1998); Petry et al. (1984); Petry and Sipes (1987); Rieben and Coulombe (2004); Tepe and Williams (1999); Wagner et al. (1993); Weidner et al. (1990) |
| Retrorsine (dehydroretronecine) | In vitro cross-linking assay of PA-exposed cells or pyrrolic PA-exposed nuclei | DNA–DNA crosslinks | Hincks et al. (1991); Reed et al. (1988) |
| Riddelliine (dehydroriddelliine) |
In vitro cross-linking assay of PA-exposed cells or pyrrolic PA-exposed nuclei |
DNA–protein crosslinks DNA–DNA crosslinks |
Hincks et al. (1991); Kim et al. (1995, 1999) |
| Senecionine (dehydrosenecionine) |
In vitro cross-linking assay of PA-exposed cells or pyrrolic PA-exposed nuclei |
DNA–protein crosslinks DNA–DNA crosslinks |
Coulombe et al. (1999); Hincks et al. (1991); Kim et al. (1995, 1999) |
| Seneciphylline (dehydroseneciphylline) |
In vitro cross-linking assay of PA-exposed cells or pyrrolic PA-exposed nuclei |
DNA–protein crosslinks DNA–DNA crosslinks |
Hincks et al. (1991); Kim et al. (1995) |
| DNA strand break Heliosupine | In vitro alkaline elution | Negative | Hincks et al. (1991) |
| Isatidin | In vitro comet assay | Positive | Uhl et al. (2000) |
| Jacobine | In vivo alkaline elution | Negative | Petry et al. (1986) |
| Latifoline | In vitro alkaline elution | Negative | Hincks et al. (1991) |
| Monocrotaline | In vitro alkaline elution | Negative | Hincks et al. (1991); Petry et al. (1984) |
| Monocrotaline | In vitro comet assay | Positive | Silva-Neto et al. (2010) |
| Retrorsine | In vitro alkaline elution | Negative | Hincks et al. (1991) |
| Riddelliine | In vitro alkaline elution | Negative | Hincks et al. (1991) |
| senecionine | In vitro alkaline elution | Negative | Hincks et al. (1991) |
| seneciphylline | In vitro alkaline elution | Negative | Hincks et al. (1991) |
| Unscheduled DNA synthesis Retrorsine | In rat hepatocytes following in vivo treatment | Positive | Griffin and Segall (1986) |
| Riddelliine | In rat and mouse hepatocytes following in vivo treatment | Positive | Chan (1993); Chan et al. (1994); Mirsalis (1987); Mirsalis et al. (1993); NTP (2003) |
| Senecionine | In rat hepatocytes following in vivo treatment | Positive | Griffin and Segall (1986) |
| Seneciphylline | In rat hepatocytes following in vivo treatment | Positive | Griffin and Segall (1986) |
Note: the pyrrolic PAs in brackets were also measured.
To identify the types of DNA adducts induced by PAs, Fu and his research group developed a 32P-postlabeling/HPLC method and determined the DNA adduct formation in vitro and in vivo using riddelliine as a model PA (Chou et al., 2003a; Xia et al., 2003; Yang et al., 2001). A set of DHP-derived DNA adducts were identified, among which two were enantiomers of DHP-derived 7′-deoxyguanosin-N2-yl adducts and the other six were DHP-modified dinucleotides. Further studies showed that different types of tumorigenic PAs and PA-containing plants induced the same set of DHP-derived DNA adducts in vivo and in vitro. Those PAs and PA-containing plants included clivorine, heliotrine, lasiocarpine, monocrotaline, retrosine, comfrey root extract, comfrey compound oil, coltsfoot root extract, Flos farfara extract and Ligularia hodgsonnii extracts (Chou and Fu, 2006; Wang et al., 2005a, b; Xia et al., 2004, 2006, 2008). Therefore, it is suggested that the formation of DHP-derived DNA adducts is common to all types of tumorigenic PAs.
The levels of the DHP-derived DNA adducts correlated closely with tumorigenic potency in the rats fed different doses of riddelliine (Chou et al., 2003c; Yang et al., 2001). Riddelliine mainly induced liver hemangiosarcomas in rats and mice (Chan et al., 2003). Liver contains two types of cells involved in tumorigenesis, endothelial and parenchymal cells, and hemangiosarcomas develop from endothelial cells. F344 rats and B6C3F1 mice were treated by gavage 5 days a week for 2 weeks with riddelliine at 1.0 mg/kg for rats and 3.0 mg/kg for mice. The treatment resulted in a significantly greater DHP-derived DNA adduct levels in the endothelial cells than those in the parenchymal cells (Chou et al., 2003c). Thus, the overall results suggest that DHP-derived DNA adducts are, at least partially, responsible for the liver tumor development (Fu et al., 2004). In addition, Fu et al. concluded that this set of DHP-derived DNA adducts has the potential to be utilized as a biomarker of PA exposure and tumorigenicity.
DNA cross-linking
PA metabolites contain two functional groups, at the C7 and the C9 positions of the necine base (Fig. 1), that are capable of binding to two sites in DNA or protein to form DNA or protein cross-linking (Table 1). The antimitotic, toxic and carcinogenic actions of PAs are thought to be caused, at least in part, by these crosslinks (Coulombe et al., 1999; Hincks et al., 1991; Kim et al., 1995, 1999).
Studies on the DNA–protein cross-linking activity of several structurally diverse PAs were conducted using cells or isolated nuclei. The pyrrolic PAs, dehydrosenecionine, dehydromonocrotaline, dehydroseneciphylline and dehydroriddelliine readily induced DNA–protein crosslinks, accounting for approximately 50% of the total cellular DNA crosslinks. The DNA–protein cross-linking potency of PAs coincided with their known toxicity potency in animals. Thus, it was concluded that DNA–protein cross-linking was probably involved in PA-related toxicity (Coulombe et al., 1999; Kim et al., 1995; Petry et al., 1984).
PA-induced DNA–DNA cross-linking was investigated in vivo and in vitro using the model cross-linking PA monocrotaline. Hepatic DNA damage induced by monocrotaline was evaluated following i.p. administration to adult male Sprague–Dawley rats. DNA–DNA crosslinks were characterized by the alkaline elution technique; and a mixture of DNA–DNA interstrand cross-links and DNA–protein cross-links was found (Petry et al., 1984). The active metabolite of monocrotaline, dehydromonocrotaline, was also demonstrated to mediate interstrand DNA cross-link formation (Tepe and Williams, 1999). A study on the types of PA-induced DNA–DNA cross-linking showed that the monocrotaline metabolites produced piperidine- and heat-resistant multiple DNA cross-links that were confirmed by electrophoresis and electron microscopy. It was proposed that the metabolites undergo rapid polymerization to a structure capable of crosslinking several fragments of DNA (Pereira et al., 1998). While one study found the DNA–DNA cross-linking by dehydromonocrotaline lacked base sequence preference (Rieben and Coulombe, 2004), another demonstrated that the metabolites preferentially cross-linked DNA duplexes containing the sequence 5’-CG (Weidner et al., 1990).
Coulombe et al. (1999) characterized the ability of different types of PAs to cross-link cellular DNA in cultured bovine kidney epithelial cells. They found that every PA tested induced DNA crosslinks. The relative potency of PAs in causing DNA cross-linking, however, varied, with an order of seneciphylline > riddelliine > retrorsine > senecionine > heliosupine > monocrotaline > latifoline > retronecine. Their studies suggest that PAs with a macrocyclic necic acid ester and an α,β-unsaturated ester function are more potent cross-linkers. In addition, the stereochemical orientation of the ester linkage was found to have no effect on biological activity (Hincks et al., 1991; Kim et al., 1999). Unfortunately, the structures of the DNA crosslink adducts have never been fully characterized; and the levels of their formation have not been correlated with the tumorigenic potencies of rodents treated with PAs. These warrant further investigation.
DNA strand breakage
Several reports have been published on PA-induced DNA strand breakage (Table 1). Hincks et al. (1991) studied DNA crosslinks and DNA strand breaks induced by several bifunctional PAs, including seneciphylline, riddelliine, retrorsine, senecionine, monocrotaline, heliosupine and latifoline, using the alkaline elution assay. None of the PAs induced detectable amounts of DNA single-strand breaks. Petry et al. (1984, 1986) confirmed that monocrotaline and Jacobine did not induced DNA single-strand breaks using the alkaline elution assay. However, isatidine caused a pronounced effect on human hepatoma cells (HepG2 cells) using the single cell gel electrophoresis assay (Comet assay). The lowest concentration that induced a significant positive effect was 500 μM (Uhl et al., 2000). The authors suggested that HepG2 cells catalyzed the activation reaction, leading to DNA strand breaks. Glial cells from the human glioblastoma cell line GL-15 were treated with 1–5000 μM monocrotaline and the DNA strand breaks were measured using the Comet assay (Silva-Neto et al., 2010). The data showed that the treatment caused significant dose–response increases in cell DNA breaks. The conflicting results from studies using the alkaline elution assay and Comet assay have been explained by the greater sensitivity of the Comet assay for detecting DNA strand breaks (Uhl et al., 2000).
Unscheduled DNA synthesis
The unscheduled DNA synthesis (UDS) test measures the DNA repair synthesis after excision and removal of a stretch of DNA containing the region of damage induced by chemical or physical agents. Therefore, this assay measures the repair of primary DNA damage like DNA adducts. Several UDS studies have been conducted to measure primary DNA damage induced by PAs (Table 1). Riddelliine induced significant elevations in UDS in rat liver (Mirsalis, 1987; Mirsalis et al., 1993). UDS was also detected in hepatocytes cultured from male and female rats and mice following 5 or 30 days of riddelliine treatment by gavage (Chan, 1993; Chan et al., 1994; NTP, 2003). Four PAs or PA metabolites, senecionine, retrorsine, seneciphylline and 19-OH-senecionine, along with four alkenals, trans-4-OH-2-hexenal, trans-4-OH-2-nonenal, nonenal and hexenal, were measured in primary cultures of rat hepatocytes using the UDS test. All eight compounds exhibited positive, dose-related responses as measured by autoradiographic detection. The similar UDS responses by PAs and alkenals suggest that trans-4-OH-2-hexenal is a toxic metabolite of the PAs (Griffin and Segall, 1986).
Chromosomal Damage Induced by PAs
Micronucleus assay
Micronucleus induction by PAs has been widely studied and the results clearly demonstrate that PAs are strong clastogenic agents, producing micronuclei in hepatocytes, bone marrow erythrocytes and peripheral blood cells (Table 2). A PA mixture of crude integerrimine and retrorsine and pure integerrimine was extracted from Senecio brasiliensis, which had been stored for more than 23 years under variable conditions of temperature and humidity and exposed to light. Both the mixture and pure PAs induced significant increases in micronucleus frequency in polychromatic erythrocytes (PCEs) of mouse bone marrow (Santos-Mello et al., 2002). Monocrotaline and Crotalaria seeds containing about 6.84% (dry weight) of monocrotaline were administered to mice in their diet for 6 days; the treatment increased the frequency of micronucleated cells in peripheral blood (MacGregor et al., 1990). Clastogenic damage was evaluated in mice following adult and transplacental exposure to heliotrine and monocrotaline using the micronucleus assays of PCE in mouse adult bone marrow and fetal liver, respectively. Both chemicals significantly increased frequencies of micronucleated PCE in the adult and fetal tissues, but heliotrine resulted in the larger increases in mean value of micronucleated PCE. Also, the induction of micronuclei was significantly higher in fetal than in adult cells. The induction of micronuclei following heliotrine treatment showed a peak expression in PCE at 18 h after injection for adult bone marrow and at 24 h for fetal liver (Sanderson and Clark, 1993). Isatidine, retrorsine and monocrotaline were found to induce micronuclei in cultured rat hepatocytes (Muller-Tegethoff et al., 1995, 1997).
Table 2.
Chromosome damage induced by pyrrolizidine alkaloids
| Agent | Testing system | Results | References |
|---|---|---|---|
| Micronucleus assay Crotalaria seeds | In vivo peripheral blood of mice | Positive | MacGregor et al. (1990) |
| Crude mixture of integerrimine, retrorsine and impurities | In vivo polychromatic erythrocytes of mouse bone marrow | Positive | Santos-Mello et al. (2002) |
| Heliotrine | In vivo polychromatic erythrocytes of mouse adult bone marrow and fetal liver | Positive | Sanderson and Clark (1993) |
| Integerrimine | In vivo polychromatic erythrocytes of mouse bone marrow | Positive | Santos-Mello et al. (2002) |
| Isatidine | in vitro rat hepatocytes | Positive | Muller-Tegethoff et al. (1997) |
| Monocrotaline | In vivo peripheral blood of mice | Positive | MacGregor et al. (1990) |
| Monocrotaline | In vivo polychromatic erythrocytes of mouse adult bone marrow and fetal liver | Positive | Sanderson and Clark (1993) |
| Monocrotaline | In vitro rat hepatocyte micronucleus | Positive | Muller-Tegethoff et al. (1995, 1997) |
| Riddelliine | In vivo peripheral blood and bone marrow of male mice | Week positive | Chan et al. (1994) |
| Riddelliine | In vivo peripheral blood of rats and mice | Negative | Chan et al. (1994); Mirsalis et al. (1993) |
| Retrorsine | In vitro rat hepatocyte micronucleus | Positive | Muller-Tegethoff et al. (1995, 1997) |
| Chromosome aberration Crotalaria retusa | Mouse bone marrow | Positive | Ribeiro et al. (1993) |
| Fulvine | Human blood cells of children suff ering from veno-occlusive disease | Positive | Martin et al. (1972) |
| Isatidine | In vitro V79 cells with or without S9 | Negative | Muller et al. (1992) |
| Isatidine | In vitro V79 cells with primary hepatocytes | Positive | Muller et al. (1992) |
| Heliotrine | In vitro V79 cells with or without S9 | Positive | Takanashi et al. (1980) |
| Heliotropium curassavicum | In vitro CHO cells with or without S9 | Positive | Carballo et al. (1992) |
| Integerrimine | Mouse bone marrow | Positive | Gimmler-Luz et al. (1990) |
| Lasiocarpine | In vitro V79 cells with or without S9 | Positive | Takanashi et al. (1980) |
| Monocrotaline | In vitro V79 cells with S9 or with primary hepatocyte activation | Positive | Muller et al. (1992) |
| Petasitenine | In vitro V79 cells with or without S9 | Positive | Takanashi et al. (1980) |
| Riddelliine | In vitro CHO cells with S9. | Positive | Chan (1993); NTP (2003) |
| Retrorsine | In vitro V79 cells with or without S9; or with primary hepatocytes | Positive | Muller et al. (1992) |
| Senkirkine | In vitro V79 cells with or without S9 | Positive | Takanashi et al. (1980) |
| Sister chromatid exchange dehydroretronecine | In vitro human lymphocytes | Positive | Ord et al. (1985) |
| Heliotrine | In vitro V79 cells with primary chick embryo hepatocyte activation | Positive | Bruggeman and van der Hoeven (1985) |
| Monocrotaline | In vitro V79 cells with primary chick embryo hepatocyte activation | Positive | Bruggeman and van der Hoeven (1985) |
| Riddelliine | In vitro CHO cells with and without S9 | Positive | Chan (1993); NTP (2003) |
| Seneciphylline | In vitro V79 cells with primary chick embryo hepatocyte activation | Positive | Bruggeman and van der Hoeven (1985) |
| Senkirkine | In vitro V79 cells with primary chick embryo hepatocyte activation | Positive | Bruggeman and van der Hoeven (1985) |
The frequency of micronucleated erythrocytes in mouse and rat peripheral blood samples was not elevated after 4 or 13 weeks of daily gavage treatments of riddelliine at doses up to 10 mg/kg for rats and 25 mg/kg for mice. A weakly positive response was noted in the peripheral blood and bone marrow of male mice administered a single 150 mg/kg riddelliine by gavage (Chan, 1993; Mirsalis et al., 1993).
Chromosomal aberration
Many PAs induce chromosomal aberrations in mammalian cells or in mouse bone marrow when they are appropriately metabolically activated (Table 2). Chromosomal aberrations were induced by riddelliine in Chinese hamster ovary (CHO) cells only in the presence of S9 (Chan, 1993; NTP, 2003). Heliotrine, lasiocarpine, petasitenine and senkirkine induced chromosomal aberrations in V79 cells (Takanashi et al., 1980). While heliotrine and petasitenine induced interchromosomal exchanges, lasiocarpine and senkirkine caused chromatid gaps. Male and female C57Bl/6 mice were treated with integerrimine and their bone marrow cells were collected for measurement of chromosomal aberrations. The chromosomal aberrations were significantly induced in a dose responsive manner. The greatest frequency of chromosomal aberrations was detected 12 h after treatment (Gimmler-Luz et al., 1990).
Muller et al. (1992) investigated chromosomal aberration induction by monocrotaline, retrorsine and isatidine in different activation systems. Two hours of PA treatment of V79 cells with S9 mix led to a strong and concentration-dependent increase for retrorsine, but a negative response for isatidine (retrorsine N-oxide) and a weak positive for monocrotaline. In contrast, an 18 h PA treatment of V79 cells in the presence of primary hepatocytes resulted in clear concentration-dependent positive responses for all three PAs. The authors postulated that primary liver cells could reduce isatidine to retrorsine whereas the S9 mix could not.
Two PA-containing plants were evaluated in the chromosomal aberration test. Ribeiro et al. (1993) found that Crotalaria retusa extracts containing monocrotaline induced chromosomal aberrations in mouse bone marrow cells. Heliotropium curassavicum is a widely employed medicinal plant that contains PAs. In order to analyze its genotoxic effects, Carballo et al. (1992) studied chromosomal aberrations induced by Heliotropium extracts in CHO cells with or without S9 mix. They found that the plant extract induced chromosomal aberrations and the induction was enhanced by the addition of an S9 fraction. The authors suggested that the toxic effects were associated with PAs and their N-oxides.
Genotoxicity studies on PAs in humans are rare. There is one report on chromosomal aberrations in the blood cells of children suffering from veno-occlusive disease, believed to have been caused by fulvine, a cyclic diester of retroneoine (Martin et al., 1972).
Sister chromatid exchanges
Sister chromatid exchange (SCE) is the exchange of genetic material between two identical sister chromatids. SCE has been associated with chromosome damage and tumor induction. Several PAs have been tested and found to be SCE inducers (Table 2). Riddelliine induced SCEs in CHO cells with and without S9 (Chan, 1993; NTP, 2003). Heliotrine, monocrotaline, seneciphylline and senkirkine were studied with the SCE assay in V79 cells. Treatment of the cells with the PAs in the presence of co-cultured primary chick embryo hepatocytes resulted in a strong induction of SCEs. The rank order found was seneciphylline > senkirkine > heliotrine > monocrotaline (Bruggeman and van der Hoeven, 1985). Dehydroretronecine, the common reactive pyrrolic metabolite of retronecine-type PAs, was measured in human lymphocytes for induction of SCE and produced a strong positive result (Ord et al., 1985).
Mutations Induced by PAs
Mutations in bacteria
The Salmonella typhimurium/mammalian microsome test has been extensively used for determining the mutagenicity of PAs (Table 3). Retrorsine was measured using TA98, TA100, TA1535 and TA1537 tester strains in the presence of S9 and the PA was mutagenic for TA1535 and TA1537, indicating that it induced both basepair substitution and frameshift mutations (Wehner et al., 1979). Dehydroretronecine also induced mutations in the S. typhimurium base substitution strain TA92 (Ord et al., 1985). The mutagenicities of clivorine, fukinotoxin, heliotrine, lasiocarpine, ligularidine and senkirkine to S. typhimurium TA100 were demonstrated by pre-incubation of the PAs with S9 mix and bacteria in a liquid medium (Yamanaka et al., 1979). Riddelliine was mutagenic in TA100 with, but not without, S9 activation. Riddelliine was negative in strains TA97, TA98 and TA1535 (Chan, 1993). An acetone extract of tansy ragwort produced positive mutagenic responses in tester strains TA98, TA100, TA1535 and TA1537, with S9 activation (White et al., 1984).
Table 3.
Mutagenicity of pyrrolizidine alkaloids
| Agent | Testing system | Results | References |
|---|---|---|---|
| In bacteria Clivorine | Salmonella typhimurium TA100 with S9 | Positive | Yamanaka et al. (1979) |
| Dyhydroretrosine | S. typhimurium TA100 and T97 | Positive | Ord et al. (1985) |
| Fukinotoxin | S. typhimurium TA100 with S9 | Positive | Yamanaka et al. (1979) |
| Heliotrine | S. typhimurium TA100 with S9 | Positive | Yamanaka et al. (1979) |
| Heliotrine | Escherichia coli WP2 and WP2 uvrA | Negative | Green and Muriel (1975) |
| Isatidine | S. typhimurium TA100 with S9 | Negative | Rubiolo et al. (1992) |
| Lasiocarpine | S. typhimurium TA100 with S9 | Positive | Yamanaka et al. (1979) |
| Ligularidine | S. typhimurium TA100 with S9 | Positive | Yamanaka et al. (1979) |
| LX201 | S. typhimurium TA100 with S9 | Positive | Yamanaka et al. (1979) |
| Monocrotaline | S. typhimurium TA100 with S9 | Negative | Rubiolo et al. (1992) |
| Monocrotaline | E. coli WP2 and WP2 uvrA | Negative | Green and Muriel (1975) |
| Retrorsine | S. typhimurium TA1535 and TA1537 with S9 | Positive | Wehner et al. (1979) |
| Retrorsine | S. typhimurium TA100 with S9 | Weakly positive | Rubiolo et al. (1992) |
| Riddelliine | S. typhimurium TA97, TA98 or TA1535 with or without S9 | Negative | Chan (1993) |
| Riddelliine | S. typhimurium TA100 with S9 | Positive | Chan (1993) |
| Senecivernine | S. typhimurium TA100 with S9 | Weakly positive | Rubiolo et al. (1992) |
| Senecionine | S. typhimurium TA100 with S9 | Negative | Rubiolo et al. (1992) |
| Seneciphylline | S. typhimurium TA100 with S9 | Weakly positive | Rubiolo et al. (1992) |
| Senecio inaequidens and | S. typhimurium TA100 with S9 | Weakly positive | Rubiolo et al. (1992) |
| S. fuchsia | S. typhimurium TA100 with S9 | Weakly positive | Rubiolo et al. (1992) |
| S. cacliastershowed | S. typhimurium TA100 with S9 | Weakly positive | Rubiolo et al. (1992) |
| Senkirkine | S. typhimurium TA100 with S9 | Positive | Yamanaka et al. (1979) |
| Tansy ragwort | S. typhimurium TA1535, TA1537, TA98 and TA100 with S9 | Positive | White et al. (1984) |
|
In Drosophila 7-Acetyl-intermedine |
Wing spot test | Positive | Frei et al. (1992) |
| 7-Acetyl-lycopsamine | Wing spot test | Positive | Frei et al. (1992) |
| Heliotrine | Wing spot test | Positive | Brink (1969); Frei et al. (1992); Sivlingham and Brink (1988). |
| Heliotrine | Sex-linked recessive lethal test | Positive | Clark (1959) |
| Indicine | Wing spot test | Positive | Frei et al. (1992) |
| indicine-N-oxide | Wing spot test | Positive | Frei et al. (1992) |
| Integerrimine | Wing spot test | Positive | Campesato et al. (1997) |
| Intermedine | Wing spot test | Positive | Frei et al. (1992) |
| Jacoline | Wing spot test | Positive | Frei et al. (1992) |
| Lycopsamine | Wing spot test | Positive | Frei et al. (1992) |
| Monocrotaline | Wing spot test | Positive | Frei et al. (1992) |
| Monocrotaline | Sex-linked recessive lethal test | Positive | Clark (1959) |
| Retrorsine | Wing spot test | Positive | Frei et al. (1992) |
| Senecionine | Wing spot test | Positive | Frei et al. (1992) |
| Seneciphylline | Wing spot test | Positive | Frei et al. (1992) |
| Seneciphylline | Sex-linked recessive lethal test | Positive | Candrian et al. (1984) |
| Senkirkine | Wing spot test | Positive | Frei et al. (1992) |
| Senkirkine | Sex-linked recessive lethal test | Positive | Candrian et al. (1984) |
| Supinine | Wing spot test | Negative | Frei et al. (1992) |
| Symlandine | Wing spot test | Positive | Frei et al. (1992) |
| Symphytine | Wing spot test | Positive | Frei et al. (1992) |
| In rodent Comfrey | Transgenic rat cII assay | Positive in liver and lung; The major types of induced-mutations are G : C → T : A and tandem base substitutions | Mei et al. (2005); Mei and Chen (2007) |
| Riddelliine | Transgenic rat cII assay | Positive in liver and showed the liver endothelial specificity; The major types of induced-mutations are G : C → T : A and tandem base substitutions | Mei et al. (2004a, b) |
| Riddelliine | Mutations in the K-ras gene in mouse liver tumor induced by riddelliine | K-ras codon 12 G : C → T : A transversion; 58% incidence vs 0% in spontaneous tumors | Hong et al. (2003) |
| Mutations in the p53 gene in mouse liver tumor induced by riddelliine | 75% incidence vs 0% in spontaneous tumors | Hong et al. (2003) |
In spite of the results described above, there were contradictory reports on the system for detection of mutagenicity of PAs. Clark (1976) reported that PAs did not produce mutagenicity in the S. typhimurium test system, even in the presence of a liver microsome preparation. Rubiolo et al. (1992) found that several PAs gave negative results in the test system. Retrorsine, senecivernine, seneciphylline and extracts from PA-containing plants Senecio inaequidens, S. fuchsia and S. cacliastershowed only showed weak mutagenic activity. The authors concluded that the S. typhimurium/mammalian microsome system was not a sensitive assay for detection of Pas’ mutagenicity unless a suitable activation enzyme system was applied.
Mutations in Drosophila
Drosophila test systems are particularly suitable for testing PAs due to the fly’s versatile metabolic bioactivation system and its excellent sensitivity to cross-linking genotoxins (Candrian et al., 1984). Heliotrine was a powerful mutagen in D. melanogaster (Brink, 1969; Clark, 1959; Sivlingham and Brink, 1988). Seneciphylline and senkirkine were found to produce sex-linked recessive lethal in males of D. melanogaster using the 3-day feeding method (Candrian et al., 1984). In addition, monocrotaline was positive in Drosophila sex-linked recessive lethal assay (Clark, 1976). Drosophila flies fed with milk from lactating rats given an oral dose of 25 mg seneciphylline/kg showed 1.2% sex-linked recessive lethal compared with 0.3% in controls (Candrian et al., 1984).
Integerrimine was positive in the somatic mutation and recombination wing spot test (SMART) in D. melanogaster. Analysis of the dose–response data indicated that 85–90% of the genotoxic events were due to mitotic recombination activity while 10–15% of them resulted from somatic mutations (Campesato et al., 1997).
Frei et al. (1992) examined 15 PAs and one PA N-oxide (indicine N-oxide) for their genotoxic potency with respect to the structure/activity relationship in the wing spot test of D. melanogaster following oral administration (Table 3). All PAs tested except the C9-monoester supinine were clearly genotoxic. The results demonstrated that the macrocyclic diester-type PAs were the most genotoxic; the open diesters PAs were intermediate; and 7-hydroxy C9-monoester types of PAs were the least active. Stereoisomeric PAs mostly showed similar activity. An increasing number of hydroxy groups in the PA molecule seemed to reduce genotoxic potency. A rank order with decreasing genotoxic potency (senkirkine as 100%) follows: senkirkine (100.0), monocrotaline (90.0), seneciphylline (54.5), senecionine (39.1), 7-acetyl-intermedine (22.5), heliotrine (13.4), retrorsine (8.3), 7-acetyl-lycopsamine (7.9), symphytine (3.8), Jacoline (1.8), symlandine (1.7), intermedine (0.49), indicine (0.27), lycopsamine (0.19), indicine-N-oxide (0.07) and supinine (0.002).
Mutations in rodents
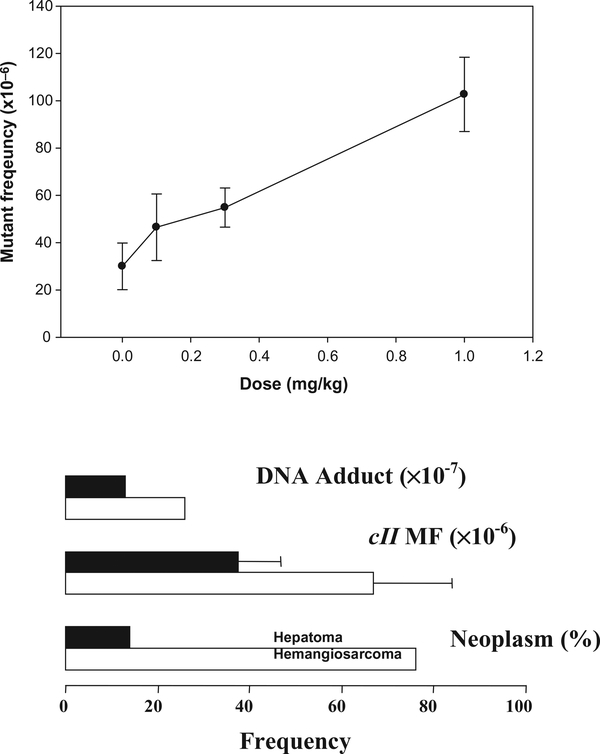
The mutagenicity of riddelliine in rat liver was investigated using Big Blue transgenic rats (Mei et al., 2004a, b). Transgenic mutation assays provide a unique opportunity for studying the induction of tissue-specific mutation, and the assays also permit quantitative measurements of mutant frequencies (MFs) in the PA-target tissues and molecular analysis of the PA-specific mutational spectra. Groups of six female transgenic Big Blue rats were gavaged with 0.1, 0.3 and 1.0 mg riddelliine per kg body weight 5 days a week for 12 weeks and sacrificed 1 day after the last treatment. The middle and high doses resulted in liver tumors in an NTP bioassay (NTP, 2003). A significant dose-dependent increase in MF was found (Fig. 4).
Figure 4.
Mutant frequencies induced by riddelliine in liver of female transgenic Big Blue rats. The upper panel shows mutant frequencies induced by riddelliine treatment in the cII genes in rat liver as a function of dose. The lower panel compares mutant frequencies with other biological consequences after riddelliine treatments in rat liver hepatocytes (solid bars) and endothelial cells (open bars). Data are from the literature (Chou et al., 2003c; Hirono et al., 1978; Mei et al., 2004a).
Riddelliine mainly induces liver hemangiosarcomas that develop from endothelial cells in rat or mouse liver (Chan et al., 2003). To identify the cell type-specific response to mutagenicity of PA, we separated the endothelial cells from the parenchymal cells in the livers of Big Blue rats treated with riddelliine (Mei et al., 2004a). While there was no difference in the cII MFs of liver parenchymal cells in control and riddelliine-treated rats, the cII MF of liver endothelial cells from treated rats was significantly greater than the cII MF of endothelial cells from control rats. The induction of mutation also correlated with induction of DNA adducts and tumors by riddelliine in rat liver (Fig. 4). These results suggest that the relatively high mutagenicity of riddelliine in rat liver endothelial cells may be partially responsible for the tumorigenic specificity of this agent.
A statistically significant difference was found between the mutational spectra from the riddelliine-treated and the control rats. The major types of mutations induced by riddelliine were G : C → T : A transversion and tandem base substitutions of GG → TT and GG → AT (Table 4). The types of mutations induced by riddelliine are consistent with riddelliine adducts involving G : C base pairs (Chou et al., 2003a). Riddelliine reacts with guanine, adenine and thymine, but not with cytosine, and the relative reactivity of guanine with DHP is greater than other bases. The GG → TT and GG → AT tandem base substitutions were believed to result from intra-strand crosslinks in adjacent guanine bases forming DHP-modified dimers (Chou et al., 2003a; Mei et al., 2004b). This unique spectrum may serve as a signature for genetic damage produced by riddelliine and other PAs. The types of mutations induced by riddelliine suggest that both mononucleotide and dinucleotide DNA adducts involving G : C base pairs are responsible for its mutagenicity (Mei et al., 2004a, b).
Table 4.
Summary of the types of mutations in cII gene from riddelliine- or comfrey-treated and control Big Blue rats
| Type of mutation | Control (%) | Treatment (%) | |||
|---|---|---|---|---|---|
| In liver | In lung | Riddelliine in liver | Comfrey in liver | Comfrey in lung | |
| G : C → C : G | 11 | 9 | 5 | 6 | 8 |
| G : C → A : T | 43 | 63 | 26 | 12 | 28 |
| G : C → T : A | 20 | 9 | 35 | 42 | 29 |
| A : T → T : A | 2 | 0 | 5 | 2 | 16 |
| A : T → C : G | 7 | 2 | 6 | 3 | 4 |
| A : T → G : C | 2 | 2 | 5 | 4 | 6 |
| Frameshift | 15 | 13 | 10 | 13 | 5 |
| Complex mutation | 0 | 2 | 0 | 1 | 0 |
| Tandem–base substitution | 0 | 0 | 8 | 17 | 4 |
Note: the data are from the literature (Mei et al., 2004b, 2005, 2007)
PA-containing comfrey is a rat liver toxin and carcinogen (Hirono et al., 1978). In order to evaluate the mechanisms underlying its carcinogenicity, we examined the mutagenicity of comfrey in the transgenic Big Blue rat model. Groups of six 6-week-old male Big Blue rats were fed either a basal diet or the comfrey diet for 12 weeks. MFs were determined for the liver and lung cII gene of the rats treated with comfrey. The MFs in both liver and lung were increased by the comfrey treatment with a much higher MF in liver compared to that in lung (Mei et al., 2005; Mei and Chen, 2007). These results correlated with the previous report that tumors were induced by comfrey in liver and liver was the major target tissue (Hirono et al., 1978). Sequencing analysis of the comfrey-induced cII mutant DNA showed the PA mutational signature, with a high induction of G : C → T : A transversions and tandem base substitutions (Table 4). Therefore, these mutational data support the hypothesis that the mutations induced by comfrey in rat liver and lung were due to the PAs in comfrey (Mei et al., 2005; Mei and Chen, 2007).
Hong et al. (2003) examined the mutations occurring in the K-ras protooncongene and p53 tumor suppressor gene in riddelliine-induced liver hemangiosarcomas. They found that 58% riddelliine-induced tumors contained K-ras codon 12 G → T mutation, a PA signature mutation, and 75% riddelliine-induced tumors contained p53 mutations, the types of which were not identified. In contrast, spontaneous hemangiosarcomas from control mice lacked mutations in both the K-ras and p53 genes. It was concluded that p53 and K-ras mutations in riddelliine-induced hemangiosarcomas most likely occurred as a result of the mutagenic effects of riddelliine (Hong et al., 2003).
SUMMARY
There have been a number of outbreaks of human poisoning as a result of ingestion of contaminated grain as well as case reports of poisoning caused by intentional ingestion of herbal medicines containing PAs. In recent years, there has been an increasing use of herbal medicines and dietary supplements to treat various chronic diseases and to promote health. Thus, humans may be exposed to PAs via these herbs or dietary supplements, such as Sympthytum spp. (comfrey), which have been deliberately ingested. Although there are no epidemiological data regarding the carcinogenicity of PAs in humans, a number of studies demonstrated that various PAs are carcinogens in experimental animals. Several PAs were classified as possibly carcinogenic to humans (group 2B) (IARC, 1976). Comparison of the total intake resulting in human toxicity with the total doses to death observed in the chronic toxicity studies in rats indicates that human beings are more susceptible and suggests that humans may survive for sufficient time to develop cancer after only a brief exposure at toxic levels or a longer exposure at a markedly lower levels (IPCS, 1988). Thus, a potential cancer risk for human beings should be seriously considered. To assess the risk to humans, it is necessary to determine whether tumorigenic PAs are mutagenic carcinogens because the selection of an appropriate model for conducting quantitative cancer risk assessment is based upon an understanding of the chemical’s mode-of-action.
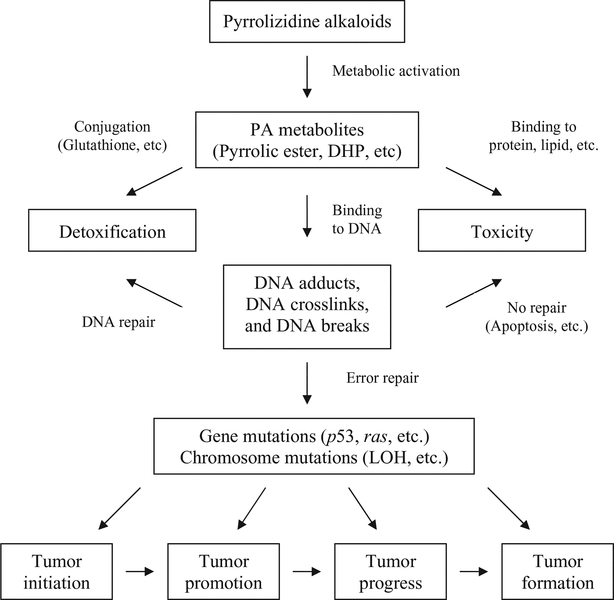
It seems that the carcinogenic activity of individual alkaloids parallels their mutagenic behavior, but not their relative hepatotoxicities (IPCS, 1988). Our review of the available information demonstrated that the tumorigenic PAs are mutagenic. The mutagenic activity is mediated by the formation of PA metabolites binding to DNA, thus resulting in DNA damage, gene mutations and chromosomal mutations. Since the mutagenicities have been found in the tumorigenic target tissues and in oncogenes of PA-induced tumors, it is reasonable to conclude that the PAs induce tumors via a mutagenic mode of action. A postulated mechanism of pyrrolizidine alkaloid carcinogenesis is shown in Fig. 5.
Figure 5.
Postulated mechanism of pyrrolizidine alkaloid carcinogenesis.
PA metabolic activation plays a major role in their mutagenicity. This is evidenced by the fact that the main genotoxic and tumorigenic target organ is the liver where the most PAs’ metabolic activation occurs. The contradictory reports between in vitro and in vivo mutagenicities of PAs also suggest the importance of PAs’ metabolism for their mutagenicities. PAs were not mutagenic or showed weak mutagenic activity in S. typhimurium, especially when suitable activation enzymes were not employed. Surprisingly, no reports were found on mutagenicity of PAs using in vitro mammalian cell assays, perhaps due to insensitivities of these in vitro systems to PAs’ mutagenic insults. In contrast to the in vitro systems, the in vivo test systems like Drosophila and transgenic rodents are particularly suitable for testing PAs due to the in vivo system’s versatile metabolic bioactivation systems. The mutagenic activities of some PAs that were identified as negative or weakly positive in bacteria were shown to be strong positives in Drosophila or rodent test systems.
A large body of evidence shows that PAs induced DNA adducts and DNA crosslinks while the results for PA-induced DNA breaks were less clear. Pyrrolic esters or DHP can bind to DNA and generate DHP-derived DNA adducts. Two of the DNA adducts were identified as enantiomers of DHP-derived 7′-deoxyguanosin-N2-yl adducts and the others were characterized as DHP-modified dinucleotides. The PA-induced DNA adducts could be formed in common by all PAs. The observation that common types of mutations, G : C → T : A and tandem base pair substitutions, were induced by different PAs strengthen this theory (Table 4). PAs have proved to be strong cross-linking agents. The bifunctional PAs are capable of forming DNA–DNA or DNA–protein crosslinks. DNA–protein crosslinks comprise about half of the total cellular DNA crosslinks. It also has been suggested that PA metabolites can undergo polymerization to form a structure capable of producing multiple DNA crosslinks. Different PAs have different potencies in causing DNA cross-linking. PAs with a macrocyclic necic acid ester and anα,β-unsaturated ester function demonstrated the most potent cross-linking activity. While DNA adducts may induce more gene mutations, DNA crosslinks tend to induce chromosome mutations.
PAs are both gene mutagens and chromosomal mutagens, perhaps more potent as chromosomal mutagens than as gene mutagens. Many PAs were positive in micronucleus, chromosomal aberration and sister chromatid exchange assays which detect chromosomal damage, and indicate the likely induction of chromosomal mutations. Although many of the micronucleus tests were conducted in surrogate tissues, bone marrow and peripheral blood cells, PAs mainly were positive most of the time, albeit weak. Chromosomal aberrations have been found in the blood cells of children suffering from veno-occlusive disease, believed to be caused by the PA fulvine. PAs were positive in many different S. typhimurium strains, although the assay was relatively insensitive to PAs’ mutagenicity. PAs induced mutations in Drosophila, primarily measured with the wing spot or sex-linked recessive lethal assays. The systems appeared to be particularly suitable for testing PA mutagenicity. The mutagenic potency for a number of PAs has been ranked in these systems. PAs induced mutations in the transgenic cII gene in rat liver and lung. Riddelliine induced higher MFs in the endothelial cells than the parenchymal cells in rat livers, which correlated with induction of hemangiosarcomas developed from rat endothelial cells. Signature mutations of PAs have been identified as G : C → T : A transversion and tandem base substitutions. This signature marker may be used as a fingerprint of PA exposure. Mutations have been detected in the p53 tumor suppressor gene and K-ras oncogene of liver tumors induced by riddelliine in mice. The signature type of mutation, G : C → T : A, was found in the oncogene.
The consistency between the induction of mutations and tumors in liver suggests that gene and chromosomal mutations are major factors in the induction of tumors by PAs. Gene and chromosomal mutations in oncogenes and tumor suppressor genes can initiate tumorigenesis. The initiated cells can be promoted and progressed into tumors under the effects of mutations induced by PAs in different genes. Gene expression studies have confirmed that exposure of PAs significantly changes genes that are involved in cancer, cell death, tissue development, cellular movement, tissue morphology, cell-to-cell signaling and interaction, and cellular growth and proliferation. (Guo et al., 2007; Mei et al., 2006, 2007). Although there is little evidence for mutagenicity of PAs in humans, mutagenicity data from studies in vitro, in vivo and in oncogenes provide strong evidence that PAs are mutagenic carcinogens.
Footnotes
This article is a US Government work and is in the public damain in the USA.
The views presented in this article do not necessarily refl ect those of the US Food and Drug Administration.
REFERENCES
- Allen JR, Hsu IC, Carstens LA. 1975. Dehydroretronecine-induced rhabdomyosarcomas in rats. Cancer Res. 35: 997–1002. [PubMed] [Google Scholar]
- Arseculeratne SN, Gunatilaka AA, Panabokke RG. 1981. Studies on medicinal plants of Sri Lanka: occurrence of pyrrolizidine alkaloids and hepatotoxic properties in some traditional medicinal herbs. J. Ethnopharmacol 4: 159–177. [DOI] [PubMed] [Google Scholar]
- Arseculeratne SN, Gunatilaka AA, Panabokke RG. 1985. Studies of medicinal plants of Sri Lanka. Part 14: toxicity of some traditional medicinal herbs. J. Ethnopharmacol 13: 323–335. [DOI] [PubMed] [Google Scholar]
- Arzt J, Mount ME. 1999. Hepatotoxicity associated with pyrrolizidine alkaloid (Crotalaria spp) ingestion in a horse on Easter Island. Vet. Hum. Toxicol 41: 96–99. [PubMed] [Google Scholar]
- Bach N, Thung SN, Schaff ner F. 1989. Comfrey herb tea-induced hepatic veno-occlusive disease. Am. J. Med 87: 97–99. [DOI] [PubMed] [Google Scholar]
- Bah M, Bye R, Pereda-Miranda R. 1994. Hepatotoxic pyrrolizidine alkaloids in the Mexican medicinal plant Packera candidissima (Asteraceae: Senecioneae). J. Ethnopharmacol 43: 19–30. [DOI] [PubMed] [Google Scholar]
- Brandange S, Luning B, Moberg C, Sjostrand E. 1970. Studies on orchidaceae alkaloids. XXIV. A pyrrolizidine alkaloid from Phalaenopsis cornucervi Rchb. f. Acta. Chem. Scand 25: 349–350. [DOI] [PubMed] [Google Scholar]
- Bras G, Jelliff e DB, Stuart KL. 1954. Veno-occlusive disease of liver with nonportal type of cirrhosis, occurring in Jamaica. AMA Arch. Pathol 57: 285–300. [PubMed] [Google Scholar]
- Brink NG. 1969. The mutagenic activity of the pyrrolizidine alkaloid heliotrine in Drosophila melanogaster. II. Chromosome rearrangements. Mutat. Res 8: 139–146. [DOI] [PubMed] [Google Scholar]
- Bruggeman IM, van der Hoeven JC. 1985. Induction of SCEs by some pyrrolizidine alkaloids in V79 Chinese hamster cells co-cultured with chick embryo hepatocytes. Mutat. Res 142: 209–212. [DOI] [PubMed] [Google Scholar]
- Campesato VR, Graf U, Reguly ML, de Andrade HH. 1997. Recombinagenic activity of integerrimine, a pyrrolizidine alkaloid from Senecio brasiliensis, in somatic cells of Drosophila melanogaster. Environ. Mol. Mutagen 29: 91–97. [PubMed] [Google Scholar]
- Candrian U, Luthy J, Graf U, Schlatter C. 1984. Mutagenic activity of the pyrrolizidine alkaloids seneciphylline and senkirkine in Drosophila and their transfer into rat milk. Food Chem. Toxicol 22: 223–225. [DOI] [PubMed] [Google Scholar]
- Candrian U, Luthy J, Schlatter C. 1985. In vivo covalent binding of retronecine-labelled [3H]seneciphylline and [3H]senecionine to DNA of rat liver, lung and kidney. Chem. Biol. Interact 54: 57–69. [DOI] [PubMed] [Google Scholar]
- Carballo M, Mudry MD, Larripa IB, Villamil E, D’Aquino M. 1992. Genotoxic action of an aqueous extract of Heliotropium curassavicum var. argentinum. Mutat. Res 279: 245–253. [DOI] [PubMed] [Google Scholar]
- Chan P 1993. NTP technical report on the toxicity studies of riddelliine (CAS no. 23246–96–0) administered by gavage to F344 rats and B6C3F1 mice. Toxic. Rep. Ser 27: 1–D9. [PubMed] [Google Scholar]
- Chan PC, Mahler J, Bucher JR, Travlos GS, Reid JB. 1994. Toxicity and carcinogenicity of riddelliine following 13 weeks of treatment to rats and mice. Toxicon 32: 891–908. [DOI] [PubMed] [Google Scholar]
- Chan PC, Haseman JK, Prejean JD, Nyska A. 2003. Toxicity and carcinogenicity of riddelliine in rats and mice. Toxicol. Lett 144: 295–311. [DOI] [PubMed] [Google Scholar]
- Cheeke PR. 1988. Toxicity and metabolism of pyrrolizidine alkaloids. J. Anim. Sci 66: 2343–2350. [DOI] [PubMed] [Google Scholar]
- Chou MW, Fu PP. 2006. Formation of DHP-derived DNA adducts in vivo from dietary supplements and chinese herbal plant extracts containing carcinogenic pyrrolizidine alkaloids. Toxicol. Ind. Health 22: 321–327. [DOI] [PubMed] [Google Scholar]
- Chou MW, Jian Y, Williams LD, Xia Q, Churchwell M, Doerge DR, Fu PP. 2003a. Identification of DNA adducts derived from riddelliine, a carcinogenic pyrrolizidine alkaloid. Chem. Res. Toxicol 16: 1130–1137. [DOI] [PubMed] [Google Scholar]
- Chou MW, Wang YP, Yan J, Yang YC, Beger RD, Williams LD, Doerge DR, Fu PP. 2003b. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol. Lett 145: 239–247. [DOI] [PubMed] [Google Scholar]
- Chou MW, Yan J, Nichols J, Xia Q, Beland FA, Chan PC, PP Fu. 2003c. Correlation of DNA adduct formation and riddelliine-induced liver tumorigenesis in F344 rats and B6C3F(1) mice. Cancer Lett. 193: 119–125. [DOI] [PubMed] [Google Scholar]
- Clark AM. 1959. Mutagenic activity of the alkaloid heliotrine in Drosophila. Nature 183: 731–732. [DOI] [PubMed] [Google Scholar]
- Clark AM. 1976. Naturally occuring mutagens. Mutat. Res 32: 361–374. [DOI] [PubMed] [Google Scholar]
- Cook JW, Duff y E, Schoental R. 1950. Primary liver tumours in rats following feeding with alkaloids of Senecio jacobaea. Br. J. Cancer 4: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe RA Jr., Drew GL, Stermitz FR. 1999. Pyrrolizidine alkaloids crosslink DNA with actin. Toxicol. Appl. Pharmacol 154: 198–202. [DOI] [PubMed] [Google Scholar]
- Culvenor CC, Edgar JA, Smith LW. 1981. Pyrrolizidine alkaloids in honey from Echium plantagineum L. J. Agric. Food Chem 29: 958–960. [DOI] [PubMed] [Google Scholar]
- Deinzer ML, Thomson PA, Burgett DM, Isaacson DL. 1977. Pyrrolizidine alkaloids: their occurrence in honey from tansy ragwort (Senecio jacobaea L.). Science 195: 497–499. [DOI] [PubMed] [Google Scholar]
- de Lanux-Van Gorder V 2000. Tansy ragwort poisoning in a horse in southern Ontario. Can. Vet. J 41: 409–410. [PMC free article] [PubMed] [Google Scholar]
- Dickinson JO, Cooke MP, King RR, Mohamed PA. 1976. Milk transfer of pyrrolizidine alkoloids in cattle. J. Am. Vet. Med. Assoc 169: 1192–1196. [PubMed] [Google Scholar]
- Dueker SR, Lame MW, Morin D, Wilson DW, Segall HJ. 1992. Guinea pig and rat hepatic microsomal metabolism of monocrotaline. Drug. Metab. Dispos 20: 275–280. [PubMed] [Google Scholar]
- Edgar JA, Lin HJ, Kumana CR, Ng MM. 1992. Pyrrolizidine alkaloid composition of three Chinese medicinal herbs, Eupatorium cannabinum, E. japonicum and Crotalaria assamica. Am. J. Chin. Med 20: 281–288. [DOI] [PubMed] [Google Scholar]
- Edgar JA, Roeder E, Molyneux RJ. 2002. Honey from plants containing pyrrolizidine alkaloids: a potential threat to health. J. Agric. Food Chem 50: 2719–2730. [DOI] [PubMed] [Google Scholar]
- Fletcher MT, McKenzie RA, Blaney BJ, Reichmann KG. 2009. Pyrrolizidine alkaloids in Crotalaria taxa from northern Australia: risk to grazing livestock. J. Agric. Food Chem 57: 311–319. [DOI] [PubMed] [Google Scholar]
- Fowler ME. 1968. Pyrrolizidine alkaloid poisoning in calves. J. Am. Vet. Med. Assoc 152: 1131–1137. [PubMed] [Google Scholar]
- Frei H, Luthy J, Brauchli J, Zweifel U, Wurgler FE, Schlatter C. 1992. Structure/activity relationships of the genotoxic potencies of sixteen pyrrolizidine alkaloids assayed for the induction of somatic mutation and recombination in wing cells of Drosophila melanogaster. Chem. Biol. Interact 83: 1–22. [DOI] [PubMed] [Google Scholar]
- Fu PP, Xia Q, Lin G, Chou MW. 2004. Pyrrolizidine alkaloids – genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab. Rev 36: 1–55. [DOI] [PubMed] [Google Scholar]
- Furuya T, Hikichi M, Iitaka Y. 1976. Fukinotoxin, a new pyrrolizidine alkaloid from Petasites japonicus. Chem. Pharm. Bull. (Tokyo) 24: 1120–1122. [DOI] [PubMed] [Google Scholar]
- Gimmler-Luz MC, Erdtmann B, Balbueno RA. 1990. The effect of the pyrrolizidine alkaloid integerrimine on the chromosomes of mouse bone marrow cells. Mutat. Res 241: 297–304. [DOI] [PubMed] [Google Scholar]
- Green MH, Muriel WJ. 1975. Use of repair-deficient strains of Escherichia coli and liver microsomes to detect and characterise DNA damage caused by pyrrolizidine alkaloids heliotrine and monocrotaline. Mutat. Res 28: 331–336. [DOI] [PubMed] [Google Scholar]
- Griffi n DS, Segall HJ. 1986. Genotoxicity and cytotoxicity of selected pyrrolizidine alkaloids, a possible alkenal metabolite of the alkaloids, and related alkenals. Toxicol. Appl. Pharmacol 86: 227–234. [DOI] [PubMed] [Google Scholar]
- Guo L, Mei N, Dial S, Fuscoe J, Chen T. 2007. Comparison of gene expression profi les altered by comfrey and riddelliine in rat liver. BMC Bioinformatics 8(suppl. 7): S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PN, Chen KK. 1970. Development of hepatic tumors in rats following ingestion of Senecio longilobus. Cancer Res. 30: 2881–2886. [PubMed] [Google Scholar]
- Hincks JR, Kim HY, Segall HJ, Molyneux RJ, Stermitz FR, Coulombe RA, Jr. 1991. DNA cross-linking in mammalian cells by pyrrolizidine alkaloids: structure–activity relationships. Toxicol. Appl. Pharmacol 111: 90–98. [DOI] [PubMed] [Google Scholar]
- Hirono I, Mori H, Culvenor CC. 1976. Carcinogenic activity of coltsfoot, Tussilago farfara l. Gann 67: 125–129. [PubMed] [Google Scholar]
- Hirono I, Mori H, Yamada K, Hirata Y, Haga M. 1977. Carcinogenic activity of petasitenine, a new pyrrolizidine alkaloid isolated from Petasites japonicus Maxim. J. Natl Cancer Inst 58: 1155–1157. [DOI] [PubMed] [Google Scholar]
- Hirono I, Mori H, Haga M. 1978. Carcinogenic activity of Symphytum offi cinale. J. Natl Cancer Inst 61: 865–869. [PubMed] [Google Scholar]
- Hirono I, Haga M, Fujii M, Matsuura S, Matsubara N, Nakayama M, Furuya T, Hikichi M, Takanashi H, Uchida E, Hosaka S, Ueno I. 1979. Induction of hepatic tumors in rats by senkirkine and symphytine. J. Natl Cancer Inst 63: 469–472. [PubMed] [Google Scholar]
- Hirono I, Ueno I, Aiso S, Yamaji T, Haga M. 1983. Carcinogenic activity of Farfugium japonicum and Senecio cannabifolius. Cancer Lett. 20: 191–198. [DOI] [PubMed] [Google Scholar]
- Hong HL, Ton TV, Devereux TR, Moomaw C, Clayton N, Chan P, Dunnick JK, Sills RC. 2003. Chemical-specifi c alterations in ras, p53, and betacatenin genes in hemangiosarcomas from B6C3F1 mice exposed to o-nitrotoluene or riddelliine for 2 years. Toxicol. Appl. Pharmacol 191: 227–234. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ, Luthy J, Zweifel U. 1986. Toxicity of comfrey–pepsin preparations. New Engl. J. Med 315: 1095. [PubMed] [Google Scholar]
- IARC. 1976. Some naturally occurring substances In IARC Monograph on the Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 10. International Agency for Research in Cancer: Lyon. [Google Scholar]
- IPCS. 1988. Pyrrolizidine alkaloids In Environmental Health Criteria, Vol. 80. World Health Organization: Geneva. [Google Scholar]
- Jago MV. 1969. The development of the hepatic megalocytosis of chronic pyrrolizidine alkaloid poisoning. Am. J. Pathol 56: 405–421. [PMC free article] [PubMed] [Google Scholar]
- Johnson WD, Robertson KA, Pounds JG, Allen JR. 1978. Dehydroretronecine-induced skin tumors in mice. J. Natl Cancer Inst 61: 85–89. [DOI] [PubMed] [Google Scholar]
- Kim HY, Stermitz FR, Coulombe RA Jr., 1995. Pyrrolizidine alkaloid-induced DNA–protein cross-links. Carcinogenesis 16: 2691–2697. [DOI] [PubMed] [Google Scholar]
- Kim HY, Stermitz FR, Li JK, Coulombe RA Jr., 1999. Comparative DNA crosslinking by activated pyrrolizidine alkaloids. Food Chem. Toxicol 37: 619–625. [DOI] [PubMed] [Google Scholar]
- Knight AP, Kimberling CV, Stermitz FR, Roby MR. 1984. Cynoglossum offi cinale (hound’s-tongue) – a cause of pyrrolizidine alkaloid poisoning in horses. J. Am. Vet. Med. Assoc 185: 647–650. [PubMed] [Google Scholar]
- Kuhara K, TakanashiH, Hirono I, Furuya T, Asada Y. 1980. Carcinogenic activity of clivorine, a pyrrolizidine alkaloid isolated from Ligularia dentata. Cancer Lett. 10: 117–122. [DOI] [PubMed] [Google Scholar]
- Kumana CR, Ng M, Lin HJ, Ko W, Wu PC Todd D. 1983. Hepatic venoocclusive disease due to toxic alkaloid herbal tea. Lancet 2: 1360–1361. [DOI] [PubMed] [Google Scholar]
- Kumana CR, Ng M, Lin HJ, Ko W, Wu PC, Todd D. 1985. Herbal tea induced hepatic veno-occlusive disease: quantification of toxic alkaloid exposure in adults. Gut 26: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafranconi WM, Huxtable RJ. 1984. Hepatic metabolism and pulmonary toxicity of monocrotaline using isolated perfused liver and lung. Biochem. Pharmacol 33: 2479–2484. [DOI] [PubMed] [Google Scholar]
- MacGregor JT, Wehr CM, Henika PR, Shelby MD. 1990. The in vivo erythrocyte micronucleus test: measurement at steady state increases assay efficiency and permits integration with toxicity studies. Fundam. Appl. Toxicol 14: 513–522. [DOI] [PubMed] [Google Scholar]
- Martin PA, Thorburn MJ, Hutchinson S, Bras G, Miller CG. 1972. Preliminary findings of chromosomal studies on rats and humans with venoocclusive disease. Br. J. Exp. Pathol 53: 374–380. [PMC free article] [PubMed] [Google Scholar]
- Mattocks AR. 1971. Hepatotoxic effects due to pyrrolizidine alkaloid N-oxides. Xenobiotica 1: 563–565. [DOI] [PubMed] [Google Scholar]
- Mattocks AR. 1980. Toxic pyrrolizidine alkaloids in comfrey. Lancet 2: 1136–1137. [DOI] [PubMed] [Google Scholar]
- Mattocks AR. 1986. Chemistry and Toxicology of Pyrrolizidine Alkaloids. Academic Press: London. [Google Scholar]
- Mattocks AR, Cabral JR. 1982. Carcinogenicity of some pyrrolic pyrrolizidine alkaloid metabolites and analogues. Cancer Lett. 17: 61–66. [DOI] [PubMed] [Google Scholar]
- Mei X, Chen T. 2007. The mutant frequencies and types of mutations induced by comfrey in the lungs of transgenic Big Blue rats. J. Food Drug Anal 15: 458–465. [Google Scholar]
- Mei N, Chou MW, Fu PP, Hefl ich RH, Chen T. 2004a. Differential mutagenicity of riddelliine in liver endothelial and parenchymal cells of transgenic big blue rats. Cancer Lett. 215: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Hefl ich RH, Chou MW, Chen T. 2004b. Mutations induced by the carcinogenic pyrrolizidine alkaloid riddelliine in the liver cII gene of transgenic big blue rats. Chem. Res. Toxicol 17: 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Guo L, Fu PP, Hefl ich RH, Chen T. 2005. Mutagenicity of comfrey (Symphytum officinale) in rat liver. Br. J. Cancer 92: 873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MeiN, Guo L, Zhang L, Shi L, Sun YA, Fung C, Moland CL, Dial SL, Fuscoe JC, Chen T. 2006. Analysis of gene expression changes in relation to toxicity and tumorigenesis in the livers of Big Blue transgenic rats fed comfrey (Symphytum officinale). BMC Bioinformatics 7(suppl. 2): S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Guo L, Liu R, Fuscoe JC, Chen T. 2007. Gene expression changes induced by the tumorigenic pyrrolizidine alkaloid riddelliine in liver of Big Blue rats. BMC Bioinformatics 8(suppl. 7): S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsalis JC. 1987. In vivo measurement of unscheduled DNA synthesis and S-phase synthesis as an indicator of hepatocarcinogenesis in rodents. Cell Biol. Toxicol 3: 165–173. [DOI] [PubMed] [Google Scholar]
- Mirsalis JC, Steinmetz KL, Blazak WF, Spalding JW. 1993. Evaluation of the potential of riddelliine to induce unscheduled DNA synthesis, S-phase synthesis, or micronuclei following in vivo treatment with multiple doses. Environ. Mol. Mutagen 21: 265–271. [DOI] [PubMed] [Google Scholar]
- Mohabbat O, Younos MS, Merzad AA, Srivastava RN, Sediq GG, Aram GN. 1976. An outbreak of hepatic veno-occlusive disease in northwestern Afghanistan. Lancet 2: 269–271. [DOI] [PubMed] [Google Scholar]
- Mori H, Kawai K, Ohbayashi F, Bunai Y, Yamada K, Hirono I. 1984. Some toxic properties of a carcinogenic pyrrolizidine alkaloid, petasitenine. J. Toxicol. Sci 9: 143–149. [DOI] [PubMed] [Google Scholar]
- Muller L, Kasper P, Kaufmann G. 1992. The clastogenic potential in vitro of pyrrolizidine alkaloids employing hepatocyte metabolism. Mutat. Res 282: 169–176. [DOI] [PubMed] [Google Scholar]
- Muller-TegethoffK, Kasper P, Muller L. 1995. Evaluation studies on the in vitro rat hepatocyte micronucleus assay. Mutat. Res 335: 293–307. [DOI] [PubMed] [Google Scholar]
- Muller-TegethoffK, Kersten B, Kasper P, Muller L. 1997. Application of the in vitro rat hepatocyte micronucleus assay in genetic toxicology testing. Mutat. Res 392: 125–138. [DOI] [PubMed] [Google Scholar]
- NTP. 2003. Toxicology and carcinogenesis studies of riddelliine (CAS no. 23246–96–0) in F344/N rats and B6C3F1 mice (gavage studies). National Toxicology Program Technical Report Series. [PubMed]
- Ord MJ, Herbert A, Mattocks AR. 1985. The ability of bifunctional and monofunctional pyrrole compounds to induce sister-chromatid exchange (SCE) in human lymphocytes and mutations in Salmonella typhimurium. Mutat. Res 149: 485–493. [DOI] [PubMed] [Google Scholar]
- Ortiz Cansado A, Crespo Valades E, Morales Blanco P, Saenz de Santamaria J, Gonzalez Campillejo JM, Ruiz Tellez T. 1995. [Veno-occlusive liver disease due to intake of Senecio vulgaris tea]. Gastroenterol. Hepatol. 18: 413–416. [PubMed] [Google Scholar]
- Pavlica D, Samuel I. 1970. Primary carcinoma of the liver in Ethiopia. A study of 38 cases proved at post-mortem examination. Br. J. Cancer 24: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira TN, Webb RI, Reilly PE, Seawright AA, Prakash AS. 1998. Dehydromonocrotaline generates sequence-selective N-7 guanine alkylation and heat and alkali stable multiple fragment DNA crosslinks. Nucleic Acids Res. 26: 5441–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JE, Jago MV, Reddy JK, Jarrett RG. 1983. Neoplasia and chronic disease associated with the prolonged administration of dehydroheliotridine to rats. J. Natl Cancer Inst 70: 381–386. [PubMed] [Google Scholar]
- Petry TW, Sipes IG. 1987. Modulation of monocrotaline-induced hepatic genotoxicity in rats. Carcinogenesis 8: 415–419. [DOI] [PubMed] [Google Scholar]
- Petry TW, Bowden GT, Huxtable RJ, Sipes IG. 1984. Characterization of hepatic DNA damage induced in rats by the pyrrolizidine alkaloid monocrotaline. Cancer Res. 44: 1505–1509. [PubMed] [Google Scholar]
- Petry TW, Bowden GT, Buhler DR, Sipes IG. 1986. Genotoxicity of the pyrrolizidine alkaloid jacobine in rats. Toxicol. Lett 32: 275–281. [DOI] [PubMed] [Google Scholar]
- Prakash AS, Pereira TN, Reilly PE, Seawright AA. 1999. Pyrrolizidine alkaloids in human diet. Mutat. Res 443: 53–67. [DOI] [PubMed] [Google Scholar]
- Rao MS, Reddy JK. 1978. Malignant neoplasms in rats fed lasiocarpine. Br. J. Cancer 37: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Jago MV, Reddy JK. 1983. Effect of calorie restriction on the fate of hyperplastic liver nodules induced by concurrent administration of lasiocarpine and thioacetamide. Hum. Toxicol 2: 15–26. [DOI] [PubMed] [Google Scholar]
- Reed RL, Ahern KG, Pearson GD, Buhler DR. 1988. Crosslinking of DNA by dehydroretronecine, a metabolite of pyrrolizidine alkaloids. Carcinogenesis 9: 1355–1361. [DOI] [PubMed] [Google Scholar]
- Ribeiro LR, Silva AR, Bautista AR, Costa SL, Sales LA, Rios AC, Salvadori DM. 1993. Clastogenic effect of extracts obtained from Crotalaria retusa L. and Crotalaria mucronata Desv. on mouse bone marrow cells. Mutat. Res 300: 253–258. [DOI] [PubMed] [Google Scholar]
- Ridker PM, McDermott WV. 1989. Comfrey herb tea and hepatic venoocclusive disease. Lancet 1: 657–658. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Ohkuma S, McDermott WV, Trey C, Huxtable RJ. 1985. Hepatic venocclusive disease associated with the consumption of pyrrolizidine-containing dietary supplements. Gastroenterology 88: 1050–1054. [DOI] [PubMed] [Google Scholar]
- Rieben WK Jr, and Coulombe RA Jr., 2004. DNA cross-linking by dehydromonocrotaline lacks apparent base sequence preference. Toxicol. Sci 82: 497–503. [DOI] [PubMed] [Google Scholar]
- Robins DJ. 1984. Pyrrolizidine alkaloids. Nat. Prod. Rep 1: 235–243. [DOI] [PubMed] [Google Scholar]
- Roeder E 1995. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie 50: 83–98. [PubMed] [Google Scholar]
- Roeder E 2000. Medicinal plants in China containing pyrrolizidine alkaloids. Pharmazie 55: 711–726. [PubMed] [Google Scholar]
- Roitman JN. 1981. Comfrey and liver damage. Lancet 1: 944. [DOI] [PubMed] [Google Scholar]
- Rubiolo P, Pieters L, Calomme M, Bicchi C, Vlietinck A, Vanden Berghe D. 1992. Mutagenicity of pyrrolizidine alkaloids in the Salmonella typhimurium/mammalian microsome system. Mutat. Res 281: 143–147. [DOI] [PubMed] [Google Scholar]
- Sanderson BJ, Clark AM. 1993. Micronuclei in adult and foetal mice exposed in vivo to heliotrine, urethane, monocrotaline and benzidine. Mutat. Res 285: 27–33. [DOI] [PubMed] [Google Scholar]
- Santos-Mello R, Deimlimg LI, Lauer Junior CM, Almeida A. 2002. Induction of micronuclei by alkaloids extracted from Senecio brasiliensis and stored for 23 years. Mutat. Res 516: 23–28. [DOI] [PubMed] [Google Scholar]
- Schoental R 1968. Toxicology and carcinogenic action of pyrrolizidine alkaloids. Cancer Res. 28: 2237–2246. [PubMed] [Google Scholar]
- Schoental R 1975. Proceedings: pancreatic islet cell and other tumours induced in rats by heliotrine – a mono-ester pyrrolizidine alkaloid; the effects of additional treatment with nicotinamide. Br. J. Cancer 31: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoental R, Cavanagh JB. 1972. Brain and spinal cord tumors in rats treated with pyrrolizidine alkaloids. J. Natl Cancer Inst 49: 665–671. [PubMed] [Google Scholar]
- Schoental R, Coady A. 1968. The hepatotoxicity of some Ethiopian and East African plants, including some used in traditional medicines. East Afr. Med. J 45: 577–580. [PubMed] [Google Scholar]
- Schoental R, Head MA. 1957. Progression of liver lesions produced in rats by temporary treatment with pyrrolizidine (senecio) alkaloids, and the effects of betaine and high casein diet. Br. J. Cancer 11: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoental R, Head MA, Peacock PR. 1954. Senecio alkaloids; primary liver tumours in rats as a result of treatment with (1) a mixture of alkaloids from S. jacobaea Lin.; (2) retrorsine; (3) isatidine. Br. J. Cancer 8: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoental R, Fowler ME, Coady A. 1970. Islet cell tumors of the pancreas found in rats given pyrrolizidine alkaloids from Amsinckia intermedia Fisch and Mey and from Heliotropium supinum L. Cancer Res. 30: 2127–2131. [PubMed] [Google Scholar]
- Schoental R, Hard GC, Gibbard S. 1971. Histopathology of renal lipomatous tumors in rats treated with the ‘natural’ products, pyrrolizidine alkaloids and unsaturated aldehydes. J. Natl Cancer Inst 47: 1037–1044. [PubMed] [Google Scholar]
- Seaman JT. 1978. Pyrrolizidine alkaloid poisoning of horses. Aust. Vet. J 54: 150. [DOI] [PubMed] [Google Scholar]
- Seaman JT. 1987. Pyrrolizidine alkaloid poisoning of sheep in New South Wales. Aust. Vet. J 64: 164–167. [DOI] [PubMed] [Google Scholar]
- Sharrock AG. 1969. Pyrrolizidine alkaloid poisoning in a horse in New South Wales. Aust. Vet. J 45: 388. [DOI] [PubMed] [Google Scholar]
- Shumaker RC, Robertson KA, Hsu IC, Allen JR. 1976. Neoplastic transformation in tissues of rats exposed to monocrotaline or dehydroretronecine. J. Natl Cancer Inst 56: 787–790. [DOI] [PubMed] [Google Scholar]
- Silva-Neto JP, Barreto RA, Pitanga BP, Souza CS, Silva VD, Silva AR, Velozo ES, Cunha SD, Batatinha MJ, Tardy M, Ribeiro CS, Costa MF, El-Bacha RS, Costa SL. 2010. Genotoxicity and morphological changes induced by the alkaloid monocrotaline, extracted from Crotalaria retusa, in a model of glial cells. Toxicon 55: 105–117. [DOI] [PubMed] [Google Scholar]
- Sivlingham R, Brink NG. 1988. Somatic mutation induced by heliotrine in Drosophila. Teratog. Carcinog. Mutagen 8: 205–213. [DOI] [PubMed] [Google Scholar]
- Sperl W, Stuppner H, Gassner I, Judmaier W, Dietze O, Vogel W. 1995. Reversible hepatic veno-occlusive disease in an infant after consumption of pyrrolizidine-containing herbal tea. Eur. J. Pediatr 154: 112–116. [DOI] [PubMed] [Google Scholar]
- Steenkamp V, Stewart MJ, Zuckerman M. 2000. Clinical and analytical aspects of pyrrolizidine poisoning caused by South African traditional medicines. Ther. Drug Monit 22: 302–306. [DOI] [PubMed] [Google Scholar]
- Stegelmeier BL, Edgar JA, Colegate SM, Gardner DR, Schoch TK, Coulombe RA, Molyneux RJ. 1999. Pyrrolizidine alkaloid plants, metabolism and toxicity. J. Nat. Toxins 8: 95–116. [PubMed] [Google Scholar]
- Svoboda DJ, Reddy JK. 1972. Malignant tumors in rats given lasiocarpine. Cancer Res. 32: 908–913. [PubMed] [Google Scholar]
- Svoboda DJ, Reddy JK. 1974. Laslocarpine-induced, transplantable squamous cell carcinoma of rat skin. J. Natl Cancer Inst 53: 1415–1418. [DOI] [PubMed] [Google Scholar]
- Takanashi H, Umeda M, Hirono I. 1980. Chromosomal aberrations and mutation in cultured mammalian cells induced by pyrrolizidine alkaloids. Mutat. Res 78: 67–77. [DOI] [PubMed] [Google Scholar]
- Tandon BN, Tandon HD, Tandon RK, Narndranathan M, Joshi YK. 1976. An epidemic of veno-occlusive disease of liver in central India. Lancet 2: 271–272. [DOI] [PubMed] [Google Scholar]
- Tepe JJ, Williams RM. 1999. Reductive activation of a hydroxylamine hemiacetal derivative of dehydromonocrotaline: the fi rst reductively activated pyrrolizidine alkaloid capable of cross-linking DNA. Angew. Chem. Int. Ed. Engl 38: 3501–3503. [DOI] [PubMed] [Google Scholar]
- Uhl M, Helma C, Knasmuller S. 2000. Evaluation of the single cell gel electrophoresis assay with human hepatoma (Hep G2) cells. Mutat. Res 468: 213–225. [DOI] [PubMed] [Google Scholar]
- van der Watt JJ, Purchase IF, Tustin RC. 1972. The chronic toxicity of retrorsine, a pyrrolizidine alkaloid, in vervet monkeys. J. Pathol 107: 279–287. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Petry TW, Roth RA. 1993. Characterization of monocrotaline pyrrole-induced DNA cross-linking in pulmonary artery endothelium. Am. J. Physiol 264: L517–522. [DOI] [PubMed] [Google Scholar]
- Wang YP, Fu PP, Chou MW. 2005a. Metabolic activation of the tumorigenic pyrrolizidine alkaloid, retrorsine, leading to DNA adduct formation in vivo. Int. J. Environ. Res. Public Health 2: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YP, Yan J, Beger RD, Fu PP, Chou MW. 2005b. Metabolic activation of the tumorigenic pyrrolizidine alkaloid, monocrotaline, leading to DNA adduct formation in vivo. Cancer Lett. 226: 27–35. [DOI] [PubMed] [Google Scholar]
- Wang YP, Yan J, Fu PP, Chou MW. 2005c. Human liver microsomal reduction of pyrrolizidine alkaloid N-oxides to form the corresponding carcinogenic parent alkaloid. Toxicol. Lett 155: 411–420. [DOI] [PubMed] [Google Scholar]
- Wehner FC, Thiel PG, van Rensburg SJ. 1979. Mutagenicity of alkaloids in the Salmonella/microsome system. Mutat. Res 66: 187–190. [DOI] [PubMed] [Google Scholar]
- Weidner MF, Sigurdsson ST, Hopkins PB. 1990. Sequence preferences of DNA interstrand cross-linking agents: dG-to-dG cross-linking at 5’-CG by structurally simplifi ed analogues of mitomycin C. Biochemistry 29: 9225–9233. [DOI] [PubMed] [Google Scholar]
- Weston CF, Cooper BT, Davies JD, Levine DF. 1987. Veno-occlusive disease of the liver secondary to ingestion of comfrey. Br. Med. J. (Clin. Res. Edn) 295: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RD, Krumperman PH, Cheeke PR, Deinzer ML, Buhler DR. 1984. Mutagenic responses of tansy ragwort (Senecio jacobaea) plant, pyrrolizidine alkaloids and metabolites in goat milk with the Salmonella/ mammalian-microsome mutagenicity test. J. Anim. Sci 58: 1245–1254. [DOI] [PubMed] [Google Scholar]
- Williams AO. 1970. Ultrastructure of rat hepatocarcinoma due to retrorsine – a pyrrolizidine alkaloid. Afr. J. Med. Sci 1: 273–283. [PubMed] [Google Scholar]
- Williams AO, Edington GM, Obakponovwe PC. 1967. Hepatocellular carcinoma in infancy and childhood in Ibadan, Western Nigeria. Br. J. Cancer 21: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DE, Reed RL, Kedzierski B, Dannan GA, Guengerich FP, Buhler DR. 1989a. Bioactivation and detoxication of the pyrrolizidine alkaloid senecionine by cytochrome P-450 enzymes in rat liver. Drug Metab. Dispos 17: 387–392. [PubMed] [Google Scholar]
- Williams DE, Reed RL, Kedzierski B, Ziegler DM, Buhler DR. 1989b. The role of flavin-containing monooxygenase in the N-oxidation of the pyrrolizidine alkaloid senecionine. Drug Metab. Dispos 17: 380–386. [PubMed] [Google Scholar]
- Wiltjer JC, Walker CE. 1974. Letter: rectal prolapses in cattle associated with pyrrolizidine alkaloid poisoning. Aust. Vet. J 50: 579–580. [DOI] [PubMed] [Google Scholar]
- Xia Q, Chou MW, Kadlubar FF, Chan PC, Fu PP. 2003. Human liver microsomal metabolism and DNA adduct formation of the tumorigenic pyrrolizidine alkaloid, riddelliine. Chem. Res. Toxicol 16: 66–73. [DOI] [PubMed] [Google Scholar]
- Xia Q, Chou MW, Lin G, Fu PP. 2004. Metabolic formation of DHP-derived DNA adducts from a representative otonecine type pyrrolizidine alkaloid clivorine and the extract of Ligularia hodgsonnii hook. Chem. Res. Toxicol 17: 702–708. [DOI] [PubMed] [Google Scholar]
- Xia Q, Chou MW, Edgar JA, Doerge DR, Fu PP. 2006. Formation of DHPderived DNA adducts from metabolic activation of the prototype heliotridine-type pyrrolizidine alkaloid, lasiocarpine. Cancer Lett. 231: 138–145. [DOI] [PubMed] [Google Scholar]
- Xia Q, Yan J, Chou MW, Fu PP. 2008. Formation of DHP-derived DNA adducts from metabolic activation of the prototype heliotridine-type pyrrolizidine alkaloid, heliotrine. Toxicol. Lett 178: 77–82. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Nagao M, Sugimura T, Furuya T, Shirai A, Matsushima T. 1979. Mutagenicity of pyrrolizidine alkaloids in the Salmonella/mammalian-microsome test. Mutat. Res 68: 211–216. [DOI] [PubMed] [Google Scholar]
- Yan J, Xia Q, Chou MW, Fu PP. 2008. Metabolic activation of retronecine and retronecine N-oxide – formation of DHP-derived DNA adducts. Toxicol. Ind. Health 24: 181–188. [DOI] [PubMed] [Google Scholar]
- Yang YC, Yan J, Doerge DR, Chan PC, Fu PP, Chou MW. 2001. Metabolic activation of the tumorigenic pyrrolizidine alkaloid, riddelliine, leading to DNA adduct formation in vivo. Chem. Res. Toxicol 14: 101–109. [DOI] [PubMed] [Google Scholar]
- Yeong ML, Swinburn B, Kennedy M, Nicholson G. 1990. Hepatic venoocclusive disease associated with comfrey ingestion. J. Gastroenterol. Hepatol 5: 211–214. [DOI] [PubMed] [Google Scholar]
- Zhao XL, Chan MY, Ogle CW. 1989. The identification of pyrrolizidine alkaloid-containing plants – a study on, 20 herbs of the Compositae family. Am. J. Chin. Med 17, 71–78. [DOI] [PubMed] [Google Scholar]